Abstract
People with advanced age have a higher susceptibility to infections and exhibit increased mortality and morbidity as the ability of the immune system to combat infections decreases with age. While innate immune cells display functional defects such as decreased phagocytosis, chemotaxis and cytokine production, adaptive immune cells exhibit reduced receptor diversity, defective antibody production and a sharp decline in naive cell populations. Successful responses to vaccination in the elderly are critical to prevent common infections such as influenza and pneumonia, but vaccine efficacy decreases in older individuals compared with young adults. Trained immunity is a newly emerging concept that showed that innate immune cells possess non-specific immunological memory established through epigenetic and metabolic reprogramming upon encountering certain pathogenic stimuli. Clinical studies suggest that trained immunity can be utilized to enhance immune responses against infections and improve the efficiency of vaccinations in adults; however, how trained immunity responses are shaped with advanced age is still an open question. In this review, we provide an overview of the age-related changes in the immune system with a focus on innate immunity, discuss current vaccination strategies for the elderly, present the concept of trained immunity and propose it as a novel approach to enhance responses against infections and vaccinations in the elderly population.
Keywords: aging, immunosenescence, inflammaging, innate immune memory, vaccination
How ‘trained immunity’ enhances immune responses
Introduction
Rapid aging of the world population is one of the most crucial social shifts taking place in the twenty-first century with an extensive impact on different fields, including economics and health care. According to the United Nations Population Division, the number of people over 60 years of age in urban areas increased by 68% between 2000 and 2015 (1). This number is predicted to grow by another 56% until 2030, reaching 1.4 billion. By 2050, the population over 60 years will more than double its current size, exceeding 2 billion people.
As humans age, their immune system undergoes age-related changes that are collectively termed immunosenescence (2). Besides other age-related conditions such as Alzheimer’s disease and cardiovascular diseases, aging of the immune system leads to increased susceptibility to infections and autoimmune diseases, and poor response to vaccination, followed by high hospitalization and increased mortality rates (3). Morbidity associated with infectious diseases in the elderly population is a significant burden on the healthcare systems and economies of countries all around the globe. Because of these reasons, counteracting immunosenescence and developing new immunization strategies for elderly people are considered priority research areas by the World Health Organization (4). Understanding the mechanisms of immunosenescence and developing counteractive measures are of great importance.
Here we describe the mechanisms of immunosenescence, with a particular emphasis on the innate immune system. We then review the impact of vaccine responses in the elderly and the current approaches to improve vaccine efficacy. Lastly, we describe the concept of trained immunity, the adaptation of innate host defense that leads to non-specific immunological memory in innate immune cells through epigenetic and metabolic reprogramming (5). We finally detail recent studies utilizing trained immunity to boost vaccine responses and propose trained immunity as a promising approach to increase vaccine efficiency in the elderly population.
Aging of the immune system: a brief overview
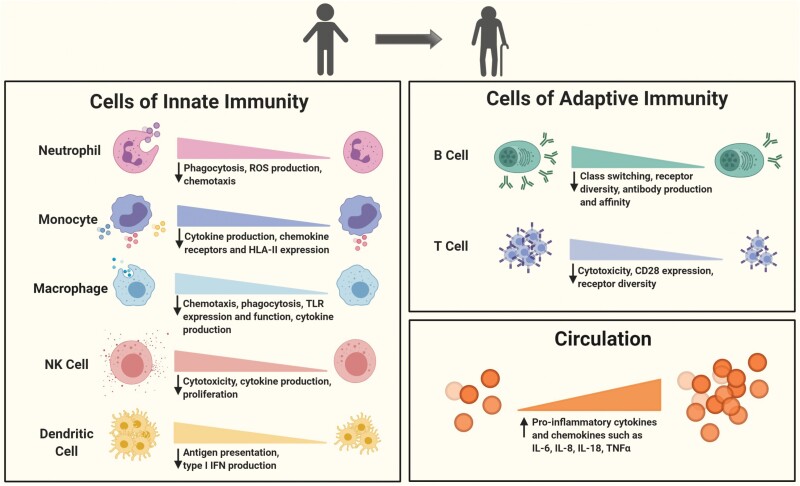
The most established features of immunosenescence—the dysregulated state of an aged immune system—include short-lived memory responses, defective response to new antigens, higher disposition to autoimmunity and the chronic low-grade systemic inflammation that is termed inflammaging (6). The main cellular culprits behind these dysregulated responses are a sharp decrease of naive T- and B-cell pools with increasing age, reduced natural killer (NK) cell cytotoxicity, impaired signaling and decreased function of some innate immune cell subsets (2) (Fig. 1).
Fig. 1.
Age-associated functional changes in the immune system. Both innate and adaptive immune systems undergo age-related alterations in terms of cell numbers and functions toward the later decades of human life. Multiple human and murine studies revealed that the cells of innate immunity such as neutrophils, monocytes, macrophages, dendritic cells and NK cells display impaired receptor expression, chemotaxis, phagocytosis, antigen presentation, cytotoxicity, ROS and cytokine production. Adaptive immune cells (B cells and T cells) experience shifts in sub-populations such as the depletion of naive cell pools and accumulation of late-differentiated effector and memory cells. Apart from those, both display reduced receptor diversity. Functionally, expression of the co-stimulatory molecule CD28 is critically diminished in T cells while B cells become weaker in class-switching and affinity maturation. Numbers of plasma cells and production of antibodies also decrease. Despite these functional down-regulations at the cellular level, levels of pro-inflammatory cytokines and chemokines are elevated in circulation with advancing age.
Lingering inflammation causes tissue damage and contributes to the development and progression of age-related diseases. Elevated circulating levels of pro-inflammatory cytokines interleukin 6 (IL-6) and tumor necrosis factor α (TNFα) along with C-reactive protein (CRP) are some of the most reliable markers of inflammaging, their circulating concentrations predicting frailty and mortality in the elderly (7, 8). Inflammaging is the result of the accumulated long-term stimulation of the innate immune system with increasing age. As the current life expectancy of humans exceeds the life span that characterized human evolution for hundreds of thousands of years, beneficial physiological responses may become damaging as humans age (9).
One of the mechanisms proposed to drive inflammaging is the accumulation of damage-associated molecular patterns (DAMPs), which are essential for effective tissue repair and inflammatory response against pathogens, but can also cause maladaptive responses and chronic disease, as disposal of the accumulating material by autophagy or mitophagy declines with age (10). Another likely source is the senescence-associated secretory phenotype (SASP) of senescent epithelial and endothelial cells which secrete pro-inflammatory cytokines and modify the response of neighboring cells (11, 12). Products of microbiota might also contribute to inflammaging. As the body ages, the gut is less efficient in sequestering microbes and their products (13). Contents of the gut microbiota change with age as well, becoming more inflammatory (14). Age-related expansion of Proteobacteria and a decline in butyrate-producing bacteria, for example, have been correlated to increased IL-6 and IL-8 levels (15).
Age-related changes in innate immunity
Hematopoietic stem cells (HSCs) in the bone marrow increase in number with age and become more likely to commit to the myeloid lineage, which gives rise to the majority of the innate immune cells including dendritic cells (DCs), monocytes, macrophages, mast cells and granulocytes (e.g. neutrophils) (16, 17). However, despite the skewing to the myeloid lineage in the bone marrow, numerous age-related declines in terms of cell number and function have been described for the cells of innate immunity. On the one hand, elderly people tend to develop low-grade systemic inflammation although their immune cells present defective capacities of migration, phagocytosis and cytokine production (18). Impaired functions of innate immunity can further exacerbate the flaws in adaptive immunity, for instance by not providing efficient antigen presentation to T cells. Here, we detail the age-related changes in different innate immune cell subsets and their consequences.
Neutrophils
Neutrophils are the most abundant type of immune cell in circulation. They internalize pathogens through phagocytosis and destroy them using reactive oxygen species (ROS) and degradative proteases, while also recruiting and activating DCs, monocytes and lymphocytes (19). Neutrophils also efficiently trap and kill extracellular pathogens by forming neutrophil extracellular traps composed of web-like structures of chromatin and proteases (20).
Many functions of neutrophils including chemotaxis, phagocytosis, ROS production, signal transduction and apoptosis have been reported to be dysfunctional in the elderly (21–25). However, neutrophil numbers are mostly preserved during aging (21). Healthy centenarians—the people aged 100 years or older—have well-conserved neutrophil functions (26). Increased activation of constitutive phosphoinositide 3-kinase (PI3K) was associated with impaired chemotaxis in the elderly (22). Expression of CD16, an Fc receptor, is low in neutrophils of people aged over 65, which potentially restricts Fc-mediated phagocytic activity (24). Intracellular killing of the phagocytosed pathogens is also defective in the elderly (27, 28). Defects in ROS production have been linked to the changing composition of cell membranes with age (29). Moreover, granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-2 have anti-apoptotic effects on neutrophils of young adults but not in adults over 65 years of age (30, 31). Increased neutrophil susceptibility to apoptosis might also contribute to the weakened response of the elderly against pathogens (3).
Monocytes
Monocytes present a large range of functions, including phagocytosis, cytokine production and antigen presentation. They circulate in the blood and migrate into tissues in response to infection or tissue damage where they can differentiate into macrophages or DCs (32). In humans, there are three major monocyte subsets with different functions, identified based on their CD14 and CD16 expression: CD14+ CD16− classical monocytes, CD14+ CD16+ intermediate monocytes and CD14dim CD16+ non-classical monocytes (33).
