Abstract
Background
As the global outbreak of COVID-19 continues to ravage the world, it is important to understand how frontline clinicians manage ventilatory support and the various limiting factors.
Methods
An online survey composed of 32 questions was developed and validated by an international expert panel.
Results
Overall, 502 respondents from 40 countries across six continents completed the survey. The mean number (±SD) of ICU beds was 64 ± 84. The most popular initial diagnostic tools used for treatment initiation were arterial blood gas (48%) and clinical presentation (37.5%), while the national COVID-19 guidelines were the most used (61.2%). High flow nasal cannula (HFNC) (53.8%), non-invasive ventilation (NIV) (47%), and invasive mechanical ventilation (IMV) (92%) were mostly used for mild, moderate, and severe COVID-19 cases, respectively. However, only 38.8%, 56.6% and 82.9% of the respondents had standard protocols for HFNC, NIV, and IMV, respectively. The most frequently used modes of IMV and NIV were volume control (VC) (36.1%) and continuous positive airway pressure/pressure support (CPAP/PS) (40.6%). About 54% of the respondents did not adhere to the recommended, regular ventilator check interval. The majority of the respondents (85.7%) used proning with IMV, with 48.4% using it for 12–16 hours, and 46.2% had tried awake proning in combination with HFNC or NIV. Increased staff workload (45.02%), lack of trained staff (44.22%) and shortage of personal protective equipment (PPE) (42.63%) were the main barriers to COVID-19 management.
Conclusion
Our results show that general clinical practices involving ventilatory support were highly heterogeneous, with limited use of standard protocols and most frontline clinicians depending on isolated and varied management guidelines. We found increased staff workload, lack of trained staff and shortage of PPE to be the main limiting factors affecting global COVID-19 ventilatory support management.
Keywords: COVID-19, ventilation, respiratory, clinical management, proning, mechanical ventilation, NIV, HFNC
Introduction
Coronavirus (COVID-19) is a viral infectious disease that has spread globally and has become a pandemic.1 According to the World Health Organization (WHO), on July 12, 2020, the number of people diagnosed with COVID-19 had exceeded 12 million, with total death cases of 561, 617, worldwide.2 Individuals diagnosed with COVID-19 are at risk of developing respiratory illness that might require ventilatory support and intensive care unit (ICU) admission.3 Studies have shown that some of confirmed cases develop acute respiratory distress syndrome (ARDS).4–7 Moreover, Grasselli et al reported that 16% of 3420 confirmed cases were admitted to the ICU.8 According to the WHO-China Joint Mission report on COVID-19, out of 55,924 confirmed laboratory cases, 6.1% were judged critical, and 13% as severe, with about 25% of critical and severe cases requiring ventilatory support.9 In general, the severity of COVID-19 and admission to ICU are linked to various comorbidities, including chronic respiratory, cardiovascular and digestive diseases.10–12 These comorbidities are also associated with an increased rate of mortality and present a challenge for ICU management of COVID-19 patients.13
In another study, Guan et al found that about 6.1% of 1099 positive cases had received mechanical ventilation (MV), in which non-invasive ventilation (NIV) was used the most, even though nosocomial transmission with NIV and high flow nasal cannula (HFNC) is still unclear.14 Development of ARDS in COVID-19 patients may indicate the need for ventilatory support management, which may vary across hospitals, countries and regions. Current guidance on ICU management of COVID-19 patients, including ventilatory support protocols, is lacking and so far, practices have been largely informed by evidence from standard intensive care management and experience with other viral respiratory infections or from direct experience with COVID-19.4
The rapid spread of the COVID-19 pandemic has made an integrated effort challenging and the sharing of best practices challenging. This has increased the need to explore and assess the current global practices regarding ventilatory support management of COVID-19 patients. Therefore, this study aims to understand which ventilation techniques critical care providers (CCPs) have used to manage adult COVID-19 patients worldwide, to shed light on the challenges that clinicians have faced, and ultimately to develop potential recommendations to mitigate this clinical challenge.
Methods
An online survey composed of 32 questions was developed based on the current emerging evidence and validated by an international expert panel, which included consultant respiratory therapists, physiotherapists, nurses, intensivists, and pulmonologists. Face and content validity were assessed after piloting this survey to 10 CCPs from different specialities. The questionnaire contained a structured response, which involved multiple-choice responses in three separate sections. Section one contained respondents’ demographic information (eg, practice type, location, number of beds, experiences and training background). Formal training in MV was defined as theoretical and practical sessions for at least six weeks or more. Section two contained questions about the general clinical management of ventilatory support in COVID-19 patients (eg, diagnostic tools, type of ventilation modalities used, and different ventilation strategies). The severity was defined based on Berlin definition of ARDS. Section three was designed to assess difficulties that could hinder ventilatory support management in COVID-19 patients. The survey was distributed via different international societies, including the Canadian Society of Respiratory Therapists, the Thoracic Society of Australia and New Zealand (TSANZ), the Association of Chartered Physiotherapists in Respiratory Care (ACPR), the World Confederation for Physical Therapy (WCPT), the Indian Association of Respiratory Care (IARC), the Saudi Society for Respiratory Care (SSRC) and Brazilian Association of Cardiorespiratory Physiotherapy and Physiotherapy in Intensive Care (ASSOBRAFIR). The International Council for Respiratory Care (ICRC) was also involved in this project and supported us in distributing our survey link to all affiliated international council members and societies. To reach more frontline clinicians in various countries, we involved representatives from different regions, including the United Kingdom (UK), Europe, the Middle East, North and South America, Australia and Asia. The survey was also advertised through other professional groups, including the Royal Brompton Hospital physiotherapists’ group, various coronavirus networks, the Italian Group for the Evaluation of interventions in Intensive Care and the official Saudi group for respiratory therapists. The survey was carried out between April 15, 2020 and June 15, 2020. Ethical approval was obtained from the Armed Forces Hospitals Eastern Region Institutional Review Board (IRB) (AFHER-IRB-2020-012), which the principal investigator affiliation (Prince Sultan Military College of Health Sciences) is under this regional committee and that approval was accepted by all other sites involved in this study. All participants provided informed consent to partake in this study and that this study complied with the Declaration of Helsinki.
Statistical Analysis
Descriptive analysis (ie, absolute values and proportions) was used to analyse responses and summarise respondents’ characteristics. Chi-square and Fisher exact tests were applied to draw comparisons between groups. The Statistical Package for the Social Sciences (SPSS) version 24 was used to analyse the collected responses. A p-value of ≤0.05 was considered statistically significant.
