Abstract
The germline is unique. It is able to perform unique jobs (meiosis), is selected for genetic changes, and it is immortal (or at least its genome is). Part of being this special also means that entry into the germline club is restricted and cells of the soma are always left out. However, recent evidence from multiple animals now suggests that somatic cells may join the club and become germline cells in an animal when the original germline is removed. This “violation” may have garnered acceptance by the observation that iPScells, originating experimentally from somatic cells of an adult, can form reproductively successful eggs and sperm, all in vitro. Each of the genes and their functions used to induce pluripotentiality are found normally in the cell and the in vitro conditions to direct germline commitment replicates conditions in vivo. Here we discuss evidence from three different animals, an ascidian, a segmented worm, and a sea urchin, that cells of a somatic cell lineage can convert to the germline in vivo. We discuss the consequences of such transitions, and provide thoughts as to how this process may have equal precision to the original germline formation of an embryo.
Keywords: Soma, germline, germ cell, cell fate conversion
Heritability in animals is limited to a select few cells, the germ cells. These cells are usually specified early in development, proceed through a proscribed process of migration, expansion, and eventually they begin meiosis and function in fertilization (Gilbert, 2016). Although cells of the soma die with the individual – for example the nerves, skin, and heart - the germline process is cyclic and its genome is immortalized by passage to the next generation (Figure 1; (McLaren, 1981; Weissmann, 1893)). How the germline cells are selected, directed, and specified in the embryo is a process of great divergence throughout evolution (C. G. Extavour & Akam, 2003; Seydoux & Braun, 2006). Some animals use cell signaling to specify the heritable cohort whereas other animals have localized materials in their eggs that direct the receiving cells to a fate of the germline. Historically, researchers have posited that selecting the germline in early development meant excluding somatic cells from entering this lineage, which would otherwise result in diverse genetic origins of the germline (L. Buss, 1987). Perhaps homogenous origins meant decreased cell competition, increased fidelity, and increased control over their development resulting in more viable offspring. The philosophy of importance of the germline, and its relationship to somatic cells is perhaps best paraphrased by Buss (L. W. Buss, 1983) from the work of August Weismann in 1893 (Weissmann, 1893): “Heritability is solely the province of the “germ plasm” whereas the soma is simply a mortal vessel of no evolutionary significance.” While it can be argued that the soma – wings, claws, noses, and pigment - is significant, if not essential for survival, selection, and mating, the importance of the germline in evolutionary change cannot be debated.
Figure 1:
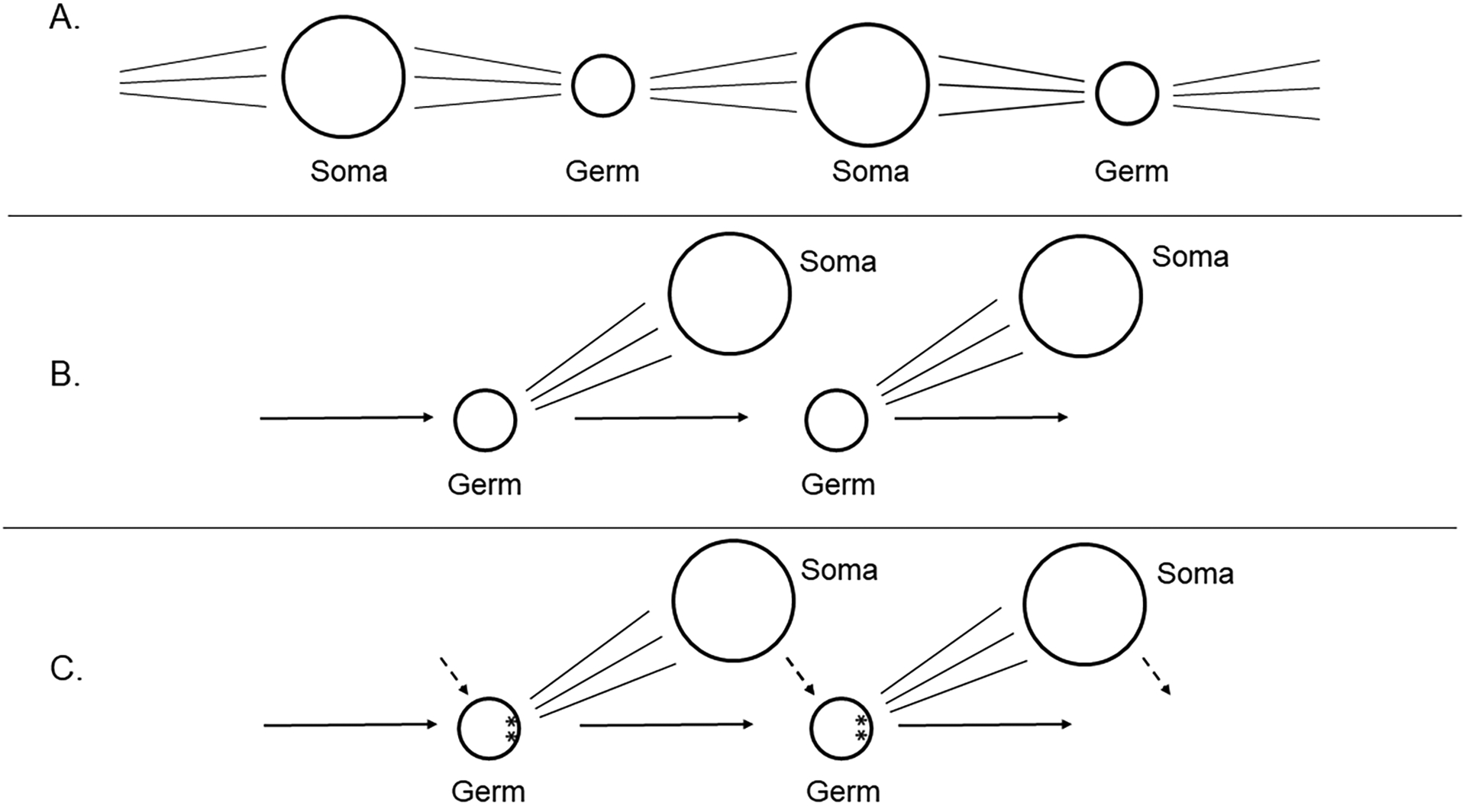
A. Darwin’s concept of transgenerational germ cells
B. Weismann’s concept of soma divergence from the germline to eliminate somatic cell contribution to heritable cells (Weismann, 1893).
C. Some germlines lead to localized information in the egg that is asymmetric and that directs germline formation in those cells that acquire it. The soma however does impact the epigenetics of the germline (dotted line), and thereby has an effect on the germline, even though it is not a direct DNA sequence contribution.
D. McLaren (McLaren, 1981) posited the epiblast cell concept in which, in contrast to C, the germline comes from one of any cells within a pluripotential cell population, all of which are capable of becoming soma or germline. McLaren further emphasized the strong influence of the soma on the germline through epigenetic mechanisms (dotted line).
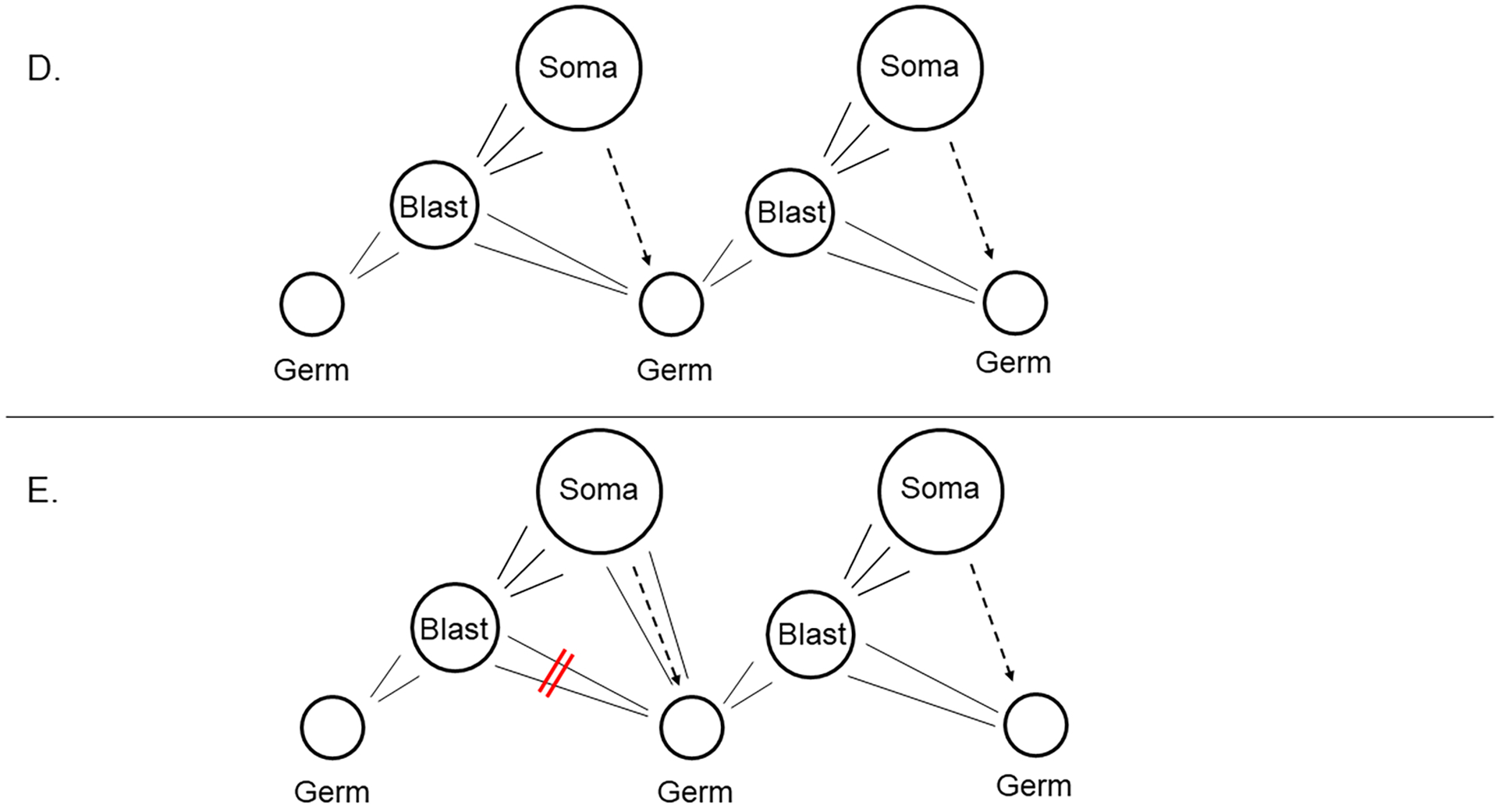
E. Were blast cells blocked from becoming germ cells, or if the precursor cells to the germ cells were lost, a replacement phenomenon from soma to germline is seen in some animals.
F. Buss (L. W. Buss, 1983) emphasized consideration of somatic mutations, which if the soma did give rise to a germ cell normally, or to germ cells in a replacement/conversion phenomena, the germ cells and subsequent offspring would be genetically distinct.
