Highlights
-
•
Malignant GCTB carries a poor prognosis.
-
•
Adequate surgical margins decreased the local recurrence (LR) rate (P = 0.006).
-
•
Secondary malignant transformation should be suspected in patients presenting with recurrence especially after 4 years.
-
•
Adjuvant chemotherapy use did not benefit survival, but was associated with increased pulmonary progression-free survival.
Keywords: Malignant, Giant cell tumor, Bone, Recurrence, Chemotherapy, Survival
Abstract
Background
Malignancy in giant cell tumor of bone (GCTB) is a rare tumor with relevant literature being sparse. In primary malignant GCTB, distinct areas of benign GCTB are juxtaposed with high-grade sarcoma, while in secondary malignant GCTB sarcoma occurs at the site of previously managed GCTB. This study assesses the distinguishing characteristics of patients with this condition, the time interval for development of secondary malignant GCTB, the outcome of treatment, and explores factors associated with oncologic outcomes.
Methods
This is a retrospective case series of patients from a prospectively collected institutional musculoskeletal oncology database. From January 1998 to December 2016, 1365 patients were managed for extremity GCTBs. 32 (2.3%) patients had malignant GCTB, including 12 with primary malignant GCTB and 20 with secondary malignant GCTB. The study population comprised 18 males and 14 females presenting at a mean age of 33.7 years (±13.0) and followed for a mean of 9.5 years (±7.4). Data were collected on patient and treatment-related factors, and the occurrence of local recurrence, metastasis, and death. The time from the diagnosis of GCTB to the secondary malignant GCTB was defined as the latent period.
Results
Malignant GCTB most commonly presents in the distal femur and proximal tibia with pain and swelling. Radiologically, they are aggressive Campanacci Grade III tumors with prominent bony destruction and soft tissue extension. In the 20 patients with secondary malignant GCTB, the tumors were osteosarcoma in 15, undifferentiated pleomorphic sarcoma in 4 patients and fibrosarcoma in one patient. The mean latent period in patients with secondary malignant GCTB was 7.9 year (±7.3). The median recurrence-free survival (RFS) of secondary malignant GCTB (latent period) and benign GCTB were 61.5 and 19 months respectively (p < 0.001), receiver operating curve analysis found 49.5 months to be the critical threshold, with a longer interval to recurrence being seen in malignant recurrence. The 5 and 10-year overall survival rate of malignant GCTB were 45.8% and 36.1% respectively. The 5-year survival rates of primary malignant GCTB and secondary malignant GCTB were 56.2% and 40.0% respectively (p = 0.188). Adequate surgical margins decreased the local recurrence (LR) rate (P = 0.006). Pulmonary metastasis developed in 69% of patients. The median distant metastasis-free survival between malignant GCTB and benign GCTB were 9 and 21 months (p = 0.002). Chemotherapy was associated with a longer pulmonary metastasis free survival (13 months Vs 6 months, P = 0.002), but not with increased overall survival (57.0% Vs 33.3%, P = 0.167).
Conclusions
Malignant GCTB carries a poor prognosis. Accurate diagnosis is critical to avoid inadequate surgical margins when treating primary malignant GCTB. Aggressive tumors and pulmonary metastasis should raise suspicion for malignant GCTB. Secondary malignant transformation should be suspected in patients presenting with recurrence especially after 4 years. Adjuvant chemotherapy use did not benefit survival, but was associated with increased pulmonary progression-free survival.
1. Introduction
Malignant giant cell tumor of bone (GCTB) is a rare sarcoma, with incidence among patients with benign GCTB estimated to be 2–11% [1], [2], [3], [4], [5]. It was first described by Stewart [6], and the distinction between primary malignant GCTB and secondary malignant GCTB was made by early investigators such as Hutter [7] and Dahlin [2]. Primary malignant GCTB is diagnosed when sarcoma is diagnosed concurrently with the initial diagnosis of GCTB, while secondary malignant GCTB is where malignancy is diagnosed at the site of GCTB previously treated with surgery or radiation.
The literature on this condition is sparse, and the insufficient data makes understanding the biological behavior of this disease challenging. Several large series have been reported including a 19 patient series from the Mayo Clinic spanning 8 decades [1], a 17 case series by Bertoni et al from the Rizzoli institute [3], an 8 patient series [8], a 26 patient series [4] from Memorial Sloan Kettering documenting a favorable prognosis, and a 29 patient series from Paris by Anract [9] documenting a poor prognosis. With the variations in the clinical behavior, treatment, and oncologic outcomes observed in these series, the behavior of malignant GCTB is unclear and it is difficult to draw conclusions to guide treatment.
We sought to explore the following questions with a retrospective study with long-term follow-up: 1. What are the epidemiological, clinical and radiologic characteristics of malignant GCTB in the extremities? 2. What are the histologic features of malignant GCTB? 3. What is the time interval for development of secondary malignant GCTB? 4. How are patients with primary and secondary malignant GCTB treated and what are the oncologic outcomes?
2. Patients and methods
This study is a retrospective case series. Following institutional review board approval for the study, the prospectively collected institutional musculoskeletal oncology database was queried to identify patients who had been diagnosed with and managed for malignant GCTB. We identified 1365 patients who had been managed for GCTBs involving the extremities during the period of January 1998 to December 2016. We identified all cases with the diagnosis of GCTB and sarcoma made either synchronously or metachronously. 32 patients met criteria for study inclusion, including 12 patients with primary malignant GCTB and 20 patients with secondary malignant GCTB. All patients underwent at least one of their surgeries at our institution, and pathologic examination of tissue obtained at surgery was reviewed by one of three pathologists.
The study population of 32 patients comprised 18 males and 14 females presenting at a mean of 33.7 years of age (±13.0) and were followed for a mean of 9.5 years (±7.4) from the diagnosis of GCTB. The mean duration of follow-up from the time of diagnosis of malignant GCTB was 4.5 years (±4.2), while the duration of follow-up of the 20 patients with secondary malignant GCTB from initial diagnosis of GCTB was 11.9 years (±7.7).
Data were collected on demographic characteristics of subjects, tumor related features, the nature of surgical and medical treatment, and the occurrence of any significant malignancy-related events (ie. Local recurrence, metastasis, death). The factors assessed for potential effect on outcomes included age at diagnosis, size of tumor (as assessed by maximum dimension in centimeters), the pathologic type of the malignant tumor, location of the tumor, and whether chemotherapy was administered. Surgical margin grading for the resection of the malignant GCTB was categorized according to the TNM system with R0 resection defined as adequate margin and inadequate margin including R1 and R2 excision [10]. For patients with secondary malignant GCTB, the time from the diagnosis of GCTB to the time of diagnosis of the secondary malignant GCTB was defined as the latent period [3]. Overall length of follow-up was defined as the interval from initial diagnosis to the last follow-up date or date of death. Survival time was defined as the time from the diagnosis of the malignancy to death or the last follow-up date.
