Abstract
Background
Due to lack of approved drugs and vaccines, the medical world has resorted to older drugs, produced for viral infections and other diseases, as a remedy to combat COVID-19. The accumulating evidence from in vitro and in vivo studies for SARS-CoV and MERS-CoV have demonstrated that several polyphenols found in plants and zinc- polyphenol clusters have been in use as herbal medicines have antiviral activities against viruses with various mechanisms.
Scope of review
Curcumin, zinc and zinc-ionophores have been considered as nutraceuticals and nutrients showing great antiviral activities with their medicinal like activities.
Major conclusions
In this work, we discussed the potential prophylactic and/or therapeutic effects of curcumin, zinc and zinc-ionophores in treatment of viral infections including COVID-19.
General significance
Curcuminoids and Zinc classified as nutraceuticals under GRAS (Generally Recognized As Safe) by FDA can provide complementary treatment for COVID 19 patients with their immunity-boosting and antiviral properties.
Highlights
-
•
Antiviral activity of Curcumin, zinc and zinc-ionophores.
-
•
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
-
•
COVID-19 (corona virus disease 2019).
-
•
Nutraceutical and nutrient.
1. Introduction
While new year celebrations take place across the world, the World Health Organization (WHO) was alerted by Chinese officials about emergence of pneumonia-like cases in Wuhan city, Hubei province, China [1]. Chronologically, the U.S Centre for Disease Control and Prevention (CDC) identified a sea food market in Wuhan suspected to be the hub of the outbreak on 1 January 2020,
China reported both the emergence of a new type of coronavirus and first known death of a 61-year-old man infected by this virus on 7 January 2020. The number of the infected people and infection related deaths unexpectedly reached to 17,205 and 361 on 3 February 2020, respectively [2,3]. Unfortunately, Dr. Li Wenliang, a Chinese doctor who first warned about the spread of the virus before it was officially recorded, died on 7 February. On 11 February 2020, name of this new virus was declared “severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the International Committee on Taxonomy of Viruses (ICTV) and the Wuhan infection was named as corona virus disease 2019 (COVID-19) by the World Health Organization (WHO) [4]. Although COVID-19 has been first observed in China, this outbreak has quickly surrounded the whole world in a short time. In fact, India, Brazil, some European countries and United States of America (USA) have been drastically affected by this pandemic compared to China. As of November19th, 2020, the latest global numbers of over 55.000.000 SARS-CoV-2 infected cases and approximate 1.350.000 deaths across the world were provided by WHO [5].
To develop a proper treatment and prevent COVID-19 outbreak, SARS-CoV-2 was isolated from COVID-19 patients and its structure was identified [6]. The genetic analysis revealed that SARS-CoV-2 is largely similar to previously emerged severe acute respiratory syndrome coronaviruses (SARS-CoV) [7]. For instance, the genome sequence of SARS-CoV-2 displays the 79.5% identity to SARS-CoV and both viruses target human alveolar epithelial cells with high specific binding of virus spike (S) protein to angiotensin converting enzyme 2 (ACE-2) receptor [7,8]. However, there is no clinically approved prophylactic or therapeutic drug and vaccine for COVID-19. It is worth to mention that various drugs, previously discovered for different viral infections and other purposes (malaria, parasite and helminths, etc.,), and certain antibiotics have been simultaneously used for treatment of COVID-19. For instance, although chloroquine and remdesivir, recent the most popular drugs, have broad-spectrum antiviral effects and are currently utilized to treat COVID-19, however their effectiveness and mechanisms have been is currently under debate [9,10]. Concisely, ritonavir and lopinavir used as Human Immunodeficiency Virus (HIV) protease inhibitors are also combined with appropriate interferons in the treatment of COVID- 19 [7], ribavirin may has potential for the treatment of COVID-19 owing to its approved antiviral activity towards respiratory syncytial virus (RSV), SARS and MERS (Middle East Respiratory Syndrome) infections [11,12]. However, all chemical drugs mentioned here exhibit some side effects and their effective antiviral activity to SARS-CoV-2 and pharmaceutical mechanisms have been not clearly documented yet.
From ancient days to today, people have consumed plants not only as food but also a medicine for viral infections owing to their secondary metabolites long while their mechanism of action is not well elucidated yet.
