Abstract
Objective
Energy metabolism abnormality is the hallmark in epithelial ovarian carcinoma (EOC). This study aimed to investigate energy metabolism pathway alterations and their regulation by the antiparasite drug ivermectin in EOC for the discovery of energy metabolism pathway-based molecular biomarker pattern and therapeutic targets in the context of predictive, preventive, and personalized medicine (PPPM) in EOC.
Methods
iTRAQ-based quantitative proteomics was used to identify mitochondrial differentially expressed proteins (mtDEPs) between human EOC and control mitochondrial samples isolated from 8 EOC and 11 control ovary tissues from gynecologic surgery of Chinese patients, respectively. Stable isotope labeling with amino acids in cell culture (SILAC)-based quantitative proteomics was used to analyze the protein expressions of energy metabolic pathways in EOC cells treated with and without ivermectin. Cell proliferation, cell cycle, apoptosis, and important molecules in energy metabolism pathway were examined before and after ivermectin treatment of different EOC cells.
Results
In total, 1198 mtDEPs were identified, and various mtDEPs were related to energy metabolism changes in EOC, with an interesting result that EOC tissues had enhanced abilities in oxidative phosphorylation (OXPHOS), Kreb’s cycle, and aerobic glycolysis, for ATP generation, with experiment-confirmed upregulations of UQCRH in OXPHOS; IDH2, CS, and OGDHL in Kreb’s cycle; and PKM2 in glycolysis pathways. Importantly, PDHB that links glycolysis with Kreb’s cycle was upregulated in EOC. SILAC-based quantitative proteomics found that the protein expression levels of energy metabolic pathways were regulated by ivermectin in EOC cells. Furthermore, ivermectin demonstrated its strong abilities to inhibit proliferation and cell cycle and promote apoptosis in EOC cells, through molecular networks to target PFKP in glycolysis; IDH2 and IDH3B in Kreb’s cycle; ND2, ND5, CYTB, and UQCRH in OXPHOS; and MCT1 and MCT4 in lactate shuttle to inhibit EOC growth.
Conclusions
Our findings revealed that the Warburg and reverse Warburg effects coexisted in human ovarian cancer tissues, provided the first multiomics-based molecular alteration spectrum of ovarian cancer energy metabolism pathways (aerobic glycolysis, Kreb’s cycle, oxidative phosphorylation, and lactate shuttle), and demonstrated that the antiparasite drug ivermectin effectively regulated these changed molecules in energy metabolism pathways and had strong capability to inhibit cell proliferation and cell cycle progression and promote cell apoptosis in ovarian cancer cells. The observed molecular changes in energy metabolism pathways bring benefits for an in-depth understanding of the molecular mechanisms of energy metabolism heterogeneity and the discovery of effective biomarkers for individualized patient stratification and predictive/prognostic assessment and therapeutic targets/drugs for personalized therapy of ovarian cancer patients.
Electronic supplementary material
The online version of this article (10.1007/s13167-020-00224-z) contains supplementary material, which is available to authorized users.
Keywords: Epithelial ovarian carcinoma, Ivermectin, Mitochondrial proteomics, Warburg effect, Reverse Warburg effect, iTRAQ-based quantitative proteomics, SILAC-based quantitative proteomics, Energy metabolism pathway, Aerobic glycolysis, Kreb’s cycle, Oxidative phosphorylation, Lactate shuttle, Molecular biomarker pattern, Early diagnosis, Prognostic assessment, Predictive preventive personalized medicine (PPPM)
Introduction
Ovarian neoplasms consist of several clinic solid tumors, and their treatment depends on tumor grade and clinical stage. Epithelial ovarian carcinoma (EOC) constitutes the majority (nearly 90%) of malignant ovarian neoplasms with high mortality [1]. Despite advances in surgery, target therapy, and chemotherapy, EOC patients still have a poor 5-year overall survival rate (~ 30%) [2]. Early-stage diagnosis is a challenging clinical problem in EOC because of its hidden location [3]. Although ultrasound and cancer antigen 125 (CA-125) can be used to monitor high-risk factor women, they still cannot achieve good clinical effects [4]. The encouraging reports from the FDA in 2017 [5] show that olaparib (AZD2281), a PARP (polyADP-ribose polymerase) inhibitor, showed its efficacy on EOC patients with BRCA1 and BRCA2 mutations [6]. Therefore, it is urgently needed to develop novel molecular biomarkers for early diagnosis, treatment, and prognosis for EOC patients [7].
Proteomics was widely used in protein identification and quantification [8, 9]. Subcellular proteome research might provide more subtle clues to protein functions [10]. The mitochondria are the center of energy metabolism in eukaryotic cells; however, they are also involved in the processes of autophagy, apoptotic, cell cycle, cellular differentiation, and oxidative stress regulations [11]. All those biological processes are closely associated with tumor relapse or metastasis. Thus, exploration of mitochondria-mediated tumorigenesis and tumor progression mechanisms should be a novel way to the next generation of cancer therapeutics [12, 13]. The mitochondrial structural and morphological alterations were observed between cancer cells and control cells, and the changed structure and morphology were presumably associated with mitochondrial differentially expressed proteins (mtDEPs) [14]. Ovarian cancer mitochondrial proteomics proved that the mitochondria may mediate energy metabolism heterogeneity and chemoresistance signaling pathway [15–17]. Mitochondrial dysfunction in cancer cells is one of the important characteristics, and mitochondria-rejuvenating drugs would prevent from tumorigenesis [18]. Quantitative mitochondrial proteomics in EOC tissues revealed multiple signaling pathway changes [16, 19].
The Warburg effect and reverse Warburg effect promote the study of energy metabolic reprogramming in cancer cells [20]. The traditional Warburg effect refers to that cancer cells tend to produce ATP via glycolysis, even in aerobic condition [21]. A previous study observed increasing activity of glycolytic enzymes [22] and decreased energy production from the Kreb’s cycle and oxidative phosphorylation (OXPHOS) [23]. However, in the novel “reverse Warburg effect” model, cancer cells could rely on both aerobic glycolysis and OXPHOS [24]. Oxidative stress is increased in cancer-associated fibroblasts (CAFs), and CAFs secrete plenty of nutriment to the surrounding cancer cells through aerobic glycolysis [25]. Monocarboxylate transporters (MCTs), including MCT1 and MCT4, form the “lactate shuttle” to accomplish metabolic symbiosis between cancer cells and CAFs [26]. Thus, Warburg and reverse Warburg effects are complementary to each other in the study of energy metabolic reprogramming [18]. The Warburg and reverse Warburg effects coexist in tumor tissues [27]. Upregulation and flexibility of both aerobic glycolysis and OXPHOS pathways in EOC cells have been shown previously. For example, expression of PKM2 induces a high glycolytic rate in ovarian cancer, and PKM2 inhibitor suppresses ovarian cancer cell migration and growth by disturbing Warburg effects [28]. A large number of evidence also shows contradictory findings with regard to the Warburg effect, including high mitochondrial activities and low ATP contribution of glycolysis in highly invasive ovarian cancer [29]. EOC cells presented metabolic flexibility but energy metabolic reprogramming in EOC cells remains unclear. It is necessary to study in-depth the energy metabolism inhibitors.
Ivermectin is an effective medication in the treatment of many kinds of parasites, through increasing cell membrane penetrability to cause paralysis and death of the parasites [30]. Ivermectin that was initially discovered from soil in Japan in 1973 was used in the clinic in 1981, which was collected from the list of essential medicines of the World Health Organization [31]. Satoshi Ōmura who discovered ivermectin received the Gairdner Global Health Award in 2014 and the Nobel Prize in 2015. Today, ivermectin shows multiple potential roles against bacteria and virus and as anticancer, which is continuously surprising scientists and researchers [32]. In 2004, a Russian group found that ivermectin had significant antiproliferative activity against human melanoma and a few other cancers [33]. A Chinese group reported that ivermectin regulated autophagy to suppress breast cancer growth, and found that ivermectin decreased the expression of p21-activated kinase 1 though the ubiquitination-mediated degradation pathway and resulted in the decreased phosphorylation level of Akt to block the Akt/mTOR signaling pathway [34]. Some studies also found that ivermectin induced oxidative damage and mitochondrial dysfunction in renal cell carcinoma, and ivermectin demonstrated the preferential toxicity to renal cell carcinoma rather than normal kidney cells [35]. Ivermectin also selectively induced cell apoptosis in chronic myeloid leukemia (CML) through regulating oxidative stress and mitochondrial dysfunction [36]. However, it is still a long way for ivermectin to be applied in cancer treatment. A study found that EOC patients with worse prognosis had higher expression of oncogene KPNB1 regulating p21, p27, and APC/C family member, and ivermectin induced death of EOC cell models by inhibition of oncogene KPNB1 [37]. However, KPNB1 was not found to be a differentially expressed protein in human EOC tissues by isobaric tag for relative and absolute quantification (iTRAQ)-quantitative tissue proteomics [38] and iTRAQ quantitative tissue mitochondrial proteomics [16]. Ivermectin also blocked human epididymis protein 4/importin-4 nuclear accumulation and PAK1-dependent growth in human ovarian cancer [39, 40]. The antitumor effect of ivermectin is attracting many researches and has made some advances. However, the accurate molecular mechanism of its antitumor effect remains unclear. This study, for the first time, focused on the effects of ivermectin on energy metabolism pathways in human EOC cells through regulating energy metabolism–related enzymes to suppress EOC cell growth.
In our long-term program of EOC mitochondrial proteomics, mtDEPs were identified in EOCs compared with controls [16], and these mtDEPs were involved in multiple signaling pathways [19]. In combination with quantitative proteomics of whole EOC tissues [38], this study revealed the molecular profiling changes of energy metabolism pathways in EOC. Here is the experimental flowchart to study mtDEPs in EOCs relative to controls (Fig. 1a). Furthermore, the effect of ivermectin on human ovarian cancer cell lines was also investigated to show the roles of ivermectin in inhibiting proliferation and cell cycle progression and promoting apoptosis in EOC cells via regulating energy metabolism pathways.
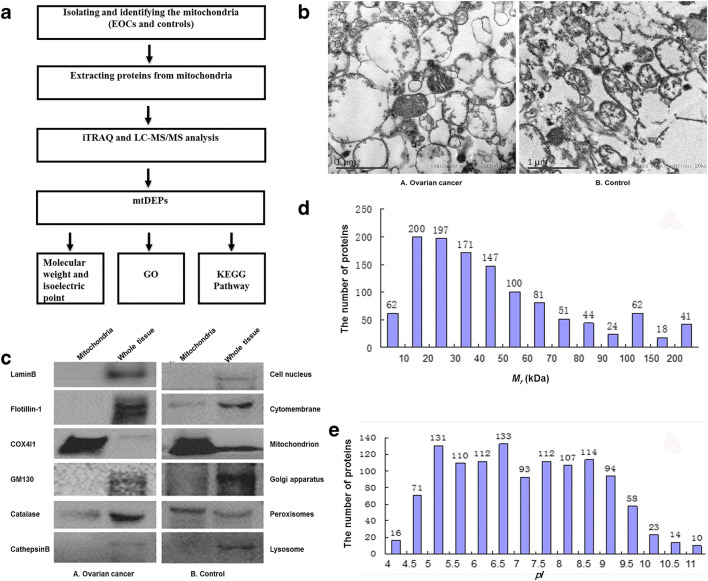
Fig. 1.
Identification of mitochondrial differentially expressed proteins in EOCs relative to controls. a Experimental flowchart to study mitochondrial differentially expressed proteins. b Electron micrograph analysis of mitochondria isolated from epithelial ovarian cancer (A) and control (B) tissues. c Organelle-specific antibody-based western blot analysis of mitochondria isolated from epithelial ovarian cancer (A) and control (B) tissues. Equal amounts of proteins were loaded onto a 10% SDS-PAGE and analyzed by western blotting with indicated antibodies against marker proteins from the cell nucleus, cytomembrane, mitochondrion, Golgi apparatus, peroxisomes, and lysosome. d Distribution status of 1198 mtDEPs according to their molecular mass (Mr). e Distribution status of 1198 mtDEPs according to their isoelectric points (pI). EOC, epithelial ovarian carcinoma; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; mtDEPs, mitochondrial differentially expressed proteins; iTRAQ, isobaric tags for relative and absolute quantitation; LC-MS/MS, liquid chromatography-tandem mass spectrometry; GO, Gene Ontology; GM130, golgin A2; KEGG, Kyoto Encyclopedia of Genes and Genomes; COX4I1, cytochrome c oxidase subunit 4I1
Materials and methods
Ovarian cancer tissue specimen and preparation of mitochondria protein samples
Eight EOC tissues and eleven control ovaries with benign gynecologic disease (Table 1) were collected during gynecologic surgery from Chinese patients from the Department of Obstetrics and Gynecology, Xiangya Hospital, Central South University, China, after approval of Xiangya Hospital Medical Ethics Committee, and informed consent was collected from Chinese patients who had never been treated with radiotherapy or chemotherapy prior to surgery. The mitochondria were isolated and purified from EOC and control tissues with differential speed centrifugation and Nycodenz density gradient centrifugation [16, 17]. The mitochondria prepared from eight EOC tissues were combined as the EOC mitochondrial sample. The mitochondria prepared from eleven control ovaries were combined as the control mitochondrial sample. The purified mitochondria were verified with electron microscopy and western blot with different antibodies specific to different subcellular organelles, including COX4I1 (mitochondrion), flotillin-1 (cytomembrane), GM130 (Golgi apparatus), catalase (peroxisomes), cathepsin B (lysosome), and lamin B (cell nucleus). The proteins were extracted from purified mitochondrial samples for iTRAQ-labeled quantitative proteomic analysis. The detailed procedure was described previously [16, 17].
Table 1.
Clinical information of EOC and control ovary tissues that were used to prepare the mitochondria
| Type of the sample | Patient no. | Age (years) | Clinical diagnosis | Pathological characteristics | Other diseases |
|---|---|---|---|---|---|
| EOC | T4 | 49 | Stage IIIC ovarian serous cystadenocarcinoma | High-grade serous adenocarcinoma; cancer cells were found in ascites; the poorly differentiated adenocarcinoma in bilateral ovaries; metastasis of cancer cells to both sides of the fallopian tube, uterine surface, omentum majus, and intestinal wall; IHC: CK7 (+), CEA (−), CA125 (−), CDX-2 (−), WT1 (+), P63 (−), P53 (+), villin (−), CK20 (−), CK20 (−), CK19 (+), and PLAP (+−) | Moderate anemia; postoperative status of appendectomy; adenomatous polyp in transverse colon; endometrial hyperplasia; cervicitis |
| T8 | 46 | Stage IIIC poorly differentiated human ovarian adenocarcinoma | Poorly differentiated adenocarcinoma in left ovary; metastasis of cancer cells to the epiploon and peritoneum; and no metastatic carcinoma to other places | Chronic vaginitis with squamous epithelial hyperplasia; uterus leiomyoma; cervicitis; endometrial hyperplasia; hepatitis B | |
| T9 | 47 | Stage IIIC ovarian serous cystadenocarcinoma | Serous cystadenocarcinoma (grades II–III and size 10 × 6.5 × 3 cm); no vascular or nerve invasiveness; metastasis of cancer cells to epiploon (size 10 × 6.5 × 3 cm); IHC: Ki67 (50%+), CA125 (+), CK (+), and CK20 (−) | Cervicitis; uterus leiomyoma; chronic salpingitis; chronic superficial gastritis | |
| T10 | 49 | Stage IIIA ovarian cancer with endometrioid adenocarcinoma plus serous adenocarcinoma | Ovary mixed moderately–poorly differentiated adenocarcinoma with endometrioid adenocarcinoma plus serous adenocarcinoma; cancer embolus in right pelvic funnel ligament; no metastatic carcinoma to other places; IHC: CA125 (+), CK7 (+), CK-Pan (+), vimentin (−), ER (+) , PR (+), P53 (−), Ki67 (60%+), desmin (−), and actin (−) | Chronic cervicitis; chronic gastritis; cholecystic polypus; depressive disorder; pulmonary infection; hypoproteinemia | |
| T16 | 52 | Stage IIIC moderately and poorly differentiated papillary serous adenocarcinoma in both ovaries | Moderately and poorly differentiated papillary serous adenocarcinoma in both ovaries without cancer embolus in vessel; cancer cells in right fallopian tube; no metastatic carcinoma to other places; a small amount of proliferative granulation tissue in pelvic cavity; dyskaryotic cell in ascites smear | Cervicitis with squamous hyperplasia; senile endometrium; postoperative status after resection of left breast | |
| T22 | 45 | Stage IIIC moderately and poorly differentiated endometrioid adenocarcinoma in right ovary | Moderately and poorly differentiated endometrioid adenocarcinoma in right ovary (size 25 × 19 × 7 cm); no vascular or neurological invasion; metastatic carcinoma in the surface of colon sigmoideum; no metastasis to other places; IHC: Ki67 (60%+), P53 (−), ER (++), PR (+), CK7 (+), CA125 (+), CK-L (−), and CD31 (+) | Cervicitis with squamous hyperplasia; deep venous thrombosis; pleural effusion; pulmonary infection; respiratory failure type I; postoperative status after cystectomy of left ovarian cysts | |
| T29 | 45 | Moderately and poorly differentiated serous ovarian carcinoma | Moderately and poorly differentiated serous ovarian carcinoma in both ovaries; no definite vascular or neurologic invasion; no metastatic carcinoma to other place; IHC: CA125 (+), ki67(30–40%+), PR (+), ER (+), villin (−), ck20 (−), CDX-2 (–), and ck7 (+) | Chronic salpingitis; chronic cervicitis | |
| T39 | 67 | Stage IIC moderately and poorly differentiated mucinous papillary ovarian adenocarcinoma | Moderately and poorly differentiated mucinous papillary ovarian adenocarcinoma without cancer embolus; cancer cells in abdominal cavity; no metastatic carcinoma to other places; IHC: ki67 (30%+), wt1 (−), pax-8 (+), p%3 (+), PR (−), ER (−), and P16 (−) | Senile endometrium; chronic cervicitis with squamous metaplasia; cervical intraepithelial neoplasia (CIN grade I); hypertension; mild anemia | |
| Con | C51 | 60 | Normal ovaries | No abnormality in bilateral ovaries; mesosalpinx cyst in the right fallopian tube | Uterine prolapse (degree II); vaginal anterior wall prolapse (degree III); vaginal posterior wall prolapse (degree I); cervical intraepithelial neoplasia (CIN grade I); cervical chronic cervicitis; senile endometrium; diabetes (type II); hypertension (grade III); bronchial asthma |
| C52 | 56 | Normal ovary (right) | Ovary serous cystadenoma (left) covering with mucous epithelial cell in special mess; mesosalpinx cyst in the right fallopian tube; no abnormality in left ovary and the left fallopian tube | Ovary serous cystadenoma (left), hypertension; pelvic inflammatory disease (sequelae phase) | |
| C54 | 50 | Normal ovaries | No abnormality was observed in bilateral ovaries; mesosalpinx cyst was observed in bilateral fallopian tubes | Cervical intraepithelial neoplasia (CIN grade III); chronic cervicitis with squamous epithelial hyperplasia and metaplasia; postoperative status of loop electrosurgical excision procedure (LEEP) for the treatment of CIN; HPV infection | |
| C55 | 49 | Normal ovaries | No abnormality was observed in bilateral ovaries and bilateral fallopian tubes; adenomyoma and multiple leiomyoma in uterus; endometrial polyp | Cervical intraepithelial neoplasia (CIN grade III); chronic cervicitis; uterine fibroids (multiple); mild anemia; liver dysfunction | |
| C60 | 53 | Normal ovaries | No abnormality was observed in bilateral ovaries and bilateral fallopian tubes | Uterine fibroids; chronic cervicitis with squamous hyperplasia; senile endometrium; fatty liver; mild anemia | |
| C66 | 44 | Normal ovaries | No abnormality was observed in bilateral ovaries and left fallopian tubes; mesosalpinx cyst in right fallopian tubes; multiple uterus leiomyoma (6.5 × 7 × 9 cm, 5 × 4 × 8 cm, 5 × 3.5 × 5 cm) | Uterine fibroids; chronic cervicitis; renal hamartoma (right side) | |
| C68 | 54 | Normal ovaries | No abnormality in bilateral ovaries, right fallopian tubes, vagina, and parametrial tissues; mesosalpinx cyst was observed in left fallopian tubes | Endometrial atypical hyperplasia (serious); fibrous tissue hyperplasia and glass-like changes in ligament tissues; hypertension; coronary heart disease; postoperative status after cholecystectomy | |
| C77 | 47 | Normal ovary (left) | No abnormality in left ovary; cystic bleb in right ovary; effusion and cystic dilation in right fallopian tube | Ovarian follicular sac (right side); adenomyosis; chronic cervicitis with squamous metaplasia; chronic vaginitis; postoperative status after resection of left ovarian cyst | |
| C79 | 44 | Normal ovary (right) | No abnormality in right ovary and bilateral fallopian tube; no cancer metastasis and enlarged lymph nodes in omentum | Ovarian serous cystadenoma (left side); postoperative status after post-hysterectomy and cystectomy of benign ovarian cysts | |
| C92 | 51 | Normal ovaries | No abnormality was observed in bilateral ovaries, bilateral fallopian tubes, and parametrial tissues | Cervical intraepithelial neoplasia (CIN grade III); uterine fibroids (multiple); senile endometrium; chronic cervicitis; hypertension (grade II); hepatic cysts | |
| C93 | 52 | Normal ovaries | White body formation in bilateral ovaries; multiple uterus leiomyoma (1 × 1 × 0.8 cm to 8 × 8 × 4 cm); mesosalpinx cyst in bilateral fallopian tubes | Multiple uterine fibroids; senile endometrium; chronic cervicitis; hyperlipemia |
All samples were from female Chinese patients
EOC, epithelial ovarian cancer; Con, control ovary, IHC, immunohistochemistry
iTRAQ-based quantitative proteomics to identify mtDEPs
The extracted mitochondrial proteins (200 μg/each sample) were treated with N-hydroxysuccinimide (SDT), followed by reduction, alkylation, digestion with trypsin, and desalination. The tryptic peptides (100 μg/each sample) were labeled with iTRAQ reagents, and each sample was labeled three times. The six labeled tryptic peptide samples were equally mixed, followed by peptide fractionation with strong cation exchange (SCX) chromatography. Each SCX-fractionated sample was subject to liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis on a Q Exactive mass spectrometer (Thermo Scientific) within a 60-min LC separation gradient to obtain MS/MS data. The MS/MS data were used to identify proteins with MASCOT search engine. The iTRAQ reporter-ion intensities were used to determine each mtDEP. The mtDEP data were subject to Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis. The detailed procedure was described previously [16, 17].
