Abstract
Carbapenem-resistant gram-negative pathogens remain an urgent public health threat, and safe, effective treatment options are limited. Although several agents are now available to combat these infections, meropenem-vaborbactam was the first to combine a novel, cyclic, boronic acid-based, β-lactamase inhibitor with a carbapenem backbone. Vaborbactam emanated from a discovery program specifically designed to identify candidate β-lactamase inhibitors with biochemical, microbiologic, and pharmacologic properties optimized for use in conjunction with a carbapenem. Meropenem was selected as the ideal carbapenem given its broad-spectrum in vitro activity, well established safety profile, and proven efficacy in the treatment of serious gram-negative infections. The combination has demonstrated potent in vitro activity against resistant gram-negative pathogens, particularly KPC-producing Klebsiella pneumoniae (MIC50 values typically ≤ 0.06 mg/l). Importantly, the pharmacokinetic (PK) profiles of the two agents are well matched, and the approved optimized dosing regimen of 4 g every 8 h (Q8h) as a 3-h infusion provides reliable probability of target attainment against the majority of commonly encountered carbapenem-resistant Enterobacteriaceae (CRE). Robust in vitro and in vivo PK/pharmacodynamic (PD) data support the ability of this dosing regimen to achieve specified PK/PD targets for both bactericidal activity and prevention of resistance among pathogens with MICs up to 8 mg/l. This concerted effort into optimizing the PK and PD parameters of both the β-lactam and β-lactamase inhibitor alone and in combination contributed to the clinical success of meropenem-vaborbactam demonstrated in phase 3 trials in patients with complicated urinary tract infections (cUTI), including acute pyelonephritis (AP), and serious CRE infections. As the use of meropenem-vaborbactam increases concomitantly with the prevalence of KPC-producing CRE, continued pharmacovigilance and antimicrobial stewardship efforts will be of upmost importance to ensure that these PK/PD efforts translate into improved patient outcomes.
Keywords: Carbapenem-resistant enterobacteriaceae, Meropenem, Pharmacodynamics, Pharmacokinetics, Vaborbactam
Plain Language Summary
Carbapenem-resistant gram-negative pathogens, specifically, Enterobacteriaceae, remain an urgent public health threat, and safe, effective treatment options are limited. The antibiotic agents meropenem and vaborbactam were selected to be combined to leverage their individual properties for efficacy against carbapenem-resistant gram-negative pathogens, the most prevalent being Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae.
Infections due to carbapenem-resistant Enterobacteriaceae (CRE) are associated with high morbidity and mortality and excess healthcare costs and have traditionally required treatment with low-efficacy, high-toxicity antimicrobials such as the polymyxins.
The authors present a review of the pre-clinical, clinical, pharmacokinetic (PK), and pharmacodynamic (PD) data on meropenem-vaborbactam, and data on difficult to treat organisms, and on special patient populations obtained post-marketing.
Pre-clinical in vitro and in vivo PK/PD data support this optimized combination of these agents with meropenem-vaborbactam demonstrating low MIC50/MIC90 values against KPC-producing Enterobacteriaceae. Phase 1 PK trials confirmed the PK parameters, including in subjects with renal impairment and in target extravascular body sites such as the pulmonary epithelial lining fluid. In vitro, the combination of meropenem-vaborbactam has shown potent activity against resistant gram-negative pathogens; importantly, this includes KPC-producing Klebsiella pneumoniae. The approved optimized dosing regimen [4 g every 8 h (Q8h) as a 3-h infusion] achieves the PK/PD targets to achieve both bactericidal activity and prevention of resistance among pathogens with MICs up to 8 mg/l. Phase 3 trials showed the clinical success of meropenem-vaborbactam in patients with complicated urinary tract infections (cUTI), including acute pyelonephritis (AP), and serious CRE infections.
Key Summary Points
| Meropenem-vaborbactam emerged from a rationale drug development program designed to combine the first cyclic, boronic acid-based β-lactamase inhibitor with a carbapenem backbone to leverage their individual properties for efficacy against carbapenem-resistant Enterobacterales. |
| The combination of meropenem and vaborbactam has shown potent in vitro activity against KPC-producing Klebsiella pneumoniae and comparable pharmacokinetic properties between the two components, even across varying degrees of renal dysfunction. |
| Robust pre-clinical and clinical data demonstrate the ability of the approved optimized dosing regimen of 4 g every 8 h as a 3-h infusion to achieve applicable PK/PD targets for bactericidal activity and the prevention of resistance against pathogens with MICs up to 8 mg/l. |
| Phase 3 trials established the clinical success of meropenem-vaborbactam in patients with complicated urinary tract infections (cUTI), including acute pyelonephritis (AP), and a small cohort with serious CRE infections. |
| Further post-marketing studies investigating the PK/PD properties and efficacy of meropenem-vaborbactam in additional special patient populations, extravascular body sites beyond urine and the intrapulmonary space, and emerging mechanisms of resistance are warranted to fully establish its place in therapy. |
Digital Features
This article is published with digital features to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.12937460.
Introduction
The now widespread dissemination and continued increase in prevalence of carbapenem-resistant gram-negative pathogens pose enormous public health concerns both nationally and internationally [1–3]. The most prominent of these carbapenem-resistant organisms are the Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae, which comprise > 90% of carbapenem-resistant Enterobacteriaceae (CRE) in the US [4]. Infections due to CRE are associated with high morbidity, mortality, and excess healthcare costs and have traditionally required treatment with low-efficacy, high-toxicity antimicrobials such as the polymyxins [5]. Fortunately, several novel β-lactam/β-lactamase inhibitor (BL/BLI) agents with potent activity against class A serine carbapenemases, including the prominent KPC enzyme, are now available and have demonstrated improved efficacy and safety compared to traditional treatment regimens [6–8].