Descriptive statistical analysis and Kaplan-Meier survival analysis was performed. Both overall survival and recurrence-free survival was analyzed. Statistical analysis was also performed comparing this study population with the patient population with benign GCTB from our institution, who have been previously studied and published [11], [12]. The characteristics compared were the latent period or time to a malignant recurrence with the interval to local recurrence in benign disease, and the interval to development of pulmonary metastasis. Functional outcomes were measured using MSTS criteria [13].
Statistical analysis was conducted using SPSS (version 21.0. IBM). Parametric data were analyzed with descriptive statistics. Categorical data were described by result frequencies. Bivariate statistical analyses were performed by chi-square/Fisher’s exact test and Wilcoxon signed ranks-test. Oncologic outcomes were estimated by Kaplan-Meier survival analysis. The cutoff value for assessing the time of recurrence was determined by the receiver operating characteristic (ROC) curve. Multivariate analysis was performed by Cox regression model using the Forward Wald Method. Statistical Significance was determined using a 95% confidence level and all statistical tests were two-tailed.
3. Results
3.1. Clinical, and radiologic characteristics
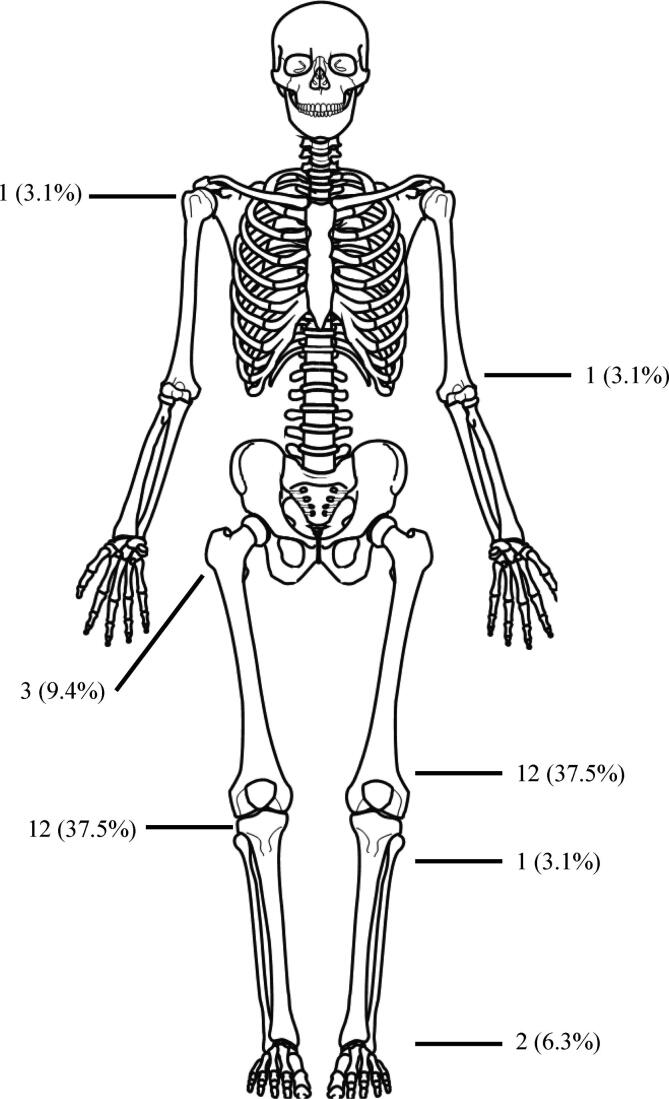
The commonest site of occurrence of the tumor was the distal femur (12 patients) with the proximal tibia (10 patients) being the second most common site (Fig. 1). All patients with primary malignant GCTB presented with pain and swelling, while patients with secondary malignant GCTB presented with varying degrees of dysfunction, and were suspected clinically and radiologically of having a local recurrence. None of the patients had received radiation therapy or denosumab. Fifteen of the twenty patients with secondary malignant GCTB were diagnosed at the time of their second surgery, while five of the twenty had undergone two prior surgeries for GCTB prior to their diagnosis of secondary malignant GCTB on their third surgery (Tables 1 and 2).
Fig. 1.
Anatomical distribution of the 32 cases of malignant GCTB. The majority of lesions affected the meta-epiphyseal region of the long bones, especially around the knee joint, which was similar to GCTB site.
Table 1.
Clinical Features and Outcomes of Primary Malignancy in Giant Cell Tumor of Bone.
| No | Gender | Age (y) | Site | Campanacci | Initial Treat | Surgery | Surgical Margin | Pathology | Chemotherapy | Radiation | Recurence | Lung Metastasis | Reoperation | Limb Salvage | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 18 | PF | III | JST | Resection* | Inadequate | UPS | N | N | N | N | N | Y | NED |
| 2 | M | 40 | PT | II | JST | Resection | Adequate | UPS | Y | N | Y | N | Amputation | N | NED |
| 3 | M | 17 | DH | II | Referral | Curettage | Inadequate# | OS | Y | N | Y | N | Resection | Y | NED |
| 4 | M | 29 | DT | II | Referral | Curettage | Inadequate# | OS | Y | N | Y | Y | Amputation | N | Death |
| 5 | M | 67 | DT | II | Referral | Curettage | Inadequate# | OS | N | N | Y | Y | Amputation | N | Death |
| 6 | F | 20 | PF | II | JST | Resection* | Inadequate | UPS | N | N | N | N | N | Y | NED |
| 7 | M | 21 | PT | III | JST | Resection | Adequate | OS | Y | N | Y | Y | Resection | Y | Death |
| 8 | M | 47 | DT | II | Referral | Curettage, | Inadequate# | OS | Y | N | Y | Y | Resection | Y | SWT |
| 9 | F | 31 | DF | III | JST | Curettage, Resection** | Adequate | OS | Y | N | N | N | N | Y | NED |
| 10 | F | 16 | PF | II | JST | Curettage, | Inadequate | OS | N | N | Y | Y | Refuse Surgery | Y | Death |
| 11 | F | 43 | PT | III | JST | Resection | Adequate | FS | N | N | N | N | N | Y | NED |
| 12 | F | 32 | DF | III | JST | Curettage, | Inadequate | OS | Y | N | Y | Y | Amputation | N | Death |
| PF: Proximal Femur; PT: Proximal Tibia; DH: Distal Humerus; DT: Distal Tibia; DF: Distal Femur; JST: Beijing Jishuitan Hospital; UPS: Undifferentiated pleomorphic sarcoma; OS: Osteosarcoma; FS: Fibrosarcoma; NED: No evidence of disease; SWT: Survival with tumor; *Tumor contamination when resection; **Reoperation in 2 weeks; #Surgery in other hospital. | |||||||||||||||
Table 2.
