Abstract
Purpose
The long-term survival rate of osteosarcoma, which is the most common type of primary malignant bone tumor, has stagnated in past decades. Acacetin is a natural flavonoid compound that has antioxidative and anti-inflammatory effects and exhibits extensive therapeutic effects on various cancers. In this study, the anticancer potential of acacetin and the underlying molecular mechanisms were examined in human osteosarcoma cells (SJSA and HOS).
Materials and Methods
HOS and SJSA cell lines were exposed to different concentrations of acacetin. Cell proliferation and viability were assessed by CCK-8 and colony-formation assays. Hoechst 33258 fluorescent staining was employed to detect apoptosis. Cell apoptosis was measured by an annexin V-FITC/PI assay by flow cytometry. The alteration in the mitochondrial membrane potential was detected by a JC-1 Assay Kit. Apoptosis-related protein expression was determined by Western blotting. Intracellular reactive oxygen species (ROS) production was detected by fluorescence microscopy and flow cytometry. Subsequently, the activation of the ROS/JNK signaling pathway was investigated.
Results
Acacetin could inhibit proliferation and induce apoptosis in SJSA and HOS cells. The acacetin treatment resulted in the activation of caspase-3, −8, and −9 and cleaved PARP. Further studies showed that acacetin-induced apoptosis was attributed to ROS. In addition, we found that acacetin induced the activation of the downstream c-Jun N-terminal kinase (JNK) signaling pathway. Subsequently, after treatment with the ROS scavenger GSH and the JNK inhibitor SP600125, the apoptosis-inducing effect triggered by acacetin was significantly attenuated.
Conclusion
The results of the present study indicate that acacetin may induce apoptosis to inhibit cell growth by activating the ROS/JNK signaling pathway in SJSA and HOS cells, suggesting that acacetin may be a promising candidate for the management of osteosarcomas.
Keywords: acacetin, osteosarcoma, apoptosis, ROS/JNK activation
Introduction
Osteosarcoma (OS) is the most common type of primary malignant bone tumor, predominately affects the rapid growth of bones in children and adolescents, and accounts for approximately 15% of all bone malignancies.1 The 5-year survival rate of OS has been greatly improved to approximately 60–70% due to the use of neoadjuvant chemotherapy in combination with surgery.2 However, the long-term survival rate of patients has not been further improved in recent decades due to local relapse or early lung metastasis even after curative excision of the primary tumor and intensive chemotherapy.3 Thus, novel pharmacological molecules or strategies for OS and the further elucidation of the underlying molecular mechanism are urgently needed to improve patient survival.4
Currently, Chinese herbal medicine monomers have received increasing attention from scientists. Due to their safety and effectiveness, Chinese herbal medicine monomers have emerged as an important source of drugs and lead compounds.5 Flavonoids are a part of our daily diet and have been extensively studied due to their pharmacological properties against many diseases, including cancer; previous studies have suggested that Chinese herbal medicine monomers can be used as chemopreventive and adjuvant agents. Acacetin (5,7-dihydroxy-40 –methoxyflavone) is a natural flavonoid compound with antioxidative and anti-inflammatory effects that exhibits extensive therapeutic effects on many cancers, including OS.5–14 However, to date, its detailed mechanism in OS has not been studied.
Apoptosis refers to the autonomic and orderly death of cells controlled by genes to maintain the stability of the internal environment. Apoptosis involves the activation, expression and regulation of a series of genes.15 Similarly, apoptosis is the main mechanism that induces OS cell death. Recent research has found that many factors play an important role in the regulation of osteosarcoma cell apoptosis.16 Although the detailed mechanism of the apoptosis process is not fully understood, it has been determined that caspase plays an essential role in the process of apoptosis. Mitochondria are the control center of cell life activities. Experiments have shown that the release of cytochrome C from mitochondria is a key step in cell apoptosis.17
Reactive oxygen species (ROS) have been proven to be a trigger or mediator of members of the mitogen-activated protein kinase (MAPK) family and play an important role in various common biochemical reactions and pathological progress.16 A moderate increase in ROS can promote cell proliferation and differentiation, while excessive ROS can interfere with cell signaling pathways by causing oxidative damage to lipids, proteins and DNA.18,19 Several apoptotic effectors, including caspases, Bcl-2, and cytochrome c, are markedly regulated by cellular ROS.20 The c-Jun-N-terminal kinase (JNK) of the MAPK family is essential for many cell processes and plays an important role in stress responses, such as inflammation and apoptosis. The targeted activation of the ROS/JNK signaling cascade to induce OS cell apoptosis plays an important role in treatment.21
In this work, we aimed to investigate the underlying molecular pathways by which acacetin induces apoptosis in OS cells via the ROS/JNK signaling cascade. Our data suggest that acacetin is a promising candidate for the management of OS.
Materials and Methods
Materials and Reagents
Acacetin was obtained from Shanghai Tongtian Biotechnology, Ltd. (Shanghai, China). A stock solution of 20 mM was generated with DMSO (Sigma, St. Louis, MO, USA) and stored in the dark at −20 °C. DMSO was included in the 0 μM group at the same concentration as in the treated cells. Doxorubicin was obtained from Shanghai Tongtian Biotechnology, Ltd. The CCK8 (Cell Counting Kit-8) and Annexin V‑FITC Apoptosis Test kits were purchased from Beyotime Biotechnology, Ltd. (Shanghai, China). Glutathione (GSH) and SP600125 were purchased from Beyotime Biotechnology, Ltd. (Shanghai, China). The antibodies against poly(ADP-ribose) polymerase (PARP), Bax, Bcl-2, JNK, phospho-JNK, c-Jun, phospho-c-Jun, Cyt-c, caspase-3, caspase-8, caspase-9 and GAPDH were purchased from Cell Signaling Technology (Beverly, MA, USA). The secondary antibodies goat anti‑rabbit immunoglobulin G‑horseradish peroxidase (IgG‑HRP) (SC‑2004) and goat anti‑mouse IgG‑HRP (SC‑2005) were purchased from Millipore (Billerica, MA, USA).
