Abstract
Background:
While traumatic brain injury (TBI) is recognized as a risk factor for dementia, there is lack of clinical tools to identify brain changes that may confer such vulnerability. Brain MRI volumetric quantification can sensitively identify brain atrophy.
Objective:
To characterize regional brain volume loss in persons with TBI presenting with cognitive impairment.
Methods:
IRB approved review of medical records in patients with cognitive decline focused on those who had documented TBI histories and brain MRI scans after TBI (n = 40, 67.7 ± 14.5 years) with volumetric quantification by applying an FDA cleared software program. TBI documentation included head trauma mechanism. Brain volumes were compared to a normative database to determine the extent of atrophy. Correlations between these regions and global tests of cognition (MMSE in n = 17, MoCA in n = 27, n = 14 in both) were performed.
Results:
Multiple regions demonstrated volume loss in TBI, particularly ventral diencephalon, putamen, and pallidum with smaller magnitude of atrophy in temporal lobes and brainstem. Lobar structures showed strongest correlations between atrophy and lower scores on MMSE and MoCA. The hippocampus, while correlated to tests of cognitive function, was the least atrophic region as a function of TBI history.
Conclusion:
Persons with TBI history exhibit show regional brain atrophy. Several of these areas, such as thalamus and temporal lobes, also correlate with cognitive function. Alzheimer’s disease atrophy was less likely given relative sparing of the hippocampi. Volumetric quantification of brain MRI in TBI warrants further investigation to further determine its clinical potential in TBI and differentiating causes of cognitive impairment.
Keywords: Magnetic resonance imaging, traumatic brain injury, volumetric quantification
INTRODUCTION
Traumatic brain injury (TBI) affects about 2.5 million persons per year in the U.S., resulting in an estimated annual cost of 76.5 billion dollars [1]. TBI of all severities, including mild TBI, are related to increased cognitive impairment as well as a higher risk for dementia [2]. Additionally, TBI may potentially reduce the age of onset and expedite the emergence of Alzheimer’s disease (AD) [3]. Repetitive, mild TBIs are also associated with chronic traumatic encephalopathy (CTE), a neurodegenerative disorder which is neuropathologically distinct from AD [4, 5].
Imaging of TBI is utilized mostly in the acute clinical care setting with non-contrast computerized tomography (CT) brain scans used to identify critical findings such as hemorrhage, hydrocephalus, herniation, and vascular injury [6]. Some patients with a remote history of TBI may have overt brain atrophy, ventricular enlargement, a cavum septum pellucidum, white matter changes, or other residual findings from TBI; however, many of these patients have visually interpreted “normal” neuroimaging [7]. Consequently, individuals with the late cognitive consequences of TBI may not have identifiable measures of brain changes on conventional imaging. Clinicians need an objective neuroimaging measure to assess whether remote TBIs are associated with cognitive complaints in later life.
Quantitative volumetric MR brain imaging, which has been utilized for AD and cognitive disorders [8, 9], may be a potential tool for assessing the chronic, delayed effects of TBI. Given that TBI is thought to increase risk for cognitive decline such as AD and CTE, our purpose was to apply readily available quantitative volumetric MRI, with normative data, to persons presenting for cognitive complaints and having a history of TBI sometime in the past. We hypothesized that these patients would have a distinct neuroimaging profile, different from age-related changes and distinct from the usual patterns seen in common dementias such AD.
METHODS
Participants
As part of an IRB approved study at UCLA (IRB# 16-001491), we retrospectively evaluated 40 MRI scans from patients who were referred for cognitive complaints and had a documented history of a traumatic brain injury by hospital records. All subjects received referral to and/or evaluation by either internal medicine, neurosurgery, geriatricians, subspecialty cognitive and behavioral neurologist (VRP or MFM), or a geriatric psychiatrist (DAM). We did not include persons with a TBI history who lacked corresponding documentation of trauma mechanism. As the data and mechanism of trauma were extracted by history, severity of classification for mild, moderate, and severe TBI were not invoked for this study.
