Abstract
The hospital wastewater imposes a potent threat to the security of human health concerning its high vulnerability towards the outbreak of several diseases. Furthermore, the outbreak of COVID-19 pandemic demanded a global attention towards monitoring viruses and other infectious pathogens in hospital wastewater and their removal. Apart from that, the presence of various recalcitrant organics, pharmaceutically active compounds (PhACs), etc. imparts a complex pollution load to water resources and ecosystem. In this review, an insight into the occurrence, persistence and removal of drug-resistant microorganisms and infectious viruses as well as other micro-pollutants have been documented. The performance of various pilot/full-scale studies have been evaluated in terms of removal of biochemical oxygen demand (BOD), chemical oxygen demand (COD), total suspended solids (TSS), PhACs, pathogens, etc. It was found that many biological processes, such as membrane bioreactor, activated sludge process, constructed wetlands, etc. provided more than 80% removal of BOD, COD, TSS, etc. However, the removal of several recalcitrant organic pollutants are less responsive to those processes and demands the application of tertiary treatments, such as adsorption, ozone treatment, UV treatment, etc. Antibiotic-resistant microorganisms, viruses were found to be persistent even after the treatment of hospital wastewater, and high dose of chlorination or UV treatment was required to inactivate them. This article circumscribes the various emerging technologies, which have been used to treat PhACs and pathogens. The present review also emphasized the global concern of the presence of SARS-CoV-2 RNA in hospital wastewater and its removal by the existing treatment facilities.
Keywords: Biological processes, Advanced oxidation processes, Antibiotic-resistant bacteria, Antibiotic-resistant genes, SARS-CoV-2 RNA, Pharmaceutically active compounds
Graphical Abstract
1. Introduction
Hospitals play a pivotal role in the well-being of humanity and facilitate research in the field of medical advancement. They help in complementing various parts of the health system and provide continuous services to tackle the complex health conditions of human beings [1]. The healthcare sector is one of the largest employers in the United States (US), with more than six million people employed at US hospitals with around 36.3 million admissions in 2018 [2]. The worth of the Indian health sector has been projected to jump from 140 billion U.S. dollars in the year 2016 to 372 billion dollars by the year 2022 [3]. With the onset of the COVID-19 pandemic, hospitals and other health care facilities have been responsible for giving a chance for survival to more than 20 million people affected by the SARS-CoV-2 virus. Concerning the ever-growing expansion of medication and health care activities in the hospital, the generation of large quantities of wastewater and its management is an impounding challenge in environmental engineering [1]. On average, hospitals in developed countries generate a significantly higher volume of wastewater as compared to hospitals in developing countries [1], [4], [5], [6], [7], [8].
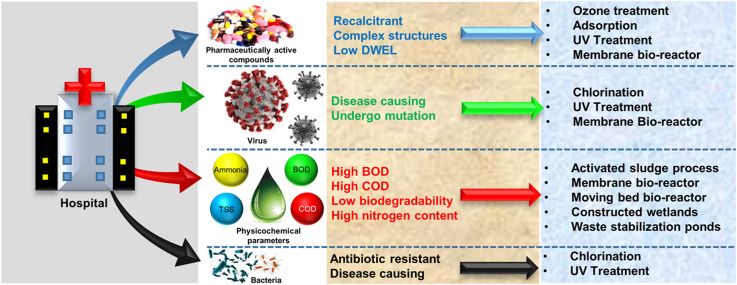
Hospital wastewater (HWW) is also characterized by the presence of various emerging contaminants, such as pharmaceutically active compounds (PhACs), several microorganisms including antibiotic-resistant bacteria (ARB), antibiotic-resistant genes (ARG), persistent viruses, etc. [9], [10], [11], [12]. Generally, HWW comprises high biochemical oxygen demand (BOD), chemical oxygen demand (COD), ammonia, and nitrogen content, and their concentration is higher compared to the domestic wastewater [13], [14]. BOD is the amount of oxygen consumed by microorganisms to decompose organic matter under aerobic conditions at a specific temperature and duration of time, while COD is the amount of oxygen equivalents consumed in the chemical oxidation of organic matter by a strong oxidant [15], [16]. Hence BOD can be referred to as the biodegradable fraction of wastewater, while COD is the measure of both biodegradable and non-biodegradable organic compounds. The ratio of BOD and COD of wastewater is referred to as the biodegradability index [16], [17]. The biodegradability index of HWW is also lower than the municipal wastewater, making them difficult to treat by conventional biological systems [13], [14], [18]. Many of the recalcitrant organic compounds present in HWW, such as PhACs, are highly toxic with very low drinking water equivalent limit (DWEL) values making them a considerable threat to the environment [19]. Viruses, ARB, and ARG continue to survive even after the treatment of HWW, and their release to the aquatic ecosystem imposes a significant threat to the environment [6], [20].
Over the years, various treatment technologies, including the biological methods, such as activated sludge process (ASP), membrane bioreactor (MBR), moving bed bioreactor (MBBR), constructed wetlands (CWs), the advanced oxidation processes, such as photocatalysis, Fenton process, etc. have been implemented to treat HWW [8], [13], [21], [22]. Many lab-based studies targeting the removal of PhACs and other recalcitrant contaminants present in HWW are reported in several works of literature, but only a handful number of pilot-scale and full-scale studies have been conducted addressing their treatment concerning HWW [8], [19], [23]. Treatment of HWW is not an easy feat, considering the vast quantities of wastewater generated having high COD, nitrogen, and PhAC content. Furthermore, the onset of COVID-19 pandemic has shifted the focus to the removal of viruses, ARG, ARB present in HWW, and this area has not been substantially addressed. Given the necessity and recent emergence of this profound health and environmental concern, the present review stems from the unavailability of comprehensive documentation in this area.
In this review, a thorough characterization of HWW has been conducted considering the variation of the characteristics of HWW in different regions. A detailed insight has been provided on the occurrence of PhACs, viruses, and several microorganisms in various HWW. A special emphasis has been given to the presence of SARS-CoV-2 and SARS-CoV in wastewater, keeping in mVerlicchiind the COVID-19 pandemic scenario. In recent years, various reviews were published on the characterization of hospital wastewater and their treatment. Khan et al. [24] reviewed the occurrence of pharmaceuticals in HWW and the performance of primary, secondary, and tertiary treatment techniques for their removal. Orias and Perrodin [25] reviewed the characteristics of hospital wastewater and its eco-toxicity. Verlicchi et al. [26] also summarized the characteristics of hospital wastewater and their treatment using conventional and advanced processes. However, most of these studies cover lab-based technologies that are still in developing stages. Performance of pilot/full-scale treatment units dedicated to the simultaneous removal of recalcitrant organic compounds, physicochemical parameters, such as BOD, COD, total suspended solids (TSS), ammonia nitrogen, total nitrogen, pathogens, etc. from HWW has not been sufficiently addressed. This review primarily focuses on the performance of various operational pilot-scale and full-scale treatment units by various biological and advanced oxidation treatment technologies dedicated to the treatment of HWW. The performance of these treatment units in terms of removal of BOD, COD, ammonia nitrogen, TSS, and PhACs has been extensively discussed. The inactivation of persistent ARG, ARB, and virus are also critically analyzed. Furthermore, the various emerging technologies to combat PhACs, ARB, ARG, such as photocatalysis, anodic oxidation, Fenton-based processes, and treatment using nanoparticles have also been discussed. A special emphasis is provided on the occurrence and removal of SARS-CoV-2 and SARS-CoV to catalyze the research on the present global need.
2. Water consumption and effluent generation from hospitals
Hospitals around the globe require large amounts of water for their proper functioning for various health care facilities. HWW, among all other healthcare waste, imposes a grave hazard to human health and the environment because of their capability to enter watersheds, pollute surface and groundwater, when inappropriately handled and disposed to hydrosphere [27]. According to the World health organization (WHO) guidelines for the proper functioning of healthcare facilities, 40–60 L/day of water is required for every inpatient. Operating theatres require around 100 L/intervention. The amount of water required for patients dealing with a severe acute respiratory syndrome or viral hemorrhagic fever is around 100–400 L/patient/day [28], [29]. This consumption of water by the hospitals leads to the generation of large volumes of wastewater [30]. The amount of wastewater generated from the hospital depends on the capacity or the number of beds available in the hospital, type and size of the healthcare facility, technical facilities available, services provided (laundry, kitchen, air-conditioning), in-house wastewater management facilities, etc. [31]. Kumari et al. [1] reported that the wastewater generated by developing countries varies from 200 to 400 L/capita/day, while in developed countries, it varies from 400 to 1200 L/capita/day. The amount of wastewater generation from various hospitals across the world has been provided in Table 1.
Table 1.
Number of in-house patients and wastewater generated daily by different hospitals across the world.
| Countries | Number of Patients | Wastewater generated (m3/d) | Wastewater generated per patient (L/patient/day) | References |
|---|---|---|---|---|
| Italy | 300 | 180 | 600 | [4] |
| Germany | 560 | 111 | 198 | [4] |
| Spain | 750 | 429 | 572 | [42] |
| Portugal | 1120 | 1000 | 892 | [7] |
| Brazil | 432 | [5] | ||
| Brazil | 325.7 | [6] | ||
| Iran | 43 | [4] | ||
| Denmark | 691 | 360 | 520 | [8] |
| Germany | 340 | 768 | 2258 | [210] |
| Germany | 580 | 200 | 344 | [8] |
| Netherlands | 1076 | 240 | 223 | [8] |
| Ethiopia | 305 | 143 | 468 | [8] |
| India | 319 | 50 | 156 | [211] |
| India | 480 | [212] | ||
| Nepal | 20 | [98] | ||
| China | 20 | [102] | ||
| Brazil | 190 | [213] | ||
| Brazil | 2000 | 219 | 109 | [6] |
| Brazil | 22,000 | 432 | 19 | [6] |
| Brazil | 320 | 220 | 687 | [8] |
| USA | 968 | [214] | ||
| Ghana | 31 | [31] | ||
| Ghana | 54 | [31] |
A hospital in Portugal, which has more than 30 clinical facilities and has a capacity of 1120 beds discharged 1000 m3 of wastewater daily [7]. An average of 30.8 L/patient/day and 54.5 L/bed/day was estimated during the sampling and analysis of two healthcare centers in Ghana [31]. On average, the hospitals in developed countries such as the United States (US), Germany, Italy, Spain, Denmark, Netherlands, and Portugal generate around 411 m3 of wastewater daily, which amounts to around 730 L/patient/day (Table 1). In comparison, the average wastewater generated by hospitals from developing or semi-developed countries, such as India, Iran, Brazil, Ethiopia, Ghana, Nepal, is around 290 m3/hospital/day and 250 L/patient/day, which is significantly less to that of the developed countries (Table 1). As per a report in 2008, the amount of wastewater generated by 19,712 hospitals in China is 1.29 × 106 m3/day, i.e., 65 m3/hospital/day [32]. The amount of wastewater generated from the hospitals only becomes a cause for concern when they are not discharged adhering to the standards and guidelines set by various organizations, such as the environment protection agency (EPA), WHO, etc. [1]. Usually, hospital effluents are discharged into the sewer systems before they are treated with municipal sewage treatment plants [21]. However, most of the sewage treatment plants are not designed to tackle bio-medical waste and persistent organic compounds, such as PhACs, personal care products, etc. [19]. Furthermore, there have been reports that many hospitals in developing countries, such as Algeria, Congo, Nepal, Pakistan, Bangladesh, Vietnam, etc. discharge their effluent into drainage systems, rivers, and lakes without any pre-treatment [21]. These hospital effluents are characterized by high COD content (120–500 mg/L), TSS (150–160 mg/L), PhACs, bacteria, viruses, etc. which can impose adverse effects on the aquatic environment [1].
3. Characteristics of hospital wastewater
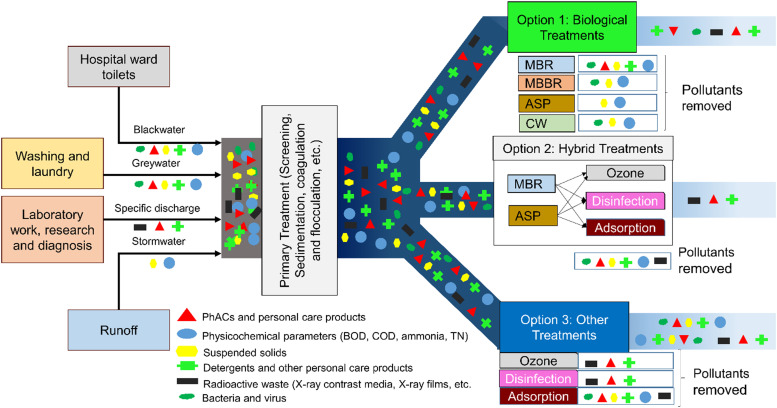
The effluent coming out of different hospitals are rich in PhACs, microorganisms, and are characterized by high COD, BOD, ammonia, nitrate, total nitrogen (TN), TSS, total organic carbon (TOC), total Kjeldahl Nitrogen (TKN), etc. Qualitative analysis of medical waste of 10 hospitals in Iran indicated that liquid waste had a 16.70% contribution to hazardous–infectious waste [33]. The discharge from hospitals can be classified into four broad categories, i.e., blackwater, greywater, stormwater, and specific discharges. The blackwater or sewage mainly comprises of the fecal matter and urine coming out of the toilets in hospital wards, which accounts for the major portion of the BOD of the wastewater [34]. The blackwater is rich in various kinds of microorganisms since fecal matter is the primary source of microorganisms in wastewater. Apart from being pathogenic, these microorganisms may also have developed antimicrobial or antibiotic resistance [35]. The fecal matters and urine also contain unmetabolized PhACs, which had been administered to the patients during treatment [26], [36]. The greywater or sullage is the water coming out of washing, bathing, laundry, and other processes like rinsing of X-Ray films, disinfection, etc. This water contains recalcitrant compounds such as surfactants, detergents, and other cytotoxic or genotoxic agents and radioactive elements [1]. The stormwater is usually lost through the drain or groundwater percolation, or reused in toilets and irrigation of hospital grounds [34]. The wastewater generated from activities pertaining to laboratory work, such as research and diagnosis, radiology department, are classified under specific discharges. This wastewater contains highly toxic substances, such as disinfectants, detergents, acids, alkalis, pharmaceutical residues, solvents, X-ray contrast media, etc. These substances are highly toxic and persistent and stay in the aqueous environment even after conventional treatment processes [1], [26], [36]. The effluents coming out of hospitals also contain toxic heavy metals, such as Cd, Cu, Ni, Hg, Sn, etc. [1], [26].
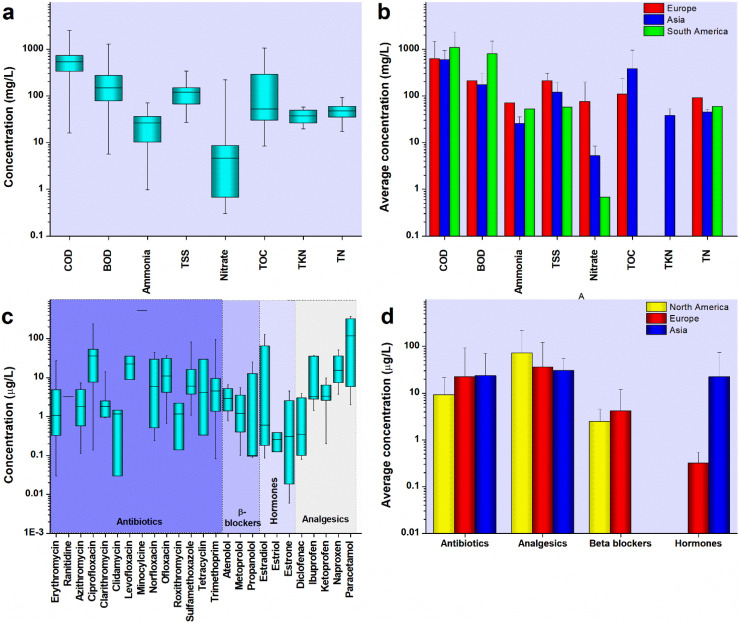
The component-specific and continent-wise concentration of various HWW parameters has been depicted in Fig. 1a and b, respectively. HWW is characterized by much lower biodegradable component as compared to domestic or municipal wastewater [13], [37], [38]. The average BOD concentration in hospital effluent of Europe and Asia was found to be around 200 mg/L, which was much lower than the BOD observed in hospital effluents of South America (Fig. 1b). A high BOD concentration of 1268 mg/L was observed in a hospital effluent of Brazil [39]. In India, the BOD concentration in some hospital effluents ranged from 92.8 mg/L to 270 mg/L with the average concentration being 153 mg/L [18], [40], [41].
Fig. 1.
Characteristics of hospital wastewater: a) range of COD, BOD, ammonia, TSS, nitrate, TOC, TKN, TN b) variation of average concentration of COD, BOD, ammonia, TSS, nitrate, TOC, TKN, TN in different continents, c) range of pharmaceutically active compounds, and d) variation of average concentration of pharmaceutically active compounds in different continents.
Data source: Tables S1 and S2 of the supplementary section.
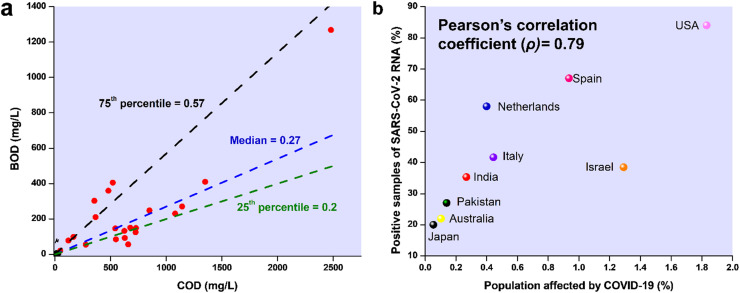
The average COD concentrations in HWW of Europe, South America, and Asia were found to be 613 mg/L, 1074 mg/L, and 591 mg/L, respectively (Fig. 1a). High COD concentrations of 2480 mg/L, 2464 mg/L, and 1142 mg/L were observed in some studies in Brazil, Spain, and India, respectively [40], [42], [43]. The average concentration of COD in HWW of South American countries was found to be higher than that of Europe and Asia (Fig. 1b). The average BOD/COD ratio for HWW around the world was found to 0.29–0.34 in Asia and Europe, respectively, which was also considerably less than the values seen in municipal wastewater, thereby making hospital effluent difficult to treat [17], [38]. The values of BOD/COD ratios have been depicted in Fig. 2a. The observed median, 25th percentile, and 75th percentile for BOD/COD ratio for hospital wastewater of various regions across the world were found to be 0.27, 0.20, and 0.57, respectively. However, high BOD/COD values of 0.75, 0.64, and 0.85 were observed in some hospital effluents of Iran, Thailand, and Brazil, respectively. The average concentration of TOC was found to be 223 mg/L, with the maximum TOC concentration of 1050 mg/L being reported from Thailand [44].