Cell Lines and Cell Culture
Human cell line osteoblasts and the human OS cell lines 143B, MG63, SJSA and HOS were obtained from Shanghai First People’s Hospital Affiliated with Shanghai Jiao Tong University School of Medicine. This study was approved by the Research Ethics Committees of Xiang’an Hospital of Xiamen University. The cells were cultured in high-glucose Dulbecco’s modified Eagle’s medium (Biological Industries, Beit-Haemek, Israel) with 10% fetal bovine serum (Biological Industries, Beit-Haemek, Israel), 100 U/mL penicillin and 100 μg/mL streptomycin (Biological Industries, Beit-Haemek, Israel) in a humidified incubator at 37 °C under an atmosphere of 95% air and 5% CO2.
Cell Viability Assay
Cell viability was measured by a CCK-8 kit according to the manufacturer’s protocol. In brief, cells were plated in 96-well plates at a density of 2×104 cells/mL overnight and then incubated with different concentrations of acacetin for 24–72 h. Subsequently, 100 μL/well of CCK-8 working fluid were added, and after a 2-h incubation, the absorbance at the wavelength of 450 nm was measured using a microplate reader (Thermo Fisher Scientific, Inc.). These data are shown as the mean ± SD. Cell viability (%) = (OD [treated] − OD [blank])/(OD [control] − OD [blank]) × 100%. Then, the IC50 values (50% inhibition concentration) were calculated using the Statistical Package for the Social Sciences (SPSS, Inc., Chicago, IL, USA). Before and after IC50, the experimental concentration of the drug is set according to the arithmetic concentration gradient.
Colony-Formation Assay
Colony formation assays were performed to assess the antiproliferative activity of acacetin in OS. The cells were plated in six-well plates at a density of 500 cells per well. In the drug treatment group, the medium was changed to fresh medium containing acacetin (20 and 40 μM) for approximately 10–14 days until the cells grew to visible colonies. The medium was discarded, and the cells were washed with PBS twice. After being fixed with 4% paraformaldehyde for 20 min and washed with PBS, the cells were stained with 0.1% crystal violet for 15 min. The colonies that comprised > 50 cells were counted.
Morphological Apoptosis
To evaluate whether acacetin exhibited cytotoxicity in OS cells through apoptosis, a Hoechst 33258 staining analysis was performed to observe the nuclear morphological changes. In total, 5×104 cells per well were seeded in 6-well plates overnight and treated with 0 or 40 μM acacetin for 24 h. The cell medium was discarded, and then, the cells were washed with PBS and fixed with 4% paraformaldehyde for 30 min at room temperature, followed by Hoechst 33258 staining for 10 min in the dark. After being washed twice with PBS, the cells were observed under a fluorescence microscope (Olympus, Japan) to assess nuclei fragmentation and chromatin condensation.
Apoptosis Analysis by Flow Cytometry
To measure apoptotic cell death induced by the acacetin treatment, an Annexin-V-FITC Apoptosis Detection Kit was employed. The cells were cultured in six-well plates at a density of 5 × 104/mL and then incubated with acacetin at different concentrations overnight at 37 °C in a 5% CO2 incubator. After the acacetin treatment, the cells were digested, washed twice with chilled PBS and resuspended in binding buffer. Then, the cells were incubated with FITC-labeled Annexin V and PI for 15 min at room temperature in the dark. The samples were analyzed by flow cytometry (Beckham, USA).
Mitochondrial Membrane Potential (MMP) Measurement
The alteration in the MMP was examined by a JC-1 Assay kit (Beyotime, Shanghai, China) according to the manufacturer’s instructions. In total, 5 × 104 cells were seeded in 6-well plates, and acacetin was used to treat the SJSA and HOS cells at different concentrations for 24 h. Then, 100 μL of the JC-1 staining solution were added to 1 mL of culture medium, and the samples were incubated for 20 min. Subsequently, the samples were analyzed by flow cytometry (Beckham, USA).
ROS Assay
The production of ROS was analyzed by DCFH–DA staining by fluorescence microscopy and flow cytometry. The cells were plated at a density of 5 × 104 cells/well in six-well plates and treated with acacetin in a dose-dependent manner. According to the manufacturer’s protocol, the cells were incubated with DCFH–DA at a final concentration of 10 μM in DMEM for 30 min at 37 °C and then washed three times. Then, the generation of intracellular ROS was observed by fluorescence microscopy (Olympus, Japan) and analyzed by flow cytometry (Beckham, USA).
Western Blotting Analysis
The cells were seeded in six-well plates at a density of 5 × 104 cells/mL per well and treated with acacetin (0, 20, 40, and 60 μM) for 24 h. After washing the cells with PBS, the cells were lysed in ice-cold RIPA for 30 min. The lysis buffer contained a mixture of protease and phosphatase inhibitors. Subsequently, the cells were centrifuged at 12,000 ×g for 15 min at 4 °C, and the supernatant was collected. A BSA Protein Assay (Beyotime, Shanghai, China) was used to quantify the protein concentration according to the manufacturer’s instructions. The proteins were separated using SDS-PAGE gels (8–12% Tris-SDS gels) at 110 V for 0.5–1.5 h and wet-transferred to PVDF membranes at 300 mA. After blocking the membranes with 5% nonfat milk for an hour at room temperature, the membranes were incubated with the primary antibody at 4 °C overnight. The membranes were washed three times with TBST buffer and then probed with an anti-rabbit (1:5000) or anti-mouse (1: 5000) secondary antibody tagged with horseradish peroxidase for 1 h at room temperature. The bound immunocomplexes were detected using ChemiDoc XRS (BioRad).
Statistical Analysis
The experiments were performed three times, and the statistical analysis was conducted using Student’s t-test with IBM SPSS 20 software (SPSS, Inc., Chicago, IL, USA). The results are expressed as the means ± standard deviation (SD), and P < 0.05 indicated a statistically significant difference.
