Abstract
Acute kidney injury (AKI) in premature neonates is common due to the administration of life-saving therapies. The impact of AKI on renal morphology and susceptibility to further renal damage is poorly understood. Recent advances in radiological imaging have allowed integration of soft tissue morphology in the intact organ, facilitating a more complete understanding of changes in tissue microstructure associated with pathology. Here, we applied magnetic resonance imaging (MRI) to detect both glomerular and vascular changes in a rabbit model of neonatal AKI, induced by indomethacin and gentamicin. Using combined spin-echo MRI and cationic ferritin enhanced gradient-echo MRI (CFE-MRI), we observed 1) an increased cortical arterial diameter in the AKI cohort compared to healthy controls, and 2) focal loss of vascular density and glomerular loss in a circumferential band ~1mm from the cortical surface. This combined use of vascular and glomerular imaging may give insight into the etiology of AKI and its impact on renal health later in life.
Keywords: nephron number, glomerulus, vasculature, magnetic resonance imaging (MRI), acute kidney injury
Introduction
The kidney is responsible for a wide range of physiological processes, including homeostasis of systemic osmolality, maintenance of acid-base status, filtration of blood, reabsorption of metabolites, and regulation of blood pressure. The vasculature and interstitium of the kidney are tightly coupled to both filter the blood and extract oxygen. As a consequence, kidney function is highly susceptible to a wide range of pathologies associated with vascular and tubulointerstitial damage. However, there is variability in the kidney microstructure, including in the number of glomeruli per kidney(1). This may determine how focal pathologies affect gross function of the organ. Tools to evaluate the microstructure throughout the kidney are critical to fully understanding renal physiology at a systems level, and inform the development of early diagnostic markers leading to therapies in an early stage of renal disease.
Renal pathology is highly heterogeneous, especially in humans. Integrating the structure and function of glomeruli with the vasculature will guide our understanding of the relationship between local and systems physiology. Recent advances in microscopy have begun to shed light on the interplay between the renal vasculature, glomerular morphology, and nephron structure. Light sheet microscopy performed in optically cleared organs is beginning to provide three dimensional (3D) maps of molecular signaling in targeted cells in conjunction with the surrounding tissue morphology in small samples (2, 3). Several new radiological imaging techniques are being developed, including x-ray computed tomography (CT) (4) and magnetic resonance imaging (MRI) (5), to connect the molecular and cellular levels in the whole, intact kidney. MRI is also providing a new view of these connections in vivo (6–11).
Recently, an MRI technique has been developed to allow for whole-kidney maps of individual glomeruli in 3D (7–9, 12–14). This technique is based on a modified form of ferritin, cationic ferritin (CF), as a contrast agent which is injected intravenously. As CF circulates in the living animal, it traverses the endothelium of the fenestrated glomerular capillaries and binds transiently to the glomerular basement membrane. The CF is detectable by gradient-echo MRI for approximately 48 hours in healthy rats (15). CF–enhanced MRI (CFE-MRI) has been used to map glomerular microstructure in various animal models of kidney disease, both ex vivo and in vivo, providing a unique view of renal microstructure and its relationship to the onset and development of tissue pathology. The development of CFE-MRI is preceded by decades of stereological work and coincides with recent investigations of nephron number in human kidneys (16–19), but with the potential for in vivo preclinical and clinical translation. In addition to its potential for in vivo and clinical use, CFE- MRI provides a wide range of soft tissue contrast that could enable a fully integrated view of the kidney during disease progression, reporting not only nephron loss, but also how glomerular loss is related to other vascular and tubular structural changes.
Here, we develop an approach to integrate vascular morphology with CFE-MRI to map the arterial changes associated with focal loss of glomeruli in a neonatal model of AKI. It is important to understand the role of the vasculature in tissue reorganization following nephrotoxin-induced acute kidney injury (AKI). Additionally, vascular imaging biomarkers may allow differentiation between the variable causes of AKI in humans, providing insight into new therapies aimed at reducing the impact of the injury or facilitating local remodeling. AKI was induced in rabbit kits using a combination of nephrotoxic medications, gentamicin and indomethacin, during a brief window of postnatal nephrogenesis. Gentamicin and indomethacin are medications commonly used in preterm infants to treat sepsis and patent ductus arteriosus, a vascular remnant of fetal circulation. A retrospective study demonstrated 86% of very low birth weight neonates received gentamicin and 43% received indomethacin in the neonatal intensive care unit(20). Neonates who received these drugs had a greater risk of developing AKI. Several studies in animals have also demonstrated nephron malformation or loss following exposure to gentamicin or indomethacin (21, 22). However, little data exists on the structural changes in the vessels in response to these nephrotoxic medications, because reconstruction of the whole vasculature using histology is challenging. Using image processing and analysis, we demonstrate the combined use of vascular imaging and CFE-MRI to examine arterial and glomerular reorganization in this rabbit model of nephrotoxin-induced AKI.
Methods
Animal preparation and labeling with CF
All animal experiments were approved by the University of Virginia Institutional Animal Care and Use Committee, according to the NIH Guide for the Care and Use of Laboratory Animals. Juvenile offspring of adult New Zealand rabbits (Robinson Services, Inc., Mosksville, NC) and Charles River (Wilmington, MA) were used.