Clinical Features and Outcomes of Secondary Malignancy in Giant Cell Tumor of Bone.
| No | Gender | Age | Site | Campanacci |
Surgical Treatment |
Surgical Margin | Latent Period | Pathology | Chemotherapy | Recurence** | Reoperation | Metastasis | Limb Salvage | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (y) | BGCTB | MGCTB | BGCTB | MGCTB | MGCTB | (M) | MGCTB | |||||||||
| 1 | F | 18 | DF | II | III | Curettage | Amputation | Adequate | 54 | OS | N | N | N | Lung | N | Death |
| 2 | F | 21 | PH | II | III | Resection | Resection | Adequate | 51 | UPS | N | N | N | Lung | Y | Death |
| 3 | M | 52 | PT | Unavailable | III | Curettage X2 | Resection | Adequate | 158 | OS | Y | Y | Amputation | Lung | N | Death |
| 4 | M | 45 | PT | II | III | Curettage X2 | Resection | Adequate | 66 | OS | N | Y | Resection | Lung | Y | Death |
| 5 | F | 25 | DF | II | III | Curettage | Resection | Adequate | 23 | UPS | N | N | N | N | Y | NED |
| 6 | F | 61 | DF | Unavailable | III | ResectionX2 | Amputation | Adequate | 308 | UPS | N | N | N | Lung&Brain | N | Death |
| 7 | F | 48 | DF | I | III | Curettage X2 | Resection | Adequate | 276 | OS | N | Y | Amputation | Lung | N | Death |
| 8 | F | 29 | DF | Unavailable | III | Curettage X2 | Resection | Adequate | 85 | OS | Y | N | N | Lung | Y | Death |
| 9 | F | 40 | DF | Unavailable | II | Curettage | Resection* | Adequate | 204 | OS | N | N | N | Lung | Y | NED |
| 10 | M | 21 | DF | II | III | Curettage | Resection | Adequate | 6 | OS | Y | N | N | Lung | Y | Death |
| 11 | F | 27 | PF* | II | III | Resection | Resection | Adequate | 19 | OS | Y | N | N | N | Y | NED |
| 12 | M | 33 | PT | I | III | Curettage | Resection | Adequate | 96 | OS | Y | N | N | Lung | Y | SWT |
| 13 | M | 43 | DF | III | III | Curettage | Amputation | Adequate | 72 | OS | N | N | N | Lung | N | Death |
| 14 | M | 38 | DF | II | III | Curettage | Resection | Adequate | 57 | OS | Y | N | N | N | Y | NED |
| 15 | F | 38 | PT | II | III | Curettage | Resection | Adequate | 8 | OS | N | N | N | Lung | Y | Death |
| 16 | M | 21 | DT | II | III | Curettage | Amputation* | Adequate | 13 | OS | Y | N | N | Lung | N | Death |
| 17 | M | 39 | PT | II | III | Curettage | Curettage | Inadequate | 52 | OS | Y | Y | Amputation | Lung&Bone | N | Death |
| 18 | M | 24 | PT | III | III | Curettage | Resection | Adequate | 56 | UPS | N | N | N | Lung | Y | Death |
| 19 | M | 40 | DF | Unavailable | III | Curettage | Resection | Adequate | 203 | UPS | Y | N | N | N | Y | NED |
| 20 | M | 31 | PT | Unavailable | III | Curettage | Resection | Adequate | 97 | FS | Y | N | N | Lung&Bone | Y | Death |
| PH: Proximal Humerus; PF: Proximal Fibula; PT: Proximal Tibia; DT: Distal Tibia; DF: Distal Femur; MTP: UPS: Undifferentiated pleomorphic sarcoma; OS: Osteosarcoma; FS: Fibrosarcoma; NED: No evidence of disease; SWT: Survival with tumor; *Reoperation in 2 weeks after Curettage; **Recurrence after MGCTB surgery. | ||||||||||||||||
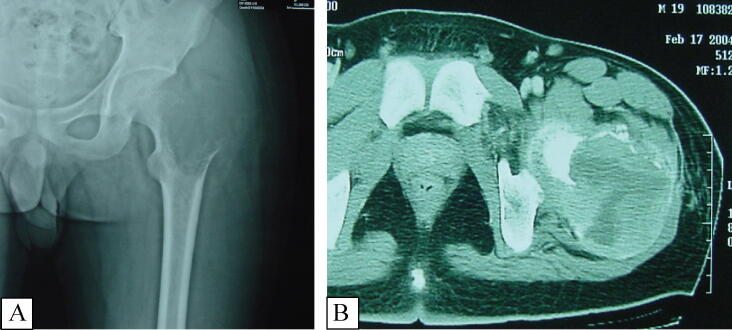
Regarding the radiologic appearance of primary malignant GCTB, all patients presented with well-circumscribed osteolytic lesions in the epiphysis. Eleven pre-operative computed tomography (CT) scans were available for review and they displayed a lack of peripheral sclerosis, homogenous matrix, and well-defined boundaries. Cortical expansion or destruction was present in all 11 of these cases and an extra-osseous soft-tissue component was noted in 5 (Fig. 2).
Fig. 2.
Male, 18yrs, Primary Malignancy in Giant Cell Tumor of Bone. Anteroposterior hip joint radiograph (Fig. 2-A) showing osteolytic lesion at the left proximal femur, illustrating the well-circumscribed margin without sclerotic rim in the epiphyses and soft tissue mass adjacent to expanded cortical bone. CT-scan (Fig. 2-B) demonstrating a more clearly visible osteolytic lesion without mineralization, soft tissue mass breaks through the cortical bone with obvious enhancement.