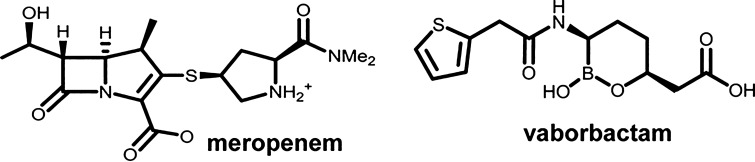
Meropenem-vaborbactam was the first of these BL/BLI agents to employ a carbapenem backbone in conjunction with a novel cyclic boronic acid-based inhibitor (Fig. 1). Vaborbactam was the first boronic acid BLI developed and was specifically designed to inhibit the KPC enzyme, but also possess activity against other class A and C β-lactamases [9]. Conversely, vaborbactam has no appreciable activity against Ambler class B metallo-β-lactamases or class D OXA carbapenemases [9, 10]. It acts as a competitive inhibitor of the KPC enzyme via formation of a reversible covalent bond, although the mean residence time in the active site is > 16 h, making it functionally irreversible and therefore providing potent, long-lasting inhibition [10]. Additionally, vaborbactam is not affected by the KPC variant, mutations in the KPC enzyme, or efflux mechanisms [10]. Given its engineered specificity for the KPC enzyme, vaborbactam was intended to be paired with a carbapenem agent stable to hydrolysis by other class A serine extended-spectrum β-lactamase (ESBL) enzymes (e.g., CTX-M, TEM, SHV) [11]. Meropenem was chosen from the carbapenems given its broad-spectrum in vitro activity, well-established safety profile, activity against Pseudomonas spp., and efficacy in the treatment of serious gram-negative infections [12]. Furthermore, initial pre-clinical and phase I pharmacokinetic (PK) studies demonstrated highly comparable PK profiles between meropenem and vaborbactam. Together, vaborbactam at 8 mg/l has been shown to reduce the MIC of meropenem up to 64-fold against KPC-producing K. pneumoniae, with the combination displaying very low MIC50 and MIC90 values of ≤ 0.06 and 1 mg/l, respectively [13]. These low MICs, coupled with the optimized PK stemming from the registered high-dose, extended infusion dosing scheme, provide > 90% simulated probability of PK/PD target attainment for treating infections and preventing resistance against pathogens with MICs up to 4–8 mg/l [14]. Accordingly, the clinical MIC breakpoints for meropenem-vaborbactam against Enterobacterales set by CLSI and recognized by the FDA are ≤ 4 mg/l, 8 mg/l, and ≥ 16 mg/l for susceptible, intermediate, and resistant [15]. No interpretive criteria are provided for other pathogens such as non-fermenting gram negatives including Pseudomonas aeruginosa and Acinetobacter baumannii, given the lack of activity of vaborbactam over meropenem alone against these organisms. This projected efficacy of meropenem-vaborbactam against susceptible pathogens has been confirmed in numerous in vitro and mammalian PK/PD studies and in patients with cUTI and serious KPC-producing CRE infections.
Fig. 1.
Chemical structures of meropenem and the novel cyclic boronic acid-based β-lactamase inhibitor vaborbactam. Me2, dimethyl. Reprinted with permission from [10]
Vaborbactam emerged from a development program designed to discover candidate BLIs with biochemical, microbiologic, and pharmacologic properties optimized for use in conjunction with a carbapenem. This rationale design process led to the approval of a potent, dose-optimized, effective treatment agent for infections due to resistant gram-negative pathogens, particularly KPC-producing K. pneumoniae. The pre-clinical and clinical PK and PD data that supported the approval of this novel compound will be reviewed herein along with relevant post-marketing data against difficult-to-treat organisms and in special patient populations. This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors. To gather relevant publications written in the English language, a literature search was performed using PubMed, clinicaltrials.gov, and Google Scholar electronic databases for articles published from 2013 to 2019. Search terms included the following: meropenem, vaborbactam, meropenem-vaborbactam, and RPX7009. Information was also gathered from referenced papers and abstracts from the Interscience Conference on Antimicrobial Agents and Chemotherapy, the European Congress of Clinical Microbiology and Infectious Diseases, IDWeek, and the American Society for Microbiology Microbe meetings.
Pharmacokinetics
A key consideration in the development of BL/BLI agents is the selection of a β-lactam that has a comparable PK profile to the inhibitor. Importantly, the BLI needs to have a half-life that is as long as or longer than the BL ensure adequate BLI inhibition and to prevent hydrolysis of unprotected meropenem over the course of the entire dosing interval. Vaborbactam was initially designed to be combined with biapenem [16, 17], but was aborted in favor of meropenem, in part because of the improved PK correlation between meropenem and vaborbactam. Pre-clinical and phase 1 PK studies support the approved high-dose, extended-infusion dosing regimen and confirm the PK comparability between vaborbactam and meropenem, including in subjects with renal impairment and in target body sites, such as the intrapulmonary epithelial lining fluid (ELF).
Initial pre-clinical, multiple-dose PK studies of vaborbactam in rats demonstrated linear PK and dose proportionality [9]. Moreover, the concentration-time profiles of vaborbactam generated in these animal studies closely mimicked those typical of BL antibiotics, displaying a high Cmax and AUC combined with a low Vd and short half-life, confirming the ability to combine it with a BL agent. Toxicology studies in dogs did not reveal any dose-limiting toxicities in multiple doses up to the human equivalent of 10 g/day, allowing first-in-human phase I studies to proceed.
In the first-in-human, single-center phase 1 study conducted in healthy Australian volunteers, vaborbactam was administered to 80 subjects in a large single- and multiple-ascending dose study [18]. Six subjects were randomized to receive doses of vaborbactam ranging from 250 to 2000 mg or placebo administered as a 3-h infusion across ten cohorts. The first six cohorts of subjects received single doses of vaborbactam ranging from 250 to 1500 mg, while the remaining four cohorts received doses ranging from 250 to 2000 mg Q8h for 7 days. Serial blood samples were obtained from 0 to 24 h post-dose, and urine was collected in intervals up to 48 h post-dose. Additionally, serum protein binding was determined via ultrafiltration in spiked serum samples at concentrations of vaborbactam ranging from 1 to 50 mg/l. The observed PK parameters after single or multiple ascending doses of vaborbactam are displayed in Fig. 2. The exposure of vaborbactam based on Cmax and AUC increased proportionally to dose after both single and repeated doses (R2 = 0.91 for the relationship of dose to AUC), demonstrating linear PK through the proposed dosing range. Additionally, the PK parameters and renal clearance of vaborbactam did not change following repeated dosing over time suggesting a lack of accumulation in plasma at an every 8 h administration schedule. Protein binding was approximately 33%, regardless of concentration.
Fig. 2.

Mean (± SD) vaborbactam plasma concentration-versus-time profiles following 3-h intravenous infusions of 250–2000 mg in healthy volunteer subjects. Reprinted with permission from [18]
In a follow-up, single-center phase 1 study also in 80 healthy Australian volunteer subjects, vaborbactam was administered alone and in combination with meropenem in single and multiple doses as a 3-h infusion across five dosing cohorts [29]. Subjects received single doses on days 1, 2, and 7 and multiple doses on days 8–14. Meropenem was administered as 1 g and 2 g doses in combination with vaborbactam at doses of 250 mg, 1 g, 1.5 g, and 2 g. Serial blood and urine samples were collected in the same fashion as in the previous study. When administered together, both meropenem and vaborbactam demonstrated linear PK and dose-proportional increases in AUC across the dosing range (Table 1). Importantly, this study demonstrated that the geometric mean half-life of meropenem and vaborbactam was 0.9–1.3 h and 1.1–1.9 h, respectively, confirming the comparability of their PK profiles in humans. Furthermore, plasma clearance and AUC were similar when administered alone, or in combination, establishing the lack of any drug-drug interactions between the components. Both agents alone and in combination were well tolerated and demonstrated virtually superimposable PK profiles at doses up to 2 g Q8h.
Table 1.
