Abstract
Vaborbactam is a novel boron-based beta-lactamase inhibitor developed to be effective against Klebsiella pneumoniae carbapenemase (KPC)-producing bacteria. This enzyme is a key driver in the global spread of β-lactam resistance among carbapenem-resistant Enterobacterales. Alone, vaborbactam has no antibacterial activity; however, the combination of meropenem-vaborbactam has enhanced activity against gram-negative organisms, particularly Enterobacterales with class A and C carbapenemases. Multiple in vitro studies evaluating isolates from various geographic regions, and over different time periods, have demonstrated the high potency of meropenem-vaborbactam against organisms containing KPC2 and KPC3. However, meropenem-vaborbactam does not have activity against OXA-48 or metallo-beta lactamases. This review covers the in vitro studies of meropenem-vaborbactam performed to date, which evaluated both large cohorts of clinical isolates and engineered isolates, to determine efficacy in various settings, including the presence of porin mutations and efflux pump upregulation.
Keywords: B-lactam, B-lactamase inhibitor, CRE, Meropenem-vaborbactam
Plain Language Summary
Meropenem-vaborbactam is a new combination antibiotic that was designed specifically for efficacy against bacteria that produce the Klebsiella pneumoniae carbapenemase (KPC) enzyme, which enables resistance to beta-lactam antibiotics. The global spread and increase of difficult-to-treat infections caused by carbapenem-resistant Enterobacterales (CRE) is in part because they produce KPC enzymes. The authors review the in vitro studies of meropenem-vaborbactam activity, which have included isolates from different geographic regions, time periods, and settings, showing that it has high potency against organisms containing KPC enzymes-KPC2 and KPC3. Meropenem-vaborbactam was tested against globally sourced isolates that carried different resistance mechanisms, including carbapenem resistance, multidrug resistant (MDR), and resistance to colistin and/or tigecycline; it inhibited activity of 99.1% Enterobacterales isolates tested at ≤ 1 µg/ml, and at ≤ 8 µg/ml it inhibited 96.5% of MDR isolates and 82% of XDR isolates. Against OXA-48 or metallo-beta lactamase enzymes, meropenem-vaborbactam has limited or no activity, so in the Asia-Pacific region where MLBs are prevalent it was least effective, but and was most effective against US strains where KPC is prevalent. In multiple studies, meropenem-vaborbactam showed strong in vitro activity against E. coli, Enterobacter spp., and K. pneumoniae. Compared to available antibiotics, against both clinical and engineered isolates, as well as engineered E. coli strains with KPC, SHV, and TEM enzymes, meropenem-vaborbactam demonstrated lower MIC values. Overall, in vitro studies of meropenem-vaborbactam have shown enhanced activity against CRE and KPC producers compared to other antibiotics, which is needed in the current CRE environment where KPC is dominant.
Key Summary Points
| Meropenem-vaborbactam as a combination has demonstrated enhanced in vitro activity against gram-negative organisms, particularly Enterobacterales with class A and C carbapenemases. |
| KPC is the most dominant strain of carbapenemase-resistant Enterobacterales (CRE). Meropenem-vaborbactam has been shown in multiple in vitro studies of globally sourced isolates to be a potent inhibitor of Enterobacterales with KPC enzymes. |
| Meropenem-vaborbactam does not have activity against OXA-48 or metallo-beta lactamases, thus was least effective in strains from the Asia-Pacific region where MLBs are prevalent and was most effective against strains from the US. |
| Compared to currently available antibiotics, meropenem-vaborbactam demonstrated lower MIC values against both clinical and engineered isolates, including engineered E. coli strains that had KPC, SHV, and TEM enzymes. |
| Sub-analysis in the TANGOII trial demonstrated meropenem-vaborbactam had a lower potential for resistance to develop. |
Digital Features
This article is published with digital features, including a summary slide and plain language summary to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.12937256.
Introduction
With the emergence of carbapenem-resistant Enterobacteriaceae (CRE) organisms, the need for new compounds with activity against these resistant isolates has become acute [1]. A successful method to overcome resistance mediated by beta-lactamase enzymes is to combine beta-lactams with β-lactamase inhibitors [2]; commonly used β-lactamase inhibitors are tazobactam, sulbactam, and clavulanic acid. The addition of these inhibitors to already established beta-lactams has produced the following combinations: piperacillin-tazobactam, ampicillin-sulbactam, and amoxicillin-clavulanate [3]. These combinations maintain the efficacy of the β-lactam class of antibiotics and expand the spectrum of activity against gram-negative pathogens [4, 5]. However, these β-lactam inhibitors are only active against some Class A, SHV, TEM, Klebsiella pneumoniae carbapenemase (KPC) enzymes; none of these β-lactamase inhibitors have activity against the emerging and more resistant Class B (NDM IMP, VIM), C (not true carbapenemase), or D (OXAs) enzymes [6].
