Abstract
Coronavirus disease 2019 (COVID-19) is an ongoing pandemic that has affected millions of individuals worldwide. Prior studies suggest that COVID-19 may be associated with an increased risk for various cardiovascular disorders, such as myocardial injury, arrhythmia, acute coronary syndrome, and venous thromboembolism. Early reports of non-COVID-19 patients have described the concurrence of takotsubo cardiomyopathy (TTC) and spontaneous coronary artery dissection (SCAD). However, the interplay between COVID-19, TTC and SCAD has not been well established. We herein propose two sets of two-hit hypotheses for the development of SCAD and TTC in the context of COVID-19. The first two-hit hypothesis explains the development of SCAD, in which TTC-associated formation of vulnerable coronary substrate serves as the first hit (predisposing factor), and COVID-19-associated inflammation and vascular disruption serves as the second hit (precipitating factor). The second two-hit hypothesis is proposed to explain the development of TTC, in which SCAD-associated formation of vulnerable myocardial substrate serves as the first hit, and COVID-19-associated sympathetic overactivity serves as the second hit. Under this conceptual framework, COVID-19 poses a double threat for the development of SCAD (among patients with underlying TTC) as well as TTC (among patients with underlying SCAD), thereby forming a reciprocal causation. This hypothesis provides a rationale for the joint assessment of TTC and SCAD in COVID-19 patients with pertinent cardiovascular manifestations.
Abbreviations: ACS, acute coronary syndrome; COVID-19, coronavirus disease 2019; SCAD, spontaneous coronary artery dissection; TTC, takotsubo cardiomyopathy
Keywords: COVID-19, Takotsubo cardiomyopathy, Spontaneous coronary artery dissection, Stress cardiomyopathy, Acute coronary syndrome
Background
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2, is an ongoing pandemic that has affected millions of individuals worldwide. COVID-19 is associated with significant morbidity and mortality, mostly due to pulmonary and cardiovascular complications. Preliminary reports have described the occurrence of takotsubo cardiomyopathy (TTC) and spontaneous coronary artery dissection (SCAD) in COVID-19 patients. However, it remains unclear whether COVID-19 patients are predisposed to TTC or SCAD.
The pathogenesis of TTC has been considered to be mediated by catecholamine excess. In a normal physiological condition, β2-adrenergic receptor activation by epinephrine leads to a positive inotropic response via the Gs protein–adenylyl cyclase–protein kinase A pathway. In contrast, at supraphysiological epinephrine levels, a process termed stimulus trafficking takes place, in which stimulation of β2 adrenergic receptor is switched from Gs protein to Gi protein signaling, resulting in a negative inotropic effect [1]. Thus, the manifestation of apical hypokinesis in TTC develops due to a higher density of β2-adrenergic receptor at the apical myocardium than the basal myocardium. With respect to SCAD, two possible mechanisms have been proposed. The first is the “inside-out” model, where the intimal tear allows blood to cross the internal elastic lamina and accumulate in the tunica media. The second is the “outside-in” model, where disruption of vasa vasorum results in hemorrhage into the tunica media. In both models, there is the formation of false lumen filled with intramural hematoma and subsequent compression of the true lumen [2], [3]. Intriguingly, both TTC and SCAD affect predominantly women and may be precipitated by emotional stress or strenuous exercise associated with sympathetic discharge. Numerous reports of non-COVID-19 patients have described the concurrence of TTC and SCAD, suggesting a potential reciprocal causal association between TTC and SCAD. This article discusses the speculative interplay between TTC and SCAD in the context of COVID-19.