Immunoaffinity verification of mtDEPs in tissue mitochondrial samples
One-dimensional gel electrophoresis (1DGE)-based western blot was used to verify mtDEPs (PFKP, PKM2, PDHB, CS, IDH2, IDH3A, IDH3B, OGDHL, ND2, ND5, CYTB, UQCRH, MCT1, and MCT4) between EOC and control mitochondrial samples. Because the mitochondria interact extensively with the actin cytoskeleton [41], β-actin was unavoidable to be contained in the isolated mitochondrial sample. Furthermore, iTRAQ quantitative proteomics found that β-actin (accession no. K4ENJ5) was equal between EOC and control mitochondrial samples [Ratio of T/N (T = tumor; N = control) = 1.06, p = 0.314] [16]. Moreover, cytochrome (Cyto), COXVI, VDAC1, and TOMM20 are commonly used as internal standard of western blot in the analysis of mitochondria; however, these proteins are all identified as differentially expressed proteins by our iTRAQ quantitative proteomics between ovarian cancer and control mitochondrial samples [16]. Therefore, β-actin was used as internal standard of western blot.
Ingenuity Pathway Analysis of ivermectin
Ingenuity Pathway Analysis (IPA) was used to reveal the relationship of ivermectin and potential target genes in energy metabolism pathways. IPA was the classical and very popular pathway network analysis software (http://www.ingenuity.com) [19]. Ivermectin and potential target genes in energy metabolism pathways were input into the IPA tools (build tool and grow tool) to create new “my pathway” and to show molecule networks.
SILAC-based protein quantification of effects of ivermectin on EOC cells
Stable isotope labeling with amino acids in cell culture (SILAC) labeling used kits from Thermo Fisher Scientific with RPMI 1640 lacking lysine (K) and arginine (R) supplemented with 100 mg/l [13C6,15N4] arginine and 100 mg/l [13C6,15N2] lysine with 10% dialyzed fetal bovine serum (BI-Biological Industries, Cromwell, CT, USA). TOV-21G OC cells were cultured with normal RPMI 1640 and heavy chain–labeled RPMI 1640. After 10 passages, TOV-21G cells cultured with heavy chain–labeled RPMI 1640 were treated with 20 μM ivermectin. TOV-21G cells were cultured with normal RPMI 1640 treated with DMSO. Cells were collected after 24 h of ivermectin treatment for protein extraction. The extracted proteins from TOV-21G cell treated with (heavy-labeling “H”) and without (light-labeling “L”) ivermectin were digested with trypsin, followed by peptide fractionation (n = 15 fractions), LC-MS/MS, and database searching to identify and quantify proteins in TOV-21G cells treated with (H) and without (L) ivermectin.
Effects of ivermectin on EOC biological behaviors
Two EOC cell lines (SKOV3 and TOV-21G) and one normal control cell line (IOSE80) were purchased from Keibai Academy of Science (Nanjing, China) and used in this study. First, CCK8 assay was used to detect the IC50 of ivermectin in SKOV3, TOV-21G, and IOSE80, with different concentration gradients (0–60 μM) of ivermectin for 24 h. Second, EdU assay was used to measure DNA synthesis in cells SKOV3 and TOV-21G after treatment with ivermectin (0 μM, 10 μM, 20 μM, and 30 μM) for 24 h. Third, clonogenic assay was used to investigate the in vitro effects of ivermectin in cells SKOV3 and TOV-21G after treatment with ivermectin (0 μM, 10 μM, 20 μM, and 30 μM) for 48 h. Fourth, flow cytometry was used to measure cell cycle and cell apoptosis changes in cells SKOV3 and TOV-21G after treatment with ivermectin (0 μM, 10 μM, 20 μM, and 30 μM) for 24 h.
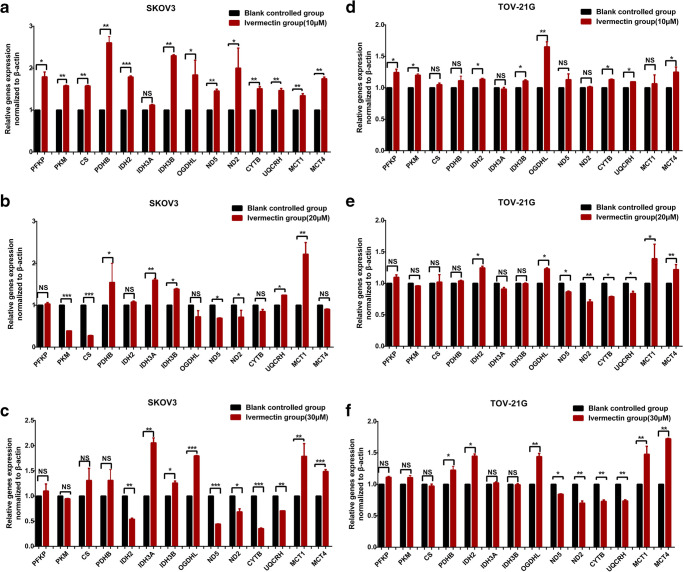
Effects of ivermectin on target genes in energy metabolism pathways
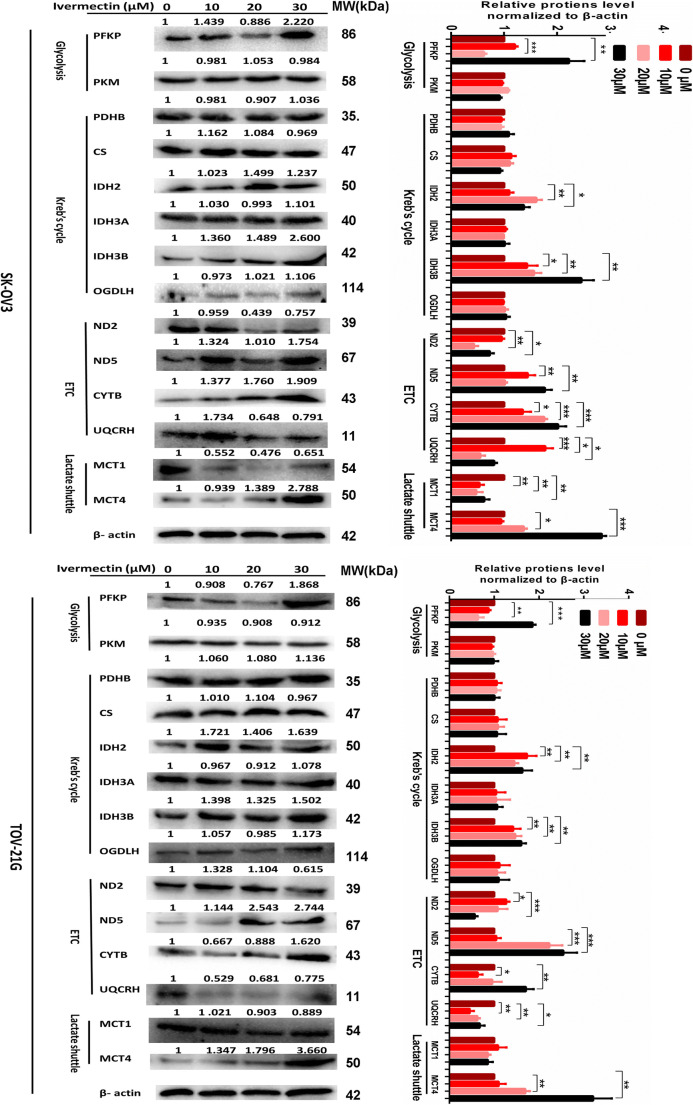
Quantitative real-time PCR (qRT-PCR) and western blot were used to measure the mRNA and protein expressions of target genes (PFKP, PKM, CS, PDHB, IDH2, IDH3A, IDH3B, OGDHL, ND2, ND5, CYTB, UQCRH, MCT1, and MCT4) in cells SKOV3 and TOV-21G after treatment with ivermectin (0 μM, 10 μM, 20 μM, and 30 μM) 24 h treatment for RNA and 48 h for protein.
Statistical analysis
For GO and KEGG enrichment analyses and IPA analysis, p values were corrected with Benjamini–Hochberg (FDR) for multiple testing. For western blot and qRT-PCR data, data were expressed as the mean ± SD, and the statistically significant level of p < 0.05 was used, with Student’s t test in SPSS 13.0 (SPSS Inc., Chicago, USA) (n = 3).
Results
Quality of the prepared mitochondrial samples
The mitochondrial samples from EOC and control tissues were prepared with differential speed centrifugation and Nycodenz density gradient centrifugation [16, 17], followed by quality evaluation with electron microscopy and western blot. Electron microscopic images showed that mitochondria were present as main organelles in the prepared EOC and control mitochondrial samples (Fig. 1b). No other organelles and cell debris were found except a small amount of peroxisomes, which demonstrated that the quality of the prepared mitochondrial samples was very good. Moreover, the quality of mitochondrial samples was also evaluated by western blotting with the antibodies of subcellular organelles’ feature proteins such as COX4I1, flotillin-1, GM130, catalase, cathepsin B, and lamin B (Fig. 1c). COX4I1 was specifically located in mitochondrion, flotillin-1 in cytomembrane, GM130 in Golgi apparatus, catalase in peroxisome, cathepsin B in lysosome, and lamin B in cell nucleus. For the whole tissue samples, all subcellular organelles were detected in EOC and control tissues. For the prepared mitochondrial samples, only mitochondria were detected as the major component in EOCs and controls, respectively (Fig. 1c), whereas the cell nucleus, Golgi apparatus, and lysosome were not detected at all. A certain amount of peroxisomes and cytomembranes were detected (Fig. 1c), which is very reasonable because mitochondria interact extensively with the cytosol cytoskeleton [41] and peroxisomes [42] to further reflect the functional complexity of mitochondria. These results clearly demonstrated that the prepared mitochondrial samples were of a very good quality.
The mtDEP profiling in EOC
In total, 1198 mtDEPs between EOC and control mitochondrial samples were determined with iTRAQ-SCX-LC-MS/MS (Supplementary Table 1) [17]. Those mtDEPs were mostly distributed within a Mr range of 10–200 kDa (Fig. 1d) and a pI range of 4–11 (Fig. 1e). No protein was detected in the area of pI < 4 and the majority of proteins were within pI 4–10, which showed good consistency of pI distribution pattern in this study compared with that of a previous study [43]. Moreover, most of mtDEPs were localized within the mitochondria. However, some DEPs were not annotated in the mitochondria but in other cellular compartments, and the reason for this observation would be that these DEPs were derived from the proteins that interacted with outer mitochondrial membrane or mitochondria-related proteins [44].
Furthermore, functional analysis revealed that those 1198 mtDEPs were involved in multiple biological processes. Especially interesting was the observation that mitochondrial ribosome and energy metabolism pathways were significantly changed. iTRAQ quantitative proteomics found 17 mitochondrial ribosome proteins were changed, including MRPL41, MRPL46, MRPL49, MRPL51, MRPL52, MRPL53, MRPL54, MRPL55, MRPS10, MRPS12, MRPS15, MRPS17, MRPS21, MRPS23, MRPS33, MRPS6, and MRPS9, which were all upregulated (Table 2). Mitochondrial ribosome was a protein complex that monitors mitochondrial translation for mRNAs encoded in mtDNA. It revealed that mitochondrial functions and its involved pathophysiological activities were unavoidably changed. A quantitative analysis of mitochondrial ribosome proteins can reveal mechanisms of mitochondrial translational control. Though most of the mitochondrial proteins are synthesized by cytoplasmic ribosomes, the crucial protein components in the electron transport chain (ETC) complexes are partially translated in the mitochondria [45]. It clearly demonstrated that the mitochondrial ribosome function was changed in EOC, which results in changes of its synthesized key protein components in the ETC complex to affect energy metabolism in EOC.
Table 2.
DEPs in ribosome-associated proteins
| Accession no. | Protein | Unique peptides | Coverage (%) | PSMs | calc. pI | MW (kDa) | Ratio (T/N) | p value (t test) |
|---|---|---|---|---|---|---|---|---|
| O15235 | 28S ribosomal protein S12, mitochondrial | 1 | 5.8 | 1 | 10.3 | 15.2 | 2.3 | 1.21E-03 |
| P82914 | 28S ribosomal protein S15, mitochondrial | 10 | 33.07 | 20 | 10.5 | 29.8 | 1.5 | 7.14E-03 |
| E9PE17 | 28S ribosomal protein S17, mitochondrial (fragment) | 4 | 51.94 | 10 | 9.8 | 14.4 | 1.8 | 1.52E-03 |
| A0A075B746 | 28S ribosomal protein S21, mitochondrial | 3 | 39.08 | 6 | 9.9 | 10.7 | 2.0 | 3.18E-03 |
| Q9Y3D9 | 28S ribosomal protein S23, mitochondrial | 7 | 40.53 | 24 | 8.9 | 21.8 | 1.8 | 1.06E-03 |
| C9JBY7 | 28S ribosomal protein S33, mitochondrial | 2 | 21.88 | 3 | 10.2 | 11.4 | 1.5 | 6.00E-03 |
| P82932 | 28S ribosomal protein S6, mitochondrial | 5 | 39.2 | 11 | 9.3 | 14.2 | 2.6 | 1.33E-03 |
| P82933 | 28S ribosomal protein S9, mitochondrial | 13 | 39.14 | 29 | 9.5 | 45.8 | 1.6 | 1.98E-02 |
| Q8IXM3 | 39S ribosomal protein L41, mitochondrial | 5 | 38.69 | 9 | 9.6 | 15.4 | 1.5 | 3.78E-02 |
| Q9H2W6 | 39S ribosomal protein L46, mitochondrial | 8 | 36.92 | 13 | 7.0 | 31.7 | 1.5 | 7.89E-03 |
| Q13405 | 39S ribosomal protein L49, mitochondrial | 5 | 30.12 | 6 | 9.5 | 19.2 | 1.6 | 2.91E-03 |
| Q4U2R6 | 39S ribosomal protein L51, mitochondrial | 1 | 5.47 | 1 | 11.3 | 15.1 | 1.6 | 8.25E-03 |
| G5E9P5 | 39S ribosomal protein L52, mitochondrial | 1 | 30 | 1 | 9.5 | 11.7 | 1.8 | 2.20E-02 |
| Q96EL3 | 39S ribosomal protein L53, mitochondrial | 4 | 43.75 | 9 | 8.8 | 12.1 | 1.6 | 5.88E-04 |
| Q6P161 | 39S ribosomal protein L54, mitochondrial | 3 | 48.55 | 6 | 9.6 | 15.8 | 1.8 | 8.11E-03 |
| X6RIW1 | 39S ribosomal protein L55, mitochondrial (fragment) | 1 | 10.53 | 2 | 11.9 | 8.6 | 1.6 | 9.50E-03 |
| P05141 | ADP/ATP translocase 2 | 6 | 49.66 | 304 | 9.7 | 32.8 | 1.8 | 1.21E-03 |
| Q6PI41 | AURKAIP1 protein (fragment) | 1 | 6.17 | 3 | 10.5 | 18.6 | 2.0 | 8.96E-04 |
| B4DP77 | cDNA FLJ57413, highly similar to Mitochondrial 28S ribosomal protein S10 | 4 | 35 | 11 | 6.4 | 18.7 | 1.6 | 1.12E-02 |
| Q96RP9 | Elongation factor G, mitochondrial | 25 | 36.22 | 56 | 7.0 | 83.4 | 1.9 | 9.21E-04 |
| P43897 | Elongation factor Ts, mitochondrial | 12 | 43.69 | 32 | 8.4 | 35.4 | 1.6 | 2.12E-03 |
| P49411 | Elongation factor Tu, mitochondrial | 29 | 63.27 | 251 | 7.6 | 49.5 | 1.5 | 2.10E-03 |
| Q96DP5 | Methionyl-tRNA formyltransferase, mitochondrial | 2 | 5.4 | 2 | 9.7 | 43.8 | 1.5 | 1.15E-04 |
| Q9UBX3 | Mitochondrial dicarboxylate carrier | 1 | 34.49 | 12 | 9.5 | 31.3 | 2.5 | 1.06E-02 |
| Q8TEM1 | Nuclear pore membrane glycoprotein 210 | 19 | 13.14 | 30 | 6.8 | 205.0 | 1.5 | 1.39E-02 |
| Q9Y5M8 | Signal recognition particle receptor subunit beta | 11 | 44.28 | 37 | 9.0 | 29.7 | 1.5 | 1.88E-02 |
| Q9BSK2 | Solute carrier family 25 member 33 | 1 | 6.85 | 3 | 9.6 | 35.4 | 1.6 | 1.40E-02 |
T/N refers to protein ratio of EOC/control
DEP, differentially expressed protein; MW, molecular weight; pI, isoelectric point; PSMs, peptide spectrum matches
Enhanced activities of three energy metabolism pathways in EOCs
A previous iTRAQ-labeled quantitative proteomic study between EOC and control whole tissues found that the key enzymes in the glycolysis pathway [38], located in the cytoplasm, were significantly upregulated in the EOC relative to control tissues. It demonstrated the increased activities of glycolysis pathway in EOC tissues, which coincided with the Warburg effect proposed in 1926 [46]. Moreover, the KEGG pathway analysis of those 1198 mtDEPs found that the Kreb’s cycle and OXPHOS pathways, located in the mitochondria, were significantly involved in the identified mtDEPs, and the key proteins (PDHB, CS, IDH2, OGDHL, and UQCRH) in the OXPHOS and Kreb’s cycle pathways were significantly upregulated. It demonstrated the increased activities of OXPHOS and Kreb’s cycle pathways in EOC tissues, namely the reverse Warburg effect [24].
