Abstract
Background:
The relation between malnutrition and pulmonary death in patients with cystic fibrosis (CF) has resulted in intensive nutritional intervention over the last few decades, leading to a significant decline in underweight and the emergence of overweight/obesity as a potential new problem.
Methods:
We performed a cross-sectional database analysis of 484 adults with CF seen at the University of Minnesota CF Center between January 2015-January 2017, to determine the prevalence and pulmonary/cardiovascular risk factors associated with overweight and obesity in this population.
Results:
Mean age was 35.2 ± 11.6 years. 5.2% were underweight (BMI<18.5 kg/m2), 62.6% normal weight (BMI ≥ 18.5–24.9 kg/m2), 25.6% overweight (BMI ≥ 25–29.9 kg/m2) and 6.6% obese (BMI ≥ 30 kg/m2). In the subgroup with severe genotypes, 25% had BMI ≥ 25 kg/m2. In the entire cohort, overweight/obese were likely to be older (OR = 1.04, p < 0.0001) and to have a mild CFTR genotype (OR = 3.33, p = 0.0003) and modestly elevated triglyceride levels (OR = 1.008, p < 0.0001). The prevalence of hypertension was higher in overweight (25%) and obese (31%) than normal (17%) or underweight (16%), p = 0.01. Total cholesterol levels were higher in overweight/obese versus normal/underweight (144–147 vs 123– 131 mg/dL, p = 0.04) as were LDL levels (70–71 vs 53–60 mg/dL, p = 0.02), but all were within the normal range. Percent predicted FEV1 was higher in overweight/obese (78–81%) versus underweight (59%) and normal (70%), p < 0.0001, and overweight/obese experienced significantly fewer acute pulmonary exacerbations.
Conclusions:
Overweight/obesity is common in adults with CF including those with severe genotypes. Lung function is better in the overweight/obese and lipid levels are within the normal range, albeit higher than in normal/underweight.
Keywords: Obesity, Cystic Fibrosis, Prevalence
1. Introduction
Cystic fibrosis (CF), the most common autosomal recessive disease in Caucasians, is caused by mutations of the CF transmembrane regulator protein (CFTR) gene. Loss of function mutations of CFTR-mediated chloride and bicarbonate transport in the apical membrane of epithelial cells lead to impaired mucociliary clearance and accumulation of mucus in various organs, resulting in chronic airway disease, pancreatic insufficiency, malabsorption, biliary cirrhosis, and infertility [1–2]. Individuals with CF are classically at risk for malnutrition, which is related to inadequate intake, increased energy expenditure, and malabsorption [3]. Malnutrition is well-known to be associated with declining pulmonary function and high morbidity and mortality in this population [4–7], and multiple studies have demonstrated that achieving an adequate weight is associated with improved pulmonary function and survival [8–9]. This has long been a focus of CF management, and European and North American CF guidelines recommend maintaining a BMI above 22 kg/m2 in females and 23 kg/m2 in males [10–11].
Intensive efforts to promote adequate nutrition in CF with a high caloric diet, pancreatic enzyme replacement therapy, fat-soluble vitamin supplementation and, more recently, CFTR modulator therapy have significantly reduced the prevalence of malnutrition. At the same time, an increase in the number of CF patients who are overweight/obese, previously almost unheard of in this population, has been recognized. In Toronto, the proportion of underweight adults with CF decreased from 20% before 1990 to 11% in the 2000s, whereas the proportion of overweight/obese patients increased from 7% in the 1980s to 18% in the 2000s [12]. In Pittsburgh, 23% of children with CF seen in 2012 were reported to be overweight or obese [13].
Overweight/obesity is a major global health concern. It is associated with high morbidity and mortality in the general population, primarily because of the risk of hyperlipidemia, hypertension, and atherosclerotic cardiovascular disease [14–15]. Atherosclerotic cardiovascular disease is rare in CF and their cholesterol levels tend to be low. Overall the CF population is relatively young, so it is not clear if overweight/obesity poses the same risk in CF as in the general population. The following study was performed to assess the prevalence of obesity/overweight in adults at the large Minnesota CF center, and to determine its association with cardiovascular risk factors and pulmonary health.
2. Methods
2.1. Patients
This cross-sectional study included adults age ≥18 years followed at the University of Minnesota Cystic Fibrosis Center from January 1st, 2015 to January 31st, 2017. All data including demographics (age, ethnicity, gender, age at CF diagnosis), body mass index (BMI), CF genotype classification, lung function, cystic fibrosis related diabetes (CFRD) status, CFRD duration, presence of hypertension, supplemental feeding (oral supplements, gastrostomy or nasogastric tube overnight drip feeding), and specific medications (pancreatic enzymes, insulin, corticosteroids, CFTR modulators) were obtained through the CF Foundation patient registry database. The lipid profile was obtained from the University of Minnesota CF Center Access database. BMI was calculated as weight (kg)/height (m2) and categorized using the WHO adult criteria, where underweight is defined as BMI <18.5 kg/m2; normal weight BMI ≥ 18.5 to 24.9 kg/m2; overweight BMI ≥ 25 to 29.9 kg/m2 and obesity BMI ≥ 30 kg/m2. CFRD was diagnosed according to standard criteria [16]. CF patients who had undergone lung transplantation were included in this analysis. Lung function was assessed as the forced expiratory volume in 1 s (FEV1), expressed as a percentage of the normal predicted values for height and sex using the Hankinson reference norms [17]. Cholesterol, HDL, and triglyceride level were measured by an enzymatic, colorimetric method with the Dimension Vista® system (Siemens Healthcare Diagnostics Inc, Newark, DE). LDL was calculated according to the Friedewald equation. Clinical and laboratory parameters from the most recent outpatient clinic visit during the study period were used in the analyses. However, for pancreatic enzymes and CFTR modulators, patients were considered to be on these medications if they took them at any time during the study period.
