Abstract
Ascorbic acid, a water-soluble antioxidant, regulates various biological processes and is thought to influence cholesterol. However, little is known about the mechanisms underpinning ascorbic acid-mediated cholesterol metabolism. Here, we determined if ascorbic acid can regulate expression of proprotein convertase subtilisin/kexin 9 (PCSK9), which binds low-density lipoprotein receptor (LDLR) leading to its intracellular degradation, to influence low-density lipoprotein (LDL) metabolism. At cellular levels, ascorbic acid inhibited PCSK9 expression in HepG2 and Huh7 cell lines. Consequently, LDLR expression and cellular LDL uptake were enhanced. Similar effects of ascorbic acid on PCSK9 and LDLR expression were observed in mouse primary hepatocytes. Mechanistically, ascorbic acid suppressed PCSK9 expression in a forkhead box O3-dependent manner. In addition, ascorbic acid increased LDLR transcription by regulating sterol regulatory element-binding protein 2. In vivo, administration of ascorbic acid reduced serum PCSK9 levels and enhanced liver LDLR expression in C57BL/6J mice. Reciprocally, lack of ascorbic acid supplementation in L-gulono-γ-lactone oxidase deficient (Gulo−/−) mice increased circulating PCSK9 and LDL levels, and decreased liver LDLR expression, whereas ascorbic acid supplementation decreased PCSK9 and increased LDLR expression, ameliorating LDL levels in Gulo−/− mice fed a high fat diet. Moreover, ascorbic acid levels were negatively correlated to PCSK9, total and LDL levels in human serum samples. Taken together, these findings suggest that ascorbic acid reduces PCSK9 expression, leading to increased LDLR expression and cellular LDL uptake. Thus, supplementation of ascorbic acid may ameliorate lipid profiles in ascorbic acid-deficient species.
Keywords: ascorbic acid, FoxO3a, LDL, LDLR, PCSK9, lipoprotein metabolism, lipoprotein receptor, low-density lipoprotein), proprotein convertase subtilisin/kexin type 9, ascorbic acid, forkhead box P3 (FOXP3).
The increased low-density lipoprotein cholesterol (LDL-cholesterol or LDL) levels in plasma is a pivotal risk factor for the initiation of cardiovascular disease, the leading cause of death in all countries (1). LDL receptor (LDLR) plays a critical role in anti-atherosclerosis by regulating LDL catabolism. The majority of excess LDL can be cleared by hepatic LDLR (2). LDLR binds LDL to mediate LDL endocytosis and degradation in lysosomes, and then recycles itself to the cell surface (3). Therefore, mutations of LDLR, either heterozygous or homozygous, result in familial hypercholesterolemia (2). It is effective to lower plasma LDL levels by enhancing hepatic LDLR expression. At the transcriptional level, LDLR expression is predominantly activated by sterol regulatory element-binding protein 2 (SREBP2), which binds to the sterol regulatory element (SRE) in the promoter of LDLR to trigger its transcription (4). In addition to SREBP2, proprotein convertase subtilisin/kexin 9 (PCSK9) regulates LDLR expression at the post-translational level (5). PCSK9 can bind LDLR to lead LDLR degradation intracellularly, resulting in suppressed LDL clearance from the circulation (6). Accordingly, reduction of PCSK9 decreases plasma LDL levels efficiently. PCSK9 is mainly synthesized and secreted from the liver, and the circulating PCSK9 has become an important target for treatment of cardiovascular diseases.
PCSK9 expression can be regulated by several transcription factors, such as hepatocyte nuclear 1α (HNF-1α), peroxisome proliferator-activated receptor γ (PPARγ), SREBP2 and forkhead box O3 (FoxO3a) (7–9). SREBP2 or HNF-1α binds to SRE or HNF1 motif to activate PCSK9 transcription (10). FoxO3a protein can interact with the insulin response element (IRE), which is overlapped by HNF1α binding motif in PCSK9 promoter, so FoxO3a blocks the binding of HNF-1α to the PCSK9 promoter, thereby resulting in inhibition of PCSK9 expression (9). Reciprocally, activated PCSK9 expression is observed in FoxO3a liver-specific deficient mice. Moreover, FoxO3a recruits Sirt6 to the proximal region of PCSK9 promoter, which also suppresses PCSK9 transcription (9).
Ascorbic acid (also known as vitamin C) is a water-soluble antioxidant that is naturally present in some kinds of food. Due to its capability to scavenge free radicals, the beneficial effects of ascorbic acid on lipid profiles have been demonstrated (11). It differs from mice, humans, and guinea pigs are prone to develop ascorbic acid deficiency as the consequence of mutation in l-gulono-γ-lactone oxidase (Gulo), the enzyme is required for the last step of ascorbic acid biosynthesis (12). Therefore, maintaining adequate levels of ascorbic acid supplement is essential to achieve normal body functions and optimal health (13). Most studies have found that chronic ascorbic acid deficiency in guinea pigs leads to increased cholesterol synthesis and attenuated cholesterol conversion into bile acids (14, 15). In addition, in Gulo and ApoE double-deficient (Gulo−/−Apoe−/−) mice, the total cholesterol (CHO) levels is decreased by increased ascorbic acid intake (16). These findings suggest that ascorbic acid supplement can influence LDL metabolism. However, the precise actions and the involved mechanisms by ascorbic acid remain unknown. In this study, we investigated if ascorbic acid can affect LDL levels and the action is completed by regulating PCSK9 and LDLR expression.
Results
Ascorbic acid inhibits PCSK9 expression in human hepatic cell lines
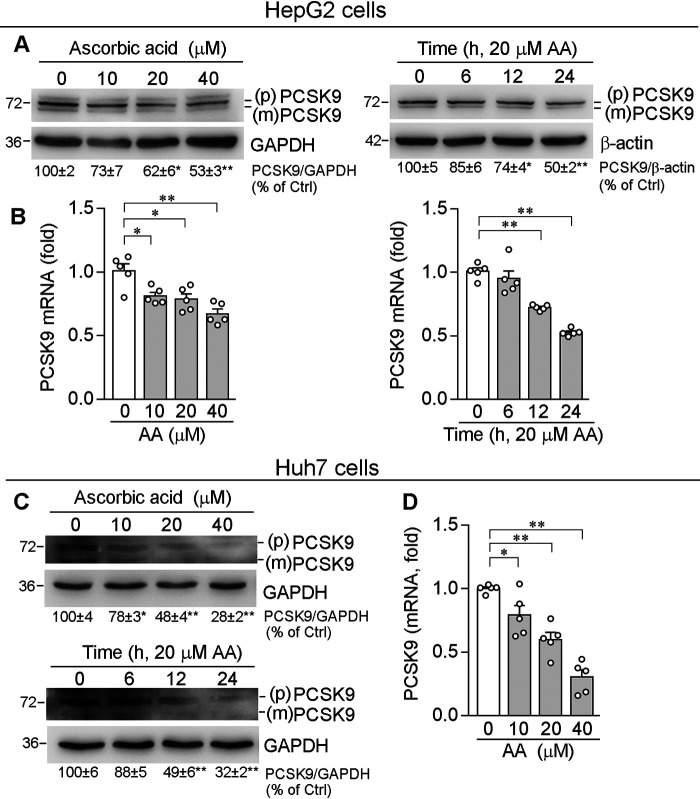
PCSK9 is mainly present in hepatocytes as a ∼72 kDa precursor and secreted from cells after autocleaved as a mature form with a molecular mass of ∼62 kDa (9, 17). We first assessed the effects of ascorbic acid on PCSK9 expression in human hepatic cell lines, HepG2 and Huh7 cells. As shown in Fig. 1A, ascorbic acid down-regulated both PCSK9 precursor and mature form in HepG2 cell in concentration- and time-dependent manners. To determine whether inhibition of PCSK9 protein expression by ascorbic acid is associated with reduced PCSK9 mRNA expression, total cellular RNA was extracted after treatment and used to determine PCSK9 mRNA levels by qRT-PCR. Consistently, ascorbic acid reduced PCSK9 mRNA expression in the similar patterns to protein (Fig. 1B), indicating reduction of PCSK9 expression by ascorbic acid can be at a transcriptional level. Similar to HepG2 cells, ascorbic acid also reduced PCSK9 protein and mRNA expression in Huh7 cells, another human hepatic cell line (Fig. 1, C and D).