Circulating monocyte numbers are stable with advancing age (21). However, the ratios of different monocyte subsets are altered. Classical monocytes are reportedly reduced, while intermediate and non-classical monocytes are increased, with age (34). Of note, expanding non-classical monocytes present lower expression of the CX3CR1 chemokine receptor and the human leukocyte antigen class II molecule HLA-DR, whereas macrophages derived from monocytes of elderly subjects display intact cytokine production (35, 36). Monocytes from subjects over 60 years old present higher Toll-like receptor 5 (TLR5) expression, produce more IL-8 and show increased phosphorylation of mitogen-activated protein kinases (MAPKs) p38 and extracellular signal-regulated kinase (ERK) upon activation with TLR ligands, but they are defective in activation of nuclear factor κB (NF-κB) (37).
Another study reported lower TLR1 expression, less ERK1/ERK2 phosphorylation upon TLR1/TLR2 activation, and reduced IL-6 and TNFα production in monocytes of people over 66 years of age (38). A recent study investigating innate immune responses in a healthy population of individuals of various ages has shown intact cytokine production capacity and normal numbers of innate immune cells in the circulation (39). Moreover, the production of some of the inflammatory cytokines was even higher in the elderly, underscoring the development of inflammaging.
Macrophages
Macrophages are phagocytic cells present in nearly all tissues where they contribute to tissue homeostasis, tissue repair and host defense (40). They exhibit high plasticity and heterogeneity, and secrete a wide variety of cytokines and chemokines upon recognition of pathogen-derived or damage-associated signals (41).
Although circulating monocyte numbers are stable through life, numbers of macrophage precursors are reportedly reduced with advancing age (42, 43). Similar to neutrophils, macrophages display age-related defects in chemotaxis, TLR expression and function, signal transduction, phagocytosis and superoxide production (21). Upon lipopolysaccharide (LPS) stimulation, peritoneal macrophages of aged mice had 70% decreased p38 MAPK and c-Jun N-terminal kinase (c-JNK) activation, which are critical for TLR-mediated responses (44, 45). Decreased expression of inducible nitric oxide synthase (iNOS) and impaired production of nitric oxide were also observed in macrophages from aged mice (46).
Aged-mouse macrophages also had less major histocompatibility complex (MHC) class II expression, lower levels of TLRs and reduced IL-6 and TNFα production upon stimulation with TLR ligands (47, 48). MHC II expression on the surface was 50% less in macrophages of old mice following interferon γ (IFNγ) stimulation (49). LPS-induced IL-1β and IL-12 production was also reduced in splenic macrophages from aged mice (50). Macrophages in aged mice were also less capable of clearing apoptotic debris (51).
Most published studies investigating age-related changes in macrophages are murine studies, because of difficulties in obtaining tissue macrophages from humans. Nevertheless, there are a few studies suggesting decreased macrophage function in the elderly. The numbers of bone marrow macrophages in the later decades of life were found to be comparable to younger adults (52). Monocyte-derived macrophages from the elderly produced less TNFα, IL-6, IL-8 and IL-1β when incubated with Streptococcus pneumoniae, even though their phagocytic ability seemed to be intact (53). This functional defect was linked to impaired PI3K–AKT (Ak-strain thymoma oncogene; also called protein kinase B) signaling. Another study with monocyte/macrophage cultures infected with dengue virus revealed lower TNFα, IL-6 and IL-1β production by cells of elderly subjects over 65 years old compared with younger adults (54).
Dendritic cells
DCs are very potent antigen-presenting cells (APCs) that are usually considered as the bridge between innate and adaptive immunity (55, 56). The two main subsets of DCs are myeloid DCs (mDCs) or conventional DCs (cDCs) of myeloid origin and plasmacytoid DCs (pDCs) of lymphoid origin, which are crucial for anti-viral defense (57).
Total peripheral DC and mDC numbers are lower in people aged over 60 years although pDC numbers remain stable (58). Thymic DCs are also reduced in the elderly and are less efficient in stimulating T cells (59). Even though pDC numbers do not change with age, they have lower type I IFN-releasing capacity due to impaired interferon regulatory factor 7 (IRF7) phosphorylation, which is associated with a reduced response to influenza virus (60). Their antigen-presentation capacity is also decreased. mDCs of elderly people have restricted migratory and phagocytic capacities (61).
People over 60 years of age present higher production of TNFα and IL-6 by mDCs upon TLR4 stimulation, despite defective AKT phosphorylation and PI3K signaling (62, 63). Also, when derived from elderly individuals, mDCs that appear to have a more mature phenotype produced less IL-12 upon LPS stimulation (58). In addition, Langerhans cells, which are specialized DCs in epidermis and are critical for skin immunity, are lower in number in elderly people and migrate less in response to TNFα (64).
NK cells
NK cells are cytotoxic cells that are heavily studied in the context of anti-tumor responses, but they also exert cytotoxic activity upon recognition of infected cells, particularly in viral infections, or cytokines such as IL-2, IL-12, IL-15 and IL-18 (65, 66).
Studies reported increased or maintained NK numbers in the elderly although proliferation rates appear to be decreased (67, 68). This is suggestive of the existence of long-lived NK cells. Recently, memory-like NK cells, defined as NKG2C+ CD57+, were indeed described in people with cytomegalovirus (CMV) infection and were also detected in CMV− individuals later (69, 70).
Despite some studies reporting preserved NK cytotoxicity, it is considered to be impaired on a per cell basis (71, 72). Low NK cytotoxicity is associated with higher infection rates and infection-related deaths in the elderly (73). Higher NK cytotoxic activity is also linked to higher antibody titers after influenza vaccination in people over 65 years of age (74). Production of IFNγ and proliferation upon IL-2 stimulation were also reduced in this group (71). The NK cell receptor repertoire was also found to be altered with age (75). Additionally, the CD56bright NK cell subset, which constitutes around 10% of peripheral NK cells, was critically diminished in the elderly (76).
Age-related changes in adaptive immunity
Antigen-specific adaptive immunity with memory-generating capabilities is crucial for responding against tumors, allergens and pathogens. The most profound changes in the immune system related with aging are observed in adaptive immunity. In the following paragraphs, we summarize the age-related defects in T cells and B cells.
T cells
T cells, through their diverse range of antigen receptors [T-cell receptors (TCRs)], recognize pathogenic or tumor-derived antigens and develop antigen-specific memory or tolerance (77). Upon recognition of antigen and receiving co-stimulatory signals, naive T cells differentiate into effector cells. Most of the effector cells are short-lived; however, a portion persist as memory cells and establish long-term immunity. The two main lineages of T cells are CD4+ helper and CD8+ cytotoxic T cells (78).
Maturation and selection of T cells take place in the thymus. Thymic involution—the gradual atrophy of the thymus with age—starts from the first year of life and progresses until the end of life (79). The thymopoietic space, where T-cell maturation occurs, is shrunk to <10% in volume by the age of 70 (80). Processes underlying this include loss of thymic epithelium, reduced IL-7 production by thymic epithelium, which is essential for the maturation of thymocytes, and defective rearrangement of the TCR β-chain (81, 82). People who had undergone thymectomy in early childhood show a premature immunosenescent phenotype (83).
The typical immunosenescent profile includes reduced output of naive T cells and a T-cell pool consisting mostly of differentiated effector cells and memory cells (84). It is important to note that most age-related changes in T-cell profiles are either only seen in, or are more pronounced in, individuals seropositive for CMV, which is a chronic infection present in almost 70% of people over 60 years of age (85, 86). Among CD8+ T cells, the CD28− effector population is markedly increased in the elderly (87, 88). In contrast, the naive CD8+ T-cell pool is depleted with age (89). Loss of CD28, which plays a critical role in T-cell activation in effector cells, is among the hallmarks of immunosenescence in T cells (90).
Furthermore, the limited number of existing CD28+ cells have a more restricted TCR repertoire and shorter telomeres in people over 65 years of age (91). Clonal expansion of CD28− CD8+ T cells was inversely correlated with antibody production against influenza vaccination (92). Because of the extreme expansion of these cells and the reduced naive T-cell output, the T-cell repertoire diversity is restricted and susceptibility to novel infections is increased (93).
The naive CD4+ T-cell pool does not undergo such a critical change as CD8+ T cells, although there is a decline in numbers (94, 95). Upon probing with novel antigens, IL-2 production by naive CD4+ T cells of elderly people was also comparable to young individuals (96), even though there is defective TCR-induced ERK signaling (97). In contrast to naive cells, central memory CD4+ cells accumulate in people over 65 years of age (94, 98). Effector memory cells, on the other hand, are found at a lower frequency in the elderly and their numbers were correlated with anti-influenza response upon vaccination (98). The accumulation of effector cells and loss of CD28 seen in CD8+ T cells are not pronounced in CD4+ cells (95).
B cells
B cells mediate humoral immunity against pathogens and allergens by producing antibodies with high specificity and affinity (99). The B-cell antibody response is one of the crucial outcomes that vaccination strategies strive to achieve. B cells develop and mature in the bone marrow. In contrast to T cells, whose output is severely affected by thymic involution, B-cell lymphopoiesis continues throughout life, but B-cell precursor numbers in the bone marrow and the antibody-producing plasma cells decrease with age (100, 101).
