Abstract
Background:
Various, often conflicting, estimates for post-operative morbidity and mortality following ALPPS have been reported in the literature, suggesting that considerable center-level variation exists. Some of this variation may be related to center volume and experience over time.
Methods:
Using data from seventeen centers who were early adopters of the ALPPS technique, we estimated the variation, by center, in standardized 90-day mortality and comprehensive complication index (CCI) for patients treated between 2012 to 2018.
Results:
We estimated that center-specific 90-day mortality following treatment with ALPPS varied from 4.2% (95% CI: 0.8, 9.9) to 29.1% (95% CI: 13.9, 50.9), and that center-specific CCI following treatment with ALPPS varied from 17.0 (95% CI: 7.5, 26.5) to 49.8 (95% CI: 38.1, 61.8). Declines in estimated 90-day mortality and CCI were observed over time, and almost all individual centers followed this trend. Patients treated at centers with a higher number of ALPPS cases performed over the prior year had a lower risk of post-operative mortality.
Discussion:
Despite considerable center-level variation in ALPPS outcomes, perioperative outcomes following ALPPS have improved over time and treatment at higher volume centers results in a lower risk of 90-day mortality. Morbidity and mortality remain concerningly high at some centers.
Introduction
Conflicting experiences and opinions have generated controversy within the hepatobiliary community about associating liver partition and portal vein ligation for staged hepatectomy (ALPPS)1. In single and multicenter case series, institutions have reported a wide range of morbidity and mortality estimates following ALPPS which has created confusion regarding the value of ALPPS2–11. While it seems intuitive that treatment at high volume hepatobiliary centers would be preferable to treatment at less experienced centers12, the extent of the variation in outcomes has not been characterized.
Compared to conventional two-staged hepatectomy with portal vein embolization/ligation, ALPPS increases the feasibility of second-stage hepatectomy completion at the cost of higher perioperative morbidity and mortality13,14. Estimates of the risk of perioperative morbidity following ALPPS has varied considerably, with some centers reporting high morbidity5–7, and others reporting minimal severe morbidity and zero perioperative mortality2,3,10,11. Multi-center and registry-based studies have estimated the 90-day mortality following ALPPS for any indication to be 9–17%8,15,16.
Considerable variation in clinical outcomes from hospital to hospital following ALPPS is not particularly surprising as it has been observed following a variety of operations, ranging in technical difficulty from appendectomy to complex thoracic and hepatobiliary surgery17–23. It is thought that hospital level variation can be partly explained by differences in patient characteristics from hospital to hospital, and by an inverse relationship between hospital volume and perioperative morbidity and mortality24–27.
In this study we use data from the international ALPPS registry to estimate the range of possible perioperative outcomes, the expected improvements over time, and the role that ALPPS volume plays in influencing patient outcomes.
Methods
We used the ALPPS registry to identify ‘early-adopting’ ALPPS centers. Specifically, we defined early-adopting centers as those which performed and recorded 3 or more cases prior to January 1st, 2013 in the ALPPS registry. Since the first formal report of ALPPS was in April 2011 28, this definition means that ‘early-adopting’ centers are those who performed and reported an average of approximately one ALPPS every six months since formal introduction of the procedure. The study was restricted to early-adopting centers to ensure that the centers would have an adequate number of cases for estimation of the center-specific trends in ALPPS outcomes over several years.
The ALPPS registry is maintained by the Department of Surgery, University of Zurich, Switzerland, approved by the Cantonal Ethics Committee of Zurich (KEK 2013–0326) and is registered at ClinicalTrials.gov (NCT01924741). Since contributing cases to the ALPPS registry is voluntary, analyses based on ALPPS registry data rely on compliance with data submission. Unfortunately, in many cases, covariate and follow-up information is incomplete (42.5% of patients do not have information on 90-day mortality). As such, to avoid selection bias due to incomplete data submission to the registry, all ‘early-adopting’ centers were contacted to update the registry by i) providing data on all ALPPS cases performed at their center since adoption of the ALPPS technique, including cases not already entered in the registry, ii) providing more comprehensive information on post-operative complications, and iii) completing follow-up data for all patients up to 90 days post surgery. This ensured that all centers included in our study had reported all ALPPS cases performed at their center, with complete (100%) covariate and follow-up information. Only anonymized data was used by the study analysts.
We used the updated ALPPS registry data for ‘early-adopting’ centers to estimate i) center-level variation in complications and 90-day mortality following ALPPS, ii) overall and center-specific time trends in complications and 90-day mortality following ALPPS, and iii) the relationship between center ALPPS procedure volume and ALPPS outcomes. We defined 90-day mortality as death due to any cause within 90 days of completion of ALPPS stage-1, and used the comprehensive complication index (CCI)29 to summarize the severity of post-operative complications. The comprehensive complication index is a continuous measure, bounded between 0 and 100, which is calculated as the sum of all complications for a given patient, weighted by the complication severity. As such, rather than ignoring complication severity, or considering only the most severe post-operative complication, the complication index captures the overall morbidity incurred by a given patient in the post-operative period. The index was calculated using all complications that occurred after stage-1 of ALPPS. The surgical teams at participating centers contributed information on all perioperative complications following ALPPS, and this information was used to compute the CCI, as described by Slankamenac et al29, for all patients. We analyzed all ALPPS cases (i.e. none were excluded) performed between 2012 and 2018 at the ‘early-adopting’ centers included in our study. The collaboration between centers in data sharing ensured no missing data.
We used a Bayesian hierarchical logistic model with minimally informative priors to estimate the standardized center variation in risk of 90-day mortality. The model included terms for baseline covariates (listed below) to adjust for center differences in case selection. Since no prior studies have estimated center-variation in ALPPS outcomes, minimally informative priors were used to produce results that depend essentially on the data alone. To describe the center-level variation in 90-day mortality, we computed the median odds ratio which is defined as the median value of the odds ratio between the center at higher risk and the center at lower risk when randomly picking two centers30,31. Markov chain Monte Carlo modeling (with 4 chains, 20 000 iterations burn-in and 20 000 saved iterations per chain) was used to derive effect estimates, posterior probabilities of 90-day mortality, and 95% credible intervals. Similarly, we used a Bayesian hierarchical logistic model to estimate the risk of 90-day mortality following ALPPS over time with a flexible function of time, in months, (restricted cubic splines with four knots) and, to adjust for differences in case selection by center or over time, the model included baseline patient covariates. Random center-level intercepts and slopes allowed for center-level variation in the association between 90-day mortality and time (month and year) of surgery. To estimate the risk of 90-day mortality for patients undergoing surgery at centers with various levels of ALPPS volume, we fit a logistic model with a flexible function of center-volume (restricted cubic splines with four knots) and baseline patient covariates. For a given patient undergoing ALPPS, center-volume was defined as the number of ALPPS cases performed at the treating center in the year prior to the patient’s surgery. For this analysis, we computed 95% confidence intervals using percentiles of a non-parametric bootstrap with 1000 iterations. Otherwise identical linear models were used to estimate the center-level variation in mean complication index, the mean comprehensive complication index by year and center, and the relationship between center-volume and mean complication index.
