Abstract
Background:
Rosacea is a chronic inflammatory skin condition whose etiology has been linked to mast cells and the antimicrobial peptide cathelicidin LL-37. Individuals with refractory disease have demonstrated clinical benefit with periodic injections of onabotulinum toxin, but the mechanism of action is unknown.
Objectives:
To investigate the molecular mechanism by which botulinum toxin improves rosacea lesions.
Methods:
Primary human and murine mast cells were pretreated with onabotulinum toxin A or B or control. Mast cell degranulation was evaluated by β-hexosaminidase activity. Expression of botulinum toxin receptor Sv2 was measured by qPCR. The presence of SNAP-25 and VAMP2 was established by immunofluorescence. In vivo rosacea model was established by intradermally injecting LL-37 with or without onabotulinum toxin A pretreatment. Mast cell degranulation was assessed in vivo by histologic counts. Rosacea biomarkers were analyzed by qPCR of mouse skin sections.
Results:
Onabotulinum toxin A and B inhibited compound 48/80-induced degranulation of both human and murine mast cells. Expression of Sv2 was established in mouse mast cells. Onabotulinum toxin A and B increased cleaved SNAP-25 and decreased VAMP2 staining in mast cells respectively. In mice, injection of onabotulinum toxin A significantly reduced LL-37-induced skin erythema, mast cell degranulation, and mRNA expression of rosacea biomarkers.
Conclusions:
These findings suggest that onabotulinum toxin reduces rosacea-associated skin inflammation by directly inhibiting mast cell degranulation. Periodic applications of onabotulinum toxin may be an effective therapy for refractory rosacea and deserves further study.
Keywords: botox, botulinum toxin, mast cell, mechanism of action, rosacea
Introduction
Rosacea is a chronic inflammatory skin disorder which is characterized by facial flushing, telangiectasia and inflammatory papules and pustules on the central area of the face[1]. Although the pathogenesis of rosacea is complicated and not yet fully understood, abnormal neurovascular signaling, dysregulation of innate immune system, and imbalance of commensal skin microbiota are considered to be involved[2]. Various factors trigger flushing in rosacea such as alcohol, heat, exercise, or spicy foods which supports the role of neurogenic inflammation in the development of the disease[2, 3]. It has been hypothesized that the activation of transient receptor potential vanilloid (TRPV) and transient receptor potential ankyrin (TRPA) on peripheral sensory neurons, by rosacea triggers, stimulates the release of vasoactive neuropeptides that cause rosacea flares[2, 4].
In addition to vascular hyper-reactivity, the dysregulation of the innate immune system through abnormal levels of cathelicidins also appears to have a central role in the pathogenesis of rosacea[5]. Cathelicidins are antimicrobial peptides in human skin that protect against infection from a variety of microbes[6]. In addition to their direct antimicrobial activity, they also promote and regulate the host immune response by several mechanisms, including angiogenesis[7], expression of extracellular matrix proteins[8], and leukocyte chemotaxis[9].
Human cathelicidin is initially secreted as a biologically inactive pro-protein called cationic antimicrobial protein 18 (CAP18)[10]. Serine proteases, such as kallikrein-related peptidase 5 (KLK5), cleave CAP18 to form active peptides in the epidermis[11]. Abnormally increased KLK5 activity causes increased expression of cathelicidin LL-37, a molecule which has been shown to induce rosacea-like skin inflammation in a mouse model[5]. Interestingly, small doses of doxycycline, a proven therapy for rosacea, inhibit matrix metalloproteinases (MMPs), which subsequently blocks the activity of KLKs, preventing the formation of active cathelicidin peptides in the epidermis[12]. Thus, cathelicidin LL-37 appears to have a central role in the pathogenesis of rosacea.
Recently, mast cells (MCs) have emerged as key enablers of cathelicidin LL-37-induced skin inflammation[13]. Specifically, LL-37 induces MC chemotaxis, degranulation, and release of proinflammatory cytokines[14, 15]. Muto el al demonstrated that MC-deficient mice did not develop rosacea-like features after LL-37 injection. Furthermore, stabilization of MCs with cromolyn sodium reduced skin inflammation and rosacea biomarkers in both humans and mice[13]. These results not only emphasized the importance of MCs in cathelicidin-induced inflammation but also provided potential therapeutic targets for rosacea.
A variety of systemic and topical treatment options are used for rosacea, such as oral tetracyclines and topical ivermectin, metronidazole, azelaic acid and brimonidine[16, 17], However, it can be challenging to treat severe flushing and persistent erythema in refractory cases of rosacea. Rosacea has been associated with serious social stigma and therefore is considered a significant psychological problem[18].
There are a couple of case reports that successfully treated refractory erythema and flushing with β-adrenergic blockers, naloxone (opiate antagonist), ondansetron (serotonin antagonist), or endoscopic thoracic sympathectomy[19-23]. Recently, intradermal botulinum toxin (BoNT) has also been investigated as a novel treatment of refractory facial erythema and flushing[24-26]. BoNT A is a potent neurotoxin produced by the bacterium Clostridium botulinum, which inhibits the release of acetylcholine (ACh) from the presynaptic vesicle[27]. BoNT not only inhibits the release of ACh but also modulates several other neuropeptides, including substance P (SP), calcitonin gene–related peptide (CGRP) and vasoactive intestinal peptide (VIP)[28, 29]. Since ACh and VIP are key mediators of vasodilation and flushing,[30, 31] inhibition of their release is a reasonable mechanism of action for BoNT in rosacea. However, the mechanism by which BoNT acts on the immune system in rosacea remains unclear.
