Abstract
Stimulated by the leading mortalities of cardiovascular diseases (CVDs), various types of cardiovascular biomaterials have been widely investigated in the past few decades. Although great therapeutic effects can be achieved by bare metal stents (BMS) and drug-eluting stents (DES) within months or years, the long-term complications such as late thrombosis and restenosis have limited their further applications. It is well accepted that rapid endothelialization is a promising approach to eliminate these complications. Convincing evidence has shown that endothelial progenitor cells (EPCs) could be mobilized into the damaged vascular sites systemically and achieve endothelial repair in situ, which significantly contributes to the re-endothelialization process. Therefore, how to effectively capture EPCs via specific molecules immobilized on biomaterials is an important point to achieve rapid endothelialization. Further, in the context of predictive, preventive, personalized medicine (PPPM), the abnormal number alteration of EPCs in circulating blood and certain inflammation responses can also serve as important indicators for predicting and preventing early cardiovascular disease. In this contribution, we mainly focused on the following sections: the definition and classification of EPCs, the mechanisms of EPCs in treating CVDs, the potential diagnostic role of EPCs in predicting CVDs, as well as the main strategies for cardiovascular biomaterials to capture EPCs.
Keywords: Cardiovascular biomaterials, Rapid endothelialization, Endothelial progenitor cells, Endothelial progenitor cell capturing stent, Predictive diagnostics, Targeted prevention, EPC-specific molecules, Predictive preventive personalized medicine (PPPM/3PM)
Introduction
Cardiovascular diseases (CVDs), mainly caused by atherosclerosis and thrombosis, have become the leading mortalities in the past few decades [1, 2]. Cardiovascular stent intervention is proved to be an effective strategy to treat CVDs in clinical [3, 4]. However, a series of complications including inflammation, late thrombosis, and restenosis after long-term intervention limit the use of traditional cardiovascular stent [5, 6]. In order to overcome these limitations, drug-eluting stents (DES) have been systematically designed and investigated in recent years. The main principle of DES design is to inhibit the excessive proliferation of smooth muscle cells (SMCs) through cytotoxic drugs [7]. Although the DES lowers the incidence of in-stent restenosis (ISR) for short-periods, researchers have confirmed that the anti-proliferative drugs eluted on the DES could influence vascular healing process in a long term, which may increase the risk of late thrombosis [8–10]. It is well known that how to minimize the risk of ISR and accelerate the endothelialization process are the two most critical points in the success of cardiovascular stent. Based on the two principles, a novel vascular technique selectively recruits endothelial progenitor cells (EPCs) by the immobilized bioactive molecules, which is so-called endothelial progenitor cell capturing stent (ECS), has been developed [11–13]. Although these novel vascular stents have shown great therapeutic effects in clinical, the subject has not been completely addressed till now, which reminds us to pay more attention to the most advanced cost-effective approach in biomedical sciences and healthcare [14]. The advanced concepts of PPPM (3 PM) has been presented by the European Association for Predictive, Preventive and Personalized Medicine (EPMA) as applicable to both “disease care” and “health care” [15]. The general report suggested that the number alteration of EPCs and some secondary complications especially inflammatory could serve as predictive judgments of potential CVDs, which provide a new strategy for the prevention and treatment of CVDs [16, 17].
In this review, we first introduce the biology of EPCs and mechanisms of EPCs in treating CVDs. More importantly, the potential role of EPCs as diagnostic biomarkers in predicting CVDs was also discussed in this review, contributing therefore to the prevention of CVDs as well as emphasizing the crucial role of EPCs in treatment of CVDs. On the other hand, we introduce the different approaches for EPCs capturing, including EPC-specific antibodies, aptamers, EPC-specific peptides, and magnetic molecules. The key factor of the subject is to maximize the number of captured EPCs through bioactive molecules. Therefore, it is essential to understand how to regulate the behaviors of EPCs and their responds toward corresponding molecules.
The definition and classification of EPCs
EPCs, the precursor of vascular endothelial cells mainly derived from bone marrow, have been firstly isolated by Asahara in 1997 [18–22]. Since then, the research on the biological characteristics and therapeutic effects of EPCs has become a new hot spot due to their potential in forming angiogenic and differentiating into functional endothelial cells (ECs) [23]. In fact, in addition to the bone marrow, umbilical cord blood, circulating blood, and arterial walls also contain EPCs but not more [24–27]. Zammaretti et al. confirmed that only about 0.01% of EPCs exist in human circulating blood [28]. Circulating EPCs (CEPCs) are mainly mobilized from non-hematopoietic tissues such as blood vessel walls and homed to the damaged endothelium and then form new layers [29–32]. The vascular endothelium, acting as a barrier between the blood and SMCs, plays a crucial role in maintaining the normal flow of blood [33, 34]. Not only can it prevent the formation of thrombus but also releases regulation mediators including nitric oxide (NO), soluble thrombomodulin, soluble E-selectin, prostacyclin, and tissue-plasminogen activators to keep vascular patency [35, 36]. More recently, a strong correlation has also been found between the status of endothelium and CVDs, which may serve as early indicators to predict cardiovascular disorders [37]. Such reliable indicators could be classified into two main categories, including (a) cytokines mentioned above and (b) EPCs. Since EPCs possess huge potential in endothelium regeneration, predicting endothelial dysfunction and achieving rapid endothelialization through EPCs are of great significance for the treatment of CVDs [38]. Although the other approach for rapid endothelialization, which is so-called EC pre-seeding, also presented promising effects, the clinical trials showed a 3-week lag phase in preventing thrombus after implantation [39, 40]. In addition, the poor engraftment of host mural cells as well as low survival rate of ECs has also hampered its further development [41]. Therefore, researches on capturing circulating EPCs through cardiovascular biomaterials have been paid much attention.
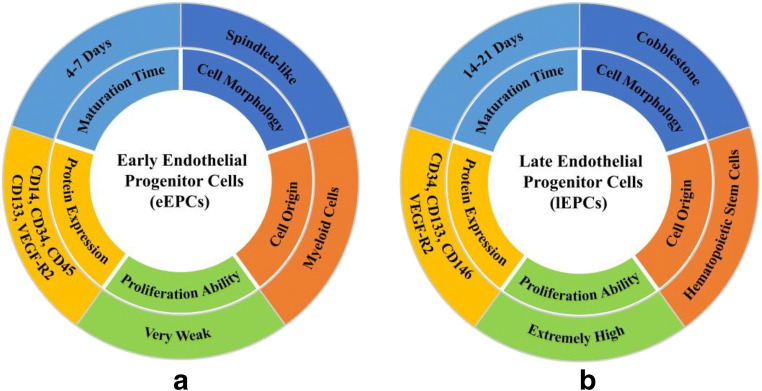
Depending upon the time of culture, EPCs can be classified into two types, which are called early EPCs (eEPCs) and late EPCs (lEPCs), respectively [42]. The reason for this division is based on their maturation time; eEPCs emerged after 4–7 days of culture while 14–21 days for lEPCs [43]. Although it is literally just a difference in culturing time, the distinctions of the two subpopulations are as follows (Fig. 1): (i) Cell origin—eEPCs, whose properties similar to CD14+ cells, are also defined them as CD14+ EPCs. Rehman et al. reported that the origin of these cells is myeloid or hematopoietic progenitor cells [44], whereas lEPCs, also named them as outgrowth endothelial cells (OECs) or CD34+ EPCs, are similar to the circulating bone marrow-derived from CD34+ hematopoietic stem cells [36]; (ii) Cell morphology—the eEPCs exhibit a spindled-like morphology while the lEPCs often express cobblestone morphology [45]; (iii) protein expression—it has already been proved that both of the subpopulations could express some identical surface makers, such as CD34, CD133, and vascular endothelial growth factor receptor 2 (VEGF-R2, also known as kinase insert domain receptor) [46, 47]. However, several other cells including dendritic cells and macrophages could also respond to VEGF-R2 while CD34 could be expressed by megakaryocytes [21, 48, 49]. Therefore, the applications of utilizing these nonspecific factors to capture EPCs remain many limitations. In addition to the abovementioned factors, most cytokines, whatever types or amount, suffer great differences between the two. Yoon et al. demonstrated that eEPCs express CD14 and CD45 whereas lEPCs do not show the expression of the antigens [50]. Their previous research also reported that lEPCs showed a higher expression level of kinase insert domain receptor (KDR) and chemokine receptor 1 (CXCR-1) compared with eEPCs [51]. On the contrary, the expression of other surface markers by lEPCs, such as WAS and LYN which is related to hematopoiesis, is less than eEPCs [52]; and (iv) cell proliferation ability and differentiation ability—Zhang et al. reported that the proliferation ability of eEPCs is so weak that they can hardly be passaged. However, lEPCs possess extremely high proliferation potential and the capacity to form capillary-like tubes, which is similar to microvascular ECs [53, 54].
Fig. 1.
a The characteristics of eEPCs and b the characteristics of lEPCs
The mechanisms of EPCs in treating CVDs
It is generally accepted that EPCs could migrate from bone marrow into circulating blood and then home into the injured vascular sites when thrombosis occurs [55]. Shantsila et al. reported that circulating EPCs are responsible for postnatal neovascularization due to the high proliferation potential [56]. Accordingly, EPCs exert their effects based on the following two mechanisms: Firstly, during the formation of vascular networks, EPCs can incorporate into new vascular tissues directly. Secondly, EPCs also release several angiogenic mediators including vascular endothelial growth factor (VEGF), NO and matrix metalloproteinase (MMPs), etc., contributing to both endothelial repair as well as neointimal formation [57, 58]. Studies on these factors confirmed that they can accelerate the endothelialization process at some degree. For instance, VEGF, an important factor for angiogenesis, can not only promote EC migration and proliferation but also accelerates thrombosis resolution [59]. Li et al. immobilized VEGF on the MePBMTM coatings through covalent bonding [60]. After culturing for 3 days, a large number of EPCs with completely expanded state were observed on MePBMTM-VEGF sample. Besides, the platelets adhered on MePBMTM-VEGF sample not only remained a dendritic shape but also presented the lowest platelet adhesion level among all the groups. The experiments demonstrated that surface modification via VEGF could simultaneously address two issues: endothelialization and preventing thrombus formation. NO is also a crucial mediator in vascular endothelialization by supporting the behaviors of ECs migration and proliferation and EPCs differentiation. On the other hand, the adhesion of platelets and SMCs could also be inhibited by NO via upregulating cyclic guanylate monophosphate (cGMP) [61]. Yang et al. developed a one-step method to construct NO-generating coatings by dipping dopamine (Dopa) and selenocystamine (SeCA) in aqueous solution [62]. The results suggested that the SeCA-Dopa coatings could enhance the migration and proliferation of ECs while simultaneously inhibit these activities of SMCs due to the generation of NO gas. MMPs, an important mediator in thrombus formation, are increasingly valued thanks to their functions in capillary formation and neovascularization [63]. Kanayasu et al. discovered that MMPs could also stimulate ECs and EPCs through degrading extracellular matrix (ECM) components [64]. Therefore, mediator regulation is an extremely important mechanism for EPCs in treating CVDs.