Laboratory studies have demonstrated that plants as “called herb medicines” have protected people from various infectious diseases with a variety of ways including boosting immune system, degradation of virus nucleotides for inhibiting the replication and prevention of virus entry to host cells [13,14]. For instance, during outbreak of SARS-CoV and MERS-CoV, various plants have been benefited to treat the virus infected patients. And they successfully inhibited virus replication and virus entry [[15], [16], [17], [18]].
We mainly focus on the discussion on food and supplements, Curcuminoids and Zinc, which have been classified as nutrients under GRAS (Generally Recognized As Safe) by FDA. The in vitro and in vivo studies have proved their efficacy and safety in the treatment of virus infected patients.
The researchers have suggested various mechanisms on prophylactic and therapeutic actions of curcuminoids and zinc used, respectively against different viral infections [19]. Polyphenols, organic compounds are found plentifully in fruits, vegetables and flowers. They produce secondary metabolites and play an effective role for protection of attack by pathogens. Also, they contribute to formation of color, flavor, odor and bitter taste in plants. In recent times, polyphenols have attracted great interest due to their protective effects against development of diseases [20,21]. Curcumin is found in Curcuma longa rhizomes as major polyphenolic component and its yellow colorant powder form commonly known as turmeric is not only consumed as food but also used as medicine especially in India and around the world.
Zn is the most abundant element in the human body after iron. It is an important component in the structure and function of many proteins and involved in 10% of human proteome. It plays role in gene transcription and the structure of many catalytic enzymes and involved in biological functions including DNA synthesis, RNA transcription, in maintenance of metabolic and immune homeostasis and many other cellular processes.
2. Similar mechanisms of curcumin and zinc in fight against COVID-19
2.1. Suppression of cytokine storm
various infections, especially viral ones induce cytokine storm which results in hypotension, hemorrhage, and, ultimately multi-organ failure owing to overexpression of certain cytokines including interleukin-1 (IL1), IL6, IL10, tumor necrosis factor-α (TNFα) [22]. Reported studies demonstrated that increases in those cytokines can be efficiently suppressed by curcumin. For instance, Abe et al. and Jain et al. separately reported that curcumin can inhibit the release of a series of cytokines such as IL1β, IL8, TNFα, monocyte chemoattractant protein-1 (MCP1) and macrophage inflammatory protein-1α (MIP1α) from monocytes and macrophages [23,24]. Additionally, the secretion of IL1 in bone marrow stromal cell, IL6 in rheumatoid synovial fibroblasts, IL8 in human esophageal epithelial cells and alveolar epithelial cells was controlled using curcumin [[25], [26], [27], [28]]. Consequently, curcumin has great potential to prevent occurrence of cytokine storm in the COVID-19 patients.
Life threatening viral infections in older individuals can be seen more frequently in Zn deficiency. The recent systematic study has shown that Zn replacement leads to significant decrease in the degree of infection by increasing IFN-alpha and decreasing TNF productions in people aged of 55–87 years [29,30]. In Zn deficiency, expression of both IL-1β and TNFα can be regulated via epigenetic and redox-mediated reaction. As aforementioned above, Zinc is a major structural element of biological macromolecules that orchestrate various cell functions. For example, physiological cell functions regulated by the zinc of cell oxidant or antioxidant balance are varied and connected to each other. Zinc reacts with sulfur in cysteines by forming a very stable sulfur–zinc bond which is quite stable in a complex cell environment and leads to either association or dissociation of the metal. Zinc itself is a redox inactive element however intrinsic redox function of thiol groups results in the release of zinc from metallothionein (MT) and other cysteine‑zinc complex containing proteins by oxidation reaction, it imparts an indirect redox activity to the zinc. Zinc can regulate direct or indirect redox signaling by the following potential mechanisms; 1) zinc acts as a major regulator for control of production of oxidants and oxidative damage caused by the metal, 2) Zinc liberated by zinc nitric oxide (NO), hydrogen peroxide (H2O2), oxidized antioxidants and thiol group containing oxidants, can dynamically associate with sulfur in cysteine the containing proteins, 3) released zinc from MTs can function as a scavenging oxidant by forming zinc-binding protein and 4) zinc also can involve in regulation of glutathione metabolism and thiol-induced redox status [31,32].