Figure 1.
Ascorbic acid decreases PCSK9 expression in hepatic cell lines. Human hepatic cell lines, HepG2 and Huh7 cells, were treated with AA at the indicated concentrations for 12 h or with 20 μm AA for the indicated times. Expression of PCSK9 protein (A and C) [proprotein (p) and mature (m) form of PCSK9] and mRNA (B and D) were determined by Western blotting and qRT-PCR, respectively. *, p < 0.05; **, p < 0.01 versus control (n = 5).
Ascorbic acid enhances LDLR expression and LDL uptake
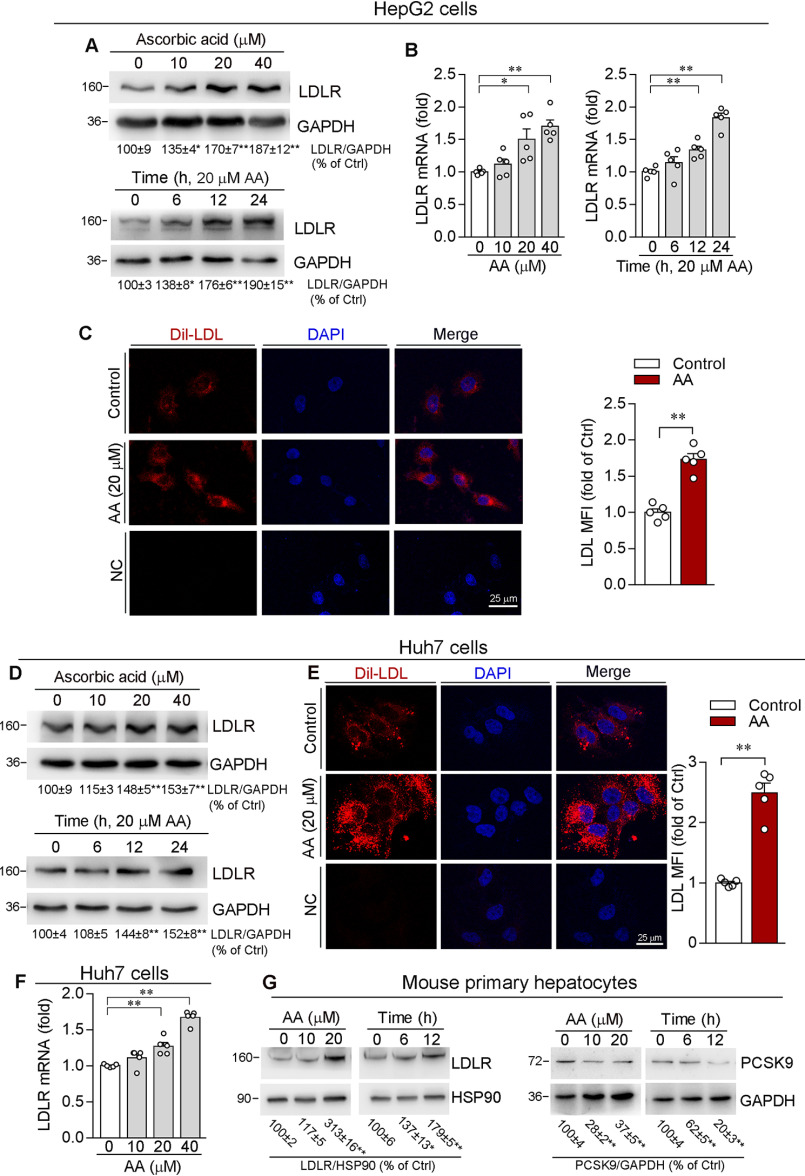
Inhibition of PCSK9 expression can result in increased LDLR protein levels (18). To detect whether ascorbic acid-reduced PCSK9 can increase LDLR protein expression, we evaluated LDLR protein levels by Western blotting, and found LDLR expression in HepG2 cells was increased by ascorbic acid in a concentration-dependent manner (Fig. 2A). In addition, ascorbic acid increased LDLR mRNA expression (Fig. 2B), indicating it may enhance LDLR levels at both transcriptional and translational levels. Meanwhile, we found a significant increase of Dil-LDL uptake by HepG2 cell in response to ascorbic acid treatment (Fig. 2C), suggesting the enhanced LDLR activity by ascorbic acid. In parallel, ascorbic acid induced LDLR protein and mRNA expression, as well as uptake of Dil-LDL by Huh7 cells (Fig. 2, D–F).
Figure 2.
Ascorbic acid enhances LDLR expression and LDL uptake by hepatic cell lines. HepG2 or Huh7 cells were treated with AA at the indicated concentrations for 12 h or with 20 μm AA for the indicated times. LDLR protein or mRNA expression was determined by Western blotting (A and D) or qRT-PCR (B and F). C and E, HepG2 or Huh7 cells were treated with 20 μm AA for 12 h. Cells were then switched to serum-free medium containing Dil-LDL (20 µg/ml) and incubated for 4 h at 37 °C, followed by photograph with a fluorescence microscope. The MFI in images was quantitatively analyzed (original magnification, ×400). G, primary hepatocytes isolated from C57BL/6J mice were treated with AA at the indicated concentrations for 12 h or with 20 μm AA for the indicated times. Expression of PCSK9 or LDLR protein was determined by Western blotting. *, p < 0.05; **, p < 0.01 versus control (n = 5).
Furthermore, we treated primary hepatocytes isolated from C57BL/6J mice with ascorbic acid. Similarly, ascorbic acid increased LDLR although decreased PCSK9 expression in murine primary hepatocytes (Fig. 2G), which indicates that regulation of PCSK9/LDLR expression by ascorbic acid is not species-dependent.
