Abstract
Introduction
Clinical trials of direct-acting antivirals for patients with decompensated cirrhosis have been conducted, but there is limited information on the medicinal applications in clinical settings. We aimed to evaluate the safety and efficacy of sofosbuvir/velpatasvir for decompensated cirrhotic patients with genotypes 1 and 2 in real-world clinical practice.
Methods
A prospective, multicenter study of 12-week sofosbuvir/velpatasvir was conducted for patients with decompensated cirrhosis at 33 institutions.
Results
The cohort included 71 patients (52 genotype 1, 19 genotype 2): 7 with Child–Pugh class A, 47 with class B, and 17 with class C (median score 8; range 5–13). The albumin–bilirubin (ALBI) score ranged from − 3.01 to − 0.45 (median − 1.58). Sixty-nine patients (97.2%) completed treatment as scheduled. The overall rate of sustained virologic response at 12 weeks post-treatment (SVR12) was 94.4% (67/71). SVR12 rates in the patients with Child–Pugh classes A, B, and C were 85.7%, 97.9%, and 88.2%, respectively. Among 22 patients with a history of hepatocellular carcinoma treatment, 20 (90.9%) achieved SVR12. The Child–Pugh score and ALBI grade significantly improved after achieving SVR12 (p = 7.19 × 10−4 and 2.42 × 10−4, respectively). Notably, the use of diuretics and branched-chain amino acid preparations significantly reduced after achieving SVR12. Adverse events were observed in 19.7% of the patients, leading to treatment discontinuation in two patients with cholecystitis and esophageal varices rupture, respectively.
Conclusion
Twelve weeks of sofosbuvir/velpatasvir in real-world clinical practice yielded high SVR rates and acceptable safety profiles in decompensated cirrhotic patients with genotypes 1 and 2. Achievement of SVR not only restored the liver functional reserve but also reduced or spared the administration of drugs for related complications.
Trial Registration
UMIN registration no, 000038587.
Electronic supplementary material
The online version of this article (10.1007/s40121-020-00329-y) contains supplementary material, which is available to authorized users.
Keywords: ALBI grade, Child–Pugh class, Decompensated cirrhosis, Hepatitis C virus, Sofosbuvir, Velpatasvir
Key Summary Points
| Why carry out this study? |
| Real-world patient cohorts may include patients with unfavorable factors or conditions, unlike the participants in phase 3 clinical trials. |
| To confirm the real-world efficacy and safety of 12-week sofosbuvir/velpatasvir (SOF/VEL) (without ribavirin), we performed a prospective multicenter study of decompensated cirrhotic patients, including those who would be ineligible for clinical trials, and addressed how sustained virologic response (SVR) achievement could have a beneficial impact on such patients within a short period in real-world clinical practice. |
| Among 71 patients, 69 (97.2%) completed treatment as scheduled, 71 (100%) had virological response at the end (or cessation) of treatment, and 67 (94.4%) achieved sustained virologic response at 12 weeks post-treatment (SVR12). |
| Twelve weeks of SOF/VEL yielded high efficacy, acceptable safety, and good tolerability, even in patients exhibiting advanced disease stages or unfavorable conditions. The achievement of SVR not only restored the liver functional reserve but also reduced or spared the administration of drugs for related complications. |
| What was learned from the study? |
| In real-world clinical practice, achieving SVR with SOF/VEL not only restored the liver functional reserve but also reduced or spared the use of diuretics and branched-chain amino acid preparations for the treatment of cirrhosis-related complications in the short term. |
| These findings suggest that direct-acting antiviral treatment such as SOF/VEL may improve health-related quality of life and prognosis and reduce physical and economic burdens of decompensated cirrhotic patients. |
Digital Features
This article is published with digital features to facilitate understanding of the article. You can access the digital features on the article’s associated Figshare page. To view digital features for this article go to 10.6084/m9.figshare.12770987.
Background
Chronic hepatitis C virus (HCV) infection causes hepatic necroinflammation and fibrogenesis, and, consequently, is a primary risk factor for liver cirrhosis and hepatocellular carcinoma (HCC). Liver fibrosis gradually progresses through chronic active hepatitis to compensated cirrhosis, and eventually to decompensated cirrhosis, which is accompanied by various portal hypertension-related complications, such as ascites, gastrointestinal hemorrhage, and hepatic encephalopathy [1]. A recent meta-analysis showed that the 2-year survival rates of patients with Child–Pugh classes B and C were 70% and 40%, respectively [1]. A Spanish cohort study reported that the mean survival of patients with decompensated cirrhosis was 4.1 years [2]. Therefore, a breakthrough treatment is urgently desired in order to improve the poor prognosis of decompensated cirrhosis.
Currently, direct-acting antiviral agents (DAAs) are the first-line standard of care for chronic HCV infection. We have previously reported that HCV elimination was achieved in 95–99% of patients with chronic hepatitis or compensated cirrhosis [3–10], although our reports excluded patients with decompensated cirrhosis. Meanwhile, several real-world studies from Europe and the United States have included decompensated cirrhotic patients, but only one phase 3 study focusing on decompensated cirrhotic patients has been reported in Asia, by Japan [11–17]. Considering that patients with hepatitis C in Japan are generally older than those in Europe and the United States [3–17], it is necessary to evaluate the efficacy and safety of DAAs for decompensated cirrhotic patients in real-world clinical practice in Japan.
DAAs available for treating HCV include NS3/4A protease inhibitors, NS5A inhibitors, and NS5B polymerase inhibitors. Generally, two or more of these different drugs are administered in combination to treat chronic HCV infection. However, given the hepatotoxicity of some NS3/4A protease inhibitors [4–10, 18], the guidelines of the European Association for the Study of Liver and the American Association for the Study of Liver Diseases recommend combining an NS5A inhibitor and an NS5B polymerase inhibitor for patients with decompensated cirrhosis [19, 20]. Indeed, clinical trials in Europe and the United States indicate that this combination yielded high sustained virologic response (SVR) rates and that the liver functional reserve improved after SVR achievement [11–14]. There have been only two phase 3 clinical studies of sofosbuvir/velpatasvir (SOF/VEL) with or without ribavirin in patients with decompensated cirrhosis [Child–Pugh class B (score of 7–9) in ASTRAL-4 or Child–Pugh score of ≤ 12 in a Japanese phase 3 study] [13, 21]. The Japanese phase 3 study reported that the addition of ribavirin did not improve the SVR rate, but rather increased the frequency and severity of adverse events (AEs) [21]. Therefore, the 12-week SOF/VEL regimen (without ribavirin) was approved in 2019 in Japan for patients with decompensated cirrhosis.