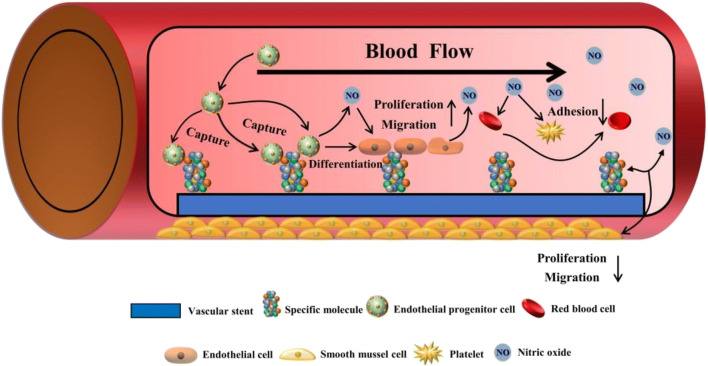
What occurs when the vascular endothelium is injured? Initially, the neighboring mature ECs were thought to replace the injured vascular sites through migration and proliferation. However, only a small number of ECs were detected in the injured endothelium [65]. It seems to imply that there may be another repair mechanism (Fig. 2). Several studies related to the phenomenon were reported both in human and animal models. For example, in mice model, a sharp increase in the number of EPCs recruited by VEGF had been discovered by Kallka and his colleges [66]. Further studies also suggested that EPCs have the capability to form capillary-like tubes in ECM environment in vitro and thereby participating in constructing vascular networks [67]. Additionally, in vivo experiments reported that cells which could secret VEGF-R2, such as immature CD133+ hematopoietic stem cells, maintained a high degree of coverage on the vascular implants, leading to the further recruitment of EPCs and re-endothelialization process [68]. Through a balloon injury model, EPCs were found to localize to neovascularization sites spontaneously. At the same time, the inhibition on SMCs activities and neointima formation were also detected [69]. The overall results not only indicated that EPCs play an important role in re-endothelialization process but also open a new approach to treat CVDs.
Fig. 2.
The schematic diagram of vascular repair by capturing circulating EPCs. After being recognized by specific molecules on cardiovascular biomaterials, the EPCs can home into the damaged sites where they differentiate into endothelial cells, thereby accelerating the re-endothelialization process. NO, released by the EPCs and mature ECs, could simultaneously inhibit the adhesion of platelets and SMCs, as well as promoting the growth of ECs
In addition to the promotion effect in repairing vascular endothelium, it is well accepted that EPCs could also exert huge influence on thrombus propagation. Several studies in human experiments have confirmed this fact. Miglionico et al. reported a clinical trial consisted of 80 patients who received ECS surgery [70]. Sixteen months clinical observation showed that the average stenosis for these patients was 2.2 ± 2.5% and the minimal lumen diameter was 3.3 ± 0.5 mm, both significantly lower than preoperative. The experiments strongly demonstrated that the ECS, with almost no formation of thrombus propagation, could simultaneously achieve resist coagulation and promote endothelialization. Accordingly, Li et al. anticipated that the phenomenon may be attributed to the effect of antithrombogenic mediators, such as NO and prostaglandin I2 (PGI2) [71]. NO, as mentioned above, can not only increase ECs proliferation but also suppresses platelet adhesion and aggravation. PGI2 is one of the most important substances secreted by EPCs, resulting in the inhibition on platelets and reduced risk of thrombus propagation [72].
The role of EPCs as potential diagnostic biomarkers in predicting CVDs
The number fluctuations of EPCs as predictive biomarkers of CVDs
Predictive diagnostics and targeted prevention, the two main aspects of PPPM, have been widely recognized by not only experts but also patients in recent years [73]. Encouraged by PPPM, the prevention of chronic symptoms related to CVDs has attracted much attention. Current data have demonstrated that several chronic conditions including peripheral arterial disease (PAD), coronary artery disease (CAD), and hypertension are strongly related to the number of EPCs [74, 75]. Therefore, the number fluctuation of EPCs could serve as an important diagnostic biomarker of these diseases, which is in great accordance with the concept of PPPM.
PAD, a serious complication caused by diabetes, threatens almost 20% elderly aged over 65 and it causes striking mortality rate [75]. Researchers have preliminary explored the relationships between number of EPCs and patients with PAD (Table 1). It was shown by Fadini that diabetes patients with PAD have decreased number of EPCs compared with those with diabetes alone [76]. Their further experiments also proofed the view that diabetes patients with PAD had lower expression levels of CD34, CD133, and KDR compared with diabetes free from PAD [77]. In order to determine the differences between subjects with and without PAD, Hayek et al. defined the CD34+/KDR+ counts as the number of EPCs [78]. The results showed that the EPCs counts in CAD patients with PAD symptom suffered a nearly 50% reduction compared with those with only CAD. Similar results were also reported by Bitterli et al. when compared with healthy subjects [79]. However, different measurement standards may cause completely different counting results. Morishita et al. and Delva et al. considered the amounts of CD34+/CD133+/KDR+ cells as the number of EPCs and reported that PAD patients seem to have an increase in both number and function levels of EPCs [80, 81]. Besides, the varieties of proteins related to EPCs mobilization including pentraxin-3 and membrane type 1 matrix metalloproteinase (MT1-MMP) were also detected. Pentraxin-3, secreted by endothelial cells, could intuitively reflect inflammation conditions of endothelium, while the expression of MT1-MMP was highly related to vascular remodeling [75, 89, 90]. The results showed that the concentrations of pentraxin-3 were upregulated while MT1-MMP was downregulated in PAD patients when compared with healthy subjects. All in all, no matter which kinds of measurement standards, it is feasible to predict PAD according to the number of EPCs.
Table 1.
A brief overview of several current studies on the relationships between EPCs and CVDs
| Study | Subjects | Types of EPCs | EPCs number | EPCs function |
|---|---|---|---|---|
| Fadini et al. [76] |
Diabetes patients with PAD Healthy |
CD34+/KDR+ | CD34+/KDR+ ↓ | No data |
| Fadini et al. [77] |
Diabetes patients with PAD Diabetes patients free from PAD |
CD34+/CD133+/KDR+ | CD34+/CD133+/KDR+ ↓ in diabetes patients with PAD | Clonogenic and adhesion ability ↓ compared with diabetes patients without PAD |
| Hayek et al. [78] |
CAD patients with PAD CAD patients free from PAD |
CD34+/KDR+ | CD34+/KDR+ ↓ in CAD patients with PAD | No data |
| Bitterli et al. [79] |
PAD patients Diabetes patients with PAD Healthy |
CD34+/KDR+ | CD34+/KDR+ ↓ in PAD patients | Proliferation ability ↓ in PAD patients |
| Morishita et al. [80] |
PAD patients Healthy |
CD34+/CD133+/KDR+ | CD34+/CD133+/KDR+ ↑ | pentraxin-3 ↑ and MT1-MMP ↓ in PAD patients |
| Delva et al. [81] |
PAD patients Healthy |
CD34+, CD133+, CD34+/KDR+ CFU-ECs | CFU-ECs ↑, CD34+ and CD133+ ↓ | Proliferation ability ↑ in PAD patients |
| Vasa et al. [82] |
CAD patients Healthy |
CD34+/KDR+ | CD34+/KDR+ ↓ | Migratory ability ↓ in CAD patients |
| Briguori et al. [83] | CAD patients | CD34+, CD133+, CD34+/KDR+, CD34+/VE-cadherin+, CFU-ECs | CD34+/KDR+, CD34+/ VE-cadherin+ and CFU-ECs ↓ in CAD progressors | Proliferation and differentiation ability ↓ in CAD progressors |
| Eizawa et al. [84] |
CAD patients Healthy |
CD34+ | CD34+ ↓ | No data |
| Pirro et al. [85] | Essential hypertension (never treated) | CD34+/KDR+ | CD34+/KDR+ ↓ | HOXA9 expression ↓ |
| Essential hypertension (treated with Ramipril) | CD34+/KDR+ ↑ | HOXA9 expression ↑ | ||
| Oliveras et al. [86] | Hypertension patients | CD34+/CD45+/CD133+ | CD34+/CD45+/CD133+ ↓ | No data |
| Budzyń et al. [87] | Resistant hypertension patients | CEPCs/CECs ↓ | Triglycerides ↑ | |
| Mild hypertension patients | CEPCs/CECs ratio | CEPCs/CECs ↓ | LDL-cholesterol and hsCRP ↑ | |
| Szpera-Goździewicz et al. [88] |
Pregnant women with chronic hypertensive Healthy |
CEPCs/CECs ratio | CEPCs/CECs ↓ | vWf expression ↓ |
Increasing evidence has confirmed that the circulating EPCs are responsible for the endothelium repair after severely damaged, which means a reduction in EPCs counts may reflect whether a patient has CAD syndromes [91]. The number and function levels of EPCs have been reported to inversely correlate with risk factors for CVD-related symptoms such as age, smoking, and hypertension [92]. Vasa et al. revealed that smoking is the primary culprit for the reduction of EPCs, while the impaired levels of EPCs migration toward VEGF may be attributed to hypertension [82]. Taking 2-month clinical results as an example, patients diagnosed with CVDs were more likely to be smokers with a high prevalence of older population [92]. These risk factors could barricade off signaling pathways of granulocyte-macrophage colony-stimulating factor (GM-CSF) and VEGF as well as those associated with cells differentiation and migration [75]. Despite the common risk factors, patients diagnosed with CAD also have individual factors such as family history. Another representative example is a study involving 45 patients with CAD syndromes; the results showed that those suffered from family history presented a slight reduction in EPCs migration, which supports family history as one of the individual factors. Therefore, the family history, as an important composition of “individualized patient profile,” could serve as an early indicator for CAD prediction [93]. With the applying of flow cytometry technique, the counts of circulating EPCs in human blood could be determined as CD34+/KDR+ cells. For this, Vasa et al. observed a 48% reduction in CAD patients compared with healthy subjects [82]. In addition to the number of CD34+/KDR+, Briguori et al. also evaluated the number of CD34+/vascular endothelial-cadherin+ (VE-cadherin+) as well as endothelial cell colony-forming units (CFU-ECs) with the purpose of clarifying the variety of EPCs levels during the CAD progression [83]. Specifically, both levels of CD34+/KDR+ and CD34+/VE-cadherin+ in CAD progressors were reduced by 37.5%, whereas those levels of CFU-ECs were also reduced by 28% compared with nonprogressors. More importantly, the author pointed out that the levels of CFU-ECs could predict CAD progression more precisely than CD34+/KDR+. Eizawa et al. also noted similar findings when considered CD34+ cell populations as an indicator for the levels of EPCs [84]. In summary, reduced levels of EPCs including the cells of CD34+, CD34+/KDR+, CD34+/VE-cadherin+, and CFU-ECs may serve as indicators to predict potential patients with CAD.