-
(i)
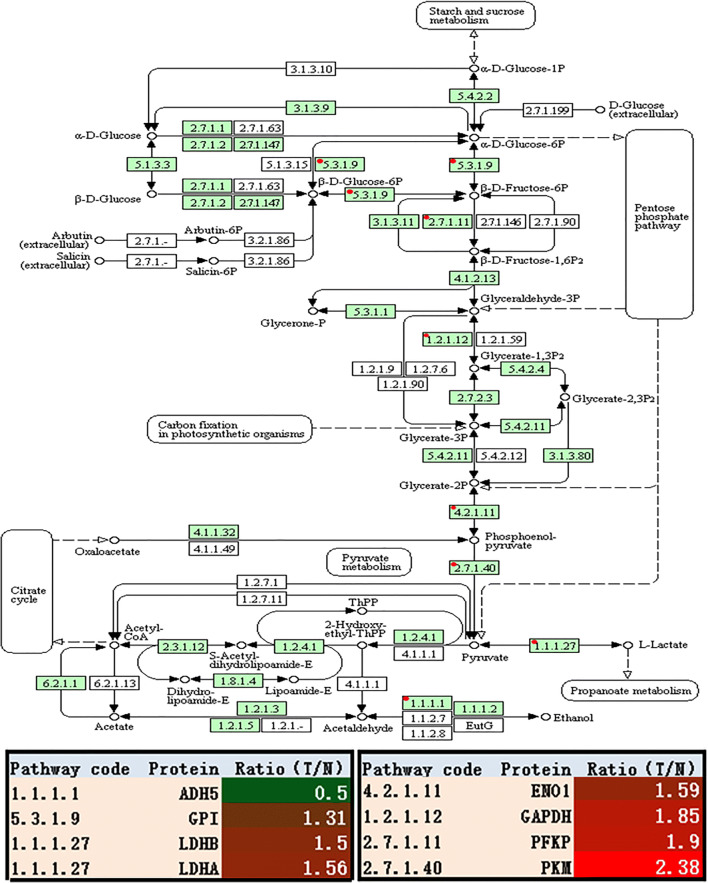
The enhanced glycolysis: iTRAQ-SCX-LC-MS/MS analysis of the whole tissue samples revealed that the glycolysis-related enzymes were significantly increased in EOC tissues relative to controls, including phosphofructokinase platelet (PFKP), pyruvate kinase muscle (PKM), lactate dehydrogenase B (LDHB), lactate dehydrogenase A (LDHA), enolase 1 (ENO1), alcohol dehydrogenase 5 class III chi polypeptide (ADH5), and glucose-6-phosphate isomerase (GPI) (Fig. 2 and Table 3) [38]. Among them, PFKP (fold change = 1.90, p = 2.28E-2) and PKM (fold change = 2.38, p = 1.50E-4) were the rate-limiting enzymes. PFKM took part in an irreversible reaction in the process of glycolysis, and it served as one of the rate-limiting enzymes. Pyruvate kinase catalyzed the final step of glycolysis to form pyruvate and ATP. GPI (fold change = 1.31, p = 2.96E-2) was not the rate-limiting enzyme of glycolysis but was one of the important regulatory enzymes. GPI protein has different functions inside and outside the cell; it was involved in the breakdown and buildup of glucose in the cytoplasm inside the cell or acted as neuroleukin outside the cell [47]. In short, those results demonstrated that EOC relied mainly on high levels of glycolysis.
-
(ii)
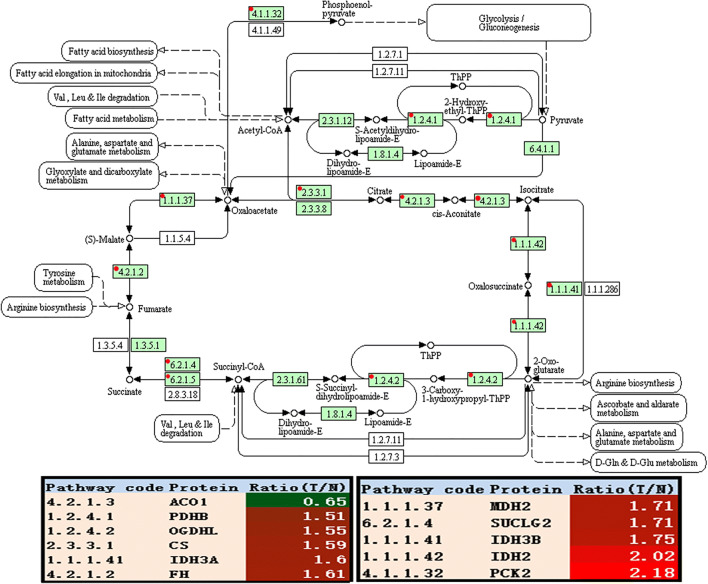
The enhanced Kreb’s cycle: Quantitative mitochondrial proteomics revealed that the related enzymes of Kreb’s cycle were significantly increased in EOCs relative to controls, including CS, IDH, OGDHL, SUCLG2, FH, MDH2, and PDHB (Fig. 3 and Table 4). Among them, CS (fold change = 1.59, p = 4.00E-3), IDH2 (fold change = 2.02, p = 2.00E-3), IDH3A (fold change = 0.56, p = 2.54E-3), IDH3B (fold change = 1.60, p = 2.20E-2), and OGDHL (fold change = 1.55, p = 1.00E-3) were the rate-limiting enzymes. It is well-known that the enzyme pyruvate dehydrogenase complex (PDC) converted pyruvate to acetyl CoA by pyruvate decarboxylation, which connected cytoplasmic glycolysis with mitochondrial Kreb’s cycle. What was notable in the PDC was that PDHB (fold change = 1.75, p = 0.008) as one subunit of PDC was obviously upregulated in EOC tissues. Those findings demonstrated that EOC had an enhanced Kreb’s cycle, which coincided with the well-known reverse Warburg effect.
-
(iii)
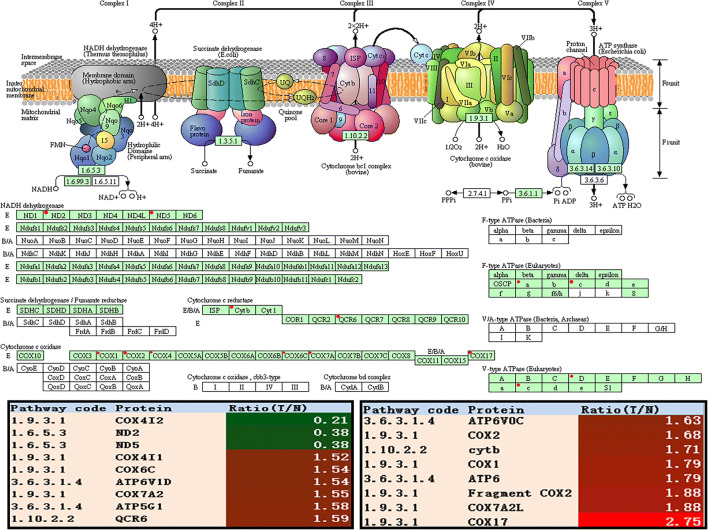
The enhanced OXPHOS: In most eukaryotes, Kreb’s cycle-generated NADH and FADH2 were fed into OXPHOS inside the mitochondria. The eukaryotic ETCs contain complex I—NADH-coenzyme Q oxidoreductase, complex II—succinate-Q oxidoreductase, complex III—Q-cytochrome c oxidoreductase, complex IV—cytochrome coxidase, and complex V—ATP synthase. The expressions of complex III subunits (CYTB and UQCRH), complex IV (COX17, COX4I2, COX6C, COX7A2L, COX7A2, COX1, and COX2), and complex V (ATP6, ATP5G1, ATP6V0C, and ATP6V1D) were significantly upregulated in EOC tissues (Fig. 4 and Table 5). It clearly demonstrated that the eukaryotic ETCs were enhanced in EOC tissues. The main function of the mitochondria was to produce ATP and ROS [48]. Although the implications of electron “leakage” was not always clear, ROS productions were increased in cancer cells compared with normal cells [49]. Thus, the increase of ROS productions in cancer cells enhanced oxidative stress in stromal CAFs, which coincided with the well-known reverse Warburg effect.
Fig. 2.
Glycolysis/gluconeogenesis pathway altered in epithelial ovarian cancer. Green rectangle with red mark means the differentially expressed proteins. Green rectangle without red mark means species-specific enzymes. White rectangle means reference pathway. The solid line means molecular interaction. The dot line means indirect effect. The circle means mostly chemical complex. ADH5, alcohol dehydrogenase 5 class III chi polypeptide; GPI, glucose-6-phosphate isomerase; LDHB, lactate dehydrogenase B; LDHA, lactate dehydrogenase A; ENO1, enolase 1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PFKP, phosphofructokinase, platelet; PKM, pyruvate kinase muscle
Table 3.
Glycolysis pathways involved DEPs operated in ovarian cancer biological system
| Pathway code | Accession no. | Protein | calc. pI | MW (kDa) | Ratio (T/N) | p value (t test) |
|---|---|---|---|---|---|---|
| 1.1.1.1 | P11766 | Alcohol dehydrogenase 5 (class III), chi polypeptide (ADH5) | 7.5 | 39.70 | 0.50 | 6.60E-05 |
| 4.2.1.11 | P06733 | Enolase 1 (ENO1) | 7.4 | 47.14 | 1.59 | 2.60E-11 |
| 5.3.1.9 | K7EQ48 | Glucose-6-phosphate isomerase (GPI) | 8.7 | 53.37 | 1.31 | 2.96E-02 |
| 1.2.1.12 | P04406 | Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | 8.5 | 36.03 | 1.85 | 2.24E-44 |
| 1.1.1.27 | P00338 | Lactate dehydrogenase A (LDHA) | 8.3 | 36.67 | 1.56 | 4.00E-04 |
| 1.1.1.27 | P07195 | Lactate dehydrogenase B (LDHB) | 6.1 | 36.62 | 1.50 | 6.40E-03 |
| 2.7.1.11 | Q01813 | Phosphofructokinase, platelet (PFKP) | 7.5 | 85.54 | 1.90 | 2.28E-02 |
| 2.7.1.40 | A0A024R5Z9 | Pyruvate kinase, muscle (PKM) | 7.7 | 58.02 | 2.38 | 1.50E-04 |
Ratio (T/N) means the ratio of tumor to control
DEPs, differentially expressed proteins; pI, isoelectric point; MW, molecular weight
Fig. 3.
Kreb’s cycle altered in ovarian cancer. Green rectangle without red mark means species-specific enzymes. White rectangle means reference pathway. The solid line means molecular interaction. The dot line means indirect effect. The circle means mostly chemical complex. ACO1, cytoplasmic aconitate hydratase; PDHB, pyruvate dehydrogenase E1 subunit beta; IDH2, isocitrate dehydrogenase (NADP(+)) 2; CS, citrate synthase; IDH3A, mitochondrial isocitrate dehydrogenase [NAD] subunit alpha; FH, fumarate hydratase; MDH2, malate dehydrogenase 2; SUCLG2, succinate–CoA ligase GDP-forming subunit beta; IDH3B, isocitrate dehydrogenase (NAD(+)) 3 noncatalytic subunit beta; OGDHL, oxoglutarate dehydrogenase L; PCK2, mitochondrial phosphoenolpyruvate carboxykinase [GTP]
Table 4.
Kreb’s cycle involved mtDEPs operated in ovarian cancer biological system
| Pathway code | Accession no. | Protein | Unique peptides | Coverage (%) | PSMs | Calc. pI | MW (kDa) | Ratio (T/N) | p value (t test) |
|---|---|---|---|---|---|---|---|---|---|
| 4.2.1.3 | P21399 | Aconitase 1 (ACO1) | 10 | 13.27 | 13 | 6.7 | 98.34 | 0.65 | 6.04E-03 |
| 4.1.1.32 | Q16822 | PCK2 protein (PCK2) | 1 | 35.00 | 31 | 7.6 | 70.68 | 2.18 | 4.48E-03 |
| 1.2.4.1 | P11177 | Pyruvate dehydrogenase E1 component subunit beta, mitochondrial (PDHB) | 14 | 52.92 | 79 | 6.7 | 39.21 | 1.51 | 3.25E-03 |
| 1.1.1.37 | Q75MT9 | Malate dehydrogenase (fragment) (MDH2) | 21 | 74.37 | 262 | 8.3 | 33.21 | 1.71 | 5.51E-03 |
| 2.3.3.1 | B4DJV2 | Citrate synthase (CS) | 13 | 26.93 | 73 | 7.9 | 50.40 | 1.59 | 4.65E-03 |
| 1.1.1.42 | P48735 | Isocitrate deh1ydrogenase (NADP), mitochondrial (IDH2) | 27 | 56.64 | 355 | 8.7 | 50.88 | 2.02 | 2.07E-03 |
| 1.1.1.41 | O43837 | Isocitrate dehydrogenase (NAD) subunit beta, mitochondrial (IDH3B) | 13 | 41.56 | 43 | 8.5 | 42.16 | 1.75 | 8.69E-03 |
| 1.1.1.41 | P50213 | Isocitrate dehydrogenase (NAD) subunit alpha, mitochondrial (IDH3A) | 18 | 47.81 | 53 | 6.9 | 39.57 | 1.60 | 2.27E-02 |
| 4.2.1.2 | P07954 | Fumarate hydratase, mitochondrial (FH) | 7 | 35.69 | 137 | 8.8 | 54.60 | 1.61 | 8.84E-03 |
| 6.2.1.4 | Q96I99 | Succinate–CoA ligase (GDP-forming) subunit beta, mitochondrial (SUCLG2) | 19 | 44.91 | 115 | 6.4 | 46.48 | 1.71 | 8.17E-04 |
| 1.2.4.2 | Q9ULD0 | 2-Oxoglutarate dehydrogenase-like, mitochondrial (OGDHL) | 13 | 26.83 | 58 | 6.7 | 114.41 | 1.55 | 1.25E-03 |
Ratio (T/N) means the ratio of tumor to control
mtDEPs, mitochondrial differentially expressed proteins; pI, isoelectric point; MW, molecular weight; PSMs, peptide spectrum matches
Fig. 4.
Oxidative phosphorylation altered in ovarian cancer. Green rectangle with red mark means the differential proteins. Green rectangle without red mark means species-specific enzymes. White rectangle means reference pathway. The solid line means molecular interaction. The dot line means indirect effect. The circle means mostly chemical complex. COX4I2, cytochrome c oxidase subunit 4I2; ND2, mitochondrially encoded NADH dehydrogenase 2; ND5, mitochondrially encoded NADH dehydrogenase 5; COX17, cytochrome c oxidase copper chaperone COX17; COX6C, cytochrome c oxidase subunit 6C; ATP6V1D, ATPase H+ transporting V1 subunit D; COX7A2, cytochrome c oxidase subunit 7A2; ATP5G1, ATP synthase membrane subunit c locus 1; QCR6, mitochondrial cytochrome b-c1 complex subunit 6; ATP6V0C, ATPase H+ transporting V0 subunit c; COX2, cytochrome c oxidase subunit II; CYTB, mitochondrially encoded cytochrome b; CYP3A4, cytochrome P450 family 3 subfamily A member 4; COX1, cytochrome c oxidase subunit; ATP6, ATP synthase F0 subunit 6; COX7A2L, cytochrome c oxidase subunit 7A2 like; COX4I1, cytochrome c oxidase subunit 4I1
Table 5.
Oxidative phosphorylation involved mtDEPs operated in ovarian cancer biological system
| Pathway code | Accession no. | Protein | Unique peptides | Coverage (%) | PSMs | Calc. pI | MW (kDa) | Ratio (T/N) | p value (t test) |
|---|---|---|---|---|---|---|---|---|---|
| 1.6.5.3 | A0A059T3A1 | NADH-ubiquinone oxidoreductase chain 2 (ND2) | 1 | 4.61 | 2 | 9.8 | 38.93 | 0.38 | 6.03E-04 |
| 1.6.5.3 | A0A096WB60 | NADH-ubiquinone oxidoreductase chain 5 (ND5) | 1 | 5.14 | 6 | 9.0 | 67.01 | 0.38 | 3.34E-04 |
| 1.10.2.2 | A0A0A0QN99 | Cytochrome b (cytb) | 1 | 4.21 | 4 | 8.0 | 42.71 | 1.71 | 7.60E-03 |
| 1.10.2.2 | P07919 | Cytochrome b-c1 complex subunit 6, mitochondrial (QCR6) | 5 | 51.65 | 18 | 4.4 | 10.73 | 1.59 | 1.63E-02 |
| 1.9.3.1 | H7C4E5 | COX17, cytochrome c oxidase copper chaperone (COX17) | 1 | 12.07 | 1 | 7.7 | 6.41 | 2.75 | 2.33E-03 |
| 1.9.3.1 | H9LP39 | Cytochrome c oxidase subunit I (COX1) | 1 | 6.04 | 1 | 6.7 | 57.01 | 1.79 | 2.44E-03 |
| 1.9.3.1 | P09669 | Cytochrome c oxidase subunit 6C (COX6C) | 7 | 52 | 30 | 10.4 | 8.78 | 1.54 | 5.97E-03 |
| 1.9.3.1 | Q96KJ9 | Cytochrome c oxidase subunit 4I2 (COX4I2) | 1 | 5.85 | 1 | 9.6 | 20.00 | 0.21 | 6.28E-04 |
| 1.9.3.1 | P13073 | Cytochrome c oxidase subunit 4 isoform 1, mitochondrial (COX4I1) | 7 | 36.09 | 48 | 9.5 | 19.56 | 1.52 | 4.37E-03 |
| 1.9.3.1 | A0A097Q0T5 | Cytochrome c oxidase subunit 2 (COX2) | 1 | 26.43 | 64 | 4.8 | 25.55 | 1.68 | 4.38E-02 |
| 1.9.3.1 | H9E7B8 | Cytochrome c oxidase subunit 2 (fragment) (COX2) | 1 | 27.27 | 65 | 4.7 | 24.84 | 1.88 | 1.97E-02 |
| 1.9.3.1 | O14548 | Cytochrome c oxidase subunit 7A2 like (COX7A2L) | 4 | 54.39 | 8 | 9.4 | 12.61 | 1.88 | 5.33E-03 |
| 1.9.3.1 | P14406 | Cytochrome c oxidase subunit 7A2 (COX7A2) | 2 | 27.71 | 15 | 9.8 | 9.39 | 1.55 | 3.89E-02 |
| 3.6.3.1.4 | P27449 | ATPase H+ transporting V0 subunit c (ATP6V0C) | 1 | 11.61 | 18 | 8.4 | 15.73 | 1.63 | 4.24E-04 |
| 3.6.3.1.4 | A0A059QB80 | ATP synthase subunit a (ATP6) | 1 | 4.42 | 17 | 10.1 | 24.74 | 1.79 | 6.63E-03 |
| 3.6.3.1.4 | G3V2V6 | ATPase H+ transporting V1 subunit D (ATP6V1D) | 1 | 7.43 | 1 | 9.5 | 17.41 | 1.54 | 8.22E-04 |
| 3.6.3.1.4 | I3L0Y5 | ATP synthase, H+ transporting, mitochondrial Fo complex subunit C1 (subunit 9) (ATP5G1) | 1 | 7.14 | 6 | 10.0 | 10.03 | 1.58 | 9.78E-03 |
Ratio (T/N) means the ratio of tumor to control
mtDEPs, mitochondrial differentially expressed proteins; pI, isoelectric point; MW, molecular weight; PSMs, peptide spectrum matches
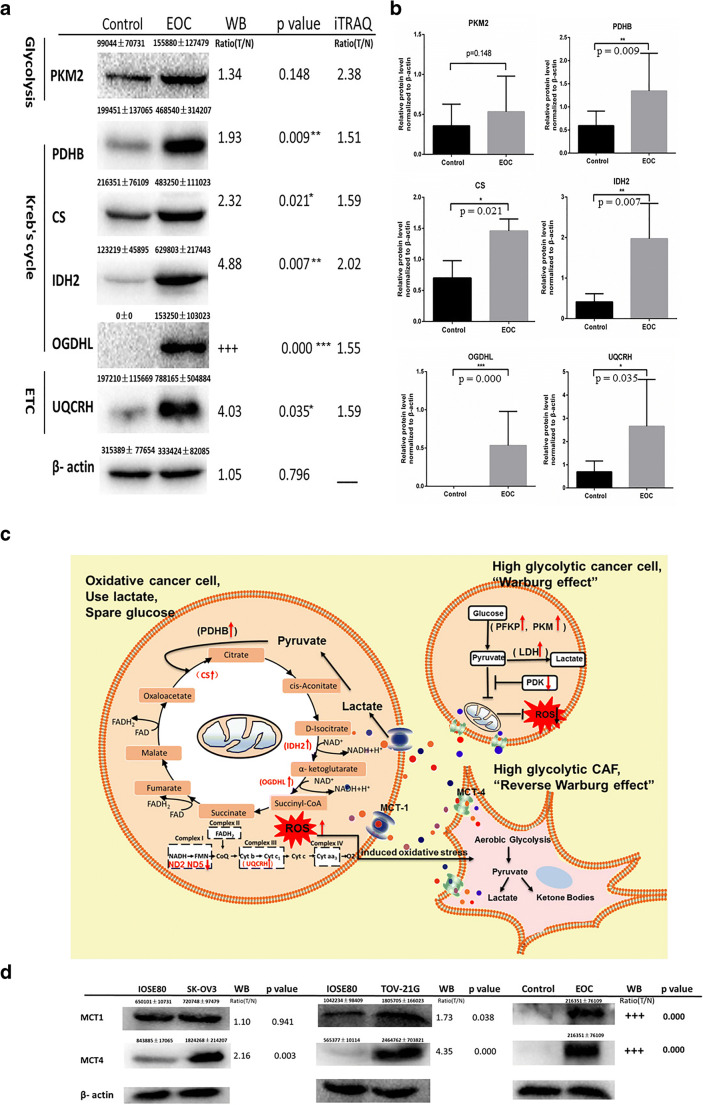
Western blot validation of the rate-limiting enzymes in energy metabolism pathways
For validation of mtDEPs identified with iTRAQ-SCX-LC-MS/MS, those rate-limiting enzymes were further analyzed with western blot, including PKM2, PDHB, CS, IDH2, OGDHL, and UQCRH, between human EOC and control mitochondrial samples. Western blot found that the protein levels of PDHB, CS, IDH2, OGDHL, and UQCRH were significantly increased in EOCs relative to controls (Fig. 5 a and b), whereas PKM2 showed a rising trend without a statistical significance. The western blot results showed a very good consistency with the results of iTRAQ quantification and also confirmed the enhanced capabilities of three energy metabolism pathways (Kreb’s cycle, OXPHOS, and glycolysis) in EOC tissues.