We developed the model of renal injury in neonatal rabbits using indomethacin and gentamicin and the biochemical and histologic evaluation of this model was recently published (23). Gentamicin and indomethacin are medications used clinically in preterm human neonates to treat deadly bacterial infections and patent ductus arteriosus, but are associated with neonatal AKI (20). In previous work, a four day course of indomethacin and gentamicin in rats induced AKI and reduced creatinine clearance (24). Here, three rabbits received pharmaceutical grade indomethacin (5 mg/kg, oral; avKare, Inc, Pulaski, TN) and gentamicin (100 mg/kg, IP injection, APP Pharmaceuticals, LLC; Schaumburg, IL) every day for four days, starting one week after birth. Three rabbits in the control group received saline.
At six weeks of age, the rabbits were sedated, a catheter was placed in the marginal ear vein, and hydrocortisone sodium succinate (10 mg/kg)(25) was administered intramuscularly. Cationic ferritin was prepared from native horse spleen ferritin (Sigma-Aldrich, St. Louis, MO) based on the method of Danon et al. CF was dialyzed using 8kDa dialysis tubing and filtered using a 22 μm filter.CF concentration was determined by Bradford assay. CF was administered as a single intravenous injection of 1.92 mg/100 grams body weight over 10-15 minutes to both the AKI and control groups.
Ninety minutes after the injection of CF, rabbits received ketamine (80 mg/kg) and xylazine (10 mg/kg) IP, followed by isofluorane for transcardiac perfusion. The left renal artery was clamped to avoid formalin fixation (this tissue was examined by transmission electron microscopy in a previously published study (23)). Physiological saline and then 10% buffered formalin phosphate were infused into the right kidney. The left kidney was stored in 2% glutaraldehyde/0.1 M cacodylate solution at 4°C for imaging.
MR Imaging
Each kidney was cut in half across the axial plane and placed in 2% glutaraldehyde/0.1 M cacodylate solution in a custom holder for imaging. Fixed kidneys were imaged using a Bruker 7T/30 MRI (Bruker, Co., Billerica, MA, USA), a quadrature RF probe with an inner diameter of 30 mm, and Siemens software for acquisition and reconstruction (Siemens, Munich, Germany). To detect CF-labeled glomeruli by CFE-MRI, we applied a gradient recalled echo (GRE) pulse sequence of echo time/repetition time (TE/TR) = 20ms/80ms with a 26 × 26 mm FOV, 192 slices, 3 averages, resolution of 64.7 × 64.7 × 170 μm3 and a flip angle of 25°, and a total acquisition time of 5 hours 10 minutes. To provide contrast of the renal vasculature in a separate scan of the same kidneys, we applied a spin-echo pulse sequence with TE/TR = 30ms/4000ms using a 26 × 26 mm FOV, 256 × 256 matrix, 96 slices, 11 averages, resolution of 101.6 × 101.6 × 200 μm3, and a total acquisition time of 3 hours 8 minutes.
Image Analysis: Vasculature
Semi-automated segmentation of the vascular images was performed in MIPAR Image Analysis Software version 2.1.5 (MIPAR Software LLC, Worthington, OH) and in Amira 6.7.0 (Thermo Fischer Scientific, Waltham, MA). The images were pre-processed in Amira. The border of the kidney was manually segmented on ~15% of the slices and propagated to the remaining slices using the interpolation tool. The resulting images were transferred to MIPAR. On a single 2D image, the kidney was windowed and leveled manually to a narrow window and a threshold was selected to generate a tissue mask. This mask was eroded by two pixels and the result was stored as Mask 1. The original image was visually windowed to accentuate bright vessels. An adaptive filter was used (120%, 12×12 pixels) on Mask 1 to generate a vascular mask, which was stored as Mask 2. The adaptive filter selects pixels with a value greater than the threshold in relation to a specified size of surrounding pixels. A bright texture filter (also referred to as a top-hat filter; grayscale opening minus the original image) was applied to the original image. This filter accentuates borders and added to the original image. An adaptive filter was then applied (54%, 14×14 pixels), in conjunction with Mask 2. The result stored as Mask 3. A Frangi filter, (thickness 1 and strength 4), which accentuates lines, was applied to the original image. An adaptive filter was then applied to the result (45%, 15×15 pixels) in conjunction with Mask 3 and labeled objects less than two pixels in area in the 2D plane were removed. All images in the volume were then processed using the recipe developed on the single slice above. The resulting vascular maps on each slice were manually corrected to eliminate any pixels outside the vessels and to include any vessels not automatically included. Three-dimensional visualization was conducted in Amira (Figures 2a, 2b). The processed images were uploaded and isosurface rendering was used to locate vessel branches. To measure arterial diameters, the arteries were manually segmented on all 2D slices in Amira. Major arteries were differentiated from veins based on their round shape and thicker walls compared to veins. We confirmed that the larger vessels were connected to the smaller cortical vessels. A 3D visualization was performed and the artery diameter was measured manually perpendicular to the artery length.
