Abstract
Purpose
The mechanisms of CC chemokine receptor 5 (CCR5) in the process of autophagy remain unknown. In this study, we examined the role of HY peptide, which is an antagonistic peptide specifically binding the second extracellular loop of CCR5, in the expression of autophagy genes and β-arrestin 2 in lung tissues of asthmatic mice.
Methods
Experimental asthmatic mice were treated with HY peptide and dexamethasone sodium phosphate (Dex). Airway inflammation, autophagy-related genes, autophagic vacuoles (AVs) and β-arrestin 2 were examined in lung tissues, and the correlation between β-arrestin 2 and LC3 expression was assessed.
Results
HY peptide and Dex treatments alleviate airway inflammation. The expression of autophagy-related genes, such as BECN1, ATG5 and LC3, was decreased in the lung tissues of the asthmatic mice. However, HY peptide and Dex treatments increased the expression of these genes as well as the formation of AVs. Additionally, the expression of the β-arrestin 2 protein was significantly increased in the HY peptide-treated group, and positive cells expressing β-arrestin 2 were mainly located in the membrane and cytoplasm of bronchial epithelial cells. The β-arrestin 2 expression was positively correlated with the expression of LC3 in the model and HY peptide-treated groups.
Conclusions
HY peptide inhibits airway inflammation, autophagic dysfunction exists in asthmatic mice, and targeting HY peptide increases the expression of autophagy-related genes. Thus, β-arrestin 2 may participate in the mechanisms underlying these processes.
Keywords: Asthma, autophagy, CC chemokine receptor 5, beta-Arrestins
INTRODUCTION
Asthma is a common chronic respiratory disease that affects 300 million people worldwide1; although this airway disease can begin at any age, the primary symptoms in most situations occur during childhood. Studies have shown that multiple inflammatory cells (e.g., neutrophils, eosinophils and lymphocytes) and cellular components participate in the development of asthma.
Autophagy plays an important role in transporting damaged organelles, unnecessary proteins and macromolecules within double-membrane compartments for lysosomal degradation.2 Autophagy has been recognized as a crucial regulator of cell survival and maintenance. However, previous studies have suggested that autophagic dysfunction is linked to the pathogenesis of various human diseases, such as cancer as well as lung and heart diseases.3 Moreover, in childhood asthma, genetic variants in the autophagy gene autophagy-related 5 (ATG5) are related to the loss of lung function and airway remodeling.4 Another study has revealed that interleukin 4 (IL-4) is critical for B cell autophagy and is associated with asthma.5 Based on these findings, we sought to further investigate whether the defects in the autophagy progress are involved in OVA-induced asthmatic mice.
Several chemokines and chemokine receptors have been suggested to be vital for allergic airway inflammatory responses in asthmatic mouse models.6 Among these factors, CC chemokine receptor 5 (CCR5) and its major ligand regulated on activation, normal T-cell expressed and secreted (RANTES) reportedly function in the recruitment of eosinophils and T cells.7 Additionally, a 32-nucleotide deletion (delta 32) within the CCR5 (CCR5∆32) gene can reduce the risk of developing severe asthma.8 However, as a chemokine receptor expressed on many inflammatory cell surfaces, the functions and effects of CCR5 on autophagy in inflammatory cells are still unrevealed. Our study addresses the hypothesis that CCR5 is highly expressed in the lung tissues of asthmatic mice, because it can be attracted by chemokines and migrate to inflammatory sites in contrast to the higher expression of CCR5 in a murine asthma model, lower autophagy levels in lung tissues will be observed, the specific mechanisms of this phenomenon are not clear. According to our previous study, however, we suppose that CCR5 down-regulates the autophagy-related signaling pathway in lung tissues. Additionally, β-arrestin 2, which is a member of the arrestin family of proteins,9 modulates CCR5 function by binding a specific area. A review of its effects in the central nervous system shows that β-arrestin 2 not only mediates G-protein-independent signaling, but also plays a role in assisting CCR5 with binding clathrin to initiate receptor endocytosis. Although β-arrestin 2 is essential for the regulation of CCR5, the role of β-arrestin 2 in asthmatic mice after HY peptide treatment has not been investigated, and the relationship between β-arrestin2 and autophagy is largely unknown.
HY peptide used in this study is an antagonistic peptide that specifically binds the second extracellular loop (ECL2) of CCR5, which antagonized the natural ligands, RANTES, macrophage inflammatory protein 1α (MIP-1α) and MIP-1β, from binding to CCR5 and blocks chemokine-induced chemotaxis migrate to specific tissues.10 The amino acid sequence of ECL2 (MRSQK EGSHY TCSPH FLHIQ YRFWK) was searched in the protein database and chemically synthesized as a target in bio-panning. It was selected by using a phage display technology from a Ph.D.-7 Library after 4 rounds.10 The sequence of the peptide displayed on the selected phage was HYIDFRW, and the binding efficiency of the phage clone to target was identified by enzyme-linked immunosorbent assay, which showed positive results. High-performance liquid chromatography analysis showed that the purity of HY peptide was 96.43% and with high solubility, together with high activity and low rates of irrelevant effects on the immune system. Our previous study screened the optional concentration of HY peptide at 2.5 mg/mL by injecting it into the tail veins of asthmatic mice and found that this peptide inhibited inflammatory cell infiltration in the lungs by down-regulating the tumor necrosis factor-α/nuclear factor-κB (NF-κB) pathway.11 Therefore, in this study, we first test the effects of HY peptide treatment on airway inflammation, and evaluate the autophagic dysfunction occurring in asthmatic mice, then verify the effects of HY peptide on autophagic gene expression and the formation of autophagic vacuoles (AVs) in an ovalbumin (OVA)-specific murine model of allergic asthma. We further examine the location and expression of β-arrestin 2 in lung tissues and the relationship between the immunohistochemistry score of β-arrestin 2 and LC3 protein expression. Overall, our study not only extends our knowledge regarding autophagy regulation in asthmatic mice after treatment with HY peptide, but also provides new insights into the pathogenesis of asthma.
MATERIAL AND METHODS
Mice and grouping
Female BALB/c mice aged 6–8 weeks were purchased from Sun Yat-Sen University Laboratory Animal Co., Ltd. (Guangzhou, China). All protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Sun Yat-Sen University (IACUC-DD-17-1207). The mice were housed at the laboratory animal center of Sun Yat-Sen University in a specific pathogen-free (SPF) environment for 1 week prior to the experiments. Forty mice were divided into the following 5 groups (n = 8 per group): the 1) control group, 2) sensitization group, 3) model group, 4) dexamethasone (Dex)-treated group (Dex group), and 5) HY peptide-treated group (HY group).
