Abstract
Purpose
Asthma is a heterogeneous airway disease occurring in children, and it has various clinical phenotypes. A clear differentiation of the clinical phenotypes can provide better asthma management and prediction of asthma prognosis. Little is currently known about asthma phenotypes in Korean children. This study was designed to identify asthma phenotypes in school-aged Korean children.
Methods
This study enrolled 674 children with physician-diagnosed asthma from the Korean childhood Asthma Study (KAS) cohort. The physicians verified the relevant histories of asthma and comorbid diseases, as well as airway lability and hyper-responsiveness from the results of pulmonary function tests and bronchial provocation tests. Questionnaires regarding the participants' baseline characteristics, their environment and self-rating of asthma control were collected at the time of enrollment. Laboratory tests were performed to assess allergy and airway inflammation. Children with asthma were classified by hierarchical cluster analysis.
Results
Of the 674 patients enrolled from the KAS cohort, 447 were included in the cluster analysis. Cluster analysis of these 447 children revealed 4 asthma phenotypes: cluster 1 (n = 216, 48.3%) which was characterized by male-dominant atopic asthma; cluster 2 (n = 79, 17.7%) which was characterized by early-onset atopic asthma with atopic dermatitis; cluster 3 (n = 47, 10.5%) which was characterized by puberty-onset, female-dominant atopic asthma with the low lung function; and cluster 4 (n = 105, 23.5%) which was characterized by early-onset, non-atopic dominant asthma.
Conclusions
The asthma phenotypes among Korean children can be classified into 4 distinct clusters. Long-term follow-up with these phenotypes will be needed to define their prognosis and response to treatment.
Keywords: Asthma, childhood, phenotype, cluster analysis
INTRODUCTION
Asthma is a heterogeneous airway disease and occurs in various clinical phenotypes. These phenotypes differ in physiological and biochemical characteristics, and the various endotypes of asthma are characterized by distinct functional and/or pathophysiological mechanisms.1,2,3,4 A clear differentiation of these clinical phenotypes and endotypes is necessary to improve asthma management, thereby improving and personalizing asthma treatment.5,6,7 Unsupervised cluster analysis is a statistical method frequently utilized in clinical studies to group participants with similar characteristics. This has also been used to identify discrete asthma phenotypes in several cohorts of patients varying in age, demographic characteristics, and underlying asthma severity.8
Childhood asthma phenotypes have been classified in children with severe asthma.9,10,11,12,13 These studies suggested the differences in severe asthma phenotypes in children stratified by onset age, atopy, sex, treatment, and lung function, suggesting that asthma is clinically heterogeneous and that new approaches are required for the classification of asthma severity. However, asthma phenotype clustering within general population-based studies is insufficient. Furthermore, because most of the previous studies have assessed patients in North America and Europe, their results may not be applicable to Asian populations, due to ethnic differences based on genetic effects.14 Analysis of the progression of childhood asthma over time and comparisons of asthma exacerbation in clusters require prospective studies to identify participants' asthma phenotypes and to suggest appropriate intervention to prevent progression to adult asthma.
Few studies to date have assessed asthma phenotypes in Korean children.15 The purpose of this study aimed to determine asthma phenotypes in school-aged Korean children by cluster analysis of the Korean childhood Asthma Study (KAS) cohort.
MATERIALS AND METHODS
Overall design of the KAS cohort
The KAS is a nationwide, 19-center prospective cohort study of 1,000 participants aged 5 to 15 years who were diagnosed with childhood asthma by pediatric allergists and pulmonologists. Children who experienced typical symptoms of asthma (i.e., wheezing, dyspnea, and chronic cough) within the recent 12 month period were included if they showed either an elevated bronchodilator response (BDR) (i.e., a ≥ 12% increase of forced expiratory volume in 1 second [FEV1] 15 minutes after inhaling 200 mcg of salbutamol) or bronchial hyper-responsiveness (BHR) (i.e., a < 16 mg/mL dose of provocative methacholine concentration causing a 20% reduction in FEV1 [PC20] or a < 635 mg dose of provocative mannitol weight causing a 15% reduction in FEV1 [PD15]).16,17 Methacholine provocation test was conducted on 498 (73.9%) participants, and mannitol provocation test was conducted on 41 (6.1%) participants. Children who exhibited interstitial lung diseases or pulmonary neoplasms were excluded. Researchers collected the participants' baseline characteristics using a set of questionnaires assessing the frequency of asthma symptoms within 3 months, and history of health-care use (≥ 1 hospitalization or emergency department [ED] visit). Pulmonary function data, including BHR, blood samples and skin prick test results for allergies, as well as additional potential variables were also evaluated. Predicted values of spirometry were based on global lung initiative reference equations.18 Evaluation of asthma severity was based on the physician's subjective clinical assessment using the National Asthma Education and Prevention Program (NAEPP) guidelines.19 No treatment intervention was involved. All participants continued to receive asthma medication as directed by the NAEPP guidelines.11 Participants' responses to treatment, including levels of asthma control and episodes of exacerbation, lung function, and changes in the body's physical and environmental factors, were evaluated at least once every 6 months during regular visits. BHR and atopy were measured every 3 years during the study period. Changes in all the above variables were compared among clusters at baseline and every 3 years during the study period. The follow-up duration of this cohort is planned as every 6 months for the next 3 to 5 years. The study methods have been detailed elsewhere.20 We included 674 of KAS children in our study from September, 2016 to August, 2018. The study was approved by the Institutional Review Boards (IRBs) of Asan Medical Center (IRB No. 2016-0914), Seoul National University Hospital (IRB No. 1607-165-779), Pusan National University Yangsan Hospital (IRB No. 05-2016-121), Inha University Hospital (IRB No. 2016-07-016-008), Seoul National University Bundang Hospital (IRB No. 10-2017-036), Chonnam National University Hospital (IRB No. 2017-201), Korea University Anam Hospital (IRB No. 2015 AN 0310), Soonchunhyang University Hospital in Seoul (IRB No. 2017-01-011-002), Bucheon St. Mary's Hospital (IRB No. HC16SNMI0056), Sungkyunkwan University Samsung Changwon Hospital (IRB No. 2017-02-006-001), Kangdong Sacred Heart Hospital (IRB No. 2016-12-007-001), The Catholic University of Korea, Uijeongbu St. Mary's Hospital (IRB No. UC16ONMI0113), Chungbuk National University Hospital (IRB No. 2016-09-003), Dankook University Hospital (IRB No. 2017-02-013), Korea University Guro Hospital (IRB No. 2016GR0336), Inje University Seoul Paik Hospital (IRB No. 2016-314), CHA Gangnam Medical Center (IRB No. GCI-16-37), National Health Insurance Service Ilsan Hospital (IRB No. NHIMC 2017-02-008), and Soonchunhyang University School of Medicine in Bucheon (IRB No 2016-08-007-009). Written informed consent was obtained from all parents and the guardians of all patients after a detailed explanation of the study.