Results
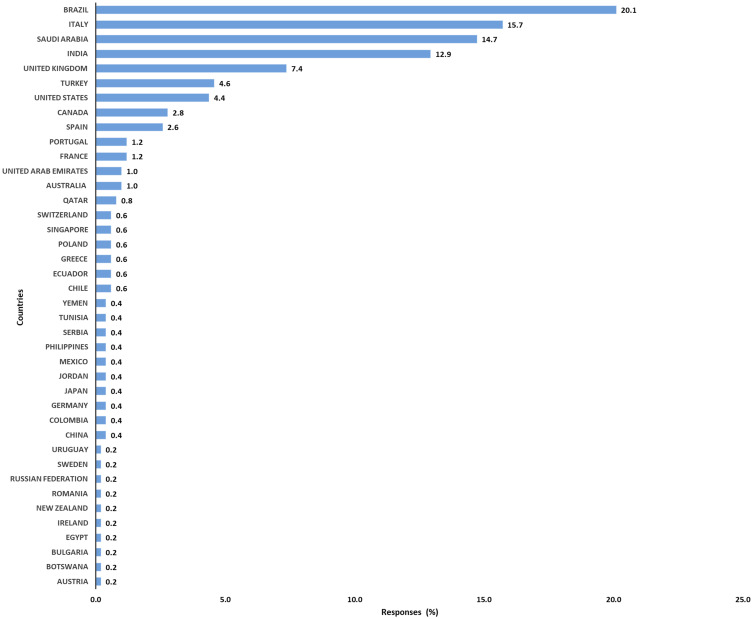
A total of 502 respondents completed the survey from 40 different countries across six continents. Most of the respondents were from Brazil (20.12%), Italy (15.74%), Saudi Arabia (14.74%) and India (12.95%) (Figure 1). In general, the highest responses were from Asia and Europe (Table 1). There were six main groups of professions out of the respondents, with respiratory therapists (34.46%), physiotherapists (24.50%) and ICU intensivists (21.51%) being the most represented. Previous training in MV was reported by 85% of the total respondents. According to the respondents, 80 (16.36%) of the hospitals had >1000 beds while the mean number (±SD) of ICU beds was 64 (±84). Table 1 describes the characteristics of all respondents.
Figure 1.
Percentage of responders per country.
Table 1.
Characteristics of Critical Care Practitioners (N= 502) *
| Characteristics | Values |
|---|---|
| Highest qualification | |
| ● Diploma | 45 (8.96%) |
| ● Bachelor | 228 (45.42%) |
| ● Master | 158 (31.47%) |
| ● PhD | 71 (14.14%) |
| Profession | |
| ● Respiratory therapists | 173 (34.46%) |
| ● Physiotherapist | 123 (24.50%) |
| ● Nurse | 8 (1.59%) |
| ● ICU Intensivist | 108 (21.51%) |
| ● Pulmonologist | 60 (11.95%) |
| ● Anesthesiologist | 30 (5.98%) |
| Continent | |
| ● Africa | 4 (0.80%) |
| ● Asia | 185 (36.85%) |
| ● Europe | 159 (31.67%) |
| ● North America | 36 (7.17%) |
| ● Oceania | 6 (1.20%) |
| ● South America | 112 (22.31%) |
| Type of hospital | |
| ● Secondary care hospital | 137 (27.29%) |
| ● Tertiary care hospital | 365 (72.71%) |
| Hospital characteristics | |
| ● <200 beds | 93 (19.02%) |
| ● 200–499 beds | 175 (35.79%) |
| ● 500–1000 beds | 141 (28.83%) |
| ● >1000 beds | 80 (16.36%) |
| ICU beds | |
| ● Total number | 31,144 |
| ● Mean (±SD) | 64 (±84) |
| ● 1–10 | 42 (8.64%) |
| ● 11–100 | 365 (75.10%) |
| ● >100 | 79 (16.26%) |
| Previous training in MV | 424 (85%) |
| Experience and training | |
| ● >20 – year experience | 94 (18.73%) |
| ● 11–20 – year experience | 144 (28.69%) |
| ● 6–10 – year experience | 115 (22.91%) |
| ● ≤5 – year experience | 149 (29.68%) |
| ● >20 – year experience and trained | 82 (19%) |
| ● 11–20 – year experience and trained | 122 (29%) |
| ● 6–10 – year experience and trained | 97 (23%) |
| ● ≤5 – year experience and trained | 123 (29%) |
Note: *All percentages are expressed corresponding to the total number of respondents.
Abbreviations: PhD, Doctor of Philosophy; ICU, intensive care unit; SD, standard deviation.
Continents, Qualifications, and Formal Training in MV
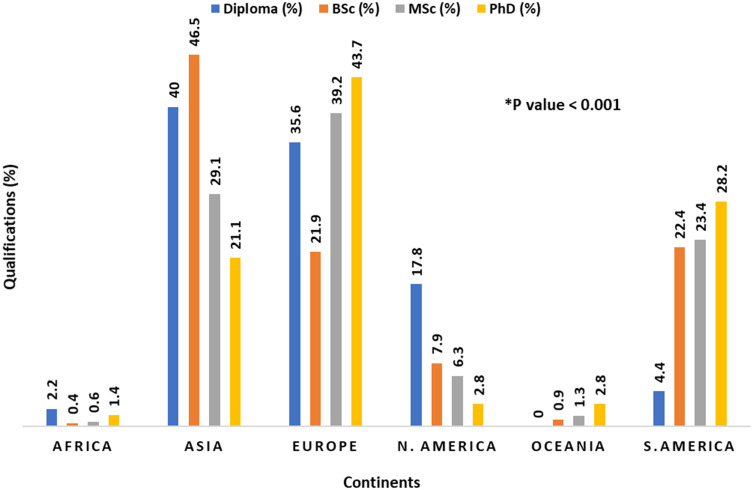
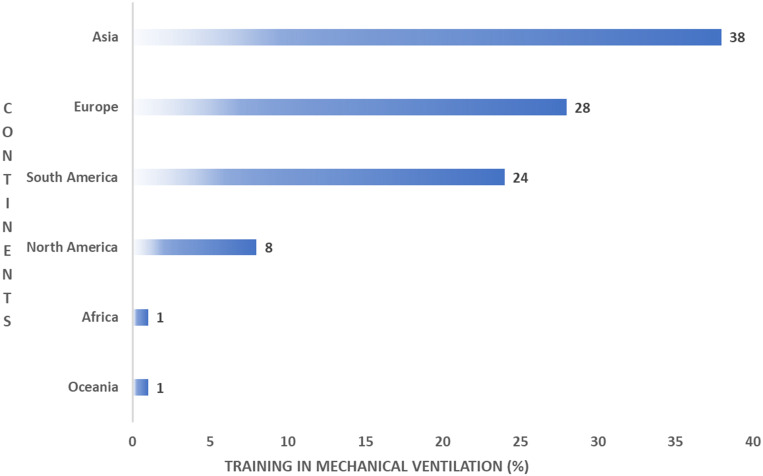
We found significant differences in academic degrees between the continents. The highest percentage of diploma holders was reported in Asia (40%), Europe (35.6%) and North America (17.8%), while the highest percentage of PhD holders was reported in continental Europe (43.7%) (Figure 2). There was no significant difference in formal training in MV between the various academic degrees (p=0.394). Interestingly, there was a significant difference in formal training in MV between the continents’ practitioners (p < 0.001). The highest percentage of non-trained professionals was reported in Europe (53.8%), followed by Asia (28.2%) (Figure 3).