CFTR genotype was classified as severe or mild based on the predicted effect on gene expression (e.g., nonsense mutation, splice junction) or protein function, and using the rate of pancreatic insufficiency among individuals with that genotype in the CFTR2 study [18]. Patients with both CFTR alleles having severe mutations (severe/severe) were considered to have a severe genotype, whereas those with at least one mild mutation (severe/mild, mild/unclassified, mild/unknown or mild/mild) were considered to have a mild genotype. Patients with severe/unclassified, severe/unknown, unclassified/unclassified, and unknown mutations were “unclassified.” For example, F508del/F508del and F508del/W1282X were classified as severe mutations, whereas F508del/R117C and G542X/4005+2T->C were classified as mild genotypes. The specific alleles defined by this classification are listed in the online supplement Table 1 in Lewis et al. [19]. This study was approved by the University of Minnesota’s Institutional Review Board.
Table 1.
Characteristics of adult patients with CF by body mass index category (N = 484).
| Underweight (N = 25) | Normal weight (N = 303) | Overweight (N = 124) | Obesity (N = 32) | P-value | |
|---|---|---|---|---|---|
| Age (years) | 30.7 ± 9.9 | 33.4 ± 10.5 | 39.6 ± 13.0a,b | 39.1 ± 11.4c,d | <0.0001 |
| BMI (kg/m2 )e | 17.7 ± 0.8 | 21.9 ± 1.8 | 27.0 ± 1.3 | 34.8 ± 5.7 | <0.0001 |
| Male | 8 (32%) | 156 (51%) | 78 (63%) | 22 (69%) | 0.006 |
| Caucasian | 23 (92%) | 292 (96%) | 117 (94%) | 30 (94%) | 0.37 |
| Age at CF diagnosis (years) | 1.8 ± 3.9 median 0.3 (0–16.5) | 5.1 ± 9.3 median 0.7 (0–70.3) | 11.4 ± 14.7a,b median 4.5 (0–64.7) | 12.5 ± 15.1a,b median 4.6 (0–42.9) | <0.0001 |
| CFTR genotype classification (n = 477) | <0.001 | ||||
| -Mild | 2 (8%) | 31 (10%) | 38 (31%) | 15 (48%) | |
| - Severe | 20 (80%) | 230 (77%) | 73 (59%) | 11 (35%) | |
| -Unclassified | 3 (12%) | 37 (12%) | 12 (10%) | 5 (16%) | |
| Homozygous F508del | 15 (60%) | 142 (48%) | 60 (49%) | 10 (32%) | 0.20 |
| History of lung transplant | 4 (16%) | 37 (12%) | 18 (15%) | 1 (3%) | 0.30 |
| Percent of patients taking pancreatic enzyme (n = 417) | 20 (100%) | 240 (91%) | 77 (73%) | 16 (57%) | <0.001 |
| Percent of patients using supplemental feeding (n = 477) | 13 (52%) (n = 25) | 105 (35%) (n = 299) | 18 (15%) (n = 121) | 2 (6%) (n = 32) | <0.001 |
| - Oral supplement | 7 (54%) | 88 (84%) | 15 (83%) | 1 (50%) | |
| -Gastrostomy tube | 4 (31%) | 9 (9%) | 3 (17%) | 1 (50%) | |
| -Nasogastric tube | 0 (0%) | 1 (1%) | 0 (0%) | 0 (0%) | |
| -Both oral and gastrostomy tube | 2 (15%) | 7 (7%) | 0 (0%) | 0 (0%) | |
| Percent of patients using corticosteroid | 108 (36%) | 44 (35%) | 8 (25%) | 0.76 | |
| - Inhaled | 8 (32%) | 40 (13%) | 20 (16%) | 3 (9%) | |
| - Oral | 3 (12%) | 155 (51%) | 60 (48%) | 21 (66%) | |
| - None | 14 (56%) | ||||
| Percent of patients who have CFRD (n = 483) | 15 (60%) | 144 (48%) | 52 (42%) | 10 (31%) | 0.11 |
| Duration of CFRD (years) | 13.6 ± 5.9 median 13.5 (3–25.5) | 13.2 ± 7.4 median 12.5 (1–41.6) | 13.5 ± 6.9 median 14.3 (1.9–26.4) | 14.9 ± 8.9 median 14.5 (5–32.4) | 0.90 |
| Duration of insulin treatment (years) | 10.9 ± 6.1 | 11.3 ± 7.2 | 10.4 ± 5.4 | 14.4 ± 8.1 | 0.46 |
| HbA1c (%) | 6.5 ± 2.2 | 6.2 ± 1.0 | 6.3 ± 1.1 | 6.3 ± 1.3 | 0.43 |
| Hypertension | 4 (16%) | 50 (17%) | 38 (31%) | 8 (25%) | 0.01 |
| Percent of patients who receive CFTR modulators | 9 (36%) | 103 (34%) | 39 (31%) | 10 (31%) | 0.94 |
| Duration of CFTR modulator treatment (years) | 1.6 ± 0.9 | 1.7 ± 0.6 | 1.7 ± 0.7 | 1.5 ± 0.8 | 0.85 |
| FEV1 (% predicted) | 58.7 ± 27.8 | 69.8 ± 23.5c | 78.0 ± 23.4a,b | 81.4 ± 18.9a,b | <0.0001 |
| Frequency of pulmonary exacerbations over 3 years | 8.4 ± 7.5 median 6.0 (0–29.0) | 5.3 ± 5.4c median 3.0 (0–32.0) | 3.8 ± 4.7a,d median 2.0 (0–30.0) | 3.2 ± 4.5a median 1.5 (0–20.0) | 0.0004 |
| Total Cholesterol (mg/dL) (n = 455) | 122.7 ± 29.2 | 130.7 ± 36.3 | 144.1 ± 37.4c,d | 146.9 ± 34.7c | 0.04 |
| HDL-C (mg/dL) (n = 455) | 49.7 ± 16.2 | 51.5 ± 17.1 | 48.9 ± 14.1d | 43.8 ± 14.1d | 0.048 |
| LDL-C (mg/dL) (n = 455) | 53.4 ± 23.7 | 59.5 ± 26.1 | 70.5 ± 31d | 71.4 ± 29.7 | 0.02 |
| Triglyceride (mg/dL) (n = 455) | 99.4 ± 47.5 | 98.2 ± 58.3 | 124.3 ± 60.6b | 158.2 ± 87.6a,b,f | <0.0001 |
| VLDL-C (mg/dL) (n = 398) | 21.7 ± 11.2 | 20.2 ± 10.6 | 23.6 ± 13.5 | 26.9 ± 17.0d | 0.09 |
P = ≤ 0.01 when compared to underweight group.
