Abstract
BACKGROUND:
Transient cognitive impairment is common in adult patients of all ages following anaesthesia and surgery. Apolipoprotein E (APOE) ε4 carriers may have a larger deterioration in short term cognitive function after major surgery compared to APOE ε4 non-carriers.
OBJECTIVES:
The aim was to examine the effect of APOE ε4 on the association between exposure to surgery and anaesthesia, and subsequent cognitive functioning. A more pronounced deterioration in cognitive function in APOE ε4 carriers was hypothesized.
DESIGN:
An observational cross-sectional and a 6-10 years longitudinal twin cohort design.
SETTING:
Survey and register study of 2,936 Danish twins aged 45-92 years.
MAIN OUTCOME MEASURES:
Cognitive function was assessed using five age-sensitive cognitive tests. In the cross-sectional study, we compared twins exposed to surgery with a reference group (unexposed). Linear regression models were used adjusting for sex and age and stratified by APOE ε4 carrier status. In the longitudinal cognitive follow-up study 1671 twins participated. Intra-pair analyses were also performed using 70 same-sexed twin pairs concordant for APOE ε4 carrier status, but discordant for major surgery.
RESULTS:
APOE ε4 carriers had lower cognitive scores compared with non-carriers, and this was statistically significant in elderly twins 70+ years of age (mean difference, −0.67; 95% CI, −1.14 to −0.17). There was no significant impact on cognitive function after surgery according to APOE ε4 carrier status in the cross-sectional study. Similarly, there was no APOE ε4 modification in the longitudinal study. Also, in the intra-pair analyses no evidence was found of lower cognitive score after major surgery compared with the non-exposed co-twins among APOE ε4 carriers.
CONCLUSIONS:
No evidence was found of more pronounced long term deterioration in cognitive function after surgery among APOE ε4 carriers, but elderly APOE ε4 carriers in general performed worse on the cognitive tests than non-carriers.
Introduction
Transient cognitive impairment is common in adult patients of all ages following surgery and anaesthesia, but older patients (≥60 years) are at a higher risk and are more likely to suffer prolonged effects 1. The aetiology of postoperative cognitive dysfunction (POCD) is not fully understood, but there is general agreement in the literature that POCD is a multifactorial condition in which both perioperative and patient-related factors are implicated; possible interplays between surgery and anaesthesia and genetic factors have also been suggested 2-5. The majority of patients with POCD improve over months to years, but a small fraction seems to be particularly susceptible as they suffer a more persistent cognitive impairment 6. Recently we found a negligibly lower cognitive functioning after major surgery in Danish twins that may lend support for this differential susceptibility hypothesis 7. Regarding long term longitudinal cognitive decline no significant differences were observed8. However, there is large variation in cognitive function and decline, and genetics partially influences both cognitive function and decline.
Although first established as a major determinant in lipoprotein metabolism and cardiovascular disease, Apolipoprotein E (APOE) has emerged as an important factor in several other biological processes including Alzheimer’s disease and cognitive function 9. Studies have shown that carrying the ε4 allele of APOE is associated with an increased risk of Alzheimer’s disease 10-12, cognitive decline in healthy ageing individuals 13, 14, and poor outcome after a number of conditions including stroke 15 and head trauma 16, 17. A possible association between the APOE ε4 allele and postoperative cognitive dysfunction (POCD) after cardiac and non-cardiac surgery has also been studied, but the literature is conflicting: A meta-analysis including both non-cardiac 2, 4, 5, 18 and cardiac surgical patients 19-23 reported a statistically significant association between the presence of APOE ε4 and POCD about one week postoperatively, suggesting that carriers of the ε4 allele may be at increased risk of POCD 24.
Of four recent studies, not included in the meta-analysis, one showed a higher incidence of POCD among APOE ε4 carriers compared with non-carriers three months postoperatively 25, whereas the three other studies found no association between APOE ε4 and POCD in a long-term perspective (two to eight years postoperatively) 26-28. However, one of the latter studies showed less improvement in cognitive function five years after cardiac surgery among APOE ε4 carriers 26, and another study including both cardiac and non-cardiac patients showed a steeper cognitive decline on the Mini-Mental State Examination (MMSE) among APOE ε4 carriers 28.
Of the original sample of 8,503 middle-aged and elderly twins included in our recent study, we sought to assess the importance of APOE genotype by including those with available genotypes. Our aim was to examine the effect by APOE ε4 on the association between exposure to surgery and anaesthesia, and subsequent cognitive functioning. We hypothesized that a more pronounced deterioration in cognitive function would occur after surgery in APOE ε4 carriers compared to non-carriers.