Fig. 2.
a) The BOD/COD ratio of effluents from various hospitals, b) correlation between percentage of population affected by COVID-19 and percentage of positive samples of SARS-CoV-2 RNA in water of different countries
Data source: Tables S1 and S3 of the supplementary section.
The average pH of hospital effluent was found to be around 7.5, with the maximum value being 8.7 in Spain and the minimum value being 6.42 in India in some studies [18], [42] (Table S1). The average concentration of suspended solids was found to be 119.7 mg/L and 209.5 mg/L in the HWW of Asia and Europe (Fig. 1b), respectively, with the maximum reported by Suarez et al. [42] in Spain (339 mg/L). The average ammonia, TN, TKN, and nitrate content in hospital effluent was 27.6 mg/L, 50.4 mg/L, 37.5 mg/L, and 34.4 mg/L, respectively (Fig. 1a). Lan et al. [45] reported high concentrations of nitrate (217 mg/L) in a hospital effluent of France. The concentrations of TSS, nitrate, and TN in hospital effluents of Europe were found to be higher than that of Asia and South America (Fig. 1b). HWW also hosts a range of PhACs, personal care products, bacteria, protozoa, viruses, etc. which have been addressed in the following sections.
3.1. Pharmaceutically active compounds
Emerging contaminants, such as PhACs are prevalent in the hospital effluent because of their excessive use in medical facilities, and their component-specific and continent-wise occurrence in hospital effluent has been depicted in Fig. 1c and d, respectively. Nevertheless, the concentration of analgesics, antibiotics, β-blockers, hormones, etc. in hospital effluent was found to be much higher as compared to their concentrations in domestic wastewater [19], [46], [47], [48]. Although there are hundreds of PhACs detected in HWW, in this review the PhACs, which are most commonly detected and whose concentrations are such that they may pose a threat to the environment, have been considered. Analgesics, such as acetaminophen, diclofenac, ketoprofen, ibuprofen, naproxen, etc. were frequently detected in various hospital effluents [19], [36], [46], [47], [48]. The average concentration of analgesics in hospital discharge was found to be more in North America, as compared to Asia and Europe (Fig. 1d). The concentration of acetaminophen was found to be 374 μg/L and accounted for 45% of the total PhAC average concentration in a hospital effluent of the US [36]. Langford and Thomas [49] reported 325 μg/L of acetaminophen in hospital effluents of Norway. Ibuprofen was found in the range of 2.8–36.5 μg/L in various hospital effluents of the US, Italy, Spain, and Norway [23], [36], [42], [49]. Diclofenac was found in concentrations ranging from 0.5 μg/L to 3 μg/L in HWW of Norway which was higher than the DWEL (0.2 μg/L to 0.3 μg/L) [19], [49]. Prasertkulsak et al. [50] reported around 3.8 μg/L of diclofenac in the hospital effluent of Thailand. Ciprofloxacin, sulfamethoxazole, trimethoprim, norfloxacin, and ofloxacin were the most frequently reported antibiotics in hospital effluents [19]. The average concentration of antibiotics in the hospital effluents of Asia and Europe were found to be in the same range (Fig. 1d). Researchers reported norfloxacin (29.6 μg/L), sulfamethoxazole (81 μg/L), and ciprofloxacin (237 μg/L) in various hospital effluents of India [48], [51]. Ciprofloxacin concentrations in some HWW samples of India (>200 μg/L) and Portugal (38.6 μg/L) were considerably higher than the acceptable DWEL values [19], [48], [51]. Traces of sulfamethoxazole were found in some hospital effluent of Portugal (8.7 μg/L) and the US (2.2 μg/L) [36], [52]. Ofloxacin, levofloxacin, erythromycin, azithromycin were among other antibiotics found in various hospital effluents [19], [36], [46], [47], [48]. Atenolol, metoprolol, and propranolol were the most common β-blockers found in the hospital effluents of North America and Europe (Table S2). Propranolol was detected in the range of 10 μg/L to 25 μg/L in a hospital effluent of Oslo, Norway [49]. Stimulants, such as caffeine were found in the range of 53 μg/L to 325 μg/L in the hospital discharges of the USA [36]. Hormones, such as estriol, estradiol, and estrone were detected in the range of 0.1 μg/L to 0.9 μg/L in hospital effluent of Iran, Korea, Belgium, and Norway [53], [54], [55], [56]. Prasertkulsak et al. [50] reported 128 μg/L of estradiol in the effluent of a hospital in Thailand. PhACs, such as carbamazepine, metformin, theobromine, theophylline, and gabapentin, was also common in hospital effluents of the US [36]. HWW was found to host a wide variety of PhACs and their metabolites, with analgesics and antibiotics being the most prevalent. Although the concentration of these compounds is not very high, they are highly toxic to biotic components of the environment. Most of the PhACs found in hospital effluent were at concentrations higher than the predicted no effect concentration (PNEC) values, while few PhACs, such as diclofenac and ciprofloxacin were found at concentrations higher than the DWEL values indicating a detrimental effect on human beings upon exposure [19].
3.2. Bacteria
Hospital effluents are a host to numerous bacteria and pathogenic microorganisms, such as Escherichia coli (E. coli), Enterococci, thermotolerant coliform, fecal coliform, etc. Liu et al. [32] reported around 2.40 × 106 to 1.19 × 1012 number/mL of bacteria and 9.0 × 104 to 2.38 × 1010 number/mL of coliform in HWW of Guangzhao, China. Other hospital effluents in China accounted for 9.9 × 103 to 1 × 107 PFU/L of bacteria and 16,000–108 PFU/L of fecal coliform [32]. Berto et al. [43] reported a total coliform concentration of 2 × 108 MPN/100 mL and a thermotolerant coliform concentration of 1.6 × 108 MPN/100 mL in Brazil. Beier et al. [57] reported E. coli, fecal coliform, and enterococci in the range of 103 to 106 MPN/100 mL. In a hospital effluent of France, the E. coli concentration varied from 8.3 × 104 CFU/mL 3 × 105 CFU/mL [58], [59]. In some hospital effluents of Sweden, the E. coli concentration was found to be in the range of 2.6 × 104 and 5.5 × 104 CFU/mL [9]. E. coli concentration of 5.4 × 106 CFU/mL was found in a HWW of Ireland [60]. Hocquet et al. [59] reported enterococci concentration of 6.5 × 106 MPN/100 mL and 1.4 × 106 MPN/100 mL in certain hospital effluents of France and the United Kingdom, respectively. In the wastewater of six hospitals located in India, the concentration of total coliform ranged from 0.92 × 103 to 2.4 × 103 MPN/100 mL, and fecal coliform ranged from 1.8 × 101 to 3.2 × 102 MPN/100 mL [18]. Although these microorganisms are present in significant numbers, the eminent danger lies in the presence of resistant bacteria, such as Proteus vulgaris, Pseudomonas aeruginosa, vancomycin-resistant enterococci, mycobacteria, etc. and resistant strains (Enterobacter sakazakii, Extended-Spectrum Beta-Lactamase (ESBL)-producing-strains) [59], [61].
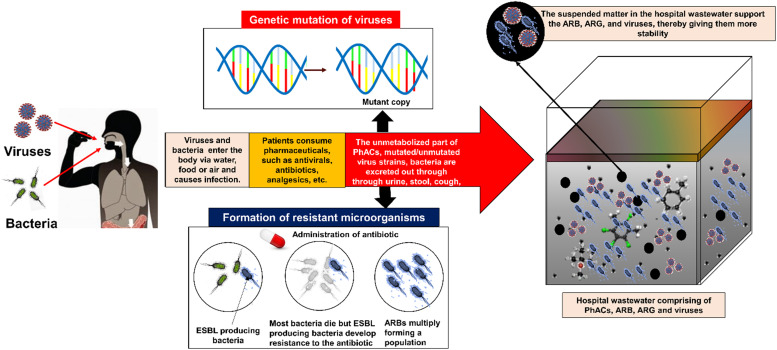
The resistance can be intrinsic or be developed due to spontaneous mutations. Intrinsic resistance belongs to those microorganisms, which can prevent the antibiotic from entering their cell-wall [62]. ESBLs are a type of enzyme that is produced by certain bacteria, such as ESBL producing E. coli (ESBLE) which makes them resistant to antibiotics. The formation of ARB and their pathways into the hospital effluent have been depicted in Fig. 3. In European countries, such as Ireland, France, Sweden, Spain, Netherlands, Poland, 3.8–13.6% of the E. coli present in hospital effluents were found to be ESBLE [59]. Chagas et al. [5] reported that out of 7.4 × 103 CFU/mL of coliforms found in a HWWs of Brazil, 38.6% were ESBL-producing coliforms. The percentage of ESBLE present in HWW was much higher compared to that in urban wastewater and discharge of wastewater treatment plant (WWTP) [59]. This was because HWW contains large amounts of antibiotics, disinfectants, etc., making the ESBL producing microorganisms resistant to them. Unlike normal E. coli, these ESBLEs produce infections in human beings that can no longer be treated by ordinary antibiotics, making them a potential cause of concern [59]. Pseudomonas aeruginosa is another multidrug-resistant pathogen found in HWW. They occur as a result of mutations or gene transfer. They can be found in the water medium, having sufficient dissolved oxygen (DO) [59]. They have the capacity to acquire resistance to multiple classes of antibiotics, thereby making infections caused by such microorganisms more complex. They have been found in HWW in the range of 4 × 103 CFU/mL, out of which 76% of them were resistant to one or more classes of antibiotics [63]. Enterococci, a very common bacteria usually found in the gastrointestinal tracts of humans and animals, have also exhibited resistance to antibiotics, and their prevalence has increased in the last few decades. In a HWW of France 6.5 × 106 CFU/mL of enterococci were detected, out of which almost all were resistant to amoxicillin [59]. A high prevalence of vancomycin-resistant enterococci was observed in hospital effluents of the United Kingdom and Portugal [64], [65], [66]. Fluoroquinolone resistant E. coli in European countries was found to increase from 25% to 50% between 2002 and 2007. Among others, Acinetobacter baumannii and Staphylococcus aureus also have shown the capacity to develop resistance to antimicrobials, such as methicillin [67]. Different phylums in HWW, such as proteobacteria, planctomycetes, nitrospirae, caldithrix, chlorobi, and acidobacteria were found to be resistant to various antibiotics, among which tetracycline was the most common [68].
Fig. 3.
Pathways of pharmaceutically active compounds, antibiotic-resistant microorganisms and viruses in hospital wastewater.
3.3. Viruses
There are more than 120 identified human enteric viruses, among which the enteroviruses (polio-, echo- and coxsackieviruses), adenoviruses, hepatitis A, rotaviruses, and human caliciviruses (noroviruses) are most prevalent in HWW [69]. The presence of viruses in HWW is a cause for major environmental and public health concerns. The outbreak of severe acute respiratory syndrome (SARS) coronavirus 2 (SARS-CoV-2) in 2019–20, leading to the global pandemic, COVID-19 is the most recent example of the threat, which viruses possess. Viruses are known to be highly stable even under adverse conditions and pose a potential threat to human health. During wastewater treatment, they may settle on the suspended matter present in the wastewater and become highly stable. The pathway of viruses into the hospital effluent has been depicted in Fig. 3. Rotavirus A, norovirus, hepatitis virus, human adenovirus, etc. have been detected in effluents from HWW treatment plants in Brazil [6]. Liu et al. [32] found that viruses may persist in HWW even if E. coli concentration is less than 50 PFU/100 mL, as they are more tolerant compared to E. coli. Ibrahim et al. [70] detected human adenovirus in the effluent of two HWW treatment units. Caudovirales, Myoviridae, Podoviridae, and Siphoviridae were commonly detected in HWWs of Israel [20], [70]. Wang et al. [71], [73] found SARS-coronavirus in HWW of China, which was persistent for up to two days at 20 °C and 14 days at 4 °C, while Gundy et al. [72] also found that there was a 99.9% reduction of the coronavirus after 12 days when the water temperature was 23 °C.
The virus, SARS-CoV-2, responsible for the pandemic in 2019–20, has been extensively studied all over the world. Since fecal shedding of SARS-CoV-2 RNA is widely reported, researchers investigated the presence of such RNA is municipal WWTPs of Spain and detected them in the untreated water [74]. The SARS-CoV-2 RNA was present in 11% of the secondary treated water samples. Wang et al. [75] confirmed the presence of SARS-CoV-2 RNA in the sewage samples of the hospital of Zhejiang University, China. In another study, SARS-CoV-2 RNA was found in high concentrations of 0.5–18.7 × 103 copies/L in the septic tanks of Wuchang Cabin Hospital, Wuhan, China [76]. This RNA persisted even after disinfection by sodium hypochlorite. The reason behind the high level of persistence may be because the virus was embedded in the stool particles. However, when the dose of sodium hypochlorite was increased, no RNA was found, but high levels of disinfection by-products were detected [76]. Ahmed et al. [77] detected 1.9–12 copies/100 mL of SARS-CoV-2 RNA in wastewater samples in Australia. In Ahmedabad, India, Kumar et al. [78] tested water samples from a WWTP receiving effluent from a hospital treating COVID-19 patients. Three genes of SARS-CoV-2, i.e., ORF1ab, N, and S genes, were detected in the influent of the WWTP. The number of copies of RNA detected increased by ten times over a period of 20 days, during which the number of COVID-19 patients also increased by two times in Ahmedabad. A similar correlation between the number of SARS-CoV-2 RNA and the number of COVID-19 patients was also observed in Australia, China, and France [76], [77], [78], [79]. Kitajima et al. [80] reported 2 × 105 and 3 × 104 copies/L SARS-CoV-2 RNA copies/L in the wastewater of Massachusetts, United States of America, and Bozeman, Montana, respectively, while wastewater in France accounted for 105 to 106 SARS-CoV-2 RNA copies/L. Haramoto et al. [82] reported 2.4 × 103 copies/L of SARS-CoV-2 RNA in the wastewater of Japan. The frequency of detection of SARS-CoV-2 RNA in the wastewater and hospital effluent samples was correlated with the percentage of people (per country) affected by the virus (Fig. 2b). The person’s correlation coefficient was calculated to be 0.79, which indicated a strong positive trend [83]. The results suggested that the occurrence of SARS-CoV-2 RNA in wastewater was directly related to the percentage of people infected. Furthermore, proper wastewater monitoring can help in identifying areas with COVID-19 infected people. Although most viruses are known to be highly stable even in adverse environmental conditions, SARS-CoV-2 was unstable in the presence of disinfectants and at a temperature higher than 20 °C [71], [84]. However, these viruses can survive when they get enveloped inside fecal particles or suspended solids. Furthermore, the entrapped virus in sewage can generate virus-laden aerosols during wastewater flushing and provide an air-borne route for the virus to transmit [85], [86].
4. Pilot/full-scale treatment systems for HWW management
4.1. Removal of BOD, COD, TSS, nitrogen, and PhACs
Over the past two decades, various treatment processes have been implemented and up-scaled to pilot or full-scale treatment system for treating HWW. The details of various pilot/full-scale treatment units have been mentioned in Table 2. The performance of the various pilot/full-scale treatment technologies in treating the different components of HWW has been discussed in the following sections and has been depicted in Fig. 4. A schematic representation of the source of different pollutants in HWW and the different pilot/full-scale treatment technologies implemented for the remediation of the pollutants have been depicted in Fig. 5. Primary treatment is required to provide a pre-treatment to the hospital wastewater. The resulting effluent can be treated using different biological processes, such as ASP, MBR, MBBR, and CWs. These biological units can also be combined with different disinfection, adsorption or advanced oxidation technologies to enhance the removal of recalcitrant organic pollutants. Tertiary treatment can also be provided directly after pre-treatment, however, the performance of these processes are usually hindered due to high organic and nutrient loading [87], [88].
Table 2.
Details of various pilot-scale and full-scale studies for remediation of hospital wastewater.
