Abstract
The use of plant and plant products in the synthesis of silver nanoparticles (AgNPs) and gold nanoparticles (AuNPs) is made possible because of the natural inherent phytochemicals responsible for the reduction of respective metallic salts to nanoparticle forms, and ensuring therapeutic applicability. In this study, synthesis of AgNPs and AuNPs was performed using two different aqueous extraction methods for Crassocephalum rubens: maceration using laboratory method of extraction (cold aqueous extract of Crassocephalum rubens (AECR)), and decoction using traditional healer's method of extraction (hot aqueous crude extract of Crassocephalum rubens (CECR)). The synthesized nanoparticles were characterized using various methods, and in vitro antioxidant potential were thereafter investigated. The characterization results indicated the formation of mostly spherical-shaped AgNPs and AuNPs with surface plasmon resonance (SPR) band of 470 nm and 540 nm, respectively. The nanoparticles possess high antioxidant potentials but AECR synthesized AuNPs exhibited the least phytochemical contents and antioxidant potential when compared to other nanoparticles. It can therefore be concluded that extraction method and nanoparticle type are important factors that could influence the antioxidant properties of the nanoparticles. Further studies using these nanoparticles as anticancer or anti-inflammatory agent in both in vitro and in vivo are underway.
Keywords: Materials science, Nanotechnology, Antioxidant, Crassocephalum rubens, Extraction, Gold nanoparticles, Silver nanoparticles
Materials science; Nanotechnology; Antioxidant; Crassocephalum rubens; Extraction; Gold nanoparticles; Silver nanoparticles.
1. Introduction
Metallic nanoparticles, including gold nanoparticles (AuNPs) and silver nanoparticles (AuNPs), have received much attention because of the catalytic, electrical, magnetic, and optical properties exhibited when compared to their respective bulk metallic state [1]. Physical and chemical methods have been employed for the synthesis of nanoparticles, but several associated limitations have led to the use of biological methods, including the use of microorganisms and plants/plant products. These limitations include time consumption during synthesis and the use of chemicals which might produce toxic nanoparticles, and are not eco-friendly [2, 3].
Plant and plant products are currently used in the synthesis of AuNPs and AgNPs, because of their naturally inherent phytochemicals. It has been shown that extract of medicinal plants may be used in the synthesis of metallic nanoparticles, most especially AuNPs and AgNPs, and several biological activities of the biosynthesized nanoparticles have been reported [4, 5, 6, 7, 8].
Crassocephalum rubens belongs to the family Asteraceae. Among the Yorubas in Nigeria, it is called Ebolo. It is found in many African countries, especially in West and Central Africa. The leaves are eaten raw or cooked in sauce. The leaves have shown to possess anticancer and hepatoprotective properties [9, 10]. It is used in the treatment of indigestion, liver and heart problems, cough, allergies, and as purgative [11]. Phytochemical such as coumarins, flavonoids, mucilage, proanthocyanidin, steroids, tannins, and reducing compounds have been found in the leaves of C. rubens [12]. Methanolic extract of the leaves of C. rubens have been reported to exhibit in vitro and in vivo antioxidant activity [13].
Decoction (using traditional) and maceration (using laboratory) methods of aqueous extraction were considered in this study because it has been reported that combination of phytochemicals in plant diet may be more efficient than isolated compounds in cancer chemoprotection [9]. In quest for finding possible solution to free radical related diseases, it is imperative to try several means to explore cost efficient, less toxic, and targeted therapies. Aqueous medium was considered in this study, as it is the most preferred medium for household consumption and traditional herbal preparation. It is, therefore, necessary to synthesize nanoparticles using aqueous medium for C. rubens extraction, and investigate the in vitro antioxidant activities of the biosynthesized AuNPs and AgNPs. This will provide preliminary information on the effect of the biosynthesized nanoparticles in vivo, and thus its potential use in nanomedicine.
2. Materials and methods
2.1. Chemicals
Chemicals including 1,1-Diphenyl-2-picryl-hydrazyl (DPPH), methanol, phosphate buffer, potassium ferricyanide (C6N6 FeK3), Trichloroacetic acid (C2HCl3O2), Iron (III) chloride (FeCl3), hydrochloric acid (HCl), nitric acid (HNO3), sodium phosphate, Ammonium molybdate ((NH4)2MoO4), Di-methylsulfoxide (CH3)2SO, and gold (III) chloride trihydrate (HAuCl4·3H2O), silver nitrate (AgNO3) were purchased from Sigma Aldrich, USA. All glassware was rinsed with aqua regia before used for the synthesis of AuNPs. All reagents used were of analytical grade.
2.2. Plant preparation
The leaves of Crassocephalum rubens were obtained from Oyi farmland, Ora-Igbomina, Osun State Nigeria. The leaves were authenticated at the Forestry Research Institute of Nigeria, Ibadan Nigeria. A sample of the plant portion was deposited at the Institute's Herbarium with voucher number FHI 112047. The leaves were plucked out from the stem, and air-dried at 25 °C. The dried leaves were then ground.
2.3. Plant extraction
Two different aqueous extraction methods (maceration and decoction) were considered for Crassocephalum rubens. In the maceration, using laboratory extraction method, 10 g of powdered dried leaves of Crassocephalum rubens was soaked in 100 mL of distilled water at room temperature for 24 h. It was then filtered using cheese cloth, followed by Whatman filter paper (number 1). This was freeze dried and the concentrate (cold aqueous extract of Crassocephalum rubens (AECR)) was stored at 4 °C until use. In the decoction, using traditional extraction method, 10 g of C. rubens leaves was boiled in 100 mL distilled water at 100 °C for 45 min. This was thereafter filtered using cheese cloth, and thereafter with Whatman filter paper (number 1), and then centrifuged at 10000 x g for 10 min, to remove undissolved particulates. The supernatant (hot aqueous crude extract of C. rubens (CECR)) was kept at 4 °C until further use.
2.4. Biosynthesis of nanoparticles
The biosynthesis of AgNPs and AuNPs was performed following methods described by Huo et al. [5] and Jin et al. [4], with some modifications.