To adjust for differences in patient characteristics over time or differences in patient selection among centers, the previously described models included terms for the following baseline covariates: BMI, age at stage 1, future liver remnant (FLR) size prior to stage 1, tumor type (colorectal liver metastases, primary liver or biliary tumors, or other), and the following individual comorbidities: diabetes, myocardial infarction, heart failure, peripheral vascular disease, stroke, chronic kidney disease, peptic ulcer disease. All continuous baseline covariates (i.e. BMI, age, FLR size) were flexibly modelled using restricted cubic splines with four knots. Then, for each individual, under each level of the exposure (e.g. treatment center, center volume in the past year) the model was used to predict the CCI or probability of 90-day mortality. The predicted CCIs or 90-day mortality probabilities were then averaged over all individuals to yield an estimate that is standardized to the study population having a distribution of baseline covariates described in Table 1. As such, all estimates in the study were adjusted for baseline risk and indication.
Table 1:
Baseline and treatment characteristics for 500 patients undergoing ALPPS
| Age (y), median (IQR) | 61 (52 – 69) |
| Height (m), median (IQR) | 1.7 (1.64 – 1.77) |
| Weight (kg), median (IQR) | 73 (64 – 83) |
| BMI (kg/m2), median (IQR) | 24.8 (22.8 – 27.6) |
| Standardized pre-stage 1 future liver remnant size, median (IQR) | 0.21 (0.16 – 0.26) |
| Non-white | 13 (2.6) |
| Other | 100 (20.0) |
| Diabetes (%) | 51 (10.2) |
| Coronary artery disease (%) | 23 (4.6) |
| Heart failure (%) | 12 (2.4) |
| Peripheral vascular disease (%) | 22 (4.4) |
| Stroke (%) | 17 (3.4) |
| COPD | 21 (4.2) |
| Chronic kidney disease (%) | 11 (2.2) |
| Peptic ulcer disease (%) | 23 (4.6) |
| Laparoscopic | 34 (6.8) |
| Partial/mini | 201 (40.2) |
Subgroup analysis
Because estimates of perioperative CCI and 90-day mortality following ALPPS will appear high if many patients in the cohort have primary hepatobiliary tumors, we also estimated the overall and center-specific perioperative outcomes in the subgroup of patients with colorectal liver metastases, as well as the relationship between ALPPS volume and perioperative outcomes among patients with colorectal liver metastases.
All analyses were conducted using R version 3.5.2. For Bayesian hierarchical modelling, brms32 was used to run Stan33.
Results