| Study number | Country | Treatment Description | Flow | HRT | SRT | Plant type | References |
|---|---|---|---|---|---|---|---|
| Study 1 | Belgium | A transportable pilot-scale subsurface flow CW (1 m3). The cubic tank was filled with a 80 cm layer of coarse Rhine gravel (8–16 mm, porosity = 40%, Macrophyte- Phragmites australis. | 200 L/day | 2 d | Pilot-scale | [96] | |
| Study 2 | Brazil | UASB followed by 3 serial anaerobic filters | 2.54 L/s | 8 h | Full-scale | [6] | |
| Study 3 | Brazil | ASP with extended aeration followed by chlorination | 5 L/s | 18 h | Full-scale | [6] | |
| Study 4 | China | MBR: 6 m3 had 2 equal parts separated by a plate. 1 hollow fiber membrane module set was submerged in each part of the reactor. Each set consisted of 24 membrane modules, total membrane area = 96 m2 | 20 m3/day | 7.2 h | 180 d | Full-scale | [102] |
| Study 5 | China | Conventional ASP (aeration) | 480 m3/day | 35 d | Full-scale | [91] | |
| Study 6 | China | Conventional ASP (aeration) | 200 m3/day | Full-scale | [91] | ||
| Study 7 | Denmark | MBBR consisted of three identical reactors of 3 L in series (M1, M2 and M3) each containing 500 AnoxKaldnes™ K5 carriers (AnoxKaldnes, Lund, Sweden), Filling ratio = 50%. The mixing was performed by aeration, Flow =. Retention time | 0.50 L/h | 6 h for each reactor | Pilot-scale | [112] | |
| Study 8 | Denmark | MBBR comprised of six reactors- M1 (900 L for BOD removal and denitrifying), M2 (900 L for nitrifying), M3A (900 L for nitrifying), M3B (900 L for nitrifying), M4 (500 L for denitrifying) and M5 (500 L for nitrifying), respectively. Filling ratio of 50% with 150,000 and 80,000 Anox K™5 carriers (AnoxKaldnes, Lund, Sweden) in the 900 L and 500 L reactors, respectively. | M1, M2, M3A, M3B = 800 L/h, M4, M5 = 300 L/h | M1, M2, M3A, M3B = 1.13 h, M4, M5 = 1.67 h | Pilot-scale | [113] | |
| Study 9 | Denmark | MBR followed by 450 mg/L of PAC | 2.2 m3/day | 35 d | Pilot-scale | [10] | |
| Study 10 | Denmark | MBR | 2.2.m3/day | 35 d | Pilot-scale | [10] | |
| Study 11 | Ethiopia | Waste Stabilization Ponds: 2 facultative ponds (667 m2), 2 maturation ponds (401 m2, 396 m2), and 1 fish pond (862 m2) | 29 d | Full-scale | [117] | ||
| Study 12 | Ethiopia | 8 horizontal subsurface flow CWs (4 m length, 1.2 m width and 0.6 m depth) with gravel and broken brick media as substrate. | 165.75 L/day | 4 d | Pilot-scale | [12], [97] | |
| Study 13 | Finland | Ultrafiltration followed by pulsed corona discharge (30 W was applied for 1 kWh /m3 of pulse energy delivered) | Pilot-scale | [119] | |||
| Study 14 | Spain | MBR (11 m3) with 10 flat sheet (FS) chloral polyethylene membranes (0.8 m2 each). Coarse bubble aeration was provided, MLSS- 8 g/L | 100 L/h | 50 h | 30 d | [100] | |
| Study 15 | France | Activated sludge incorporated with biofilms followed by ultrafiltration, Dissolved oxygen: 1–4.5 mg/L | 100 L/day | 22 h | 20 d | [90] | |
| Study 16 | Germany | The unit comprised of a MBR: Membrane area per module = 320 m2, Total membrane area = 1600 m2 | 130 m3/day | 31.3 h | Pilot-scale | [57] | |
| Study 17 | Germany | MBR comprising of Mesh, primary settling tank (21 m3: HRT= 1 h), Oxic/anaerobic chamber (56 m3, suspended solid concentration= 10–12 g/L), microfiltration (102 m3) followed by NF/RO | 130 m3/day | Pilot-scale | [104] | ||
| Study 18 | Greece | Pre-treatment (grit-removal), a mix tank, and a biological secondary treatment- Aeration tank (600 m3) followed by disinfection (chlorine dose 10–20 mg/L) | 6 h | 11 d | Full-scale | [89] | |
| Study 19 | India | Conventional ASP followed by high pressure filtration (26 pounds/cm2) and chlorination (5% hypochlorite- 35 L per 0.3 million L of water. | Pilot-scale | [101] | |||
| Study 20 | India | Horizontal sub surface flow CW (1.5 m length, 0.65 m width and 0.5 m depth) | 10 m3/day | Pilot-scale | [41] | ||
| Study 21 | Indonesia | Aerated Fixed Film bio filter Reactor followed by ozone reactor | Pilot-scale | [118] | |||
| Study 22 | Italy | Submerged MBR with UF shallow fiber membranes. biomass content (10–12 kg/m3) | 90 L/h | 14 h | 50 d | Pilot-scale | [103] |
| Study 23 | Iran | 2 sets of cylindrical columns made of Plexiglass were used as MBBR reactors. Packing= 70%, Packing material for column 1- Kaldnes (K1) (Pakan Ghatreh, Iran) Packing material for column 2- lightweight expanded clay aggregate (LECA). Column’s dimension- inside diameter, height, overflow height, total volume, and effective volume were 30 cm, 150 cm, 130 cm, 105 L, and 91 L, respectively. Air was supplied from bottom of the columns using an air, MLSS = 3000 mg/L. | 0.001–0.003 L/s | 24 h | Full-scale | [114] | |
| Study 24 | Iran | ASP (Aerobic and anaerobic zones) | Pilot-scale | [92] | |||
| Study 25 | Korea | Chemical flocculation followed by activated carbon adsorption | Full-scale | [207] | |||
| Study 26 | Luxembourg | MBR followed by UV (10 kW Medium pressure lamp, 1.11 gH2O2/L. | 3.33 m3/day | Pilot-scale | [105] | ||
| Study 27 | Nepal | The system consists of a septic tank (16.7 m3), followed by a horizontal flow CW (140 m2) with 0.65–0.75 m depth and a vertical flow CW bed (120 m2) with 1 m depth. | 20 m3/day | Full-scale | [98] | ||
| Study 28 | Saudi Arabia | ASP (Aerobic Tank) followed by sand filtration and chlorination process. | 904 m3/day | Full-scale | [93] | ||
| Study 29 | Saudi Arabia | ASP (Aeration Tank with 3 blowers) followed by sand filtration and chlorination process. | 622 m3/day | Full-scale | [93] | ||
| Study 30 | Switzerland | Primary clarifier followed by MBR ( Chamber 1 is oxic and chamber 2 is anoxic). | 1.2 m3/day | Pilot-scale | [87] | ||
| Study 31 | Switzerland | Ozonation- 1.08 gO3/g Dissolved organic carbon | 12–23 L/h | Pilot-scale | [88] | ||
| Study 32 | Switzerland | PAC- 23 mg/L | 180 L/day | Pilot-scale | [88] | ||
| Study 33 | Switzerland | UV- 2400 J/m2 | 600 L/h | Pilot-Scale | [88] | ||
| Study 34 | Switzerland | MBR ( Chamber 1 is oxic and chamber 2 is anoxic) followed by Ozonation- 1.08 gO3/g | Pilot-scale | [88] | |||
| Study 35 | Switzerland | MBR ( Chamber 1 is oxic and chamber 2 is anoxic) followed by PAC- 23 mg/L | Pilot-scale | [88] | |||
| Study 36 | Switzerland | MBR ( Chamber 1 is oxic and chamber 2 is anoxic) followed by UV- 2400 J/m2 | Pilot-scale | [88] | |||
| Study 37 | Thailand | Vertical flow CW (1.5 m length, 0.6 m width and 0.6 m depth), The media bed contained sand, pea gravel and gravel with respective height of 0.1, 0.2, and 0.4 m from top to bottom. | 75–85 L/day | 5 d | Pilot-scale | [99] | |
| Study 38 | Thailand | MBR with aeration supplied at 340 L/min | 500 L/h | 3 h | Pilot-scale | [50] | |
| Study 39 | Vietnam | Physical, chemical treatment followed by ASP | Full-scale | [11] | |||
| Study 40 | Vietnam | ASP followed by filtration | Full-scale | [11] |
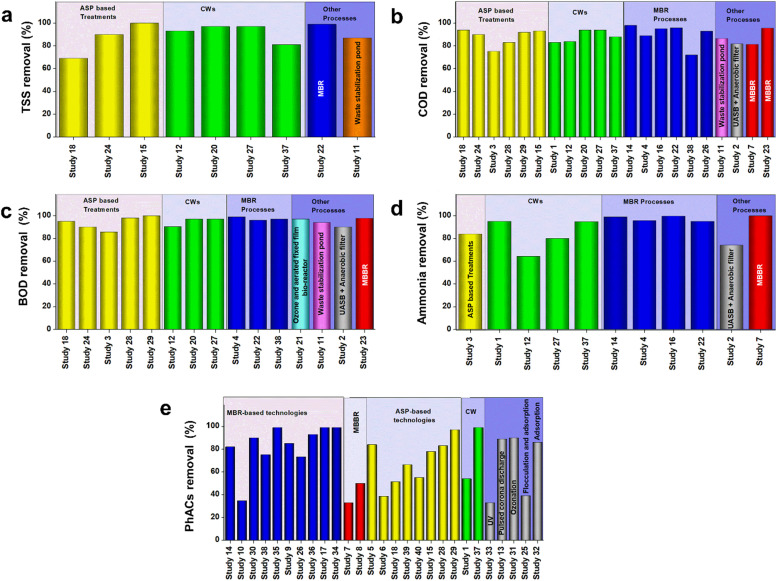
Fig. 4.
Performance of pilot-scale and full-scale studies in terms of a) TSS removal b) COD removal, c) BOD removal, d) ammonia removal, and e) PhACs removal from hospital wastewater
Data source: References in Table 2.
Fig. 5.
Schematic representation of different pilot/full-scale treatment units implemented for removing various pollutants in hospital wastewater generated from different sources.
4.1.1. Activated sludge processes
The remediation of HWW using ASP has been widely practiced in countries all over the world (Table 2). Kosma et al. [89] implemented a full-scale HWW treatment system in Greece comprising of a grit chamber, mix tanks, aeration tank, and disinfection (Table 2). The effluent from the aeration tank was treated with chlorine, and the average removal of PhAC obtained was 51.45%. Amongst the PhACs, diclofenac was found to exhibit negative removal [89]. Mousaab et al. [90] combined HDPE biofilms and ultrafiltration with ASP to treat wastewater having PhACs (Table 2). The system provided a removal percentage of around 100%, 93%, and 91% for TSS, COD, and TN, respectively. The average PhAC removal was found to be 78%. However, diclofenac, trimethoprim, and hydrochlorothiazide showed low removal of 30%, 21%, and 11%, respectively. Mousaab et al. [90] also observed that there was a significant increase in the performance of the system when the HDPE biofilms were incorporated. Yuan et al. [91] studied the performance of two conventional full-scale ASPs in HWW treatment plants in China. The average PhAC removal percentage obtained for the two ASPs were 84% and 39%, respectively. Although compounds, such as olanzapine (93–98%) and andrisperidone (72–95%), showed high removal percentages, lorazepam, oxazepam, carbamazepine, clozapine, sulpiride, and quetiapine were found to be resistant to degradation due to their complex structures [91]. Furthermore, negative removal of PhACs was also observed for compounds, such as lorazepam, oxazepam, zaleplon, sulfide, etc. in one of the ASPs. This may be the result of the conjugates of the parent compound present in the WWTP effluent return back to their parent form after undergoing enzymatic modifications in the treatment system [91]. Lien et al. [11] studied the PhACs removal from HWW of Vietnam. Two full-scale treatment units were considered for the study. The first unit comprised of physical and chemical treatment followed by a conventional ASP, and it provided an average PhAC removal of 66.3%. The second unit comprised of an additional sand filtration unit following the ASP and provided an average PhAC removal percentage of 55.2% [11]. Prado et al. [6] observed the performance of a full-scale ASP with extended aeration and chlorination to treat HWW in Brazil. The removal percentages of COD, BOD, and ammonia of the combined system were 75.3%, 85.7%, and 84%, respectively. In Iran, Azar et al. [92] achieved greater than 90% removal for TSS, COD, BOD, nitrite, and nitrate using an ASP comprising of aerobic and anaerobic zones. Al Qarni et al. [93] studied the performances of two ASPs for the treatment of HWW. The ASPs comprised of only aeration units and were followed by sand filtration and chlorination. The average PhACs removal percentage of the two treatment systems were 83% and 97%, respectively. Although more than 80% removal was achieved, negative removal was observed for nitrite and nitrate in both the systems [93]. This may be due to the fact that there was no anaerobic unit to denitrify the produced nitrate and nitrite. The aeration unit converted the present ammonia to nitrate, thereby increasing the concentration of nitrate in the effluent [93], [94].
It can be observed from Fig. 4e, that the average PhACs removal resulting from ASP based technologies varied from 40% to 99%. Furthermore, there was a significant increase in PhAC removal when chlorination was combined with ASP. This may be because the presence of chlorine in water releases various radicals with high oxidizing potential, which helps in the degradation of the complex PhACs. The average TSS removal from all the ASP-based studies was found to be higher than 90% (Fig. 4a). The removal percentages of BOD, COD, and ammonia were also found to be around 80% and higher (Fig. 4b, c, and d), suggesting that the conventional ASP, if properly modified or provided with necessary pre-treatment can be an effective solution for remediating hospital effluent.
4.1.2. Constructed wetlands
CWs are rapidly gaining popularity in the field of wastewater treatment because of their versatility and robust nature [94], [95]. Although CWs require a large amount of land and regular maintenance of the macrophytes, various removal mechanisms, such as phytoremediation, filtration, microbial degradation, adsorption, etc. occur simultaneously in CWs, making them a suitable option for HWW management. Along with the efficient removal of BOD, COD, etc., CWs have been known to degrade recalcitrant organic pollutants as well [94]. Auvinen et al. [96] implemented a transportable pilot-scale subsurface flow CW (1 m3) to treat HWW in Belgium. The main features of this CW have been mentioned in Table 2. This system achieved a COD and ammonia removal of 83% and 95%, respectively. However, negative removal for nitrate was observed, which was due to the conversion of ammonia to nitrate [96]. In another study conducted by Khan et al. [24] using constructed horizontal subsurface flow CWs (5 m long, 0.65 m wide, and 0.5 m deep) in India, similar negative removal for nitrate was observed. However, Khan et al. [41] achieved greater than 90% removal for TSS, COD, and BOD. The average removal percentage for PhACs was 54% [41]. Dires et al. [97] studied the performance of horizontal subsurface flow CWs (4 m long, 1.2 m wide, and 0.6 m deep) to treat hospital effluent (Table 2). The TSS, COD, BOD and ammonia removal obtained were 93.2%, 83.7%, 90.4%, and 64.3%, respectively. Shrestha et al. [98] combined a horizontal subsurface flow CW, having an area of 140 m2 with a vertical subsurface flow CW, having an area of 120 m2 to treat hospital effluent coming in Nepal. The wastewater entering the CWs were passed through a septic tank. The final removal percentage for TSS, COD, BOD, ammonia were 97%, 94%, 97%, and 80%, respectively [98]. Vo et al. [99] assessed the performance of a vertical flow CW to remove TSS, COD, ammonia, TN, and the paracetamol. Vo et al. [99] observed more than 99% removal of paracetamol and more than 80% removal for TSS, COD, and ammonia. However, only 22% of TN was removed, indicating incomplete denitrification of nitrate and nitrite.
The performance of CWs in terms of COD, BOD, and TSS removal was comparable to other treatment methods (Fig. 4). The removal of ammonia was a significant drawback, primarily in the case of horizontal subsurface flow CWs. This was primarily because there is insufficient dissolved oxygen present in such systems, thereby preventing complete nitrification of ammonia by aerobic microorganisms [94]. However, due to the prevailing anaerobic conditions, the horizontal subsurface flow CWs are efficient in denitrification. In the case of vertical flow CWs, effective ammonia removal could be achieved but due to the lack of denitrifying conditions, complete removal of TN could not be achieved. This drawback can be addressed by using hybrid flow CWs or combing a horizontal flow CW with a vertical flow CW because such systems provide the advantages of both horizontal and vertical flow CWs. This setup was implemented by Shrestha et al. [98].
4.1.3. Membrane bioreactors
MBR is a combination of biological treatment processes and membrane-based solid-liquid separation by microfiltration or ultrafiltration. They have gained significant attention in recent times because of their efficiency and low foot-print as compared to other treatment processes, such as CWs [50], [100], [101]. Prasertkulsak et al. [50] implemented aeration at 340 L/min to the pilot-scale MBR unit and achieved an average PhAC removal of 75.13% after a HRT of 3 h. PhACs, such as estradiol, trimethoprim, and ibuprofen, were almost completely removed, but carbamazepine and diclofenac showed very little removal in this system. Wen et al. [102] used a submerged MBR to treat hospital effluent of China and achieved more than 90% removal of BOD and ammonia. In another study, a pilot-scale submerged MBR with shallow ultrafiltration fiber membranes to treat HWW. This system provided more than 95% removal of TSS, BOD, and ammonia after a HRT of 14 h [103]. Cartagena et al. [100] used MBR based treatment to achieve more than 98% COD removal, 99% ammonia removal, and 82% TN removal. Furthermore, the system could also remove around 78–82% of the PhACs.
Kovalova et al. [87] treated hospital effluent in Switzerland using a pilot-scale MBR set-up comprising of one oxic and one anoxic chamber. The treatment unit handled a flow of 1.2 m3/day, and a primary clarifier was provided after the MBR unit. Although the average removal of PhACs was greater than 90%, the average removal of iodinated X-ray contrast media was significantly low (2%). X-ray compounds, such as phenazone and oseltamivir showed high negative removal percentage of −158% and −42%, respectively [87]. In order to remove the X-ray contrast media, Kovalova et al. [88] combined the MBR set-up separately with ozone treatment, UV treatment, and adsorption by powdered activated carbon (PAC). The removal percentage of PhACs and X-ray contrast media increased to 99% and 51%, respectively, when the effluent of the MBR was subjected to ozone treatment using 1.08 gO3/gDOC. The removal percentage of X-ray contrast media further increases to 62%, when the effluent from MBR was subjected to adsorption by PAC (dose= 23 mg/L) instead of ozone treatment. When the MBR effluent was subjected to UV treatment (2400 J/m2), degradation of X-ray contrast media increased to 66%, but the average removal of PhACs dropped to 93% [88].
In another study, a very low average PhAC removal of around 34% using an MBR pilot unit. However, PhACs, such as oxcarbamazepine, paracetamol, sulfadiazine, and sulfamethoxazole, were almost completely removed [10]. In order to increase the performance of the system, the effluent of the MBR was further treated with PAC at a dose of 450 mg/L. The adsorption enhanced the PhAC removal percentage to around 80–90% [10]. In another study, more than 95% removal for COD and ammonia was observed using an MBR after 31.3 h retention time [57]. Beier et al. [104] observed the removal of PhACs from hospital effluent using another MBR based treatment system combined with reverse osmosis. The system was able to achieve greater than 99% removal of PhACs.
A combination of MBR and UV treatment to treat hospital effluent of Luxembourg. This pilot-scale treatment unit handled a flow of about 3.33 m3/day [105]. The wastewater was subjected to the radiation of a 10 kW UV medium-pressure lamp, and hydrogen peroxide was also added to enhance the performance of the system. A removal percentage of 90% COD and 70% TN was achieved from this system. Furthermore, an average removal percentage of 73% of PhACs was observed. However, some PhACs like erythromycin and ifosfamide showed almost no removal [105].
MBR based systems could effectively remove BOD, COD, ammonia, and TSS from HWW (Fig. 4). It was also found that MBR systems can effectively remove PhACs. When they were used in the absence of any additional advanced treatment, an average removal of around 60% was observed for PhACs (Fig. S1). The performance of the MBRs further increased when the effluent from the MBR was subjected to UV treatment or adsorption. However, the maximum removal of PhACs was observed when the MBR was combined with ozone treatment or reverse osmosis (Fig. S1). MBR based technologies were found to be more effective as compared to other treatment methods demonstrating high removal of BOD, COD, TSS, ammonia, and PhAC (Fig. 4). However, MBR based technologies are subjected to clogging and fouling of the membrane. As a result, they need regular cleaning with chemicals, and maintaining them is a costly affair. Fouling of membrane brings down the performance of the MBRs [106], [107], [108], [109]. This problem can be addressed by aeration, gas-scrubbing, or regular backwashing [110], [111].
4.1.4. Moving bed bioreactor
MBBR works on the principle of biologically treating wastewater involving microorganisms present in both suspended and attached conditions [94]. Casas et al. [112] implemented a pilot-scale MBBR treatment unit using three identical reactors in series to treat HWW in Denmark. The reactors were filled up to 50% of their volume using carriers (Table 2). Aeration was provided at a rate of 0.50 L/h for proper mixing, and a retention time of 6 h was provided for the reactors [112]. Although Casas et al. [112] attained more than 99% removal for ammonium, there was negative nitrate removal. This was due to the conversion of ammonium to nitrate, and the presence of excess aerobic conditions, denitrification of nitrate could not occur. The average removal percentage for PhACs was only 33%, whereas sulfamethoxazole showed negative removal. However, ibuprofen and clindamycin showed more than 90% removal [112]. Ooi et al. [113] conducted another pilot study with six reactors with each reactor specially designed for particular purposes, such as denitrification and nitrification. However, the average removal percentage of PhACs was around 50% [113]. Shokoohi et al. [114] implemented a pilot-scale treatment unit comprising of two cylindrical columns as MBBRs to treat hospital effluent in Iran. This treatment unit could effectively reduce COD and BOD of wastewater by more than 95% [114]. It is evident from Fig. 4 that MBBR can perform effectively in terms of BOD, COD, and ammonia removal. However, denitrification is a major drawback for which additional denitrifying units have to be implemented to take into consideration the excess nitrate. Furthermore, the average PhAC removal of MBBR was also found to be less as compared to other treatment units (Fig. 4). This may be because the PhACs are toxic and kill the microorganisms, thereby limiting biological degradation [19]. Furthermore, loss of biofilms is a major problem associated with MBBR processes which can affect the performance of the systems [115], [116].