Results
Acacetin Inhibits Cell Proliferation in OS Cells
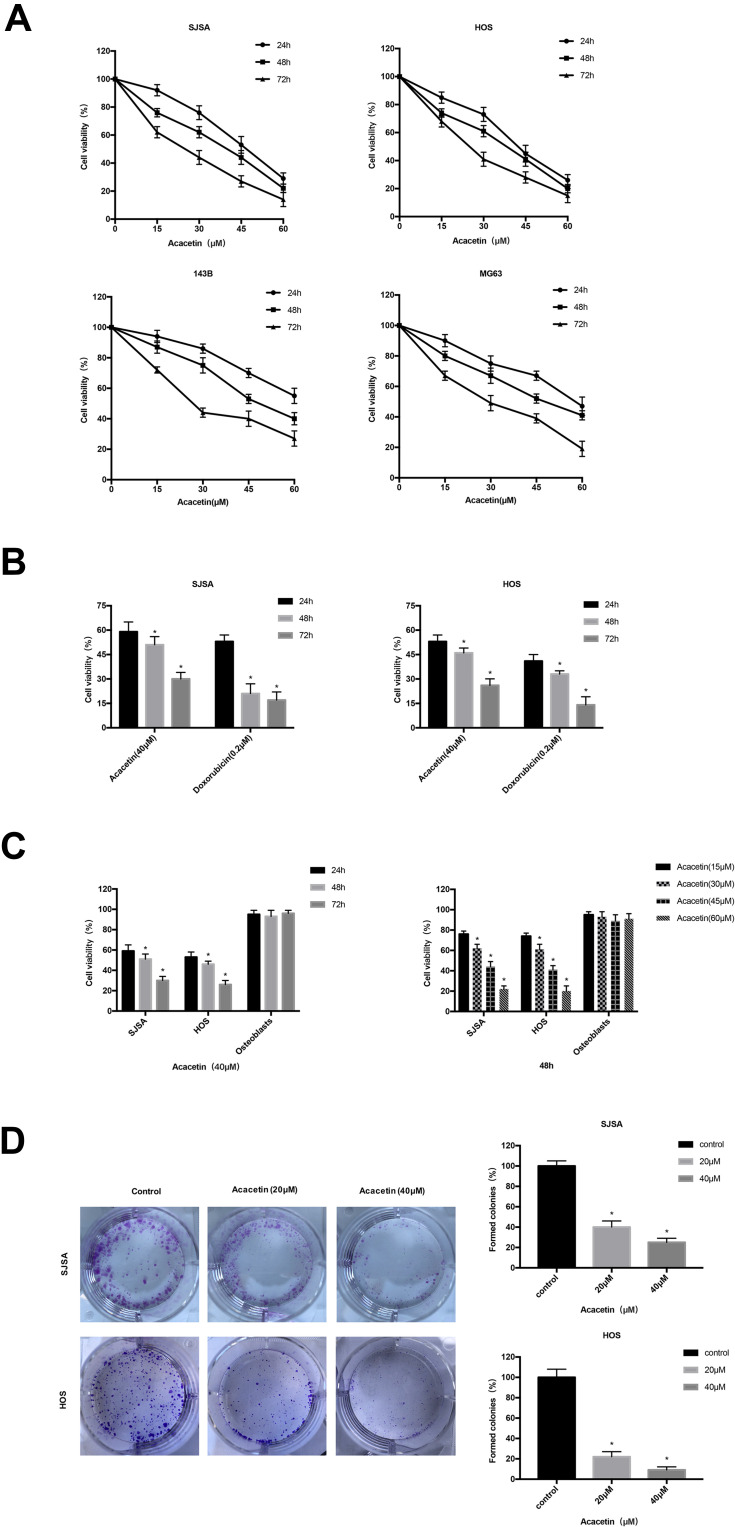
To investigate the antitumor effect of acacetin in OS, we treated 143B, MG63, SJSA and HOS OS cells with acacetin for 24, 48 or 72 h at drug concentrations of 0, 15, 30, 45, and 60 μM, and the cell vitality was measured with a CCK-8 Apoptosis Test kit. As shown in Figure 1A, as the drug concentration increased, the viability of both cell lines was significantly inhibited in an obvious dose- and time-dependent manner. The IC50 values were 47.31 μM (24 h), 42.98 μM (48 h), and 28.91 μM (72 h) in the SJSA cells and 43.13 μM (24 h), 39.78 μM (48 h), and 28.72 μM (72 h) in the HOS cells. Doxorubicin is a chemotherapy drug widely used for OS. To investigate the effectiveness of acacetin, we treated SJSA and HOS OS cells with 40 μM acacetin and a therapeutic concentration of doxorubicin (0.2 μM) for 24, 48 or 72 h, and the cell vitality was measured with a CCK-8 Apoptosis Test Kit. The results indicated that both acacetin and doxorubicin induced a time‐dependent decrease in cell viability, although to different degrees (Figure 1B). Subsequently, to evaluate the safety of acacetin, we used osteoblast cells as a negative control; we treated SJSA, HOS and osteoblast cells with 40 μM acacetin for 24, 48 or 72h or acacetin concentrations of 15, 30, 45, and 60 μM for 48 h, and the cell vitality was measured with a CCK-8 Apoptosis Test kit. As shown in Figure 1C, acacetin had a minimal effect on the osteoblasts’ cell viability. In addition, the colony formation test confirmed the anti-proliferative effect of acacetin in the SJSA and HOS OS cells. These results show that compared with the untreated cells, the treatment with acacetin significantly reduced the number of colonies in a dose-dependent manner (Figure 1D). These results indicate that treatment with acacetin inhibits the viability of OS cells.
Figure 1.
Acacetin inhibits cell proliferation in OS cells. (A) Acacetin inhibits human osteosarcoma cell proliferation. 143B, MG63, SJSA and HOS cells were treated with acacetin (0, 15, 30, 45, and 60 μM) for the indicated times (24, 48 and 72 h), followed by assessment of the inhibition rate by the CCK-8 assay (n=3). (B) After the SJSA and HOS cells were treated with 40mL of acacetin for the indicated times (24, 48 and 72 h), the cell viability was measured with CCK-8 assay and compared with the doxorubicin. (C) SJSA, HOS and osteoblast cells were treated with 40 μM acacetin for the indicated times (24, 48 and 72 h) or treated with increased concentrations of acacetin for 48 h and the cell viability was measured with CCK-8 assay. (D) Colony-formation assay of SJSA and HOS cells treated with the DMSO or acacetin. The histogram shows the ratio of clone formation. Control group was normalized to 100%. *P<0.05, significantly different compared with control.
