Abstract
Introduction
The global target for 2020 is that ≥90% of people living with HIV (PLHIV) receiving antiretroviral therapy (ART) will achieve viral load suppression (VLS). We examined VLS and its determinants among adults receiving ART for at least four months.
Methods
We analysed data from the population‐based HIV impact assessment (PHIA) surveys in Eswatini, Lesotho, Malawi, Zambia and Zimbabwe (2015 to 2017). PHIA surveys are nationally representative, cross‐sectional household surveys. Data collection included structured interviews, home‐based HIV testing and laboratory testing. Blood samples from PLHIV were analysed for HIV RNA, CD4 counts and recent exposure to antiretroviral drugs (ARVs). We calculated representative estimates for the prevalence of VLS (viral load <1000 copies/mL), nonsuppressed viral load (NVL; viral load ≥1000 copies/mL), virologic failure (VF; ARVs present and viral load ≥1000 copies/mL), interrupted ART (ARVs absent and viral load ≥1000 copies/mL) and rates of switching to second‐line ART (protease inhibitors present) among PLHIV aged 15 to 59 years who participated in the PHIA surveys in Eswatini, Lesotho, Malawi, Zambia and Zimbabwe, initiated ART at least four months before the survey and were receiving ART at the time of the survey (according to self‐report or ARV testing). We calculated odds ratios and incidence rate ratios for factors associated with NVL, VF, interrupted ART, and switching to second‐line ART.
Results
We included 9200 adults receiving ART of whom 88.8% had VLS and 11.2% had NVL including 8.2% who experienced VF and 3.0% who interrupted ART. Younger age, male sex, less education, suboptimal adherence, receiving nevirapine, HIV non‐disclosure, never having married and residing in Zimbabwe, Lesotho or Zambia were associated with higher odds of NVL. Among people with NVL, marriage, female sex, shorter ART duration, higher CD4 count and alcohol use were associated with lower odds for VF and higher odds for interrupted ART. Many people with VF (44.8%) had CD4 counts <200 cells/µL, but few (0.31% per year) switched to second‐line ART.
Conclusions
Countries are approaching global VLS targets for adults. Treatment support, in particular for younger adults, and people with higher CD4 counts, and switching of people to protease inhibitor‐ or integrase inhibitor‐based regimens may further reduce NVL prevalence.
Keywords: HIV, ARV, viral suppression, 90‐90‐90 targets, second‐line ART, sub‐Saharan Africa
1. INTRODUCTION
The Joint United Nations Programme on HIV/AIDS (UNAIDS) has set a target for 90% of people on ART to have viral load suppression (VLS) by 2020 [1]. Maintaining VLS keeps HIV‐positive patients healthy and reduces the risk of HIV transmission [2, 3, 4, 5]. Consistent retention and adherence to an effective ART regimen are essential for maintaining VLS, but inadequate retention and adherence and use of ineffective ART regimens are common, leading to reduced individual and public health benefits of ART [6, 7, 8].
The World Health Organization (WHO) recommends routine viral load (VL) monitoring of patients receiving ART [9]. Recently, VL monitoring has been scaled up in resource‐limited settings; however, in most sub‐Saharan African countries, access remains limited. The proportion of patients who had received at least one VL test by mid‐2016 was 19% in Malawi, 11% in Côte d’Ivoire, 49% in Kenya, 43% in Namibia, 9% in Tanzania and 22% in Uganda [10]. For settings with limited VL testing capacity, WHO recommends targeted VL testing of patients with suspected treatment failure [9]. In settings without access to VL testing, healthcare workers rely on immunological or clinical criteria to detect ART failure [6]. The diagnostic accuracy of these criteria for identifying patients with virologic failure (VF) is poor, and patients may not receive adherence support, may remain on ineffective ART regimens or may be switched to second‐line ART unnecessarily [6]. Even where VL testing has been implemented, patients may not benefit from clinical management if results are not used for clinical decision‐making [6, 11].
We analysed nationally representative data from the population‐based HIV impact assessment (PHIA) surveys in Eswatini (formerly called Swaziland), Lesotho, Malawi, Zambia and Zimbabwe to examine VLS and its determinants (interrupting ART, VF and switching to second‐line ART) among adults receiving ART for at least four months.
2. METHODS
2.1. Survey design
The PHIA surveys are nationally representative, cross‐sectional household surveys. PHIA surveys use a two‐stage cluster‐based sampling design. The surveys measure HIV incidence, HIV prevalence and VLS prevalence among PLHIV and progress towards each of the UNAIDS 90‐90‐90 targets. The survey was conducted between 2015 and 2017 in the five countries included in our analysis.
Data collection included structured household and individual interviews, home‐based HIV testing using each country’s national rapid testing algorithm with immediate return of results and further laboratory testing. HIV‐positive blood specimens were tested for plasma VL. If an insufficient volume of plasma was collected HIV VL testing was performed on dried blood spots (DBS). Positive specimens were also tested for CD4 cell counts and the presence of selected antiretroviral drugs (ARVs). In each country, samples were tested for the first‐line ARVs efavirenz (EFV) and nevirapine (NVP) and the second‐line ARV lopinavir (LPV). Malawi and Zambia also tested samples for the second‐line ARV atazanavir (ATV). A qualitative high‐performance liquid chromatography/tandem mass spectrometry assay was performed to detect antiretroviral drug (ARV) in DBS. A concentration of 0.02 μg/mL was used as the cut‐off. More details of laboratory and survey methods are given in the appendix (Text S1) and in survey reports [12, 13, 14, 15, 16] which are available online at https://phia.icap.columbia.edu/resource_categories/final‐reports/.
2.2. Eligibility
We included HIV‐positive adults aged 15 to 59 years who participated in the five PHIA surveys, who consented to biomarker testing, who provided complete data on HIV awareness and treatment status and who were classified as receiving ART at the time of the survey either by reporting current ART use or by having detectable blood levels of selected ARVs. We excluded people who reported receiving ART for less than four months to account for people with high baseline VL who may not have had VLS at the time of data collection.
2.3. Measures
As per WHO definitions, we defined VLS as VL < 1000 copies/mL and NVL as VL ≥ 1000 copies/mL [9]. People with NVL were classified as either experiencing VF if any single ARV was detected in blood samples or as having interrupted ART if no ARVs were detected. We classified regimens as EFV‐based if EFV was detected, as NVP‐based if NVP was detected, and as protease inhibitor (PI)‐based if either LPV or ATV was detected. We classified non‐nucleoside reverse‐transcriptase inhibitors (NNRTI)‐based regimens as first‐line ART and PI‐based regimens as second‐line ART [9]. CD4 cell count was categorized as <100, 100 to 199, 200 to 349, 350 to 499 and ≥500 cells/µL. VL was categorized as <40, 40‐999 and ≥1000 copies/mL. DBS VL samples could not be categorized and were excluded from this analysis.
We calculated ART duration as the date of the survey interview minus the self‐reported ART start date. We categorized ART duration as <1, 1 to 2, 2 to 5, 6 to 10 and >10 years. We defined adherence to ART, based on self‐reported data, as optimal if a participant reported missing less than two doses in the last 30 days or suboptimal if the participant missed two or more doses in the last 30 days.
We used an asset‐based wealth index and categorized the wealth index scores into quintiles [17]. Households that received cash transfer, assistance for school fees, material support for education or income generation support in cash or other forms in the last 12 months were considered to have received economic support. Work for which individuals received a salary, cash or in‐kind payment in the last 12 months was classified as paid work. Alcohol use was assessed with the AUDIT‐C three‐item questionnaire. We used the cut‐off AUDIT‐C score of ≥4 in men and ≥3 in women to classify people screening positive for hazardous drinking [18, 19].
2.4. Statistical analysis
We estimated the prevalence of VLS and NVL among people receiving ART for at least four months and the prevalence of VF and interrupted ART among those with NVL. In a sensitivity analysis, we examined how the exclusion of people who received ART for less than four months affected NVL prevalence. We used bivariate and multivariable logistic regression to calculate odds ratios (OR) for factors associated with NVL, interrupted ART and VF. Logistic regression models accounted for stratification and clustering in the sample design. We estimated variance using the Taylor series linearization method.
