Abstract
Cytosolic malate dehydrogenase (MDH) is a key enzyme that regulates the interconversion between malate and oxaloacetate (OAA). However, its role in modulating storage compound accumulation in maize endosperm is largely unknown. Here, we characterized a novel naturally occurring maize mdh4‐1 mutant, which produces small, opaque kernels and exhibits reduced starch but enhanced lysine content. Map‐based cloning, functional complementation and allelism analyses identified ZmMdh4 as the causal gene. Enzymatic assays demonstrated that ZmMDH4 predominantly catalyses the conversion from OAA to malate. In comparison, the activity of the mutant enzyme, which lacks one glutamic acid (Glu), was completed abolished, demonstrating that the Glu residue was essential for ZmMDH4 function. Knocking down ZmMdh4 in vivo led to a substantial metabolic shift towards glycolysis and a dramatic disruption in the activity of the mitochondrial complex I, which was correlated with transcriptomic alterations. Taken together, these results demonstrate that ZmMdh4 regulates the balance between mitochondrial respiration and glycolysis, ATP production and endosperm development, through a yet unknown feedback regulatory mechanism in mitochondria.
Keywords: Maize, cytosolic malate dehydrogenase 4, energetics, starch, zein, mitochondrion
Introduction
Owing to its high value as animal feed, raw industrial material, food for human consumption and fuel through bioethanol production (Ranum et al., 2014), maize (Zea mays L.) is widely distributed and cultivated throughout the world. Maize endosperm accounts for over 75% of the kernel dry weight and mainly contains starch and protein. Maize starchy endosperm mutants often exhibit changes in the structure and/or accumulation of starch granules and/or protein bodies (PBs), thus affecting grain yield and nutritional quality. Examples include the opaque (o) and floury (fl) mutants. o1, o10, fl1 and fl4 mutants have abnormal PBs and noticeable changes in the levels of zeins and alcohol‐soluble prolamins, which are the main components of PBs (Holding et al., 2007; Larkins and Hurkman, 1978; Lending and Larkins, 1989; Wang et al., 2014a; Wang et al., 2012; Yao et al., 2016). Several other opaque/floury mutants, such as o2, o6, o7, o11, fl2, fl3, Mucronate, De*‐B30, Mto140 and ocd1, display reduced zeins and a compensatory increase in non‐zeins, which result in elevated lysine content (Coleman et al., 1997; Feng et al., 2018; Holding et al., 2010; Kim et al., 2006; Kim et al., 2004; Li et al., 2017; Schmidt et al., 1992; Wang et al., 2011; Wang et al., 2014b; Yang et al., 2018). In case of o5, however, the opaque phenotype is caused by morphological alterations in starch granules rather than changes in protein and/or amino acid composition (Hunter et al., 2002; Myers et al., 2011). These alterations are due to mutations in the structural or regulatory protein genes of zeins. opaque/floury mutants, such as o2, o11, fl2, fl3, Mucronate and De*‐B30, involve genes that regulate zein biosynthesis (Coleman et al., 1997; Feng et al., 2018; Kim et al., 2006; Kim et al., 2004; Li et al., 2017; Schmidt et al., 1992); whereas, other opaque/floury mutants, such as o1, o10, fl1 and fl4, are controlled by genes that participate in the assembly of zeins into PBs (Holding et al., 2007; Wang et al., 2014a; Wang et al., 2012; Yao et al., 2016). In addition, the o6, o7, Mto140 and ocd1 mutants are impaired in amino acid metabolism or other primary metabolic pathways (Holding et al., 2010; Wang et al., 2011; Yang et al., 2018). For example, O6 (also known as Pro1) and Mto140 encode Δ1‐pyrroline‐5‐carboxylate synthetase and arogenate dehydrogenase 1 that catalyse proline and tyrosine biosynthesis, respectively. Mutations of these two genes resulted in restricted proline and tyrosine supply for zein biosynthesis (Holding et al., 2010; Wang et al., 2014b). O7 and Ocd1, however, are oxalyl‐CoA synthetase and oxalyl‐CoA decarboxylase genes that are involved in oxalate degradation. These mutations affect amino acid levels and zein accumulation (Wang et al., 2011; Yang et al., 2018) through an unknown mechanism. Together, these published data suggest a link between amino acid metabolism, zein biosynthesis and endosperm texture. Thus, the cloning and characterization of additional kernel mutants will deepen our understanding of how protein and/or starch biosynthesis are regulated in maize endosperm.
To this end, we isolated a starchy kernel mutant with altered starch, zein and lysine content in the endosperm. The mutant phenotype was found to be the result of a 3‐bp deletion in the cytosolic NAD‐dependent malate dehydrogenase 4 (Mdh4) gene. MDHs are oxidoreductases that catalyse the reversible interconversion between malate and OAA in the cytosol and other organelles, including glyoxysomes, mitochondria, peroxisomes and chloroplasts. In Arabidopsis thaliana, the plastidial NAD‐specific MDH shuttles malate and OAA in non‐photosynthetic tissues and its null mutant is embryo lethal (Beeler et al., 2014; Selinski et al., 2014). In contrast, NADP‐MDH is important for adjusting the ATP/NADPH ratio in light malate valve and loss of NADP‐MDH has no or little effect on growth (Hebbelmann et al., 2012; Heyno et al., 2014). Overexpressing maize NADP‐dependent MDH in A. thaliana confers salt tolerance (Kandoi et al., 2018). Mitochondrial NAD‐MDHs play a critical role in the tricarboxylic acid (TCA) cycle and null mutants of the two NAD‐MDH isoforms display viable but abnormal plant development (Sew et al., 2016; Tomaz et al., 2010). Peroxisomal NAD‐MDHs mainly generate NAD+ for the β‐oxidation of fatty acids and double mutant of the two isoforms fail to efficiently mobilize triacylglycerols (Pracharoenwattana et al., 2007, 2010). Although loss‐of‐function mutants of the plastidial, mitochondrial and peroxisomal MDH isoforms have been characterized, the phenotypic expression of cytosolic MDH of A. thaliana mutants is largely unknown. Cytosolic MDH mediates malate biosynthesis in the cytosol and has been reported to participate in plant and cell growth in apple (Wang et al., 2016; Yao et al., 2011a, 2011b). Its overexpression confers abiotic stress tolerance, such as cold and salt exposure, in apple trees by modulating redox homeostasis via malate accumulation (Wang et al., 2016; Yao et al., 2011a, 2011b). Recently, a NAD‐dependent cytosolic MDH (flo16) has been reported in rice, revealing a potential role of cytosolic MDHs on reserve storage and seed development in the grass family (Teng et al., 2019). The mutant exhibits reduced ATP production and enhanced oxidation via a reduction in the activities of starch biosynthetic enzymes, leading to decreased starch accumulation in rice endosperm. Conversely, the overexpression of cytosolic MDH significantly improves grain weight (Teng et al., 2019). Taken together, these data point to a role of cytosolic MDHs in regulating starch biosynthesis and seed development in grass species.
The maize genome contains four NAD‐dependent MDH isoforms and one NADP‐dependent MDH isoform (Goodman et al., 1981). However, data concerning the roles of these isoforms are limited. Of these, ZmMdh1 (Zm00001d009640), ZmMdh2 (Zm00001d039089) and ZmMdh3 (Zm00001d044042) are localized to mitochondria, ZmMdh4 (Zm00001d032695) and ZmMdh5 (Zm00001d014030) to the cytosol, and ZmMdh6 (Zm00001d031899) to plastids (Goodman et al., 1981; Newton and Schwartz, 1980). The cytosolic isoforms ZmMdh4 and ZmMdh5 are a result of gene duplication and reside on different chromosomes (Mcmillin and Scandalios, 1980). In maize, at least one of the two mitochondrial MDHs needs to be present to ensure normal kernel development (Goodman et al., 1981), suggesting that MDH activity is indispensable for maize kernel and plant development under non‐stress conditions. By contrast, the null homozygous mutants of either or both cytosolic MDHs are viable and can grow to maturity (Goodman et al., 1981), suggesting a potential regulatory role in endosperm development and storage reserve accumulation.
In this study, we isolated the maize kernel mutant, mdh4‐1, which shows a deformed endosperm, reduced starch and zein content, and enhanced lysine content. Map‐based cloning, genetic transformation and allelism analyses confirmed cytosolic ZmMdh4 as the causal gene. A 3 base pairs (bps) deletion in exon 7 of ZmMdh4 was validated as the functional mutational site. This mutation renders the ZmMDH4 enzyme almost inactive for converting OAA to malate, likely by altering the tertiary structure of the enzyme. The mutant shows elevated Zmmdh4 transcript and protein levels, presumably to compensate for the reduction in enzymatic activity. Combined with the transcriptome and metabolome data of immature kernels at 12 days after pollination (DAP), we propose a regulatory model of ZmMdh4 on storage reserve accumulation in maize.