Pharmacokinetic parameters of meropenem and vaborbactam following single or multiple doses in healthy volunteer subjects
| Cohort/dose and treatment | n | Cmax (μg/ml) | t1/2 (h) | AUC0–∞ (μg h/ml), single dose | AUC0–t (μg h/ml), multiple dose | Vss (liters) | CLT (liter/h) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Single dose | Multiple dose | Single dose | Multiple dose | Single dose | Multiple dose | Single dose | Multiple dose | ||||
| Meropenem PK | |||||||||||
| 1/1000:250 | |||||||||||
| Meropenem alone | 32 | 16.9 (44.7) | 16.5 (58.0) | 1.0 (42.8) | 1.0 (55.5) | 51.6 (43.3) | 49.3 (55.7) | 24.9 (52.7) | 25.7 (77.3) | 19.4 (43.3) | 20.3 (55.6) |
| Combination | 16 | 18.6 (32.4) | 15.7 (34.5) | 1.0 (28.8) | 1.0 (33.4) | 56.1 (30.5) | 47.1 (32.5) | 25.2 (49.5) | 29.4 (62.8) | 17.8 (30.5) | 21.2 (32.8) |
| 2/1000:1000 | |||||||||||
| Meropenem alone | 13 | 18.6 (47.4) | 16.4 (38.4) | 1.0 (35.3) | 0.9 (20.3) | 58.1 (47.7) | 49.9 (39.3) | 23.2 (49.1) | 23.2 (34.4) | 17.2 (47.7) | 20.1 (39.2) |
| Combination | 10 | 19.9 (46.6) | 17.0 (32.1) | 1.1 (45.2) | 0.9 (24.0) | 64.1 (51.0) | 53.9 (36.5) | 20.8 (50.7) | 23.1 (44.2) | 15.6 (51.0) | 18.5 (36.2) |
| 3/1000:1500 | |||||||||||
| Meropenem alone | 19 | 20.9 (34.8) | 23.3 (59.1) | 0.89 (31.5) | 0.87 (33.2) | 64.6 (38.2) | 67. (55.9) | 18.9 (40.1) | 17.2 (51.5) | 15.5 (38.2) | 14.9 (55.9) |
| Combination | 14 | 20.9 (34.8) | 23.3 (59.1) | 0.89 (31.5) | 0.87 (33.2) | 64.6 (38.2) | 67. (55.9) | 21.3 (47.8) | 18.5 (52.7) | 15.6 (48.4) | 15.5 (48.0) |
| 4/1000:2000 | |||||||||||
| Meropenem alone | 19 | 17.3 (37.4) | 15.9 (43.9) | 1.0 (47.5) | 1.0 (52.6) | 53.7 (43.0) | 50.7 (32.7) | 25.0 (47.0) | 25.2 (42.4) | 18.6 (43.0) | 19.7 (32.7) |
| Combination | 14 | 17.5 (23.7) | 15.8 (29.2) | 0.9 (45.5) | 1.1 (39.8) | 55.0 (27.0) | 47.9 (20.7) | 23.8 (29.7) | 25.5 (31.8) | 18.2 (27.0) | 20.9 (20.7) |
| 5/2000:2000 | |||||||||||
| Meropenem alone | 20 | 40.9 (53.6) | 46.0 (59.6) | 1.1 (54.90 | 1.0 (46.0) | 125.7 (48.9) | 133.7 (56.4) | 22.2 (52.8) | 20.2 (47.9) | 15.9 (48.9) | 15.0 (56.4) |
| Combination | 16 | 45.7 (35.7) | 42.5 (48.5) | 1.3 (82.6) | 1.2 (56.5) | 139.3 (45.8) | 135.7 (46.8) | 21.7 (38.2) | 21.0 (42.5) | 14.4 (45.8) | 14.9 (45.8) |
| Vaborbactam PK | |||||||||||
| 1/1000:250 | |||||||||||
| Vaborbactam alone | 40 | 5.2 (41.8) | 4.9 (37.0) | 1.1 (57.2) | 1.1 (50.3) | 17.2 (42.7) | 16.2 (46.8) | 22.2 (48.6) | 23.2 (46.0) | 14.5 (42.7) | 15.3 (45.9) |
| Combination | 16 | 5.3 (40.2) | 4.6 (39.5) | 1.1 (52.0) | 1.2 (38.6) | 17.0 (38.3) | 14.6 (39.8) | 24.3 (49.8) | 27.0 (66.1) | 14.7 (38.3) | 16.9 (39.4) |
| 2/1000:1000 | |||||||||||
| Vaborbactam alone | 5 | 21.7 (42.3) | 1.5 (69.6) | 75.4 (48.0) | 21.9 (59.0) | 13.3 (48.0) | |||||
| Combination | 10 | 23.3 (46.1) | 19.9 (29.7) | 1.5 (49.8) | 1.3 (61.8) | 79.4 (46.2) | 68.4 (39.0) | 20.2 (41.8) | 22.5 (29.3) | 12.6 (46.2) | 14.8 (37.4) |
| 3/1000:1500 | |||||||||||
| Vaborbactam alone | 7 | 36.3 (41.0) | 1.2 (49.3) | 117.8 (36.6) | 21.1 (68.4) | 12.7 (36.6) | |||||
| Combination | 14 | 36.9 (37.0) | 32.6 (32.5) | 1.3 (44.7) | 1.4 (61.2) | 124.2 (40.4) | 114.7 (38.4) | 19.9 (38.2) | 19.4 (36.7) | 12.1 (40.4) | 13.3 (37.3) |
| 4/1000:2000 | |||||||||||
| Vaborbactam alone | 7 | 38.0 (28.8) | 1.3 (54.8) | 125.8 (35.1) | 22.8 (28.3) | 15.9 (35.0) | |||||
| Combination | 14 | 40.0 (22.7) | 34.7 (34.6) | 1.4 (46.7) | 1.5 (56.3) | 132.8 (29.2) | 113.2 (29.8) | 23.8 (26.3) | 26.8 (35.0) | 15.1 (29.2) | 17.9 (28.7) |
| 5/2000:2000 | |||||||||||
| Vaborbactam alone | 8 | 49.5 (57.4) | 1.4 (37.7) | 151.9 (54.5) | 21.8 (43.5) | 13.2 (54.5) | |||||
| Combination | 16 | 50.1 (42.6) | 54.7 (47.1) | 1.9 (61.9) | 1.6 (50.5) | 165.3 (45.2) | 192.6 (45.0) | 22.0 (41.0) | 19.3 (36.8) | 12.1 (45.2) | 10.7 (43.1) |
AUC0–t area under the plasma concentration-time curve from time zero to the last measurable concentration, AUCinf area under the plasma concentration-time curve from time zero to infinity, CLT total clearance, Cmax maximum drug concentration in serum, fe fraction of administered dose excreted over the urine collection interval, expressed as a percent, t1/2 terminal half-life over the sampling period, Vss steady-state volume of distribution
Reprinted with permission from [19]
aDoses are expressed as meropenem (mg):vaborbactam (mg)
bValues shown are geometric means (geometric coefficient of variation)
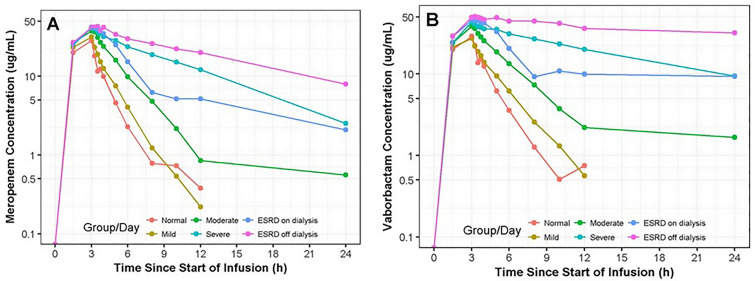
Given the predominant renal elimination of both meropenem and vaborbactam, the PK and tolerability of the combination was studied in a phase 1, multicenter (DaVita Clinical Research, USA) trial of 41 subjects with chronic renal impairment including those with end stage renal disease (ESRD) on hemodialysis (HD) [20]. In this study, subjects received only a single fixed dose of 1 g meropenem plus 1 g vaborbactam as a 3-h infusion, except for the ESRD subjects on HD, who received a dose both on and off dialysis. Subjects were enrolled into five study cohorts based on renal function as assessed by estimated glomerular filtration rate (eGFR) according to the modification of diet in renal disease (MDRD) equation: groups 1, 2, and 3 had mild (eGFR 60–89 ml/min/1.73 m2; n = 8), moderate (eGFR 30–59 ml/min/1.73 m2; n = 8), and severe renal impairment (eGFR < 30 ml/min/1.73 m2; n = 8), respectively, while group 4 consisted of subjects with normal renal function (creatinine clearance ≥ 90 ml/min; n = 8) matched to groups 1, 2, and 3 on age (± 10 years), sex, and body mass index (± 20%). Group 5 were subjects receiving HD (n = 9) and