Seventeen ‘early-adopting’ ALPPS centers performed a total of 500 cases between 2012 and 2018. Individual centers performed a median of 29 cases over the time period (IQR: 18 – 39). Baseline and treatment characteristics for the 500 individuals are summarized in Table 1. Stage 2 of ALPPS was completed in 474 patients (94.8%). The group of surgeons who performed the ALPPS procedures at each included center performed, per year, a median of 165 (IQR: 110 – 299) liver surgery cases, including a median of 80 (IQR: 30 – 110) minor liver resections, 50 (IQR: 25 – 80) major liver resections, 65 (IQR: 44 – 76) liver transplants at 8 (47%) centers which perform liver transplant. A median of 2 surgeons (IQR 1–3) performed ALPPS at each center. The overall 90-day mortality in the cohort was 13.4%, but was 8.0% in the 286-patient subset with colorectal liver metastases. The average CCI in the cohort was 32.5, but was 28.0 in the subset with colorectal liver metastases. Average center-level yearly number of liver surgery cases performed was weakly correlated with center average comprehensive complication index (r=−0.19) and 90-day mortality (r=−0.07) in correlational analysis that was unadjusted for baseline patient characteristics (see the following paragraphs for estimates of the relationship between ALPPS volume and outcome with adjustment for baseline covariates).
We estimated that the 90-day mortality for treatment at a particular center, adjusted for baseline covariates by standardizing to the distribution of baseline covariates in Table 1, varied from 4.2% (95% CI: 0.8, 9.9) to 29.1% (95% CI: 13.9, 50.9), and that the CCI for treatment at a particular center, similarly adjusted, varied from 17.0 (95% CI: 7.5, 26.5) to 49.8 (95% CI: 38.1, 61.8). The median odds ratio for 90-day mortality was 3.1 (95% CI: 1.6–6.9). The estimated risk of 90-day mortality and CCI at each of the 17 centers is displayed in Figure 1.
Figure 1 -.
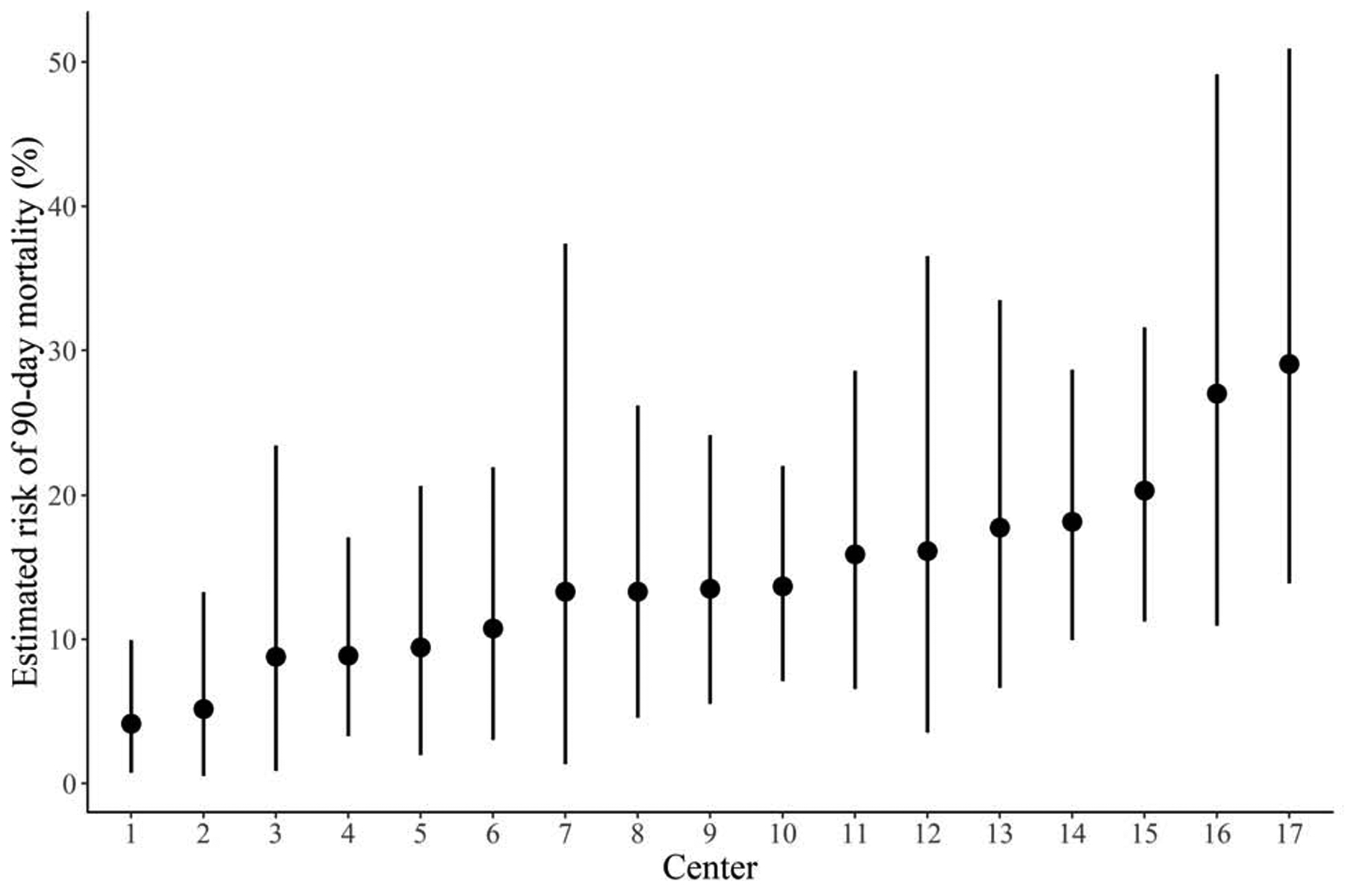
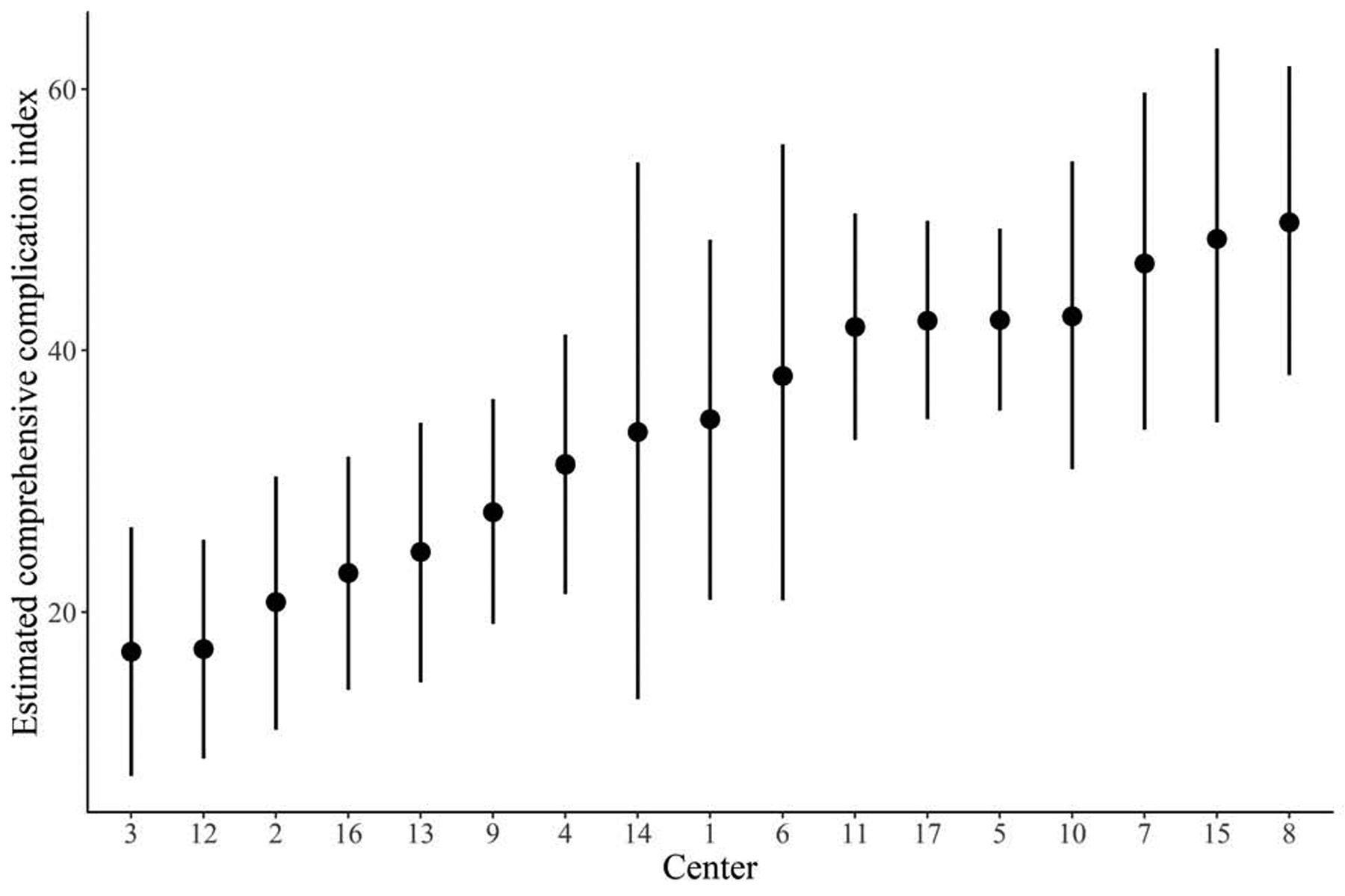
Estimated risk of 90-day mortality (A) and estimated comprehensive complication index (B) at each of the 17 centers over the entire study period, with 95% credible intervals
The estimated risk of 90-day mortality for treatment with ALPPS in 2018, adjusted for baseline covariates by standardizing to the distribution of baseline covariates in Table 1, was 9.2% (95% CI: 4.5, 15.5) compared to 17.9% (95% CI: 8.8, 29.7) in 2012. The estimated CCI for treatment with ALPPS in 2012, similarly adjusted, was 27.0 (95% CI: 20.4, 33.7) compared to 35.9 (95% CI: 27.3, 44.4) in 2018. The estimated relationship between 90-day mortality and CCI for treatment with ALPPS over the study period, varying by center, is displayed in Figure 2.
Figure 2 -.