We used purposeful selection of covariates to build our final regression models [20]. We considered characteristics of ART and sociodemographic and behavioural characteristics in bivariate analysis. Variables associated with the outcome at a significance level of <0.20 in bivariate analysis [21, 22] and variables that had been reported as important determinants of VLS or interruption of ART (i.e. wealth quintile, education and ART duration) [23, 24, 25, 26, 27, 28, 29] were considered in multivariable analysis. Variables not significant at the level <0.05 in multivariable analysis were successively eliminated from multivariable models starting with the least significant variable. After eliminating a variable from the model, we compared the estimates of all coefficients that remained in the smaller model to their respective estimates from the larger model and added the eliminated variable back into the model if an estimate changed by more than 20%. We checked for interactions between independent variables that remained in the final model. We imputed missing data for all variables that were considered in multivariable analysis using multiple imputation with chained equations, ran multivariable analysis on 25 imputed datasets and pooled results using Rubin’s rules [30, 31, 32, 33]. Imputation models included all variables that were considered in multivariable analysis, NVL, VF, interruption of ART, CD4 cell count, region and survey weights.
We calculated rates of switching to second‐line ART as the number of participants who switched divided by the person‐years on first‐line ART. We had no data on the timing of switching to second‐line ART but assumed that participants switched after 1.85 years, the median time from initiation of first‐line ART to switching to second‐line ART in programs in resource‐limited settings without access to routine VL monitoring [34]. We calculated incidence rate ratios (IRR) comparing switching rates by country, age, sex, CD4 count and urban or rural residence using multivariable Poisson regression. In a sensitivity analysis, we assumed that participants switched at the earliest and latest possible times (i.e. six months after initiating first‐line ART and on the day of the survey). We excluded participants with unknown ART start date and unknown ART regimen from analysis of switching rates and factors associated with switching. To ensure national population‐level representativeness of the survey, all analyses were weighted for selection probability, non‐response and non‐coverage using survey weights. We report weighted percentages and unweighted numbers of participants in all analyses if not stated otherwise. Statistical analysis was done using Stata (Version 15, Stata Corporation, College Station, TX, USA).
2.5. Ethical considerations
Local ethics committees and Institutional Review Boards approved the PHIA surveys. All participants provided written informed consent. Anonymized data were used for statistical analyses. More details on research ethics are given in the appendix (Text S2).
3. RESULTS
3.1. Inclusion and characteristics of participants
Out of the 13 852 adults, aged 15 to 59 years, who tested HIV‐positive during the five PHIA surveys, 10 031 (68%) had initiated ART. Of those who had initiated ART, 121 (1.5%) had stopped ART before the survey, and 9910 (98.5%) were still receiving ART at the time of the survey. Out of those still on ART at the time of the survey, we included 9200 (93.2%) participants who were on ART for at least four months (Figure 1). Most were women (64.2%) and resided in rural areas (59.5%). The median age was 39 years (interquartile range [IQR], 32 to 46 years). Most (61.5%) were married or cohabitating. Few (6.9%) reported being unaware of their HIV‐positive status or being HIV negative. Most (69.0%) had disclosed their HIV status to a family member (Table 1, Table S1). The prevalence of participants who screened positive for problematic or hazardous drinking or active alcohol use disorder was 8.3% (Table S2).
Figure 1.
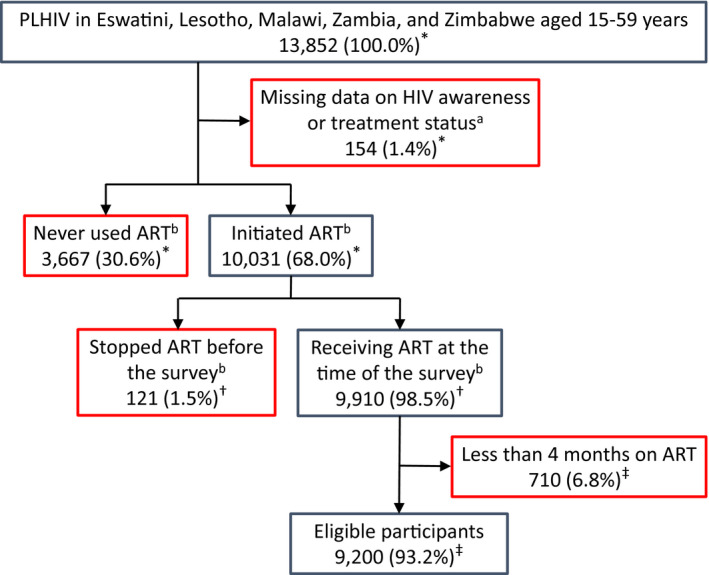
Flow diagram of inclusion of HIV‐positive adults aged 15 to 59 years on antiretroviral therapy (ART) for at least four months who participated in the Population‐based HIV Impact Assessment (PHIA) survey in Eswatini, Lesotho, Malawi, Zambia and Zimbabwe (2015 to 2017). Data are unweighted numbers of participants and weighted percentages. Participants shown in red boxes were excluded. aWe excluded participants with missing data on HIV awareness status, ART use, HIV RNA or ARV testing. bWe assessed ART use based on reported past and current ART use to treat HIV infection and ARV testing results. Participants who reported no past or current ART use and who had no detectable ARV blood levels were classified as having never used ART. Participants who reported past or current ART use or who had detectable ARV blood levels were classified as having initiated ART. Participants who reported past but no current ART use and who had no detectable ARV blood levels were classified as having ceased ART before the survey. Participants who reported receiving ART at the time of the survey, or who had detectable ARV blood levels were classified as receiving ART at the time of the survey. *Denominators for percentages are PLHIV in Eswatini, Lesotho, Malawi, Zambia and Zimbabwe aged 15 to 59 years. †Denominators for percentages are participants who initiated ART. ‡Denominators for percentages are participants who were on ART at the time of the survey. ART, antiretroviral therapy; ARV, antiretroviral drugs; PLHIV, people living with HIV.
Table 1.
Sociodemographic characteristics of HIV‐positive adults receiving antiretroviral therapy in Lesotho, Malawi, Eswatini, Zambia and Zimbabwe (2015 to 2017)
| Characteristic | Lesotho | Malawi | Eswatini | Zambia | Zimbabwe | Total |
|---|---|---|---|---|---|---|
| N = 2177 | N = 1406 | N = 2025 | N = 1424 | N = 2168 | N = 9200 | |
| Residence, n (%) | 2177 (100.0) | 1406 (100.0) | 2025 (100.0) | 1424 (100.0) | 2168 (100.0) | 9200 (100.0) |
| Urban | 991 (49.8) | 644 (24.9) | 487 (29.1) | 868 (62.3) | 656 (35.8) | 3646 (40.5) |
| Rural | 1186 (50.2) | 762 (75.1) | 1538 (70.9) | 556 (37.7) | 1512 (64.2) | 5554 (59.5) |
| Sex, n (%) | 2177 (100.0) | 1406 (100.0) | 2025 (100.0) | 1424 (100.0) | 2168 (100.0) | 9200 (100.0) |
| Male | 646 (38.0) | 401 (35.6) | 587 (30.3) | 414 (35.8) | 660 (36.3) | 2708 (35.8) |
| Female | 1531 (62.0) | 1005 (64.4) | 1438 (69.7) | 1010 (64.2) | 1508 (63.7) | 6492 (64.2) |
| Age in years, n (%) | 2177 (100.0) | 1406 (100.0) | 2025 (100.0) | 1424 (100.0) | 2168 (100.0) | 9200 (100.0) |
| 15 to 19 | 56 (2.7) | 22 (2.0) | 70 (3.3) | 29 (2.6) | 83 (4.6) | 260 (3.1) |
| 20 to 24 | 116 (4.7) | 76 (4.3) | 124 (6.1) | 65 (4.9) | 85 (4.4) | 466 (4.6) |
| 25 to 44 | 1293 (61.6) | 923 (65.0) | 1294 (67.0) | 895 (62.2) | 1287 (61.4) | 5692 (62.9) |
| 45 to 59 | 712 (30.9) | 385 (28.7) | 537 (23.6) | 435 (30.3) | 713 (29.7) | 2782 (29.3) |
| Median (IQR) | 39 (32 to −47) | 39 (32 to 45) | 37 (30 to 44) | 40 (33 to 46) | 39 (32 to 46) | 39 (32 to 46) |
| Wealth quintile, n (%) | 2174 (100.0) | 1406 (100.0) | 2024 (100.0) | 1413 (100.0) | 2168 (100.0) | 9185 (100.0) |
| Lowest | 440 (17.4) | 147 (14.1) | 512 (23.1) | 119 (8.2) | 554 (21.1) | 1772 (15.8) |