Acacetin Induces Apoptosis in OS Cells
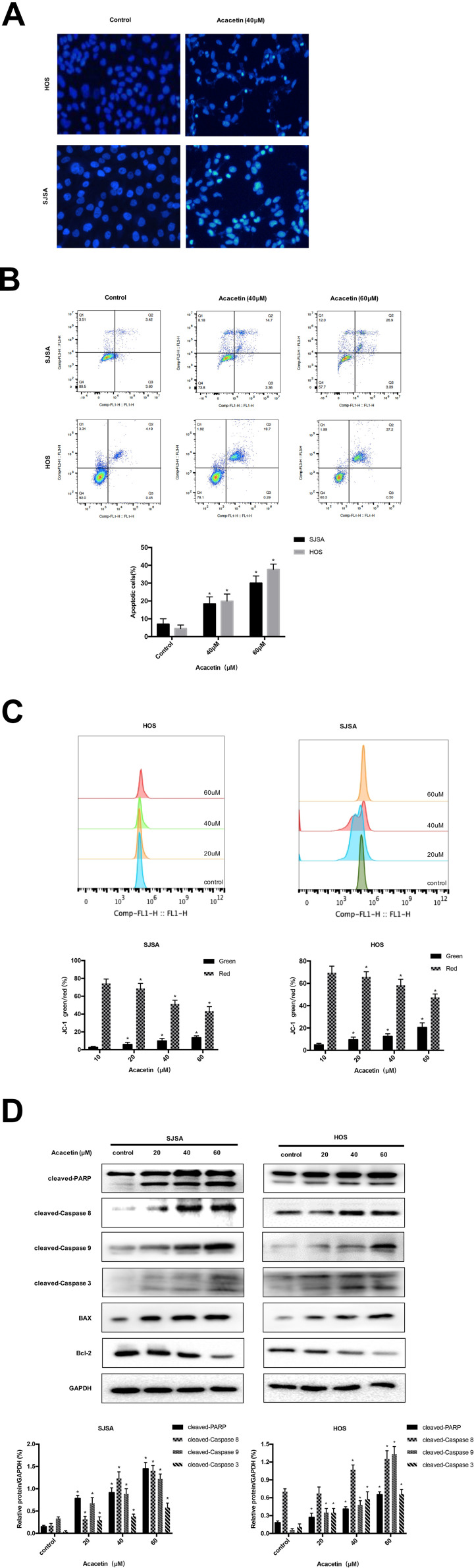
To further explore the characteristics of acacetin’s inhibitory effect on the viability of OS cells, Hoechst 33258 staining was used to evaluate the acacetin-dependent cell morphological changes. After the treatment with 40 μM acacetin for 24 h, both the HOS and SJSA cells showed cell shrinkage, chromatin condensation and nuclear fragmentation (Figure 2A). Consistent with the Hoechst 33258 staining results, flow cytometry with FITC/PI double staining also confirmed the role of acacetin in inducing apoptosis in OS cells. As shown in Figure 2B, compared with the control, early apoptosis in the SJSA and HOS cells increased in a dose-dependent manner after the 24-h acacetin treatment. To confirm that mitochondria are involved in acacetin-induced apoptosis, we used the fluorescent mitochondrial probe JC-1 to measure the mitochondrial membrane potential. Compared with the control group, the experimental group exhibited a significant transition from red to green fluorescence using flow cytometry (Figure 2C), indicating that acacetin induced mitochondrial depolarization in the OS cells. To further determine whether the extrinsic or intrinsic pathway mediates apoptosis induced by acacetin, we used Western blotting to explore the expression of downstream apoptosis-related proteins. As shown in Figure 2D, caspase-3, −8, −9, Bax and PARP and the expression of Bax were significantly increased, while the expression of Bcl-2 was decreased. In summary, these data indicate that acacetin induces apoptosis by activating extrinsic and intrinsic pathways.
Figure 2.
Acacetin induces apoptosis of OS cells. (A) Acacetin induced apoptosis in human osteosarcoma cells. Apoptotic nuclear morphological changes were evaluated by Hoechst 33258 staining and observed under a fluorescence microscope. (B) Acacetin induces apoptosis in HOS and SJSA cells. HOS and SJSA cells were treated with acacetin (0, 40 and 60 μM) for 24 h, and apoptotic cells were analyzed by flow cytometry. The histograms indicate that the percentage of total apoptosis. Results are shown as mean±S.D. from three independent experiments. (C) The mitochondrial membrane potential after acacetin treatment was measured using JC-1 staining by flow cytometry. The histograms indicate the ratio of green and red fluorescence. The results are shown as the mean±S.D. from three independent experiments. (D) Cells were treated with various concentrations of acacetin for 24 h. The apoptosis-related proteins cleaved PARP, caspase-3, −8, and −9, Bax and Bcl-2 were analyzed by Western blotting. *P < 0.05, significantly different compared with the untreated control group.