Avibactam is a diazabicyclooctane inhibitor, which is not based on the beta-lactam class and uses a urea core. Originally developed to target class A enzymes, Avibactam was found to have activity against enzymes in classes A, C, and a subset of D, specifically against KPC [7]. The combination of ceftazidime and avibactam is approved by the Federal Drug Administration (FDA) for the treatment of complicated intra-abdominal infections, complicated urinary tract infections, hospital-acquired bacterial pneumonia, and ventilator-associated bacterial pneumonia. Unfortunately, development of resistant organisms after treatment with ceftazidime-avibactam has already been reported [8–11], fueling the need for and highlighting the importance of continued drug discovery and development.
Vaborbactam is a novel β-lactamase inhibitor based on a boron ring structure, giving it superior efficacy compared to older β-lactamase inhibitors and the recently approved avibactam [12]. Boronic acid has long been known as a safe compound with therapeutic potential for treatment of various diseases processes [13, 14]. Thus, it is not surprising that the development of a β-lactamase inhibitor with a boron core would be a potent agent. Boronic acids are unique in that the covalent structures that are formed with serine hydrolases can take the tetrahedral structure of either the acylation and deacylation states.
Vaborbactam contains a cyclic α-acylaminoboronic acid, which forms a boronic ester ring that forces a preferred conformation for binding structure that results in greater potency [15]. Fortunately, it does not have any activity against mammalian serine proteases [15]. The compound was designed to form an aromatic ring, which would increase the affinity for β-lactamases as well as maintain the formed structure as the bond is reversible. Several candidate structures were docked with Classes A, C, and D β-lactamases and evaluated; the structure that had favorable pre-covalent and covalent bonding for Classes A and C enzyme active sites was selected for further analysis. Subsequently, analogs of this structure were evaluated to determine the N-acyl-substituent relationships to the potentiation of cephalosporins and carbapenems. The most potent analog was selected, and additional testing with crystallography confirmed visualization of a covalent bond between the serine residue of enzymes (amp C and CTX-m-15) with the inhibitor’s boron atom. The selected analog was initially entitled RPX7009 and later named vaborbactam.
Of note, vaborbactam tested alone against Enterobacterales showed no activity with minimum inhibitory concentration (MIC)50 and MIC90 values of > 64 ug/ml, indicating a lack of antibacterial activity [16].
The formulation of vaborbactam was tested with cephalosporins and aztreonam, but carbapenems produced the most potent combination, and meropenem with vaborbactam proved to be the most effective with the maximum potentiation [12, 17, 18]. Meropenem-vaborbactam was specifically designed to be effective against multidrug-resistant organisms, Enterobacterales-producing extended spectrum beta lactamases (ESBL), and carbapenemase-producing bacteria, such as (KPC) [16, 19–22]. This meropenem-vaborbactam combination maintains the broad spectrum of activity of meropenem, which includes many antibiotic-resistant gram-negative bacteria, and also utilizes the potent carbapenemase and β-lactamase inhibitor actions of vaborbactam, enhancing its range and potency of antimicrobial activity [16, 19]. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Microbiological Properties of Meropenem-Vaborbactam
Gram-Positive Organisms
The combination meropenem-vaborbactam has not been evaluated in vitro against gram-positive organisms, but retains the gram-positive activity of meropenem; however, it has limited coverage against methicillin-resistant Staphylococcus aureus (MRSA), Enterococcus faecalis, and Enterococcus faecium [23–25].
Gram-Negative Organisms
Meropenem and vaborbactam individually have minimal and no activity, respectively, against KPC isolates [16]; however, the combination meropenem-vaborbactam is highly effective against gram-negative Enterobacterales, particularly those with KPC enzymes. This has been demonstrated by in vivo animal studies [15]. Moreover, several studies have evaluated meropenem-vaborbactam efficacy against clinical strains using reference broth microdilution techniques. Vaborbactam is a narrow-spectrum beta-lactamase inhibitor, which does not infer any additional protection against Class C/AmpC hyper production, which tends to cause resistance in Pseudomonas. However, one in vitro study suggested that vaborbactam may enhance activity against Pseudomonas aeruginosa, although the full findings have not yet been published [26]. Of note, meropenem-vaborbactam has no improved activity against Acinetobacter baumannii and is not effective against the metallo-beta-lactamase (MBL) (Class B)-containing Enterobacterales [17, 27, 28].
Castanheira et al. evaluated the effectiveness of meropenem-vaborbactam against 315 clinical isolates of serine carbapenemase-producing Enterobacterales collected over a period of 13 years from multiple countries [16]. The long time interval and geographic variety were purposely chosen to diversify the test population. The majority of the isolates evaluated were K. pneumoniae (66%), and the most common resistance genes detected among the Enterobacterales were blaKPC-2 (46%) and blaKPC-3 (37%). When the isolates were tested alone against meropenem, only 2.2% of the entire collection were susceptible at the CLSI (Clinical & Laboratory Standards Institute) breakpoint of ≤ 1 for meropenem [29]. The combination of meropenem (≤ 2 µg/ml) with increasing concentrations of vaborbactam ranging from 4 to 32 µg/ml inhibited 90.2–98.1% of the isolates tested. Vaborbactam increased the activity of meropenem by at least 64 fold. When focusing on KPC-producing isolates, meropenem-vaborbactam with an inhibitor concentration of 8 µg/ml inhibited 96.6% K.pneumoniae and 100% Escherichia coli, Enterobacter cloacae, Klebsiella oxytoca, Serratia marcescens, and Citrobacter freundii isolates. In addition, 98.7% of isolates that carried additional beta-lactamases were suppressed with the combination. Of the seven isolates that exhibited intrinsic resistance to meropenem-vaborbactam MICs ≥ 16 µg/ml, four carried MBLs for VIM in addition to KPC, and the remaining three isolates showed alterations in expression of outer membrane porin (OMP) and efflux pump mechanisms (Table 1).