Figure 2.
Academic degrees per continent.
Figure 3.
Formal training in MV per continent.
Experience, Professions, and Formal Training in MV
Although there were no significant differences in formal training in MV between the different professions, the highest percentage of formal training was reported in the respiratory therapist profession (36.3%), followed by physiotherapists (23.1%) and ICU intensivists (21.2%). Interestingly, the highest number of non-trained professionals was also found among physiotherapists (32.1%), respiratory therapists (24.4%), and ICU intensivists (23.1%).
We found a significant difference in years of experience between the professions (p < 0.001). ICU intensivists had more experience (>20 years) than any other profession 37.2% (35/94) followed by pulmonologists 19.1% (18/94) and respiratory therapists 18.1% (17/94). Interestingly, respiratory therapists also had the highest number of professionals with ≤5 years experience 60.4% (90/149). However, there were no significant differences in formal training in MV between the different years of experience (p = 0.84) in all professions. No significant differences in years of experience, training and qualifications were found between the tertiary and secondary hospitals. However, 68.8% (31/45) of staff in the tertiary hospital were reported to have a higher number of diploma degree.
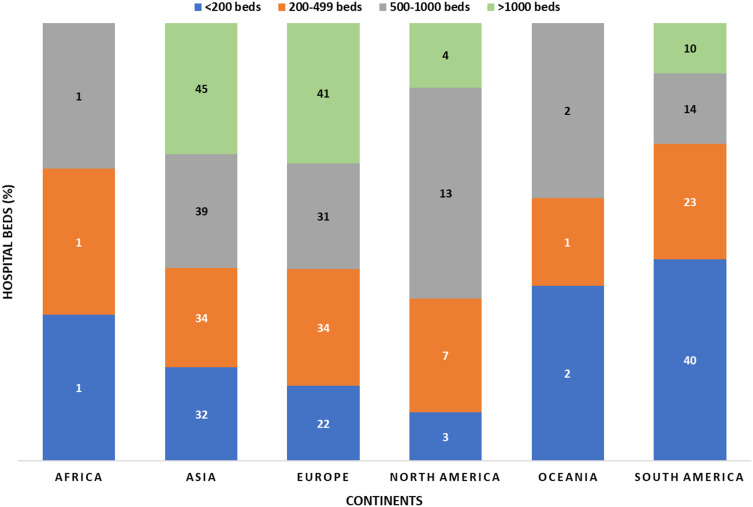
Hospital Beds, ICU Beds and Continents
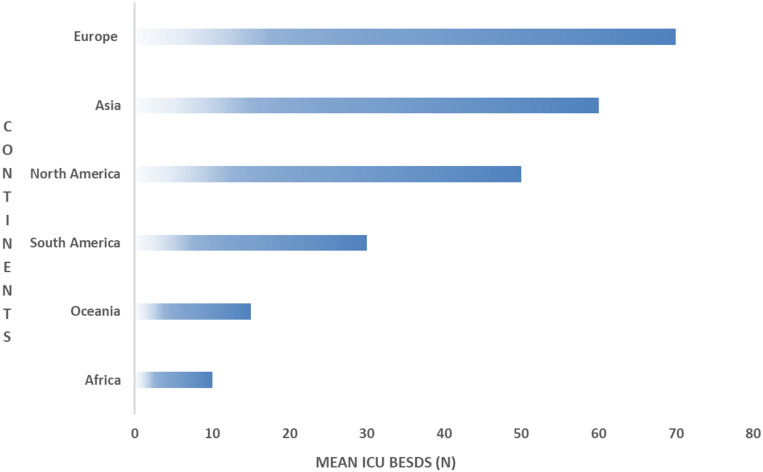
There were significant differences in the hospital bed capacities between continents, where the highest numbers of beds in hospitals (>1000) were found in Asia 36/80 (45%) followed by Europe 33/80 (41.3%) and South America 8/80 (10%) (Figure 4). While there was no significant difference in the means of ICU beds between the continents (p = 0.09), we found that North America had the highest mean ICU beds (82.58 ± 66.74), followed by Asia (72.69 ± 78.95) (Figure 5).
Figure 4.
Mean ICU beds per continent.
Figure 5.
Total beds per hospital in all continents.
Factors for MV Initiation and Monitoring of COVID-19 Patients
We found that arterial blood gas (ABG) (48%) and clinical presentation (37.5%) of the patients were the most commonly used diagnostic tools to initiate ventilator support in COVID-19 patients. Of the total respondents, only 38.8% and 56.6% had protocols available for HFNC and NIV modalities, respectively. The most commonly used guidelines by the respondents during this pandemic were the national guidelines (61.2%), followed by WHO (47.6%) and Society of Critical Care Medicine (SCCM) (39.2%) guidelines. HFNC (53.8%) and NIV (47%) were frequently used in mild and moderate cases, respectively, while IMV (92%) was the most popular technique for the management of severe cases. The most-reported rationales for using HFNC in mild cases were based on diagnostic tools (16.7%), available protocols (15.9%), and fewer aerosol-generating particles (11.1%) respectively. Similarly, the most commonly reported rationales for using NIV in moderate cases were based on diagnostic tools (25.4%), available protocols (19.5%), and the knowledge and skills of CCPs (8%). Rationales for the use of IMV in severe cases were similar to those of HFNC application in mild cases. We found that most of the respondents monitored the ventilators as needed (53.8%) instead of using a standardised, regular system check (Table 2).
Table 2.