Figure 2:

Rabbit vasculature in 3D magnetic resonance images and histology. (a) Nodes were identified at intersecting vessels from the 3D spin-echo images. (b) Nodes were connected to vessels, which were associated with the renal artery to separate arteries from veins and render the arterial structure in 3D. Histology of the renal cortex in healthy rabbits (c) and (d) rabbits with acute kidney injury (AKI). Glomeruli are marked by a yellow circle, convoluted tubules are marked by the red arrow, interlobar arteries are filled in red, and veins are marked by the green arrow.(e) A three-dimensional map of rabbit vasculature was segmented by automated adaptive thresholding. The tagnified image shows Individual interlobar arteries and veins in the cortex with sizes measured in 3D(f).
Image Analysis: Glomeruli
The resolution (x, y, z) of each image was increased by linear interpolation to 12.7 × 12.7 × 25 microns using Amira (FEI, Bordeaux, France). The medulla was segmented manually and the images were processed in MIPAR using adaptive thresholding. The glomeruli are shown as dark spots in Figure 1–c. If the pixel is <50% of its local 20×20 window average, then that pixel would be included as part of the glomerulus. Glomeruli were segmented and measured using custom scripts in MATLAB, (The Mathworks, Natick, MA), described previously (8).
Figure 1:

Magnetic resonance imaging (MRI) of the rabbit kidney. Representative 2D slices from 3D MRI datasets are shown. In spin-echo MRI images, the vessels appear bright in healthy rabbits (a) and rabbits six weeks after acute kidney injury (AKI) (b). Cationized ferritin (CF)–enhanced MRI (CFE-MRI) with a gradient echo pulse sequence is used to detect individual glomeruli throughout the kidney in healthy (c) and (d) (AKI) rabbits. The white arrows in (a) and (b) indicate arcuate arteries. The black arrow in (a) indicates interlobular artery. White arrows in (c) indicate regions of normal glomerular density and indicate regions of reduced glomerular density in (d)
Histology
We performed a histological evaluation of the kidneys to compare with our MRI measurements. Following MRI, the kidney was cut into quarters and one quarter was exhaustively sectioned at 50 μm thickness in the coronal plane using a Leica VT100S vibratome. A random number generator was used to select the first section and every 20th section was placed in phosphate buffered saline. Samples were blocked in 2% bovine serum albumin followed by 1% Triton X-100. The samples were incubated with wheat germ agglutinin conjugated to Alexa-555 (WGA-555; Life Technologies, Carlsbad, CA) overnight and mounted with ProLong Diamond with 4’6-diamidino-2-phenylindole (LifeTechnologies). A Zeiss LSM710 confocal microscope running Zen 2012 was used to acquire images as previously described (19). Images were stitched in Zen (Thornwood, NY) and exported as stacks of tiff images for analysis.
The histology images were imported and displayed in Amira in 2D. The renal interlobular arteries were distinguished from interlobular veins by their round shape, thicker wall than the thin-walled veins as well as a contraction artifact between the artery and surrounding renal interstitium. In order to avoid partial-volume artificial increase in vessel diameter, only round arteries were measured as they pass through plane. Small and large diameters were measured to include the surrounding contraction artifact (Figure 2c–d). The small and large diameters were averaged together to obtain the arterial diameter.
Statistical Analyses
The arterial diameters and glomerular numbers of the normal rabbits and those with AKI were analyzed in Matlab (MathWorks, Natick, MA) using a one-way ANOVA test. Statistical significance was determined at a p=0.05 level.
Results
We performed 3D spin-echo MRI and CFE-MRI to establish our ability to detect both the renal vasculature and the glomeruli using this combination of contrast techniques. As shown in the 2D slices from the 3D dataset in Figure 1a–b, spin-echo MRI revealed vascular structures, enhanced in the images due to water present in the vessel lumen. The renal vasculature up to the interlobar arteries were readily detected and were connected in 3D to the major renal arteries and veins. CFE-MRI of the same kidneys (Figure 1c–d) provided a map of the renal glomeruli, visible as dark spots throughout the renal cortex. Importantly, there were differences in the spatial maps of the cortical vasculature and the glomeruli in the AKI cohort of rabbits. Vessels in the AKI cohort were less dense approximately 1 mm from the cortical surface compared to healthy control animals, and glomerular density was lower in these same regions of the same kidneys, compared to the densely CF-labeled cortex in healthy controls. A summary of the glomerular numbers and vessel diameters for each animal is included in Table 1.
Table 1:
Glomerular number and volume by MRI and stereology.
| ID | Body weight (kg) | Kidney weight (g) | MRI Nglom | Vessel diameter (mm) | |
|---|---|---|---|---|---|
| AKI | 1 | 0.70 | 8.6 | 74,034 | 0.26±0.04 |
| 2 | 0.86 | 10.3 | 97,334 | 0.23±0.06 | |
| 3 | 0.75 | 9.3 | 86,815 | 0.23±0.04 | |
| controls | 1 | 1.31 | 12.7 | 167,017 | 0.15±0.02 |
| 2 | 1.40 | 16.1 | 262,636 | 0.14±0.02 | |
| 3 | 1.50 | 17.7 | 201,419 | 0.15±0.03 |
To visualize the renal vasculature, we performed a semi-automated image segmentation on one slice, which was then automatically propagated to all image slices. The resulting vascular tree (both arteries and veins) was then rendered in 3D. Manual segmentation of the vessels was then performed to separate arteries from veins. Connected vascular nodes were mapped back to connected arteries, as shown in Figure 2a–b. From this, we mapped arterial diameter in 3D, as shown in Figure 2e, with vascular diameters represented by a color map. With this image resolution, we were unable to distinguish the arterioles perfusing the glomeruli, but the cortical interlobar arteries were readily measured, as seen in Figure 2f. We further confirmed vessel size from 2D histological sections, where the approximate diameters of the vessels were measured (Figure 2c–d).