P = ≤ 0.01 when compared to normal weight group.
P = ≤ 0.05 when compared to underweight group.
P = ≤ 0.05 when compared to normal weight group.
P = ≤ 0.001 for all group comparisons.
P = ≤ 0.05 when compared to overweight groupBMI; Body mass index, CFRD; Cystic fibrosis related diabetes, CFTR: Cystic fibrosis transmembrane regulator, FEV1; forced expiratory volume in 1 s.
2.2. Statistical analysis
Baseline characteristics were summarized and presented as mean ± SD or median (minimum and maximum) as appropriate for continuous variables, and frequency and percentage for categorical variables. In bi-variate analysis, groups were compared using the analysis of variance (ANOVA) with Tukey’s multiple comparisons adjustment for continuous variables and Chi-square test (or Fisher’s exact test if indicated) for categorical variables. Multi-variate analyses were conducted using logistic regression models. Variables that were significant at the 0.10 level in bi-variate analysis were included in multi-variate analysis. The backward model selection method was used to select the best set of factors associated with overweight and obesity. The Loess method and weighted least squares were used to examine the relationship between BMI and FEV1% predicted and to determine the BMI cut-off at which BMI is no longer associated with increased FEV1% predicted. Statistical analyses were performed in SAS 9.3 (SAS Institute, Cary, NC). P values < 0.05 were considered statistically significant.
3. Results
3.1. Prevalence of overweight/obesity
A total of 484 adults with CF were included. Mean age was 35.2 ± 11.6 years, 95% were white, and 55% were male. Eighty-five percent of patients were pancreatic insufficient, 46% had CFRD, 15% had history of lung transplantation and 29% were on supplemental feeding (Table 1). The majority of patients in this cohort (70%) had severe CFTR genotypes.
In the entire cohort, mean BMI was 23.9 ± 4.4 kg/m2. Only 5.2% of patients were underweight, and 62.6% had normal weight. Thus, the University of Minnesota CF Center has been highly effective in minimizing malnutrition in the CF population. However, 25.6% of patients were overweight and 6.6% were obese. As might be expected, patients in the overweight and obese categories were more likely to be older, male, diagnosed with CF at a later age, pancreatic sufficient, and to have a mild CFTR genotype compared to patients who were normal or underweight (Table 1). The prevalence of a severe CFTR genotype in patients with a BMI ≥ 25 kg/m2 was 56%, while 80% of patients who were underweight had a severe CFTR genotype. Sixty percent of overweight/obese and 100% of underweight CF patients were on pancreatic enzyme therapy. Underweight/normal weight patients were more likely to be on supplemental feeding compared to overweight/obese patients (36.4% vs 13.0%; P < 0.0001) (Table 1). Of the overweight/obese patients on supplemental feeding, the majority used oral supplements (80%), with 4 patients in this group using gastrostomy tube. The indication for gastrostomy tube was to support nutritional status during exacerbation of chronic pulmonary disease secondary to infection. History of lung transplant, use of corticosteroids, prevalence and duration of diabetes, duration of insulin treatment, and treatment with CFTR modulators were not different amongst BMI categories.
In a subgroup analysis of patients with a severe CFTR genotype, 25% had a BMI ≥ 25 kg/m2. The mean BMI for the severe CFTR genotype group was similar to the total cohort at 23.2 ± 3.7 kg/m2. Patients in this group who were overweight or obese were more likely to be older and male. Almost all of them were diagnosed with CF at a very early age and were pancreatic insufficient (Table 2). Thus, overweight/obesity was highly prevalent even in this group of CF patients with “classic” severe disease.
Table 2.