Variable selection
Cluster-defining variables were selected from the KAS baseline variables based on clinical meaningfulness and minimal missing data. From an initial list of clinical variables, 12 were selected as representative of each child's objective factors associated with increased asthma burdens as inputs for the clustering algorithm. The final set of variables used in the cluster analyses was as follows: 1) sex; 2) age; 3) current diagnosis of allergic rhinitis (AR) (exhibiting AR symptoms within the last 12 months and a diagnosis of AR by clinicians at baseline); 4) current diagnosis of atopic dermatitis (AD) (exhibiting AD symptoms within the last 12 months and a diagnosis of AD by clinicians at baseline); 5) lifetime history of AD diagnosis; 6) history of acute bronchiolitis; 7) puberty stage21; 8) age at asthma onset; 9) PC20 from the methacholine challenge test performed according to the American Thoracic Society guidelines16; 10) atopy defined as a positive response to at least 1 allergen on skin prick tests; 11) baseline predicted FEV1 (%); and 12) frequency of asthma symptoms.
The outcome variables evaluated included age at the onset of asthma symptoms, asthma severity, methacholine PC20 (mg/mL), body mass index (BMI, kg/m2), puberty stage based on Tanner stage,22 use of inhaled corticosteroid (ICS), use of controller medications, any emergency department visit or hospitalization due to asthma exacerbation during the previous 12 months, any use of systemic corticosteroid burst due to asthma exacerbation during the previous 12 months, and pre- and postbronchodilator lung function at baseline and 6 months.23
Cluster and statistical analyses
Cluster analysis was performed at baseline as cross-sectional variables using a hierarchical clustering algorithm with the Ward minimum variance method, which has the advantage of minimizing the total within-cluster variance.24 Stepwise discriminant analysis of the cluster variables was performed to determine the strongest predictors of cluster assignment (Supplementary Table S1).25 Missing values were excluded from the analysis without missing value imputation. Differences among clusters were compared by ANOVA for continuous variables and χ2 tests for categorical variables, with Bonferroni correction adjustment for multiple comparisons. Variables with significant (P < 0.05) differences among clusters were considered as candidate distinguishing features. Data were analyzed using commercially available statistical software, the Statistical Analysis System (SAS) version 9.4 (SAS Inc., Cary, NC, USA).
RESULTS
The baseline characteristics of all 674 patients are presented in Table 1. Participants who missed one or more of the cluster variables were excluded. Among these 674 patients, 447 were available for cluster analysis (Table 1, Supplementary Table S2). There were significant differences in puberty rate, atopy rate, and baseline FEV1 (% predicted) between 447 participants included in the cluster analysis and 227 participants excluded from the analysis due to missing values in any of the variables. Other variables showed no statistically significant differences (Table 2).
Table 1. Baseline characteristics of study participants in total and according to cluster analysis.
| Characteristics | KAS in total (n = 674) | |
|---|---|---|
| Age (yr) | 9.0 ± 2.6 | |
| Male | 445/674 (66.0) | |
| Current AR diagnosis | 530/669 (79.2) | |
| Current AD diagnosis | 146/670 (21.8) | |
| Lifetime history of AD | 261/662 (39.4) | |
| History of acute bronchiolitis | 225/651 (34.6) | |
| Puberty | ||
| I | 481/659 (73.0) | |
| II | 105/659 (15.9) | |
| III | 40/659 (6.1) | |
| IV | 22/659 (3.3) | |
| V | 11/659 (1.7) | |
| Age at asthma symptom onset (yr) | ||
| < 3 | 109/659 (16.5) | |
| ≥ 3, < 6 | 223/659 (33.8) | |
| ≥ 6, < 9 | 181/659 (27.5) | |
| ≥ 9, < 12 | 103/659 (15.6) | |
| ≥ 12 | 43/659 (6.5) | |
| Asthma severity | ||
| Mild intermittent | 251/667 (37.6) | |
| Mild persistent | 260/667 (39.0) | |
| Moderate persistent | 152/667 (22.8) | |
| Severe persistent | 4/667 (0.6) | |
| Frequency of asthma symptoms | ||
| None | 154/644 (23.9) | |
| < 1/month | 197/644 (30.6) | |
| ≥ 1/month, < week | 148/644 (23.0) | |
| ≥ 1/week, < 2/week | 59/644 (9.2) | |
| ≥ 2/week, < 1/day | 53/644 (8.2) | |
| ≥ 1/day | 33/644 (5.1) | |
| Methacholine (PC20, mg/mL), mean (range) | 1.9 (0.5–8.0) | |
| Atopy (≥ 1 on skin prick test) | 501/674 (74.3) | |
| Baseline FEV1 (% predicted) | 90.4 ± 16.2 | |
| Bronchodilator response (%) | 6.6 ± 9.0 | |
Data are presented as number (%) or mean ± standard deviation, unless otherwise indicated.
P < 0.05 by χ2 tests.
KAS, Korean childhood Asthma Study; AR, allergic rhinitis; AD, atopic dermatitis; PC20, provocative methacholine concentration causing a 20% reduction in FEV1; FEV1, forced expiratory volume in 1 second.