Our group has recently demonstrated that human and mouse MCs express SNARE (soluble N-ethylmaleimide-sensitive-factor attachment protein receptor) proteins, including synaptosomal-associated protein-25 (SNAP-25) and vesicle-associated membrane protein (VAMP)[32]. SNAREs are the main components of a protein complex involved in the docking and fusion of vesicles with the presynaptic membrane. On sensory nerve endings, BoNT A and B block the exocytosis of vesicles containing neuropeptides by cleaving SNAP and VAMP respectively[33, 34]. The presence of SNAREs on MCs suggests that BoNT’s mechanism of action in the treatment of rosacea may extend beyond the regulation of neuronal activity. We hypothesized that BoNT could improve erythema and flushing in rosacea by direct inhibition of MC degranulation, a key step in the development of the disease[13]. In the present study, we further investigate this novel mechanism.
Materials and methods
Mouse experiments
C57BL/6 mice were bred at our facility. Institutional Animal Care and Use Committee (IACUC) of UC San Diego approved all animal experiments. Mice were shaved 24 hours before intradermal injection of 0.5 units of botulinum toxin A (BoNT A 25 pg in 100 μl PBS, ALLERGAN), 12 hours later, intradermally injected with 50 μl of 320 μM cathelicidin LL-37 (AnaSpec) or phosphate-buffered saline (PBS) (n=3) twice a day for 2 days. Images of clinical lesions were processed with Image J software, and the area of cutaneous erythema was calculated. Skin biopsies were taken after 72 hours of observation, and tissues were immersed immediately into RNA stabilization reagent (RNAlater; QIAGEN) and stored at −80°C.
Mast Cells
Primary murine MCs (mMCs) were generated by extracting bone marrow cells from the femurs of 5- to 8-week-old mice and culturing cells in Roswell Park Memorial Institute (RPMI) 1640 medium (Invitrogen) supplemented with 10% inactivated fetal bovine serum (FBS) (Thermo Fisher), 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (pH 7.4), 4 mM L-glutamine, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, 100 IU/ml penicillin and 100 μg/ml streptomycin. Recombinant murine interleukin-3 (IL-3) (1 ng/ml, R&D Systems) and recombinant murine stem cell factor (20 ng/ml, R&D Systems) were also included. After 4 weeks, mMCs were consistently generated as confirmed by the expression of CD117 (c-Kit) and FcεRI; cell maturation was confirmed by metachromatic staining with toluidine blue. The purity of mMCs was greater than 98%[35].
Primary human MCs (hMCs) were derived from human cord blood CD34+CD45+ cells (Astarte Biologics) according to Kirshenbaum and Metcalfe[36]. Briefly, CD34+CD45+ cells were cultured in serum-free culture media (Stemline II, Sigma) containing recombinant human stem cell factor (100 ng/ml, R&D Systems), recombinant human IL-6 (100 ng/ml, R&D Systems), and recombinant human IL-3 (20 ng/ml, R&D Systems, first week only). After 10 weeks, hMCs were consistently generated as confirmed by the expression of CD117 and FcεRI. Cell maturation was confirmed by metachromatic staining with toluidine blue. The purity of hMCs was greater than 98%.
Real-time quantitative RT-PCR
Total RNA was isolated using the RNeasy Mini Kit (QIAGEN), and 0.5 μg of total RNA was used for cDNA synthesis using the iSCRIPT cDNA Synthesis Kit (Bio-Rad). Real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR) was performed in an ABI 7300 Real-Time PCR system (Applied Biosystems). We used the comparative ΔΔCT method to determine the quantification of gene expression, normalized the target gene expression in the test samples to the endogenous reference glyceraldehyde 3-phosphate dehydrogenase (GAPDH) level and reported them as the fold difference relative to GAPDH gene expression in untreated baseline control[37]. TaqMan gene expression assays (Applied Biosystems) were used to analyze the expressions of mouse Mmp9, mouse Trpv2, mouse Klk5, and mouse chymase (Cma1). SYBR Green gene expression assays (Bio-Rad) were used to analyze the expressions of mouse synaptic vesicle glycoprotein 2a (Sv2a), mouse Sv2b, and mouse Sv2c. The following primer sequences were used: mouse Sv2a forward, 5′-TCGTCCTTCGTCCAGGGTTA-3′, and reverse, 5′-ATGAATCGTTTTAATGTGGGTCAC-3′; mouse Sv2b forward, 5′-CACTGTCTACAGGATACCGC-3′, and reverse, 5 ′-CCCACTATGACAATCTGCTAG-3 mouse Sv2c forward, 5′-TGAGATGCTTCAACTACCCAGTCAGG-3′, and reverse, 5′-CCTTTTGCACATTCCTAGTTAGCAG-3′ [38]; mouse Gapdh forward, 5’-CTTAGCACCCCTGGCCAAG-3’, and reverse, 5’-TGGTCATGAGTCCTTCCACG-3’. We performed all the assays in triplicate and repeated the experiments at least three times.