were not matched to the other cohorts. These subjects received a dose of study drug prior to and after a 5-h dialysis session on separate occasions. Serial blood and urine samples were collected to 24 and 48 h post-dose, respectively, along with hourly dialysate samples during the 5-h HD session for subjects in group 5. As expected, overall exposure of both meropenem and vaborbactam increased as renal function declined (Fig. 3). The average AUC0-∞ for meropenem increased approximately 4.5-fold from 87.1 mg/l in subjects with normal renal function to 397 mg/l in subjects with several renal impairment, whereas for vaborbactam, the exposure increased almost 8-fold from 99.4 to 781 mg/l across the same renal groups (Table 2) [20]. Although equally proportional changes in exposure across renal impairment groups would be ideal, it is crucial for efficacy that vaborbactam is cleared more slowly than meropenem during renal impairment and not vice versa, which could potentially leave meropenem vulnerable to KPC hydrolysis. The non-proportional changes in exposure are likely due to the negligible, non-renal clearance of vaborbactam, making it more dependent on renal clearance for elimination than meropenem. This trend was also observed in patients on HD where the average AUC0–∞ of vaborbactam was almost fivefold higher off HD vs. on compared to meropenem that was 2.2-fold higher. HD effectively removed both components, with 38% and 53% of the dose of meropenem and vaborbactam, respectively, recovered in dialysate. These data support a proportional dose reduction, and therefore a fixed-dose combination, of meropenem-vaborbactam with the understanding that vaborbactam will accumulate to a greater extent than meropenem after repeated doses. Importantly, there was no evidence of increasing adverse events in frequency or severity correlative to a decline in renal function attributed to either agent. These three phase 1 studies helped to establish the foundation for the dosing recommendations for patients with normal and impaired renal function that have subsequently demonstrated success in two phase 3 clinical trials [21] and led to FDA approval for the treatment of cUTI and AP in adult patients [22]. It is important to note that meropenem-vaborbactam was one of the first antimicrobials approved with recommendations for the use of the Modification of Diet in Renal Disease (MDRD) Study equation for the estimation of renal function and corresponding dosage adjustments, in lieu of the more commonly utilized Cockcroft-Gault equation [23, 24]. As these equations are not uniformly interchangeable, it is important for clinicians to understand which equation is recommended in the product labeling and the patient populations in which these equations may produce the most discrepant results [25].
Fig. 3.
Median meropenem (a) and vaborbactam (b) plasma concentration-vs.-time profiles following 3-h intravenous infusion of 1 g meropenem/1 g vaborbactam in subjects with normal renal function and chronic renal impairment. Reprinted with permission from [20]
Table 2.
Plasma pharmacokinetic parameters (mean ± SD) of meropenem and vaborbactam after a single 1 g meropenem/1 g vaborbactam dose stratified by renal function group
| Drug and renal study group | Cmax (μg/ml) | AUC0–∞ (μg h/ml) | AUC0–t (μg h/ml) | t1/2 (h) | CLNR (l/h) | CLR (l/h) | CLT (l/h) | Vss (l) |
|---|---|---|---|---|---|---|---|---|
| Meropenem | ||||||||
| Normal (n = 8) | 27.5 ± 7.03 | 87.1 ± 26.7 | 86.4 ± 26.6 | 1.30 ± 0.254 | 4.79 ± 2.18 | 7.69 ± 2.33 | 12.5 ± 3.83 | 19.2 ± 2.69 |
| Mild (n = 8) | 32.7 ± 9.1 | 112 ± 40.6 | 111 ± 40.1 | 1.43 ± 0.179 | 4.11 ± 1.30 | 5.55 ± 2.06 | 9.66 ± 2.42 | 16.5 ± 3.22 |
| Moderate (n = 8) | 40.5 ± 6.27 | 181 ± 59.4 | 177 ± 57.2 | 2.19 ± 0.867 | 2.71 ± 0.811 | 3.34 ± 1.19 | 6.05 ± 1.83 | 16.6 ± 2.21 |
| Severe (n = 8) | 45.5 ± 13.0 | 397 ± 98.0 | 362 ± 83.3 | 6.10 ± 2.59 | 1.69 ± 0.376 | 0.964 ± 0.375 | 2.65 ± 0.606 | 21.6 ± 6.69 |
| ESRD on dialysis (n = 9) | 44.7 ± 8.40 | 280 ± 58.7 | 274 ± 56.8 | 9.33 ± 1.94 | NA | NA | 3.72 ± 0.764 | 30.0 ± 7.18 |
| ESRD off dialysis (n = 8) | 47.7 ± 11.7 | 629 ± 206 | 606 ± 196 | 9.45 ± 1.75 | NA | NA | 1.73 ± 0.512 | 22.2 ± 6.33 |
| Vaborbactam | ||||||||
| Normal (n = 8) | 27.8 ± 6.34 | 99.4 ± 33.0 | 98.0 ± 32.2 | 1.65 ± 0.352 | 0.554 ± 1.69 | 10.5 ± 2.77 | 11.1 ± 3.60 | 20.5 ± 3.53 |
| Mild (n = 8) | 30.1 ± 8.31 | 116 ± 35.9 | 114 ± 34.5 | 1.88 ± 0.274 | 1.67 ± 1.52 | 7.51 ± 1.99 | 9.17 ± 1.98 | 20.8 ± 3.73 |
| Moderate (n = 8) | 42.4 ± 7.45 | 238 ± 108 | 229 ± 99.9 | 3.45 ± 1.71 | 0.642 ± 0.402 | 4.25 ± 1.66 | 4.89 ± 1.78 | 18.8 ± 3.02 |
| Severe (n = 8) | 46.4 ± 12.3 | 781 ± 279 | 532 ± 110 | 13.5 ± 8.71 | 0.288 ± 0.271 | 1.13 ± 0.505 | 1.42 ± 0.465 | 23.3 ± 7.24 |
| ESRD on dialysis (n = 9) | 50.1 ± 10.2 | 1100 ± 539 | 533 ± 124 | 55.2 ± 33.6 | NA | NA | 1.22 ± 0.799 | 59.1 ± 16.8 |
| ESRD off dialysis (n = 8) | 56.5 ± 13.9 | 5220 ± 4620 | 1640 ± 567 | 79.3 ± 76.2 | NA | NA | 0.405 ± 0.329 | 22.2 ± 5.47 |
Cmax maximum observed plasma concentration, AUC0–∞ area under the plasma concentration-time curve from time zero to infinity, AUC0–t area under the plasma concentration-time curve from time zero to the last quantifiable concentration in plasma, t1/2 elimination half-life, CLNR nonrenal clearance, CLR renal clearance, CLT total plasma clearance, Vss steady-state volume of distribution, ESRD end-stage renal disease. NA not available
Reprinted with permission from [20]
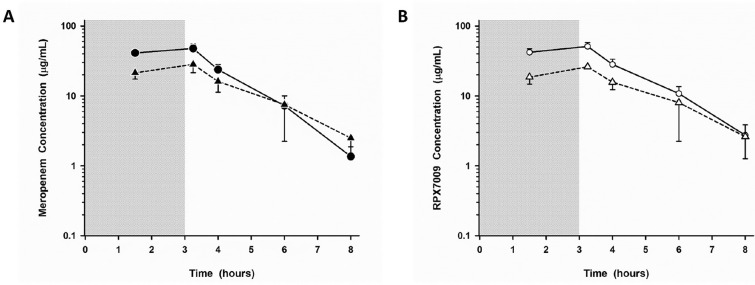