4.1.5. Other pilot/full-scale treatment units
Over the past few decades, researchers have been doing extensive research to treat hospital effluent. Although technologies based on ASP, MBR, MBBR, and CWs were more popular, various other treatment units have also been implemented to tackle HWW. Prado et al. [6] studied the performance of an up-flow anaerobic sludge blanket (UASB) followed by a filtration unit. The HRT provided for the UASB digestion was 8 h, and the effluent coming out of it was passed through three anaerobic filters in series. This full-scale treatment unit was successful in achieving 82% COD reduction, 90% BOD reduction, and 74% ammonia reduction [6].
Hunachew and Getachew [117] used waste stabilization ponds (WSP) as a full-scale treatment unit to treat hospital effluent in Ethiopia. The system achieved a removal percentage of 87%, 86%, and 94% for TSS, COD, and BOD, respectively, after an HRT of 29 days. However, there was a drastic increase in the concentration of total ammonia in the final effluent. This could be accounted for the rise in the pH of the effluent, which led to the conversion of ammonium ions to ammonia gas [117]. The reduction of TN by 54.5% and nitrate by 68% along with more than 80% removal for TSS, BOD, and COD indicated that this system is robust, it can be used to treat large volumes of wastewater with high loading.
In Indonesia, an aerated fixed film bio-filter was followed by an ozone reactor (pilot-scale) for the treatment of PhACs [118]. Almost complete removal of PhACs was observed using this treatment, indicating ozone treatment is essential to enhance the degradation of PhACs. Similarly, Kovalova et al. [88] used ozone treatment to evaluate the removal of PhACs and X-ray contrast media. 90% removal of PhACs and 50% removal of X-ray contrast media was achieved at an ozone dose of 1.08 g O3/g DOC. Kovalova et al. [88] further tested the performance of UV treatment (2400 J/m2 UV) and adsorption (PAC dose = 23 mg/L) and obtained 33% and 86%, respectively of PhAC removal and 65% and 61% of X-Ray contrast media, respectively. It can be seen that UV treatment was not as efficient as ozone treatment and adsorption for degrading PhACs. Furthermore, ozone treatment, UV treatment, adsorption are not self-sufficient treatment technologies to completely remove recalcitrant organic pollutants. However, when they were combined with a MBR system, the removal percentages get significantly improved [88]. Sim et al. [207] used chemical flocculation followed by adsorption using activated carbon in a full-scale HWW treatment unit in Korea [207]. It was observed that in spite of using adsorption using activated carbon, the average removal percentage of the PhACs was only 39%. This may be due to the low log kow values of the PhACs, making them hydrophilic in nature and preventing them from getting adsorbed [19].
Amongst other advanced oxidation processes, plasma discharge has gained substantial popularity in the past few years. Although such processes require high initial cost and skilled maintenance, they have been known to be highly effective in treating PhACs. However, the electrical energy required for these processes was found to be considerably low as compared to other advanced oxidation processes, such as photocatalysis, anodic oxidation, etc. [19]. Ajo et al. [119] used a pilot-scale treatment unit comprising of pulsed corona discharge (PCD) and ultrafiltration to treat hospital effluents of Finland. At 30 W power and a frequency of 840 Hz, most of the PhACs got degraded. The average removal percentage of the PhACs obtained was 89% with ibuprofen and caffeine, showing 50% and 19% removal, respectively [119].
4.2. Removal of bacteria
Most of the technologies employed for HWW treatment have been designed specifically to eliminate microorganisms and the pathogen indicators, such as fecal bacteria, E. coli, total coliforms, etc. Chitnis et al. [101] studied the performance of an ASP combined with high-pressure filtration (26 pounds/cm2) and chlorination (5% hypochlorite- 35 L per 0.3 million L of water) to treat HWW in India. This system could efficiently reduce the E. coli, total coliform, and enterococci count by 99% [101]. Similarly, other pilot/full-scale treatment units have achieved similar removal in terms of removal of microorganisms. More than 99% removal of total coliforms was obtained using MBR, WSP, ASP and CWs [10], [12], [92], [98], [117]. Similar results were obtained in terms of removal of E. coli, fecal bacteria, and total enterococci using MBR based technologies, ASP and CWs [10], [12], [57], [92], [98], [102], [103].
Although HWW treatment units have shown promise in terms of reduction of microbial load, the proportion of antibiotic-resistant microorganisms increase after the treatment [59]. Hocquet et al. [59] reported that there was a significant increase in ESBL producing E. coli in the effluent of the WWTP effluent. After conducting a thorough literature survey, Hocquet et al. [59] concluded that the ratio of ESBL producing E. coli to normal E. coli in the wastewater increased after undergoing treatment. On the other hand, the proportion of vancomycin producing enterococci was not altered after going through treatment units, and the proportion of multidrug-resistant P. aeruginosa was found to decrease in WWTP effluent. Although a portion of the antibiotic-resistant microorganisms has been removed in WWTPs, some strains of the resistant microorganisms are released to the environment, which may pose a serious threat to aquatic organisms [63].
Various treatment strategies have also been implemented to remove ARB and ARG from water and wastewater [62]. Due to an insufficient number of studies related to the removal of ARG and ARB from HWW, studies pertaining to the removal of ARB and ARG from all kinds of water matrices have also been highlighted in this work. Disinfection was found to be very efficient in terms of drastically reducing the number of ARB and ARG in wastewater [120], [121], [122], [123], [124], [125]. In order to inactivate the ARG inside the bacteria cells, the disinfectant should be able to pass through the cell envelope without getting bound to other cellular constituents. A sufficient quantity of disinfectant should be present to react with the ARG containing DNA, thereby making disinfectant dose a vital factor in the efficient inactivation of ARG [126], [127]. During chlorination, various oxidizing radicals are generated, which help in the inactivation of the DNA [128], [129]. Yuan et al. [130] varied the dose of chlorine from 15 to 300 mg Cl2 min/L to study its effect on the removal of ARB. A minimum dose of 60 mg Cl2 min/L was required to inactivate sulfadiazine- and erythromycin-resistant bacteria. Furthermore, only 15 mg Cl2 min/L was found to be sufficient for other types of bacteria present in the water [130]. However, a detailed quantitative real-time investigation by Yuan et al. [130] revealed that chlorination alone could not effectively remove ARG. More than 40% of erythromycin and tetracycline-resistant genes were persistent in wastewater even after chlorination. Auerbach et al. [131] revealed that UV irradiation was also not effective in removing ARG. Zhang et al. [120] studied the inactivation of ARG by only chlorine, only UV irradiation, and sequential UV/chlorination and found that the inactivation of ARG was directly proportional to chlorine dose and contact time. The required chlorine dose for the inactivation of different microorganisms has been depicted in Fig. 6. The maximum inactivation achieved was in the range of 1.30–1.49 log unit at 30 mg/L chlorine dose [120]. High intensity of UV irradiation (249.5 mJ/cm2) was also required for the irradiation to be absorbed into the RNA and DNA, thereby inactivating the ARG. The synergistic effect of chlorination and UV irradiation was also prominent as it led to better inactivation of ARG. Guo et al. [132] observed that at a dose of 80 mg Cl2 min/L, the frequency of ARG transfer was greatly suppressed. Fenton-based process, ozone treatment, and electron beam treatment have also been implemented to inactivate ARB and ARG [133], [134], [135], [136]. An absorbed dose of 0.5 kGy of the electron beam was required for 90% removal of ARG and ARB [62], [134]. The performance of ozone treatment, electron beam treatment, and Fenton processes can be further increased by the addition of hydrogen peroxide [62]. Inactivation of ARG and ARB by photocatalysis have also been studied extensively [137], [138], [139]. The generation of hydroxyl radicals, superoxide radicals, and holes take part in oxidizing the ARG and ARB. An increase in the dose of photocatalysts led to decreased survival fractions of the bacteria [139]. Although advanced oxidation processes have shown considerable promise in terms of ARG and ARB inactivation, these processes have proved to be expensive and up-scaling them to pilot/full-scale treatment system is still a major challenge [19]. Alternatively, biological processes, such as CWs and MBR have been efficient in terms of ARG and ARB inactivation [10], [12], [140], [141], [142], [143].
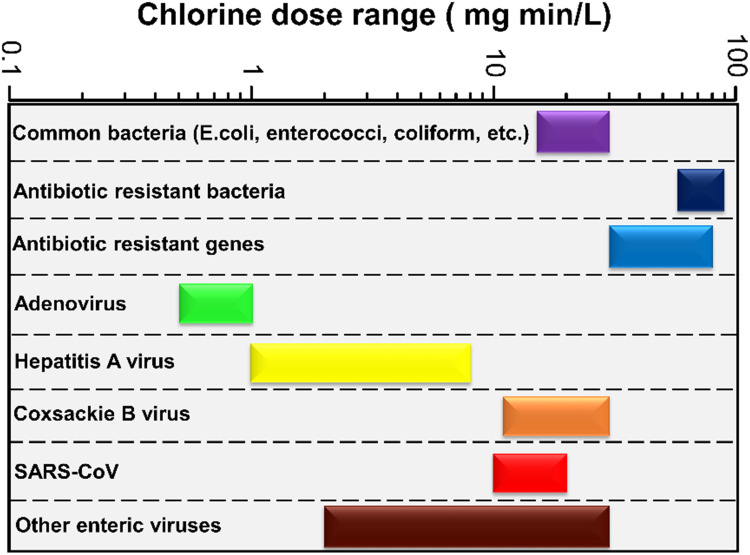
Fig. 6.
Chlorine dose required for efficient inactivation of antibiotic-resistant bacteria, antibiotic-resistant genes, viruses and other microorganisms
Data source: Table S4 of the supplementary section.
Huang et al. [140] used a vertical upflow CW to remove ARG. After a HRT of 5 days, around 99% removal of ARGs was obtained. Chen et al. [141] studied the removal of ARGs using horizontal subsurface CWs and attained around 95–99% removal after a HRT of 1.5 days. In another study, involving horizontal subsurface flow CW, 87–97% removal of ARG, such as tetA, tetB, and tetM was achieved. However, only 50% removal of ARG, sul1 was achieved [142]. The high removal of ARGs in CWs may be due to exposure to sunlight, resulting in photolysis and aerobic degradation [62]. Dires et al. [12] reported that 100% of the Salmonella isolates found in hospital effluent of Ethiopia were resistant to ampicillin and 75% of them were resistant to doxycycline, erythromycin, ceftazidime, cefoxitin, and chloramphenicol. 82% of E. coli were found to be resistant to ampicillin, and around 73% were found to be resistant to cotrimoxazole and amoxicillin-clavulanic acid [12]. After the HWW was treated using horizontal subsurface flow CWs, Dires et al. [12] observed about 93% reduction in the number of ARB. Nielsen et al. [10] achieved 100% removal of ARB from the HWW using only MBR treatment. Le et al. [143] also implemented ASP and MBR to remove ARB and ARG from wastewater. Although there was a decline in the number of ARB in the effluent of the ASP, complete removal was not achieved. However, no traces of ARB were found in the effluent of the MBR. On the other hand, the ARG present in MBR effluent was less than the detection limit of the instrument used to measure [143].
4.3. Inactivation of virus
Viruses have been found in varying quantities in treated HWW. Their numbers vary with other microbial cells in the ratio of 10:1, and the viral DNA represents 0.1% of the total DNA in the microorganisms [68]. Viruses are small infectious agents having the size of 10–200 nm in cross-section and usually get adsorbed on to the surface of the suspended solids, thereby making them more stable [32], [69]. Furthermore, they are protected by layers of fat or protein [144]. Their persistence in the treated HWW was further substantialized by Petrovich et al. [20] as they found viruses belonging to the families Myoviridae, Podoviridae, and Siphoviridae, present in the effluent of a pilot-scale ASP-based treatment unit (oxic and anoxic) in Israel. Prado et al. [6] studied the performance of two full-scale treatment units and assessed their performance in terms of virus removal. The first unit comprised of a UASB reactor followed by anaerobic filtration with a HRT of 8 h. The average viral load in the effluent was 2.8 × 103, 2.4 × 103, and 1.9 × 103 for human adenovirus, norovirus, and rotavirus, respectively. The second treatment unit was an ASP-based process with a HRT of 18 h and the viral load of the effluent was 8.1 × 102, 2.8 × 104, 1.4 × 103, and 1.2 × 105 for norovirus, hepatitis A virus, human adenovirus, and rotavirus, respectively [1], [6]. Verbyla and Mihelcic [145] found out that various WSPs used for the treatment of wastewater could only achieve one log 10 reduction of viruses after 15–20 days of HRT. Ibrahim et al. [146] reported an increase in the frequency of adenovirus after treating HWW of Tunisia using natural oxidizing lagoons and rotating bio disks. However, around 90% removal of sapovirus was achieved using the natural oxidizing lagoons and rotating bio disks [146], [147].
Few biological treatment units have been efficient in removing viruses from wastewater. Lv et al. [148] used submerged MBR and achieved almost complete removal of phage T4 (a model virus). Two different membrane modules were used for this study. In the case of the 0.22 µm module, the cake layer, the gel layer, and the membrane contributed to phage removal in 6.3, 3.1, and 1.7 log-scale, respectively. On the other hand, for the 0.1 µm module, the membrane alone was responsible for the maximum removal of phage [148]. MBR based treatment technologies have been effective in terms of virus removal as the dynamic layer on the membrane surface helps in rejection of virus, and the activated sludge helps in the inactivation of the virus [32]. Virus exclusion by membrane-based technologies, such as reverse osmosis, microfiltration, and ultrafiltration, is achieved by size exclusion mechanism. The physicochemical properties of the membrane, surface properties of the virus, and their electrostatic or hydrophobic interaction with the aqueous solution also play a significant role in the removal of viruses by membrane processes [69]. Researchers have obtained significant amount of virus removal using reverse osmosis, ultrafiltration and microfiltration [149], [150], [151], [152], [153], [154], [155], [156], [157]. The ‘capture cone’ is referred to the passage of the virus through the holes in the membrane surface, and it was found to decrease with the increase in transmembrane pressure [152]. It was observed that the aged reverse osmosis membrane helped in the adsorption of a virus surrogate (MS2 phage), and the rejection of MS2 phage was found to be in the order of 4 log-scale, in spite of damage to the membrane [157].
Viruses are also susceptible to chlorination, and UV treatment and a chlorine dose of 2 mg min/L to 30 mg min/L was found to be sufficient to obtain 4 log removal values [69]. High doses of UV (>186 mJ/cm2) could also attain the 4 log removal values for adenovirus, which is known to be one of the most resistant viruses. Virus removal in the range of 8 log removal and 10 log removal was also achieved using such traditional disinfection methods in Australia and the US, respectively [69]. In order to attain 4 log removal of adenovirus, hepatitis A, and coxsackie B virus, 0.5–1.0 mg min/L, 1.0–8.0 mg min/L, and 11.0–30.0 mg min/L of free chlorine was required (pH = 6–9 and temperature = 5–20 °C), respectively [158]. On the other hand, while using monochloramine a dose of 1000–8000 mg min/L, 1000–2000 mg min/L, and 700–3000 mg min/L was required to attain 4 log removal of adenovirus, hepatitis A, and coxsackie B virus (pH = 7–8 and temperature = 5–15 °C) [158]. The required chlorine dose for the inactivation of different viruses has been depicted in Fig. 6.
The presence of SARS-CoV-2 RNA in HWW and municipal wastewater has gained attention due to the recent outbreak of the COVID-19 pandemic. They have been reported in HWW, domestic sewage, effluents of WWTPs, raw municipal wastewater, pasteurized settled sewage, etc. [159]. The coronavirus is a virus that is enveloped in a protective layer of fat, thereby giving its stability. However, disinfectants tear apart the fat layer making them susceptible [144]. Over the years, many researchers have reported that the disinfection of the wastewater has proved to be efficient in terms of the removal of the SARS-CoV-2 virus [159]. Wang et al. [73] used disinfection by chlorine and chlorine dioxide to remove SARS-CoV from HWW of China. It was reported that free chlorine was more adept in removing SARS-CoV than chlorine dioxide and that a dose of 10 mg/L resulted in around 100% inactivation of SARS-CoV [73]. Zhang et al. [76] reported the persistence of SARS-CoV-2 RNA even after 12 h of treatment and that free chlorine was detected only up to 1.5 h after treatment. As a result, 6700 g/m3 of sodium hypochlorite was added for complete inactivation of the SARS-CoV-2 virus [76]. Lesimple et al. [160] studied the removal of various viruses having sizes less than SARS-CoV-2 and suggested the use of reverse osmosis, nanofiltration, ultrafiltration, MBR, etc. for efficient removal of SARS-CoV-2 RNA from wastewater. Ghernaout and Elboughdiri [161] suggested that merging plasma discharge, electrocoagulation could enhance the removal of SARS-CoV-2 virus from wastewater, while Ciejka et al. [162] used nano/microspheres of N-(2-hydroxypropyl)−3-trimethyl chitosan for adsorption of around 99% of the virus.
5. Emerging technologies for removal of PhACs and various pathogens
ARG, ARB, viruses, recalcitrant organic compounds, such as PhACs, personal care products, X-ray contrast media form an integral part of the HWW. The presence of these components makes the HWW less biodegradable, toxic, and difficult to treat [163]. Over the past decade, research has been focused on the removal of PhACs, ARG, ARB, and other recalcitrant organic compounds by various emerging technologies, such as photocatalytic degradation, photolysis, anodic oxidation, Fenton’s processes, treatment using nanoparticles, etc. [19], [24], [62], [163], [164], [165], [166]. These systems have been highly efficient in removing HWW specific contaminants and can be up-scaled for in-situ treatment of hospital effluents.