The hypothesis
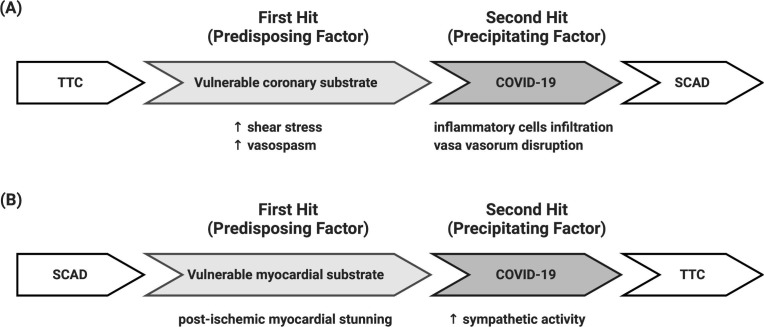
As illustrated in Fig. 1 , we propose that the development of SCAD or TTC could be explained by a two-hit hypothesis, in which the first hit represents the predisposing factor and the second hit represents the precipitating factor. It is speculated that formation of an anatomical substrate (such as vulnerable coronary artery or vulnerable myocardium) is a prerequisite for the subsequent development of SCAD or TTC, and the propensity of disease manifestation is increased by COVID-19. The anatomical coronary substrate is defined as the coronary artery wall tissue with altered structural properties characterized by inflammatory reactions (e.g., eosinophilic infiltrates) in the adventitia that predispose the coronary artery to dissection. The anatomical myocardium substrate is defined as the myocardial tissue with altered structural properties characterized by ischemia-related reactions that predispose the myocardium to wall motion abnormalities. For the development of SCAD, TTC-associated formation of vulnerable coronary substrate (due to increased shear stress and vasospasm) serves as the first hit, and COVID-19-associated inflammatory cells infiltration and vasa vasorum disruption serve as the second hit (Fig. 1, Panel A). Conversely, for the development of TTC, SCAD-associated formation of vulnerable myocardial substrate (due to post-ischemic myocardial stunning) serves as the first hit, and COVID-19-associated sympathetic overactivity serves as the second hit (Fig. 1, Panel B). Under this conceptual framework, COVID-19 could pose a double threat for the development of SCAD (among patients with underlying TTC) as well as TTC (among patients with underlying SCAD) through distinctive mechanisms, and may support the reciprocal causation between SCAD and TTC.
Fig. 1.
Two-hit hypothesis for the development of SCAD in patients with TTC (Panel A) and development of TTC in patients with SCAD (Panel B).
Empirical data
Takotsubo cardiomyopathy in COVID-19
Reported cases of TTC in patients with COVID-19 are summarized in Table 1 . A total of six cases of TTC have been reported in individuals with confirmed COVID-19. All of the patients were female, with an average age of 72 years. They presented with different symptoms such as chest pain, fever, chill, cough, myalgia, and altered mental status. TTC diagnosis was confirmed with echocardiography and managed with medical therapy. The pathophysiology of COVID-19-associated TTC may share some features with non-infectious cardiomyopathy and viral myocarditis. The first theory emphasizes the role of high sympathetic activity in the pathogenesis of TTC, similar to non-infectious cardiomyopathy. Because of the COVID-19 pandemic, psychological and physical stress posed on the community can activate the hypothalamic‑pituitary‑adrenal axis, resulting in hypercortisolism state, and also active sympathetic system resulting in increased sympathetic activity. Overstimulation of the sympathetic nervous system has a negative inotropic effect on myocyte contraction through a process called stimulus trafficking, in which epinephrine stimulation of β2 adrenergic receptor, leads to Gi protein activation and myocardial stunning. The other mechanism of TTC in COVID-19 patients is through cytokine storm syndrome, similar to viral myocarditis. Increased number of inflammatory cytokines (such as IL-6 and IL-8), chemokines (such as CXCL1), and inflammatory markers (such as ferritin and D-dimer) can lead to a systemic and localized inflammatory state. Macrophage infiltration and elevated inflammatory markers may also cause direct myocardial injury and lead to TTC [4], [5]. For definitive diagnosis of TTC, left ventriculography or echocardiography is required [6], [7].
Table 1.
Reported cases of takotsubo cardiomyopathy in patients with COVID-19.