Asthma model
The mice in the sensitization and model groups were sensitized on days 1 and 6 by intraperitoneal injection of 100 µL of phosphate-buffered saline (PBS) containing 50 µg of OVA (S7951 Grade V; Sigma-Aldrich, St. Louis, MO, USA), which was mixed with 10% aluminum hydroxide gel. On day 12, the mice in the model group were further challenged with 100 µg of OVA in 50 µL of PBS through nasal drops after transient anesthetia up to day 15 (Supplementary Fig. S1). The mice in the sensitization group were challenged with sterile saline (0.9% NaCl) alone. In the control group, the mice were sensitized and challenged with 0.9% NaCl.
Administration of HY peptide and Dex
HY peptide was synthesized by the GL Biochem Ltd. (Shanghai, China), and Dex was purchased from the Sun Yat-Sen Memorial Hospital (Guangzhou, China). Each agent was diluted with 0.9% NaCl to a final concentration of 2.5 mg/mL and 3 mg/mL, respectively. From days 16 to 22, the treatment groups received either HY peptide or Dex in 0.2-mL volumes via tail vein injection.
Histopathological examination of lung tissues
Twelve hours after the final drug administration, the mice were euthanized with sodium pentobarbital overdoses, and lung tissues were isolated to conduct histopathological examination. Paraffin-embedded lung tissues (5 µm) were stained with hematoxylin and eosin (H&E) to evaluate tissue inflammation. Ten different fields were randomly chosen for each lung section under a microscope to assess the degree of peripheral inflammation of the bronchus according to Cho's method12 and calculate the number of inflammatory cells directly.
Grade 1: absence of peribronchial inflammatory cells
Grade 2: a few scattered inflammatory cells involving less than 25% of the circumference of the bronchus
Grade 3: involving approximately 25%–75% of the circumference of the bronchus
Grade 4: 1 definite layer of inflammatory cells completely surrounding the bronchus
Grade 5: 2 definite layers of inflammatory cells completely surrounding the bronchus
Grade 6: 3 or more definite layers of inflammatory cells completely surrounding the bronchus.
The final grades ranged from a minimum grade of 0 to a maximum grade of 6. Inflammatory cells were classified as neutrophils, eosinophils and lymphocytes.
Transmission electron microscopic examination
The lung tissues were immediately fixed with 2.5% glutaraldehyde in PBS and stored at 4°C until embedding; then, the samples were stained with 1% millipore-filtered uranyl acetate. After embedding the lung tissues in LX-112 medium and cutting ultrathin sections, the sections were stained with 4% uranyl acetate and lead citrate. The images were obtained under a Tecnai Spirit Biotwin electron microscope (FEI Company, Hillsboro, OR, USA).
Immunohistochemical 2-step detection of β-arrestin 2 expression
The tissue sections were dewaxed, rehydrated and blocked with 3% H2O2 before the antigen retrieval was performed with 10 mM citrate buffer (pH 6.0) in a microwave, followed by incubation with an anti-β-arrestin 2 antibody (sc-57381; Santa Cruz Biotechnology, Dallas, TX, USA) overnight at 4°C. The samples were incubated with a secondary biotinylated anti-immunoglobulin, followed by horseradish peroxidase-conjugated streptavidin (PV-6001; Zsbio, Beijing, China) for 20 minutes at room temperature. After washing, the sections were counterstained with hematoxylin. The primary antibody was replaced with PBS as a negative control to distinguish nonspecific binding. The semiquantitative score assessments were based on the reference to Ye et al.13 and added by the following 2 parts. 1) The scores of the percentage of positive cells. We counted 100 cells in each high-power field, then randomly selected 10 fields in each slice and took the average scores. The corresponding relationship between the scores and percentages of positive cells was as follows:< 5% scored as 0, 5%–25% scored as 1, 26%–50% scored as 2, 51%–75% scored as 3, and > 75% scored as 4. 2) The scores of the staining degrees: uncolored scored as 0, light yellow scored as 1, brown yellow scored as 2, and brown scored as 3. This part was estimated by the characteristics of most positive cells. Ultimately, the scores were classified as 4 categories negative scored as 0, weakly positive scored as 1–3, positive scored as 4–5, and strongly positive scored as 6–8.
Real-time polymerase chain reaction (PCR)
All primers were synthesized by Sangon Biotech Ltd. (Shanghai, China). Also, β-actin was used as an endogenous control, and the sequences of the additional primes were as follows:BECN1, forward: 5′-GGGTCACCATCCAGGAAC-3′, reverse: 5′-CACCATCCTGGCGAG-3′; ATG5, forward: 5′-GCGGTTGAGGCTCAC-3′, reverse: 5′-GGATATTCCATGAGT-3′; LC3, forward: 5′- GCGCTTGCAGCT-3′, reverse: 5′- GTACACTTCGGAGA-3′; and ACTB, forward: 5′-CCACCATGTACCCAGGCATT-3′, reverse: 5′-AGGGTGTAAAACGCAGCTCA-3′. Total RNA was extracted from the lung tissues using TRIzol (Takara Biotechnology, Dalian, China). RNA was then reverse-transcribed to cDNA using Prime Script RT Master Mix (Takara Biotechnology). The relative quantitative levels of mRNA were measured using SYBR Green Ex Taq™ (Takara Biotechnology), and PCR was performed according to the following conditions: initial denaturation at 95°C for 10 seconds, followed by 40 cycles of 95°C for 5 seconds and 60°C for 32 seconds. The comparative transcript levels were analyzed using the cycle threshold (∆∆Ct) method.
Western blot analysis
The protein lysates from the lung tissues were harvested in ice-cold lysis buffer (20 mM Tris pH 7.5, 1% TritonX-100, 150 mM NaCl, 2.5 mM sodium pyrophosphate, 1 mM ethylenediaminetetraacetic acid, 1% Na3VO4, and 0.5 µg/mL leupeptin) (Beyotime, Beijing, China) with 1 mM PMSF. After measuring the concentration of protein via the BCA Protein Assay Kit (Beyotime), each protein extract was subsequently subjected to 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride membranes (Millipore, Burlington, MA, USA). Then, 5% nonfat milk in TBST (50 mM Tris-HCl, 150 mM NaCl, pH 7.5, and 0.1% Tween 20) was used to block nonspecific binding. After 2 hours, the membranes were incubated with primary antibodies, including Beclin1 (3495S; Cell Signaling Technology, Danvers, MA, USA), ATG5 (A0731; Sigma-Aldrich), LC3 (L8919; Sigma-Aldrich), and β-arrestin 2 (sc-57381; Santa Cruz Biotechnology,) overnight at 4°C. Then, the blots were incubated with the corresponding HRP-conjugated secondary antibodies (WBULLS0500; Millipore) for 1 hour at room temperature. Finally, the protein bands were detected by enzyme-linked chemiluminescence. The relative quality of the proteins was analyzed using ImageJ 1.38× software. GAPDH (abs830030; Absin, Shanghai, China) was used as an internal control.