In this prospective, multicenter study, we aimed to evaluate the safety and efficacy of 12-week SOF/VEL (without ribavirin) for patients with decompensated cirrhosis, including those who would be ineligible for clinical trial settings (primary objective). In addition, we assessed how SVR achievement could have beneficial impacts, such as restoration of liver functional reserve and reduction in drug doses for cirrhosis-related complications, within a short period in real-world clinical practice (secondary objective).
Methods
Patients
This was a prospective, multicenter (n = 33) study conducted by the KTK49 Liver Study Group in Japan (UMIN registration no. 000038587). Decompensated cirrhotic patients with genotypes 1 and 2 were recruited and received 12-week SOF/VEL between March 2019 and December 2019. Patients consented to participate in the study and met the following inclusion criteria: (1) serum HCV ribonucleic acid (RNA) levels > 1.2 log IU/mL; (2) 18 years or over; and (3) decompensated liver cirrhosis (Child–Pugh classes B or C) at the time of entry into the study. The exclusion criteria were: (1) malignant tumors; (2) co-infection with human immunodeficiency virus or hepatitis B virus; (3) contraindicated concomitant drugs against SOF/VEL; and (4) estimated glomerular filtration rate (eGFR) of < 30 mL/min/1.73 m2. This study was approved by the ethics committee of Nippon Medical School (IRB #R1-05-1129) and each institution. This study was performed in accordance with the 2013 Helsinki Declaration. All patients consented to provide their data. Written informed consent was obtained from each patient before enrollment.
Treatment Protocol and Laboratory Tests
Patients received one tablet of EPUCLUSA® per os once daily (400 mg SOF and 100 mg VEL; Gilead Sciences, Foster City, CA, USA) for 12 weeks. Physical, hematological, and biochemical examinations were performed at treatment initiation, and every 4 weeks during the treatment and follow-up periods.
AEs including laboratory abnormalities were graded according to the Common Terminology Criteria for Adverse Events v.5.0, as presented by the National Cancer Institute Cancer Therapy Evaluation Program [22].
Serum HCV RNA levels were measured using a real-time polymerase chain reaction (PCR)-based method (COBAS TaqMan HCV Test 2.0; Roche Molecular Systems, Pleasanton, CA, USA). The lower limit of quantification was 1.2 log IU/mL. HCV genotype was determined using PCR with genotype-specific primers for amplifying the core gene sequence [23].
Definition of Treatment Responses
SVR12 was defined as undetectable serum HCV RNA at 12 weeks post-treatment. Patients with undetectable serum HCV RNA at the end of treatment were considered to have achieved the end-of-treatment response (EOT). Patients with EOT in whom viral RNA reappeared after treatment completion were considered to have relapsed.
Evaluation of the Resistance-Associated Substitutions
NS5A substitutions were determined using direct sequencing techniques. Extracted RNA samples from the serum were reverse-transcribed and amplified via the nested PCR method, using outer primers for the NS5A region: 5′-ATGAACCGRCTGATAGCGTT-3′ [NS5A H-F1; sense, nucleotide (nt) 6075–6094, numbered according to HCV-J (accession no. D90208)] and 5′-CTAGCTGAAGAGCTGGCCAA-3′ (NS5AH-R1; antisense, nt 6969–6988), and inner primers: 5′-TCCCCYACRCACTATGTGCC-3′ (NS5A H-F2; sense, nt6117–6136) and 5′-CGCTTYGCCGTRTCTGCTGT-3′ (NS5A H-R2; antisense, nt 6921–6940). The conditions of the first and second PCR rounds were the same: 95 °C for 2 min, followed by 40 cycles at 98 °C for 10 s, 55 °C for 15 s, and 68 °C for 1 min. The PCR products were then sequenced on an automated DNA sequencer (3730xl DNA Analyzer; Thermo Fisher Scientific, Waltham, MA, USA). The resistance-associated amino acid positions were referenced as in previous reports [24]. Subtype-specific reference strains were Con1 (AJ 238799) for genotype 1b, JFH-1 (AB047639) for genotype 2a, and HC-J8 (D10988) for genotype 2b.
Evaluation of the Liver Functional Reserve
The Child–Pugh classification was used to assess the liver functional reserve. In this study, the original version was used for the Child–Pugh classification [25], where ascites was graded as follows: 1 = none, 2 = mild, and 3 = moderate. Moreover, the albumin–bilirubin (ALBI) grading system was adopted because it is known that liver functional reserve can be categorized in more detail compare to the Child–Pugh classification. The ALBI score was calculated based on serum albumin and total bilirubin values using the following formula: ALBI score = [log10 bilirubin (µmol/L) × 0.66] + [albumin (g/L) × − 0.085]. The ALBI grade was defined by the following scores: ≤ − 2.60 for grade 1, between − 2.60 and − 1.39 for grade 2, and > − 1.39 for grade 3. Furthermore, the middle ALBI grade (grade 2) was subdivided into 2a and 2b [designated as the modified ALBI (mALBI) grade] by an ALBI score of − 2.270, corresponding to the cutoff value for a 15-min indocyanine green retention rate of 30% [26].
Statistical Analyses
Continuous variables are presented as medians and ranges in parentheses. Categorical variables are presented as numbers and percentages. The Wilcoxon signed-rank test was used to evaluate the time-course changes in serum albumin, total bilirubin, eGFR, prothrombin time, Child–Pugh class/score, and ALBI grade/score. The Fisher’s exact test, chi-square test, and Tukey’s multiple comparison test were used to compare groups, as appropriate. The Cochran–Armitage trend test was used to evaluate associations between a variable with two categories and a variable with multiple categories. A p value of < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics v.26.0 (IBM Japan, Tokyo, Japan) and JMP v.12.0.1 (SAS Institute, Cary, NC, USA).