2.4.1. Synthesis and characterization of green synthesized AgNPs using extracts of C. rubens leaves
Green synthesis of AgNPs was carried out by adding 10 mL AECR (0.1 g extract/mL distilled water) or CECR (from section 2.3 above) to a beaker containing 90 mL aqueous solution of AgNO3 (1 mM) solution on a hot plate at 50 °C, and continuously stirred for 20 min using a magnetic stirrer. The synthesized nanoparticles were centrifuged twice at 15,000 x g for 15 min after each washing with distilled water. This was done to remove unbound and excess plant materials in the mixture. The pellet was left overnight at room temperature to air dry, and then kept at 4 °C until further use.
The reaction mixture used in the synthesis of AgNPs was optimized by using five different volumes of AECR/CECR (0.1 g extract/mL) to aqueous solutions of AgNO3 (1 mM) at synthesis ratio 10:90, 20:80, 30:70, 40:60, 50:50, respectively. The addition was performed in a single step. This is because bio-reduction of metal ions usually occur readily in solution, resulting in stable nanoparticles [14]. The concentration (10 mL, 0.1 g extract/mL) of AECR/CECR in 90 mL aqueous solutions of AgNO3 (1 mM) was used, as increased volumes resulted in aggregation of the nanoparticles.
2.4.2. Synthesis and characterization of green synthesized AuNPs using extracts of C. rubens leaves
Either 10 mL of AECR (0.1 g extract/mL distilled water) or CECR (from section 2.3 above) was added to a beaker containing aqueous solution of gold (III) chloride trihydrate (190 mL, 1 mM) on a hot plate at 50 °C, and the reaction was continuously stirred for 10 min using a magnetic stirrer. The resultant nanoparticles were centrifuged twice at 15,000 x g for 15 min, after each washing with distilled water, to remove unbound and excess plant materials in the mixture. The pellet was thereafter left overnight at room temperature to air dry and kept at 4 °C until further use.
The reaction mixture ratio was optimized by measuring five different volumes of AECR/CECR (0.1 g extract/mL) were added to aqueous solutions of gold (III) chloride trihydrate (1 mM) at synthesis ratio 10:190, 20:180, 30:170, 40:160, 50:150, respectively. The addition was performed in a single step. This is because bio-reduction of metal ions usually occur readily in solution, resulting in stable nanoparticles [14]. The concentration (10 mL, 0.1 g extract/mL) of AECR/CECR in 190 mL aqueous solutions of gold (III) chloride trihydrate (1 mM) was used, as increased volumes resulted in aggregation of the nanoparticles.
2.4.3. Characterization of synthesized AuNPs and AgNPs
The ultraviolet-visible (UV-Visible) spectroscopy of the AgNPs and AuNPs was performed using UV-Vis spectrophotometer, to assess the wavelength and stability of the nanoparticles. The sizes, shapes, and morphology of the synthesized AgNPs and AuNPs were investigated by transmission electron microscopy (TEM) and scanning electron microscopy (SEM). The presence of functional groups was detected by the Fourier transform infrared (FTIR) spectroscopy using dried pellets of these nanoparticles.
2.5. Quantitative phytochemical analysis
2.5.1. Total phenolic contents
The total phenolic contents (TPC) of the extract and nanoparticles was determined using Folin Ciocalteu's reagent, as described by [15]. Each sample (100 μL, 100 μg/mL) or gallic acid (100 μL, 100 μg/mL) was mixed with 500 μL Folin Ciocalteu's reagent and 1.5 mL sodium carbonate (20%). The mixture was shaken thoroughly and made up to 10 mL with distilled water. The mixture was allowed to stand for 2 h. The absorbance was determined at 765 nm against a blank containing all reagents without samples or gallic acid at the same conditions. All determinations were carried out in duplicates. The total phenolic contents were calculated from standard curve obtained using varying concentrations of gallic acid (5–20 μg/mL), and result presented in mg gallic acid equivalent (GAE) per g sample.
2.5.2. Total flavonoid contents
The total flavonoid contents (TFC) of the extract and nanoparticles were measured by aluminum chloride colorimetric assay according to a method described by Zhishen et al. [16], with slight modifications. Extract or nanoparticles (1.0 mL, 105 μg/mL) was mixed with 1.0 mL AlCl3 (5%). The mixture was allowed to stand at room temperature for 5 min, after which 2.0 mL NaNO2 (7%) was added. Thereafter, 1.0 mL sodium hydroxide (1%) was added to the reaction mixture, and the absorbance was read at 510 nm against a blank. Results were calculated from standard curve obtained using varying concentrations of quercetin (5–20 μg/mL) in methanol and expressed in mg of quercetin equivalent (QE) per g sample.
2.6. In vitro antioxidants determination of nanoparticles and extract
2.6.1. 1,1-Diphenyl-2-picrythydrazyl (DPPH) radical scavenging assay
The DPPH• assay was performed according to a method described by Shirwaikar et al. [17], with some modifications. Two mL solution of 0.1 mM DPPH• (in methanol) was added to 2 mL of various concentrations of each nanoparticle, plant extract, or ascorbic acid (standard). An equal amount of DPPH• and methanol served as control. The reaction mixture was kept in the dark at 30 °C for 20 min, and the absorbance recorded read at 517 nm. The experiment was performed in duplicates. The % scavenging activity was determined by Eq. (1):
| % DPPH radical scavenging activity = [(Abs of control –Abs of sample) / (Abs of control)] x 100 | (1) |
where Abs of control = absorbance of DPPH radical + methanol, Abs of sample = absorbance of DPPH radical + sample/standard.
2.6.2. Inhibition of lipid peroxidation
The ability of the nanoparticles to inhibit lipid peroxidation was carried out according to a method described by Ruberto et al. [18], with some modifications. Briefly, 0.1 mL of egg yolk homogenate (10% v/v) was added to 0.5 mL of varying concentrations of the nanoparticles, extract or ascorbic acid (standard) in test tubes. The volume in each test tube was made up to 1 mL with distilled water. Afterwards, 0.05 mL ferrous sulfate was added and incubated at 37 °C for 30 min. Then, 0.5 mL acetic acid-thiobarbituric acid reagent prepared in dimethyl sulfoxide was added. The resulting mixture was incubated at 95 °C for 1 h. The mixture was then allowed to cool and centrifuged at 650 × g for 5 min. The absorbance of the supernatant was read at 532 nm, and the percentage inhibition was calculated using Eq. (2) below:
| % Inhibition = (Abs of blank – Abs of sample)/Abs of blank × 100. | (2) |
2.7. Statistical analysis
Data were analyzed using SPSS software package for Windows, and values were expressed as Mean ± SD. One-way analysis of variance (ANOVA) was used to determine the levels of significance, followed by multiple comparison by Tukey's tests. P values <0.05 was considered as statistically significant.