The diameters of the interlobular arteries were compared between AKI and control cohorts and between MRI and histology. Results are shown in Figure 3. In both MRI and histology, the average diameter of the arteries in cortex was significantly larger in the AKI cohort than in healthy controls (p<0.05). Arteries measured by MRI were systematically ~50 μm larger in diameter than by histology (Figure 3b and d). We attribute this to the lower resolution of MRI, which reduces precision at the vessel walls.
Figure 3:
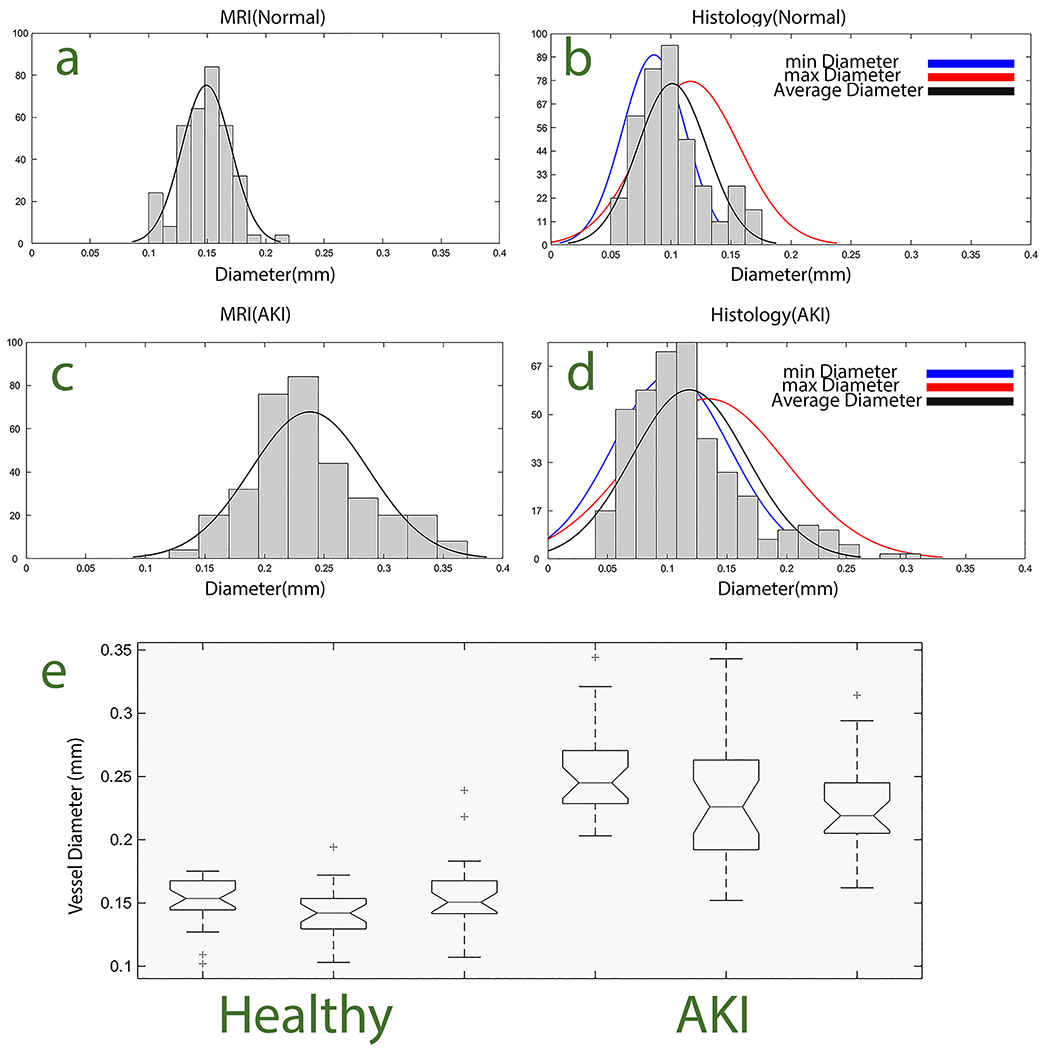
Increased interlobular arterial diameter in rabbits six weeks after acute kidney injury (AKI). Histograms of arterial diameters are shown, computed from (a,c) Magnetic resonance imaging (MRI) and (b,d) histology in health rabbits and rabbits after AKI. (e) Box and whisker plot from ANOVA analysis confirmed statistically significant (p<0.05) differences in arterial diameters between healthy and AKI cohorts measured by MRI. Blue, red, and black lines show the distributions of minimum, maximum, and average arterial diameters, respectively.