Results
A novel naturally occurring mdh4‐1 mutant displays small and opaque kernels, reduced starch content and elevated lysine levels
mdh4‐1 was originally isolated as a small kernel mutant with a deformed endosperm crown (Figure 1a), which appeared 12 DAP on segregating F2 ears. These mutants were characterized by the relatively smaller kernel size and delayed development (Figure S1). At maturity, the 100‐kernel weight of homozygous mutant kernels was only 55.1% of the wild type (WT), and the length, width and thickness were 10.8%, 16.5% and 29.8%, lower than those of the WT, respectively (P < 0.001, Student's t‐test; Figure 1b and Figure S2a‐b). In addition, the longitudinal sections of the kernels showed that the mutant embryo was abnormal and smaller in size compared with that of the WT (Figure 1c). Upon quantification of the storage constituents, a 6.9% and 14.5% reduction in total starch and amylose content, respectively, was observed in mature mutant kernels as compared to the WT (Figure 1d). Furthermore, 12 DAP mdh4‐1 endosperms accumulated a much lower level of zein protein than WT (Figure 1e). At maturity, the mutant endosperms had an increased level of non‐zein proteins (Figure S2c‐d), which was accompanied by an increase in lysine, one of the major components of non‐zeins, by approximately 1.4‐fold (Figure 1f, Table S2).
Figure 1.

Phenotypic comparison between WT and the mdh4‐1 mutant. (a) Segregation of Zheng58 × mdh4‐1 F2 seeds. The red arrows indicate mdh4‐1 kernels; scale bar, 1 cm. (b) 100‐kernel weight of the WT vs. mdh4‐1, with five replicates. (c) Comparison between the longitudinal sections of WT and mdh4‐1 kernels. En, endosperm; Em, embryo; scale bar 2 mm. (d) Starch and amylose content in WT vs. mdh4‐1 endosperms with four replicates. (e) Zein proteins in 12 DAP endosperms of WT and mdh4‐1. (f) Total lysine content in WT vs. mdh4‐1 mature kernels with three replicates. (g) Representative examples of germination of the WT and mdh4‐1 seeds; scale bar, 1 cm. (h) Germination rate of WT vs. mdh4‐1 with three replicates. (i) Radical length of WT vs.mdh4‐1 seedlings with three replicates. (j) Representative examples of WT and mdh4‐1 seedlings 13 days after germination (DAG); scale bar, 5 cm. (k) Height and root length of WT vs. mdh4‐1 seedlings 13 DAG, with ten replicates. (l) Representative examples of mature WT and mdh4‐1 plants; scale bar, 30 cm. (m) Plant height and ear length of mature WT vs. mdh4‐1 plants, with 15 replicates. In all bar graphs, values are represented as means ± SE, **P < 0.01, ***P < 0.001 denote statistically significant differences between WT and mdh4‐1 (Student's t‐test).
In addition to the kernel phenotypes, the mdh4‐1 mutant also exhibited various growth defects. For example, it exhibited a 50% lower germination rate than the WT (Figure 1h), and radicle root length of the mutant was much shorter than that of the WT (Figure 1g, i). Moreover, the mutant seedlings produced less and shorter roots than WT seedlings at 13 days after germination (DAG) (Figure 1j‐k). Lastly, mutant plant height and ear length were also reduced in comparison to the WT (Figure 1l‐m). These observations suggest that the mdh4‐1 mutation exerts pleiotropic effects on plant growth and kernel development.
The mdh4‐1 mutant exhibits delayed endosperm and embryo development
To further dissect the starchy‐like kernel phenotype, sections of mature and developing mdh4‐1 and WT kernels were examined. Under a scanning electron microscope (SEM), mature mutant kernels displayed irregularly shaped starch granules with reduced surrounding matrix, whereas the starch granules in mature WT kernels aggregated together and were surrounded by a dense matrix (Figure 2a). The paraffin sections showed that mdh4‐1 embryos developed much more slowly than those of the WT. Notably, the shoot and root apical meristems, and leaf primordia were clearly visible in the WT embryos, but were still indistinguishable in mutant embryos at 12 DAP (Figure 2b). Additionally, starchy endosperm development was severely retarded in the mutant as compared with the WT, with the starchy endosperm occupying only 2/3 of the kernel as to 100% occupancy in the WT (Figure 2c). At 16 DAP, the mutant embryo was deformed and the endosperm had developed into three blocks (Figure 2c). These observations support the notion that embryo and endosperm development were delayed in the mdh4‐1 mutant. In addition, reduced ingrowth in the cells of the basal endosperm transfer layer (BETL) in mdh4‐1 kernels as compared to the WT kernels was observed, suggesting impaired transmission of nutritional constituents from the maternal tissue to endosperm cells (Figure 2b‐c).
Figure 2.

Cytological characterization of WT and mdh4‐1 seeds at different stages. (a) Scanning electron microscopy (SEM) images of mature WT and mdh4 endosperms; scale bars, 10 µm. (b) Paraffin sections of 12 DAP and (c) 16 DAP of WT and mdh4‐1 kernels. Scale bars of whole seed and basal endosperm transfer layer (BETL, red rectangle), 1 mm and 200 µm, respectively. En, endosperm; Em, embryo. (d) Transmission electron microscopy (TEM) images of WT and the mdh4‐1 mutant seeds 18 DAP. Scale bars in left and right panels, 5 µm and 1 µm, respectively. (e) Number and (f) size of starch grains in WT vs. mdh4‐1 endosperms at 18 DAP with eight replicates. En, endosperm; Em, embryo; SG, starch granule; PB, protein body; M, mitochondria. Values are represented as means ± SE, **P < 0.01, ***P < 0.001 (Student's t‐test).
The starchy endosperm cells were examined by transmission electron microscopy (TEM), and it was found that the shape and number of PBs were similar between 18 DAP WT and mutant endosperms (Figure 2d). However, mdh4‐1 kernels exhibited smaller starch granules than the WT (Figure 2e‐f), which may be related to the loosely packed starch granules and reduced 100‐kernel weight of the mutant kernels (Figures 1b and 2a).
Map‐based cloning of mdh4‐1
For genetic analysis, mdh4‐1 was crossed with a widely used Chinese elite inbred line, Zheng58, and the resulting F1 progeny was self‐pollinated to generate an F2 population (Figure 1a). The ratio of WT to mutant F2 kernels was roughly 3:1, suggesting the existence of a single recessive mutation. We randomly selected and grew F2 kernels that showed the WT phenotype and collected and analysed 100 F3 ears. The ratio of non‐segregating (n = 30, homozygote) to segregating ears (n = 70, heterozygote) was approximately 1:2 (χ 2 = 0.5 < = 3.84). In addition, the segregation of normal to mutant F3 kernels obtained from segregating F2 ears followed a 3:1 ratio (normal:mdh4‐1 = 5494:1833, χ 2 = 0.002 < = 3.84; Table S1). Similar segregation ratios were observed in the other five F2 segregating populations constructed by crossing mdh4‐1 with different inbred lines (Table S1 and Figure S2e). Collectively, these results indicate that the mutant phenotype of mdh4‐1 is caused by a single recessive locus.
Preliminary genetic mapping using 419 F2 individuals placed the target gene between the simple sequence repeat (SSR) markers umc1245 and umc2181 (Table S3) on bin1.08 of chromosome 1. The interval was further narrowed down to a ~224‐kb region between markers C362 (two recombinants) and Indel‐98 (five recombinants) using an F2 population consisting of 34,080 individuals (Figure 3a). The 224‐kb target region contains two predicted open reading frames (ORFs), Zm00001d032695 and Zm00001d032699, in the B73 reference genome (RefGen V4; Figure 3a). The genomic sequences of these two genes were compared between mdh4‐1 and Zheng58 and several single nucleotide polymorphisms (SNPs) were identified, as well as insertions and deletions (InDel). However, only the 3‐bp deletion in exon 7 of Zm00001d032695 was found exclusively in the mdh4‐1 mutant (Figure 3b; Figure S3). Zm00001d032695 is annotated as a cytosolic malate dehydrogenase 4 in Gramene (http://www.gramene.org/Zea_mays/) and was therefore named ZmMdh4. ZmMdh4 is ~5.4‐kb in length with seven exons (Figure 3b), and the full‐length cDNA of ZmMdh4 is estimated to be 1381‐bp. The deduced protein translation contains 332 amino acids, with a molecular mass of ~36 kD. It has a predicted NAD‐dependent cytoplasmic malate dehydrogenase domain (Figure 3c). The polymorphic sites of ZmMdh4 were analysed in an association panel consisting of 540 inbred lines to identify the functional polymorphisms associated with kernel characteristics (http://www.maizego.org/Resources.html; Table S4). Three SNPs in the 5' UTR were found to associate with lysine content and/or kernel thickness (Figure 3b and Table S5). Based on these results, ZmMdh4 was selected as the candidate gene for further validation and characterization.
Figure 3.