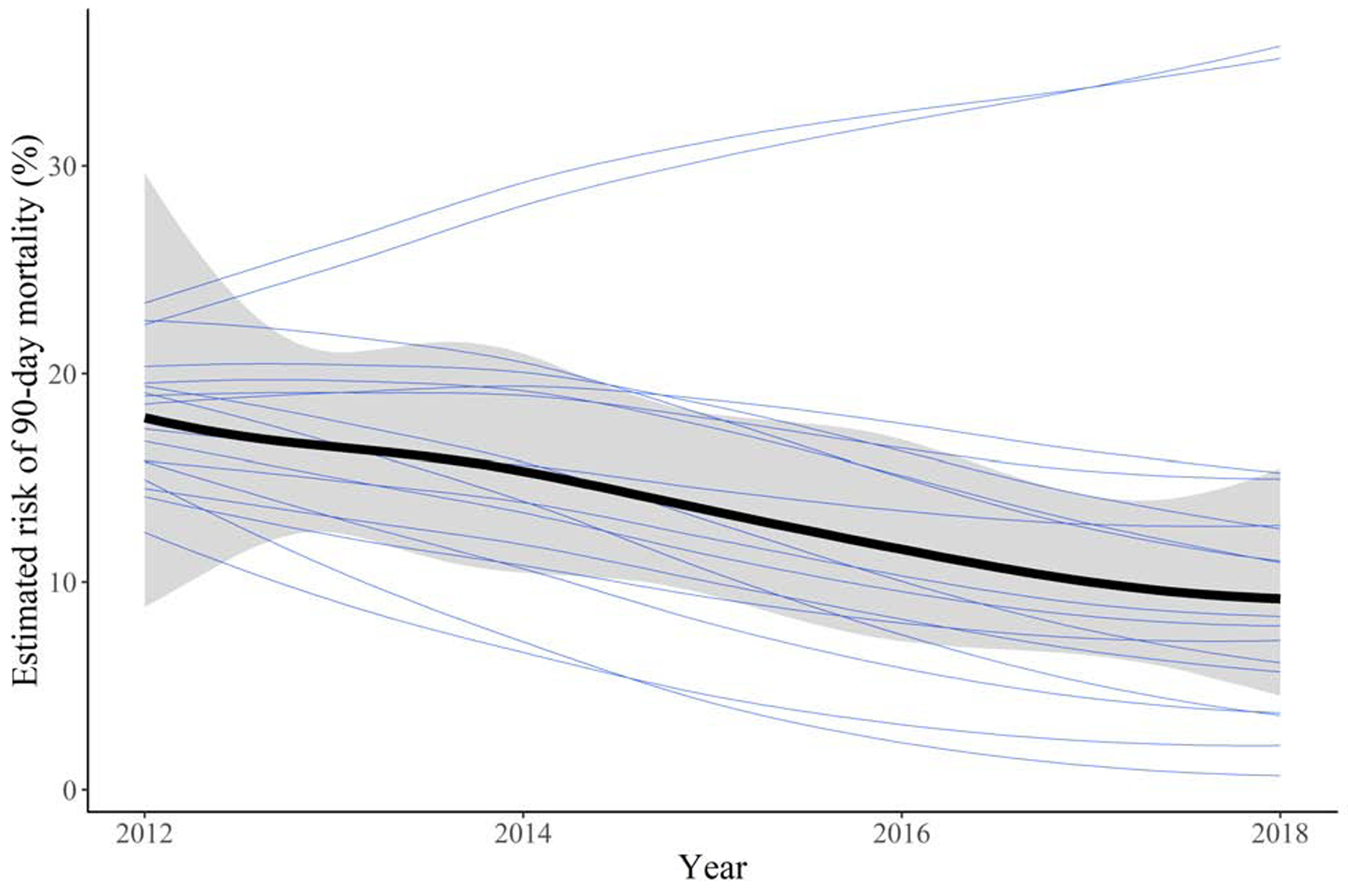
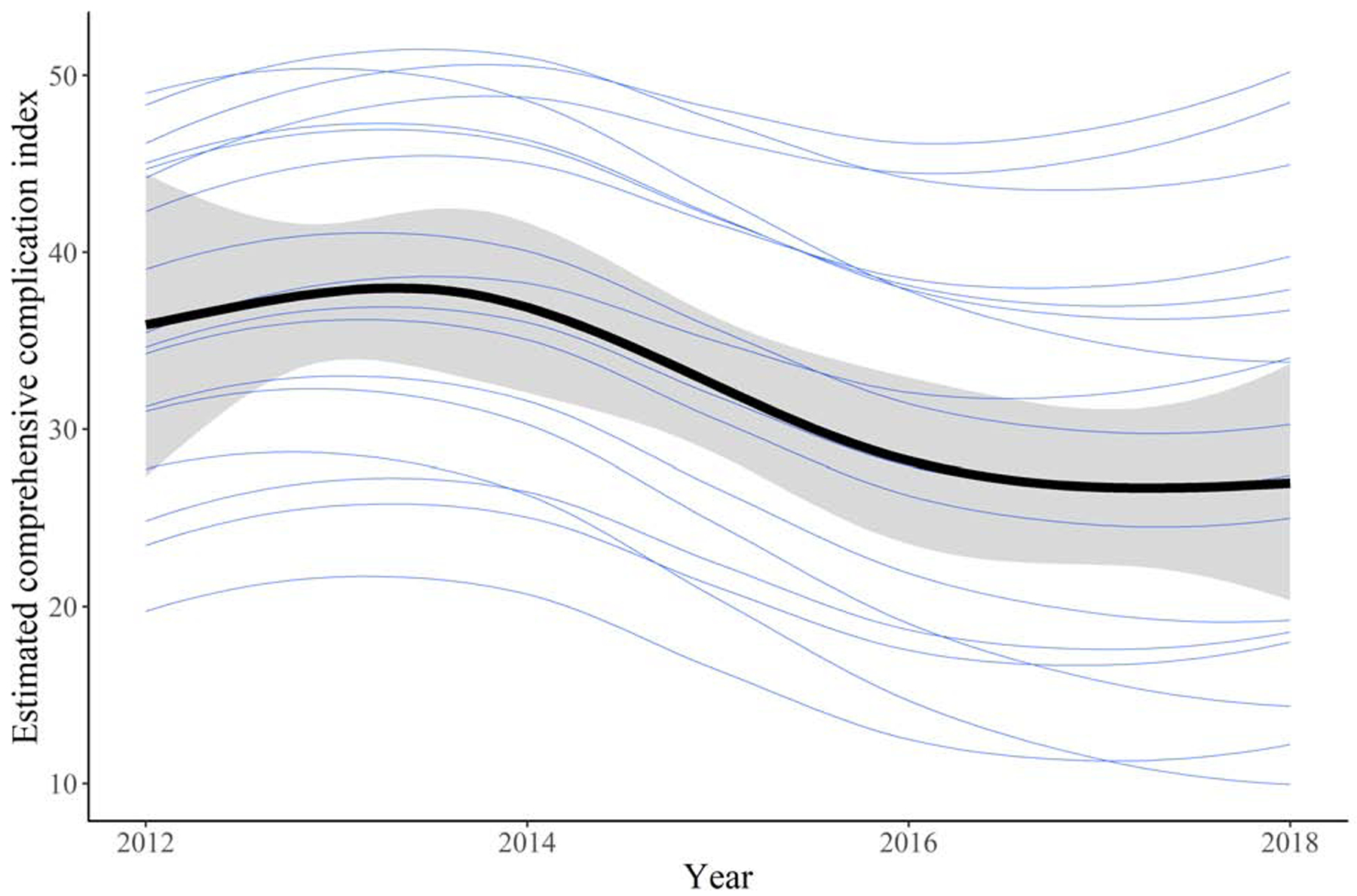
Estimate of the relationship between month and year of treatment and 90-day mortality (A) or comprehensive complication index (B), varying by center. The thicker line represents the overall trend and its 95% credible interval, and each of the other lines represents the individual centers.