It is considered that MT protects intracellular zinc homeostasis as an essential zinc binding protein. Zinc can be dissociated from thiol‑zinc clusters in MTs through the induction of NO, H2O2, oxidized antioxidants and thiol-based oxidants. While zinc can highly be consumed in oxidative status, it is less available in reducing the environment. MTs and other cysteine containing proteins may potentially act as storage vehicles for zinc and “redox sensor” for cellular environments as well [[33], [34], [35]].
Today, number of COVID 19 cases and mortality rate especially in the elderly and chronic disease group has been increasing day after day owing to aggressive infection of SARS-CoV-2. Considering the increase in Zn deficiency in the elderly population and some chronic diseases, perhaps Zn replacement as a part of routine treatment to these patients seem to be a suitable option to both support the normal immune response and benefit from the antiviral effects of Zn [36]. Zn deficiency affects both the natural and acquired immune system which can be boosted with Zn replacement [37].
Inflammatory response occurs after viral pathogens are recognized by Toll-like receptors or several cytoplasmic receptors such as RIGI, MDA5, IFI16 located on the cell surface or endosomal. In this process, there are three types of IFN oscillations (type I (IFN-α and IFN-β), type II (IFN-γ), type III (IFN-λs)). Although Type I and Type III IFNs have similar function, the type I IFN type III IFN responses are seen everywhere in the body and in the liver, gastrointestinal and pulmonary epithelial cells, respectively. Zn plays an important role in the release of IFNs, cytokine response and binding to the receptor. Unlike Type I IFNs, Zn inhibits the binding of Type III-IFN to the receptor. SOCS-1 and SOCS3 proteins that have an IFN response and inhibit the cytokine response, which prevent IL6-mediated over-inflammatory and antiviral response. ZIP-14, which provides entry into the Zn cell following the onset of the inflammatory response, is required for SOCS-3 expression and a Zn-mediated mechanism is also needed to limit the inflammatory response [31]. Therefore, Zn may also be important in suppressing the excess inflammatory response.
2.2. Inhibition of entry into the host cell
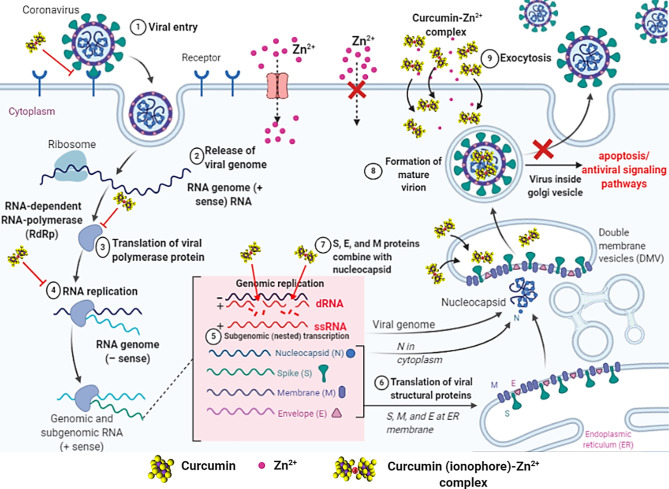
It is reported that curcumin has antiviral activity with multi-mechanisms [[38], [39], [40], [41], [42]]. These diverse mechanisms against different viruses could serve as a model to use it against SARS-CoV-2. One of the most interesting and proven mechanism of curcumin relies on blockade of viral cell entry to host cell, which is a very promising action to control any virus [43,44]. Both virus SARS-CoV-2 and SARS-CoV follow the same pathway like angiotensin-converting enzyme 2 (ACE-2) for internalization host alveolar epithelial cells [45]. Angiotensin converting enzyme 2 (ACE-2) has been described as a functional receptor of COVID-19, therefore its gene has been cloned and expressed for more information on mechanisms in the entry and also for pathogenesis of eukaryotic cells [46]. It refers that ACE-2 receptor can be targeted to develop prophylactic and/or therapeutic strategy in treatment of COVID-19 [47]. A recent molecular simulation study showed how curcumin binds to receptor binding domain (RBD) of viral spike S-protein (S-Protein) on SARS-CoV-2 and alter binding o S-Protein to ACE-2 receptor on host cell for prevention of virus entry (Fig. 1 Step 1) [48]. A further research can be conducted on potential prophylactic effect of curcuminoid on SARS-CoV-2.