Acacetin Stimulates the JNK/c-Jun Pathway by Inducing ROS Generation in OS Cells
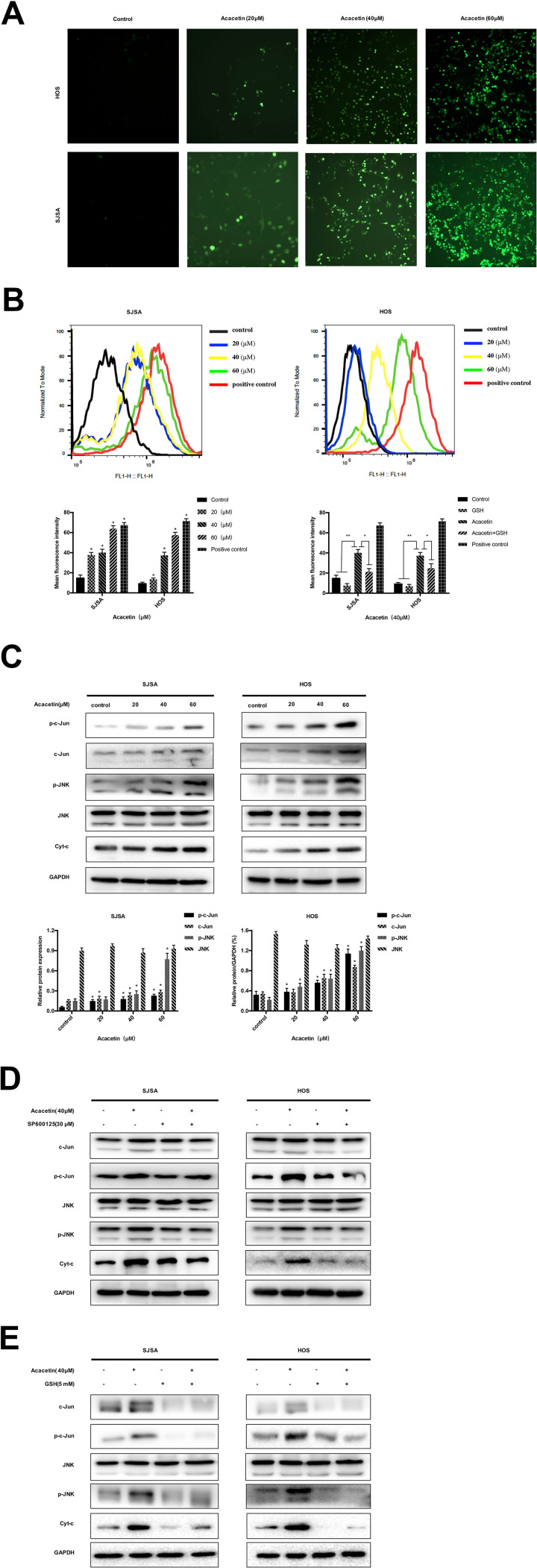
The generation of ROS in the cells was analyzed by DCFH–DA staining with fluorescence microscopy and flow cytometry. Figure 3A and B demonstrate that the exposure of the cells to acacetin resulted in a dramatic increase in the fluorescent signal that could be markedly suppressed by GSH. Then, we explored the effect of acacetin on the JNK/c-Jun signaling pathway. As shown in Figure 3C, acacetin induced phosphorylation of JNK and c-Jun in a concentration-dependent manner. However, the JNK inhibitor SP600125 effectively inhibited the activation of the JNK pathway (Figure 3D). ROS act as inducers or mediators of the activation of the JNK/c-Jun signaling pathway. In our further experiments, the pretreatment with GSH, which is a scavenger of ROS, significantly reversed the phosphorylation of JNK and c-Jun in the OS cells (Figure 3E). The above results indicate that acacetin activates the ROS/JNK signaling pathway in SJSA and HOS cells.
Figure 3.
Acacetin stimulates the JNK/c-Jun pathway by inducing ROS generation in OS cells. (A) Cells were treated with increased concentrations of acacetin for 24 h, followed by loading with 10 μM DCFH–DA for 30 min. The level of ROS was determined by fluorescence microscopy. (B) Fluorescence intensity was detected using flow cytometry. Cells were preincubated with GSH (5 mM) for 2 h and then treated with acacetin for 24 h. Fluorescent intensity was detected by flow cytometry. Results are presented as mean±S.D. from three independent experiments. (C) Cells were treated with various concentrations of acacetin for 24 h. The expression of p-JNK, JNK, p-c-Jun, Jun and Cyt-c was analyzed by Western blotting. (D and E) SJSA and HOS were preincubated with SP600125 (30 μM) or GSH (5 mM) for 2 h and then treated with acacetin for 24 h. Levels of p-JNK, JNK, p-c-Jun, Jun and Cyt-c were analyzed by Western blotting. *P<0.05 and **P<0.001, significantly different compared with the control.
Acacetin Induces Apoptosis by Activating the ROS/JNK Pathway in OS Cells
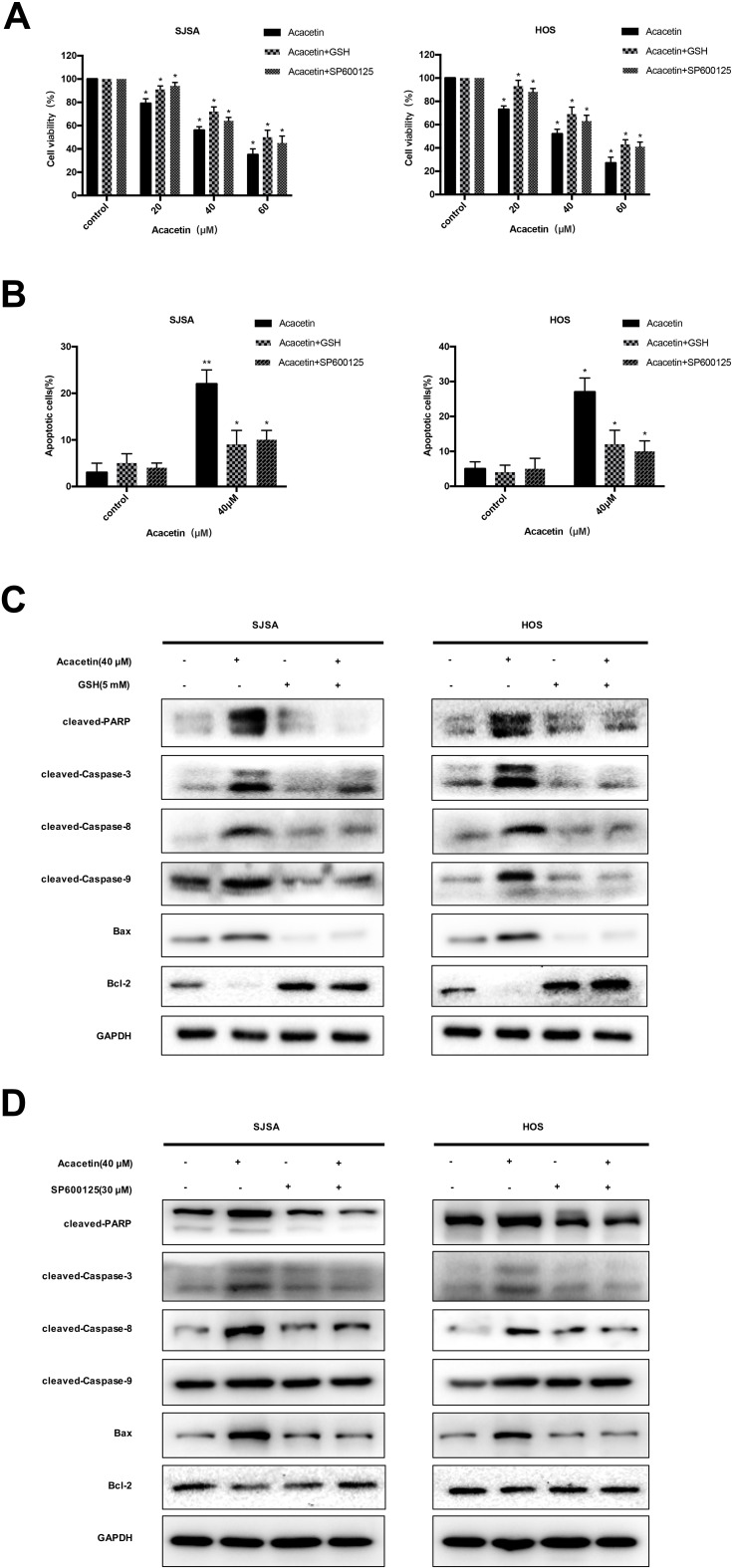
ROS have high biological activity and can participate in JNK signaling as a second messenger. ROS can activate JNK through signal proteins, such as ASK1, Src kinase, GSTπ, MLK3, RIP-TRAF2 complex, and MKPs, which may mediate antioxidant responses to induce apoptosis. We examined whether acacetin-induced apoptosis involves ROS production and JNK activation in OS cells. The cells were first pretreated with GSH and SP600125 and then treated with acacetin for 24 h. The results of the CCK8 analysis showed that the inhibition of ROS or JNK could effectively attenuate the inhibitory effect of acacetin on the growth of the OS cells (Figure 4A). The flow cytometric analysis demonstrated that GSH and SP600125 attenuated acacetin-induced apoptosis (Figure 4B). In addition, the Western blotting analysis showed that the pretreatment with GSH and SP600125 reduced the level of apoptosis-related proteins (Figure 4C and D). Thus, acacetin induces osteosarcoma cell apoptosis by activating the ROS/JNK signaling pathway.
Figure 4.
Acacetin induces apoptosis by activating the ROS/JNK signaling cascade in OS cells. Cells were preincubated with SP600125 (30 μM) or GSH (5 mM) for 2 h and then treated with acacetin for 24 h. (A) Cell viability was measured by CCK8 assay. (B) Flow cytometric analysis demonstrated that GSH and SP600125 attenuated the acacetin-induced apoptosis. Results are presented as the mean ± SD from three independent experiments. (C and D) The expression of apoptosis-related protein was measured by Western blotting. *P<0.05 and **P<0.001, significantly different compared with control.