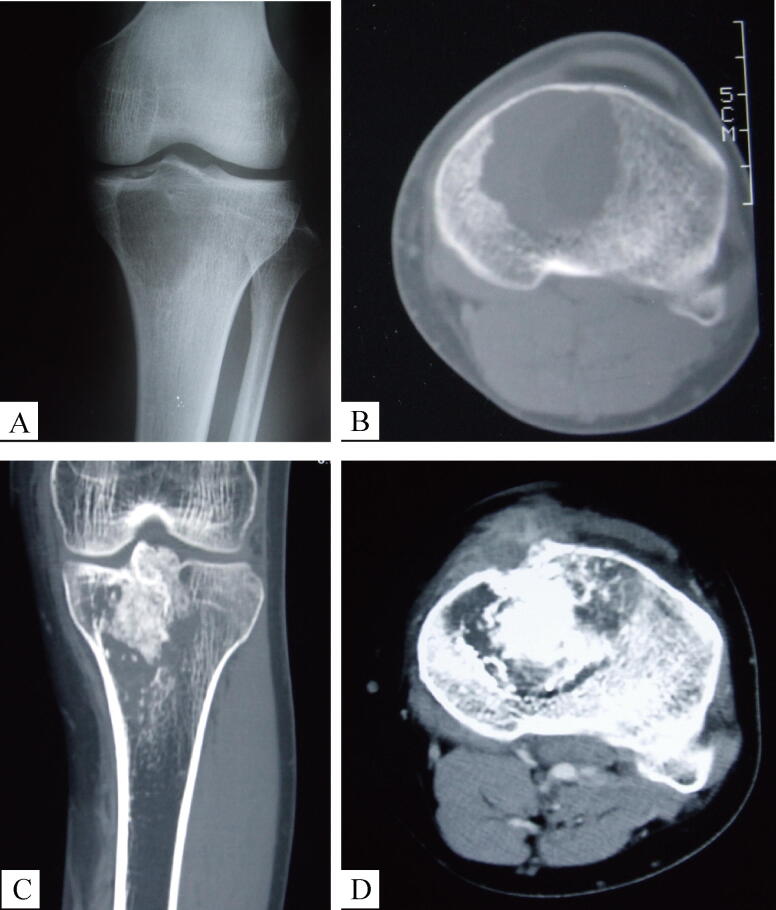
Regarding the radiologic appearance of secondary malignant GCTB, 19 of the 20 patients presented with Campanacci grade III GCTB on presentation of the secondary malignancy. The radiographs of the initial benign GCTB were available for review in 14 of these 19 patients and were graded as grade II in 10, grade I in 2, and grade III in 2 (Wilcoxon Signed Ranks Test, P = 0.001). Following malignant transformation, the radiologic studies displayed aggressive features with ill-defined margins, cortical destruction and an extraosseous soft tissue mass. The appearance of the tumor was varied: 5 cases showed mainly lytic lesions, 2 cases displayed significant ossification and calcification, while 13 cases demonstrated a heterogenous appearance. All 20 lesions showed enhancement on contrast CT scanning (Fig. 3).
Fig. 3.
Male, 33yrs, Secondary Malignancy in Giant Cell Tumhttps://elsevier.proofcentral.com/en-us/landing-page.html?token=aa2295e304800f3831fd002effa727or of Bone. Anteroposterior knee joint radiograph (Fig. 3-A from 2001) showing osteolytic lesion at the medial tibial platform, CT-scan (Fig. 3-B from 2001) illustrating the well-circumscribed lesion without cortical destruction. This lesion was treated with curettage and bone graft, complaint of pain and swelling eight years later, a local “recurrence” was diagnosed. Coronal (Fig. 3-C from 2009) and axial of CT-scan (Fig. 3-D from 2009) demonstrating a sclerotic and destructive relapse involving the tibial platform, with cortical bone breakthrough, soft tissue mass involvement and significant enhancement in contrast CT-scan.
3.2. Histologic features
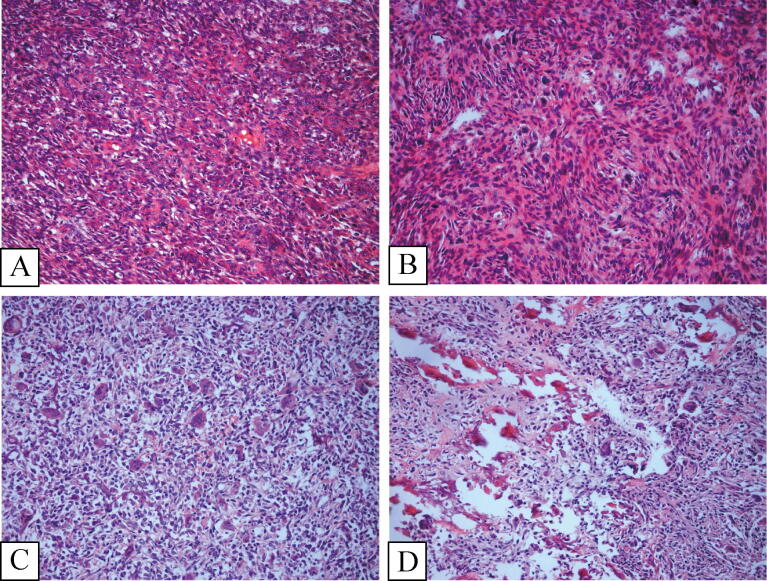
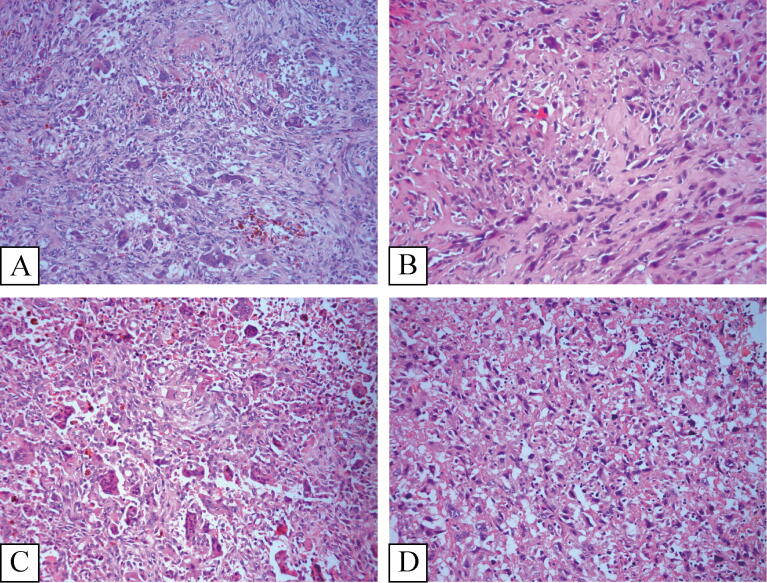
In primary malignant GCTB, tumors displayed both distinct areas of benign GCTB juxtaposed with high-grade sarcoma. In eight of twelve cases, osteoid matrix production was observed leading to the diagnosis of osteosarcoma, three cases were diagnosed with fibrosarcoma demonstrating uniformly spindle shaped cells arranged in a fascicular or “herringbone” pattern with a variable amount of collagen production, and the last case was diagnosed with undifferentiated pleomorphic sarcoma (UPS) comprising a mixture of spindle cells, histiocytoid and pleomorphic cells. The nuclei of the sarcomatous cells was quite atypical, and a characteristic storiform pattern was commonly seen in the fibroblastic areas (Fig. 4).