Fig. 1.
Cartoon showing the activity of the curcumin-Zn2+ complex in 1, 3, 4 and 8th steps at viral replication cycle of SARS-CoV-2.
Zn is a trace element with a broad antimicrobial activity. The mode of antiviral action of Zn has been extensively studied on different viruses such as human immunodeficiency virus (HIV), infectious gastroenteritis virus (TGEV), vaccinia virus, respiratory syncytial virus (RSV), SARS-CoV [36,[49], [50], [51]]. Unlike to action curcumin, the reported study demonstrated that overexpression of ACE-2 activity in rat lungs can be reduced using certain concentration of Zinc as a food supplement [52,53]. Then, binding affinity of the virus to ACE-2 receptor though S-Protein is affectively speculated and SARS-CoV-2 cellular entry can be blocked.
2.3. Inhibition of viral replication
RNA viruses form endosomal double membrane vesicles (DMV) structures during their proliferation in the host cell. Hereby, they transcribe mRNA and Genomic RNAs in RNA dependent RNA polymerase (RdRp) activity [54]. In this process, both RdRp enzyme and new generation transcripts will be protected from antiviral defense systems in cytoplasm [54]. Unlike other RNA viruses, numerous DMVs in coronavirus-infected cells bind to each other to form modified broad folded areas, including ER [55]. The curcumin can be attached to RdRp (Fig. 1 Step 3) and as well as to dRNA (formed during the genomic transcription (Fig. 1 Step 4) of new generation virions) thanks to polyphenol structure, thereby enables the activation of cellular signaling pathways such as apoptosis (Fig. 1 Step 8) [56]. There are other mechanisms that increase the chances of curcumin being effective in COVID-19 therapy. Curcumin is effective in inhibiting inosine monophosphate dehydrogenase (IMPDH), which catalyzes the rate-limiting step in de novo biosynthesis of guanin nucleotides during viral replication [57]. In addition, a study using Newcastle Disease Virus (NDV), an enveloped virus such as SARS-CoV-2, has been explored to irreversibly inhibit plaque formation in enveloped viruses [58].
Zn supplementation is important in systemic antiviral response as well as specifically inhibiting viral replication [59]. It is worth to mention that it has been previously shown that zinc can inhibit SARs-CoV RNA polymerase [60]. There are studies showing that when Zn2+ cations used in combination with different Zn ionophores much effectively inhibits RdRp SARS-CoV RNA dependent RNA polymerase [61]. For SARS-CoV-1, Zn caused inhibition of RdRp elongation. (Fig. 1 Step 3) and led to reduction in template binding [55]. Furthermore, halting the RNA replication of different RNA viruses were also achieved zinc and zinc ionophores [[61], [62], [63], [64], [65]].
It should be kept in mind that recent study has revealed a conserved, but cryptic epitope shared between SARS-CoV-2 and SARS-CoV which suggest the antiviral compounds which has activity against SARS-CoV might show activity against SARS-CoV-2.
3. An ion carrier for fighting SARS-CoV-2: Curcumin-zinc ionophore
The recent work proposed that zinc ionophore (pyrithione) could be much more effective in inhibiting SARS-CoV replication compared to Zn itself owing to its low cellular uptake. Many enzymes s targeted by polyphenols are linked to Zn. Polyphenols form chelates with Zn cations called Zn ionophores which increase the permeability of cell lipid membrane structures [48]. Dabbagh-Bazarbachi et al. showed that dietary plant polyphenols such as the quercetin and epigallocatechin-gallate give a chelate with Zn ions. These complexes might be acting as Zn ionophores and polyphenols transport Zn cations across the plasma membrane [66]. Zn applications are associated with the increase of Zn passage in the endosome, triggering of autophagy and apoptosis by inducing endosomic ZNT (zinc transporter family) pathways on endosome membranes in mammalian cells [67].