We compared both vessel-enhanced MRI with histology to determine whether there were vascular changes associated with the observed glomerular loss in cortex in the AKI cohort. Figure 4 shows 2D slices of segmented arteries from the 3D spin-echo MRI dataset, along with stained histologic sections of the same kidneys. One striking feature of the MRI was a low arterial density in the cortical region where we had observed the glomerular loss. From the 2D MRI and histology this loss appeared as a lack of contrast in the image. We therefore examined the 3D reconstructions of the vasculature and glomeruli, shown in Figure 5. The 3D reconstructions showed regions of glomeruli in superficial cortex. These surrounded the region with glomerular loss in the animals with AKI. The superficial glomeruli appeared perfused by arteries that penetrated the cortex from major branches of the arcuate arteries. Vessel diameter and glomerular number were inversely correlated (Figure 5e).
Figure 4 |.

Rabbit vasculature imaged with magnetic resonance imaging (MRI) and histology in normal rabbits (a,e,f) and rabbits with nephrotoxin-induced acute kidney injury (AKI) (c,g,h) rabbits. Spin echo (SE) images (a,c) highlighting vessels as bright objects. Segmented vascular images (b,d) of kidney cortex. Comparison histology slides for normal(e,f) and for AKI (g,h).
Figure 5:

3D vascular images in ex vivo healthy (a) and acute kidney injury (AKI) (c) rabbits. Color-coded maps of vascular diameter indicate large arteries in the normal kidney (a) and shorter and thicker arteries in the AKI. Arterial loss with AKI is indicated by the white arrow (c). 3D maps of glomeruli indicate reduced glomerular density in AKI (d) compared to controls (b). Figure 5-e shows that glomerular number and vessel diameter were highly correlated in healthy (R2 =0.71) and AKI (R2 = 0.75) cohorts.
Discussion
In this work, we have examined the development of vascular and glomerular pathology in a rabbit model of neonatal AKI, using 3D MRI. Although, indomethacin and gentamicin have been established as nephrotoxic in the developing kidney of the neonatal rabbit (23), to our knowledge no studies have examined the long-term effect of the combination of these drugs on the vasculature integrated with glomerular morphology of the whole kidney. We have demonstrated that a short exposure to commonly encountered medications during an active period of nephrogenesis results in a 50% reduction of glomeruli by MRI, along with significant vascular changes, with differences in the spatial maps of the cortical vasculature and the glomeruli.
Preterm birth is common, with nearly 10% of births occurring prior to a gestational age of 37 weeks (26). The familiar co-morbidities that manifest in many organs of the preterm neonates such as retinopathy of prematurity (27), intraventricular hemorrhage (28), patent ductus arteriosus and necrotizing enterocolitis (29), have a common origin: alterations in the vasculature (30). Early vascular compromises incurred by the preterm infant contribute to the increased risk for “adult-onset” medical conditions such as renal disease, hypertension and diabetes (31, 32). The highly vascularized kidney is particularly vulnerable to the effects of preterm birth with 60% of glomerular development occurring in the third trimester (33). During this period of active nephrogenesis, fluctuations in tissue oxygenation (34), renal perfusion, and exposures to medications (35, 36) can lead to a reduced glomerular number. In children, radiologic evaluations of the vasculature are limited due radiation exposure and sedation effects. There are not tools to evaluate the interaction of the vasculature and effect on nephrogenesis or glomerular maintenance in animal models. Furthermore, there are no clinically tools that can integrate the vascular and glomerular compartments to map simultaneous changes. As the survival rates of the most preterm infants improve (37), it is critical to focus on how to improve the morbidity associated with impairment of ongoing organogenesis in an ex utero environment, where the effects of preterm birth and kidney disease may be detectable years after birth (31, 32). Clinicians need tools to predict which preterm neonates will develop renal disease as adults. Tools to assess the kidney in 3D and integrate the major compartments of the kidney, including the vasculature and glomeruli, could provide a way to improve long term kidney outcomes in preterm neonates and limit the progression of progression in preterm neonates.
In this model of neonatal AKI with vascular and glomerular changes, reduced vascular density and larger vessel diameter may result from several processes. The outer cortical vessels may have pruned their branches because there were no glomeruli to perfuse. Alternatively, the indomethacin may have induced renal vasoconstriction, halting glomerulogenesis and resulting in a peripheral loss of glomeruli or loss of glomerular development. The MR images demonstrate a normal appearing layer of glomeruli distal to the injured area, suggesting nephrogenesis did not completely end during drug administration. Indomethacin induces vascular constriction. Guignard concluded that the impairment in renal function in newborn rabbits exposed to indomethacin is hemodynamically mediated supported by an increased renal vascular resistance and consequent decrease in both glomerular filtration rate and renal blood flow (38). Indomethacin is used in the neonatal intensive care unit as a prophylactic medication against intraventricular hemorrhage and as a therapy for patent ductus arteriosus. Nonsteroidal anti-inflammatory drugs reduce the production of prostaglandins by inhibiting cyclooxygenase. Five bioactive prostanoids (39) are derived from the cyclooxygenase-mediated metabolism of arachidonic acid and actively produced in the kidney to influence vascular tone. Intrarenal infusion of prostaglandin E2, I2, and D2 result in increased renal blood flow, renin release, sodium excretion, and free-water clearance through vasodilation (40). The vasodilatory influence of prostaglandin E is critically important in the setting of compromised renal perfusion and similarly important given the physiology of the fetal and neonatal kidney which have a low glomerular filtration rate and renal perfusion pressure. The glomerular filtration rate of a neonate is supported by a delicate balance of vasodilatory and vasoconstrictive forces of the pre- and post-glomerular arterioles (41). Future experiments are needed to trace the fate of the vessels and glomeruli in vivo to determine the mechanism of the larger, but sparser vessels in the AKI group and differentiate glomerular loss from impaired nephrogenesis in the areas of lower glomerular density.