Similar to T cells, accumulation of memory B cells with restricted receptor diversity was reported in the elderly (102). Impaired class-switching and somatic recombination along with lower diversity of antibodies are also observed in this group, leading to weak antibody responses with low affinity (103). Age-related alterations in number and size of germinal centers, where B cells proliferate and undergo somatic hypermutation, partly contributed by sub-optimal T-cell help underlie these defects (104, 105). The percentage of switched memory B cells, which have been positively correlated with influenza vaccine responses, also declines significantly with age (106–108). This population has very short telomeres in the elderly compared with younger individuals (109). In contrast, late exhausted memory B cells are expanded in the elderly, filling up the immunological space (109). Another age-related change is the increase of auto-antibodies in the elderly, likely contributing to prevalence of autoimmune diseases (110).
Vaccine responses in the elderly and current improvement strategies
In order to prevent and reduce the number of infections in elderly people, vaccines are the most cost-effective and safe approach. However, the overall vaccination efficiency of currently available vaccines remains low in the elderly population, because of the impaired ability of their immune system to respond to immune stimulation (111).
Influenza is one of the major infections worldwide, and it represents a considerable threat for vulnerable populations such as elderly and young children. There are up to 500 000 deaths reported every year in people aged >65 years because of influenza (112). Along with increased risk of hospitalization and deaths linked with influenza-associated respiratory diseases, vaccine efficiency is also lower at 17–53% in the elderly, compared with the 70–90% efficacy in young adults (113). Suggested reasons for the impaired influenza vaccine response included decreased somatic mutations in B cells (114), an increased regulatory T cell (Treg) population (115), impaired expression of the co-stimulatory molecule CD28 in T cells (116), the reduced antigen-presenting capacity of pDCs (60) and low NK cell cytotoxicity (21).
Currently, there are two commonly available influenza vaccines: inactivated vaccines and live-attenuated vaccines. A high-dose inactivated vaccine with 60 µg hemagglutinin (HA) antigen from each strain demonstrated improved antibody responses with 24.2% more efficiency in people over 65 years of age compared with the 15 µg standard dose (117–119). In 2019, a high-dose influenza vaccine was approved by the Food and Drug Administration (FDA) for use in people older than 65 years, reported as well-tolerated and more effective (120). Nonetheless, vaccination of elderly with the high-dose vaccine still induced lower antibody responses and Th1 T-cell responses in comparison with young adults vaccinated with the standard dose (121).
Another study demonstrated that intra-dermal injection instead of intramuscular injection significantly improved antibody titers in people over 65 years of age; however, intra-dermal injection of the high-dose (60 µg) influenza vaccine was not significantly different than that of the normal dose (15 µg) in terms of protection (119).
Adjuvanting the vaccines is another promising strategy to boost immune responses in the elderly. Adjuvants are a crucial part of vaccines, contributing to better vaccine responses by increasing antigen presentation and activating the innate immune system (122). Considering that antigen presentation, responsiveness and chemotaxis of immune cells are mostly impaired in old individuals, improvements in adjuvant systems would increase the efficacy of vaccinations.
MF59® (Fluad), an emulsion-based adjuvant, was significantly immunogenic, and it reduced influenza-related hospitalizations by 25% in the elderly in comparison with non-adjuvanted influenza vaccine (123–125). MF59 has been reported to increase viral antigen uptake and antigen presentation, hence enhancing immunization efficacy. Additionally, the MF59-adjuvanted subunit influenza vaccine induced antibody responses against non-specific seasonal viral strains (126). TLR ligands are also utilized as adjuvants. A phase 2b/3 trial demonstrated that topical application of the synthetic TLR7/TLR8 agonist imiquimod prior to intra-dermal trivalent influenza vaccination significantly elevated the immunogenicity of vaccine in the elderly (127).
Streptococcus pneumoniae is another prevalent cause of severe infections in the elderly that might result in several complications such as upper respiratory disease, bacteremia and meningitis (128). There are two commonly used vaccines: a 23-valent pneumococcal polysaccharide vaccine (PPSV23), which is mostly used for adults and the elderly; and a 13-valent pneumococcal conjugate vaccine (PCV13) for children older than 2 years of age (129).
Although PPV23 has been recommended for a long time to vaccinate the elderly, a meta-analysis assessing vaccine efficiency showed that PPV23 had a moderate effect on invasive pneumococcal disease while it was not potent against pneumococcal pneumonia (130). On the other hand, PCV13 has been reported as partly effective against pneumococcal diseases in old individuals; however, age still influences the potency of PCV13 with efficacy of 65% and 40% in 65-year-old and 75-year-old participants, respectively (131). A study argued that the combination of PCV13 with PPV23 possibly enhances protection in the elderly; however, clinical data demonstrating elevated antibody production and reduced disease incidence are still missing (132).
Varicella zoster virus (VZV) is another important pathogen affecting the elderly. This virus remains latent in the nerve cells of infected individuals after an episode of chickenpox in early life (133). Herpes zoster or shingles is caused by reactivation of latent VZV, and the risk of developing shingles increases with age because of the reduced activity of cell-based immunity (134); therefore, most of the cases that require hospitalization are people older than 50 years (135).
Two vaccines are licensed for usage against shingles: a live-attenuated vaccine (Zostavax™) developed by Merck; and a subunit zoster vaccine (Shingrix™) formulated by GSK. A double-blind, placebo-controlled study with people older than 60 years showed that the live-attenuated vaccine lowered the burden of illness by 61.1% and prevalence of herpes zoster by 51.3% (136).
The novel adjuvant AS01b, consisting of MPL (3-O-desacyl-monophosphoryl lipid A), a TLR4 agonist as a derivative of LPS from Salmonella minnesota, and saponin QS-21, has been shown to effectively promote antigen presentation and CD4+ T-cell-mediated immune responses, and demonstrated high efficacy in combination with different vaccines in clinical trials (137). An inactivated vaccine utilizing AS01b as a liposome-based adjuvant exhibited promising results in elderly people, with 97.2% efficacy in people over 50 years of age (138). Of note, the vaccine potency did not decrease with age; the efficiency in people older than 70 years of age is similar to that in people between 50 and 70 years old. Additionally, vaccine-induced antibody production was still higher than the pre-vaccination level even after 9 years (139). A phase II trial comparing the AS01b-adjuvanted vaccine with non-adjuvanted vaccine reported that immunogenicity of the viral subunit vaccine increased with the adjuvant in a dose-dependent manner (140). The very special behavior of this AS01-containing vaccine with high efficacy in the elderly provides a potential tool to investigate the mechanisms needed to induce proper vaccination responses in the elderly and gives hope that similar levels of efficacy may be achieved with other vaccines as well.
Trained immunity and vaccination in the elderly
For a long time, the development of immunological memory was solely attributed to adaptive immunity, which is maintained by antigen-specific long-lasting memory lymphocytes upon recognition of a pathogen. On the other hand, innate immune responses are mediated by non-specific effector molecules and have been considered as being devoid of memory properties. However, recent studies consistently reported the capacity of the innate immune system to develop memory-like features (141–144).
Our group and others showed that, following an insult with certain infections or vaccinations, members of the innate immune system, for example monocytes, DCs and NK cells, exhibit enhanced responsiveness to a second infection that might be the same or a different pathogen. This phenomenon was later termed as ‘trained immunity’ or ‘innate immune memory’ (144). Although the concept of trained immunity was first demonstrated and mostly studied in monocytes, there is evidence that memory-like properties are also present in other innate immune cells. For instance, ex vivo stimulation of human NK cells with heterologous pathogens 3 months after Bacillus Calmette–Guérin (BCG)—a live-attenuated vaccine against tuberculosis (145)—results in enhanced pro-inflammatory cytokine production but not IFNγ production compared with before vaccination (146).
Notably, BCG neither induced NK cell expansion nor altered the expression of NK cell markers. A recent study suggested that DCs from immunized mice showed a long-term memory response upon fungal challenge that was mediated by specific epigenetic modifications (147).
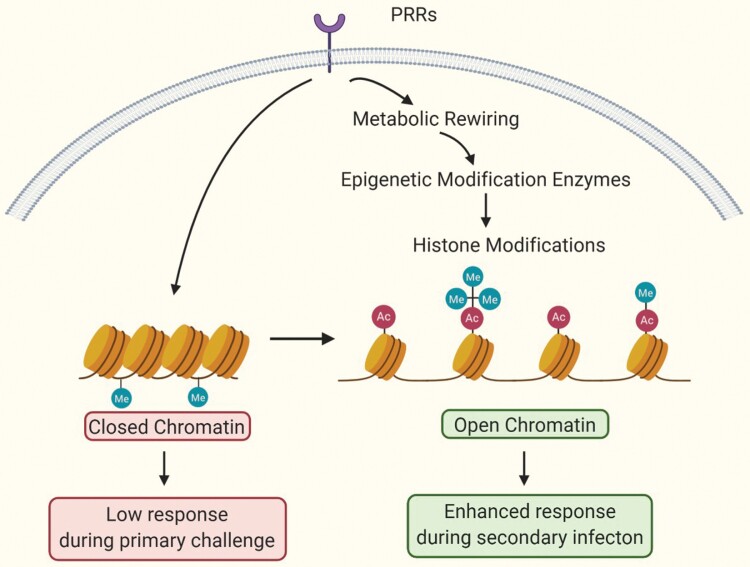
The underlying mechanisms of trained immunity are explained by epigenetic and metabolic reprogramming (Fig. 2). Immunological signal pathways, for example pattern-recognition receptors (PRRs) engaged with DAMPs or bacterial products, induce epigenetic changes (i.e. increase in H3K4me3, H3K4me and H3K27Ac and removal of H3K9me3) at the promoter and enhancer sites of genes coding for pro-inflammatory cytokines and metabolic rewiring such as up-regulation of glycolysis, cholesterol synthesis and glutaminolysis (148–151). Certain metabolites of these pathways, such as α-ketoglutarate and fumarate, subsequently modulate the activities of epigenetic remodeling enzymes, such as histone demethylases or histone acetyltransferases (5). As a result, increased chromatin accessibility of pro-inflammatory genes eventually leads to elevated pro-inflammatory cytokine production when a secondary challenge occurs. Another remarkable finding is that memory-like properties can persist for a long time beyond the limited life spans of immune cells, owing to the reprogramming of HSCs and myeloid progenitors in the bone marrow (152, 153).