Fig. 5.
Western blot analysis to validate results of iTRAQ labeling. a, b Mitochondrial proteins of EOC and control tissues were analyzed by WB using antibodies against PKM2, PDHB, CS, IDH2, OGDHL, and UQCRH. The levels of PKM2, PDHB, CS, IDH2, OGDHL, and UQCRH were normalized relative to β-actin. Data represent mean values ± SD. c Warburg effect and the reverse Warburg effect. Parenchymal cells showed metabolic heterogeneity. Some cancer cells were high glycolytic cancer cell consisting with “Warburg effect,” and the other cancer cells were oxidative cancer cell consisting with “the reverse Warburg effect.” Tumor cells and stroma cells (especially CAFs) have metabolic symbiosis; thus, cancer cell induced oxidative stress of CAFs by secreting ROS and enhanced aerobic glycolysis in CAFs. In turn, CAFs produced lots of nourishment, which was “eaten” up by the cancer cells to produce ATP. *p < 0.05, **p < 0.01, ***p < 0.001. iTRAQ, isobaric tags for relative and absolute quantitation; EOC, epithelial ovarian carcinoma; WB, western blot; ROS, reactive oxygen species; PKM2, pyruvate kinase M2; PDHB, pyruvate dehydrogenase E1 subunit beta; CS, citrate synthase; IDH2, isocitrate dehydrogenase (NADP(+)) 2; OGDHL, oxoglutarate dehydrogenase L; UQCRH, ubiquinol-cytochrome c reductase hinge protein; CAFs, cancer-associated fibroblasts; PDK, pyruvate dehydrogenase (acetyl-transferring)] kinase; MCT1, solute carrier family 16 member 1; MCT4, solute carrier family 16 member 4
The Warburg and reverse Warburg effects in EOCs
Some cancer cells mainly depended on aerobic glycolysis, whereas other cancer cells depended mainly on OXPHOS for energy supply. The evidence proved that “Warburg effect” and “reverse Warburg effect” coexisted in a population of cancer cells such as a cancer tissue. Cancer cells released ROS into extracellular interstitium, which resulted in CAFs in a state of stress and produced lots of nourishment for ATP generation through Kreb’s cycle and OXPHOS (Fig. 5c). Our previous study [17] found that MCT1 and MCT4, which link the oxidative cancer cells and the high glycolytic cancer cells/CAFs, were significantly upregulated in EOC cells (SKOV3 and TOV21G) compared with ISOE80 normal cells by qRT-PCR analysis, with the fold change of MCT-1 in SKOV3 cells (fold change = 3.70, p = 0.009) and in TOV21G cells (fold change = 2.67, p = 0.005) and with the fold change of MCT-4 in SKOV3 cells (fold change = 5.93, p = 0.002) and in TOV21G cells (fold change = 10.38, p = 0.00001). The western blot results showed a very good consistency with the results of qPCR quantification and also confirmed significantly increased levels of MCT1 and MCT4 in EOCs (both tissue and cell samples) relative to controls (Fig. 5d).
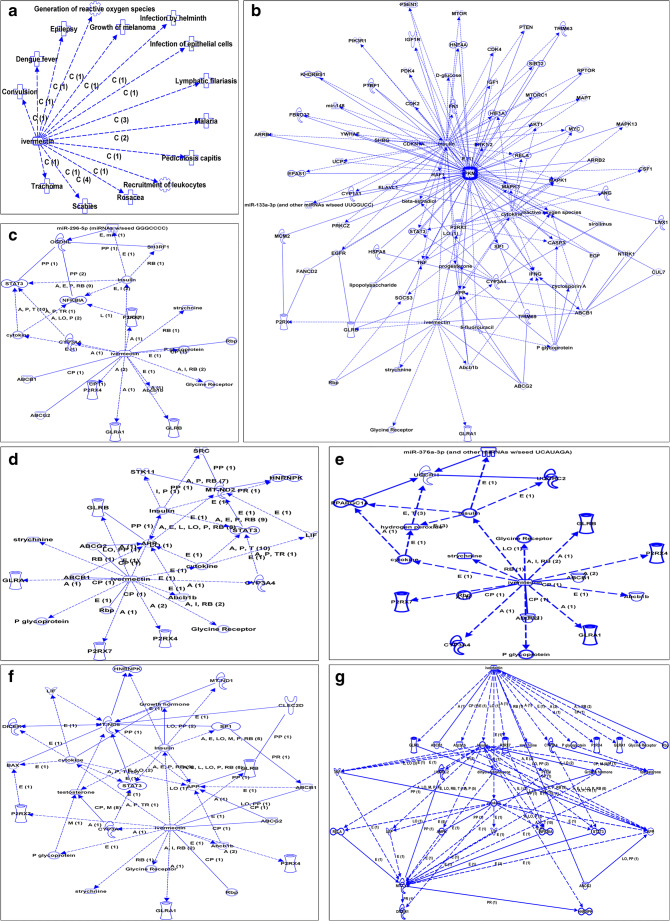
IPA analysis indicated the association of the antiparasite drug ivermectin with production of ROS and energy metabolism
Disease and function analysis of ivermectin based on the IPA database showed that ivermectin was not only a broad-spectrum antiparasite drug but also associated with cancer treatment and production of ROS (Fig. 6a). Biomolecular network analysis of ivermectin based on the IPA database showed that ivermectin regulated enzymes (PKM, OGDHL, ND2, ND5, CytB, and UQCRH) in energy metabolism pathways through other molecules (Fig. 6b–g), which showed that ivermectin might have an impact on energy metabolism of cancer cells. Other molecules included the ivermectin directly regulated molecules such as CYP3A4, Rbp, GLRB, P2RX4, P2RX7, ABCB1, ABCG2, Abcb1b, P glycoprotein, cytokine, insulin, and strychnine, and the ivermectin indirectly regulated molecules such as APP, TNF, ERK1/2, MAPK1, MAPK13, MAPK3, NFKBIA, reactive oxygen species, STAT3, and testosterone (Supplementary Table 2).
Fig. 6.
IPA analysis revealed that ivermectin was associated with production of ROS and energy metabolism. a Disease and function analysis of ivermectin based on IPA software. b Biomolecular networks analysis of ivermectin based on IPA software showed that ivermectin regulated PKM. c Biomolecular networks analysis of ivermectin based on IPA software showed that ivermectin regulated OGDHL. d Biomolecular networks analysis of ivermectin based on IPA software showed that ivermectin regulated ND2. e Biomolecular networks analysis of ivermectin based on IPA software showed that ivermectin regulated ND5. f Biomolecular networks analysis of ivermectin based on IPA software showed that ivermectin regulated UQCRH. IPA, Ingenuity Pathway Analysis; ROS, reactive oxygen species; PKM, pyruvate kinase muscle; OGDHL, oxoglutarate dehydrogenase L; ND2, mitochondrially encoded NADH dehydrogenase 2; ND5, mitochondrially encoded NADH dehydrogenase 5; UQCRH, ubiquinol-cytochrome c reductase hinge protein
Ivermectin-mediated key molecular changes in energy metabolism pathways of EOC
It is significant to explore ivermectin-mediated enzymes in energy metabolism pathways in EOCs. SILAC-based quantitative proteomics was used to analyze the protein expressions of energy metabolic pathways in ovarian cancer cells treated with (SILAC: H) and without (SILAC: L) 20 μM ivermectin for 24 h (Table 6). The results revealed that the glycolysis-related enzymes were significantly altered in EOC cells treated with vs. without ivermectin, including ADH5 (ratio H/L = 0.45, Q = 0.000), ENO1 (ratio H/L = 0.44, Q = 0.000), GPI (ratio H/L = 0.44, Q = 1.000), GAPDH (ratio H/L = /, which means the protein with expressed value 0 in both H and L groups; Q = 0.000), LDHA (ratio H/L = 0.34, Q = 1.000), LDHB (ratio H/L = 0.42, Q = 0.000), PFKP (ratio H/L = 0.54, Q = 0.000), and PKM (ratio H/L = +, which means the protein expressed in the H group but not in the L group; Q = 0.00745). The related enzymes of the Kreb’s cycle were also significantly altered in EOC cells treated with vs. without ivermectin, including ACON (ratio H/L = −, which means the protein expressed in the L group but not in the H group; Q = 0.000), PCK2 (ratio H/L = 0.56, Q = 0.000), PDHB (ratio H/L = 0.46, Q = 0.000), MDH2 (ratio H/L = 0.42, Q = 0.000), CS (ratio H/L = 0.45, Q = 0.000), IDH2 (ratio H/L = 0.46, Q = 0.000), IDH3A (ratio H/L = 0.40, Q = 0.000), IDH3B (ratio H/L = 0.41, Q = 0.000), SUCLG2 (ratio H/L = 0.41, Q = 0.000), and OGDHL (ratio H/L = 0.56, Q = 0.000). The related enzymes of OXPHOS were also significantly altered in EOC cells treated with vs. without ivermectin, including CYTB (ratio H/L = 0.55, Q = 0.00359), UQCRH (ratio H/L = 0.51, Q = 0.000), COX17 (ratio H/L = 0.36, Q = 0.000), COX1 (ratio H/L = 0.38, Q = 0.000789), COX6C (ratio H/L = 0.34, Q = 0.000), COX4I1 (ratio H/L = 0.40, Q = 0.000), COX2 (ratio H/L = 0.38, Q = 0.000), COX7A2L (ratio H/L = 17.81, Q = 0.000534), COX7A2 (ratio H/L = 0.32, Q = 0.000), ATP6V0C (ratio H/L = 0.47, Q = 1.000), and ATP6 (ratio H/L = 0.73, Q = 0.000). The lactate shuttle [MCT1 (ratio H/L = 0.53, Q = 0.000) and MCT4 (ratio H/L = 0.38, Q = 0.000)] were also changed in EOC cells treated with vs. without ivermectin.
Table 6.
SILAC-based quantitative proteomics analysis of the protein expressions of energy metabolic pathways in ovarian cancer cells TOV-21G treated with (SILAC: H) and without (SILAC: L) 20 μM ivermectin for 24 h, and verified with qPCR and western blot
| Pathway | Protein ID | Gene name | Protein name | Peptides | Unique peptides | Sequence coverage [%] | Mol. weight [kDa] | Sequence length | Score | Q value | Intensity H | Intensity L | Ratio H/L |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycolysis pathway | PFKAP | PFKP | ATP-dependent 6-phosphofructokinase, platelet type | 31 | 28 | 46.7 | 85.6 | 784 | 323.3 | 0.00E+00 | 14,226,000,000 | 25,587,000,000 | 0.54 |
| H3BQ34 | PKM | Pyruvate kinase | 1 | 1 | 0.0 | 30.7 | 281 | 1.9 | 7.46E-03 | 10,727,000 | 0 | + | |
| ODPB | PDHB | Pyruvate dehydrogenase E1 component subunit beta, mitochondrial | 9 | 9 | 34.0 | 39.2 | 359 | 67.1 | 0.00E+00 | 407,280,000 | 1,649,500,000 | 0.46 | |
| K4EN11 | GAPDH | GAPDH (fragment) | 1 | 1 | 48.1 | 2.8 | 27 | 6.3 | 0.00E+00 | 0 | 0 | / | |
| ENOA | ENO1 | Alpha-enolase | 21 | 11 | 65.9 | 47.2 | 434 | 323.3 | 0.00E+00 | 54,687,000,000 | 125,660,000,000 | 0.44 | |
| F5GXY2 | LDHA | L-lactate dehydrogenase A chain (fragment) | 15 | 1 | 82.1 | 17.3 | 156 | − 2.0 | 1.00E+00 | 10,379,000 | 29,470,000 | 0.34 | |
| Q5U077 | LDHB | L-lactate dehydrogenase | 17 | 8 | 49.4 | 36.6 | 334 | 160.4 | 0.00E+00 | 27,852,000,000 | 66,990,000,000 | 0.42 | |
| A0A0A0MTS2 | GPI | Glucose-6-phosphate isomerase (fragment) | 21 | 1 | 48.0 | 64.8 | 573 | − 2.0 | 1.00E+00 | 56,685,000 | 138,520,000 | 0.44 | |
| Q6IRT1 | ADH5 | S-(hydroxymethyl)glutathione dehydrogenase | 16 | 16 | 52.9 | 39.7 | 374 | 115.0 | 0.00E+00 | 1,308,100,000 | 3,513,700,000 | 0.45 | |
| B3KUV2 | ACSS2 | cDNA FLJ40707 fis, clone THYMU2026835, highly similar to acetyl-coenzyme A synthetase, cytoplasmic | 2 | 2 | 6.6 | 45.5 | 409 | 1.8 | 9.53E-03 | 9,455,200 | 25,758,000 | 0.73 | |
| H3BRS6 | ADPGK | ADP-dependent glucokinase (fragment) | 2 | 2 | 13.2 | 21.8 | 204 | 3.1 | 5.31E-04 | 11,465,000 | 18,413,000 | 0.69 | |
| AL1B1 | ALDH1B1 | Aldehyde dehydrogenase X, mitochondrial | 6 | 5 | 17.0 | 57.2 | 517 | 26.0 | 0.00E+00 | 69,821,000 | 196,750,000 | 0.45 | |
| ALDH2 | ALDH2 | Aldehyde dehydrogenase, mitochondrial | 18 | 17 | 48.4 | 56.4 | 517 | 68.0 | 0.00E+00 | 812,240,000 | 1,822,600,000 | 0.44 | |
| AL3A2 | ALDH3A2 | Aldehyde dehydrogenase family 3 member A2 | 10 | 10 | 26.0 | 51.9 | 461 | 30.5 | 0.00E+00 | 225,000,000 | 394,360,000 | 0.55 | |
| AL9A1 | ALDH9A1 | 4-Trimethylaminobutyraldehyde dehydrogenase | 18 | 18 | 39.3 | 53.8 | 494 | 77.3 | 0.00E+00 | 529,020,000 | 1,322,400,000 | 0.48 | |
| A0A024QZ64 | ALDOC | Fructose-bisphosphate aldolase | 12 | 8 | 45.1 | 39.5 | 364 | 109.3 | 0.00E+00 | 1,104,800,000 | 2,650,700,000 | 0.43 | |
| H0YDD4 | DLAT | Acetyltransferase component of pyruvate dehydrogenase complex (fragment) | 8 | 8 | 21.1 | 51.2 | 479 | 74.8 | 0.00E+00 | 530,720,000 | 1,251,100,000 | 0.46 | |
| A0A024R713 | DLD | Dihydrolipoyl dehydrogenase | 13 | 13 | 37.7 | 48.9 | 459 | 35.1 | 0.00E+00 | 632,170,000 | 1,843,800,000 | 0.52 | |
| Q6FHV6 | ENO2 | ENO2 protein | 13 | 11 | 50.2 | 47.3 | 434 | 175.6 | 0.00E+00 | 618,190,000 | 2,887,100,000 | 0.26 | |
| ENOB | ENO3 | Beta-enolase | 4 | 2 | 19.4 | 47.0 | 434 | 6.7 | 0.00E+00 | 215,810,000 | 482,340,000 | 0.59 | |
| B4DG62 | HK1 | cDNA FLJ56506, highly similar to hexokinase-1 | 29 | 22 | 36.0 | 102.3 | 915 | 109.9 | 0.00E+00 | 1,617,000,000 | 4,075,800,000 | 0.53 | |
| HKDC1 | HKDC1 | Hexokinase HKDC1 | 15 | 11 | 17.7 | 102.5 | 917 | 57.9 | 0.00E+00 | 132,850,000 | 568,430,000 | 0.30 | |
| PCKGC | PCK1 | Phosphoenolpyruvate carboxykinase, cytosolic [GTP] | 8 | 6 | 17.0 | 69.2 | 622 | 15.3 | 0.00E+00 | 1,267,700 | 160,370,000 | 0.07 | |
| A0A384MTT2 | PCK2 | Epididymis secretory sperm binding protein | 12 | 10 | 25.8 | 70.7 | 640 | 61.9 | 0.00E+00 | 403,190,000 | 1,032,500,000 | 0.56 | |
| A0A024RBX9 | PDHA1 | Pyruvate dehydrogenase E1 component subunit alpha | 11 | 11 | 35.1 | 43.3 | 390 | 102.8 | 0.00E+00 | 457,490,000 | 1,353,000,000 | 0.49 | |
| PFKAL | PFKL | ATP-dependent 6-phosphofructokinase, liver type | 16 | 12 | 27.1 | 85.0 | 780 | 161.1 | 0.00E+00 | 1,242,500,000 | 2,567,300,000 | 0.52 | |
| A0A024R0Y5 | PFKM | ATP-dependent 6-phosphofructokinase | 20 | 17 | 35.5 | 85.2 | 780 | 323.3 | 0.00E+00 | 1,677,600,000 | 3,768,800,000 | 0.47 | |
| Q6P6D7 | PGAM1 | Phosphoglycerate mutase | 15 | 15 | 70.5 | 28.8 | 254 | 226.1 | 0.00E+00 | 11,906,000,000 | 30,409,000,000 | 0.36 | |
| A0A3B3ITK7 | PGM1 | Phosphoglucomutase-1 | 18 | 18 | 40.2 | 64.0 | 584 | 109.1 | 0.00E+00 | 721,450,000 | 1,641,900,000 | 0.43 | |
| PGM2 | PGM2 | Phosphoglucomutase-2 | 9 | 9 | 19.8 | 68.3 | 612 | 13.7 | 0.00E+00 | 144,180,000 | 423,580,000 | 0.40 | |
| A0A024R5Z9 | PKM2 | Pyruvate kinase | 39 | 1 | 71.6 | 58.1 | 531 | − 2.0 | 1.00E+00 | 35,541,000 | 125,430,000 | 0.54 | |
| Kreb’s cycle | ODPB | PDHB | Pyruvate dehydrogenase E1 component subunit beta, mitochondrial | 9 | 9 | 34.0 | 39.2 | 359 | 67.1 | 0.00E+00 | 407,280,000 | 1,649,500,000 | 0.46 |
| B4DJV2 | CS | Citrate synthase | 15 | 14 | 43.0 | 50.4 | 453 | 76.8 | 0.00E+00 | 2,428,500,000 | 5,338,700,000 | 0.45 | |
| IDHP | IDH2 | Isocitrate dehydrogenase [NADP], mitochondrial | 18 | 17 | 48.0 | 50.9 | 452 | 174.3 | 0.00E+00 | 1,281,200,000 | 2,994,300,000 | 0.46 | |
| IDH3A | IDH3A | Isocitrate dehydrogenase [NAD] subunit alpha, mitochondrial | 10 | 10 | 36.4 | 34.5 | 316 | 90.6 | 0.00E+00 | 268,600,000 | 1,119,300,000 | 0.40 | |
| A0A087WZN1 | IDH3B | Isocitrate dehydrogenase [NAD] subunit, mitochondrial | 8 | 8 | 26.6 | 42.4 | 387 | 26.8 | 0.00E+00 | 142,630,000 | 477,180,000 | 0.41 | |
| OGDHL | OGDHL | 2-Oxoglutarate dehydrogenase-like, mitochondrial | 10 | 5 | 10.9 | 114.5 | 1010 | 12.7 | 0.00E+00 | 17,707,000 | 119,970,000 | 0.56 | |
| O75944 | ACON | Aconitase (fragment) | 16 | 3 | 33.5 | 65.3 | 600 | 12.1 | 0.00E+00 | 0 | 48,304,000 | − | |