Table 1.
In vitro activity of meropenem-vaborbactam against clinical isolates from various cohorts
| No. of isolates tested | Meropenem-vaborbactam (8 µg/ml) | Ref | |||
|---|---|---|---|---|---|
| MIC (µg/ml) | |||||
| Range | 50% | 90% | |||
| Enterobacterales | 10,426 | ≤ 0.015 to > 32 | ≤ 0.015 | 0.06 | [17] |
| Enterobacterales (KPC) | 991 | ≤ 0.03 to > 32 | 0.06 | 1 | [19] |
| Escherichia coli | 4238 | ≤ 0.015 to 32 | ≤ 0.015 | ≤ 0.015 | [17] |
| Escherichia coli (KPC) | 35 | ≤ 0.03 to > 32 | ≤ 0.03 | ≤ 0.03 | [19] |
| Escherichia coli (KPC) | 21 | ≤ 0.06 | ≤ 0.06 | ≤ 0.06 | [16] |
| Klebsiella pneumoniae | 2010 | ≤ 0.015 to > 32 | 0.03 | 0.12 | [17] |
| Klebsiella pneumoniae (KPC) | 878 | ≤ 0.03 to 0.12 | 0.12 | 1 | [19] |
| Klebsiella pneumoniae (KPC) | 208 | ≤ 0.06 to > 64 | ≤ 0.06 | 1 | [16] |
| Klebsiella pneumoniae (KPC) | 121 | ≤ 0.004/8 to > 64/8 | .03 | .5 | [27] |
| Klebsiella oxytoca | 429 | ≤ 0.015 to 16 | 0.03 | 0.03 | [17] |
| Klebsiella oxytoca (KPC) | 19 | ≤ 0.03 to 0.12 | ≤ 0.03 | 0.25 | [19] |
| Klebsiella oxytoca (KPC) | 14 | ≤ 0.06 to 2 | ≤ 0.06 | 0.5 | [16] |
| Enterobacter cloacae species complex | 950 | ≤ 0.015 to 8 | ≤ 0.015 | 0.03 | [17] |
| E. cloacae (serine carpbapenemase) | 39 | ≤ 0.06 to 4 | ≤ 0.06 | 0.25 | [16] |
| Enterobacter aerogenes | 355 | ≤ 0.015 to 2 | 0.03 | 0.03 | [17] |
| Enterobacter species (KPC) | 29 | ≤ 0.03 to 0.12 | ≤ 0.03 | 0.12 | [19] |
| Citrobacter freundii species complex | 276 | ≤ 0.015 to 8 | ≤ 0.015 | 0.03 | [17] |
| Citrobacter freundii (KPC) | 12 | ≤ 0.06 to 0.25 | ≤ 0.06 | 0.12 | [16] |
| Citrobacter koseri | 194 | ≤ 0.015 to 0.03 | ≤ 0.015 | 0.03 | [17] |
| Citrobacter species | 13 | ≤ 0.03 to 0.12 | ≤ 0.03 | 0.06 | [19] |
| Proteus mirabilis | 525 | ≤ 0.015 to 1 | 0.06 | 0.12 | [17] |
| Indole-positive Proteeae spp. | 585 | ≤ 0.015 to > 32 | 0.06 | 0.06 | [17] |
| Serratia marcescens | 666 | ≤ 0.015 to 32 | 0.03 | 0.06 | [17] |
| Serratia marcescens (KPC) | 16 | ≤ 0.03 to 2 | 0.06 | 1 | [19] |
| CRE | 265 | ≤ 0.015 to > 32 | 0.5 | 32 | [17] |
| CRE | 330 | ≤ 0.015 to > 32 | 0.5 | 32 | [30] |
| KPC producers | 135 | ≤ 0.015 to 8 | 12 | 0.5 | [17] |
| KPC producers | 206 | ≤ 0.015 to 32 | 0.25 | 1 | [27, 30] |
| Non-KPC-producing CRE | 129 | ≤ 0.015 to > 32 | 4 | > 32 | [17] |
| Non-KPC-producing CRE | 121 | 0.25 to > 32 | 16 | > 32 | |
| Carbapenemase-negative isolates | 63 | ≤ 0.015 to 32 | 1 | 4 | [17] |
| Carbapenemase-negative isolates | 38 | 0.25 to 32 | 2 | 16 | [30] |
| MDR | 1210 | ≤ 0.015 to > 32 | 0.03 | 1 | [17] |
| XDR | 161 | ≤ 0.015 to > 32 | 0.5 | 32 | [17] |
| MBL producer | 41 | 1 to > 32 | 32 | > 32 | [17] |
| MBL producer | 52 | 1 to > 32 | 32 | > 32 | [30] |
| Pseudomonas aeruginosa | 2604 | ≤ 0.015 to > 32 | 0.5 | 8 | [17] |
| Pseudomonas aeruginosa | 98 | 0.25/8 to 64/8 | 8 | 32 | [27] |
Optimal vaborbactam concentration was 8 µg/ml, based on in vitro activity achieving adequate susceptibility at 97.8% of the tested isolates. Increasing dose did not achieve significantly higher susceptibility. More importantly, this concentration is roughly equivalent to the concentration of the FDA-approved dose of 2 g for vaborbactam (Table 2).