As part of their medical evaluation, subjects were screened for history of known dementia or other disorders that would influence cognition including metastatic cancer to the brain, primary brain malignancy, or large territorial ischemic infarcts. We also used the medical records to identify history of vascular disease such as hypertension and type 2 diabetes mellitus. For all subjects, time of MRI scan from injury was extracted from their medical records and rangedfromasrecentas1monthtoyears.Wedefined four categories of time of MRI scan from injury: 1) less than 6 months, 2) 6 months but less than 1 year, 3) 1 year or longer but less than 5 years, and 4) 5 years or longer. Cognitive evaluations at intake included either the Mini-Mental State Examination (MMSE) [10] (n = 17), the Montreal Cognitive Assessment (MoCA) [11] (n = 27), or both (n = 14). The 10 subjects who did not receive MMSE or MoCA received heterogeneous neuropsychological tests that included both commercial vendors (CNS Vital Signs, n = 2, and Web Neuro, n = 1), a conventional neuropsychological test battery at UCLA (n = 5) and mental status examinations that documented cognitive decline or impairment (n = 2). They were not included for analysis with the brain regions due to the heterogeneity of these methods. Subject demographics, including mechanism of TBI are detailed in Table 1 (average age in Men: 59.5 ± 16.5; Women 68 ± 11.9; p = 0.05).
Table 1.
Subject information
| Variable (n = 40) | Mean ± σ or Percentage |
|---|---|
| Age | 67.7 ± 14.5 (Range: 25–85 y) |
| Gender | Women (57%), Men (43%) |
| Mechanism of TBI | Fall (48%), MVC (30%), Contact Sports (10%), Direct Blow (7%), Blast Injury (5%) |
| Time from Injury | Less than 6 months (10%), 6 months to less than 1 year (17.5%), 1 year or longer but less than 5 years (35%), 5 or more years (37.5%) |
| Vascular disease(HTN, T2DM, CAD)* | 70% |
| MMSE | 24.1 ± 5.9 (Median: 27, Range: 12–30) |
| MoCA | 21.5 ± 5.9 (Median: 23, Range: 6–29) |
TBI, traumatic brain injury, HTN, hypertension; T2DM, type 2 diabetes mellitus; CA, coronary artery disease; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; MVC, motor vehicle collision.
MRI methods
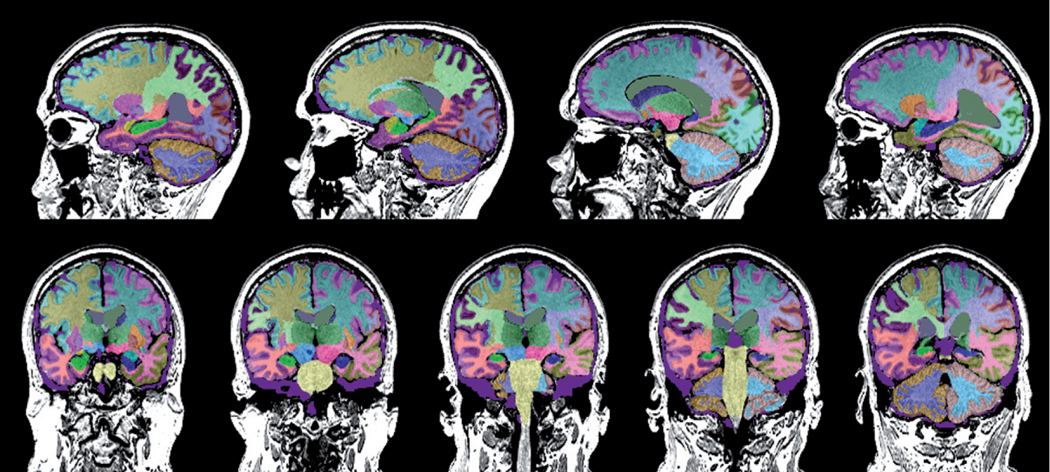
Each subject had an MRI of the brain including a 3D volumetric MPRAGE sequence on a 3.0 Tesla Siemens Scanner. All subjects were then analyzed with an FDA cleared volumetric program (Neuroreader) as detailed in prior work [10]. For each scan, 45 brain structures were quantified including the hippocampus, lobar structures, subcortical regions (thalamus, caudate, putamen, etc.), ventral diencephalon, midbrain, ventricular, and white matter volumes with an atlas-based segmentation. Total gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) volumes were also computed. Total intracranial volume (TIV) was calculated by summing GM, WM, and CSF volumes, and separate computations were done correcting individual brain structural volumes for this total head size metric. For each brain region, the following metrics were calculated: 1) region of interest volume in ml; 2) the ratio of a region of interest volume to TIV; 3) the number of standard deviations from the normative database (NR index); 4) the number for standard deviations from the mean scaled between −2 and +2 (Z-score); and 5) percentile of comparison to the normative database. The normative database was drawn from control subjects from the Alzheimer’s Disease Neuroimaging Initiative (n = 231) with an age range of 60–90 and 53% women, 47% men. All normative database comparisons for computing Z-scores and percentiles were adjusted for age, gender, and TIV in a multiple regression model. A typical example of a Neuroreader segmented brain is noted in Fig. 1.