Characteristics of adult patients with CF who had a severe CFTR genotype, by body mass index category (N = 334).
| Underweight (N = 20) | Normal weight (N = 230) | Overweight (N = 73) | Obesity (N = 11) | P-value | |
|---|---|---|---|---|---|
| Age (years) | 31.6 ± 10.3 | 32.6 ± 9.6 | 37.2 ± 11.3 | 42.4 ± 8.0a | 0.0002 |
| BMI (kg/m2)b | 17.6 ± 0.9 | 21.9 ± 1.7 | 26.9 ± 1.3 | 34.4 ± 6.2 | <0.0001 |
| Male | 5 (25%) | 126 (55%) | 50 (68%) | 7 (64%) | 0.0045 |
| Caucasian | 19 (95%) | 224 (97%) | 71 (97%) | 9 (82%) | 0.07 |
| Age at CF diagnosis (years) | 1.4 ± 3.7 median 0.3 (0–16.5) | 2.6 ± 4.8 median 0.5 (0–32.1) | 3.5 ± 6.5 median 0.6 (0–33.3) | 2.3 ± 3.9 median 0.4 (0–11.8) | 0.38 |
| Homozygous F508del | 15 (75%) | 141 (61%) | 60 (82%) | 10 (91%) | 0.002 |
| History of lung transplant | 4 (20%) | 29 (13%) | 11 (15%) | 0 (0%) | 0.45 |
| Percent of patients taking pancreatic enzymes (n = 280) | 16 (100%) (n = 16) | 195 (99%) (n = 197) | 56 (97%) (n = 58) | 9 (100%) (n = 9) | 0.47 |
| Percent of patients using corticosteroids | 0.96 | ||||
| -Inhaled | 6 (30%) | 86 (37%) | 25 (34%) | 4 (36%) | |
| -Oral | 3 (15%) | 28 (12%) | 11 (15%) | 2 (18%) | |
| -None | 11 (55%) | 116 (50%) | 37 (51%) | 5 (45%) | |
| Percent of patients who have CFRD | 12 (60%) | 128 (56%) | 45 (62%) | 6 (55%) | 0.82 |
| Duration of CFRD (years) | 13.8 ± 6.2 median 13.1 (3–25.5) | 13.3 ± 7.1 median 12.5 (1.5–41.6) | 13.9 ± 7.1 median 14.9 (1.9–26.4) | 19.5 ± 8.6 median 18.0 (7.5–32.4) | 0.22 |
| Duration of insulin treatment (years) | 11.1 ± 6.4 | 11.1 ± 6.7 | 10.7 ± 5.7 | 17.2 ± 8.3 | 0.15 |
| HbA1c (%) | 6.8 ± 2.4 | 6.3 ± 1.0 | 6.7 ± 1.2 | 6.7 ± 1.2 | 0.08 |
| Hypertension | 4 (20%) | 36 (16%) | 25 (34%) | 3 (27%) | 0.006 |
| Percent of patients who receive CFTR modulators | 9 (45%) | 101 (44%) | 38 (52%) | 8 (73%) | 0.21 |
| Duration of CFTR modulator treatment (years) | 1.6 ± 0.9 | 1.7 ± 0.6 | 1.7 ± 0.6 | 1.8 ± 0.7 | 0.91 |
| FEV1 (% predicted) | 59.6 ± 26.7 | 69.0 ± 23.5 | 75.5 ± 22.7c | 73.2 ± 18.3 | 0.04 |
| Frequency of pulmonary exacerbations over 3 years | 8.2 ± 7.2 median 5.5 (0–29.0) | 5.9 ± 5.8 median 4.0 (0–32.0) | 4.2 ± 3.8d median 3.0 (0–19.0) | 6.5 ± 6.1 median 5.0 (0–20.0) | 0.02 |
| Total Cholesterol (mg/dL) (n = 324) | 122.6 ± 29.1 | 127.3 ± 36.5 | 130.2 ± 28.3 | 135.5 ± 27.8 | 0.71 |
| HDL-C (mg/dL) (n = 324) | 50.8 ± 17.5 | 49.9 ± 16.6 | 47.5 ± 14.6 | 52.4 ± 16.1 | 0.63 |
| LDL-C (mg/dL) (n = 324) | 50.9 ± 21.5 | 57.3 ± 25.6 | 58.6 ± 23.6 | 55.5 ± 23.5 | 0.68 |
| Triglyceride (mg/dL) (n = 324) | 106.9 ± 50.7 | 100.3 ± 59.8 | 121.0 ± 57.7e | 137.0 ± 63.0 | 0.022 |
| VLDL (mg/dL) (n = 291) | 23.3 ± 12.4 | 20.4 ± 10.4 | 23.2 ± 12.6 | 26.2 ± 15.0 | 0.13 |
P = 0.023 when compared to underweight group.
P = < 0.001 for all group comparisons.
P = 0.036 when compared to underweight group.
P = 0.024 when compared to underweight group.
P = 0.046 when compared to normal weight groupBMI; Body mass index, CFRD; Cystic fibrosis related diabetes, CFTR: Cystic fibrosis transmembrane regulator protein, FEV1; forced expiratory volume in 1 s.
3.2. Cardiovascular risk factors
The prevalence of hypertension in our CF cohort was 31% in overweight and 25% in obese patients compared to 17% in normal weight CF adults (P = 0.01). Overweight and obese patients had statistically higher total cholesterol, LDL-C and triglyceride levels compared to underweight and normal weight patients after adjusting for sex and age (Table 1). HDL-C levels were significantly lower in overweight/obese compared to normal weight patients. However, it should be noted that all cholesterol values remained within the normal range across all BMI categories, and the triglyceride elevation seen in the overweight/obese group was modest.
In the severe CFTR genotype subgroup, only triglyceride levels were statistically higher in overweight/obese compared to normal weight patients. Other lipid values including total cholesterol, LDL-C, VLDL and HDL-C were similar between BMI groups (Table 2).
Lung transplantation adversely impacted the cardiovascular risk profile in CF. Transplanted patients had a markedly increased prevalence of hypertension (86.7% vs 11.3%; P < 0.0001) and higher total cholesterol (155.5 ± 39.9 mg/dL vs 131.6 ± 35.3 mg/dl; P < 0.0001) and triglyceride (157.9 ± 69.5 mg/dL vs 101.3 ± 58.4 mg/dl; P < 0.0001) levels compared to non-transplanted patients. Interestingly, HDL-C was also elevated in transplanted recipients (58.6 ± 19.7 mg/dL vs 49.0 ± 15.3 mg/dl; P = 0.0006).