| Second | 493 (21.3) | 172 (17.0) | 457 (21.7) | 157 (10.8) | 436 (18.6) | 1715 (16.7) |
| Middle | 480 (22.5) | 193 (18.3) | 429 (20.7) | 295 (20.2) | 399 (19.2) | 1796 (19.6) |
| Fourth | 400 (20.0) | 287 (23.4) | 349 (19.2) | 371 (26.0) | 393 (21.7) | 1800 (22.9) |
| Highest | 361 (18.8) | 607 (27.1) | 277 (15.3) | 471 (34.8) | 386 (19.3) | 2102 (24.9) |
| Education, n (%) | 2176 (100.0) | 1405 (100.0) | 2022 (100.0) | 1424 (100.0) | 2168 (100.0) | 9195 (100.0) |
| No or primary | 1309 (59.1) | 999 (77.5) | 890 (41.3) | 649 (44.0) | 891 (36.9) | 4738 (51.7) |
| Secondary or higher | 867 (40.9) | 406 (22.5) | 1132 (58.7) | 775 (56.0) | 1277 (63.1) | 4457 (48.3) |
| Marital status, n (%) | 2171 (100.0) | 1404 (100.0) | 2012 (100.0) | 1420 (100.0) | 2163 (100.0) | 9170 (100.0) |
| Never married | 330 (15.5) | 76 (4.6) | 654 (33.0) | 133 (10.2) | 208 (10.0) | 1401 (10.6) |
| Married/living together | 1130 (54.4) | 909 (65.9) | 1019 (50.5) | 873 (63.1) | 1287 (61.0) | 5218 (61.5) |
| Divorced, separated or widowed | 711 (30.1) | 419 (29.5) | 339 (16.5) | 414 (26.7) | 668 (29.0) | 2551 (27.9) |
| Economic support, n (%) | 2177 (100.0) | 1406 (100.0) | 2025 (100.0) | 1424 (100.0) | 2168 (100.0) | 9200 (100.0) |
| No | 1720 (80.4) | 1302 (91.1) | 1204 (60.7) | 1364 (95.8) | 1871 (87.1) | 7461 (88.1) |
| Yes | 457 (19.6) | 104 (8.9) | 821 (39.3) | 60 (4.2) | 297 (12.9) | 1739 (11.9) |
| Paid work, n (%) | 2177 (100.0) | 1406 (100.0) | 2025 (100.0) | 1421 (100.0) | 2167 (100.0) | 9196 (100.0) |
| Yes | 871 (43.9) | 480 (32.8) | 936 (48.5) | 537 (40.2) | 763 (37.4) | 3587 (38.2) |
| No | 1306 (56.1) | 926 (67.2) | 1089 (51.5) | 884 (59.8) | 1404 (62.6) | 5609 (61.8) |
| Disclosure to a family member, n (%) | 2177 (100.0) | 1406 (100.0) | 2025 (100.0) | 1424 (100.0) | 2168 (100.0) | 9200 (100.0) |
| No | 540 (25.9) | 344 (24.2) | 660 (32.6) | 291 (20.5) | 507 (24.9) | 2342 (24.2) |
| Yes | 1525 (68.1) | 977 (69.4) | 1307 (64.1) | 1005 (70.1) | 1544 (68.8) | 6358 (69.0) |
| Unknown (unaware of HIV status) | 112 (5.9) | 85 (6.4) | 58 (3.3) | 128 (9.3) | 117 (6.3) | 500 (6.9) |
IQR, interquartile range; N, Number of eligible participants; n, number of participants providing a valid response (weighted %).
3.2. Characteristics of and response to ART
Median duration on ART was reported as four years (IQR 2 to 7 years). Based on ARV testing, most participants (91.3%) received an EFV‐based first‐line regimen and 90.6% reported optimal adherence. The proportion of participants who received second‐line regimens ranged from 0.3% in Zimbabwe to 3.4% in Eswatini. The median CD4 count was 467 cells/µL (IQR 315 to 641 cells/µL). Overall, 20.5% (1837/8972) of participants who received a plasma viral load test had a detectable VL of ≥40 copies/mL. The prevalence of VLS was 88.8% (n = 8236), 11.2% (n = 964) had NVL including 8.2% (n = 686) who had evidence of VF and 3.0% (n = 278) of interrupted ART (Table 2). In the sensitivity analysis including of participants who received ART for less than four months, NVL prevalence was 12.4% (298/2416) in Lesotho, 8.8% (131/1498) in Malawi, 9.0% in Swaziland (193/2175), 10.8% (161/1542) in Zambia, 15.1% (314/2279) in Zimbabwe and 11.7% (1097/9910) across all five countries.
Table 2.
Characteristics of and response to antiretroviral therapy (ART) among HIV‐positive adults in Lesotho, Malawi, Eswatini, Zambia and Zimbabwe (2015 to 2017)
| Lesotho | Malawi | Eswatini | Zambia | Zimbabwe | Total | |
|---|---|---|---|---|---|---|
| N = 2177 | N = 1406 | N = 2025 | N = 1424 | N = 2168 | N = 9200 | |
| Duration on ART, n (%) | 2177 (100.0) | 1406 (100.0) | 2025 (100.0) | 1424 (100.0) | 2168 (100.0) | 9200 (100.0) |
| 4 to 12 months | 336 (15.6) | 144 (10.2) | 251 (12.8) | 169 (12.1) | 216 (10.7) | 1116 (11.5) |
| 1 to 2 years | 285 (13.3) | 188 (13.3) | 283 (13.8) | 186 (13.5) | 275 (12.4) | 1217 (13.1) |
| 2 to 5 years | 574 (25.9) | 434 (30.8) | 613 (30.0) | 383 (26.3) | 734 (33.6) | 2738 (30.1) |
| 5 to 10 years | 630 (28.3) | 417 (29.4) | 609 (29.9) | 415 (28.1) | 702 (31.2) | 2773 (29.6) |
| >10 years | 153 (7.0) | 104 (7.1) | 162 (7.5) | 108 (7.9) | 81 (3.8) | 608 (6.2) |
| Missing | 199 (9.9) | 119 (9.3) | 107 (5.9) | 163 (12.0) | 160 (8.3) | 748 (9.5) |
| Median (IQR) | 4 (1 to 7) | 4 (2 to 7) | 4 (2 to 7) | 4 (2 to 7) | 4 (2 to 6) | 4 (2 to 7) |
| ART regimen, n (%) | 2076 (100.0) | 1357 (100.0) | 1956 (100.0) | 1365 (100.0) | 2049 (100.0) | 8803 (100.0) |
| EFV‐based | 1750 (84.7) | 1257 (93.1) | 1477 (76.2) | 1223 (89.3) | 1970 (96.3) | 7677 (91.3) |
| NVP‐based | 303 (14.1) | 87 (6.2) | 412 (20.4) | 98 (7.4) | 74 (3.4) | 974 (7.2) |
| LPV‐based | 23 (1.2) | 0 (0.0) | 67 (3.4) | 42 (3.1) | 5 (0.3) | 137 (1.2) |
| ATV‐based | NA | 13 (0.7) | NA | 2 (0.1) | NA | 15 (0.2) |
| Adherence, n (%) | 2022 (100.0) | 1299 (100.0) | 1881 (100.0) | 1266 (100.0) | 2019 (100.0) | 8487 (100.0) |
| Optimal | 1769 (87.1) | 1142 (87.4) | 1692 (90.1) | 1154 (90.7) | 1908 (94.2) | 7665 (90.6) |
| Suboptimal | 253 (12.9) | 157 (12.6) | 189 (9.9) | 112 (9.3) | 111 (5.8) | 822 (9.4) |
| CD4 count per µL, n (%) | 2177 (100.0) | 1405 (100.0) | 2024 (100.0) | 1420 (100.0) | 2166 (100.0) | 9192 (100.0) |
| <100 | 52 (2.6) | 34 (2.4) | 25 (1.3) | 35 (2.6) | 74 (3.9) | 220 (2.9) |
| 100 to 199 | 120 (6.1) | 91 (7.5) | 68 (3.5) | 105 (7.9) | 177 (9.4) | 561 (7.8) |
| 200 to 349 | 321 (15.4) | 236 (16.4) | 249 (12.5) | 273 (19.5) | 503 (23.7) | 1582 (19.3) |
| 350 to 499 | 462 (21.9) | 342 (24.5) | 436 (21.5) | 405 (28.1) | 573 (25.2) | 2218 (25.2) |
| ≥500 | 1222 (54.0) | 702 (49.2) | 1246 (61.2) | 602 (41.9) | 839 (37.8) | 4611 (44.8) |
| Median (IQR) | 528 (360 to 701) | 497 (338 to 652) | 571 (409 to 769) | 457 (311 to 622) | 422 (278 to 591) | 467 (315 to 641) |
| Viral load in copies/mL, n (%) a | 2099 (100.0) | 1399 (100.0) | 1973 (100.0) | 1343 (100.0) | 2158 (100.0) | 8972 (100.0) |
| <40 | 1627 (77.2) | 1212 (85.7) | 1577 (79.3) | 1055 (78.6) | 1664 (75.8) | 7135 (79.5) |
| 40 to 999 | 238 (11.6) | 69 (5.6) | 232 (12.3) | 153 (11.1) | 206 (9.6) | 898 (9.2) |
| ≥1000 | 234 (11.2) | 118 (8.7) | 164 (8.4) | 135 (10.3) | 288 (14.6) | 939 (11.3) |
| ART outcome, n (%) | 2177 (100.0) | 1406 (100.0) | 2025 (100.0) | 1424 (100.0) | 2168 (100.0) | 9200 (100.0) |
| Viral load suppression | 1929 (88.5) | 1287 (91.3) | 1858 (91.7) | 1285 (90.1) | 1877 (85.3) | 8236 (88.8) |
| Nonsuppressed viral load | 248 (11.5) | 119 (8.7) | 167 (8.3) | 139 (9.9) | 291 (14.7) | 964 (11.2) |
| Virologic failure | 174 (8.0) | 75 (5.8) | 116 (5.7) | 99 (7.2) | 222 (11.5) | 686 (8.2) |
| Interrupted ART | 74 (3.5) | 44 (2.9) | 51 (2.6) | 40 (2.8) | 69 (3.2) | 278 (3.0) |
Vial load suppression: viral load < 1000 copies/mL; nonsuppressed viral load: viral load ≥ 1000 copies/mL. Virologic failure: nonsuppressed viral load and antiretroviral drugs detected; interrupted ART: nonsuppressed viral load and no antiretroviral drugs detected. Adherence was defined as optimal if a participant reported missing less than two doses of ART in the last 30 days and suboptimal if a participant reported missing two or more doses in the last 30 days. N, number of eligible participants; n, number of participants providing a valid response (weighted %). IQR, interquartile range; ART, antiretroviral therapy; EFV, efavirenz; NVP, nevirapine; LPV, lopinavir; ATV, atazanavir.