Fig. 2.
Overview of fundamental mechanisms in trained immunity. Certain infections and vaccinations alter metabolic pathways, leading to histone modifications that enable chromatin regions to be more open for transcription. Increased gene expression results in improved responses against pathogens during secondary infection.
Trained immunity can be used as an effective way to boost vaccine responses by conferring wide protection against a diverse range of pathogens (154). For instance, trained immunity induced by β-glucan protected mice against bacterial infections causing peritonitis, enteritis and pneumonia by increasing inflammatory monocyte and granulocyte numbers and IL-1β production (155).
Clinical trials and epidemiological studies revealed that certain vaccines such as vaccinia, BCG and measles have non-specific protective effects (156, 157). Among them, BCG is the most extensively studied vaccine for its heterologous protective effects. It has been used for treatment and decreasing the progression of non-muscle invasive bladder cancer for >40 years, although its mode of action has not been fully understood yet (158–160). The wide range of protection conferred by BCG is mainly attributed to increased cytokine production as a result of metabolic and epigenetic reprogramming of innate immune cells. It is also important to point out that in addition to induction of trained immunity, BCG and other live attenuated vaccines can induce heterologous adaptive immune responses, such as Th1-dependent IFNγ production (161, 162). It is conceivable that the complete beneficial effects of BCG vaccination are due to a combination of trained immunity and heterologous T-cell immunity. In addition, it is important to note that BCG vaccination prior to influenza and childhood vaccines can also act as an adjuvant and enhance antibody responses; however, the mechanisms in play are yet to be established (163, 164).
Evidence from several animal and human studies suggests that BCG vaccination is also effective to protect against Leishmania spp. and Plasmodium falciparum infections (165). In a double-blinded, placebo-controlled study, individuals vaccinated with BCG 1 month before experimental viral infection induced by yellow fever vaccine displayed less viremia in their blood compared with people vaccinated with placebo. Protection against yellow fever virus was reported to be associated with epigenetic modifications of monocytes, and high IL-1β production was inversely related to viremia (166). In a clinical trial investigating protective effects of BCG on malaria infection, BCG-vaccinated subjects 5 weeks prior to controlled malaria infection presented early activation of NK cells and monocytes which were correlated with lower parasitemia (167).
Remarkably, heterologous protection by BCG was not limited to an enhanced innate immune/trained immunity response. It has been shown that BCG vaccination induced heterologous Th1 and Th17 responses even 1 year after immunization (168). Another study from our group demonstrated that BCG vaccine could be used to improve the beneficial effects of diphtheria tetanus pertussis (DTP) and influenza vaccines. BCG vaccination prevented the immunosuppressive effects of acellular diphtheria tetanus pertussis combined vaccine (DTaP) and induced trained immunity in adults when it was given concurrently with or 3 months after DTaP (169). BCG vaccination 2 weeks before trivalent influenza vaccination significantly boosted HA-inhibiting antibody production in healthy adults. Moreover, BCG-priming induced higher production of pro-inflammatory cytokines after ex vivo stimulation of peripheral blood mononuclear cells (PBMCs) with unrelated pathogens such as Candida albicans and Staphylococcus aureus (164).
Although literature for trained immunity in the elderly is very scarce, a few studies in the elderly suggest that not only children and adults, but also the elderly, might benefit from protection against heterologous infections. It has been recently shown that BCG-vaccinated individuals in Guinea-Bissau who are older than 50 years of age displayed increased pro-inflammatory cytokine production following ex vivo stimulation with heterologous stimuli 2 months after vaccination (170). Considering the impaired ability of innate immune cells to respond against infections in the elderly, this study suggests that trained immunity could indeed be induced in elderly people and might be utilized as a powerful tool to increase vaccine responses and protect this vulnerable population from various infections by counteracting the effects of immunosenescence.
Another clinical study, in which participants between 60 and 75 years old received BCG once a month for 3 months, demonstrated that BCG vaccination significantly prevented acute upper respiratory tract infections while increasing IFNγ and IL-10 production (171). Furthermore, the scar diameter at the vaccination site was correlated to the circulating IFNγ levels. Another study performed in Japan with elderly people indicated a lower risk of pneumonia following immunization with BCG (172).
Utilizing the trained-immunity response to increase resistance and defense against infections is advantageous in many settings. First of all, since trained immunity confers a broad range of protection, it might be useful in illnesses in which secondary infections or co-infections play a role. As an example, bacterial infections following influenza can worsen the outcome by increasing morbidity and mortality (173, 174). As viruses frequently undergo mutations, conventional vaccines remain ineffective in some cases. Therefore, trained immunity can be employed to protect people from newly emerged bacterial or viral strains. Lastly, clinical conditions such as immunoparalysis could be rescued by inducing trained immunity (175).
Improving innate immune responses to provide protection is crucial for vulnerable populations such as the elderly and people with immune deficiencies. In a recent review by Sánchez-Ramón et al., approaches to employ trained immunity in vaccine formulations were explicitly discussed (154). According to that, it was suggested that trained immunity inducers can be used as immunostimulants and adjuvants, the former promoting innate and adaptive immune responses leading to enhanced protection against bystander pathogens, while the latter delivered with a specific antigen further enhance adaptive immune response against that specific pathogen.
It is important to note that trained immunity might be damaging in situations where people have excessive inflammation as a result of endogenous and exogenous stimuli, and thus vaccines based on trained immunity should be mainly aimed for groups at high risk of infections. Indeed, people with atherosclerosis and hyper-IgD syndrome have been shown to have chronic inflammation due to continuously active trained immunity (176, 177).
The prolonged presence of certain DAMPs induces reprogramming of innate immune cells by providing a basis for sustained low-grade and chronic inflammation. For instance, pre-incubation of splenocytes with high-mobility group box protein 1 (HMGB1) was shown to increase TNFα production after secondary infection, indicating that HMGB1 might prime the cells to protect against infections (178). Another molecule, oxidized low-density lipoprotein (oxLDL), leads to epigenetic reprogramming of monocytes, eventually causing long-term elevated pro-inflammatory cytokine production (179). Similarly, pre-treatment of healthy PBMCs with soluble uric acid induced cytokine secretion that was mediated by histone methylation (180). Along with advanced age, accumulation of DAMPs—for example, HMGB1, sodium monourate and uric acid crystals—results in sterile inflammation, which is one of the underlying causes of several diseases including but not limited to atherosclerosis, cardiovascular diseases, gout and ischemia–reperfusion injury (10, 181, 182).
Nevertheless, our group demonstrated that BCG vaccination lowers systemic inflammation by decreasing circulating inflammatory markers in healthy individuals while enhancing cellular responses (L. C. J. de Bree et al., unpublished data); therefore, it would serve to reduce chronic inflammation while overcoming functional impairments at a cellular level.
Conclusions
Age-related alterations in the immune system result in high susceptibility to infections, increased risk of hospitalization and mortality. Defects in adaptive immunity underlie the markedly low vaccine efficiency in the elderly. Additionally, many functional defects in chemotaxis, phagocytosis, antigen presentation, ROS production, TLR signaling and cytokine production are present in aged innate immune cells such as neutrophils, monocytes, macrophages, DCs and NK cells. Despite reduced cellular functions, a systemic increase in inflammatory markers, so-called inflammaging, is observed in aged individuals.
In addition to numerous efforts underway to develop new vaccines with higher efficacy in the elderly, novel approaches targeting innate immunity to improve host responses are crucial to evade the consequences of the aged immune system. It is an emerging concept that innate immune cells can manifest memory-like properties that are not antigen-specific and exhibit enhanced responsiveness upon later challenges with heterologous stimuli. This concept of ‘trained immunity’ has been reported to enhance immunization efficiency. However, whether trained immune responses change as people age is yet to be explored. Further investigation is crucial to understand if and how trained immunity can be employed to protect the elderly from a broad range of infections. Besides the possibility that impaired innate immune cell functions could be reversed by inducing trained immunity, recent data suggest that BCG down-regulates circulating inflammatory markers, which would help alleviate the detrimental effects of inflammaging in the elderly. Therefore, it would be worthwhile to explore the potential of trained immunity for overcoming age-related immune dysregulation and protecting the vulnerable elderly population against infections.
Funding
M.G.N. is supported by Spinoza grant from the Netherlands Organization for Scientific Research and a European Research Council Advanced Grant (#833247).
Acknowledgements
Figures 1 and 2 were created with Biorender.com.
Conflicts of interest statement: M.G.N. is a scientific founder of TTxD. The other authors declare that they have no conflicts of interest.