Table 2. Demographic characteristics of traits across clusters.
| Characteristics | Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | P value | Bonferroni | ||
|---|---|---|---|---|---|---|---|---|
| Age | 8.8 ± 2.1 | 8.9 ± 2.2 | 13.2 ± 1.8 | 7.5 ± 2.0 | < 0.0001 | 3 vs. 2, 1, 4 | ||
| Male | 156 (72.2) | 56 (70.9) | 10 (21.3) | 64 (61.0) | < 0.0001 | 1, 2 vs. 4 | ||
| Asthma history | < 0.0001 | 1, 2, 4 vs. 3 | ||||||
| Age of asthma onset (yr) | ||||||||
| < 3 | 35 (16.2) | 18 (22.8) | 0 (0) | 22 (21.0) | ||||
| ≥ 3, < 6 | 69 (31.9) | 33 (41.8) | 6 (12.8) | 44 (41.9) | ||||
| ≥ 6, < 9 | 76 (35.2) | 22 (27.9) | 6 (12.8) | 23 (21.9) | ||||
| ≥ 9, < 12 | 33 (15.3) | 5 (6.3) | 14 (29.8) | 14 (13.3) | ||||
| ≥ 12 | 3 (1.4) | 1 (1.3) | 21 (44.7) | 2 (1.9) | ||||
| History of acute bronchiolitis | 83 (38.4) | 29 (36.7) | 4 (8.5) | 31 (29.5) | 0.001 | 1, 2, 4 vs. 3 | ||
| Atopic features | ||||||||
| Current AR diagnosis | 215 (99.5) | 68 (86.1) | 37 (78.7) | 38 (36.2) | < 0.0001 | 1, 2 vs. 3 | ||
| Current AD diagnosis | 5 (2.3) | 78 (98.7) | 11 (23.4) | 0 (0.0) | < 0.0001 | 2, 3 vs. 4 | ||
| Lifetime history of AD diagnosis | 57 (26.4) | 78 (98.7) | 21 (44.7) | 15 (14.3) | < 0.0001 | 1 vs. 2, 3, 4 | ||
| Positive skin test response, atopy (%) | 209 (96.8) | 66 (83.5) | 42 (89.4) | 33 (31.4) | < 0.0001 | 2, 3 vs. 4 | ||
| Total serum IgE levels, kU/L | 347.2 (115.6–1,043.2) | 415.7 (129.0–1,339.4) | 347.2 (64.7–1,863.1) | 149.9 (38.9–578.3) | < 0.0001 | 1 vs. 2, 3 | ||
| Anthropomorphic features | ||||||||
| Tanner stage | < 0.0001 | 2, 4 vs. 3 | ||||||
| I | 178 (82.4) | 68 (86.1) | 2 (4.3) | 95 (90.5) | ||||
| II | 30 (13.9) | 9 (11.4) | 11 (23.4) | 9 (8.6) | ||||
| III | 6 (2.8) | 2 (2.5) | 11 (23.4) | 1 (1.0) | ||||
| IV | 2 (0.9) | 0 (0.0) | 16 (34.04) | 0 (0.0) | ||||
| V | 0 (0.0) | 0 (0.0) | 7 (14.89) | 0 (0.0) | ||||
| BMI (kg/m2) | 18.7 ± 3.6 | 18.3 ± 3.6 | 20.7 ± 3.9 | 17.8 ± 3.0 | < 0.0001 | 3 vs. 1, 2, 4 | ||
All P values for the multiple comparisons were < 0.008 (the adjusted P value based on the Bonferroni correction). Data are presented as number (%) or mean ± standard deviation, unless otherwise indicated.
AR, allergic rhinitis; AD, atopic dermatitis; IgE, immunoglobulin E; BMI, body mass index.
Cluster analysis
The hierarchical clustering algorithm approach identified 4 clusters distinguished by age, sex, current diagnosis of AR, current diagnosis of AD, lifetime diagnosis of AD, history of acute bronchiolitis, puberty stage, age at asthma onset, methacholine PC20, atopy, baseline predicted FEV1 (%), and frequency of asthma symptoms. Clusters also differed by medication use and other healthcare (Table 3), and lung function (Table 4).
Table 3. Asthma severity, medication, and healthcare use across clusters.
| Variables | Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | P value | Bonferroni | |
|---|---|---|---|---|---|---|---|
| Asthma severity | 0.0002 | 2 vs. 3, 4 | |||||
| Mild intermittent | 86 (40.0) | 47 (59.5) | 11 (23.4) | 30 (28.6) | |||
| Mild persistent | 87 (40.5) | 17 (21.5) | 20 (42.6) | 54 (51.4) | |||
| Moderate persistent | 40 (18.6) | 15 (19.0) | 16 (34.0) | 21 (20.0) | |||
| Severe persistent | 2 (0.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Frequency of asthma symptoms | 0.024 | 1, 2, 4 vs. 3 | |||||
| None | 59 (27.3) | 19 (24.1) | 8 (17.0) | 26 (24.8) | |||
| < 1/month | 69 (31.9) | 26 (32.9) | 14 (29.8) | 32 (30.5) | |||
| ≥ 1/month, < 1/week | 45 (20.8) | 23 (29.1) | 5 (10.6) | 29 (27.6) | |||
| ≥ 1/week, < 2/week | 20 (9.3) | 2 (2.5) | 8 (17.0) | 7 (6.7) | |||
| ≥ 2/week, < 1/day | 18 (8.3) | 5 (6.3) | 6 (12.8) | 6 (5.7) | |||
| ≥ 1/day | 5 (2.3) | 4 (5.1) | 6 (12.8) | 5 (4.8) | |||
| Use of ICS | 0.006 | 2 vs. 3 | |||||
| Low-dose ICS | 107 (50.5) | 29 (37.2) | 30 (63.8) | 56 (53.9) | |||
| Medium-dose ICS | 30 (14.2) | 8 (10.3) | 11 (23.4) | 13 (12.5) | |||
| High-dose ICS | 1 (0.5) | 0 (0.0) | 0 (0.0) | 1 (1.0) | |||
| Controller medications | 0.003 | 2 vs. 3, 4 | |||||
| None | 60 (28.0) | 34 (43.0) | 5 (10.6) | 26 (25.0) | |||
| Montelukast only | 16 (7.5) | 5 (6.3) | 2 (4.3) | 9 (8.7) | |||
| ICS only | 49 (22.9) | 24 (30.4) | 10 (21.3) | 21 (20.2) | |||
| ICS+montelukast | 21 (9.8) | 4 (5.1) | 5 (10.6) | 16 (15.4) | |||
| ICS+LABA | 20 (9.4) | 4 (5.1) | 7 (14.9) | 12 (11.5) | |||
| ICS+LABA+montelukast | 48 (22.4) | 8 (10.1) | 18 (38.3) | 20 (19.2) | |||
| At least one systemic corticosteroid | 70/214 (32.7) | 22/77 (28.6) | 11/45 (24.4) | 33/104 (31.7) | 0.697 | ||
| Healthcare use | 0.447 | ||||||
| None | 170 (78.7) | 63 (79.8) | 35 (74.5) | 75 (71.4) | |||
| ≥ 1 hospitalization or ED visit | 46 (21.3) | 16 (20.3) | 12 (25.5) | 30 (28.6) | |||
All P values for the multiple comparisons were < 0.008 (the adjusted P value based on the Bonferroni correction). Data are presented as number (%).