Factors Considered in the Clinical Management of COVID-19 Patients and the Maintenance of Ventilators (N= 502)*
| Characteristics | Values |
|---|---|
| Initial diagnostic tool used for therapy initiation | |
| ● Arterial Blood Gas | 241 (48%) |
| ● Chest Imaging | 42 (8.4%) |
| ● Clinical Presentation | 188 (37.5%) |
| ● All of the Above | 31 (6.2%) |
| Available protocols | |
| ● HFNC | 195 (38.8%) |
| ● NIV | 284 (56.6%) |
| ● IMV | 416 (82.9%) |
| Used COVID-19 guidelines | |
| ● Own practice | 197 (39.2%) |
| ● National | 307 (61.2%) |
| ● WHO | 239 (47.6%) |
| ● AARC | 128 (25.5%) |
| ● NICE | 63 (12.5%) |
| ● ANZICS | 29 (5.8%) |
| ● SCCM | 197 (39.2%) |
| Initial ventilation strategy based on severity | |
| MILD | |
|
270 (53.8%) |
|
123 (24.5%) |
|
24 (4.8%) |
| Moderate | |
|
87 (17.3%) |
|
236 (47%) |
|
130 (25.9%) |
| SEVERE | |
|
5 (1%) |
|
15 (3%) |
|
462 (92%) |
| Ventilator management | |
| Suctioning system used | |
|
35 (7%) |
|
467 (93%) |
| Ventilator system check | |
|
270 (53.8%) |
|
53 (10.6%) |
|
67 (13.3%) |
|
112 (22.3%) |
Note: *All percentages are expressed corresponding to the total number of respondents.
Abbreviations: AARC, American Association for Respiratory Care; ANZICS, Australian and New Zealand Intensive Care Society; HFNC, high flow nasal cannula; IMV, invasive mechanical ventilation; NICE, National Institute for Health and Care Excellence; NIV, noninvasive ventilation; SCCM, Society of Critical Care Medicine; WHO, World health Organization.
General Clinical Management of Ventilatory Support in COVID-19 Patients
The initial flow setting commonly used for HFNC was between 30 and 45 L/m (29.9%) and 61.6% of the respondents combined humidification with this therapy. CPAP/PS (40.6%) and full-face masks (38.2%) were the most commonly used mode and interface with NIV, respectively, while in IMV, VC (36.1%) and PC (34.9%) were the most commonly used modes for COVID-19 patients. Heat and moisture exchanger (HME) (70.9%) was also reported as the most popular humidifier used in IMV (Table 3). Lower tidal volume (VT) ventilation and higher positive end-expiratory pressure (PEEP) strategy were the most commonly used with the ARDS/COVID-19 patients. Out of the 430 respondents who combined proning with IMV, 48.4% proned for a duration of 12 to 16 hours/day. In addition, where proning was not used, the most common limitation was the lack of staff training (14.9%). In total, 46.2% of the respondents had tried awake proning with both HFNC or NIV with COVID-19 patients. We found a significant difference in the percentage of respondents who had tried awake proning between the continents (p < 0.001), where Europe (44%) had the highest percentage, followed by Asia (27.2%) and South America (18.5%). Only 12.2% had used nitric oxide (Table 3). There was a statistically significant difference among continents in frequent use of nitric oxide, use of inhaled pulmonary vasodilator and systemic corticosteroid (p < 0.001). Europe (24/61) 39% and Asia (22/61) 36% were frequently used nitric oxide with COVID-19 patients. Likewise, Asia (79/191) 41% and Europe (51/191) 27% were mostly used inhaled pulmonary vasodilator. Indeed, use of systemic corticosteroid was commonly used in Europe (122/323) 38%, Asia (101/323) 31%, followed by South America (73/323) 23%.
Table 3.
General Management of COVID-19 Using Ventilatory Support (N= 502)*
| Characteristics | Values |
|---|---|
| Initial flow setting of HFNC | |
| ● Below 30 L/m | 90 (17.9%) |
| ● From 30 to 45 L/m | 150 (29.9%) |
| ● More than 45 L/m | 80 (15.9%) |
| Use of humidification with HFNC | 309 (61.6%) |
| NIV modes | |
|
161 (32.1%) |
|
204 (40.6%) |
|
43 (8.6%) |
|
19 (3.8%) |
| NIV interfaces frequently used | |
|
192 (38.2%) |
|
67 (13.3%) |
|
19 (3.8%) |
|
154 (30.7%) |
| IMV modes | |
| ● APRV | 39 (7.8%) |
| ● PC | 175 (34.9%) |
| ● PRVC | 107 (21.3%) |
| ● VC | 181 (36.1%) |
| Humidifier type used with IMV | |
| ● Heated circuit | 135 (26.9%) |
| ● HEPA | 11 (2.2%) |
| ● HME | 356 (70.9%) |
| Ventilation strategy used in IMV | |
| ● Low VT ventilation (VT: 4–8 ml/kg of predicted body) | 481 (96%) |
| ● Higher VT ventilation (VT>8 ml/kg of predicted body weigh) | 20 (4%) |
| PEEP strategy | |
| ● Lower PEEP (PEEP levels <10 cm H2O) | 182 (36.3%) |
| ● Higher PEEP (PEEP levels >10 cm H2O) | 320 (63.7%) |
| Use of prone with IMV | 430 (85.7%) |
| Prone duration | |
|
74 (14.7%) |
|
243 (48.4%) |
| ● >16 hours/day | 116 (23.1%) |
| Reasons for not proning | |
| ● Limited resources | 61 (12.2%) |
| ● Lack of staff training | 71 (14.9%) |
| ● Complications | 67 (13.3%) |
| ● Not indicated | 64 (12.7%) |
| Tried awake prone positioning | 232 (46.2%) |
| Frequent use of nitric oxide | 61 (12.2%) |
| Use inhaled pulmonary vasodilator | 191 (38%) |
| Use of recruitment manoeuvres | 349 (69.5%) |
| Recruitment manoeuvres used | |
| ● Stepwise PEEP adjustment | 214 (42.6%) |
| ● Inspiratory hold | 146 (29.1%) |
| Use of (VV) ECMO | 97 (19.3%) |
| Use of systemic corticosteroids | 323 (64.3%) |
Note: *All percentages are expressed corresponding to the total number of respondents.
Abbreviations: APRV, airway pressure release ventilation; BIPAP, bilevel positive airway pressure; CPAP/PS, continuous positive airway pressure/pressure support; HME, heat and moisture exchanger; HEPA, high-efficiency particulate air; PEEP, positive end-expiratory pressure; PC, pressure control; PRVC, pressure regulated volume control; Vt, tidal volume; VV, venovenous; VC, volume control.