Results
Patient Characteristics
This study involved 71 patients with decompensated cirrhosis, comprising 38 males and 33 females, with a median age of 65 (43–86) years (Table 1). Fifty-two and 19 patients were infected with genotype 1 and 2, respectively. The median values of albumin, total bilirubin, and prothrombin time were 2.9 (1.9–4.5) g/dL, 1.5 (0.5–5.2) mg/dL, and 64.0 (18.0–110.0) %, respectively. The median Child–Pugh score was 8 (5–13) points. Seven patients were Child–Pugh class A (score 5–6), 47 were class B (score 7–9), and 17 were class C (score 10–15). All the patients with Child–Pugh class A had previous decompensated events, and their Child–Pugh scores improved after branched-chain amino acid (BCAA) and/or diuretics administration; consequently, the Child–Pugh class improved to A at the initiation of SOF/VEL. ALBI grades 1, 2a, 2b, and 3 were registered in 1, 4, 37, and 29 patients, respectively. Among the 71 patients, 45 (63.4%) had esophageal varices, and 12 were treated by an endoscopic procedure before SOF/VEL initiation. Twenty-two (31.0%) patients underwent previous treatment for HCC (radiofrequency ablation in 9 patients, surgical resection in 3, transcatheter arterial chemoembolization in 5, chemotherapy in 1, heavy ion therapy in 1, and proton therapy 1; all performed 2 months to 13 years before the entry into this study). None of the patients had evidence of HCC on imaging modalities at the initiation of SOF/VEL. Forty (56.3%) patients were administered BCAA for hypoalbuminemia and hepatic encephalopathy, and 48 (67.6%) patients were administered diuretics (furosemide, spironolactone, and/or tolvaptan) for hepatic edema. Other clinical characteristics at the initiation of SOF/VEL are shown in Table 1.
Table 1.
Baseline characteristics of the 71 patients
| Variable | N = 71 |
|---|---|
| Gender (male/female) | 38/33 |
| Age (years) | 65 (43–86) |
| White blood cell (/mm3) | 3420 (1500–7800) |
| Hemoglobin (g/dL) | 11.7 (7.9–16.0) |
| Platelets (× 103/mm3) | 77 (29–186) |
| AST (U/L) | 55 (20–152) |
| ALT (U/L) | 31 (11–119) |
| Serum albumin (g/dL) | 2.9 (1.9–4.5) |
| Total bilirubin (mg/dL) | 1.5 (0.5–5.2) |
| Prothrombin time (%) | 64.0 (18.0–110.0) |
| Child–Pugh score | 8 (5–13) |
| Child–Pugh class (A/B/C) | 7/47/17 |
| ALBI score | − 1.58 (− 3.01 to − 0.45) |
| ALBI grade (1/2a/2b/3) | 1/4/37/29 |
| Ascites (none/mild/moderate or severe) | 35/30/6 |
| Hepatic encephalopathy (absent/mild) | 48/23 |
| Administration of BCAA (no/yes) | 31/40 |
| Administration of diuretics (no/yes) | 23/48 |
| Esophageal varices (absent /present/unknown) | 20/45/6 |
| Alpha-fetoprotein (ng/mL) | 7.0 (1.3–2031.8) |
| HCV RNA (log IU/mL) | 5.7 (3.0–7.4) |
| HCV genotype (1b/2a/2b) | 52/17/2 |
| History of IFN-free DAAs (naïve/experienced) | 70/1 |
| History of HCC treatment (no/yes) | 49/22 |
AST aspartate aminotransferase; ALT alanine aminotransferase; BCAA branched chain amino acid; HCV hepatitis C virus; IFN interferon; DAAs direct-acting antivirals; HCC hepatocellular carcinoma
Virologic Responses
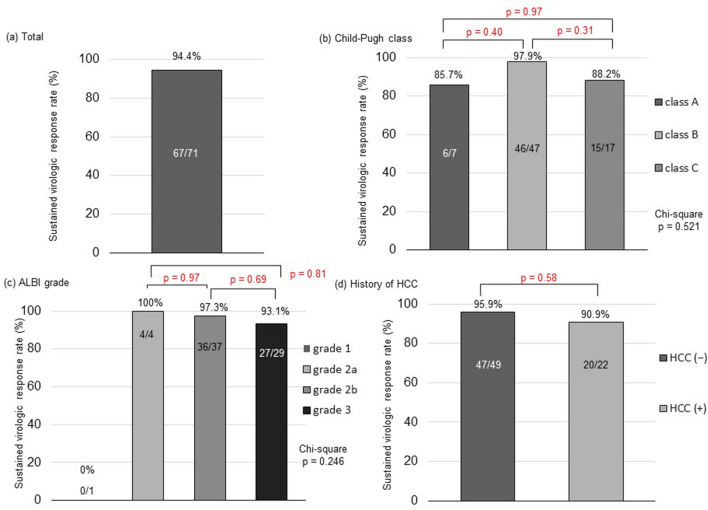
Among the 71 patients, 69 completed the treatment as scheduled; the remaining 2 patients prematurely discontinued treatment due to AEs (as described below). The overall SVR12 rate, based on intention-to-treat analysis, was 94.4% (67/71; Fig. 1a). Four patients did not achieve SVR12—two relapsed, and one discontinued treatment at week 9 (when serum HCV RNA was undetectable) due to acute cholecystitis and died of sepsis 3 weeks later; thus, the final virologic response was undetermined. The final patient that did not achieve SVR12 completed the treatment and achieved SVR4, but developed spontaneous bacterial peritonitis and died of liver failure at week 10 after the completion of treatment. The two deaths exhibited no viremia during treatment; thus, all the 71 patients achieved EOT. According to the Child–Pugh classification, the SVR12 rates were 85.7% (6/7) for class A, 97.9% (46/47) for class B, and 88.2% (15/17) for class C (Fig. 1b). The SVR12 patients with Child–Pugh class C included one individual with a Child–Pugh score of 13, who would have been excluded from the Japanese phase 3 trial [21]. According to the ALBI grading system, the SVR12 rates were 0% (0/1) for grade 1, 100% (4/4) for grade 2a, 97.3% (36/37) for grade 2b, and 93.1% (27/29) for grade 3 (Fig. 1c). Among the 22 patients with HCC treatment history, 20 (90.9%) achieved SVR12 (Fig. 1d), while the remaining 2 patients relapsed (both were naïve to DAA) (Table S1). Of the 20 patients with SVR12, 4 developed recurrent HCC during the follow-up period: 1 underwent radiofrequency ablation for solitary lesion, 1 underwent CyberKnife treatment for solitary lesion, 1 underwent hepatic arterial infusion chemotherapy using a reservoir for portal vein thrombosis, and 1 is awaiting liver transplantation due to deteriorated liver function despite a solitary lesion. Sixty-six of the 70 DAA-naïve patients (94.3%) achieved SVR12, while 1 DAA-experienced patient (prior treatment: asunaprevir plus daclatasvir) achieved SVR12.
Fig. 1.