Weismann emphasized the concept of divergence in cell fates within the soma that would exclude those cells from the germline (Weismann, 1893). This segregation is important because once the somatic cell lineages separate developmentally from the germ cell lineages, the genome of the somatic cells can no longer be inherited by the next generation. Thereafter, mutations occurring in the “exposed” somatic lineages are not passed on to the offspring, whereas inheritance of the genome resulting only from the “protected” germline may result in increased fitness (L. Buss, 1987). One argument for selection of the germline early in development is to regulate it distinctly from the adjacent soma and to minimize errors in DNA replication by minimizing the number of cell replications for the germline. This is a reasonable argument for, say a female human, who will make “only” about 7 million oogonia (Baker, 1971). But, for some animals that make even larger numbers of sperm/eggs, the argument is not convincing. Consider a human testis, which makes roughly 1000 sperm cells per every heartbeat for decades in the male-heritable cell (Amann & Howards, 1980). Here, prolonged, rapid cell divisions are standard, whereas most somatic cells never reach such a production rate. Reducing cell divisions in the germline early in the embryo only makes sense if the soma has significantly higher rates of mutation. No evidence, though, is reported for differences in error rates per cell division between soma and germline in the embryo, although adult male germline errors may be somewhat less than in adult male somatic cells (Drake, Charlesworth, Charlesworth, & Crow, 1998).
Alternative arguments for segregating the germline early in development are to limit the signaling pathway(s) impinging on the germline cells, and to synchronize gametogenic progression with the season for nutritional availability. In some respects the early germline cells are “time capsules” – by virtue of their pluripotentiality for making gametes and most other cell types, and strict control may be advantageous to minimize aberrant growth elsewhere in the organism. This essential strategy would be enhanced also were all the germline cells homogeneous in origin, and not from a variety of converts, including somatic cell conversions. Indeed, competition between early germ cells has been observed (C. Extavour & Garcia-Bellido, 2001) and is counter to fitness.
Can loss of germline cells in an organism, however, result in a new germline converting from a normally non-germline, perhaps somatic source? Here we examine three animal models in which the embryo, larva, and/or adult is capable of making a new germline following loss of the canonical germ cell lineage. This is followed by speculation as to how this lineage replacement is achieved.
Many recent reviews are available that discuss the mechanisms of germline specification in animal embryos, and the reader is directed to them for understanding this part of germline functionality (for example (Kobayashi & Surani, 2018; Makar & Sasaki, 2019; Zhang & Chambers, 2019)). Here, we will summarize these concepts only in so much as needed to contextualize mechanisms of converting to a germline. We will resist here also discussing the germline in for example Hydra and Planaria. These are remarkably regenerative animals capable of restoring full adult functionality, including the germline, from a small number of cells derived from an adult (Reddien, 2018; Tanaka & Reddien, 2011). Adding to these amazing phenomena are plants in which a single protoplast from an adult can give rise to a fully functional, and reproductively competent, adult (Steward, 1970). A recent monograph reviews the concept of germlines in plants (Lanfear, 2018) and this theme is not considered further here.
Instead, we emphasize here a conversion process in which the vast majority of the adult, larva, or embryo is intact, and within which a new lineage for the germline forms from what otherwise would have become somatic tissue. Were somatic cell mutation significant in these organisms, then the offspring of animals from somatic cells converted to germ cells would necessarily be genetically distinct from the original germline that was replaced by the somatic cells (Figure 1F). In this discussion, we are not referring to making new gametes seasonally, in which an animal has no evidence of gametes for part of the year, although this could be useful to consider when examining reproductive control synchronized with nutrition, mating season etc. Instead, the question addressed here is loss of the germline – followed by replacement with a new germline. With the advent of iPScells from which fibroblasts are able to make eggs and sperm in vitro, germline cells can come from a non-germline origin without change in the sequence of DNA of the cell (K. Hayashi, Hikabe, Obata, & Hirao, 2017; Takahashi & Yamanaka, 2006). It should be noted that iPSc-derived gametes are poorly successful (Hikabe et al., 2016). Is this because of genetic or epigenetic alterations that make them less successful? Or are the culture conditions less than optimal environmental influences on the cells? We may learn more from animals that are capable normally of re-establishing a new germline from otherwise a somatic cell lineage.
We wrestled with the terminology of the process examined herein, whereby a new germline is made following depletion of the original germline. We considered the terms germline regeneration, replacement, recovery, re-specification, trans-specification, and many other less scientific terms. None of these terms, though, reflected accurately the process that occurs in the diverse organisms so far revealing this mechanism. We also considered the term transfating to refer to this process. The term transfating (or transfate) is also used, for example, when the primary mesenchyme cells of the sea urchin embryo are removed, and are followed by conversion of a distinct mesodermal lineage to acquire a skeletogenic fate that it would normally not have (Ettensohn & McClay, 1988). Transfating however, does imply having one differentiated cell fate that changes to a distinct differentiated fate. Currently, however, we do not know the extent of commitment, or of differentiation, of the non-germline lineage at the time when it becomes the new germline. Ultimately, we settled on the terminology of “somatic cell to germline conversion” to explicitly emphasize the key features of origin (soma), the resulting fate (germline), and the process (conversion).
Somatic cell to Germ cell lineage conversion in the solitary ascidian, Ciona robusta.
The solitary ascidian, Ciona robusta (formerly known as Ciona intestinalis type A), is known to be capable of recovering from germline loss by re-specifying new germline cells (Takamura, Fujimura, & Yamaguchi, 2002). In Ciona robusta, the original germline cells are specified during embryogenesis by incorporating the post-plasm, a specialized cytoplasm localized at the posterior pole of early embryos. The post plasm accumulates a variety of maternal RNAs including an evolutionarily conserved germline gene Vasa, which is partitioned into the posterior-most blastomeres called B7.6 cells at the 64-cell stage (M. Shirae-Kurabayashi et al., 2006; Yamada, 2006; S. Yoshida, Marikawa, & Satoh, 1996). Since the B7.6 cells inherit the post plasm, these cells had been thought to be the primordial germ cells (PGCs). In 2006, Shirae-Kurabayashi et al. (M. Shirae-Kurabayashi et al., 2006) examined whether the B7.6 cells are bona fide PGCs by lineage tracing experiments by injecting fluorescent dye into the newly formed B7.6 cells in combination with immunostaining for Vasa protein. During gastrulation, B7.6 cells underdo asymmetric cell division to produce two types of daughter cells: the B8.11 cells, and the B8.12 cells (Figure 2). Among them, only the B8.12 cells maintain the high Vasa protein expression and undergo cell divisions to produce 8–16 descendants. These B8.12 cell descendants are finally associated with the primordial gonad in the juvenile. On the other hand, B8.11 cells lose Vasa protein expression, perhaps lose their nucleus, and associate with the hindgut near the anus. These findings strongly suggest that B8.12 cells are bona fide PGCs formed in a post-plasm dependent manner. However, it has been reported that ascidian larvae in which the B8.12 cell descendants are removed can form a gonad containing Vasa-positive cells at later stages (Takamura et al., 2002). These investigators surgically removed 60–75% of the larval tail containing B8.12 cell descendants and found that the tail-cut larvae metamorphose into morphologically normal juvenile lacking Vasa-positive cells. Several days after metamorphosis, however, a few Vasa-positive cells reappeared, and the tail-cut animals formed functional gametes within the gonad (Maki Shirae-Kurabayashi & Nakamura, 2018; Takamura et al., 2002; K. Yoshida et al., 2017). These observations suggest that Ciona robusta can replace its germline with – most likely – a somatic lineage. The results also suggest that this animal retains a mode of germline specification involving cell signaling mechanisms (inductive specification), in addition to the acquired mechanism used in the early embryo. The mechanism of germline formation by cell interactions (induction) appears to be the ancestral mechanism of germline formation in embryos, so perhaps these animals have retained an ancient program that is reactivated upon loss of the germline formed by a derived, inherited mechanism. This series of events appears to have commonality with the other organisms known to go through germline conversions.