Table 2.
Establish breakpoints for meropenem-vaborbactam against enterobacterales
| Minimum inhibitory concentrations (µg/ml) | Disk diffusion (zone diameters in mm) | |||||
|---|---|---|---|---|---|---|
| S | I | R | S | I | R | |
| FDA [36] | ≤ 4/8 | 8/8 | ≥ 16/8 | ≥ 17 | 14–16 | ≤ 13 |
| CLSI [29] | ≤ 4/8 | 8/8 | ≥ 16/8 | ≥ 18 | 15–17 | ≤ 14 |
| EUCAST [37] | ≤ 8/8 | – | > 8/8 | * | * | * |
*EUCAST disk diffusion in preparation
Subsequently, Castenheira et al. published a larger study that evaluated 14,304 worldwide contemporary gram-negative clinical isolates against meropenem-vaborbactam and comparator antibiotics [17]. The isolates were collected in 2014 from 82 hospitals as part of the SENTRY Antimicrobial Surveillance Program and included isolates that carried different resistance mechanisms so that some of the isolates were carbapenem resistant while others were multidrug resistant (MDR), including resistance to colistin and/or tigecycline. Meropenem effectively inhibited activity of 97.3% of Enterobacterales isolates tested at ≤ 1 µg/ml. This value increased to 99.1% when the same isolates were tested against meropenem-vaborbactam at the same concentration and 99.6% for a concentration of ≤ 8 µg/ml. There were 265 CRE isolates in this cohort, of which the majority (79.6%) were K. pneumoniae. One hundred thirty-five of the 265 screened CRE isolates carried blaKPC genes. Meropenem-vaborbactam had the most activity of the β-lactam agents tested against these CRE isolates and inhibited 84.2% of isolates at ≤ 8 µg/ml, and against comparator agents high susceptibility rates were seen: amikacin (56.2%) and colistin (70.3%). Only tigecycline had higher susceptibility at 99.2% (Fig. 1).
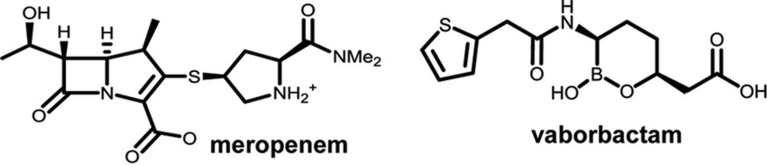
Fig. 1.
Chemical structures of meropenem and vaborbactam. Me 2 dimethyl [12]. Reused with permission under the ‘Creative Commons CC BY’ license from [12]. Copyright © 2017, American Society for Microbiology
The meropenem-vaborbactam MIC50/MIC90 for MDR isolates was 0.03/1 µg/ml and for extensively drug-resistant (XDR) isolates was 0.5/32 µg/ml. Meropenem-vaborbactam at ≤ 8 µg/ml inhibited 96.5% of MDR isolates and 82% of XDR isolates. Similar to the previous analysis, the study confirmed that meropenem-vaborbactam has limited activity against MBLs. Among the 15.5% of the CRE isolates that contained MBLs, approximately two-thirds had the NDM-1 gene. OXA-48 also accounted for 10.2% of the CRE isolates. Meropenem-vaborbactam was most effective in strains from the US and least effective in strains from Asia–Pacific countries, likely due to the region's higher prevalence of MBLs. There were only seven isolates carrying carbapenemases from the Asia–Pacific region.
The same investigators utilized a 2015 worldwide collection of 11,559 Enterobacterales isolates to evaluate meropenem-vaborbactam activity, finding that meropenem-vaborbactam demonstrated enhanced activity against CRE and KPC producers compared to other antibiotics [30]. Meropenem alone inhibited 96.9% of all the Enterobacterales tested, which increased to 99.3% when the isolates were tested against the combination of meropenem-vaborbactam. Overall, 97% of K. pneumoniae isolates were inhibited by meropenem-vaborbactam. Of the 330 CRE isolates in the cohort, the majority (80.6%) were K. pneumoniae, 88.5% had carbapenemase genes, 15.7% had MBL genes, and 13.3% had OXA-48 genes. Meropenem-vaborbactam at ≤ 4/8 µg/ml inhibited 73.9% of the CRE Enterobacterales and only 31.4% of the non-KPC gene CRE isolates. The one KPC isolate (0.5%) that was not inhibited by meropenem-vaborbactam contained a porin mutation.