As KPC-producing K. pneumoniae frequently infects the lungs [26] and the phase 3 development plan for meropenem-vaborbactam initially included a trial in patients with hospital-acquired (HABP) and ventilator-associated bacterial pneumonia (VABP) (NCT03006679), it was critical to ensure that adequate concentrations of each agent reached the intrapulmonary space after the proposed clinical trial dose of 4 g (2 g meropenem plus 2 g vaborbactam) every 8 h as a 3-h infusion. As such, the plasma and intrapulmonary PK of meropenem and vaborbactam were evaluated in a single-center phase 1 study of 25 healthy US volunteers after receiving 2 g of meropenem and 2 g of vaborbactam IV Q8h by a 3-h infusion for three doses [27]. Serial plasma sampling was performed to 8 h post-dose, and a single bronchoscopy and bronchoalveolar lavage (BAL) were performed at 1.5, 3.25, 4, 6, or 8 h after the start of the last infusion. Plasma concentrations of meropenem and vaborbactam were comparable to those observed in the aforementioned phase 1 studies. The concentration-time profiles of meropenem and vaborbactam in the epithelial lining fluid (ELF) were nearly identical (Fig. 4), equating to mean AUC % penetration ratios from plasma:ELF of 63% and 65% for meropenem and vaborbactam, respectively. When only unbound plasma concentrations were considered, penetration was 65% and 79% for meropenem and vaborbactam, respectively. Importantly, the ELF concentrations of meropenem-vaborbactam remained consistently several fold higher than the MIC90 of KPC-producing K. pneumoniae. Meropenem concentrations were consistently unmeasurable in alveolar macrophages while vaborbactam concentrations ranged from 1.26 to 93.9 mg/l. Taken together, these data supported the progression of meropenem-vaborbactam into phase 3 clinical trials of patients with serious CRE infections, including HABP/VABP.
Fig. 4.
Mean (± SD) plasma (filled and open circles; solid line) and epithelial lining fluid (ELF) (filled and open triangles; dashed lines) concentration-versus-time profiles of meropenem (a) and vaborbactam (b) at 1.5, 3.25, 4, 6, and 8 h after the third dose of meropenem 2 g/vaborbactam 2 g administered as a 3-h intravenous infusion. The shaded region represents the 3-h infusion period. The y axis is in the log scale. Reprinted with permission from [27]
Finally, many patients infected with CRE are critically ill and experience acute kidney injury, necessitating continuous renal replacement therapy (CRRT) [26], which can further exacerbate the already deranged antimicrobial PK profile of these patients. Consequently, it is important to understand the extent to which antimicrobials are removed by the CRRT circuit to optimize dosing for these patients. Sime et al. evaluated the effect of different CRRT settings and filter adsorptions during in vitro simulated continuous venovenous hemofiltration (CVVH) on the PK of meropenem and vaborbactam [28]. The model utilized a Prismaflex CRRT system and a human blood-crystalloid mixture spiked with meropenem and vaborbactam. Serial blood and effluent samples were obtained from 30 to 180 min after the drug was added to the system. Two types of hemofilters were utilized (AN69 ST hollow fiber acrylonitrile and sodium methallyl sulfonate copolymer filter), and the point of dilution of replacement fluid was varied (pre- or post-filter) along with the flow rate (1, 2, and 4 l/h) to assess the impact on drug removal. Neither meropenem (7–10%) nor vaborbactam (2%) was significantly adsorbed to either filter tested. Sieving coefficients were consistently higher for meropenem than vaborbactam (> 1 vs. ~ 0.8), corresponding primarily to the differences in protein binding between the compounds (33% vs. 2%). Clearance was also consistently higher for meropenem than for vaborbactam, similar to that observed in HD subjects, and was significantly affected by the effluent flow rate such that doses may be scaled accordingly, e.g., 1 g (0.5 g meropenem/0.5 g vaborbactam) Q8h for low effluent flow rates (1–2 l/h) and 2 g (1 g meropenem/1 g vaborbactam) Q8h for high effluent flow rates (3–4 l/h). These findings were supported by a clinical report detailing the PK of meropenem-vaborbactam in a patient receiving continuous venovenous hemodialysis (CVVHD), which demonstrated that a dose of 2 g (1 g meropenem/1 g vaborbactam) Q8h over 3 h achieved minimum plasma concentrations at 8 h of 15.8 mg/l and 31.8 mg/l for meropenem and vaborbactam, respectively, at an effluent flow rate of 3 l/h [29].
Pharmacodynamics
As discussed, vaborbactam was purposefully designed to be combined with a carbapenem to provide potent, in vitro activity against KPC-producing CRE and the PK was optimized to be almost identical to that of meropenem when administered as a high-dose, extended infusion regimen. The specificity of vaborbactam and long residence time in the active site of the KPC enzyme, combined with an agent already stable to hydrolysis by most serine β-lactamases, give meropenem-vaborbactam potent activity against KPC-producing Enterobacteriaceae with typical MIC50 values ≤ 0.06 mg/l. This robust in vitro activity combined with the almost superimposable PK profiles and optimized dosing of 2 g Q8h as a 3-h infusion provides meropenem-vaborbactam with potent bactericidal activity and a high threshold for resistance development. The pre-clinical in vitro and in vivo pharmacodynamic (PD) data support this optimized combination and have advanced our understanding of the mechanism of action and PK/PD index of efficacy for β-lactamase inhibitors.