Dried blood spot viral load test results (n = 228) could not be classified and were excluded.
3.3. Characteristics of participants with NVL
Table 3 shows the characteristics of participants with NVL. One‐quarter of participants with NVL (26.7%) had evidence of interrupted ART, and three‐quarters (73.3%) were experiencing VF. Most participants with NVL (59.8%) were women. Median age of participants with NVL was 36 years (IQR, 29 to 43 years). Median CD4 count among participants with VF was 222 cells/µL (IQR 116 to 381 cells/µL), and median duration on ART was 4.5 years (IQR 2.6 to 6.9). Only 1.8% of participants with VF received second‐line ART.
Table 3.
Characteristics of adults on antiretroviral therapy with nonsuppressed viral load: Results from the Lesotho, Malawi, Eswatini, Zambia and Zimbabwe Population‐based HIV Impact Assessment survey (2015 to 2017)
| Interrupted ART | Virologic failure | Nonsuppressed viral load | |
|---|---|---|---|
| N = 278 [26.7%] | N = 686 [73.3%] | N = 964 [100.0%] | |
| Sex, n (%) | 278 (100.0) | 686 (100.0) | 964 (100.0) |
| Male | 68 (28.9) | 246 (44.3) | 314 (40.2) |
| Female | 210 (71.1) | 440 (55.7) | 650 (59.8) |
| Age in years, n (%) | 278 (100.0) | 686 (100.0) | 964 (100.0) |
| 15 to 19 | 19 (6.8) | 45 (5.7) | 64 (6.0) |
| 20 to 24 | 36 (10.8) | 58 (7.4) | 94 (8.3) |
| 25 to 44 | 184 (66.4) | 439 (66.5) | 623 (66.5) |
| 45 to 59 | 39 (15.9) | 144 (20.4) | 183 (19.2) |
| Median (IQR) | 34 (27 to 41) | 37 (30 to 43) | 36 (29 to 43) |
| Marital status, n (%) | 277 (100.0) | 681 (100.0) | 958 (100.0) |
| Never married | 60 (13.1) | 148 (17.5) | 208 (16.4) |
| Married or living together | 149 (56.6) | 360 (60.5) | 509 (59.5) |
| Divorced, separated or widowed | 68 (30.2) | 173 (21.9) | 241 (24.2) |
| AUDIT‐C score, n (%) | 202 (100.0) | 506 (100.0) | 708 (100.0) |
| 0 | 171 (82.4) | 417 (81.5) | 588 (81.7) |
| 1 to 2 | 13 (6.9) | 43 (9.3) | 56 (8.7) |
| 3 to 4 | 5 (3.7) | 26 (4.9) | 31 (4.6) |
| 5 to 8 | 9 (4.0) | 18 (4.0) | 27 (4.0) |
| 9 to 12 | 4 (3.0) | 2 (0.3) | 6 (1.0) |
| Antiretroviral drug detected, n (%) | 278 (100.0) | 686 (100.0) | 964 (100.0) |
| EFV | 0 (0.0) | 567 (87.6) | 567 (64.2) |
| NVP | 0 (0.0) | 105 (10.7) | 105 (7.8) |
| LPV | 0 (0.0) | 12 (1.4) | 12 (1.0) |
| ATV | 0 (0.0) | 2 (0.4) | 2 (0.3) |
| No ARVs detected | 278 (100.0) | 0 (0.0) | 278 (26.7) |
| Duration on ART, n (%) | 271 (100.0) | 564 (100.0) | 835 (100.0) |
| 4 to 12 months | 58 (18.7) | 53 (10.1) | 111 (12.7) |
| 1 to 2 years | 53 (19.9) | 65 (10.1) | 118 (13.1) |
| 2 to 5 years | 83 (30.4) | 191 (34.8) | 274 (33.5) |
| 5 to 10 years | 59 (23.1) | 204 (36.4) | 263 (32.4) |
| >10 years | 18 (8.0) | 51 (8.6) | 69 (8.4) |
| Median (IQR) | 2.9 (1.2 to 5.8) | 4.5 (2.6 to 6.9) | 4.2 (1.9 to 6.6) |
| CD4 cell count per µL, n (%) | 276 (100.0) | 685 (100.0) | 961 (100.0) |
| <100 | 33 (12.7) | 128 (21.7) | 161 (19.3) |
| 100 to 199 | 52 (21.2) | 139 (23.1) | 191 (22.6) |
| 200 to 349 | 68 (22.0) | 170 (25.7) | 238 (24.7) |
| 350 to 499 | 61 (21.8) | 129 (16.4) | 190 (17.8) |
| ≥500 | 62 (22.4) | 119 (13.2) | 181 (15.6) |
| Median (IQR) | 300 (165 to 497) | 222 (116 to 381) | 237 (126 to 414) |
| Viral load log10 copies/mL, n (%) | |||
| 3 to 4 | 59 (20.2) | 254 (35.9) | 313 (31.7) |
| 4 to 5 | 152 (56.0) | 299 (44.0) | 451 (47.2) |
| 5 to 6 | 61 (22.0) | 125 (19.1) | 186 (19.9) |
| ≥6 | 6 (1.9) | 8 (1.0) | 14 (1.2) |
| Median (IQR) | 4.5 (4.2 to 5.0) | 4.3 (3.8 to 4.8) | 4.4 (3.8 to 4.9) |
Nonsuppressed viral load was defined as viral load ≥1000 copies/mL. ART, antiretroviral therapy; EFV, efavirenz; IQR, interquartile range; LPV, lopinavir: ATV, atazanavir; N, number of eligible participants; n, number of participants providing a valid response (weighted col %) [weighted row %]; NVP, nevirapine.
3.4. Factors associated with NVL
The adjusted odds ratios (aOR) for NVL for participants in Zimbabwe (aOR 2.61; 95% confidence interval [CI]: 2.03 to 3.35), Lesotho (aOR 1.61; 95% CI: 1.26 to 2.05), and Zambia (aOR 1.52; 95% CI: 1.14 to 2.02) were higher than in Eswatini. The odds for NVL in people aged 15 to 24 years were 2 to 3 times higher than in those aged 45 to 59 years. The odds of NVL were slightly increased in men (aOR 1.28; 95% CI: 1.05 to 1.55), in participants with low educational levels (aOR 1.27; 95% CI: 1.06 to 1.52), in those who had never married (aOR 1.42; 95% CI: 1.02 to 1.98) and in those who had not disclosed their HIV status to a family member (aOR 1.32; 95% CI: 1.06 to 1.66). Participants receiving NVP‐based ART had higher odds for NVL (aOR 1.84; 95% CI: 1.34 to 2.51) than those receiving EFV‐based regimens. Odds for NVL were also higher in participants who reported sub‐optimal adherence (aOR 1.81; 95% CI: 1.37 to 2.39; Table 4).