Materials and methods
Study population
The study sample consists of participants in two Danish population-based surveys, the Study of Middle-Aged Danish Twins (MADT) (n=4,314) and the Longitudinal Study of Aging Danish Twins (LSADT) (n=4,731), previously described in detail elsewhere 29, 30 (see table 1 for summery details of the combined MADT and LSADT sample). In brief, participants in the two studies were ascertained through the Danish Twin Registry, which comprises all twins born since 1870 and considered representative of the general population in Denmark 29, 31. The MADT was initiated in 1998 and represented a random sample of 120 intact twin pairs from each birth cohort from 1931 to 1952. The LSADT was initiated in 1995 and included Danish twins aged 75 years or older and residing in Denmark by January 1995. Additional cohorts were added in 1997, 1999 and 2001 and the minimum inclusion age was progressively reduced to 70 years: Twins born between 1920 and 1923 (i.e. at least age 73 years at the beginning of 1997) were added in 1997; twins born 1924 to 1928 (i.e. at least age 70 years at the beginning of 1999) were added in 1999, and twins born between 1929 and 1930 (i.e. at least age 70 years at the beginning of 2001) were added in 2001. The MADT and the LSADT are comparable with regard to the design and data collection instruments, and both studies include assessment of cognitive function and a collection of blood samples. A total of 8,527 individuals (4,309 from the MADT (99.9%) and 4,218 from the LSADT (89.2%)), completed the cognitive assessment. Twin zygosity was classified using questionnaire-based zygosity assessment32. Participants who had undergone neurosurgery (10 from the MADT and 14 from the LSADT) were excluded reducing the sample sizes to 4,299 and 4,204, respectively; and of these, a total of 2,936 (1,914 from the MADT and 1,022 from the LSADT) were genotyped for APOE. This sample subset is referred to as the total genetic sample and those remaining from the combined sample of LSADT and MADT are referred to as the sample without genetic information (see Table 1 for details). In the longitudinal analyses cognitive change was measured, individual-level change scores were calculated as the difference between the composite cognitive score at the first and the second assessment so that a negative change scores indicated cognitive decline. First assessments were for MADT participants in 1998, second assessment were for MADT participants performed in 2008-2011. For LSADT participants first assessment was in 1995-1999 and second assessment 6 years later in either of the following assessments in 2001, 2003 or 2005. The calculated cognitive decline is named the slope in cognitive score (Table 1). All methods were carried out in accordance with relevant guidelines and regulations. Written informed consents were obtained from all participants, and all surveys and use of survey information were approved by The Regional Committees on Health Research Ethics for Southern Denmark (VF 20040241).
Table 1.
Characteristics of participants from the Study of Middle-Aged and elderly Twins according to APOE ε4 genotyping status, in totals and by surgery exposure group
| Surgical procedure groups |
||||||
|---|---|---|---|---|---|---|
| Characteristics | Totals | Major surgery* | Minor surgery | Knee/hip replacement |
Other surgery | No surgery |
| n (%) | ||||||
| Total genetic sample | 2936(35) | 549(36) | 605(36) | 46(27) | 758(36) | 978(33) |
| APOE ε4 non-carriers | 2013(24) | 370(24) | 412(24) | 32(19) | 526(25) | 673(23) |
| APOE ε4 carriers | 923(11) | 179(12) | 193(11) | 14(8) | 232(11) | 305(10) |
| Sample without genetic information | 5567(65) | 997(64) | 1124(65) | 125(73) | 1334(64) | 1987(67) |
| Female, n (% of all) | ||||||
| APOE ε4 non-carriers | 1148(25) | 272(27) | 219(26) | 19(17) | 312(27) | 326(22) |
| APOE ε4 carriers | 495(11) | 135(13) | 92(11) | 10(9) | 125(11) | 133(9) |
| Sample without genetic information | 2917(64) | 604(60) | 526(63) | 83(74) | 707(62) | 997(68) |
| Age†, year | ||||||
| APOE ε4 non-carriers | 62.2(11.6) | 61.8(11.6) | 60.6(11) | 69.3(10.1) | 62(12.1) | 63.1(11.5) |
| APOE ε4 carriers | 61.9(11.4) | 62.7(11.3) | 59.5(10) | 77.5(3.5) | 61.1(11.8) | 63(11.6) |
| Sample without genetic information | 69.5(11) | 70.8(10.7) | 68(11.1) | 74.1(7.7) | 69.3(11.5) | 69.4(10.8) |
| Cognitive score± | ||||||
| APOE ε4 non-carriers | −1.8(3.6) | −1.7(3.4) | −1.6(3.7) | −3(3.5) | −1.7(3.7) | −2(3.4) |
| APOE ε4 carriers | −1.9(3.7) | −1.8(3.9) | −1.8(3.5) | −3.7(2.8) | −1.8(3.8) | −2(3.5) |
| Sample without genetic information | −3.2(3.9) | −3.7(4) | −2.9(4.1) | −3.4(3.9) | −3.2(4) | −3.1(3.8) |
| Slope in cognitive score± | ||||||
| APOE ε4 non-carriers | −0.78(2.7) | −0.63(2.8) | −0.78(2.7) | −0.77(2.4) | −0.64(2.6) | −0.81(2.7) |
| APOE ε4 carriers | −0.78(2.8) | −0.58(2.8) | −0.79(3.2) | −1.81(2.5) | 0.33(2.5) | −0.84(2.7) |
Including cardiac, thoracic, laparotomy, central and peripheral vascular and fracture surgeries.
Median (IQR).
Mean (SD)
Exposure assessment
Information on surgical exposure was obtained from the Danish National Patient Register by linkage between the intake examination data of the MADT and the LSADT and the Danish National Patient Register using a unique civil registration number. The Danish National Patient Register was established in 1977 and contains data on all hospital admissions including data on surgeries and diagnoses and from 1995, data on outpatients and emergency patients were also added 33.