Statistical analysis
The results are presented as the means ± standard deviations. One-way analysis of variance was used to assess the differences among the groups, followed by the (Least-Significant Difference, LSD)test to determine the least significance difference among the groups. The statistical relevance was determined using Spearman's correlation analysis. P values < 0.05 were considered statistically significant in all tests.
RESULTS
Effect of HY peptide treatment on inflammation of lung tissues
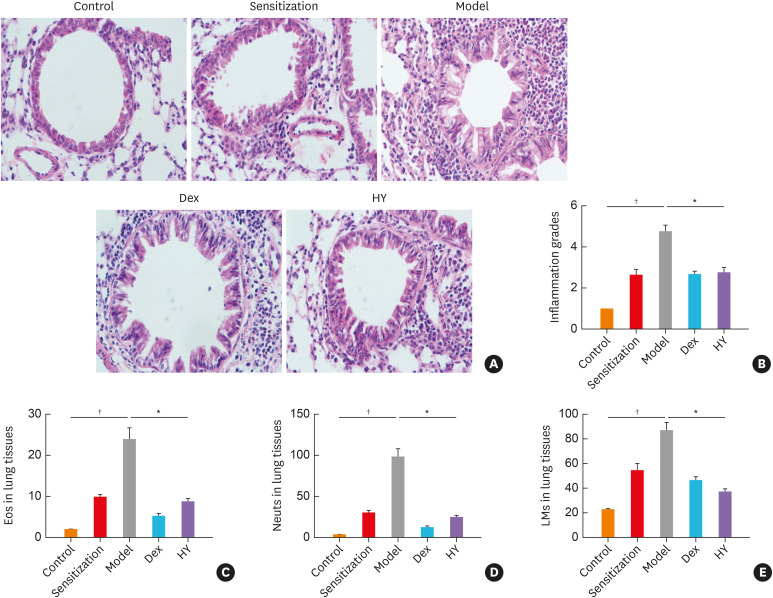
We first examined whether the HY peptide treatment relieved airway inflammation in asthmatic mice induced by OVA sensitization and challenge using Dex as a positive control. In the model group, H&E staining revealed that the airways were not only surrounded by numerous inflammatory cells (e.g., neutrophils, eosinophils and lymphocytes), but were also extensively damaged. However, this infiltration was alleviated after treatment with HY peptide and Dex (Fig. 1A). Then, we compared the inflammation scores and the classification of inflammatory cells in the lung tissues of the model and HY groups. The inflammation scores (Fig. 1B) and the percentages of inflammatory cells (Fig. 1C-E) were higher in the model group compared to the HY group.
Fig. 1. HY peptide treatment reduced established airway inflammation. (A) H&E-stained lung histology (original magnification: 400×). (B) Semiquantitative analyses of the scores in inflammatory cells. Classification of the inflammatory cells in lung tissues. (C) eosinophils, (D) neutrophils, and (E) lymphocytes. Values are means ± standard deviations (n = 8 per group).
Control, control group; Sensitization, sensitization group; Model, model group; Dex, dexamethasone-treated group; HY, HY peptide-treated group; Eos, eosinophils; Neuts, neutrophils; LMs, lymphocytes.
*P < 0.05 vs. control; †P < 0.05 vs. model.
Effect of HY peptide treatment on the mRNA expression of autophagy-related genes in lung tissues
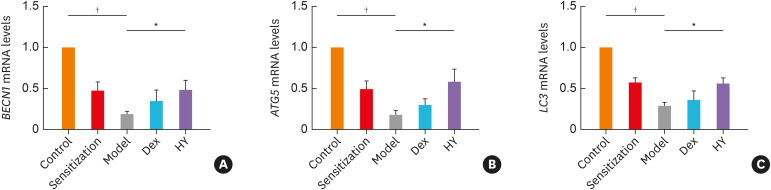
The expression of BECN1 mRNA, ATG5 mRNA and LC3 mRNA decreased more by approximately 0.19-, 0.17- and 0.28-fold, respectively, in the model group than in the control group (P < 0.001) (Supplementary Table S1), whereas these levels increased in the HY and Dex groups, but no difference was found between the Dex and model groups (Fig. 2A). The mRNA levels of BECN1, ATG5 and LC3 in the HY group increased by approximately 2.53-fold (P = 0.036), 3.41-fold (P = 0.007) and 1.96-fold (P = 0.013), respectively, and no difference in enhanced mRNA expression levels of autophagy-related genes were found between the HY and Dex groups (Supplementary Table S1). These results demonstrated that autophagic dysfunction may participate in asthma and that HY peptide could enhance the mRNA expression of autophagy-related genes.
Fig. 2. Targeting HY peptide enhanced the mRNA expression levels of autophagy-related genes in lung tissues. The expression of (A) BECN1, (B) ATG5, (C) LC3 in lung tissues of each group following HY peptide treatment. Values are means ± standard deviations (n = 8 per group).
Control, control group; Sensitization, sensitization group; Model, model group; Dex, dexamethasone-treated group; HY, HY peptide-treated group.
*P < 0.05 vs. control; †P < 0.05 vs. model.
Effect of HY peptide treatment on the protein expression of autophagy-related genes in lung tissues
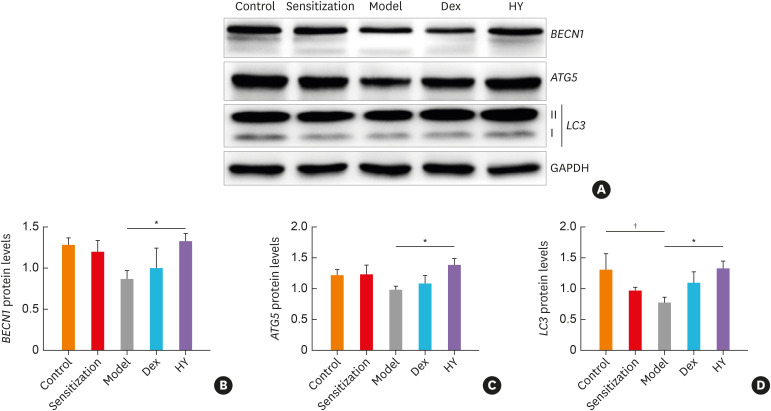
Consistent with the HY peptide treatment which increased the mRNA expression of autophagy-related genes, we observed that the total protein expression levels of Beclin1, ATG5 and LC3 decreased more by approximately 0.67-fold (P = 0.064), 0.8-fold (P = 0.156) and 0.6-fold (P = 0.032), respectively, in the model group than in the control group, whereas these levels increased after treatment with HY peptide or Dex (Fig. 3A and B), but the results were the same as those of the mRNA expression levels in the Dex group. The Beclin1, ATG5 and LC3 protein levels increased more in the HY group nearly up to 1.53-fold (P = 0.042), 1.42-fold (P = 0.020) and 1.71-fold (P = 0.026), respectively, than in the model group (Supplementary Table S2). Thus, targeting HY peptide induced the protein levels of autophagy-related genes in the asthma murine model.