Lapuebla et al. evaluated 4500 gram-negative clinical isolates from 11 New York City hospitals against meropenem-vaborbactam, meropenem, and other antibiotics [27]. Meropenem-vaborbactam had strong in vitro activity against E. coli, Enterobacter spp., and K. pneumoniae and enhanced activity in KPC-producing CR isolates. Among E. coli isolates blaKPC was the only carbapenemase gene detected, and all isolates were susceptible to meropenem-vaborbactam at ≤ 8 µg/ml. Eighty-eight percent of K. pneumoniae isolates were susceptible to meropenem, and 99.8% were susceptible to meropenem-vaborbactam (with a vaborbactam concentration of 8 µg/ml). Meropenem-vaborbactam had activity against 119/121 (98.3%) of the KPC K. pneumoniae strains. In addition, 26 K. pneumoniae isolates with known resistance mechanisms expressing blaKPC, acrB, and ompK36 were tested; only 2 (both with decreased expression of ompK36) had elevated MIC > 64 µg/ml to meropenem, which decreased to 1 and 2 with the addition of vaborbactam. All 211 isolates of Enterobacter species, 7 of which had the blaKPC gene, were sensitive to the combination of meropenem-vaborbactam. Evaluation of A. baumannii and P. aeruginosa revealed no significant change among the non-susceptible meropenem isolates when tested against meropenem-vaborbactam, which was expected.
Anaerobic Organisms
Combination meropenem-vaborbactam retains the anaerobic activity of meropenem [31].
Mechanisms of Resistance and Lower Potential for Development of Resistance
Carbapenem-resistant Enterobacterales often have multiple mechanisms of resistance, such as porin-mutations or efflux pumps, in addition to carbapenemase-producing enzymes. To evaluate its potency in various situations, meropenem-vaborbactam was tested against engineered strains of β-lactamase-producing E. coli and K. pneumoniae with and without additional mutations [12]. The study demonstrated that an E. coli strain without any beta-lactamase inhibitor had an MIC of ≤ 0.03 µg/ml for both meropenem and meropenem-vaborbactam, which was ≤ 0.125 µg/ml for both aztreonam ± beta-lactamase inhibitor and ceftazidime ± beta-lactamase inhibitor (beta-lactamase inhibitors tested were vaborbactam, tazobactam, and clavulanic acid). The trend for lower MIC values with meropenem-vaborbactam was also observed with engineered E. coli strains that had KPC, SHV, and TEM enzymes. Moreover, the addition of vaborbactam to ceftazidime and aztreonam restored the activity against strains that contained class A carbapenemases with lower MIC values compared to the other β-lactamase inhibitors. For extended-spectrum β-lactamase (ESBL) genes TEM and SHV, the MIC value was lower with the addition of vaborbactam to the tested beta-lactam compared to the β-lactam alone; however, tazobactam and clavulanic acid proved to be more potent than vaborbactam. In the engineered K. pneumoniae isolates, the investigators produced isolates that had both a porin mutation and a β-lactamase, resulting in increased MIC values for meropenem (compared to wild-type strains), which decreased with the addition of vaborbactam for KPC (64 µg/ml → 2 µg/ml), SHV (0.5 µg/ml → 0.25 µg/ml), and ampC (4 µg/ml → 1 µg/ml), but not for OXA-48 (64 µg/ml → 64 µg/ml), which was expected. Further evaluation of the effects of mutations of porin proteins (Ompk35 and Ompk36) and efflux pumps (AcrAB) revealed that efflux pumps alone had minimal effect, but the combination of one porin and efflux pump mutations resulted in a two- to four-fold increase of MIC, and the combination of both porin mutations resulted in 16-fold increased MIC. The study again demonstrated the lack of potency of meropenem-vaborbactam against NDM and VIM strains.
Another recently published study reported two newly described loss-of-function mutations for porin production. KvrA loss reduced OmpK35 and OmpK36 porin production, again demonstrating the importance of porin mutations on the effect of reducing susceptibility to meropenem-vaborbactam in a KPC-3-producing K. pneumoniae isolate [32].
Another in vitro study utilized CRE genotypes and laboratory-engineered E. coli isolates harboring mutant blaKPC genes, which are associated with ceftazidime-avibactam resistance, to identify meropenem-vaborbactam susceptibility and MICs [33].
Ninety-eight percent (117/120) of CRE isolates were susceptible to meropenem-vaborbactam (MICs ≤ 4 µg/ml), and median MICs were lower for meropenem-vaborbactam compared to ceftazidime-avibactam (0.034 µg/ml and 1). All blaKPC-harboring K. pneumoniae isolates were susceptible to meropenem-vaborbactam, and the addition of vaborbactam reduced the MICs in 78% of isolates (14/18). Both wild-type and variant KPC enzymes were inhibited by meropenem-vaborbactam. However, in KPC-producing K. pneumoniae isolates, meropenem-vaborbactam MICs were higher in those isolates that displayed mutant ompK36 genes (n = 26) compared to wild-type ompK36 genes (n = 54) (0.25 versus 0.03 µg/ml; P < 0.0001).