References
- 1. United Nations. 2015. World Population Ageing. Department of Economic and Social Affairs United Nations, New York City, NY. [Google Scholar]
- 2. Grubeck-Loebenstein, B, Della Bella, S, Iorio, A M, Michel, J P, Pawelec, G and Solana, R. 2009. Immunosenescence and vaccine failure in the elderly. Aging Clin. Exp. Res. 21:201. [DOI] [PubMed] [Google Scholar]
- 3. Crooke, S N, Ovsyannikova, I G, Poland, G A and Kennedy, R B. 2019. Immunosenescence: a systems-level overview of immune cell biology and strategies for improving vaccine responses. Exp. Gerontol. 124:110632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thomas-Crusells, J, McElhaney, J E and Aguado, M T. 2012. Report of the ad-hoc consultation on aging and immunization for a future WHO research agenda on life-course immunization. Vaccine 30:6007. [DOI] [PubMed] [Google Scholar]
- 5. Netea, M G, Domínguez-Andrés, J, Barreiro, L Bet al. 2020. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 20:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goronzy, J J and Weyand, C M. 2013. Understanding immunosenescence to improve responses to vaccines. Nat. Immunol. 14:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Puzianowska-Kuźnicka, M, Owczarz, M, Wieczorowska-Tobis, Ket al. 2016. Interleukin-6 and C-reactive protein, successful aging, and mortality: the PolSenior study. Immun. Ageing 13:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xia, S, Zhang, X, Zheng, Set al. 2016. An update on inflamm-aging: mechanisms, prevention, and treatment. J. Immunol. Res. 2016:8426874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Franceschi, C, Garagnani, P, Parini, P, Giuliani, C and Santoro, A. 2018. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 14:576. [DOI] [PubMed] [Google Scholar]
- 10. Franceschi, C, Garagnani, P, Vitale, G, Capri, M and Salvioli, S. 2017. Inflammaging and ‘Garb-aging’. Trends Endocrinol. Metab. 28:199. [DOI] [PubMed] [Google Scholar]
- 11. Coppé, J P, Desprez, P Y, Krtolica, A and Campisi, J. 2010. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Campisi, J and d’Adda di Fagagna, F. 2007. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 8:729. [DOI] [PubMed] [Google Scholar]
- 13. Biagi, E, Candela, M, Franceschi, C and Brigidi, P. 2011. The aging gut microbiota: new perspectives. Ageing Res. Rev. 10:428. [DOI] [PubMed] [Google Scholar]
- 14. Biagi, E, Candela, M, Fairweather-Tait, S, Franceschi, C and Brigidi, P. 2012. Aging of the human metaorganism: the microbial counterpart. Age (Dordr.) 34:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Biagi, E, Nylund, L, Candela, Met al. 2010. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One 5:e10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pang, W W, Price, E A, Sahoo, Det al. 2011. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc. Natl Acad. Sci. USA 108:20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beerman, I, Bhattacharya, D, Zandi, Set al. 2010. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc. Natl Acad. Sci. USA 107:5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fulop, T, Larbi, A, Dupuis, Get al. 2017. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front. Immunol. 8:1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nathan, C. 2006. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6:173. [DOI] [PubMed] [Google Scholar]
- 20. Brinkmann, V, Reichard, U, Goosmann, Cet al. 2004. Neutrophil extracellular traps kill bacteria. Science 303:1532. [DOI] [PubMed] [Google Scholar]
- 21. Solana, R, Pawelec, G and Tarazona, R. 2006. Aging and innate immunity. Immunity 24:491. [DOI] [PubMed] [Google Scholar]
- 22. Sapey, E, Greenwood, H, Walton, Get al. 2014. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood 123:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wenisch, C, Patruta, S, Daxböck, F, Krause, R and Hörl, W. 2000. Effect of age on human neutrophil function. J. Leukoc. Biol. 67:40. [DOI] [PubMed] [Google Scholar]
- 24. Butcher, S K, Chahal, H, Nayak, Let al. 2001. Senescence in innate immune responses: reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J. Leukoc. Biol. 70:881. [PubMed] [Google Scholar]
- 25. Sauce, D, Dong, Y, Campillo-Gimenez, Let al. 2017. Reduced oxidative burst by primed neutrophils in the elderly individuals is associated with increased levels of the CD16bright/CD62Ldim immunosuppressive subset. J. Gerontol. A Biol. Sci. Med. Sci. 72:163. [DOI] [PubMed] [Google Scholar]
- 26. Alonso-Fernández, P, Puerto, M, Maté, I, Ribera, J M and de la Fuente, M. 2008. Neutrophils of centenarians show function levels similar to those of young adults. J. Am. Geriatr. Soc. 56:2244. [DOI] [PubMed] [Google Scholar]
- 27. Schröder, A K and Rink, L. 2003. Neutrophil immunity of the elderly. Mech. Ageing Dev. 124:419. [DOI] [PubMed] [Google Scholar]
- 28. Göçer, P, Gürer, U S, Erten, Net al. 2005. Comparison of polymorphonuclear leukocyte functions in elderly patients and healthy young volunteers. Med. Princ. Pract. 14:382. [DOI] [PubMed] [Google Scholar]
- 29. Fulop, T, Larbi, A, Douziech, Net al. 2004. Signal transduction and functional changes in neutrophils with aging. Aging Cell 3:217. [DOI] [PubMed] [Google Scholar]
- 30. Fortin, C F, Larbi, A, Dupuis, G, Lesur, O and Fülöp, T, Jr. 2007. GM-CSF activates the Jak/STAT pathway to rescue polymorphonuclear neutrophils from spontaneous apoptosis in young but not elderly individuals. Biogerontology 8:173. [DOI] [PubMed] [Google Scholar]
- 31. Fülöp, T, Jr, Fouquet, C, Allaire, Pet al. 1997. Changes in apoptosis of human polymorphonuclear granulocytes with aging. Mech. Ageing Dev. 96:15. [DOI] [PubMed] [Google Scholar]
- 32. Geissmann, F, Manz, M G, Jung, S, Sieweke, M H, Merad, M and Ley, K. 2010. Development of monocytes, macrophages, and dendritic cells. Science 327:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ziegler-Heitbrock, L, Ancuta, P, Crowe, Set al. 2010. Nomenclature of monocytes and dendritic cells in blood. Blood 116:e74. [DOI] [PubMed] [Google Scholar]
- 34. Hearps, A C, Martin, G E, Angelovich, T Aet al. 2012. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell 11:867. [DOI] [PubMed] [Google Scholar]
- 35. Seidler, S, Zimmermann, H W, Bartneck, M, Trautwein, C and Tacke, F. 2010. Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunol. 11:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Villanueva, J L, Solana, R, Alonso, M C and Peña, J. 1990. Changes in the expression of HLA-class II antigens on peripheral blood monocytes from aged humans. Dis. Markers 8:85. [PubMed] [Google Scholar]
- 37. Qian, F, Wang, X, Zhang, Let al. 2012. Age-associated elevation in TLR5 leads to increased inflammatory responses in the elderly. Aging Cell 11:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nyugen, J, Agrawal, S, Gollapudi, S and Gupta, S. 2010. Impaired functions of peripheral blood monocyte subpopulations in aged humans. J. Clin. Immunol. 30:806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ter Horst, R, Jaeger, M, Smeekens, S Pet al. 2016. Host and environmental factors influencing individual human cytokine responses. Cell 167:1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wynn, T A, Chawla, A and Pollard, J W. 2013. Macrophage biology in development, homeostasis and disease. Nature 496:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gordon, S. 2007. The macrophage: past, present and future. Eur. J. Immunol. 37(Suppl. 1):S9. [DOI] [PubMed] [Google Scholar]
- 42. Plowden, J, Renshaw-Hoelscher, M, Engleman, C, Katz, J and Sambhara, S. 2004. Innate immunity in aging: impact on macrophage function. Aging Cell 3:161. [DOI] [PubMed] [Google Scholar]
- 43. Takahashi, I, Ohmoto, E, Aoyama, Set al. 1985. Monocyte chemiluminescence and macrophage precursors in the aged. Acta Med. Okayama 39:447. [DOI] [PubMed] [Google Scholar]
- 44. Boehmer, E D, Goral, J, Faunce, D E and Kovacs, E J. 2004. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J. Leukoc. Biol. 75:342. [DOI] [PubMed] [Google Scholar]
- 45. Dong, C, Davis, R J and Flavell, R A. 2002. MAP kinases in the immune response. Annu. Rev. Immunol. 20:55. [DOI] [PubMed] [Google Scholar]
- 46. Kissin, E, Tomasi, M, McCartney-Francis, N, Gibbs, C L and Smith, P D. 1997. Age-related decline in murine macrophage production of nitric oxide. J. Infect. Dis. 175:1004. [DOI] [PubMed] [Google Scholar]