Fig. 1.
Sagittal and coronal MRI images segmented by Neuroreader for one of the subjects in this study are displayed. Different colors correspond to different regions on different sides of the brain.
A full list of structures obtained is detailed in Fig. 2.
Fig. 2.
List of structures volumetrically measured by Neuroreader.
Data analysis
All statistical analyses were conducted using SPSS (Version 25, IBM, Armonk NY). For the purposes of this evaluation, regions of two or more unscaled standard deviations below the control mean (NR index of ≥ −2) were considered abnormal. These standard deviations were averaged across all subjects. Because these averages consisted of single subject comparisons to the normative database, multiple comparison corrections were not invoked for this analysis. Interaction terms were calculated between TBI and vascular disease status and TBI and age to determine if the volume loss in relation to TBI varied as a function of age or vascular disease in multiple regression models. Separate correlations between TBI mechanism and abnormally low volume regions were also applied. Additionally, correlations were modeled between MoCA and MMSE scores and computed volumes as a proportion of TIV. Finally, we correlated time from injury to brain volumes, adjusting for age, gender, mechanism of injury, and vascular disease.
RESULTS
Table 2 displays a ranked table of the average negative NR indices. Other metrics, including the percentile, are also included. Regions with positive NR regions are not included in the table but included the parietal and occipital lobes as well as cerebellum.
Table 2.
Abnormally low regional brain volumes in TBI group
| Structure | NR Index | Volume (mL) | Volume/TIV (%) | Z-score | Percentile |
|---|---|---|---|---|---|
| Total Gray Matter | −12.9 | 492.3 | 26.7 | −0.9 | 27.6 |
| Ventral Diencephalon | −4.6 | 9.8 | 0.5 | −0.3 | 38.4 |
| Right Ventral Diencephalon | −4.7 | 4.9 | 0.2 | −0.3 | 37.9 |
| Left Ventral Diencephalon | −4.2 | 4.9 | 0.2 | −0.3 | 39.2 |
| Putamen | −4.3 | 8.3 | 0.4 | −0.3 | 39.2 |
| Right Putamen | −3.8 | 4.1 | 0.2 | −0.3 | 40.5 |
| Left Putamen | −4.6 | 4.2 | 0.2 | −0.3 | 38.6 |
| Pallidum | −3.7 | 3 | 0.1 | −0.2 | 40.5 |
| Left Thalamus | −2.6 | 8.9 | 0.5 | −0.1 | 43.3 |
| Temporal Lobes | −1.7 | 253.9 | 11.1 | −0.1 | 45.9 |
| Right Caudate | −1.4 | 3.6 | 0.2 | −0.1 | 46 |
| Brainstem | −0.7 | 19.5 | 1.1 | 0.8 | 48 |
| Frontal Lobe | −0.7 | 493 | 20.2 | 0.1 | 48.8 |
| Amygdala | −0.5 | 3.93 | 0.2 | −0.03 | 49 |
| Hippocampus | −0.4 | 9.6 | 0.4 | −0.02 | 49.2 |
Figure 3 highlights the disparity between the most severe regional volume loss in the ventral diencephalon in contrast with the relatively preserved volume in the hippocampus. The black bars show the lower ventral diencephalon NR indices compared to the hippocampus. The gray bars show the highlight the lower percentile compared to normative database in the ventral diencephalon compared to the higher percentile in the hippocampus.