| Author | Age/Sex | PMH | Presentation | Echocardiographic findings | COVID-19 diagnosis | Treatment |
|---|---|---|---|---|---|---|
| Nguyen [27] | 71/F | Hypertension, hypercholesterolemia, normotensive hydrocephalus treated with a VP shunt | Dyspnea | The ventriculogram showed regional wall motion abnormality unrelated to the coronary lesions, compatible with a median TTC | The nasopharyngeal swab PCR test was positive for a SARS-CoV-2 infection. | Two drug-eluting stents were implanted |
| Chadha [28] | 85/F | Negative | Sudden onset substernal chest pain | Urgent cardiac catheterization which showed hemodynamically non-significant coronary artery disease but the left ventriculogram revealed basal hyperkinesis and apical ballooning, consistent with TTC. The echocardiogram confirmed the same findings with ejection fraction noted to be around 35%. | N/A | N/A |
| Sattar [29] | 67/F | Hypertension, type 2 diabetes mellitus | Fever, chills, cough, malaise, and myalgias for 2 weeks | TTE demonstrated LVEF of 30% with diffuse anterior wall and apical akinesia and apical ballooning. | Nasopharyngeal swab for SARS CoV-2 RT-PCR returned positive. | The TTC was managed with DAPT using aspirin and clopidogrel and high dose statin therapy. Atrial fibrillation was managed with amiodarone loading dose, and then 200 mg once daily. Due to the increased risk of thromboembolism from TTC, COVID-19, and AF, patient was started on rivaroxaban 20 mg once daily. |
| Kariyanna [30] | 72/F | Obesity, diabetes, hypertension, hyperlipidemia | Dry cough and loss of appetite over the past 3–4 days altered mental status. | Transthoracic echocardiography revealed diffuse hypokinesis with distinct regional wall motion abnormalities. There was apical dyskinesis or apical systolic ballooning. | SARS-CoV-2 PCR assay using M2000 platform was positive for COVID-19 for nasopharyngeal swab. | COVID 19 was managed with azithromycin and Plaquenil. Aztreonam and gentamicin was added for possible bacterial infection. Patient subsequently developed cardiogenic shock on day 4 of hospitalization and was started on multiple vasopressors and inotropic agents (vasopressin, dopamine, norepinephrine, epinephrine and dobutamine). |
| Tsao [4] | 59/F | Obesity | Fevers, chills, fatigue, myalgias, and cough. | Transthoracic echocardiogram demonstrated severe hypokinesis of the mid-left ventricular cavity, with normal-to-hyperdynamic contractility of basal and apical left ventricular segments and a moderately reduced biplane ejection fraction of 36%. | SARS-CoV-2 RT-PCR test performed on her nasopharyngeal swab specimen returned positive. | Intravenous norepinephrine and vasopressin administration for profound hypoxemic respiratory failure and vasodilatory shock. Multiple episodes of monomorphic ventricular tachycardia responding to lidocaine. Sarilumab was used for COVID treatment. |
| Giannitsi [5] | 79/F | Hypertension | Chest pain | Left ventriculography illustrated severe hypokinesia in the mid-apical segments, hyperdynamic basal segments, and impaired left ventricular systolic function with an ejection fraction of 35%. | N/A | ACE-inhibitor, aspirin, beta-blocker and statin |
Abbreviations: ACE: angiotensin converting enzyme, AF: atrial fibrillation, DAPT: dual antiplatelet treatment, LAD: left anterior descending, LVEF: left ventricle ejection fraction, PCR: polymerase chain reaction, PMH: past medical history, RT-PCR: real time polymerase chain reaction, SARS-CoV-2: severe acute respiratory syndrome coronavirus 2, TTC: takotsubo cardiomyopathy, TTE: transthoracic echocardiogram, VP shunt: ventriculoperitoneal shunt.
Spontaneous coronary artery dissection in COVID-19
Reported cases of SCAD in patients with COVID-19 are summarized in Table 2 . So far, only four cases of SCAD have been reported in patients with positive COVID-19 test, including three men (75%) and one woman (25%), with an average age of 53 years. They presented with chest pain with or without cough and fever. Lack of cardiovascular risk factors, and the presents of a stressor helps to differentiate SCAD from atherosclerotic coronary artery disease (ASCAD), but for definitive diagnosis of SCAD, coronary angiography is needed [8], [9]. Clinical course and follow-up survey with coronary angiography, left ventriculography, and/or echocardiography may be needed for differentiation. SCAD was managed with medical therapy (75%) or percutaneous coronary intervention (PCI) (25%). Multiple mechanisms have been implicated in the pathogenesis of SCAD among COVID-19 patients. There have been several case reports of acute coronary syndrome (ACS) following acute respiratory viral infections (such as influenza and coronavirus outbreaks), especially in patients with a history of cardiac disease. It has been suggested that systemic inflammation in response to the infection or localized vessel inflammation can be the underlying mechanism for the coronary event [10], [11]. Among patients with ACS, there is greater inflammatory activity and inflammatory infiltrates such as neutrophils, T-cells, and macrophages in the atherosclerotic plaques. Also, in patients with acute systemic infection, there is enhanced infiltration of macrophages and T-cell in the adventitia, and dendritic cell in the intima and media layers of coronary vessels [12], [13]. In the context of COVID-19, one of the potential mechanisms for SCAD is that SARS-CoV-2 viral infection can lead to T-cell activation and infiltration in adventitia and periadventitial fat, which in turn produce more cytokines and proteases, thereby increasing the risk of plaque rupture or erosion and subsequent dissection (inside-out mechanism of SCAD) [14]. Another mechanism is that SARS-CoV-2 may stimulate angiogenesis and lead to proliferation of the vasa vasorum. The newly formed vasa vasorum is relatively leaky, fragile, and prone to disruption, which results in intramural hematoma (outside-in mechanism of SCAD) [15]. Vasa vasorum can also serve as the conduit for the entry of inflammatory cells into the tunica media and adventitia, facilitating inflammation and disruption of vasa vasorum [16].