3. Results and discussion
3.1. Visual observation and characterization of the biosynthesized AgNPs and AuNPs
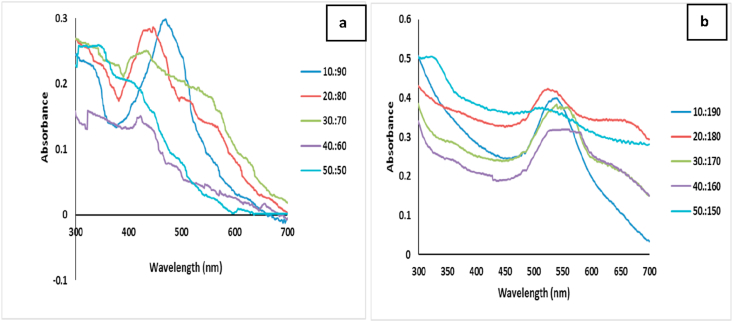
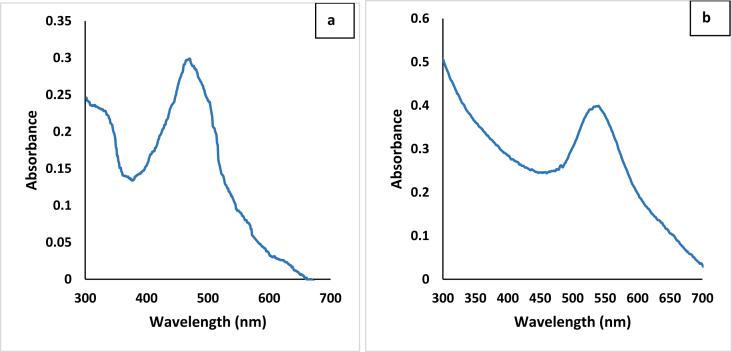
Increased volumes of the plant extract resulted in aggregation of the nanoparticles within 24 h and broader SPR bands (Figure 1a and b). The 20:80, 30:70, 40:60 and 50:50 (plant extract: AgNO3 solution), and 20:180, 30:170, 40:160, 50:150 (plant extract: gold (III) chloride trihydrate solution) produced aggregation of the nanoparticles upon formation, and were not considered further in this study. Agglomeration of nanoparticles have been reported to produce more irregular shapes, which could be linked to increase concentration of reducing agent [19]. The combination of 10 mL of AECR/CECR and 90 mL aqueous solution of AgNO3 (1 mM) or 10 mL of AECR/CECR and 190 mL aqueous solution of gold (III) chloride trihydrate (1 mM) were more suited for the synthesis of AgNPs and AuNPs with sharp intense peaks at 470 and 540 nm, respectively (Figure 2a and b).
Figure 1.
UV-Visible spectrum of C. rubens synthesized nanoparticles of different volume of C. rubens extract to (a) aqueous solution of AgNO3 (1 mM) at 10:90, 20:80, 30:70, 40:60, 50:50 (b) aqueous solution of gold (III) chloride trihydrate (1 mM) at 10:190, 20:180, 30:170, 40:160, 50:150.
Figure 2.
UV-Visible spectrum of C. rubens synthesized (a) silver nanoparticles, (b) gold nanoparticles.
The formation of AgNPs and AuNPs from extracts of C. rubens were monitored by a reaction change in colour to light brown within 30 min for AgNPs, and to purple within 20 min for AuNPs. These colour changes suggest reduction and the synthesis of the nanoparticles, which are related to the surface plasmon resonance (SPR) band of both AgNPs and AuNPs [5]. The SPR bands of the biosynthesized AgNPs and AuNPs using C. rubens were within the range of previously synthesized AgNPs [5, 20], and AuNPs [5, 21, 22] from various medicinal plants.
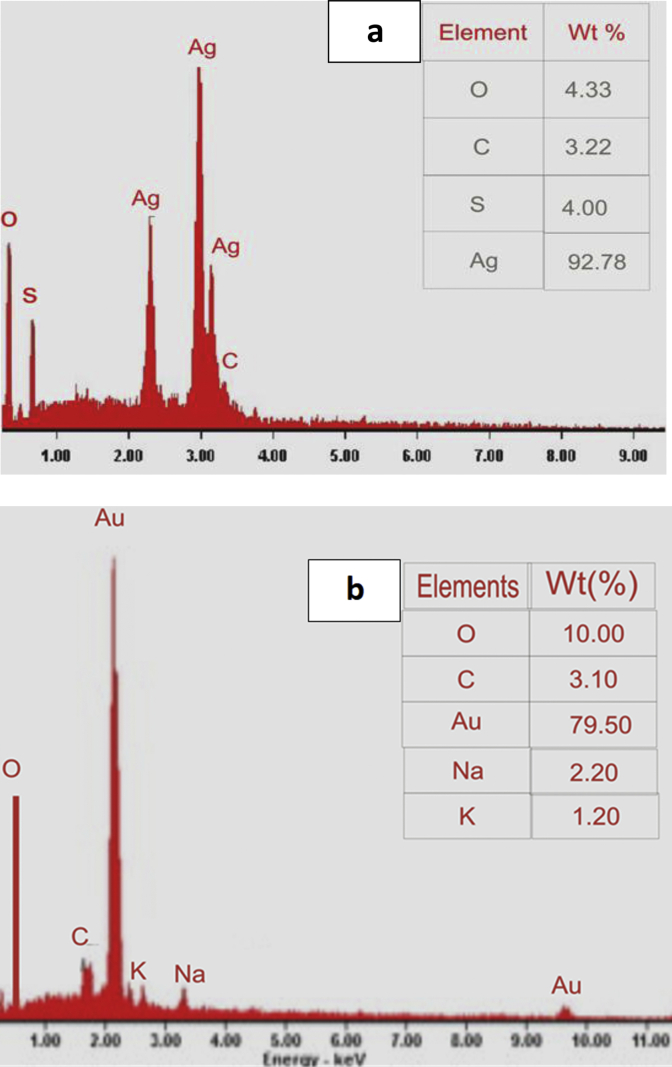
Further, elemental composition of synthesized AgNPs and AuNPs was determined by energy dispersive X-ray (EDX) spectrum, and higher percentage of silver signals when compared to other elements was revealed (Figure 3a and b, respectively). The AgNPs spectrum reveals strong signals (Figure 3a) in the silver region (92.78%), which indicates the formation of AgNPs. However, elements such as oxygen, sulphur and carbon are present at low concentrations, which could have resulted from the compounds present in the extract. For the AuNPs, it was noted that gold (79.50%) was the major element, with some other elements at low percentages (Figure 3b), which might be from the compounds from the extract. This indicates the reduction of gold ions to elemental gold. Strong signal of gold metal have reportedly been observed around 2 keV, which is a characteristic peak of AuNPs [4, 23], and around 3 keV for silver metal, which is a characteristic peak of silver nanocrystalline [5, 24].