Fig. 3.
Neuroreader (NR) index and percentiles of not able regions. See Table 2 for full list of regions.
There were no statistically significant interactions between TBI status and vascular disease. There were statistically significant interactions between age and TBI related atrophy such as the level of volume loss increased in older persons for total gray matter volume (t = −3.7, p = 001), ventral diencephalon (t = −7.2, p < 0.001), and putamen (t = −5.7, p < 0.001). There was a statistically significant interaction between gender and TBI status in total gray matter (t = −2.1, p = 0.04), ventral diencephalon (t = −2.1, p = 0.04), putamen (t = −2.9, p = 0.006), and left thalamus (t = −3.1, p = 0.003). These findings suggest that in these regions the findings vary as a function of gender with men with TBI having a higher degree of volume loss than women with TBI. It was noted that women experienced TBI more often than men in falls and motor vehicle collisions (χ = 9.56, p = 0.05), but men accounted for all of the blast injuries and direct blows. There were no statistically significant differences between brain volumes and mechanism of TBI nor was there an interaction effect.
Table 3 shows statistically significant correlations between the MMSE and MoCA scores and Neuroreader quantified regions. We did not correct for TIV as it reduced the variance. Overall, the average MMSE and MoCA scores met diagnostic criteria for cognitive impairment (average MMSE = 24.1 ± 5.9, average MoCA = 21.5 ± 5.9). The MMSE and MoCA scores were highly correlated (r = −0.79, p < 0.001). There were no statistically significant correlations between trauma mechanism and MMSE or MoCA scores nor was there an interaction effect. There were no statistically significantly correlations between time from injury and brain volumes.
Table 3.
Correlations between MMSE, MoCA, and quantified regions
| Structure | Correlation with MMSE p (n = 17) | Correlation with MoCA p (n = 27) |
|---|---|---|
| Whole Brain Matter | 0.585, 0.014 | 0.459, 0.016 |
| Grey Matter | 0.266, 0.302 | 0.306, 0.121 |
| White Matter | 0.441, 0.077 | 0.237, 0.234 |
| Hippocampus | 0.481, 0.051 | 0.365, 0.061 |
| Right Hippocampus | 0.433, 0.083 | 0.351, 0.073 |
| Left Hippocampus | 0.495, 0.043 | 0.369, 0.058 |
| Amygdala | 0.515, 0.035 | 0.453, 0.018 |
| Right Amygdala | 0.424, 0.089 | 0.392, 0.043 |
| Left Amygdala | 0.559, 0.020 | 0.485, 0.010 |
| Putamen | 0.547, 0.023 | 0.341, 0.082 |
| Right Putamen | 0.559, 0.020 | 0.361, 0.064 |
| Left Putamen | 0.515, 0.034 | 0.307, 0.119 |
| Thalamus | 0.733, 0.001 | 0.614, 0.001 |
| Right Thalamus | 0.728, 0.001 | 0.624, 0.001 |
| Left Thalamus | 0.715, 0.001 | 0.584, 0.001 |
| Ventral Diencephalon | 0.603, 0.010 | 0.332, 0.090 |
| Right Ventral Diencephalon | 0.598, 0.011 | 0.359, 0.066 |
| Left Ventral Diencephalon | 0.592, 0.012 | 0.299, 0.129 |
| Pallidum | 0.413, 0.099 | 0.231, 0.245 |
| Right Pallidum | 0.401, 0.111 | 0.209, 0.296 |
| Left Pallidum | 0.415, 0.098 | 0.240, 0.229 |
| Caudate | 0.206, 0.428 | 0.038, 0.851 |
| Right Caudate | 0.277, 0.282 | 0.073, 0.717 |
| Left Caudate | 0.127, 0.628 | 0.002, 0.991 |
| Brainstem | 0.553, 0.021 | 0.185, 0.356 |