3.3. Lung function
In the natural history of CF, the majority of patients eventually die from respiratory failure. The mean percent predicted FEV1 in this cohort was 72.1 ± 23.9%. FEV1 was significantly higher (better) in patients who were obese and overweight compared to normal and underweight patients after adjusting for sex and age. There was no significant difference in lung function between the overweight and obese groups. FEV1% predicted positively correlated with BMI, but there was no further improvement in FEV1% predicted beyond a BMI of 28–29 kg/m2 (Fig. 1). Patients with normal weight had significantly more pulmonary exacerbations than patients who were overweight, and those who were underweight experienced the greatest frequency of pulmonary exacerbations compared to all groups. There was a weak inverse relationship between BMI and frequency of pulmonary exacerbations (Spearman R = −0.24;P ≤ 0.0001).
Fig. 1.
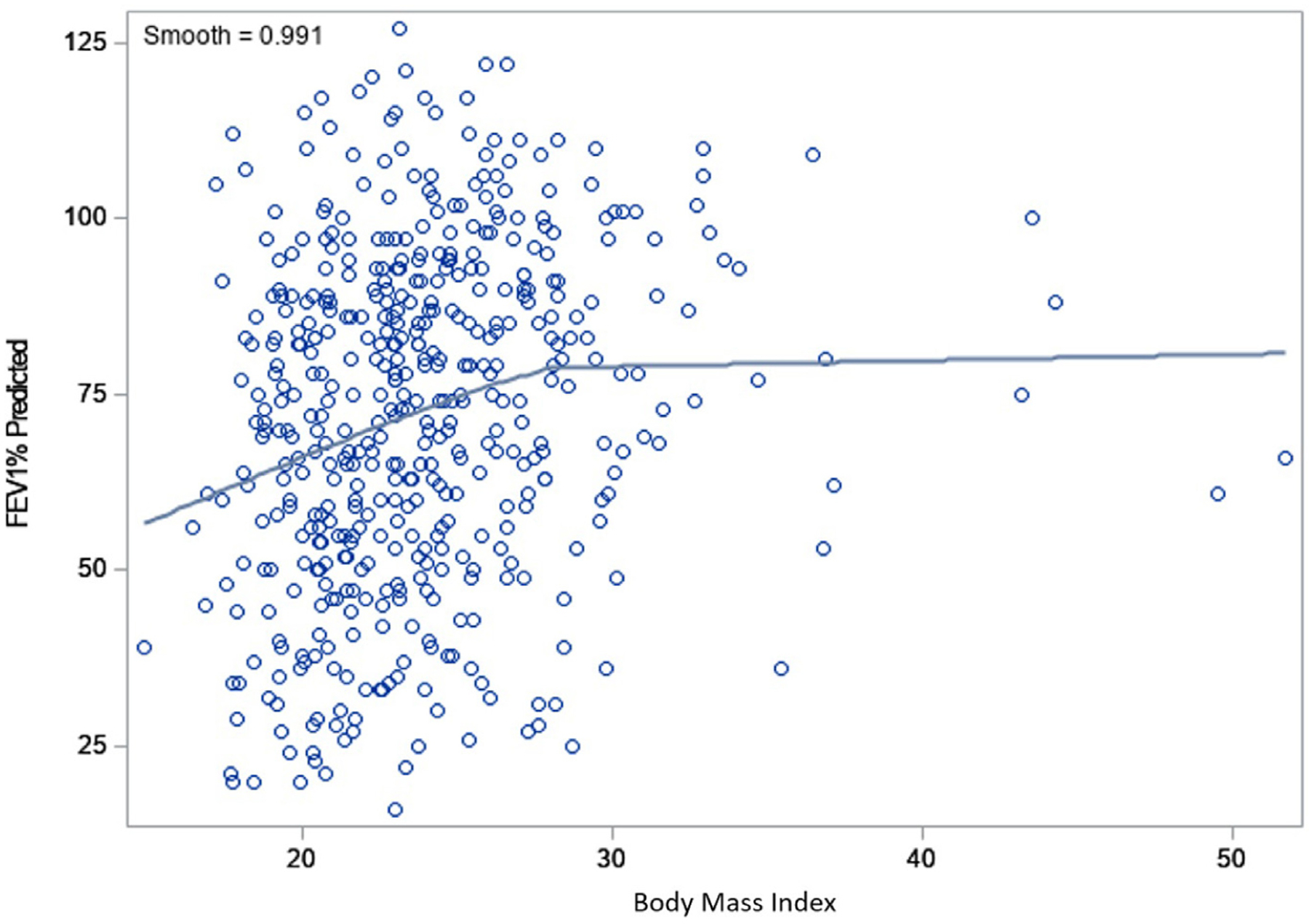
Loess fit curve between body mass index (BMI) and FEV1% predicted in all study cohort. BMI; Body mass index, FEV1% predicted; forced expiratory volume in 1 s expressed as a percentage of the normal predicted values for height and sex.
Multivariate regression analyses showed the best set of factors associated with overweight and obesity (BMI ≥ 25 kg/m2) for the entire cohort of patients with CF included age (OR = 1.04;P < 0.0001), mild CFTR genotype (OR = 3.33;P = 0.0003), and triglyceride levels (OR = 1.0 08; P < 0.0 0 01). (Table 3), while the best set of factors associated with overweight and obesity in patients with severe CFTR genotypes were male gender (OR = 2.00; P = 0.014), age (OR = 1.04; P = 0.002), homozygous F508del (OR = 3.149; P = 0.0 0 06), and triglyceride level (OR = 1.005;P = 0.016) (Table 3).