Figure 3.
Energy dispersive X-ray spectrum of C. rubens synthesized (a) silver nanoparticles, (b) gold nanoparticles.
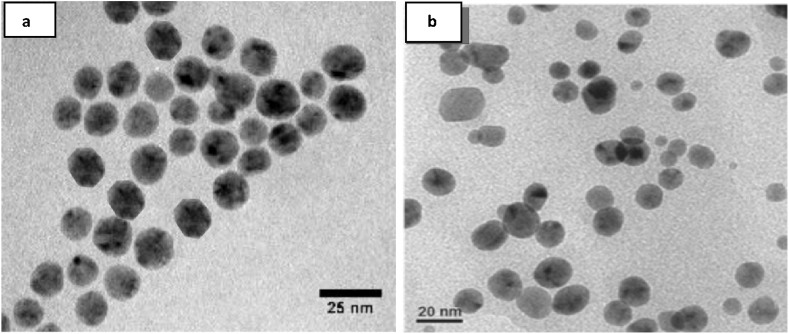
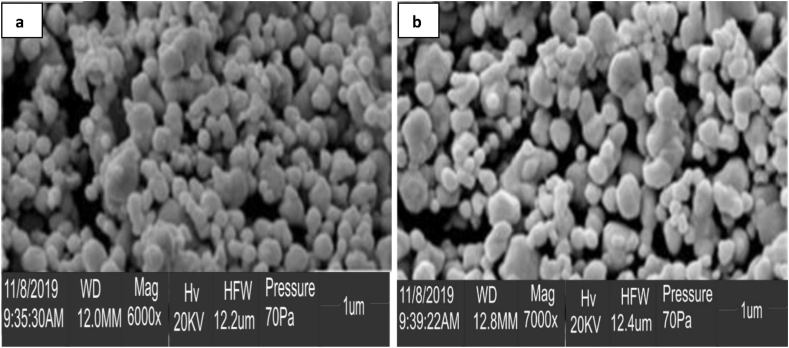
The TEM and SEM images (Figures 4 and 5) revealed the shape, size, and morphology features of the biological synthesized AuNPs and AgNPs. The size range was found to be 15–25 nm for AgNPs, and presented mostly spherical shapes and some hexagonal shapes. On the other hand, the size of AuNPs was ranging from 10 – 20 nm in diameter, and were mostly spherical in shape (Figure 4 a, b). Polydispersed nanoparticles were also noted by the TEM and SEM images, which could result from the presence of several reducing phytochemicals in the extract of C. rubens. Similar observations have previously been reported [4, 25].
Figure 4.
TEM images of C. rubens synthesized (a) AgNPs, (b) AuNPs.
Figure 5.
SEM images of C. rubens synthesized (a) AgNPs, (b) AuNPs.
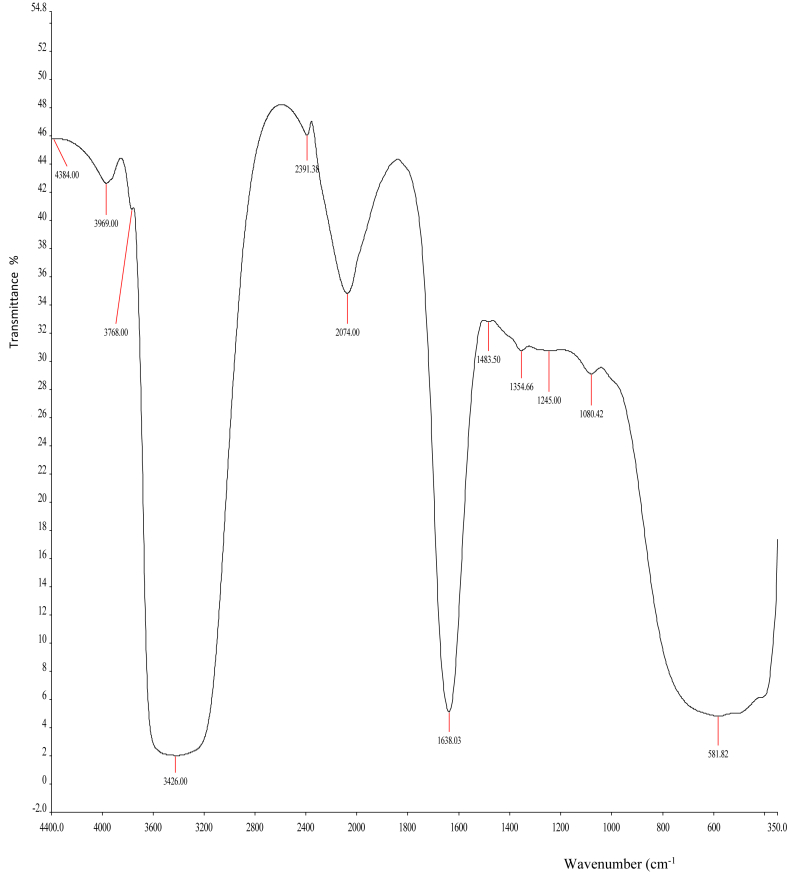
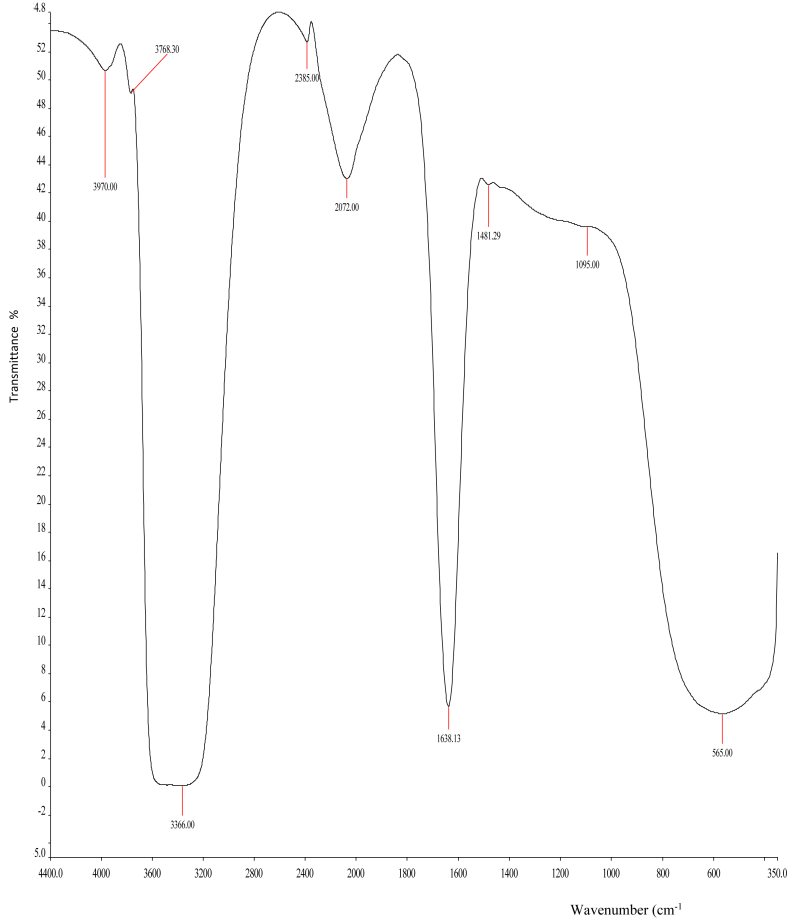
As shown in Figures 6 and 7, the FTIR spectra of CECR and CECR-AuNPS, respectively, were recorded in the frequency range between 4400 and 350 cm−1 in the % transmittance (%T). It was shown that there were slight shifts in the FTIR peaks of C. rubens leaves extract (4384, 3969, 