Fig. 3. HY peptide treatment induced the protein expression levels of autophagy-related genes in lung tissues of each group. (A) Representative western blot showing BECN1, ATG5 and LC3 in lung tissues from asthmatic mice following the HY peptide treatment. GAPDH serves as the reference standard. Blot is representative of 3 separate experiments. Fold-change in (B) BECN1, (C) ATG5, (D) LC3 expression following by treating with HY peptide. Values are means ± standard deviations (n = 8 per group).
Control, control group; Sensitization, sensitization group; Model, model group; Dex, dexamethasone-treated group; HY, HY peptide-treated group.
*P < 0.05 vs. control; †P < 0.05 vs. model.
Effect of the HY peptide treatment on AVs in lung tissues
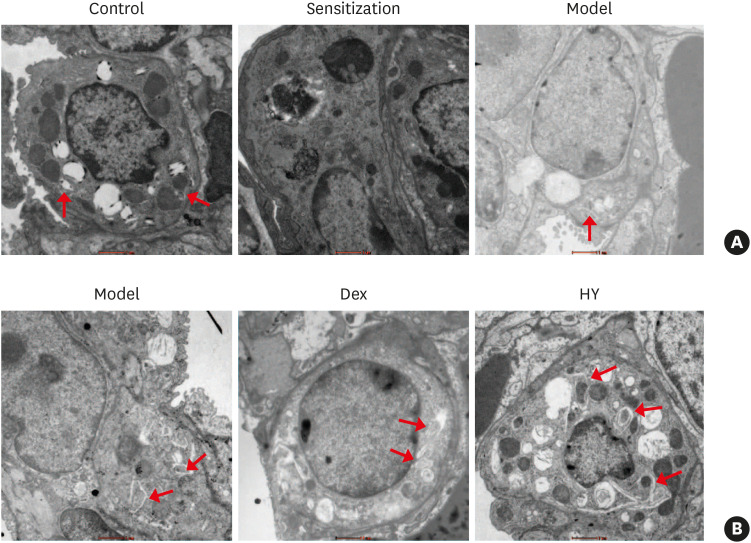
Double-membrane AVs have been recognized as the most important hallmarks of autophagy, which involves damaged organelles and cytosolic elements.14 Therefore, transmission electron microscopy was used to observe the location and formation of AVs in lung tissues. In our study, we observed that AVs were mainly located in type 2 alveolar epithelial cells (ATII) and were slightly less prevalent in the asthmatic mice than in the control mice (Fig. 4A), indicating that the formation of AVs was decreased in the asthmatic mice. Following HY peptide and Dex treatments, mature double-membrane autophagosomes were observed which became more prominent in the HY group and were located in ATII (Fig. 4B). These findings further indicated that HY peptide affected and increased the formation of AVs in lung tissues, although these results were not as robust as those observed in the expression levels of autophagy-related proteins and mRNA.
Fig. 4. Effect of HY peptide treatment on AVs in lung tissues. (A) AVs mainly located in ATII, as well as the formation of AVs in lung tissues of asthmatic mice, were decreased under TEM. (B) Electron microscopic examination of AVs after treatment with HY peptide in lung tissues. The red arrows indicate the presence of AVs (scale bar = 1 µm).
AV, autophagic vacuole; Control, control group; Sensitization, sensitization group; Model, model group; Dex, dexamethasone-treated group; HY, HY peptide-treated group; TEM, transmission electron microscopy.
Expression and subcellular localization of β-arrestin 2 in lung tissues
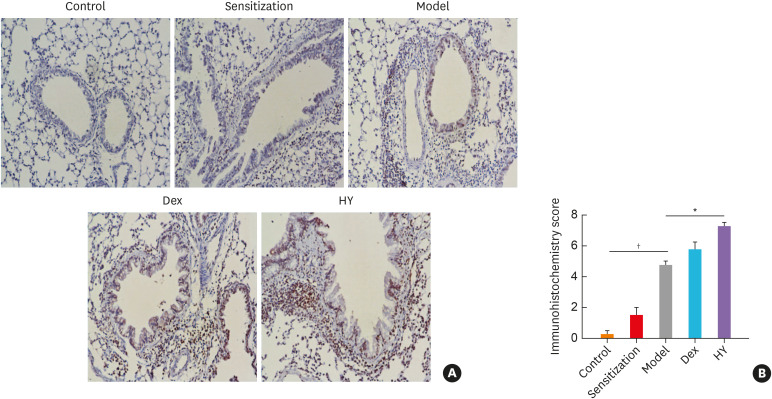
Our immunohistochemical examination revealed that β-arrestin 2 was mainly expressed in the membrane and cytoplasm and that the β-arrestin 2 positivity was mainly observed in the bronchial epithelial cells, while some positive cells were also observed among the inflammatory cells (Fig. 5A). The β-arrestin 2 expression measured using a semiquantitative assessment tool was strongly positive in the HY group, whereas it was weakly positive in the model group (Fig. 5B).
Fig. 5. The location and expression of β-arrestin 2 in lung tissues of each group. (A) The β-arrestin 2 was primarily located in the bronchial epithelial cells and expressed in the membrane and cytoplasm (original magnification: 200×). (B) Semiquantitative scores of β-arrestin 2 calculated via immunohistochemistry. Values are means ± standard deviations (n = 8 per group).
Control, control group; Sensitization, sensitization group; Model, model group; Dex, dexamethasone-treated group; HY, HY peptide-treated group.
*P < 0.05 vs. control; †P < 0.05 vs. model.
Effect of HY peptide treatment on β-arrestin 2 expression
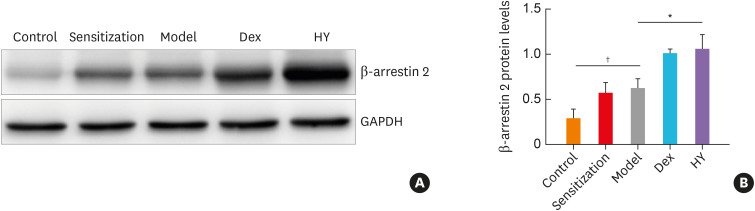
To further examine the effect of HY peptide treatment on β-arrestin 2 in a murine asthma model, we determined the protein expression level of β-arrestin 2, which acts as a scaffold and adaptor of CCR5. The western blot analysis showed that the expression of β-arrestin 2 was significantly more enhanced in the HY and Dex groups than in the model group (Fig. 6A and B), and the protein level of β-arrestin 2 increased by nearly 1.68-fold (P = 0.012) and 1.63-fold (P = 0.023), respectively, in the HY and Dex groups. These data demonstrated that β-arrestin 2 may be regulated by HY peptide.
Fig. 6. The protein level of β-arrestin 2 in a murine model of asthma model. (A) Western blot analysis of β-arrestin 2 expression in the lung tissues from all groups. Glyceraldehyde 3-phosphate dehydrogenase serves as the reference standard. Blots are representative of 3 separate experiments. (B) Fold-changes in β-arrestin 2 expression after treating with HY peptide. Values are m means ± SDs (n=8 per group).