Ascorbic acid attenuates PCSK9 expression by activating FoxO3a
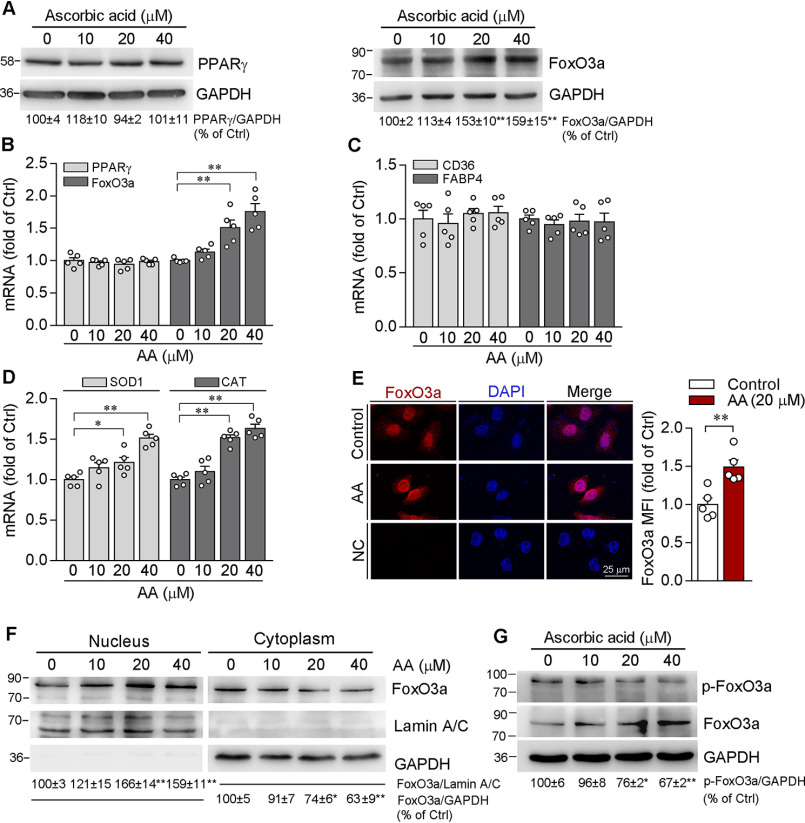
To identify the molecular mechanism by which ascorbic acid inhibits PCSK9 expression in hepatocytes, we detected effects of ascorbic acid on transcription factors responsible for PCSK9 expression, such as PPARγ and FoxO3a. Although it has been reported that ascorbic acid deficiency up-regulates PPARγ in the liver of senescence marker protein 30-deficient mice (19), we observed that both PPARγ protein and mRNA levels were not altered by ascorbic acid (Fig. 3, A and B). In contrast, ascorbic acid clearly increased FoxO3a protein and mRNA expression (Fig. 3, A and B). We further examined the transcriptional activity of PPARγ and FoxO3a. Consistently, mRNA levels of CD36 and fatty acid-binding protein 4 (FABP4), the downstream genes of PPARγ (20), were not affected by ascorbic acid either (Fig. 3C). In contrast, mRNA levels of superoxide dismutase 1 (SOD1) and catalase (CAT), the downstream genes of FoxO3a (21), were obviously enhanced by ascorbic acid (Fig. 3D). Furthermore, increased FoxO3a nuclear translocation was determined in cells treated with ascorbic acid (Fig. 3, E and F). To investigate if ascorbic acid can decrease FoxO3a phosphorylation to enhance its nuclear translocation because the phosphorylated FoxO3a retains in the cytoplasm (22), we determined total and phosphorylated FoxO3a levels, and found ascorbic acid increased total FoxO3a but decreased phosphorylated FoxO3a levels (Fig. 3G).
Figure 3.
Induction of FoxO3a expression and nuclear translocation by ascorbic acid. A and B, HepG2 cells were treated with AA at the indicated concentrations for 12 h. Expression of PPARγ and FoxO3a protein (A) and mRNA (B) were determined by Western blotting and qRT-PCR, respectively. C and D, expression of CD36, FABP4, SOD1, and CAT mRNA was examined by qRT-PCR. E, HepG2 cells were treated with 20 µm AA for 12 h. FoxO3a expression was determined by immunofluorescent staining followed by quantifying MFI in images (original magnification, ×400). F, HepG2 cells were treated with AA at the indicated concentrations for 12 h, followed by extraction of nuclear and cytosolic proteins and determination of FoxO3a expression by Western blotting. G, HepG2 cells were treated with AA at the indicated concentrations for 12 h, and expression of FoxO3a and phosphorylated FoxO3a (p-FoxO3a) was determined by Western blotting. *, p < 0.05; **, p < 0.01 versus control (n = 5).
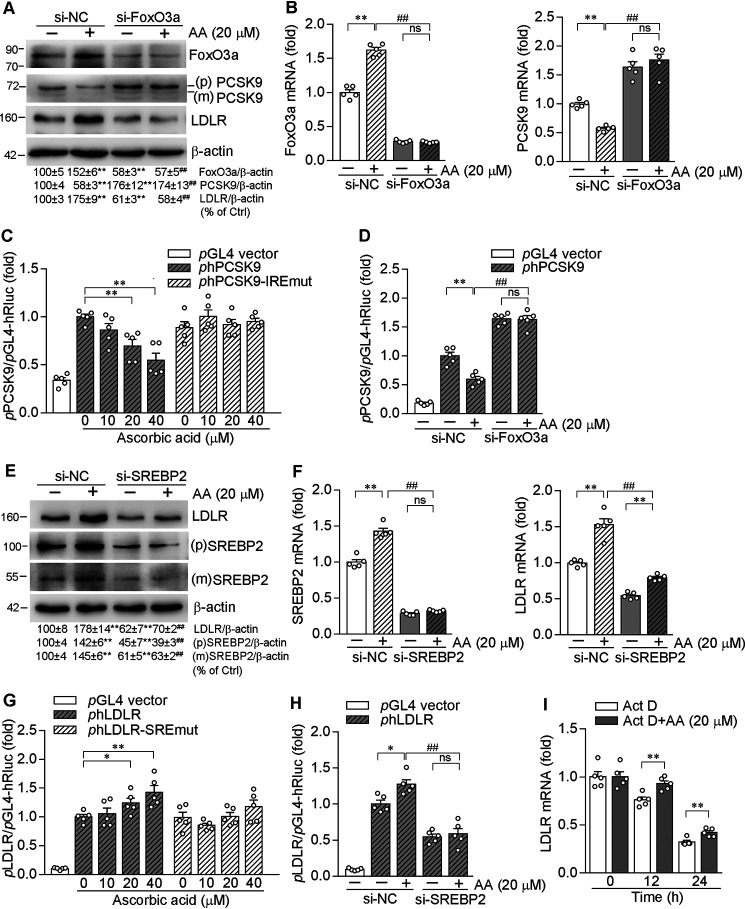
To further determine the role of FoxO3a in ascorbic acid-inhibited PCSK9 expression, we used FoxO3a siRNA to inhibit FoxO3a protein and mRNA expression (Fig. 4, A and B). As expected, inhibition of FoxO3a expression not only increased PCSK9 expression, but also abolished the effect of ascorbic acid on PCSK9 expression (Fig. 4, A and B). Moreover, FoxO3a siRNA attenuated ascorbic acid-induced LDLR expression (Fig. 4A), which should be due to the failure of PCSK9 inhibition in the presence of FoxO3a siRNA.