Curcumin acts as natural zinc ionophores and can promote the cellular uptake of zinc and can be used with zinc to increase the effectiveness of these compounds in the inhibition of the virus. ZnT transporters are known to function as Zn2+ / H + modifiers, transporting Zn from the cytoplasm to the extracellular space or intracellular compartments [68]. In a study, Zn accumulation was demonstrated in vesicles in cells expressing ZnT-2 in Zn applications [69]. Therefore, natural compounds identified as zinc ionophores can be used in conjunction with zinc supplementation to act antivirally against many RNA viruses, including SARS-CoV-2. Thus, both nutraceutical and food supplement can act as promising weapons to develop preventative and therapeutic strategies against COVID-19 pandemic.
4. Conclusion and perspectives
Clinical studies have suggested that curcumin, zinc and zinc-ionophores have great potentials for their antiviral activities towards viral infections. Hence these food supplements can be used as complementary power in treatment of COVID-19 with various mechanisms including substantial individual immunity support, inhibition of RNA replication of the SARS-COV-2 and prevention of the virus entry into cell. Although food supplements with their medicinal properties seem to help treatment of COVID-19, food supplement-drug interaction should be considered in terms of increasing toxicity and drug efficiency.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgements
This work is supported by grants awarded by the Erciyes University Scientific Research Office (VA-06-09). We thank to Associate Professor Basri Gulbakan for assistance in revision of the manuscript.
References
- 1.Gralinski L.E., Menachery V.D. Return of the 1. Coronavirus: 2019-nCoV. Viruses. 2020;12(2):135. doi: 10.3390/v12020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int. J. Biol. Sci. 2020;16(10):1708. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burki T.K. Coronavirus in China. The Lancet Respir Med. 2020;8(3):238. doi: 10.1016/S2213-2600(20)30056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization WHO Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020
- 5.https://covid19.who.int/ (08.30.2020)
- 6.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Du B., Li L., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. 2020. Clinical Characteristics of 2019 Novel Coronavirus Infection in China. MedRxiv. [Google Scholar]
- 7.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Diaz G., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S., Lu X., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zumla A., Chan J.F., Azhar E.I., Hui D.S., Yuen K.Y. Coronaviruses – drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordan P.C., Stevens S.K., Deval J. Nucleosides for the treatment of respiratory RNA virus infections. Antivir Chem Chemother. 2018;26 doi: 10.1177/2040206618764483. 2040206618764483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li G., Clercq E.D. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 14.Li T., Peng T. Traditional Chinese herbal medicine as a source of molecules with antiviral activity. Antivir. Res. 2013;97:1–9. doi: 10.1016/j.antiviral.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S.Y., Chen C., Zhang H.Q., Guo H.Y., Wang H., Wang L., Zhang X., Hua S.N., Yu J., Xiao P.G., Li R.S., Tan X. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir. Res. 2005;67(1):18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau K.M., Lee K.M., Koon C.M., Cheung C.S.F., Lau C.P., Ho H.M., Lee M.Y.H., Au S.W.N., Cheng C.H.K., Lau C.B.S., Tsui S.K.W., Wan D.C.C., Waye M.M.Y., Wong K.B., Wong C.K., Lam C.W.K., Leung P.C., Fung K.P. Immunomodulatory and anti-SARS activities of Houttuynia cordata. J. Ethnopharmacol. 2008;118(1):79–85. doi: 10.1016/j.jep.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redeploying plant defencesNat. Plants. 2020;6:177. doi: 10.1038/s41477-020-0628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy A., Sarkar B., Celik C., Ghosh A., Basu U., Jana M., Jana A., Gencay A., Can Sezgin G., Ildiz N., Dam P., Mandal A.K., Ocsoy I. Can concomitant use of zinc and curcumin with other immunity-boosting nutraceuticals be the arsenal against COVID-19? Phytother. Res. 2020:1–4. doi: 10.1002/ptr.6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009;2(5):270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beckman C.H. Phenolic-storing cells: keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiol. Mol. Plant Pathol. 2000;57:101–110. [Google Scholar]