ICS, inhaled corticosteroid; LABA, long acting beta-2 agonist; ED, emergency department.
Table 4. Pulmonary function variables across clusters.
| Variables | Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | P value | Bonferroni | |
|---|---|---|---|---|---|---|---|
| Prebronchodilator pulmonary function | |||||||
| FEV1 (% predicted) | 94.3 ± 14.3 | 94.0 ± 13.0 | 84.6 ± 15.9* | 95.9 ± 11.6 | 0.001 | 4, 1, 2 vs. 3 | |
| FVC (% predicted) | 100.6 ± 13.1 | 99.0 ± 12.2 | 90.3 ± 13.7* | 101.3 ± 12.0 | < 0.0001 | 4, 1, 2 vs. 3 | |
| FEV1/FVC | 93.8 ± 8.9 | 95.1 ± 8.0 | 94.3 ± 17.6 | 95.0 ± 9.4 | 0.456 | ||
| MMEF (% predicted) | 82.4 ± 26.1 | 86.1 ± 24.1 | 75.8 ± 28.3 | 84.2 ± 21.4 | 0.304 | ||
| Postbronchodilator pulmonary function | |||||||
| FEV1 (% predicted) | 98.9 ± 14.5 | 99.0 ± 14.4 | 90.2 ± 16.0* | 101.0 ± 12.7 | 0.007 | 4 vs. 3 | |
| FVC (% predicted) | 100.6 ± 13.2 | 99.0 ± 13.5 | 93.9 ± 11.7 | 102.9 ± 12.7 | 0.015 | ||
| MMEF (% predicted) | 104.2 ± 32.2 | 109.0 ± 30.7 | 90.2 ± 33.2 | 107.9 ± 28.0 | 0.090 | ||
| Airway responsiveness | |||||||
| Methacholine PC20 | 2.5 (0.6–10.5) | 1.8 (0.6–5.5) | 2.1 (0.5–8.2) | 1.4 (0.4–5.2) | 0.004 | 1 vs. 4 | |
| Bronchodilator response (%) | 7.1 ± 10.0 | 4.1 ± 5.7 | 10.6 ± 9.9 | 5.6 ± 7.9 | 0.011 | 3 vs. 2 | |
All P values for the multiple comparisons were < 0.008 (the adjusted P value based on the Bonferroni correction). Data are presented as number (%) or mean ± standard deviation, unless otherwise indicated.
FEV1, forced expiratory volume in 1 second; FVC, forced volume vital capacity; MMEF, maximal mid-expiratory flow; PC20, provocative methacholine concentration causing a 20% reduction in FEV1.
Cluster 1
Cluster 1 compromised 216 (156 [72.2%], boys; 60 [27.8%], girls) children (45.8%). Cluster 1 was characterized by male-dominant atopic asthma. Their mean age was 8.8 ± 2.1 years. Additionally, 215 (99.5%) children also had current AR, and 209 (96.8%) were positive on skin prick tests, with a geometric mean IgE concentration of 347.2 kU/L (Table 2). Of these 216 children, 86 (40.0%) and 87 (40.5%) had mild intermittent type and mild persistent type asthma, respectively. The frequency of asthma symptoms was none or less than once per month in 128 (59.3%) children, and 156 (72.0%) used controller medication (Table 3). Despite having BHR to methacholine (PC20, 2.46 mg/mL; range of one standard deviation [SD], 0.58–10.49 mg/mL), these children had relatively preserved pulmonary function, with a predicted forced volume vital capacity (FVC) 100.6 ± 13.1%, a predicted FEV1 94.3 ± 14.3%, a FEV1/FVC 93.8 ± 8.9, and a predicted maximal mid-expiratory flow (MMEF) 82.4 ± 26.1%. The average BDR was 7.1 ± 10.0% (Table 4).
Cluster 2
Cluster 2 comprised 79 children (36.6%) (mean age, 8.9 ± 2.1 years) and was characterized as having early-onset atopic asthma with AD. This group had an earlier onset of asthma symptoms with 78 (98.7%) having a significant rate of AD, and 66 (83.5%) having atopy. Because most patients in this cluster experienced asthma onset before the age of 6 years, and most had current AD (n = 78, 98.7%), lifetime history of AD (n = 78, 98.7%), and current AR (n = 68, 86.1%), it is highly likely that the disease in this group is equivalent to atopic march. Children in this group also had a high rate of atopy (n = 66, 83.5%), with a geometric mean IgE concentration of 415.7 kU/L (range of one SD, 129.0–1,339.4 kU/L) (Table 2). This cluster had the least severe type of asthma, with 47 (59.5%) having the mild intermittent type and 34 (43.0%) children did not use controller medications. The proportion of these patients requiring hospitalization or ED visit was the lowest among the 4 clusters, although the differences were not statistically significant among the clusters (Table 3). Their pulmonary function, including FEV1, FVC, MMEF, and post-BDRs, was similar to that observed in cluster 1. The geometric mean of BHR to methacholine PC20 was 1.80 mg/mL (range of one SD, 0.6–5.5 mg/mL) and BDR was 4.1 ± 5.7% (Table 4).