Figure 4.
Ascorbic acid inhibits PCSK9 transcription in a Foxo3a-dependent manner and enhances LDLR transcription by activating SREBP2. HepG2 cells were transfected with control siRNA (si-NC, 25 nm) or siRNA against FoxO3a (si-FoxO3a, 25 nm) for 48 h, followed by treatment with AA (20 µm) or vehicle in fresh medium for 12 h. Expression of FoxO3a, PCSK9, and LDLR protein (A), and FoxO3a and PCSK9 mRNA (B) were determined by Western blotting and qRT-PCR, respectively. C and G, 293T cells were transfected with DNA for normal human PCSK9 promoter (phPCSK9), PCSK9 promoter with IRE mutation (phPCSK9-IREmut); LDLR promoter (phLDLR) or LDLR promoter with SRE mutation (phLDLR-SREmut) plus Renilla luciferase (for internal normalization), respectively. After transfection and indicated treatment overnight, activity of firefly and Renilla luciferases in cellular lysate were determined using the Dual-Luciferase Reporter Assay system. D and H, 293T cells were transfected with control siRNA (si-NC, 25 nm) or siRNA against FoxO3a (si-FoxO3a, 25 nm) or siRNA against SREBP2 (si-SREBP2, 25 nm) for 48 h, respectively. Cells were then transfected with DNA for normal human PCSK9 promoter (phPCSK9, D) or for normal human LDLR promoter (phLDLR, H). After transfection and indicated treatment overnight, activity of firefly and Renilla luciferases in cellular lysate were determined using the Dual-Luciferase Reporter Assay system. E and F, HepG2 cells were transfected with control siRNA (si-NC, 25 nm) or siRNA against SREBP2 (si-SREBP2, 25 nm) for 48 h, followed by treatment with AA (20 µm) or vehicle in fresh medium for 12 h. Protein levels of LDLR, SREBP2 precursor (p), and mature form (m) were determined by Western blotting (E). The normalized intensity of mature SREBP2 ((m) SREBP2)) versus β-actin was calculated. Expression of LDLR and SREBP2 mRNA was examined by qRT-PCR (F). I, HepG2 cells were pre-treated with actinomycin D (Act D, 10 nm) for 2 h and then treated with AA (20 μm) or vehicle for the indicated times. Expression of LDLR mRNA was determined by qRT-PCR. *, p <0.05; **, p < 0.01 versus corresponding controls or as indicated (n = 5); ##, p < 0.01 versus si-NC plus AA (n = 5).
It has been reported that PCSK9 promoter contains a FoxO3a binding motif, the IRE, with a conserved sequence of TGTTTA (from −381 to −376) (9). To clarify the role of FoxO3a in regulation of PCSK9 transcription by ascorbic acid, we constructed a normal human PCSK9 promoter and the promoter with IRE mutation (-381CGTCTT-376, the underlined letters are mutated nucleotides). We found that ascorbic acid reduced PCSK9 promoter activity in a concentration-dependent manner (Fig. 4C). However, mutation in IRE attenuated the inhibitory effect of ascorbic acid on PCSK9 transcription. In addition, FoxO3a siRNA abolished ascorbic acid-inhibited PCSK9 promoter activity (Fig. 4D). These results collectively suggest that FoxO3a plays a central role in suppressing PCSK9 transcription by ascorbic acid.
SREBP2 signaling also correlates to activation of LDLR expression by ascorbic acid
Although inhibition of PCSK9 resulted in increased LDLR protein levels, we also observed induction of LDLR mRNA by ascorbic acid at high concentrations (Fig. 2, B and F). Thus, in addition to the regulation by PCSK9, ascorbic acid may activate LDLR expression by other pathways. SREBP2 is the transcription factor activating LDLR expression at the transcriptional level. We initially determined the effect of ascorbic acid on SREBP2 expression and maturation, and observed that ascorbic acid enhanced SREBP2 expression and maturation (Fig. 4E).
To clarify the role of SREBP2 in regulating LDLR transcription by ascorbic acid, we used SREBP2 siRNA to inhibit SREBP2 expression. As expected, inhibition of SREBP2 expression abolished the enhancement of LDLR protein and mRNA expression by ascorbic acid (Fig. 4, E and F). Correspondingly, we found ascorbic acid enhanced LDLR promoter activity at a high concentration (Fig. 4G, left). However, the activation was abolished when SRE in LDLR promoter was mutated (Fig. 4G, right). Moreover, SREBP2 siRNA attenuated ascorbic acid-enhanced LDLR promoter activity (Fig. 4H).
In addition, we determined the effect of ascorbic acid on stability of LDLR mRNA. Treatment of HepG2 cells with actinomycin D inhibited RNA transcription, therefore, reduced LDLR mRNA levels were determined with time of actinomycin D treatment due to the mRNA degradation. In contrast, compared with actinomycin D alone, co-treatment with ascorbic acid increased LDLR mRNA levels (Fig. 4I), suggesting ascorbic acid reduces LDLR mRNA degradation or increases its stability. Therefore, the data above suggest that in addition to activation by reduced PCSK9, ascorbic acid also activates LDLR expression by enhancing its stability and SREBP2 transcriptional activity.