The addition of vaborbactam at 8 µg/ml against E.Coli isolates containing wild-type blaKPC or mutant blaKPC lowered the meropenem MICs 2- to 512-fold, which resulted in meropenem-vaborbactam MICs of 0.03 µg/ml. Thus, meropenem-vaborbactam has demonstrated in vitro activity against CRE, including isolates resistant to ceftazidime-avibactam.
The in vitro activity of meropenem-vaborbactam against 991 KPC-positive Enterobacterales isolates, collected globally from 2014 to 2015 [19], was compared to the activity of seven other antibiotic agents. Although the overall susceptibility of the meropenem-vaborbactam was similar to ceftazidime-avibactam (99% vs. 98.2%), meropenem-vaborbactam achieved potency at lower MIC90 values of 1 µg/ml compared to ceftazidime-avibactam at 4 µg/ml. Similar results were seen between meropenem-vaborbactam and ceftazidime-avibactam when organisms were evaluated at the species level using MIC90 values (µg/ml); K. pneumoniae (1 µg/ml vs. 4 µg/ml), E. coli (≤ 0.03 µg/ml vs. 1.0 µg/ml), Enterobacter (0.12 µg/ml vs. 2 µg/ml), K. oxytoca (0.25 µg/ml vs. 4 µg/ml), S. marcescens (1 µg/ml vs. 2 µg/ml) and Citrobacter spp (0.06 µg/ml vs. 2 µg/ml), respectively. Interestingly, further evaluation of isolates resistant to meropenem-vaborbactam and ceftazidime-avibactam revealed only 20.8% cross-resistance. Meropenem-vaborbactam was also superior to tigecycline, gentamicin, and polymixin B.
Importantly, the lower potential for resistance to meropenem-vaborbactam to develop has been demonstrated clinically. In a sub-analysis of the Targeting Antibiotic Non-susceptible Gram-negative Organisms (TANGO) II trial, 1 patient of 25 (4%) with CRE K. pneumoniae treated with meropenem-vaborbactam for 6 days failed therapy with an increase in MIC from 0.25 µg/ml to 1.0 µg/ml, retaining activity against meropenem-vaborbactam. Of the four patients treated with ceftazidime-avibactam monotherapy, one (25%) had an increase in the MIC value of K. pneumoniae from 0.5 µg/ml to > 128 µg/ml, resulting in non-susceptibility [34]. In a different retrospective study of patients treated for CRE infections, post-hoc analysis revealed that 3 of 105 patients treated with ceftazidime-avibactam vs. no patients of the 26 treated with meropenem-vaborbactam had a recurrent infection due to development of a resistance organism [35]. Although these evaluations are of small cohort of patients, it is an important observation demonstrating the lower potential for developing resistance.
In an effort to explore optimal combinations of antimicrobial agents with activity against serine and MBL-producing CROs, the activity of aztreonam plus ceftazidime-avibactam and aztreonam plus meropenem-vaborbactam against clinical E. coli and K. pneumoniae strains coproducing NDM and one or more serine β-lactamases was evaluated and compared in a recent in vitro study [6]. The addition of aztreonam with each of these combination agents resulted in synergistic activity against enterobacteriaceae strains that co-produce NDM and at least one serine β-lactamase, with the anticipated exception of aztreonam plus meropenem-vaborbactam having no activity against OXA-48-like-producing enterobacteriaceae strains. Future studies are needed to confirm and expand upon these findings, but these findings suggest that these combinations could be a possible treatment in patients with aztreonam-resistant NDM and serine- β-lactamase-producing enterobacteriaceae infections.
Conclusion
In conclusion, meropenem-vaborbactam is a potent inhibitor of Enterobacterales with KPC enzymes. It exhibits lower MIC values with in vitro testing against both clinical and engineered isolates compared to currently available antibiotics. Although the combination does not provide any additional coverage for Pseudomonas, Acinetobacter, or Enterobacterales that have MBL enzymes, it is a welcome and needed agent in the current CRE environment, which consists of predominantly KPC-containing strains.
Acknowledgements
Funding
No funding was provided for the research or writing of this manuscript. The Rapid Service Fees were fully funded by Melinta Therapeutics.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Tanaya Bhowmick and Melvin P. Weinstein have no conflicts to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Lai CC, Chen CC, Tang HJ. Meropenem-vaborbactam in the treatment of acute bacterial infections. J Clin Med. 2019;8(10):1650. [DOI] [PMC free article] [PubMed]
- 2.Drawz SM, Papp-Wallace KM, Bonomo RA. New beta-lactamase inhibitors: a therapeutic renaissance in an MDR world. Antimicrob Agents Chemother. 2014;58(4):1835–1846. doi: 10.1128/AAC.00826-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Letourneau A. Combination beta-lactamase inhibitors, carbapenems, and monobactams. In: Hooper D, Bloom A (Ed.) UpToDate, Waltham, MA. 2020.