The exposure group consisted of participants who had at least one surgical procedure in the period between 1977 and the date of intake cognitive examination, which is a period of up to 24 years. The exposure group was stratified by type of surgical procedure according to our previous defined classifications 7: (1) major surgeries, including cardiac, thoracic, laparotomy, central and peripheral vascular and major fracture surgeries, (2) knee and hip replacements, (3) minor surgeries, and (4) other surgeries. Minor surgeries included all outpatient procedures, surgeries followed by less than two days of hospitalization, and, independently of the first two criteria, surgery codes representing eye, skin, endoscopic procedures along with biopsies and other small surgical procedures. The classification was performed by two experienced anaesthesiologists (T.G.H. and L.S.R.), who, independently of one another, went through the records of surgical codes in the study sample for the period from 1977 and until intake examination. If surgical codes were inconsistent a joint agreement was made.
Participants who had more than one surgical procedure were assigned to the groups using the following algorithm: (1) major surgery if any, (2) knee and hip replacement if any, but no major surgery, and (3) other surgeries if any, but neither major nor knee and hip replacement surgery. The reference group in all analyses comprised participants with no surgical procedures from 1977 and until intake examination.
Outcome assessment
The outcome was cognitive function evaluated by a five-component test battery at study intake. The test battery consists of the following specific tasks selected to be sensitive to normative age changes and easily administered by lay interviewers: (1) a category fluency task, in which individuals were asked to name as many animals as they could during a one-minute interval, (2) forward and (3) backward digit span, and (4) immediate and (5) delayed recall of a 12-item list. Justified by correlations in the range of 0.33 to 0.46 34, an overall composite measure of cognitive functioning was computed by adding the five standardized scores (using mean and SD from participants aged 45 to 49 years) of each of the five-component tasks 35. The composite cognitive score has been used in a series of previous studies 34-37 and is shown to have a high internal consistency reliability (0.75) and to be moderately stable over a two-year interval (0.60) 37.
Apolipoprotein
In the MADT, the APOE-genotyping was performed on two randomly selected samples from the 1998 wave one with age restriction of a maximum of 55 years used in previous aging studies (n=736) and another sample from the remaining participants (n=1,185) otherwise they were selected at random 38. In the LSADT, the APOE-genotyping was performed on randomly selected samples from the 1997 (n=703) and 1999 (n=349) waves. The figures did not strictly match the equivalents in Table 1 due to missing cognitive scores (MADT: n=2, LSADT: n=28) and exclusion of participants with neurosurgery (MADT: n=5, LSADT: n=2).
For some of the LSADT-participants, blood was donated two (n=406) and four years (n=34) after measurement of intake cognitive abilities, making the inclusion of these participants in the study conditional upon survival until time of blood collection. To investigate for selection bias, we performed statistical analyses both including and discarding the 440 delayed blood donors. For statistical analyses exclusion of LSADT-participants (N=440, aged 70+) cognitive abilities did not change the results markedly from those obtained using the full sample thus the analyses were made using the entire sample.
Genotyping of the APOE variants rs429358 and rs7412 was carried out using either custom-made primers and probes (LSADT), or predesigned TaqMan® SNP Genotyping Assays (Applied Biosystems, Foster City, CA, USA) (MADT) 39.
Statistical Analysis
Descriptive statistics were expressed as means and standard deviations (SDs) for continuous variables and as numbers and percentages or interquartile ranges (IQR) for categories.
To explore whether APOE ε4 modifies the association between surgery and anaesthesia and cognitive functioning, we stratified the study sample into two groups: APOE ε4+, including APOE ε2ε4, ε3ε4 and ε4ε4, and APOE ε4− (reference group), including APOE ε2ε2, ε2ε3 and ε3ε3. Within the APOE ε4 strata, the composite cognitive scores were compared between twins exposed to at least one surgery (major, minor surgery, knee and hip replacement surgery, and other surgeries) before examination of cognitive functioning and twins not exposed, using multivariate linear regression models adjusting for sex and age at examination. The effect estimates were compared across the APOE ε4 strata, and if they appeared to be different, an interaction term between APOE and surgery was introduced in a linear regression model including surgery, cognitive score, sex and age, to test for statistical significance of the interaction. The within-pair dependency of twin individuals was considered by estimating the standard errors using the cluster option of STATA (release 13; STATA Corp., USA). For the longitudinal analyses the difference between follow up and baseline was calculated. Model assumptions were checked by residual plots and quantile-quantile plots of residuals.
Intra-pair analysis
To take preoperative cognition and confounding from shared environmental and genetic factors into account, we performed APOE ε4-stratified intra-pair analyses in twin pairs discordant for major surgery, i.e. one twin in a pair had a history of major surgery and the other had no surgery. Only same-sexed and APOE ε4-concordant pairs were included. Among the 2,936 participants with genetic information, a total of 70 such pairs (35 monozygotic and 35 dizygotic) were identified and included in the analyses. Stratifying on APOE ε4 status, 24 pairs were APOE ε4 carriers and 46 were APOE ε4 non-carriers.
In the intra-pair analyses, the proportion of pairs in which the co-twin who had been exposed to anaesthesia and major surgery did also have the lower cognitive score was calculated including 95% CI using the binominal distribution. In addition, we assessed the numerical differences in cognitive score within pairs using a Bland Altman plot and tested whether the mean difference in the cognitive score within pairs was equal to zero using a one-sample t-test.