Sustained virologic response rates according to each category. a A sustained virologic response rate in a total of 71 patients with decompensated liver cirrhosis based on intention-to-treat analysis. b Comparison of sustained virologic response rates among patients with Child–Pugh classes A, B, and C. c Comparison of sustained virologic response rates among patients with ALBI grades 1, 2a, 2b, and 3. d Comparison of sustained virologic response rates between patients with and without a history of hepatocellular carcinoma (HCC)
We sequentially analyzed the resistance-associated substitutions (RASs) in the NS5A region of the two patients who relapsed. In one patient (genotype 1b), no RASs were detected at position L31, Q54, and Y93 at baseline; however, L31V, F37L, and Y93N emerged at relapse. In the other patient (genotype 2b), Q30K and L31M had already existed at baseline; thereafter, both Q30K and L31M remained at relapse. Neither relapser had NS5B RASs (including S282T) at baseline or relapse.
Time-Course Changes in the Liver Functional Reserve
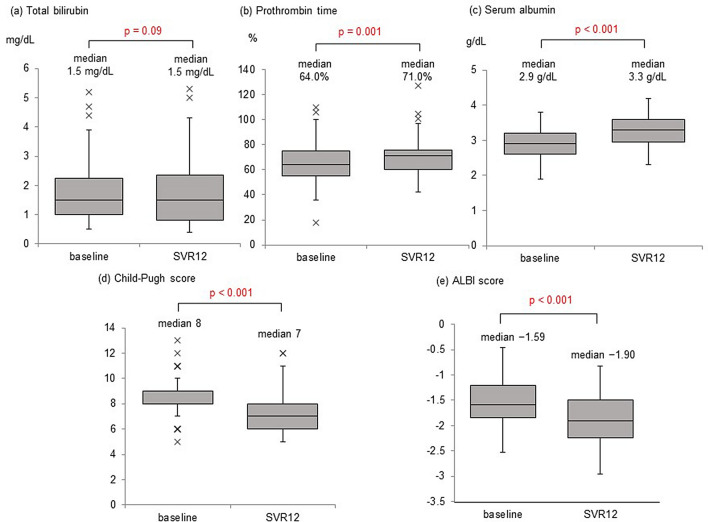
Changes in the biochemical and liver functional reserve parameters from baseline to 12 weeks post-treatment were analyzed in the 67 patients who achieved SVR12. The median total bilirubin levels did not change (p = 0.09; Fig. 2a). The median prothrombin time significantly improved from 64.0 to 71.0% (p = 0.001; Fig. 2b). The median albumin levels significantly increased from 2.9 to 3.3 g/dL (p < 0.001; Fig. 2c).
Fig. 2.
Time-course changes in the biochemical and hepatic functional reserve parameters. Time-course changes from baseline to 12-week post-treatment in a total bilirubin levels, b prothrombin time, c serum albumin levels, d Child–Pugh score, and e ALBI score among patients who achieved sustained virologic response at 12 weeks post-treatment (SVR12)
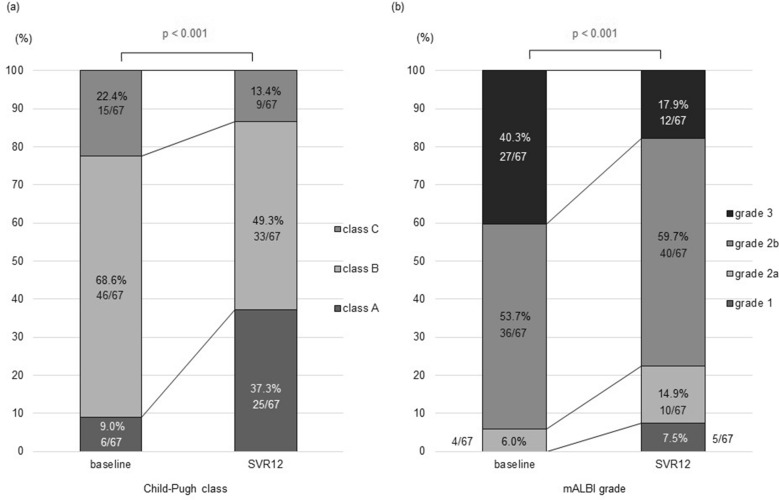
The median Child–Pugh score significantly improved, from 8 points at baseline to 7 points at 12 weeks post-treatment (p < 0.001; Fig. 2d). The proportion of Child–Pugh class A patients increased from 9.0 to 37.3%, while that of classes B and C patients decreased from 68.6 to 49.3% and from 22.4 to 13.4%, respectively. Accordingly, the liver functional reserve based on the Child–Pugh classification significantly improved in the patients who achieved SVR12 (p < 0.001; Fig. 3a). Among the 17 class C patients, 4 improved to class B and 2 improved to class A. Among the 47 class B patients, 17 improved to class A (Fig. S1). No patients showed a deterioration in Child–Pugh class, but three patients exhibited worse scores after achieving SVR12 (i.e., 7–8, 8–9, and 11–12).
Fig. 3.
Time-course changes in the distribution of the Child–Pugh class and ALBI grade. a Child–Pugh class and b ALBI grade significantly improved from baseline to 12-week post-treatment in patients who achieved sustained virologic response at 12 weeks post-treatment (SVR12) (p = 7.19 × 10−4 and 2.42 × 10−4, respectively, by the Cochran–Armitage trend test)
The median ALBI grade scores also improved from − 1.59 at baseline to − 1.90 at 12 weeks post-treatment (p < 0.001; Fig. 2e). Grade 1 increased from 0.0 to 7.5%, grade 2a increased from 6.0 to 14.9%, and grade 2b increased from 53.7 to 59.7%. In contrast, grade 3 decreased from 40.3 to 17.9%. Accordingly, the liver functional reserve based on the mALBI grade also significantly improved in patients who achieved SVR12 (p < 0.001; Fig. 3b). Among the 29 grade 3 patients, 15 improved to grade 2b and one improved to grade 2a. Among the 37 grade 2b patients, e8ight improved to grade 2a and 3 improved to grade 1. Two patients with grade 2a improved to grade 1. However, two patients deteriorated mALBI grades despite achieving SVR12: one from grade 2b to grade 3 and the other from grade 2a to grade 2b (Fig. S2). Both patients were administered tolvaptan for refractory ascites, and the former underwent a transjugular intrahepatic portosystemic shunt procedure due to non-responsiveness to tolvaptan.
Taken together, improvements in these surrogate parameters indicate that the achievement of SVR12 with SOF/VEL could restore the liver function reserve during the short term of 24 weeks in patients with decompensated cirrhosis. However, the ALBI grade and score deteriorated in some patients with refractory ascites, despite achieving SVR12.