Mast cell degranulation assay
5 × 104 hMCs or 1 × 105 mMCs were pretreated with BoNT A or B (0.5-5 pM) for 24 hours. After being washed 2 times with PBS, MC degranulation was assessed by measuring the activity of β-hexosaminidase in the supernatants[39-41] of 1 × 105 mMCs in 200 μl Tyrode’s buffer (0.1% BSA, 0.1% glucose, 2 mmol/l MgCl2, 137.5 mmol/l NaCl, 12 mmol/l NaHCO3, 2.6 mmol/l KCl, pH 7.4) or 5 × 104 hMCs in 100 μl saline phosphate buffer (0.9 % NaCl, 10 mM NaH2PO4, 45 mM glucose) incubated for 30 min at 37°C with Compound 48/80 (10 μg/ml, Sigma) which was used to promote MC degranulation in an IgE-independent manner[42]. For each sample assayed, MC supernatant aliquots (20 μl) were mixed with substrate solution (100 μl), which consisted of 1 mM 4-methylumbelliferyl-2-acetamide-2-deoxy-β-D-glucopyranoside (Calbiochem) in 0.1 M sodium citrate buffer (pH 4.5), and were incubated for 2 hours at 37°C in the dark. The reaction was then stopped by the addition of 12 μl of 0.2 M glycine (pH 10.7). The reaction mixtures were excited at 365 nm and measured at 460 nm in a fluorescence plate reader (Gemini EM microplate spectrofluorometer, Molecular Devices). To determine the total cellular content of this enzyme, an equivalent number of cells were lysed with 1% triton-X-100 (Sigma). Release of β-hexosaminidase was calculated as the percentage of the total enzyme content.
Histologic analysis of mast cell degranulation
To assess the degree of dermal mast cell degranulation in vivo, a quantitative analysis was performed. Animal skin samples were collected, fixed with buffered formalin, and embedded in paraffin. After deparaffinizing and rehydrating the sections, sections were stained with toluidine blue (RICCA Chemical) to identify MCs[43]. After staining, randomly selected sections were examined under light microscopy. For each section, the total, intact, and degranulating mast cells in the dermis were counted per 20 fields of view using a magnification of ×200. A mast cell was considered to be degranulating if four or more extruded granules were visible adjacent to the cell or if the cell outline appeared disrupted[44]. In contrast, intact mast cells demonstrate a deep blue (orthochromatic) staining. All photos were captured at original magnifications of ×400.
Fluorescence images
Mast cells were treated with 0.1 pM BoNT A or B for 24 hours, then attached to a glass slide by using Shandon Cytospin 2 cytocentrifuge (Thermo Fisher). The cells were stained with 1 μg/ml anti-BoNT A-cleaved-SNAP-25 antibody and anti-VAMP2 antibody (Synaptic Systems) according to the manufacturer’s instructions. Slides were mounted in ProLong anti-fade reagent with 4′,6-diamidino-2-phenylindole (DAPI) (Molecular Probes). We imaged the cells using the Bx51 research microscope (Olympus) and X-Cite 120 fluorescence illumination systems (EXFO Photonic Solutions). All isotype controls showed negative staining (data not shown). The fluorescence intensity of each cell was measured using Image J software (NIH) and the corrected total cell fluorescence (CTCF) was calculated.
Statistical analyses
All data are presented as the mean±SD. At least three independent experiments were performed to assess the reproducibility of the different experiments. Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software). For 48/80-induced mast cell degranulation, histologic mast cell degranulation, and gene expression experiments, results were compared using one-way ANOVA with post hoc Tukey’s tests. For comparisons of CTCF and area of erythema on skin, results were analyzed with Student's t-test. For all statistical tests, P-values<0.05 were considered statistically significant (*P<0.05, **P<0.01, ***p<0.001).
Results
Mast cell degranulation is blocked by onabotulinum toxin in vitro
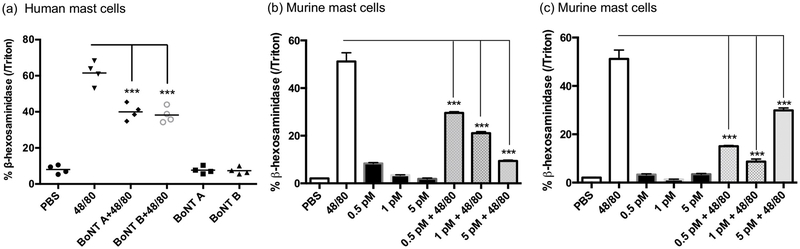
To confirm the interaction between BoNT and MCs, primary hMCs and mMCs were treated in vitro with compound 48/80, and β-hexosaminidase release was measured as a marker for MC degranulation. In comparison to controls, pretreatment of both hMCs and mMCs with BoNT A or B for 24 hours significantly decreased MC degranulation. Interestingly, BoNT A had a dose-dependent effect on MC function (Fig. 1).
Fig 1. Mast cell degranulation is blocked by onabotulinum toxin in vitro.
(a) Human mast cell (MC) degranulation was assessed by measuring release of β-hexosaminidase. The cells were pretreated with 5 pM botulinum toxin (BoNT) A or BoNT B for 24 h, then treated with compound 48/80 (10 μg/ml) for 30 min; (b-c) Murine MC degranulation was assessed by measuring release of β-hexosaminidase. The cells were pretreated with 0.5-5 pM BoNT A (b) or BoNT B (c) for 24 h, then treated with compound 48/80 (10 μg/ml) for 30 min. ***P<0.001 (n = 3).