Ascorbic acid stimulates LDLR expression and restrains PCSK9 secretion from mouse liver
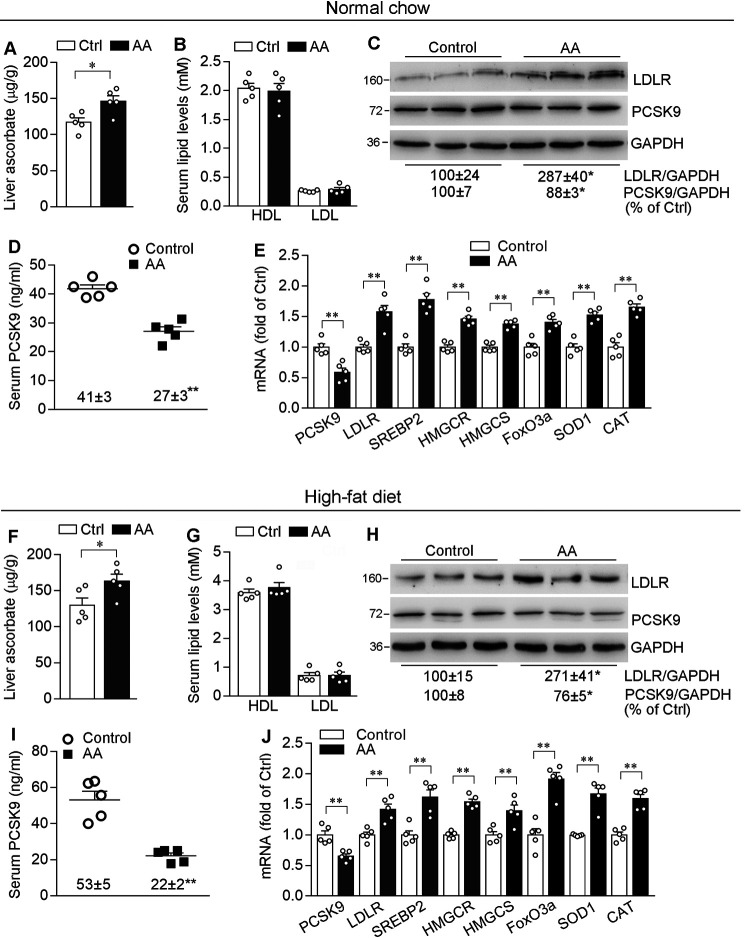
To determine the physiological relevance of ascorbic acid on PCSK9/LDLR expression, C57BL/6J mice fed either normal chow or a high-fat diet (HFD, containing 21% fat and 0.5% cholesterol) were i.p. injected ascorbic acid solution or vehicle (saline) daily for 1 week. Although WT mice do not depend on exogenous ascorbic acid supplement, as shown in Fig. 5, A and F, injection with ascorbic acid for 1 week still moderately increased ascorbic acid levels in the liver. Compared with control mice, ascorbic acid treatment did not cause differences to the animals, such as the ratio of liver weight to body weight and lipid profiles (Fig. 5, B and G).
Figure 5.
Ascorbic acid activates LDLR expression in the liver and reduces PCSK9 levels in serum of C57BL/6J mice. A–J, ∼8-week–old male C57BL/6J mice in 4 groups (5 mice/group) were fed normal chow or a HFD (0.5% cholesterol plus 21% fat), and i.p. injected ascorbic acid (100 mg/day/kg bodyweight, AA group) or saline (Control group) daily for 1 week. At the end of treatment, mouse liver and blood samples were collected and used for following assays. A and F, determination of ascorbic acid content in the liver; B and G, determination of HDL-cholesterol (HDL) and LDL-cholesterol (LDL) levels in serum. C and H, expression of LDLR and PCSK9 protein in the liver was determined by Western blotting. D and I, serum PCSK9 levels were determined by ELISA. E and J, expression of PCSK9, LDLR, SREBP2, HMGCR, HMGCS, FoxO3a, SOD1, and CAT mRNA was determined by qRT-PCR. *, p < 0.05; **, p < 0.01 versus corresponding control (n = 5).
Similar to results in in vitro studies, PCSK9 levels in the liver of mice fed normal chow were decreased by ascorbic acid (Fig. 5, C and E). PCSK9 is mainly synthesized in the liver and is rapidly secreted into plasma after its maturation through a self-engaged autocatalytic cleavage (17). Thus, we evaluated the secretion of PCSK9 using an ELISA kit, and found ascorbic acid also significantly reduced circulating PCSK9 levels compared with control mice (27 ± 3 versus 41 ± 3 ng/ml, Fig. 5D). Consistently, ascorbic acid inhibited PCSK9 expression and secretion in HFD-fed mice (Fig. 5, H and I). Consequently, ascorbic acid treatment significantly enhanced LDLR expression in the liver (Fig. 5, C, E, H, and J). In addition to LDLR, associated with changes of FoxO3a and SREBP2 expression by ascorbic acid, expression of the target genes of FoxO3a (SOD1 and CAT) and SREBP2 (HMG-CoA reductase (HMGCR), HMG-CoA synthase (HMGCS)) was also activated (Fig. 5, E and J).
Negative correlation between ascorbic acid and PCSK9 or LDL levels in Gulo−/− mouse and human serum
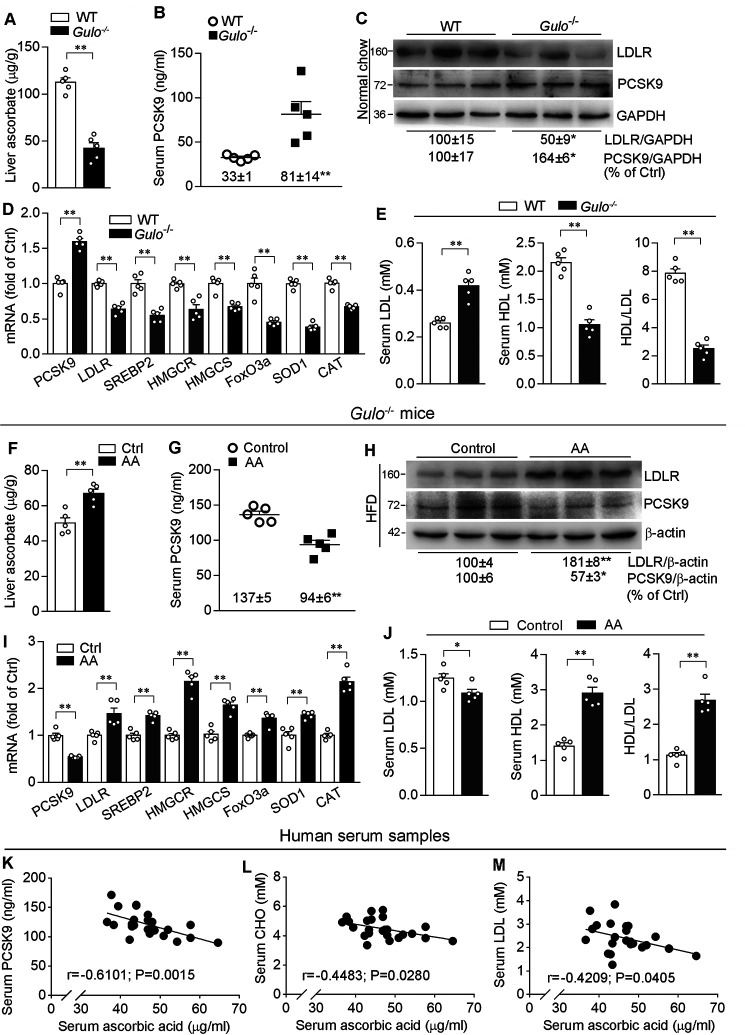
Naturally, C57BL/6J mice are able to synthesize ascorbic acid. They are not a hyperlipidemic model even though they are fed HFD. Therefore, technically it is not easy to manipulate ascorbic acid levels in vivo to influence LDL levels in mice naturally. To further clarify the effect of ascorbic acid on cholesterol metabolism in vivo, we investigated the effect of ascorbic acid deficiency on regulation of the LDL metabolism in Gulo−/− mice, the animals are fully dependent on exogenous ascorbic acid supplement.