Table 3.
Multivariate logistic regression analysis of clinical variables and overweight/obesity in all study cohort (N = 484) and in patients with severe CFTR genotype (N = 334).
| Odds ratio | 95% confidence interval | P value | |
|---|---|---|---|
| All study cohort (N = 484) | |||
| Age | 1.04 | 1.02, 1.06 | <0.0001 |
| CFTR genotype classification | |||
| - mild vs severe | 3.33 | 1.92, 5.80 | 0.0001 |
| - unclassified vs severe | 1.09 | 0.55, 2.19 | 0.15 |
| Triglyceride | 1.008 | 1.004, 1.011 | <0.0001 |
| Patients with severe CFTR genotype (N = 334) | |||
| Sex male vs female | 2.00 | 1.15, 3.48 | 0.014 |
| Age | 1.04 | 1.02, 1.07 | 0.002 |
| Homozygous F508 | 3.14 | 1.63, 6.04 | 0.0006 |
| Triglyceride | 1.005 | 1.001, 1.009 | 0.016 |
CFTR: Cystic fibrosis transmembrane regulator.
4. Discussion
Overweight and obesity have become quite prevalent in CF, occurring in one-third of all adult CF patients at the University of Minnesota. It is particularly striking that 25% of those with severe CF mutations fall into this weight category. Only 5% of patients in the total CF population were underweight, and, as expected, this was associated with worse clinical status.
In the general population, overweight/obesity is associated with significant risk of death from atherosclerotic cardiovascular disease [14–15]. Our data do not support a similar association in CF. While cholesterol levels were statistically higher in this group, they were still within normal limits. Triglyceride levels were modestly elevated and there was a higher prevalence of hypertension, but importantly, no patient (including the 30 [19%] patients age ≥50 years) had known cardiovascular disease. It is particularly relevant that overweight/obesity appeared to be associated with an improved prognosis in CF as lung function and episodes of acute pulmonary exacerbation were better in this group. However, there was no further benefit of higher BMI on lung function, beyond a BMI threshold of 28–29 kg/m². This finding is in line with results from previous studies [12,20].
With the emphasis on nutritional augmentation [10–11], BMI in CF has increased over the past 30 years. Stephenson et al. observed an average BMI of 20.7 ± 2.7 kg/m2 in the 1980s compared to 22.3 ± 3.4 kg/m2 in the 2000s in a Toronto cohort of adults with CF followed from 1985 to 2011 [12]. Ten percent and 13% of adult patients were reported to have a BMI exceeding 25 kg/m2 in a 2002 UK registry [20] and a 2004 Canadian database [21], respectively, and in 23% of Pittsburgh pediatric patients in 2012 [13]. Our observed prevalence of combined overweight and obesity (32.2%) was remarkably greater than that previously reported from the United States and other European countries. Differences in the observed prevalence of overweight and obesity between the various studies (which included patients with similar gene mutations) might be explained by improvement in pulmonary and nutritional interventions over the years, and recently, by new CFTR modifier drugs that are changing the disease phenotype [22].
CFTR modifier drugs have been reported to increase weight in CF, sometimes dramatically [23]. In this cohort, we did not observe an association between body weight and treatment with CFTR modulators. However, since lumacaftor/ivacaftor was approved in July 2015, CF patients in the current study had been on this medication for a relatively short period (on average approximately 1.6 years), which could have limited our ability to examine the chronic impact of this CFTR modulator on body weight. The prevalence of overweight and obesity in our cohort is only 5–6% behind that found in the general population of US adults based on the 2015–2016 National Health and Nutrition Examination Survey (NHANES) [24], suggesting that soon the rate of overweight and obesity in CF may mirror that of the general US population.
This study cannot answer the “chicken and egg” relation between overweight/obesity and clinical status. Overweight/obesity was associated with improved pulmonary function and reduced risk of acute pulmonary exacerbation. Is it just that a milder genotype (known to be associated with less severe pulmonary disease) allows patients to achieve a more “normal” (general population) rate of overweight/obesity, or could overweight obesity be uniquely protective in CF? These data suggest that both may be true. Patients with less severe disease (mild CFTR mutation, later age of CF diagnosis and preserved pancreatic exocrine function and pulmonary function) were more likely to be overweight/obese, as might be expected [12,21,25]. More interesting is the fact that 54% of overweight/obese patients had severe CFTR genotypes, and the severe genotype subgroup, overweight/obesity was also associated with better pulmonary function. Thus, if there is no other important morbidity associated with excess weight in CF, it may actually be beneficial.
In the general population, excess morbidity and mortality in the overweight/obese population is related to atherosclerotic cardiovascular disease. Overweight (31%) and obese (25%) patients in our CF cohort were more likely to have hypertension as compared to normal weight CF adults (17%). We also confirmed previous studies suggesting that overweight/obese patients with CF have significantly higher levels of total cholesterol, LDL-cholesterol [12,20–21 and triglyceride compared to those of normal/underweight [12,26]. This was largely a statistical consideration since cholesterol levels are generally abnormally low in the CF population. Thus, average total cholesterol levels (mg/dL) were in the 140′s (compared to 120′s) and LDL levels (mg/dL) in the 70′s (compared to 50′s) in the overweight/obese group, levels that are not typically associated with cardiovascular risk. Triglyceride levels were also significantly higher in overweight/obese CF patients but only in the 130–140 mg/dL range. One limitation of this analysis is that only about half of the triglyceride levels could be verified as fasting, as triglyceride levels can be elevated in the post prandial state. Thus, triglyceride levels may actually be lower than we have reported. Cardiovascular diseases are extremely rare in this population and no CF patient in the literature has yet been reported to have died from atherosclerotic cardiovascular disease, despite the fact that about 5% of CF population is now over 50 years of age [27]. While there are no data to date to suggest that overweight and obesity currently predispose CF patients to increased mortality, it is yet to be determined whether persistent metabolic risk over time could translate into development of cardiovascular disease similar to the general population.