| A0A384MTT2 | PCK2 | Epididymis secretory sperm binding protein | 12 | 10 | 25.8 | 70.7 | 640 | 61.9 | 0.00E+00 | 403,190,000 | 1,032,500,000 | 0.56 | |
| Q0QF37 | MDH2 | Malate dehydrogenase (fragment) | 17 | 17 | 70.5 | 32.0 | 305 | 323.3 | 0.00E+00 | 5,856,200,000 | 14,406,000,000 | 0.42 | |
| A0A024R325 | SUCLG2 | Succinate–CoA ligase [GDP-forming] subunit beta, mitochondrial | 8 | 8 | 25.5 | 46.5 | 432 | 42.3 | 0.00E+00 | 232,210,000 | 779,800,000 | 0.41 | |
| Q71UF1 | ACO2 | Aconitate hydratase, mitochondrial | 24 | 0 | 38.6 | 85.6 | 780 | − 2.0 | 1.00E+00 | 0 | 12,950,000 | − | |
| A0A024R1Y2 | ACLY | ATP-citrate synthase | 37 | 37 | 42.4 | 119.8 | 1091 | 183.5 | 0.00E+00 | 2,033,900,000 | 4,490,700,000 | 0.46 | |
| H0YDD4 | DLAT | Acetyltransferase component of pyruvate dehydrogenase complex (fragment) | 8 | 8 | 21.1 | 51.2 | 479 | 74.8 | 0.00E+00 | 530,720,000 | 1,251,100,000 | 0.46 | |
| A0A024R713 | DLD | Dihydrolipoyl dehydrogenase | 13 | 13 | 37.7 | 48.9 | 459 | 35.1 | 0.00E+00 | 632,170,000 | 1,843,800,000 | 0.52 | |
| Q6IBS5 | DLST | DLST protein | 7 | 7 | 20.5 | 48.8 | 453 | 44.5 | 0.00E+00 | 601,540,000 | 1,338,700,000 | 0.53 | |
| A0A0S2Z4C3 | FH | Epididymis secretory sperm binding protein (fragment) | 14 | 14 | 35.5 | 54.6 | 510 | 161.7 | 0.00E+00 | 1,498,700,000 | 3,849,500,000 | 0.43 | |
| IDH3G | IDH3G | Isocitrate dehydrogenase [NAD] subunit gamma, mitochondrial | 7 | 7 | 27.7 | 42.8 | 393 | 42.3 | 0.00E+00 | 55,446,000 | 230,380,000 | 0.54 | |
| ODO1 | OGDH | 2-Oxoglutarate dehydrogenase, mitochondrial | 23 | 18 | 31.5 | 115.9 | 1023 | 82.1 | 0.00E+00 | 325,090,000 | 949,610,000 | 0.43 | |
| A0A494C101 | PC | Pyruvate carboxylase, mitochondrial (fragment) | 2 | 2 | 5.2 | 53.5 | 483 | 3.0 | 7.83E-04 | 3,454,600 | 19,685,000 | 0.28 | |
| PCKGC | PCK1 | Phosphoenolpyruvate carboxykinase, cytosolic [GTP] | 8 | 6 | 17.0 | 69.2 | 622 | 15.3 | 0.00E+00 | 1,267,700 | 160,370,000 | 0.07 | |
| A0A024RBX9 | PDHA1 | Pyruvate dehydrogenase E1 component subunit alpha | 11 | 11 | 35.1 | 43.3 | 390 | 102.8 | 0.00E+00 | 457,490,000 | 1,353,000,000 | 0.49 | |
| A0A024QZ30 | SDHA | Succinate dehydrogenase [ubiquinone] flavoprotein subunit, mitochondrial | 20 | 20 | 44.9 | 72.7 | 664 | 200.0 | 0.00E+00 | 1,096,500,000 | 2,950,800,000 | 0.44 | |
| SDHB | SDHB | Succinate dehydrogenase [ubiquinone] iron-sulfur subunit, mitochondrial | 6 | 2 | 24.3 | 31.6 | 280 | 17.3 | 0.00E+00 | 143,030,000 | 516,360,000 | 0.37 | |
| D3DVH1 | SDHC | Succinate dehydrogenase complex, subunit C, integral membrane protein, 15 kDa, isoform CRAa | 3 | 3 | 35.2 | 11.3 | 105 | 6.8 | 0.00E+00 | 68,060,000 | 130,150,000 | 0.46 | |
| B7ZAF6 | SUCLA2 | Succinate–CoA ligase [ADP-forming] subunit beta, mitochondrial | 9 | 9 | 36.2 | 36.0 | 329 | 127.1 | 0.00E+00 | 114,900,000 | 726,200,000 | 0.33 | |
| Q6IAL5 | SUCLG1 | Succinate–CoA ligase [ADP/GDP-forming] subunit alpha, mitochondrial | 8 | 8 | 30.3 | 35.0 | 333 | 45.9 | 0.00E+00 | 261,580,000 | 1,105,700,000 | 0.34 | |
| Oxidative phosphorylation | D2Y6X2 | ND5 | NADH dehydrogenase subunit 5 (fragment) | 1 | 1 | 26.8 | 4.5 | 41 | 3.2 | 5.33E-04 | 4,125,200 | 24,591,000 | 0.41 |
| A0A1B0TCA9 | CYTB | Cytochrome b (fragment) | 1 | 1 | 2.8 | 35.7 | 318 | 2.2 | 3.59E-03 | 53,396,000 | 59,765,000 | 0.55 | |
| Q567R0 | UQCRH | UQCRH protein | 1 | 1 | 21.2 | 10.0 | 85 | 37.0 | 0.00E+00 | 252,050,000 | 546,900,000 | 0.51 | |
| C9J8T6 | COX17 | Cytochrome c oxidase copper chaperone | 1 | 1 | 16.3 | 10.9 | 98 | 3.5 | 0.00E+00 | 7,643,200 | 53,020,000 | 0.36 | |
| Q6FGA0 | COX7A2L | COX7A2L protein | 2 | 2 | 19.3 | 12.6 | 114 | 3.2 | 5.34E-04 | 22,802,000 | 1,280,400 | 17.81 | |
| U3L4G0 | ATP6 | ATP synthase subunit a | 2 | 2 | 11.1 | 24.8 | 226 | 3.7 | 0.00E+00 | 193,600,000 | 355,900,000 | 0.73 | |
| X2C5C9 | COX1 | Cytochrome c oxidase subunit 1 | 2 | 2 | 5.8 | 41.6 | 379 | 3.0 | 7.89E-04 | 21,347,000 | 57,543,000 | 0.38 | |
| A0A346M047 | COX2 | Cytochrome c oxidase subunit II (fragment) | 7 | 1 | 46.4 | 17.0 | 151 | 22.8 | 0.00E+00 | 1,046,600,000 | 2,406,000,000 | 0.38 | |
| H3BNI4 | ATP6V0C | V-type proton ATPase proteolipid subunit | 1 | 1 | 8.9 | 11.6 | 112 | − 2.0 | 1.00E+00 | 11,819,000 | 33,788,000 | 0.47 | |
| Q496I0 | COX7A2 | COX7A2 protein | 2 | 2 | 27.7 | 9.4 | 83 | 10.2 | 0.00E+00 | 223,550,000 | 687,660,000 | 0.32 | |
| COX6C | COX6C | Cytochrome c oxidase subunit 6C | 2 | 2 | 40.0 | 8.8 | 75 | 5.0 | 0.00E+00 | 24,314,000 | 57,899,000 | 0.34 | |
| COX41 | COX4I1 | Cytochrome c oxidase subunit 4 isoform 1, mitochondrial | 8 | 8 | 45.6 | 19.6 | 169 | 28.4 | 0.00E+00 | 1,057,000,000 | 2,524,600,000 | 0.40 | |
| AT12A | ATP12A | Potassium-transporting ATPase alpha chain 2 | 6 | 1 | 5.3 | 115.5 | 1039 | − 2.0 | 1.00E+00 | 25,608,000 | 48,153,000 | 0.50 | |
| ATPG | ATP5F1C | ATP synthase subunit gamma, mitochondrial | 11 | 1 | 36.8 | 32.2 | 291 | 102.1 | 0.00E+00 | 610,730,000 | 1,752,800,000 | 0.61 | |
| ATPD | ATP5F1D | ATP synthase subunit delta, mitochondrial | 3 | 3 | 22.6 | 17.5 | 168 | 22.0 | 0.00E+00 | 173,290,000 | 378,660,000 | 0.59 | |
| ATP5I | ATP5ME | ATP synthase subunit e, mitochondrial | 4 | 4 | 42.0 | 7.9 | 69 | 10.4 | 0.00E+00 | 139,860,000 | 416,580,000 | 0.28 | |
| ATPK | ATP5MF | ATP synthase subunit f, mitochondrial | 3 | 3 | 39.4 | 10.9 | 94 | 15.6 | 0.00E+00 | 146,600,000 | 369,660,000 | 0.57 | |
| E9PN17 | ATP5MG | ATP synthase subunit g, mitochondrial | 3 | 3 | 48.7 | 8.5 | 76 | 15.1 | 0.00E+00 | 501,810,000 | 1,039,800,000 | 0.45 | |
| Q5QNZ2 | ATP5PB | ATP synthase F(0) complex subunit B1, mitochondrial | 9 | 9 | 49.7 | 22.3 | 195 | 323.3 | 0.00E+00 | 1,074,900,000 | 2,486,300,000 | 0.46 | |
| ATP5H | ATP5PD | ATP synthase subunit d, mitochondrial | 8 | 8 | 59.0 | 18.5 | 161 | 78.9 | 0.00E+00 | 525,070,000 | 965,510,000 | 0.39 | |
| ATPO | ATP5PO | ATP synthase subunit O, mitochondrial | 8 | 8 | 54.5 | 23.3 | 213 | 82.2 | 0.00E+00 | 1,495,600,000 | 3,024,300,000 | 0.56 | |
| VPP1 | ATP6V0A1 | V-type proton ATPase 116 kDa subunit a isoform 1 | 10 | 9 | 14.8 | 95.8 | 831 | 51.9 | 0.00E+00 | 98,038,000 | 557,300,000 | 0.35 | |
| R4GN72 | ATP6V0D1 | V-type proton ATPase subunit d 1 | 7 | 7 | 25.5 | 31.7 | 274 | 21.2 | 0.00E+00 | 258,530,000 | 806,720,000 | 0.31 | |
| VATA | ATP6V1A | V-type proton ATPase catalytic subunit A | 18 | 18 | 41.3 | 68.3 | 617 | 277.6 | 0.00E+00 | 1,191,800,000 | 3,218,800,000 | 0.37 | |
| VATB2 | ATP6V1B2 | V-type proton ATPase subunit B, brain isoform | 15 | 15 | 45.6 | 56.5 | 511 | 127.2 | 0.00E+00 | 583,390,000 | 2,310,600,000 | 0.35 | |
| A0A024R9I0 | ATP6V1C1 | V-type proton ATPase subunit C | 11 | 11 | 27.7 | 43.9 | 382 | 36.3 | 0.00E+00 | 105,790,000 | 524,300,000 | 0.36 | |
| Q53Y06 | ATP6V1E1 | ATPase, H+ transporting, lysosomal 31 kDa, V1 subunit E isoform 1 | 6 | 6 | 25.2 | 26.1 | 226 | 38.1 | 0.00E+00 | 226,190,000 | 457,540,000 | 0.46 | |
| A4D1K0 | ATP6V1F | V-type proton ATPase subunit F | 3 | 3 | 33.6 | 13.4 | 119 | 7.7 | 0.00E+00 | 72,747,000 | 287,010,000 | 0.62 | |
| A0A024R883 | ATP6V1G1 | V-type proton ATPase subunit G | 3 | 3 | 29.7 | 13.8 | 118 | 22.3 | 0.00E+00 | 121,660,000 | 150,890,000 | 0.58 | |
| A0A024R7X3 | ATP6V1H | V-type proton ATPase subunit H | 6 | 6 | 17.6 | 54.2 | 465 | 26.3 | 0.00E+00 | 33,783,000 | 166,330,000 | 0.37 | |
| COX15 | COX15 | Cytochrome c oxidase assembly protein COX15 homolog | 4 | 4 | 11.0 | 46.0 | 410 | 6.5 | 0.00E+00 | 52,507,000 | 160,350,000 | 0.47 | |
| A0A343FH12 | COX3 | Cytochrome c oxidase subunit 3 | 2 | 2 | 13.4 | 30.0 | 261 | 3.6 | 0.00E+00 | 251,800,000 | 620,670,000 | 0.40 | |
| H3BNX8 | COX5A | Cytochrome c oxidase subunit 5A, mitochondrial | 3 | 3 | 20.3 | 17.2 | 153 | 7.1 | 0.00E+00 | 489,640,000 | 1,451,700,000 | 0.67 | |
| COX5B | COX5B | Cytochrome c oxidase subunit 5B, mitochondrial | 4 | 4 | 24.8 | 13.7 | 129 | 9.1 | 0.00E+00 | 237,370,000 | 754,990,000 | 0.33 | |
| CX6B1 | COX6B1 | Cytochrome c oxidase subunit 6B1 | 4 | 4 | 57.0 | 10.2 | 86 | 32.2 | 0.00E+00 | 278,190,000 | 1,028,600,000 | 0.28 | |
| CY1 | CYC1 | Cytochrome c1, heme protein, mitochondrial | 8 | 8 | 31.1 | 34.5 | 315 | 80.4 | 0.00E+00 | 426,190,000 | 876,770,000 | 0.51 | |
| Q5T1Z0 | LHPP | Phospholysine phosphohistidine inorganic pyrophosphate phosphatase | 1 | 1 | 14.2 | 22.9 | 212 | 1.8 | 9.74E-03 | 0 | 20,832,000 | − | |
| D8VCQ0 | ND4 | NADH-ubiquinone oxidoreductase chain 4 (fragment) | 1 | 1 | 3.8 | 29.6 | 266 | 2.2 | 3.36E-03 | 3,604,800 | 7,493,900 | 0.44 | |
| Q7Z518 | NDUFA10 | NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 10, mitochondrial | 5 | 5 | 18.6 | 40.7 | 354 | 14.3 | 0.00E+00 | 46,879,000 | 194,320,000 | 0.31 | |
| NDUAD | NDUFA13 | NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 13 | 4 | 4 | 33.3 | 16.7 | 144 | 12.4 | 0.00E+00 | 43,461,000 | 261,780,000 | 0.34 | |
| NDUA2 | NDUFA2 | NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 2 | 2 | 2 | 31.3 | 10.9 | 99 | 5.9 | 0.00E+00 | 35,677,000 | 165,030,000 | 0.24 | |
| NDUA4 | NDUFA4 | Cytochrome c oxidase subunit NDUFA4 | 2 | 2 | 27.2 | 9.4 | 81 | 30.4 | 0.00E+00 | 99,542,000 | 1,041,400,000 | 0.28 | |
| NDUA5 | NDUFA5 | NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 5 | 3 | 3 | 35.3 | 13.5 | 116 | 20.4 | 0.00E+00 | 126,370,000 | 440,540,000 | 0.46 | |
| NDUA8 | NDUFA8 | NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 8 | 6 | 6 | 48.8 | 20.1 | 172 | 15.3 | 0.00E+00 | 75,771,000 | 226,560,000 | 0.33 | |
| NDUA9 | NDUFA9 | NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 9, mitochondrial | 9 | 9 | 30.5 | 38.4 | 338 | 22.1 | 0.00E+00 | 38,134,000 | 212,250,000 | 0.38 | |
| H3BNK3 | NDUFAB1 | Acyl carrier protein (fragment) | 1 | 1 | 12.6 | 12.1 | 111 | 20.7 | 0.00E+00 | 91,383,000 | 220,140,000 | 0.41 | |
| NDUB1 | NDUFB1 | NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 1 | 3 | 3 | 34.5 | 7.0 | 58 | 5.4 | 0.00E+00 | 52,572,000 | 104,780,000 | 0.46 | |
| H3BPJ9 | NDUFB10 | NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 10 | 4 | 4 | 31.7 | 19.3 | 161 | 22.9 | 0.00E+00 | 68,400,000 | 353,550,000 | 0.41 | |
| NDUBB | NDUFB11 | NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 11, mitochondrial | 3 | 3 | 33.5 | 17.9 | 158 | 16.2 | 0.00E+00 | 40,408,000 | 192,110,000 | 0.31 | |
| C9JKQ2 | NDUFB3 | NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 3 (fragment) | 2 | 2 | 27.7 | 7.6 | 65 | 3.0 | 7.84E-04 | 19,660,000 | 91,217,000 | 0.33 | |
| NDUB4 | NDUFB4 | NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 4 | 2 | 2 | 21.7 | 15.2 | 129 | 6.3 | 0.00E+00 | 15,764,000 | 129,660,000 | 0.40 | |
| NDUB8 | NDUFB8 | NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 8, mitochondrial | 3 | 3 | 22.6 | 21.8 | 186 | 9.8 | 0.00E+00 | 38,897,000 | 134,110,000 | 0.34 | |
| A0A3B3IT57 | NDUFB9 | NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 9 | 3 | 3 | 34.1 | 17.2 | 138 | 13.8 | 0.00E+00 | 47,909,000 | 178,250,000 | 0.26 | |
| E5KRK5 | NDUFS1 | Mitochondrial NADH-ubiquinone oxidoreductase 75 kDa subunit | 18 | 18 | 33.4 | 79.5 | 727 | 146.6 | 0.00E+00 | 87,635,000 | 1,424,000,000 | 0.27 | |
| NDUS2 | NDUFS2 | NADH dehydrogenase [ubiquinone] iron-sulfur protein 2, mitochondrial | 9 | 9 | 26.6 | 46.4 | 406 | 23.2 | 0.00E+00 | 255,210,000 | 555,800,000 | 0.40 | |
| NDUS3 | NDUFS3 | NADH dehydrogenase [ubiquinone] iron-sulfur protein 3, mitochondrial | 8 | 8 | 39.4 | 30.2 | 264 | 62.5 | 0.00E+00 | 303,550,000 | 1,007,100,000 | 0.38 | |
| H0Y9M8 | NDUFS4 | NADH dehydrogenase [ubiquinone] iron-sulfur protein 4, mitochondrial (fragment) | 1 | 1 | 12.9 | 13.5 | 116 | 4.1 | 0.00E+00 | 22,776,000 | 124,620,000 | 0.20 | |
| Q6IBA0 | NDUFS5 | NADH dehydrogenase (ubiquinone) Fe-S protein 5, 15 kDa (NADH-coenzyme Q reductase) | 5 | 5 | 45.3 | 12.5 | 106 | 6.0 | 0.00E+00 | 13,539,000 | 83,631,000 | 0.36 | |
| B7Z4P1 | NDUFS7 | cDNA FLJ58024, highly similar to NADH-ubiquinone oxidoreductase 20 kDa subunit, mitochondrial | 1 | 1 | 6.1 | 15.8 | 148 | 2.2 | 3.38E-03 | 89,666,000 | 146,530,000 | 1.06 | |
| E9PKH6 | NDUFS8 | NADH dehydrogenase [ubiquinone] iron-sulfur protein 8, mitochondrial (fragment) | 2 | 2 | 19.6 | 15.9 | 138 | 6.0 | 0.00E+00 | 25,384,000 | 70,988,000 | 0.38 | |
| G3V0I5 | NDUFV1 | NADH dehydrogenase [ubiquinone] flavoprotein 1, mitochondrial | 4 | 4 | 12.3 | 50.1 | 457 | 9.4 | 0.00E+00 | 25,550,000 | 98,424,000 | 0.34 | |
| Q9UEH5 | NDUFV2 | 24-kDa subunit of complex I (fragment) | 4 | 4 | 23.4 | 25.4 | 231 | 16.5 | 0.00E+00 | 120,810,000 | 407,130,000 | 0.33 | |
| IPYR2 | PPA2 | Inorganic pyrophosphatase 2, mitochondrial | 9 | 3 | 38.6 | 37.9 | 334 | 38.4 | 0.00E+00 | 815,730,000 | 1,743,800,000 | 0.42 | |
| A0A024QZ30 | SDHA | Succinate dehydrogenase [ubiquinone] flavoprotein subunit, mitochondrial | 20 | 20 | 44.9 | 72.7 | 664 | 200.0 | 0.00E+00 | 1,096,500,000 | 2,950,800,000 | 0.44 | |
| SDHB | SDHB | Succinate dehydrogenase [ubiquinone] iron-sulfur subunit, mitochondrial | 6 | 2 | 24.3 | 31.6 | 280 | 17.3 | 0.00E+00 | 143,030,000 | 516,360,000 | 0.37 | |