Fig. 4.
Primary malignancy in giant cell tumor of Bone. (Fig. 4-A) and (Fig. 4-B) came from one case. (Fig. 4-C) and (Fig. 4-D) came from another case. (Fig. 4-A) and (Fig. 4-C) showed the classical giant call tumor respectively (Fig. 4-B). Illustrated the spindle cell sarcoma, mitosis was obviously visible. (Fig. 4-D) Demonstrated the osteosarcoma, the residual giant cells and classical giant cells tumor region adjacent to osteosarcoma area (H&E × 200).
In secondary malignant GCTB, the malignant tumor predominated with residual benign GCTB not being present. Histologically, the secondary malignant GCTBs were difficult to distinguish from primary sarcoma and as such the clinical history of benign GCTB was crucial for diagnosis (Fig. 5). The tumors that occurred in these 20 patients were osteosarcomas in 15, UPS in 4 patients and fibrosarcoma in one patient.
Fig. 5.
Secondary malignant giant cell tumor of Bone. (Fig. 5-A) and (Fig. 5-B) came from one case. (Fig. 5-C) and (Fig. 5-D) came from another case. (Fig. 5-A) and (Fig. 5-C) showed the classical giant call tumor from the initial lesion respectively. (Fig. 5-B) Illustrated the osteosarcoma in the recurrence lesion. (Fig. 5-D) Demonstrated the high-grade undifferentiated pleomorphic sarcoma in the recurrence lesion (H&E × 200).
3.3. Interval for development of secondary malignant GCTB
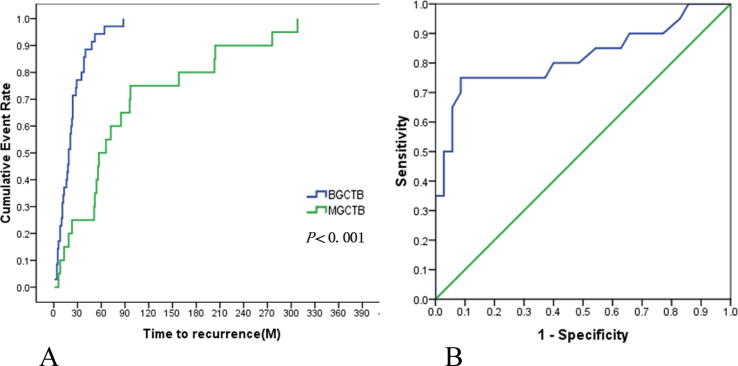
The mean latent period in patients with secondary malignant GCTBs was 7.9 year (±7.3). With the latent period defined as a malignant recurrence, the median recurrence-free survival (RFS) was 57 months in the secondary malignant GCTB group and 19 months in the benign GCTB group (log-rank test, P < 0.001) (Fig. 6-A). Data were analyzed to determine a critical threshold distinguishing malignant recurrence in secondary malignant GCTB and local recurrence in benign GCTB. The area under the curve (AUC) was 0.820 (95%CI: 0.689, 0.951) (Fig. 6-B) and 49.5 months was the critical threshold of distinguishing these two distinct “recurrence” patterns, with longer intervals predominating in secondary malignant GCTB.
Fig. 6.
Kaplan-Meier Estimates of the Recurrence-free survival (P < 0.001), difference between BGCTB recurrence [11] and BGCTB malignant transformation displayed by “recurrence” (6-A). The ROC curve of RFS in BGCTB (AUC = 0.82), contributing to identify the benign recurrence or malignant change (6-B).
3.4. Management and oncologic outcomes
Eight of twelve patients with primary malignant GCTB were diagnosed and received all their treatment at our institution. Seven of eight patients were initially diagnosed as benign GCTB on core biopsy prior to undergoing surgery, and only one was diagnosed with primary malignant GCTB on biopsy prior to definitive surgery. Five patients, including the patient diagnosed with malignancy prior to definitive surgery, were managed with wide resection of the tumor owing to the extent of bony destruction. Two of these five patients developed local recurrences at 18 and 6 months following the initial surgery. The former underwent amputation for local control of the recurrence and had no further recurrences. The latter underwent wide resection of the recurrence, but subsequently developed pulmonary metastasis and succumbed to the disease. The remaining 3 patients underwent intralesional curettage for initial management. Following the diagnosis of the malignancy, one patient underwent wide resection of the involved bone and tumor bed promptly, one patient deferred further management until local recurrence developed and underwent an amputation 6 months later, and the final patient refused surgery, developed local recurrence at 6 months and subsequently succumbed to pulmonary metastasis. Four of the twelve patients with primary malignant GCTB were referred from other hospitals when malignancy was diagnosed. These 4 patients had undergone intralesional curettage and were diagnosed with malignant GCTB after having developed a local recurrence in under 6 months. 2 patients underwent wide resection and 2 patients underwent amputation. The latter 2 patients died of pulmonary metastasis at 11 and 24 months post operatively.
The majority (85%) of patients with secondary malignant GCTB received initial treatment at other hospitals and were referred for management of their recurrent tumors. For their initial surgery. 17 of 20 patients underwent intralesional curettage and 3 patients underwent wide resection. Core biopsies identified secondary malignant GCTB in 10 patients prior to definitive surgery. 3 patients underwent neoadjuvant chemotherapy followed by wide resection and 7 patients underwent wide resection following biopsy. In the other 10 patients, malignancy was diagnosed following surgery for management of the recurrence. 7 patients under wide resection, and 3 patients underwent intralesional curettage. Of the 3 patients who underwent intralesional curettage, two underwent surgery promptly following the malignant diagnosis: One underwent resection 2 weeks later, one underwent amputation 3 weeks later and subsequently died of pulmonary metastasis 21 months later, the last patient underwent adjuvant radiotherapy, developed a local recurrence 60 months later for which an amputation was performed, and subsequently died from pulmonary metastasis. Of the 7 patients who underwent resection with the subsequent diagnosis of secondary malignant GCTB, 3 of 7 patients developed local recurrence at 6, 6 and 42 months respectively. All three underwent surgery for control of the local recurrence with 2 undergoing amputation. All 3 patients died of lung metastasis later at 13, 23, and 90 months. 21 patients ultimately underwent limb salvage surgery, with the mean MSTS score was (83.5 ± 7.0) % at the end of follow-up.