Discussion
OS is the most common primary malignant bone tumor. For decades, the therapeutic effect of chemotherapy combined with surgery has not improved. Due to resistance to traditional chemotherapy and the inherent side effects of drugs, new therapeutic strategies for OS are urgently needed.4 Currently, natural plant extracts are widely used in antitumor research investigating various human cancers due to their good bioactivities.22–24
Flavonoids, which are widely present in plants, such as vegetables and fruits, are polyphenolic compounds that contain multiple substances and have shown antitumor activity in various human tumors.25 However, the pharmacological activity of acacetin, which is a type of flavonoid, in tumors is controversial. Studies have reported its cell proliferation inhibitory activity in prostate cancer,26 lung cancer,27 and breast cancer.28 Another study showed that low concentrations of acacetin can promote proliferation in breast cancer,29 which prompted us to investigate whether acacetin inhibits proliferation in OS. In this study, we conducted in vitro experiments and demonstrated that acacetin has a good antiproliferative effect on OS that is mainly achieved by promoting apoptosis in OS cells.
Apoptosis is an important mechanism through which many drugs achieve antitumor effects. The following two main pathways are involved in apoptosis in vivo: extrinsic and intrinsic pathways. The extrinsic pathway is also known as the death receptor pathway. Antitumor drugs can induce the binding of cell membrane surface death receptors and their ligands, such as Fas/Fas-L and DIL/DIL-R, to cause self-cleavage and the activation of caspase-8, and cleaved caspase-8 can activate the downstream effector caspase-3 and eventually trigger cell apoptosis.30,31 The endogenous pathway, namely, the mitochondrial pathway, can induce the release of cytochrome C from mitochondria to the cytoplasm, which binds apoptosis-related factor 1 (apaf-1), recruits pro-caspase-9 to form apoptotic corpuscles, and finally cleaves and activates caspase-9, which can also activate caspase-3, thereby triggering cell apoptosis.30,32 The acacetin treatment induced the conversion of red fluorescence to green fluorescence, indicating a decrease in MMP as revealed by flow cytometry after the JC-1 staining. The Bcl-2 family comprises proapoptotic and antiapoptotic members, such as Bax and Bcl-2. The regulation of certain proteins in the Bcl-2 protein family is a key factor affecting mitochondrial membrane permeability.33 PARP is an important ribozyme that is involved in the repair of DNA damage by cytotoxic drugs and is the main cleavage target of caspase-3, whose cleavage is conducive to the occurrence of apoptosis.34,35 The results of the Western blot analysis showed that acacetin induced the generation of apoptotic cells through the induction of DNA fragmentation and the activation of PARP, caspase-3, −8, and −9. In addition, the downregulation of Bcl-2 was accompanied by the overexpression of Bax in the OS cells treated with acacetin. Combined with the dose-dependent inhibition of OS cell activity by acacetin, these results indicate that the anti-OS effect of acacetin was achieved through the endogenous and exogenous apoptotic pathways.