| Frontal Lobe | 0.518, 0.033 | 0.334, 0.089 |
| Right Frontal Lobe | 0.526, 0.030 | 0.358, 0.067 |
| Left Frontal Lobe | 0.506, 0.038 | 0.307, 0.119 |
| Parietal Lobe | 0.584, 0.014 | 0.512, 0.006 |
| Right Parietal Lobe | 0.583, 0.014 | 0.519, 0.006 |
| Left Parietal Lobe | 0.573, 0.016 | 0.496, 0.009 |
| Occipital Lobe | 0.364, 0.150 | 0.465, 0.014 |
| Right Occipital Lobe | 0.359, 0.157 | 0.506, 0.007 |
| Left Occipital Lobe | 0.357, 0.159 | 0.405, 0.036 |
| Temporal Lobe | 0.658, 0.004 | 0.547, 0.003 |
| Right Temporal Lobe | 0.653, 0.005 | 0.578, 0.002 |
| Left Temporal Lobe | 0.628, 0.007 | 0.489, 0.010 |
| Cerebellum | 0.483, 0.050 | 0.229, 0.250 |
| Right Cerebellum | 0.489, 0.046 | 0.213, 0.286 |
| Left Cerebellum | 0.470, 0.057 | 0.242, 0.224 |
| Cerebrospinal fluid | −0.046, 0.861 | −0.361, 0.064 |
| Lateral Ventricle | −0.211, 0.416 | −0.278, 0.161 |
| Right Lateral Ventricle | −0.177, 0.496 | −0.282, 0.154 |
| Left Lateral Ventricle | −0.237, 0.360 | −0.269, 0.175 |
DISCUSSION
TBI may be related to brain atrophy and subsequent cognitive decline [14]. Affected regions shown in prior studies include total gray matter volume [15], putamen [16], and ventral diencephalon [13, 17]. Prior work has suggested that the presence of TBI can accelerate age-related volume loss [18]. Other investigators have found a gender-based interaction with brain volumes after TBI with greater decreases in women as compared to men [19]. In this finding, our results differ as we found higher atrophy in men than in women despite the greater number and older age of the women. While women have been shown to have worse outcomes after TBI than men [20], it has also been suggested that men may have an intrinsically higher vulnerability to TBI than women [21]. In this study, we noted a trend toward statistical significance for women experiencing TBI from falls and motor vehicle collisions more often than men. By contrast, men accounted for all of the blast injuries and direct blows. These differences in mechanism of TBI may thus explain why men experienced more volume loss. Critically, the lack of interaction between vascular disease and TBI volume loss suggests that the influence of TBI on brain volumes does not vary as a function of co-morbid vascular disease.
We also found statistically significant correlations between MMSE and MoCA scores and Neuroreader computed brain regional volumes important for cognition including the frontal, temporal, and parietal lobes and weaker correlations in the hippocampus. Previous studies have suggested that brain volumes can correlate with tests of even global cognitive function [22, 23], but evidence of this in persons with TBI is lacking. Thus, our study adds to previously understood relationships in a patient group that experiences impaired cognition. The fact that the most abnormal regions were those not correlated with MMSE or MoCA, such as the ventral diencephalon, suggests that domain specific neuropsychological tests may be needed to detect abnormalities already apparent on quantified volumetric MR imaging. Thus, both quantified volumetric MRI and neuropsychological tests are best used in conjunction in evaluating TBI as both types of data are complementary.