Figure 2:
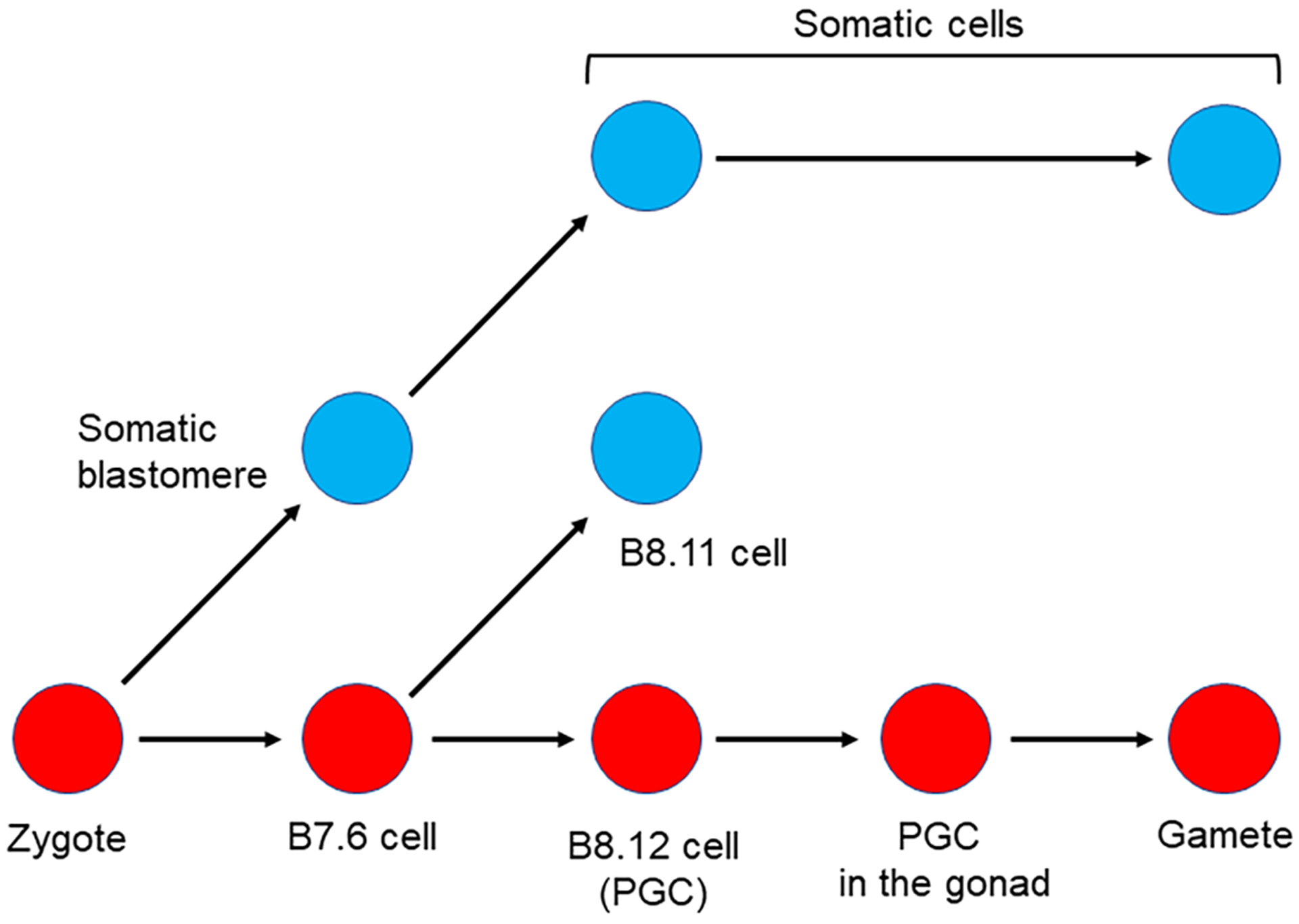
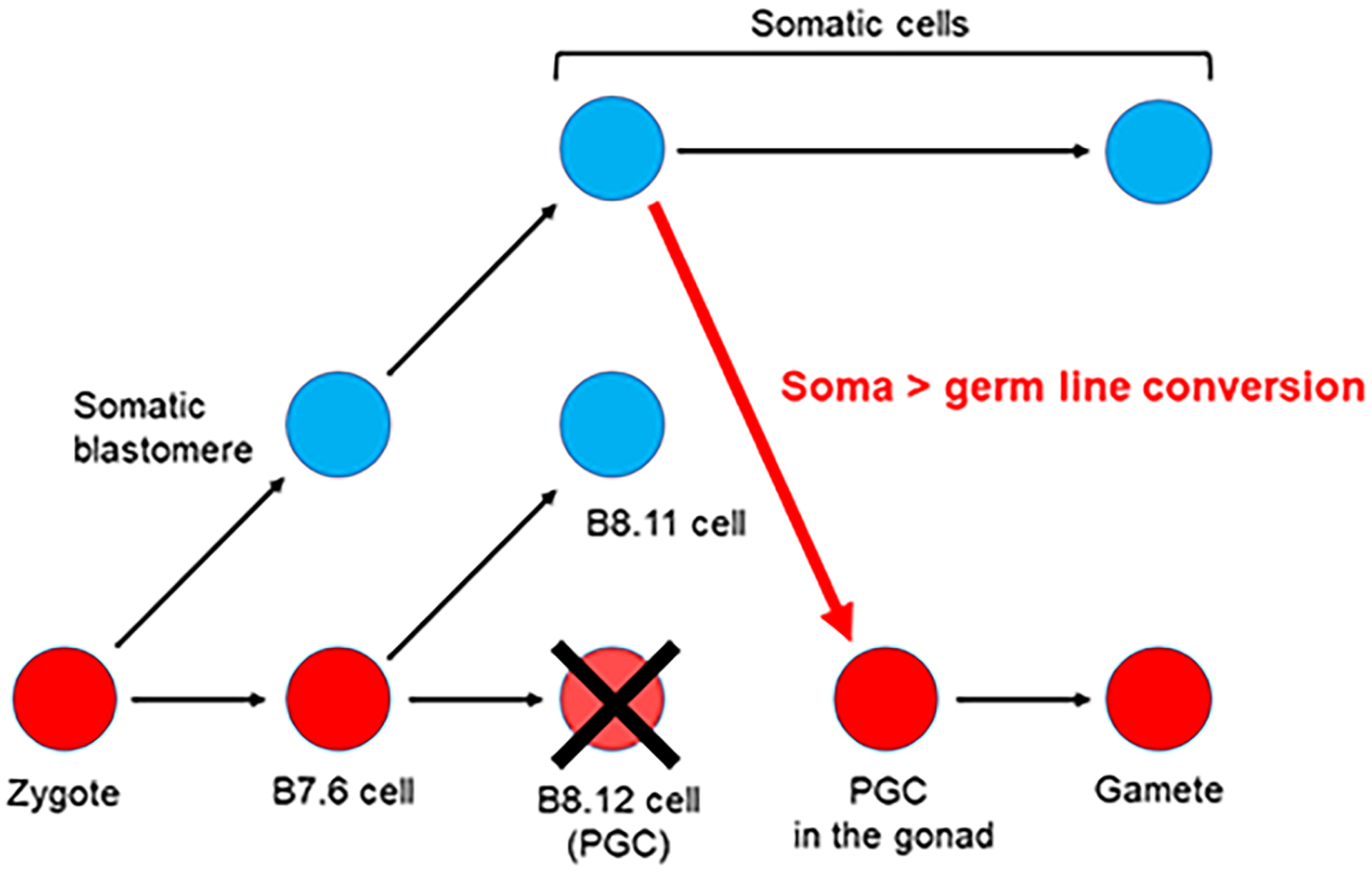
Somatic cell to Germ cell conversion in the ascidian Ciona robusta.
Germline cells (red circles) and somatic cells (blue circles) are segregated during the gastrula stage when B7.6 cells undergo asymmetric division to produce B8.12 and B8.11 cells. The B8.12 cells migrate into the gonad and differentiate into gametes. If tissues including the B8.12 descendants are surgically removed during larval stages, some somatic cells presumably convert (red arrow), thereby enabling recovery from loss of the original germline cells, B8.12 cells (Dannenberg & Seaver, 2018; Maki Shirae-Kurabayashi & Nakamura, 2018).
Many unanswered questions remain regarding somatic cell conversion to a germ cell lineage in Ciona robusta. These include for example, what are the molecular mechanisms by which the somatic cells are re-specified as new germline cells? What is the source, the cell type, of the new germline cells? A stem cell population? Or a dedifferentiated somatic cell? Further, how is the conversion triggered? Is it only by removal of the original germline cells? Or is it by a more general wounding process? In 2017, Yoshida et al. (K. Yoshida et al., 2017) provided important clues for understanding the conversion. They reported an efficient and convenient TALEN-mediated mutagenesis by electroporating TALEN expression vectors instead of injecting TALEN mRNA into the animal. Generally, it is difficult to induce germline mutations by electroporation of TALEN expression vectors due to the lack of promoters that can effectively drive the expression in germline cells. To overcome this difficulty, Yoshida et al. induced mutations into somatic cells by making use of TALEN expression vectors under the control of tissue-specific promoters, and then removed germline cells to trigger the re-specification of new germline cells from these mutated somatic cells (K. Yoshida et al., 2017). Surprisingly, germline mutations were efficiently induced when the TALEN expression was driven by promoters specific for epidermis, neural cells, or muscle cells, implying that these somatic cell types, or a stem cell contributing to these somatic cells, can contribute to the somatic cell conversion to a germline. Moreover, although germline mutagenesis efficiency was decreased, the germline mutations were detected even if the original germline cells were intact. Therefore, it is plausible that the converting mechanism occurs without the removal of the original germline cells. Does this mean that Ciona robusta has two distinct germline specification mechanisms enhancing proper germline development and successful reproduction? Is this a case of evolutionary strategy in shifting germline determination mechanisms? The above information provides important insights for a better understanding of somatic cell to germ cell conversion in other animals and the evolutionary relationship between two distinct modes of germline specification.
Somatic cell to Germ cell lineage conversion in the polychaete Capitella teleta.
Recent studies showed nicely that the polychaete annelid Capitella teleta was able to replace its germline after removal of the germline precursor (Dannenberg & Seaver, 2018; Meyer, Boyle, Martindale, & Seaver, 2010). C. teleta belongs to the superphylum of Lophotrochozoa in which most members utilize a mode of early embryogenesis called spiral cleavage, characterized by a series of asymmetric cell divisions. Spiralian embryos are particularly amenable to cell fate mapping; each cell can be identified based on their location, their size, and their time of birth. A fate map of C. teleta has been published (Meyer et al., 2010). One cell of particular importance is 4d (4 little dee) which generates the anus and the multipotent progenitor cells (MPCs), the stem cells that give rise to the germ cells and to somatic stem cells (Figure 3). These MPCs are identified by the presence of germline markers (Vasa, Piwi and Nanos) (Dill & Seaver, 2008; Giani, Yamaguchi, Boyle, & Seaver, 2011). They form a cluster that contains 8 to 20 germline cells at the late larval stage, 20 to 55 germline cells one week after metamorphosis, and 30 to 55 germline cells two weeks after metamorphosis. An adult C. teleta possesses about 75 germline cells in the MPC cluster [6]. During early embryogenesis though, the 4d cell is small and difficult to target, even for laser ablation. To test if the germline could be replaced in this animal, investigators decided instead to target its larger parent cell, 3D (3 large Dee). After laser deletion of this 3D cell, MPC clusters were detected in about 13% of the 9 day old larvae. Importantly, all the juveniles (two weeks post-metamorphosis) arising from embryos in which the 3D cell was deleted, replaced their germline. Most of the resulting adults (males, females and hermaphrodites), developed reproductive structures and produced offspring. These data provide the first evidence that C. teleta can fully replace its germline after removal of the embryonic germline precursor cell. Multiple somatic to germline conversion events seem to occur: first, a compensation in early development leading to MPC formation in a small number of larvae and a second event leading to a full replacement of the germline between the larval stage and the two week old juveniles.
Figure 3:
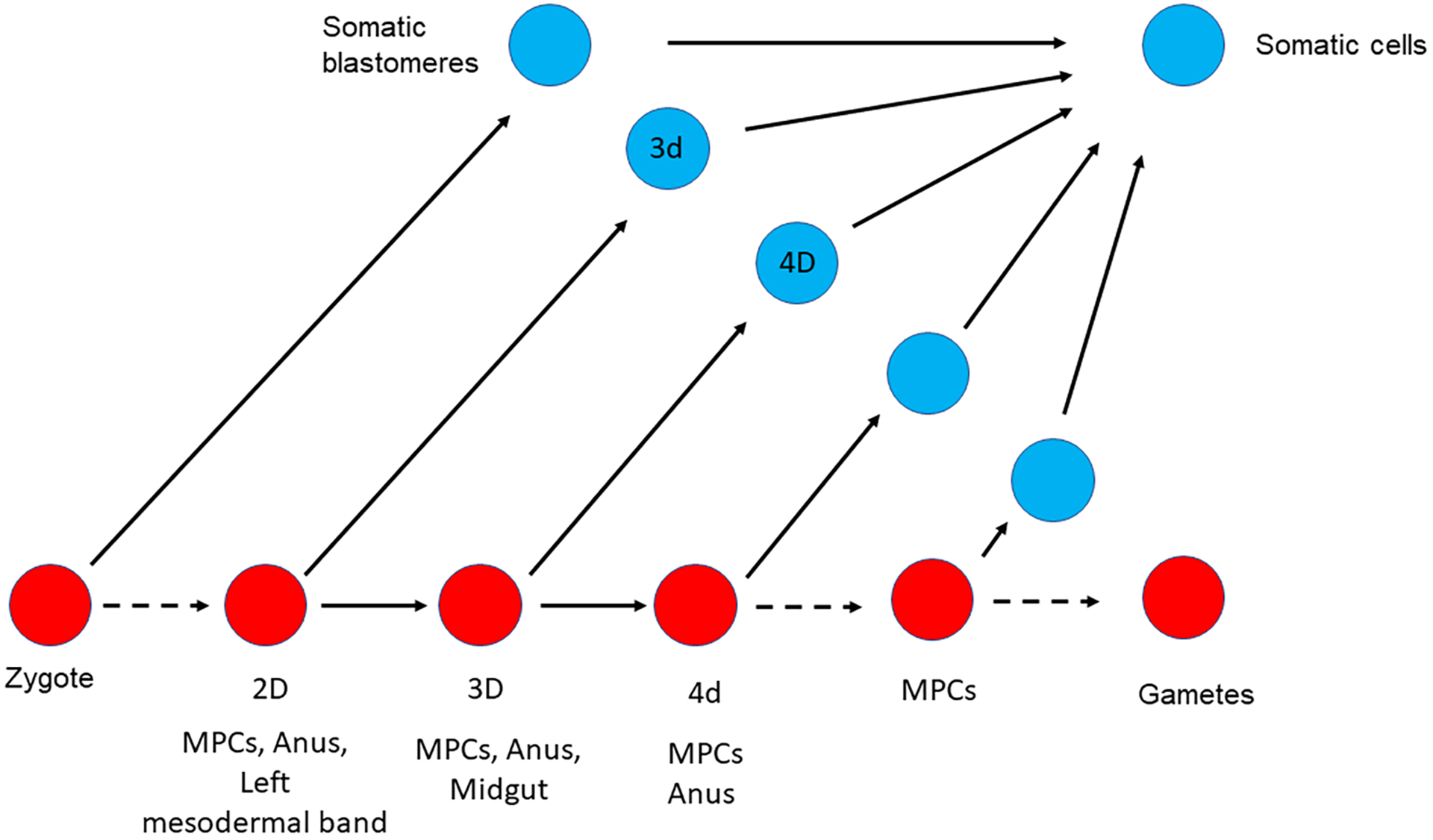

Somatic cell to Germ cell conversion in Capitella teleta.