There are several limitations to this study. First, CFE-MRI has been performed on kidneys ex vivo here, at a single time point. MRI can also be applied in vivo (9, 11). However, a detailed ex vivo study was critical to define the location and extent of the glomerular and vascular damage. Future work will focus on optimizing both hardware and MRI acquisition to image rabbit models similarly in vivo. Vascular and glomerular imaging were not performed during the same imaging session. Thus, specific vessels were not aligned with specific glomeruli. While this was not a limitation for the current study because the glomerular loss during AKI was uniform throughout the cortex, future work will require both data set acquisitions during the same session in heterogeneous disease. Another limitation is that vascular measurements by spin-echo MRI are limited to vessels on the order of the image resolution. This is apparent in Figure 3, where the diameter of the vessels detected by MRI is the diameter of the largest vessels measured by histology. This may limit sensitivity to changes in smaller vessels in this or other models, or may limit distinction of arteries in veins in some pathologies. Further work is required to ensure that smaller capillary diameters are not confounded by nearby lymphatic vessels. Our imaging resolution was limited by the available hardware, including imaging field strength, radiofrequency coils, and gradients. With further development, the resolution of both vascular imaging and CFE-MRI can be refined to increase resolution to the level of smaller capillaries, which are more readily detected in optical microscopy. Increasing spatial resolution may not lead to practical measurement of small capillaries because of the increase in imaging time required to achieve adequate signal. Finally, the development and relevance of this model to human disease requires further investigation. Despite accumulating evidence of the association of chronic kidney disease following preterm birth (42–48), histologic comparisons of animal models to the kidney tissue from preterm neonates with AKI is problematic. Kidney biopsies are not standard of care in neonates born preterm.
In conclusion, the combination of spin-echo MRI and CFE-MRI can be used to evaluate renal vascular and glomerular changes associated with AKI. This technology will useful to interrogate mechanisms of disease, track nephron fate and assess nephron protective strategies in a wide range of disease models and potentially for translation to diagnostics in humans.
Acknowledgements
The authors acknowledge the molecular imaging core at the University of Virginia (UVa) and J. Roy for his insight and technical assistance in imaging, along with J. Gatesman and the UVa veterinary staff.
Funding Sources
We gratefully acknowledge our funding sources: R01DK110622 (KB and JRC), R01DK111861 (KB and JRC), and American Society of Nephrology Carl W. Gottschalk Research Scholar Grant (JRC).
This work used the Bruker ClinScan MRI in the Molecular Imaging Core, which was purchased with support from NIH grant 1S10RR019911-01 and is supported by the University of Virginia School of Medicine. JJD is supported in part by an ASNR/RSNA Research Scholar Award.