3768, 3426, 2391.38, 2074, 1638.03, 1483.5, 1354.66, 1245, 1080.42, 581.82 cm−1) and the synthesized AuNPs (3970, 3763.3, 3366, 2385, 2072, 1638.13, 1481.29, 1095, 565 cm−1). The absence of some peaks (4384, 1354.66 and 1245 cm−1) in the synthesized AuNPs compared to the CECR, and the slight shifts noted in the peaks suggests the involvement of some functional groups in the reduction process. The bands from 4384 up to 3426 cm−1 in the FTIR spectra corresponds to O–H stretching vibration, which indicates the presence of alcohol and phenol. It was reported that hydroxyl groups (O–H) have stronger binding ability with gold ions [26]. The peaks at 582.82 and 565 cm−1 corresponds to C–H bond.
Figure 6.
FTIR spectra of C. rubens crude extract.
Figure 7.
FTIR spectra of CECR-AuNPs.
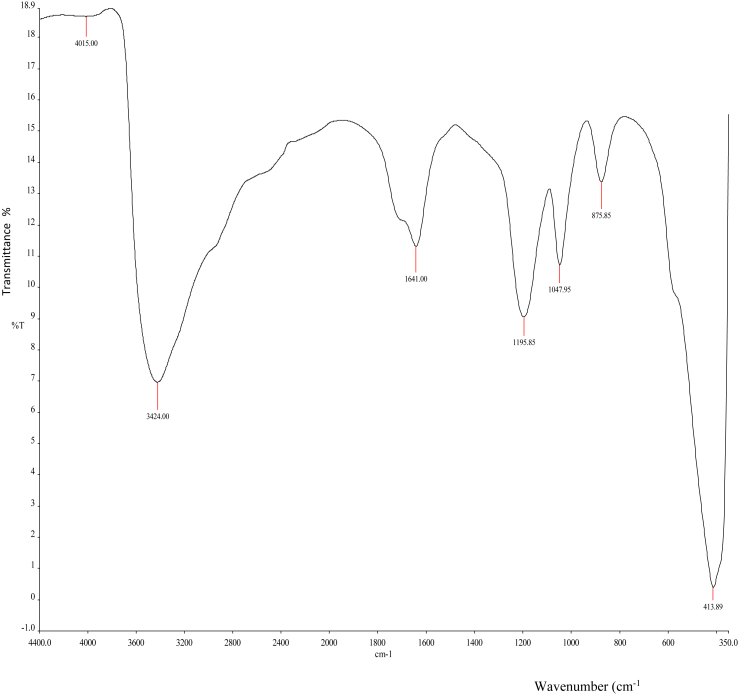
The FTIR spectra of CECR-AgNPs is shown in Figure 8 at the frequency range between 4400 and 350 cm−1 in the % transmittance (%T). It was noted that there were shifts in the FTIR peaks of C. rubens synthesized AgNPs (4015, 3424, 1641, 1195.85, 1047.95, 875.85, 413.89 cm−1) when compared to C. rubens leaves extract (Figure 6). This suggests the presence of various functional groups responsible for the reduction of silver ion to the nanoparticles.
Figure 8.
FTIR spectra of CECR-AgNPs.