Germline cells are represented with red circles, somatic cells are represented with blue circles. (A) Normal germline formation. (B) After laser deletion of either 3D, or 2D, some somatic cells presumably convert to form a new germline (Dannenberg & Seaver, 2018).
To identify the cell(s) involved in this conversion, the investigators next targeted by laser ablation the 2D cell, the parent of 3D. The lineage rising from the 2D cell gives rise to the MPCs, the anus, and the left mesodermal band. Larvae resulting from this 2D deletion did not form any MPCs but instead, showed a broad ectopic expression of nanos and vasa mRNA in the mesoderm. However, most of the resulting two week old juveniles had either multiple small MPC clusters (less than 25 cells per cluster) or multiple normal size clusters (30 to 55 cells) and did not retain any ectopic expression of Vasa in the mesoderm. These juveniles gave rise to fertile adults (males and females) suggesting that the ablation of the 2D cell during early embryogenesis is not sufficient to prevent the post metamorphosis germline conversion in this animal.
While more experiments will be required to fully identify the origin of the new germ cells in C.teleta, the best candidates are currently the left and the right mesodermal precursors, 3d and 3c respectively. Deletion of the cell 2D (precursor of both 4d and 3d) led to the absence of MPCs at the larva stage, suggesting that the cell 3d could be the origin of the new germline observed in the larvae after ablation of the cell 3D. However, later in development, most of the juveniles resulting from the 2D ablation had MPCs suggesting a different origin of the germline at this stage (potentially from the right mesodermal precursor 3c). Interestingly, the broad ectopic expression of Vasa mRNA observed after deletion of 2D (germline and left mesodermal precursors) was not observed after deletion of the cell 2C (parent of the right mesodermal precursor 3c), suggesting that the loss of a mesodermal precursor is not sufficient to induce this ectopic expression. This broad expression of Vasa results instead from the loss of both a mesodermal precursor and the germline. While the identity of the cells that form the new MPCs is still under investigation, it is interesting to note that this conversion event coincides with the approximate time when the animal is also able to regenerate its posterior end [6, 8], suggesting an important link to more than solely the germline. While the observations are strong, the mechanisms remain unknown.
Somatic cell to Germ cell lineage conversion in echinoderms.
Echinoderms are a diverse phylum of marine creatures characterized by pentameric body symmetry and a spiny epidermis (Hyman, 1955). The spines are as extreme as a foot long (the sea urchin Diadema antillarum), to small fuzzy spines (the sand dollar Dendraster execenticus), to mere nubbins (Patiria miniata and Apostichopus japonicas; sea star and sea cucumber, respectively). Most of the species develop to adulthood through a larval stage (S. purpuratus) whereas others develop directly through embryogenesis from a large (~0.5 mm) egg to a juvenile without a clear larval stage and without feeding (Heliocidaris erythrogramma) (Ettensohn, Wessel, & Wray, 2004).
The origins of the germline in echinoderms have been examined in a few species only, and tested in even fewer. Overall, the phylum appears to have mechanisms both of dependence on inductive cell interactions, and of localized information in the egg to specify the germline. Sea stars for example, appear to specify their germline by cell interactions that include asymmetric signaling by the TGF-β factor, Nodal. The sea star P. miniata (and its close relative P. pectinifera) makes a pouch off the invaginated endoderm late in gastrulation. This posterior, endodermal evagination pouch (posterior enterocoel; PE) is believed to be the source of the germline for the adult (Wessel, Fresques, Kiyomoto, Yajima, & Zazueta, 2014). This conclusion is based on two main experimental results. One is the selective accumulation of canonical germline factors (Vasa, Nanos, Piwi) in the PE. This result also has been tested and is similar in other echinoderms, including a sea cucumber and brittle star embryos (Inoue, 1992) – and suggests that most echinoderms use this type of germline morphology and mechanism (induction), to make the germline. The second line of support for the PE as the germline in sea stars comes from removal experiments. Microsurgical removal of the PE results in ~50% decrease in germline cells when measured late in larval development (Inoue, 1992). Does a 50% decrease in germ cell numbers mean there is a second source of the germline each contributing 50%? No accumulation of the germline factors has been reported in a second site in sea stars, but perhaps there are pre-germline cells yet to express such genes, but whose normal fate is the germline. Alternatively, perhaps the PE is the true, and sole source of germ cells and removal of the PE in early larvae results in a conversion mechanism from non-germline cells. Currently though, no transgenerational lineage tracing has been successful to test cell fates of the PE and the possible breadth of germline appearance in any echinoderm.
Germline factors in echinoderms show marked post-transcriptional regulation. In the sea star, Vasa and Nanos proteins are present throughout early development even though the mRNA of Nanos is not detectable after meiosis until early in gastrulation. At that time, a ring of both Vasa and Nanos mRNAs is prevalent around the blastopore and the invaginating gut. Late in gastrulation these factors become restricted to a tight circumferential band around the gut, and then are gradually restricted to the left side, into the forming PE (T. Fresques, Zazueta-Novoa, Reich, & Wessel, 2014; Juliano & Wessel, 2009). This latter restriction process appears to be a consequence of both decreased transcription and increased mRNA turnover, both resulting from right-sided Nodal signaling (T. M. Fresques & Wessel, 2018).
The germline factors in sea urchins use even more diverse regulatory steps. While Nanos2 mRNA and protein accumulate exclusively in the PGCs shortly after their appearance (Juliano et al., 2006; Voronina et al., 2008), much of this selectively appears to be post-transcriptional (Wessel, Brayboy, et al., 2014). Indeed, transcription appears to be promiscuous, being expressed in many cells, if not all cells of the early embryo. Through post-transcriptional degradation of the Nanos2 mRNA, and of the Nanos2 protein, the only detectable accumulation in the embryo is in the small micromeres, the PGCs, 4 cells resulting from two unequal cell divisions in the vegetal pole (Oulhen & Wessel, 2016). The mechanism for Nanos2 mRNA degradation is not known, but it does require a 400bp element within the 3’UTR of Nanos2, referred to as GNARLE (Global Nanos Associated RNA Lability Element) (Oulhen et al., 2013). Any RNA containing this element is degraded outside of the PGCs but retained within the PGCs. The protein also is degraded outside of the PGCs, and this selectivity appears to result from a 45 amino acid Nanos2 degron located just N-terminal of the zinc-finger domains (Oulhen & Wessel, 2016). This small peptide, when attached to another protein, will result in that protein also rapidly degrading throughout the embryo. Although the mechanism of recognition or degradation of the Nanos2 protein is not known, we do know it is not by conventional ubiquitylation. Mutating each of the 7-lysines in the Nanos2 protein does not affect protein retention. Thus, injecting an mRNA with GFP-linked to the Nanos2 open reading frame, and its 3’UTR containing GNARLE, results in selective retention of GFP in the PGCs (Oulhen & Wessel, 2016).
Vasa regulation is equally convoluted in this embryo. Vasa protein and mRNA are present in early embryos uniformly and with the birth of the PGCs at the 32 cell stage, the protein quickly becomes restricted to the PGCs (Voronina et al., 2008). The mRNA however, remains uniform throughout the embryo until the beginning of gastrulation. Thus, the embryo has contrasted expression with uniform maternal Vasa mRNA, but tightly restricted Vasa protein. Through several experimental approaches, we now conclude that Vasa mRNA is continuously translated throughout the embryo, but the protein is degraded in all cells except the PGCs (Gustafson, Yajima, Juliano, & Wessel, 2011). The mechanism of recognition and degradation is more clear than in the case of Nanos2. Vasa outside of the germline is recognized by the E3 ubiquitin ligase Gustavus [10, 11] and is rapidly degraded by proteasomes. A small segment at the N-terminus of Vasa appears necessary for the recognition, and/or ubiquitylation, directing the protein for degradation. Removal of this region results in accumulation of Vasa throughout the embryo (Gustafson et al., 2011). The theme for each of the germline factors thus is post-transcriptional, and even post-translational regulation. Perhaps this gives cells in the embryo poised abilities for a wider variety of developmental signals, transitions, and fates.
The germline of this animal can be replaced by cells during embryogenesis whose fate is normally somatic cells ((Voronina et al., 2008; Yajima & Wessel, 2011); Figure 4). The micromeres are the precursors of the small (PGCs) and large (skeleton) micromeres, resulting from asymmetric cleavage divisions. When the micromeres are removed at the 16 cell stage, the adult still makes a larval and adult skeleton, and the adult makes gametes. Even though the micromeres are such strong inducers of endomesoderm fate, these micromere-less embryos still gastrulate. Moreover, even though these animals lack their original PGCs, adults are fully gravid for both eggs and sperm (Ransick, Cameron, & Davidson, 1996; Yajima & Wessel, 2011). However, removal of the small micromeres, the PGCs that form at the 5th cell division, results in normal looking development and adults complete with a gonad, but no germ cells ((Yajima & Wessel, 2011);Figure 5).