Cluster 3
Cluster 3 was the smallest one, comprising 47 (37 [78.7%], girls; 10 [21.3%] boys) children (10.0%). Children in this cluster, termed puberty-onset, female-dominant atopic asthma, were older than those in the other clusters (mean age 13.2 ± 1.8 years). Moreover, this cluster was differentiated by late symptom onset, with 21 children (44.7%) experiencing symptom onset at age ≥ 12 years, and 45 (95.7%) having entered puberty (Tanner stages II–V). Forty-two children (89.4%) in this group had atopic features, with a geometric mean IgE concentration of 347.2 kU/L (range of one SD, 64.7–1863.1 kU/L). This cluster showed the highest BMI (kg/m2) among the 4 cluster, but the value was within normal ranges (Table 2). Sixteen children (34.0%) had moderate persistent asthma, with this group containing the most severe cases, with the highest frequency of asthma symptoms, use of controller medications (n = 42, 89.4%), and proportion who used combination treatments (n = 30, 63.8%) among the 4 clusters. Twelve children in this group (25.5%) experienced asthma symptoms more than twice weekly or more than once daily (Table 3). This cluster was distinguished by the lowest pulmonary function, including the lowest predicted FEV1 (84.6 ± 15.9%) and FVC (90.3 ± 13.7%), although both were within normal ranges. This group also showed the lowest postbronchodilator pulmonary function, including a predicted FEV1 and FVC values of 90.2 ± 16.0% and 93.9 ± 11.7%, respectively. The geometric mean of BHR to methacholine PC20 was 2.1 mg/mL (range of 1 SD, 0.5–8.2 mg/mL) and the mean of BDR was the highest among 4 groups (10.6 ± 9.9%) (Table 4).
Cluster 4
Cluster 4 comprised 105 children (22.2%), termed the early-onset, non-atopic dominant asthma group. Their mean age was 7.5 ± 2.0 years, and most children in this group first experienced asthma symptoms at the preschool age (n = 66, 62.9%). None of the patients in this cluster had a history of current AD, with this cluster having the lowest prevalence of AR (n = 38, 36.2%) and skin prick test reactivity (n = 33, 31.4%) among the 4 clusters. Their geometric mean IgE concentration was 149.9 kU/L (range of one SD, 38.9–579.3 KU/L), which was lower than those of the 3 other clusters (Table 2). The asthma symptom frequency in this group was similar to that in clusters 1 and 2, with 11 (10.5%) children experiencing asthma symptoms more than twice weekly. The proportion of patients in this cluster using non-controller medications (n = 26, 25.0%) was similar to that in cluster 1, and the proportion using controller medication (n = 56, 53.9%) was higher than that in cluster 2, with most of these patients using low-dose ICS (Table 3). Despite having BHR to methacholine (PC20, 1.40 mg/mL; range of 1 SD, 0.4–5.2 mg/mL) with BDR 5.6 ± 7.9%, their pulmonary function was preserved, with the highest predicted FEV1 (95.9 ± 11.6%) (Table 4). The frequency of hospitalization or ED visit at least once during the previous year was similar in all 4 clusters (Table 3).
Major determinants of cluster assignment
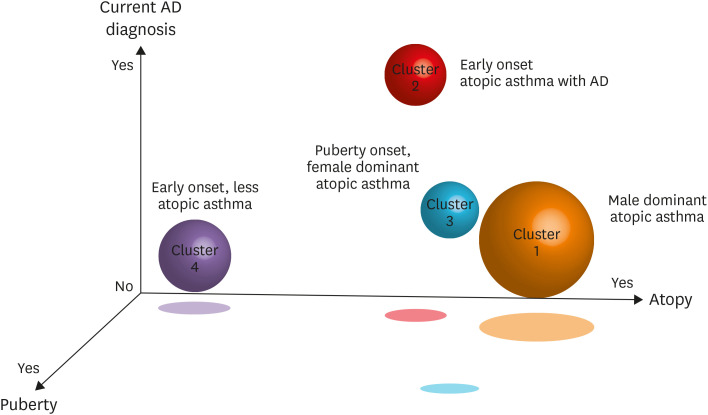
Seven variables, including current diagnosis of AD (P < 0.001), puberty (P < 0.001), positive skin test response (atopy, P < 0.001), current diagnosis of AR (P < 0.001), age at asthma onset (P < 0.001), baseline % predicted FEV1 (P < 0.001), and sex (P < 0.001), were identified as the strongest determinants of cluster assignment in this study (Wilks λ = 0.025; F = 157.18; P < 0.0001). These 7 variables resulted in the correct classification of 93.1% of the original participants (Table 2, Fig. 1).
Figure. Distribution of the four clusters around the main variables of the three axes, current diagnosis of atopic dermatitis (AD), puberty, and atopy. Cluster 1, male-dominant atopic asthma; cluster 2, early onset atopic asthma with AD; cluster 3, puberty-onset, female-dominant atopic asthma; cluster 4, early onset, non-atopic dominant asthma.
Changes in lung function variables from baseline to 6 months across clusters
The results of pulmonary function tests during a follow-up period of 6 months are shown in Table 5. Similar to the results of initial pulmonary function tests, all pulmonary function variables were preserved 6 months later. FEV1 in cluster 3 increased from 84.6% to 91.4%, likely reflecting an improvement in the degree of airway obstruction after enrollment in this study. However, prebronchodilator FVC (93.8 ± 8.6%), postbronchodilator FEV1 (92.8 ± 8.3%), and FVC (94.0 ± 8.0%) remained significantly lower in cluster 3 than in the other clusters.