Mapping and genotyping of ZmMdh4. (a) Fine mapping of ZmMdh4 using the F2 populations derived from Zheng58 and mdh4‐1. The ZmMdh4 locus was mapped to a ~224 kb region on chromosome 1. The number of recombinants and the population size are shown on the left and right of each marker, respectively. (b) The gene structure of ZmMdh4. Allelic mutants are indicated by red letters. The black and white boxes indicate exons and introns, respectively. The bold black line indicates the promoter and the gray boxes indicate the untranslated regions (UTRs). (c) A schematic diagram of the ZmMDH4 protein, with conserved domains indicated. (d) Mdh4‐OE/Mdh4 × mdh4‐1 F2 ear. Mdh4‐OE denotes the Mdh4 overexpression line. The red arrow indicates a mdh4‐1 mutant kernel. Scale bar, 2 cm. (e‐f) Genotyping of randomly selected kernels. (e) Marker Exon7‐L/Exon7‐R for identifying homozygous ZmMdh4. (f) Marker CUB‐R/CUB‐F for identifying positive ZmMdh4‐overexpressing individuals. Lanes 1‐24 and 25‐35 show the genotyping results of the normal and mutant kernels, respectively. Lane 36 shows the ZZC01 control. “‐” and “+” represent the blank (H2O) and the CUB‐ZmMdh4 vector control, respectively. M represents the marker. (g) Differences in the phenotypic expression of Mdh4‐OE lines at ten‐day after germination (DAG). WT‐, non‐transgenic lines with ZmMdh4 gene; mdh4+, positive transgenic lines with ZmMdh4 gene; mdh4‐, negative transgenic lines with ZmMdh4 gene. The scale bar, 5 cm.
To validate whether ZmMdh4 is the causal genetic basis for the observed mdh4‐1 kernel phenotype, the ZmMdh4 coding sequence was overexpressed using the constitutive ubiquitin (Ubi) promoter. The resulting transgenic line (Mdh4‐OE) was crossed with a homozygous mdh4‐1 (−/−) mutant, and F1 was self‐pollinated to generate F2 seeds (Figure 3d). Twenty‐four normal (WT) and 11 mutant F2 kernels were selected randomly and genotyped with markers detecting the 3‐bp deletion in mdh4‐1 (−/−) and the Mdh4‐OE transgene. Four of the 24 normal kernels had the homozygous mdh4‐1 (−/−) genotype with Mdh4‐OE (Figure 3e‐f). Additionally, they showed phenotypic characteristics analogous to those of the normal kernels at the seedling stage (Figure 3g). These results indicate that Mdh4‐OE could rescue the mdh4‐1 mutant phenotype. Together, these data confirmed that ZmMdh4 was the causal gene of the mdh4‐1 mutant.
Knocking out ZmMdh4, but not ZmMdh5, phenocopies mdh4‐1 phenotypes
To further confirm ZmMdh4 as the causal gene of the mdh4‐1 mutant phenotype, Mu‐inserted ZmMdh4 mutants were screened from the Maize Genetics Stock Center. However, the only Mu‐mutant identified had the Mu insertion in the non‐coding region of ZmMdh4, which exhibited no obvious kernel phenotype. Thus, CRISPR/Cas9 was used to generate ZmMdh4 loss‐of‐function lines with a specific guide RNA (gRNA) using ZZC01. Sequencing of the CRISPR/cas9‐mediated ZmMdh4‐edited transgenic individuals resulted in the identification of two mutants, mdh4‐2 and mdh4‐3, that carry null mutations in the ZmMdh4 gene (Figure 4a). The T1 individuals of mdh4‐2 and mdh4‐3 were self‐pollinated for two consecutive generations to produce homozygous T3 progeny (Figure 4b). Allelism tests were performed by crossing the homozygous mdh4‐2 (−/−) and mdh4‐3 (−/−) T3 plants with both the homozygous mdh4‐1 (−/−) and heterozygous (+/−) individuals, respectively. The results show that the F2 kernels of the mdh4‐2 (−/−) × mdh4‐1 (−/−) cross all phenocopied homozygous mdh4‐1 (−/−) kernels (Figure 4c), and the mdh4‐2 (−/−) × (+/−) and F2 kernels exhibited a mutant:WT ratio of 1:1 (Figure 4d and Table S6). Similar results were obtained with mdh4‐3 (−/−) derived F2 kernels. These findings confirmed that the CRISPR/cas9‐mediated mutations were allelic to the naturally occurring mdh4‐1 mutation. In support of this conclusion, kernel size and 100‐kernel weight of the mdh4‐2 (−/−) and mdh4‐3 (−/−) T3 individuals were found to be comparable to those of the mdh4‐1 mutant (Figure S4a‐c). In addition, the germination rate, seedling and adult height, as well as root length of the two cas9 lines, were reduced by more than 50% as compared with the control, similar to that observed for mdh4‐1 (Figure S4d‐i). These results further supported ZmMdh4 as the target gene.
Figure 4.

Validation of the ZmMdh4 transgenic lines. (a) Alignment of the genomic and amino acid sequences of the positive Cas9‐edited transgenic plants and the control ZZC01. The bold letters in black denote the sgRNA target sequence. (b) Comparison of the mdh4‐2 and mdh4‐3 ears with ZZC01. Scale bars, 2 cm. (c) Allelism test using homozygous mdh4‐1, mdh4‐2, and mdh4‐3. The scale bars are 2 cm. (d) Allelism test using heterozygous mdh4‐1 and homozygous mdh4‐2 and mdh4‐3 mutants. The scale bars, 2 cm. The red arrows indicate mutant kernels. (e) Representative kernels from T2 Cas9‐Mdh4 (+/‐) Mdh5 (+/‐); scale bars, 1 cm, (f) Linkage analysis of Zmmdh5 with randomly selected normal kernels from the mdh4‐2 T2 generation. (g) Representative mdh4‐1 × mdh4‐2 and mdh4‐1 F2 ears; scale bars, 2 cm. (h) Linkage analysis of ZmMdh5 with randomly selected kernels from the mdh4‐1 × mdh4‐2 F2 ear.
Due to the high sequence similarity between ZmMdh5 and ZmMdh4 (McMillin and Scandalios, 1980, Gramene; http://ensembl.gramene.org/Zea_mays), both genes were knocked out by CRISPR/cas9 in mdh4‐2 and mdh4‐3. To test whether the cas9‐mediated mutations in ZmMdh5 had contributed to the mutant phenotype, Zmmdh5‐linked markers were assayed. They did not co‐segregate with kernel phenotype in the T2 generation (Figure 4e‐f), suggesting that the ZmMdh5 mutations were not causal of the mutant kernel phenotype in the two cas9 lines. Further, the F2 kernels derived by crossing mdh4‐1 (Mdh5 (+/+) mdh4 (−/−)) with mdh4‐2 (Mdh5 (+/−) mdh4 (−/−)) all exhibited the mutant phenotype (Figure 4g‐h). These results suggest that the ZmMdh5 cas9 mutations were unrelated to the mutant kernel phenotype, and ZmMdh4 was the target gene.
ZmMdh4 is constitutively expressed and its protein is localized to the cytoplasm
Using qPCR, ZmMdh4 expression was quantified in the root, stem, leaf, embryo, and endosperm of the WT and mdh4‐1 mutant. ZmMdh4 was found to be constitutively expressed in all tested tissues, with the highest expression level in the leaf (Figure 5a). During kernel filling, ZmMdh4 expression was mainly detected during the early stages of endosperm development and its transcript level was higher in the mutant than the WT (Figure 5b). Consistent with this result, Western blot analysis with the MDH4 antibody indicated a relatively higher ZmMDH4 protein level in mdh4‐1 kernels even during late endosperm development (Figure 5c).
Figure 5.
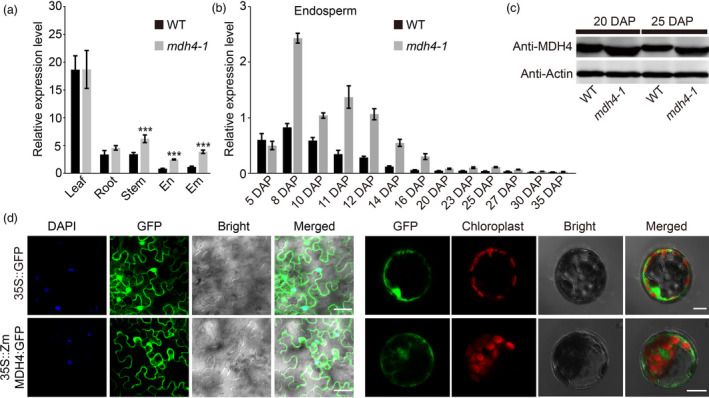
Expression pattern and subcellular localization of ZmMdh4. (a) Quantitative PCR analysis of ZmMdh4 relative transcript abundance in various tissues from WT vs. mdh4‐1 mutant plants. Values are represented as means ± SE of three biological replicates. *** P < 0.001, t‐test. (b) ZmMdh4 expression during endosperm development. (c) The abundance of ZmMDH4 protein in the WT vs. mdh4‐1 mutant. (d) Subcellular localization of ZmMDH4 in Nicotiana benthamiana leaves and Arabidopsis mesophyll protoplasts; scale bars are 100 μm.