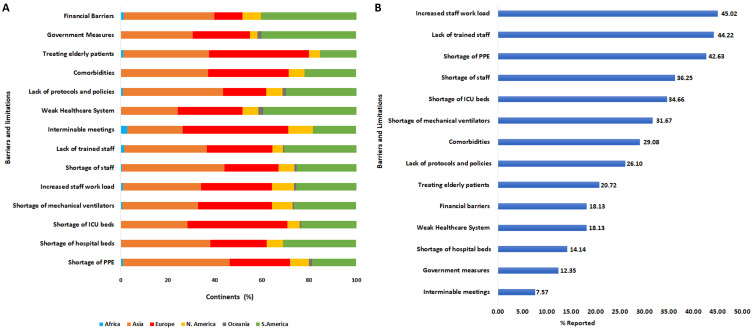
Barriers and Limitations of COVID-19 Clinical Management
Various limitations were reported to be associated with the clinical management of COVID-19 in this study. Of these, increased staff workload (45%), lack of trained staff (44%) and shortage of PPE (43%) were consecutively the three most reported limitations. Shortage of staff and ICU beds was also high on the list of limitations reported (Figure 6A).
Figure 6.
Barriers and limitations of COVID-19 clinical management. (A) represents the (%) of barriers and limitations per continent. (B) represents the (%) of barriers and limitations corresponding to the overall responses.
Although there was no significant difference (p = 0.27) in terms of increased staff workload between the six continents, we found that Asia (33.2%), Europe (30.1%) and South America (25.7%) had the highest percentage of increased workload among healthcare workers.
There was a statistically significant difference (p < 0.001) in the reported lack of trained staff between all included continents, with the highest percentage reported from Asia (35.1%), followed by South America (30.6%). The limitation from shortage of PPE between the continents was also significantly different (p = 0.01); this limitation was most commonly reported by healthcare workers from Asia (45.3%), followed by Europe (25.7%) (Figure 6B).
Discussion
To the best of our knowledge, this is the first study to report the global clinical ventilatory support practices and barriers encountered by healthcare workers handling COVID-19 patients. Our results show that general clinical practices involving ventilatory support lack uniformity, with limited use of standard protocols, and most healthcare workers work outside the general treatment guidelines. We found that of the many barriers encountered, increased staff workload, lack of trained staff and shortage of PPE were the major impediments to efficient treatment.
A variety of factors, including training and professional expertise, influences the optimal management of mechanically ventilated patients.15 As a positive aspect, most respondents (85%) reported previous formal training in MV, consisting of theoretical and practical sessions for at least six weeks or more. This previous training may have been important in enhancing confidence, knowledge and performance to support the critical care team in offering ventilation support to adult COVID-19 patients.
Despite formal training in MV being similar among practitioners, continental Asia and Europe, which had higher number of diploma holders, also had higher proportions of CCPs that are untrained in MV. Mismanagement of ventilatory support due to lack of training could lead to further complications, including ventilator-associated pneumonia, patient-ventilator asynchrony and barotrauma.16,17 Global emergency strategies to maximise the capacity of the health care workforce, such as relaxing staffing requirements and redeploying health workers to high-need areas, could further exacerbate the problem if proper rapid training mechanisms are not in place.18
Most of the responses came from respiratory therapists, physiotherapists, and intensivists. We were able to identify that intensivists had the longest professional experience (>20 years), while CCPs with <5 years experience were mostly respiratory therapists. Despite intercontinental differences in professional qualifications, our findings outline that there are healthcare workers actively engaged in ventilatory support management of COVID-19 patients worldwide. In terms of care settings, most respondents (72.7%) were from tertiary care hospitals, in which we identified the highest percentage of diplomas. This is not in agreement with the WHO definition of tertiary hospitals, which should have highly specialised staff and technical equipment.19
Our findings indicate that ICU beds and trained practitioners were limited across all continents. At a regional level, this lack of ICU bed capacity could be resolved by transforming general hospitals into critical care hospitals. At the hospital level, hospitals must innovate processes to transform general wards into ICUs to augment their capacities.20 This may be needed across all continents to cope with the high demand imposed by the rate of spread of the SARS CoV-2.21–27 The increase in ICU bed capacity by hospitals should, therefore, be guided by simulations and mathematical models, which incorporate variables, such as population demographics and public measures, to predict the expected number of COVID-19 cases and the proportion that might need admission to ICU.23,24 While there was no significant difference in the mean of ICU beds between the continents (p = 0.09), the availability of critical care beds differs across continents. This conforms with previous findings by Jason et al, who found similar heterogeneity with fewer ICU bed numbers reported in lower-income compared to higher-income countries.26 Better economic status and financial solvency of health-care systems play a major role in ICU bed expansion.26,28 Moderate-income and high-income countries can afford the high cost of preparing ICUs from scratch within a short period.29,30 However, improving the quality of critical care and ICU outcomes is still a challenge that many health care systems face, even in moderate-income and high-income countries.24,29 This is because a sudden increase in the ICU bed capacity could conflict with the availability of well-trained health care professionals to guarantee standardised critical care.31
Due to the lack of universally accepted clinical guidelines for the management of adult COVID-19 patients requiring ventilatory support, there is a need for a collaborative effort by global healthcare authorities to create a multidisciplinary task force to solve this issue. The present study also surveyed factors considered for MV initiation and ventilation techniques used in different countries. ABG shows the systemic levels of oxygenation and ventilation than clinical presentation alone,32 and this could explain why ABG was preferred laboratory criterion rather than clinical presentation alone for initiating ventilatory support in COVID-19 patients. Of the three techniques evaluated, the survey respondents preferred to use the HFNC (54%) and NIV technique (47%), for mild and moderate cases, respectively. This finding is consistent with previous work that found that HFNC was preferred as a first-line ventilatory technique, followed by NIV and IMV.33 Interestingly, the most common modality used with mild cases in both Asia and Europe was HFNC despite the limited availability of protocols in both continents. This raises a concern, especially since the HFNC procedure can generate hazardous levels of aerosols if not adequately managed based on standard protocols.34 Providing airborne precautions along with other precautionary measures could be challenging during the COVID-19 pandemic due to the shortage of PPE and isolation rooms.35,36 Surprisingly, intubation was still considered in some of the mild cases with mild symptoms. In moderate cases of COVID-19, it was reported that NIV was the preferred method of delivering ventilatory support, although close monitoring must be provided since the patient’s status might rapidly deteriorate.37
Suctioning during MV is a fundamental procedure that is necessary to keep airway patency by removing endotracheal secretions. Here, the majority of respondents used a closed suction system with COVID-19 patients, which was essential, especially with patients who required high PEEP and to minimize aerosol/droplet generation.38 However, some respondents also used an open suction system, which might increase the chance of environmental contamination.38 Despite the existence of recommendations for ventilator check frequency, our survey found that the majority of respondents did not adhere to the recommended, regular ventilator check interval.39,40
Clinical management of ventilatory and respiratory support was different between respondents in many areas. Only 63.7% reported using HFNC, although current evidence suggests that there are benefits of HFNC in reducing the need for intubation and IMV.41 Simulation studies found that HFNC aerosols are smaller and travel greater distances with higher set inspiratory flow.41 According to recent guidelines, a set inspiratory flow not greater than 30 L/min is recommended to minimise the risk of viral infection.42 However, 72% of respondents used a set inspiratory flow of more than 30 L/min, contravening the standard recommendation. Concerning NIV interfaces, full-face and oronasal masks were the most used with COVID-19 patients, with only 13% of the CCPs using helmets. Compared to full-face masks, using helmets with infectious diseases is the safer option due to the negligible leakage and dispersion caused, which is needed to prevent nosocomial infection.33,43 Owing to their availability, however, conventional facemask interfaces are likely to be the most widely used.