Hypertension (particularly chronic hypertension), one of the major risk factors for cardiovascular, is emerged as the strongest indicator of EPCs migratory impairments [82]. By utilizing the above mentioned quantifying methods, although decreasing number of EPCs from hypertension patients were observed, we must acknowledge that these methods could not reflect the balance between regeneration and degradation of vascular endothelium [85, 86]. Therefore, a number of studies have focused on the ratio between CEPCs and circulating endothelial cells (CECs). The detachment of CECs from vascular walls could be attributed to various kinds of physiological mechanisms, such as apoptosis, mechanical damage, and the lack of anchoring proteins [94]. The number of CECs is thereby considered to be a diagnostic biomarker for endothelial dysfunction. On the contrary, CEPCs are mainly originated from the bone marrow and tend to home into the damaged endothelium and then form new layers. Therefore, the number of CEPCs represents the capacity of endothelial regeneration at some degree. With this quantifying method, Budzyń et al. reported a significant lower CEPCs/CECs ratio in hypertension patients compared with healthy subjects, which indicated an insufficient process of endothelial regeneration in hypertension conditions [87, 95]. Moreover, it was shown that a CEPCs/CECs ratio below 2.72 highly indicated a potential risk of hypertension. Szpera-Goździewicz et al. compared the levels of CEPCs/CECs between the healthy control and the pregnant patients with different hypertension disorders. It seemed that patients with chronic hypertension tend to present the lowest number of CEPCs and the highest number of CECs, leading to the lowest value of CEPCs/CECs among all the groups [88]. Although further studies are required to focus on the detailed mechanisms of EPCs in predicting hypertension, the accumulated data has already showed us a promising future of EPCs in predicting hypertension.
The inflammation related to EPCs as predictive biomarkers of CVDs
Recently, certain secondary complications especially inflammatory have been considered a link between macrophages and EPCs. That is because certain subpopulations of EPCs can be academically classified as immune cells [96]. For example, of the many known cells, circulatory angiogenic cells, eEPCs, and CFU-ECs are the representative derived from monocyte lineage, while angiogenic T cells are one of the subpopulations of CFU-ECs [96]. Previous studies have demonstrated that systemic inflammation could serve as an impetus for homing of EPCs, which plays an essential role in the regeneration of injured endothelium [97]. Under these circumstances, certain inflammatory responses were proposed as another predictive biomarker of CVDs. Holmen et al. noted that the inflammatory endothelium will lead to the detachment of inflammatory endothelial cells (IECs) from injury sites, and those cells may in turn contribute to persistent vascular damage by inducing the dysfunction of EPCs [98]. Besides, an inverse correlation should be noted between inflammatory cytokines (e.g., macrophage inflammatory protein-1δ, E-Selectin, TNF-α) and EPCs levels in essential hypertension patients, suggesting the recruitment of EPCs in the process of endothelial regeneration may be inhibited by macrophages. The shift in macrophage phenotype from an inflammatory type to a common can be achieved through the interactions with EPCs, by which the inflammatory symptoms as well as the release of cytokines could be inhibited [99]. In addition to these general inflammatory responses, more emphasizes should be placed on the symptoms of individuals. Since personalized prevention is the most effective way to prevent cardiovascular disease. However, the role of EPCs in immune system is not well defined, which means further studies are required to provide a theoretical basis for personalized prevention.
The different approaches for cardiovascular biomaterials to capture EPCs
To improve the therapeutic effect of ECS, recent studies have paid much attention on surface modification via biomolecules. In order to achieve rapid endothelialization, EPC-specific antibodies, aptamers, peptides and magnetic molecules, and four main types of modified biomolecules have been widely utilized on cardiovascular biomaterials [100].
Applications of specific antibodies in EPCs capturing
Antibodies, a class of immunoglobulins that can specifically bind to antigens, are produced by human body due to the stimulation of antigen. Several types of antibodies such as anti-CD34, anti-CD133, and anti-CD146 have been applied to ECS due to their specific functions in cell recognition. Wu et al. immobilized heparin, Arg-Glu-Asp-Val (REDV) peptide, and anti-CD34 on Mg-Zn-Y-Nd surface which was alkali treated and silanized before, hoping to construct a multifunctional surface with superior blood compatibility and cytocompatibility [101]. The results indicated that the Hep/REDV/anti-CD34 coatings exhibited the biggest amount of NO release after 12-h culture, which markedly promoted ECs adhesion and proliferation. Besides, owing to the recognition ability of anti-CD34, the Hep/REDV/anti-CD34 coatings also presented much more EPCs attachment compared with other groups. Although the anti-CD34 modified surface had a good performance in capturing EPCs, convincing evidence found by Chen and co-workers has confirmed that the attachment of SMCs on anti-CD34 surface was no less than BMS, suggesting that the anti-CD34 surface was unable to prevent the adhesion and proliferation of SMCs [102]. An opposite case is that anti-CD34 was covalently prepared onto the polyethylene glycol (PEG) based Ti surface which was alkali heated and silanized in advance, improving the attachment of EPCs and inhibiting SMCs as well [103]. However, after cultivation for 3 and 5 days, the number of EPCs on the anti-CD34 coated surface is far less than the bare substrate. That means the coating may even prevent the proliferation of EPCs. Aiming to improve the capture efficiency of EPCs, Chen et al. also explored the influence of anti-CD34 orientation on biological effect [104]. Compared with the random immobilization, the oriented immobilized anti-CD34 not only had higher capture ability and efficiency but also expressed almost 3.48 times immunological binding activity. Unfortunately, the remained problem of SMCs attachment was still unresolved. Numerous clinical experiments have also demonstrated that anti-CD34 is defective to capture EPCs due to its nonspecific recognition [105]. For example, CD34 could also be expressed on hematopoietic stem cells, eEPCs and even on palates, which may lead to severe restenosis after surgery.
Based on this reason, researchers have focused on novel antibodies such as CD133, CD146, and anti-VE-cadherin. Duan et al. constructed a CD133/VEGF coating on a dopamine-hyaluronic acid/heparin-based layer [106]. Regardless of the static or stress conditions, the co-immobilized surface had the capability of re-endothelialization by enhancing the capture ratio of EPCs. In addition, with the cultivation time going on, the mRNA expression level of CD133 on co-immobilized surface suffered a great loss, while the mRNA of VEGF-R2, CD31, VE-cadherin, and von Willebrand factor (vWF) expressed by ECs were significantly enhanced, indicating that the co-immobilized surface could support the differentiation from EPCs into ECs as well. Wawrzyńska et al. constructed a biocompatible surface via immobilizing anti-CD133 on a 316 L SS surface to control the cell behaviors [7]. The results showed that the anti-CD133 modified surface not only exhibited the strong promotion effect on EPCs adhesion but also dramatically reduced the risk of neointima hyperplasia and ISR by inhibiting the proliferation of SMCs. Although it is convinced that the anti-CD133-coated stent is better in capturing EPCs compared with anti-CD34, the number of cells that are sensitive to CD133 is too small, leading to the limited response to CD133. Recently, Park et al. prepared anti-CD146 combined with nanostructured SiNf on a CoCr stent, aiming at achieving rapid endothelialization and preventing neointima formation [107]. By using a porcine model, the modified stent presented an increased cell capture by approximately 8 times compared with the bare stent. The in vivo results also presented that the modified stent had the lowest neointimal ratio among all the groups. Both in vitro and in vivo experiments demonstrated that the co-existence of anti-CD146 and SiNf could target EPCs precisely and achieve rapid endothelialization. A similar case is that VE-cadherin was immobilized on PEG-coated stent, promoting endothelialization as well as preventing restenosis [108].
Applications of aptamers in EPCs capturing
Aptamers, a sequence of single-stranded RNA/DNA oligonucleotides with high affinity toward targets, are synthesized via a process named “systematic evolution of ligands by exponential enrichment” (SELEX) [109]. Aptamers were noticed due to their superior features including strong selective targeting abilities and super chemical stability. Even under biological conditions of extreme pH or temperature, they could still maintain more sensitive affinity than antibodies. Additionally, aptamers are more convenient to be synthesized and modified compared with antibodies [110]. Based on these superior features, plenty of studies are conceived to modify ECS by immobilizing aptamers.
With the help of SELEX technology, the ssDNA aptamers with high affinity to EPCs were prepared on star- PEG coatings by Hoffmann [111]. By using a porcine model, they demonstrated that the EPCs which were recognized by aptamers could differentiate into ECs within 10 days. Dopamine, well known for its mussel inspired functions, has been widely applied to construct a rich in amine surface [112]. Qi et al. used plasma polymerized allylamine (PPAam) platforms to absorb DNA aptamers through electrostatic interaction [113]. After co-culturing for several hours, the ratio of EPCs/SMCs on PPAam-DNA surface was nearly 2 times than that of the PPAam and 316 L SS. Similar results were also observed in the case of EPCs/ECs. The ratio of PPAam-DNA increased by about 150% compared with the PPAam and 316 L SS, and no significant difference was found between the control groups, indicating that the modified surface had extremely high affinity in capturing EPCs under static conditions. To further investigate the EPCs-capture ability under dynamic conditions, Li et al. and Deng et al. immobilized DNA aptamers onto a dopamine-coated surface and then placed them in a flow chamber [114, 115]. After 4 h, both of their samples modified with DNA aptamers captured the largest number of EPCs among all the groups. These results suggested that the aptamers have great potential as bioactive molecules in realizing rapid endothelialization. However, aptamers are not always specificity in cell interaction. There is evidence that the capture ability may be affected by the affinity range. Yoon et al. prepared three clones of CD31 aptamer at a concentration of micro-meters range on EPCs and 293FT cells, aiming to test the feasibility of CD31 aptamer in visualizing EPCs [116]. But the nonspecific recognition was detected on both surfaces, which suggested that the appropriate affinity range plays an important role in specific interaction. Unfortunately, this field has not been valued in most studies. Under these circumstances, continued exploration in aptamers specificity, as an important point to improve the therapeutic effect of CVDs, was required to be done in further studies.