At last follow-up, 13 patients were alive. The 5 and 10-year Kaplan-Meier survival estimates were 45.8% and 36.1% with a median follow-up of 2.1 years. The 5-year survival estimates of primary malignant GCTB and secondary malignant GCTB were 56.2% and 40.0% respectively (log rank, P = 0.188). Local recurrence occurred in 7 of 9 cases with inadequate margins and 5 of 24 patients with adequate margin (Fisher's Exact Test P = 0.006). Logistic regression found 12.6 times higher odds of local recurrence when margins were inadequate (p = 0.008)
The incidence of lung metastases was high in malignant GCTB with 22 of 32 patients (69%) developing them, 19 of which developed them only after malignant GCTB was diagnosed. The mean interval to development of pulmonary metastasis was shorter in malignant GCTB, being 9 months as compared to 21 months in benign GCTB patients with pulmonary metastasis (P = 0.002). Regarding adjuvant chemotherapy, 17 patients received adjuvant chemotherapy in addition to surgery and the other 15 patients underwent surgery alone. The 5-year Kaplan-Meier survival estimate was 57.0% and 33.3% in the chemotherapy and non-chemotherapy groups respectively, (P = 0.167). Chemotherapy did have an effect on development of pulmonary metastasis, with median pulmonary metastasis free survival in patients who received chemotherapy being 13 months and opposed to 6 months in patients who underwent surgery alone (P = 0.002). Multivariate Cox regression analysis found the development of pulmonary metastasis to be the only variable associated with survival, with 77 times higher odds of mortality.
4. Discussion
The diagnosis of malignant GCTB is challenging, it is rare and occurs concurrently with another tumor or at the site of previously treated benign GCTB [5], [14]. Misdiagnosis of malignant GCTB has significant prognostic implications owing to the difference in prognosis compared to benign GCTB. As such a clearer understanding of the incidence patterns and behavior of malignant GCTB is crucial to identify and manage these patients better.
In our study, there was a slight male predilection in the distribution of malignant GCTB with a male-to-female ratio of 1.28. This high male-to-female ratio was similarly observed by Bertoni et al. [3] who observed a 3.25 ratio but in contrast Domovitov et al. [4] observed a ratio of 0.625. In benign GCTB, the reported gender preponderance is similarly varied with a female preponderance observed in an American population [2] while a male preponderance having been observed in studies of East Asian populations [11].
The distribution of malignant GCTB by anatomical location in this study was similar to that observed by other authors [3], [4] and to benign GCTB with 78.1% of tumors occurring around the knee. Our study found a slightly higher mean age at diagnosis of malignant GCTB of 33.7 years as compared to that of 31.4 years in benign GCTB [11]. Domovitov et al. [4] also noted this, with the mean age at diagnosis of malignant GCTB being 38 years vs 33 in patients with benign GCTB. In contrast, several studies [3], [8] have documented a even higher age of diagnosis with Bertoni et al. [3] reporting the mean age of diagnosis of 46 years in that study. In all studies, there was however a wide range in the age of diagnosis.
The clinical and radiographic presentation of malignant and benign GCTB are similar and no features clearly distinguished them in this study. Malignant GCTB rarely presents as Campanacci grade I [4] and this was noted in our study as well. Secondary malignant GCTB is difficult to distinguish from recurrent benign GCTB radiologically, and all the secondary tumors in this study had grade III disease at the time of diagnosis of the malignancy.
Osteosarcoma was the most common histology in both primary and secondary malignant GCTB in our study echoing the literature [3], [5]. Fibrosarcoma occurred in only one patient in contrast to the findings of Rock et al. [1] where fibrosarcoma was three times more common than osteosarcoma. Misdiagnosis histologically is another challenge; giant cell rich osteosarcoma can appear similar morphologically, and in secondary malignant GCTB there may not be significant areas of conventional benign GCTB appreciable microscopically. H3F3A G34W immunohistochemistry has been proposed as a marker that can be helpful [15], [16]. The marker is found in both benign and malignant GCTB and has been proposed by Amary et al. [15] to be reliable in distinguishing benign and malignant GCTB from other tumors. For the diagnosis of origin from GCTB, H3F3A mutation DNA sequencing analysis or immunohistochemical detection of the specific antibody (H3.3) against the H3F3A G34W mutation can further confirm the diagnosis. Both these methods have their limitations, DNA sequencing analysis can detect other rare H3F3A mutation, but it is limited byspecimen quality and the quantity of tumor cells. Immunohistochemistry has lower requirements for specimen quality, but it is specific for the H3.3 antibody and cannot detect the other mutation types. When H3.3 immunohistochemistry or DNA sequencing analysis are used to diagnose GCTB, one should be aware that even if H3.3 is negative and the sequencing shows the tumor to be wild type for H3F3A G34W GCTB cannot be excluded [17]. Therefore, as an advanced molecular diagnostic tool, H3F3A mutation detection is an auxiliary diagnostic indicator of GCTB.
While no patients in our study were treated with denosumab prior to diagnosis, the increasing use of denosumab in unresectable GCTB [18] and as a neoadjuvant therapy [19] can however complicate histologic diagnosis. The morphologic appearance of denosumab-treated GCTB resembles osteosarcoma with the combination of cellularity, atypia, and haphazard bone deposition [20], and eliciting the history of denosumab administration is crucial to avoid misdiagnosis. The reports of denosumab-treated GCTB undergoing malignant transformation [21], [22] warrant research to clarify if this represent true malignant transformation or initial histologic misdiagnosis.
In our study, we found a mean latent period of 7.9 years in patients who developed secondary malignant GCTB and the Kaplan-Meier estimate of median time to malignancy recurrence was 4.75 years. The interval from the diagnosis of benign GCTB to the development of secondary malignant GCTB has been reported to be longer than the typical interval for benign recurrence, and our study findings concur with this. Where secondary malignancy is diagnosed with a short latent period prior to the malignant recurrence, the possibility that the primary lesion was malignant to begin with and not benign GCTB should be considered. While late recurrence is a known phenomenon in benign GCTB [23], the majority of recurrences occur within 2 years [11]. Bertoni et al. [3] observed no secondary malignant GCTB patients with a latent period of less than 3 years, and recommended a heightened awareness of the possibility of malignant GCTB when evaluating patients with a recurrence of GCTB more than 3 years after the initial management. In seeking to assess whether a specific latent period could be used to distinguish between benign from malignant disease in the setting of recurrent GCTB, ROC curve analysis found a critical threshold of 49.5 months or 4.1 years with a longer latent period indicating a high risk of secondary malignant GCTB. The findings of our study concur with the findings of Bertoni et al. and there should be a high index of suspicion of potential malignancy in patients being worked up for recurrence more than 4 years after initial surgery for benign GCTB.