- 47. Herrero, C, Sebastián, C, Marqués, Let al. 2002. Immunosenescence of macrophages: reduced MHC class II gene expression. Exp. Gerontol. 37:389. [DOI] [PubMed] [Google Scholar]
- 48. Renshaw, M, Rockwell, J, Engleman, C, Gewirtz, A, Katz, J and Sambhara, S. 2002. Cutting edge: impaired Toll-like receptor expression and function in aging. J. Immunol. 169:4697. [DOI] [PubMed] [Google Scholar]
- 49. Herrero, C, Marqués, L, Lloberas, J and Celada, A. 2001. IFN-gamma-dependent transcription of MHC class II IA is impaired in macrophages from aged mice. J. Clin. Invest. 107:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chelvarajan, R L, Collins, S M, Van Willigen, J M and Bondada, S. 2005. The unresponsiveness of aged mice to polysaccharide antigens is a result of a defect in macrophage function. J. Leukoc. Biol. 77:503. [DOI] [PubMed] [Google Scholar]
- 51. Aprahamian, T, Takemura, Y, Goukassian, D and Walsh, K. 2008. Ageing is associated with diminished apoptotic cell clearance in vivo. Clin. Exp. Immunol. 152:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ogawa, T, Kitagawa, M and Hirokawa, K. 2000. Age-related changes of human bone marrow: a histometric estimation of proliferative cells, apoptotic cells, T cells, B cells and macrophages. Mech. Ageing Dev. 117:57. [DOI] [PubMed] [Google Scholar]
- 53. Verschoor, C P, Johnstone, J, Loeb, M, Bramson, J L and Bowdish, D M. 2014. Anti-pneumococcal deficits of monocyte-derived macrophages from the advanced-age, frail elderly and related impairments in PI3K-AKT signaling. Hum. Immunol. 75:1192. [DOI] [PubMed] [Google Scholar]
- 54. Valero, N, Mosquera, J, Levy, A, Añez, G, Marcucci, R and Alvarez-Mon, M. 2014. Differential induction of cytokines by human neonatal, adult, and elderly monocyte/macrophages infected with dengue virus. Viral Immunol. 27:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rossi, M and Young, J W. 2005. Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J. Immunol. 175:1373. [DOI] [PubMed] [Google Scholar]
- 56. Steinman, R M and Nussenzweig, M C. 2002. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc. Natl Acad. Sci. USA 99:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mildner, A and Jung, S. 2014. Development and function of dendritic cell subsets. Immunity 40:642. [DOI] [PubMed] [Google Scholar]
- 58. Della Bella, S, Bierti, L, Presicce, Pet al. 2007. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin. Immunol. 122:220. [DOI] [PubMed] [Google Scholar]
- 59. Varas, A, Sacedón, R, Hernandez-López, Cet al. 2003. Age-dependent changes in thymic macrophages and dendritic cells. Microsc. Res. Tech. 62:501. [DOI] [PubMed] [Google Scholar]
- 60. Sridharan, A, Esposo, M, Kaushal, Ket al. 2011. Age-associated impaired plasmacytoid dendritic cell functions lead to decreased CD4 and CD8 T cell immunity. Age (Dordr.) 33:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Agrawal, A and Gupta, S. 2011. Impact of aging on dendritic cell functions in humans. Ageing Res. Rev. 10:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Agrawal, A, Agrawal, S, Cao, J N, Su, H, Osann, K and Gupta, S. 2007. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J. Immunol. 178:6912. [DOI] [PubMed] [Google Scholar]
- 63. Janssen, N, Derhovanessian, E, Demuth, I, Arnaout, F, Steinhagen-Thiessen, E and Pawelec, G. 2016. Responses of dendritic cells to TLR-4 stimulation are maintained in the elderly and resist the effects of CMV infection seen in the young. J. Gerontol. A Biol. Sci. Med. Sci. 71:1117. [DOI] [PubMed] [Google Scholar]
- 64. Bhushan, M, Cumberbatch, M, Dearman, R J, Andrew, S M, Kimber, I and Griffiths, C E. 2002. Tumour necrosis factor-alpha-induced migration of human Langerhans cells: the influence of ageing. Br. J. Dermatol. 146:32. [DOI] [PubMed] [Google Scholar]
- 65. Hamerman, J A, Ogasawara, K and Lanier, L L. 2005. NK cells in innate immunity. Curr. Opin. Immunol. 17:29. [DOI] [PubMed] [Google Scholar]
- 66. Abel, A M, Yang, C, Thakar, M S and Malarkannan, S. 2018. Natural killer cells: development, maturation, and clinical utilization. Front. Immunol. 9:1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Panda, A, Arjona, A, Sapey, Eet al. 2009. Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol. 30:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang, Y, Wallace, D L, de Lara, C Met al. 2007. In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology 121:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bayard, C, Lepetitcorps, H, Roux, Aet al. 2016. Coordinated expansion of both memory T cells and NK cells in response to CMV infection in humans. Eur. J. Immunol. 46:1168. [DOI] [PubMed] [Google Scholar]
- 70. Lopez-Vergès, S, Milush, J M, Schwartz, B Set al. 2011. Expansion of a unique CD57⁺NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc. Natl Acad. Sci. USA 108:14725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Solana, R and Mariani, E. 2000. NK and NK/T cells in human senescence. Vaccine 18:1613. [DOI] [PubMed] [Google Scholar]
- 72. Ogata, K, Yokose, N, Tamura, Het al. 1997. Natural killer cells in the late decades of human life. Clin. Immunol. Immunopathol. 84:269. [DOI] [PubMed] [Google Scholar]
- 73. Ogata, K, An, E, Shioi, Yet al. 2001. Association between natural killer cell activity and infection in immunologically normal elderly people. Clin. Exp. Immunol. 124:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Myśliwska, J, Trzonkowski, P, Szmit, E, Brydak, L B, Machała, M and Myśliwski, A. 2004. Immunomodulating effect of influenza vaccination in the elderly differing in health status. Exp. Gerontol. 39:1447. [DOI] [PubMed] [Google Scholar]
- 75. Almeida-Oliveira, A, Smith-Carvalho, M, Porto, L Cet al. 2011. Age-related changes in natural killer cell receptors from childhood through old age. Hum. Immunol. 72:319. [DOI] [PubMed] [Google Scholar]
- 76. Le Garff-Tavernier, M, Béziat, V, Decocq, Jet al. 2010. Human NK cells display major phenotypic and functional changes over the life span. Aging Cell 9:527. [DOI] [PubMed] [Google Scholar]
- 77. Kumar, B V, Connors, T J and Farber, D L. 2018. Human T cell development, localization, and function throughout life. Immunity 48:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Germain, R N. 2002. T-cell development and the CD4-CD8 lineage decision. Nat. Rev. Immunol. 2:309. [DOI] [PubMed] [Google Scholar]
- 79. Steinmann, G G, Klaus, B and Müller-Hermelink, H K. 1985. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand. J. Immunol. 22:563. [DOI] [PubMed] [Google Scholar]
- 80. Flores, K G, Li, J, Sempowski, G D, Haynes, B F and Hale, L P. 1999. Analysis of the human thymic perivascular space during aging. J. Clin. Invest. 104:1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Andrew, D and Aspinall, R. 2002. Age-associated thymic atrophy is linked to a decline in IL-7 production. Exp. Gerontol. 37:455. [DOI] [PubMed] [Google Scholar]
- 82. Agarwal, S and Busse, P J. 2010. Innate and adaptive immunosenescence. Ann. Allergy Asthma Immunol. 104:183. [DOI] [PubMed] [Google Scholar]
- 83. Sauce, D, Larsen, M, Fastenackels, Set al. 2009. Evidence of premature immune aging in patients thymectomized during early childhood. J. Clin. Invest. 119:3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Weinberger, B, Herndler-Brandstetter, D, Schwanninger, A, Weiskopf, D and Grubeck-Loebenstein, B. 2008. Biology of immune responses to vaccines in elderly persons. Clin. Infect. Dis. 46:1078. [DOI] [PubMed] [Google Scholar]
- 85. Chidrawar, S, Khan, N, Wei, Wet al. 2009. Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clin. Exp. Immunol. 155:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Derhovanessian, E, Maier, A B, Beck, Ret al. 2010. Hallmark features of immunosenescence are absent in familial longevity. J. Immunol. 185:4618. [DOI] [PubMed] [Google Scholar]
- 87. Olsson, J, Wikby, A, Johansson, B, Löfgren, S, Nilsson, B O and Ferguson, F G. 2000. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech. Ageing Dev. 121:187. [DOI] [PubMed] [Google Scholar]
- 88. Wikby, A, Johansson, B, Olsson, J, Löfgren, S, Nilsson, B O and Ferguson, F. 2002. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp. Gerontol. 37:445. [DOI] [PubMed] [Google Scholar]