5.1. Photocatalytic treatment
Photocatalytic treatment involves the use of materials (photocatalysts) having a low bandgap, which are excited by photons from a given light source. When the photons have an energy greater than the bandgap of the photocatalysts, electron-hole pairs are generated. The generated holes react with the water molecules to generate hydroxyl radicals, which in turn reacts with the organic contaminants to degrade them [19], [166], [167], [168], [169]. The other reactive species generated during photocatalysis, such as superoxide radicals, singlet oxygen, and holes also have a redox potential and can actively take part in photocatalytic degradation [19], [62]. Photocatalytic treatment can effectively bring down the concentration of PhACs by around 90%. Furthermore, the reaction time in photocatalysis is also very less as compared to many biological processes [19], [166]. The performance of the photocatalytic process depends on several parameters, such as the type of catalyst used, the light source, the physicochemical properties of the PhACs, etc. [19]. Researchers have reported high removal of around 99%, 100%, 90%, 88%, 100%, 95%, 100%, 95%, 90%, for various PhACs such as ciprofloxacin, erythromycin, trimethoprim, tetracycline, sulfamethoxazole, paracetamol, naproxen, atenolol, metoprolol, respectively [19], [167], [170], [171], [172], [173], [174], [175]. Photocatalytic processes have also been known to inactivate ARB as well. UV radiation is effective for the inactivation of viruses, ARB, and ARG [62]. Tsai et al. [139] investigated the removal of methicillin-resistant S. aureus, multidrug-resistant Acinetabacter baumannii, vancomycin-resistant Enterococcus faecalis, S. aureus, A. baumannii, E. faecalis, and E. coli using titanium dioxide-based photocatalyst. The photocatalytic degradation could oxidize the bacteria and the number of bacteria was reduced by 1–3 log units [139]. Inactivation of different ARB and E. coli was also achieved by Kangwansupamonkon et al. [177] and Xiong and Hu [176] using different photocatalyst in presence of UV irradiation. The properties of photocatalysts to simultaneously degrade PhACs and oxidize microorganisms make the process a lucrative option that can be up-scaled for HWW management [164].
5.2. Fenton oxidation
Various studies have been carried out to degrade PhACs and microorganisms using Fenton-based processes [19], [62]. Hydroxyl radicals are the primary oxidizing radical in Fenton-based processes, which are generated resulting from the reaction between hydrogen peroxide and Fe+2/Fe+3 [19], [62]. One significant advantage of Fenton processes over photocatalysis is that the consumed catalyst in Fenton processes can be regenerated using photo-radiation or electrolytic forces [19]. Mondal et al. [178] observed 99.3% removal of ciprofloxacin in presence of zero-valent iron and H2O2. Real et al. [179] obtained almost complete removal of atenolol and ketoprofen in the presence of Fe 2+ and H2O2. Veloutsou et al. [180] used a Hg lamp to provide photo radiation of 290 nm in presence of Fe 2+ and H2O2 to obtain almost complete removal of atenolol. Alizadeh Fard and Barkdoll [181] used iron electrodes and provided a current intensity of 300 mA to obtain 100% removal of ketoprofen [181]. Karaolia et al. [136] investigated the performance of solar-Fenton oxidation in terms of the inactivation of ARB and achieved a 5 log reduction of ARB. In another study, 2.42–3.48 log reduction of ARGs was observed using Fenton based processes. Fenton-based processes have been efficient in the removal of PhACs and microorganism in an average reaction time of 2 h [19], [62]. However, a significant drawback of this process is that the Fenton-based processes show better performance in acidic medium (pH of 3) [19], [62].
5.3. Anodic oxidation
In anodic oxidation, water is oxidized to form hydroxyl radicals using high O2 evolution overvoltage anodes (Pt, PbO2, SnO2, BDD) [19]. The hydroxide radicals along with other oxidizing agents, such as peroxydisulfate, hypochlorous acid, biphosphate, peroxydicarbonate, etc. which are generated due to the presence of sulfate ions, chlorine ions, phosphate ions, carbonate ions, etc., also take part in the degradation of the PhACs and microorganisms [19], [182]. Boron doped diamond anode is the most commonly used anode in anodic oxidation. The anodic oxidation processes involving boron-doped diamond as the anode could effectively degrade erythromycin, paracetamol, naproxen, and trimethoprim by more than 95% [183], [184], [185], [186]. Among other electrodes, Wang et al. [187] used SnO2–Sb/Ti electrode to degrade around 99% of ciprofloxacin, while García-Gómez et al. [188] achieved more than 88% removal carbamazepine using Ti/PbO2 anodes. Jeong et al. [182] found out that the hydroxyl radicals generated during the anodic oxidation process are one of the major species responsible for the inactivation of E. coli in a chlorine-free environment. Furthermore, the inactivation of E. coli was promoted at lower temperatures [182]. In another study, a mixed metal oxide anode was used to effectively achieve log 2 reduction of Deinococcus geothermalis, Pseudoxanthomonas taiwanensis, and Meiothermus silvanus [189]. The electrical energy per order required to remove PhACs and inactivate ARB and other microorganisms was found to be less as compared to photocatalysis, ultrasound treatment, and other processes, which makes anodic oxidation a good alternative to address the HWW specific contaminants [19].
5.4. Treatment using nanoparticles
The anti-microbial property of various nanoparticles, such as silver nanoparticles, copper oxide nanoparticles, zinc oxide nanoparticles, iron oxide nanoparticles, etc. have proved to be effective in inactivating ARB and ARG [62], [190], [191], [192]. Furthermore, nanoparticles are characterized by high surface area, which facilitates the adsorption of PhACs and other contaminants present in wastewater. The various functional groups present in synthesized adsorbents also enhance the adsorption of organic contaminants [19], [193], [194], [195]. Rajendran and Sen [195] achieved around 90% removal of carbamazepine using biosynthesized hematite nanoparticles. Ali et al. [196] used composite iron nanoparticles to achieve 92% removal of ibuprofen. Metal-organic frameworks have also been efficient in terms of the removal of PhACs [193], [197]. Although nanoparticles are efficient in adsorption of PhACs, the PhACs are only transferred from the aqueous phase to a solid phase and they are not completely removed from the environment. Proper disposal of sludge is essential for the treatment of wastewater using adsorption [19], [194], [198].
6. Challenges in HWW management
One of the major challenges in the field of HWW management is the monitoring and detection of the pollutants [199]. PhACs and other recalcitrant organic compounds are present in the HWW is the range of ng/L to μg/L, which require highly sensitive to quantify [19], [36], [169], [200], [201]. Proper detection of such contaminants in necessary to implement proper legislations for HWW management [199]. Only a few guidelines pertaining to hospital waste management, such as “Effluent Guidelines and Standards (CFR 40) (NPDES) (National Pollutant Discharge Elimination System)” by EPA, 2015, “Safe management of wastes from healthcare activities” by WHO, 2013, etc. exist [1], [199]. However, these guidelines do not provide any standards for specific pollutants, such as PhAC, personal care products, etc. Lack of such legislations has led to increased disparity in the characteristics of the effluent from one country to another. Hospital effluents are often discharged into the urban sewer system, where they mix with other effluents from different sectors, before undergoing treatment in a sewage treatment plant. This practice is common in many developed and developing countries [21], [199]. However, in many other developing countries, hospital effluents are discharged into rivers, lakes, and drainage systems, without any prior treatment [21], [199]. In Pakistan, proper wastewater treatment facility lacked in many of the hospitals [202]. In Taiwan, few hospitals discharge their effluent in to the rivers with only scarce treatment [203]. In Indonesia, many of the hospitals discharge their effluent using infiltration wells or in to receiving water bodies [118]. The wastewater coming out of the hospitals without undergoing any specific or in-situ treatment comprises of PhACs and other recalcitrant components. These components, which are specific to hospital wastewater upon mixing with the municipal wastewater, make the municipal wastewater more complex and difficult to be treated. Many of the sewage treatment plants are not able to completely treat such complex wastewater because they are not designed to tackle the recalcitrant organic compounds [143], [200]. Hence, strict legislations should be implemented, which provides pollutant specific discharge standards and mandates the on-site treatment of HWW. General awareness among the public and participation of the community is integral for the proper management of HWW. However, only a small fraction of the general population understands the benefits of proper HWW management and shows interest in participating in activities related to HWW management, which causes a major hindrance to efficient HWW management [204], [205]. The recalcitrant organic compounds are often completely not removed in biological treatment methods. Due to the inconsistent chemical, physical and biological properties of the PhACs and other recalcitrant organic compounds, different removal mechanisms have to occur, in order to enhance their removal. As a result, they were found to be removed effectively when tertiary treatment processes, such as a membrane process, adsorption process or oxidation process followed the biological systems [10], [88], [89], [103], [206], [207]. There are various emerging technologies, such as photocatalysis, anodic oxidation, adsorption, Fenton oxidation, etc. which can effectively remove PhACs, personal care products, ARGs, ARB, and other recalcitrant compounds. However, most of these studies are lab-scale and conducted on synthetic wastewater. As a result, more research is required on these technologies before they can be implemented in the field [166], [174], [208], [209].
7. Summary of findings
Hospitals are significant contributors to a large amount of complex wastewater to inland surface water and municipal sewer. Furthermore, it was found that hospitals in developed countries generated much higher quantities of wastewater than developing countries. HWW comprises a wide range of contaminants, such as recalcitrant PhACs, viruses, ARG, ARB, and high nutrient content. A low biodegradability index makes the treatment of HWW more challenging. PhACs, such as diclofenac and ciprofloxacin, were present in HWW at concentrations higher than the DWEL. Among the pilot/full-scale treatment units considered in this study, MBR and ASP were found to be the most efficient. The performance of these systems got further enhanced if an additional unit, such as ozone treatment, adsorption, chlorination, reverse osmosis, nanofiltration, etc. was incorporated into the system. It was also found that the advanced treatment systems, such as adsorption, UV, or ozone treatment, could only be used as a polishing unit, and these systems alone could not produce desirable outcomes if the wastewater is not pre-treated.
Apart from the PhACs, pathogenic entities were found to be present in large numbers. Although common microorganisms, such as E. coli, fecal bacteria, coliforms were efficiently removed by WWTPs, the ARG and ARB were more persistent and required high doses of chlorination (30–80 mg min/L) or UV-treatment for their removal. SARS-CoV-2 was found in HWW and wastewater of almost all the countries severely affected by the COVID-19 pandemic. It was found that the percentage of positive samples tested for SARS-CoV-2 was directly proportional to the percentage of people affected by COVID-19 in a particular country. SARS-CoV could be inactivated at chlorine dose of 10 mg min/L which was much higher as compared to the chlorine dose required to inactivate hepatitis A virus, adenovirus, and other enteric viruses.
Treatment of HWW using domestic wastewater treatment plants should not be favored as many unique parameters in HWW cannot be removed by conventional system. Effective removal of PhACs, viruses, ARG, ARB should be given priority to secure public health. The present study summarizes various pilot/full-scale treatment units employed in HWW treatment. However, all the discussed units have certain drawbacks, such as incomplete removal of PhACs, nitrate, resistant microorganisms, high maintenance, and operational cost, etc., which should be properly addressed. Furthermore, many of the advanced treatment technologies are cost-intensive. MBRs are susceptible to membrane fouling and clogging. Regular maintenance involving chemical cleaning, back-washing of membranes need to be carried out for proper functioning of these systems. Regular monitoring of the biomass and the wastewater quality is essential for efficient functioning of these systems. The emerging technologies, such as photocatalysis, anodic oxidation, Fenton oxidation, and adsorption require more research before they can be implemented on a field-scale. Overcoming all these technological challenges along with the associated social barriers can significantly improve the HWW management sector. This review can catalyze the research in the field of treatment of HWW and the development of innovative technologies for the efficient management of emerging contaminants and pathogenic entities.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Editor: Teik Thye Lim
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jece.2020.104812.
Appendix A. Supplementary material
Supplementary material.
.
References
- 1.Kumari A., Maurya N.S., Tiwari B. Curr. Dev. Biotechnol. Bioeng. Elsevier; 2020. Hospital wastewater treatment scenario around the globe; pp. 549–570. [DOI] [Google Scholar]
- 2.J. Elflein, • Total hospitals number U.S. 1975–2018 | Statista, 2020. 〈https://www.statista.com/statistics/185843/number-of-all-hospitals-in-the-us-since-2001/〉 (accessed August 9, 2020).
- 3.S. Keelery, • India − healthcare sector size 2008–2022 | Statista, 2020. 〈https://www.statista.com/statistics/701556/healthcare-sector-size-india/〉 (accessed August 9, 2020).
- 4.Carraro E., Bonetta S., Bertino C., Lorenzi E., Bonetta S., Gilli G. Hospital effluents management: chemical, physical, microbiological risks and legislation in different countries. J. Environ. Manag. 2016;168:185–199. doi: 10.1016/j.jenvman.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Chagas T.P.G., Seki L.M., Cury J.C., Oliveira J.A.L., Dávila A.M.R., Silva D.M., Asensi M.D. Multiresistance, beta-lactamase-encoding genes and bacterial diversity in hospital wastewater in Rio de Janeiro, Brazil. J. Appl. Microbiol. 2011;111:572–581. doi: 10.1111/j.1365-2672.2011.05072.x. [DOI] [PubMed] [Google Scholar]
- 6.Prado T., Silva D.M., Guilayn W.C., Rose T.L., Gaspar A.M.C., Miagostovich M.P. Quantification and molecular characterization of enteric viruses detected in effluents from two hospital wastewater treatment plants. Water Res. 2011;45:1287–1297. doi: 10.1016/j.watres.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Varela A.R., André S., Nunes O.C., Manaia C.M. Insights into the relationship between antimicrobial residues and bacterial populations ina hospital-urban wastewater treatment plant system. Water Res. 2014;54:327–336. doi: 10.1016/j.watres.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Mozaz S., Lucas D., Barceló D. The Handbook of Environmental Chemistry Series. Springer Verlag; 2018. Full-scale plants for dedicated treatment of hospital effluents; pp. 189–208. [DOI] [Google Scholar]
- 9.Kwak Y.K., Colque P., Byfors S., Giske C.G., Möllby R., Kühn I. Surveillance of antimicrobial resistance among Escherichia coli in wastewater in Stockholm during 1 year: Does it reflect the resistance trends in the society? Int. J. Antimicrob. Agents. 2015;45:25–32. doi: 10.1016/j.ijantimicag.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen U., Hastrup C., Klausen M.M., Pedersen B.M., Kristensen G.H., Jansen J.L.C., Bak S.N., Tuerk J. Removal of APIs and bacteria from hospital wastewater by MBR plus O 3, O3 + H2O2, PAC or ClO2. Water Sci. Technol. 2013;67:854–862. doi: 10.2166/wst.2012.645. [DOI] [PubMed] [Google Scholar]
- 11.Lien L., Hoa N., Chuc N., Thoa N., Phuc H., Diwan V., Dat N., Tamhankar A., Lundborg C. Antibiotics in wastewater of a rural and an urban hospital before and after wastewater treatment, and the relationship with antibiotic use—a one year study from Vietnam. IJERPH. 2016;13:588. doi: 10.3390/ijerph13060588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dires S., Birhanu T., Ambelu A., Sahilu G. Antibiotic resistant bacteria removal of subsurface flow constructed wetlands from hospital wastewater. J. Environ. Chem. Eng. 2018;6:4265–4272. doi: 10.1016/j.jece.2018.06.034. [DOI] [Google Scholar]
- 13.Verlicchi P., Al Aukidy M., Zambello E. What have we learned from worldwide experiences on the management and treatment of hospital effluent? − an overview and a discussion on perspectives. Sci. Total Environ. 2015;514:467–491. doi: 10.1016/j.scitotenv.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tchobanoglous G., Burton F.L., Stensel H.D. Wastewater Engineering: Treatment and Reuse. 4th ed. McGraw-Hill Education,; Boston: 2004. [Google Scholar]
- 15.Hu Z., Grasso D. Encyclopedia of Analytical Science. Second ed.., 2004. Water analysis − chemical oxygen demand; pp. 325–330. [DOI] [Google Scholar]
- 16.Tchobanoglous G., Burton F.L., Stensel H.D. Wastewater Engineering: Treatment and Reuse. McGraw-Hill Education,; Boston: 2003. [Google Scholar]
- 17.Sun Y., Chen Z., Wu G., Wu Q., Zhang F., Niu Z., Hu H.Y. Characteristics of water quality of municipal wastewater treatment plants in China: implications for resources utilization and management. J. Clean. Prod. 2016;131:1–9. doi: 10.1016/j.jclepro.2016.05.068. [DOI] [Google Scholar]
- 18.Periasamy D., Sundaram A. A novel approach for pathogen reduction in wastewater treatment. J. Environ. Health Sci. Eng. 2013;11:1–9. doi: 10.1186/2052-336x-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Majumder A., Gupta B., Gupta A.K. Pharmaceutically active compounds in aqueous environment: a status, toxicity and insights of remediation. Environ. Res. 2019;176 doi: 10.1016/J.ENVRES.2019.108542. [DOI] [PubMed] [Google Scholar]
- 20.Petrovich M.L., Zilberman A., Kaplan A., Eliraz G.R., Wang Y., Langenfeld K., Duhaime M., Wigginton K., Poretsky R., Avisar D., Wells G.F. Microbial and viral communities and their antibiotic resistance genes throughout a hospital wastewater treatment system. Front. Microbiol. 2020;11:153. doi: 10.3389/fmicb.2020.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al Aukidy M., Al Chalabi S., Verlicchi P. The Handbook of Environmental Chemistry Series. Springer Verlag; 2018. Hospital wastewater treatments adopted in Asia, Africa, and Australia; pp. 171–188. [DOI] [Google Scholar]
- 22.Kümmerer K. Drugs in the environment: emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources − a review. Chemosphere. 2001;45:957–969. doi: 10.1016/S0045-6535(01)00144-8. [DOI] [PubMed] [Google Scholar]
- 23.Verlicchi P., Al Aukidy M., Galletti A., Petrovic M., Barceló D. Hospital effluent: investigation of the concentrations and distribution of pharmaceuticals and environmental risk assessment. Sci. Total Environ. 2012;430:109–118. doi: 10.1016/J.SCITOTENV.2012.04.055. [DOI] [PubMed] [Google Scholar]
- 24.Khan N., Ahmed S., Farooqi I.H., Ali I., Vambol V., Changani F., Yousefi M., Vambol S., Khan S.U., Khan A.H. Occurrence, sources and conventional treatment techniques for various antibiotics present in hospital wastewaters: a critical review. TrAC Trends Anal. Chem. 2020;129 doi: 10.1016/j.trac.2020.115921. [DOI] [Google Scholar]
- 25.Orias F., Perrodin Y. Characterisation of the ecotoxicity of hospital effluents: a review. Sci. Total Environ. 2013;454–455:250–276. doi: 10.1016/j.scitotenv.2013.02.064. [DOI] [PubMed] [Google Scholar]
- 26.Verlicchi P., Galletti A., Petrovic M., BarcelÓ D. Hospital effluents as a source of emerging pollutants: an overview of micropollutants and sustainable treatment options. J. Hydrol. 2010;389:416–428. doi: 10.1016/j.jhydrol.2010.06.005. [DOI] [Google Scholar]
- 27.Biswal S. Liquid biomedical waste management: an emerging concern for physicians. Muller J. Med. Sci. Res. 2013;4:99. doi: 10.4103/0975-9727.118238. [DOI] [Google Scholar]
- 28.S. Mehtar, Guide to infection control in the hospital, 2018.