Time-Course Changes in Drug Administration for Complications in Patients with Decompensated Cirrhosis
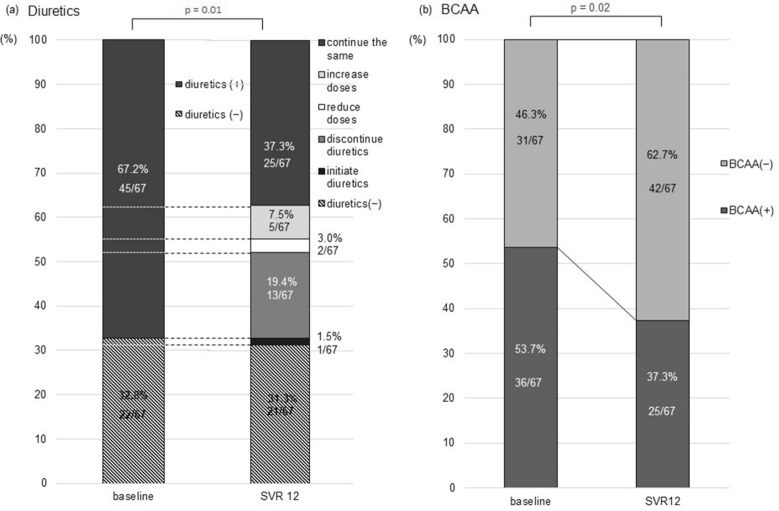
Given that the serum albumin levels, Child–Pugh scores/classes, and ALBI grades/scores improved in patients who achieved SVR12, we investigated if the diuretic doses and BCAAs were reduced or discontinued with SVR12. Among the 67 patients who achieved SVR12, 45 (67.2%) had been administered diuretics and 22 (32.8%) were diuretics free at baseline. Of the 45 patients receiving diuretics, 13 discontinued diuretic use and 2 reduced their doses with the achievement of SVR12. Twenty-five patients continued the same dosages, and five patients increased their doses. One patient initiated diuretic treatment despite achieving SVR12. Thus, the proportion of patients who maintained, increased, or initiated diuretic use during the study period significantly decreased to 46.3% (31/67) at 12 weeks post-treatment (p = 0.01; Fig. 4a). The proportion of patients receiving BCAA treatment significantly decreased from 53.7% (36/67) at baseline to 37.3% (25/67) at 12 weeks post-treatment. Thus, 11 of the 36 patients receiving BCAAs spared BCAAs (p = 0.02; Fig. 4b). These results suggest that achieving SVR12 with SOF/VEL could improve the severity of cirrhosis-related complications, thereby reducing or sparing drug administration dosages for the treatment of complications in patients with decompensated cirrhosis. Such results have not been reported before.
Fig. 4.
Time-course changes in the administration dosages of drugs for the treatment of complications. a Changes in administration doses of diuretics for hepatic edema. The proportion of patients who received the same, increased the doses or initiated diuretics significantly decreased after achieving sustained virologic response at 12-week post-treatment (SVR12) (p = 1.46 × 10−2 by the Cochran–Armitage trend test). b Changes in administration of branched chain amino acid (BCAA) for hepatic encephalopathy and/or hypoalbuminemia. The proportion of patients who continued to receive BCAA treatment was significantly reduced from baseline to 12-week post-treatment among patients who achieved SVR12 (p = 2.44 × 10−2 by the Cochrane–Armitage trend test)
Adverse Events
Overall, AEs were observed during the treatment and follow-up periods in 19.7% (14/71) of the patients. The incidence was higher in the Child–Pugh class C group [35.3% (6/17)] than in the Child–Pugh class A/B group [14.8% (8/54)], although not statistically significant (p = 0.08; Table 2). Serious AEs occurred more frequently in the former [11.8% (2/17)] than in the latter [3.8% (2/54)], although not statistically significant (p = 0.241). AEs leading to treatment discontinuation were observed in two patients with Child–Pugh class B: acute cholecystitis in one (as described above) and esophageal varices rupture in the other (as described below). Two patients died during the follow-up period (as described above): one Child–Pugh class B patient had diabetes mellitus and cholecystolithiasis at baseline and developed acute cholecystitis and later died of sepsis; and the other Child–Pugh class C patients had total bilirubin and albumin levels that fluctuated at 3.2–3.8 mg/dL and 2.3–3.0 g/dL, respectively, during the treatment period and developed spontaneous bacterial peritonitis and consequently died of liver failure. Two patients with Child–Pugh class B and ALBI grade 3 developed encephalopathy grade 2 during the treatment period, but rapidly recovered with hydration and achieved SVR12 after treatment completion. After achieving SVR12, both patients improved the liver functional reserve: Child–Pugh B to A in one and ALBI grade 3 to 2b in both. Two patients developed variceal bleeding during the treatment period, but both achieved SVR12: one patient showed varices grade 3 with red signs at baseline and discontinued treatment at week 8 (as described above); and the other patient exhibited varices grade 2 without red signs at baseline and completed the treatment. Neither patient underwent endoscopic treatment before SOF/VEL, albeit the 12 patients who had undergone endoscopic treatment for esophageal varices did not develop variceal rupture. No patients had elevated transaminase, but one patient experienced increased total bilirubin from 0.5 to 2.3 mg/dL (grade 1). The median eGFR levels did not vary from the baseline to 12 weeks post-treatment (70.0 mL/min/1.73 m2 for both; p = 0.11; Fig. S3).
Table 2.