Results
Characteristics of the middle-aged and elderly Danish twin sample are displayed in Table 1, in total and by surgical exposure groups, along with characteristics of the samples without genetic information. The cognitive score was higher for those who had genetic information but not when adjusted for age (mean difference, 0.14; 95% CI, −0.02; 0.30). The distribution of APOE ε4 carrier status was comparable across surgical exposure groups (Table 1), implying no potential confounding of APOE ε4 carrier status on the association between surgery and cognitive functioning. Also, no deviance in Hardy-Weinberg equilibrium was observed (supplementary table 1).
In the combined twin group APOE ε4 carriers had a slightly lower, but not significantly, cognitive score compared with non-carriers (mean difference, −0.13; 95% CI, −0.38; 0.12). APOE ε4 carriers had a significantly lower cognitive score compared with APOE ε4 non-carriers among elderly twins aged 70+ (mean difference, −0.66; 95% CI, −1.08 ; −0.24), which is a moderate effect of only approximately 1/5 of a standard deviation (SD=3.6), while among middle-aged twins no significant difference was observed (mean difference, 0.16; 95% CI, −0.16; 0.47).
In participants exposed to surgery there were no indication of lower cognitive score than the non-operated among APOE ε4 carriers or non-carriers. Moreover, no modifiable effects by APOE ε4 carrier were observed as indicated by the interaction (all p-values > 0.05) (Table 2), and when the 6 to 10 years longitudinal cognitive decline was investigated none of the reported cognitive slopes reached statistical significance (Table 3).
Table 2.
Mean differences in cross-sectional cognitive composite score between participants having major, minor, knee and hip replacement, or other surgeries and the group not exposed to surgery, by APOEε4 status
| Differences in cognitive score (95% CI)* |
|||||
|---|---|---|---|---|---|
| Surgical procedure groups | n | APOEε4 non-carriers | N | APOEε4 carriers | |
| No surgery | 673 | Reference | 305 | Reference | |
| Any surgery | 1340 | −0.04(−0.45 to 0.38) | 618 | 0.04(−0.60 to 0.69) | p=0.76 |
| Major† | 370 | −0.04(−0.45 to 0.38) | 179 | 0.04(−0.60 to 0.69) | p=1.00 |
| Minor | 412 | 0.01(−0.41 to 0.40) | 193 | −0.32(−0.88 to 0.24) | p=0.54 |
| Knee/hip replacement | 32 | −0.33(−1.47 to 0.81) | 14 | 0.56(−0.82 to 1.93) | p=0.67 |
| Other | 526 | 0.12(−0.36 to 0.39) | 232 | −0.07 (−0.64 to 0.50) | p=0.86 |
Multiple linear regression analyses, adjusted for sex and age at intake examination.
Including cardiac, thoracic, laparotomy, central and peripheral vascular and fracture surgeries. P-values are for interaction between APOE and surgery. APOE = apolipoprotein
Table 3.
Mean longitudinal 6-10 years differences (slope) in cognitive composite score between participants having major, minor, knee and hip replacement, or other surgeries and the group not exposed to surgery, by APOEε4 status
| Differences in cognitive score (95% CI)* |
|||||
|---|---|---|---|---|---|
| Surgical procedure groups | n | APOEε4 non-carriers | N | APOEε4 carriers | |
| No surgery | 736 | Reference | 316 | Reference | |
| Any surgery | 422 | 0.08 (−0.27 to 0.42) | 197 | 0.08 (−0.45 to 0.61) | p=0.78 |
| Major† | 78 | 0.22 (−0.44 to 0.89) | 34 | 0.11 (−0.90 to 1.11) | p=0.89 |
| Minor | 243 | 0.01 (−0.41 to 0.42) | 125 | −0.19 (−0.66 to 0.62) | p=0.99 |
| Knee/hip replacement | 27 | 0.89 (−0.85 to 1.02) | 12 | −1.21 (−2.64 to 0.22) | p=0.26 |
| Other | 74 | 0.14 (−0.49 to 0.78) | 26 | 1.08 (0.09 to 2.08) | p=0.08 |
Multiple linear regression analyses, adjusted for sex and age at intake examination.
Including cardiac, thoracic, laparotomy, central and peripheral vascular and fracture surgeries. P-values are for interaction between APOE and surgery APOE = apolipoprotein
The study uses twin cohorts, so we also performed an APOE ε4-stratified intra-pair twin analysis. Here one twin had a history of major surgery and the other had no surgery. In the APOE ε4 non-carrier group, the intra-pair analysis of all 46 surgery-discordant pairs revealed that the surgery exposed twin had a lower cognitive score than the co-twin in 35 % (95% CI: 20 % to 49 %) of the pairs, i.e. it was significantly more common to have the higher cognitive score when exposed to surgery than it was to have the lower cognitive score. When twin pairs were restricted according to the magnitude of the intra-pair differences in cognitive score to the top 75, 50, and 25%, respectively, the proportion of surgically exposed twins having the lower score was reduced even further. In the APOE ε4 carrier group, we found that the cognitive score was independent of surgery, as the twin exposed to surgery had the lower cognitive score in 50% of the pairs (Figure 1).
Figure 1.