3.2. Phytochemical analysis and in vitro antioxidant activities of biosynthesized AuNPs and AgNPS
3.2.1. Phytochemical screening
Phytochemicals have been reported to have a major impact in the biosynthesis of nanoparticles using medicinal plants. The total phenolics and flavonoids contents were estimated and compared. It was noted that CECR-AuNPs possessed significantly (p < 0.05) higher TPC and TFC when compared to AECR-AuNPs and the AgNPs (Table 1), although, the CECR had significantly (p < 0.05) higher TPC and TFC compared to CECR-AuNPs and other nanoparticles. These differences might be linked to the involvement of these phytochemicals in the reduction of gold or silver ions to the nanoparticles [27, 28], and also acting as capping agents. These was also confirmed by the FTIR spectra of the samples (Figures 6, 7, and 8). It was also noted that CECR possessed higher TPC and TFC when compared to AECR.
Table 1.
Total phenolics and total flavonoid contents.
| Phenolics (mg gallic acid equivalent/g sample) | Flavonoids (mg quercetin equivalent/g sample) | |
|---|---|---|
| CECR-AgNPs | 19.4 ± 2.3a | 5.5 ± 1.4a |
| CECR-AuNPs | 26.0 ± 0.2b | 15.5 ± 0.8b |
| AECR-AgNPs | 15.6 ± 1.7a | 19.4 ± 0.1bc |
| AECR-AuNPs | 3.5 ± 1.2c | 12.6 ± 0.3b |
| AECR | 37.5 ± 0.3d | 23.2 ± 1.0cd |
| CECR | 42.7 ± 2.2e | 27.0 ± 4.3d |
Values represent Mean ± SD (n = 3). p < 0.05, at different alphabet in the same column.
CECR-AgNPs (Crude extract of Crassocephalum rubens-silver nanoparticles); CECR-AuNPs (Crude extract of Crassocephalum rubens-gold nanoparticles); AECR-AgNPs (Aqueous extract of Crassocephalum rubens-silver nanoparticles); AECR-AuNPs (Aqueous extract of Crassocephalum rubens-gold nanoparticles); CECR (Crude extract of Crassocephalum rubens).
3.2.2. In vitro antioxidant activities
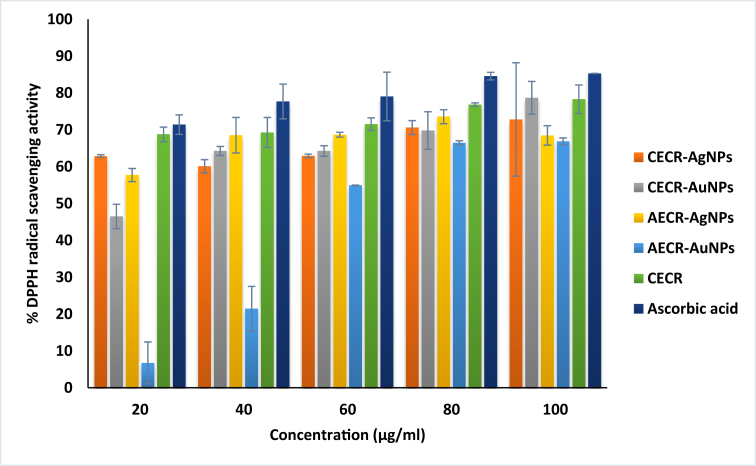
The free radicals released during various biological processes are involved in pathogenicity. The antioxidant activities of nanoparticles and CECR were carried out against DPPH radical. As shown in Figure 9, the extract (CECR) and the nanoparticles exhibited good DPPH radical scavenging activity at all tested concentrations in a dose dependent manner, except for AECR-AuNPs that rarely exhibited DPPH radical scavenging activity at lower concentrations (20 and 40 μg/mL). At the highest tested concentration (100 μg/mL), the exhibition of DPPH radical scavenging activity was in the following order: Ascorbic acid (85.30%) > CECR-AuNPs (78.69%) > CECR (78.29%) > CECR-AgNPs (72.81%) > AECR-AgNPs (68.47%) > AECR-AuNPs (66.87%) (Figure 9). Noted differences in the activities could be linked to the extraction method, indicating higher antioxidant potential with traditional extraction of the C. rubens leaves. These phytochemicals also contributed to the stabilizing of the nanoparticles. Higher DPPH radical scavenging activity was reported in Glycyrrhiza uralensis synthesized silver chloride nanoparticles (Gu-AgClNPs) when compared to Glycyrrhiza uralensis synthesized gold nanoparticles (Gu-AuNPs) [5]. Contradictions noted compared with our study could be due to differences in phytochemicals present in both plants (G. uralensis and C. rubens).
Figure 9.
Diphenyl-2-picryl-hydrazyl (DPPH) radical scavenging activities of samples.
Values represent Mean ± SD (n = 3). CECR-AgNPs (Crude extract of Crassocephalum rubens-silver nanoparticles); CECR-AuNPs (Crude extract of Crassocephalum rubens-gold nanoparticles); AECR-AgNPs (Aqueous extract of Crassocephalum rubens-silver nanoparticles); AECR-AuNPs (Aqueous extract of Crassocephalum rubens-gold nanoparticles); CECR (Crude extract of Crassocephalum rubens).