Table 4.
Factors associated with nonsuppressed viral load among HIV‐positive participants in the Population‐based HIV Impact Assessment survey in Eswatini, Lesotho, Malawi, Zambia and Zimbabwe (2015 to 2017)
| Nonsuppressed viral load | ||||
|---|---|---|---|---|
| Bivariate analysis | Multivariable analysis | |||
| OR (95% CI) | P‐value | aOR (95% CI) | P‐value | |
| Characteristics of participants | ||||
| Country of residence | <0.001 | <0.001 | ||
| Lesotho | 1.43 (1.14 to 1.79) | 1.61 (1.26 to 2.05) | ||
| Malawi | 1.05 (0.78 to 1.44) | 1.25 (0.90 to 1.74) | ||
| Eswatini | 1.00 | 1.00 | ||
| Zambia | 1.22 (0.94 to 1.57) | 1.52 (1.14 to 2.02) | ||
| Zimbabwe | 1.90 (1.53 to 2.35) | 2.61 (2.03 to 3.35) | ||
| Age, years | <0.001 | <0.001 | ||
| 15 to 19 | 3.47 (2.23 to 5.41) | 2.15 (1.23 to 3.78) | ||
| 20 to 24 | 3.17 (2.17 to 4.62) | 3.13 (2.08 to 4.71) | ||
| 25 to 44 | 1.70 (1.34 to 2.14) | 1.81 (1.42 to 2.32) | ||
| 45 to 59 | 1.00 | 1.00 | ||
| Sex | 0.022 | 0.013 | ||
| Male | 1.24 (1.03 to 1.48) | 1.28 (1.05 to 1.55) | ||
| Female | 1.00 | 1.00 | ||
| Marital status | <0.001 | 0.110 | ||
| Never married | 1.72 (1.33 to 2.23) | 1.42 (1.02 to 1.98) | ||
| Married or living together | 1.00 | 1.00 | ||
| Divorced, separated or widowed | 0.89 (0.72 to 1.09) | 1.02 (0.82 to 1.27) | ||
| Education | 0.977 | 0.010 | ||
| No or primary | 1.00 (0.83 to 1.20) | 1.27 (1.06 to 1.52) | ||
| Secondary or higher | 1.00 | 1.00 | ||
| Disclosure to a family member | 0.003 | 0.014 | ||
| No | 1.29 (1.04 to 1.61) | 1.32 (1.06 to 1.66) | ||
| Yes | 1.00 | 1.00 | ||
| Not self‐reported positive | 1.64 (1.16 to 2.32) | 1.39 (0.96 to 2.02) | ||
| Characteristics of antiretroviral therapy | ||||
| Antiretroviral regimen | 0.003 | 0.001 | ||
| EFV‐based | 1.00 | 1.00 | ||
| NVP‐based | 1.66 (1.23 to 2.24) | 1.84 (1.34 to 2.51) | ||
| PI‐based | 1.65 (0.80 to 3.39) | 1.86 (0.88 to 3.89) | ||
| Adherence | <0.001 | <0.001 | ||
| Optimal | 1.00 | 1.00 | ||
| Suboptimal | 1.91 (1.46 to 2.50) | 1.81 (1.37 to 2.39) | ||
Multivariable analysis adjusted for country, age, sex, marital status, education, disclosure of HIV status to a family member, antiretroviral regimen and adherence. Nonsuppressed viral load was defined as viral load ≥1000 copies/mL. P‐values of categorical variables are for joint test for significance. aOR, adjusted odds ratios; ART, antiretroviral therapy; EFV, efavirenz; NVP, nevirapine; OR, odds ratio; PI, protease inhibitor.
3.5. Factors associated with VF and interrupted ART among participants with NVL
Table 5 shows aORs comparing the odds for VF among participants with NVL. The adjusted odds for VF among participants aged 15 to 19 years were lower (aOR 0.32; 95% CI: 0.14 to 0.76) than in those aged 44 to 59 years. Women had almost half the odds (aOR 0.54; 95% CI: 0.29 to 0.99) of VF compared to men. Married (aOR 0.53; 95% CI: 0.31 to 0.93) and divorced participants (aOR 0.37; 95% CI: 0.19 to 0.71) had lower odds for VF than participants who never married. The odds for VF were 66% (aOR 0.34; 95% CI: 0.17 to 0.71) and 68% (aOR 0.32; 95% CI: 0.17 to 0.59) lower in participants receiving ART for <1 year and one to two years, respectively, compared to those receiving ART for five to ten years. Participants with a CD4 count of 350 to 500 cells/µL (aOR 0.48 95% CI: 0.27 to 0.85) and those with a CD4 count ≥500 cells/µL (aOR 0.40 95% CI: 0.19 to 0.81) had lower odds of VF than those with a CD4 cell count <100 cells/µL. Factors associated with VF were inversely associated with interrupted ART because participants with NVL either had VF or interrupted ART.
Table 5.
Factors associated with virologic failure among participants with nonsuppressed viral load in Eswatini, Lesotho, Malawi, Zambia and Zimbabwe (2015 to 2017)
| Virologic failure | ||||
|---|---|---|---|---|
| Bivariate analysis | Multivariable analysis | |||
| OR (95% CI) | P‐value | aOR (95% CI) | P‐value | |
| Sociodemographic characteristics | ||||
| Age in years | 0.250 | 0.056 | ||
| 15 to 19 | 0.66 (0.30 to 1.47) | 0.32 (0.14 to 0.76) | ||
| 20 to 24 | 0.53 (0.28 to 1.01) | 0.54 (0.24 to 1.18) | ||
| 25 to 44 | 0.78 (0.49 to 1.23) | 0.89 (0.56 to 1.42) | ||
| 45 to 59 | 1.00 | 1.00 | ||
| Sex | 0.002 | 0.047 | ||
| Male | 1.00 | 1.00 | ||
| Female | 0.51 (0.34 to 0.78) | 0.54 (0.29 to 0.99) | ||
| Marital status | 0.043 | 0.012 | ||
| Never married | 1.00 | 1.00 | ||
| Married or living together | 0.80 (0.53 to 1.20) | 0.53 (0.31 to 0.93) | ||
| Divorced, separated or widowed | 0.54 (0.34 to 0.88) | 0.37 (0.19 to 0.7) | ||
| Antiretroviral therapy characteristics | ||||
| Duration on ART in years | 0.001 | <0.001 | ||
| <1 | 0.39 (0.20 to 0.76) | 0.34 (0.17 to 0.71) | ||
| 1 to 2 | 0.37 (0.21 to 0.65) | 0.32 (0.17 to 0.59) | ||
| 2 to 5 | 0.74 (0.47 to 1.17) | 0.75 (0.45 to 1.24) | ||
| 5 to 10 | 1.00 | 1.00 | ||
| >10 | 0.69 (0.33 to 1.40) | 0.62 (0.30 to 1.26) | ||
| CD4 cell count per µL | 0.004 | 0.048 | ||
| <100 | 1.00 | 1.00 | ||
| 100 to 199 | 0.65 (0.40 to 1.05) | 0.59 (0.37 to 0.95) | ||
| 200 to 349 | 0.69 (0.38 to 1.25) | 0.67 (0.37 to 1.21) | ||
| 350 to 499 | 0.44 (0.26 to 0.76) | 0.48 (0.27 to 0.85) | ||
| ≥500 | 0.35 (0.19 to 0.63) | 0.40 (0.19 to 0.81) | ||
| Behavioural characteristics | ||||
| AUDIT‐C score | <0.001 | 0.001 | ||
| 0 | 1.00 | 1.00 | ||
| 1 to 2 | 1.33 (0.67 to 2.63) | 1.01 (0.41 to 2.47) | ||
| 3 to 4 | 1.26 (0.76 to 2.11) | 0.93 (0.46 to 1.87) | ||
| 5 to 8 | 1.00 (0.55 to 1.81) | 0.55 (0.26 to 1.20) | ||
| 9 to 12 | 0.10 (0.04 to 0.28) | 0.09 (0.03 to 0.28) | ||
Nonsuppressed viral load was defined as a viral load of ≥1000 copies/mL. Multivariable analysis adjusted for all variables shown in the table. P‐values of categorical variables are for joint test for significance. aOR, adjusted odds ratios; ART, antiretroviral therapy; ARV, antiretroviral medication; OR, odds ratio.