A moderate increase in ROS can promote cell proliferation and differentiation, while excessive ROS can interfere with cell signal transduction pathways by causing oxidative damage to lipids, proteins and DNA and play an important role in various common biochemical reactions and pathological progress.36 In the present study, acacetin increased the production of ROS in SJSA and HOS osteosarcoma cells in a concentration-dependent manner, and the pretreatment of the cells with the ROS inhibitor GSH significantly reversed the cell proliferation inhibition and apoptosis induction effects of acacetin. In addition, caspase-3, −8, −9, Bax and PARP and the expression of Bax were significantly increased and the expression of Bcl-2 was decreased, indicating that the induction of ROS may activate DNA damage and lead to OS cell apoptosis. Previous studies have shown that the JNK signaling pathway could transduce oxidative stress signals and induce cell apoptosis under various stress stimuli. In this study, we found that JNK and c-Jun phosphorylation was significantly enhanced after the treatment with acacetin. Confirmed using the JNK inhibitor SP600125, JNK activation is associated with the regulation of acacetin-induced apoptosis. Furthermore, JNK can sometimes appear upstream of ROS and play a biological role by promoting the production or aggregation of ROS. However, in this study, the inhibition of ROS prevented the activation of JNK, suggesting that ROS production was upstream of JNK. According to previous reports in the literature, in response to various stress stimuli, the JNK proteins are activated by a series of phosphorylation. Subsequently, JNK phosphorylates and activates the transcription factor activator protein-1 (AP-1) and the Bcl-2 family. These proteins control various cellular responses, including proliferation, differentiation, expression of other proteins and cell death. The downstream molecules activated by JNK include c-Jun, ELK1, SMAD4, p53, ATF-2 and HSF1, and those inhibited by JNK activation include NFATC1, NFAT4 and STAT3.37 Consistent with the results of previous studies, our experiments confirmed that p-JNK, c-Jun and p-c-Jun increased in a dose-dependent manner. Based on the above results, we believe that JNK, which acts as a downstream target of ROS, participates in acacetin-induced apoptosis. Therefore, the mechanism underlying the antitumor effect mediated by acacetin is related to the activation of the ROS/JNK signaling pathway.
Our study elucidated the detailed mechanism of OS cell apoptosis induced by acacetin. However, some possible limitations to this study should be acknowledged. Although we demonstrated the anticancer properties of acacetin in this study, the in vivo antitumor effect of acacetin must be evaluated. Furthermore, further studies are required to explore combined therapy involving acacetin and other established treatments to enhance the antitumor effects. In conclusion, this study revealed the antitumor effects of acacetin, and the findings of the present study may contribute to an improved understanding of the benefits and clinical applications of acacetin therapy.
Conclusion
The results of the present study indicate that acacetin may induce apoptosis to inhibit cell growth by activating the ROS/JNK signaling pathway in SJSA and HOS cells, suggesting that acacetin may be a promising candidate for the management of OS.
Acknowledgments
This work was supported by Program of the National Natural Science Foundation of China (grant numbers 82074233) and Scientific Research Foundation for Advanced Talents, Xiang’an hospital of Xiamen university (PM201809170009). The authors thank the members of their colleagues for making this study possible.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Pushpam D, Garg V. Management of refractory pediatric sarcoma: current challenges and future prospects. Onco Targets Ther. 2020;13:5093–5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Czarnecka AM, Synoradzki K, Firlej W, et al. Molecular biology of osteosarcoma. Cancers (Basel). 2020;12(8):2130. doi: 10.3390/cancers12082130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lilienthal I, Herold N. Targeting molecular mechanisms underlying treatment efficacy and resistance in osteosarcoma: a review of current and future strategies. Int J Mol Sci. 2020;21(18):6885. doi: 10.3390/ijms21186885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marec-Berard P, Dalban C, Gaspar N, et al. A multicentric randomized Phase II clinical trial evaluating high-dose thiotepa as adjuvant treatment to standard chemotherapy in patients with resectable relapsed osteosarcoma. Eur J Cancer. 2020;125(p):58–68. doi: 10.1016/j.ejca.2019.11.007 [DOI] [PubMed] [Google Scholar]
- 5.Yuan YL, Jiang N, Li Z-Y, et al. Polyphyllin VI induces apoptosis and autophagy in human osteosarcoma cells by modulation of ROS/JNK activation. Drug Des Devel Ther. 2019;13:3091–3103. doi: 10.2147/DDDT.S194961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun F, Li D, Wang C, et al. Acacetin-induced cell apoptosis in head and neck squamous cell carcinoma cells: evidence for the role of muscarinic M3 receptor. Phytother Res. 2019;33(5):1551–1561. doi: 10.1002/ptr.6343 [DOI] [PubMed] [Google Scholar]
- 7.Pan M-H, Lai C-S, Hsu P-C, Wang. Acacetin induces apoptosis in human gastric carcinoma cells accompanied by activation of caspase cascades and production of reactive oxygen species. J Agri Food Chem, 2005;53:620–630 [DOI] [PubMed] [Google Scholar]
- 8.Singh RP, Agrawal P, Yim D, Agarwal C, Agarwal RJC. Acacetin inhibits cell growth and cell cycle progression, and induces apoptosis in human prostate cancer cells: structure–activity relationship with linarin and linarin acetate. 2005;26:845–854. [DOI] [PubMed] [Google Scholar]
- 9.Hsu Y-L, Kuo P-L, Lin -C-CJBP. Acacetin inhibits the proliferation of Hep G2 by blocking cell cycle progression and inducing apoptosis. 2004;67:823–829. [DOI] [PubMed] [Google Scholar]