In Gramene (http://ensembl.gramene.org/Zea_mays), ZmMDH4 is predicted to be localized in the cytoplasm. To verify this prediction, the full‐length CDS of ZmMdh4 was C‐terminally fused to the enhanced green fluorescent protein (eGFP) and transiently expressed under the control of the CaMV 35S promoter in Nicotiana benthamiana epidermal leaf cells and A. thaliana mesophyll protoplasts. In both approaches, the fusion protein was found localized in the cytoplasm (Figure 5d), thus confirming the localization of ZmMDH4 to the cytoplasm. On the contrary, free GFP signal was detected in the nuclei and the cytoplasm.
ZmMDH4 predominantly catalyzes the conversion from OAA to malate
The 3‐bp deletion in the mdh4‐1 mutant results in a Glu deletion at position 322 in the C‐terminus of the MDH4 protein, which is distant from the active site (located at 156–168aa; Uniprot database, http://www.Uniprot.org). However, this deletion may alter the overall conformational structure of the ZmMDH4 protein. Specifically, Arg97 and Lys98, the spatial neighbours of Glu322, reside in a loop (88‐103aa) associated with substrate binding (SWISS‐MODEL algorithm; Waterhouse et al., 2018). The +/− salt bridge between Arg97 and Glu322 draws the loop and helix9 together in the WT MDH4 enzyme. In the mutant enzyme, however, the +/− salt bridge is abolished by the change of Lys98, thereby potentially loosening the MDH4 structure by preventing the interaction between the loop and helix 9 (Figure 6a).
Figure 6.

Enzymatic characterization of ZmMDH4. (a) Predicted 3D structure of the WT and mutant ZmMDH4. (b) SDS‐PAGE analysis of the His‐tagged WT and mdh4‐1 mutant proteins. M, protein standard with molecular weights listed on the left. (c) Determination of WT and mutant ZmMDH4 oxidoreductase activity by detecting the change in NADH levels at 340 nm, with four replicates. A total of 5 μg protein was used for initial loading.
To examine the effect of Glu322 deletion on MDH4 function, purified WT and mutant MDH4 protein heterologously expressed in bacteria were used in enzymatic assays to monitor changes in NADH levels (Figure 6b‐c). Very little change in NADH levels was observed during the malate‐to‐OAA conversion for both enzymes but a substantial decrease in NADH with the WT MDH4 enzyme during the OAA‐to‐malate conversion, suggesting that MDH4 mainly catalyses the reaction from OAA to malate (Figure 6c). Consistent with our hypothesis, the mutant enzyme almost completely lost its activity for catalysing the OAA‐to‐malate conversion, as no obvious change in NADH was observed (Figure 6c). Thus, 3‐bp deletion in exon 7 significantly impacts MDH4 activity presumably by causing a conformational change in MDH4 tertiary structure.
Disruption of ZmMdh4 leads to changes in cellular energetics and impairment of mitochondrial complex I and II function
Because the conversion of OAA to malate is a key step in the TCA cycle, the amount of ATP and metabolites associated with energy metabolism in 12 DAP mdh4‐1 and WT kernels were quantified. Specifically, this was undertaken to investigate if the lack in ZmMDH4 activity affected energy production. The levels of lactate, aconitate, 3‐phosphoglycerate (3PG), phosphoenolpyruvate (PEP) and cyclic‐AMP increased significantly by >1.5‐fold in mdh4‐1 compared with the WT, whereas those of NAD+, NADH, pyruvate and alpha‐ketoglutaric acid (α‐KG acid) were reduced by >1.5‐fold (Figure 7a‐b). These data suggest that both glycolysis and the TCA cycle were altered. By contrast, the ratio of NAD+/NADH, which reflects the redox state, increased by 2.7‐fold in the mdh4‐1 mutant as compared to the WT (Figure 7c). Taken together, these results suggest that the TCA cycle is impaired in the mdh4‐1 mutant. This is further supported by the observation that ATP content decreased ~40% in the 12 DAP mutant kernels compared with the WT (Figure 7d). Consistently, we observed reduced NADH dehydrogenase activity and the disassociation of the mitochondrial ATP‐producing I + III super‐complex in 15 DAP mutant kernels compared with the WT, as fewer I + III super‐complexes were observed in the mutant than WT (Figure 7e). Further supporting evidence came from the substantial decreases in NAD7 and SDH1 proteins, as well as an increase in CYC1 protein in 15 DAP mutant kernels (Figure 7f). These results are consistent with the vacuolization of the mutant mitochondria in 15 DAP kernels (Figure 7g), suggesting that the disruption of ZmMDH4 led to mitochondria dysfunction and reduced ATP production.
Figure 7.

Targeted metabolomic analysis of WT and mdh4‐1 kernels. (a) Fold change of energy metabolite levels in mdh4‐1 kernels over WT. (b) Relative content of lactate of mdh4‐1 as compared to WT. (c) Fold change of the NAD+/NADH ratio in mdh4‐1 as compared to WT. (d) Relative ATP content in the developing endosperm of WT and mdh4‐1 12 and 15 DAP, with three replicates. (e) BN‐PAGE assay of mitochondrial complex assembly and in‐gel NADH enzyme activity from immature kernels of the mdh4‐1 mutant and WT at 15 DAP. M, protein standard. (f) Western blot with NAD7, SDH1, and CYC1 antibodies against protein isolated from WT and mdh4‐1 kernels. (g) The ultrastructure of mitochondrion (M) in mdh4‐1 and WT kernel cells; scale bar, 500 nm. (h) Fold differences in overall amino acid content of mdh4‐1 as compared to WT kernels. Error bars indicate the SE of three biological replicates. *P < 0.05, **P < 0.01, ***P < 0.001 (Student's t‐test).
The loss of ZmMDH4 activity in mutant kernels also affected the levels of amino acids derived from the intermediates of the TCA cycle (Figure 7h). For example, we detected the accumulation of amino acids derived from the Asp‐derived pathway and those from the Glu‐derived pathway that competes with Pro, an important component of the zein protein, for the Glu substrate (Figure 7h). These results indicate that the mutant endosperm had developmental defects and alterations in central metabolism.
Coupling metabolic changes to transcriptomic alterations upon ZmMDH4 depletion
To further explore the molecular basis of the metabolic changes in the mdh4‐1 mutant, 12 DAP mutant and WT kernels were subjected to RNA‐seq analysis. A total of 23 594 transcripts were detected and retrieved, among which 413 were differentially expressed [log2 (fold change) > 0.78 or <−1 and false discovery rate (FDR) < 0.01] between the WT and the mdh4‐1 mutant. Of the differentially expressed genes (DEGs), 291 were up‐regulated and 122 were down‐regulated in the mdh4‐1 mutant as compared to WT (Figure 8a). Of those, 309 DEGs could be functionally annotated by BLAST searches against the UniProt database (http://www.uniprot.org) and were analysed by an online Gene Ontology (GO) software (agriGO, Tian et al., 2017). The significantly enriched terms included carbohydrate metabolic process (GO: 0005975), oxidation‐reduction process (GO: 0055114) and nutrient reservoir activity (GO: 0045735) (Figure 8b; Table S7).
Figure 8.

Transcriptomic analysis of WT and mdh4‐1 kernels. (a) The numbers of differentially expressed genes in mdh4‐1 compared with WT. (b) The GO terms of functionally annotated DEGs. (c) The selected genes were verified by qPCR with three replicates. Values are represented as means ± SE, ***P < 0.001 (Student's t‐test). (d) Heat map showing differentially expressed genes implicated in Zein and starch biosynthesis.
Several genes were randomly selected and tested with quantitative real‐time PCR (qPCR) to validate the RNA‐seq results. Consistent with the RNA‐seq data, the transcript levels of Zm00001d018971 (O2), Zm00001d044129 (Sh2), Zm00001d048813 (zein‐alfa), Zm00001d050032 (Bt2) and Zm00001d052079 (LKR/SDH) were lower, and those of Zm00001d033805, Zm00001d039346, Zm00001d041671 (BETL3) and Zm00001d047253 (SuSy) were higher in mdh4‐1 than in WT (Figure 8c; Table S7). DEGs under carbohydrate metabolic processes (GO: 0005975) and starch biosynthesis and metabolic processes (GO: 0019252 and GO: 0005982) were enriched (Table S7). Key genes involved in starch and amylose biosynthesis, including Zm00001d044129 (Sh2), Zm00001d050032 (Bt2), Zm00001d033937 (GBSSI) and Zm00001d045462 (wx1), were significantly down‐regulated in the mdh4‐1 mutant. DEGs under nutrient reservoir activity (GO: 0045735) were found to be mainly involved in zein protein biosynthesis (21/24). These DEGs were also significantly down‐regulated the mdh4‐1 mutant (Figure 8d; Table S7). The changed expression levels of genes related to starch and protein biosynthesis suggest a role of ZmMdh4 in storage reserve accumulation.