3.6. Rates of and factors associated with switching to second‐line ART
Overall, 131 (1.4%) of 8066 participants with known date of ART initiation and known regimen switched to second‐line ART, for an annual switching rate of 0.31% (95% CI: 0.25 to 0.40). Annual switching rates were 0.22% (95% CI: 0.14 to 0.36) in Lesotho, 0.15% (95% CI: 0.08 to 0.35) in Malawi, 0.72% (95% CI: 0.54 to 0.96) in Eswatini, 0.73% (95% CI: 0.52 to 1.04) in Zambia and 0.06% (95% CI: 0.02 to 0.33) in Zimbabwe. Compared to Eswatini, adjusted switching rates were more than 10 times lower in Zimbabwe (IRR, 0.07; 95% CI: 0.02 to 0.24), five times lower in Malawi (IRR 0.19; 95% CI: 0.09 to 0.44), more than three times lower in Lesotho (IRR 0.28; 95% CI: 0.15 to 0.52) and similar in Zambia (IRR 0.86; 95% CI: 0.57 to 1.32).
People with CD4 counts <100 cells/µL were more than three times (IRR 3.52; 95% CI: 1.05 to 11.76) and those with CD4 counts of 100 to 199 cells/µL were more than two times (IRR 2.29; 95% CI: 1.05 to 5.01) as likely to switch to second‐line ART than those with CD4 count of ≥500 cells/µL. Age, sex and residence (rural or urban) were not associated with switching rates.
Analysis of switching rates was not sensitive to assumptions on the timing of switching. The overall annual switching rate remained 0.31% whether we assumed that participants switched six months after initiation of first‐line ART or on the day of the survey (Table S3).
4. DISCUSSION
These population‐based nationally representative surveys showed that these five southern African countries are approaching UNAIDS VLS targets despite limited access to VL monitoring. We found that 88.8% of adults on ART achieved VLS (VL < 1000 copies/mL), while 11.2% had NVL (VL ≥ 1000 copies/mL). Sociodemographic characteristics, current ART regimen, adherence and HIV status disclosure were associated with NVL. One‐quarter of participants with NVL had no detectable blood concentrations of ARVs and were classified as having interrupted ART, while three‐quarters of participants with NVL had evidence of recent ARV exposure and were classified as experiencing VF. Marriage, female sex, shorter ART duration, higher CD4 count and alcohol use were associated with higher odds for interrupted ART and lower odds for VF. Almost half of the people with VF were severely immunosuppressed although they received ART for several years. Switching rates varied considerably between countries, but even in countries with the highest switching rates, very few patients had switched to second‐line therapy.
Low switching rates, as documented in this study, indicate substantial gaps in monitoring of ART and/or clinical management of patients with treatment failure. HIV cohort studies from low‐income and middle‐income countries showed that each year about 3% of adults have a confirmed virologic treatment failure of first‐line ART and require switching to second‐line ART [6, 35]. We found nationally representative switching rates far below the annual failure rate of 3%: annual switching rates in our study ranged from 0.06% to 0.73%. Limited access to VL monitoring likely explains these low switching rates. When PHIA data were collected, the five countries mainly relied on CD4 monitoring and clinical monitoring with limited access to targeted VL testing [10, 36, 37]. Without routine VL monitoring, treatment failure often remains undetected and patients are not switched to second‐line ART, or patients are switched late and at low CD4 cell counts [6]. Our study demonstrates the limitations of clinical and immunological monitoring of ART and supports country‐level efforts to scale‐up VL testing for early detection of NVL.
Once patients with NVL have been identified, healthcare providers need to distinguish between patients who require adherence support and those who need a new ART regimen. We noted that more than one‐quarter of participants with NVL tested negative for recent exposure to ARVs, indicating that temporary interruption of ART was the likely reason for NVL in these individuals. Young women, those with high CD4 cell counts, and those who were on ART for <2 years were more likely to interrupt ART. Suboptimal retention and adherence among women who started ART during pregnancy under Option B+ guidelines which recommend rapid ART initiation for pregnant and breastfeeding women may explain these findings [38, 39, 40]. In line with previous data, our study suggests that a substantial proportion of people with NVL interrupted ART and may benefit from adherence interventions. The ANRS 12110 trial showed that one‐third of patients with NVL had no viral resistance [41]. The DART trial showed that one‐third of patients with NVL re‐suppressed without changing their ART regimen [42]. A systematic review of five observational studies from South Africa, Eswatini, Thailand and France showed that more than 70% of patients with NVL re‐suppressed following adherence intervention [43]. In agreement with earlier reports, our study supports WHO guidelines to offer adherence support and confirmatory VL testing to patients with a first NVL to avoid unnecessary switching of ART [9]. Our study also underlines the importance of adherence support for patients who start ART with high CD4 cell counts, especially during the initial phase of treatment.
Optimizing ART regimens likely can support countries’ efforts to increase VLS rates among people receiving ART. In December 2018, WHO recommended dolutegravir (DTG), tenofovir (TDF), and either lamivudine or emtricitabine (XTC) as the preferred first‐line regimen, and low‐ and middle‐income countries are transitioning from the current first‐line regimen TDF/XTC/EFV to the new DTG‐based regimen [44]. DTG is safer and has a higher genetic barrier to resistance than EFV. Data from randomized controlled trials support switching people with undetectable VL from an EFV‐based regimen to a DTG‐based regimen; however, currently, no evidence supports this switch for those with detectable or unknown VL [45]. Our finding that about 20% of people receiving ART had a detectable VL > 40 copies/mL underlines the need for data to support switching from a failing current first‐line treatment to effective second‐line treatments. However, the effectiveness of switching from TDF/XTC/EFV to TDF/XTC/DTG needs to be evaluated. Our data suggest that men, individuals who never married, and those with a poor immunologic response to long‐term ART are at increased risk of VF and should be monitored carefully after switching to DTG.
Our study has several strengths and limitations. The national representativeness of our findings and the richness of collected sociodemographic, behavioural and laboratory data including measures on viral suppression and ARV detection are important strengths of our study. Our findings have to be considered in the view of several limitations. Our definition of VF did not rely on drug resistance data, and our ability to distinguish between patients with NVL due to inadequate adherence, drug resistance or both was limited. We classified participants as having interrupted or failing ART based on self‐report of current ART use and laboratory testing for detectable concentrations of selected ARVs. Both data sources have limitations that may lead to misclassification. Self‐reported data on ART might be susceptible to recall and social desirability bias. Likewise, laboratory errors could result in misclassification. We also acknowledge that our estimates for the prevalence of participants who interrupted ART do not include individuals who stopped ART before the survey. In our study, only 1.5% of the participants with a history of ART use reported having stopped ART before the survey. HIV cohort studies found higher rates of treatment discontinuation [8, 46, 47, 48]. PHIA estimates for treatment discontinuation might be an underestimate because PLHIV who stopped ART might underreport prior ART use or awareness of their own HIV status. The PHIA survey only included people who were alive. High mortality among individuals who remain on failing first‐line regimens might partially explain the low NVL prevalence among those who survived and were included in the survey [6, 49]. Finally, the rates of switching to second‐line ART in Lesotho, Eswatini and Zimbabwe may be underestimates because samples from these countries were tested for LPV but not ATV, although both drugs are WHO‐recommended second‐line treatments [9]. However, the ARVs tested were selected according to national treatment guidelines, and we assumed very few people in Lesotho, Eswatini and Zimbabwe would have received ATV.
5. CONCLUSIONS
The five countries included in our analysis have low rates of NVL and are approaching UNAIDS VLS targets, despite limited access to VL monitoring and possible suboptimal clinical management of patients with NVL. Treatment support, in particular for younger adults, and people with higher CD4 counts, and switching of patients to PI‐ or DTG‐based regimens may further reduce NVL prevalence.
COMPETING INTERESTS
The authors have no conflicts of interest to declare.
AUTHORS’ CONTRIBUTIONS
AH, ER and JJ conceptualized the study. AH did statistical analysis and wrote the first draft of the manuscript which was revised by ER and JJ. All authors contributed to interpretation of data and provided critical inputs in the draft manuscript. ER, HA, AJH, NP, SJ, SS, AL, HP, AS, JR, KF, EK, GB, DW, BP, KS, DB, TK, SB, GM, OM, BB, KS, LM, KT, TA, KB, AV and JJ contributed to design and implementation of PHIA surveys. All authors have read and approved the final manuscript.
Supporting information
Text S1. Laboratory methods for processing of HIV‐positive specimens
Text S2. Ethical oversight over the Population‐based HIV Impact Assessment (PHIA) surveys in participating countries.