Mast cells express SNAP-25 and VAMP2
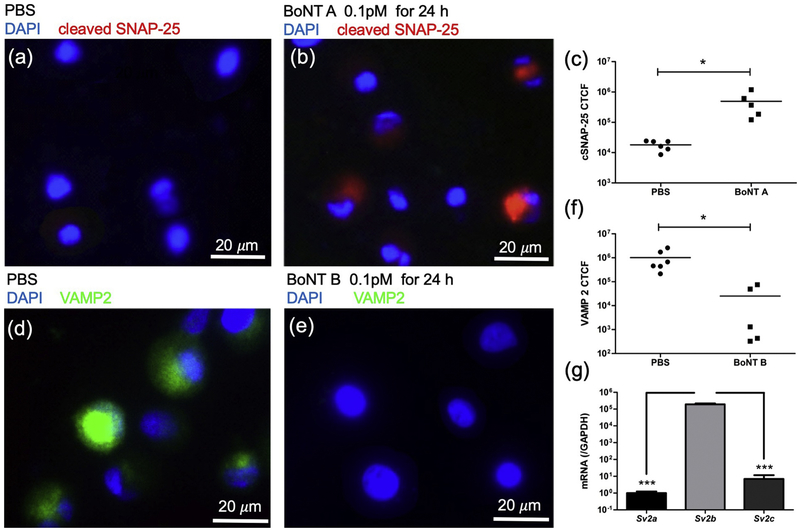
To verify the presence of SNARE proteins on MCs, immunostaining for SNAP-25 was performed on mMCs with or without BoNT A pretreatment. The SNAP-25 antibody used only recognizes the cleaved product of SNAP-25 (cSNAP-25) and not the full-length molecule. In comparison to control, pretreatment of the mMCs with BoNT showed a quantitative increase in cSNAP-25 staining (Fig. 2a-c). The VAMP2 antibody used in the present study detects the intact molecule. Therefore, the reduction of VAMP2 protein expression was used as a measure of VAMP2 cleavage by BoNT B. Following pretreatment with BoNT B, mMCs demonstrated a decrease in VAMP2 staining. Therefore, VAMP2 cleavage was significantly greater in the BoNT B treated cells in comparison to the control group (Fig. 2d-f).
Fig 2. Mast cells express SNAP-25, VAMP2, and SV2.
Immunostaining for cleaved synaptosomal-associated protein-25 (SNAP-25) (a-c) or vesicle-associated membrane protein 2 (VAMP2) (d-f) was performed on mouse MCs. (a) Cells treated with PBS; (b) Cells treated with 0.1 pM BoNT A for 24 h; (d) Cells treated with PBS; (e) Cells treated with 0.1 pM BoNT B for 24 h; (c, f) Corrected total cell fluorescence (CTCF) intensity for cells treated with PBS or BoNT A or B. *P<0.05 (n = 5-6). Expression of the three isoforms of synaptic vesicle glycoprotein 2 (Sv2) in mouse MCs were measured by RT-qPCR; (g) Although all isoforms were detected, Sv2b was expressed at significantly higher levels. ***P<0.001 (n = 3).
Mast cells express BoNT receptor SV2
It has been shown that BoNT enters neurons by binding to synaptic vesicle proteins, such as synaptic vesicle glycoprotein 2 (SV2), and subsequently taking advantage of the physiological recycling of synaptic vesicles[45]. To investigate the presence of BoNT receptors on primary mMCs, we measured the mRNA expression of the three isoforms of Sv2 by RT-qPCR. Although all isoforms were present, Sv2b demonstrated significantly higher expression (Fig. 2g). We subsequently examined how BoNT treatment affects the expression of Sv2 isoforms and found no appreciable differences (data not shown).
Onabotulinum toxin reduces LL-37-induced skin inflammation
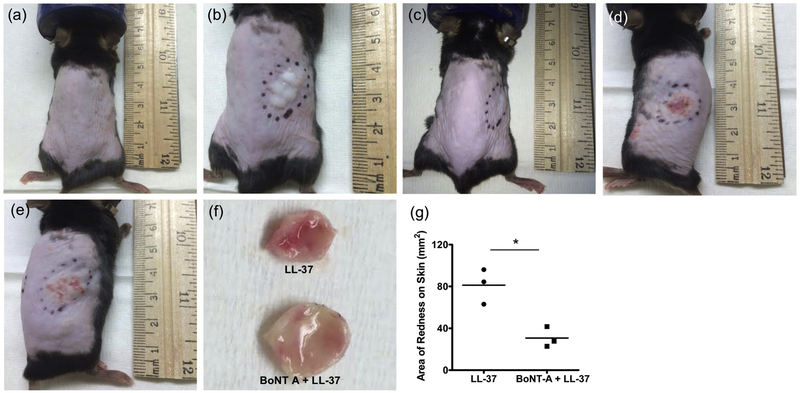
To investigate the clinical effects of BoNT on rosacea inflammation, we used a well-established mouse model of rosacea-like inflammation[5]. We injected cathelicidin LL-37 intradermally into C57BL/6 mice twice a day for two consecutive days and compared the resulting inflammation with mice that were pretreated with BoNT A. Our clinical endpoint observation at 72 hours demonstrated visibly and quantitatively reduced inflammation in the skin of the pretreatment group (Fig. 3).
Fig 3. Onabotulinum toxin reduces LL-37-induced skin inflammation in mice.