The primary strength of this study is the size and richness of a large, prospectively collected adult CF population database at an accredited CF Center. This study has several limitations in addition to that mentioned for triglyceride measurement. Because the data were analyzed cross-sectionally, causal relationships cannot be inferred. The results from this single center study may not be generalizable to other CF populations, in particular because of the decades-long emphasis at the University of Minnesota on a high-calorie diet for people with CF. BMI, a tool used to classify patients’ nutritional status, has its limitations since it does not reflect body composition— fat mass, muscle mass and fat distribution may influence metabolic and overall health and could be more important than BMI per se in CF patients. The diagnosis of hypertension was obtained from the CF Foundation patient registry database; therefore, it was not possible to verify what blood pressure threshold was used by individual providers to diagnose hypertension.
Nutritional counselling for the CF patients has historically focused on improving energy intake to achieve recommended calorie targets of 120–150% above the daily recommended intake for the general population [10–11]. Given the increasing prevalence of overweight/obesity in patients with CF, modern nutritional advice should take into consideration the patient’s body weight category and potential cardiovascular risk factors. The 2017 Nutrition Guidelines for CF in Australia and New Zealand provide a framework for identification, assessment and management of dietary interventions in CF according to BMI in adults or BMI percentiles in children [28]. While acknowledging the lack of evidence for the management of overweight and obesity in the CF population, these guidelines suggest that nutritional consideration may shift from a high fat, high energy diet to a more nutrient dense, moderate energy diet in CF patients with a BMI > 27 kg/m² or a BMI percentile in the overweight/obese range [28]. These guidelines also recommend that nutritional counseling should take into account body composition, as CF patients are known to have sarcopenia and are at risk of losing lean body mass if they do not consume adequate calories [28]. It is unclear at this time whether weight management recommendations for the general population can be generalized to overweight and obese patients with CF, and thus this needs to be approached with caution.
In summary, the low prevalence of undernutrition, previously a source of major morbidity in adults with CF, reflects successful comprehensive multidisciplinary care over the last few decades. However, this success has been accompanied by a significant rise in obesity and overweight, which is beginning to resemble the global trend of obesity in the general population. Whether or not this is harmful in CF remains to be determined since at present overweight/obese patients appear to have better pulmonary function than their normal and underweight peers. As there does not appear to be any further pulmonary advantage to being obese compared to simply being overweight, it seems reasonable to recommend a BMI < 30 kg/m2 for adults with CF. Further longitudinal studies should address the consequences associated with overweight/obesity in adults and children with CF, to inform appropriate nutritional approaches and treatment plans. In the mean-time, nutritional counselling for CF patients who are overweight or obese should be implemented carefully.
Acknowledgments
The authors would like to thank Ms. Catherine Moen from Department of Pediatrics, University of Minnesota for her assistance on database retrieval and technical support.
Funding
A. Moheet has grant support by Cystic Fibrosis Foundation through the “MOHEET16GE0-EnVision CF: Emerging Leaders in CF Endocrinology Program”.
Footnotes
Declaration of Competing Interest
T. Harindhanavudhi, A. Moheet, A. Moran and Q. Wang have no competing interests. J. Dunitz has received grants from Vertex Pharmaceuticals Inc, Hillrom, Proteostasis Therapeutics, Inc, and Cystic Fibrosis Foundation independent from the submitted work.
References
- [1].Elborn JS. Cystic fibrosis. Lancet 2016;388:2519–31. doi: 10.1016/S0140-6736(16)00576-6. [DOI] [PubMed] [Google Scholar]
- [2].Li L, Somerset S. Digestive system dysfunction in cystic fibrosis: challenges for nutrition therapy. Dig Liver Dis 2014;46:865–74. [DOI] [PubMed] [Google Scholar]
- [3].Culhane S, George C, Pearo B, Spoede E. Malnutrition in cystic fibrosis: a review. Nutr Clin Pract 2013;28:676–83. [DOI] [PubMed] [Google Scholar]
- [4].Steinkamp G, Wiedemann B. Relationship between nutritional status and lung function in cystic fibrosis: cross sectional and longitudinal analyses from the German CF quality assurance (CFQA) project. Thorax 2002;57:596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jarad NA, Higgs S, Jeffcote T, Giles K. Factors associated with reduced FEV1 in adult patients with cystic fibrosis in a relatively affluent area. Chron Respir Dis 2005;2:133–7. doi: 10.1191/1479972305cd065oa. [DOI] [PubMed] [Google Scholar]
- [6].Kerem E, Viviani L, Zolin A, MacNeill S, Hatziagorou E, Ellemunter H, et al. Factors associated with FEV1 decline in cystic fibrosis: analysis of the ECFS patient registry. Eur Respir J 2014;43:125–33. doi: 10.1183/09031936.00166412. [DOI] [PubMed] [Google Scholar]