Applications of specific peptides in EPCs capturing
ECM, a friendly microenvironment for EPCs, plays an important role in promoting cell interaction, differentiation, and proliferation [117]. In addition to the above functions in cell behaviors, the ECM provides biochemical and mechanical supports for cells as well [118]. Typically, ECM is mainly composed of proteins and small bioactive molecules. As a consequence, remodeling a functional surface with ECM-derived proteins and small peptides is an ideal strategy to achieve rapid endothelialization. However, surface modification with proteins may cause a series of implications including proteolytic decomposition and conformational changes [119]. On the contrary, such serious implications can be minimized by the peptides due to the more stable structure [120]. Thus, it is widely accepted that peptides modification could realize better cell interaction in comparison with proteins.
The tripeptide sequence Arg-Gly-Asp (RGD), which can be specifically recognized by cell mediators such as αvβ3 and α5β1, has been widely applied to improve the recruitment of EPCs. In order to evaluate the effects of a cyclic Arg-Gly-Asp peptide (cRGD) in recruiting EPCs, Blindt et al. used a newly polymer coating to load cRGD onto the stainless steel [121]. The dynamic capture experiments showed that the cRGD-modified stents captured a great number of EPCs while much fewer for bovine serum albumin-coated stents. Although the cRGD at a concentration of 100 μg/ml resulted in an increase in attached cell number compared with 1 μg/ml, the gap between the two was almost negligible. Similar tendency was also observed in the EPC- and SMC-specific recognition experiments. It means that they had not determined an appropriate amount of cRGD loaded on the stents. Further research by Le Saux et al. confirmed that RGD at a density of 0.1 fmol/cm2 is the lowest density to promote ECs adhesion and that 10 fmol/cm2 is required for the cells spreading on the RGD modified surface [122]. Based on this theory, Royer et al. immobilized GRGDS at a density of 1.9 ± 0.1 pmol/mm2 to fabricate a polyethylene terephthalate (PET) micro-patterned surface in which sitagliptin was combined [123]. Regardless of the different sizes of patterns, the GRGDS/Sita surface largely reduced the number of CD 34 positive cells by about 50%, indicating that the combination of the two peptides could induce the differentiation of EPCs into ECs. However, due to the general adhesive property of GRGD peptide, it is impossible to avoid nonspecific interactions between GRGD and other cells or proteins [124].
In order to solve this problem, Hao et al. then developed a novel cyclic peptide named LXW7 which mainly achieves specific recognition to EPCs/ECs via αvβ3 integrin [125]. Contrary to the typical cRGD peptide, LXW7 possesses highly binding selectively to EPCs/ECs, low affinity to platelets, as well as no affinity to monocytes, which can be attributed to its stable cyclic structure. To further evaluate the guidance of LXW7 on EPCs, Hao et al. immobilized LXW7 on polycaprolactone/poly (L-lactic acid) (PCL/PLLA) surface through a click chemistry method [126]. The results showed that endothelial colony forming cells (ECFCs), a subpopulation of EPCs, were specifically captured from circulating blood by the modified surface. While at the same time, a mouse model also demonstrated that the modified surface was able to minimize thrombus formation and thereby maintaining vascular patency, which indicated an excellent ability in promoting re-endothelialization.
TPS is another EPC-specific peptide and has been used as an aptamer for recruiting EPCs in the past few years. Chen et al. used a two-step condensation reaction to prepare dopamine and TPS peptide onto a titanium (Ti) surface [127]. The modified surface not only showed obvious inhibition effects on platelets attachment but also supported the adhesion and proliferation of EPCs. Through a CCK-8 assay and in vivo experiments, similar results were also observed on a TPS and bovine serum albumin co-immobilized Ti surface [128]. It seems that the incorporation of TPS peptide and bioactive molecules could construct a suitable microenvironment for EPCs. In addition, other EPC-adhesive peptides, such as hemocompatible peptide-1 (HCP-1) and YIGSR, have also been proved to exert an outstanding effect on recruiting EPCs [40, 129].
Applications of magnetic molecules in EPCs capturing
Magnetic molecules, which are effective in tracking and capturing EPCs, act through an external magnetic field (EMF) to localize EPCs to the injured vessels [130]. Based on this theory, superparamagnetic iron oxide nanoparticles (SPION) have been widely applied in surface modification. Wilhelm et al. reported that the biological behaviors of EPCs could be influenced by particle sizes as well as the concentrations of SPION [131]. Since then, a series of exploration have been reported on the impacts of the above two factors on tracking EPCs. Carenza et al. reported that the capture efficiency for the large aggregated nanoparticles was seven times higher compared with those of the dispersed group, with almost equivalent cell viabilities and functions [132]. As for the best choice for the SPION concentration, it was initially assumed that the concentrations ranged within 0–50 μg/ml were safe for biological activities of EPCs [133, 134]. Later, with no significant weaken in proliferation potential and cell viabilities between labeled and unlabeled EPCs, the safe concentration was extended to 70 μg/ml by Wei and co-workers [135]. However, several studies also obtained satisfactory results at a concentration of 100 μg/ml, which seem to imply that the safest concentration of SPION for EPCs tracking remains unclear.
In addition, researchers have paid more attention to the development of new SPION materials. Zhang et al. successfully prepared a 10-nm thick single silica layer to wrap around a SPION core [136], while the silica-coated SPION in rat models significantly increased the aggregations of EPCs around the infracted area where an EMF was applied, accompanied by an obviously enhanced density of capillaries and reduced area of infraction. More importantly, it seems to be the first report that an applied EMF can significantly facilitate the retention of EPCs in the ischemic myocardium. Recently, a novel method combined the SPION with specific antibodies was confirmed to have high affinity towards EPCs. Aiming at attracting EPCs to the injury sites via EMF to achieve re-endothelization, Chen et al. modified Fe3O4 stent with anti-CD34 and citric acid (CA) [137]. As expected, compared with the group modified with single anti-CD34 or CA, the incorporation of the two significantly increased the affinity of EPCs toward iron stent, indicating the excellent ability of SPION in attracting EPCs. To further detect the effects of EMF on dynamic EPCs capturing, the iron samples were placed in a flow chamber at a flow rate of 1 m/s. Three hours later, only a few cells were observed on the iron sample with no application of EMF. On the contrary, the stent under a 300-mT EMF was almost completely covered by EPCs. Therefore, this approach showed no inhibition effects on EPCs and possessed ability to guide the EPCs to the desired region and thereby achieve re-endothelization.
Conclusions and expert recommendations
Depending upon the high proliferation potential, repairing the damaged endothelium by EPCs has been confirmed as a promising approach to accelerate re-endothelialization. There is no doubt that a better comprehension in EPCs biology could direct the modification strategies for cardiovascular biomaterials, but more importantly, it also plays a key role in the prevention and treatment of CVDs. Besides, applying the number fluctuations of EPCs in our bodies not only provide us early diagnostics and personalized prevention strategies but also practice the most advanced cost-effective approach in biomedical sciences and healthcare. Personalized prevention will definitely become an important strategy for the prevention and treatment of CVDs in the future. These tasks should be developed based on the number and function levels of EPCs in individualized patient. However, we must admit the current limitations associated to the methods in EPCs quantification. First, almost all the studies have a common limitation; that is, the number of subjects involved was too small. This factor may cause the experimental results to be non-universal. Secondly, it is widely accepted that the standardized criteria for isolating and quantifying EPCs are lacking, leading to the undesired alterations in functions of EPCs and thereby affecting experimental results in varying degrees. Therefore, we implicate that the standardized criteria as a significant yet underestimated factor for EPCs quantification should be noted in further studies.
The present review mainly focused on the basic introduction of EPCs, the mechanisms of EPCs in treating CVDs, and the potential diagnostic role of EPCs in predicting CVDs as well as capturing EPCs with some specific molecules including EPC-specific antibodies, aptamers, peptides, and magnetic molecules. Although numerous results showed that the re-endothelialization process can be accelerated at some degree, the affinity of these modified biomaterials toward EPCs still needs to be improved to meet the clinical requirement. Thus, how to enhance the combination efficiency between biomaterials and EPCs remains the main subject for further studies.
In order to address the unresolved issue, the following perspectives for future research may deserve more attention:
Shear stress to enhance the adhesion between EPCs and cardiovascular biomaterials. Accumulating evidence has proved that the adhesion of EPCs to a surface could be enhanced by pretreating with unidirectional laminar shear stress, but more importantly, the preimplantation adhesion of EPCs also largely promotes the regenerative potential of EPCs, including the capability of homing injuries, forming capillary-like tubes, as well as differentiation into mature ECs and thereby accelerating the process of endothelialization. Unfortunately, the perspective was hardly considered in many current studies and deserves more attention in future research.
Modifications to minimize the adhesion of plasma proteins. EPCs are captured by the biomaterials through interactions with the bioactive sites provided by the specific molecules. However, the redundant plasma proteins such as fibrinogen may take the lead in binding with the bioactive sites, subsequently weakening the affinity of biomaterials toward EPCs. Therefore, both the amounts of absorbed plasma proteins and capture efficiency of EPCs should be considered in future research.
Abbreviations
- CVDs
Cardiovascular diseases
- BMS
Bare metal stents
- DES
drug-eluting stents
- EPCs
Endothelial progenitor cells
- PPPM
Predictive, preventive and personalized medicine
- SMCs
Smooth muscle cells
- ISR
In-stent restenosis
- ECS
Endothelial progenitor cell capturing stent
- ECs
Endothelial cells
- CEPCs
Circulating endothelial progenitor cells
- NO
Nitric oxide
- eEPCs
Early endothelial progenitor cells
- lEPCs
Late endothelial progenitor cells
- OECs
Outgrowth endothelial cells
- KDR
Kinase insert domain receptor
- CXCR-1
Chemokine receptor 1
- MMPs
Matrix metalloproteinase
- VEGF
Vascular endothelial growth factor
- cGMP
Cyclic guanylate monophosphate
- Dopa
Dopamine
- SeCA
Selenocystamine
- ECM
Extracellular matrix
- VEGF-R2
Vascular endothelial growth factor receptor 2
- PGI2
Prostaglandin I2
- PAD
Peripheral arterial disease
- CAD
Coronary artery disease
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- VE-cadherin+
Vascular endothelial-cadherin+
- CFU-ECs
Endothelial cell colony-forming units
- CECs
Circulating endothelial cells
- lECs
Inflammatory endothelial cells
- REDV
Arg-Glu-Asp-Val
- PEG
Polyethylene glycol
- vWF
Von Willebrand factor
- SELEX
Systematic evolution of ligands by exponential enrichment
- PPAam
Plasma polymerized allylamine
- RGD
Arg-Gly-Asp
- cRGD
Cyclic Arg-Gly-Asp
- PCL
Polycaprolactone
- PLLA
Poly (L-lactic acid)
- ECFCs
Endothelial colony forming cells
- TPS
TPSLEQRTVYAK
- HCP-1
Hemocompatible peptide-1
- EMF
External magnetic field
- SPION
Superparamagnetic iron oxide nanoparticles
- CA
Citric acid
Funding
This research was funded by National Key Research and Development Program of China (2017YFB0702500, 2018YFC1106703 and 2016YFC1102403), and Top Doctor Program of Zhengzhou University (grant number 32210475).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lijie Xiang, Email: ljxiang@suda.edu.cn.