The estimated risk of 90-day mortality was 17.5% (95% CI: 7.5, 31.7) for treatment at centers which performed no ALPPS cases in the prior year, 13.7% (95% CI: 8.2, 20.1) for treatment at centers which performed 10 ALPPS cases in the prior year, and 10.1% (95% CI: 1.7, 30.9) for treatment at centers which performed 20 ALPPS cases in the prior year after adjustment for baseline covariates by standardizing the estimate to the distribution of baseline covariates in Table 1. Improved CCI was also observed but this trend plateaued and slightly increased at higher levels of ALPPS volume. The estimates of the association between center-volume and CCI, and between center-volume and 90-day mortality are summarized in Table 2 and displayed in Figure 3.
Table 2:
Estimated risk of 90-day mortality and estimated comprehensive complication index following treatment at centers with various levels of ALPPS volume in the prior year
| 0 cases in the prior year | 17.5% (95% CI: 7.5, 31.7) | 38.8 (95% CI: 29.6, 49.1) |
| 5 cases in the prior year | 12.5% (95% CI: 8.1, 17.3) | 30.7 (95% CI: 26.5, 35.0) |
| 10 cases in the prior year | 13.7% (95% CI: 8.2, 20.1) | 32.4 (95% CI: 27.0, 38.3) |
| 15 cases in the prior year | 12.0% (95% CI: 5.6, 21.0) | 33.2 (95% CI: 25.9, 41.2) |
| 20 cases in the prior year | 10.1% (95% CI: 1.7, 30.9) | 33.9 (95% CI: 16.4, 52.0) |
Figure 3 -.
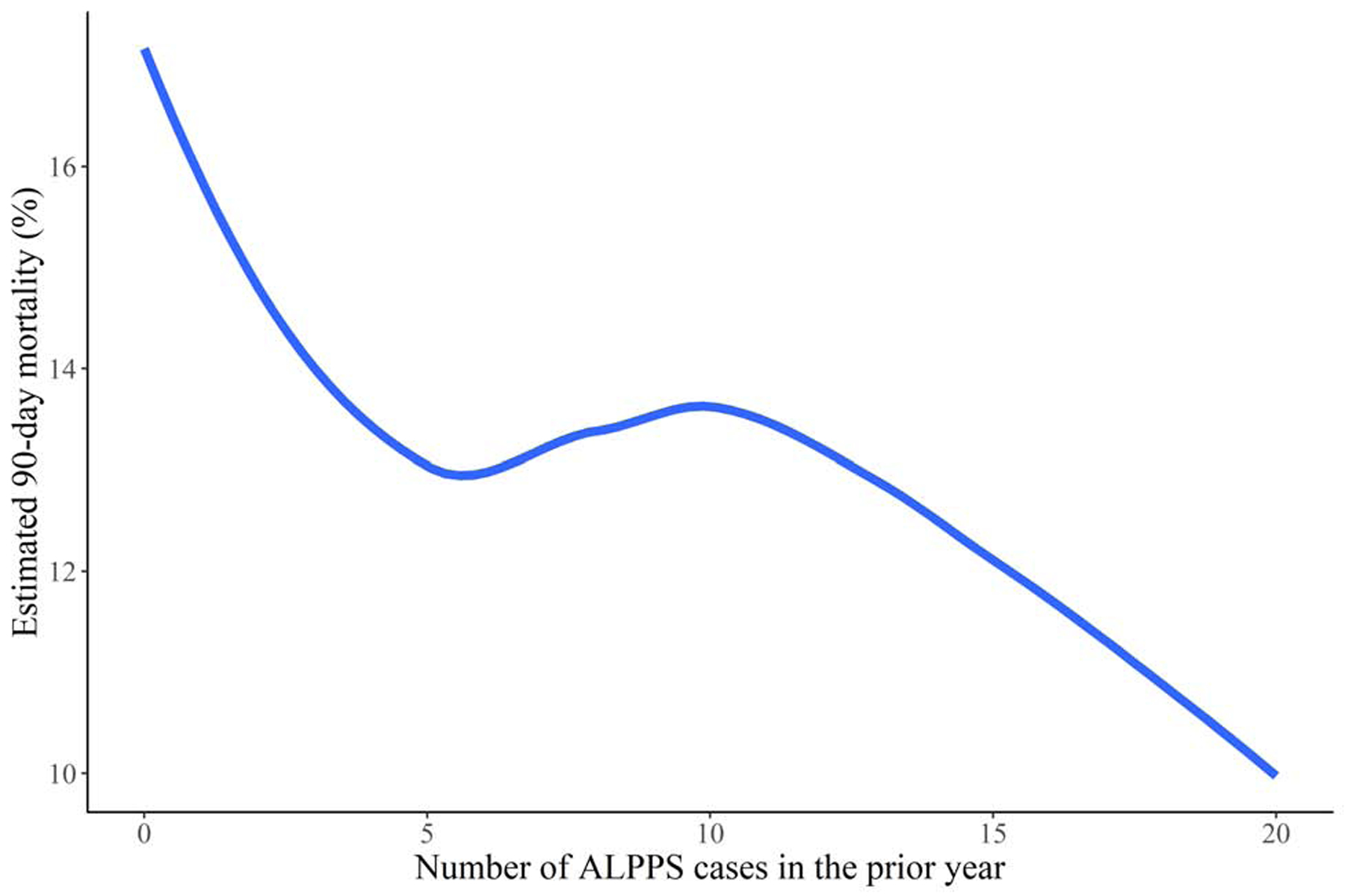
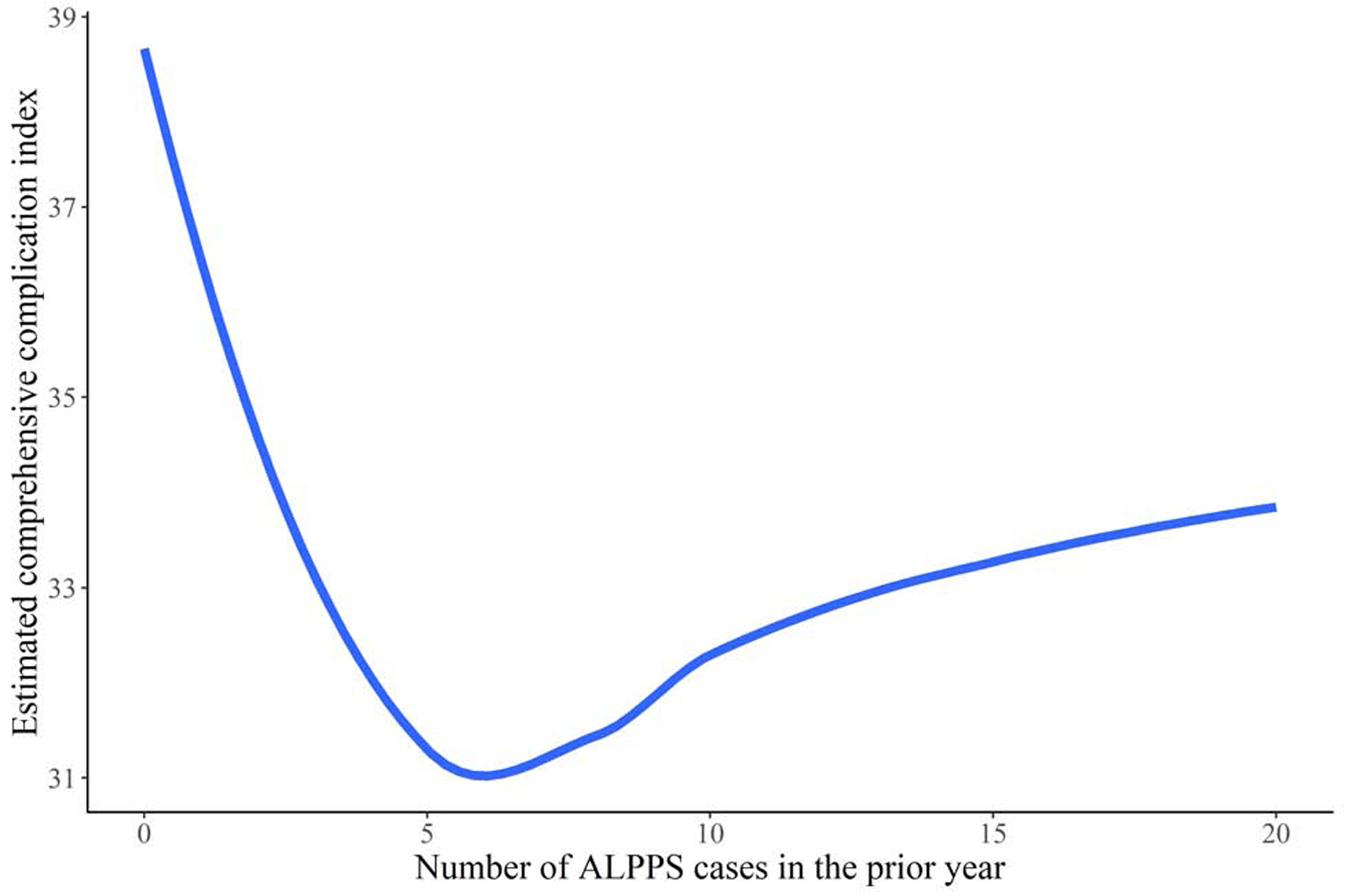
Estimated risk of 90-day mortality (A) and comprehensive complication index (B) for patients undergoing ALPPS at centers with various levels of ALPPS volume over the prior year
Subgroup analysis – perioperative outcomes for patients with colorectal liver metastases
Among patients with colorectal liver metastases, we estimated that the 90-day mortality for treatment at a particular center varied from 2.2% (95% CI: 0.3, 6.1) to 21.0% (95% CI: 7.5, 42.8), and that the CCI for treatment at a particular center varied from 12.0 (95% CI: 2.5, 21.2) to 44.9 (95% CI: 33.0, 56.8). Among patients with colorectal liver metastases, the estimated risk of 90-day mortality was 10.9% (95% CI: 6.8, 32.4) for treatment at centers which performed no ALPPS cases in the prior year, 8.2% (95% CI: 8.4, 17.5) for treatment at centers which performed 10 ALPPS cases in the prior year, and 5.7% (95% CI: 2.1, 32.0) for treatment at centers which performed 20 ALPPS cases in the prior year.
Discussion