The major implication of this study is the ability of volumetric quantification to detect brain atrophy following TBI among older patients who present with cognitive complaints [25, 26]. Additionally non-atrophy related findings of TBI such as microbleeds or significant white matter sheer injury are seen only 34% of the time on conventional MR sequences [27]. Furthermore, the fact that we did not find predominant hippocampal or temporal-parietal atrophy in this group compared to other regions suggests that TBI related brain damage is distinct, and that the mechanisms of TBI-related cognitive decline are potentially distinguishable from those for AD and other dementias. The abnormally low ventral diencephalon volumes, for example, do correspond to prior work suggesting that CTE neuropathology deposits in this area [17]. Thus, while volumetric quantification is not currently recommended in standard clinical practice guidelines for even mild TBI [28], it does hold potential future applications for this population.
There are several potential limitations of this study. First, we do not establish causation between the low brain volumes and cognitive impairment or the mechanism of TBI of these patients. Second, the assessment of TBI was retrospective and, consequently, fraught with problems with the recall of specific details of TBIs, as is usual for this type of study. Third, our study is additionally limited in its generalization to the overall population by the relatively small sample size. Larger samples sizes for various mechanisms may demonstrate specific differences as suggested in prior work [29]. The lack of TBI severity grading is another limitation. It is possible that the study findings may only apply to moderate or severe TBI; however, atrophy can be seen even in mild TBI with one study showing increasing atrophy with loss of consciousness [30]. Additionally, post-traumatic impairment of memory function also predicts increased atrophy [31]. Different 3T magnets were also used in this study. While this could be a potential limitation, Neuroreader has produced reproducible high quality regional brain segmentation regardless of vendor or field strength [12, 32]. The younger ages in our cohort compared to the normal database raises the possibility that atrophy may have been underestimated. Another limitation of this study is the differing sensitivities of MMSE and MoCA, with one study showing greater sensitivity of MoCA compared to MMSE in detecting cognitive impairment in relation to TBI [24]. Finally, we do not evaluate distinct white matter tracts in this study and did not find abnormally low white matter volumes in the TBI group. However prior work has shown the importance of white matter analyses in capturing the cognitive deficits in TBI [33]. Thus, future studies should incorporate combined longitudinal volumetric and white matter diffusion MR imaging analyses for maximal sensitivity in detecting TBI related brain abnormalities.
This study demonstrates total reduction of gray matter volume in TBI compared to controls with focal areas showing greater areas of volume loss. This work may form the basis for future studies that not only utilize these regions for improved accuracy of TBI related brain damage but may also serve as biomarkers for treatment response for cognitive rehabilitation programs [34]. Future studies with clinical trials will be necessary for such additional validation.
ACKNOWLEDGMENTS
Supported by McLoughlin Cognitive Health Gift Fund. Dr. Raji is supported in his research by grants from the WUSTL NIH KL2 Grant (KL2 TR000450 – ICTS Multidisciplinary Clinical Research Career Development Program) and the Radiological Society of North America Research Scholar Grant and the Foundation of the American Society of Neuroradiology Boerger Research Fund for Alzheimer’s Disease and Neurocognitive Disorders.
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/19-0708r1).