- 4.Adam D. Beta-lactam/beta-lactamase inhibitor combinations in empiric management of pediatric infections. J Int Med Res. 2002;30(Suppl 1):10A–A19. doi: 10.1177/14732300020300S103. [DOI] [PubMed] [Google Scholar]
- 5.Finegold S. In vitro efficacy of betalactam/beta-lactamase inhibitor combinations against bacteria involved in mixed infections. Int J Antimicrob Agents. 1999;12:S9–S14. doi: 10.1016/S0924-8579(99)00086-2. [DOI] [PubMed] [Google Scholar]
- 6.Biagi M, Wu T, Lee M, Patel S, Butler D, Wenzler E. Exploring aztreonam in combination with ceftazidime-avibactam or meropenem-vaborbactam as potential treatments for metallo- and serine-β-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2019;63(12):e01426–e1519. doi: 10.1128/AAC.01426-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shields RK, Clancy CJ, Hao B, et al. Effects of Klebsiella pneumoniae carbapenemase subtypes, extended-spectrum beta-lactamases, and porin mutations on the in vitro activity of ceftazidime-avibactam against carbapenem-resistant K. pneumoniae. Antimicrob Agents Chemother. 2015;59(9):5793–7. [DOI] [PMC free article] [PubMed]
- 8.Shields RK, Potoski BA, Haidar G, et al. Clinical outcomes, drug toxicity, and emergence of ceftazidime–avibactam resistance among patients treated for carbapenem-resistant enterobacteriaceae Infections. Clin Infect Dis. 2016;63(12):1615–1618. doi: 10.1093/cid/ciw636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shields RK, Nguyen MH, Press EG, Chen L, Kreiswirth BN, Clancy CJ. Emergence of ceftazidime–avibactam resistance and restoration of carbapenem susceptibility in klebsiella pneumoniae carbapenemase-producing K pneumoniae: a case report and review of literature. Open Forum Infect Dis. 2017;4(3):ofx101. [DOI] [PMC free article] [PubMed]
- 10.Shields RK, Nguyen MH, Chen L, Press EG, Kreiswirth BN, Clancy CJ. Pneumonia and renal replacement therapy are risk factors for ceftazidime–avibactam treatment failures and resistance among patients with carbapenem-resistant Enterobacteriaceae infections. Antimicrob Agents Chemother. 2018;62(5):e02497–17. [DOI] [PMC free article] [PubMed]
- 11.Kawai A, McElheny CL, Iovleva A, et al. Structural basis of reduced susceptibility to ceftazidime–avibactam and cefiderocol in enterobacter cloacae due to AmpC R2 loop deletion. Antimicrob Agents Chemother. 2020:e00198–20. [DOI] [PMC free article] [PubMed]
- 12.Lomovskaya O, Sun D, Rubio-Aparicio D, et al. Vaborbactam: spectrum of beta-lactamase inhibition and impact of resistance mechanisms on activity in Enterobacteriaceae. Antimicrob Agents Chemother. 2017;61(11):e01443–e1517. doi: 10.1128/AAC.01443-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker SJ, Ding CZ, Akama T, Zhang YK, Hernandez V, Xia Y. Therapeutic potential of boron-containing compounds. Future Med Chem. 2009;1(7):1275–1288. doi: 10.4155/fmc.09.71. [DOI] [PubMed] [Google Scholar]
- 14.Beesley T, Gascoyne N, Knott-Hunziker V, et al. The inhibition of class C beta-lactamases by boronic acids. Biochem J. 1983;209(1):229–233. doi: 10.1042/bj2090229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hecker SJ, Reddy KR, Totrov M, et al. Discovery of a cyclic boronic acid β-lactamase inhibitor (RPX7009) with utility vs Class A serine carbapenemases. J Med Chem. 2015;58(9):3682–3692. doi: 10.1021/acs.jmedchem.5b00127. [DOI] [PubMed] [Google Scholar]
- 16.Castanheira M, Rhomberg PR, Flamm RK, Jones RN. Effect of the β-lactamase inhibitor vaborbactam combined with meropenem against serine carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2016;60(9):5454–5458. doi: 10.1128/AAC.00711-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castanheira M, Huband MD, Mendes RE, Flamm RK. Meropenem-vaborbactam tested against contemporary gram-negative isolates collected worldwide during 2014, including carbapenem-resistant, KPC-producing, multidrug-resistant, and extensively drug-resistant enterobacteriaceae. Antimicrob Agents Chemother. 2017;61(9):e00567–e617. doi: 10.1128/AAC.00567-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livermore DM, Mushtaq S. Activity of biapenem (RPX2003) combined with the boronate β-lactamase inhibitor RPX7009 against carbapenem-resistant Enterobacteriaceae. J Antimicrob Chemother. 2013;68(8):1825–1831. doi: 10.1093/jac/dkt118. [DOI] [PubMed] [Google Scholar]