Cathelicidin LL-37 50 μl of 320 μM was injected intradermally into C57BL/6 mice twice a day for two consecutive days with or without 0.5 unit of BoNT A pretreatment. (a) Control (injected with PBS); (b) Immediately after LL-37 intradermal injection; (c) 12 h after BoNT A pre-treatment and just before LL-37 injection; (d-e) Three days after LL-37 injection without (d) or with (e) BoNT A pretreatment; (f) Collected skin tissues from LL-37 treated mice without and with BoNT A pretreatment; (g) Image J software quantification of area of erythematous skin on LL-37 treated mice without and with BoNT A pretreatment. *P<0.05 (n = 3).
Dermal mast cell degranulation is blocked by onabotulinum toxin in vivo
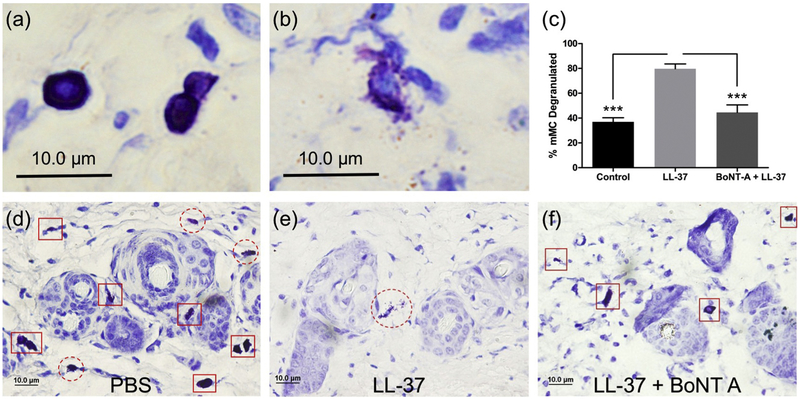
Skin biopsies from each mouse were subsequently taken to assess the degree of mast cell degranulation in vivo. In comparison to mice only treated with cathelicidin LL-37, the BoNT A pretreatment group showed a significantly decreased percentage of dermal mast cells that were degranulating (Fig. 4). Furthermore, the degree of mast cell degranulation in the pretreatment group approached that of the control group.
Fig 4. Mast cell degranulation is blocked by onabotulinum toxin in vivo.
Intact (a) and degranulating (b) dermal MCs were counted in murine skin to assess the effect of BoNT A in vivo. Representative toluidine blue staining of skin tissue taken from mice treated with PBS (d) or cathelicidin LL-37 without (e) or with (f) BoNT A pretreatment; Intact and degranulating mast cells are indicated by red square and red dashed red circle outlines respectively; (c) Quantification of the percentage of dermal mast cells that are degranulating. ***P<0,001 (n = 20 fields of view).
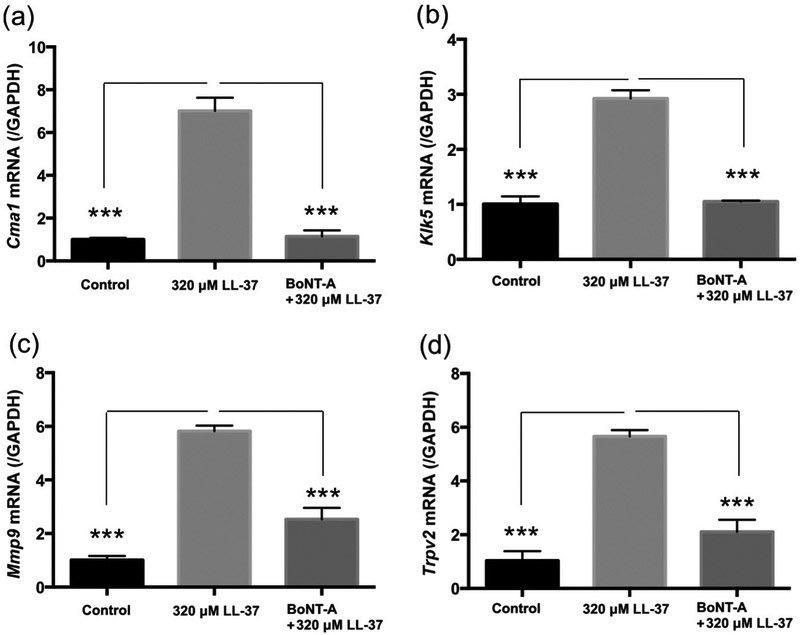
Onabotulinum toxin reduces expression of rosacea biomarkers
Chymase, KLK5, matrix metallopeptidase 9, and transient receptor potential cation channel proteins are involved in the mast cell immune response and have been previously shown to be reliable biomarkers for rosacea [4, 5, 12, 13]. To quantitatively assess the clinical response to BoNT A, we measured the expression of Cmal, Klk5, Mmp9, and Trpv2 in skin tissue taken from each mouse. In comparison to mice only treated with cathelicidin LL-37, the BoNT A pretreatment group showed a significantly decreased expression of these rosacea biomarkers (Fig. 5).
Fig 5. Onabotulinum toxin inhibits the expression of rosacea biomarkers in LL-37-induced rosacea mouse model.
LL-37 50 μl of 320 μM was injected intradermally into C57BL/6 mice twice a day for two consecutive days with or without 0.5 unit of BoNT A pre-treatment. Control mice were injected with PBS. Cmal (chymase) (a), Klk5 (kallikrein related-peptidase 5) (b), Mmp9 (matrix metallopeptidase 9) (c), and Trpv2 (transient receptor potential cation channel, subfamily V, member 2) (d) mRNA expressions in the skin were measured by RT-qPCR. ***P<0.001 (n = 3).