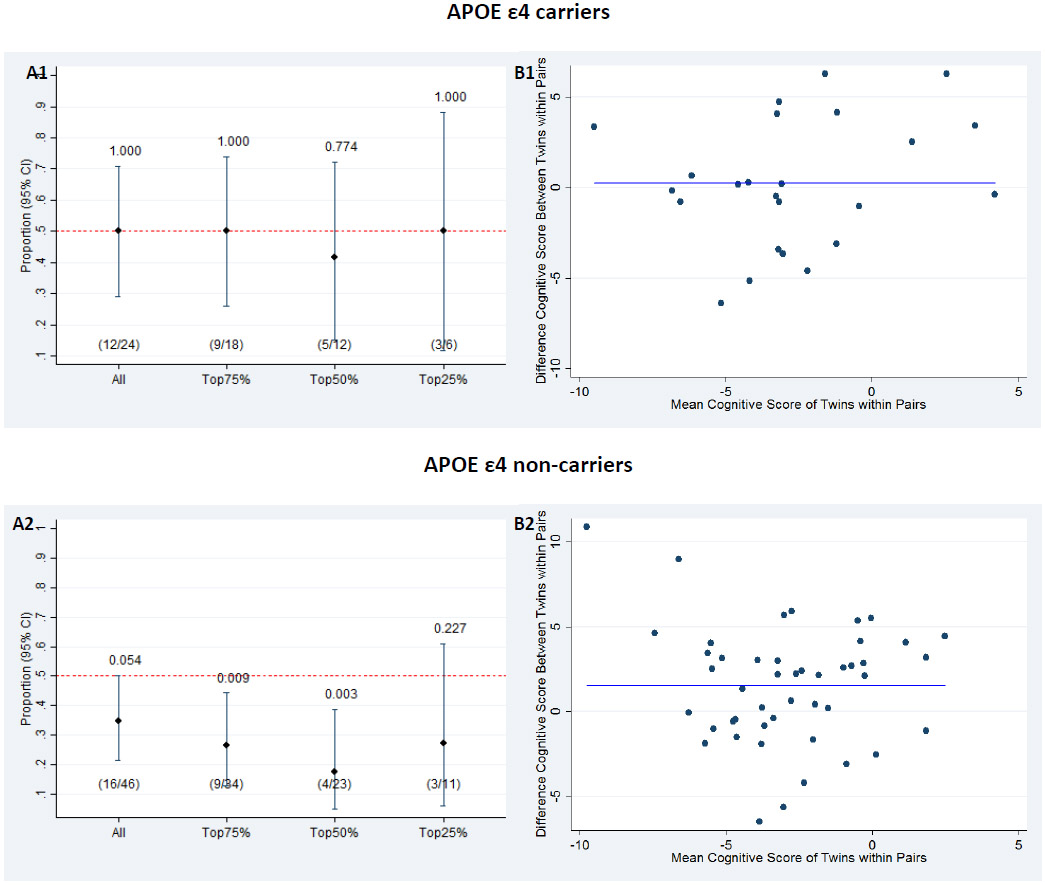
(A) Proportion of twin pairs in which the twin exposed to major surgery had a lower cognitive score for all twin pairs and stratified according to the magnitude of intra-pair difference in cognitive score, separately for APOE ε4 carriers (A1) and non-carriers (A2). Above each line is the p-value from the binomial test, which tests whether the proportion equals to 0.5. (B) Bland Altman plot of difference in cognitive score between the twins (twin exposed to major surgery – twin not exposed to major surgery), separately for APOE ε4 carriers (B1) and non-carriers (B2). The solid blue lines represent the mean difference in cognitive score between twins exposed to major surgery and twins without surgery (mean 0.10 (B1) and 0.42 (B2)).
Discussion
In this study, we examined effect modification by APOE ε4 carrier status on the association between exposure to surgery and anaesthesia, and cognitive functioning among 2,936 middle-aged and elderly Danish twins. Our findings did not support the hypothesis that being an APOE ε4 carrier would increase the negative impact on cognitive function after anaesthesia and surgery.
Furthermore, our recent finding of a negligibly lower cognitive score after major surgery than after no surgery using the larger sample 7 could not be replicated in this genotyped subsample. As expected, we found a significantly lower cognitive score among APOE ε4 carriers compared with non-carriers in the group aged 70 years and above.
The possibilities to directly compare our findings with the findings from other studies are restricted by the limited number of studies that include information about APOE status and postoperative cognitive function as well as by the fact that the studies differed in several aspects, i.e. by different designs, study population, length of follow up and cognitive measures. We identified three other studies investigating effect modification by APOE ε4 carrier status on the association between surgery and anaesthesia, and cognition functioning 27, 28, 40. Schenning et al. reported a modifying effect of APOE ε4 carrier status contributing to postsurgical cognitive decline measured by the MMSE test in a study with a mean follow up of seven years after surgery 28. However, we did not find any such effect, and neither did Patel et al.27 or Sprung et al. 40.
In addition, a recent meta-analysis 24 including two studies 2, 23 found no association between APOE ε4 and cognitive decline one year after surgery (OR 1.15, 95% CI 0.71 to 1.86), and neither did six additional studies on cognitive impairment following one to eight years after non-cardiac 26, 41, 42 and cardiac surgery 43, 44.
The study by Bartels and co-workers did not include a reference group, but merely a surgical group stratified on cardiac and non-cardiac surgery, showed less cognitive improvement five years following cardiac surgery among patients carrying the ε4 allele 26. This might reflect the finding of a general APOE ε4 adverse effect on cognition also present in the elderly participants in our study, although the Bartels study showed no association between APOE ε4 allele status and change in cognition in the non-cardiac surgery group.
Our study population was in Hardy Weinberg equilibrium, and allele frequencies and distributions of APOE genotypes were in line with a previous report on allele frequencies in another Danish study 45.