Most respondents reported using CPAP/PS mode with NIV (40.6%), which highlights the hypoxic respiratory failure nature of the COVID-19 disease process. When IMV is used, the majority (36.1%) used VC-based ventilation, which in this mode the pressure is variable based on lung mechanics and may cause barotrauma to the lung if not carefully monitored.44 PC mode was noted to be used frequently (34.9%) by respondents, which is a safe mode of ventilation, but volume is variable and dependant on lung mechanics, and therefore frequent alteration of settings is needed. Frequent adjustment is potentially hazardous to the therapist due to increased exposure to SARS COV-2 or increased risk of spreading it. Pressure regulated volume control (PRVC) delivers a set volume within safe pressure limits; therefore, it limits settings adjustment,45 but it was noted that only 21.3% of respondents used this mode of ventilation. The airway pressure release ventilation (APRV) was used the least (7.8%), although it may be a good option for treating COVID-19 patients as it works by increasing the mean airway pressure and limiting the expiratory time in order to minimize derecruitment. This increases functional residual capacity (FRC), but APRV needs special ventilators, well-trained and experienced clinicians, which could limit its use due to lack of resources, training and protocols.46,47
Low VT ventilation strategy was frequently used internationally (96%), in line with recent critical care guidelines.4 Most respondents (63.7%) indicated using “high PEEP low fraction of inspired oxygen (FiO2)” strategy in treating ARDS of COVID-19 patients, although it has been found previously that there is no difference in using high FiO2/low PEEP or low FiO2/high PEEP in treating ARDS patients.48 Further studies are needed to develop an evidence-based protocol of initiating, managing, weaning and discontinuing IMV, as mortality rates are high.49–51
Proning intubated patients with severely hypoxemic respiratory failure is an effective intervention in reducing mortality.52 The major barrier to proning among respondents was found to be the lack of training, which includes appropriate proning technique and management of ventilatory support. In COVID-19 patients, there is still a lack of evidence for proning effectiveness, but there are 50 trials currently registered at clinicaltrials.gov studying the role of proning as part of COVID-19 treatment in many modalities, including awake or coupled with other therapies. Interestingly, 46% of respondents attempted awake pronation of COVID-19 patients, with most of the attempts in Europe, which indicates that local European guidelines have indications and contraindications for awake prone positioning of COVID-19 patients. This indicates the feasibility of this technique, although the actual effectiveness of the technique remains under-studied.53 Nitric oxide and inhaled pulmonary vasodilators were not widely used internationally. Remarkably, the majority of the CCPs (41%) used stepwise PEEP adjustment compared to the recommended traditional method (inspiratory hold, 29%). This poses a clinical concern as this strategy is not recommended due to increased mortality.4,54 More than half of the CCPs (64%) had used systematic corticosteroids with COVID-19 patients, which indicates increased severity accompanied by ARDS.
We report for the first time the limitations and barriers in the management of COVID-19 patients by healthcare workers. Most respondents reported increased workload, poor training and lack of PPE to be the main limitations associated with COVID-19 patients’ management. This agrees with recent reports where increased workload was reported as one of the main factors affecting how healthcare workers deal with patients with infectious respiratory diseases.55 Increased workload-to-staff ratio increased the mortality rate of critically ill patients.56 Previous studies also confirmed the lack of general staff training on both the use of PPE, and the use and maintenance of ventilator support systems as major issues in the handling of pandemics.13 Indisputably, the shortage of PPE and other important clinical resources including MVs, hospital beds, and ICU beds among others has been well documented in various reports.13,36
Respondents from Asia were the most likely to report poor training, higher workload, shortage of staff, hospital beds, PPE, lack of protocols and comorbidities as the main barriers to the management of COVID-19 patients. This may be due to the disproportionate escalation in the rate of infection in Asia, resulting in shortage of healthcare resources and subsequent increased mortality.57,58 Respondents from Europe mostly reported the lack of ICU beds, interminable meetings, and treatment of elderly patients as the main barriers. Despite having the best healthcare systems globally, the infection and mortality rates due to COVID-19 were disproportionately higher in Europe because of the aforementioned reasons.59,60 Respondents from South America, on the other hand, mostly reported weak healthcare systems and financial barriers as the main limitations. These results confirm previous reports on the fragility of healthcare systems in Latin America, which has been exacerbated by the recent socioeconomic crisis in the region.61,62 Generally, most of these barriers or limitations are modifiable, while the reminder needs a holistic approach for better management of patient-related factors such as multimorbidity.63
In general, there are significant differences in the types of limitations between continents which is linked to regional lapses in the handling of the COVID-19 pandemic and outcomes. Critical evaluation and resolution of those modifiable limitations would help regions around the world to better prepare for future pandemics. To our knowledge, the strength of this global study is that it is the first to explore and compare critical care management of COVID-19 patients in different countries across continents that have been profoundly affected by the pandemic. For the first time, this study included multidisciplinary international representatives with relevant expertise in ventilatory support management, who provided multidisciplinary perspectives. This increased the generalisability of our findings. However, the present study has important limitations worth highlighting. Although a diverse sample of different medical health care specialties in terms of academic qualifications and practice experience was recruited from different countries across continents, the findings reported cannot be extrapolated because different countries have used different care strategies to fight the pandemic. Due to the nature of COVID-19, the number of respondents was limited and mostly from five countries. The response rate was not available because our official partners distributed the survey through their social networking websites.