The hollow fiber infection model (HFIM) is the premier in vitro model for studying the PD of antimicrobial agents, as it allows for simulation of human PK profiles, precise determination of exposure-response relationships, and long simulated durations of therapy. Meropenem-vaborbactam has been evaluated in several HFIM experiments, the first of which examined its activity against 17 meropenem-resistant Enterobacteriaceae isolates (1 Escherichia coli, 3 Enterobacter cloacae, 13 Klebsiella pneumoniae) [30], 10 of which were susceptible to the combination and 7 non-susceptible. Multiple dosage regimens based on the phase 1 PK data were simulated, including the approved dose of 4 g Q8h as a 3-h infusion. This simulated dosing regimen provided meropenem concentrations > 8 mg/l and 16 mg/l for 75% and 40–50% of the dosing interval, respectively, and a vaborbactam AUC of 317 mg·h/l. Over the course of the 32-h HFIM experiment, this simulated dosing regimen produced bactericidal activity [6 log10 CFU/ml decrease from the starting inoculum (108 CFU/ml)] and suppressed the development of resistance for all strains with MICs up to 8 mg/l, regardless of bacterial species or mechanisms of resistance.
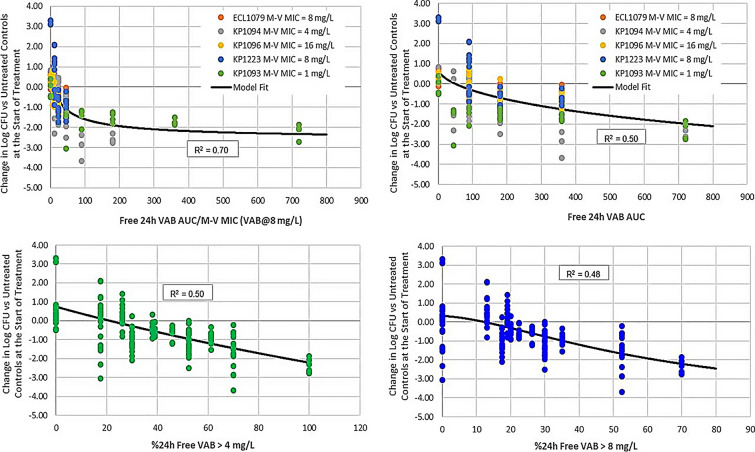
The same dosing regimen was further validated in a tandem, in vitro HFIM/in vivo neutropenic mouse thigh infection model experiment [31]. In this study, 13 strains of K. pneumoniae, 3 E. cloacae, and 1 E. coli clinical isolate were evaluated in the HFIM, and 4 clinical K. pneumoniae and 1 E. cloacae were included in the neutropenic thigh model. The studied pathogens all produced either KPC-2 or KPC-3 with or without mutations in the ompk35 or ompk36 porin channels and had a range of phenotypic susceptibility to meropenem-vaborbactam (MIC range ≤ 0.06–64 mg/l). Notably in the murine thigh infection model, the PK/PD index that best correlated with bacterial kill was the ratio of the 24-h free vaborbactam AUC/meropenem-vaborbactam MIC (R2 = 0.70; Fig. 5), and the thresholds required for bacteriostasis, 1 log, and 2 log reduction in CFU/thigh were 9, 38, and 220, respectively. This index was also supported by the HFIM model, which demonstrated thresholds of 12, 18, and 25 required for stasis, 1 log, and 2 log kill, respectively. As our understanding of the mechanism of action and PD parameters that govern BLI continues to improve, it is crucial that each newly developed BLI undergo a thorough PK/PD evaluation in combination with several potential BL agents of interest in order to establish the PK/PD driver of efficacy and determine thresholds for bactericidality and suppression of resistance for that specific BL-BLI combination. This is highlighted by the discordance in PK/PD drivers observed for avibactam compared to vaborbactam and relebactam [32] despite their similar spectrums of activity and may contribute, in part, to the emergence of resistance observed, albeit infrequently, during clinical use of ceftazidime-avibactam [33]. As use of meropenem-vaborbactam increases, future real-world clinical outcomes data will help determine if these PK/PD advantages translate into improved outcomes for these difficult-to-treat infections.
Fig. 5.
PK/PD driver of efficacy for meropenem-vaborbactam against five clinical CRE strains in the neutropenic mouse thigh infection model. Reprinted with permission from [31]
As meropenem-vaborbactam was to be studied in patients with cUTI and AP in the phase 3 trial, a murine model of pyelonephritis was performed to evaluate its activity against CRE [34]. Two KPC-producing strains of K. pneumoniae and one E. coli were utilized, all of which had MICs to meropenem-vaborbactam of ≤ 0.06 mg/l. A total of 16 mice were infected via transurethral inoculation, which established confirmed pyelonephritis with average starting bacterial titers of ~ 3–7 log CFU/kidney. Meropenem-vaborbactam at the human simulated dose of 4 g Q8h as a 3-h infusion significantly reduced kidney bacterial titers by 1–2 log CFU compared to untreated controls and to meropenem alone. In addition to cUTI, the efficacy of meropenem-vaborbactam was established in a mouse lung infection model to help support its use in the phase 3 HABP/VABP and CRE trials [35]. In this study, 2 KPC-producing K. pneumoniae clinical isolates were utilized with MICs to meropenem-vaborbactam of ≤ 0.06, and the dosing was designed to simulate the 4 g Q8h as a 3-h infusion dose established in humans. Similar to the thigh infection model, meropenem alone had no activity against either strain and allowed 0.3–1 log CFU of bacterial growth, while the combination of meropenem-vaborbactam achieved 1.8–2.9 log CFU killing against both strains.
In addition to KPC-producing Enterobacteriaceae, the fixed 4 g Q8h as a 3-h infusion dose of meropenem-vaborbactam has been evaluated in an in vivo neutropenic mouse thigh model against six clinical strains of P. aeruginosa and 3 Acinetobacter baumannii [36]. Unlike for Enterobacteriaceae, the addition of vaborbactam to meropenem did not decrease the MIC for these non-fermenting strains, and MICs ranged from 2 to 8 mg/l. Against P. aeruginosa, meropenem-vaborbactam produced > 1.5 log reductions in CFU/thigh and was significantly more effective than meropenem alone for 50% of strains. Against A. baumannii, bacterial killing was nearly identical between meropenem alone and meropenem-vaborbactam, with both producing 2–4 log reductions in CFU/thigh. Importantly, despite the lack of established breakpoints for non-Enterobacteriaceae species, meropenem-vaborbactam achieved at least a 1 log reduction in CFU/thigh against seven of the nine strains tested, despite having MICs up to 16 mg/l. Additionally, meropenem-vaborbactam was more active than meropenem alone against the six P. aeruginosa strains despite having the same MIC, suggesting vaborbactam may inhibit a β-lactamase induced after exposure to meropenem.