Table 5. Pulmonary function variables after 6 months across clusters.
| Variables | Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | P value | Bonferroni | |
|---|---|---|---|---|---|---|---|
| Prebronchodilator pulmonary function | |||||||
| FEV1 (% predicted) | 96.5 ± 12.5 | 95.8 ± 10.1 | 91.4 ± 7.9 | 96.9 ± 13.5 | 0.186 | ||
| FVC (% predicted) | 101.0 ± 10.7 | 100.3 ± 9.8 | 93.8 ± 8.6 | 103.4 ± 12.1 | 0.008 | 4, 1 vs. 3 | |
| FEV1/FVC | 95.9 ± 11.8 | 95.8 ± 7.8 | 97.8 ± 7.6 | 93.7 ± 7.4 | 0.263 | ||
| MMEF (% predicted) | 87.4 ± 28.9 | 84.9 ± 21.7 | 93.7 ± 22.3 | 85.3 ± 23.7 | 0.558 | ||
| Postbronchodilator pulmonary function | |||||||
| FEV1 (% predicted) | 101.1 ± 11.1 | 100.6 ± 9.9 | 92.8 ± 8.3 | 101.3 ± 15.2 | 0.122 | ||
| FVC (% predicted) | 101.6 ± 10.1 | 100.2 ± 11.1 | 94.0 ± 8.0 | 103.6 ± 12.3 | 0.043 | ||
| MMEF (% predicted) | 105.7 ± 28.6 | 106.7 ± 24.8 | 100.9 ± 22.8 | 109.8 ± 32.3 | 0.880 | ||
| Bronchodilator response (%) | 6.0 ± 8.8 | 5.3 ± 6.1 | 3.1 ± 8.4 | 7.2 ± 7.8 | 0.516 | ||
All P values for the multiple comparisons were < 0.008 (the adjusted P value based on the Bonferroni correction). Data are presented as mean ± standard deviation.
FEV1, forced expiratory volume in 1 second; FVC, forced volume vital capacity; MMEF, maximal mid-expiratory flow.
DISCUSSION
To the best of our knowledge, this is the first nationwide study showing that asthma phenotypes in Korean children could be classified into 4 distinct clusters: cluster 1 comprising male-dominant atopic asthma: cluster 2, early-onset atopic asthma with AD: cluster 3, puberty-onset, female-dominant atopic asthma: and cluster 4, early-onset, non-atopic dominant asthma. These findings demonstrated that childhood asthma could be classified into distinctive phenotypes, consistent with the clinical characteristics partly revealed in previous studies.6,7,26,27
Several previous studies on asthma clusters have demonstrated the heterogeneity of childhood asthma.7,9,10,11,13,15,20 In the Severe Asthma Research Program (SARP) cohort, asthma duration, number of asthma controller medications, and baseline lung function were major determinants of the asthma phenotype in cluster analysis. Because this study recruited only patients with severe asthma, all of those with childhood asthma had atopic disease, with reduced lung function.13 As our present study recruited and analyzed a nationwide cohort of Korean patients with childhood asthma, the percentage of patients with severe asthma was lower than the percentages of patients with mild persistent and mild intermittent asthma. The rates of hospitalization and ED visits were low and showed no differences across the clusters. The rates of severe asthma did not differ significantly among the 4 clusters. Almost all children in the KAS cohort showed preserved lung function. These differences were probably due to the differences in asthma severity and ethnicity between the SARP cohort and ours.28 Additionally, the SARP cohort did not include any patients with a low degree of atopy, whereas a few patients in cluster 4 in the present study had atopic features.
In the present study, cluster 1 comprised patients with clinically typical early-onset childhood asthma with allergic sensitization, a condition that affects boys more than girls in childhood. Male dominance and atopy were key characteristics as previously reported.1,9,29,30 The clinical course of these patients with a typical childhood asthma phenotype in Korea will be followed up in the future. Cluster 2 was characterized by a significantly high rate of lifetime history of AD diagnosis (98.7%) and current AR diagnosis (86.1%), with early-onset asthma, indicative of atopic march. Compared with the other groups, however, AD was not a risk factor for more exacerbations or less well-controlled asthma. Rather, children in cluster 2 showed relatively mild symptoms of asthma, with fewer using controller medications. Previous study results have differed regarding the association between AD and asthma severity. For example, oral corticosteroid use and ED visits were significantly higher in children with than without eczema symptoms.31 However, other studies suggested that early AD alone did not increase the risk of current wheeze and BHR in 7-year-old children, and that only 3.1% of children with asthma showed allergic march.32,33 Furthermore, the genetic susceptibility regions for asthma and AD showed little overlap, suggesting that different genes may be involved in the pathogenesis of these atopic disorders.34 AD may not be associated with asthma severity, suggesting the need for further evaluation and assessment.
Cluster 3 in this study, characterized by puberty-onset, female-dominant atopic asthma, showed the most frequent asthma symptoms, including relatively lower lung function, among all clusters. Because patients in this cluster were older than those in the other clusters, with a high proportion having entered puberty, patients in the other clusters have the potential to enter cluster 3 with age. However, this cluster seems to be a distinct group rather than a progression from other clusters, because the patients were older at asthma onset than those in the other groups. Sex hormones may be associated with the development of asthma symptoms during puberty, suggesting that sex hormones may play an important role in the pathogenesis of asthma.35 Dysanaptic airway growth may also partially account for the physiological differences between men and women, because the small airway resistance is greater in women than in men.36 Recent studies explain that the androgen surge with puberty is likely to confer protective effects on lung growth in both males and females.37,38 However, estrogens may have deleterious effects on lung growth in females extending into adult development.37,39 Although the exact mechanisms are unclear, asthma symptoms were more predominant and severe in female than male adolescents at the onset of puberty, indicating that puberty is a critical stage in asthma symptom progression.26 These studies may help explain the female dominant asthma phenotype during adolescence, and likewise inform lung growth and asthma severity with subsequent maturation into adulthood. Thus, attention should be paid to newly diagnosed asthma in adolescent girls, with asthma control and lung function assessed regularly. Furthermore, this cluster showed the highest BMI among the 4 groups, although there was no statistical significance in the analysis with BMI percentile considering their age. These findings may help predict more important variables in patients with persistently uncontrolled asthma. Interestingly, children in cluster 3 showed the low-lung function, as assessed by FVC and FEV1, both at baseline and after bronchodilator administration. This group also showed the same tendency of the low lung function after 6 months as those at baseline. These pulmonary function results are specific characteristics of cluster 3, which may be associated with an air-trapping phenotype.40,41 This can only be interpreted as a characteristic of cluster 3, but we can carefully interpret this result to indicate that the baseline cluster's characteristic is maintained during a period of 6 months. Therefore, it suggests that cluster analysis is divided into groups with their distinct characteristics rather than temporal cross-sectional statistical results. Further studies are required to assess the role of sex hormones and sex-specific dysanaptic airway growth, and the effects of obesity on pulmonary physiology, immunology, and pathology of asthma.