Table 2.
Reported cases of spontaneous coronary artery dissection in patients with COVID-19.
| Author | Age/Sex | PMH | Presentation | Angiography findings | COVID diagnosis | Treatment |
|---|---|---|---|---|---|---|
| Kumar [26] | 48/F | Migraine, hyperlipidemia | Severe retrosternal chest pain | SCAD (LAD); tapered compression of 70% was noted at the mid through distal LAD with reconstitution at the apex. Delayed contrast washout was noted in the proximal LAD indicating the proximal extent of the dissection. | Positive nasopharyngeal swab | Medical therapy (aspirin 81 mg daily and clopidogrel 75 mg daily) |
| Courand [31] | 55/M | Peripheral artery disease | Cough, fever, dyspnea, chest pain | SCAD (RCA); total occlusion of the posterior descending artery with epicardial collateral from the left anterior descending artery. In the mid-right coronary artery, a spontaneous dissecting coronary hematoma was observed with an intimal tear. | Positive PCR | Conservative management (aspirin, statins, and beta-blockers) |
| Albiero [32] | 70/M | Smoking, hypertension, type 2 diabetes, prior PCI | Persistent severe chest pain | SCAD (LAD); moderate in-stent restenosis on LCx-OM and a moderate RCA stenosis. The culprit lesion was the proximal LAD that presented with a very complex and unusual plaque morphology, indicative of a coronary artery dissection. | Positive RT-PCR | PCI/discharged with: aspirin 100 mg/day, clopidogrel 75 mg/day, pantoprazole 40 mg/day, atorvastatin 40 mg/day, bisoprolol 1.25 mg/day, and metformin 850 mg three times a day. |
| Fernandez Gasso [33] | 39/M | Unremarkable | Fever, cough, myalgia, oppressive chest pain, and dyspnea at rest | SCAD (LAD, OM): large and severe lumen narrowing in the first OM bordered by normal segments compatible with type 2A SCAD; moderate lumen narrowing in the distal LAD that extended to the end of the artery compatible with type 2B SCAD; a mild fistula between the proximal segment of the RCA and the pulmonary artery. | Positive nasopharyngeal and oropharyngeal swabs | Conservative therapy |
Abbreviations: LAD: left anterior descending, LCx: left circumflex artery, OM: obtuse marginal, PCI: percutaneous coronary intervention, PCR: polymerase chain reaction, RCA: right coronary artery, RT-PCR: real time polymerase chain reaction, SCAD: spontaneous coronary artery dissection
Interplay between TTC, SCAD, and COVID-19
Prior to the COVID-19 pandemic, several reports have described the concurrence of TTC and SCAD [17], [18], [19], [20]. In the setting of TTC, vigorous contraction of the left ventricular base in conjunction with adjacent dyskinetic segments could form a prerequisite anatomical substrate for the causation of SCAD. The coronary dissection plane may develop as a result of excessive movement of the epicardial vessels and increased shear stress on the vessel wall at the hinge point between the hyperdynamic and akinetic myocardium [21]. It has also been speculated that the coronary arteries traversing the anterior or anterolateral wall would be more vulnerable to dissection as this region marks the transition point of the hyperdynamic basal segment and the remaining hypokinetic left ventricular segments [21]. In addition, the vulnerable coronary substrate may occur as a consequence of coronary vasospasm due to catecholamine excess in TTC. With the vulnerable coronary substrate, further insults associated with COVID-19 (such as inflammatory cell infiltration and vasa vasorum rupture) could subsequently trigger the dissection of the coronary artery. On the contrary, post-ischemic myocardial stunning associated with SCAD could render the myocardium more susceptible to wall motion abnormalities, thus forming the vulnerable myocardial substrate. Under the influence of sympathetic overactivity associated with COVID-19, TTC may manifest due to hypokinesia in stunned myocardium coupled with hyperkinesia in the stimulated myocardium. Interestingly, it is noteworthy that both TTC and SCAD patients faced enormous “stresses” (e.g., emotional, sickness-related, or drug-related) that may cause or potentiate catecholamine surge and inflammatory cells infiltration. Moreover, COVID-19 (either the disease itself or the fear it caused) may also serve as a stressor for the development of TTC and/or SCAD. In this framework, it could be speculated that COVID-19 not only triggers the development of SCAD among patients with underlying TTC, but also manifests the development of TTC among patients with underlying SCAD, thereby serving as a precipitating factor that supports the reciprocal causation between TTC and SCAD.