After removal of ascorbic acid from the drinking water for 3 weeks, compared with WT mice, Gulo deficiency reduced ascorbic acid more than 60% in the liver (Fig. 6A). Associated with reduced ascorbic acid, serum PCSK9 levels and hepatic PCSK9 expression were increased (Fig. 6, B-D), which resulted in reduced hepatic LDLR expression in Gulo−/− mice (Fig. 6, C and D). Correspondingly, serum LDL levels were significantly increased, whereas HDL levels were decreased, thereby resulting in decreased ratio of HDL to LDL (Fig. 6E).
Figure 6.
Ascorbic acid ameliorates lipid profiles in Gulo−/− mice associated with regulation of PCSK9 and LDLR expressions. A–E, the ∼8-week–old male C57BL/6J (WT) or Gulo−/− (C57BL/6J background) mice (5 mice/group) were fed normal chow without ascorbic acid supplementation for 3 weeks. F–J, male Gulo−/− mice (∼8-week–old) fed HFD were randomly divided into two groups (5 mice/group), and i.p. injected with ascorbic acid (100 mg/day/kg bodyweight, AA group) or the same volume of saline (Control group) for 2 weeks. After treatment, mouse liver and blood samples were collected and used for the following assays. A and F, determination of ascorbic acid content in the liver. B and G, determination of serum PCSK9 levels. C and H, expression of LDLR and PCSK9 protein was measured by Western blotting. D and I, expression of PCSK9, LDLR, SREBP2, HMGCR, HMGCS, FoxO3a, SOD1, and CAT mRNA was determined by qRT-PCR. E and J, determination of serum LDL, HDL levels and calculation of the ratio of HDL to LDL. *, p < 0.05; **, p < 0.01 versus corresponding control (n = 5); K–M, PCSK9, CHO, LDL, and ascorbic acid levels in human serum samples (n = 24) were determined. K, serum PCSK9 levels are plotted against ascorbic acid levels in serum; Serum ascorbic acid levels are also plotted against CHO (L) and LDL (M) levels, respectively.
In contrast to ascorbic acid deficiency, injection of HFD-fed Gulo−/− mice with ascorbic acid solution for 2 weeks substantially increased ascorbic acid levels in the liver (Fig. 6F). Associated with increased hepatic ascorbic acid levels, serum PCSK9 levels and hepatic PCSK9 expression were reduced (Fig. 6, G-I). Meanwhile, hepatic LDLR expression was activated (Fig. 6, H and I). Consequently, serum LDL levels were reduced, whereas HDL levels and the ratio of HDL to LDL were increased by exogenous ascorbic acid supplement in Gulo−/− mice (Fig. 6J). In addition, we observed reduced SREBP2 and FoxO3a activity (decreased HMGCR, HMGCS, SOD1, and CAT, Fig. 6D) by ascorbic acid deficiency, and activated both (activated HMGCR, HMGCS, SOD1 and CAT, Fig. 6I) by ascorbic acid supplement in Gulo−/− mice fed HFD. Taken together, these results suggest the importance of hepatic ascorbic acid in maintaining cholesterol homeostasis.
The correlation between PCSK9 and circulating cholesterol levels has been well established (11, 23, 24). Our results above suggest that ascorbic acid deficiency increased PCSK9 expression, which can make a substantial contribution to increased LDL levels in Gulo−/− mice. Similar to guinea pigs or Gulo−/− mice, human beings are not able to produce ascorbic acid either, and our life solely depends on the exogenous ascorbic acid supplement. Therefore, we speculated that ascorbic acid levels in humans could be one of determinants for circulating PCSK9, LDL and total CHO levels. To determine it, we collected serum samples form 24 volunteers and determined levels of CHO, LDL, PCSK9, and ascorbic acid. Consistent with the previous study, serum CHO and LDL levels were positively correlated to PCSK9 levels (data not shown). Interestingly, we observed that PCSK9 levels were negatively correlated to ascorbic acid levels in human serum (Fig. 6K). Furthermore, serum ascorbic acid levels were negatively correlated to serum CHO and LDL levels (Fig. 6, L and M), suggesting the role of ascorbic acid in regulating PCSK9 expression and cholesterol metabolism in humans.
Discussion
As a water-soluble antioxidant, ascorbic acid has multiple biological functions. It is essential for collagen synthesis and enzyme activity maintenance (13, 25). Besides scurvy, many other pathogenesis of ascorbic acid deficiency have been observed. Lack of ascorbic acid production in mice exacerbates diabetic kidney injury, suggesting that compensation for ascorbic acid loss is an effective treatment for diabetic kidney injury (26). The ascorbic acid deficiency also enhances tumor necrosis factor α-induced insulin resistance (27).
In vascular biology, ascorbic acid deficiency can reduce collagen content of atherosclerotic plaques, resulting in formation of the vulnerable plaques (16). Physiological concentrations of ascorbic acid inhibits LDL oxidation in vitro (28). In addition, low levels of ascorbic acid cause dyslipidemia in guinea pigs, especially when fed a high-cholesterol or -fat diet (14). Many studies suggest that advisable intake of ascorbic acid has a positive effect on lipid profiles. Ascorbic acid deficiency is a prevalent complication in hemodialysis patients due to its loss during dialysis sessions (29). Thus, supplementing ascorbic acid to hemodialysis patients for 3 months can significantly increase serum ascorbic acid levels, associated with increased HDL and reduced CHO, LDL, and triglyceride levels (29). Supplementing more than 500 mg/day of ascorbic acid for a minimum 28 days can result in a significant decrease in serum LDL or triglyceride levels in patients with hypercholesterolemia (30). Ascorbic acid supplement also decreases triglyceride and LDL levels in type 2 diabetic patients (31). In addition, C57BL/6J mice that received a HFD supplement with ascorbic acid (1% w/w) for 15 weeks had reduced serum LDL levels by 50%, suggesting that ascorbic acid may suppress HFD-induced visceral obesity and nonalcoholic fatty liver disease (32). In HFD-fed Gulo−/−Apoe−/− mice, supplement of ascorbic acid substantially increased serum ascorbic acid levels, which is associated with obviously reduced plasma CHO levels (16). In our study, we found that ascorbic acid withdrawal resulted in substantial reduction of ascorbic acid in Gulo−/− mice (62.2%, Fig. 6A). Associated with decreased ascorbic acid levels, increased LDL and decreased HDL levels were determined in Gulo−/− mice compared with C57BL/6J mice (Fig. 6E). However, exogenous ascorbic acid supplement failed to reduce LDL levels in C57BL/6J mice (Fig. 5, B and G), although LDLR expression was activated in the liver (Fig. 5, C and H). This may be due to that C57BL/6J mice are naturally able to produce ascorbic acid, which results in that the animals have a constant high ascorbic acid level, whereas the short-term exogenous supplement of ascorbic acid to C57BL/6J mice is hard to manipulate ascorbic acid levels substantially (27.8%, Fig. 5A). In addition, C57BL/6J mice are not a hypercholesterolemia model because increased CHO levels by HFD feeding is mainly contributed by increased HDL but not LDL levels (Fig. 5, B and G). In contrast, supplement of ascorbic acid to deficient mice or patients can clearly ameliorate lipid profiles (16, 29–32). The amelioration of lipid profiles by ascorbic acid supplement should be attributed to inhibition of PCSK9 expression and activation of LDLR expression in the liver (Fig. 6, F-J).