REFERENCES
- [1].DoD Worldwide Numbers for TBI, Last updated February 13, 2019, Accessed on February 13, 2019. [Google Scholar]
- [2].Barnes DE, Byers AL, Gardner RC, Seal KH, Boscardin WJ, Yaffe K (2018) Association of mild traumatic brain injury with and without loss of consciousness with dementia in US military veterans. JAMA Neurol 75, 1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mendez MF, Paholpak P, Lin A, Zhang JY, Teng E (2015) Prevalence of traumatic brain injury in early versus lateonset Alzheimer’s disease. J Alzheimers Dis 47, 985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH (2005) Chronic traumatic encephalopathy in a National Football League player. Neurosurgery 57, 128134. [DOI] [PubMed] [Google Scholar]
- [5].Omalu BI, DeKosky ST, Hamilton RL, Minster RL, Kamboh MI, Shakir AM, Wecht CH (2006) Chronic traumatic encephalopathy in a National Football League player: Part II. Neurosurgery 59, 1086–1092; discussion 1092–1093. [DOI] [PubMed] [Google Scholar]
- [6].Shetty VS, Reis MN, Aulino JM, Berger KL, Broder J, Choudhri AF, Kendi AT, Kessler MM, Kirsch CF, Luttrull MD, Mechtler LL, Prall JA, Raksin PB, Roth CJ, Sharma A, West OC, Wintermark M, Cornelius RS, Bykowski J (2016) ACR appropriateness criteria head trauma. J Am Coll Radiol 13, 668–679. [DOI] [PubMed] [Google Scholar]
- [7].(2016) Management of Concussion-mild Traumatic Brain Injury (mTBI). VA/DoD Clinical Practice Guidelines. [Google Scholar]
- [8].Jack CR, Shiung MM, Gunter JL, O’Brien PC, Weigand SD, Knopman DS, Boeve BF, Ivnik RJ, Smith GE, Cha RH, Tangalos EG, Petersen RC (2004) Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology 62, 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Elliott C, Masliah E, Ryan L, Silverberg N (2018) NIAAA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state: A practical method grading the cognitive state of patients for the clinician. Psychiatr Res 12, 189–198. [DOI] [PubMed] [Google Scholar]
- [11].Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53, 695–699. [DOI] [PubMed] [Google Scholar]
- [12].Ahdidan J, Raji CA, DeYoe EA, Mathis J, Noe KO, Rimestad J, Kjeldsen TK, Mosegaard J, Becker JT, Lopez O (2015) Quantitative neuroimaging software for clinical assessment of hippocampal volumes on MR imaging. J Alzheimers Dis 49, 723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Raji CA, Merrill DA, Barrio JR, Omalu B, Small GW (2016) Progressive focal gray matter volume loss in a former high school football player: A possible magnetic resonance imaging volumetric signature for chronic traumatic encephalopathy. Am J Geriatr Psychiatry 24, 784–790. [DOI] [PubMed] [Google Scholar]
- [14].Ross DE (2011) Review of longitudinal studies of MRI brain volumetry in patients with traumatic brain injury. Brain Inj 25, 1271–1278. [DOI] [PubMed] [Google Scholar]
- [15].Sussman D, da Costa L, Chakravarty MM, Pang EW, Taylor MJ, Dunkley BT (2017) Concussion induces focal and widespread neuromorphological changes. NeurosciLett 650, 52–59. [DOI] [PubMed] [Google Scholar]
- [16].Gooijers J, Chalavi S, Beeckmans K, Michiels K, Lafosse C, Sunaert S, Swinnen SP (2016) Subcortical volume loss in the thalamus, putamen, and pallidum, induced by traumatic brain injury, is associated with motor performance deficits. Neurorehabil Neural Repair 30, 603–614. [DOI] [PubMed] [Google Scholar]
- [17].Barrio JR, Small GW, Wong KP, Huang SC, Liu J, Merrill DA, Giza CC, Fitzsimmons RP, Omalu B, Bailes J, Kepe V (2015) In vivo characterization of chronic traumatic encephalopathy using [F-18]FDDNP PET brain imaging. Proc Natl Acad Sci U S A 112, E2039–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Santhanam P, Wilson SH, Oakes TR, Weaver LK (2018) Accelerated age-related cortical thinning in mild traumatic brain injury. Brain Behav 9, e01161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bazarian JJ, Blyth B, Mookerjee S, He H, McDermott MP (2010) Sex differences in outcome after mild traumatic brain injury. J Neurotrauma 27, 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vagnerova K, Koerner IP, Hurn PD (2008) Gender and the injured brain. Anesth Analg 107, 201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li H, Ruan J, Xie Z, Wang H, Liu W (2007) Investigation of the critical geometric characteristics of living human skulls utilising medical image analysis techniques. Int J Veh Saf 2, 345. [Google Scholar]