- 89. Almanzar, G, Schwaiger, S, Jenewein, Bet al. 2005. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J. Virol. 79:3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Moro-García, M A, Alonso-Arias, R and López-Larrea, C. 2013. When aging reaches CD4+ T-cells: phenotypic and functional changes. Front. Immunol. 4:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pfister, G, Weiskopf, D, Lazuardi, Let al. 2006. Naive T cells in the elderly: are they still there? Ann. NY Acad. Sci. 1067:152. [DOI] [PubMed] [Google Scholar]
- 92. Saurwein-Teissl, M, Lung, T L, Marx, Fet al. 2002. Lack of antibody production following immunization in old age: association with CD8(+)CD28(-) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J. Immunol. 168:5893. [DOI] [PubMed] [Google Scholar]
- 93. Weng, N P. 2006. Aging of the immune system: how much can the adaptive immune system adapt? Immunity 24:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Koch, S, Larbi, A, Derhovanessian, E, Ozcelik, D, Naumova, E and Pawelec, G. 2008. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun. Ageing 5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Czesnikiewicz-Guzik, M, Lee, W W, Cui, Det al. 2008. T cell subset-specific susceptibility to aging. Clin. Immunol. 127:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gomez, I, Marx, F, Gould, E A and Grubeck-Loebenstein, B. 2004. T cells from elderly persons respond to neoantigenic stimulation with an unimpaired IL-2 production and an enhanced differentiation into effector cells. Exp. Gerontol. 39:597. [DOI] [PubMed] [Google Scholar]
- 97. Li, G, Yu, M, Lee, W Wet al. 2012. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat. Med. 18:1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kang, I, Hong, M S, Nolasco, Het al. 2004. Age-associated change in the frequency of memory CD4+ T cells impairs long term CD4+ T cell responses to influenza vaccine. J. Immunol. 173:673. [DOI] [PubMed] [Google Scholar]
- 99. Ademokun, A, Wu, Y C and Dunn-Walters, D. 2010. The ageing B cell population: composition and function. Biogerontology 11:125. [DOI] [PubMed] [Google Scholar]
- 100. Frasca, D, Landin, A M, Riley, R L and Blomberg, B B. 2008. Mechanisms for decreased function of B cells in aged mice and humans. J. Immunol. 180:2741. [DOI] [PubMed] [Google Scholar]
- 101. Caraux, A, Klein, B, Paiva, Bet al. ; Myeloma Stem Cell Network . 2010. Circulating human B and plasma cells. Age-associated changes in counts and detailed characterization of circulating normal CD138- and CD138+ plasma cells. Haematologica 95:1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Siegrist, C A and Aspinall, R. 2009. B-cell responses to vaccination at the extremes of age. Nat. Rev. Immunol. 9:185. [DOI] [PubMed] [Google Scholar]
- 103. Frasca, D, Riley, R L and Blomberg, B B. 2005. Humoral immune response and B-cell functions including immunoglobulin class switch are downregulated in aged mice and humans. Semin. Immunol. 17:378. [DOI] [PubMed] [Google Scholar]
- 104. Johnson, S A and Cambier, J C. 2004. Ageing, autoimmunity and arthritis: senescence of the B cell compartment - implications for humoral immunity. Arthritis Res. Ther. 6:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Eaton, S M, Burns, E M, Kusser, K, Randall, T D and Haynes, L. 2004. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J. Exp. Med. 200:1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Frasca, D, Diaz, A, Romero, Met al. 2012. Unique biomarkers for B-cell function predict the serum response to pandemic H1N1 influenza vaccine. Int. Immunol. 24:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Frasca, D, Landin, A M, Lechner, S Cet al. 2008. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J. Immunol. 180:5283. [DOI] [PubMed] [Google Scholar]
- 108. Shi, Y, Yamazaki, T, Okubo, Y, Uehara, Y, Sugane, K and Agematsu, K. 2005. Regulation of aged humoral immune defense against pneumococcal bacteria by IgM memory B cell. J. Immunol. 175:3262. [DOI] [PubMed] [Google Scholar]
- 109. Colonna-Romano, G, Bulati, M, Aquino, Aet al. 2009. A double-negative (IgD-CD27-) B cell population is increased in the peripheral blood of elderly people. Mech. Ageing Dev. 130:681. [DOI] [PubMed] [Google Scholar]
- 110. Ma, S, Wang, C, Mao, X and Hao, Y. 2019. B cell dysfunction associated with aging and autoimmune diseases. Front. Immunol. 10:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Weinberger, B. 2018. Vaccines for the elderly: current use and future challenges. Immun. Ageing 15:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Haq, K and McElhaney, J E. 2014. Immunosenescence: influenza vaccination and the elderly. Curr. Opin. Immunol. 29:38. [DOI] [PubMed] [Google Scholar]
- 113. Goodwin, K, Viboud, C and Simonsen, L. 2006. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 24:1159. [DOI] [PubMed] [Google Scholar]
- 114. Henry, C, Zheng, N Y, Huang, Met al. 2019. Influenza Virus vaccination elicits poorly adapted B cell responses in elderly individuals. Cell Host Microbe 25:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Herrero-Fernández, I, Rosado-Sánchez, I, Álvarez-Ríos, A Iet al. 2019. Effect of homeostatic T-cell proliferation in the vaccine responsiveness against influenza in elderly people. Immun. Ageing 16:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Goronzy, J J, Fulbright, J W, Crowson, C S, Poland, G A, O’Fallon, W M and Weyand, C M. 2001. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J. Virol. 75:12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. DiazGranados, C A, Dunning, A J, Kimmel, Met al. 2014. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N. Engl. J. Med. 371:635. [DOI] [PubMed] [Google Scholar]
- 118. DiazGranados, C A, Dunning, A J, Jordanov, E, Landolfi, V, Denis, M and Talbot, H K. 2013. High-dose trivalent influenza vaccine compared to standard dose vaccine in elderly adults: safety, immunogenicity and relative efficacy during the 2009-2010 season. Vaccine 31:861. [DOI] [PubMed] [Google Scholar]
- 119. Tsang, P, Gorse, G J, Strout, C Bet al. 2014. Immunogenicity and safety of Fluzone(®) intradermal and high-dose influenza vaccines in older adults ≥65 years of age: a randomized, controlled, phase II trial. Vaccine 32:2507. [DOI] [PubMed] [Google Scholar]
- 120. U.S. Food and Drug Administration (FDA). 2019. Fluzone, fluzone high-dose and fluzone intradermal. Available at: https://www.fda.gov/vaccines-blood-biologics/vaccines/fluzone-fluzone-high-dose-and-fluzone-intradermal.
- 121. Chen, W H, Cross, A S, Edelman, R, Sztein, M B, Blackwelder, W C and Pasetti, M F. 2011. Antibody and Th1-type cell-mediated immune responses in elderly and young adults immunized with the standard or a high dose influenza vaccine. Vaccine 29:2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Leroux-Roels, G. 2010. Unmet needs in modern vaccinology: adjuvants to improve the immune response. Vaccine 28(Suppl. 3):C25. [DOI] [PubMed] [Google Scholar]
- 123. Tsai, T F. 2013. Fluad®-MF59®-adjuvanted influenza vaccine in older adults. Infect. Chemother. 45:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Puig Barberà, J and González Vidal, D. 2007. MF59-adjuvanted subunit influenza vaccine: an improved interpandemic influenza vaccine for vulnerable populations. Expert Rev. Vaccines 6:659. [DOI] [PubMed] [Google Scholar]
- 125. Camilloni, B, Basileo, M, Valente, S, Nunzi, E and Iorio, A M. 2015. Immunogenicity of intramuscular MF59-adjuvanted and intradermal administered influenza enhanced vaccines in subjects aged over 60: a literature review. Hum. Vaccin. Immunother. 11:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ansaldi, F, Zancolli, M, Durando, Pet al. 2010. Antibody response against heterogeneous circulating influenza virus strains elicited by MF59- and non-adjuvanted vaccines during seasons with good or partial matching between vaccine strain and clinical isolates. Vaccine 28:4123. [DOI] [PubMed] [Google Scholar]
- 127. Hung, I F, Zhang, A J, To, K Ket al. 2016. Topical imiquimod before intradermal trivalent influenza vaccine for protection against heterologous non-vaccine and antigenically drifted viruses: a single-centre, double-blind, randomised, controlled phase 2b/3 trial. Lancet Infect. Dis. 16:209. [DOI] [PubMed] [Google Scholar]
- 128. Fedson, D S, Scott, J A and Scott, G. 1999. The burden of pneumococcal disease among adults in developed and developing countries: what is and is not known. Vaccine 17(Suppl. 1):S11. [DOI] [PubMed] [Google Scholar]
- 129. Centers for Disease Control and Prevention (CDC). 2019. Pneumococcal Vaccination. Available at: www.cdc.gov/pneumococcal/vaccination.html.