Jingan Li, Email: lijingan@zzu.edu.cn.
References
- 1.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: The sixth joint task force of the european society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Atherosclerosis. 2016;252:207–274. doi: 10.1016/j.atherosclerosis.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Zheng B, Wang P, Wang X, Zhang B, Shi Q, Xi T, Chen M, Guan S. Enhanced in vitro and in vivo performance of Mg-Zn-Y-Nd alloy achieved with APTES pretreatment for drug-eluting vascular stent application. ACS Appl Mater Interfaces. 2016;8(28):17842–17858. doi: 10.1021/acsami.6b05038. [DOI] [PubMed] [Google Scholar]
- 3.Borhani S, Hassanajili S, Ahmadi Tafti SH, Rabbani S. Cardiovascular stents: overview, evolution, and next generation. Prog Biomater. 2018;7(3):175–205. doi: 10.1007/s40204-018-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuster V. Top 10 cardiovascular therapies and interventions for the next decade. Nat Rev Cardiol. 2014;11(11):671–683. doi: 10.1038/nrcardio.2014.137. [DOI] [PubMed] [Google Scholar]
- 5.Cyrus T, Wickline SA, Lanza GM. Nanotechnology in interventional cardiology. Wiley Interdiscip Rev-Nanomed Nanobiotechnol. 2012;4(1):82–95. doi: 10.1002/wnan.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischell TA. Editorial: the “JET” technique for provisional side-branch stenting back to the future, and in the right direction. J Interv Cardiol. 2017;30(6):535–536. doi: 10.1111/joic.12453. [DOI] [PubMed] [Google Scholar]
- 7.Wawrzynska M, Duda M, Wysokinska E, Strzadala L, Bialy D, Ulatowska-Jarza A, et al. Functionalized CD133 antibody coated stent surface simultaneously promotes EPCs adhesion and inhibits smooth muscle cell proliferation-a novel approach to prevent in-stent restenosis. Colloids Surf, B. 2019;174:587–597. doi: 10.1016/j.colsurfb.2018.11.061. [DOI] [PubMed] [Google Scholar]
- 8.Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Sophia V, Morger C, et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007;369(9562):667–678. doi: 10.1016/s0140-6736(07)60314-6. [DOI] [PubMed] [Google Scholar]
- 9.Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, Gold HK, Virmani R. Pathological correlates of late drug-eluting stent thrombosis - strut coverage as a marker of endothelialization. Circulation. 2007;115(18):2435–2441. doi: 10.1161/circulationaha.107.693739. [DOI] [PubMed] [Google Scholar]
- 10.Luscher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC, et al. Drug-eluting stent and coronary thrombosis - biological mechanisms and clinical implications. Circulation. 2007;115(8):1051–1058. doi: 10.1161/Circulationaha.106.675934. [DOI] [PubMed] [Google Scholar]
- 11.Veleva AN, Cooper SL, Patterson C. Selection and initial characterization of novel peptide ligands that bind specifically to human blood outgrowth endothelial cells. Biotechnol Bioeng. 2007;98(1):306–312. doi: 10.1002/bit.21420. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Chen Y, Liu T, Wang X, Liu Y, Wang Y, Chen J, Huang N. Covalent co-immobilization of heparin/laminin complex that with different concentration ratio on titanium surface for selectively direction of platelets and vascular cells behavior. Appl Surf Sci. 2014;317:776–786. doi: 10.1016/j.apsusc.2014.07.129. [DOI] [Google Scholar]
- 13.Woudstra P, De Winter RJ, Beijk MA. Next-generation DES: the COMBO dual therapy stent with genous endothelial progenitor capturing technology and an abluminal sirolimus matrix. Expert Rev Med Devices. 2014;11(2):121–135. doi: 10.1586/17434440.2014.882046. [DOI] [PubMed] [Google Scholar]
- 14.Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, Krapfenbauer K, Mozaffari MS, Costigliola V. Medicine in the early twenty-first century: paradigm and anticipation - EPMA position paper 2016. EPMA J. 2016;7:23. doi: 10.1186/s13167-016-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berger JS, Haskell L, Ting W, Lurie F, Chang S-C, Mueller LA, Elder K, Rich K, Crivera C, Schein JR, Alas V. Evaluation of machine learning methodology for the prediction of healthcare resource utilization and healthcare costs in patients with critical limb ischemia-is preventive and personalized approach on the horizon? EPMA J. 2020;11(1):53–64. doi: 10.1007/s13167-019-00196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferda J, Baxa J, Ferdova E, Kucera R, Topolcan O, Molacek J. Abdominal aortic aneurysm in prostate cancer patients: the “road map” from incidental detection to advanced predictive, preventive, and personalized approach utilizing common follow-up for both pathologies. EPMA J. 2019;10(4):415–423. doi: 10.1007/s13167-019-00193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golubnitschaja O, Costigliola V, EPMA. General report & recommendations in predictive, preventive and personalised medicine 2012: white paper of the European Association for Predictive, Preventive and Personalised Medicine. EPMA J. 2012;3(1):14. 10.1186/1878-5085-3-14. [DOI] [PMC free article] [PubMed]
- 18.Asahara T, Kawamoto A, Masuda H. Concise review: circulating endothelial progenitor cells for vascular medicine. Stem Cells. 2011;29(11):1650–1655. doi: 10.1002/stem.745. [DOI] [PubMed] [Google Scholar]
- 19.Asahara T, Murohara T, Sullivan A, Silver M, Vanderzee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 20.Chong MSK, Ng WK, Chan JKY. Concise review: endothelial progenitor cells in regenerative medicine: applications and challenges. Stem Cells Transl Med. 2016;5(4):530–538. doi: 10.5966/sctm.2015-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel J, Seppanen EJ, Rodero MP, Wong HY, Donovan P, Neufeld Z, Fisk NM, Francois M, Khosrotehrani K. Functional definition of progenitors versus mature endothelial cells reveals key SoxF-dependent differentiation process. Circulation. 2017;135(8):786–805. doi: 10.1161/circulationaha.116.024754. [DOI] [PubMed] [Google Scholar]
- 22.Urbich C, Dimmeler S. Endothelial progenitor cells - characterization and role in vascular biology. Circ Res. 2004;95(4):343–353. doi: 10.1161/01.Res.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 23.Ravindranath RR, Romaschin A, Thompson M. In vitro and in vivo cell-capture strategies using cardiac stent technology - a review. Clin Biochem. 2016;49(1–2):186–191. doi: 10.1016/j.clinbiochem.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Doyle B, Caplice N. A new source of endothelial progenitor cells - vascular biology redefined? Trends Biotechnol. 2005;23(9):444–446. doi: 10.1016/j.tibtech.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Finney MR, Greco NJ, Haynesworth SE, Martin JM, Hedrick DP, Swan JZ, Winter DG, Kadereit S, Joseph ME, Fu P, Pompili VJ, Laughlin MJ. Direct comparison of umbilical cord blood versus bone marrow-derived endothelial precursor cells in mediating neovascularization in response to vascular ischemia. Biol Blood Marrow Transplant. 2006;12(5):585–593. doi: 10.1016/j.bbmt.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 26.Pacilli A, Pasquinelli G. Vascular wall resident progenitor cells a review. Exp Cell Res. 2009;315(6):901–914. doi: 10.1016/j.yexcr.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 27.Wu H, Riha GM, Yang H, Li M, Yao Q, Chen C. Differentiation and proliferation of endothelial progenitor cells from canine peripheral blood mononuclear cells. J Surg Res. 2005;126(2):193–198. doi: 10.1016/j.jss.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 28.Zammaretti P, Zisch AH. Adult ‘endothelial progenitor cells’ - renewing vasculature. Int J Biochem Cell Biol. 2005;37(3):493–503. doi: 10.1016/j.biocel.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Fu G, Yu Z, Chen Y, Chen Y, Tian F, Yang X. Direct adsorption of anti-CD34 antibodies on the nano-porous stent surface to enhance endothelialization. Acta Cardiol Sin. 2016;32(3):273–280. doi: 10.6515/acs20150813a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu Y, Davison F, Zhang Z, Xu Q. Endothelial replacement and angiogenesis in arteriosclerotic lesions of allografts are contributed by circulating progenitor cells. Circulation. 2003;108(25):3122–3127. doi: 10.1161/01.Cir.0000105722.96112.67. [DOI] [PubMed] [Google Scholar]
- 31.Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105(7):2783–2786. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 32.Medina RJ, Barber CL, Sabatier F, Dignat George F, Melero Martin JM, Khosrotehrani K, et al. Endothelial progenitors: a consensus statement on nomenclature. Stem Cells Transl Med. 2017;6(5):1316–1320. doi: 10.1002/sctm.16-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butruk-Raszeja BA, Dresler MS, Kuzminska A, Ciach T. Endothelialization of polyurethanes: surface silanization and immobilization of REDV peptide. Colloids Surf, B. 2016;144:335–343. doi: 10.1016/j.colsurfb.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Chen H, Zhao Y, Xiong K, Li J, Chen J, Yang P, Huang N. Multifunctional coating based on EPC-specific peptide and phospholipid polymers for potential applications in cardiovascular implants fate. J Mater Chem. 2016;4(48):7870–7881. doi: 10.1039/c6tb01811d. [DOI] [PubMed] [Google Scholar]
- 35.Otsuka F, Finn AV, Yazdani SK, Nakano M, Kolodgie FD, Virmani R. The importance of the endothelium in atherothrombosis and coronary stenting. Nat Rev Cardiol. 2012;9(8):439–453. doi: 10.1038/nrcardio.2012.64. [DOI] [PubMed] [Google Scholar]
- 36.Zhang K, Liu T, Li J, Chen J, Wang J, Huang N. Surface modification of implanted cardiovascular metal stents: from antithrombosis and antirestenosis to endothelialization. J Biomed Mater Res A. 2014;102(2):588–609. doi: 10.1002/jbm.a.34714. [DOI] [PubMed] [Google Scholar]