Adverse events
| Adverse events | Total (n = 71) | Child–Pugh class A or B (n = 54) | Child–Pugh class C (n = 17) | p value |
|---|---|---|---|---|
| Any adverse events | 14 (19.7%) | 8 (14.8%) | 6 (35.3%) | 0.08 |
| Death | 2 (2.8%) | 1 (1.9%) | 1 (5.9%) | 0.42 |
| Serious adverse events (CTCAE grade ≥ 4) | 4 (5.6%) | 2 (3.8%) | 2 (11.8%) | 0.24 |
| Adverse events leading to treatment discontinuation | 2 (2.8%) | 2 (3.8%) | 0 (0.0%) | 1.00 |
| CTCAE grade | ||||
| Grade 1 | 5 (7.0%) | 3 (5.7%) | 2 (11.8%) | 0.59 |
| Grade 2 | 2 (2.8%) | 1 (1.9%) | 1 (5.9%) | 0.42 |
| Grade 3 | 3 (4.2%) | 2 (3.8%) | 1 (5.9%) | 0.57 |
| Grade 4 | 2 (2.8%) | 1 (1.9%) | 1 (5.9%) | 0.42 |
| Grade 5 | 2 (2.8%) | 1 (1.9%) | 1 (5.9%) | 0.42 |
| Adverse events | ||||
| Nosebleed | 1 (1.4%) | 0 (0.0%) | 1 (5.9%) | 0.24 |
| Dysgeusia | 1 (1.4%) | 1 (1.9%) | 0 (0.0%) | 1.00 |
| Hepatic encephalopathy | 2 (2.8%) | 2 (3.8%) | 0 (0.0%) | 1.00 |
| Progression of diabetes mellitus | 1 (1.4%) | 0 (0.0%) | 1 (5.9%) | 0.24 |
| Esophageal varices rupture | 2 (2.8%) | 1 (1.9%) | 1 (5.9%) | 0.42 |
| Acute cholecystitis | 1 (1.4%) | 1 (1.9%) | 0 (0.0%) | 1.00 |
| Deterioration of ascites | 2 (2.8%) | 1 (1.9%) | 1 (5.9%) | 0.42 |
| Fatigue | 1 (1.4%) | 0 (0.0%) | 1 (5.9%) | 0.24 |
| Pharynx discharge | 1 (1.4%) | 1 (1.9%) | 0 (0.0%) | 1.00 |
| Liver failure | 1 (1.4%) | 0 (0.0%) | 1 (5.9%) | 0.24 |
| Laboratory abnormalities | ||||
| Total bilirubin elevation | 1 (1.4%) | 1 (1.9%) | 0 (0.0%) | 1.00 |
Adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v.4.0, as presented by the National Cancer Institute Cancer Therapy Evaluation Program
Discussion
To date, there have been only two phase 3 clinical trials of SOF/VEL with or without ribavirin for patients with decompensated cirrhosis: one from the United States (ASTRAL-4) for those with Child–Pugh class B (score of 7–9) [13]; and the other from Japan for those with Child–Pugh score ≤ 12 [21]. Our prospective, real-world, multicenter study included patients with more advanced disease stages or unfavorable conditions; however, it achieved high effectiveness, acceptable safety profiles, and good tolerability of 12-week SOF/VEL in decompensated cirrhotic patients with genotypes 1 and 2 (Child–Pugh score ≤ 13) in real-world clinical practice. The SVR rate of 94.4% (67/71) in our study is similar to those of 12-week SOF/VEL without ribavirin in the ASTRAL-4 study [88.8% (16/18) for genotype 1b and 100% (4/4) for genotype 2] and in the Japan phase 3 study (92.2%; 47/51) [21]. Real-world study cohorts may include patients with more unfavorable factors or conditions, unlike the participants in phase 3 clinical trials. For instance, our study cohort included one patient with a Child–Pugh score of 13, who would have been ineligible for the previous phase 3 studies [13, 21]. Nevertheless, this individual completed the treatment safely and improved to score 12 after achieving SVR. Likewise, 31.0% of the patients had undergone prior treatment modalities for HCC, and 91.3% of those patients achieved SVR after treatment completion (some of whom would have been ineligible for the above studies [13, 21]. In many patients, the liver functional reserve was restored in the short period, suggesting that the achievement of SVR might provide more treatment options for recurrent HCC. However, deteriorated liver functional reserve was observed in some patients despite achieving SVR; hence, a better understanding of the limitations of the treatment (e.g., identification of such patients) is required.
Previous and our studies have indicated that the achievement of SVR with 12-week SOF/VEL could retrieve the liver functional reserve over a short time of 24 weeks even for patients with decompensated cirrhosis [21]. Notably, we suggest that the restored liver functional reserve could improve cirrhosis-related complications and consequently reduce or spare the doses of drug, such as diuretics for hepatic edema and BCAA preparations for hepatic encephalopathy and hypoalbuminemia. This is the first report to clarify such beneficial effects of VEL/SOF on complications and related treatments. Given our previous report on the poor prognosis of cirrhotic patients receiving high-dose diuretics for hepatic edema [27], reduced or spared diuretic use may lead to improvements in the mid- and long-term prognosis of patients with decompensated cirrhosis, especially those with hepatic edema.
An advantage of the SOF/VEL regimen is that a protease inhibitor, which may cause hepatopathy, is not included. We also observed no increases in the transaminase levels in this study. Increased total bilirubin was observed in one patient, but the relationship between this result and the drugs is unclear; perhaps, the treatment was administered during naturally-occurring deterioration.
In our real-world cohort, treatment was prematurely discontinued due to cholecystitis and esophageal varices rupture. Specifically, patients with advanced decompensated cirrhosis may be vulnerable to acute infection, which can be difficult to treat or control. Uncontrollable or rapidly progressive infections could impair the functioning of the liver and other organs, resulting in multiple organ failure. In our study, acute cholecystitis followed by sepsis and spontaneous bacterial peritonitis caused or triggered patient deaths. Therefore, physicians and patients must pay scrupulous attention to HCV and acute infections. The Japanese phase 3 study also reported esophageal varices rupture during treatment [21]; in our study, the two patients with variceal rupture had esophageal varices ≥ grade 2 with or without red signs and had not undergone endoscopic treatment before SOF/VEL. These results suggest that advanced treatment for esophageal varices should be considered for such patients prior to SOF/VEL. Our results indicate no association between SOF/VEL and acute infections or esophageal varices rupture.
The current study has some limitations. First, the sample size was small, although it did exceed the sample size of previous phase 3 studies [13, 21]. Second, this was an open-label study. Third, the mid- and long-term effects of achieving SVR with SOF/VEL on the liver functional reserve, health-related quality of life, and prognosis remain unclear. Finally, the RASs at baseline and relapse were analyzed in patients with virologic failure, but the pre-existing RASs to SOF/VEL were not analyzed in all the patients.
Conclusion
This prospective, multicenter study demonstrated the high efficacy, acceptable safety, and good tolerability of 12-week SOF/VEL in decompensated cirrhotic patients with genotypes 1 and 2 in real-world clinical practice. The achievement of SVR not only restored the liver functional reserve but also reduced or spared the use of diuretics and BCAA preparations for the treatment of complications over a short term of 24 weeks.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1 (S1). Changes in the Child–Pugh class among patients who achieved sustained virologic response at 12 weeks post-treatment (SVR12). Among 17 patients with Child–Pugh class C, 4 improved to class B, and 2 improved to class A. Among 47 patients with Child–Pugh class B, 17 improved to class A. No patients deteriorated Child–Pugh class (JPG 40 kb)
Supplementary Fig. 2. (S2). Changes in ALBI grade among patients who achieved sustained virologic response at 12 weeks post-treatment (SVR12). Among 29 patients with ALBI grade 3, 15 improved to grade 2b and 1 patient improved to grade 2a. Among 37 patients with ALBI grade 2b, 8 improved to grade 2a, and 3 patients improved to grade 1. Two patients with ALBI grade 2a improved to grade 1. However, one patient with ALBI grade 2b deteriorated to grade 3 and one patient with ALBI grade 2a deteriorated to grade 2b, despite having achieved SVR12 (JPG 52 kb)
Supplementary Fig. 3 (S3). Time-course changes in the eGFR level. Among patients who achieved sustained virologic response at 12 weeks post-treatment (SVR12), eGFR (estimated glomerular filtration rate) values did not significantly change between the baseline and 12-week post-treatment (JPG 25 kb)
Acknowledgements
We thank the participants of the study.