Footnotes
Disclosure
K. M. Bennett and J. R. Charlton own Sindri Technologies, LLC. K.M. Bennett owns Nephrodiagnostics, LLC.
References
- 1.Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE. Human nephron number: implications for health and disease. Pediatr Nephrol. 2011;26(9):1529–33. [DOI] [PubMed] [Google Scholar]
- 2.Chung K, Wallace J, Kim SY, Kalyanasundaram S, Andalman AS, Davidson TJ, et al. Structural and molecular interrogation of intact biological systems. Nature. 2013;497(7449):332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang J, Brenna C, Khan AUM, Daniele C, Rudolf R, Heuveline V, et al. A cationic near infrared fluorescent agent and ethyl-cinnamate tissue clearing protocol for vascular staining and imaging. Sci Rep. 2019;9(1):521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie L, Koukos G, Barck K, Foreman O, Lee WP, Brendza R, et al. Micro-CT imaging and structural analysis of glomeruli in a model of Adriamycin-induced nephropathy. Am J Physiol Renal Physiol. 2019;316(1):F76–F89. [DOI] [PubMed] [Google Scholar]
- 5.Xie L, Bennett KM, Liu C, Johnson GA, Zhang JL, Lee VS. MRI tools for assessment of microstructure and nephron function of the kidney. Am J Physiol Renal Physiol. 2016;311(6):F1109–F24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie L, Dibb R, Cofer GP, Li W, Nicholls PJ, Johnson GA, et al. Susceptibility tensor imaging of the kidney and its microstructural underpinnings. Magn Reson Med. 2015;73(3):1270–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beeman SC, Cullen-McEwen LA, Puelles VG, Zhang M, Wu T, Baldelomar EJ, et al. MRI-based glomerular morphology and pathology in whole human kidneys. Am J Physiol Renal Physiol. 2014;306(11):F1381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldelomar EJ, Charlton JR, Beeman SC, Hann BD, Cullen-McEwen L, Pearl VM, et al. Phenotyping by magnetic resonance imaging nondestructively measures glomerular number and volume distribution in mice with and without nephron reduction. Kidney Int. 2016;89(2):498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldelomar EJ, Charlton JR, Beeman SC, Bennett KM. Measuring rat kidney glomerular number and size in vivo with MRI. Am J Physiol Renal Physiol. 2018;314(3):F399–F406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian C, Yu X, Chen DY, Dodd S, Bouraoud N, Pothayee N, et al. Wireless amplified nuclear MR detector (WAND) for high-spatial-resolution MR imaging of internal organs: preclinical demonstration in a rodent model. Radiology. 2013;268(1):228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baldelomar EJ, Charlton JR, deRonde KA, Bennett KM. In vivo measurement of kidney glomerular number and size in healthy and Os(/+) mice using MRI. Am J Physiol Renal Physiol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett KM, Zhou H, Sumner JP, Dodd SJ, Bouraoud N, Doi K, et al. MRI of the basement membrane using charged nanoparticles as contrast agents. Magn Reson Med. 2008;60(3):564–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beeman SC, Zhang M, Gubhaju L, Wu T, Bertram JF, Frakes DH, et al. Measuring glomerular number and size in perfused kidneys using MRI. Am J Physiol Renal Physiol. 2011;300(6):F1454–7. [DOI] [PubMed] [Google Scholar]
- 14.Heilmann M, Neudecker S, Wolf I, Gubhaju L, Sticht C, Schock-Kusch D, et al. Quantification of glomerular number and size distribution in normal rat kidneys using magnetic resonance imaging. Nephrol Dial Transplant. 2012;27(1):100–7. [DOI] [PubMed] [Google Scholar]
- 15.Beeman SC, Georges JF, Bennett KM. Toxicity, biodistribution, and ex vivo MRI detection of intravenously injected cationized ferritin. Magn Reson Med. 2013;69(3):853–61. [DOI] [PubMed] [Google Scholar]
- 16.Bertram JF, Cullen-McEwen LA, Egan GF, Gretz N, Baldelomar E, Beeman SC, et al. Why and how we determine nephron number. Pediatr Nephrol. 2014;29(4):575–80. [DOI] [PubMed] [Google Scholar]
- 17.Bertram JF. Analyzing renal glomeruli with the new stereology. Int Rev Cytol. 1995;161:111–72. [DOI] [PubMed] [Google Scholar]
- 18.Kanzaki G, Puelles VG, Cullen-McEwen LA, Hoy WE, Okabayashi Y, Tsuboi N, et al. New insights on glomerular hyperfiltration: a Japanese autopsy study. JCI Insight. 2017;2(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE. Human nephron number: implications for health and disease. Pediatric nephrology (Berlin, Germany). 2011;26(9):1529–33. [DOI] [PubMed] [Google Scholar]
- 20.Rhone ET, Carmody JB, Swanson JR, Charlton JR. Nephrotoxic medication exposure in very low birth weight infants. J Matern Fetal Neonatal Med. 2014;27(14):1485–90. [DOI] [PubMed] [Google Scholar]
- 21.Gilbert T, Lelievre-Pegorier M, Merlet-Benichou C. Long-term effects of mild oligonephronia induced in utero by gentamicin in the rat. Pediatr Res. 1991;30(5):450–6. [DOI] [PubMed] [Google Scholar]
- 22.Kent AL, Koina ME, Gubhaju L, Cullen-McEwen LA, Bertram JF, Lynnhtun J, et al. Indomethacin administered early in the postnatal period results in reduced glomerular number in the adult rat. Am J Physiol Renal Physiol. 2014;307(10):F1105–10. [DOI] [PubMed] [Google Scholar]
- 23.Charlton JR, Baldelomar EJ, deRonde K, Cathro HP, Charlton NP, Criswell S, et al. Nephron loss detected by MRI following neonatal acute kidney injury in rabbits. Pediatr Res. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosaka EM, Santos OF, Seguro AC, Vattimo MF. Effect of cyclooxygenase inhibitors on gentamicin-induced nephrotoxicity in rats. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas. 2004;37(7):979–85. [DOI] [PubMed] [Google Scholar]
- 25.Charlton JR, Pearl VM, Denotti AR, Lee JB, Swaminathan S, Scindia YM, et al. Biocompatibility of ferritin-based nanoparticles as targeted MRI contrast agents. Nanomedicine : nanotechnology, biology, and medicine. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.March of Dimes.