Airway hyper-responsiveness is considered a marker of asthma, independent of the atopic status, and it should be considered a parallel pathological process that can lead to subsequent symptoms and clinical evidence of asthma in children without evidence of atopy. Non-atopic asthma has a milder and shorter prognosis than atopic asthma.42 In the present study, the early-onset non-atopic dominant asthma group (cluster 4) showed the second highest asthma severity among the 4 clusters. Furthermore, several of these patients required combination therapy, probably because this cluster included some patients with atopy and those with non-atopic asthma suffering from neutrophil-dominant steroid-refractory recurrent wheeze,43,44 which was previously reported in other studies. These findings suggest that the prognosis of patients with non-atopic dominant early-onset asthma varies among the clusters. Longitudinal monitoring of this group may reveal factors common to adults with non-atopic asthma.
This study had several notable limitations. Because the current cluster analysis was based on cross-sectional data, with pulmonary function monitored after 6 months, it is necessary to determine whether these 4 clusters show dynamic changes across clusters or are fixed at follow-up. Long-term follow-up, which includes assessment of treatment patterns and responses to treatment, is also required. Another limitation may be the small sample size and the relatively short-term follow-up period in this study. Assessments of larger patient populations are required to identify critical variables, including disease subtypes, environmental factors, and biomarkers, which would allow the differentiation of asthma subtypes at any cross-sectional time point. Additionally, we evaluated asthma onset and history of acute bronchiolitis based on parent-reported questionnaires dependent on remote memory recall, suggesting a possibility of reporting bias. Nevertheless, this method is commonly used in epidemiological research on children, and the questionnaires we used have proven reliable and been validated in previous studies.45 This is the first nationwide prospective childhood asthma cohort study in Korea. Because we evaluated childhood asthma using well-validated measures, including pulmonary function tests, methacholine tests, skin prick tests, and serum total IgE concentrations in patients with physician-diagnosed asthma, the results represented a real-world assessment of childhood asthma in Korea. Finally, the participants in the current study will be evaluated every 6 months, enabling long-term predictions of prognosis and response to treatment in children with various asthma phenotypes.
In conclusion, the asthma phenotype in Korean children can be classified into 4 distinct clusters. Further challenges to the optimal use of clustering methodologies include tailoring models to individual data sets as well as incorporating genetic, epigenetic, and more detailed molecular-level data.
ACKNOWLEDGMENTS
This study was supported by a research fund from the Research of Korea Centers for Disease Control and Prevention, Republic of Korea (2016-ER6703-00, 2019-ER6701-00).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Results of stepwise linear discriminant analysis of the 12 variables used in cluster analysis
Baseline characteristics of total subjects (n = 674) and subjects who are included and not included in cluster analysis
References
- 1.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore WC, Peters SP. Severe asthma: an overview. J Allergy Clin Immunol. 2006;117:487–494. doi: 10.1016/j.jaci.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 3.Yoo Y. Phenotypes and endotypes of severe asthma in children. Korean J Pediatr. 2013;56:191–195. doi: 10.3345/kjp.2013.56.5.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee E, Song DJ, Kim WK, Suh DI, Baek HS, Shin M, et al. Associated factors for asthma severity in Korean children: a Korean childhood asthma study. Allergy Asthma Immunol Res. 2020;12:86–98. doi: 10.4168/aair.2020.12.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiley J, Smith R, Noel P. Asthma phenotypes. Curr Opin Pulm Med. 2007;13:19–23. doi: 10.1097/MCP.0b013e328011b84b. [DOI] [PubMed] [Google Scholar]
- 6.Reddy MB, Covar RA. Asthma phenotypes in childhood. Curr Opin Allergy Clin Immunol. 2016;16:127–134. doi: 10.1097/ACI.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 7.Lee E, Hong SJ. Phenotypes of allergic diseases in children and their application in clinical situations. Korean J Pediatr. 2019;62:325–333. doi: 10.3345/kjp.2018.07395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deliu M, Sperrin M, Belgrave D, Custovic A. Identification of Asthma Subtypes Using Clustering Methodologies. Pulm Ther. 2016;2:19–41. doi: 10.1007/s41030-016-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the severe asthma research program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howrylak JA, Fuhlbrigge AL, Strunk RC, Zeiger RS, Weiss ST, Raby BA, et al. Classification of childhood asthma phenotypes and long-term clinical responses to inhaled anti-inflammatory medications. J Allergy Clin Immunol. 2014;133:1289–1300. doi: 10.1016/j.jaci.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schatz M, Hsu JW, Zeiger RS, Chen W, Dorenbaum A, Chipps BE, et al. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2014;133:1549–1556. doi: 10.1016/j.jaci.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Just J, Gouvis-Echraghi R, Rouve S, Wanin S, Moreau D, Annesi-Maesano I. Two novel, severe asthma phenotypes identified during childhood using a clustering approach. Eur Respir J. 2012;40:55–60. doi: 10.1183/09031936.00123411. [DOI] [PubMed] [Google Scholar]
- 13.Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, et al. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol. 2011;127:382–389.e1. doi: 10.1016/j.jaci.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lester LA, Rich SS, Blumenthal MN, Togias A, Murphy S, Malveaux F, et al. Ethnic differences in asthma and associated phenotypes: collaborative study on the genetics of asthma. J Allergy Clin Immunol. 2001;108:357–362. doi: 10.1067/mai.2001.117796. [DOI] [PubMed] [Google Scholar]