KTK49 Liver Study Group Members: Masanori Atsukawa, Akihito Tsubota, Chisa Kondo, Hidenori Toyoda, Makoto Nakamuta, Koichi Takaguchi, Tsunamasa Watanabe, Atsushi Hiraoka, Haruki Uojima, Toru Ishikawa, Motoh Iwasa, Toshifumi Tada, Akito Nozaki, Makoto Chuma, Shinya Fukunishi, Akira Asai, Toru Asano, Chikara Ogawa, Hiroshi Abe, Naoki Hotta, Toshihide Shima, Etsuko Iio, Shigeru Mikami, Yoshihiko Tachi, Shinichi Fujioka, Hironao Okubo, Noritomo Shimada, Joji Tani, Isao Hidaka, Akio Moriya, Kunihiko Tsuji, Takehiro Akahane, Naoki Yamashita, Tomomi Okubo, Taeang Arai, Kiyoshi Morita, Kazuhito Kawata, Yasuhito Tanaka, Takeshi Okanoue, Shin Maeda, Takashi Kumada, Katsuhiko Iwakiri, Akemi Tsutsui, Notio Itokawa, Keizo Kato, Tsunekazu Oikawa, Isao Sakaida, Asahiro Morishita.
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
MA, AT, HT, KT, TM, NM and SM contributed to the concept of this study. MA and AT equally contributed to manuscript writing. CK and AT performed the statistical data analyses. MA, AT, HT, KT, TW, AH, HU, TI, MI, TT, AK, MC, SF, AA, CO, HA, TS, EI, SM, YT, SF, HO, NS, JT, IH, AM, KT, TA, NY, TO, TA, KM, KK, YT, YO, SM and KI contributed to patient’s recruitment, enrollment, clinical assessments, and data collection. All authors approved the final manuscript.
Disclosures
Masanori Atsukawa, Hidenori Toyoda, Koichi Takaguchi and Takashi Kumada received lecture fees from Gilead Sciences. Akihito Tsubota, Chisa Kondo, Makoto Nakamuta, Tsunamasa Watanabe, Atsushi Hiraoka, Haruki Uojima, Toru Ishikawa, Motoh Iwasa, Toshifumi Tada, Akito Nozaki, Makoto Chuma, Shinya Fukunishi, Akira Asai, Toru Asano, Chikara Ogawa, Hiroshi Abe, Naoki Hotta, Toshihide Shima, Etsuko Iio, Shigeru Mikami, Yoshihiko Tachi, Shinichi Fujioka, Hironao Okubo, Noritomo Shimada, Joji Tani, Isao Hidaka, Akio Moriya, Kunihiko Tsuji, Takehiro Akahane, Naoki Yamashita, Tomomi Okubo, Taeang Arai, Kiyoshi Morita, Kazuhito Kawata, Yasuhito Tanaka, Takeshi Okanoue, Shin Maeda and Katsuhiko Iwakiri have nothing to disclose.
Compliance with Ethics Guidelines
This study was approved by the ethics committee of Nippon Medical School (IRB #R1-05-1129. All documents approved by Nippon Medical School (Atsukawa M) were sent to each institution and approved by each institution under the same IRB name. This study was performed in accordance with the 2013 Helsinki Declaration. All patients consented to provide their data. Written informed consent was obtained from each patient before enrollment.
Data Availability
The datasets are available from the corresponding author on reasonable request.
Footnotes
Digital Features
To view digital features for this article go to 10.6084/m9.figshare.12770987.
The members of KTK49 Liver Study Group are listed in Acknowledgements.
Akihito Tsubota and Masanori Atsukawa contributed equally to the preparation of this manuscript.
Contributor Information
Masanori Atsukawa, Email: momogachi@yahoo.co.jp.
Akihito Tsubota, Email: atsubo@jikei.ac.jp.
References
- 1.D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(1):217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Mar J, Martínez-Baz I, Ibarrondo O, et al. Survival and clinical events related to end-stage liver disease associated with HCV prior to the era of all oral direct-acting antiviral treatments. Expert Rev Gastroenterol Hepatol. 2019;13(7):699–708. doi: 10.1080/17474124.2017.1383155. [DOI] [PubMed] [Google Scholar]
- 3.Okubo T, Atsukawa M, Tsubota A, et al. Efficacy and safety of ledipasvir/sofosbuvir for genotype 1b chronic hepatitis C patients with moderate renal impairment. Hepatol Int. 2018;12(2):133–142. doi: 10.1007/s12072-018-9859-9. [DOI] [PubMed] [Google Scholar]
- 4.Arai T, Atsukawa M, Tsubota A, et al. Efficacy and safety of ombitasvir/paritaprevir/ritonavir combination therapy for genotype 1b chronic hepatitis C patients complicated with chronic kidney disease. Hepatol Res. 2018;48(7):549–555. doi: 10.1111/hepr.13058. [DOI] [PubMed] [Google Scholar]
- 5.Atsukawa M, Tsubota A, Toyoda H, et al. Efficacy and safety of ombitasvir/paritaprevir/ritonavir and ribavirin for chronic hepatitis patients infected with genotype 2a in Japan. Hepatol Res. 2019;49(4):369–376. doi: 10.1111/hepr.13292. [DOI] [PubMed] [Google Scholar]
- 6.Atsukawa M, Tsubota A, Toyoda H, et al. Efficacy and safety of elbasvir/grazoprevir for Japanese patients with genotype 1b chronic hepatitis C complicated by chronic kidney disease, including those undergoing hemodialysis: a post hoc analysis of a multicenter study. J Gastroenterol Hepatol. 2019;34(2):364–369. doi: 10.1111/jgh.14447. [DOI] [PubMed] [Google Scholar]
- 7.Atsukawa M, Tsubota A, Toyoda H, et al. The efficacy and safety of glecaprevir plus pibrentasvir in 141 patients with severe renal impairment: a prospective, multicenter study. Aliment Pharmacol Ther. 2019;49(9):1230–1241. doi: 10.1111/apt.15218. [DOI] [PubMed] [Google Scholar]
- 8.Toyoda H, Atsukawa M, Watanabe T, et al. Real-world experience of 12-week direct-acting antiviral regimen of glecaprevir and pibrentasvir in patients with chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2020;35(5):855–861. doi: 10.1111/jgh.14874. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda H, Watanabe T, Atsukawa M, et al. Evaluation of 8-week glecaprevir/pibrentasvir treatment in direct-acting antiviral-naïve noncirrhotic HCV genotype 1 and 2infected patients in a real-world setting in Japan. J Viral Hepat. 2019;26(11):1266–1275. doi: 10.1111/jvh.13170. [DOI] [PubMed] [Google Scholar]