Major strengths of the present study were the representative nature of the study sample derived from population-based surveys retrieved from the Danish Twin Registry 29 and the nationwide coverage and completeness of the Danish National Patient Register 33, 46 that minimizes the risk of selection bias. Furthermore, the register-based information on exposure left no room for recall bias, which was stressed as a potential limitation in a recent study using self-reported exposure information 28. Comparing twin pairs is on the other hand a very powerful analysis that can detect moderate environmental effects using few pairs of twin 47 as was done in the present work for APOE ε4 carriers (npairs=24) (Figure 1). However, we could not confirm our hypothesis that surgery is associated with lower cognitive function among the APOE ε4 carriers. In contrast, the association between surgery and better cognitive function found among APOE ε4 non-carriers in the twin pair design is likely to be a chance finding (npairs=46). A cautious alternative explanation might be selection, e.g. both twins in a pair needed to be alive and especially the twins who went through surgery are survivors of a major disease. Over all the twin paired analyses suggests that there are no strong or moderate environmental effects from major surgery or general anesthesia on subsequent long term cognitive function.
The large sample size of more than 2,900 genotyped participants was an additional strength, which enabled us to stratify our analysis on age and surgery making our exposure groups more homogeneous.
A potential limitation of our study is that the cognitive composite score primarily includes the memory component, and differences in other or more specific cognitive domains may thus have been overlooked; hence, alternative measures of cognitive ability should be considered, subjective cognitive function or capacity for activities of daily living in future studies 48. It is noteworthy, though, that the cognitive composite score has shown to be very age-sensitive with a difference of 2.5 SD from age 45 to 90 years 35. In addition, our notable finding of a significant association between APOE ε4 and cognitive function among participants aged 70 or above, but not in the younger group, was supported by a recent meta-analysis in which the observed differences in several cognitive measures between APOE ε4 carriers and APOE ε4 non-carriers increase at older ages 49.
As preoperative cognitive trajectory is suggested as one of the most important determinants of postoperative cognitive trajectory 50, the absence of preoperative cognitive assessments in the current study is a potential limitation. We cannot entirely exclude that a true association between surgery and anaesthesia, and cognitive function as well as an effect modification by APOE ε4 may have been masked by not taking preoperative cognitive function into consideration. However, the use of a co-twin design including 70 pairs takes to some extent preoperative cognition into account as the intra-pair similarity of cognitive function is high 51. The co-twin design did also control for common environmental factors and genetics minimizing the risk of unmeasured confounding.
Even though we argued that APOE-genotyping was performed on randomly selected samples from two populations-based surveys minimizing the risk of selection bias, participants with genetic information were generally younger (partly explained by the age restriction in one of the samples) in comparison with those without genetic information (Table 1), and in the group aged 70 years and above, participants had slightly higher age-adjusted cognitive score than non-participants.
An effect of APOE ε4 on the risk of Alzheimer’s disease has been evident for decades 10 and is also associated with cognitive decline 52, 53. We found no significant evidence on the longitudinal cognitive decline for the APOE ε4 carriers than for non carriers which may be due to lack of sufficient power.
Conclusion
Our data revealed a general APOE ε4 adverse effect on cognition in the participants aged 70 and above, but this was independent of surgical exposure status. We found no evidence that APOE ε4 carrier status was associated with a more pronounced deterioration in cognitive function after surgery in middle-aged and elderly patients.
Supplementary Material
Acknowledgements
Assistance with the study: none.
Financial support and sponsorship: This work was not directly supported, but the Danish Twin Registry and the Danish Aging Research Center have received grants from the National Program for Research Infrastructure, the Danish Agency for Science Technology and Innovation, Copenhagen, Denmark, 2007 (grant no. 09-063256); the VELUX Foundation, Copenhagen, Denmark; and the U.S. National Institutes of Health, Bethesda, Maryland (grant no. P01 AG08761). Dr. Rasmussen has received funding from the Tryg Foundation, Virum, Denmark. The funding sources were involved neither in the design, analysis, and preparation of the article nor in the decision to submit the article for publication.
Footnotes
Conflict of interest: None.
Presentation: None declared.