Using data from the first multicenter cohort on ALPPS with complete outcome data from seventeen diverse ALPPS centers, including 500 cases over six years, we estimated that treatment at some centers (the best performing ones) resulted in considerably lower 90-day mortality and fewer complications than treatment at others (the worst performing ones). Moreover, we estimated that treatment at centers with a higher volume of ALPPS cases within the past year resulted in a lower risk of mortality. Centers have reported better outcomes following ALPPS in recent years compared to earlier years, and almost all centers (except two of 17) have shown a trend, over time, towards a reduced risk of 90-day mortality and reduced complications following ALPPS even accounting for changes in patient selection. Perioperative morbidity and mortality are high for indications other than colorectal liver metastases, and is concerningly high at some centers.
The LIGRO trial, which was conducted at Scandinavian centers with experience in complex liver surgery, and ALPPS in particular, estimated that the risk of major complications and 90-day mortality is similar when well-selected patients (i.e. stable disease on chemotherapy and no major comorbidities) with a future liver remnant/standardized total liver volume ratio of <30% are treated with ALPPS compared to when they are treated with two-staged hepatectomy; but, that treatment with ALPPS improves the rate of second-stage hepatectomy completion34. The latter finding is unsurprising since classical two-staged hepatectomy cannot be completed in approximately 30% of cases due to inadequate FLR growth, or, more commonly, tumor progression during the inter-stage period35,36.
In contrast, the former finding from the LIGRO trial, that ALPPS does not result in a higher complication or mortality rate compared to two-staged hepatectomy is unexpected because, during the first several years of ALPPS adoption, various case series were published reporting high morbidity and mortality4–7, with larger, multi-center and registry based, studies also raising concerns about perioperative outcomes 8,15,16. However, much lower perioperative morbidity and mortality rates were reported by institutions at which ALPPS was generally only offered to patients with colorectal liver metastases2,3,9–11.
More recently, due to more careful patient selection – particularly, exclusion of patients with primary hepatobiliary cancers – the initially high morbidity and mortality has appeared to improve over time16,37. But, beyond refinements in patient selection, some have proposed that a learning curve likely exists for ALPPS, as it does for other complex hepatobiliary operations38–40. Our estimates suggest that, even accounting for differences in patient selection over time or across centers and differences in surgical approach, morbidity and mortality outcomes following ALPPS have improved considerably. This supports the hypothesis that short-term post-operative ALPPS outcomes can be expected to improve over time at centers which adopt the technique. While more data on long-term outcomes following ALPPS is still forthcoming, initial results in patients with colorectal liver metastases have been encouraging41,42.