Consequences of the hypothesis and discussion
SCAD or TTC may occur as an extrapulmonary manifestation in the setting of COVID-19. COVID‐19 testing prior to cardiac catheterization or imaging in suspected cases with unknown COVID-19 status may be considered. Based on the study by Duran et al., approximately 55.8% of the angiographically-confirmed SCAD patients who underwent left ventriculography were found to have a concomitant TTC [21]. The high prevalence of coexistence suggests the need of a high index of clinical suspicion and diagnostic modalities (such as angiography, left ventriculography, or echocardiography) when encountering SCAD or TTC. In uncomplicated cases of SCAD and TTC, uneventful recovery (e.g., spontaneous healing of dissected coronary arteries and restoration of left ventricular function) can be usually expected. Given that COVID-19 is associated with greater mortality and the potential risk of both SCAD and TTC, it is advisable to screen for SCAD in the presence of TTC in order to improve patient outcomes. In this regard, luminal geometry should be scrutinized to rule out SCAD among COVID-19 patients presenting with TTC who undergo coronary angiography. Similarly, among COVID-19 patients presenting with SCAD, it may be prudent to perform echocardiography or ventriculography to rule out wall motion abnormalities associated with TTC. Further studies are required to elucidate the mechanistic interplay between COVID-19, TTC, and SCAD.
With respect to the management of TTC, the latest international consensus statements on stress cardiomyopathy recommended supportive care with a goal of preventing complications as well as recurrence. The treatment strategy, therefore, differs based on the clinical experience and should focus on the aforementioned goals. Acute treatment of TTC manages the heart failure and therefore consists of beta-blockers as well as medications targeting the angiotensin pathway. Long-term treatment mostly comprised of administering an angiotensin-converting enzyme inhibitor or angiotensin II receptor blockers. In case of concomitant atherosclerotic disease, statins and aspirin should also be included as part of the treatment regimen [22]. The European Society of Cardiology (ESC) taskforce statement on TTC suggested no or a short course of medical therapy in mild cases as well as early mechanical support in the cases with circulatory failure [23]. The reported cases of COVID-19 induced TTC have been managed conservatively with the goal of reducing the complications [5], [24]. In addition, in of the COVID-19 case that hydroxychloroquine was administered as the COVID-19 treatment, it was later discontinued due to its potential adverse effect on the cardiomyopathy as well as increasing the risk of QT interval prolongation that is a known complication of TTC [24].
The American Heart Association (AHA) and ESC practice guidelines recommended early invasive treatment strategies with revascularization of the involved vessel as the foundation of acute coronary syndrome (ACS) management and advocated that this strategy has been associated with a better long-term outcomes and lower complication rates. However, current literature lacks any randomized controlled trial study comparing the efficacy of invasive versus conservative managements in the ACS caused by SCAD. The latest AHA statement regarding SCAD recommended conservative therapy only in clinically stable cases and in the absence of left main or proximal two-vessel coronary artery dissection [9]. However, a meta-analysis of observational studies reported similar in-hospital and long-term outcomes in SCAD patients who were managed medically comparted to those who received early revascularization in the absence of aforementioned criteria [25]. Patients with COVID-19–induced SCAD has been managed conservatively by dual antiplatelet therapy as well as beta-blockers and anti-arrhythmic medications to prevent the dysrhythmia [26]. Future studies should investigate the optimal treatment for SCAD and TTC in the context of COVID-19.