- 29.WHO | World Health Organization, (2020). 〈https://www.who.int/〉 (accessed August 31, 2020).
- 30.Emmanuel E., Pierre M.G., Perrodin Y. Groundwater contamination by microbiological and chemical substances released from hospital wastewater: health risk assessment for drinking water consumers. Environ. Int. 2009;35:718–726. doi: 10.1016/j.envint.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Wiafe S., Nooni I., Appiah Boateng K., Nlasia M.S., Fianko S. Clinical liquid waste management in three Ghanaian healthcare facilities – a case study of Sunyani Municipality. Br. J. Environ. Sci. 2016;4:11–34. [Google Scholar]
- 32.Liu Q., Zhou Y., Chen L., Zheng X. Application of MBR for hospital wastewater treatment in China. Desalination. 2010;250:605–608. doi: 10.1016/j.desal.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taghipour H., Mosaferi M. Characterization of medical waste from hospitals in Tabriz, Iran. Sci. Total Environ. 2009;407:1527–1535. doi: 10.1016/j.scitotenv.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 34.Carraro E., Bonetta S., Bonetta S. The Handbook of Environmental Chemistry Series. Springer Verlag; 2018. Hospital wastewater: existing regulations and current trends in management; pp. 1–16. [DOI] [Google Scholar]
- 35.Šunta U., Žitnik M., Finocchiaro N.C., Bulc T.G., Torkar K.G. Faecal indicator bacteria and antibiotic-resistant β-lactamase producing Escherichia coli in blackwater: a pilot study. Arh. Hig. Rada Toksikol. 2019;70:140–148. doi: 10.2478/aiht-2019-70-3212. [DOI] [PubMed] [Google Scholar]
- 36.Oliveira T.S., Murphy M., Mendola N., Wong V., Carlson D., Waring L. Characterization of pharmaceuticals and personal care products in hospital effluent and waste water influent/effluent by direct-injection LC-MS-MS. Sci. Total Environ. 2015;518–519:459–478. doi: 10.1016/J.SCITOTENV.2015.02.104. [DOI] [PubMed] [Google Scholar]
- 37.Álvarez-Torrellas S., Peres J.A., Gil-Álvarez V., Ovejero G., García J. Effective adsorption of non-biodegradable pharmaceuticals from hospital wastewater with different carbon materials. Chem. Eng. J. 2017;320:319–329. doi: 10.1016/j.cej.2017.03.077. [DOI] [Google Scholar]
- 38.Judd S.J. The status of industrial and municipal effluent treatment with membrane bioreactor technology. Chem. Eng. J. 2016;305:37–45. doi: 10.1016/j.cej.2015.08.141. [DOI] [Google Scholar]
- 39.Wilde M.L., Montipó S., Martins A.F. Degradation of β-blockers in hospital wastewater by means of ozonation and Fe2+/ozonation. Water Res. 2014;48:280–295. doi: 10.1016/J.WATRES.2013.09.039. [DOI] [PubMed] [Google Scholar]
- 40.Khan A.H., Khan N.A., Ahmed S., Dhingra A., Singh C.P., Khan S.U., Mohammadi A.A., Changani F., Yousefi M., alam Shamshad, Vambol S., Vambol V., Khursheed A., Ali I. Application of advanced oxidation processes followed by different treatment technologies for hospital wastewater treatment. J. Clean. Prod. 2020;269 doi: 10.1016/j.jclepro.2020.122411. [DOI] [Google Scholar]
- 41.Khan N.A., El Morabet R., Khan R.A., Ahmed S., Dhingra A., Alsubih M., Khan A.R. Horizontal sub surface flow Constructed Wetlands coupled with tubesettler for hospital wastewater treatment. J. Environ. Manag. 2020;267 doi: 10.1016/j.jenvman.2020.110627. [DOI] [PubMed] [Google Scholar]
- 42.Suarez S., Lema J.M., Omil F. Pre-treatment of hospital wastewater by coagulation-flocculation and flotation. Bioresour. Technol. 2009;100:2138–2146. doi: 10.1016/j.biortech.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 43.Berto J., Rochenbach G.C., Barreiros M.A.B., Corrêa A.X.R., Peluso-Silva S., Radetski C.M. Physico-chemical, microbiological and ecotoxicological evaluation of a septic tank/Fenton reaction combination for the treatment of hospital wastewaters. Ecotoxicol. Environ. Saf. 2009;72:1076–1081. doi: 10.1016/j.ecoenv.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Kajitvichyanukul P., Suntronvipart N. Evaluation of biodegradability and oxidation degree of hospital wastewater using photo-Fenton process as the pretreatment method. J. Hazard. Mater. 2006;138:384–391. doi: 10.1016/j.jhazmat.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 45.Lan Y., Groenen-Serrano K., Coetsier C., Causserand C. Nanofiltration performances after membrane bioreactor for hospital wastewater treatment: fouling mechanisms and the quantitative link between stable fluxes and the water matrix. Water Res. 2018;146:77–87. doi: 10.1016/j.watres.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Kasprzyk-Hordern B., Dinsdale R.M., Guwy A.J. The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK. Water Res. 2008;42:3498–3518. doi: 10.1016/j.watres.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 47.K’oreje K.O., Vergeynst L., Ombaka D., De Wispelaere P., Okoth M., Van Langenhove H., Demeestere K. Occurrence patterns of pharmaceutical residues in wastewater, surface water and groundwater of Nairobi and Kisumu city, Kenya. Chemosphere. 2016;149:238–244. doi: 10.1016/j.chemosphere.2016.01.095. [DOI] [PubMed] [Google Scholar]
- 48.Balakrishna K., Rath A., Praveenkumarreddy Y., Guruge K.S., Subedi B. A review of the occurrence of pharmaceuticals and personal care products in Indian water bodies. Ecotoxicol. Environ. Saf. 2017;137:113–120. doi: 10.1016/J.ECOENV.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 49.Langford K.H., Thomas K.V. Determination of pharmaceutical compounds in hospital effluents and their contribution to wastewater treatment works. Environ. Int. 2009;35:766–770. doi: 10.1016/j.envint.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 50.Prasertkulsak S., Chiemchaisri C., Chiemchaisri W., Itonaga T., Yamamoto K. Removals of pharmaceutical compounds from hospital wastewater in membrane bioreactor operated under short hydraulic retention time. Chemosphere. 2016;150:624–631. doi: 10.1016/j.chemosphere.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 51.Diwan V., Tamhankar A.J., Khandal R.K., Sen S., Aggarwal M., Marothi Y., Iyer R.V., Sundblad-Tonderski K., Stålsby- Lundborg C. Antibiotics and antibiotic-resistant bacteria in waters associated with a hospital in Ujjain, India. BMC Public Health. 2010;10:414. doi: 10.1186/1471-2458-10-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santos L.H.M.L.M., Gros M., Rodriguez-Mozaz S., Delerue-Matos C., Pena A., Barceló D., Montenegro M.C.B.S.M. Contribution of hospital effluents to the load of pharmaceuticals in urban wastewaters: identification of ecologically relevant pharmaceuticals. Sci. Total Environ. 461–. 2013;462:302–316. doi: 10.1016/J.SCITOTENV.2013.04.077. [DOI] [PubMed] [Google Scholar]
- 53.Pauwels B., Noppe H., De Brabander H., Verstraete W. Comparison of steroid hormone concentrations in domestic and hospital wastewater treatment plants. J. Environ. Eng. 2008;134:933–936. doi: 10.1061/ASCE0733-93722008134:11933. [DOI] [Google Scholar]
- 54.Sim W.-J., Lee J.-W., Lee E.-S., Shin S.-K., Hwang S.-R., Oh J.-E. Occurrence and distribution of pharmaceuticals in wastewater from households, livestock farms, hospitals and pharmaceutical manufactures. Chemosphere. 2011;82:179–186. doi: 10.1016/J.CHEMOSPHERE.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 55.Amin M.M., Bina B., Ebrahimi A., Yavari Z., Mohammadi F., Rahimi S. The occurrence, fate, and distribution of natural and synthetic hormones in different types of wastewater treatment plants in Iran. Chin. J. Chem. Eng. 2018;26:1132–1139. doi: 10.1016/J.CJCHE.2017.09.005. [DOI] [Google Scholar]
- 56.Avber M., S€ J., Heath E. Dynamics of steroid estrogen daily concentrations in hospital effluent and connected waste water treatment plant. J. Environ. Monit. Cite. 2011;13:2221–2226. doi: 10.1039/c1em10147a. [DOI] [PubMed] [Google Scholar]
- 57.Beier S., Cramer C., Mauer C., Köster S., Schröder H.F., Pinnekamp J. MBR technology: a promising approach for the (pre-)treatment of hospital wastewater. Water Sci. Technol. 2012;65:1648–1653. doi: 10.2166/wst.2012.880. [DOI] [PubMed] [Google Scholar]
- 58.Bréchet C., Guyeux C., Talon D., Hocquet D., Plantin J., Sauget M., Thouverez M., Cholley P., Bertrand X. Wastewater treatment plants release large amounts of Extended-Spectrum β-Lactamase-Producing Escherichia coli Into the environment. Clin. Infect. Dis. 2014;58:1658–1665. doi: 10.1093/cid/ciu190. [DOI] [PubMed] [Google Scholar]
- 59.Hocquet D., Muller A., Bertrand X. What happens in hospitals does not stay in hospitals: antibiotic-resistant bacteria in hospital wastewater systems. J. Hosp. Infect. 2016;93:395–402. doi: 10.1016/j.jhin.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 60.Galvin S., Boyle F., Hickey P., Vellinga A., Morris D., Cormican M. Enumeration and characterization of antimicrobial-resistant Escherichia coli bacteria in effluent from municipal, hospital, and secondary treatment facility sources. Appl. Environ. Microbiol. 2010;76:4772–4779. doi: 10.1128/AEM.02898-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boillot C., Bazin C., Tissot-Guerraz F., Droguet J., Perraud M., Cetre J.C., Trepo D., Perrodin Y. Daily physicochemical, microbiological and ecotoxicological fluctuations of a hospital effluent according to technical and care activities. Sci. Total Environ. 2008;403:113–129. doi: 10.1016/j.scitotenv.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 62.Sharma V.K., Johnson N., Cizmas L., McDonald T.J., Kim H. A review of the influence of treatment strategies on antibiotic resistant bacteria and antibiotic resistance genes. Chemosphere. 2016;150:702–714. doi: 10.1016/j.chemosphere.2015.12.084. [DOI] [PubMed] [Google Scholar]
- 63.Slekovec C., Plantin J., Cholley P., Thouverez M., Talon D., Bertrand X., Hocquet D. Tracking down antibiotic-resistant pseudomonas aeruginosa Isolates in a wastewater network. PLoS One. 2012;7 doi: 10.1371/journal.pone.0049300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caplin J.L., Hanlon G.W., Taylor H.D. Presence of vancomycin and ampicillin-resistant Enterococcus faecium of epidemic clonal complex-17 in wastewaters from the south coast of England. Environ. Microbiol. 2008;10:885–892. doi: 10.1111/j.1462-2920.2007.01507.x. [DOI] [PubMed] [Google Scholar]
- 65.Varela A.R., Ferro G., Vredenburg J., Yanik M., Vieira L., Rizzo L., Lameiras C., Manaia C.M. Vancomycin resistant enterococci: from the hospital effluent to the urban wastewater treatment plant. Sci. Total Environ. 2013;450–451:155–161. doi: 10.1016/j.scitotenv.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 66.Narciso-Da-Rocha C., Varela A.R., Schwartz T., Nunes O.C., Manaia C.M. BlaTEM and vanA as indicator genes of antibiotic resistance contamination in a hospital-urban wastewater treatment plant system. J. Glob. Antimicrob. Resist. 2014;2:309–315. doi: 10.1016/j.jgar.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 67.Harris S.J., Cormican M., Cummins E. Antimicrobial residues and antimicrobial-resistant bacteria: impact on the microbial environment and risk to human health − a review. Human Ecol. Risk Assess. Int. J. 2012;18:767–809. doi: 10.1080/10807039.2012.688702. [DOI] [Google Scholar]
- 68.Talan A., Tyagi R.D. Current Developments in Biotechnology and Bioengineering. Elsevier; 2020. Fate of pathogens and viruses in hospital wastewater and their treatment methods; pp. 149–175. [DOI] [Google Scholar]
- 69.Pype M.L., Lawrence M.G., Keller J., Gernjak W. Reverse osmosis integrity monitoring in water reuse: the challenge to verify virus removal − a review. Water Res. 2016;98:384–395. doi: 10.1016/j.watres.2016.04.040. [DOI] [PubMed] [Google Scholar]
- 70.Ibrahim C., Hassen A., Pothier P., Mejri S., Hammami S. Molecular detection and genoty[1] S. Profile, S. Hammami, S. Mejri, C. Ibrahim, I. Mehri, A. Hassen, P. Pothier, Removal of human astroviruses from hospital wastewater by two biological treatment methods: natural oxidizing lagoons and rotating biodisks. Environ. Sci. Pollut. Res. 2018;25:10977–10987. doi: 10.1007/s11356-018-1399-2. [DOI] [PubMed] [Google Scholar]
- 71.Wang X.W., Li J.S., Jin M., Zhen B., Kong Q.X., Song N., Xiao W.J., Yin J., Wei W., Wang G.J., Si B.Y., Guo B.Z., Liu C., Ou G.R., Wang M.N., Fang T.Y., Chao F.H., Li J.W. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol. Methods. 2005;126:171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food Environ. Virol. 2009;1:10–14. doi: 10.1007/s12560-008-9001-6. [DOI] [Google Scholar]
- 73.Wang X.W., Li J.S., Guo T.K., Zhen B., Kong Q.X., Yi B., Li Z., Song N., Jin M., Xiao W.J., Zhu X.M., Gu C.Q., Yin J., Wei W., Yao W., Liu C., Li J.F., Ou G.R., Wang M.N., Fang T.Y., Wang G.J., Qiu Y.H., Wu H.H., Chao F.H., Li J.W. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan Hospital and the 309th Hospital. J. Virol. Methods. 2005;128:156–161. doi: 10.1016/j.jviromet.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang J., Feng H., Zhang S., Ni Z., Ni L., Chen Y., Zhuo L., Zhong Z., Qu T. SARS-CoV-2 RNA detection of hospital isolation wards hygiene monitoring during the Coronavirus Disease 2019 outbreak in a Chinese hospital. Int. J. Infect. Dis. 2020;94:103–106. doi: 10.1016/j.ijid.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang D., Ling H., Huang X., Li J., Li W., Yi C., Zhang T., Jiang Y., He Y., Deng S., Zhang X., Wang X., Liu Y., Li G., Qu J. Potential spreading risks and disinfection challenges of medical wastewater by the presence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of Fangcang Hospital. Sci. Total Environ. 2020;741 doi: 10.1016/j.scitotenv.2020.140445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: A proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., Joshi C.G. The first proof of the capability of wastewater surveillance for COVID-19 in India through the detection of the genetic material of SARS-CoV-2. MedRxiv. 2020 doi: 10.1101/2020.06.16.20133215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kitajima M., Iker B.C., Rachmadi A.T., Haramoto E., Gerba C.P. Quantification and genetic analysis of Salivirus/Klassevirus in wastewater in Arizona, USA. Food Environ. Virol. 2014;6:213–216. doi: 10.1007/s12560-014-9148-2. [DOI] [PubMed] [Google Scholar]
- 80.Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Haramoto E., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ratnasari D., Nazir F., Toresano L.O.H.Z., Pawiro S.A., Soejoko D.S. The correlation between effective renal plasma flow (ERPF) and glomerular filtration rate (GFR) with renal scintigraphy 99mTc-DTPA study. J. Phys.: Conf. Ser. 2016;694 doi: 10.1088/1742-6596/694/1/012062. [DOI] [Google Scholar]
- 84.Race M., Ferraro A., Galdiero E., Guida M., Núñez-Delgado A., Pirozzi F., Siciliano A., Fabbricino M. Current emerging SARS-CoV-2 pandemic: Potential direct/indirect negative impacts of virus persistence and related therapeutic drugs on the aquatic compartments. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qu G., Li X., Hu L., Jiang G. An imperative need for research on the role of environmental factors in transmission of Novel Coronavirus (COVID-19) Environ. Sci. Technol. 2020;54:3730–3732. doi: 10.1021/acs.est.0c01102. [DOI] [PubMed] [Google Scholar]
- 86.Arslan M., Xu B., Gamal El-Din M. Transmission of SARS-CoV-2 via fecal-oral and aerosols–borne routes: environmental dynamics and implications for wastewater management in underprivileged societies. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kovalova L., Siegrist H., Singer H., Wittmer A., McArdell C.S. Hospital wastewater treatment by membrane bioreactor: performance and efficiency for organic micropollutant elimination. Environ. Sci. Technol. 2012;46:1536–1545. doi: 10.1021/es203495d. [DOI] [PubMed] [Google Scholar]
- 88.Kovalova L., Siegrist H., Von Gunten U., Eugster J., Hagenbuch M., Wittmer A., Moser R., McArdell C.S. Elimination of micropollutants during post-treatment of hospital wastewater with powdered activated carbon, ozone, and UV. Environ. Sci. Technol. 2013;47:7899–7908. doi: 10.1021/es400708w. [DOI] [PubMed] [Google Scholar]
- 89.Kosma C.I., Lambropoulou D.A., Albanis T.A. Occurrence and removal of PPCPs in municipal and hospital wastewaters in Greece. J. Hazard. Mater. 2010;179:804–817. doi: 10.1016/j.jhazmat.2010.03.075. [DOI] [PubMed] [Google Scholar]
- 90.Mousaab A., Claire C., Magali C., Christophe D. Upgrading the performances of ultrafiltration membrane system coupled with activated sludge reactor by addition of biofilm supports for the treatment of hospital effluents. Chem. Eng. J. 2015;262:456–463. doi: 10.1016/j.cej.2014.09.069. [DOI] [Google Scholar]
- 91.Yuan S., Jiang X., Xia X., Zhang H., Zheng S. Detection, occurrence and fate of 22 psychiatric pharmaceuticals in psychiatric hospital and municipal wastewater treatment plants in Beijing, China. Chemosphere. 2013;90:2520–2525. doi: 10.1016/j.chemosphere.2012.10.089. [DOI] [PubMed] [Google Scholar]