Control, control group; Sensitization, sensitization group; Model, model group; Dex, dexamethasone-treated group; HY, HY peptide-treated group.
*P < 0.05 vs. control; †P < 0.05 vs. model.
Correlations between β-arrestin 2 and LC3 expressions
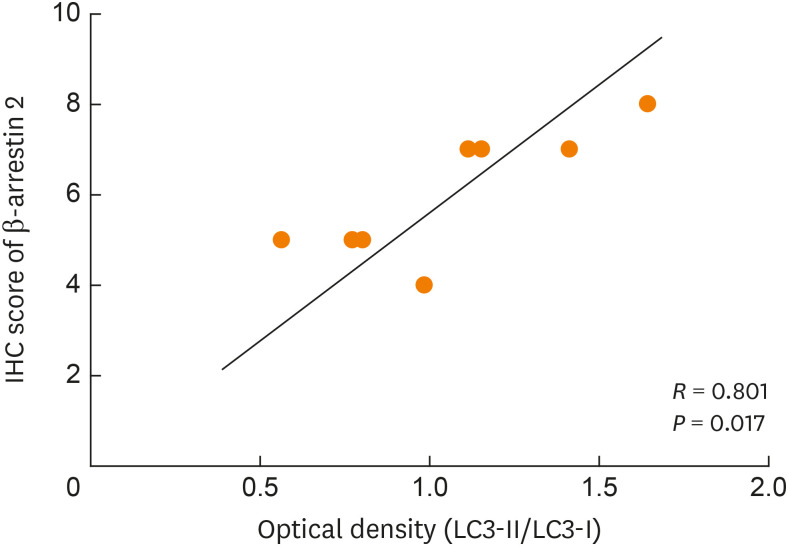
β-arrestin 2 is a central regulator of the GPCR signaling, which is known to be involved in both ligand-induced CCR5 desensitization and endocytosis.15 LC3 acts as an indicator of autophagy because of the conversion from LC3-I to LC3-II that occurs during autophagosome formation; therefore, the ratio of LC3-II/LC3-I represents the level of autophagy. Thus, the correlation between the semiquantitative scores of β-arrestin 2 and LC3 expressions in lung tissues were assessed using Spearman's correlation coefficients. The scores of β-arrestin 2 were positively correlated with the expression of LC3 (P = 0.017, R = 0.801) in the model and HY groups (Fig. 7). Although we did not explore the mechanism between β-arrestin 2 and LC3, we found a tight link between them by using Spearman's correlation analysis.
Fig. 7. Spearman's correlation analysis demonstrated a strong positively correlation between the scores of β-arrestin 2 and the expression of LC3.
IHC, immunohistochemistry.
DISCUSSION
Asthma is a complex disorder with different cellular and molecular responses. Currently, most therapies for asthma adopt anti-inflammatory drugs16; however, these methods still present significant challenges especially for steroid-resistant asthma and airway remodeling changes. To overcome these problems, inhibiting the initial recruitment of T cells and inflammatory cells to the airways represents a novel therapeutic approach. Studies have shown that CCR5 influences asthma by recruiting inflammatory cells to airways.17 In a chronic fungal asthma model, Schuh et al.18 compared CCR5−/− and CCR5+/+ mice under the same conditions, and found that CCR5-/- mice exhibited significantly less peribronchial T cell and eosinophil accumulation and airway remodeling features, while CCR5+/+ mice had opposite results. In addition, Suzaki et al.19 examined the mRNA expression of CCR5 and CCR3 in lung tissues of asthmatic mice, and found that the expression levels of these genes increased. However, when they used the small-molecule compound TAK-779, which was developed as an anti-HIV agent, to block CCR5 and CCR3, their data demonstrated that TAK-779 could significantly alleviate airway dysfunction and prevent the development of pulmonary inflammation in asthmatic mice by down-regulating the expression of the cytokines CCR5, CCR3 and Th1. Our study evaluated the inflammation grades and counted the classification of inflammatory cells in all groups. Consistent with our previous findings, in the model group, H&E staining revealed that the airways were not only surrounded by numerous inflammatory cells (e.g., neutrophils, eosinophils and lymphocytes), but were also extensively damaged. Nevertheless, this infiltration, as well as damage to bronchial walls and epithelial cells, was alleviated after treatment with HY peptide and Dex. We also found that the inflammation grades and the percentages of inflammatory cells were lower in the HY group than in the model group. Therefore, we demonstrated that HY peptide treatment reversed airway inflammation in asthmatic mice to a large extent, which implies that antagonism of CCR5 could represent a novel therapeutic target for asthma. Compared with Suzaki's experiment, there are 2 main innovation points in our study. For one thing, since we used an antagonistic peptide specifically binding the second extracellular loop of CCR5,20 which had a more accurate target and was more specific than TAK-779,21,22 this method is more in line with the trend of precision medicine in the future. For the other, in our study, mice were administered a peptide (HY peptide) rather than a small-molecule compound (TAK-779),23 which displayed higher activity and better selectivity, and more importantly, had no drug interactions with others,10 reducing non-specific effects to a large extent. Therefore, the drug used in our study demonstrated 2 different aspects compared with the previous one, and had its own innovation and implementation.
Autophagy is a fundamental cell fate pathway that has essential functions in many lung diseases, including chronic obstructive pulmonary disease, acute lung injury and asthma.24 It participates in the pathogenesis of asthma involved in genetic susceptibility and adaptive immune response. A genetic study conducted by Poon et al.25 demonstrated that single nucleotide polymorphism rs12212740 G>A of the ATG5 gene was associated with asthma and low forced expiratory volume in the first second, and they included 1,338 patients with known asthma and analyzed 5 different autophagy genes. Another study of 2 pediatric cohorts by Martin et al.4 revealed that the minor allele (A) of ATG5 rs12201458 was related to a decreased risk of childhood asthma, whereas the minor allele (G) of ATG5 rs510432 was associated with an increased risk of childhood asthma. Meanwhile, autophagy also plays an important role in the adaptive immune response of asthma. First, since autophagy is essential for the development and survival of lymphocytes,26 autophagy-deficient B cells and T cells show impaired regulation and differentiation. Secondly, autophagy is required for both central and peripheral tolerance to self-antigens, and another study revealed that ATG5 deficiency in the murine thymus not only reduces the amounts of peripheral T cells, but also attenuates their ability to proliferate and produce tolerance after stimulation with an antigen.27 Thirdly, since autophagy activity has been shown to be significantly influenced by cytokines,28 it can be inhibited by the Th2 cytokines IL-4 and IL-13 and improved by the Th1 cytokine interferon-γ. However, the specific role of autophagy in asthma is still controversial. Suzuki et al.29 showed that pulmonary CD11c+ cells lacking autophagy may still contribute to the process of neutrophilic airway inflammation. Additionally, in IL-13-treated airway epithelial cells, the depletion of ATG5 or ATG14 results in a decrease in mucin 5AC (MUC5AC) secretion and goblet cell hypertrophy.30 Furthermore, Gu et al.31 concluded that the level of autophagy is more decreased in OVA-induced murine models than in PBS-treated controls as in our study. We also confirmed that autophagic dysfunction occurs in asthmatic mice. Moreover, the double-membrane AVs were slightly more decreased in the model group than in the control group and were found in ATII which has immune function. We did not study sufficient mice in each group to achieve statistical significance of these AVs.