Discussion
ZmMdh4 is indispensable for kernel development
Gooman and associates have reported that the kernels of homozygous Zmmdh4 and Zmmdh5 mutants were viable and could germinate and develop to normal mature plants (Goodman et al., 1981). In this study, both the natural mdh4‐1 mutant and the cas9 mdh4‐2 and mdh4‐3 lines could develop into relatively normal plants but exhibited poor seed germination, retarded vegetative and reproductive growth compared with controls (Figures 1, 4a‐b; S4). It is worth noting that ZmMhd5, the only paralog of ZmMhd4, is unlinked to the mutant kernel phenotype (Figure 4e‐h). Our findings point to an indispensable role for ZmMdh4 in kernel and plant development. Previous studies have reported a positive correlation between the transcript level and enzymatic activity of cytosolic MDHs in apple and cotton (Imran et al., 2017; Yao et al., 2011b). Taken together with the lack of obvious morphological and developmental defects in the mdh4‐1 and mdh4‐cas9 lines, these results indicate the existence of other genetic factors that are functionally redundant to ZmMdh4.
ZmMDH4 is indispensable for ATP production
In non‐photosynthetic organs, such as the endosperm, glycolysis in cytoplasm and the TCA cycle in mitochondria are major sources for ATP. The cytosolic MDHs, which catalyse the reversible conversion between malate and OAA, contribute to the partitioning of metabolic flux between glycolysis and the TCA cycle (Selinski and Scheibe, 2019), thereby regulate ATP production. Consistent with this function, the loss of ZmMDH4 activity caused substantial changes in the levels of glycolysis and TCA cycle‐related metabolites in mdh4‐1 (Figure 7a‐b and h). For example, an increase in lactate level was observed, as well as a reduction in ATP content in mdh4‐1 compared with the WT (Figure 7b, d), implying enhanced glycolysis and a role of a malate/OAA shuttle in regulating ATP production (Scheibe, 2004). As a result, the mitochondria of mdh4‐1 were vacuolated, NADH oxidase activity was reduced, and the mitochondrial I + III super‐complex was disassociated (Figure 7c‐g). These data are in line with a previous report that the rice flo16 mutant, which was determined to be caused by a mutated cytosolic MDH, had reduced ATP production (Teng et al., 2019). These data suggest that cytosolic ZmMDH maintains mitochondrial complex activity, ATP production and the homeostasis of glycolysis and the TCA cycle.
ZmMDH4 affects starch and zein synthesis in the endosperm
In the cytoplasm, pyruvate phosphate dikinase (PPDK), which reversibly converts PEP to pyruvate, also involved in the malate metabolic pathway. Knockout of maize endosperm PPDK (cytosolic pdk2) results in opaque kernel characteristics, elevated glycolysis metabolites, reduced ATP content, but unaffected starch and zein contents (Lappe et al., 2018). However, mutations in ZmMdh4 cause an elevation in glycolysis metabolites and a reduction in ATP content, with a concomitant decrease in starch and zein contents (Figures 1d‐e, 7a‐d). These results suggest that different regulators might be involved in cytosolic pdk2 and ZmMdh4 expression to balance glycolysis and the TCA cycle, as indicated that PPDK is the direct target of O2 and MDH is the target of thioredoxin‐h1 (Hara et al., 2006; Lappe et al., 2018; Manicacci et al., 2009). Loss‐function of the cytosolic Mdh leads to increased oxidation, which subsequently results in a reductive ADP‐glucose pyrophosphorylase (AGP, the committed enzyme of starch biosynthesis) activation state, thereby reducing starch content in tomato plastids (Centeno et al., 2011) and in rice endosperm (Teng et al., 2019). These findings are consistent with increased NAD+/NADH levels, as well as the reduced expression levels of Bt2 and Sh2, which encode the large and small subunits of AGP observed in the mdh4‐1 (Figures 7c and 8c). Thus, it can be inferred that restricted AGP activity causes reduced starch accumulation in mdh4‐1 mutants. Another reason could be related to compromised ATP production, which would directly impair the differentiation of BETL cells, or the activity of endosperm cells (Figure 2b‐c). This would inhibit the biosynthesis and deposition of storage compounds, resulting in small kernels.
Zeins are the most abundant seed storage proteins (>60%; Wu and Messing, 2014) that determine the nutritional quality of maize grain (Frizzi et al., 2010; Hunter et al., 2002). Published data have demonstrated that mutants with reduced zein contents, especially α‐zein, such as the o2, o7, ocd1 and fl2 mutants, accumulate lysine, an essential amino acid whose levels in maize grains is not well balanced for human and animal consumption, to compensate (Coleman et al., 1997; Kemper et al., 1999; Miclaus et al., 2011; Wang et al., 2011; Yang et al., 2018). Analogous to that observed with these mutants, the biosynthesis of zein (especially 19‐ and 22‐kD α‐zein) was restricted in the mdh4‐1 mutant and the lysine content was increased in the mdh4‐1 mutant; this was further supported by the down‐regulation of all DEGs involved in zein biosynthesis and lysine degradation, and enhanced levels of aspartate for lysine and glutamine biosynthesis, which competes with glutamate for zein biosynthesis in the mdh4‐1 (Figures 1e‐f, 8c‐d). Collectively, these results demonstrate that ZmMdh4 influences TCA‐derived substrate supply for protein biosynthesis, though the gap between ZmMDH4 activity and the final seed phenotype needs to be comprehensively investigated.
In summary, we cloned the ZmMdh4 gene that encodes cytosolic malate dehydrogenase in maize. The 3‐bp deletion in exon 7 eliminates ZmMDH4 enzymatic activity, resulting in reduced ATP supply and an elevated oxidation level, perturbing AGP activity and starch production in the endosperm. Concomitantly, impaired TCA cycle alters the substrate availability for amino acid biosynthesis, which influences the proportions of zein and non‐zein protein by modulating the expression levels of related genes in the endosperm as depicted in our proposed model (Figure 9).
Figure 9.

A proposed regulatory model of ZmMdh4 in maize endosperm development. SuS1, sucrose synthase1; Bt2, brittle2; Sh2, shrunken‐2; GBSSI, granule‐bound starch synthase I; Mdh4, malate dehydrogenase 4; NADP‐ME, NADP malic enzyme; LDH1, lactate dehydrogenase1; LKR/SDH, lysine‐ketoglutarate reductase/saccharopine dehydrogenase; Gln2, glutamine synthetase 2; Gld1, glutamate decarboxylase 1; PPDK2, pyruvate phosphate dikinase; G6P, glucose‐6‐phosphate; G1P, glucose‐1‐phosphate; 3PG, 3‐phosphoglycerate; OAA, oxaloacetate; PEP, phosphoenolpyruvate; AGP, ADP‐glucose pyrophosphorylase.
Methods
Plant materials
mdh4‐1 is a naturally occurring mutant isolated during maize breeding and was crossed with Zheng58, PH6WC, HCL645, Qi319, Lx9801 and D1798Z to generate F1 hybrids. The resulting F1 plants were self‐pollinated to construct six F2 mapping populations. The mdh4 mutant, Zheng58, PH6WC, HCL645, Qi319, Lx9801, D1798Z and the F2 populations were grown at the research farm of Henan Agricultural University, Zhengzhou, China (113°42′ E, 34°48′ N). The near‐isogenic lines (NILs) of mdh4‐1 were produced by self‐crossing heterozygous F2 individuals, derived from the by crossing Zheng58 with mdh4‐1, for eight generations under background and foreground marker‐assisted selection. The ears and kernels of the F2 plants and NILs were collected from no less than three individuals at 5, 8, 10, 11, 12, 14, 16, 20, 23, 25, 27, 30 and 35 DAP.
Cytological section preparation
To prepare the paraffin sections of kernels, immature seeds were fixed overnight at 4 °C in a formalin‐acetic acid‐alcohol (FAA) solution containing 50% ethanol, 5% acetic acid and 3.7% formaldehyde. The fixed materials were then dehydrated in an ethanol gradient series (50, 70, 85, 95 and 100% ethanol). Afterwards, the samples were treated with xylene, embedded in paraffin wax via infiltration and cut into 6–10 µm‐thick sections under Leica RM2235 (Germany). The sections were stained with toluidine blue (Sinopharm Chemical Reagent Co., Ltd) and examined under the Lecia M165FC stereomicroscope (Germany). The endosperm structures of mature and immature seeds of different developmental stages were observed by scanning electron microscopy (SEM) and transmission electron microscopy (TEM), respectively (Wu and Messing, 2010; Zhang et al., 2016).
Map‐based cloning of ZmMdh4
A total of 34 080 F2 individuals were used for map‐based cloning. The genotypes of key F2 recombinants were verified by examining the corresponding F3 seeds from each F2 ear. Table S3 lists the primers used. Additional polymorphic markers were developed by comparing genomic sequence near the ZmMdh4 locus between mdh4‐1 and Zheng58.
Vector construction and gene transformation
To construct the CRISPR/Cas9 vector, the recombinant PBUE411 vector was designed to produce mutations within the coding region of ZmMdh4, using a small guide RNA (sgRNA) alongside the Cas9 endonuclease gene. To obtain ZmMdh4 overexpression lines (Mdh4‐OE), the CDS driven by a Ubi promoter was cloned into a CUB skeleton vector. The CRISPR/Cas9 and overexpression constructs were introduced into agrobacterium strain EHA105 and used to transform the immature embryos of maize inbred line ZZC01, by co‐cultivation at the Life Science and Technology Center of China National Seed Group Co. Ltd (Wuhan, China). Positive transgenic plants were confirmed by amplifying the Bar gene and the knock‐out regions by PCR using ZmMdh4‐specific primers CUB‐F/CUB‐R, which span ZmMdh4 and the CUB vector. A co‐dominant functional marker for the 3‐bp InDel in exon 7, Exon7‐L/Exon7‐R, was developed to identify the homozygous mdh4‐1 genotype. All primers used for vector construction and gene transformation are listed in Table S3.