References
- 1.Monk TG, Weldon BC, Garvan CW et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 2008; 108:18–30. [DOI] [PubMed] [Google Scholar]
- 2.McDonagh DL, Mathew JP, White WD et al. Cognitive Function after Major Noncardiac Surgery, Apolipoprotein E4 Genotype, and Biomarkers of Brain Injury. Anesthesiology 2010; 112:852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinmetz J, Jespersgaard C, Dalhoff K et al. Cytochrome P450 polymorphism and postoperative cognitive dysfunction. Minerva anestesiologica 2012; 78:303–309. [PubMed] [Google Scholar]
- 4.Cai Y, Hu H, Liu P et al. Association between the apolipoprotein E4 and postoperative cognitive dysfunction in elderly patients undergoing intravenous anesthesia and inhalation anesthesia. Anesthesiology 2012; 116:84–93. [DOI] [PubMed] [Google Scholar]
- 5.Abildstrom H, Christiansen M, Siersma VD, Rasmussen LS, Investigators I. Apolipoprotein E genotype and cognitive dysfunction after noncardiac surgery. Anesthesiology 2004; 101:855–861. [DOI] [PubMed] [Google Scholar]
- 6.Abildstrom H, Rasmussen LS, Rentowl P et al. Cognitive dysfunction 1-2 years after non-cardiac surgery in the elderly. ISPOCD group. International Study of Post-Operative Cognitive Dysfunction. Acta anaesthesiologica Scandinavica 2000; 44:1246–1251. [DOI] [PubMed] [Google Scholar]
- 7.Dokkedal U, Hansen TG, Rasmussen LS, Mengel-From J, Christensen K. Cognitive Functioning after Surgery in Middle-aged and Elderly Danish Twins. Anesthesiology 2016; 124:312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dokkedal U, Wod M, Thinggaard M et al. No impact of surgery on cognitive function: a longitudinal study of middle-aged Danish twins. Ann Epidemiol 2018; 28:95–101.e101. [DOI] [PubMed] [Google Scholar]
- 9.Mahley RW, Rall SC Jr. Apolipoprotein E: far more than a lipid transport protein. Annual review of genomics and human genetics 2000; 1:507–537. [DOI] [PubMed] [Google Scholar]
- 10.Corder EH, Saunders AM, Strittmatter WJ et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science (New York, NY) 1993; 261:921–923. [DOI] [PubMed] [Google Scholar]
- 11.Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 2013; 9:106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poirier J, Davignon J, Bouthillier D, Kogan S, Bertrand P, Gauthier S. Apolipoprotein E polymorphism and Alzheimer's disease. Lancet 1993; 342:697–699. [DOI] [PubMed] [Google Scholar]
- 13.Feskens EJ, Havekes LM, Kalmijn S, de Knijff P, Launer LJ, Kromhout D. Apolipoprotein e4 allele and cognitive decline in elderly men. BMJ 1994; 309:1202–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiepers OJ, Harris SE, Gow AJ et al. APOE E4 status predicts age-related cognitive decline in the ninth decade: longitudinal follow-up of the Lothian Birth Cohort 1921. Molecular psychiatry 2012; 17:315–324. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Gonzalez NA, Sudlow CL. Effects of apolipoprotein E genotype on outcome after ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage. Journal of neurology, neurosurgery, and psychiatry 2006; 77:1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teasdale GM, Nicoll JA, Murray G, Fiddes M. Association of apolipoprotein E polymorphism with outcome after head injury. Lancet 1997; 350:1069–1071. [DOI] [PubMed] [Google Scholar]
- 17.Laskowitz DT, Horsburgh K, Roses AD. Apolipoprotein E and the CNS response to injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 1998; 18:465–471. [DOI] [PubMed] [Google Scholar]
- 18.Rentowl P, Hanning CD. Odour identification as a marker for postoperative cognitive dysfunction: a pilot study. Anaesthesia 2004; 59:337–343. [DOI] [PubMed] [Google Scholar]
- 19.Askar FZ, Cetin HY, Kumral E et al. Apolipoprotein E epsilon4 allele and neurobehavioral status after on-pump coronary artery bypass grafting. Journal of cardiac surgery 2005; 20:501–505. [DOI] [PubMed] [Google Scholar]
- 20.Bryson GL, Wyand A, Wozny D, Rees L, Taljaard M, Nathan H. A prospective cohort study evaluating associations among delirium, postoperative cognitive dysfunction, and apolipoprotein E genotype following open aortic repair. Canadian journal of anaesthesia = Journal canadien d'anesthesie 2011; 58:246–255. [DOI] [PubMed] [Google Scholar]
- 21.Heyer EJ, Wilson DA, Sahlein DH et al. APOE-epsilon4 predisposes to cognitive dysfunction following uncomplicated carotid endarterectomy. Neurology 2005; 65:1759–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lelis RG, Krieger JE, Pereira AC et al. Apolipoprotein E4 genotype increases the risk of postoperative cognitive dysfunction in patients undergoing coronary artery bypass graft surgery. The Journal of cardiovascular surgery 2006; 47:451–456. [PubMed] [Google Scholar]
- 23.Stewart A, Katznelson R, Kraeva N et al. Genetic variation and cognitive dysfunction one year after cardiac surgery. Anaesthesia 2013; 68:571–575. [DOI] [PubMed] [Google Scholar]
- 24.Cao L, Wang K, Gu T, Du B, Song J. Association between APOE epsilon 4 allele and postoperative cognitive dysfunction: a meta-analysis. The International journal of neuroscience 2014; 124:478–485. [DOI] [PubMed] [Google Scholar]