Table S1. Characteristics of HIV status disclosure among adults on antiretroviral therapy who participated in the Population‐based HIV Impact Assessment (PHIA) survey in Lesotho, Malawi, Eswatini, Zambia and Zimbabwe (2015 to 2017)
Table S2. Prevalence of alcohol use disorder among adults on antiretroviral therapy who participated in the Population‐based HIV Impact Assessment (PHIA) survey in Malawi, Eswatini, Zambia, and Zimbabwe (2015 to 2017)
Table S3. Sensitivity analysis of rates of switching to second‐line ART among HIV‐positive adults who participated in the Population‐based HIV Impact Assessment (PHIA) survey in Lesotho, Malawi, Eswatini, Zambia, and Zimbabwe (2015 to 2017)
ACKNOWLEDGEMENTS
PHIA is conducted under the leadership of the respective countries’ Ministries of Health, U.S. Centers for Disease Control and Prevention (CDC) and ICAP at Columbia University (http://www.icap.columbia.edu/).
Collaborating institutions: Eswatini: Ministry of Health; CDC; Central Statistical Office. Lesotho: Ministry of Health; CDC; Bureau of Statistics; National University of Lesotho. Malawi: Ministry of Health; CDC; Centre for Social Research; Chancellor College, University of Malawi; National AIDS Commission; National Statistics Office; Blantyre Health Research and Training Trust. Zambia: Ministry of Health; CDC; Central Statistical Office; Tropical Diseases Research Centre University of Zambia; University Teaching Hospital; National HIV/AIDS/STI/TB Council; Zambian National Public Health Institute. Zimbabwe: Ministry of Health and Child Care, CDC; National Statistics Agency; the Biomedical Research and Training Institute; Lancet Laboratories. South Africa: University of Cape Town (UCT). USA: ICAP at Columbia University; CDC Atlanta; Johns Hopkins University Laboratories Statistical Center for HIV/AIDS Research and Prevention; WESTAT.
FUNDING
This research was supported by the President's Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) through the following grants: U2GGH001226, U2GGH000994 and 6NU2GGH001271 (formerly 5U2GGH001271). AH was supported by a Swiss National Science Foundation (SNF) Early Postdoc Mobility Fellowship (grant number: P2BEP3_178602).
DISCLAIMER
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies.
Haas, A. D. , Radin, E. , Avi, H. , Jahn, A. , Philip, N. M. , Jonnalagadda, S. , Saito, S. , Low, A. , Patel, H. , Schwitters, A. , Rogers, J. H. , Frederix, K. , Kim, E. , Bello, G. , Williams, D. B. , Parekh, B. , Sachathep, K. , Barradas, D. T. , Kalua, T. , Birhanu, S. , Musuka, G. , Mugurungi, O. , Tippett Barr, B. A. , Sleeman, K. , Mulenga, L. B. , Thin, K. , Ao, T. , Brown, K. , Voetsch, A. C. and Justman, J. J. Prevalence of nonsuppressed viral load and associated factors among HIV‐positive adults receiving antiretroviral therapy in Eswatini, Lesotho, Malawi, Zambia and Zimbabwe (2015 to 2017): results from population‐based nationally representative surveys. J Int AIDS Soc. 2020; 23(00):e25631
Contributor Information
Andreas D Haas, Email: andreas.haas@ispm.unibe.ch.
Elizabeth Radin, Email: er2743@cumc.columbia.edu.
Avi J Hakim, Email: hxv8@cdc.gov.
Andreas Jahn, Email: drandreasjahn@gmail.com.
Neena M Philip, Email: nmp6@cumc.columbia.edu.
Sasi Jonnalagadda, Email: wau4@cdc.gov.
Suzue Saito, Email: ss1117@cumc.columbia.edu.
Andrea Low, Email: al3546@cumc.columbia.edu.
Hetal Patel, Email: byg7@cdc.gov.
Amee M Schwitters, Email: efn6@cdc.gov.
John H Rogers, Email: yet6@cdc.gov.
Koen Frederix, Email: frederixk@icap.org.ls.
Evelyn Kim, Email: gvh5@cdc.gov.
George Bello, Email: gbello@itech-malawi.org.
Daniel B Williams, Email: hfe7@cdc.gov.
Bharat Parekh, Email: bsp1@cdc.gov.
Karampreet Sachathep, Email: ks3265@cumc.columbia.edu.
Danielle T Barradas, Email: cue4@cdc.gov.
Thokozani Kalua, Email: thokokalua@gmail.com.
Sehin Birhanu, Email: vjw2@cdc.gov.
Godfrey Musuka, Email: gm2660@cumc.columbia.edu.
Owen Mugurungi, Email: mugurungi@gmail.com.
Beth A Tippett Barr, Email: hkc5@cdc.gov.
Katrina Sleeman, Email: hhk6@cdc.gov.
Lloyd B Mulenga, Email: lbmulenga@yahoo.com.
Kyaw Thin, Email: dr.kyawthin@gmail.com.
Trong T Ao, Email: tfa8@cdc.gov.
Kristin Brown, Email: aoi2@cdc.gov.
Andrew C Voetsch, Email: aav6@cdc.gov.
Jessica E Justman, Email: jj2158@cumc.columbia.edu.
REFERENCES
- 1. Joint United Nations Programme on HIV/AIDS (UNAIDS) . 90‐90‐90. An ambitious treatment target to help end the AIDS epidemic. UNAIDS; 2014.
- 2. Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta‐analysis. AIDS. 2009;23(11):1397–404. [DOI] [PubMed] [Google Scholar]
- 3. May MT, Gompels M, Delpech V, Porter K, Orkin C, Kegg S, et al. Impact on life expectancy of HIV‐1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS. 2014;28(8):1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnson LF, May MT, Dorrington RE, Cornell M, Boulle A, Egger M, et al. Estimating the impact of antiretroviral treatment on adult mortality trends in South Africa: A mathematical modelling study. Suthar AB, editor. PLOS Med. 2017;14:e1002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV‐1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haas AD, Keiser O, Balestre E, Brown S, Bissagnene E, Chimbetete C, et al. Monitoring and switching of first‐line antiretroviral therapy in adult treatment cohorts in sub‐Saharan Africa: Collaborative analysis. Lancet HIV. 2015;2(7):e271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor‐based HIV therapy and virologic outcomes. Ann Intern Med. 2007;146(8):564–73. [DOI] [PubMed] [Google Scholar]
- 8. Haas AD, Zaniewski E, Anderegg N, Ford N, Fox MP, Vinikoor M, et al. Retention and mortality on antiretroviral therapy in sub‐Saharan Africa: collaborative analyses of HIV treatment programmes. J Int AIDS Soc. 2018;21:e25084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection Recommendations for a public health approach, 2nd edn Geneva: World Health Organization; 2016. [PubMed] [Google Scholar]
- 10. Lecher S, Williams J, Fonjungo PN, Kim AA, Ellenberger D, Zhang G, et al. Progress with scale‐up of HIV viral load monitoring ‐ seven Sub‐Saharan African countries, January 2015‐June 2016. Morb Mortal Wkly Rep. 2016;65(47):1332–5. [DOI] [PubMed] [Google Scholar]
- 11. Peter T, Zeh C, Katz Z, Elbireer A, Alemayehu B, Vojnov L, et al. Scaling up HIV viral load ‐ lessons from the large‐scale implementation of HIV early infant diagnosis and CD4 testing. J Int AIDS Soc. 2017;20:e25008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Government of the Kingdom of Eswatini . Swaziland HIV Incidence Measurement Survey 2 (SHIMS2) 2016–2017. Final Report. Mbabane: Government of the Kingdom of Eswatini; 2019. [Google Scholar]
- 13. Ministry of Health, Lesotho, Centers for Disease Control and Prevention (CDC), and ICAP at Columbia University . Lesotho Population‐based HIV Impact Assessment (LePHIA) 2016–2017: Final Report. Maseru, Lesotho, Atlanta, Georgia, and NewYork, NewYork, USA: Ministry of Health, CDC, and ICAP; 2019. [Google Scholar]
- 14. Ministry of Health, Malawi . Malawi Population‐Based HIV Impact Assessment (MPHIA) 2015–2016: Final Report. Lilongwe: Ministry of Health; 2018. [Google Scholar]
- 15. Ministry of Health, Zambia . Zambia Population‐based HIV Impact Assessment (ZAMPHIA) 2016. Final Report. Lusaka: Ministry of Health; 2019. [Google Scholar]
- 16. Ministry of Health and Child Care (MOHCC), Zimbabwe . Zimbabwe Population‐based HIV Impact Assessment (ZIMPHIA) 2015–2016: Final Report. Harare: MOHCC; 2019. [Google Scholar]
- 17. Rutstein SO, Johnson K The DHS Wealth Index. DHS Comp Reports No 6. 2004; 1–71.