| D3DVH1 | SDHC | Succinate dehydrogenase complex, subunit C, integral membrane protein, 15 kDa, isoform CRAa | 3 | 3 | 35.2 | 11.3 | 105 | 6.8 | 0.00E+00 | 68,060,000 | 130,150,000 | 0.46 | |
| A0A024R5E5 | TCIRG1 | V-type proton ATPase subunit a | 8 | 7 | 11.8 | 93.0 | 830 | 29.0 | 0.00E+00 | 139,450,000 | 227,300,000 | 0.73 | |
| QCR9 | UQCR10 | Cytochrome b-c1 complex subunit 9 | 2 | 2 | 38.1 | 7.3 | 63 | 12.3 | 0.00E+00 | 260,330,000 | 491,890,000 | 0.52 | |
| QCR7 | UQCRB | Cytochrome b-c1 complex subunit 7 | 4 | 4 | 36.0 | 13.5 | 111 | 13.2 | 0.00E+00 | 208,330,000 | 523,300,000 | 0.37 | |
| QCR1 | UQCRC1 | Cytochrome b-c1 complex subunit 1, mitochondrial | 15 | 15 | 45.8 | 52.6 | 480 | 205.2 | 0.00E+00 | 1,326,400,000 | 3,772,100,000 | 0.43 | |
| QCR2 | UQCRC2 | Cytochrome b-c1 complex subunit 2, mitochondrial | 15 | 15 | 43.9 | 48.4 | 453 | 142.7 | 0.00E+00 | 1,718,400,000 | 3,636,100,000 | 0.43 | |
| A0A384NPX8 | UQCRFS1 | Cytochrome b-c1 complex subunit Rieske, mitochondrial | 4 | 4 | 15.3 | 29.7 | 274 | 16.3 | 0.00E+00 | 79,904,000 | 211,540,000 | 0.34 | |
| QCR8 | UQCRQ | Cytochrome b-c1 complex subunit 8 | 2 | 2 | 26.7 | 8.3 | 75 | 8.3 | 0.00E+00 | 88,536,000 | 237,890,000 | 0.57 | |
| Lactate shuttle | B4E106 | MCT1 | cDNA FLJ53399, highly similar to monocarboxylate transporter 1 | 2 | 2 | 6.7 | 51.9 | 480 | 21.3 | 0.00E+00 | 23,799,000 | 115,420,000 | 0.53 |
| MOT4 | MCT4 | Monocarboxylate transporter 4 | 7 | 7 | 16.1 | 49.469 | 465 | 43.453 | 0.00E+00 | 818,320,000 | 2,103,700,000 | 0.38 |
− means the protein expressed in the L group but not in the H group. + means the protein expressed in the H group but not in the L group. / means the protein with expressed value 0 in both the H and L groups. Ratio H/L means the ratio of the ivermectin-treated group (SILAC: H) to the no ivermectin-treated group (SILAC: L)
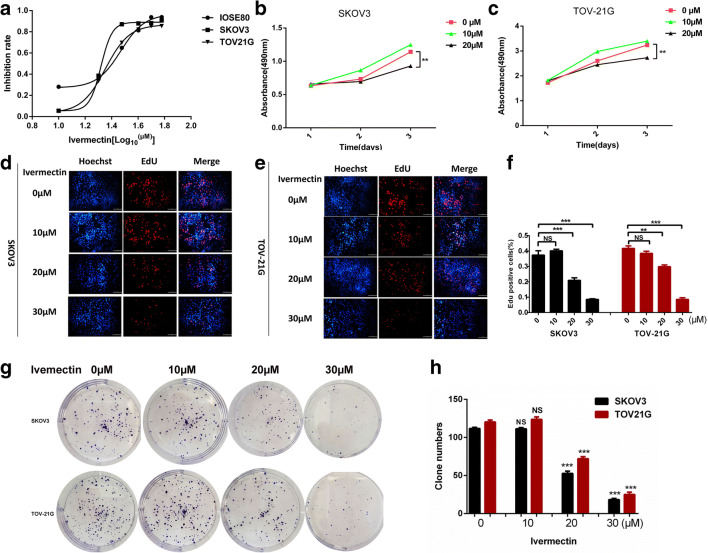
Ivermectin inhibited the proliferation of EOC cells in vitro
The anticancer ability of ivermectin was measured with CCK8 assay before and after ivermectin treatment of EOC cells SKOV3 and TOV-21G and normal control cells IOSE80. After ivermectin treatment for 24 h, the viability of EOC cells was significantly decreased with an inhibition rate from 0, 28.1, 35.5, 64.9, 81.4, 93.7 to 93.8% for the control cells IOSE80; from 0, 5.6, 38.5, 87.1, 87.9, 88.2 to 90.7% for SKOV3; and from 0, 5.2, 33.6, 69.7, 82.1, 85.3 to 85.4% for TOV-21G, corresponding to the ivermectin concentration from 0, 10, 20, 30, 40 μM, 50 to 60 μM, which had a dose-dependent relationship (Fig. 7a). IC50 (29.46 μM) of the control cells IOSE80 was significantly higher than that of EOC cells (20.85 μM in SKOV3 and 22.54 in TOV-21G). Consistently, 20 μM ivermectin (which was close and slightly lower than their IC50) significantly suppressed cell proliferation in SKOV3 and TOV-21G cells as evidenced by CCK8 cell proliferation test (Fig. 7b, c), EdU cell proliferation test (Fig. 7d–f), and reduced clonogenic survival (Fig. 7g, h) in ivermectin-treated cells compared with controls (0 μM ivermectin), which had a time-dependent relationship (Fig. 7b, c). Further analysis found that 10 μM ivermectin (which was much lower than their IC50) did not suppress cell proliferation in SKOV3 and TO-21G cells (Fig. 7b–h), and 30 μM ivermectin (which was much higher than their IC50) caused cell death in SKOV3 and TO-21G cells (Fig. 7d–h). These results clearly demonstrated that 20 μM ivermectin was a suitable dose and significantly inhibited in vitro proliferation and growth of ovarian cancer cells.
Fig. 7.
Ivermectin inhibited ovarian cancer cells proliferation in vitro. a Cell viability was measured by the CCK8 assay in IOSE80, SKOV3, and TOV-21G cells treated with the different concentrations of ivermectin for 24 h (n = 3, X = Log (ivermectin concentration)). b CCK8 cell proliferation test on SKOV3 (n = 3). c CCK8 cell proliferation test on TOV-21G (n = 3). d EdU cell proliferation test on SKOV3. e EdU cell proliferation test on TOV-21G. f Histogram statistics of EdU cell proliferation test on SKOV3 and TOV-21G (n = 3). g Clonogenic survival test on SKOV3 and TOV-21G. h Histogram statistics of clonogenic survival test on SKOV3 and TOV-21G (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001
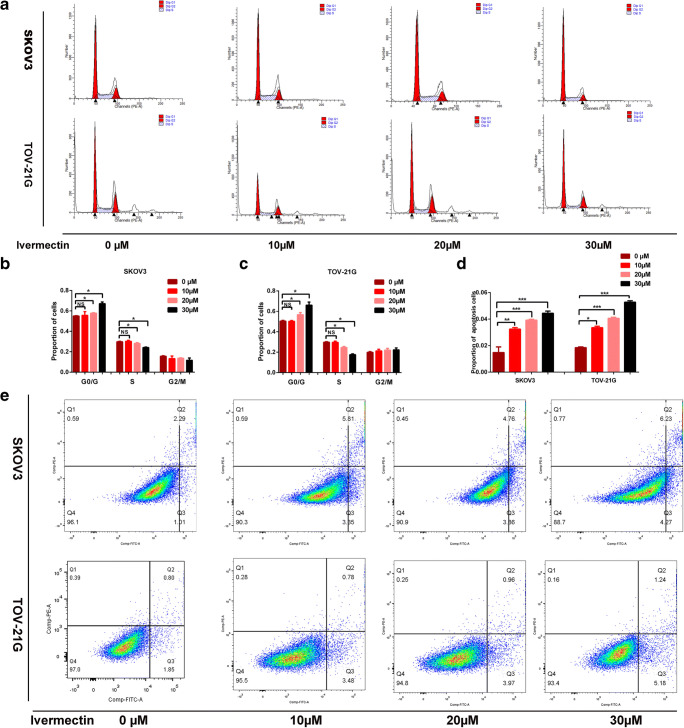
Ivermectin inhibited cell cycle progression and promoted EOC cell apoptosis
To gain insights into the mechanism that ivermectin inhibited EOC cell proliferation, differences in cell cycle distributions were analyzed after treatments with different concentrations of ivermectin (0, 10, 20, and 30 μM) for 24 h with fluorescence-activated cell sorting (FACS). The results found that significant G0/G arrest was observed in the high drug concentration (20 μM and 30 μM) groups compared with the control (0 μM) and low drug concentration (10 μM) groups (Fig. 8a–c) and that the proportion of cells was significantly increased in G0/G phase, significantly decreased in S phase, and no change in G2/M phase in the 20- and 30-μM ivermectin groups compared with the 0- and 10-μM ivermectin groups (Fig. 8b, c). These data strongly demonstrated that ivermectin inhibited cell proliferation by blocking cell cycle progression from G0/G to S phase. Furthermore, the apoptosis was measured with FACS in EOC cells that were stained with PI and annexin V. The results showed that the proportion of apoptosis cells was significantly increased in the 10-, 20-, and 30-μM drug concentration groups compared with the control (0 μM) groups, and the proportion of apoptosis cells was increased with the increased drug concentrations (Fig. 8d, e). These findings clearly demonstrated that ivermectin inhibited EOC cell cycle progression from G0/G to S phases and promoted its apoptosis.
Fig. 8.
Ivermectin inhibits cell cycle progression and promotes EOC cell apoptosis. a Differences in cell cycle distributions following ivermectin at multiple drug concentrations (0 μM, 10 μM, 20 μM, and 30 μM) by fluorescence-activated cell sorting (FACS). b Histogram statistics of cell cycle distributions on SKOV3 (n = 3). c Histogram statistics of cell cycle distributions on TOV-21G (n = 3). d Histogram statistics of apoptosis cell percentage on SKOV3 and TOV-21G (n = 3). e Apoptosis cell percentage following ivermectin at multiple drug concentrations (0 μM, 10 μM, 20 μM, and 30 μM) by fluorescence-activated cell sorting (FACS). *p < 0.05, **p < 0.01, ***p < 0.001. EOC, epithelial ovarian carcinoma
Ivermectin affected energy metabolism pathways for its anticancer effects through targeting PFKP, IDH2, IDH3B, ND2, ND5, CYTB, UQCRH, MCT1, and MCT4 in the energy metabolism pathways
To further investigate the molecular mechanisms that ivermectin inhibited proliferation and promoted apoptosis in EOC cells, EOC cells treated with ivermectin (10 μM, 20 μM, and 30 μM) and control cells (within 0.1% DMSO) were established. The mRNA expressions of target genes (PFKP, PKM, CS, PDHB, IDH2, IDH3A, IDH3B, OGDHL, ND2, ND5, CYTB, UQCRH, MCT1, and MCT4) were analyzed by qRT-PCR (Fig. 9a–f). A significant change was found for the mRNA expressions of target genes in energy metabolism pathways. Furthermore, western blot revealed that the protein expressions of target genes were significantly changed in ivermectin-treated EOC cells, including PFKP, IDH2, IDH3A, IDH3B, ND2, ND5, CYTB, UQCRH, MCT1, and MCT4 (Fig. 10). For the first time, these findings clearly demonstrated that ivermectin significantly changed the expressions of key molecules in energy metabolism pathways, which indicated that ivermectin might regulate ovarian cancer energy metabolism pathways.
Fig. 9.
Ivermectin affects energy metabolism for its anticancer efficiency through targeting PFKP, IDH2, IDH3B, ND2, ND5, CYTB, UQCRH, MCT1, and MCT4 at the mRNA levels analyzed with qPCR. a–f EOC cells adding ivermectin (10 μM, 20 μM, and 30 μM) and control cell lines (within 0.1% DMSO) were verified by RT-PCR after treatment to identify energy metabolism enzymes and lactate shuttle (MCT1 and MCT2) mRNA expressions (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001. PFKP, phosphofructokinase platelet; IDH2, isocitrate dehydrogenase (NADP(+)) 2; IDH3B, isocitrate dehydrogenase (NAD(+)) 3 noncatalytic subunit beta; ND2, mitochondrially encoded NADH dehydrogenase 2; ND5, mitochondrially encoded NADH dehydrogenase 5; UQCRH, ubiquinol-cytochrome c reductase hinge protein; MCT1, solute carrier family 16 member 1; MCT4, solute carrier family 16 member 4; EOC, epithelial ovarian carcinoma; CYTB, mitochondrially encoded cytochrome b; DMSO, dimethyl sulfoxide; qRT-PCR, quantitative real-time PCR
Fig. 10.
Ivermectin affects energy metabolism for its anticancer efficiency through targeting PFKP, IDH2, IDH3B, ND2, ND5, CYTB, UQCRH, MCT1, and MCT4 at the protein levels analyzed with western blot. EOC cells adding ivermectin (10 μM, 20 μM, and 30 μM) and control cell lines (within 0.1% DMSO) were verified by western blot to detect the protein expression of FPKP, PKM, PDHB,CS, IDH2, IDH3A, IDH3B, OGDHL, ND2, ND5, CYTB, UQCRH, MCT1, and MCT4 (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001. PFK, phosphofructokinase platelet; IDH2, isocitrate dehydrogenase (NADP(+)) 2, IDH3A, mitochondrial isocitrate dehydrogenase [NAD] subunit alpha; IDH3B, isocitrate dehydrogenase (NAD(+)) 3 noncatalytic subunit beta; ND2, mitochondrially encoded NADH dehydrogenase 2; ND5, mitochondrially encoded NADH dehydrogenase 5; UQCRH:, ubiquinol-cytochrome c reductase hinge protein; MCT1, solute carrier family 16 member 1; MCT4, solute carrier family 16 member 4; EOC, epithelial ovarian carcinoma; CYTB, mitochondrially encoded cytochrome b; DMSO, dimethyl sulfoxide; PKM, pyruvate kinase muscle; PDHB, pyruvate dehydrogenase E1 subunit beta; CS, citrate synthase; OGDHL, oxoglutarate dehydrogenase L
Discussion
EOC is a type of ovarian malignant neoplasms with high mortality in women and unclear molecular mechanisms [50]. Cancer cell metabolism alteration became one of the research hot spots. Cancer cells in tumor tissues are highly heterogeneous. For tumor energy metabolism, the coexistence of Warburg and reverse Warburg effects in cancer tissues has become the common sense [20, 24, 27]. However, the landscape of detailed molecular profiling changes in energy metabolism pathways remains unclear in EOC. This study made the advances in clarifying the molecular profiling changes in EOC energy metabolism pathways, including glycolysis, Kreb’s cycle, oxidative phosphorylation, and lactate shuttle.
Cancer cells needed more fuel to maintain high growth rate compared with normal cells [51]. In 1956, Warburg found that the principal energy supplies of cancer cells were from aerobic glycolysis [21]. “Warburg effect” played a leading role for energy generations in cancer cells, and many researchers were engaged in developing drugs against the anti-Warburg effect. It is well-known that splicing and posttranslational modifications (PTMs) were tightly associated with diseases [52, 53]. PKM2 was closely related to energy metabolic reprogramming, because the activity of PKM2 was regulated by many ways including PTMs [54]. PKM2 might be associated with the Warburg effect. It was reported that PKM2 modification might lead to increased glucose consumption [22]. Thus, anti-Warburg effect drugs would be potential inhibitors for cancer treatment. For example, one kind of anti-Warburg effect agents, the erastin-like agent, could effectively decrease lactate formation in cancer cells to prevent mitochondrial depolarization [55].
However, the interactions between cancer cells and stromal cells, or cancer cells and cancer cells, were completely ignored according to the Warburg effect. The reverse Warburg effect took into consideration the tumor microenvironment, which complemented the Warburg effect in terms of energy metabolism. In the novel reverse Warburg effect model, energy metabolism involved high aerobic glycolysis in cancer cells and the neighboring stromal fibroblasts and increasing OXPHOS process in other cancer cells. The interaction among those cells relied on the “lactate shuttle” (MCT1 and MCT4). Energy-rich metabolites were transported through the “lactate shuttle” from high aerobic glycolysis cells to increasing OXPHOS cancer cells [24]. Thus, the coexistence of the Warburg effect and reverse Warburg effect made the regulatory mechanism of energy metabolism more reasonable. The Warburg effect and reverse Warburg effect could not suppress each other, and the new reverse Warburg effect theory could not replace the Warburg effect. In a word, the coexistence of two effects reflected the heterogeneity and plasticity of energy metabolism in cancer [56]. The detailed mechanisms of energy metabolism heterogeneity still remain unclear. The questions were as follows: How was the metabolic coupling processed? Which was competing for the leading role in the cancer energy metabolism? What kind of drugs could block both the Warburg and reverse Warburg effects?