Figure 4:
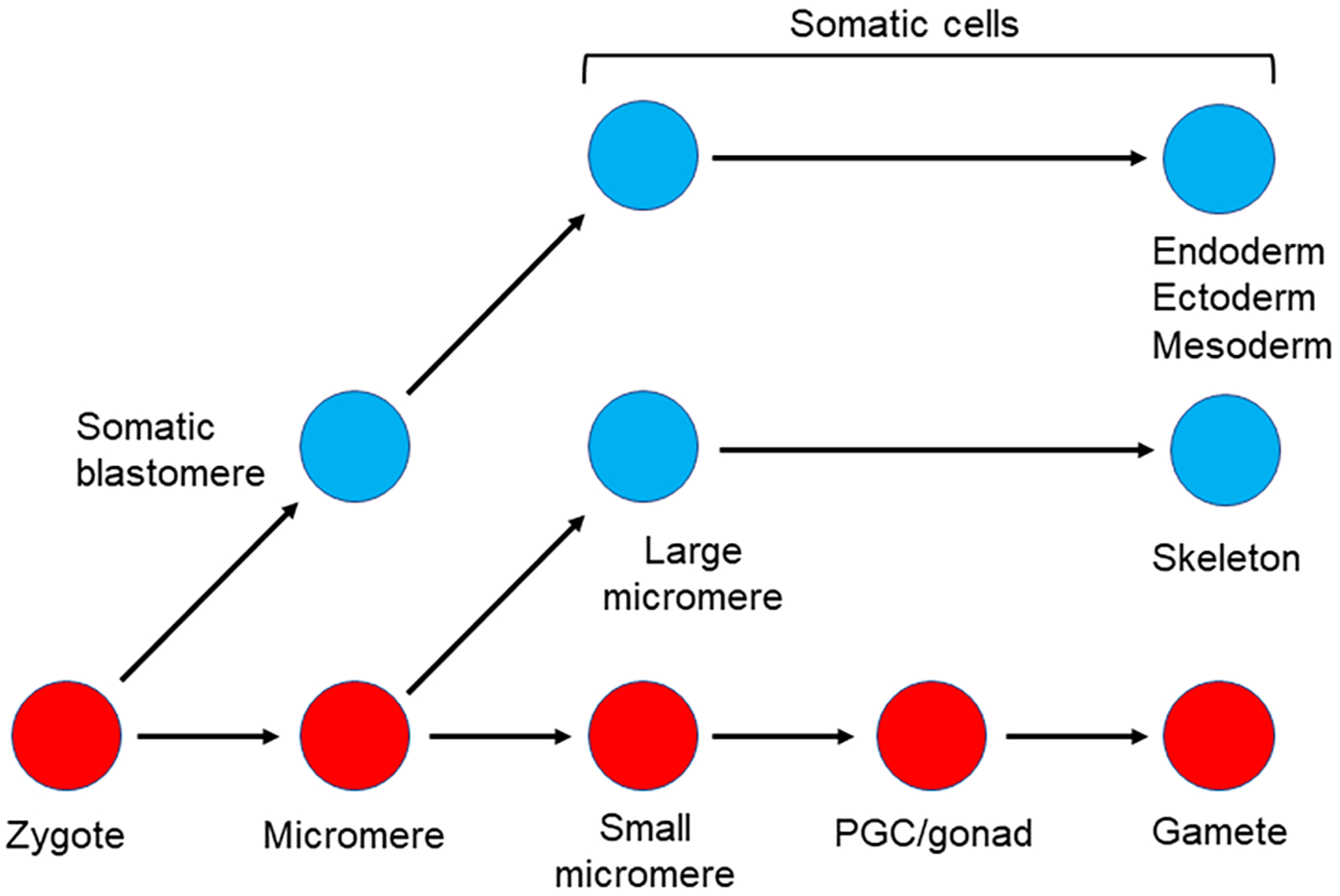
Somatic cell to Germ cell conversion in the sea urchin.
A) Lineage diagram of the germline and relevant somatic cells early in development in the sea urchin.
B) Removal of the micromeres (but not the small micromeres) results in a conversion of cells that normally give rise to somatic cells (potentially Veg2 cells?) to a new germ cell lineage (Oulhen et al., 2019; Voronina et al., 2008).
Figure 5:
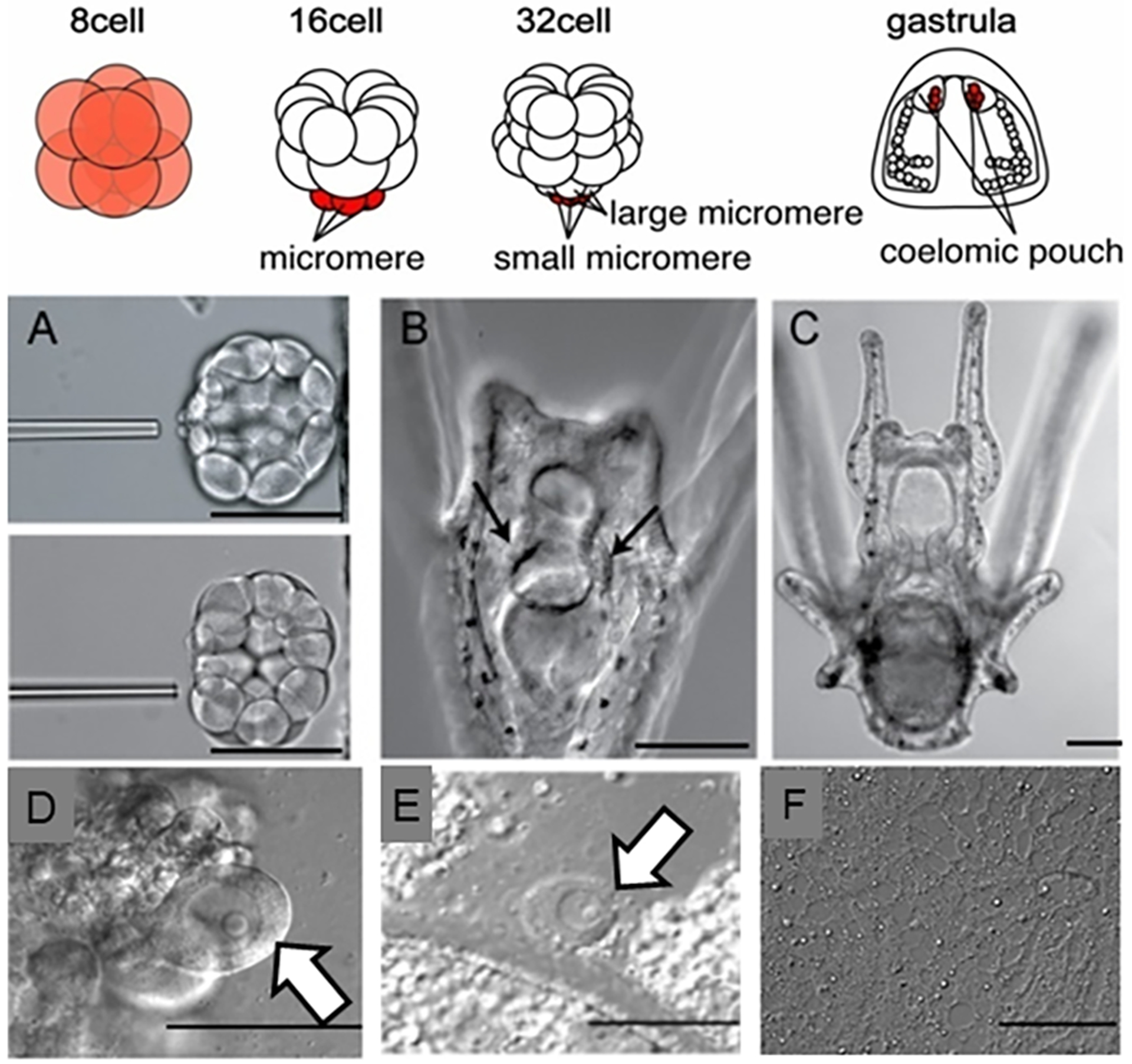
Somatic cell conversion to a germ cell lineage in the sea urchin. Top diagram shows early embryonic development in a sea urchin (for example Strongylocentrotus purpuratus, Lytechinus variegatus). The early embryo (8-cell) has uniform Vasa protein and mRNA throughout, which gradually becomes restricted to the germline (small micromeres) that are displaced to the tip of the archenteron following gastrulation.
A) Removal of the small micromeres from a 32 cell stage embryo.
B-C) Larvae develop normally without the small micromeres, and metamorphose into juveniles, which will make gonads (not shown), but no gametes (F). Molecular analysis (not shown; (Yajima & Wessel, 2011)) showed that these gonads had no germline factors expressed.
D) Oocytes with large germinal vesicle and nucleolus in a non-manipulated animal.
E) If the micromeres are removed at the 16 cell stage instead of the small micromeres, the animal makes gametes as in the non-manipulated animal (modified from (Yajima & Wessel, 2011)).
F) No gametes are produced if the small micromeres are deleted, although all other tissues, including the gonads, appear normal.
We now know some of the pieces of this germline puzzle. First, when micromeres are removed, Vasa protein resulting from maternal mRNA increases throughout the embryo. The amount of Vasa increase over normal embryos is approximately 10-fold, perhaps resulting from inhibition of the Gustavus degradation mechanism (Gustafson et al., 2011; Voronina et al., 2008). Using a morpholino targeting Gustavus results in embryos that phenocopy the micromere-removed embryos for Vasa expression (Figure 6). This uniform and abundant Vasa protein then becomes restricted to the foregut and oral ectoderm in larvae, presumably trending toward a new germline population, but subsequent expression has not been tested. A mechanism for repressing Gustavus activity in the embryo is unknown, but since the micromeres are major signaling cells, it is speculated that Gustavus is hooked into these pathways, and removal of the signal by micromere deletion results in its repression. The signaling mechanism is postulated to include Wnt and Notch/Delta signaling, emanating from the micromeres (McClay, 2011; McClay, Peterson, Range, Winter-Vann, & Ferkowicz, 2000).