Our estimates also provide evidence that, despite improvements over time, considerable variation in perioperative outcomes exists between centers and two centers had estimated morbidity and mortality that increased over time. While considerable hospital variation in clinical outcomes has been observed following a variety of operations, much of the literature evaluating hospital level variation has focused on the inverse effect of hospital volume on perioperative complications and mortality24–27 and less work has been dedicated to describing the overall hospital to hospital variation in adverse perioperative outcomes. For ALPPS, we estimated that treatment at centers who performed a higher number of ALPPS cases within the prior year resulted in more favorable post-operative outcomes compared to treatment at centers with fewer ALPPS cases. In the absence of more granular data on overall liver surgery volume, we did not consider whether treatment at centers who performed a higher number of all liver cases had a similar effect, although it is likely that centers with a high volume of complex liver surgery develop expertise which can be applied to ALPPS.
Interestingly, center-specific 90-day mortality did not correlate perfectly with center-specific morbidity, and while mortality decreased considerably with higher levels of ALPPS volume, the same relationship was not observed for CCI. This suggests that reductions in mortality may be possible even if overall morbidity remains relatively high.
While we standardized our estimates over the distribution of baseline patient covariates to account for differences in patient selection criteria and surgical approach, it is possible that data on some important covariates was unmeasured. This would mean that our findings might be partially explained away by differences in patient prognostic characteristics rather than improvements in technique and perioperative management over time or between centers. Further, our study relied on accurate data from the included centers. Measurement error, particularly differences in complication recording from center to center, may have impacted the analysis if present. Specifically, while all centers used the same complication severity classification system (in order to facilitate calculation of the CCI), inter-rater reliability is expected to be imperfect for complication recording. Additionally, for some estimates, uncertainty intervals were wide since some centers performed a limited number of ALPPS cases. Lastly, our estimates were obtained using data from ‘early-adopting’ centers interested in contributing to the ALPPS registry. If these centers are not representative of non-contributing centers, then estimates from this study will not be transportable to other settings.
However, while other studies reporting outcomes following ALPPS have been criticized for having a highly selected cohort of patients, in this study, inclusion of a diverse set of ALPPS-performing centers with a wide range of outcomes yields outcome estimates that are more generalizable. Moreover, cooperation between seventeen centers to provide up to date and complete comprehensive data on all ALPPS cases conducted at their institutions reduced the risk of bias due to missing data or measurement error which has been present in prior multi-institutional ALPPS studies in which patients with missing outcome data have been excluded.
Conclusions
Perioperative outcomes following ALPPS vary considerably from center to center, even among early-adopting centers, with some centers performing ALPPS with very high morbidity and mortality. ALPPS outcomes generally improve over time, although concerningly, not every center followed this trend. Treatment at centers with higher recent ALPPS volume results in a lower risk of 90-day mortality than treatment at lower volume centers, and the outcomes of the best performing centers suggest that ALPPS can be performed effectively by experienced surgical teams. While morbidity and mortality following ALPPS for indications besides colorectal liver metastases remains concerningly high, ALPPS for colorectal liver metastases can be performed with acceptable perioperative outcomes at some centers. Improved outcomes over time for almost all included centers imply that there exists a learning curve, as is thought to exist with other complex hepatobiliary operations, and that centers who adopt the ALPPS technique can expect reduced morbidity and mortality over time.
Acknowledgments
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under award number F32 AG064831-01 (Arin L. Madenci).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olthof PB, Schnitzbauer AA, Schadde E. The HPB controversy of the decade: 2007–2017 – Ten years of ALPPS. Eur J Surg Oncol. 2018;44:1624–1627. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez-Alejandro R, Bertens KA, Pineda-Solis K, et al. Can we improve the morbidity and mortality associated with the associating liver partition with portal vein ligation for staged hepatectomy (ALPPS) procedure in the management of colorectal liver metastases? Surgery. 2015;157:194–201. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez FA, Ardiles V, de Santibañes M, et al. Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy Offers High Oncological Feasibility With Adequate Patient Safety: A Prospective Study at a Single Center. Ann Surg. 2015;261:723–732. [DOI] [PubMed] [Google Scholar]
- 4.Ratti F, Cipriani F, Gagliano A, et al. Defining indications to ALPPS procedure: technical aspects and open issues. Updat Surg. 2013;66:41–49. [DOI] [PubMed] [Google Scholar]
- 5.Torres OJM, Fernandes E de SM, Oliveira CVC, et al. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): the Brazilian experience. Arq Bras Cir Dig. 2013;26:40–43. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Girotti P, Königsrainer I, et al. ALPPS in Right Trisectionectomy: a Safe Procedure to Avoid Postoperative Liver Failure? J Gastrointest Surg. 2013;17:956–61. [DOI] [PubMed] [Google Scholar]
- 7.Nadalin S, Capobianco I, Li J, Girotti P, Königsrainer I, Königsrainer A. Indications and limits for associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). Lessons Learned from 15 cases at a single centre. Z Gastroenterol. 2014;52:35–42. [DOI] [PubMed] [Google Scholar]
- 8.Schadde E, Ardiles V, Slankamenac K, et al. ALPPS Offers a Better Chance of Complete Resection in Patients with Primarily Unresectable Liver Tumors Compared with Conventional-Staged Hepatectomies: Results of a Multicenter Analysis. World J Surg. 2014;38:1510–1519. [DOI] [PubMed] [Google Scholar]
- 9.Sala S, Ardiles V, Ulla M, et al. Our initial experience with ALPPS technique: encouraging results. Updat Surg. 2012;64:167–172. [DOI] [PubMed] [Google Scholar]
- 10.Oldhafer KJ, Donati M, Jenner RM, et al. ALPPS for Patients with Colorectal Liver Metastases: Effective Liver Hypertrophy, but Early Tumor Recurrence. World J Surg. 2013;38:1504–1509. [DOI] [PubMed] [Google Scholar]