- 27.Romagnoli C Risk factors and growth factors in ROP. Early Hum Dev. 2009;85(10 Suppl):S79–82. [DOI] [PubMed] [Google Scholar]
- 28.Ballabh P Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nankervis CA, Giannone PJ, Reber KM. The neonatal intestinal vasculature: contributing factors to necrotizing enterocolitis. Semin Perinatol. 2008;32(2):83–91. [DOI] [PubMed] [Google Scholar]
- 30.Ligi I, Grandvuillemin I, Andres V, Dignat-George F, Simeoni U. Low birth weight infants and the developmental programming of hypertension: a focus on vascular factors. Semin Perinatol. 2010;34(3):188–92. [DOI] [PubMed] [Google Scholar]
- 31.Raju TNK, Buist AS, Blaisdell CJ, Moxey-Mims M, Saigal S. Adults born preterm: a review of general health and system-specific outcomes. Acta Paediatrica (Oslo, Norway : 1992). 2017;106(9):1409–37. [DOI] [PubMed] [Google Scholar]
- 32.Raju TNK, Pemberton VL, Saigal S, Blaisdell CJ, Moxey-Mims M, Buist S, et al. Long-Term Healthcare Outcomes of Preterm Birth: An Executive Summary of a Conference Sponsored by the National Institutes of Health. J Pediatr. 2017;181:309–18.e1. [DOI] [PubMed] [Google Scholar]
- 33.Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Laboratory investigation; a journal of technical methods and pathology. 1991;64(6):777–84. [PubMed] [Google Scholar]
- 34.Hemker SL, Sims-Lucas S, Ho J. Role of hypoxia during nephrogenesis. Pediatr Nephrol. 2016;31(10):1571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kent AL, Brown L, Broom M, Broomfield A, Dahlstrom JE. Increased urinary podocytes following indomethacin suggests drug-induced glomerular injury. Pediatric nephrology (Berlin, Germany). 2012;27(7):1111–7. [DOI] [PubMed] [Google Scholar]
- 36.Kent AL, Koina ME, Gubhaju L, Cullen-McEwen LA, Bertram JF, Lynnhtun J, et al. Indomethacin administered early in the postnatal period results in reduced glomerular number in the adult rat. American journal of physiologyRenal physiology. 2014;307(10):F1105–10. [DOI] [PubMed] [Google Scholar]
- 37.Marlow N, Bennett C, Draper ES, Hennessy EM, Morgan AS, Costeloe KL. Perinatal outcomes for extremely preterm babies in relation to place of birth in England: the EPICure 2 study. Archives of disease in childhoodFetal and neonatal edition. 2014;99(3):F181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guignard JP. The adverse renal effects of prostaglandin-synthesis inhibitors in the newborn rabbit. Semin Perinatol. 2002;26(6):398–405. [DOI] [PubMed] [Google Scholar]
- 39.Apiwattanakul N, Sekine T, Chairoungdua A, Kanai Y, Nakajima N, Sophasan S, et al. Transport properties of nonsteroidal anti-inflammatory drugs by organic anion transporter 1 expressed in Xenopus laevis oocytes. Mol Pharmacol. 1999;55(5):847–54. [PubMed] [Google Scholar]
- 40.Smith WL. Prostanoid biosynthesis and mechanisms of action. Am J Physiol. 1992;263(2 Pt 2):F181–91. [DOI] [PubMed] [Google Scholar]
- 41.Guignard JP, Gouyon JB, John EG. Vasoactive factors in the immature kidney. Pediatr Nephrol. 1991;5(4):443–6. [DOI] [PubMed] [Google Scholar]
- 42.White SL, Perkovic V, Cass A, Chang CL, Poulter NR, Spector T, et al. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. American Journal of Kidney Diseases : The Official Journal of the National Kidney Foundation. 2009;54(2):248–61. [DOI] [PubMed] [Google Scholar]
- 43.Hsu CW, Yamamoto KT, Henry RK, De Roos AJ, Flynn JT. Prenatal Risk Factors for Childhood CKD. Journal of the American Society of Nephrology : JASN. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khalsa DD, Beydoun HA, Carmody JB. Prevalence of chronic kidney disease risk factors among low birth weight adolescents. Pediatric nephrology (Berlin, Germany). 2016;31(9):1509–16. [DOI] [PubMed] [Google Scholar]
- 45.Crump C, Sundquist J, Winkleby MA, Sundquist K. Preterm birth and risk of chronic kidney disease from childhood into mid-adulthood: national cohort study. BMJ. 2019;365:l1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harer MW, Pope CF, Conaway MR, Charlton JR. Follow-up of Acute kidney injury in Neonates during Childhood Years (FANCY): a prospective cohort study. Pediatric nephrology (Berlin, Germany). 2017;32(6):1067–76. [DOI] [PubMed] [Google Scholar]
- 47.Nishizaki N, Hirano D, Nishizaki Y, Fujinaga S, Nagata S, Ohtomo Y, et al. Increased urinary angiotensinogen is an effective marker of chronic renal impairment in very low birth weight children. Clinical and experimental nephrology. 2014;18(4):642–8. [DOI] [PubMed] [Google Scholar]
- 48.Kwinta P, Klimek M, Drozdz D, Grudzien A, Jagla M, Zasada M, et al. Assessment of long-term renal complications in extremely low birth weight children. Pediatric nephrology (Berlin, Germany). 2011;26(7):1095–103. [DOI] [PMC free article] [PubMed] [Google Scholar]