Figure 6:
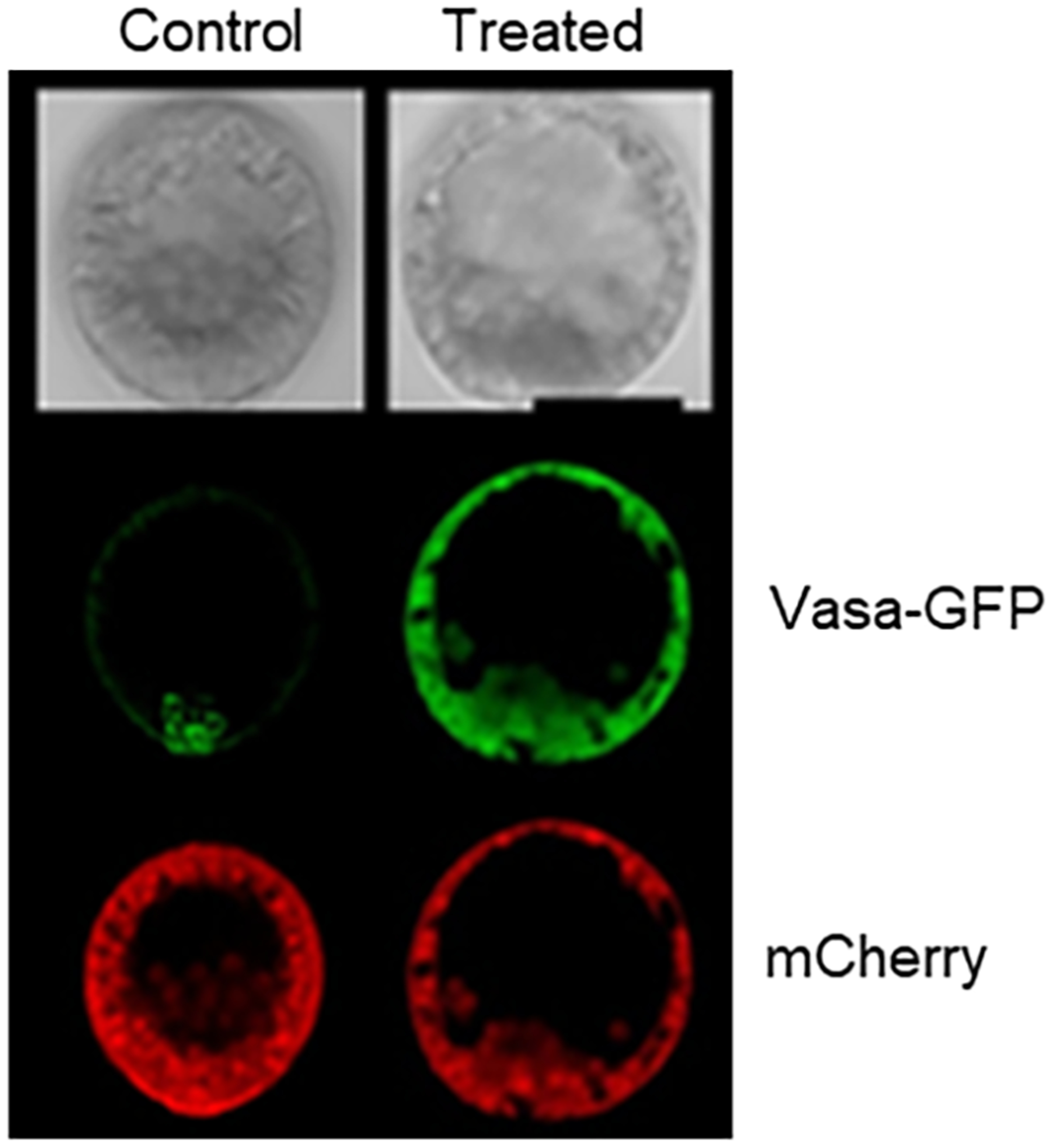
Injecting Vasa-GFP mRNA into a zygote along with mRNA for mCherry results in uniform mCherry expression, but highly selective Vasa-GFP accumulation in the germline cells (left column, control). A similar experiment is shown in the right column but in this case, either the Vasa sequence is missing a small section of its N-terminus that is thought to get ubiquitylated, or the proteasome is inhibited, or Gustavus (E3-ligase that binds Vasa) is knocked down (treated column). In each of these cases, both mCherry and Vasa-GFP uniformly accumulate throughout the embryo (adapted from (Gustafson et al., 2011)). Embryos are 100 microns in diameter.
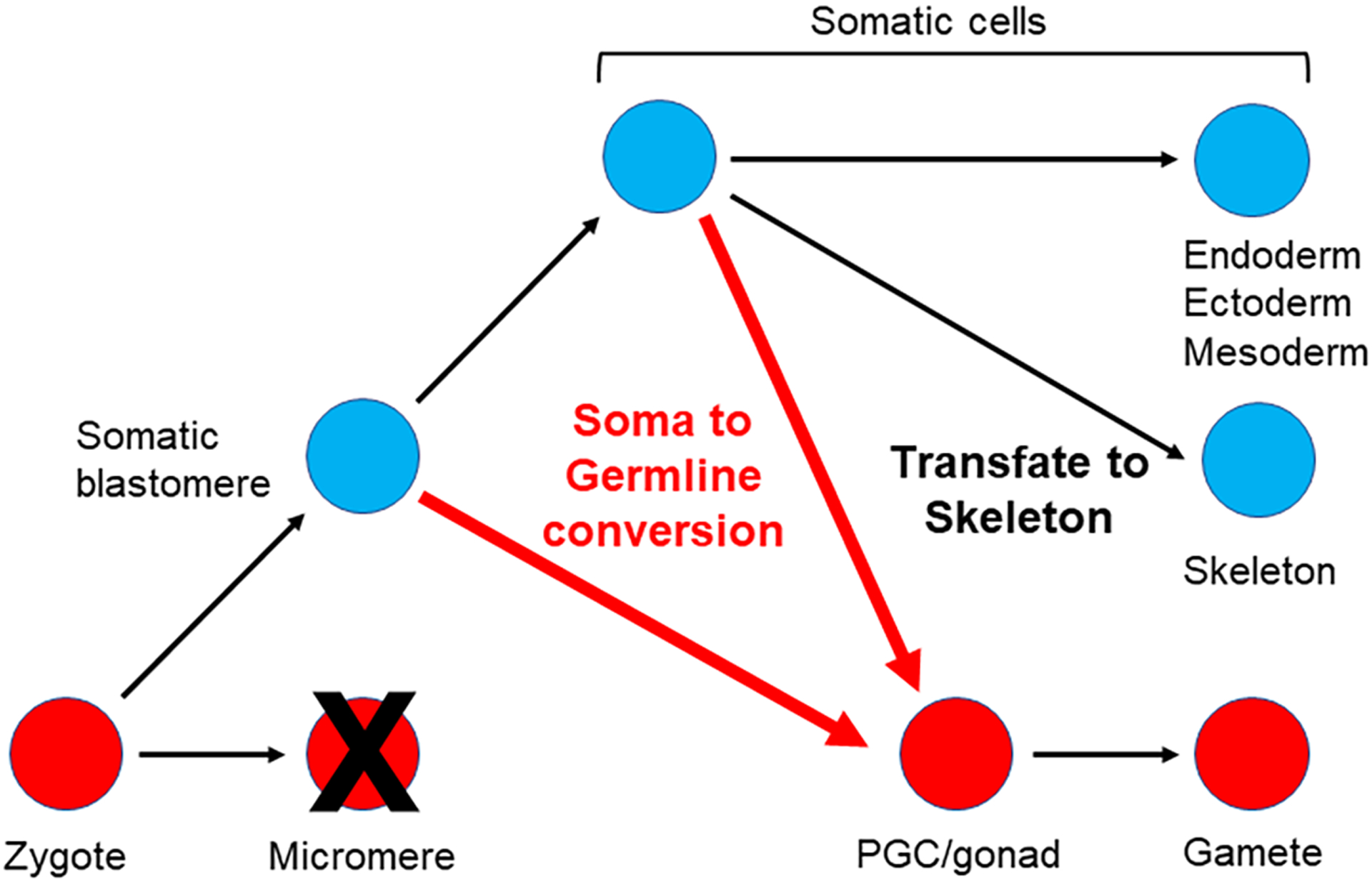
We find it striking that sea urchin embryos lacking micromeres appear to phenocopy the sea star embryo in their Vasa expression. Embryos from both classes of echinoderms show uniform Vasa protein expression throughout the embryo. We speculate that the sea urchin, the taxon containing derived features of asymmetric divisions, larval skeleton, and extensive pigment relative to other echinoderm larvae, may have retained pieces of an ancestral program of germline specification seen for example in sea stars (Figure 7).
Figure 7:
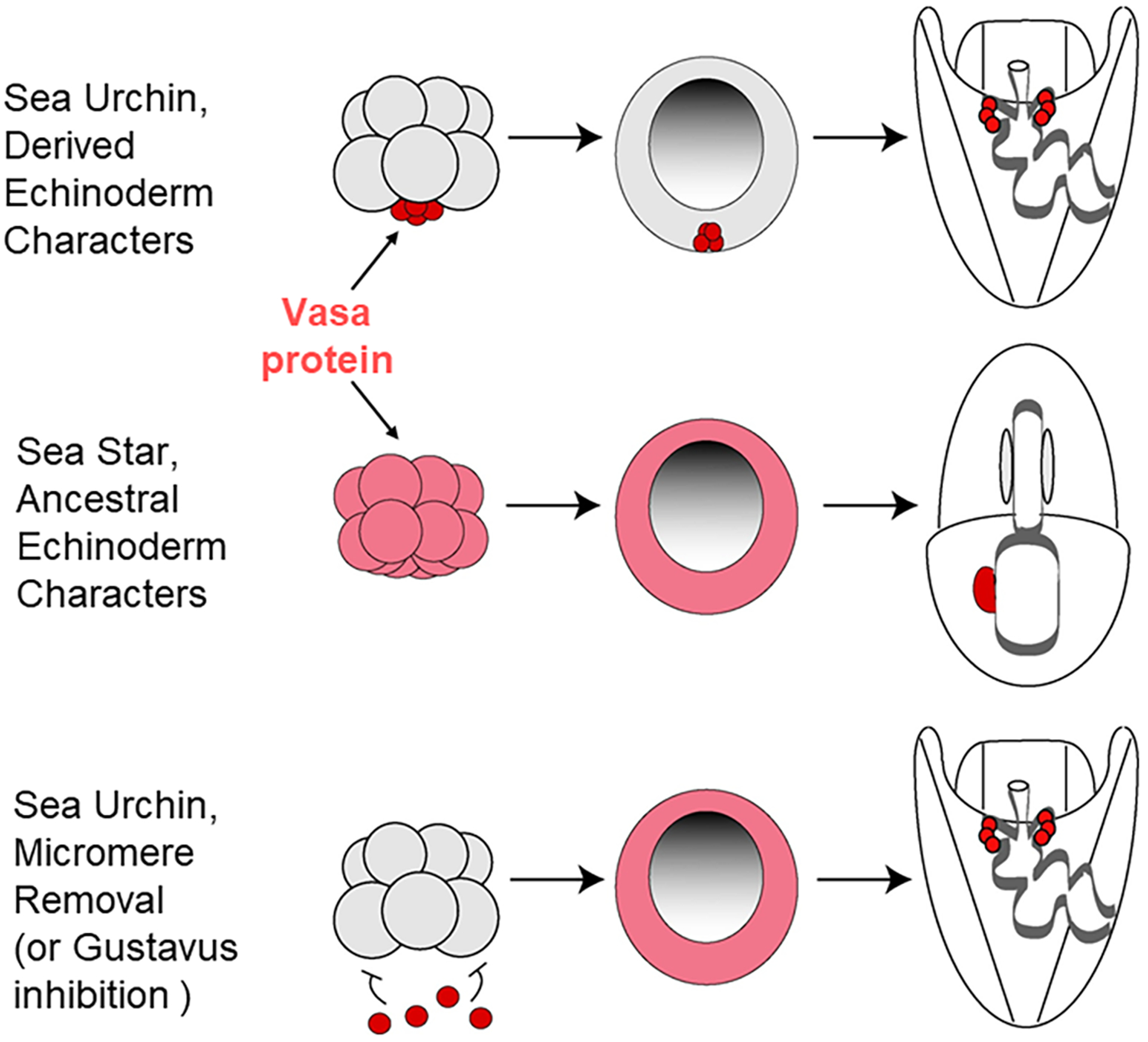
Somatic cell to germ cell conversion in the sea urchin appears to follow an ancestral program seen in sea stars, at least so far as Vasa expression is seen. Top row, Vasa accumulation in sea urchin embryos, middle row, in sea star embryos, and the bottom row shows the impact on Vasa accumulation when the micromeres are removed from the embryo. The lack of repressive micromere signaling results in a large upregulation of Vasa protein accumulation throughout the embryo, followed by a gradual restriction to the tip of the gut. Modified from (Juliano & Wessel, 2009).