- 92.Azar A., Jelogir A., Bidhendi G., Mehrdadi N., Zaredar N., Poshtegal M. Investigation of optimal method for hospital wastewater treatment. J. Food Agric. Environ. 2010;8:1199–1202. [Google Scholar]
- 93.Al Qarni H., Collier P., O’Keeffe J., Akunna J. Investigating the removal of some pharmaceutical compounds in hospital wastewater treatment plants operating in Saudi Arabia. Environ. Sci. Pollut. Res. 2016;23:13003–13014. doi: 10.1007/s11356-016-6389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jain M., Majumder A., Ghosal P., Gupta A.K. A review on treatment of petroleum refinery and petrochemical plant wastewater: a special emphasis on constructed wetlands. J. Environ. Manag. 2020;272 doi: 10.1016/j.jenvman.2020.111057. [DOI] [PubMed] [Google Scholar]
- 95.Varma M., Gupta A.K., Ghosal P.S., Majumder A. A review on performance of constructed wetlands in tropical and cold climate: Insights of mechanism, role of influencing factors, and system modification in low temperature. Sci. Total Environ. 2021;755 doi: 10.1016/j.scitotenv.2020.142540. [DOI] [PubMed] [Google Scholar]
- 96.Auvinen H., Havran I., Hubau L., Vanseveren L., Gebhardt W., Linnemann V., Van Oirschot D., Du Laing G., Rousseau D.P.L. Removal of pharmaceuticals by a pilot aerated sub-surface flow constructed wetland treating municipal and hospital wastewater. Ecol. Eng. 2017;100:157–164. doi: 10.1016/j.ecoleng.2016.12.031. [DOI] [Google Scholar]
- 97.Dires S., Birhanu T., Ambelu A. Use of broken brick to enhance the removal of nutrients in subsurface flow constructed wetlands receiving hospital wastewater. Water Sci. Technol. 2019;79:156–164. doi: 10.2166/WST.2019.037. [DOI] [PubMed] [Google Scholar]
- 98.Shrestha R.R., Haberl R., Laber J. Water Science and Technology. IWA Publishing; 2001. Constructed Wetland technology transfer to Nepal; pp. 345–350. [DOI] [PubMed] [Google Scholar]
- 99.Vo H.N.P., Koottatep T., Chapagain S.K., Panuvatvanich A., Polprasert C., Nguyen T.M.H., Chaiwong C., Nguyen N.L. Removal and monitoring acetaminophen-contaminated hospital wastewater by vertical flow constructed wetland and peroxidase enzymes. J. Environ. Manag. 2019;250 doi: 10.1016/j.jenvman.2019.109526. [DOI] [PubMed] [Google Scholar]
- 100.Cartagena P., Kaddouri M. El, Cases V., Trapote A., Prats D. Reduction of emerging micropollutants, organic matter, nutrients and salinity from real wastewater by combined MBR-NF/RO treatment. Sep. Purif. Technol. 2013;110:132–143. doi: 10.1016/j.seppur.2013.03.024. [DOI] [Google Scholar]
- 101.Chitnis V., Chitnis S., Vaidya K., Ravikant S., Patil S., Chitnis D.S. Bacterial population changes in hospital effluent treatment plant in central India. Water Res. 2004;38:441–447. doi: 10.1016/j.watres.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 102.Wen X., Ding H., Huang X., Liu R. Treatment of hospital wastewater using a submerged membrane bioreactor. Process Biochem. 2004;39:1427–1431. doi: 10.1016/S0032-9592(03)00277-2. [DOI] [Google Scholar]
- 103.Verlicchi P., Galletti A., Masotti L. Management of hospital wastewaters: the case of the effluent of a large hospital situated in a small town. Water Sci. Technol. 2010;61:2507–2519. doi: 10.2166/wst.2010.138. [DOI] [PubMed] [Google Scholar]
- 104.Beier S., Köster S., Veltmann K., Schröder H.F., Pinnekamp J. Treatment of hospital wastewater effluent by nanofiltration and reverse osmosis. Water Sci. Technol. 2010;61:1691–1698. doi: 10.2166/wst.2010.119. [DOI] [PubMed] [Google Scholar]
- 105.Köhler C., Venditti S., Igos E., Klepiszewski K., Benetto E., Cornelissen A. Elimination of pharmaceutical residues in biologically pre-treated hospital wastewater using advanced UV irradiation technology: a comparative assessment. J. Hazard. Mater. 2012;239–240:70–77. doi: 10.1016/j.jhazmat.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 106.Mutamim N.S.A., Noor Z.Z., Hassan M.A.A., Olsson G. Application of membrane bioreactor technology in treating high strength industrial wastewater: a performance review. Desalination. 2012;305:1–11. doi: 10.1016/j.desal.2012.07.033. [DOI] [Google Scholar]
- 107.Zsirai T., Buzatu P., Aerts P., Judd S. Efficacy of relaxation, backflushing, chemical cleaning and clogging removal for an immersed hollow fibre membrane bioreactor. Water Res. 2012;46:4499–4507. doi: 10.1016/j.watres.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 108.Perreault F., Jaramillo H., Xie M., Ude M., Nghiem L.D., Elimelech M. Biofouling mitigation in forward osmosis using graphene oxide functionalized thin-film composite membranes. Environ. Sci. Technol. 2016;50:5840–5848. doi: 10.1021/acs.est.5b06364. [DOI] [PubMed] [Google Scholar]
- 109.Bouhabila E.H., Ben Aïm R., Buisson H. Fouling characterisation in membrane bioreactors. Sep. Purif. Technol. 2001;22–23:123–132. doi: 10.1016/S1383-5866(00)00156-8. [DOI] [Google Scholar]
- 110.King C. Membranes and wastewater: modular treatment systems offer solutions. Filtr. Sep. 2007;44:18–20. doi: 10.1016/S0015-1882(07)70143-X. [DOI] [Google Scholar]
- 111.Pang H., Wu P., Li L., Yu Z., Zhang Z. Effective biodegradation of organic matter and biogas reuse in a novel integrated modular anaerobic system for rural wastewater treatment: a pilot case study. Chem. Eng. 2017;119:131–139. doi: 10.1016/j.cep.2017.04.006. [DOI] [Google Scholar]
- 112.Casas M.E., Chhetri R.K., Ooi G., Hansen K.M.S., Litty K., Christensson M., Kragelund C., Andersen H.R., Bester K. Biodegradation of pharmaceuticals in hospital wastewater by staged Moving Bed Biofilm Reactors (MBBR) Water Res. 2015;83:293–302. doi: 10.1016/j.watres.2015.06.042. [DOI] [PubMed] [Google Scholar]
- 113.Ooi G.T.H., Tang K., Chhetri R.K., Kaarsholm K.M.S., Sundmark K., Kragelund C., Litty K., Christensen A., Lindholst S., Sund C., Christensson M., Bester K., Andersen H.R. Biological removal of pharmaceuticals from hospital wastewater in a pilot-scale staged moving bed biofilm reactor (MBBR) utilising nitrifying and denitrifying processes. Bioresour. Technol. 2018;267:677–687. doi: 10.1016/j.biortech.2018.07.077. [DOI] [PubMed] [Google Scholar]
- 114.Shokoohi R., Asgari G., Leili M., Khiadani M., Foroughi M., Sedighi M. Hemmat, modelling of moving bed biofilm reactor (MBBR) efficiency on hospital wastewater (HW) treatment: a comprehensive analysis on BOD and COD removal. Int. J. Environ. Sci. Technol. 2017;14:841–852. doi: 10.1007/s13762-017-1255-9. [DOI] [Google Scholar]
- 115.Hoang V., Delatolla R., Abujamel T., Mottawea W., Gadbois A., Laflamme E., Stintzi A. Nitrifying moving bed biofilm reactor (MBBR) biofilm and biomass response to long term exposure to 1°C. Water Res. 2014;49:215–224. doi: 10.1016/j.watres.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 116.Barwal A., Chaudhary R. To study the performance of biocarriers in moving bed biofilm reactor (MBBR) technology and kinetics of biofilm for retrofitting the existing aerobic treatment systems: a review. Rev. Environ. Sci. Biotechnol. 2014;13:285–299. doi: 10.1007/s11157-014-9333-7. [DOI] [Google Scholar]
- 117.Hunachew B., Getachew R. Assessment of waste stabilization ponds for the treatment of hospital wastewater: the case of Hawassa University Referral Hospital. World Appl. Sci. J. 2011;15:142–150. 〈https://www.researchgate.net/publication/278882875〉 accessed July 23, 2020. [Google Scholar]
- 118.Prayitno, Kusuma Z., Yanuwiadi B., Laksmono R.W., Kamahara H., Daimon H. Hospital wastewater treatment using aerated fixed film Biofilter--ozonation (Af2b/O3) Adv. Environ. Biol. 2014:1251–1260. [Google Scholar]
- 119.Ajo P., Preis S., Vornamo T., Mänttäri M., Kallioinen M., Louhi-Kultanen M. Hospital wastewater treatment with pilot-scale pulsed corona discharge for removal of pharmaceutical residues. J. Environ. Chem. Eng. 2018;6:1569–1577. doi: 10.1016/J.JECE.2018.02.007. [DOI] [Google Scholar]
- 120.Zhang Y., Zhuang Y., Geng J., Zhang Y., Ding L., Xu K. Inactivation of antibiotic resistance genes in municipal wastewater by chlorination, ultraviolet, and ozonation disinfection. Environ. Sci. Pollut. Res. 2015;22:7037–7044. doi: 10.1007/s11356-014-3919-z. [DOI] [PubMed] [Google Scholar]
- 121.Al-Jassim N., Ansari M.I., Harb M., Hong P.Y. Removal of bacterial contaminants and antibiotic resistance genes by conventional wastewater treatment processes in Saudi Arabia: is the treated wastewater safe to reuse for agricultural irrigation? Water Res. 2015;73:277–290. doi: 10.1016/j.watres.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 122.Fiorentino A., Ferro G., Alferez M.C., Polo-López M.I., Fernández-Ibañez P., Rizzo L. Inactivation and regrowth of multidrug resistant bacteria in urban wastewater after disinfection by solar-driven and chlorination processes. J. Photochem. Photobiol. B Biol. 2015;148:43–50. doi: 10.1016/j.jphotobiol.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 123.Childress H., Sullivan B., Kaur J., Karthikeyan R. Effects of ultraviolet light disinfection on tetracycline-resistant bacteria in wastewater effluents. J. Water Health. 2014;12:404–409. doi: 10.2166/wh.2013.257. [DOI] [PubMed] [Google Scholar]
- 124.Pang Y., Huang J., Xi J., Hu H., Zhu Y. Effect of ultraviolet irradiation and chlorination on ampicillin-resistant Escherichia coli and its ampicillin resistance gene. Front. Environ. Sci. Eng. 2016;10:522–530. doi: 10.1007/s11783-015-0779-9. [DOI] [Google Scholar]
- 125.Jia S., Shi P., Hu Q., Li B., Zhang T., Zhang X.X. Bacterial community shift drives antibiotic resistance promotion during drinking water chlorination. Environ. Sci. Technol. 2015;49:12271–12279. doi: 10.1021/acs.est.5b03521. [DOI] [PubMed] [Google Scholar]
- 126.Dodd M.C. Potential impacts of disinfection processes on elimination and deactivation of antibiotic resistance genes during water and wastewater treatment. J. Environ. Monit. 2012;14:1754–1771. doi: 10.1039/c2em00006g. [DOI] [PubMed] [Google Scholar]
- 127.Cho M., Kim J., Kim J.Y., Yoon J., Kim J.H. Mechanisms of Escherichia coli inactivation by several disinfectants. Water Res. 2010;44:3410–3418. doi: 10.1016/j.watres.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 128.Carrell Morris J. The acid ionization constant of HOCl from 5 to 35°. J. Phys. Chem. 1966;70:3798–3805. doi: 10.1021/j100884a007. [DOI] [Google Scholar]
- 129.Deborde M., von Gunten U. Reactions of chlorine with inorganic and organic compounds during water treatment-Kinetics and mechanisms: a critical review. Water Res. 2008;42:13–51. doi: 10.1016/j.watres.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 130.Bin Yuan Q., Guo M.T., Yang J. Fate of antibiotic resistant bacteria and genes during wastewater chlorination: Implication for antibiotic resistance control. PLoS One. 2015;10 doi: 10.1371/journal.pone.0119403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Auerbach E.A., Seyfried E.E., McMahon K.D. Tetracycline resistance genes in activated sludge wastewater treatment plants. Water Res. 2007;41:1143–1151. doi: 10.1016/j.watres.2006.11.045. [DOI] [PubMed] [Google Scholar]
- 132.Guo M.T., Bin Yuan Q., Yang J. Distinguishing effects of ultraviolet exposure and chlorination on the horizontal transfer of antibiotic resistance genes in municipal wastewater. Environ. Sci. Technol. 2015;49:5771–5778. doi: 10.1021/acs.est.5b00644. [DOI] [PubMed] [Google Scholar]
- 133.Cengiz M., Uslu M.O., Balcioglu I. Treatment of E. coli HB101 and the tetM gene by Fenton’s reagent and ozone in cow manure. J. Environ. Manag. 2010;91:2590–2593. doi: 10.1016/j.jenvman.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 134.Oh J., Medriano C.A., Kim S. The effect of tetracycline in the antibiotic resistance gene transfer before and after ozone disinfection. Desalin. Water Treat. 2016;57:646–650. doi: 10.1080/19443994.2014.986828. [DOI] [Google Scholar]
- 135.Pliego G., Zazo J.A., Garcia-Muñoz P., Munoz M., Casas J.A., Rodriguez J.J. Trends in the intensification of the Fenton process for wastewater treatment: an overview. Crit. Rev. Environ. Sci. Technol. 2015;45:2611–2692. doi: 10.1080/10643389.2015.1025646. [DOI] [Google Scholar]
- 136.Karaolia P., Michael I., García-Fernández I., Agüera A., Malato S., Fernández-Ibáñez P., Fatta-Kassinos D. Reduction of clarithromycin and sulfamethoxazole-resistant Enterococcus by pilot-scale solar-driven Fenton oxidation. Sci. Total Environ. 2014;468–469:19–27. doi: 10.1016/j.scitotenv.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 137.Etacheri V., Di Valentin C., Schneider J., Bahnemann D., Pillai S.C. Visible-light activation of TiO2 photocatalysts: advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015;25:1–29. doi: 10.1016/j.jphotochemrev.2015.08.003. [DOI] [Google Scholar]
- 138.Dunlop P.S.M., Ciavola M., Rizzo L., McDowell D.A., Byrne J.A. Effect of photocatalysis on the transfer of antibiotic resistance genes in urban wastewater. Catal. Today. 2015;240:55–60. doi: 10.1016/j.cattod.2014.03.049. [DOI] [Google Scholar]
- 139.Tsai T.M., Chang H.H., Chang K.C., Liu Y.L., Tseng C.C. A comparative study of the bactericidal effect of photocatalytic oxidation by TiO2 on antibiotic-resistant and antibiotic-sensitive bacteria. J. Chem. Technol. Biotechnol. 2010;85:1642–1653. doi: 10.1002/jctb.2476. [DOI] [Google Scholar]
- 140.Huang X., Liu C., Li K., Su J., Zhu G., Liu L. Performance of vertical up-flow constructed wetlands on swine wastewater containing tetracyclines and tet genes. Water Res. 2015;70:109–117. doi: 10.1016/j.watres.2014.11.048. [DOI] [PubMed] [Google Scholar]
- 141.Chen J., Liu Y.S., Su H.C., Ying G.G., Liu F., Liu S.S., He L.Y., Chen Z.F., Yang Y.Q., Chen F.R. Removal of antibiotics and antibiotic resistance genes in rural wastewater by an integrated constructed wetland. Environ. Sci. Pollut. Res. 2015;22:1794–1803. doi: 10.1007/s11356-014-2800-4. [DOI] [PubMed] [Google Scholar]
- 142.Nõlvak H., Truu M., Tiirik K., Oopkaup K., Sildvee T., Kaasik A., Mander Ü., Truu J. Dynamics of antibiotic resistance genes and their relationships with system treatment efficiency in a horizontal subsurface flow constructed wetland. Sci. Total Environ. 2013;461–462:636–644. doi: 10.1016/j.scitotenv.2013.05.052. [DOI] [PubMed] [Google Scholar]
- 143.Le T.H., Ng C., Tran N.H., Chen H., Gin K.Y.H. Removal of antibiotic residues, antibiotic resistant bacteria and antibiotic resistance genes in municipal wastewater by membrane bioreactor systems. Water Res. 2018;145:498–508. doi: 10.1016/j.watres.2018.08.060. [DOI] [PubMed] [Google Scholar]
- 144.Service R. Does disinfecting surfaces really prevent the spread of coronavirus? Science. 2020 doi: 10.1126/science.abb7058. [DOI] [Google Scholar]
- 145.Verbyla M.E., Mihelcic J.R. A review of virus removal in wastewater treatment pond systems. Water Res. 2015;71:107–124. doi: 10.1016/j.watres.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 146.C. Ibrahim, I. Mehri, S. Hammami, S. Mejri, A. Hassen, P. Pothier, Removal of human astroviruses from hospital wastewater by two biological treatment methods: natural oxidizing lagoons and rotating biodisks, 2017. 10.5004/dwt.2017.21356. [DOI]
- 147.Ibrahim C., Hammami S., Chérif N., Mejri S., Pothier P., Hassen A. Detection of Sapoviruses in two biological lines of Tunisian hospital wastewater treatment. Int. J. Environ. Health Res. 2019;29:400–413. doi: 10.1080/09603123.2018.1546835. [DOI] [PubMed] [Google Scholar]
- 148.Lv W., Zheng X., Yang M., Zhang Y., Liu Y., Liu J. Virus removal performance and mechanism of a submerged membrane bioreactor. Process Biochem. 2006;41:299–304. doi: 10.1016/j.procbio.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lovins W.A., Taylor J.S., Hong S.K. Micro-organism rejection by membrane systems. Environ. Eng. Sci. 2002;19:453–465. doi: 10.1089/109287502320963436. [DOI] [Google Scholar]
- 150.Farahbakhsh K., Smith D.W. Removal of coliphages in secondary effluent by microfiltration − mechanisms of removal and impact of operating parameters. Water Res. 2004;38:585–592. doi: 10.1016/j.watres.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 151.Wickramasinghe S.R., Stump E.D., Grzenia D.L., Husson S.M., Pellegrino J. Understanding virus filtration membrane performance. J. Memb. Sci. 2010;365:160–169. doi: 10.1016/j.memsci.2010.09.002. [DOI] [Google Scholar]
- 152.Pontius F.W., Crimaldi J.P., Amy G.L. Virus passage through compromised low-pressure membranes: a particle tracking model. J. Memb. Sci. 2011;379:249–259. doi: 10.1016/j.memsci.2011.05.066. [DOI] [Google Scholar]