- 37.Abebe W, Mozaffari M. Endothelial dysfunction in diabetes: potential application of circulating markers as advanced diagnostic and prognostic tools. EPMA J. 2010;1(1):32–45. doi: 10.1007/s13167-010-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Böhm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353(10):999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 39.Poole-Warren LA, Schindhelm K, Graham AR, Slowiaczek PR, Noble KR. Performance of small diameter synthetic vascular prostheses with confluent autologous endothelial cell linings. J Biomed Mater Res. 1996;30(2):221–229. doi: 10.1002/(sici)1097-4636(199602)30:2<221::Aid-jbm12>3.0.Co;2-p. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Munisso MC, Mahara A, Kambe Y, Yamaoka T. Anti-platelet adhesion and in situ capture of circulating endothelial progenitor cells on ePTFE surface modified with poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC) and hemocompatible peptide 1 (HCP-1) Colloids Surf, B. 2020;193:111113. doi: 10.1016/j.colsurfb.2020.111113. [DOI] [PubMed] [Google Scholar]
- 41.Li Q, Wang Z, Zhang S, Zheng W, Zhao Q, Zhang J, Wang L, Wang S, Kong D. Functionalization of the surface of electrospun poly (epsilon-caprolactone) mats using zwitterionic poly (carboxybetaine methacrylate) and cell-specific peptide for endothelial progenitor cells capture. Mater Sci Eng C-Mater Biol Appl. 2013;33(3):1646–1653. doi: 10.1016/j.msec.2012.12.074. [DOI] [PubMed] [Google Scholar]
- 42.Patel J, Donovan P, Khosrotehrani K. Concise review: functional definition of endothelial progenitor cells: a molecular perspective. Stem Cells Transl Med. 2016;5(10):1302–1306. doi: 10.5966/sctm.2016-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang H-M, Hur J, Yoon C-H, Park K-W, Lee C-S, Kim J-Y, et al. A novel subset of T cells (angiogenic T cells) facilitates EPC differentiation and has clinical relevance. Am J Cardiol. 2007;100(8A):80L-L. [Google Scholar]
- 44.Rehman J, Li JL, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107(8):1164–1169. doi: 10.1161/01.Cir.0000058702.69484.A0. [DOI] [PubMed] [Google Scholar]
- 45.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104(9):2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 46.Madonna R, De Caterina R. Circulating endothelial progenitor cells: Do they live up to their name? Vasc Pharmacol. 2015;67–69:2–5. doi: 10.1016/j.vph.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 47.Peichev M, Naiyer AJ, Pereira D, Zhu ZP, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MAS, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95(3):952–958. doi: 10.1182/blood.V95.3.952.003k27_952_958. [DOI] [PubMed] [Google Scholar]
- 48.Agudo J, Ruzo A, Tung N, Salmon H, Leboeuf M, Hashimoto D, Becker C, Garrett-Sinha LA, Baccarini A, Merad M, Brown BD. The miR-126-VEGFR2 axis controls the innate response to pathogen-associated nucleic acids. Nat Immunol. 2014;15(1):54–62. doi: 10.1038/ni.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elias I, Franckhauser S, Ferre T, Vila L, Tafuro S, Munoz S, Roca C, Ramos D, Pujol A, Riu E, Ruberte J, Bosch F. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes. 2012;61(7):1801–1813. doi: 10.2337/db11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, Yang HK, Oh BH, Park YB, Kim HS. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112(11):1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 51.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24(2):288–293. doi: 10.1161/01.Atv.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 52.Kaushik K, Das A. Endothelial progenitor cell therapy for chronic wound tissue regeneration. Cytotherapy. 2019;21(11):1137–1150. doi: 10.1016/j.jcyt.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Ravishankar P, Zeballos MA, Balachandran K. Isolation of endothelial progenitor cells from human umbilical cord blood. J Vis Exp. 2017;127:e56021. doi: 10.3791/56021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wils J, Favre J, Bellien J. Modulating putative endothelial progenitor cells for the treatment of endothelial dysfunction and cardiovascular complications in diabetes. Pharmacol Ther. 2017;170:98–115. doi: 10.1016/j.pharmthera.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 55.Zampetaki A, Kirton JP, Xu Q. Vascular repair by endothelial progenitor cells. Cardiovasc Res. 2008;78(3):413–421. doi: 10.1093/cvr/cvn081. [DOI] [PubMed] [Google Scholar]
- 56.Shantsila E, Watson T, Lip GY. Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol. 2007;49(7):741–752. doi: 10.1016/j.jacc.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 57.Asahara T, Takahashi T, Masuda H, Kalka C, Chen DH, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18(14):3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cai X, Chen Z, Pan X, Xia L, Chen P, Yang Y, Hu H, Zhang J, Li K, Ge J, Yu K, Zhuang J. Inhibition of angiogenesis, fibrosis and thrombosis by tetramethylpyrazine: mechanisms contributing to the SDF-1/CXCR4 axis. PLoS One. 2014;9(2):e88176. doi: 10.1371/journal.pone.0088176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meng Q, Wang W, Yu X, Li W, Kong L, Qian A, Li C, Li X. Upregulation of microRNA-126 contributes to endothelial progenitor cell function in deep vein thrombosis via its target PIK3R2. J Cell Biochem. 2015;116(8):1613–1623. doi: 10.1002/jcb.25115. [DOI] [PubMed] [Google Scholar]
- 60.Li P, Luo Z, Li X, Wang R, Chen H, Zhao Y, Wang J, Huang N. Preparation, evaluation and functionalization of biomimetic block copolymer coatings for potential applications in cardiovascular implants. Appl Surf Sci. 2020;502(1):144085–144096. doi: 10.1016/j.apsusc.2019.144085. [DOI] [Google Scholar]
- 61.Wang S, Zhu S, Zhang X, Li J, Guan S. Effects of degradation products of biomedical magnesium alloys on nitric oxide release from vascular endothelial cells. Med Gas Res. 2019;9(3):153–159. doi: 10.4103/2045-9912.266991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang Z, Yang Y, Zhang L, Xiong K, Li X, Zhang F, Wang J, Zhao X, Huang N. Mussel-inspired catalytic selenocystamine-dopamine coatings for long-term generation of therapeutic gas on cardiovascular stents. Biomaterials. 2018;178:1–10. doi: 10.1016/j.biomaterials.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 63.Deatrick KB, Eliason JL, Lynch EM, Moore AJ, Dewyer NA, Varma MR, Pearce CG, Upchurch GR, Jr, Wakefield TW, Henke PK. Vein wall remodeling after deep vein thrombosis involves matrix metalloproteinases and late fibrosis in a mouse model. J Vasc Surg. 2005;42(1):140–148. doi: 10.1016/j.jvs.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 64.Kanayasu-Toyoda T, Tanaka T, Ishii-Watabe A, Kitagawa H, Matsuyama A, Uchida E, Yamaguchi T. Angiogenic role of MMP-2/9 expressed on the cell surface of early endothelial progenitor cells/myeloid angiogenic cells. J Cell Physiol. 2015;230(11):2763–2775. doi: 10.1002/jcp.25002. [DOI] [PubMed] [Google Scholar]
- 65.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85(3):221–228. doi: 10.1161/01.Res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 66.Kalka C, Tehrani H, Laudenberg B, Vale PR, Isner JM, Asahara T, Symes JF. VEGF gene transfer mobilizes endothelial progenitor cells in patients with inoperable coronary disease. Ann Thorac Surg. 2000;70(3):829–834. doi: 10.1016/s0003-4975(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 67.Kebir A, Harhouri K, Guillet B, Liu JW, Foucault-Bertaud A, Lamy E, Kaspi E, Elganfoud N, Vely F́́, Sabatier F, Sampol J́, Pisano P, Kruithof EKO, Bardin N, Dignat-George F̧, Blot-Chabaud M. CD146 short isoform increases the proangiogenic potential of endothelial progenitor cells in vitro and in vivo. Circ Res. 2010;107(1):66–75. doi: 10.1161/circresaha.109.213827. [DOI] [PubMed] [Google Scholar]
- 68.Dimmeler S, Zeiher AM. Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis? J Mol Med. 2004;82(10):671–677. doi: 10.1007/s00109-004-0580-x. [DOI] [PubMed] [Google Scholar]
- 69.Kong DL, Melo LG, Gnecchi M, Zhang LN, Mostoslavsky G, Liew CC, Pratt RE, Dzau VJ. Cytokine-induced mobilization of circulating endothelial progenitor cells enhances repair of injured arteries. Circulation. 2004;110(14):2039–2046. doi: 10.1161/01.Cir.0000143161.01901.Bd. [DOI] [PubMed] [Google Scholar]
- 70.Miglionico M, Patti G, D'ambrosio A, Di Sciascio G. Percutaneous coronary intervention utilizing a new endothelial progenitor cells antibody-coated stent: a prospective single-center registry in high-risk patients. Catheter Cardiovasc Interv. 2008;71(5):600–604. doi: 10.1002/ccd.21437. [DOI] [PubMed] [Google Scholar]
- 71.Li W, Li X. Endothelial progenitor cells accelerate the resolution of deep vein thrombosis. Vasc Pharmacol. 2016;83:10–16. doi: 10.1016/j.vph.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 72.Yu Y, Ricciotti E, Scalia R, Tang SY, Grant G, Yu Z, et al. Vascular COX-2 modulates blood pressure and thrombosis in mice. Sci Transl Med. 2012;4(132):132ra54. doi: 10.1126/scitranslmed.3003787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Polivka J, Jr, Polivka J, Pesta M, Rohan V, Celedova L, Mahajani S, Topolcan O, Golubnitschaja O. Risks associated with the stroke predisposition at young age: facts and hypotheses in light of individualized predictive and preventive approach. EPMA J. 2019;10(1):81–99. doi: 10.1007/s13167-019-00162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Imanishi T, Kobayashi K, Hano T, Nishio I. Effect of estrogen on differentiation and senescence in endothelial progenitor cells derived from bone marrow in spontaneously hypertensive rats. Hypertens Res. 2005;28(9):763–772. doi: 10.1291/hypres.28.763. [DOI] [PubMed] [Google Scholar]