Despite the clear beneficial effects of ascorbic acid on lipid metabolism, the underlying mechanisms have not been fully elucidated. PCSK9 plays a critical role in cholesterol homeostasis. Lowering PCSK9 levels results in an increase of intracellular LDLR protein abundance and contributes to metabolism/reduction of LDL (33). Amelioration of lipid metabolism by ascorbic acid implies that the action may be completed, at least in part, by inhibiting PCSK9 expression. Indeed, in this study we found that PCSK9 levels were negatively correlated to ascorbic acid levels, which was associated with the positive correlation to CHO and LDL levels in both human and Gulo−/− mouse serum (Fig. 6). At the molecular level, we determined ascorbic acid decreased PCSK9 expression and increased LDLR expression in HepG2 or Huh7 cells in vitro (Figs. 1 and 2) and in the liver (Figs. 5 and 6). SRE is present in the PCSK9 promoter and activation of SREBP2 increases PCSK9 gene expression (34). Although ascorbic acid activated SREBP2, it significantly attenuated PCSK9 protein and mRNA levels (Fig. 1), indicating that inhibition of PCSK9 expression by ascorbic acid is independent of SREBP2 pathway. Recently, FoxO3a has been identified as an important regulator of lipid metabolism and liver functions (35, 36). FoxO3a suppresses PCSK9 transcription by blocking the binding of HNF-1α with the promoter (9). In this study, we determined that ascorbic acid activated FoxO3a expression/transcriptional activity, whereas had little effect on either PPARγ expression and its transcriptional activity (Fig. 3), suggesting FoxO3a is the major player in ascorbic acid-inhibited PCSK9 (Fig. 4, A-D).
The post-translational regulation of LDLR expression is primarily controlled by PCSK9. Interestingly, in addition to protein levels, we also found increased LDLR mRNA levels by ascorbic acid (Fig. 2, B and F), suggesting an additional mechanism involved in ascorbic acid-activated LDLR expression. Indeed, we observed that ascorbic acid enhanced SREBP2 transcriptional activity. It also increased LDLR mRNA stability (Fig. 4, E-I). Therefore, ascorbic acid activates LDLR expression by regulating multiple pathways.
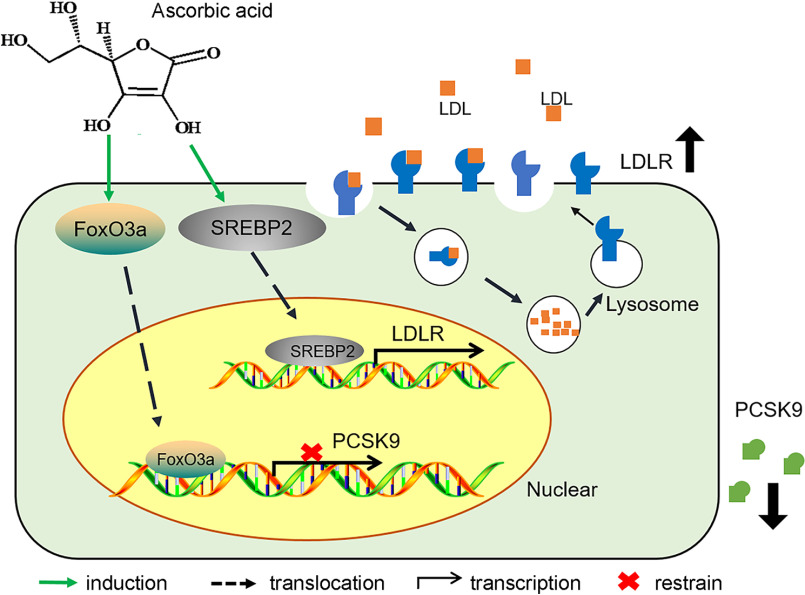
In conclusion, we found that ascorbic acid reduced PCSK9 expression/secretion and enhanced LDLR expression as well as the underlying mechanisms (Fig. 7). Importantly, we validated that human serum PCSK9 and LDL levels were negatively correlated to ascorbic acid levels. Our study provides the molecular mechanisms by which supplement of ascorbic acid can be helpful in improving lipid profiles in species who are not able to produce ascorbic acid endogenously.
Figure 7.
Identifying the role of ascorbic acid in regulating PCSK9-LDLR pathway. Treatment of hepatocytes or mice with ascorbic acid activates FoxO3a, which results in inhibition of PCSK9 transcription and activation of LDLR protein expression. In addition, ascorbic acid is able to activate the SREBP2 pathway, which leads to activation of LDLR transcription. These two pathways work together to enhance LDLR activity and ameliorate cholesterol metabolism, particularly in the species relying on exogenous ascorbic acid supplement.
Experimental procedures
Materials
Ascorbic acid was obtained from Sinopharm Chemical Reagent (Shanghai, China). HepG2, Huh7, and 293T cells were obtained from ATCC (Rockville, MD, USA). Rabbit anti-PCSK9 (catalog number 55206-1-AP), LDLR (catalog number 10785-1-AP), SREBP2 (catalog number 14508-1-AP), PPARγ (catalog number 16643-1-AP), FoxO3a (catalog number 10849-1-AP), HSP90 (catalog number 13171-1-AP) polyclonal antibodies and FITC-conjugated goat anti-rabbit IgG (H + L) antibody (catalog number SA00003-2) were purchased from Proteintech (Chicago, USA). Rabbit anti-Lamin A/C (catalog number A0249), phosphorylated FoxO3a (p-FoxO3a) (catalog number AP0351), β-actin (catalog number AC026) polyclonal antibodies and mouse anti-GAPDH (catalog number AC033) mAb were purchased from Abclonal (Boston, USA). Goat anti-rabbit IgG (H + L)-HRP (catalog number LK2001) and goat anti-mouse IgG (H + L)-HRP (catalog number LK2003) were purchased from Sungene Biotech (Tianjin, China). The Immobilon® Western Chemiluminescent HRP Substrate was purchased from Millipore (MA, USA). Mouse PCSK9 ELISA assay kit (catalog number SEK50251) and human PCSK9 ELISA assay kit (catalog number SEK10594) were purchased from Sino Biological (Beijing, China). Human 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanide perchlorate (Dil)-LDL complex (catalog number 20614ES76) was purchased from Yeasen (Shanghai, China). Actinomycin D (catalog number HY-17559) was obtained from MedChem Express (NJ, USA). Ascorbic acid assay kit (catalog number TC2033) was purchased from Leagene Biotechnology Co., Ltd. (Beijing, China).