Disclosure
The work is not funded. The authors declare no conflicts of interest.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.110410.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Pasupula D.K., Patthipati V.S., Javed A., Malleshappa S.K.S. Takotsubo cardiomyopathy: understanding the pathophysiology of selective left ventricular involvement. Cureus. 2019;11 doi: 10.7759/cureus.5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfonso F. Spontaneous coronary artery dissection: new insights from the tip of the iceberg? Circulation. 2012;126(6):667–670. doi: 10.1161/CIRCULATIONAHA.112.122093. [DOI] [PubMed] [Google Scholar]
- 3.Saw J., Mancini G.J., Humphries K.H. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol. 2016;68:297–312. doi: 10.1016/j.jacc.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 4.Tsao C.W., Strom J.B., Chang J.D., Manning W.J. COVID-19–associated stress (Takotsubo) cardiomyopathy. Circ Cardiovasc Imag. 2020;13 doi: 10.1161/CIRCIMAGING.120.011222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giannitsi S., Tsinivizov P., Poulimenos L.E. [Case Report] Stress induced (Takotsubo) cardiomyopathy triggered by the COVID-19 pandemic. Experimental and Therapeutic Medicine. 2020;20:2812–2814. doi: 10.3892/etm.2020.8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prasad A., Lerman A., Rihal C.S. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408–417. doi: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Patel S.M., Lennon R.J., Prasad A. Regional wall motion abnormality in apical ballooning syndrome (Takotsubo/stress cardiomyopathy): importance of biplane left ventriculography for differentiating from spontaneously aborted anterior myocardial infarction. Int J Cardiovasc Imaging. 2012;28:687–694. doi: 10.1007/s10554-011-9911-5. [DOI] [PubMed] [Google Scholar]
- 8.Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheterization Cardiovasc Intervent. 2014;84:1115–1122. doi: 10.1002/ccd.25293. [DOI] [PubMed] [Google Scholar]
- 9.Hayes S.N., Kim E.S., Saw J. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation. 2018;137:e523–e557. doi: 10.1161/CIR.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwong J.C., Schwartz K.L., Campitelli M.A. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378:345–353. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 11.Madjid M., Miller C.C., Zarubaev V.V. Influenza epidemics and acute respiratory disease activity are associated with a surge in autopsy-confirmed coronary heart disease death: results from 8 years of autopsies in 34 892 subjects. Eur Heart J. 2007;28:1205–1210. doi: 10.1093/eurheartj/ehm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corrales-Medina V.F., Madjid M., Musher D.M. Role of acute infection in triggering acute coronary syndromes. Lancet Infect Dis. 2010;10:83–92. doi: 10.1016/S1473-3099(09)70331-7. [DOI] [PubMed] [Google Scholar]
- 13.Madjid M., Vela D., Khalili-Tabrizi H., Casscells S.W., Litovsky S. Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries: clues to the triggering effect of acute infections on acute coronary syndromes. Tex Heart Inst J. 2007;34:11. [PMC free article] [PubMed] [Google Scholar]
- 14.Adlam D., Alfonso F., Maas A., Vrints C., Committee W. European Society of Cardiology, acute cardiovascular care association, SCAD study group: a position paper on spontaneous coronary artery dissection. Eur Heart J. 2018;39:3353. doi: 10.1093/eurheartj/ehy080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pitliya A., Datta S., Kalayci A. Eosinophilic inflammation in spontaneous coronary artery dissection: a potential therapeutic target? Med Hypotheses. 2018;121:91–94. doi: 10.1016/j.mehy.2018.09.039. [DOI] [PubMed] [Google Scholar]
- 16.Meizlish M., Pine A., Goshua G. Circulating markers of angiogenesis and endotheliopathy in COVID-19. medRxiv. 2020 doi: 10.1177/2045894020966547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassan S.Y., Themudo R., Maret E. Spontaneous coronary artery dissection and takotsubo syndrome: the chicken or the egg causality dilemma. Catheter Cardiovasc Interv. 2017;89:1215–1218. doi: 10.1002/ccd.26956. [DOI] [PubMed] [Google Scholar]