Discussion
The treatment of rosacea typically involves a combination of behavioral modification and therapeutic intervention. However, satisfactory improvement of symptoms is uncommon, necessitating the investigation of new therapies. BoNT is a neuromuscular blocking agent that was initially approved by the Food and Drug Administration (FDA) in 1989 for blepharospasm, hemifacial spasm, and strabismus[46]. Since then, there have been many additional indications, including axillary hyperhidrosis, cervical dystonia, chronic migraine, limb spasticity, and overactive bladder, in addition to several cosmetic uses[46, 47]. Recently, several individuals with papulopustular rosacea have demonstrated clinical improvement with intradermal injections of BoNT A, but the mechanism of action has not been investigated[24-26].
To our best knowledge, this is the first study to investigate the direct action of BoNT on MCs in rosacea. Interestingly, one prior study described BoNT activity on MC infiltration in wound healing. The number of infiltrated MCs was markedly fewer in the skin pretreated with BoNT A compared to the control group[48]. Similarly, in a NC/Nga mouse model of atopic dermatitis, the skin pretreated with intradermal BoNT injection had less MC infiltration compared to control[49]. We confirmed this observation in the LL-37-induced rosacea mouse model (data not shown).
Here, we demonstrated the direct effect of BoNT on MC degranulation in the absence of its neuromodulating activity. Both BoNT A and B decreased MC degranulation in both mouse and human MCs in vitro. To investigate the interaction between BoNT and MCs, we established the presence of BoNT’s receptor SV2 on MCs. To confirm the direct action of BoNT on MCs, VAMP2 and SNAP-25, which are cleaved by BoNT A and B respectively, were measured. Immunostaining showed decreased cleavage of SNAP-25 and VAMP2 in MCs pretreated with BoNT which supports our hypothesis that BoNT directly inhibits MC degranulation. Since MC degranulation has a critical role in promoting LL-37 rosacea inflammation, its inhibition by BoNT should contribute to rosacea improvement.
Thus, to validate the effect of BoNT on rosacea in vivo, we used an LL-37-induced rosacea mouse model. Since BoNT B is only FDA-approved for cervical dystonia by intramuscular injection and since BoNT A is more widely administered by intradermal injection, only BoNT A was utilized in our in vivo experiments. Mice pretreated with intradermal BoNT A showed significantly less erythema compared to the control. Additionally, the pretreatment group showed an almost two-fold reduction in dermal mast cell degranulation in comparison to LL-37-injected mice.
Cathelicidin, cutaneous serine proteases, and MMPs are involved in the mast cell immune response and are known as reliable biomarkers in rosacea[5, 12]. Chymase is a serine protease that is abundant in MCs and is used as marker of MC activity; KLK5 is the predominant serine protease in epidermis that is responsible for cathelicidin cleavage, leading to increased LL-37 expression in rosacea[5]; MMP9 is needed to activate KLK5 by cleaving its preproenzyme form[2]. TRPV is closely related to sensory pathologies, and it has been hypothesized that the activation of TRPV and TRPA on peripheral sensory neurons by rosacea triggers stimulates the release of vasoactive neuropeptides that cause rosacea flares[4]. Additionally, TRPV2 and TRPV4 are co-localized with MCs in rosacea skin[50]. In BoNT-pretreated mice, these biomarkers were significantly suppressed compared to the control upon LL-37 administration, quantitatively supporting its clinical protective effect[12].
In summary, the results of this study suggest that the mechanism of botulinum toxin in the treatment of rosacea involves the blockage of MC degranulation by cleaving SNARE proteins within the cell. Therefore, not only does BoNT target the neurogenic inflammatory component of rosacea, but it also has direct inhibitory effects on MCs. These multiple targets of BoNT may offer therapeutic advantages over currently available remedies. Since intradermal injections are invasive and require periodic office visits, a well-formulated topical BoNT may be appropriate for further study in the treatment of rosacea.
Acknowledgments
Funding: This study was funded by grants from the National Institute of Health and the National Rosacea Society.