- 11.Røsok BI, Björnsson B, Sparrelid E, et al. Scandinavian multicenter study on the safety and feasibility of the associating liver partition and portal vein ligation for staged hepatectomy procedure. Surgery. 2016;159:1279–1286. [DOI] [PubMed] [Google Scholar]
- 12.Reames BN, Ghaferi AA, Birkmeyer JD, et al. Hospital Volume and Operative Mortality in the Modern Era. Ann Surg. 2014;260:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eshmuminov D, Raptis DA, Linecker M, et al. Meta-analysis of associating liver partition with portal vein ligation and portal vein occlusion for two-stage hepatectomy: ALPPS versus portal vein occlusion for staged hepatectomy. Br J Surg. 2016;103:1768–1782. [DOI] [PubMed] [Google Scholar]
- 14.Moris D, Ronnekleiv-Kelly S, Kostakis ID, et al. Operative Results and Oncologic Outcomes of Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS) Versus Two-Stage Hepatectomy (TSH) in Patients with Unresectable Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. World J Surg 2018;42:806–815. [DOI] [PubMed] [Google Scholar]
- 15.Schadde E, Ardiles V, Robles-Campos R, et al. Early Survival and Safety of ALPPS: First Report of the International ALPPS Registry. Ann Surg. 2014;260:829–838. [DOI] [PubMed] [Google Scholar]
- 16.Linecker M, Björnsson B, Stavrou GA, et al. Risk Adjustment in ALPPS Is Associated With a Dramatic Decrease in Early Mortality and Morbidity: Ann Surg. 2017;266:779–786. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins PC, Oerline MK, Mullard AJ, et al. Hospital variation in outcomes following appendectomy in a regional quality improvement program. Am J Surg. 2016;212:857–862. [DOI] [PubMed] [Google Scholar]
- 18.Desai A, Bekelis K, Ball PA, et al. Variation in Outcomes Across Centers After Surgery for Lumbar Stenosis and Degenerative Spondylolisthesis in the Spine Patient Outcomes Research Trial. Spine. 2013;38:678–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng Z, Hanna N, Onukwugha E, et al. Hospital Center Effect for Laparoscopic Colectomy Among Elderly Stage I-III Colon Cancer Patients: Ann Surg. 2014;259:924–929. [DOI] [PubMed] [Google Scholar]
- 20.Spolverato G, Ejaz A, Hyder O, et al. Failure to rescue as a source of variation in hospital mortality after hepatic surgery: Failure to rescue after liver resection. Br J Surg. 2014;101:836–846. [DOI] [PubMed] [Google Scholar]
- 21.Thomas J, Hanby A, Pinder SE, et al. Adverse surgical outcomes in screen-detected ductal carcinoma in situ of the breast. Eur J Cancer. 2014;50:1880–1890. [DOI] [PubMed] [Google Scholar]
- 22.Vinocur JM, Menk JS, Connett J, et al. Surgical Volume and Center Effects on Early Mortality After Pediatric Cardiac Surgery: 25-Year North American Experience From a Multi-institutional Registry. Pediatr Cardiol. 2013;34:1226–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs JP, O’Brien SM, Pasquali SK, et al. Variation in Outcomes for Risk-Stratified Pediatric Cardiac Surgical Operations: An Analysis of the STS Congenital Heart Surgery Database. Ann Thorac Surg. 2012;94:564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richardson AJ, Pang TCY, Johnston E, et al. The Volume Effect in Liver Surgery—A Systematic Review and Meta-analysis. J Gastrointest Surg. 2013;17:1984–1996. [DOI] [PubMed] [Google Scholar]
- 25.Hata T, Motoi F, Ishida M, et al. Effect of Hospital Volume on Surgical Outcomes After Pancreaticoduodenectomy: A Systematic Review and Meta-analysis. Ann Surg. 2016;263:664–672. [DOI] [PubMed] [Google Scholar]
- 26.Birkmeyer JD, Siewers AE, Finlayson EVA, et al. Hospital Volume and Surgical Mortality in the United States. N Engl J Med. 2002;346:1128–1137. [DOI] [PubMed] [Google Scholar]
- 27.LaRiviere CA, McAteer JP, Huaco JA, et al. Outcomes in pediatric surgery by hospital volume: a population-based comparison. Pediatr Surg Int. 2013;29:561–570. [DOI] [PubMed] [Google Scholar]
- 28.Baumgart J, Lang S, Lang H. A new method for induction of liver hypertrophy prior to right trisectionectomy: a report of three cases. HPB (Oxford). 2011;13(suppl 2):1–145. HPB (Oxford). [Google Scholar]
- 29.Slankamenac K, Graf R, Barkun J, et al. The Comprehensive Complication Index: A Novel Continuous Scale to Measure Surgical Morbidity. Ann Surg. 2013;258:1–7. [DOI] [PubMed] [Google Scholar]
- 30.Larsen K. Appropriate Assessment of Neighborhood Effects on Individual Health: Integrating Random and Fixed Effects in Multilevel Logistic Regression. Am J Epidemiol. 2005;161:81–88. [DOI] [PubMed] [Google Scholar]
- 31.Merlo J. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bürkner P-C. Advanced Bayesian Multilevel Modeling with the R Package brms. R J. 2018;10:395. [Google Scholar]
- 33.Carpenter B, Gelman A, Hoffman MD, Lee D, Goodrich B, Betancourt M, et al. Stan : A Probabilistic Programming Language. J Stat Softw [Internet]. 2017. [cited 2019 Jul 7];76 Available from: http://www.jstatsoft.org/v76/i01/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandström P, Røsok BI, Sparrelid E, et al. ALPPS Improves Resectability Compared With Conventional Two-stage Hepatectomy in Patients With Advanced Colorectal Liver Metastasis: Results From a Scandinavian Multicenter Randomized Controlled Trial (LIGRO Trial). Ann Surg. 2018;267:833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lam VWT, Laurence JM, Johnston E, et al. A systematic review of two-stage hepatectomy in patients with initially unresectable colorectal liver metastases. HPB. 2013;15:483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chua TC, Liauw W, Chu F, et al. Summary outcomes of two-stage resection for advanced colorectal liver metastases: Two-Stage Resection of CLM. J Surg Oncol. 2013;107:211–216. [DOI] [PubMed] [Google Scholar]
- 37.Oldhafer KJ, Stavrou GA, van Gulik TM. ALPPS—Where Do We Stand, Where Do We Go?: Eight Recommendations From the First International Expert Meeting. Ann Surg. 2016;263:839–841. [DOI] [PubMed] [Google Scholar]
- 38.Kluger MD, Vigano L, Barroso R, et al. The learning curve in laparoscopic major liver resection. J Hepato-Biliary-Pancreat Sci. 2013;20:131–136. [DOI] [PubMed] [Google Scholar]
- 39.Vigano L, Laurent A, Tayar C, et al. The Learning Curve in Laparoscopic Liver Resection: Improved Feasibility and Reproducibility. Ann Surg. 2009;250:772–782. [DOI] [PubMed] [Google Scholar]
- 40.Fisher WE, Hodges SE, Wu M-F, et al. Assessment of the learning curve for pancreaticoduodenectomy. Am J Surg. 2012;203:684–690. [DOI] [PubMed] [Google Scholar]
- 41.Björnsson B, Sparrelid E, Røsok B, et al. Associating liver partition and portal vein ligation for staged hepatectomy in patients with colorectal liver metastases – Intermediate oncological results. Eur J Surg Oncol. 2016;42:531–537. [DOI] [PubMed] [Google Scholar]
- 42.Wanis KN, Ardiles V, Alvarez FA, et al. Intermediate-term survival and quality of life outcomes in patients with advanced colorectal liver metastases undergoing associating liver partition and portal vein ligation for staged hepatectomy. Surgery. 2018;163:691–697. [DOI] [PubMed] [Google Scholar]