As a common 7-transmembrane structure receptor, CCR5 can transmit signals to cells by coupling with and activating G proteins in addition to directly participating in inflammatory responses. Venuti et al.32 discovered that natural CCR5 antibodies induce a CCR5-negative phenotype in T cells after binding the first extracellular loop of CCR5. This mechanism mainly depends on the activation of signaling pathways, which then form a stable “signalosome.” Autophagy represents one of the most important methods of removing damaged endoplasmic reticulum and mitochondria under oxidative conditions.33 It is unknown whether the functions and expression levels of autophagy are influenced by HY peptide. To explore the effects of HY peptide treatment on autophagy, we evaluated the therapeutic effects of Dex and HY peptide on autophagy in asthmatic mice, respectively, and determined whether they influence the pathway of autophagy. Dex plays a role in preventing inflammation by reducing the survival of certain inflammatory cells such as neutrophils and eosinophils. A previous study conducted by Harr et al.34 showed that dexamethasone induced autophagy in a murine T-cell lymphoma cell line. However, another study35 reported that dexamethasone did not change autophagy levels in peripheral blood eosinophils from patients with severe asthma, but decreased autophagic protein expression in healthy airway epithelial cells. These findings suggested that the effects of dexamethasone on autophagy may vary among different conditions and experiment subjects. In our study, we also showed a slight increase in autophagy after treatment with Dex, but there was no significant difference between the Dex and model groups. HY peptide administration more enhanced the expression of autophagy-related genes and AV formation in lung tissues compared with the model group and demonstrated that HY peptide treatment caused higher levels of autophagy in the asthmatic mice than dexamethasone treatment, which further confirmed the therapeutic effects of HY peptide in modulating asthma.
To obtain definitive evidence of the possible mechanism, the role of β-arrestin 2 was investigated in an asthma model. Although β-arrestin 2 has emerged as an essential regulator of chemotaxis,36 its role in chemotaxis varies among cell types and receptors to the inflammatory model system. Research has suggested that β-arrestin 2 accelerates the process of asthma by stimulating the production of IL-17 and CD4+ T lymphocytes in murine asthma models.37 An in vivo study demonstrated that chemokine (C-X-C motif) receptor 2-induced neutrophil recruitment to inflammation tissues was increased in β-arrestin2−/− mice.38 In polymicrobial sepsis, β-arrestin 2 regulated the inflammatory response negatively.39 However, Walker et al.40 reported that T-cells lacking β-arrestin 2 showed neither impaired migration in vitro nor other inflammatory features of asthma by establishing β-arrestin 2-deficient mice. These studies partially suggest that β-arrestin 2 may regulate the pathology of asthma. Meanwhile, a study by Ye et al. 13 showed that CCR5 and β-arrestin 2 had an opposite effect in inflammatory bowel disease (IBD): the expression of CCR5 was higher in active IBD, while β-arrestin 2 was expressed at a lower level. In our study, although the numbers of inflammatory cells were decreased in the Dex and HY groups, the protein level of β-arrestin 2 was enhanced, and strong positive expressions were observed in both groups; however, the exact reason for this difference remains to be determined. One possible explanation may be that a “signalosome” was formed with the help of β-arrestin 2 and that β-arrestin 2 probably acts as a negative regulator of CCR5-mediated signaling.32 HY peptide antagonizes the ligands of CCR5 and eventually induces the expression of β-arrestin 2. Another possibility that HY peptide direct induced the production of β-arrestin 2 rather than antagonized CCR5, albeit without strong evidence for this assumption. Because of the different cell phenotypes and receptor-dependent manners, β-arrestin 2 negatively modulated inflammatory cells in lung tissues, consistent with a previously published study.41 Thus, we conclude that β-arrestin 2 can participate in the development of asthma likely via CCR5-activated signaling pathways. Besides, Dex treatment also enhanced the expression of β-arrestin 2, but showed a different total effect on asthma, whose causes may be related to the inconsistency of its target and signaling pathway compared to the HY peptide treatment. A study with an antidepressant medication revealed that Dex through its interaction with the glucocorticoid receptor increased the levels of β-arrestin 2, which associated with the enhancement of MAPK/ERK1/2 signaling pathway.42 This finding provided direct evidence of the β-arrestin 2-Dex complex. Although the relationship between Dex and β-arrestin 2 in asthma has not been reported, we suspected that Dex influenced the levels of β-arrestin 2 by activating a different pathway. Likewise, Dex can affect not only white blood cells but also airway smooth muscle and epithelial function,43 which can lead to an up-regulation of β-arrestin 2 in lung tissues of asthmatic mice directly or indirectly. Further studies are necessary to determine the ultimate effect of enhancing β-arrestin 2 on HY peptide and Dex-dependent signaling pathways.
Finally, we assessed the possible correlation between β-arrestin 2 and autophagy to further determine whether the up-regulation of autophagy after the treatment with HY peptide may also be related to β-arrestin 2. Few studies have focused on this issue, except for 1 study conducted by Liu et al.44 These authors found that β-arrestin 2 mediated podocyte autophagy by negatively regulating ATG12-ATG5 conjugation in diabetic nephropathy, providing direct evidence of the possible relationship between β-arrestin 2 and the autophagosomal process. Additionally, accumulating evidence supports that β-arrestin 2 can act as a scaffold and adapter to ultimately initiate complex signal pathways such as NF-κB cascades and class III phosphoinositide 3-kinase (PI3K).45 PI3K is associated with and regulated by Beclin1, which plays an essential role in the initiation of autophagosome formation. NF-κB increases autophagosomal maturation by inducing the expression of DNA-regulated autophagy modulator protein 1.46 These results indicate that β-arrestin 2 regulates autophagy by interacting with such signaling pathways positively or negatively. In our study, we found a significant association between the immunohistochemistry scores of β-arrestin 2 and LC3 expression in lung tissues. Although we did not explore its precise mechanism, our findings still indicate that β-arrestin 2 may be closely associated with autophagy in asthma to some extent.
In summary, we demonstrated that the autophagic process was involved in asthmatic mice and affected by HY peptide; this peptide partially enhanced the expression of autophagy and β-arrestin 2 in mice. Targeting HY peptide may regulate β-arrestin 2 and further influence the process of autophagy. Taken together, our findings offer a potential therapeutic target for the treatment of asthma.