What cells replace the small micromeres as the germline when the micromeres are removed from the embryo? It is not clear yet, but a second population of Nanos2-containing cells was recently identified and appears to be a strong candidate for this role (Oulhen, Swartz, Wang, Wikramanayake, & Wessel, 2019). These Nanos2-positive cells are part of the Veg2 tier of cells in a sea urchin embryo, and these cells normally give rise to endomesoderm and reside adjacent to the small micromeres/PGCs. The Veg2-Nanos2 positive cells exhibit reduced levels of metabolic activity relative to other somatic cells of the embryo (for example protein synthesis; Figure 8). These cells begin Nanos2 accumulation well after the PGCs (24hrs, vs 8 hrs post-fertilization), are at the leading edge of invagination, precisely where the Vasa restriction occurs upon micromere depletion, and they have a distinct mechanism of Nanos2 transcriptional regulation. While the PGCs use a Wnt signaling pathway and β-catenin transcriptional event, the Veg2 cells instead rely on FoxY through the Notch/Delta pathway to transcribe Nanos2. We do not yet know how long these cells retain Nanos2, but phenotypically, they are our best candidate for replacing lost PGCs from early development.
Figure 8:

Germ cells in the sea urchin become quiescent shortly following their birth. This quiescence includes transcription, cell cycle, mitochondrial activity, and protein synthesis. Adjacent to the germline are cells derived from the Veg2 tier of cells, that normally give rise to endomesoderm. The Veg2 cells exhibit intermediate levels of quiescence to the PGCs and ectoderm for example protein synthesis (A, quantified in B; A’ shows protein synthesis assay merged Vasa immunolabeling to define the germ cells). The Veg2 cells begin nanos2 accumulation well after the PGCs do, (C, C’, C”; 24hrs, vs 8 hrs post-fertilization), are at the leading edge of invagination precisely where the Vasa restriction occurs upon micromere depletion, and they have a distinct mechanism of Nanos2 transcriptional regulation. The asterisk (*) in A indicates the site of the germline labeled by Vasa antibody, and the dashed lines ( - - - ) indicate the border of the Veg2 tier of cells from the ectoderm. C) At this stage in development (24hrs) Nanos2 mRNA (green) is expressed outside the germline (red immunolabeling of Vasa). Nuclei (DAPI) is in blue and scale bar is 20 microns (Oulhen, Swartz, Laird, Mascaro, & Wessel, 2017; Oulhen et al., 2019; Oulhen & Wessel, 2017).
An alternative source of cells for germline conversion are blastocoelar cells. These cells are putatively mesodermally derived and an array of these morphologically distinct cells migrate throughout the blastocoel during and after gastrulation (Tamboline & Burke, 1992). Of particular importance here is that when the primary mesenchyme cells, the skeletogenic cells of the larvae, are removed, a cohort of the blastocoelar cells transfate to become skeletogenic (Ettensohn & McClay, 1988). Recent evidence suggests that the VEGF program may be responsible for normally repressing blastocoelar cell conversion to skeletogenic cells (Ettensohn & Adomako-Ankomah, 2019). Although this excellent example of transfating in the sea urchin is skeletogenic and not germline, it does expose the awesome power of sea urchin fate switching. For sure this example is not of a terminally differentiated cell that leaps tall buildings, or hurdles high Waddington peaks (Waddington, 1957) to reach a new fate, but it does speak to significant changes in developmental potentials in this embryo.
It is important in the future to better identify the embryo response to loss of the micromeres, the precursor cells of the PGCs. Many outstanding questions remain – 1) what genes are activated (or repressed) by removal of the micromeres, the main signaling center of the embryo? 2) what proteins change germline factor accumulation as a result of micromere removal? 3) What transition may have occurred in the last common ancestor of the sea urchins and sea stars such that the origin of the PGCs in these taxa are so distinct? 4) and finally, one that resonates throughout the animal examples given here, is how upon germline removal that a signal is given and received to, in some cases, distant sites throughout the embryo to change fate to a germline?
How many animals are capable of this somatic-to-germline conversion? We currently do not know how prevalent this mechanism is, but we speculate that many animals would do so for the reason that the three animals examined here are from vastly different phylogenetic positions. We would be surprising were such a mechanism to be infrequent, or to be retained so sporadically, given the fluid nature of germline determination mechanisms in animal phylogeny. Instead, we think more and more animals representing many different phyla will show various forms of somatic cell to germ cell conversions. The constraint for this test may be that the manipulation of the animal cannot be a genetic manipulation of the germline factors. Nor can the test be a germ cell promoter driving cell death in the PGCs. Instead, the depletion of the endogenous PGCs must not interfere with gene activity for germ cell function since the converting cells likely use much the same machinery to become the converting germline as the endogenous cells do to become the original germline. Microsurgical manipulations are key for such a test of response to PGC removal although this experimental strategy may limit many animals from testing. We do know however, that not all animal are capable of the conversion phenotype. For example, the pole cells of Drosophila melanogaster have been removed microsurgically and the embryos were cultured to adulthood. Development and maturation is normal, but the adults are sterile (for example (Illmensee & Mahowald, 1974) and references therein). Thus, individual testing of animals by similar means of microsurgical removal will enable a direct experimental comparison for prevalence of this skill set.
It is important to consider also the opposite phenomenon, that is, can germline cells become somatic cells? Two examples are shown here to suggest that cell fate conversion from the germline to the soma is possible, but perhaps not prevalent. In Drosophila melanogaster, for example, PGCs that have been manipulated to lack maternal Nanos function and major apoptosis genes (for example H99 deletion; (Y. Hayashi, Hayashi, & Kobayashi, 2004)). When these pole cells are transplanted into normal embryos, some of them lose Vasa expression and are incorporated into somatic tissues (midgut, gastric caeca and trachea). These cells begin to express midgut marker genes and the cell-lineage was traced by using β-gal expressing only in donor PGCs. Some of transplanted PGCs migrated to the gonad, but they never contribute to functional gametes. PGCs lacking maternal Nanos alone do not reach the gonad, because they are eliminated by apoptosis, as are most PGCs that do not reach the gonad within a specified developmental timeframe.
In a mouse, the PGCs do not appear capable of contributing directly to somatic cell tissues (Leitch et al., 2014). PGCs injected into preimplantation embryos for example did not integrate into the embryo and contribute to the soma. It appears that the PGCs are unipotent and normally have one fate only - reaching the gonad and forming eggs or sperm. Normally though PGCs undergo apoptosis if they do not reach the gonad. PGCs do, however, have a latent potential that is revealed in the multilineage teratocarcinomas seen in many mammals, presumably resulting from loss of apoptotic functionality (Oosterhuis & Looijenga, 2019).
What is similar between the three animal models of somatic cell to germ cell conversion analyzed here and what might one look for in other animals? 1) Conversion appears to require inductive mechanisms for this process to occur, regardless of how the original germline cells are specified. 2) Once the conversion program is activated, the transitioning cells appear to utilize the same germline factors used by other animals, and used by the original germline specification mechanism. In testing various animals for conversion then, genetically knocking out a germline factor in the original germline will likely incapacitate a subsequent conversion process also. Thus, for animals with strong utility in genetic manipulations, one need use care in how the original germline is removed in order to test the extent of conversion potential. 3) Each of the animals examined herein appear to specify their original germline by acquired, localized materials in early development, and then activate a separate, perhaps ancestral inductive mechanism for conversion. This implies that they have retained pieces of a germline fate program using inductive interactions that may have predated the extant animal. Not all animals have retained this ability though. Drosophilia melanogaster, for example, specifies its germline by acquisition of maternally deposited mRNAs and proteins, yet when its germline, the pole cells are removed from the embryo, the adult is sterile (C. G. Extavour & Akam, 2003; Illmensee & Mahowald, 1974). Thus, hypothetical retention of an ancestral inductive protein is not a consistent feature of extant embryos using inherited mechanisms of germline determination. 4) The converted cells, even when specified at a site distinct from the original germline, are still able to transit areas of the embryo/larvae and populate the gonad. Finally, 5) It appears as if distant signaling is activated. That is, the endogenous germline forms at a site distinct from the site of the cells that are converted. Further, as seen in the case of the sea urchin, the embryo responds broadly and extensively from the site of the original germline (Voronina et al., 2008). How does the embryo/larvae signal loss of the germline, and how does it become restricted to this new “replacement” site? Clearly, this process of conversion is more than changing the fate of a few somatic cells, and instead suggests that larger scale, intercellular signaling, induces broad changes in the fates, pathways, and physiology of the animal.