- 25.Shoair OA, Grasso Ii MP, Lahaye LA, Daniel R, Biddle CJ, Slattum PW. Incidence and risk factors for postoperative cognitive dysfunction in older adults undergoing major noncardiac surgery: A prospective study. J Anaesthesiol Clin Pharmacol 2015; 31:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartels K, Li YJ, Li YW et al. Apolipoprotein epsilon 4 genotype is associated with less improvement in cognitive function five years after cardiac surgery: a retrospective cohort study. Canadian journal of anaesthesia = Journal canadien d'anesthesie 2015; 62:618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel D, Lunn AD, Smith AD, Lehmann DJ, Dorrington KL. Cognitive decline in the elderly after surgery and anaesthesia: results from the Oxford Project to Investigate Memory and Ageing (OPTIMA) cohort. Anaesthesia 2016; 71:1144–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schenning KJ, Murchison CF, Mattek NC, Silbert LC, Kaye JA, Quinn JF. Surgery is associated with ventricular enlargement as well as cognitive and functional decline. Alzheimer's & dementia : the journal of the Alzheimer's Association 2016; 12:590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skytthe A, Christiansen L, Kyvik KO et al. The Danish Twin Registry: linking surveys, national registers, and biological information. Twin research and human genetics : the official journal of the International Society for Twin Studies 2013; 16:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christensen K, Holm NV, McGue M, Corder L, Vaupel JW. A Danish population-based twin study on general health in the elderly. Journal of aging and health 1999; 11:49–64. [DOI] [PubMed] [Google Scholar]
- 31.Skytthe A, Kyvik K, Holm NV, Vaupel JW, Christensen K. The Danish Twin Registry: 127 birth cohorts of twins. Twin Res 2002; 5:352–357. [DOI] [PubMed] [Google Scholar]
- 32.Christiansen L, Frederiksen H, Schousboe K et al. Age- and sex-differences in the validity of questionnaire-based zygosity in twins. Twin Res 2003; 6:275–278. [DOI] [PubMed] [Google Scholar]
- 33.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scandinavian journal of public health 2011; 39:30–33. [DOI] [PubMed] [Google Scholar]
- 34.McGue M, Christensen K. The heritability of cognitive functioning in very old adults: evidence from Danish twins aged 75 years and older. Psychology and aging 2001; 16:272–280. [DOI] [PubMed] [Google Scholar]
- 35.Vestergaard S, Thinggaard M, Jeune B, Vaupel JW, McGue M, Christensen K. Physical and mental decline and yet rather happy? A study of Danes aged 45 and older. Aging & mental health 2015; 19:400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGue M, Christensen K. The heritability of level and rate-of-change in cognitive functioning in Danish twins aged 70 years and older. Experimental aging research 2002; 28:435–451. [DOI] [PubMed] [Google Scholar]
- 37.McGue M, Christensen K. Growing old but not growing apart: twin similarity in the latter half of the lifespan. Behav Genet 2013; 43:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soerensen M, Dato S, Tan Q et al. Evidence from case-control and longitudinal studies supports associations of genetic variation in APOE, CETP, and IL6 with human longevity. Age (Dordr) 2013; 35:487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersen I, Pedersen NL, Rantanen T et al. GxE Interaction Influences Trajectories of Hand Grip Strength. Behavior genetics 2016; 46:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sprung J, Roberts RO, Knopman DS et al. Association of Mild Cognitive Impairment With Exposure to General Anesthesia for Surgical and Nonsurgical Procedures: A Population-Based Study. Mayo Clinic proceedings 2016; 91:208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ancelin ML, de Roquefeuil G, Scali J et al. Long-term post-operative cognitive decline in the elderly: the effects of anesthesia type, apolipoprotein E genotype, and clinical antecedents. Journal of Alzheimer's disease : JAD 2010; 22 Suppl 3:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel N, Minhas JS, Chung EM. Risk Factors Associated with Cognitive Decline after Cardiac Surgery: A Systematic Review. Cardiovascular psychiatry and neurology 2015; 2015:370612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selnes OA, Grega MA, Bailey MM et al. Neurocognitive outcomes 3 years after coronary artery bypass graft surgery: a controlled study. The Annals of thoracic surgery 2007; 84:1885–1896. [DOI] [PubMed] [Google Scholar]
- 44.Silbert BS, Evered LA, Scott DA, Cowie TF. The apolipoprotein E epsilon4 allele is not associated with cognitive dysfunction in cardiac surgery. The Annals of thoracic surgery 2008; 86:841–847. [DOI] [PubMed] [Google Scholar]
- 45.Gerdes LU, Klausen IC, Sihm I, Faergeman O. Apolipoprotein E polymorphism in a Danish population compared to findings in 45 other study populations around the world. Genetic epidemiology 1992; 9:155–167. [DOI] [PubMed] [Google Scholar]
- 46.Andersen TF, Madsen M, Jorgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register. A valuable source of data for modern health sciences. Danish medical bulletin 1999; 46:263–268. [PubMed] [Google Scholar]
- 47.Mengel-From J, Ronne ME, Carlsen AL et al. Circulating, Cell-Free Micro-RNA Profiles Reflect Discordant Development of Dementia in Monozygotic Twins. J Alzheimers Dis 2018; 63:591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evered L, Silbert B, Knopman DS et al. Recommendations for the Nomenclature of Cognitive Change Associated with Anaesthesia and Surgery-2018. Anesthesiology 2018; 129:872–879. [DOI] [PubMed] [Google Scholar]
- 49.Wisdom NM, Callahan JL, Hawkins KA. The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiology of aging 2011; 32:63–74. [DOI] [PubMed] [Google Scholar]
- 50.Nadelson MR, Sanders RD, Avidan MS. Perioperative cognitive trajectory in adults. British journal of anaesthesia 2014; 112:440–451. [DOI] [PubMed] [Google Scholar]
- 51.Deary IJ, Johnson W, Houlihan LM. Genetic foundations of human intelligence. Human genetics 2009; 126:215–232. [DOI] [PubMed] [Google Scholar]
- 52.Liu F, Pardo LM, Schuur M et al. The apolipoprotein E gene and its age-specific effects on cognitive function. Neurobiology of aging 2010; 31:1831–1833. [DOI] [PubMed] [Google Scholar]
- 53.Small BJ, Rosnick CB, Fratiglioni L, Backman L. Apolipoprotein E and cognitive performance: a meta-analysis. Psychology and aging 2004; 19:592–600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