This study found that many key protein molecules were significantly changed in three energy metabolism pathways (glycolysis, Kreb’s cycle, and OXPHOS) in EOC. Interestingly, the results demonstrated the enhanced ability of those three energy pathways for ATP generation, with the upregulation of rate-limiting enzyme subunits (PKM, CS, IDH2, and OGDHL). Furthermore, immunoaffinity experiments confirmed the upregulated PKM2 in the glycolysis pathway; the upregulated CS, IDH2, and OGDHL in the Kreb’s cycle pathway; and the upregulated UQCRH in OXPHOS. PDHB was significantly upregulated in EOC tissues, which catalyzed pyruvate into acetyl-CoA to link glycolysis with the Kreb’s cycle. These results clearly demonstrated that EOC relied on both aerobic glycolysis and OXPHOS to produce energy, namely energy metabolism heterogeneity in EOC tissues. The reason why defective complex I was performing a principal role in urinary system oncocytomas and complex III deficiency caused by cytochrome b mutations was frequently occurring in thyroid oncocytomas remains to be determined [57]. It suggested that different cancers had totally different pathways. Cancer cells could proceed with high aerobic glycolysis or increased OXPHOS to produce ATP [58]. Even though the “Warburg effect” was very popular, more and more evidence indicated its limitations. The Warburg effect merely emphasized metabolic symbiosis between cancer cells and stroma cells in the microenvironment, with evidence that glycolysis only offered < 50% ATPs for some human cancer cells. Especially, in gynecological cancer cells, such as MCFs and HeLa, OXPHOS had the main position in producing energy [59]. Moreover, OXPHOS and aerobic glycolysis were not always completely independent of one another. To some extent, alterations of the tumor microenvironment (in normoxia and in hypoxia) affected the status of OXPHOS and aerobic glycolysis to produce ATPs [27]. Moreover, a study found that glycolysis inhibitor had an unsatisfactory curative effect, such as drug resistance or strong side effects [60]. On the other hand, recent clinical research found that the mitochondria operated more efficiently in gynecological tumor cells and that those kinds of cancers might be sensitive to OXPHOS inhibitors [61]. Here, some scientists suggested to focus on metabolic target drug, and the real potential method might be combination therapy to block both glycolysis and mitochondrial OXPHOS pathways.
Today, the antiparasite drug ivermectin remains a relatively unknown drug and has been extended to various disease models [62]. Recently, ivermectin has broken through the bondage of traditional clinical medication, and its abilities to inhibit tumor growth in several types of cancers, including ovarian cancer, breast cancer, and colon cancer, have been reported [34]. However, the mechanisms of its anticancer effects remain unclear. IPA-based network analysis of ivermectin (Fig. 6) revealed that ivermectin could regulate the target molecules PKM, OGDHL, ND2, ND5, CYTB, and UQCRH in various ways. For example, ivermectin could regulate PKM, OGDHL, CYTB, ND2, ND5, and UQCRH by influencing the localization of insulin and controlling the expression of cytokine. Additionally, ivermectin could regulate CYTB, ND2, ND5, and PKM by affecting the chemical protein interactions of ABCB1 and ABCG2. Interestingly, PKM as a key enzyme involved in glycolysis could be regulated by ivermectin through insulin and also could be regulated by ivermectin through downstream target genes of insulin. Also, SILAC quantitative proteomics revealed that the molecular profiling in glycolysis, Kreb’s cycle, oxidative phosphorylation, and lactate shuttle pathways was extensively affected by ivermectin (Table 6). The experimental evidence suggested that the molecular mechanisms were complex between ivermectin and energy metabolism pathways. This present study clearly showed that ivermectin suppressed the energy metabolism system by affecting energy metabolism pathways via targeting PFKP, IDH2, IDH3B, ND2, ND5, CYTB, UQCRH, MCT1, and MCT4 (Figs. 9 and 10), and thereby activated apoptosis, promoted cell cycle arrest, and inhibited cell proliferation (Figs. 7 and 8).
In summary, these findings provided novel insights into the energy metabolism pathway changes in EOC and the antitumor effects of ivermectin via targeting energy metabolism pathways in EOC. These altered molecules and their regulators in EOC energy metabolism pathways were the precious resource in the field of ovarian cancer energy metabolism, which offers increasing promise in the in-depth understanding of EOC energy metabolism and the discovery of energy metabolism-based molecular biomarker pattern and novel antitumor targets/drugs to effectively treat EOC in the context of predictive, preventive, and personalized medicine (PPPM) practice.
Strength and limitations
Energy metabolism abnormality is the important pathophysiological characteristics in EOC. This study revealed the changes of key proteins in the Kreb’s cycle and oxidative phosphorylation pathways with quantitative mitochondrial proteomics of EOC tissues, the changes of key proteins in the glycolysis pathway with quantitative proteomics of EOC tissues, and the changes of key proteins in lactate shuttle with quantitative proteomics of EOC tissues. These changed key proteins in these energy metabolism pathways were significantly regulated by the drug ivermectin, and ivermectin can inhibit EOC cell proliferation, suppress cell cycle progression, and promote EOC cell apoptosis. These findings, for the first time, provide the complete landscape of molecule profiling changes at the level of protein in the energy metabolism pathways in EOC tissues and their regulation by the drug ivermectin in EOC cell models. These changed molecule profiles in energy metabolism pathways in individualized ovarian cancer patients are the potential biomarker pattern for predictive/prognostic diagnosis, patient stratification, and personalized management of EOC patients, and/or therapeutic targets for effective personalized therapy of EOC patients. Therefore, the main strength of this study is that the landscape of molecule profiling changes at the protein level in energy metabolism pathways was revealed with multiple proteomics strategies in EOC tissues, and these changed molecules can be regulated by the drug ivermectin.
However, one must realize that energy metabolism reprogramming is very complex in EOC, and the findings of this study offer important clues to deeply study energy metabolism abnormality in EOC. The following aspects are proposed to further study EOC energy metabolism abnormality: (i) most of the changed molecules in the energy metabolism pathways are the enzymes, and we will further investigate the activity change of those enzymes. (ii) The mitochondria play important roles in EOC energy metabolism, and we will further investigate the functions and activities of the mitochondria in EOC with multiple methods such the sea horse experiment. (iii) We will further investigate the regulatory mechanism system of EOC energy metabolism abnormality, including lncRNAs such as SNHG3 [17, 63], microRNAs such as miRNA-186-5p [17], RNA-binding proteins such as EIF4A3 [17, 63], and drugs such as ivermectin [63] (including the results of this present study). (iv) The changed molecules in the EOC energy metabolism pathways and their regulatory molecules will be the potential biomarker pattern and effective therapeutic targets for the management of EOC patients in the context of PPPM practice.
Moreover, one must also note that the SILAC quantification results of ivermectin-treated TOV-21G cells (Table 6) were not fully consistent with PCR and western blot quantification results of ivermectin-treated TOV-21G (Figs. 9 and 10), which might be due to several factors: (i) There are lots of proteoforms with much different abundance that are derived from the same one gene, because of many factors such as alternative RNA splicing and protein PTMs [52, 53, 64]. Protein is the umbrella term for all proteoforms encoded by the same gene [65, 66]. (ii) Each proteoform should have its corresponding specific antibody. The commercially available antibodies used in this study were not the proteoform-specific antibodies. (iii) In the process from gene to proteoforms, there are lots of alternative RNA splicing and PTMs [52, 65, 66], which might result in much difference between the gene and protein (exactly speaking, proteoforms). It can be evidenced by the difference between mRNA and protein expressions of vimentin and multidrug resistance-associated protein 1 (MRP1) in lung squamous carcinoma in our other study [67]. The final abundance of a proteoform is determined by the balance between protein synthesis and degradation system at a given condition. Thus, the abundance of a protein (exactly, proteoform) is dynamically changed with the given conditions. Therefore, in order to accurately reveal the effect of ivermectin on the key molecules in EOC energy metabolism pathways, we propose in future studies to investigate the dose-dependent effect (e.g., 0, 5, 10, 20, 30, 40, and 50 μM ivermectin treatment) and time-dependent effect (e.g., 0, 6, 12, 24, 48, and 72 h after a given ivermectin treatment) of ivermectin on each key molecule in EOC energy metabolism pathways at the levels of mRNA and protein. Anyway, our study clearly demonstrated that ivermectin regulated a wide range of key molecules in energy metabolism pathways—glycolysis, Kreb’s cycle, oxidative phosphorylation, and lactate shuttle, which suggests that energy metabolism pathways might be the drug target of ivermectin for its anticancer effects on EOC.
Conclusion and recommendation
Energy metabolism abnormality is the hallmark in EOC. For the first time, this study used iTRAQ quantitative proteomics and mitochondrial proteomics approaches to reveal the changed molecule landscape of energy metabolism pathways in ovarian cancer, with the upregulated key protein molecules PKM2 in glycolysis; IDH2, CS, and OGDHL in Kreb’s cycle; UQCRH in oxidative phosphorylation; and MCT1 and MCT4 in lactate shuttle pathways, and SILAC quantitative proteomics, immunoaffinity blot, and RT-qPCR to reveal the antiparasite drug ivermectin effectively inhibited cell proliferation, suppressed cell cycle progression, and promoted cell apoptosis of EOC cells through targeting the key protein molecules PFKP and PKM2 in glycolysis; IDH2 and IDH3B in Kreb’s cycle; ND2, ND5, CYTB, and UQCRH in OXPHOS; and MCT1 and MCT4 in lactate shuttle. We concluded that (i) the Warburg and reverse Warburg effects coexisted in human ovarian cancer tissues, with the changed molecular profile of energy metabolism pathways; (ii) ivermectin had the antitumor capability in ovarian cancer cells through targeting energy metabolism pathways; and (iii) those altered key molecules in the energy metabolism pathways are the potential molecular pattern biomarkers for patient individualized stratification, predictive/prognostic diagnosis, and personalized treatment of ovarian cancer patients and are therapeutic targets for personalized therapy of ovarian cancer. Therefore, those findings provide new scientific evidence about the understanding of energy metabolism abnormality of ovarian cancer and the anticancer ability of ivermectin and amplifying its clinical applications.
We recommend to strengthen the study of energy metabolism pathways in ovarian cancer with different omics strategies. Multiomics is the effective approach to study energy metabolism abnormality and reveal the molecular profiling changes in energy metabolism pathways—glycolysis, Kreb’s cycle, oxidative phosphorylation, and lactate shuttle, and their regulators including lncRNAs such as SNHG3, microRNAs such as miRNA-186-5p, RNA-binding proteins such as EIF4A3, and drugs such as ivermectin. Energy metabolism pathway network-based molecule pattern biomarkers and therapeutic targets have more important scientific merits in EOC in the context of PPPM practice. We also suggest that it is essential to deeply study the functions and activities of those changed molecules in energy metabolism pathways of ovarian cancers for maximum and precise application of these changed molecules in clinical practice.
We propose the following for further PPPM development and practical application based on those changed energy metabolism pathways in ovarian cancer:
-
(i)
Warburg effect and reverse Warburg effect. This study found the coexistence of the Warburg and reverse Warburg effects in ovarian cancer tissues with the evidence of four enhanced energy metabolism pathways (upregulations of UQCRH in OXPHOS, IDH2, CS, and OGDHL in Kreb’s cycle; PKM2 in glycolysis pathways; and MCT1 and MCT4 in lactate shuttle), which clearly indicated the complexity of energy metabolism in ovarian cancer. Thus, one must have a systematic and comprehensive viewpoint to consider the energy metabolism abnormality of ovarian cancer. Also, one must realize that in ovarian cancer tissue, some cells mainly rely on the Warburg effect—the enhanced glycolysis, and some cells mainly rely on the reverse Warburg effect—the enhanced OXPHOS and Kreb’s cycle. The ratio of cells in the states of Warburg effect and of reverse Warburg effect, namely the pattern of the altered molecules in four energy metabolism pathways, can be used as biomarkers for patient stratification, predictive/prognostic assessment, and therapeutic targets of ovarian cancer [68].
-
(ii)
Energy metabolism-based therapeutic targets and drugs. Based on the coexistence of the Warburg and reverse Warburg effects in ovarian cancer tissues and those corresponding changed key molecules in energy pathways, some effective targeted drugs can be designed for ovarian cancer management for targeted prevention and therapy. For example, flavonoids had the anti-Warburg effect through targeting PKM2 in glycolysis pathway, HK2, GLUT1, and HIF-1 to modulate key pathways involved in the Warburg phenotype to cut Gordian knot of cancer cell metabolism [69]. This study found that ivermectin effectively inhibited cell proliferation and cell cycle progression and promoted cell apoptosis in ovarian cancer cells, through molecular networks to target PFKP and PKM2 in glycolysis; IDH2 and IDH3B in Kreb’s cycle; ND2, ND5, CYTB, and UQCRH in OXPHOS; and MCT1 and MCT4 in lactate shuttle to inhibit ovarian cancer growth. Therefore, flavonoids and ivermectin might be two types of potential drugs to directly and/or indirectly target energy metabolism pathways for ovarian cancer prevention and therapy. Also, measurement of these key molecule changes of energy metabolism pathways can be used as prognostic assessment for preventive response and therapeutic response and patient stratification [70].
-
(iii)
Crucial roles of multiomics. Multiomics offers the great potential for the identification of the altered molecule profiling in energy metabolism pathways of ovarian cancers [71, 72]. For example, iTRAQ quantitative proteomics of whole tissues effectively identified the molecular change profiling of glycolysis pathway in ovarian cancer (Table 3). The iTRAQ quantitative mitochondrial proteomics effectively identified the molecular change profiling of Kreb’s cycle and OXPHOS pathways in ovarian cancers (Tables 4 and 5). Quantitative transcriptomics effectively identified the regulatory mechanism of the changed energy metabolism pathways in ovarian cancer [17]. SILC quantitative proteomics effectively identified the molecular changed profiling of four energy metabolism pathways after drug-treated ovarian cancer cells (Table 6). Therefore, multiomics is a powerful tool to identify changes in the molecular pattern of energy metabolism pathways in the context of PPPM practice in ovarian cancer [71, 73].
-
(iv)
Application of individualized patient profiling. Energy metabolism pathway-based molecular pattern changes in combination with individualized patient profiling [70] will precisely stratify patients for personalized treatment and predictive/prognostic assessment of ovarian cancer patients.
Electronic supplementary material
(XLS 326 kb)
(PDF 43 kb)
Abbreviations
- 1DGE
one-dimensional gel electrophoresis
- 3P medicine
predictive, preventive, and personalized medicine (PPPM)
- ABCB1
ATP binding cassette subfamily B member 1
- Abcb1b
ATP-binding cassette, subfamily B (MDR/TAP), member 1B
- ABCG2
ATP-binding cassette subfamily G member 2
- ACO1
cytoplasmic aconitate hydratase
- ADH5
alcohol dehydrogenase 5 class III chi polypeptide
- Akt
AKT serine/threonine kinase 1
- APC
APC regulator of WNT signaling pathway
- APP
amyloid beta precursor protein
- ATP
adenosine triphosphate
- ATP5G1
ATP synthase membrane subunit c locus 1
- ATP6
ATP synthase F0 subunit 6
- ATP6V0C
ATPase H+ transporting V0 subunit c
- ATP6V1D
ATPase H+ transporting V1 subunit D
- AZD2281
olaparib
- BRCA1
BRCA1 DNA repair associated
- BRCA2
BRCA2 DNA repair associated
- CA-125
cancer antigen 125
- CAFs
cancer-associated fibroblasts
- CCK8
Cell Counting Kit-8
- CML
chronic myeloid leukemia
- CoA
acetyl-coenzyme A
- COX1
cytochrome c oxidase subunit
- COX17
cytochrome c oxidase copper chaperone COX17
- COX2
cytochrome c oxidase subunit II
- COX4I1
cytochrome c oxidase subunit 4I1
- COX4I2
cytochrome c oxidase subunit 4I2
- COX6C
cytochrome c oxidase subunit 6C
- COX7A2
cytochrome c oxidase subunit 7A2
- COX7A2L
cytochrome c oxidase subunit 7A2-like
- CS
citrate synthase
- CYP3A4
cytochrome P450 family 3 subfamily A member 4
- CYTB
mitochondrially encoded cytochrome b
- DMSO
dimethyl sulfoxide
- DNA
deoxyribonucleic acid
- EdU
5-ethynyl-2′-deoxyuridine
- EIF4A3
eukaryotic translation initiation factor 4A3
- ENO1
enolase 1
- EOC
epithelial ovarian carcinoma
- ERK1/2
mitogen-activated protein kinase 3
- ETC
electron transport chain
- FACS
fluorescence-activated cell sorting
- FADH2
2,4-dienoyl-CoA reductase
- FDA
Food and Drug Administration
- FH
fumarate hydratase
- G0/G
G0/G cell cycle phase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GLRB
glycine receptor beta
- GM130
golgin A2
- GO
Gene Ontology
- GPI
glucose-6-phosphate isomerase
- IC50
the half maximal inhibitory concentration
- IDH2
isocitrate dehydrogenase (NADP(+)) 2
- IDH3A
isocitrate dehydrogenase [NAD] subunit alpha, mitochondrial
- IDH3B
isocitrate dehydrogenase (NAD(+)) 3 noncatalytic subunit beta
- IPA
Ingenuity Pathway Analysis
- iTRAQ
isobaric tags for relative and absolute quantitation
- K
lysine
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- KPNB1
karyopherin subunit beta 1
- Kreb’s cycle
tricarboxylic acid cycle
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- LDHA
lactate dehydrogenase A
- LDHB
lactate dehydrogenase B
- lncRNA
long noncoding RNAs
- MAPK1
mitogen-activated protein kinase 1
- MAPK13
mitogen-activated protein kinase 13
- MAPK3
mitogen-activated protein kinase 3
- MCT1
solute carrier family 16 member 1
- MCT4
solute carrier family 16 member 4
- MCTs
monocarboxylate transporters
- MDH2
malate dehydrogenase 2
- miRNA
microRNA
- Mr
protein molecular weight
- mRNA
messenger RNA
- MRP1
multidrug resistance-associated protein 1
- MRPL41
mitochondrial ribosomal protein L41
- MRPL46
mitochondrial ribosomal protein L41
- MRPL49
mitochondrial ribosomal protein L49
- MRPL51
mitochondrial ribosomal protein L51
- MRPL52
mitochondrial ribosomal protein L52
- MRPL53
mitochondrial ribosomal protein L53
- MRPL54
mitochondrial ribosomal protein L54
- MRPL55
mitochondrial ribosomal protein L55
- MRPS10
mitochondrial ribosomal protein S10
- MRPS12
mitochondrial ribosomal protein S12
- MRPS15
mitochondrial ribosomal protein S15
- MRPS17
mitochondrial ribosomal protein S17
- MRPS21
mitochondrial ribosomal protein S21
- MRPS23
mitochondrial ribosomal protein S23
- MRPS33
mitochondrial ribosomal protein S33
- MRPS6
mitochondrial ribosomal protein S6
- MRPS9
mitochondrial ribosomal protein S9
- mtDEPs
mitochondrial differentially expressed proteins
- mTOR
mechanistic target of rapamycin kinase
- NADH
mitochondrially encoded NADH dehydrogenase 1
- ND2
mitochondrially encoded NADH dehydrogenase 2
- ND5
mitochondrially encoded NADH dehydrogenase 5
- NFKBIA
NFKB inhibitor alpha
- OGDHL
oxoglutarate dehydrogenase L
- OXPHOS
oxidative phosphorylation
- p21
cyclin-dependent kinase inhibitor 1A
- p27
cyclin-dependent kinase inhibitor 1B
- P2RX4
purinergic receptor P2X 4
- P2RX7
purinergic receptor P2X 7
- PAK1
p21 (RAC1)-activated kinase 1
- PARP
polyADP-ribose polymerase inhibitor
- PCK2
phosphoenolpyruvate carboxykinase [GTP], mitochondrial
- PDC
pyruvate dehydrogenase complex
- PDHB
pyruvate dehydrogenase E1 subunit beta
- PFKP
phosphofructokinase, platelet
- pI
isoelectric point
- PKM
pyruvate kinase muscle
- PKM2
pyruvate kinase M2
- PPPM
predictive, preventive, and personalized medicine
- PTMs
posttranslational modifications
- QCR6
mitochondrial cytochrome b-c1 complex subunit 6
- qRT-PCR
quantitative real-time PCR
- R
arginine
- Rbp
SURP and G-patch domain containing 1
- RNA
ribonucleic acid
- ROS
reactive oxygen species
- SCX
strong cation exchange chromatography
- SD
standard deviation
- SDT
N-hydroxysuccinimide
- SILAC
stable isotope labeling with amino acids in cell culture
- SNHG3
small nucleolar RNA host gene 3
- STAT3
signal transducer and activator of transcription 3
- SUCLG2
succinate–CoA ligase GDP-forming subunit beta
- TNF
tumor necrosis factor
- TOMM20
translocase of outer mitochondrial membrane 20
- UQCRH
ubiquinol-cytochrome c reductase hinge protein
- VDAC1
voltage-dependent anion channel 1
Author contributions
N.L. carried out the cell experiments, analyzed the data, prepared the figures and tables, and wrote the manuscript. H.L. collected the samples, prepared the mitochondrial samples, and participated in data analysis and table preparation. Y.W. participated in western blot experiments. L.C. collected tumor tissue samples and performed clinical diagnosis. X.Z. conceived the concept, designed the experiments and manuscript, instructed experiments and data analysis, coordinated and obtained the mitochondrial iTRAQ quantitative proteomic data, supervised the results, wrote and critically revised the manuscript, and was responsible for its financial support and the corresponding works. All authors approved the final manuscript.