- 10.Shim H-Y, et al. Acacetin-induced apoptosis of human breast cancer MCF-7 cells involves caspase cascade, mitochondria-mediated death signaling and SAPK/JNK1/2-c-Jun activation. Mol Cells. 2007;24. [PubMed] [Google Scholar]
- 11.Shen K-H, Park JH, HD Paik, et al. Acacetin, a flavonoid, inhibits the invasion and migration of human prostate cancer DU145 cells via inactivation of the p38 MAPK signaling pathway. 2010;333:279. [DOI] [PubMed] [Google Scholar]
- 12.Rettig EM, D’Souza G. Epidemiology of head and neck cancer. Surg Oncol Clinics. 2015;24:379–396. [DOI] [PubMed] [Google Scholar]
- 13.Kim C-D, Cha J-D, Li S, et al. The mechanism of acacetin-induced apoptosis on oral squamous cell carcinoma. 2015;60:1283–1298. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Teng J, Ma L, et al. Flavonoids isolated from the flowers of limonium bicolor and their in vitro antitumor evaluation. Pharmacogn Mag. 2017;13(50):222–225. doi: 10.4103/0973-1296.204566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgess DJ. Apoptosis: refined and lethal. Nat Rev Cancer. 2013;13(2):79. doi: 10.1038/nrc3462 [DOI] [PubMed] [Google Scholar]
- 16.Li B, Zhou P, Xu K, et al. Metformin induces cell cycle arrest, apoptosis and autophagy through ROS/JNK signaling pathway in human osteosarcoma. Int J Biol Sci. 2020;16(1):74–84. doi: 10.7150/ijbs.33787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagata S. Apoptosis and clearance of apoptotic cells. Annu Rev Immunol. 2018;36:489–517. doi: 10.1146/annurev-immunol-042617-053010 [DOI] [PubMed] [Google Scholar]
- 18.Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10:1343–1374. doi: 10.1089/ars.2007.1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Wang K, Lei Y, et al. Redox signaling: potential arbitrator of autophagy and apoptosis in therapeutic response. Free Radic Biol Med. 2015;89:452–465. doi: 10.1016/j.freeradbiomed.2015.08.030 [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Zhang T, Sun W, et al. Erianin induces G2/M-phase arrest, apoptosis, and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death Dis. 2016;7(6):e2247. doi: 10.1038/cddis.2016.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shajahan AN, Dobbin ZC, Hickman FE, Dakshanamurthy S, Clarke R. Tyrosine-phosphorylated caveolin-1 (Tyr-14) increases sensitivity to paclitaxel by inhibiting BCL2 and BCLxL proteins via c-Jun N-terminal kinase (JNK). J Biol Chem. 2012;287(21):17682–17692. doi: 10.1074/jbc.M111.304022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleinsimon S, Kauczor G, Jaeger S, et al. ViscumTT induces apoptosis and alters IAP expression in osteosarcoma in vitro and has synergistic action when combined with different chemotherapeutic drugs. BMC Complement Altern Med. 2017;17(1):26. doi: 10.1186/s12906-016-1545-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Rimawi F, et al. Anticancer activity, antioxidant activity, and phenolic and flavonoids content of wild tragopogon porrifolius plant extracts. Evid Based Complement Alternat Med. 2016;9612490-9612490:2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu F-Y, WQ Shang, Yu J, et al. The antitumor activity study of ginsenosides and metabolites in lung cancer cell. Am J Transl Res. 2016;8:1708–1718. [PMC free article] [PubMed] [Google Scholar]
- 25.Liu H, Gao Y, Dong Y, et al. Flavonoids active against osteosarcoma: a review of the molecular mechanisms involved. Curr Pharm Des. 2017;23(13):1993–2001. doi: 10.2174/1381612823666170214115718 [DOI] [PubMed] [Google Scholar]
- 26.Kim HR, Park CG, Jung JY. Acacetin (5,7-dihydroxy-4ʹ-methoxyflavone) exhibits in vitro and in vivo anticancer activity through the suppression of NF-κB/Akt signaling in prostate cancer cells. Int J Mol Med. 2014;33:317–324. doi: 10.3892/ijmm.2013.1571 [DOI] [PubMed] [Google Scholar]
- 27.Hsu Y-L, Kuo P-L, Liu C-F, Lin -C-C. Acacetin-induced cell cycle arrest and apoptosis in human non-small cell lung cancer A549 cells. Cancer Lett. 2004;212:53–60. doi: 10.1016/j.canlet.2004.02.019 [DOI] [PubMed] [Google Scholar]
- 28.Zhang H-W, Hu -J-J, Fu R-Q, et al. Flavonoids inhibit cell proliferation and induce apoptosis and autophagy through downregulation of PI3Kγ mediated PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in human breast cancer cells. Sci Rep. 2018;8(1):11255. doi: 10.1038/s41598-018-29308-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren H, Ma J, Si L, et al. Low dose of acacetin promotes breast cancer MCF-7 cells proliferation through the Activation of ERK/PI3K/AKT and cyclin signaling pathway. Recent Pat Anticancer Drug Discov. 2018;13:368–377. doi: 10.2174/1574892813666180420154012 [DOI] [PubMed] [Google Scholar]
- 30.Zou H, Li Y, Liu X, Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549 [DOI] [PubMed] [Google Scholar]
- 31.Debatin K-M, Krammer PH. Death receptors in chemotherapy and cancer. Oncogene. 2004;23:2950–2966. doi: 10.1038/sj.onc.1207558 [DOI] [PubMed] [Google Scholar]
- 32.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/S0092-8674(04)00046-7 [DOI] [PubMed] [Google Scholar]
- 33.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. [DOI] [PubMed] [Google Scholar]
- 34.Zhou L, Wang S, Cao L, Ren X, Li Y, Shao J, Xu L. Lead acetate induces apoptosis in Leydig cells by activating PPARγ/caspase-3/PARP pathway. Int J Environ Health Res. 2019;1–11. [DOI] [PubMed] [Google Scholar]
- 35.KY L, Cell KWJ. PARP inhibitors for cancer therapy. 2017;169:183. [DOI] [PubMed] [Google Scholar]
- 36.Gupta RK, Patel AK, Shah N, et al. Oxidative stress and antioxidants in disease and cancer: a review. Asian Pac j Cancer Prev. 2014;15:4405–4409. doi: 10.7314/APJCP.2014.15.11.4405 [DOI] [PubMed] [Google Scholar]
- 37.Kumar A, Singh UK, Kini SG, et al. JNK pathway signaling: a novel and smarter therapeutic targets for various biological diseases. Future Med Chem. 2015;7(15):2065–2086. doi: 10.4155/fmc.15.132 [DOI] [PubMed] [Google Scholar]