- [22].Paul R, Lane EM, Tate DF, Heaps J, Romo DM, Akbudak E, Niehoff J, Conturo TE (2011) Neuroimaging signatures and cognitive correlates of the Montreal Cognitive Assessment screen in a nonclinical elderly sample. Arch Clin Neuropsychol 26, 454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dinomais M, Celle S, Duval GT, Roche F, Henni S, Bartha R, Beauchet O, Annweiler C (2016) Anatomic correlation of the Mini-Mental State Examination: A voxel-based morphometric study in older adults. PLoS One 11, e0162889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang H, Zhang X-N, Zhang H-L, Huang L, Chi Q-Q, Zhang X, Yun X-P (2016) Differences in cognitive profiles between traumatic brain injury and stroke: A comparison of the Montreal Cognitive Assessment and Mini-Mental State Examination. Chin J Traumatol 19, 271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ross DE, Ochs AL, Seabaugh JM, Shrader CR, the Alzheimer’s Disease Neuroimaging Initiative (2013) Man versus machine: Comparison of radiologists’ interpretations and NeuroQuant ® volumetric analyses of brain MRIs in patients with traumatic brain injury. J Neuropsychiatry Clin Neurosci 25, 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ross DE, Ochs AL, DeSmit ME, Seabaugh JM, Havranek MD, Alzheimer’s Disease Neuroimaging Initiative (2015) Man versus machine part 2: Comparison of radiologists’ interpretations and NeuroQuant measures of brain asymmetry and progressive atrophy in patients with traumatic brain injury. J Neuropsychiatry Clin Neurosci 27, 147–152. [DOI] [PubMed] [Google Scholar]
- [27].Buttram SDW, Garcia-Filion P, Miller J, Youssfi M, Danielle Brown S, Dalton HJ, David Adelson P (2015) Computed tomography vs magnetic resonance imaging for identifying acute lesions in pediatric traumatic brain injury. Hosp Pediatr 5, 79–84. [DOI] [PubMed] [Google Scholar]
- [28].Wintermark M, Sanelli PC, Anzai Y, Tsiouris AJ, Whitlow CT, American College of Radiology Head Injury Institute (2015) Imaging evidence and recommendations for traumatic brain injury: Advanced neuro- and neurovascular imaging techniques. AJNR Am J Neuroradiol 36, E1–E11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mendez MF, Owens EM, Reza Berenji G, Peppers DC, Liang LJ, Licht EA (2013) Mild traumatic brain injury from primary blast vs. blunt forces: Post-concussion consequences and functional neuroimaging. Neurorehabilitation 32, 397–407. [DOI] [PubMed] [Google Scholar]
- [30].MacKenzie JD, Siddiqi F, Babb JS, Bagley LJ, Mannon LJ, Sinson GP, Grossman RI (2002) Brain atrophy in mild or moderate traumatic brain injury: A longitudinal quantitative analysis. AJNR Am J Neuroradiol 23, 1509–1515. [PMC free article] [PubMed] [Google Scholar]
- [31].Wilde EA, Bigler ED, Pedroza C, Ryser DK (2006) Posttraumatic amnesia predicts long-term cerebral atrophy in traumatic brain injury. Brain Inj 20, 695–699. [DOI] [PubMed] [Google Scholar]
- [32].Mettenburg JM, Branstetter BF, Wiley CA, Lee P, Richardson RM (2019) Improved detection of subtle mesial temporal sclerosis: Validation of a commercially available software for automated segmentation of hippocampal volume. Am J Neuroradiol 40, 440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yuh EL, Mukherjee P, Lingsma HF, Yue JK, Ferguson AR, Gordon WA, Valadka AB, Schnyer DM, Okonkwo DO, Maas AI, Manley GT, TRACK-TBI Investigators (2013) Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann Neurol 73, 224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Galetto V, Sacco K (2017) Neuroplastic changes induced by cognitive rehabilitation in traumatic brain injury: A review. Neurorehabil Neural Repair 31, 800–813. [DOI] [PubMed] [Google Scholar]