Funding
The authors acknowledge the financial support from the Shandong First Medical University Talent Introduction Funds (to X.Z.) and the Hunan Provincial Hundred Talent Plan (to X.Z.).
Compliance with ethical standards
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable
Ethical approval
All the patients were informed about the purposes of the study and, consequently, have signed their “consent of the patient.” All investigations conformed to the principles outlined in the Declaration of Helsinki and were performed with permission by the responsible Medical Ethics Committee of Xiangya Hospital, Central South University, China.
Footnotes
Abbreviations for all particular genes and proteins can be found at the following link: https://www.ncbi.nlm.nih.gov/gene/.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chan JK, Cheung MK, Husain A, Teng NN, West D, Whittemore AS, Berek JS, Osann K. Patterns and progress in ovarian cancer over 14 years. Obstet Gynecol. 2006;108:521–528. doi: 10.1097/01.AOG.0000231680.58221.a7. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Gadducci A, Cosio S, Zola P, Landoni F, Maggino T, Sartori E. Surveillance procedures for patients treated for epithelial ovarian cancer: a review of the literature. Int J Gynecol Cancer. 2007;17:21–31. doi: 10.1111/j.1525-1438.2007.00826.x. [DOI] [PubMed] [Google Scholar]
- 4.Smith RA, Manassaram-Baptiste D, Brooks D, Doroshenk M, Fedewa S, Saslow D, Brawley OW, Wender R. Cancer screening in the United States, 2015: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2015;65:30–54. doi: 10.3322/caac.21261. [DOI] [PubMed] [Google Scholar]
- 5.Buchtel KM, Vogel Postula KJ, Weiss S, Williams C, Pineda M, Weissman SM. FDA approval of PARP inhibitors and the impact on genetic counseling and genetic testing practices. J Genet Couns. 2018;27(1):131–139. doi: 10.1007/s10897-017-0130-7. [DOI] [PubMed] [Google Scholar]
- 6.Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355:1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacob F, Meier M, Caduff R, Goldstein D, Pochechueva T, Hacker N, Fink D, Heinzelmann-Schwarz V. No benefit from combining HE4 and CA125 as ovarian tumor markers in a clinical setting. Gynecol Oncol. 2011;121:487–491. doi: 10.1016/j.ygyno.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Vanni S. Omics of prion diseases. Prog Mol Biol Transl Sci. 2017;150:409–431. doi: 10.1016/bs.pmbts.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Zhan X, Wang X, Long Y, Desiderio DM. Heterogeneity analysis of the proteomes in clinically nonfunctional pituitary adenomas. BMC Med Genet. 2014;7:69. doi: 10.1186/s12920-014-0069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sala S, Van Troys M, Medves S, Catillon M, Timmerman E, Staes A, Schaffner-Reckinger E, Gevaert K. Expanding the interactome of TES by exploiting TES modules with different subcellular localizations. J Proteome Res. 2017;16:2054–2071. doi: 10.1021/acs.jproteome.7b00034. [DOI] [PubMed] [Google Scholar]
- 11.Sassano ML, van Vliet AR, Agostinis P. Mitochondria-associated membranes as networking platforms and regulators of cancer cell fate. Front Oncol. 2017;7:174. doi: 10.3389/fonc.2017.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strickertsson JAB, Desler C, Rasmussen LJ. Bacterial infection increases risk of carcinogenesis by targeting mitochondria. Semin Cancer Biol. 2017;47:95–100. doi: 10.1016/j.semcancer.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Zhang J, Li B, He QY. Proteomic analysis of mitochondria: biological and clinical progresses in cancer. Expert Rev Proteomics. 2017;14:891–903. doi: 10.1080/14789450.2017.1374180. [DOI] [PubMed] [Google Scholar]
- 14.Mintz HA, Yawn DH, Safer B, Bresnick E, Liebelt AG, Blailock ZR, Rabin ER, Schwartz A. Morphological and biochemical studies of isolated mitochondria from fetal, neonatal, and adult liver and from neoplastic tissues. J Cell Biol. 1967;34:513–523. doi: 10.1083/jcb.34.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen M, Huang H, He H, Ying W, Liu X, Dai Z, Yin J, Mao N, Qian X, Pan L. Quantitative proteomic analysis of mitochondria from human ovarian cancer cells and their paclitaxel-resistant sublines. Cancer Sci. 2015;106:1075–1083. doi: 10.1111/cas.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li N, Li H, Cao L, Zhan X. Quantitative analysis of the mitochondrial proteome in human ovarian carcinomas. Endocr Relat Cancer. 2018;25:909–931. doi: 10.1530/erc-18-0243. [DOI] [PubMed] [Google Scholar]
- 17.Li N, Zhan X, Zhan X. The lncRNA SNHG3 regulates energy metabolism of ovarian cancer by an analysis of mitochondrial proteomes. Gynecol Oncol. 2018;150:343–354. doi: 10.1016/j.ygyno.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Vyas S, Zaganjor E, Haigis MC. Mitochondria and cancer. Cell. 2016;166:555–566. doi: 10.1016/j.cell.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li N, Zhan X. Signaling pathway network alterations in human ovarian cancers identified with quantitative mitochondrial proteomics. EPMA J. 2019;10:153–172. doi: 10.1007/s13167-019-00170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim A. Mitochondria in cancer energy metabolism: culprits or bystanders? Toxicol Res. 2015;31:323–330. doi: 10.5487/tr.2015.31.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 22.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 23.Birsoy K, Wang T, Chen WW, Freinkman E, Abu-Remaileh M, Sabatini DM. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell. 2015;162:540–551. doi: 10.1016/j.cell.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 25.Bar-Or D, Carrick M, Tanner A, 2nd, Lieser MJ, Rael LT, Brody E. Overcoming the Warburg effect: is it the key to survival in sepsis? J Crit Care. 2018;43:197–201. doi: 10.1016/j.jcrc.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Whitaker-Menezes D, Martinez-Outschoorn UE, Lin Z, Ertel A, Flomenberg N, Witkiewicz AK, Birbe RC, Howell A, Palides S, Gandara R, Pestell RG, Sotgia F, Philp NJ, Lisanti MP. Evidence for a stromal-epithelial "lactate shuttle" in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle. 2011;10:1772–1783. doi: 10.4161/cc.10.11.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu XD, Shao SX, Jiang HP, Cao YW, Wang YH, Yang XC, Wang YL, Wang XS, Niu HT. Warburg effect or reverse Warburg effect? A review of cancer metabolism. Oncol Res Treat. 2015;38:117–122. doi: 10.1159/000375435. [DOI] [PubMed] [Google Scholar]
- 28.Chao TK, Huang TS, Liao YP, Huang RL, Su PH, Shen HY, Lai HC, Wang YC. Pyruvate kinase M2 is a poor prognostic marker of and a therapeutic target in ovarian cancer. PLoS One. 2017;12:e0182166. doi: 10.1371/journal.pone.0182166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suh DH, Kim HS, Kim B, Song YS. Metabolic orchestration between cancer cells and tumor microenvironment as a co-evolutionary source of chemoresistance in ovarian cancer: a therapeutic implication. Biochem Pharmacol. 2014;92:43–54. doi: 10.1016/j.bcp.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Chosidow O, Bernigaud C, Do-Pham G. High-dose ivermectin in malaria and other parasitic diseases: a new step in the development of a neglected drug. Parasite. 2018;25:33. doi: 10.1051/parasite/2018039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Omura S. A splendid gift from the earth: the origins and impact of the Avermectins (Nobel lecture) Angew Chem Int Ed Eng. 2016;55:10190–10209. doi: 10.1002/anie.201602164. [DOI] [PubMed] [Google Scholar]
- 32.Crump A. Ivermectin: enigmatic multifaceted 'wonder' drug continues to surprise and exceed expectations. J Antibiot (Tokyo) 2017;70:495–505. doi: 10.1038/ja.2017.11. [DOI] [PubMed] [Google Scholar]
- 33.Drinyaev VA, Mosin VA, Kruglyak EB, Novik TS, Sterlina TS, Ermakova NV, Kublik LN, Levitman M, Shaposhnikova VV, Korystov YN. Antitumor effect of avermectins. Eur J Pharmacol. 2004;501:19–23. doi: 10.1016/j.ejphar.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Dou Q, Chen HN, Wang K, Yuan K, Lei Y, Li K, Lan J, Chen Y, Huang Z, Xie N, Zhang L, Xiang R, Nice EC, Wei Y, Huang C. Ivermectin induces cytostatic autophagy by blocking the PAK1/Akt axis in breast cancer. Cancer Res. 2016;76:4457–4469. doi: 10.1158/0008-5472.can-15-2887. [DOI] [PubMed] [Google Scholar]
- 35.Zhu M, Li Y, Zhou Z. Antibiotic ivermectin preferentially targets renal cancer through inducing mitochondrial dysfunction and oxidative damage. Biochem Biophys Res Commun. 2017;492:373–378. doi: 10.1016/j.bbrc.2017.08.097. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Xu Y, Wan H, Hu J. Antibiotic ivermectin selectively induces apoptosis in chronic myeloid leukemia through inducing mitochondrial dysfunction and oxidative stress. Biochem Biophys Res Commun. 2018;497:241–247. doi: 10.1016/j.bbrc.2018.02.063. [DOI] [PubMed] [Google Scholar]
- 37.Kodama M, Kodama T. In vivo loss-of-function screens identify KPNB1 as a new druggable oncogene in epithelial ovarian cancer. Proc Natl Acad Sci U S A. 2017;114:E7301–e7310. doi: 10.1073/pnas.1705441114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang LN, Tong SW, Hu HD, Ye F, Li SL, Ren H, Zhang DZ, Xiang R, Yang YX. Quantitative proteome analysis of ovarian cancer tissues using a iTRAQ approach. J Cell Biochem. 2012;113:3762–3772. doi: 10.1002/jcb.24250. [DOI] [PubMed] [Google Scholar]
- 39.Lokich E, Singh RK, Han A, Romano N, Yano N, Kim K, Moore RG. HE4 expression is associated with hormonal elements and mediated by importin-dependent nuclear translocation. Sci Rep. 2014;4:5500. doi: 10.1038/srep05500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hashimoto H, Messerli SM, Sudo T, Maruta H. Ivermectin inactivates the kinase PAK1 and blocks the PAK1-dependent growth of human ovarian cancer and NF2 tumor cell lines. Drug Discov Ther. 2009;3:243–246. [PubMed] [Google Scholar]
- 41.Bartolak-Suki E, Imsirovic J, Nishibori Y, Krishnan R, Suki B. Regulation of mitochondrial structure and dynamics by the cytoskeleton and mechanical factors. Int J Mol Sci. 2017;18. 10.3390/ijms18081812. [DOI] [PMC free article] [PubMed]
- 42.Schrader M, Costello J, Godinho LF, Islinger M. Peroxisome-mitochondria interplay and disease. J Inherit Metab Dis. 2015;38(4):681–702. doi: 10.1007/s10545-015-9819-7. [DOI] [PubMed] [Google Scholar]
- 43.Rezaul K, Wu L, Mayya V, Hwang SI, Han D. A systematic characterization of mitochondrial proteome from human T leukemia cells. Mol Cell Proteomics. 2005;4:169–181. doi: 10.1074/mcp.M400115-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bragoszewski P, Wasilewski M, Sakowska P, Gornicka A, Bottinger L, Qiu J, Wiedemann N, Chacinska A. Retro-translocation of mitochondrial intermembrane space proteins. Proc Natl Acad Sci U S A. 2015;112:7713–7718. doi: 10.1073/pnas.1504615112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnston IG, Williams BP. Evolutionary inference across eukaryotes identifies specific pressures favoring mitochondrial gene retention. Cell Syst. 2016;2:101–111. doi: 10.1016/j.cels.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 46.Tekade RK, Sun X. The Warburg effect and glucose-derived cancer theranostics. Drug Discov Today. 2017;22:1637–1653. doi: 10.1016/j.drudis.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe H, Takehana K, Date M, Shinozaki T, Raz A. Tumor cell autocrine motility factor is the neuroleukin/phosphohexose isomerase polypeptide. Cancer Res. 1996;56:2960–2963. [PubMed] [Google Scholar]
- 48.Pavlides S, Vera I, Gandara R, Sneddon S, Pestell RG, Mercier I, Martinez-Outschoorn UE, Whitaker-Menezes D, Howell A, Sotgia F, Lisanti MP. Warburg meets autophagy: cancer-associated fibroblasts accelerate tumor growth and metastasis via oxidative stress, mitophagy, and aerobic glycolysis. Antioxid Redox Signal. 2012;16:1264–1284. doi: 10.1089/ars.2011.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sullivan LB, Chandel NS. Mitochondrial reactive oxygen species and cancer. Cancer Metab. 2014;2:17. doi: 10.1186/2049-3002-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakhuja S, Yun H, Pisu M, Akinyemiju T. Availability of healthcare resources and epithelial ovarian cancer stage of diagnosis and mortality among Blacks and Whites. J Ovarian Res. 2017;10:57. doi: 10.1186/s13048-017-0352-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Min HY, Lee HY. Oncogene-driven metabolic alterations in cancer. Biomol Ther (Seoul) 2018;26:45–56. doi: 10.4062/biomolther.2017.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhan X, Long Y, Lu M. Exploration of variations in proteome and metabolome for predictive diagnostics and personalized treatment algorithms: innovative approach and examples for potential clinical application. J Proteome. 2018;188:30–40. doi: 10.1016/j.jprot.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 53.Qian S, Yang Y, Li N, Cheng T, Wang X, Liu J, Li X, Desiderio DM, Zhan X. Prolactin variants in human pituitaries and pituitary adenomas identified with two-dimensional gel electrophoresis and mass spectrometry. Front Endocrinol (Lausanne) 2018;9:468. doi: 10.3389/fendo.2018.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kobierzycki C, Piotrowska A, Latkowski K, Zabel M, Nowak-Markwitz E, Spaczynski M, Kedzia W, Pula B, Podhorska-Okolow M, Dziegiel P. Correlation of pyruvate kinase M2 expression with clinicopathological data in ovarian cancer. Anticancer Res. 2018;38:295–300. doi: 10.21873/anticanres.12221. [DOI] [PubMed] [Google Scholar]
- 55.DeHart DN, Lemasters JJ, Maldonado EN. Erastin-like anti-Warburg agents prevent mitochondrial depolarization induced by free tubulin and decrease lactate formation in cancer cells. SLAS Discov. 2018;23:23–33. doi: 10.1177/2472555217731556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshida GJ. Metabolic reprogramming: the emerging concept and associated therapeutic strategies. J Exp Clin Cancer Res. 2015;34:111. doi: 10.1186/s13046-015-0221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Godinot C, de Laplanche E, Hervouet E, Simonnet H. Actuality of Warburg's views in our understanding of renal cancer metabolism. J Bioenerg Biomembr. 2007;39:235–241. doi: 10.1007/s10863-007-9088-8. [DOI] [PubMed] [Google Scholar]
- 58.Lee M, Yoon JH. Metabolic interplay between glycolysis and mitochondrial oxidation: the reverse Warburg effect and its therapeutic implication. World J Biol Chem. 2015;6:148–161. doi: 10.4331/wjbc.v6.i3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hernandez-Resendiz I, Roman-Rosales A, Garcia-Villa E, Lopez-Macay A, Pineda E, Saavedra E, Gallardo-Perez JC, Alvarez-Rios E, Gariglio P, Moreno-Sanchez R, Rodriguez-Enriquez S. Dual regulation of energy metabolism by p53 in human cervix and breast cancer cells. Biochim Biophys Acta. 1853;2015:3266–3278. doi: 10.1016/j.bbamcr.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 60.Suganuma K, Miwa H, Imai N, Shikami M, Gotou M, Goto M, Mizuno S, Takahashi M, Yamamoto H, Hiramatsu A, Wakabayashi M, Watarai M, Hanamura I, Imamura A, Mihara H, Nitta M. Energy metabolism of leukemia cells: glycolysis versus oxidative phosphorylation. Leuk Lymphoma. 2010;51:2112–2119. doi: 10.3109/10428194.2010.512966. [DOI] [PubMed] [Google Scholar]
- 61.Klepinin A, Ounpuu L, Guzun R, Chekulayev V, Timohhina N, Tepp K, Shevchuk I, Schlattner U, Kaambre T. Simple oxygraphic analysis for the presence of adenylate kinase 1 and 2 in normal and tumor cells. J Bioenerg Biomembr. 2016;48:531–548. doi: 10.1007/s10863-016-9687-3. [DOI] [PubMed] [Google Scholar]
- 62.Jin L, Feng X, Rong H, Pan Z, Inaba Y, Qiu L, Zheng W, Lin S, Wang R, Wang Z, Wang S, Liu H, Li S, Xie W, Li Y. The antiparasitic drug ivermectin is a novel FXR ligand that regulates metabolism. Nat Commun. 2013;4:1937. doi: 10.1038/ncomms2924. [DOI] [PubMed] [Google Scholar]
- 63.Li N, Zhan X. Anti-parasite drug ivermectin can suppress ovarian cancer by regulating lncRNA-EIF4A3-mRNA axes. EPMA J. 2020;11:289–309. doi: 10.1007/s13167-020-00209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhan X, Giorgianni F, Desiderio DM. Proteomics analysis of growth hormone isoforms in the human pituitary. Proteomics. 2005;5:1228–1241. doi: 10.1002/pmic.200400987. [DOI] [PubMed] [Google Scholar]
- 65.Zhan X, Li B, Zhan X, Schlüter H, Jungblut PR, Coorssen JR. Innovating the concept and practice of two-dimensional gel electrophoresis in the analysis of proteomes at the proteoform level. Proteomes. 2019;7(4):36. doi: 10.3390/proteomes7040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhan X, Li N, Zhan X, Qian S. Revival of 2DE-LC/MS in proteomics and its potential for large-scale study of human proteoforms. Med One. 2018;3:e180008. doi: 10.20900/mo.20180008. [DOI] [Google Scholar]
- 67.Lu M. Wei Chen, Zhuang W, Zhan X. Label-free quantitative identification of abnormally ubiquitinated proteins as useful biomarkers for human lung squamous cell carcinomas. EPMA J. 2020;11(1):73–94. doi: 10.1007/s13167-019-00197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Janssens JP, Schuster K, Voss A. Preventive, predictive, and personalized medicine for effective and affordable cancer care. EPMA J. 2018;9(2):113–123. doi: 10.1007/s13167-018-0130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Samec M, Liskova A, Koklesova L, Samuel SM, Zhai K, Buhrmann C, Varghese E, Abotaleb M, Qaradakhi T, Zulli A, Kello M, Mojzis J, Zubor P, Kwon TK, Shakibaei M, Büsselberg D, Sarria GR, Golubnitschaja O, Kubatka P. Flavonoids against the Warburg phenotype-concepts of predictive, preventive and personalised medicine to cut the Gordian knot of cancer cell metabolism. EPMA J. 2020;11(3):377–398. doi: 10.1007/s13167-020-00217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Golubnitschaja O, Flammer J. Individualised patient profile: clinical utility of Flammer syndrome phenotype and general lessons for predictive, preventive and personalised medicine. EPMA J. 2018;9(1):15–20. doi: 10.1007/s13167-018-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu M, Zhan X. The crucial role of multiomic approach in cancer research and clinically relevant outcomes. EPMA J. 2018;9(1):77–102. doi: 10.1007/s13167-018-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li N, Zhan X. Identification of clinical trait-related lncRNA and mRNA biomarkers with weighted gene co-expression network analysis as useful tool for personalized medicine in ovarian cancer. EPMA J. 2019;10(3):273–290. doi: 10.1007/s13167-019-00175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheng T, Zhan X. Pattern recognition for predictive, preventive, and personalized medicine in cancer. EPMA J. 2017;8(1):51–60. doi: 10.1007/s13167-017-0083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS 326 kb)
(PDF 43 kb)