Abbreviations:
- ACh
acetylcholine
- BoNT
botulinum toxin
- CAP
cationic antimicrobial protein
- CMA
chymase
- KLK
kallikrein-related peptidase
- MMP
matrix metalloproteinases
- SNAP
Synaptosomal-associated protein
- SNARE
Soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- TRPA
transient receptor potential ankyrin
- TRPV
transient receptor potential vanilloid
- VAMP
vesicle-associated membrane protein
Footnotes
Disclosures: The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gallo RL, Granstein RD, Kang S, Mannis M, Steinhoff M, Tan J, et al. Standard classification and pathophysiology of rosacea: The 2017 update by the National Rosacea Society Expert Committee. Journal of the American Academy of Dermatology. 2018. January;78(1):148–55. [DOI] [PubMed] [Google Scholar]
- 2.Two AM, Wu W, Gallo RL, Hata TR. Rosacea: part I. Introduction, categorization, histology, pathogenesis, and risk factors. J Am Acad Dermatol. 2015. May;72(5):749–58; quiz 59-60. [DOI] [PubMed] [Google Scholar]
- 3.Choi JE, Di Nardo A. Skin neurogenic inflammation. Semin Immunopathol. 2018. May;40(3):249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mascarenhas NL, Wang Z, Chang YL, Di Nardo A. TRPV4 Mediates Mast Cell Activation in Cathelicidin-Induced Rosacea Inflammation. J Invest Dermatol. 2017. April;137(4):972–5. [DOI] [PubMed] [Google Scholar]
- 5.Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007. August; 13 (8):975–80. [DOI] [PubMed] [Google Scholar]
- 6.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001. November 22;414(6862):454–7. [DOI] [PubMed] [Google Scholar]
- 7.Koczulla R, von Degenfeld G, Kupatt C, Krotz F, Zahler S, Gloe T, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003. June;111(11):1665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park HJ, Cho DH, Kim HJ, Lee JY, Cho BK, Bang SI, et al. Collagen synthesis is suppressed in dermal fibroblasts by the human antimicrobial peptide LL-37. J Invest Dermatol. 2009. April;129(4):843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang D, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. The Journal of experimental medicine. 2000. October 2; 192(7): 1069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaiou M, Nizet V, Gallo RL. Antimicrobial and protease inhibitory functions of the human cathelicidin (hCAP18/LL-37) prosequence. J Invest Dermatol. 2003. May;120(5):810–6. [DOI] [PubMed] [Google Scholar]
- 11.Yamasaki K, Schauber J, Coda A, Lin H, Dorschner RA, Schechter NM, et al. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006. October;20(12):2068–80. [DOI] [PubMed] [Google Scholar]
- 12.Di Nardo A, Holmes AD, Muto Y, Huang EY, Preston N, Winkelman WJ, et al. Improved clinical outcome and biomarkers in adults with papulopustular rosacea treated with doxycycline modified-release capsules in a randomized trial. Journal of the American Academy of Dermatology. 2016. June;74(6): 1086–92. [DOI] [PubMed] [Google Scholar]
- 13.Muto Y, Wang Z, Vanderberghe M, Two A, Gallo RL, Di Nardo A. Mast cells are key mediators of cathelicidin-initiated skin inflammation in rosacea. The Journal of investigative dermatology. 2014. November; 134(11):2728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiemann F, Brandt E, Gross R, Lindner B, Mittelstadt J, Sommerhoff CP, et al. The cathelicidin LL-37 activates human mast cells and is degraded by mast cell tryptase: counter-regulation by CXCL4. Journal of immunology (Baltimore, Md : 1950). 2009. August 15; 183(4):2223–31. [DOI] [PubMed] [Google Scholar]
- 15.Yoshioka M, Fukuishi N, Kubo Y, Yamanobe H, Ohsaki K, Kawasoe Y, et al. Human cathelicidin CAP18/LL-37 changes mast cell function toward innate immunity. Biological & pharmaceutical bulletin. 2008. February;31(2):212–6. [DOI] [PubMed] [Google Scholar]
- 16.Holmes AD, Steinhoff M. Integrative concepts of rosacea pathophysiology, clinical presentation and new therapeutics. Experimental dermatology. 2016. July 04. [DOI] [PubMed] [Google Scholar]
- 17.Two AM, Wu W, Gallo RL, Hata TR. Rosacea: part II. Topical and systemic therapies in the treatment of rosacea. J Am Acad Dermatol. 2015. May;72(5):761–70; quiz 71-2. [DOI] [PubMed] [Google Scholar]
- 18.Huynh TT. Burden of Disease: The Psychosocial Impact of Rosacea on a Patient's Quality of Life. Am Health Drug Benefits. 2013. July;6(6):348–54. [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu CC, Lee JY. Pronounced facial flushing and persistent erythema of rosacea effectively treated by carvedilol, a nonselective beta-adrenergic blocker. Journal of the American Academy of Dermatology. 2012. September;67(3):491–3. [DOI] [PubMed] [Google Scholar]
- 20.Schram AM, James WD. Neurogenic rosacea treated with endoscopic thoracic sympathectomy. Archives of dermatology. 2012. February;148(2):270–1. [DOI] [PubMed] [Google Scholar]
- 21.Wollina U The response of erythematous rosacea to ondansetron. The British journal of dermatology. 1999. March;140(3):561–2. [DOI] [PubMed] [Google Scholar]
- 22.Wilkin JK. Effect of subdepressor clonidine on flushing reactions in rosacea. Change in malar thermal circulation index during provoked flushing reactions. Archives of dermatology. 1983. March;119(3):211–4. [PubMed] [Google Scholar]
- 23.Bernstein JE, Soltani K. Alcohol-induced rosacea flushing blocked by naloxone. The British journal of dermatology. 1982. July;107(1):59–61. [DOI] [PubMed] [Google Scholar]