- 22.Sordillo P.P., Helson L. Curcumin suppression of cytokine release and cytokine storm. A potential therapy for patients with Ebola and other severe viral infections. in vivo. 2015;29(1):1–4. [PubMed] [Google Scholar]
- 23.Abe Y., Hashimoto S., Horie T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol. Res. 1999;39:41–47. doi: 10.1006/phrs.1998.0404. [DOI] [PubMed] [Google Scholar]
- 24.Jain S.K., Rains J., Croad J., Larson B., Jones K. Curcumin supplementation lowers TNFα, IL6, IL8, and MCP1 secretion in high glucose-treated cultured monocytes and blood levels of TNFα, IL6, MCP1, glucose, and glycosylated hemoglobin in diabetic rats. Antioxid. Redox Signal. 2009;11:241–249. doi: 10.1089/ars.2008.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kloesch B., Becker T., Dietersdorfer E., Kiener H., Steiner G. Anti-inflammatory and apoptotic effects of the polyphenol curcumin on human fibroblast-like synoviocytes. Int. Immunopharmacol. 2013;15:400–405. doi: 10.1016/j.intimp.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Raflee P., Nelson V.M., Manley S., Wellner M., Floer M., Binion D.G., Shaker R. Effect of curcumin on acidic pH-induced expression of IL6 and IL8 in human esophageal epithelial cells (HET1A): role of PKC, MAPKs, and NFĸB. Amer J Physiol-Gastrointest Liver Physiol. 2009;296:G388–G398. doi: 10.1152/ajpgi.90428.2008. [DOI] [PubMed] [Google Scholar]
- 27.Biswas S.K., McClure D., Jimenez L.A., Megson I.L., Rahman I. Curcumin induces glutathione biosynthesis and inhibits NFĸB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxid. Redox Signal. 2005;7:32–41. doi: 10.1089/ars.2005.7.32. [DOI] [PubMed] [Google Scholar]
- 28.Xu Y.X., Pindolia K.R., Janakiraman N., Chapman R.A., Gautam S.C. Curcumin inhibits IL1α and TNFα induction of AP1 and NFĸB DNA-binding activity in bone marrow stromal cells. Hematopathol Mol Hematol. 1998;11:49–62. [PubMed] [Google Scholar]
- 29.Lonnerdal B. Dietary factors influencing zinc absorption. J. Nutr. 2000;130:1378–1383. doi: 10.1093/jn/130.5.1378S. [DOI] [PubMed] [Google Scholar]
- 30.Prasad A.S., Beck F.W., Bao B., Fitzgerald J.T., Snell D.C., Steinberg J.D., Cardozo L.J. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am. J. Clin. Nutr. 2007;85(3):837–844. doi: 10.1093/ajcn/85.3.837. [DOI] [PubMed] [Google Scholar]
- 31.Oteiza P.I. Zinc and the modulation of redox homeostasis. Free Radic. Biol. Med. 2012;53(9):1748–1759. doi: 10.1016/j.freeradbiomed.2012.08.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krężel A., Hao Q., Maret W. The zinc/thiolate redox biochemistry of metallothionein and the control of zinc ion fluctuations in cell signaling. Arch. Biochem. 2007;463(2):188–200. doi: 10.1016/j.abb.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 33.Giles N.M., Watts A.B., Giles G.I., Fry F.H., Littlechild J.A., Jacob C. Metal and redox modulation of cysteine protein function. Chem. Biol. 2003;10(8):677–693. doi: 10.1016/s1074-5521(03)00174-1. [DOI] [PubMed] [Google Scholar]
- 34.Maret W. Redox biochemistry of mammalian metallothioneins. J. Biol. Inorg. 2011;16(7):1079–1086. doi: 10.1007/s00775-011-0800-0. [DOI] [PubMed] [Google Scholar]
- 35.Maret W. The function of zinc metallothionein: a link between cellular zinc and redox state. J. Nutr. 2000;130(5):1455S–1458S. doi: 10.1093/jn/130.5.1455S. [DOI] [PubMed] [Google Scholar]