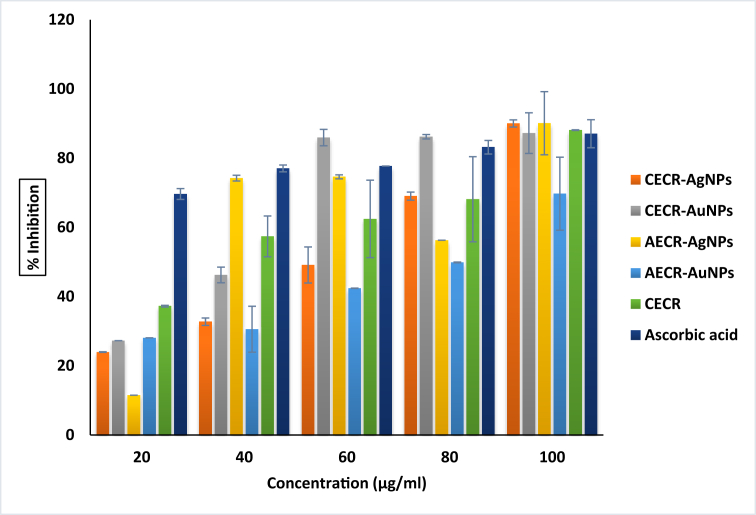
From the results in Figure 10, the CECR and the nanoparticles have the ability to inhibit lipid peroxidation in a dose-dependent manner, like the standard antioxidant (ascorbic acid). Only the AECR-AuNPs showed reduced inhibition of lipid peroxidation at all concentrations. Inhibition of lipid peroxidation at the highest concentration tested is in the following order: AECR-AgNPs (90%) > CECR-AgNPs (90%) > CECR (88%) > CECR-AuNPs (87.22%) > Ascorbic acid (87.05%) > AECR-AuNPs (69.71%) (Figure 10). It can be suggested that the extract of C. rubens and the synthesized nanoparticles could act as reducing agents, and have antioxidant defense capabilities, thereby inhibiting peroxidation of lipids. These properties are reportedly found with ascorbic acid, and is regarded as powerful scavenger of free radicals [29]. The result from this study supports the findings of Ruttkay-Nedecky et al. [30], where biological method of synthesis (green synthesis) of silver nanoparticles using green tea and coffee extracts showed higher antioxidant and free radical quenching abilities when compared to citrate reduction method.
Figure 10.
% Inhibition of lipid peroxidation.
Values represent Mean ± SD (n = 3). CECR-AgNPs (Crude extract of Crassocephalum rubens-silver nanoparticles); CECR-AuNPs (Crude extract of Crassocephalum rubens-gold nanoparticles); AECR-AgNPs (Aqueous extract of Crassocephalum rubens-silver nanoparticles); AECR-AuNPs (Aqueous extract of Crassocephalum rubens-gold nanoparticles); CECR (Crude extract of Crassocephalum rubens).
4. Conclusions
From the results obtained in this study, it can be concluded that the leaves of Crassocephalum rubens contains a wide variety of secondary metabolites that could serve as reducing and capping agent in the synthesis of nanoparticles. These compounds also enhance the antioxidant capabilities of the synthesized nanoparticles based on the in vitro antioxidant assays performed. Overall, it can be deduced from this study that AECR-AuNPs presented the least antioxidant capabilities to scavenge free radicals. As observed in this study, extraction method is a major factor that influences antioxidant properties of the nanoparticles. It can therefore be suggested that extraction of C. rubens through the decoction method is more suitable than the maceration method for the synthesis of AuNPs, while either of the methods for the synthesis of AgNPs. Further studies would be carried out to investigate the biomedical applications of these nanoparticles, including the anti-inflammatory and anticancer potentials in vivo.
Declarations
Author contribution statement
Olusola B. Adewale: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Kayode A. Egbeyemi: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Joan O. Onwuelu, Sotonye S. Potts-Johnson, Jonathan Johnson: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Scholastica O. Anadozie: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Adewale O. Fadaka: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Olukemi A. Osukoya, Bukola T. Aluko: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Tajudeen O. Obafemi: Conceived and designed the experiments; Wrote the paper.
Amos Onasanya: Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors acknowledge the technical support from Mr J.O. Awe and Mr O.B. Afolabi.
References
- 1.Khatami M., Sharifi I., Nobre M.A.L., Zafarnia N., Aflatoonian M.R. Waste-grass-mediated green synthesis of silver nanoparticles and evaluation of their anticancer, antifungal and antibacterial activity. Green Chem. Lett. Rev. 2018;11(2):125–134. [Google Scholar]
- 2.Yahyaei B., Nouri M., Bakherad S., Hassani M., Pourali P. Effects of biologically produced gold nanoparticles: toxicity assessment in different rat organs after intraperitoneal injection. Amb. Express. 2019;9(1):38. doi: 10.1186/s13568-019-0762-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Latha D., Sampurnam S., Arulvasu C., Prabu P., Govindaraju K., Narayanan V. Biosynthesis and characterization of gold nanoparticle from Justicia adhatoda and its catalytic activity. Mater. Today: Proceedings. 2018;5(2, Part 3):8968–8972. [Google Scholar]
- 4.Jin X., Simeon N.C., Palma J., Kim D., Ngabire D., Kim N.-H., Tarte N.H., Kim G.-D. Anticancer activity of Sasa borealis leaf extract-mediated gold nanoparticles AU - Patil, Maheshkumar Prakash. Artif. Cells Nanomed. Biotechnol. 2018;46(1):82–88. doi: 10.1080/21691401.2017.1293675. [DOI] [PubMed] [Google Scholar]
- 5.Huo Y., Singh P., Kim Y.J., Soshnikova V., Kang J., Markus J., Ahn S., Castro-Aceituno V., Mathiyalagan R., Chokkalingam M., Bae K.S., Yang D.C. Biological synthesis of gold and silver chloride nanoparticles by Glycyrrhiza uralensis and in vitro applications. Artif. Cells Nanomed. Biotechnol. 2018;46(2):303–312. doi: 10.1080/21691401.2017.1307213. [DOI] [PubMed] [Google Scholar]
- 6.Lakshmanan G., Sathiyaseelan A., Kalaichelvan P.T., Murugesan K. Plant-mediated synthesis of silver nanoparticles using fruit extract of Cleome viscosa L.: assessment of their antibacterial and anticancer activity. Karbala Int. J. Modern Sci. 2018;4(1):61–68. [Google Scholar]
- 7.Chahardoli A., Karimi N., Sadeghi F., Fattahi A. Green approach for synthesis of gold nanoparticles from Nigella arvensis leaf extract and evaluation of their antibacterial, antioxidant, cytotoxicity and catalytic activities. Artif. Cells Nanomed. Biotechnol. 2018;46(3):579–588. doi: 10.1080/21691401.2017.1332634. [DOI] [PubMed] [Google Scholar]