- [7].Hauschild DB, Rosa AF, Ventura JC, Barbosa E, Moreira EAM, Ludwig Neto N, Moreno YMF. Association of nutritional status with lung function and morbidity in children and adolescents with cystic fibrosis: a 36-month cohort study. Rev Paul Pediatr 2018;36:8. doi: 10.1590/1984-0462/;2018;36;1;00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Konstan MW, Butler SM, Wohl ME, Stoddard M, Matousek R, Wagener JS, Johnson CA, Morgan WJ. Investigators and coordinators of the epidemiologic study of cystic fibrosis. growth and nutritional indexes in early life predict pulmonary function in cystic fibrosis. J Pediatr 2003;142:624–33. [DOI] [PubMed] [Google Scholar]
- [9].Yen EH, Quinton H, Borowitz D. Better nutritional status in early childhood is associated with improved clinical outcomes and survival in patients with cystic fibrosis. J Pediatr 2013;162:530–5. [DOI] [PubMed] [Google Scholar]
- [10].Castellani C, Duff A, Bell S, Heijerman H, Munck A, Ratjen F, et al. ECFS best practice guidelines: the 2018 revision. J Cyst Fibrosis 2018;17:153–78. doi: 10.1016/j.jcf.2018.02.006. [DOI] [PubMed] [Google Scholar]
- [11].Turck D, Braegger C, Colombo C, Declercq D, Morton A, Pancheva R. ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin Nutr 2016;35:557–77. doi: 10.1016/j.clnu.2016.03.004. [DOI] [PubMed] [Google Scholar]
- [12].Stephenson AL, Mannik LA, Walsh S, Brotherwood M, Robert R, Darling PB, et al. Longitudinal trends in nutritional status and the relation between lung function and BMI in cystic fibrosis: a population-based cohort study. Am J Clin Nutr 2013;97:872–7. [DOI] [PubMed] [Google Scholar]
- [13].Hanna RM, Weiner DJ. Overweight and obesity in patients with cystic fibrosis: a center-based analysis. Pediatr Pulmonol 2015;50:35–41. [DOI] [PubMed] [Google Scholar]
- [14].Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med 2010;363:2211–19. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Di Angelantonio E, Bhupathiraju ShN, Wormser D, Gao P, Kaptoge S, Berrington de Gonzalez A, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016;388:776–86. doi: 10.1016/S0140-6736(16)30175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Moran A, Brunzell C, Cohen RC, Katz M, Marshall BC, Onady G, et al. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the american diabetes association and a clinical practice guideline of the cystic fibrosis foundation, endorsed by the pediatric endocrine society. Diabetes Care 2010;33:2697–708. doi: 10.2337/dc10-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population.. Am J Respir Crit Care Med 1999;159:179–87. [DOI] [PubMed] [Google Scholar]
- [18].Sosnay PR, Siklosi KR, Van Goor F, Kaniecki K, Yu H, Sharma N, Ramalho AS, et al. Defining the disease liability of variants in the cystic fibrosis transmembrane conductance regulator gene. Nat Genet 2013;45:1160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lewis C, Blackman S, Nelson A, Oberdorfer E, Wells D, Dunitz J, et al. Diabetes-related mortality in adults with cystic fibrosis: role of genotype and sex. Am J Respir Crit Care Med 2015;191:194–200. doi: 10.1164/rccm.201403-0576OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kastner-Cole D, Palmer CN, Ogston SA, et al. Overweight and obesity in deltaF508 homozygous cystic fibrosis. J Pediatr 2005;147:402–4. [DOI] [PubMed] [Google Scholar]
- [21].Coderre L, Fadainia C, Belson L, Belisle V, Ziai S, Maillhot G, et al. LDL-cholesterol and insulin are independently associated with body mass index in adult cystic fibrosis patients. J Cyst Fibros 2012;11:393–7. doi: 10.1016/j.jcf.2012.03.006. [DOI] [PubMed] [Google Scholar]
- [22].Rowe SM, Daines C, Ringshausen FC, Kerem E, Wilson J, Tullis E, et al. Tezacaftor-Ivacaftor in residual-function heterozygotes with cystic fibrosis. N Engl J Med 2017;377:2024–35. doi: 10.1056/NEJMoa1709847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Borowitz D, Lubarsky B, Wilschanski M, Munck A, Gelfond D, Bodewes F, Schwarzenberg SJ. Nutritional status improved in cystic fibrosis patients with the G551D mutation after treatment with ivacaftor. Dig Dis Sci 2016;61((1) January):198–207. doi: 10.1007/s10620-015-3834-2. [DOI] [PubMed] [Google Scholar]
- [24].Fryar CD, Carroll M, Ogden C. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960–1962 through 2015–2016. National Center for Health Statistics; https://www.cdc.gov/nchs/data/hestat/obesity_adult_15_16/obesity_adult_15_16.htm [assessed 21 January 2019] [Google Scholar]
- [25].Dray X, Kanaan R, Bienvenu T, Desmazes-Dufeu N, Dusser D, Marteau P, et al. Malnutrition in adults with cystic fibrosis. Eur J Clin Nutr 2005;59:152–4. doi: 10.1038/sj.ejcn.1602039. [DOI] [PubMed] [Google Scholar]
- [26].Rhodes B, Nash E, Tullis E, Pencharz P, Brotherwood M, Dupuis A, Stephenson A. Prevalence of dyslipidemia in adults with cystic fibrosis. J Cyst Fibros 2010;9:24–8. doi: 10.1016/j.jcf.2009.09.002. [DOI] [PubMed] [Google Scholar]
- [27].https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2017-Patient-Registry-Annual-Data-Report.pdf [assess June 12, 2019]
- [28].Saxby N, Painter C, Kench A, King S, Crowder T, van der Haak N. and the australian and new zealand cystic fibrosis nutrition guideline authorship group. Nutrition guidelines for cystic fibrosis in Australia and New Zealand. Bell Scott C, editor. Sydney: Thoracic Society of Australia and New Zealand; 2017. [DOI] [PubMed] [Google Scholar]