Malignant GCTB is an aggressive disease. The 5 and 10-year Kaplan-Meier survival estimates of 45.8% and 36.1% in our study are poorer than osteosarcoma, which was the main sarcoma subtype in over 70% of our study. The challenges in diagnosis of malignant GCTB have significant implications in their surgical management. In primary malignant GCTB, the juxtaposition of benign disease with malignant disease frequently leads to intralesional curettage as the initial treatment as it is common for the biopsy to sample only the benign component. In secondary malignant GCTB, the malignant transformation mimics an aggressive recurrence and thus similarly can be misdiagnosed and mismanaged at the initial surgery. In both these situations, the prerequisite for local control, adequate surgical margins [13], is rarely met and can be more challenging to obtain in subsequent procedures. Our study found a higher rate of recurrence in patients initially managed with inadequate margins, echoing this principle of orthopaedic oncology. The importance of accurate diagnosis cannot be overstated.
With respect to oncologic outcomes, no benefit on overall survival was found with adjuvant chemotherapy in this study. A significant difference in pulmonary metastasis-free survival was however found when comparing patients who did and did not receive chemotherapy. Antract [9] reported the better one-year survival in patients who underwent surgery and received chemotherapy when compared to those who underwent surgery alone, however this benefit was not seen in five-year survival. The role of chemotherapy for malignant GCTB remains controversial and there is still insufficient evidence of survival benefit. Only one patient underwent radiation and died of metastasis in present study, the effectiveness data of radiation is limited and inconclusive and Chen et al. [24] suggested that RT should not be recommended as a regular therapeutic method for malignant GCTB but Lin et al. [25] considered as independent prognostic factors among patients.
This study has its limitations. The total number of cases included in this study are still relatively small when compared to studies of other diseases and malignancies. This limits its power to identify associations between various factors and patient outcomes. It is also a retrospective observational study and assessment of the effect of a variety of interventions is confounded by the variety of factors that may have contributed to the selection of different interventions for the subgroups of patients receiving them. The mean duration of follow-up of 4.5 years is also relatively short for a condition where late events are known to occur. In this series, molecular diagnosis of H3F3A G34W mutation was not evaluated owing to many of the cases in the series predating the introduction of H3F3A G34W and the retrospective nature of this study. With respect to the possibility of misdiagnosis of primary tumors in cases of secondary malignant GCTB without the use of this molecular tool. Should this have been a significant issue, reduction in the observed mean latent period to malignant recurrence would be the expected effect of misdiagnosed cases. While this possibility cannot be completely excluded, if it had occurred it would in fact lend greater weight to our observation of a longer latent period being observed in malignant recurrence for secondary malignant GCTB. These limitations notwithstanding, this work is the study of a relatively large number of patients with a rare variant of an uncommon tumor managed a single centre with medium term follow-up.
5. Conclusion
In conclusion, the prognoses of both primary and secondary malignant GCTB are not optimistic. Initial diagnosis is critical for appropriate treatment of primary malignant GCTB and correlating radiologic imaging with histologic findings is crucial. When evaluating patients suspected of recurrent GCTB, 4 years can be applied as a threshold value above which there should be a high index of suspicion for malignant transformation. A more aggressive radiologic appearance and the development of pulmonary metastasis should also increase the index of suspicion for malignant GCTB. Achieving adequate margins in surgery decreased the recurrent rate in malignant GCTBs, and surgery and chemotherapy may be superior to surgery alone in delaying the onset of pulmonary metastasis, although no overall survival benefit was found in this study.
Declaration of Competing Interest
Dr. G Douglas Letson is the Consultant for Stryker Orthopedics, The other authors declare that there are no conflicts of interest.
Source of funding
This research was supported by Chinese Society of Clinical Oncology (CSCO) Research Foundation (Y-2019GCTB-002, Y-young2019-070), National Natural Science Foundation of China (51973021), Beijing JST Research Funding (ZR-201902, YGQ-201925).
CRediT authorship contribution statement
Weifeng Liu: Conceptualization, Data curation, Formal analysis, Investigation, Visualization, Methodology, Writing - original draft, Writing - review & editing. Chung Ming Chan: Conceptualization, Data curation, Investigation, Validation, Writing - original draft, Writing - review & editing. Lihua Gong: Conceptualization, Data curation, Investigation, Methodology, Writing - original draft, Writing - review & editing. Marilyn M Bui: Conceptualization, Data curation, Investigation, Writing - review & editing. Gang Han: Conceptualization, Methodology, Writing - review & editing. G. Douglas Letson: Conceptualization, Investigation, Writing - review & editing. Yongkun Yang: Conceptualization, Data curation, Writing - review & editing. Xiaohui Niu: Conceptualization, Methodology, Project administration, Resources, Supervision, Writing - review & editing.
Acknowledgements
The authors thank David Cheong MD who reviewed the manuscript, Zhen Huang MD, Yuan Li MD, Hairong Xu MD, Lin Hao MD, Qing Zhang MD, Tao Jin MD, for their contribution to the data collection of this series.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jbo.2020.100334.
Contributor Information
Weifeng Liu, Email: liuweifengjst@126.com.
Chung Ming Chan, Email: chancm@ortho.ufl.edu.
Lihua Gong, Email: lhgong2005@126.com.
Marilyn M Bui, Email: Marilyn.Bui@moffitt.org.
Gang Han, Email: gang.han@tamu.edu.
G. Douglas Letson, Email: douglas.letson@moffitt.org.
Yongkun Yang, Email: Yangykhhh@163.com.