- 24.Park KY, Hyun MY, Jeong SY, Kim BJ, Kim MN, Hong CK. Botulinum toxin for the treatment of refractory erythema and flushing of rosacea. Dermatology. 2015;230(4):299–301. [DOI] [PubMed] [Google Scholar]
- 25.Dayan SH, Pritzker RN, Arkins JP. A new treatment regimen for rosacea: onabotulinumtoxinA. J Drugs Dermatol. 2012. December;11(12):e76–9. [PubMed] [Google Scholar]
- 26.Dayan SH, Ashourian N, Cho K. A Pilot, Double-Blind, Placebo-Controlled Study to Assess the Efficacy and Safety of IncobotulinumtoxinA Injections in the Treatment of Rosacea. J Drugs Dermatol. 2017. June 1;16(6):549–54. [PubMed] [Google Scholar]
- 27.Huang W, Foster JA, Rogachefsky AS. Pharmacology of botulinum toxin. Journal of the American Academy of Dermatology. 2000. August;43(2 Pt 1):249–59. [DOI] [PubMed] [Google Scholar]
- 28.Meng J, Wang J, Lawrence G, Dolly JO. Synaptobrevin I mediates exocytosis of CGRP from sensory neurons and inhibition by botulinum toxins reflects their anti-nociceptive potential. Journal of cell science. 2007. August 15; 120(Pt 16):2864–74. [DOI] [PubMed] [Google Scholar]
- 29.Aoki KR. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology. 2005. October;26(5):785–93. [DOI] [PubMed] [Google Scholar]
- 30.Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilatation in young and aged human skin. The Journal of physiology. 2005. March 15;563(Pt 3):965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilkins BW, Chung LH, Tublitz NJ, Wong BJ, Minson CT. Mechanisms of vasoactive intestinal peptide-mediated vasodilation in human skin. Journal of applied physiology (Bethesda, Md : 1985). 2004. October;97(4): 1291–8. [DOI] [PubMed] [Google Scholar]
- 32.Ramachandran R, Marino MJ, Paul S, Wang Z, Mascarenhas NL, Pellett S, et al. A Study and Review of Effects of Botulinum Toxins on Mast Cell Dependent and Independent Pruritus. Toxins. 2018. March 23;10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zanetti G, Azarnia Tehran D, Pirazzini M, Binz T, Shone CC, Fillo S, et al. Inhibition of botulinum neurotoxins interchain disulfide bond reduction prevents the peripheral neuroparalysis of botulism. Biochemical pharmacology. 2015. December 1;98(3):522–30. [DOI] [PubMed] [Google Scholar]
- 34.Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiological reviews. 2000. April;80(2):717–66. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Lai Y, Bernard JJ, Macleod DT, Cogen AL, Moss B, et al. Skin mast cells protect mice against vaccinia virus by triggering mast cell receptor S1PR2 and releasing antimicrobial peptides. J Immunol. 2012. January 1;188(l):345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirshenbaum AS, Metcalfe DD. Growth of human mast cells from bone marrow and peripheral blood-derived CD34+pluripotent progenitor cells. Methods Mol Biol. 2006;315:105–12. [DOI] [PubMed] [Google Scholar]
- 37.Luu-The V, Paquet N, Calvo E, Cumps J. Improved real-time RT-PCR method for high-throughput measurements using second derivative calculation and double correction. Biotechniques. 2005. February;38(2):287–93. [DOI] [PubMed] [Google Scholar]
- 38.Peng C, Zhu G, Liu X, Li H. Mutant Huntingtin Causes a Selective Decrease in the Expression of Synaptic Vesicle Protein 2C. Neurosci Bull. 2018. October;34(5):747–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schick B, Austen KF. Modulation of chymase-mediated rat serosal mast cell degranulation by trypsin or diisopropyl fluorophosphate. Immunology. 1989. March;66(3):434–8. [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki K, Verma IM. Phosphorylation of SNAP-23 by IkappaB kinase 2 regulates mast cell degranulation. Cell. 2008. August 8;134(3):485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartz LB, Austen KF, Wasserman SI. Immunologic release of beta-hexosaminidase and beta-glucuronidase from purified rat serosal mast cells. Journal of immunology (Baltimore, Md : 1950). 1979. October; 123(4): 1445–50. [PubMed] [Google Scholar]
- 42.Rothschild AM. Mechanisms of histamine release by compound 48-80. British journal of pharmacology. 1970. January;38(l):253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith EW, Atkinson WB. Simple procedure for identification and rapid counting of mast cells in tissue sections. Science. 1956. May;123(3204):941–2. [DOI] [PubMed] [Google Scholar]
- 44.Dyson M, Luke DA. Induction of mast cell degranulation in skin by ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control. 1986;33(2):194–201. [DOI] [PubMed] [Google Scholar]
- 45.Dong M, Yeh F, Tepp WH, Dean C, Johnson EA, Janz R, et al. SV2 is the protein receptor for botulinum neurotoxin A. Science. 2006. April;312(5773):592–6. [DOI] [PubMed] [Google Scholar]
- 46.Botox [product insert]. Irvine, CA: Allergan Inc; 2017. [Google Scholar]
- 47.Botox Cosmetic [product insert]. Irvine, CA: Allergan Inc; 2017. [Google Scholar]
- 48.Park TH. The effects of botulinum toxin A on mast cell activity: preliminary results. Burns : journal of the International Society for Burn Injuries. 2013. June;39(4):816–7. [DOI] [PubMed] [Google Scholar]
- 49.Han SB, Kim H, Cho SH, Chung JH, Kim HS. Protective Effect of Botulinum Toxin Type A Against Atopic Dermatitis-Like Skin Lesions in NC/Nga Mice. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al]. 2017. April 24. [DOI] [PubMed] [Google Scholar]
- 50.Sulk M, Seeliger S, Aubert J, Schwab VD, Cevikbas F, Rivier M, et al. Distribution and expression of non-neuronal transient receptor potential (TRPV) ion channels in rosacea. J Invest Dermatol. 2012. April; 132(4): 1253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]