- 130. Melegaro, A and Edmunds, W J. 2004. The 23-valent pneumococcal polysaccharide vaccine. Part I. Efficacy of PPV in the elderly: a comparison of meta-analyses. Eur. J. Epidemiol. 19:353. [DOI] [PubMed] [Google Scholar]
- 131. van Werkhoven, C H, Huijts, S M, Bolkenbaas, M, Grobbee, D E and Bonten, M J. 2015. The impact of age on the efficacy of 13-valent pneumococcal conjugate vaccine in elderly. Clin. Infect. Dis. 61:1835. [DOI] [PubMed] [Google Scholar]
- 132. Hayward, S, Thompson, L A and McEachern, A. 2016. Is 13-valent pneumococcal conjugate vaccine (PCV13) combined with 23-valent pneumococcal polysaccharide vaccine (PPSV23) superior to PPSV23 alone for reducing incidence or severity of pneumonia in older adults? A Clin-IQ. J. Patient Cent. Res. Rev. 3:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Arvin, A M. 1996. Varicella-zoster virus. Clin. Microbiol. Rev. 9:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Oxman, M N. 2009. Herpes zoster pathogenesis and cell-mediated immunity and immunosenescence. J. Am. Osteopath. Assoc. 109(6Suppl. 2):S13. [PubMed] [Google Scholar]
- 135. Kawai, K, Gebremeskel, B G and Acosta, C J. 2014. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open 4:e004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Oxman, M N, Levin, M J, Johnson, G Ret al. ; Shingles Prevention Study Group . 2005. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N. Engl. J. Med. 352:2271. [DOI] [PubMed] [Google Scholar]
- 137. Didierlaurent, A M, Laupèze, B, Di Pasquale, A, Hergli, N, Collignon, C and Garçon, N. 2017. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 16:55. [DOI] [PubMed] [Google Scholar]
- 138. Lal, H, Cunningham, A L, Godeaux, Oet al. ; ZOE-50 Study Group . 2015. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N. Engl. J. Med. 372:2087. [DOI] [PubMed] [Google Scholar]
- 139. Schwarz, T F, Volpe, S, Catteau, Get al. 2018. Persistence of immune response to an adjuvanted varicella-zoster virus subunit vaccine for up to year nine in older adults. Hum. Vaccin. Immunother. 14:1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Chlibek, R, Bayas, J M, Collins, Het al. 2013. Safety and immunogenicity of an AS01-adjuvanted varicella-zoster virus subunit candidate vaccine against herpes zoster in adults >=50 years of age. J. Infect. Dis. 208:1953. [DOI] [PubMed] [Google Scholar]
- 141. Milutinović, B and Kurtz, J. 2016. Immune memory in invertebrates. Semin. Immunol. 28:328. [DOI] [PubMed] [Google Scholar]
- 142. Quintin, J, Saeed, S, Martens, J H Aet al. 2012. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Netea, M G, Quintin, J and van der Meer, J W. 2011. Trained immunity: a memory for innate host defense. Cell Host Microbe 9:355. [DOI] [PubMed] [Google Scholar]
- 144. Netea, M G, Joosten, L A, Latz, Eet al. 2016. Trained immunity: a program of innate immune memory in health and disease. Science 352:aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Dockrell, H M and Smith, S G. 2017. What have we learnt about BCG vaccination in the last 20 years? Front. Immunol. 8:1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kleinnijenhuis, J, Quintin, J, Preijers, Fet al. 2014. BCG-induced trained immunity in NK cells: role for non-specific protection to infection. Clin. Immunol. 155:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Hole, C R, Wager, C M L, Castro-Lopez, Net al. 2019. Induction of memory-like dendritic cell responses in vivo. Nat. Commun. 10:2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Arts, R J, Joosten, L A and Netea, M G. 2016. Immunometabolic circuits in trained immunity. Semin. Immunol. 28:425. [DOI] [PubMed] [Google Scholar]
- 149. van der Heijden, C D C C, Noz, M P, Joosten, L A B, Netea, M G, Riksen, N P and Keating, S T. 2018. Epigenetics and trained immunity. Antioxid. Redox Signal. 29:1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Arts, R J, Novakovic, B, Ter Horst, Ret al. 2016. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 24:807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Arts, R J W, Carvalho, A, La Rocca, Cet al. 2016. Immunometabolic pathways in BCG-induced trained immunity. Cell Rep. 17:2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Mitroulis, I, Ruppova, K, Wang, Bet al. 2018. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 172:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kaufmann, E, Sanz, J, Dunn, J Let al. 2018. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 172:176. [DOI] [PubMed] [Google Scholar]
- 154. Sánchez-Ramón, S, Conejero, L, Netea, M G, Sancho, D, Palomares, Ó and Subiza, J L. 2018. Trained immunity-based vaccines: a new paradigm for the development of broad-spectrum anti-infectious formulations. Front. Immunol. 9:2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Ciarlo, E, Heinonen, T, Theroude, Cet al. 2019. Trained immunity confers broad-spectrum protection against bacterial infections. J. Infect. Dis. [Epub ahead of print]. doi: 10.1093/infdis/jiz692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Benn, C S, Netea, M G, Selin, L K and Aaby, P. 2013. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. 34:431. [DOI] [PubMed] [Google Scholar]
- 157. de Bree, L C J, Koeken, V A C M, Joosten, L A Bet al. 2018. Non-specific effects of vaccines: current evidence and potential implications. Semin. Immunol. 39:35. [DOI] [PubMed] [Google Scholar]
- 158. Alhunaidi, O and Zlotta, A R. 2019. The use of intravesical BCG in urothelial carcinoma of the bladder. Ecancermedicalscience 13:905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Fuge, O, Vasdev, N, Allchorne, P and Green, J S. 2015. Immunotherapy for bladder cancer. Res. Rep. Urol. 7:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Kamat, A M, Flaig, T W, Grossman, H Bet al. 2015. Expert consensus document: consensus statement on best practice management regarding the use of intravesical immunotherapy with BCG for bladder cancer. Nat. Rev. Urol. 12:225. [DOI] [PubMed] [Google Scholar]
- 161. Kleinnijenhuis, J, Quintin, J, Preijers, Fet al. 2012. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl Acad. Sci. USA 109:17537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Gil, A, Kenney, L L, Mishra, R, Watkin, L B, Aslan, N and Selin, L K. 2015. Vaccination and heterologous immunity: educating the immune system. Trans. R. Soc. Trop. Med. Hyg. 109:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Ritz, N, Mui, M, Balloch, A and Curtis, N. 2013. Non-specific effect of Bacille Calmette-Guérin vaccine on the immune response to routine immunisations. Vaccine 31:3098. [DOI] [PubMed] [Google Scholar]
- 164. Leentjens, J, Kox, M, Stokman, Ret al. 2015. BCG vaccination enhances the immunogenicity of subsequent influenza vaccination in healthy volunteers: a randomized, placebo-controlled pilot study. J. Infect. Dis. 212:1930. [DOI] [PubMed] [Google Scholar]
- 165. Dos Santos, J C, Vilela Teodoro Silva, M, Ribeiro-Dias, F and Joosten, L A B. 2019. Non-specific effects of BCG in protozoal infections: tegumentary leishmaniasis and malaria. Clin. Microbiol. Infect. 25:1479. [DOI] [PubMed] [Google Scholar]
- 166. Arts, R J W, Moorlag, S J C F M, Novakovic, Bet al. 2018. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe 23:89. [DOI] [PubMed] [Google Scholar]
- 167. Walk, J, de Bree, L C J, Graumans, Wet al. 2019. Outcomes of controlled human malaria infection after BCG vaccination. Nat. Commun. 10:874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Kleinnijenhuis, J, Quintin, J, Preijers, Fet al. 2014. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 6:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Blok, B A, de Bree, L C J, Diavatopoulos, D Aet al. 2020. Interacting, nonspecific, immunological effects of Bacille Calmette-Guerin and tetanus-diphtheria-pertussis inactivated polio vaccinations: an explorative, randomized trial. Clin. Infect. Dis.70:455. [DOI] [PubMed] [Google Scholar]
- 170. Berendsen, M, Bles, P, de Bree, Cet al. 2020. BCG vaccination induces innate immune training in adults over 50 years of age. Optimmunize: improving the beneficial effects of vaccines, Cambridge, UK. Abstract no: P3. Available at: https://coursesandconferences.wellcomegenomecampus.org/wp-content/uploads/2020/02/Optimmunize-Abstract-Book-v2.pdf
- 171. Wardhana, R, Datau, E A, Sultana, A, Mandang, V V and Jim, E. 2011. The efficacy of Bacillus Calmette-Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta Med. Indones. 43:185. [PubMed] [Google Scholar]
- 172. Ohrui, T, Nakayama, K, Fukushima, T, Chiba, H and Sasaki, H. 2005. [Prevention of elderly pneumonia by pneumococcal, influenza and BCG vaccinations]. Nihon Ronen Igakkai Zasshi 42:34. [DOI] [PubMed] [Google Scholar]
- 173. Morris, D E, Cleary, D W and Clarke, S C. 2017. Secondary bacterial infections associated with influenza pandemics. Front. Microbiol. 8:1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. MacIntyre, C R, Chughtai, A A, Barnes, Met al. 2018. The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza a(H1N1)pdm09. BMC Infect. Dis. 18:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Novakovic, B, Habibi, E, Wang, S Yet al. 2016. β-Glucan reverses the epigenetic state of LPS-induced immunological tolerance. Cell 167:1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Leentjens, J, Bekkering, S, Joosten, L A B, Netea, M G, Burgner, D P and Riksen, N P. 2018. Trained innate immunity as a novel mechanism linking infection and the development of atherosclerosis. Circ. Res. 122:664. [DOI] [PubMed] [Google Scholar]
- 177. Bekkering, S, Arts, R J W, Novakovic, Bet al. 2018. Metabolic induction of trained immunity through the mevalonate pathway. Cell 172:135. [DOI] [PubMed] [Google Scholar]
- 178. Valdés-Ferrer, S I, Rosas-Ballina, M, Olofsson, P Set al. 2013. High-mobility group box 1 mediates persistent splenocyte priming in sepsis survivors: evidence from a murine model. Shock 40:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Bekkering, S, Quintin, J, Joosten, L A, van der Meer, J W, Netea, M G and Riksen, N P. 2014. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler. Thromb. Vasc. Biol. 34:1731. [DOI] [PubMed] [Google Scholar]
- 180. Crișan, T O, Cleophas, M C, Oosting, Met al. 2016. Soluble uric acid primes TLR-induced proinflammatory cytokine production by human primary cells via inhibition of IL-1Ra. Ann. Rheum. Dis. 75:755. [DOI] [PubMed] [Google Scholar]
- 181. Gong, T, Liu, L, Jiang, W and Zhou, R. 2020. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 20:95. [DOI] [PubMed] [Google Scholar]
- 182. Rock, K L, Latz, E, Ontiveros, F and Kono, H. 2010. The sterile inflammatory response. Annu. Rev. Immunol. 28:321. [DOI] [PMC free article] [PubMed] [Google Scholar]