Starch, protein and total amino acid determination
A minimum of 20 endosperms from mature kernels of the WT and mdh4‐1 mutant were pulverized into fine powder using a pulverizer. For each sample, 50 mg flour was used to measure starch content using the Megazyme kit (K‐TSTA; Megazyme). SDS‐PAGE was used to analyse the accumulation patterns of zein and non‐zein proteins in both the WT and mdh4‐1 mutant following previously published procedures (Liu et al., 2016; Zhang et al., 2016). Total amino acids (free amino acids and protein‐bound amino acids) in the mature kernels were analysed according to the method of Wang et al. (2011) with three replicates.
RNA extraction and quantitative real‐time PCR (qPCR)
Total RNA was extracted from immature endosperms, embryos and other tissues using TransZol Plant (Transgen). Five hundred nanogram of total RNA was used for first‐strand cDNA synthesis using HiScript® QRT SuperMix for qPCR (+gDNA wiper) (Vazyme). All qPCR analyses were carried out in a Bio‐Rad iQ5 system (Bio‐Rad iQ5 Real Time PCR, ABI 7500) using the SYBR Green I kit (Vazyme). The 2−ΔΔCT method was used to calculate the relative transcript level of the target gene with ZmActin (Zm00001d010159) as the endogenous control. The PCR program was conducted as follows: (1) 5 min at 94 °C; (2) 40 cycles of 10 s at 95 °C, 30 s at 58 °C. The 20‐μL reaction volumes contained 2 μL cDNA, 0.4 μL L/R primers (10 μm), 10 μL 2 × qPCR SYBR Green I mix and 7.2 μL double‐distilled water. Statistically significant differences in gene expression levels were analysed by Student's t‐test. All primers used for qPCR are listed in Table S3.
Subcellular localization of ZmMDH4
The CDS of ZmMdh4 was fused with the enhanced GFP (eGFP) reporter gene and cloned into the pCAMBIA1300 vector (35S::ZmMDH4:GFP). pCAMBIA1300‐35S‐GFP vector not containing the ZmMdh4 gene was used as the free GFP control (35S::GFP). The 35S::GFP and 35S::ZmMDH4:GFP constructs were transiently expressed in Nicotiana benthamiana (N. benthamiana) leaves and Arabidopsis mesophyll protoplasts as described by Li et al. (2017) and Yang et al. (2018). GFP signal was observed and imaged using a confocal microscope (FV1000, Olympus). All primers used for the subcellular localization analysis are listed in Table S3.
Protein extraction and Western blot
Endosperm proteins were isolated using the method described by Wu and Messing (2012), separated on a 15% SDS PAGE by electrophoresis, and transferred to a polyvinylidene difluoride (PVDF) membrane (Bio‐Rad). The membrane was then incubated with commercial MDH4 (AS153065, Agrisera) and ACTIN (Abclonal, China) antibodies and visualized using the Tanon‐5200 system (Tanon Science & Technology Co., Ltd.). The MDH4 and ACTIN antibodies were 1:2000 and 1:5000 diluted, respectively (Yang et al., 2018). The NAD7 (PHY1077S, Phytoab), SDH1 (PHY0558S, Phytoab) and CYC1 (PHY0566S, Phytoab) antibodies were 1:3,000 diluted, while the α‐tubulin antibody (AS10680, Agrisera) was 1:10 000 diluted.
Enzymatic assay of ZmMDH4
The open reading frames (ORFs) of ZmMdh4 and Zmmdh4 were amplified by PCR with gene‐specific primers (Table S3) and separately cloned into the pET‐28a vector using a One Step Cloning Kit (Vazyme). The resulting ZmMdh4 and Zmmdh4 constructs were each transformed into the Escherichia coli (E. coli) strain BL21 (DE3). 2 mL of the transformed cells was used to inoculate 200 mL LB media in a 500 mL conical flask and cultured at 37 °C until OD600 reached 0.4–0.6, when 0.5 mm isopropyl‐β‐d‐thiogalactoside (IPTG) was added to induce protein expression. After culturing at 16 °C overnight, the cells were collected by centrifugation and then resuspended in 10 mL lysis buffer [50 mm Tris‐HCl pH 8.0, 50 mm NaCl, 5% glycerol, and 5 mm imidazole, 0.1 mm phenylmethanesulfonyl fluoride (PMSF) and 0.0037% β‐mercaptoethanol]. Next, the cells were disrupted by sonicating for 30 min and centrifuged at 5752 g for 50 min. The supernatant was added to the Ni‐NTA resin, which was pre‐equilibrated with 10 mL Lysis buffer, and incubated with 6 mL lysis buffer to rinse the resin. Finally, the proteins were eluted by a series of elution buffers (Lysis buffer + imidazole) containing 10 mm, 50 mm, 100 mm and 200 mm imidazole. The eluted protein fractions were pooled and dialysed with a 10 kDa ultrafiltration device to remove imidazole and salt, evaluated for purity via SDS‐PAGE electrophoresis, and purified by dialysis buffer (50 mm Tris‐HCl pH 8.0, 20 mm NaCl, 5% glycerol and 0.037% β‐mercaptoethanol). The purified proteins were quantified using a calibration curve based on the absorbance at 280 nm (A280) with BSA as a standard.
The 1 mL oxidative/reductive catalytic reaction included 250 mm HEPES buffer (PH 8.0), 2 mm MgCl2, 0.25 mm NAD+/NADH, 2.5 mm OAA or malate (Solarbio, Beijing, China), and 5 μg (1 μg/μL) of each protein sample. The change in NADH concentration (A340) of each reaction was monitored for 6 min by a spectrophotometer (DU®730, Nucleic Acid/Protein Analyzer, BeckMan) at 1 min intervals.
Determination of amino acids and energy metabolites
12‐DAP kernels of the WT and mdh4‐1 mutant were collected with three biological replications, snap frozen in liquid nitrogen, and stored at −80 °C until use. More than 3 g of kernels were ground and 55 mg fine powder from each sample was mixed with 1 mL of pre‐cooled methanol/acetonitrile/H2O solution and vortexed for 30 s. The mixture was then sonicated for 30 min on ice and left at −20 °C for 1 h to allow protein precipitation. Afterwards, the mixture was centrifuged for 15 min at 14 000 g at 4 °C, and the proteins were vacuum dried using a lyophilizer (FD‐1D‐80, BILON, Shanghai). The dried protein extracts were then dissolved in 100 μL 1:1 (v:v) mixture of acetonitrile:H2O and centrifuged at 14 000 g at 4 °C for 15 min. Targeted metabolic analysis was performed using the LC‐MS/MS system at Shanghai Applied Protein Technology Co. Ltd. Electrospray ionization was conducted with an Agilent 1290 Infinity chromatography system and AB SCIEX QTRAP 5500 mass spectrometer.
ATP content was determined using an ATP Assay Kit (Beyotime) following the manufacturer's instructions; three biological replicates were analysed for each sample. Briefly, 100 mg fresh endosperm of WT and mdh4‐1 was homogenized in 1 mL pre‐cooled lysis buffer and centrifuged at 12 000 g for 5 min at 4 °C. The supernatant was used to determine ATP content. ATP standard solutions of various concentrations (0, 0.025, 0.05, 0.1, 0.2, 0.5, 1 µm) were prepared to generate an ATP calibration curve. The supernatant and ATP standards were separately mixed with the ATP detection solution (working concentration) provided in the Kit in a 1:9 ratio. Luminescence was detected by a Tecan Spark 20M microplate reader (Shanghai), and the ATP content of each sample was calculated based on the calibration curve.
BN‐PAGE and the determination of mitochondrial complex activity
The mitochondria were separated from 15 DAP kernels using the Plant mitochondria DNA Extraction Kit (Beijing biolab technology co. LTD) with minor modifications. Briefly, about 400mg kernel was ground in liquid nitrogen and 1.6 mL plant cell lysis buffer (0.5% β‐mercaptoethanol) was added to each sample. The samples were mixed and centrifuged at 1000 g for 5 min at 4 °C. Then, the supernatant was transferred to a new tube and centrifuged at 16 000 g for 10 min at 4 °C. Crude mitochondria were resuspended in cleanout fluid and centrifuged at 1000 g for 5 min at 4 °C. The resulting supernatant was centrifuged at 16 000 g for 10 min at 4 °C to collect highly purified mitochondria and the pellet was resuspended in 100 µL B25G20 solution. BN‐PAGE and in‐gel activity assay of mitochondrial complexes were performed as described by Chen et al. (2017).