- 75.Lee PSS, Poh KK. Endothelial progenitor cells in cardiovascular diseases. World J Stem Cells. 2014;6(3):355–366. doi: 10.4252/wjsc.v6.i3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A, Agostini C, Avogaro A. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45(9):1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 77.Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, Grego F, Vigili de Kreutzenberg S, Tiengo A, Agostini C, Avogaro A. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006;26(9):2140–2146. doi: 10.1161/01.Atv.0000237750.44469.88. [DOI] [PubMed] [Google Scholar]
- 78.Hayek SS, Macnamara J, Tahhan AS, Awad M, Yadalam A, Ko Y-A, et al. Circulating progenitor cells identify peripheral arterial disease in patients with coronary artery disease. Circ Res. 2016;119(4):564–571. doi: 10.1161/circresaha.116.308802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bitterli L, Afan S, Buehler S, Disanto S, Zwahlen M, Schmidlin K, et al. Endothelial progenitor cells as a biological marker of peripheral artery disease. Vasc Med. 2016;21(1):3–11. doi: 10.1177/1358863x15611225. [DOI] [PubMed] [Google Scholar]
- 80.Morishita T, Uzui H, Nakano A, Mitsuke Y, Geshi T, Ueda T, Lee JD. Number of endothelial progenitor cells in peripheral artery disease as a marker of severity and association with pentraxin-3, malondialdehyde-modified low-density lipoprotein and membrane type-1 matrix metalloproteinase. J Atheroscler Thromb. 2012;19(2):149–158. doi: 10.5551/jat.10074. [DOI] [PubMed] [Google Scholar]
- 81.Delva P, De Marchi S, Prior M, Degan M, Lechi A, Trettene M, et al. Endothelial progenitor cells in patients with severe peripheral arterial disease. Endothelium. 2008;15(5–6):246–53. 10.1080/10623320802487718. [DOI] [PubMed]
- 82.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89(1):E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 83.Briguori C, Testa U, Riccioni R, Colombo A, Petrucci E, Condorelli G, Mariani G, D’Andrea D, de Micco F, Rivera NV, Puca AA, Peschle C, Condorelli G. Correlations between progression of coronary artery disease and circulating endothelial progenitor cells. FASEB J. 2010;24(6):1981–1988. doi: 10.1096/fj.09-138198. [DOI] [PubMed] [Google Scholar]
- 84.Eizawa T, Ikeda U, Murakami Y, Matsui K, Yoshioka T, Takahashi M, Muroi K, Shimada K. Decrease in circulating endothelial progenitor cells in patients with stable coronary artery disease. Heart. 2004;90(6):685–686. doi: 10.1136/hrt.2002.008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pirro M, Schillaci G, Menecali C, Bagaglia F, Paltriccia R, Vaudo G, Mannarino MR, Mannarino E. Reduced number of circulating endothelial progenitors and HOXA9 expression in CD34+cells of hypertensive patients. J Hypertens. 2007;25(10):2093–2099. doi: 10.1097/HJH.0b013e32828e506d. [DOI] [PubMed] [Google Scholar]
- 86.Oliveras A, Soler MJ, Martinez-Estrada OM, Vazquez S, Marco-Feliu D, Vila JS, et al. Endothelial progenitor cells are reduced in refractory hypertension. J Hum Hypertens. 2008;22(3):183–190. doi: 10.1038/sj.jhh.1002304. [DOI] [PubMed] [Google Scholar]
- 87.Budzyn M, Gryszczynka B, Boruczkowski M, Kaczmarek M, Begier-Krasinska B, Osinska A, et al. The potential role of circulating endothelial cells and endothelial progenitor cells in the prediction of left ventricular hypertrophy in hypertensive patients. Front Physiol. 2019;10. 10.3389/fphys.2019.01005. [DOI] [PMC free article] [PubMed]
- 88.Szpera-Gozdziewicz A, Majcherek M, Boruczkowski M, Gozdziewicz T, Dworacki G, Wicherek L, et al. Circulating endothelial cells, circulating endothelial progenitor cells, and von Willebrand factor in pregnancies complicated by hypertensive disorders. Am J Reprod Immunol. 2017;77(3):e12625. doi: 10.1111/aji.12625. [DOI] [PubMed] [Google Scholar]
- 89.Miyaki A, Maeda S, Yoshizawa M, Misono M, Sasai H, Shimojo N, Tanaka K, Ajisaka R. Is pentraxin 3 involved in obesity-induced decrease in arterial distensibility. J Atheroscler Thromb. 2010;17(3):278–284. doi: 10.5551/jat.2741. [DOI] [PubMed] [Google Scholar]
- 90.Rajavashisth TB, Xu XP, Jovinge S, Meisel S, Xu XO, Chai NN, Fishbein MC, Kaul S, Cercek B, Sharifi B, Shah PK. Membrane type 1 matrix metalloproteinase expression in human atherosclerotic plaques: evidence for activation by proinflammatory mediators. Circulation. 1999;99(24):3103–3109. doi: 10.1161/01.Cir.99.24.3103. [DOI] [PubMed] [Google Scholar]
- 91.Balistreri CR, Buffa S, Pisano C, Lio D, Ruvolo G, Mazzesi G. Are endothelial progenitor cells the real solution for cardiovascular diseases? Focus on controversies and perspectives. Biomed Res Int. 2015;835934:1–17. doi: 10.1155/2015/835934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kaihan AB, Hishida M, Imaizumi T, Okazaki M, Kaihan AN, Katsuno T, Taguchi A, Yasuda Y, Tsuboi N, Kosugi T, Maruyama S. Circulating levels of CD34(+) cells predict longterm cardiovascular outcomes in patients on maintenance hemodialysis. PLoS One. 2019;14(10):e0223390. doi: 10.1371/journal.pone.0223390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Golubnitschaja O, Flammer J. Individualised patient profile: clinical utility of Flammer syndrome phenotype and general lessons for predictive, preventive and personalised medicine. EPMA J. 2018;9(1):15–20. doi: 10.1007/s13167-018-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sabatier F, Camoin-Jau L, Anfosso F, Sampol J, Dignat-George F. Circulating endothelial cells, microparticles and progenitors: key players towards the definition of vascular competence. J Cell Mol Med. 2009;13(3):454–471. doi: 10.1111/j.1582-4934.2008.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Budzyn M, Gryszczynka B, Boruczkowski M, Kaczmarek M, Begier-Krasinska B, Osinska A, et al. The endothelial status reflected by circulating endothelial cells, circulating endothelial progenitor cells and soluble thrombomodulin in patients with mild and resistant hypertension. Vasc Pharmacol. 2019;113:77–85. doi: 10.1016/j.vph.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 96.Luo S, Xia W, Chen C, Robinson EA, Tao J. Endothelial progenitor cells and hypertension: current concepts and future implications. Clin Sci. 2016;130(22):2029–2042. doi: 10.1042/cs20160587. [DOI] [PubMed] [Google Scholar]
- 97.Eirin A, Zhu X-Y, Woollard JR, Herrmann SM, Gloviczki ML, Saad A, Juncos LA, Calhoun DA, Rule AD, Lerman A, Textor SC, Lerman LO. Increased circulating inflammatory endothelial cells in blacks with essential hypertension. Hypertension. 2013;62(3):585–591. doi: 10.1161/hypertensionaha.113.01621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Holmen C, Elsheikh E, Stenvinkel P, Qureshi AR, Pettersson E, Jalkanen S, et al. Circulating inflammatory endothelial cells contribute to endothelial progenitor cell dysfunction in patients with vasculitis and kidney involvement. J Am Soc Nephrol. 2005;16(10):3110–3120. doi: 10.1681/asn.2005040347. [DOI] [PubMed] [Google Scholar]
- 99.Eirin A, Zhu X-Y, Li Z, Ebrahimi B, Zhang X, Tang H, Korsmo MJ, Chade AR, Grande JP, Ward CJ, Simari RD, Lerman A, Textor SC, Lerman LO. Endothelial outgrowth cells shift macrophage phenotype and improve kidney viability in swine renal artery stenosis. Arterioscler Thromb Vasc Biol. 2013;33(5):1006–1013. doi: 10.1161/atvbaha.113.301164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rotmans JI, Verhagen HJM, Velema E, De Kleijn DPV, Van Den Heuvel M, Kastelein JJP, et al. Local overexpression of C-type natriuretic peptide ameliorates vascular adaptation of porcine hemodialysis grafts. Kidney Int. 2004;65(5):1897–1905. doi: 10.1111/j.1523-1755.2004.00598.x. [DOI] [PubMed] [Google Scholar]
- 101.Wu Y, Chang L, Li J, Wang L, Guan S. Conjugating heparin, Arg-Glu-Asp-Val peptide, and anti-CD34 to the silanic Mg-Zn-Y-Nd alloy for better endothelialization. J Biomater Appl. 2020;35(2):158–168. doi: 10.1177/0885328220926655. [DOI] [PubMed] [Google Scholar]
- 102.Chen J, Li Q, Xu J, Zhang L, Maitz MF, Li J. Thromboresistant and rapid-endothelialization effects of dopamine and staphylococcal protein A mediated anti-CD34 coating on 316L stainless steel for cardiovascular devices. J Mater Chem. 2015;3(13):2615–2623. doi: 10.1039/c4tb01825g. [DOI] [PubMed] [Google Scholar]
- 103.Chen J, Cao J, Wang J, Maitz MF, Guo L, Zhao Y, Li Q, Xiong K, Huang N. Biofunctionalization of titanium with PEG and anti-CD34 for hemocompatibility and stimulated endothelialization. J Colloid Interface Sci. 2012;368:636–647. doi: 10.1016/j.jcis.2011.11.039. [DOI] [PubMed] [Google Scholar]
- 104.Chen J, Li Q, Li J, Maitz MF. The effect of anti-CD34 antibody orientation control on endothelial progenitor cell capturing cardiovascular devices. J Bioact Compat Polym. 2016;31(6):583–599. doi: 10.1177/0883911516637376. [DOI] [Google Scholar]