- 36.Kaushik N., Subramani C., Anang S., Muthumohan R., Nayak B., Ranjith-Kumar C.T., Surjit M. Zinc salts block hepatitis E virus replication by inhibiting the activity of viral RNA-dependent RNA polymerase. J. Virol. 2017;91(21) doi: 10.1128/JVI.00754-17. e00754-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The role of zinc in antiviral immunity. Adv. Nutr. 2019;10(4):696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richart S.M., Li Y.L., Mizushina Y., Chang Y.Y., Chung T.Y., Chen G.H., Tzen J.T.C., Shia K.S., Hsu W.L. Synergic effect of curcumin and its structural analogue (Monoacetylcurcumin) on anti-influenza virus infection. J. Food Drug Anal. 2018;26:1015–1023. doi: 10.1016/j.jfda.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du T., Shi Y., Xiao S., Li N., Zhao Q., Zhang A., Nan Y., Mu Y., Sun Y., Wu C., Zhang H., Zhou E.M. Curcumin is a promising inhibitor of genotype 2 porcine reproductive and respiratory syndrome virus infection. BMC Vet. Res. 2017;13:298. doi: 10.1186/s12917-017-1218-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Umar S., Shah M.A.A., Munir M.T., Yaqoob M., Fiaz M., Anjum S., Kaboudi K., Bouzouaia M., Younus M., Nisa Q., Iqbal M., Umar W. Synergistic effects of thymoquinone and curcumin on immune response and anti-viral activity against avian influenza virus (H9N2) in turkeys. Poult. Sci. 2016;95:1513–1520. doi: 10.3382/ps/pew069. [DOI] [PubMed] [Google Scholar]
- 41.Chen D.Y., Shien J.H., Tiley L., Chiou S.S., Wang S.Y., Chang T.J., Lee Y.J., Chan K.W., Hsu W.L. Curcumin inhibits influenza virus infection and haemagglutination activity. Food Chem. 2010;119:1346–1351. [Google Scholar]
- 42.Kim K., Kim K.H., Kim H.Y., Cho H.K., Sakamoto N., Cheong J. Curcumin inhibits hepatitis C virus replication via suppressing the Akt-SREBP-1 pathway. FEBS Lett. 2010;584:707–712. doi: 10.1016/j.febslet.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 43.Colpitts C.C., Schang L.M., Rachmawati H., Frentzen A., Pfaender S., Behrendt P., Brown R.J.P., Bankwitz D., Steinmann J., Ott M., Meuleman P., Rice C.M., Ploss A., Pietschmann T., Steinmann E. Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells. Gut. 2014;63:1137–1149. doi: 10.1136/gutjnl-2012-304299. [DOI] [PubMed] [Google Scholar]
- 44.39Mounce, B. C, Cesaro T., Carrau L., Vallet T., Vignuzzi M. Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antiviral Res. 2017;142:148–157. doi: 10.1016/j.antiviral.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 45.Hoffmann M., Kleine-Weber H., Krüger N., Mueller M.A., Drosten C., Pöhlmann S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. BioRxiv. 2020 [Google Scholar]
- 46.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Jianwei W., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1–12. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dandapat J., Jena A.B., Kanungo N., Nayak V., Chainy G. 2020. Catechin and Curcumin interact with corona (2019-nCoV/SARS-CoV2) viral S protein and ACE2 of human cell membrane: insights from Computational study and implication for intervention. [DOI] [Google Scholar]
- 49.Te Velthuis A.J., van den Worm S.H., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn2+ inhibits coronavirus and Arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghaffari H., Tavakoli A., Moradi A., Tabarraei A., Bokharaei-Salim F., Zahmatkeshan M., Farahmand M., Javanmard D., Kiani S.J., Esghaei M., Pirhajati-Mahabadi V., Monavari S.H., Ataei-Pirkooh A., Pirhajati-Mahabadi V. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine. J. Biomed. Sci. 2019;26:70. doi: 10.1186/s12929-019-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson J.K., Harris F.L., Ping X.D., Gauthier T.W., Brown L.A.S. Role of zinc insufficiency in fetal alveolar macrophage dysfunction and RSV exacerbation associated with fetal ethanol exposure. Alcohol. 2019;80:5–16. doi: 10.1016/j.alcohol.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Speth R., Carrera E., Jean-Baptiste M., Joachim A., Linares A. Concentration-dependent effects of zinc on angiotensin-converting enzyme-2 activity. The FASEB J. 2014;28(1_supplement) 1067–4. [Google Scholar]