Combining the available PK and PD data, Bhavnani et al. investigated the probability of target attainment (PTA) of the 4 g Q8h as a 3-h infusion dose of meropenem-vaborbactam using data from the multicenter phase 3 cUTI study [37]. This included data from 175 microbiologically evaluable patients, of which 154 patients had an Enterobacteriaceae-confirmed isolate at baseline. High percentages of successful response were observed across study visits (93–100% clinical response; 76.3–100% microbiologic response; 79–100% overall response). A pre-clinical, free-drug plasma meropenem %T > MIC target ≥ 45% was achieved by 96.6% and 98.7% of all patients with cUTI and in the subset with Enterobacteriaceae, respectively. Over 90% of all patients with an Enterobactericeae cUTI at baseline achieved free-drug meropenem %T > MIC of 100% and a median (min–max), day 1, free-drug vaborbactam AUC:meropenem-vaborbactam MIC ratio of 7567 (17.24–26,033), given that the baseline meropenem-vaborbactam MIC90 was ≤ 0.06 mg/l. Of the three patients with KPC-producing Enterobacteriaceae, all achieved 100% T > MIC and a free drug plasma vaborbactam AUC:MIC of ≥ 2252, far exceeding the necessary threshold determined in pre-clinical in vitro and in vivo evaluations [37]. Conversely, of the four non-KPC-producing carbapenem-resistant pathogens with meropenem-vaborbactam MICs ≥ 32 mg/l isolated from three patients with AP, the day 1 free-drug vaborbactam AUC:meropenem-vaborbactam MIC ratio stasis target of nine, established in the murine thigh infection model, was achieved against only two of four isolates, although all three patients ultimately achieved a successful clinical response. In concordance with the high rates of PK/PD PTA observed in the cUTI trial, none of the 25 patients who received meropenem-vaborbactam monotherapy in the CRE trial developed resistance during the study period [8], although this requires continued surveillance during use outside the clinical trial setting.
Discussion and Conclusion
CRE continue to threaten our remaining antibiotic armamentarium and contribute to excess morbidity, mortality, and healthcare costs. Fortunately, several new BL/BLI agents are now available to combat these CRE infections, including meropenem-vaborbactam. Vaborbactam was designed specifically to inhibit the KPC enzyme and to be combined with a carbapenem intrinsically stable to other class A β-lactamases. Meropenem demonstrated potent in vitro activity when combined with vaborbactam and their concentration-time PK profiles were analogous. The PK/PD of the combination was then rationally optimized to be delivered as a high-dose, extended-infusion regimen to maximize efficacy and minimize the development of resistance. Pre-clinical in vitro and in vivo PK/PD data provide initial evidence of this successful optimization, with meropenem-vaborbactam demonstrating low MIC50/MIC90 values against KPC-producing Enterobacteriaceae and correspondingly high % PTA. Phase 1 PK trials of vaborbactam alone and in combination with meropenem confirmed the comparable PK parameters, including in subjects with renal impairment and in target extravascular body sites like the pulmonary ELF. Together, these PK and PD data were used to justify the 4 g Q8h as a 3-h infusion dosing scheme used in the two phase 3 clinical trials, which solidified the efficacy and safety of meropenem-vaborbactam for patients with cUTI or AP and showed promise in a cohort of those with serious CRE infections.
The development program for vaborbactam can serve as an example for how to rationally design a BLI for a specific resistance mechanism and optimally combine it with a BL that complements its in vitro activity and PK profile. A deep understanding and appreciation of the importance of optimizing PK/PD parameters when treating multidrug-resistant gram-negative infections was apparent during the development of meropenem-vaborbactam as evidenced by the wealth of robust pre-clinical and clinical PK and PD data available. These data along with efficacy in phase 3 trials supported the approval of meropenem-vaborbactam, and the focus on PK/PD optimization during the developmental process will hopefully extend its lifespan as a viable treatment option for KPC-producing CRE infections. Future post-marketing studies should help establish its specific place in therapy by continuing to investigate novel mechanisms of resistance, PK/PD properties in extravascular body sites beyond urine and ELF and efficacy in special patient populations.
Acknowledgements
Funding
The Rapid Service Fees for this work were paid for by Melinta Therapeutics, Inc. Eric Wenzler received no funding or sponsorship for publication of this article. Patrick J. Scoble received consulting fees for this manuscript.
Editorial Assistance
The authors would like to thank Nicolette Theriault (GST Micro LLC) for providing editorial assistance. No funding was provided for this assistance.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Eric Wenzler serves on the speaker’s bureau for Melinta Therapeutics, Astellas Pharma and Allergan Plc and on the advisory board for GenMark Diagnostics and Shionogi & Co. Patrick J. Scoble serves as a consultant for Iterum Pharmaceuticals LTD, Shionogi & Co. and Spero Therapeutics.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Footnotes
Digital Features
To view digital features for this article go to 10.6084/m9.figshare.12937460.
References
- 1.Centers for Disease Control and Prevention. 2018. www.cdc.gov/hai/organisms/cre/trackingcre.html. Accessed 19 Jul 2019.
- 2.Nordmann P, Naas T, Poirel L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17(10):1791–1798. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother. 2011;55(11):4943–4960. doi: 10.1128/AAC.00296-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollett S, Miller S, Hindler J, Uslan D, Carvalho M, Humphries RM. Phenotypic and molecular characteristics of carbapenem-resistant Enterobacteriaceae in a health care system in Los Angeles, California, from 2011 to 2013. J Clin Microbiol. 2014;52(11):4003–4009. doi: 10.1128/JCM.01397-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falagas ME, Lourida P, Poulikakos P, Rafailidis PI, Tansarli GS. Antibiotic treatment of infections due to carbapenem-resistant Enterobacteriaceae: systematic evaluation of the available evidence. Antimicrob Agents Chemother. 2014;58(2):654–663. doi: 10.1128/AAC.01222-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shields RK, Nguyen MH, Chen L, et al. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother. 2017;61(8):e00883–17. doi: 10.1128/AAC.00883-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Duin D, Lok JJ, Earley M, et al. Colistin vs. Ceftazidime-avibactam in the Treatment of Infections due to Carbapenem-Resistant Enterobacteriaceae. Clin Infect Dis. 2018;66(2):163–171. doi: 10.1093/cid/cix783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G, et al. Effect and safety of Meropenem-Vaborbactam versus best-available therapy in patients with Carbapenem-resistant Enterobacteriaceae infections: The TANGO II randomized clinical trial. Infect Dis Ther. 2018;7(4):439–455. doi: 10.1007/s40121-018-0214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hecker SJ, Reddy KR, Totrov M, et al. Discovery of a cyclic boronic acid β-lactamase inhibitor (RPX7009) with utility vs class A serine carbapenemases. J Med Chem. 2015;58(9):3682–3692. doi: 10.1021/acs.jmedchem.5b00127. [DOI] [PubMed] [Google Scholar]