- 15.Lee E, Lee SH, Kwon JW, Kim YH, Yoon J, Cho HJ, et al. Persistent asthma phenotype related with late-onset, high atopy, and low socioeconomic status in school-aged Korean children. BMC Pulm Med. 2017;17:45. doi: 10.1186/s12890-017-0387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 17.Brannan JD, Anderson SD, Perry CP, Freed-Martens R, Lassig AR, Charlton B Aridol Study Group. The safety and efficacy of inhaled dry powder mannitol as a bronchial provocation test for airway hyperresponsiveness: a phase 3 comparison study with hypertonic (4.5%) saline. Respir Res. 2005;6:144. doi: 10.1186/1465-9921-6-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deschildre A, Pin I, El Abd K, Belmin-Larrar S, El Mourad S, Thumerelle C, et al. Asthma control assessment in a pediatric population: comparison between GINA/NAEPP guidelines, Childhood Asthma Control Test (C-ACT), and physician's rating. Allergy. 2014;69:784–790. doi: 10.1111/all.12402. [DOI] [PubMed] [Google Scholar]
- 20.In Suh D, Song DJ, Baek HS, Shin M, Yoo Y, Kwon JW, et al. Korean childhood asthma study (KAS): a prospective, observational cohort of Korean asthmatic children. BMC Pulm Med. 2019;19:64. doi: 10.1186/s12890-019-0829-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts C. Tanner's Puberty Scale: exploring the historical entanglements of children, scientific photography and sex. Sexualities. 2016;19:328–346. [Google Scholar]
- 22.Emmanuel M, Bokor BR. Tanner stages. Treasure Island (FL): StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 23.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 24.Ward JH., Jr Hierarchical grouping to optimize an objective function. J Am Stat Assoc. 1963;58:236–244. [Google Scholar]
- 25.Fisher RA. The use of multiple measurements in taxonomic problems. Ann Eug. 1936;7:179–188. [Google Scholar]
- 26.Fu L, Freishtat RJ, Gordish-Dressman H, Teach SJ, Resca L, Hoffman EP, et al. Natural progression of childhood asthma symptoms and strong influence of sex and puberty. Ann Am Thorac Soc. 2014;11:939–944. doi: 10.1513/AnnalsATS.201402-084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Just J, Bourgoin-Heck M, Amat F. Clinical phenotypes in asthma during childhood. Clin Exp Allergy. 2017;47:848–855. doi: 10.1111/cea.12939. [DOI] [PubMed] [Google Scholar]
- 28.Sarpong EM, Miller GE. Racial and ethnic differences in childhood asthma treatment in the United States. Health Serv Res. 2013;48:2014–2036. doi: 10.1111/1475-6773.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almqvist C, Worm M, Leynaert B working group of GA2LEN WP 2.5 Gender. Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy. 2008;63:47–57. doi: 10.1111/j.1398-9995.2007.01524.x. [DOI] [PubMed] [Google Scholar]
- 30.Schatz M, Rosenwasser L. The allergic asthma phenotype. J Allergy Clin Immunol Pract. 2014;2:645–648. doi: 10.1016/j.jaip.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Arabkhazaeli A, Vijverberg SJ, van Erp FC, Raaijmakers JA, van der Ent CK, Maitland van der Zee AH. Characteristics and severity of asthma in children with and without atopic conditions: a cross-sectional study. BMC Pediatr. 2015;15:172. doi: 10.1186/s12887-015-0481-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Illi S, von Mutius E, Lau S, Nickel R, Grüber C, Niggemann B, et al. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J Allergy Clin Immunol. 2004;113:925–931. doi: 10.1016/j.jaci.2004.01.778. [DOI] [PubMed] [Google Scholar]
- 33.Redlich CA. Skin exposure and asthma: is there a connection? Proc Am Thorac Soc. 2010;7:134–137. doi: 10.1513/pats.201002-025RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffjan S, Epplen JT. The genetics of atopic dermatitis: recent findings and future options. J Mol Med (Berl) 2005;83:682–692. doi: 10.1007/s00109-005-0672-2. [DOI] [PubMed] [Google Scholar]
- 35.Balzano G, Fuschillo S, Melillo G, Bonini S. Asthma and sex hormones. Allergy. 2001;56:13–20. doi: 10.1034/j.1398-9995.2001.00128.x. [DOI] [PubMed] [Google Scholar]
- 36.Sheel AW, Dominelli PB, Molgat-Seon Y. Revisiting dysanapsis: sex-based differences in airways and the mechanics of breathing during exercise. Exp Physiol. 2016;101:213–218. doi: 10.1113/EP085366. [DOI] [PubMed] [Google Scholar]
- 37.DeBoer MD, Phillips BR, Mauger DT, Zein J, Erzurum SC, Fitzpatrick AM, et al. Effects of endogenous sex hormones on lung function and symptom control in adolescents with asthma. BMC Pulm Med. 2018;18:58. doi: 10.1186/s12890-018-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zein JG, Udeh BL, Teague WG, Koroukian SM, Schlitz NK, Bleecker ER, et al. Impact of age and sex on outcomes and hospital cost of acute asthma in the United States, 2011–2012. PLoS One. 2016;11:e0157301. doi: 10.1371/journal.pone.0157301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zein JG, Erzurum SC. Asthma is different in women. Curr Allergy Asthma Rep. 2015;15:28. doi: 10.1007/s11882-015-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sorkness RL, Zoratti EM, Kattan M, Gergen PJ, Evans MD, Visness CM, et al. Obstruction phenotype as a predictor of asthma severity and instability in children. J Allergy Clin Immunol. 2018;142:1090–1099.e4. doi: 10.1016/j.jaci.2017.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sorkness RL, Zoratti EM, Kattan M, Gergen PJ, Evans MD, Visness CM, et al. Obstruction phenotype as a predictor of asthma severity and instability in children. J Allergy Clin Immunol. 2018;142:1090–1099.e4. doi: 10.1016/j.jaci.2017.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henderson J, Granell R, Heron J, Sherriff A, Simpson A, Woodcock A, et al. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid-childhood. Thorax. 2008;63:974–980. doi: 10.1136/thx.2007.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drews AC, Pizzichini MM, Pizzichini E, Pereira MU, Pitrez PM, Jones MH, et al. Neutrophilic airway inflammation is a main feature of induced sputum in nonatopic asthmatic children. Allergy. 2009;64:1597–1601. doi: 10.1111/j.1398-9995.2009.02057.x. [DOI] [PubMed] [Google Scholar]
- 44.Guiddir T, Saint-Pierre P, Purenne-Denis E, Lambert N, Laoudi Y, Couderc R, et al. Neutrophilic steroid-refractory recurrent wheeze and eosinophilic steroid-refractory asthma in children. J Allergy Clin Immunol Pract. 2017;5:1351–1361.e2. doi: 10.1016/j.jaip.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 45.Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of stepwise linear discriminant analysis of the 12 variables used in cluster analysis
Baseline characteristics of total subjects (n = 674) and subjects who are included and not included in cluster analysis