RNA‐seq analysis
Total RNA was extracted from 12 DAP endosperms of the mdh4‐1 mutant and WT with RNAprep Pure Plant Kit (Tiangen). The VAHTSTM Stranded mRNA‐seq Library Prep Kit for Illumina® (Vazyme) was used to construct the RNA‐seq libraries. Clean reads were obtained using the Illumina HiSeq X Ten platform (JiakangBio, Wuhan, China) and mapped to the B73 reference genome (RefGen_V4) using Bowtie2. Gene expression level was converted to Fragments per Kilobase Million (FPKM) for each transcript model. Differentially expressed genes (DEGs) were selected by the following criteria: log2(fold change) > 0.78 or <1, false‐discovery rate (FDR) <0.05, as calculated by the DEseq2 software, and P‐value < 0.05. GO enrichment analysis of the DEGs was performed using an online version of agriGO (Tian et al., 2017).
Conflict of interest
This study did not involve human participants and/or animals. All authors declare no financial or commercial conflicts of interest.
Author contributions
Z.F. and J.T. designed and supervised this study. Y.C., H.Z., R.T., H.Y., C.S., L.W. and W.Z. performed the experiments. Z.F., Y.C., Z.G. and X.Z performed the data analysis. Z.F., Y.C. and J.T. prepared the manuscript with inputs from other authors.
Supporting information
Figure S1 Dynamic development of the kernels on F2 ears. The red arrows indicate mutant kernels.
Figure S2 Phenotype of mdh4‐1 kernels and kernel segregation in other genetic backgrounds.
Figure S3 Multisequence alignment showing the distribution of the 3‐bp Indel in teosinte and 55 diverse maize inbred lines.
Figure S4 Phenotypic characteristics of transgenic lines.
Table S1 Field evaluation of normal and mdh4‐1 kernel segregation in different F2 populations.
Table S2 Amino acid level determination in mature WT and mdh4‐1 kernels.
Table S3 Primers used in this study.
Table S4 ZmMdh4 polymorphisms identified in the association panel.
Table S5 Association analysis of ZmMdh4.
Table S6 Allelism test using heterozygous mdh4‐1 and homozygous mdh4‐2 and mdh4‐3 T3 plants.
Table S7 Gene ontology classifications of DEGs with functional annotation.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (grant no. 2016YFD0101003), the National Natural Science Foundation of China (grant no. 91735306 and 91335205), the Central Plains Scholars Project of Henan Province (grant no. 172101510004) and Henan Natural Science Foundation (grant no. 13A210469). We also thank the TEM Center of Henan University of Chinese Medicine for their technical assistance.
Chen, Y. , Fu, Z. , Zhang, H. , Tian, R. , Yang, H. , Sun, C. , Wang, L. , Zhang, W. , Guo, Z. , Zhang, X. and Tang, J. (2020) Cytosolic malate dehydrogenase 4 modulates cellular energetics and storage reserve accumulation in maize endosperm. Plant Biotechnol. J., 10.1111/pbi.13416
Contributor Information
Zhiyuan Fu, Email: fuzhiyuan2004@163.com.
Jihua Tang, Email: tangjihua1@163.com.
References
- Beeler, S. , Liu, H.C. , Stadler, M. , Schreier, T. , Eicke, S. , Lue, W.L. , Truernit, E. et al. (2014) Plastidial NAD‐dependent malate dehydrogenase is critical for embryo development and heterotrophic metabolism in Arabidopsis . Plant Physiol. 164, 1175–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centeno, D.C. , Osorio, S. , Nunes‐Nesi, A. , Bertolo, A.L. , Carneiro, R.T. , Araújo, W.L. , Steinhauser, M.C. et al.(2011) Malate plays a crucial role in starch metabolism, ripening, and soluble solid content of tomato fruit and affects postharvest softening. Plant Cell, 23, 162–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. , Feng, F. , Qi, W. , Xu, L. , Yao, D. , Wang, Q. and Song, R. (2017) Dek35 encodes a PPR protein that affects cis‐splicing of mitochondrial nad4 intron 1 and seed development in maize. Mol. Plant, 10, 427–441. [DOI] [PubMed] [Google Scholar]
- Coleman, C.E. , Clore, A.M. , Ranch, J.P. , Higgins, R. , Lopes, M.A. and Larkins, B.A. (1997) Expression of a mutant alpha‐zein creates the floury2 phenotype in transgenic maize. Proc. Natl. Acad. Sci. USA, 94, 7094–7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, F. , Qi, W. , Lv, Y. , Yan, S. , Xu, L. , Yang, W. , Yuan, Y. et al. (2018) OPAQUE11 is a central hub of the regulatory network for maize endosperm development and nutrient metabolism. Plant Cell, 30, 375–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzi, A. , Caldo, R.A. , Morrell, J.A. , Meng, W. , Lutfiyya, L.L. , Brown, W.E. , Malvar, T.M. et al. (2010) Compositional and transcriptional analyses of reduced zein kernels derived from the opaque2 mutation and RNAi suppression. Plant Mol. Biol. 73, 569–585. [DOI] [PubMed] [Google Scholar]
- Goodman, M.M. , Newton, K.J. and Stuber, C.W. (1981) Malate dehydrogenase viability of cytosolic nulls and lethality of mitochondrial nulls in maize. Proc. Natl. Acad. Sci. USA, 78, 1783–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara, S. , Motohashi, K. , Arisaka, F. , Romano, P.G.N. , Hosoya‐Matsuda, N. , Kikuchi, N. , Fusada, N. et al. (2006) Thioredoxin‐h1 reduces and reactivates the oxidized cytosolic malate dehydrogenase dimer in higher plants. J. Biol. Chem. 281, 32065–32071. [DOI] [PubMed] [Google Scholar]
- Hebbelmann, I. , Selinski, J. , Wehmeyer, C. , Goss, T. , Voss, I. , Mulo, P. , Kangasjärvi, S. et al. (2012) Multiple strategies to prevent oxidative stress in Arabidopsis plants lacking the malate valve enzyme NADP‐malate dehydrogenase. J. Exp. Bot. 63, 1445–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyno, E. , Innocenti, G. , Lemaire, S.D. , Issakidis‐Bourguet, E. and Krieger‐Liszkay, A. (2014) Putative role of the malate valve enzyme NADP‐malate dehydrogenase in H2O2 signalling in Arabidopsis . Philos. Trans. R Soc. Lond. B Biol. Sci. 369, 20130228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holding, D. , Otegui, M. , Li, B. , Meeley, R. , Dam, T. , Hunter, B.G. , Jung, R. et al. (2007) The maize floury1 gene encodes a novel endoplasmic reticulum protein involved in zein protein body formation. Plant Cell, 19, 2569–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holding, D.R. , Meeley, R.B. , Hazebroek, J. , Selinger, D. , Gruis, F. , Jung, R. and Larkins, B.A. (2010) Identification and characterization of the maize arogenate dehydrogenase gene family. J. Exp. Bot. 61, 3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, B.G. , Beatty, M.K. , Singletary, G.W. , Hamaker, B.R. , Dilkes, B.P. , Larkins, B.A. and Jung, R. (2002) Maize opaque endosperm mutations create extensive changes in patterns of gene expression. Plant Cell, 14, 2591–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imran, M. , Zhang, B. , Tang, K. and Liu, J. (2017) Molecular characterization of a cytosolic malate dehydrogenase gene (GhcMDH1) from cotton. Chem. Res. Chin. Univ. 33, 87–93. [Google Scholar]
- Kandoi, D. , Mohanty, S. and Tripathy, B.C. (2018) Overexpression of plastidic maize NADP‐malate dehydrogenase (ZmNADP‐MDH) in Arabidopsis thaliana confers tolerance to salt stress. Protoplasma, 255, 547–563. [DOI] [PubMed] [Google Scholar]
- Kemper, E.L. , Neto, G.C. , Papes, F. , Moraes, K.C. , Leite, A. and Arruda, P. (1999) The role of opaque2 in the control of lysine‐degrading activities in developing maize endosperm. Plant Cell, 11, 1981–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, C.S. , Hunter, B.G. , Jeffery, K. , Boston, R.S. , Sarah, Y. , Rudolf, J. and Larkins, B.A. (2004) A defective signal peptide in a 19‐kD alpha‐zein protein causes the unfolded protein response and an opaque endosperm phenotype in the maize De*‐B30 mutant. Plant Physiol. 134, 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, C. , Gibbon, B. , Gillikin, J. , Larkins, B. , Boston, R. and Jung, R. (2006) The maize Mucronate mutation is a deletion in the 16‐kDa gamma‐zein gene that induces the unfolded protein response. Plant J. 48, 440–451. [DOI] [PubMed] [Google Scholar]