- 153.Arkhangelsky E., Gitis V. Effect of transmembrane pressure on rejection of viruses by ultrafiltration membranes. Sep. Purif. Technol. 2008;62:619–628. doi: 10.1016/j.seppur.2008.03.013. [DOI] [Google Scholar]
- 154.Van Voorthuizen E.M., Ashbolt N.J., Schäfer A.I. Role of hydrophobic and electrostatic interactions for initial enteric virus retention by MF membranes. J. Memb. Sci. 2001;194:69–79. doi: 10.1016/S0376-7388(01)00522-1. [DOI] [Google Scholar]
- 155.Adham S., Gagliardo P., Smith D., Ross D., Gramith K., Trussell R. Monitoring the integrity of reverse osmosis membranes. Desalination. 1998;119:143–150. doi: 10.1016/S0011-9164(98)00134-9. [DOI] [Google Scholar]
- 156.Mi B., Eaton C.L., Kim J.H., Colvin C.K., Lozier J.C., Mariñas B.J. Removal of biological and non-biological viral surrogates by spiral-wound reverse osmosis membrane elements with intact and compromised integrity. Water Res. 2004;38:3821–3832. doi: 10.1016/j.watres.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 157.Pype M.L., Donose B.C., Martí L., Patureau D., Wery N., Gernjak W. Virus removal and integrity in aged RO membranes. Water Res. 2016;90:167–175. doi: 10.1016/j.watres.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 158.Rachmadi A.T., Kitajima M., Kato T., Kato H., Okabe S., Sano D. Required chlorination doses to fulfill the credit value for disinfection of enteric viruses in water: a critical review. Environ. Sci. Technol. 2020;54:2068–2077. doi: 10.1021/acs.est.9b01685. [DOI] [PubMed] [Google Scholar]
- 159.Mandal P., Gupta A.K., Dubey B.K. A review on presence, survival, disinfection/removal methods of coronavirus in wastewater and progress of wastewater-based epidemiology. J. Environ. Chem. Eng. 2020;8 doi: 10.1016/j.jece.2020.104317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lesimple A., Jasim S.Y., Johnson D.J., Hilal N. The role of wastewater treatment plants as tools for SARS-CoV-2 early detection and removal. J. Water Process Eng. 2020;38 doi: 10.1016/j.jwpe.2020.101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ghernaout D., Elboughdiri N. Disinfecting water: plasma discharge for removing coronaviruses. OALib. 2020;07:1–29. doi: 10.4236/oalib.1106314. [DOI] [Google Scholar]
- 162.Ciejka J., Wolski K., Nowakowska M., Pyrc K., Szczubiałka K. Biopolymeric nano/microspheres for selective and reversible adsorption of coronaviruses. Mater. Sci. Eng. C. 2017;76:735–742. doi: 10.1016/j.msec.2017.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Khan N.A., Khan S.U., Ahmed S., Farooqi I.H., Yousefi M., Mohammadi A.A., Changani F. Recent trends in disposal and treatment technologies of emerging-pollutants − a critical review. TrAC Trends Anal. Chem. 2020;122 doi: 10.1016/j.trac.2019.115744. [DOI] [Google Scholar]
- 164.Uyguner Demirel C.S., Birben N.C., Bekbolet M. A comprehensive review on the use of second generation TiO2 photocatalysts: microorganism inactivation. Chemosphere. 2018;211:420–448. doi: 10.1016/j.chemosphere.2018.07.121. [DOI] [PubMed] [Google Scholar]
- 165.Zhang Y., Zhuang Y., Geng J., Ren H., Xu K., Ding L. Reduction of antibiotic resistance genes in municipal wastewater effluent by advanced oxidation processes. Sci. Total Environ. 2016;550:184–191. doi: 10.1016/j.scitotenv.2016.01.078. [DOI] [PubMed] [Google Scholar]
- 166.Kanakaraju D., Glass B.D., Oelgemöller M. Advanced oxidation process-mediated removal of pharmaceuticals from water: a review. J. Environ. Manag. 2018;219:189–207. doi: 10.1016/J.JENVMAN.2018.04.103. [DOI] [PubMed] [Google Scholar]
- 167.Kanakaraju D., Motti C.A., Glass B.D., Oelgemöller M. TiO2photocatalysis of naproxen: effect of the water matrix, anions and diclofenac on degradation rates. Chemosphere. 2015;139:579–588. doi: 10.1016/j.chemosphere.2015.07.070. [DOI] [PubMed] [Google Scholar]
- 168.Gupta B., Gupta A.K., Tiwary C.S., Ghosal P.S. A multivariate modeling and experimental realization of photocatalytic system of engineered S–C3N4/ZnO hybrid for ciprofloxacin removal: influencing factors and degradation pathways. Environ. Res. 2020 doi: 10.1016/j.envres.2020.110390. [DOI] [PubMed] [Google Scholar]
- 169.Majumder A., Gupta A.K. Enhanced photocatalytic degradation of 17β-estradiol by polythiophene modified Al-doped ZnO: optimization of synthesis parameters using multivariate optimization techniques. J. Environ. Chem. Eng. 2020;8 doi: 10.1016/j.jece.2020.104463. [DOI] [Google Scholar]
- 170.An T., Yang H., Li G., Song W., Cooper W.J., Nie X. Kinetics and mechanism of advanced oxidation processes (AOPs) in degradation of ciprofloxacin in water. Appl. Catal. B Environ. 2010;94:288–294. doi: 10.1016/J.APCATB.2009.12.002. [DOI] [Google Scholar]
- 171.Ambrosetti B., Campanella L., Palmisano R. Degradation of antibiotics in aqueous solution by photocatalytic process: comparing the efficiency in the use of ZnO or TiO2. J. Environ. Sci. Eng. A. 2015;4:273–281. doi: 10.17265/2162-5298/2015.06.001. [DOI] [Google Scholar]
- 172.Molinari A., Sarti E., Marchetti N., Pasti L. Degradation of emerging concern contaminants in water by heterogeneous photocatalysis with Na4W10O32. Appl. Catal. B Environ. 2017;203:9–17. doi: 10.1016/j.apcatb.2016.09.031. [DOI] [Google Scholar]
- 173.Zhu Z., Lu Z., Wang D., Tang X., Yan Y., Shi W., Wang Y., Gao N., Yao X., Dong H. Construction of high-dispersed Ag/Fe3O4/g-C3N4 photocatalyst by selective photo-deposition and improved photocatalytic activity. Appl. Catal. B Environ. 2016;182:115–122. doi: 10.1016/j.apcatb.2015.09.029. [DOI] [Google Scholar]
- 174.Xekoukoulotakis N.P., Drosou C., Brebou C., Chatzisymeon E., Hapeshi E., Fatta-Kassinos D., Mantzavinos D. Kinetics of UV-A/TiO2 photocatalytic degradation and mineralization of the antibiotic sulfamethoxazole in aqueous matrices. Catal. Today. 2011;161:163–168. doi: 10.1016/j.cattod.2010.09.027. [DOI] [Google Scholar]
- 175.Rimoldi L., Meroni D., Falletta E., Pifferi V., Falciola L., Cappelletti G., Ardizzone S. Emerging pollutant mixture mineralization by TiO2 photocatalysts. The role of the water medium. Photochem. Photobiol. Sci. 2017;16:60–66. doi: 10.1039/C6PP00214E. [DOI] [PubMed] [Google Scholar]
- 176.Xiong P., Hu J. Inactivation/reactivation of antibiotic-resistant bacteria by a novel UVA/LED/TiO2 system. Water Res. 2013;47:4547–4555. doi: 10.1016/j.watres.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 177.Kangwansupamonkon W., Lauruengtana V., Surassmo S., Ruktanonchai U. Antibacterial effect of apatite-coated titanium dioxide for textiles applications. Nanomed. Nanotechnol. Biol. Med. 2009;5:240–249. doi: 10.1016/j.nano.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 178.Mondal S.K., Saha A.K., Sinha A. Removal of ciprofloxacin using modified advanced oxidation processes: kinetics, pathways and process optimization. J. Clean. Prod. 2018;171:1203–1214. doi: 10.1016/J.JCLEPRO.2017.10.091. [DOI] [Google Scholar]
- 179.Real F.J., Benitez F.J., Acero J.L., Sagasti J.J.P., Casas F. Kinetics of the chemical oxidation of the Pharmaceuticals Primidone, Ketoprofen, and Diatrizoate in ultrapure and natural waters. Ind. Eng. Chem. Res. 2009;48:3380–3388. doi: 10.1021/ie801762p. [DOI] [Google Scholar]
- 180.Veloutsou S., Bizani E., Fytianos K. Photo-Fenton decomposition of beta-blockers atenolol and metoprolol; study and optimization of system parameters and identification of intermediates. Chemosphere. 2014;107:180–186. doi: 10.1016/j.chemosphere.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 181.Alizadeh Fard M., Barkdoll B. Effects of oxalate and persulfate addition to Electrofenton and Electrofenton-Fenton processes for oxidation of Ketoprofen: Determination of reactive species and mass balance analysis. Electrochim. Acta. 2018;265:209–220. doi: 10.1016/j.electacta.2018.01.153. [DOI] [Google Scholar]
- 182.Jeong J., Kim J.Y., Yoon J. The role of reactive oxygen species in the electrochemical inactivation of microorganisms. Environ. Sci. Technol. 2006;40:6117–6122. doi: 10.1021/es0604313. [DOI] [PubMed] [Google Scholar]
- 183.Menapace H.M., Diaz N., Weiss S. Electrochemical treatment of pharmaceutical wastewater by combining anodic oxidation with ozonation. J. Environ. Sci. Health Part A Toxic/Hazardous Substain. Environ. Eng. 2008;43:961–968. doi: 10.1080/10934520801974558. [DOI] [PubMed] [Google Scholar]
- 184.González T., Domínguez J.R., Palo P., Sánchez-Martín J., Cuerda-Correa E.M. Development and optimization of the BDD-electrochemical oxidation of the antibiotic trimethoprim in aqueous solution. Desalination. 2011;280:197–202. doi: 10.1016/j.desal.2011.07.012. [DOI] [Google Scholar]
- 185.Brillas E., Sirés I., Arias C., Cabot P.L., Centellas F., Rodríguez R.M., Garrido J.A. Mineralization of paracetamol in aqueous medium by anodic oxidation with a boron-doped diamond electrode. Chemosphere. 2005;58:399–406. doi: 10.1016/j.chemosphere.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 186.Pérez G., Fernández-Alba A.R., Urtiaga A.M., Ortiz I. Electro-oxidation of reverse osmosis concentrates generated in tertiary water treatment. Water Res. 2010;44:2763–2772. doi: 10.1016/j.watres.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 187.Wang Y., Shen C., Zhang M., Zhang B., Yu Y. The electrochemical degradation of ciprofloxacin using a SnO 2 -Sb / Ti anode: influencing factors, reaction pathways and energy demand. Chem. Eng. J. 2016;296:79–89. doi: 10.1016/j.cej.2016.03.093. [DOI] [Google Scholar]
- 188.García-Gómez C., Drogui P., Zaviska F., Seyhi B., Gortáres-Moroyoqui P., Buelna G., Neira-Sáenz C., Estrada-alvarado M., Ulloa-Mercado R.G. Experimental design methodology applied to electrochemical oxidation of carbamazepine using Ti/PbO2 and Ti/BDD electrodes. J. Electroanal. Chem. 2014;732:1–10. doi: 10.1016/j.jelechem.2014.08.032. [DOI] [Google Scholar]
- 189.Särkkä H., Vepsäläinen M., Pulliainen M., Sillanpää M. Electrochemical inactivation of paper mill bacteria with mixed metal oxide electrode. J. Hazard. Mater. 2008;156:208–213. doi: 10.1016/j.jhazmat.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 190.Dallas P., Sharma V.K., Zboril R. Silver polymeric nanocomposites as advanced antimicrobial agents: classification, synthetic paths, applications, and perspectives. Adv. Colloid Interface Sci. 2011;166:119–135. doi: 10.1016/j.cis.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 191.Sharma V.K., Siskova K.M., Zboril R., Gardea-Torresdey J.L. Organic-coated silver nanoparticles in biological and environmental conditions: fate, stability and toxicity. Adv. Colloid Interface Sci. 2014;204:15–34. doi: 10.1016/j.cis.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 192.Ren G., Hu D., Cheng E.W.C., Vargas-Reus M.A., Reip P., Allaker R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents. 2009;33:587–590. doi: 10.1016/j.ijantimicag.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 193.Seo P.W., Bhadra B.N., Ahmed I., Khan N.A., Jhung S.H. Adsorptive removal of pharmaceuticals and personal care products from water with functionalized metal-organic frameworks: remarkable adsorbents with hydrogen-bonding abilities. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep34462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Majumder A., Ramrakhiani L., Mukherjee D., Mishra U., Halder A., Mandal A.K., Ghosh S. Green synthesis of iron oxide nanoparticles for arsenic remediation in water and sludge utilization. Clean Technol. Environ. Policy. 2019;21:795–813. doi: 10.1007/s10098-019-01669-1. [DOI] [Google Scholar]
- 195.Rajendran K., Sen S. Adsorptive removal of carbamazepine using biosynthesized hematite nanoparticles. Environ. Nanotechnol. Monit. Manag. 2018;9:122–127. doi: 10.1016/j.enmm.2018.01.001. [DOI] [Google Scholar]
- 196.Ali I., Al-Othman Z.A., Alwarthan A. Synthesis of composite iron nano adsorbent and removal of ibuprofen drug residue from water. J. Mol. Liq. 2016;219:858–864. doi: 10.1016/j.molliq.2016.04.031. [DOI] [Google Scholar]
- 197.Huang L., Shen R., Shuai Q. Adsorptive removal of pharmaceuticals from water using metal-organic frameworks: a review. J. Environ. Manag. 2021;277 doi: 10.1016/j.jenvman.2020.111389. [DOI] [PubMed] [Google Scholar]
- 198.Ramrakhiani L., Halder A., Majumder A., Mandal A.K., Majumdar S., Ghosh S. Industrial waste derived biosorbent for toxic metal remediation: mechanism studies and spent biosorbent management. Chem. Eng. J. 2017;308:1048–1064. doi: 10.1016/J.CEJ.2016.09.145. [DOI] [Google Scholar]
- 199.Verlicchi P. Final remarks and perspectives on the management and treatment of hospital effluents. Handb. Environ. Chem. 2018:231–238. doi: 10.1007/698_2017_56. [DOI] [Google Scholar]
- 200.Tran N.H., Reinhard M., Yew-Hoong Gin K. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2017;133:182–207. doi: 10.1016/j.watres.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 201.Gupta B., Gupta A.K., Ghosal P.S., Tiwary C.S. Photo-induced degradation of bio-toxic Ciprofloxacin using the porous 3D hybrid architecture of an atomically thin sulfur-doped g-C3N4/ZnO nanosheet. Environ. Res. 2020;183 doi: 10.1016/j.envres.2020.109154. [DOI] [PubMed] [Google Scholar]
- 202.Ashfaq M., Khan K.N., Rasool S., Mustafa G., Saif-Ur-Rehman M., Nazar M.F., Sun Q., Yu C.P. Occurrence and ecological risk assessment of fluoroquinolone antibiotics in hospital waste of Lahore, Pakistan. Environ. Toxicol. Pharmacol. 2016;42:16–22. doi: 10.1016/j.etap.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 203.Lin A.Y.C., Wang X.H., Lin C.F. Impact of wastewaters and hospital effluents on the occurrence of controlled substances in surface waters. Chemosphere. 2010;81:562–570. doi: 10.1016/j.chemosphere.2010.08.051. [DOI] [PubMed] [Google Scholar]
- 204.D. Daniels, J. Krosnick, T. Tompson, Public opinion on environmental policy in the United States, 2011. 〈http://comm.stanford.edu/faculty/krosnick/docs/2011/daniels_krosnick_web_appendix.pdf〉 (accessed September 25, 2020).
- 205.C.J. Bosso, D.L. Guber, Maintaining presence: environmental advocacy and the permanent campaign, 2005.
- 206.Beier S., Cramer C., Köster S., Mauer C., Palmowski L., Schröder H.F., Pinnekamp J. Full scale membrane bioreactor treatment of hospital wastewater as forerunner for hot-spot wastewater treatment solutions in high density urban areas. Water Sci. Technol. 2011;63:66–71. doi: 10.2166/wst.2011.010. [DOI] [PubMed] [Google Scholar]
- 207.Sim W.J., Kim H.Y., Choi S.D., Kwon J.H., Oh J.E. Evaluation of pharmaceuticals and personal care products with emphasis on anthelmintics in human sanitary waste, sewage, hospital wastewater, livestock wastewater and receiving water. J. Hazard. Mater. 2013;248–249:219–227. doi: 10.1016/j.jhazmat.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 208.Oturan M.A., Aaron J.J. Advanced oxidation processes in water/wastewater treatment: principles and applications. A review. Crit. Rev. Environ. Sci. Technol. 2014;44:2577–2641. doi: 10.1080/10643389.2013.829765. [DOI] [Google Scholar]
- 209.Li L., Long Y., Chen Y., Wang S., Wang L.L., Zhang S., Jiang F. Facile synthesis of Fe/Bi2SiO5 nanocomposite with enhanced photocatalytic activity for degradation of 17β-Estradiol(E2) Solid State Sci. 2018;83:143–151. doi: 10.1016/j.solidstatesciences.2018.07.008. [DOI] [Google Scholar]
- 210.Maletz S., Floehr T., Beier S., Klümper C., Brouwer A., Behnisch P., Higley E., Giesy J.P., Hecker M., Gebhardt W., Linnemann V., Pinnekamp J., Hollert H. In vitro characterization of the effectiveness of enhanced sewage treatment processes to eliminate endocrine activity of hospital effluents. Water Res. 2013;47:1545–1557. doi: 10.1016/j.watres.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 211.Sharma P., Mathur N., Singh A., Sogani M., Bhatnagar P., Atri R., Pareek S. Monitoring hospital wastewaters for their probable genotoxicity and mutagenicity. Environ. Monit. Assess. 2015;187:1–9. doi: 10.1007/s10661-014-4180-0. [DOI] [PubMed] [Google Scholar]
- 212.Gautam A.K., Kumar S., Sabumon P.C. Preliminary study of physico-chemical treatment options for hospital wastewater. J. Environ. Manag. 2007;83:298–306. doi: 10.1016/j.jenvman.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 213.De Almeida C.A.A., Brenner C.G.B., Minetto L., Mallmann C.A., Martins A.F. Determination of anti-anxiety and anti-epileptic drugs in hospital effluent and a preliminary risk assessment. Chemosphere. 2013;93:2349–2355. doi: 10.1016/j.chemosphere.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 214.Emmanuel E., Perrodin Y., Keck G., Blanchard J.M., Vermande P. Ecotoxicological risk assessment of hospital wastewater: a proposed framework for raw effluents discharging into urban sewer network. J. Hazard. Mater. 2005;117:1–11. doi: 10.1016/j.jhazmat.2004.08.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.