- 105.Hoyt J, Lerman A, Lennon RJ, Rihal CS, Prasad A. Left anterior descending artery length and coronary atherosclerosis in apical ballooning syndrome (Takotsubo/stress induced cardiomyopathy) Int J Cardiol. 2010;145(1):112–115. doi: 10.1016/j.ijcard.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 106.Takahashi M, Matsuoka Y, Sumide K, Nakatsuka R, Fujioka T, Kohno H, Sasaki Y, Matsui K, Asano H, Kaneko K, Sonoda Y. CD133 is a positive marker for a distinct class of primitive human cord blood-derived CD34-negative hematopoietic stem cells. Leukemia. 2014;28(6):1308–1315. doi: 10.1038/leu.2013.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Park K-S, Kang SN, Kim DH, Kim H-B, Im KS, Park W, Hong YJ, Han DK, Joung YK. Late endothelial progenitor cell-capture stents with CD146 antibody and nanostructure reduce in-stent restenosis and thrombosis. Acta Biomater. 2020;111:91–101. doi: 10.1016/j.actbio.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 108.Kang CK, Lim WH, Kyeong S, Choe WS, Kim HS, Jun BH, Lee YS. Fabrication of biofunctional stents with endothelial progenitor cell specificity for vascular re-endothelialization. Colloids Surf, B. 2013;102:744–751. doi: 10.1016/j.colsurfb.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 109.Tan KX, Danquah MK, Sidhu A, Yon LS, Ongkudon CM. Aptamer-mediated polymeric vehicles for enhanced cell-targeted drug delivery. Curr Drug Targets. 2018;19(3):248–258. doi: 10.2174/1389450117666160617120926. [DOI] [PubMed] [Google Scholar]
- 110.Tan KX, Pan S, Jeevanandam J, Danquah MK. Cardiovascular therapies utilizing targeted delivery of nanomedicines and aptamers. Int J Pharm. 2019;558:413–425. doi: 10.1016/j.ijpharm.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 111.Hoffmann J, Paul A, Harwardt M, Groll J, Reeswinkel T, Klee D, Moeller M, Fischer H, Walker T, Greiner T, Ziemer G, Wendel HP. Immobilized DNA aptamers used as potent attractors for porcine endothelial precursor cells. J Biomed Mater Res A. 2008;84A(3):614–621. doi: 10.1002/jbm.a.31309. [DOI] [PubMed] [Google Scholar]
- 112.Chen L, Li JA, Chang J, Jin S, Wu D, Yan H, Wang XF, Guan SK. Mg-Zn-Y-Nd coated with citric acid and dopamine by layer-by-layer self-assembly to improve surface biocompatibility. Sci China Technol Sci. 2018;61(8):1228–1237. doi: 10.1007/s11431-017-9190-2. [DOI] [Google Scholar]
- 113.Qi P, Yan W, Yang Y, Li Y, Fan Y, Chen J, Yang Z, Tu Q, Huang N. Immobilization of DNA aptamers via plasma polymerized allylamine film to construct an endothelial progenitor cell-capture surface. Colloids Surf, B. 2015;126:70–79. doi: 10.1016/j.colsurfb.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 114.Deng J, Yuan S, Li X, Wang K, Xie L, Li N, Wang J, Huang N. Heparin/DNA aptamer co-assembled multifunctional catecholamine coating for EPC capture and improved hemocompatibility of vascular devices. Mater Sci Eng C-Mater Biol Appl. 2017;79:305–314. doi: 10.1016/j.msec.2017.05.057. [DOI] [PubMed] [Google Scholar]
- 115.Deng J, Yuan S, Wang J, Luo R, Chen S, Wang J, et al. Fabrication of endothelial progenitor cell capture surface via DNA aptamer modifying dopamine/polyethyleneimine copolymer film. Appl Surf Sci. 2016;386:138–150. doi: 10.1016/j.apsusc.2016.06.015. [DOI] [Google Scholar]
- 116.Tan A, Goh D, Farhatnia Y, Natasha G, Lim J, Teoh SH, et al. An anti-CD34 antibody-functionalized clinical-grade POSS-PCU nanocomposite polymer for cardiovascular stent coating applications: a preliminary assessment of endothelial progenitor cell capture and hemocompatibility. PLoS One. 2013;8(10):e77112. doi: 10.1371/journal.pone.0077112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Qi P, Maitz MF, Huang N. Surface modification of cardiovascular materials and implants. Surf Coat Technol. 2013;233:80–90. doi: 10.1016/j.surfcoat.2013.02.008. [DOI] [Google Scholar]
- 118.Ventura RD, Padalhin AR, Park CM, Lee BT. Enhanced decellularization technique of porcine dermal ECM for tissue engineering applications. Mater Sci Eng C-Mater Biol Appl. 2019;104:109841. doi: 10.1016/j.msec.2019.109841. [DOI] [PubMed] [Google Scholar]
- 119.Colin T, Durrieu M-C, Joie J, Lei Y, Mammeri Y, Poignard C, et al. Modeling of the migration of endothelial cells on bioactive micropatterned polymers. Math Biosci Eng. 2013;10(4):997–1015. doi: 10.3934/mbe.2013.10.997. [DOI] [PubMed] [Google Scholar]
- 120.Royer C, Guay-Begin A-A, Chanseau C, Chevallier P, Bordenave L, Laroche G, et al. Bioactive micropatterning of biomaterials for induction of endothelial progenitor cell differentiation: acceleration of in situ endothelialization. J Biomed Mater Res A. 2020;108(7):1479–1492. doi: 10.1002/jbm.a.36918. [DOI] [PubMed] [Google Scholar]
- 121.Blindt R, Vogt F, Astafieva I, Fach C, Hristov M, Krott N, Seitz B, Kapurniotu A, Kwok C, Dewor M, Bosserhoff AK, Bernhagen J, Hanrath P, Hoffmann R, Weber C. A novel drug-eluting stent coated with an integrin-binding cyclic Arg-Gly-Asp peptide inhibits neointimal hyperplasia by recruiting endothelial progenitor cells. J Am Coll Cardiol. 2006;47(9):1786–1795. doi: 10.1016/j.jacc.2005.11.081. [DOI] [PubMed] [Google Scholar]
- 122.Chollet C, Chanseau C, Remy M, Guignandon A, Bareille R, Labrugere C, et al. The effect of RGD density on osteoblast and endothelial cell behavior on RGD-grafted polyethylene terephthalate surfaces. Biomaterials. 2009;30(5):711–720. doi: 10.1016/j.biomaterials.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 123.Ji Y, Wei Y, Liu X, Wang J, Ren K, Ji J. Zwitterionic polycarboxybetaine coating functionalized with REDV peptide to improve selectivity for endothelial cells. J Biomed Mater Res A. 2012;100A(6):1387–1397. doi: 10.1002/jbm.a.34077. [DOI] [PubMed] [Google Scholar]
- 124.Qin Z. The use of THP-1 cells as a model for mimicking the function and regulation of monocytes and macrophages in the vasculature. Atherosclerosis. 2012;221(1):2–11. doi: 10.1016/j.atherosclerosis.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 125.Hao D, Xiao W, Liu R, Kumar P, Li Y, Zhou P, Guo F, Farmer DL, Lam KS, Wang F, Wang A. Discovery and characterization of a potent and specific peptide ligand targeting endothelial progenitor cells and endothelial cells for tissue regeneration. ACS Chem Biol. 2017;12(4):1075–1086. doi: 10.1021/acschembio.7b00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hao D, Fan Y, Xiao W, Liu R, Pivetti C, Walimbe T, Guo F, Zhang X, Farmer DL, Wang F, Panitch A, Lam KS, Wang A. Rapid endothelialization of small diameter vascular grafts by a bioactive integrin-binding ligand specifically targeting endothelial progenitor cells and endothelial cells. Acta Biomater. 2020;108:178–193. doi: 10.1016/j.actbio.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen H, Li X, Zhao Y, Li J, Chen J, Yang P, Maitz MF, Huang N. Construction of a multifunctional coating consisting of phospholipids and endothelial progenitor cell-specific peptides on titanium substrates. Appl Surf Sci. 2015;347:169–177. doi: 10.1016/j.apsusc.2015.02.032. [DOI] [Google Scholar]
- 128.Chen Z, Li Q, Chen J, Luo R, Maitz MF, Huang N. Immobilization of serum albumin and peptide aptamer for EPC on polydopamine coated titanium surface for enhanced in-situ self-endothelialization. Mater Sci Eng C-Mater Biol Appl. 2016;60:219–229. doi: 10.1016/j.msec.2015.11.044. [DOI] [PubMed] [Google Scholar]
- 129.Jun HW, West JL. Modification of polyurethaneurea with PEG and YIGSR peptide to enhance endothelialization without platelet adhesion. J Biomed Mater Res Part B. 2005;72B(1):131–139. doi: 10.1002/jbm.b.30135. [DOI] [PubMed] [Google Scholar]
- 130.Avci-Adali M, Ziemer G, Wendel HP. Induction of EPC homing on biofunctionalized vascular grafts for rapid in vivo self-endothelialization - a review of current strategies. Biotechnol Adv. 2010;28(1):119–129. doi: 10.1016/j.biotechadv.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 131.Wilhelm C, Gazeau F. Universal cell labelling with anionic magnetic nanoparticles. Biomaterials. 2008;29(22):3161–3174. doi: 10.1016/j.biomaterials.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 132.Carenza E, Barcelo V, Morancho A, Montaner J, Rosell A, Roig A. Rapid synthesis of water-dispersible superparamagnetic iron oxide nanoparticles by a microwave-assisted route for safe labeling of endothelial progenitor cells. Acta Biomater. 2014;10(8):3775–3785. doi: 10.1016/j.actbio.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 133.Arbab AS, Bashaw LA, Miller BR, Jordan EK, Bulte JWM, Frank JA. Intracytoplasmic tagging of cells with ferumoxides and transfection agent for cellular magnetic resonance imaging after cell transplantation: methods and techniques. Transplantation. 2003;76(7):1123–1130. doi: 10.1097/01.Tp.0000089237.39220.83. [DOI] [PubMed] [Google Scholar]
- 134.Himes N, Min JY, Lee R, Brown C, Shea J, Huang XL, Xiao YF, Morgan JP, Burstein D, Oettgen P. In vivo MRI of embryonic stem cells in a mouse model of myocardial infarction. Magn Reson Med. 2004;52(5):1214–1219. doi: 10.1002/mrm.20220. [DOI] [PubMed] [Google Scholar]
- 135.Wei M, Wen D, Wang X, Huan Y, Yang Y, Xu J, et al. Experimental study of endothelial progenitor cells labeled with superparamagnetic iron oxide in vitro. Mol Med Rep. 2015;11(5):3814–3819. doi: 10.3892/mmr.2014.3122. [DOI] [PubMed] [Google Scholar]
- 136.Zhang B, Jiang H, Chen J, Hu Q, Yang S, Liu X. Silica-coated magnetic nanoparticles labeled endothelial progenitor cells alleviate ischemic myocardial injury and improve long-term cardiac function with magnetic field guidance in rats with myocardial infarction. J Cell Physiol. 2019;234(10):18544–18559. doi: 10.1002/jcp.28492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen J, Wang S, Wu Z, Wei Z, Zhang W, Li W. Anti-CD34-grafted magnetic nanoparticles promote endothelial progenitor cell adhesion on an iron stent for rapid endothelialization. ACS Omega. 2019;4(21):19469–77. 10.1021/acsomega.9b03016. [DOI] [PMC free article] [PubMed]