- 18. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT‐C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–95. [DOI] [PubMed] [Google Scholar]
- 19. Bradley KA, Bush KR, Epler AJ, Dobie DJ, Davis TM, Sporleder JL, et al. Two brief alcohol‐screening tests From the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Arch Intern Med. 2003;163(7):821–9. [DOI] [PubMed] [Google Scholar]
- 20. Gortmaker SL, Hosmer DW, Lemeshow S. Applied Logistic Regression. Contemporary Sociology. 1994;23:159. [Google Scholar]
- 21. Robert BB, Afifi AA. Comparison of Stopping Rules in Forward “Stepwise” Regression. J Am Stat Assoc. 1977;72(357):46–53. [Google Scholar]
- 22. Mickey RM, Greenland S. The impact for confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129(1):125–37. [DOI] [PubMed] [Google Scholar]
- 23. Hare AQ, Ordóñez CE, Johnson BA, Del Rio C, Kearns RA, Wu B, et al. Gender‐specific risk factors for virologic failure in KwaZulu‐Natal: automobile ownership and financial insecurity. AIDS Behav. 2014;18(11):2219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flynn AG, Anguzu G, Mubiru F, Kiragga AN, Kamya M, Meya DB, et al. Socioeconomic position and ten‐year survival and virologic outcomes in a Ugandan HIV cohort receiving antiretroviral therapy. PLoS One. 2017;12(12):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Babo YD, Alemie GA, Fentaye FW. Predictors of first‐line antiretroviral therapy failure amongst HIV‐infected adult clients at Woldia Hospital, Northeast Ethiopia. PLoS One. 2017;12(11):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mekuria LA, Nieuwkerk PT, Yalew AW, Sprangers MA, Prins JM. High level of virological suppression among HIV‐infected adults receiving combination antiretroviral therapy in Addis Ababa, Ethiopia. Antivir Ther. 2016;21(5):385–96. [DOI] [PubMed] [Google Scholar]
- 27. Labhardt ND, Bader J, Ramoeletsi M, Kamele M, Lejone TI, Cheleboi M, et al. Clinical and socio‐demographic predictors for virologic failure in rural Southern Africa: preliminary findings from CART‐1. J Int AIDS Soc. 2014;17 4 Suppl 3:19666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aibibula W, Cox J, Hamelin A‐M, McLinden T, Klein MB, Brassard P. Association Between Food Insecurity and HIV Viral Suppression: A Systematic Review and Meta‐Analysis. AIDS Behav. 2017;21(3):754–65. [DOI] [PubMed] [Google Scholar]
- 29. Billioux VG, Grabowski MK, Ssekasanvu J, Reynolds SJ, Berman A, Bazaale J, et al. HIV viral suppression and geospatial patterns of HIV antiretroviral therapy treatment facility use in Rakai, Uganda. AIDS. 2018;32(6):819–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rubin DBD.Multiple Imputation for Nonresponse in Surveys [Internet]. Rubin DB. editor. Proceedings of the Survey Research Merhods Section of rhe American Statistical Association. New York; USA: John Wiley & Sons, Inc.; 1987: 20–34. (Wiley Series in Probability and Statistics). [Google Scholar]
- 31. White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30(4):377–99. [DOI] [PubMed] [Google Scholar]
- 32. Carpenter J, Kenward M. Multiple imputation and its application. Chichester: John Wiley & Sons; 2013. [Google Scholar]
- 33. Sterne JAC, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. ART‐LINC of IeDEA Study Group , Keiser O, Tweya H, Boulle A, Braitstein P, Schecter M, et al. Switching to second‐line antiretroviral therapy in resource‐limited settings: comparison of programmes with and without viral load monitoring. AIDS. 2009;23(14):1867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kyaw NTT, Harries AD, Kumar AMV, Oo MM, Kyaw KWY, Win T, et al. High rate of virological failure and low rate of switching to second‐line treatment among adolescents and adults living with HIV on first‐line ART in Myanmar, 2005–2015. De Socio GV, editor. PLoS One. 2017;12:e0171780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ministry Of Health And Child Care (MoHCC) . Zimbabwe HIV viral load scale‐up plan: 2015–2018. [cited 2020 Oct 21]. Available from: https://depts.washington.edu/edgh/zw/vl/project‐resources/Viral_Load_Plan.pdf
- 37. El‐Sadr WM, Rabkin M, Nkengasong J, Birx DL. Realizing the potential of routine viral load testing in sub‐Saharan Africa. J Int AIDS Soc. 2017;20:e25010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haas AD, Msukwa MT, Egger M, Tenthani L, Tweya H, Jahn A, et al. Adherence to Antiretroviral Therapy During and After Pregnancy: Cohort Study on Women Receiving Care in Malawi’s Option B+ Program. Clin Infect Dis. 2016;63(9):1227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Haas AD, Tenthani L, Msukwa MT, Tal K, Jahn A, Gadabu OJ, et al. Retention in care during the first 3 years of antiretroviral therapy for women in Malawi’s option B+ programme: an observational cohort study. Lancet HIV. 2016;3(4):e175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. World Health Organization . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. Geneva: WHO; 2013. [PubMed] [Google Scholar]
- 41. Laurent C, Kouanfack C, Laborde‐Balen G, Aghokeng AF, Mbougua JBT, Boyer S, et al. Monitoring of HIV viral loads, CD4 cell counts, and clinical assessments versus clinical monitoring alone for antiretroviral therapy in rural district hospitals in Cameroon (Stratall ANRS 12110/ESTHER): a randomised non‐inferiority trial. Lancet Infect Dis. 2011;11(11):825–33. [DOI] [PubMed] [Google Scholar]
- 42. Gupta RK, Goodall RL, Ranopa M, Kityo C, Munderi P, Lyagoba F, et al. High rate of HIV resuppression after viral failure on first‐line antiretroviral therapy in the absence of switch to second‐line therapy. Clin Infect Dis. 2014;58(7):1023–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bonner K, Mezochow A, Roberts T, Ford N, Cohn J. Viral load monitoring as a tool to reinforce adherence: a systematic review. J Acquir Immune Defic Syndr. 2013;64(1):74–8. [DOI] [PubMed] [Google Scholar]
- 44. World Health Organization . Interim guidelines. Updated recommedations on first‐line and second‐line antiretroviral regimens and post‐exposure prophylaxis and recommendations on early infant diagnosis of HIV. Geneva: WHO; 2018. [Google Scholar]
- 45. Vitoria M, Hill A, Ford N, Doherty M, Clayden P, Venter F, et al. The transition to dolutegravir and other new antiretrovirals in low‐income and middle‐income countries: what are the issues? AIDS. 2018;32(12):1551–61. [DOI] [PubMed] [Google Scholar]
- 46. Chammartin F, Zürcher K, Keiser O, Weigel R, Chu K, Kiragga AN, et al. Outcomes of Patients Lost to Follow‐up in African Antiretroviral Therapy Programs: Individual Patient Data Meta‐analysis. Clin Infect Dis. 2018;67(11):1643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zürcher K, Mooser A, Anderegg N, Tymejczyk O, Couvillon MJ, Nash D, et al. Outcomes of HIV‐positive patients lost to follow‐up in African treatment programmes. Trop Med Int Health. 2017;22(4):375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Geng EH, Odeny TA, Lyamuya R, Nakiwogga‐Muwanga A, Diero L, Bwana M, et al. Retention in care and patient‐reported reasons for undocumented transfer or stopping care among HIV‐infected patients on antiretroviral therapy in Eastern Africa: application of a sampling‐based approach. Clin Infect Dis. 2016;62(7):935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bell‐Gorrod H, Fox MP, Boulle A, Prozesky H, Wood R, Tanser F, et al. The impact of delayed switch to second‐line antiretroviral therapy on mortality, depending on failure time definition and CD4 count at failure. Am J Epidemiol. 2020;189(8):811‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Text S1. Laboratory methods for processing of HIV‐positive specimens
Text S2. Ethical oversight over the Population‐based HIV Impact Assessment (PHIA) surveys in participating countries.
Table S1. Characteristics of HIV status disclosure among adults on antiretroviral therapy who participated in the Population‐based HIV Impact Assessment (PHIA) survey in Lesotho, Malawi, Eswatini, Zambia and Zimbabwe (2015 to 2017)
Table S2. Prevalence of alcohol use disorder among adults on antiretroviral therapy who participated in the Population‐based HIV Impact Assessment (PHIA) survey in Malawi, Eswatini, Zambia, and Zimbabwe (2015 to 2017)
Table S3. Sensitivity analysis of rates of switching to second‐line ART among HIV‐positive adults who participated in the Population‐based HIV Impact Assessment (PHIA) survey in Lesotho, Malawi, Eswatini, Zambia, and Zimbabwe (2015 to 2017)