However, our results have important clinical and research implications. They highlight the current global practices, including strengths and limitations encountered by frontline healthcare professionals who manage COVID-19 patients needing ventilatory support. This will inform better management in future, which could be of considerable benefit to both clinicians and COVID-19 patients. Future research should focus on producing a clear and integrated guidance for ventilatory support management of COVID-19 patients and find solutions to the major barriers and limitations found in this survey.
Conclusion
Our data from 40 countries across six continents presents the first report on the clinical ventilatory support practices and barriers encountered by healthcare workers handling COVID-19 patients globally. Our results show that general clinical practices involving ventilatory support are highly heterogeneous, with limited use of standard protocols and most frontline clinicians depending on isolated and varied management guidelines. We found increased staff workload, lack of trained staff and shortage of PPE to be the main limiting factors affecting global COVID-19 ventilatory support management.
Acknowledgments
We acknowledge all health care providers who take the time to participate in this international survey. We thank the GiViTI Italian ICU network and all the international societies that supported us in distributing and promoting the survey.
Disclosure
DDR received research equipment support (Draeger) and working as consultant in (Philips and Mallinckrodt) and non-financial support from Draeger, outside the submitted work. CO has a patent 102016000114357 with royalties paid from Intersurgical SpA. The authors report no other competing interests in this work.
References
- 1.Hamid S, Mir MY, Rohela GK. Noval coronavirus disease (COVID-19): a pandemic (epidemiology, pathogenesis and potential therapeutics). New Microbes New Infect. 2020;35:100679. doi: 10.1016/j.nmni.2020.100679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Coronavirus disease (COVID-19) situation report-155; [updated July12, 2020]. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200712-covid-19-sitrep-174.pdf?sfvrsn=5d1c1b2c_2. Accessed November05, 2020.
- 3.World Health Organization. Clinical Management of COVID-19: Interim Guidance. World Health Organization; 2020. [Google Scholar]
- 4.Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with Coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Llancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545–1546. doi: 10.1001/jama.2020.4031 [DOI] [PubMed] [Google Scholar]
- 9.Mission W-CJ. Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19); 2020. [updated June24, 2020]. Available from: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf.
- 10.Alqahtani JS, Oyelade T, Aldhahir AM, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One. 2020;15(5):e0233147. doi: 10.1371/journal.pone.0233147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oyelade T, Alqahtani J, Canciani G. Prognosis of COVID-19 in patients with liver and kidney diseases: an early systematic review and meta-analysis. Trop Med Infect Dis. 2020;5(2):80. doi: 10.3390/tropicalmed5020080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phua J, Weng L, Ling L, Egi M, Lim C-M, Divatia JV. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8(5):506–517. doi: 10.1016/S2213-2600(20)30161-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciullo A, Yee J, Frey JA, et al. Telepresent mechanical ventilation training versus traditional instruction: a simulation-based pilot study. BMJ STEL. 2019;5(1):8–14. doi: 10.1136/bmjstel-2017-000254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alqahtani JS, AlAhmari MD, Alshamrani KH, et al. Patient-ventilator asynchrony in critical care settings: national outcomes of ventilator waveform analysis. Heart Lung. 2020;49(5):630–636. doi: 10.1016/j.hrtlng.2020.04.002 [DOI] [PubMed] [Google Scholar]
- 17.Klompas M. Potential strategies to prevent ventilator-associated events. Am J Respir Crit Care Med. 2015;192(12):1420–1430. doi: 10.1164/rccm.201506-1161CI [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Strengthening the health system response to COVID-19: technical guidance# 1: maintaining the delivery of essential health care services while mobilizing the health workforce for the COVID-19 response, 18 April 2020: World Health Organization. Regional Office for Europe; [cited June05, 2020]. Available from: https://www.euro.who.int/__data/assets/pdf_file/0007/436354/strengthening-health-systems-response-COVID-19-technical-guidance-1.pdf. [Google Scholar]
- 19.World Health Organization. Disease control priorities in developing countries; 2008. [cited June05, 2020]. Available from: https://www.who.int/management/facility/ReferralDefinitions.pdf.
- 20.Arabi YM, Murthy S, Webb S. COVID-19: a novel coronavirus and a novel challenge for critical care. Intensive Care Med. 2020;1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie J, Tong Z, Guan X, Du B, Qiu H, Slutsky AS. Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensive Care Med. 2020;1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He H, Hu C, Xiong N, Liu C, Huang X. How to transform a general hospital into an “infectious disease hospital” during the epidemic of COVID-19. Crit Care. 2020;24(1):1–2. doi: 10.1186/s13054-020-02864-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moghadas SM, Shoukat A, Fitzpatrick MC, et al. Projecting hospital utilization during the COVID-19 outbreaks in the United States. Proc Natl Acad Sci. 2020;117(16):9122–9126. doi: 10.1073/pnas.2004064117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395(10231):1225–1228. doi: 10.1016/S0140-6736(20)30627-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alban A, Chick SE, Dongelmans DA, Vlaar AP, Sent D, Group S. ICU capacity management during the COVID-19 pandemic using a process simulation. Intensive Care Med. 2020;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phua J, Faruq MO, Kulkarni AP, et al. Critical care bed capacity in Asian countries and regions. Crit Care Med. 2020;48(5):654–662.31923030 [Google Scholar]
- 27.Michael Le Grange J, James DS, Robert Jeppe Davis J. Capacity building during COVID-19: utilising South Africa’s underutilised international medical graduates. SAMJ. 2020;110(5). [DOI] [PubMed] [Google Scholar]
- 28.Rhodes A, Ferdinande P, Flaatten H, Guidet B, Metnitz P, Moreno R. The variability of critical care bed numbers in Europe. Intensive Care Med. 2012;38(10):1647–1653. doi: 10.1007/s00134-012-2627-8 [DOI] [PubMed] [Google Scholar]
- 29.Dondorp AM, Iyer SS, Schultz MJ. Critical care in resource-restricted settings. JAMA. 2016;315(8):753–754. doi: 10.1001/jama.2016.0976 [DOI] [PubMed] [Google Scholar]