- 53.Chilvers M.A., McKean M., Rutman A., Myint B.S. Silverman M and O'Callaghan C: the effects of coronavirus on human nasal ciliated respiratory epithelium. Eur. Respir. J. 2001;18:965–970. doi: 10.1183/09031936.01.00093001. [DOI] [PubMed] [Google Scholar]
- 54.Blanchard E., Roingeard P. Virus-induced double-membrane vesicles. Cell. Microbiol. 2015;1:45–50. doi: 10.1111/cmi.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knoops K., Kikkert M., Worm S.H., Zevenhoven-Dobbe J., Meer Y., Koster A.J., Mommaas A.M., Snijder E.J. SARS-Coronavirus Replication is Supported by a Reticulovesicular Network of Modified Endoplasmic Reticulum. PLoS Biol. 2008;6(9) doi: 10.1371/journal.pbio.0060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nafisi S., Adelzadeh M., Norouzi Z., Sarbolouki M.N. Curcumin binding to DNA and RNA. DNA Cell Biol. 2009;28(4):201–208. doi: 10.1089/dna.2008.0840. [DOI] [PubMed] [Google Scholar]
- 57.Chen T.Y., Chen D.Y., Wen H.W., Ou J.L., Chiou S.S., Chen J.M., Wong M.L., Hsu W.L. Inhibition of enveloped viruses infectivity by curcumin. PLoS One. 2013:8(5). doi: 10.1371/journal.pone.0062482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lonnerdal B. Dietary factors influencing zinc absorption. J. Nutr. 2000;130:1378–1383. doi: 10.1093/jn/130.5.1378S. [DOI] [PubMed] [Google Scholar]
- 59.Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The role of zinc in antiviral immunity. Adv. Nutr. 2019;10(4):696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Te Velthuis A.J., van den Worm S.H., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11) doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xue J., Moyer A., Peng B., Wu J., Hannafon B.N., Ding W.Q. Chloroquine is a zinc ionophore. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0109180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krenn B.M., Gaudernak E., Holzer B., Lanke K., Van Kuppeveld F.J.M., Seipelt J. Antiviral activity of the zinc ionophores pyrithione and hinokitiol against picornavirus infections. J. Virol. 2009;83(1):58–64. doi: 10.1128/JVI.01543-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lanke K., Krenn B.M., Melchers W.J.G., Seipelt J., van Kuppeveld F.J.M. PDTC inhibits picornavirus polyprotein processing and RNA replication by transporting zinc ions into cells. J. Gen. Virol. 2007;88:1206–1217. doi: 10.1099/vir.0.82634-0. [DOI] [PubMed] [Google Scholar]
- 64.Korant B.D., Kauer J.C., Butterworth B.E. Zinc ions inhibit replication of rhinoviruses. Nature. 1974;248:588–590. doi: 10.1038/248588a0. [DOI] [PubMed] [Google Scholar]
- 65.Si X., McManus B.M., Zhang J., Yuan J., Cheung C., Esfandiarei M., Suarez A., Morgan A., Luo H. Pyrrolidine dithiocarbamate reduces coxsackievirus B3 replication through inhibition of the ubiquitin-proteasome pathway. J. Virol. 2005;79(13):8014–8023. doi: 10.1128/JVI.79.13.8014-8023.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.E, H, Lergeaud G., Quesada I.M., Ortiz M., O'Sullivan C.K., Fernández-Larrea J.B. Zinc ionophore activity of quercetin and epigallocatechin-gallate: from Hepa 1–6 cells to a liposome model. J Agric Food Chem. 2014;62:8085–8093. doi: 10.1021/jf5014633. [DOI] [PubMed] [Google Scholar]
- 67.John E., Laskow T.C., Buchser W.J., Pitt B.R., Basse P.H., Butterfield L.H., Kalinski P., Lotze M.T. Zinc in innate and adaptive tumor immunity. J. Transl. Med. 2010;8:118. doi: 10.1186/1479-5876-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sareen R., Jain N., Dhar K.L. Curcumin–Zn (II) complex for enhanced solubility and stability: an approach for improved delivery and pharmacodynamic effects. Pharm. Dev. Technol. 2016;21(5):630–635. doi: 10.3109/10837450.2015.1041042. [DOI] [PubMed] [Google Scholar]
- 69.Palmiter R.D., Cole T.B., Findley S.D. ZnT-2, a mammalian protein that confers resistance to zinc by facilitating vesicular sequestration. EMBO J. 1996;15(8):1784–1791. [PMC free article] [PubMed] [Google Scholar]