- Lappe, R.R. , Baier, J.W. , Boehlein, S.K. , Huffman, R. , Lin, Q. , Wattebled, F. , Settles, A.M. et al. (2018) Functions of maize genes encoding pyruvate phosphate dikinase in developing endosperm. Proc. Natl. Acad. Sci. USA, 115, E24–E33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins, B.A. and Hurkman, W.J. (1978) Synthesis and deposition of zein in protein bodies of maize endosperm. Plant Physiol. 62, 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lending, C.R. and Larkins, B.A. (1989) Changes in the zein composition of protein bodies during maize endosperm development. Plant Cell, 1, 1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. , Wang, J. , Ye, J. , Zheng, X. , Xiang, X. , Li, C. , Fu, M. et al. (2017) The maize imprinted gene floury3 encodes a PLATZ protein required for tRNA and 5S rRNA transcription through interaction with RNA polymerase. Plant Cell, 29, 2661–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. , Shi, J. , Sun, C. , Gong, H. , Fan, X. , Qiu, F. , Huang, X. et al. (2016) Gene duplication confers enhanced expression of 27‐kDa γ‐zein for endosperm modification in quality protein maize. Proc. Natl. Acad. Sci. USA, 113, 4964–4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manicacci, D. , Camuskulandaivelu, L. , Fourmann, M. , Arar, C. , Barrault, S. , Rousselet, A. , Feminias, N. et al. (2009) Epistatic interactions between Opaque2 transcriptional activator and its target gene cyPpdk1 control kernel trait variation in maize. Plant Physiol. 150, 506–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillin, D.E. and Scandalios, J.G. (1980) Duplicated cytosolic malate dehydrogenase genes in Zea mays . Proc. Natl. Acad. Sci. USA, 77, 4866–4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miclaus, M. , Wu, Y. , Xu, J.H. , Dooner, H.K. and Messing, J. (2011) The maize high‐lysine mutant opaque7 is defective in an acyl‐CoA synthetase‐like protein. Genetics, 189, 1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, A.M. , James, M.G. , Qiaohui, L. , Gibum, Y. , Stinard, P.S. , Hennen‐Bierwagen, T.A. and Becraft, P.W. (2011) Maize opaque5 encodes monogalactosyldiacylglycerol synthase and specifically affects galactolipids necessary for amyloplast and chloroplast function. Plant Cell, 23, 2331–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton, K.J. and Schwartz, D. (1980) Genetic basis of the major malate dehydrogenase isozymes in maize. Genetics, 95, 425–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pracharoenwattana, I. , Cornah, J.E. and Smith, S.M. (2007) Arabidopsis peroxisomal malate dehydrogenase functions in beta‐oxidation but not in the glyoxylate cycle. Plant J. 50, 381–390. [DOI] [PubMed] [Google Scholar]
- Pracharoenwattana, I. , Zhou, W. and Smith, S.M. (2010) Fatty acid beta‐oxidation in germinating Arabidopsis seeds is supported by peroxisomal hydroxypyruvate reductase when malate dehydrogenase is absent. Plant Mol. Biol. 72, 101–109. [DOI] [PubMed] [Google Scholar]
- Ranum, P. , Peñarosas, J.P. , Garciacasal, M.N. and Pachón, H. (2014) Global maize production, utilization, and consumption. Ann. N. Acad. Sci. 1312, 105–112. [DOI] [PubMed] [Google Scholar]
- Scheibe, R. (2004) Malate valves to balance cellular energy supply. Physiol. Plant. 120, 21–26. [DOI] [PubMed] [Google Scholar]
- Schmidt, R.J. , Ketudat, M. , Aukerman, M.J. and Hoschek, G. (1992) Opaque‐2 is a transcriptional activator that recognizes a specific target site in 22‐kD zein genes. Plant Cell, 4, 689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinski, J. and Scheibe, R. (2019) Malate valves: old shuttles with new perspectives. Plant Biol. 21(Suppl. 1), 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinski, J. , König, N. , Wellmeyer, B. , Hanke, G.T. , Linke, V. , Neuhaus, H.E. and Scheibe, R. (2014) The plastid‐localized NAD‐dependent malate sehydrogenase is crucial for energy homeostasis in developing Arabidopsis thaliana seeds. Mol. Plant, 7, 170–186. [DOI] [PubMed] [Google Scholar]
- Sew, Y.S. , Ströher, E. , Fenske, R. and Millar, A.H. (2016) Loss of mitochondrial malate dehydrogenase activity alters seed metabolism impairing seed maturation and post‐germination growth in Arabidopsis . Plant Physiol. 171, 849–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng, X. , Zhong, M. , Zhu, X. , Wang, C. , Ren, Y. , Wang, Y. , Zhang, H. et al. (2019) FLOURY ENDOSPERM16 encoding encoding a NAD‐dependent cytosolic malate dehydrogenase plays an important role in starch synthesis and seed development in rice. Plant Biotechnol. J. 17, 1914–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, T. , Liu, Y. , Yan, H. , You, Q. , Yi, X. , Du, Z. , Xu, W. et al. (2017) agriGo v2.0: a GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 45, W122–W129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaz, T. , Bagard, M. , Pracharoenwattana, I. , Lindén, P. , Lee, C.P. , Carroll, A.J. , Ströher, E. et al. (2010) Mitochondrial malate dehydrogenase lowers leaf respiration and alters photorespiration and plant growth in Arabidopsis . Plant Physiol. 154, 1143–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, G. , Sun, X. , Wang, G. , Wang, F. , Gao, Q. , Sun, X. , Tang, Y. et al. (2011) Opaque7 encodes an acyl‐activating enzyme‐like protein that affects storage protein synthesis in maize endosperm. Genetics, 189, 1281–1295. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang, G. , Wang, F. , Wang, G. , Wang, F. , Zhang, X. , Zhong, M. , Zhang, J. et al. (2012) Opaque1 encodes a myosin XI motor protein that is required for endoplasmic reticulum motility and protein body formation in maize endosperm. Plant Cell, 24, 3447–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, G. , Qi, W. , Wu, Q. , Yao, D. , Zhang, J. , Zhu, J. , Tang, Y. et al. (2014a) Identification and characterization of maize floury4 as a novel semidominant opaque mutant that disrupts protein body assembly. Plant Physiol. 165, 582–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, G. , Zhang, J. , Fan, X. , Sun, X. , Qin, H. , Xu, N. , Zhong, M. et al. (2014b) Proline responding1 plays a critical role in regulating general protein synthesis and the cell cycle in maize. Plant Cell, 26, 2582–2600. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang, Q.J. , Sun, H. , Dong, Q.L. , Sun, T.Y. , Jin, Z.X. , Hao, Y.J. and Yao, Y.X. (2016) The enhancement of tolerance to salt and cold stresses by modifying the redox state and salicylic acid content via the cytosolic malate dehydrogenase gene in transgenic apple plants. Plant Biotechnol. J. 14, 1986–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse, A. , Bertoni, M. , Bienert, S. , Studer, G. , Tauriello, G. , Gumienny, R. , Heer, F.T. et al. (2018) SWISS‐MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. and Messing, J. (2010) RNA interference‐mediated change in protein body morphology and seed opacity through loss of different zein proteins. Plant Physiol. 153, 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. and Messing, J. (2012) RNA interference can rebalance the nitrogen sink of maize seeds without losing hard endosperm. PloS ONE 7, e32850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. and Messing, J. (2014) Proteome balancing of the maize seed for higher nutritional value. Front. Plant Sci. 5, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J. , Fu, M. , Ji, C. , Huang, Y. and Wu, Y. (2018) Maize oxalyl‐COA decarboxylase1 degrades oxalate and affects the seed metabolome and nutritional quality. Plant Cell, 30, 2447–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, Y.X. , Dong, Q.L. , Zhai, H. , You, C.X. and Hao, Y.J. (2011a) The functions of an apple cytosolic malate dehydrogenase gene in growth and tolerance to cold and salt stresses. Plant Physiol. Biochem. 49, 257–264. [DOI] [PubMed] [Google Scholar]
- Yao, Y.X. , Li, M. , Zhai, H. , You, C.X. and Hao, Y.J. (2011b) Isolation and characterization of an apple cytosolic malate dehydrogenase gene reveal its function in malate synthesis. J. Plant Physiol. 168, 474–480. [DOI] [PubMed] [Google Scholar]
- Yao, D. , Qi, W. , Li, X. , Yang, Q. , Yan, S. , Ling, H. , Wang, G. et al. (2016) Maize opaque10 encodes a cereal‐specific protein that is essential for the proper distribution of zeins in endosperm protein bodies. Plos Genet. 12, e1006270. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang, Z. , Zheng, X. , Yang, J. , Messing, J. and Wu, Y. (2016) Maize endosperm‐specific transcription factors O2 and PBF network the regulation of protein and starch synthesis. Proc. Natl. Acad. Sci. USA, 113, 10842–10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Dynamic development of the kernels on F2 ears. The red arrows indicate mutant kernels.
Figure S2 Phenotype of mdh4‐1 kernels and kernel segregation in other genetic backgrounds.
Figure S3 Multisequence alignment showing the distribution of the 3‐bp Indel in teosinte and 55 diverse maize inbred lines.
Figure S4 Phenotypic characteristics of transgenic lines.
Table S1 Field evaluation of normal and mdh4‐1 kernel segregation in different F2 populations.
Table S2 Amino acid level determination in mature WT and mdh4‐1 kernels.
Table S3 Primers used in this study.
Table S4 ZmMdh4 polymorphisms identified in the association panel.
Table S5 Association analysis of ZmMdh4.
Table S6 Allelism test using heterozygous mdh4‐1 and homozygous mdh4‐2 and mdh4‐3 T3 plants.
Table S7 Gene ontology classifications of DEGs with functional annotation.