- 30.White DB, Lo B. A framework for rationing ventilators and critical care beds during the COVID-19 pandemic. JAMA. 2020;323(18):1773–1774. doi: 10.1001/jama.2020.5046 [DOI] [PubMed] [Google Scholar]
- 31.Bedford J, Enria D, Giesecke J, et al. COVID-19: towards controlling of a pandemic. Lancet. 2020;395(10229):1015–1018. doi: 10.1016/S0140-6736(20)30673-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):6. doi: 10.1097/CCM.0000000000004363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winck JC, Ambrosino N. COVID-19 pandemic and non invasive respiratory management: every Goliath needs a David. An evidence based evaluation of problems. Pulmonology. 2020;26(4):213–220. doi: 10.1016/j.pulmoe.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health O. Clinical Management of Severe Acute Respiratory Infection (Sari) When Covid-19 Disease is Suspected: Interim Guidance. Geneva: World Health Organization; 2020. Contract No.: WHO/2019-nCoV/clinical/2020.4. [Google Scholar]
- 35.Qiu H, Tong Z, Ma P, et al. Intensive Care During the Coronavirus Epidemic. Springer; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie J, Tong Z, Guan X, Du B, Qiu H, Slutsky AS. Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensive Care Med. 2020;46(5):837–840. doi: 10.1007/s00134-020-05979-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karim HM, Burns KE, Ciobanu LD, El-Khatib M, Nicolini A, Vargas N, Hernández-Gilsoul T, Skoczyński S, Falcone VA, Arnal JM, Bach J. Noninvasive ventilation: education and training. A narrative analysis and an international consensus document. Advances in respiratory medicine. 2019;87(1):36. [DOI] [PubMed] [Google Scholar]
- 38.Torres A, Ewig S. editors. Nosocomial and Ventilator-Associated Pneumonia. 2011;168. [Google Scholar]
- 39.AARC clinical practice guideline. Patient-ventilator system checks. American Association for Respiratory Care. Respir Care. 1992;37(8):882–886. [PubMed] [Google Scholar]
- 40.Guidelines for standards of care for patients with acute respiratory failure on mechanical ventilatory support. Task force on guidelines; Society of Critical Care Medicine. Crit Care Med. 1991;19(2):275–278. [DOI] [PubMed] [Google Scholar]
- 41.Agarwal A, Basmaji J, Muttalib F, et al. High-flow nasal cannula for acute hypoxemic respiratory failure in patients with COVID-19: systematic reviews of effectiveness and its risks of aerosolization, dispersion, and infection transmission. Can J Anaesth. 2020;1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas P, Baldwin C, Bissett B, et al. Physiotherapy management for COVID-19 in the acute hospital setting: clinical practice recommendations. J Physiother. 2020;66(2):73–82. doi: 10.1016/j.jphys.2020.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hui DS, Chow BK, Lo T, et al. Exhaled air dispersion during noninvasive ventilation via helmets and a total facemask. Chest. 2015;147(5):1336–1343. doi: 10.1378/chest.14-1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haas CF. Mechanical ventilation with lung protective strategies: what works? Crit Care Clin. 2011;27(3):469–486. doi: 10.1016/j.ccc.2011.05.008 [DOI] [PubMed] [Google Scholar]
- 45.MacIntyre NR, Sessler CN. Are there benefits or harm from pressure targeting during lung-protective ventilation? Respir Care. 2010;55(2):175. [PubMed] [Google Scholar]
- 46.Daoud EG. Airway pressure release ventilation. Ann Thorac Med. 2007;2(4):176–179. doi: 10.4103/1817-1737.36556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nieman GF, Gatto LA, Andrews P, et al. Prevention and treatment of acute lung injury with time-controlled adaptive ventilation: physiologically informed modification of airway pressure release ventilation. Ann Intensive Care. 2020;10(1):3. doi: 10.1186/s13613-019-0619-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336. [DOI] [PubMed] [Google Scholar]
- 49.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934. doi: 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koulouras V, Papathanakos G, Papathanasiou A, Nakos G. Efficacy of prone position in acute respiratory distress syndrome patients: a pathophysiology-based review. World J Crit Care Med. 2016;5(2):121–136. doi: 10.5492/wjccm.v5.i2.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sartini C, Tresoldi M, Scarpellini P, et al. Respiratory parameters in patients with COVID-19 after using noninvasive ventilation in the prone position outside the intensive care unit. JAMA. 2020;323(22):2338–2340. doi: 10.1001/jama.2020.7861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sreedharan JK, Alqahtani JS. Driving pressure: clinical applications and implications in the intensive care units. Indian J Respir Care. 2018;7(2):62. doi: 10.4103/ijrc.ijrc_12_18 [DOI] [Google Scholar]
- 55.Houghton C, Meskell P, Delaney H, et al. Barriers and facilitators to healthcare workers’ adherence with infection prevention and control (IPC) guidelines for respiratory infectious diseases: a rapid qualitative evidence synthesis. Cochrane Database Syst Rev. 2020;(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee A, Cheung YSL, Joynt GM, Leung CCH, Wong WT, Gomersall CD. Are high nurse workload/staffing ratios associated with decreased survival in critically ill patients? A cohort study. Ann Intensive Care. 2017;7(1):46. doi: 10.1186/s13613-017-0269-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hopman J, Allegranzi B, Mehtar S. Managing COVID-19 in low- and middle-income countries. JAMA. 2020;323(16):1549–1550. doi: 10.1001/jama.2020.4169 [DOI] [PubMed] [Google Scholar]
- 58.Ji Y, Ma Z, Peppelenbosch MP, Pan Q. Potential association between COVID-19 mortality and health-care resource availability. Lancet Glob Health. 2020;8(4):e480. doi: 10.1016/S2214-109X(20)30068-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Assessment RR. Coronavirus Disease 2019 (COVID-19) in the EU/EEA and the UK–Ninth Update. Stockholm: European Centre for Disease Prevention and Control; 2020. [Google Scholar]
- 60.Gale RP. Can a disease be conquered by extensive publications, reading guidelines and interminable meetings? Leukemia. 2020;34(8):1977–1978. doi: 10.1038/s41375-020-0897-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lancet T. The unfolding migrant crisis in Latin America. Lancet (London). 2019;394(10213):1966. doi: 10.1016/S0140-6736(19)32934-4 [DOI] [PubMed] [Google Scholar]
- 62.Rodriguez-Morales AJ, Gallego V, Escalera-Antezana JP, et al. COVID-19 in Latin America: the implications of the first confirmed case in Brazil. Travel Med Infect Dis. 2020;35:101613. doi: 10.1016/j.tmaid.2020.101613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alqahtani JS, Njoku CM, Bereznicki B, et al. Risk factors for all-cause hospital readmission following exacerbation of COPD: a systematic review and meta-analysis. Eur Respir J. 2020;29(156):190166. doi: 10.1183/16000617.0166-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]