- 10.Nozaki A, Atsukawa M, Kondo C, KTK49 Liver Study Group et al. The effectiveness and safety of glecaprevir/pibrentasvir in chronic hepatitis C patients with refractory factors in the real world: a comprehensive analysis of a prospective multicenter study. Hepatol Int. 2020;14(2):225–238. doi: 10.1007/s12072-020-10019-z. [DOI] [PubMed] [Google Scholar]
- 11.Charlton M, Everson GT, Flamm SL, SOLAR-1 Investigators et al. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology. 2015;149(3):649–659. doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Manns M, Samuel D, Gane EJ, SOLAR-2 investigators et al. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis. 2016;16(6):685–697. doi: 10.1016/S1473-3099(16)00052-9. [DOI] [PubMed] [Google Scholar]
- 13.Curry MP, O'Leary JG, Bzowej N, ASTRAL-4 Investigators et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015;373(27):2618–2628. doi: 10.1056/NEJMoa1512614. [DOI] [PubMed] [Google Scholar]
- 14.Foster GR, Irving WL, Cheung MC, HCV Research, UK et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64(6):1224–1231. doi: 10.1016/j.jhep.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 15.Belli LS, Berenguer M, Cortesi PA, European Liver and Intestine Association (ELITA) et al. Delisting of liver transplant candidates with chronic hepatitis C after viral eradication: a European study. J Hepatol. 2016;65(3):524–531. doi: 10.1016/j.jhep.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Fernández Carrillo C, Lens S, Llop E, et al. Treatment of hepatitis C virus infection in patients with cirrhosis and predictive value of model for end-stage liver disease: analysis of data from the Hepa-C registry. Hepatology. 2017;65(6):1810–1822. doi: 10.1002/hep.29097. [DOI] [PubMed] [Google Scholar]
- 17.McCaughan GW, Thwaites PA, Roberts SK, Australian Liver Association Clinical Research Network et al. Sofosbuvir and daclatasvir therapy in patients with hepatitis C-related advanced decompensated liver disease (MELD ≥ 15) Aliment Pharmacol Ther. 2018;47(3):401–411. doi: 10.1111/apt.14404. [DOI] [PubMed] [Google Scholar]
- 18.Kato K, Shimada N, Atsukawa M, et al. Single nucleotide polymorphisms associated with elevated alanine aminotransferase in patients receiving asunaprevir plus daclatasvir combination therapy for chronic hepatitis C. PLoS ONE. 2019;14(7):e0219022. doi: 10.1371/journal.pone.0219022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.EASL Recommendations on Treatment of Hepatitis C European Association for the study of the liver. J Hepatol. 2018;69(2):461–511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 20.Hepatitis C Guidance Guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. AASLD/IDSA HCV Guid Panel Hepatol. 2015;62(3):932–954. doi: 10.1002/hep.27950. [DOI] [PubMed] [Google Scholar]
- 21.Takehara T, Sakamoto N, Nishiguchi S, et al. Efficacy and safety of sofosbuvir-velpatasvir with or without ribavirin in HCV-infected Japanese patients with decompensated cirrhosis: an open-label phase 3 trial. J Gastroenterol. 2019;54(1):87–95. doi: 10.1007/s00535-018-1503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Cancer Institute Division of Cancer Treatment & Diagnosis. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50. Accessed 27 Mar 2020.
- 23.Ohno O, Mizokami M, Wu RR, et al. New hepatitis C virus (HCV) genotyping system that allows for identification of HCV genotypes 1a, 1b, 2a, 2b, 3a, 3b, 4, 5a, and 6a. J Clin Microbiol. 1997;35(1):201–207. doi: 10.1128/JCM.35.1.201-207.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dvory-Sobol H, Han B, Lu J, et al. In vitro resistance profile of hepatitis C virus NS5A inhibitor velpatasvir in genotypes 1 to 6. J Viral Hepat. 2019;26(8):991–1001. doi: 10.1111/jvh.13116. [DOI] [PubMed] [Google Scholar]
- 25.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 26.Hiraoka A, Michitaka K, Kumada T, et al. Validation and potential of albumin–bilirubin grade and prognostication in a nationwide survey of 46,681 hepatocellular carcinoma patients in Japan: the need for a more detailed evaluation of hepatic function. Liver Cancer. 2017;6(4):325–336. doi: 10.1159/000479984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atsukawa M, Tsubota A, Takaguchi K, et al. Analysis of factors associated with the prognosis of cirrhotic patients who were treated with tolvaptan for hepatic edema. J Gastroenterol Hepatol. 2020;35(7):1229–1237. doi: 10.1111/jgh.14965. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1 (S1). Changes in the Child–Pugh class among patients who achieved sustained virologic response at 12 weeks post-treatment (SVR12). Among 17 patients with Child–Pugh class C, 4 improved to class B, and 2 improved to class A. Among 47 patients with Child–Pugh class B, 17 improved to class A. No patients deteriorated Child–Pugh class (JPG 40 kb)
Supplementary Fig. 2. (S2). Changes in ALBI grade among patients who achieved sustained virologic response at 12 weeks post-treatment (SVR12). Among 29 patients with ALBI grade 3, 15 improved to grade 2b and 1 patient improved to grade 2a. Among 37 patients with ALBI grade 2b, 8 improved to grade 2a, and 3 patients improved to grade 1. Two patients with ALBI grade 2a improved to grade 1. However, one patient with ALBI grade 2b deteriorated to grade 3 and one patient with ALBI grade 2a deteriorated to grade 2b, despite having achieved SVR12 (JPG 52 kb)
Supplementary Fig. 3 (S3). Time-course changes in the eGFR level. Among patients who achieved sustained virologic response at 12 weeks post-treatment (SVR12), eGFR (estimated glomerular filtration rate) values did not significantly change between the baseline and 12-week post-treatment (JPG 25 kb)
Data Availability Statement
The datasets are available from the corresponding author on reasonable request.