- 19.Hackel MA, Lomovskaya O, Dudley MN, Karlowsky JA, Sahm DF. In vitro activity of meropenem-vaborbactam against clinical isolates of KPC-positive Enterobacteriaceae. Antimicrob Agents Chemother. 2018;62(1):e01904–e1917. doi: 10.1128/AAC.01904-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinn PM, Chen DJ, Gihring TM, et al. In vitro evaluation of meropenem-vaborbactam against clinical CRE isolates at a tertiary care center with low KPC-mediated carbapenem resistance. Diagn Microbiol Infect Dis. 2019;93(3):258–260. doi: 10.1016/j.diagmicrobio.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 21.Sabet M, Tarazi Z, Griffith DC. Activity of meropenem-vaborbactam against pseudomonas aeruginosa and acinetobacter baumannii in a neutropenic mouse thigh infection model. Antimicrob Agents Chemother. 2018;63(1):e01665–e1718. doi: 10.1128/AAC.01665-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savov E, Trifonova A, Kovachka K, Kjosseva E, Strateva T. Antimicrobial in vitro activities of ceftazidime-avibactam, meropenem-vaborbactam and plazomicin against multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa—a pilot Bulgarian study. Infect Dis (Lond) 2019;51(11–12):870–873. doi: 10.1080/23744235.2019.1653491. [DOI] [PubMed] [Google Scholar]
- 23.Baldwin CM, Lyseng-Williamson KA, Keam SJ. Meropenem. Drugs. 2008;68(6):803–838. doi: 10.2165/00003495-200868060-00006. [DOI] [PubMed] [Google Scholar]
- 24.Lutsar I, Chazallon C, Trafojer U, et al. Meropenem vs standard of care for treatment of neonatal late onset sepsis (NeoMero1): a randomised controlled trial. PLoS ONE. 2020;15(3):e0229380. doi: 10.1371/journal.pone.0229380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhanel GG, Wiebe R, Dilay L, et al. Comparative review of the carbapenems. Drugs. 2007;67(7):1027–1052. doi: 10.2165/00003495-200767070-00006. [DOI] [PubMed] [Google Scholar]
- 26.Patel TS, Kaye KS, Krishnan J, et al. 521. Comparative in vitro activity of meropenem/vaborbactam and meropenem against a collection of real-world clinical isolates of Pseudomonas aeruginosa. Open Forum Infect Di. 2019;6(Supplement_2):S251–S.
- 27.Lapuebla A, Abdallah M, Olafisoye O, et al. Activity of meropenem combined with RPX7009, a novel beta-lactamase inhibitor, against gram-negative clinical isolates in New York City. Antimicrob Agents Chemother. 2015;59(8):4856–4860. doi: 10.1128/AAC.00843-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castanheira M, Doyle TB, Kantro V, Mendes RE, Shortridge D. Meropenem-vaborbactam activity against carbapenem-resistant enterobacterales isolates collected in U.S. Hospitals during 2016 to 2018. Antimicrob Agents Chemother. 2020;64(2):e01951–19. [DOI] [PMC free article] [PubMed]
- 29.CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. CLSI Supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute. 2020.
- 30.Pfaller MA, Huband MD, Mendes RE, Flamm RK, Castanheira M. In vitro activity of meropenem/vaborbactam and characterisation of carbapenem resistance mechanisms among carbapenem-resistant Enterobacteriaceae from the 2015 meropenem/vaborbactam surveillance programme. Int J Antimicrob Agents. 2018;52(2):144–150. doi: 10.1016/j.ijantimicag.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein EJC, Citron DM, Tyrrell KL, Merriam CV. In vitro activity of biapenem plus RPX7009, a carbapenem combined with a serine β-lactamase inhibitor, against anaerobic bacteria. Antimicrob Agents Chemother. 2013;57(6):2620–2630. doi: 10.1128/AAC.02418-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dulyayangkul P, Wan Nur Ismah WAK, Douglas EJA, Avison MB. Mutation of KVR causes OmpK35 and OmpK36 Porin Downregulation and reduced meropenem-vaborbactam susceptibility in KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2020;64(7):e02208–19. [DOI] [PMC free article] [PubMed]
- 33.Wilson WR, Kline EG, Jones CE, et al. Effects of KPC variant and Porin genotype on the in vitro activity of meropenem-vaborbactam against carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother. 2019;63(3):e02048–e2118. doi: 10.1128/AAC.02048-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lomovskaya O, Castanheira M, Vazquez J, et al. Assessment of MIC increases with meropenem-vaborbactam and ceftazidime-avibactam in TANGO II (a Phase 3 Study of the Treatment of CRE Infections). Abstract No.1874. Open Forum Infect Dis. 2017;4(suppl_1):S540–S.
- 35.Ackley R, Roshdy D, Meredith J, et al. Meropenem-vaborbactam versus ceftazidime-avibactam for treatment of carbapenem-resistant enterobacteriaceae infections. Antimicrob Agents Chemother. 2020;64(5):e02313–e2319. doi: 10.1128/AAC.02313-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The Medicines Company. VABOMERE™ (meropenem and vaborbactam) for injection, for intravenous use (Package Insert). 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209776lbl.pdf. Accessed 22 Aug 2019.
- 37.The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters, version 10.0. 2020.