- 18.Hassan S.Y., Henareh L. Spontaneous coronary artery dissection triggered post-ischemic myocardial stunning and takotsubo syndrome: two different names for the same condition. Cardiovasc Revasc Med. 2013;14:109–112. doi: 10.1016/j.carrev.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Hassan S.Y., Bohm F. The causal link between spontaneous coronary artery dissection and takotsubo syndrome: a case presented with both conditions. Int J Cardiol. 2016;203:828–831. doi: 10.1016/j.ijcard.2015.11.047. [DOI] [PubMed] [Google Scholar]
- 20.Bakhit A., Bin Abdulhak A. Spontaneous coronary dissection misdiagnosed as Takotsubo's Cardiomyopathy. Int J Cardiol. 2016;225:384–386. doi: 10.1016/j.ijcard.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Duran J.M., Naderi S., Vidula M. Spontaneous coronary artery dissection and its association with takotsubo syndrome: novel insights from a tertiary center registry. Catheter Cardiovasc Interv. 2020;95:485–491. doi: 10.1002/ccd.28314. [DOI] [PubMed] [Google Scholar]
- 22.Ghadri J.-R., Wittstein I.S., Prasad A. International expert consensus document on Takotsubo syndrome (part II): diagnostic workup, outcome, and management. Eur Heart J. 2018;39:2047–2062. doi: 10.1093/eurheartj/ehy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyon A.R., Bossone E., Schneider B. Current state of knowledge on Takotsubo syndrome: a Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2016;18:8–27. doi: 10.1002/ejhf.424. [DOI] [PubMed] [Google Scholar]
- 24.Minhas A.S., Scheel P., Garibaldi B. Takotsubo syndrome in the setting of COVID-19. JACC: Case Rep. 2020;2(9):1321–1325. doi: 10.1016/j.jaccas.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jamil A., Tajrishi F.Z., Kahe F. Spontaneous coronary artery dissection managed with a conservative or revascularization approach: a meta-analysis. J Cardiovasc Med. 2020;21:42–50. doi: 10.2459/JCM.0000000000000891. [DOI] [PubMed] [Google Scholar]
- 26.Kumar K., Vogt J.C., Divanji P.H., Cigarroa J.E. Spontaneous coronary artery dissection of the left anterior descending artery in a patient with COVID-19 infection. Catheteriz Cardiovasc Intervent. 2020 doi: 10.1002/ccd.28960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen D., Nguyen T., De Bels D., Rodriguez J.C. A case of Takotsubo cardiomyopathy with COVID 19. Eur Heart J Cardiovasc Imag. 2020 doi: 10.1093/ehjci/jeaa152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chadha S. ‘COVID-19 pandemic’ anxiety induced tako-tsubo cardiomyopathy. QJM: Int J Med. 2020 doi: 10.1093/qjmed/hcaa135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sattar Y., Connerney M., Ullah W. COVID-19 presenting as takotsubo cardiomyopathy complicated with atrial fibrillation. Int J Cardiol Heart Vasculature. 2020;29 doi: 10.1016/j.ijcha.2020.100580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kariyanna P.T., Chandrakumar H.P., Jayarangaiah A. Apical takotsubo cardiomyopathy in a COVID-19 patient presenting with stroke: a case report and pathophysiologic insights. Am J Med Case Rep. 2020;8:350. [Google Scholar]
- 31.Courand P.-Y., Harbaoui B., Bonnet M., Lantelme P. Spontaneous coronary artery dissection in a patient with COVID-19. JACC: Cardiovasc Intervent. 2020;13(12):e107–e108. doi: 10.1016/j.jcin.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albiero R., Seresini G. Atherosclerotic spontaneous coronary artery dissection (A-SCAD) in a patient with COVID-19: case report and possible mechanisms. Eur Heart J: Case Rep. 2020 doi: 10.1093/ehjcr/ytaa133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandez Gasso L., Maneiro Melon N.M., Sarnago Cebada F., Solis J., Garcia Tejada J. Multivessel spontaneous coronary artery dissection presenting in a patient with severe acute SARS-CoV-2 respiratory infection. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.