- 8.Khattak U., Ullah R., Khan S., Afriq S., Rauf A., Hassanien M. Synthesis, characteristics and biological activities of silver nanoparticles from Euphorbia dracunculoides. EurAsia J. BioSci. 2019;13(2):2249–2260. [Google Scholar]
- 9.Alhassan S.O., Atawodi S.E.-O. Chemopreventive effect of dietary inclusion with Crassocephalum rubens (Juss ex Jacq) leaf on N-methyl-N-nitrosourea (MNU)-induced colorectal carcinogenesis in Wistar rats. J. Funct. Foods. 2019;63:103589. [Google Scholar]
- 10.Adewale O.B., Onasanya A., Anadozie S.O., Abu M.F., Akintan I.A., Ogbole C.J., Olayide, Afolabi O.B., Jaiyesimi K.F., Ajiboye B.O., Fadaka A.O. Evaluation of acute and subacute toxicity of aqueous extract of Crassocephalum rubens leaves in rats. J. Ethnopharmacol. 2016;188:153–158. doi: 10.1016/j.jep.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Ojo F.M., Adenegan-Alakinde T.A. Phytochemical studies of four indigenous vegetables commonly consumed in ile-ife, South-West Nigeria. Int. J. Curr. Sci. 2017;21(6):E6–13. [Google Scholar]
- 12.Adjatin A., Dansi A., Badoussi E., Loko Y., Dansi M., Gbaguidi F., Azokpota P., Ahissou H., Akoègninou A., Akpagana K. Phytochemical screening and toxicity studies of Crassocephalum rubens (Juss. ex Jacq.) S. Moore and Crassocephalum crepidioides (Benth.) S. Moore consumed as vegetable in Benin. J. Chem. Pharmaceut. Res. 2013;5(6):160–167. [Google Scholar]
- 13.Omoregie E., Osagie A., Iruolaje E. In vitro antioxidant activity and the effect of methanolic extracts of some local plants on nutritionally stressed rats. Pharmacologyonline. 2011;1:23–56. [Google Scholar]
- 14.Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011;13:2638–2650. [Google Scholar]
- 15.Kumaran A., Karunakaran R.J. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. Lwt - Food Sci. Technol. 2007;40(2):344–352. [Google Scholar]
- 16.Zhishen J., Mengcheng T., Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64(4):555–559. [Google Scholar]
- 17.Shirwaikar A., Shirwaikar A., Kuppusamy R., Samraj P. In vitro antioxidant studies on the benzyl tetra isoquinoline alkaloid berberine. Biol. Pharm. Bull. 2006;29:1906–1910. doi: 10.1248/bpb.29.1906. [DOI] [PubMed] [Google Scholar]
- 18.Ruberto G., Baratta M.T., Deans S.G., Dorman H.J. Antioxidant and antimicrobial activity of Foeniculum vulgare and Crithmum maritimum essential oils. Planta Med. 2000;66(8):687–693. doi: 10.1055/s-2000-9773. [DOI] [PubMed] [Google Scholar]
- 19.Lee K.X., Shameli K., Yew Y.P., Teow S.Y., Jahangirian H., Rafiee-Moghaddam R., Webster T.J. Recent developments in the facile bio-synthesis of gold nanoparticles (AuNPs) and their biomedical applications. Int. J. Nanomed. 2020;15:275–300. doi: 10.2147/IJN.S233789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banala R.R., Nagati V.B., Karnati P.R. Green synthesis and characterization of Carica papaya leaf extract coated silver nanoparticles through X-ray diffraction, electron microscopy and evaluation of bactericidal properties. Saudi J. Biol. Sci. 2015;22(5):637–644. doi: 10.1016/j.sjbs.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jafarizad A., Safaee K., Gharibian S., Omidi Y., Ekinci D. Biosynthesis and in-vitro study of gold nanoparticles using mentha and Pelargonium extracts. Proc. Mater. Sci. 2015;11:224–230. [Google Scholar]
- 22.Elia P., Zach R., Hazan S., Kolusheva S., Porat Ze, Zeiri Y. Green synthesis of gold nanoparticles using plant extracts as reducing agents. Int. J. Nanomed. 2014;9:4007–4021. doi: 10.2147/IJN.S57343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan S., Bakht J., Syed F. Green synthesis of gold nanoparticles using Acer pentapomicum leaves extract its characterization, antibacterial, antifungal and antioxidant bioassay. Dig. J. Nanomater. Bios. 2018;2(13):579–589. [Google Scholar]
- 24.John Quiawan M., Billacura M., Canalita D. Green synthesis of silver nanoparticles using fresh leaf extracts of Crassocephalum crepidioides (benth.) S. Moore: evaluation of its antimicrobial property and partial characterization. Sci. Int. 2017;29:13–17. [Google Scholar]
- 25.Ramakrishna M., Babu D.R., Gengan R.M., Chandra S., Rao G.N. Green synthesis of gold nanoparticles using marine algae and evaluation of their catalytic activity. J. Nanostructure Chem. 2016;6(1):1–13. [Google Scholar]
- 26.Rajeshkumar S. Anticancer activity of eco-friendly gold nanoparticles against lung and liver cancer cells. J. Genet. Eng. Biotechnol. 2016;14(1):195–202. doi: 10.1016/j.jgeb.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barai A.C., Paul K., Dey A., Manna S., Roy S., Bag B.G., Mukhopadhyay C. Green synthesis of Nerium oleander-conjugated gold nanoparticles and study of its in vitro anticancer activity on MCF-7 cell lines and catalytic activity. Nano Convergence. 2018;5(1):10. doi: 10.1186/s40580-018-0142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aljabali A., Akkam Y., Al Zoubi M., Al-Batayneh K., Al-Trad B., Abo Alrob O., Alkilany A., Benamara M., Evans D. Synthesis of gold nanoparticles using leaf extract of ziziphus zizyphus and their antimicrobial activity. Nanomaterials. 2018;8(3):174. doi: 10.3390/nano8030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santo A., Zhu H., Li Y.R. Free radicals: from health to disease. Reactive Oxygen Species. 2016;2(4):245–263. [Google Scholar]
- 30.Ruttkay-Nedecky B., Skalickova S., Kepinska M., Cihalova K., Docekalova M., Stankova M., Uhlirova D., Fernandez C., Sochor J., Milnerowicz H., Beklova M., Kizek R. Development of new silver nanoparticles suitable for materials with antimicrobial properties. J. Nanosci. Nanotechnol. 2019;19(5):2762–2769. doi: 10.1166/jnn.2019.15867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.