ACKNOWLEDGMENTS
This work was supported by the Natural Science Foundation of Guangdong Province, China (2015A030313027, 2016A03031343) and Guangzhou Science and Technology Program Key projects (20180301004).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
mRNA expression of Beclin1, ATG5, LC3 in each group (n = 8)
Proteins expression of Beclin1, ATG5, LC3 in each group (n = 8)
Schematic diagram of the experimental protocol for BALB/c mice sensitization and challenge with OVA, and drugs administration.
References
- 1.Martinez FD, Vercelli D. Asthma. Lancet. 2013;382:1360–1372. doi: 10.1016/S0140-6736(13)61536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014;20:460–473. doi: 10.1089/ars.2013.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeki AA, Yeganeh B, Kenyon NJ, Post M, Ghavami S. Autophagy in airway diseases: a new frontier in human asthma? Allergy. 2016;71:5–14. doi: 10.1111/all.12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin LJ, Gupta J, Jyothula SS, Butsch Kovacic M, Biagini Myers JM, Patterson TL, et al. Functional variant in the autophagy-related 5 gene promotor is associated with childhood asthma. PLoS One. 2012;7:e33454. doi: 10.1371/journal.pone.0033454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia F, Deng C, Jiang Y, Qu Y, Deng J, Cai Z, et al. IL4 (interleukin 4) induces autophagy in B cells leading to exacerbated asthma. Autophagy. 2018;14:450–464. doi: 10.1080/15548627.2017.1421884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chvatchko Y, Proudfoot AE, Buser R, Juillard P, Alouani S, Kosco-Vilbois M, et al. Inhibition of airway inflammation by amino-terminally modified RANTES/CC chemokine ligand 5 analogues is not mediated through CCR3. J Immunol. 2003;171:5498–5506. doi: 10.4049/jimmunol.171.10.5498. [DOI] [PubMed] [Google Scholar]
- 7.Raport CJ, Gosling J, Schweickart VL, Gray PW, Charo IF. Molecular cloning and functional characterization of a novel human CC chemokine receptor (CCR5) for RANTES, MIP-1beta, and MIP-1alpha. J Biol Chem. 1996;271:17161–17166. doi: 10.1074/jbc.271.29.17161. [DOI] [PubMed] [Google Scholar]
- 8.Gomulska M, Rusin G, Gwiazdak P. Prevalence of CCR5-delta32 mutation in asthmatic and non-asthmatic subjects from department of medicine, JUCM, Cracow. Folia Med Cracov. 2014;54:5–13. [PubMed] [Google Scholar]
- 9.Gao H, Sun Y, Wu Y, Luan B, Wang Y, Qu B, et al. Identification of β-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-κB pathways. Mol Cell. 2004;14:303–317. doi: 10.1016/s1097-2765(04)00216-3. [DOI] [PubMed] [Google Scholar]
- 10.Liu SX, Hu M, Ye XY, Huang HR, Zhong YQ. Panning and identification of antagonistic active peptides specifically bin-ding to the first and second extracellular membrane loops of rat CCR5 by technique of phage display peptide library. Chin J Pathophysiol. 2015;31:1225–1230. [Google Scholar]
- 11.Liang RR, Li WJ, Liu J, Shen XM, Huang HR. Effects of antagonistic peptide specifically binding to second extracellular loop of CCR5 on inflammatory cell infiltration and TNF-α expression in lung tissues of asthmatic mice induced by OVA. Chin J Pathophysiol. 2017;33:596–602. [Google Scholar]
- 12.Cho JY, Miller M, Baek KJ, Castaneda D, Nayar J, Roman M, et al. Immunostimulatory DNA sequences inhibit respiratory syncytial viral load, airway inflammation, and mucus secretion. J Allergy Clin Immunol. 2001;108:697–702. doi: 10.1067/mai.2001.119918. [DOI] [PubMed] [Google Scholar]
- 13.Ye X, Liu S, Hu M, Song Y, Huang H, Zhong Y. CCR5 expression in inflammatory bowel disease and its correlation with inflammatory cells and β-arrestin2 expression. Scand J Gastroenterol. 2017;52:551–557. doi: 10.1080/00365521.2017.1281435. [DOI] [PubMed] [Google Scholar]
- 14.Zhou JS, Zhao Y, Zhou HB, Wang Y, Wu YF, Li ZY, et al. Autophagy plays an essential role in cigarette smoke-induced expression of MUC5AC in airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2016;310:L1042–52. doi: 10.1152/ajplung.00418.2015. [DOI] [PubMed] [Google Scholar]
- 15.Lederman MM, Penn-Nicholson A, Cho M, Mosier D. Biology of CCR5 and its role in HIV infection and treatment. JAMA. 2006;296:815–826. doi: 10.1001/jama.296.7.815. [DOI] [PubMed] [Google Scholar]
- 16.Kim MH, Rhee CK, Shim JS, Park SY, Yoo KH, Kim BY, et al. Inhaled corticosteroids in asthma and the risk of pneumonia. Allergy Asthma Immunol Res. 2019;11:795–805. doi: 10.4168/aair.2019.11.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker JK, Ahumada A, Frank B, Gaspard R, Berman K, Quackenbush J, et al. Multistrain genetic comparisons reveal CCR5 as a receptor involved in airway hyperresponsiveness. Am J Respir Cell Mol Biol. 2006;34:711–718. doi: 10.1165/rcmb.2005-0314OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuh JM, Blease K, Hogaboam CM. The role of CC chemokine receptor 5 (CCR5) and RANTES/CCL5 during chronic fungal asthma in mice. FASEB J. 2002;16:228–230. doi: 10.1096/fj.01-0528fje. [DOI] [PubMed] [Google Scholar]
- 19.Suzaki Y, Hamada K, Nomi T, Ito T, Sho M, Kai Y, et al. A small-molecule compound targeting CCR5 and CXCR3 prevents airway hyperresponsiveness and inflammation. Eur Respir J. 2008;31:783–789. doi: 10.1183/09031936.00111507. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Liang R, Xie A, Shi W, Huang H, Zhong Y. Antagonistic peptides that specifically bind to the first and second extracellular loops of CCR5 and anti-IL-23p19 antibody reduce airway inflammation by suppressing the IL-23/Th17 signaling pathway. Mediators Inflamm. 2020;2020:1719467. doi: 10.1155/2020/1719467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baba M, Nishimura O, Kanzaki N, Okamoto M, Sawada H, Iizawa Y, et al. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc Natl Acad Sci U S A. 1999;96:5698–5703. doi: 10.1073/pnas.96.10.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao P, Zhou XY, Yashiro-Ohtani Y, Yang YF, Sugimoto N, Ono S, et al. The unique target specificity of a nonpeptide chemokine receptor antagonist: selective blockade of two Th1 chemokine receptors CCR5 and CXCR3. J Leukoc Biol. 2003;73:273–280. doi: 10.1189/jlb.0602269. [DOI] [PubMed] [Google Scholar]