- 10.Lomovskaya O, Sun D, Rubio-Aparicio D, et al. Vaborbactam: spectrum of beta-lactamase inhibition and impact of resistance mechanisms on activity in Enterobacteriaceae. Antimicrob Agents Chemother. 2017;61(11):e01443–17. doi: 10.1128/AAC.01443-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bush K. Past and present perspectives on β-lactamases. Antimicrob Agents Chemother. 2018;62(10):e01076–e1118. doi: 10.1128/AAC.01076-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baldwin CM, Lyseng-Williamson KA, Keam SJ. Meropenem: a review of its use in the treatment of serious bacterial infections. Drugs. 2008;68(6):803–838. doi: 10.2165/00003495-200868060-00006. [DOI] [PubMed] [Google Scholar]
- 13.Castanheira M, Rhomberg PR, Flamm RK, Jones RN. Effect of the β-lactamase inhibitor vaborbactam combined with meropenem against serine Carbapenemase-Producing Enterobacteriaceae. Antimicrob Agents and Chemother. 2016;60(9):5454–5458. doi: 10.1128/AAC.00711-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun D, Rubio-Aparicio D, Nelson K, Dudley MN, Lomovskaya O. Meropenem-vaborbactam resistance selection, resistance prevention, and molecular mechanisms in mutants of KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2017;61(12):e01694–e1717. doi: 10.1128/AAC.01694-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute . Performance standards for antimicrobial susceptibility testing: thirtieth informational supplement M100–S30. Wayne: CLSI; 2020. [Google Scholar]
- 16.Goldstein EJ, Citron DM, Tyrrell KL, Merriam CV. In vitro activity of Biapenem plus RPX7009, a carbapenem combined with a serine beta-lactamase inhibitor, against anaerobic bacteria. Antimicrob Agents Chemother. 2013;57(6):2620–2630. doi: 10.1128/AAC.02418-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livermore DM, Mushtaq S. Activity of biapenem (RPX2003) combined with the boronate beta-lactamase inhibitor RPX7009 against carbapenem-resistant Enterobacteriaceae. J Antimicrob Chemother. 2013;68(8):1825–1831. doi: 10.1093/jac/dkt118. [DOI] [PubMed] [Google Scholar]
- 18.Griffith DC, Loutit JS, Morgan EE, Durso S, Dudley MN. Phase 1 study of the safety, tolerability, and pharmacokinetics of the β-lactamase inhibitor vaborbactam (RPX7009) in healthy adult subjects. Antimicrob Agents Chemother. 2016;60(10):6326–6332. doi: 10.1128/AAC.00568-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubino CM, Bhavnani SM, Loutit JS, et al. Phase 1 study of the safety, tolerability, and pharmacokinetics of Vaborbactam and Meropenem alone and in combination following single and multiple doses in healthy adult subjects. Antimicrob Agents Chemother. 2018;62(4):e02228–e2317. doi: 10.1128/AAC.02228-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubino CM, Bhavnani SM, Loutit JS, Lohse B, Dudley MN, Griffith DC. Single-dose pharmacokinetics and safety of Meropenem-Vaborbactam in subjects with chronic renal impairment. Antimicrob Agents and Chemother. 2018;62(3):e02103–e2117. doi: 10.1128/AAC.02103-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaye KS, Bhowmick T, Metallidis S, et al. Effect of Meropenem-Vaborbactam vs Piperacillin-Tazobactam on clinical cure or improvement and microbial eradication in complicated urinary tract infection: The TANGO I randomized clinical trial. JAMA. 2018;319(8):788–799. doi: 10.1001/jama.2018.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.VABOMERE (meropenem/vaborbactam) Prescribing information. The medicines company. August 2017. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209776lbl.pdf. Accessed 19 Jul 2019.
- 23.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 25.Lew AK, Crass RL, Eschenauer G. Evolution of equations for estimating renal function and their application to the dosing of new antimicrobials. Ann Pharmacother. 2019;54(5):496–503. doi: 10.1177/1060028019890346. [DOI] [PubMed] [Google Scholar]
- 26.Alexander EL, Loutit J, Tumbarello M, et al. Carbapenem-resistant Enterobacteriaceae infections: results from a retrospective series and implications for the design of prospective clinical trials. Open Forum Infect Dis. 2017;4(2):ofx063. doi: 10.1093/ofid/ofx063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wenzler E, Gotfried MH, Loutit JS, et al. Meropenem-RPX7009 concentrations in plasma, epithelial lining fluid, and alveolar macrophages of healthy adult subjects. Antimicrob Agents Chemother. 2015;59(12):7232–7239. doi: 10.1128/AAC.01713-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sime FB, Pandey S, Karamujic N, et al. Ex vivo characterization of effects of renal replacement therapy modalities and settings on pharmacokinetics of meropenem and vaborbactam. Antimicrob Agents Chemother. 2018;62(10):e01306–e1318. doi: 10.1128/AAC.01306-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kufel WD, Eranki AP, Paolino KM, Call A, Miller CD, Mogle BT. In vivo pharmacokinetic analysis of meropenem/vaborbactam during continuous venovenous haemodialysis. J Antimicrob Chemother. 2019;74(7):2117–2118. doi: 10.1093/jac/dkz103. [DOI] [PubMed] [Google Scholar]
- 30.Sabet M, Tarazi Z, Rubio-Aparicio D, et al. Activity of simulated human dosage regimens of meropenem and vaborbactam against carbapenem-resistant Enterobacteriaceae in an in vitro hollow-fiber model. Antimicrob Agents Chemother. 2018;62(2):e01969–17. doi: 10.1128/AAC.01969-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffith DC, Sabet M, Tarazi Z, Lomovskaya O, Dudley MN. Pharmacokinetics/pharmacodynamics of vaborbactam, a novel beta-lactamase inhibitor, in combination with meropenem. Antimicrob Agents Chemother. 2019;63(1):e01659–18. doi: 10.1128/AAC.01659-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu J, Racine F, Wismer MK, et al. Exploring the pharmacokinetic/pharmacodynamic relationship of relebactam (MK-7655) in combination with Imipenem in a hollow-fiber infection model. Antimicrob Agents Chemother. 2018;62(5):e02323–e2417. doi: 10.1128/AAC.02323-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shields RK, Potoski BA, Haidar G, et al. Clinical outcomes, drug toxicity, and emergence of Ceftazidime-Avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis. 2016;63(12):1615–1618. doi: 10.1093/cid/ciw636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss WJ, Pulse ME, Nguyen P, et al. Activity of meropenem-vaborbactam against Carbapenem-resistant Enterobacteriaceae in a murine model of pyelonephritis. Antimicrob Agents Chemother. 2018;62(1):e01439–17. doi: 10.1128/AAC.01439-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabet M, Tarazi Z, Nolan T, et al. Activity of meropenem-vaborbactam in mouse models of infection due to KPC-producing Carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother. 2018;62(1):e01446–17. doi: 10.1128/AAC.01446-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabet M, Tarazi Z, Griffith DC. Activity of Meropenem-vaborbactam against Pseudomonas aeruginosa and Acinetobacter baumannii in a neutropenic mouse thigh infection model. Antimicrob Agents Chemother. 2019;63(1):e01665–e01718. doi: 10.1128/AAC.01665-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhavnani SM, Hammel JP, Rubino CM, et al. Meropenem–Vaborbactam Pharmacokinetic/pharmacodynamic analyses for efficacy based on data from patients enrolled in phase 3 studies [abstract no. 2834 plus poster]. In: 2nd ASM Microbe; 2017; New Orleans.