Xiaohui Niu, Email: niuxiaohui@263.net.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Rock M.G., Sim F.H., Unni K.K., Witrak G.A., Frassica F.J., Schray M.F., Beabout J.W., Dahlin D.C. Secondary malignant giant-cell tumor of bone. Clinicopathological assessment of nineteen patients. J. Bone Joint Surg. Am. 1986;68:1073–1079. [PubMed] [Google Scholar]
- 2.Dahlin D.C., Cupps R.E., Johnson E.W. Giant-cell tumor: a study of 195 cases. Cancer. 1970;25:1061–1070. doi: 10.1002/1097-0142(197005)25:5<1061::aid-cncr2820250509>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 3.Bertoni F., Bacchini P., Staals E.L. Malignancy in giant cell tumor of bone. Cancer. 2003;97:2520–2529. doi: 10.1002/cncr.11359. [DOI] [PubMed] [Google Scholar]
- 4.Domovitov S.V., Healey J.H. Primary malignant giant-cell tumor of bone has high survival rate. Ann. Surg. Oncol. 2010;17:694–701. doi: 10.1245/s10434-009-0803-z. [DOI] [PubMed] [Google Scholar]
- 5.Palmerini E., Picci P., Reichardt P., Downey G. Malignancy in giant cell tumor of bone: a review of the literature. Technol. Cancer Res. Treat. 2019;18 doi: 10.1177/1533033819840000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart F.W., Coley B.L., Farrow J.H. Malignant giant cell tumor of bone. Am. J. Pathol. 1938;14:515–536. [PMC free article] [PubMed] [Google Scholar]
- 7.Hutter R.V., Worcester J.N., Francis K.C., Foote F.W., Stewart F.W. Benign and malignant giant cell tumors of bone. A clinicopathological analysis of the natural history of the disease. Cancer. 1962;15:653–690. doi: 10.1002/1097-0142(196207/08)15:4<653::aid-cncr2820150402>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 8.Nascimento A.G., Huvos A.G., Marcove R.C. Primary malignant giant cell tumor of bone: a study of eight cases and review of the literature. Cancer. 1979;44:1393–1402. doi: 10.1002/1097-0142(197910)44:4<1393::aid-cncr2820440433>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 9.Anract P., De Pinieux G., Cottias P., Pouillart P., Forest M., Tomeno B. Malignant giant-cell tumours of bone. Clinico-pathological types and prognosis: a review of 29 cases. Int. Orthop. 1998;22:19–26. doi: 10.1007/s002640050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wittekind C., Compton C.C., Greene F.L., Sobin L.H. TNM residual tumor classification revisited. Cancer. 2002;94:2511–2516. doi: 10.1002/cncr.10492. [DOI] [PubMed] [Google Scholar]
- 11.Niu X., Zhang Q., Hao L., Ding Y., Li Y., Xu H., Liu W. Giant cell tumor of the extremity: retrospective analysis of 621 Chinese patients from one institution. J. Bone Joint Surg. Am. 2012;94:461–467. doi: 10.2106/JBJS.J.01922. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y., Huang Z., Niu X., Xu H., Li Y., Liu W. Clinical characteristics and risk factors analysis of lung metastasis of benign giant cell tumor of bone. J. Bone Oncol. 2017;7:23–28. doi: 10.1016/j.jbo.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enneking W.F., Dunham W., Gebhardt M.C., Malawar M., Pritchard D.J. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin. Orthop. 1993:241–246. [PubMed] [Google Scholar]
- 14.Gong L., Liu W., Sun X., Sajdik C., Tian X., Niu X., Huang X. Histological and clinical characteristics of malignant giant cell tumor of bone. Virchows Arch. Int. J. Pathol. 2012;460:327–334. doi: 10.1007/s00428-012-1198-y. [DOI] [PubMed] [Google Scholar]
- 15.Amary F., Berisha F., Ye H., Gupta M., Gutteridge A., Baumhoer D., Gibbons R., Tirabosco R., O’Donnell P., Flanagan A.M. H3F3A (Histone 3.3) G34W immunohistochemistry: a reliable marker defining benign and malignant giant cell tumor of bone. Am. J. Surg. Pathol. 2017;41:1059–1068. doi: 10.1097/PAS.0000000000000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsukamoto Y., Futani H., Kihara T., Watanabe T., Kumanishi S., Matsuo S., Hirota S., Ueda T., Yamamoto H., Yoshiya S. An extremely rare case of primary malignancy in giant cell tumor of bone, arising in the right femur and harboring H3F3A mutation. Pathol. Res. Pract. 2018;214:1504–1509. doi: 10.1016/j.prp.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 17.K.-I. Yoshida, Y. Nakano, M. Honda-Kitahara, S. Wakai, T. Motoi, K. Ogura, N. Sano, T. Shibata, T. Okuma, S. Iwata, A. Kawai, K. Ichimura, A. Yoshida, Absence of H3F3A mutation in a subset of malignant giant cell tumor of bone, Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc. 32 (2019) 1751–1761. https://doi.org/10.1038/s41379-019-0318-5. [DOI] [PubMed]
- 18.Palmerini E., Chawla N.S., Ferrari S., Sudan M., Picci P., Marchesi E., Leopardi M.P., Syed I., Sankhala K.K., Parthasarathy P., Mendanha W.E., Pierini M., Paioli A., Chawla S.P. Denosumab in advanced/unresectable giant-cell tumour of bone (GCTB): For how long? Eur. J. Cancer Oxf. Engl. 2017;1990(76):118–124. doi: 10.1016/j.ejca.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 19.Rutkowski P., Ferrari S., Grimer R.J., Stalley P.D., Dijkstra S.P.D., Pienkowski A., Vaz G., Wunder J.S., Seeger L.L., Feng A., Roberts Z.J., Bach B.A. Surgical downstaging in an open-label phase II trial of denosumab in patients with giant cell tumor of bone. Ann. Surg. Oncol. 2015;22:2860–2868. doi: 10.1245/s10434-015-4634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wojcik J., Rosenberg A.E., Bredella M.A., Choy E., Hornicek F.J., Nielsen G.P., Deshpande V. Denosumab-treated giant cell tumor of bone exhibits morphologic overlap with malignant giant cell tumor of bone. Am. J. Surg. Pathol. 2016;40:72–80. doi: 10.1097/PAS.0000000000000506. [DOI] [PubMed] [Google Scholar]
- 21.Aponte-Tinao L.A., Piuzzi N.S., Roitman P., Farfalli G.L. A high-grade sarcoma arising in a patient with recurrent benign giant cell tumor of the proximal tibia while receiving treatment with denosumab. Clin. Orthop. 2015;473:3050–3055. doi: 10.1007/s11999-015-4249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Errani C., Tsukamoto S., Mavrogenis A.F. How safe and effective is denosumab for bone giant cell tumour? Int. Orthop. 2017;41:2397–2400. doi: 10.1007/s00264-017-3536-9. [DOI] [PubMed] [Google Scholar]
- 23.Scully S.P., Mott M.P., Temple H.T., O’Keefe R.J., O’Donnell R.J., Mankin H.J. Late recurrence of giant-cell tumor of bone. A report of four cases. J. Bone Joint Surg. Am. 1994;76:1231–1233. doi: 10.2106/00004623-199408000-00013. [DOI] [PubMed] [Google Scholar]
- 24.W. Chen, Z. Yan, V. Tirumala, Malignant giant cell tumor of bone or soft tissue treated by surgery with or without radiotherapy, J. Orthop. Res. n/a (n.d.). https://doi.org/10.1002/jor.24698. [DOI] [PubMed]
- 25.Lin J.-L., Wu Y.-H., Shi Y.-F., Lin H., Nisar M., Meftah Z., Xu C., Chen J.-X., Wang X.-Y. Survival and prognosis in malignant giant cell tumor of bone: a population-based analysis from 1984 to 2013. J. Bone Oncol. 2019;19 doi: 10.1016/j.jbo.2019.100260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.