- 23.Akashi S, Sho M, Kashizuka H, Hamada K, Ikeda N, Kuzumoto Y, et al. A novel small-molecule compound targeting CCR5 and CXCR3 prevents acute and chronic allograft rejection. Transplantation. 2005;80:378–384. doi: 10.1097/01.tp.0000166338.99933.e1. [DOI] [PubMed] [Google Scholar]
- 24.Nakahira K, Choi AM. Autophagy: a potential therapeutic target in lung diseases. Am J Physiol Lung Cell Mol Physiol. 2013;305:L93–107. doi: 10.1152/ajplung.00072.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poon AH, Chouiali F, Tse SM, Litonjua AA, Hussain SN, Baglole CJ, et al. Genetic and histologic evidence for autophagy in asthma pathogenesis. J Allergy Clin Immunol. 2012;129:569–571. doi: 10.1016/j.jaci.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puleston DJ, Simon AK. Autophagy in the immune system. Immunology. 2014;141:1–8. doi: 10.1111/imm.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nedjic J, Aichinger M, Emmerich J, Mizushima N, Klein L. Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature. 2008;455:396–400. doi: 10.1038/nature07208. [DOI] [PubMed] [Google Scholar]
- 28.Xu Y, Eissa NT. Autophagy in innate and adaptive immunity. Proc Am Thorac Soc. 2010;7:22–28. doi: 10.1513/pats.200909-103JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki Y, Maazi H, Sankaranarayanan I, Lam J, Khoo B, Soroosh P, et al. Lack of autophagy induces steroid-resistant airway inflammation. J Allergy Clin Immunol. 2016;137:1382–1389.e9. doi: 10.1016/j.jaci.2015.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dickinson JD, Alevy Y, Malvin NP, Patel KK, Gunsten SP, Holtzman MJ, et al. IL13 activates autophagy to regulate secretion in airway epithelial cells. Autophagy. 2016;12:397–409. doi: 10.1080/15548627.2015.1056967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu W, Cui R, Ding T, Li X, Peng J, Xu W, et al. Simvastatin alleviates airway inflammation and remodelling through up-regulation of autophagy in mouse models of asthma. Respirology. 2017;22:533–541. doi: 10.1111/resp.12926. [DOI] [PubMed] [Google Scholar]
- 32.Venuti A, Pastori C, Pennisi R, Riva A, Sciortino MT, Lopalco L. Class B β-arrestin2-dependent CCR5 signalosome retention with natural antibodies to CCR5. Sci Rep. 2016;6:39382. doi: 10.1038/srep39382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jyothula SS, Eissa NT. Autophagy and role in asthma. Curr Opin Pulm Med. 2013;19:30–35. doi: 10.1097/MCP.0b013e32835b1150. [DOI] [PubMed] [Google Scholar]
- 34.Harr MW, McColl KS, Zhong F, Molitoris JK, Distelhorst CW. Glucocorticoids downregulate Fyn and inhibit IP3-mediated calcium signaling to promote autophagy in T lymphocytes. Autophagy. 2010;6:912–921. doi: 10.4161/auto.6.7.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ban GY, Pham DL, Trinh TH, Lee SI, Suh DH, Yang EM, et al. Autophagy mechanisms in sputum and peripheral blood cells of patients with severe asthma: a new therapeutic target. Clin Exp Allergy. 2016;46:48–59. doi: 10.1111/cea.12585. [DOI] [PubMed] [Google Scholar]
- 36.DeFea KA. Stop that cell! β-Arrestin-dependent chemotaxis: a tale of localized actin assembly and receptor desensitization. Annu Rev Physiol. 2007;69:535–560. doi: 10.1146/annurev.physiol.69.022405.154804. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Wang GY, Liu SK, Yang MY, Ma LB, Li K, et al. β-arrestin2 stimulates interleukin-17 production and expression of CD4+ T lymphocytes in a murine asthma model. Iran J Allergy Asthma Immunol. 2011;10:171–182. [PubMed] [Google Scholar]
- 38.Su Y, Raghuwanshi SK, Yu Y, Nanney LB, Richardson RM, Richmond A. Altered CXCR2 signaling in beta-arrestin-2-deficient mouse models. J Immunol. 2005;175:5396–5402. doi: 10.4049/jimmunol.175.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan H, Bitto A, Zingarelli B, Luttrell LM, Borg K, Halushka PV, et al. Beta-arrestin 2 negatively regulates sepsis-induced inflammation. Immunology. 2010;130:344–351. doi: 10.1111/j.1365-2567.2009.03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker JK, Fong AM, Lawson BL, Savov JD, Patel DD, Schwartz DA, et al. β-Arrestin-2 regulates the development of allergic asthma. J Clin Invest. 2003;112:566–574. doi: 10.1172/JCI17265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basher F, Fan H, Zingarelli B, Borg KT, Luttrell LM, Tempel GE, et al. β-Arrestin 2: a negative regulator of inflammatory responses in polymorphonuclear leukocytes. Int J Clin Exp Med. 2008;1:32–41. [PMC free article] [PubMed] [Google Scholar]
- 42.Lucki A, Klein E, Karry R, Ben-Shachar D. Dexamethasone in the presence of desipramine enhances MAPK/ERK1/2 signaling possibly via its interference with β-arrestin. J Neural Transm (Vienna) 2014;121:289–298. doi: 10.1007/s00702-013-1099-5. [DOI] [PubMed] [Google Scholar]
- 43.Kaur M, Chivers JE, Giembycz MA, Newton R. Long-acting β2-adrenoceptor agonists synergistically enhance glucocorticoid-dependent transcription in human airway epithelial and smooth muscle cells. Mol Pharmacol. 2008;73:203–214. doi: 10.1124/mol.107.040121. [DOI] [PubMed] [Google Scholar]
- 44.Liu J, Li QX, Wang XJ, Zhang C, Duan YQ, Wang ZY, et al. β-Arrestins promote podocyte injury by inhibition of autophagy in diabetic nephropathy. Cell Death Dis. 2016;7:e2183. doi: 10.1038/cddis.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spiegel A. Cell signaling. β-Arrestin--not just for G protein-coupled receptors. Science. 2003;301:1338–1339. doi: 10.1126/science.1089552. [DOI] [PubMed] [Google Scholar]
- 46.Cadwell K. Crosstalk between autophagy and inflammatory signalling pathways: balancing defence and homeostasis. Nat Rev Immunol. 2016;16:661–675. doi: 10.1038/nri.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
mRNA expression of Beclin1, ATG5, LC3 in each group (n = 8)
Proteins expression of Beclin1, ATG5, LC3 in each group (n = 8)
Schematic diagram of the experimental protocol for BALB/c mice sensitization and challenge with OVA, and drugs administration.