References
- Amann RP, & Howards SS (1980). Daily spermatozoal production and epididymal spermatozoal reserves of the human male. J Urol, 124(2), 211–215. doi: 10.1016/s0022-5347(17)55377-x [DOI] [PubMed] [Google Scholar]
- Baker TG (1971). Radiosensitivity of mammalian oocytes with particular reference to the human female. Am J Obstet Gynecol, 110(5), 746–761. doi: 10.1016/0002-9378(71)90271-7 [DOI] [PubMed] [Google Scholar]
- Buss L (1987). The Evolution of Individuality: Princeton University Press. [Google Scholar]
- Buss LW (1983). Evolution, development, and the units of selection. Proc Natl Acad Sci U S A, 80(5), 1387–1391. doi: 10.1073/pnas.80.5.1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg LC, & Seaver EC (2018). Regeneration of the germline in the annelid Capitella teleta. Dev Biol, 440(2), 74–87. doi: 10.1016/j.ydbio.2018.05.004 [DOI] [PubMed] [Google Scholar]
- Dill KK, & Seaver EC (2008). Vasa and nanos are coexpressed in somatic and germ line tissue from early embryonic cleavage stages through adulthood in the polychaete Capitella sp. I. Dev Genes Evol, 218(9), 453–463. doi: 10.1007/s00427-008-0236-x [DOI] [PubMed] [Google Scholar]
- Drake JW, Charlesworth B, Charlesworth D, & Crow JF (1998). Rates of spontaneous mutation. Genetics, 148(4), 1667–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettensohn CA, & Adomako-Ankomah A (2019). The evolution of a new cell type was associated with competition for a signaling ligand. Plos Biology, 17(9), e3000460. doi: 10.1371/journal.pbio.3000460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettensohn CA, & McClay DR (1988). Cell lineage conversion in the sea urchin embryo. Dev Biol, 125(2), 396–409. [DOI] [PubMed] [Google Scholar]
- Ettensohn CA, Wessel GM, & Wray GA (2004). The invertebrate deuterostomes: an introduction to their phylogeny, reproduction, development, and genomics. Methods Cell Biol, 74, 1–13. [DOI] [PubMed] [Google Scholar]
- Extavour C, & Garcia-Bellido A (2001). Germ cell selection in genetic mosaics in Drosophila melanogaster. Proc Natl Acad Sci U S A, 98(20), 11341–11346. doi: 10.1073/pnas.201409198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Extavour CG, & Akam M (2003). Mechanisms of germ cell specification across the metazoans: epigenesis and preformation. Development, 130(24), 5869–5884. doi: 10.1242/dev.00804 [DOI] [PubMed] [Google Scholar]
- Fresques T, Zazueta-Novoa V, Reich A, & Wessel GM (2014). Selective accumulation of germ-line associated gene products in early development of the sea star and distinct differences from germ-line development in the sea urchin. Dev Dyn, 243(4), 568–587. doi: 10.1002/dvdy.24038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresques TM, & Wessel GM (2018). Nodal induces sequential restriction of germ cell factors during primordial germ cell specification. Development, 145(2). doi: 10.1242/dev.155663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giani VC Jr., Yamaguchi E, Boyle MJ, & Seaver EC (2011). Somatic and germline expression of piwi during development and regeneration in the marine polychaete annelid Capitella teleta. Evodevo, 2, 10. doi: 10.1186/2041-9139-2-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SFB, M. JF(2016). Developmental Biology: Sinauer. [Google Scholar]
- Gustafson EA, Yajima M, Juliano CE, & Wessel GM (2011). Post-translational regulation by gustavus contributes to selective Vasa protein accumulation in multipotent cells during embryogenesis. Dev Biol, 349(2), 440–450. doi: 10.1016/j.ydbio.2010.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Hikabe O, Obata Y, & Hirao Y (2017). Reconstitution of mouse oogenesis in a dish from pluripotent stem cells. Nat Protoc, 12(9), 1733–1744. doi: 10.1038/nprot.2017.070 [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Hayashi M, & Kobayashi S (2004). Nanos suppresses somatic cell fate in Drosophila germ line. Proc Natl Acad Sci U S A, 101(28), 10338–10342. doi: 10.1073/pnas.0401647101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikabe O, Hamazaki N, Nagamatsu G, Obata Y, Hirao Y, Hamada N, … Hayashi K (2016). Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature, 539(7628), 299–303. doi: 10.1038/nature20104 [DOI] [PubMed] [Google Scholar]
- Hyman H (1955). The Invertebrates: Echinodermata (Vol. 4). New York: McGraw-Hill Education. [Google Scholar]
- Illmensee K, & Mahowald AP (1974). Transplantation of posterior polar plasm in Drosophila. Induction of germ cells at the anterior pole of the egg. Proc Natl Acad Sci U S A, 71(4), 1016–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue C, Kiyomoto M, Shirai H (1992). Germ cell differentiation in starfish: The posterior enterocoel as the origin of germ cells in Asterina pectinifera. Development Growth & Differentiation, 34(4), 413–418. [DOI] [PubMed] [Google Scholar]
- Juliano CE, Voronina E, Stack C, Aldrich M, Cameron AR, & Wessel GM (2006). Germ line determinants are not localized early in sea urchin development, but do accumulate in the small micromere lineage. Dev Biol, 300(1), 406–415. doi: 10.1016/j.ydbio.2006.07.035 [DOI] [PubMed] [Google Scholar]
- Juliano CE, & Wessel GM (2009). An evolutionary transition of Vasa regulation in echinoderms. Evolution & Development, 11(5), 560–573. doi: 10.1111/j.1525-142X.2009.00362.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, & Surani MA (2018). On the origin of the human germline. Development, 145(16). doi: 10.1242/dev.150433 [DOI] [PubMed] [Google Scholar]
- Lanfear R (2018). Do plants have a segregated germline? Plos Biology, 16(5), e2005439. doi: 10.1371/journal.pbio.2005439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch HG, Okamura D, Durcova-Hills G, Stewart CL, Gardner RL, Matsui Y, & Papaioannou VE (2014). On the fate of primordial germ cells injected into early mouse embryos. Dev Biol, 385(2), 155–159. doi: 10.1016/j.ydbio.2013.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makar K, & Sasaki K (2019). Roadmap of germline development and in vitro gametogenesis from pluripotent stem cells. Andrology. doi: 10.1111/andr.12726 [DOI] [PubMed] [Google Scholar]
- McClay DR (2011). Evolutionary crossroads in developmental biology: sea urchins. Development, 138(13), 2639–2648. doi: 10.1242/dev.048967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClay DR, Peterson RE, Range RC, Winter-Vann AM, & Ferkowicz MJ (2000). A micromere induction signal is activated by beta-catenin and acts through notch to initiate specification of secondary mesenchyme cells in the sea urchin embryo. Development, 127(23), 5113–5122. [DOI] [PubMed] [Google Scholar]
- McLaren A (1981). Germ Cells and Soma: A New Look at an Old Probelm. New York: Yale University Press. [Google Scholar]
- Meyer NP, Boyle MJ, Martindale MQ, & Seaver EC (2010). A comprehensive fate map by intracellular injection of identified blastomeres in the marine polychaete Capitella teleta. Evodevo, 1(1), 8. doi: 10.1186/2041-9139-1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterhuis JW, & Looijenga LHJ (2019). Human germ cell tumours from a developmental perspective. Nat Rev Cancer, 19(9), 522–537. doi: 10.1038/s41568-019-0178-9 [DOI] [PubMed] [Google Scholar]
- Oulhen N, Swartz SZ, Laird J, Mascaro A, & Wessel GM (2017). Transient translational quiescence in primordial germ cells. Development, 144(7), 1201–1210. doi: 10.1242/dev.144170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhen N, Swartz SZ, Wang LY, Wikramanayake A, & Wessel GM (2019). Distinct transcriptional regulation of Nanos2 in the germ line and soma by the Wnt and delta/notch pathways. Dev Biol, 452(1), 34–42. doi: 10.1016/j.ydbio.2019.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhen N, & Wessel G (2017). A quiet space during rush hour: Quiescence in primordial germ cells. Stem Cell Res, 25, 296–299. doi: 10.1016/j.scr.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhen N, & Wessel GM (2016). Differential Nanos 2 protein stability results in selective germ cell accumulation in the sea urchin. Develomental Biology, 418, 146–156 doi:doi: 10.1016/j.ydbio.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhen N, Yoshida T, Yajima M, Song JL, Sakuma T, Sakamoto N, … Wessel GM (2013). The 3’UTR of nanos2 directs enrichment in the germ cell lineage of the sea urchin. Dev Biol, 377(1), 275–283. doi: 10.1016/j.ydbio.2013.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransick A, Cameron RA, & Davidson EH (1996). Postembryonic segregation of the germ line in sea urchins in relation to indirect development. Proc Natl Acad Sci U S A, 93(13), 6759–6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien PW (2018). The Cellular and Molecular Basis for Planarian Regeneration. Cell, 175(2), 327–345. doi: 10.1016/j.cell.2018.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydoux G, & Braun RE (2006). Pathway to totipotency: lessons from germ cells. Cell, 127(5), 891–904. doi: 10.1016/j.cell.2006.11.016 [DOI] [PubMed] [Google Scholar]
- Shirae-Kurabayashi M, & Nakamura A (2018). Germ-Cell Formation in Solitary Ascidians: Coexistence of Preformation and Epigenesis. In (pp. 3–18). [Google Scholar]
- Shirae-Kurabayashi M, Nishikata T, Takamura K, Tanaka KJ, Nakamoto C, & Nakamura A (2006). Dynamic redistribution of vasa homolog and exclusion of somatic cell determinants during germ cell specification in Ciona intestinalis. Development, 133(14), 2683–2693. doi: 10.1242/dev.02446 [DOI] [PubMed] [Google Scholar]
- Steward FC (1970). From cultured cells to whole plants: the induction and control of their growth and morphogenesis. Proc R Soc Lond B Biol Sci, 175(1038), 1–30. doi: 10.1098/rspb.1970.0009 [DOI] [PubMed] [Google Scholar]
- Takahashi K, & Yamanaka S (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126(4), 663–676. doi: 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Takamura K, Fujimura M, & Yamaguchi Y (2002). Primordial germ cells originate from the endodermal strand cells in the ascidian Ciona intestinalis. Dev Genes Evol, 212(1), 11–18. doi: 10.1007/s00427-001-0204-1 [DOI] [PubMed] [Google Scholar]
- Tamboline CR, & Burke RD (1992). Secondary mesenchyme of the sea urchin embryo: ontogeny of blastocoelar cells. J Exp Zool, 262(1), 51–60. doi: 10.1002/jez.1402620108 [DOI] [PubMed] [Google Scholar]
- Tanaka EM, & Reddien PW (2011). The cellular basis for animal regeneration. Dev Cell, 21(1), 172–185. doi: 10.1016/j.devcel.2011.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronina E, Lopez M, Juliano CE, Gustafson E, Song JL, Extavour C, … Wessel G (2008). Vasa protein expression is restricted to the small micromeres of the sea urchin, but is inducible in other lineages early in development. Dev Biol, 314(2), 276–286. doi: 10.1016/j.ydbio.2007.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington CH (1957). The Strategy of the Genes London: Allen & Unwin. [Google Scholar]
- Weismann A (1893). The Germ-plasm: a theory of heredity. Translated by W. Newton Parker and Harriet Rönnfeldt. New York: Scribner. [Google Scholar]
- Weissmann A (1893). Das Keimplasma Eine Theorie der Vererbung. Nature, 47(1212), 265–266. doi: 10.1038/047265a0 [DOI] [Google Scholar]
- Wessel GM, Brayboy L, Fresques T, Gustafson EA, Oulhen N, Ramos I, … Zazueta V (2014). The biology of the germ line in echinoderms. Molecular Reproduction and Development, 81(8), 679–711. doi: 10.1002/mrd.22223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel GM, Fresques T, Kiyomoto M, Yajima M, & Zazueta V (2014). Origin and development of the germ line in sea stars. Genesis, 52(5), 367–377. doi: 10.1002/dvg.22772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima M, & Wessel GM (2011). Small micromeres contribute to the germline in the sea urchin. Development, 138(2), 237–243. doi: 10.1242/dev.054940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada L (2006). Embryonic expression profiles and conserved localization mechanisms of pem/postplasmic mRNAs of two species of ascidian, Ciona intestinalis and Ciona savignyi. Dev Biol, 296(2), 524–536. doi: 10.1016/j.ydbio.2006.05.018 [DOI] [PubMed] [Google Scholar]
- Yoshida K, Hozumi A, Treen N, Sakuma T, Yamamoto T, Shirae-Kurabayashi M, & Sasakura Y (2017). Germ cell regeneration-mediated, enhanced mutagenesis in the ascidian Ciona intestinalis reveals flexible germ cell formation from different somatic cells. Dev Biol, 423(2), 111–125. doi: 10.1016/j.ydbio.2017.01.022 [DOI] [PubMed] [Google Scholar]
- Yoshida S, Marikawa Y, & Satoh N (1996). Posterior end mark, a novel maternal gene encoding a localized factor in the ascidian embryo. Development, 122(7), 2005–2012. [DOI] [PubMed] [Google Scholar]
- Zhang M, & Chambers I (2019). Segregation of the mouse germline and soma. Cell Cycle, 18(22), 3064–3071. doi: 10.1080/15384101.2019.1672466 [DOI] [PMC free article] [PubMed] [Google Scholar]