Cell culture
HepG2 or Huh7 cells, the human hepatic cell lines, were cultured in high glucose Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (FBS), 50 µg/ml penicillin and streptomycin, and 2 mm glutamine. Human embryonic kidney 293T cell line was cultured in RPMI 1640 medium containing 10% FBS, 50 µg/ml of penicillin/streptomycin, and 2 mm glutamine. Mouse primary hepatocytes were isolated from C57BL/6J mice by a collagenase perfusion method as described (37). All the cells were cultured in a humidified atmosphere incubator at 37 °C and 5% CO2.
Western blotting and immunofluorescent staining
The cells were dispensed in a 6-well culture plate at a density of 5 × 105 cells/well. After reaching ∼80% confluence, cells received treatment in medium containing 2% FBS. Expression of LDLR, PCSK9, PPARγ, SREBP2, phosphorylated FoxO3a and FoxO3a protein in total cellular extract was determined by Western blotting as described (38). To prepare nuclear and cytoplasmic proteins, cells were harvested using the Nuclear and Cytoplasmic Protein Extraction Kit (Keygen Biotech, Nanjing, China) according to the manufacturer's protocol. FoxO3a expression in cells was also determined by immunofluorescent staining as described (39). The samples were observed and photographed with a fluorescence microscope after staining. After capture, the mean fluorescence intensity (MFI) of all immunofluorescent images was calculated as described (40) using morphometry software (IP Laboratory, Scanalytics, Rockville, MD) by a person who was blinded to the treatment. For Western blotting, bands from five repeated experiments were scanned and the density of band was quantified with statistical analysis.
Real-time quantitative RT-PCR (qRT-PCR)
Total RNA was isolated from cells or a piece of liver using the Total RNApure reagent (Zomanbio, Beijing, China) according to the manufacturer's instructions. Total RNA (1 µg) was converted into cDNA using the reverse transcription kit (Vazyme, Nanjing, China). The real time PCR was then performed using a SYBR Green PCR master mix with primers listed in Table 1. mRNA expression of genes was normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA in the corresponding samples.
Table 1.
Sequences of primers for qRT-PCR
| Gene | Forward | Backward |
|---|---|---|
| hLDLRa | AGGAGACGTGCTTGTCTGTC | CTGAGCCGTTGTCGCAGT |
| hPCSK9 | AGTTGCCCCATGTCGACTAC | GAGATACACCTCCACCAGGC |
| hFoxO3a | CAGGCTCAGTGTACCCCATT | AAGCCACCTGAAATCACACC |
| hPPARγ | TTCAGAAATGCCTTGCAGTGG | AGCTTCTCCTTCTCGGCCTG |
| hCD36 | TCACTGCGACATGATTAATGGTAC | ACGTCGGATTCAAATACAGCATAGAT |
| hFABP4 | GCGAACTTCAGTCCAGGTCAAC | ACGAGAGGATGATAAACTGGTGG |
| hSOD1 | TAATGCTTCCCCACACCTTC | CTAGCGAGTTATGGCGACGA |
| hCAT | AGTGATCGGGGGATTCCAGA | GAGGGGTACTTTCCTGTGGC |
| hSREBP2 | CCCTGGGAGACATCGACGA | CGTTGCACTGAAGGGTCCA |
| hGAPDH | GGTGGTCTCCTCTGACTTCAACA | GTTGCTGTAGCCAAATTCGTTGT |
| mLDLR | GAGGAACTGGCGGCTGAA | GTGCTGGATGGGGAGGTCT |
| mPCSK9 | TATGAAGAGCTGATGCTCGC | CACAATGTAGGTTCCTGGCA |
| mFoxO3a | GGGGAGTTTGGTCAATCAGA | GCCTGAGAGAGAGTCCGAGA |
| mSREBP2 | CTGCAGCCTCAAGTGCAAAG | CAGTGTGCCATTGGCTGTCT |
| mHMGCR | CCGGCAACAACAAGATCTGTG | ATGTACAGGATGGCGATGCA |
| mHMGCS | GCGGCTAGAAGTTGGAACAG | AGCATATCGTCCATCCCAAG |
| mSOD1 | GCCTTGTGTATTGTCCCCAT | ACCATCCACTTCGAGCAGAA |
| mCAT | TATCTCCTATTGGGTTCCCG | CCGCAATCCTACACCATGTC |
| mGAPDH | ACCCAGAAGACTGTGGATGG | ACACATTGGGGGTAGGAACA |
ah, Homo sapiens; m, Mus musculus.
LDL uptake assay
To analyze LDLR activity in HepG2 or Huh7 cells, cells (1 × 105 cells/well) were cultured in glass bottom cell culture dishes (Corning, NY, USA) overnight. Cells were then treated with vehicle or ascorbic acid (20 μm) for 12 h in 2% serum medium. Cells were switched into serum-free medium containing Dil-LDL (20 µg/ml) and incubated for 4 h at 37 °C. After washing twice with PBS and covered by Vectashield mounting medium for 30 min, cells were stained with 4′,6-diamidino-2-phenylindole solution for the nucleus. Finally, cells were observed and photographed with a fluorescence microscope.
Luciferase reporter assay
Human PCSK9 and LDLR promoters were constructed as described (41). The human PCSK9 promoter with mutation of FoxO3a binding site or IRE (named as phPCSK9-IREmut) and the human LDLR promoter with SRE mutation (named as phLDLR-SREmut) were constructed using the TransStartFastPfu DNA Polymerase Kit (TransGen Biotech, Beijing, China) with primers including the corresponding IRE or SRE mutations (Table 2), respectively. The PCR product was digested and then ligated into pGL4 luciferase reporter vector. The promoter activity was determined using the Dual-Luciferase® Reporter Assay System (Promega, Madison, WI, USA) (41).
Table 2.
Sequences of the primers for construction of promoter DNA. The sequence of WT IRE in human PCSK9 promoter is TGTTTA (from −381 to −376). The sequences of SRE of human LDLR are ATCACCCCAC. The underlined letters indicate the mutated nucleotides.
| Promoter | Forward | Backward |
|---|---|---|
| hPCSK9 | CACGCTCGAGGGTGAGAGGCGGGAGAGG | ACGTAAGCTTCGCAGCGGTGGAAGGTG |
| hPCSK9-IREmut | GGGTTCCGTTAACGTCTTATCAGATAGGATCGT | ACGATCCTATCTGATAAGACGTTAACGGAACCC |
| hLDLR | GCGGTACCCCTTTTGAGGCAGAGAGGACA | ACCTCGAGGGGCTCCCTCTCAACCTATTC |
| hLDLR-SREmut | TGAAGACATTTGAAAATCACGGCACTGCAAACTCC | GGGGAGGAGTTTGCAGTGCCGTGATTTTCAAATGT |
siRNA transfection
The siRNA against human FoxO3a (si-FOXO3a, catalog number siG12109131035-1-5) or SREBP2 (si-SREBP2, catalog number siB180112102225-1-5) and the corresponding scrambled siRNA were purchased from RiboBio Biotechnology (Guangzhou, China). The cells were dispensed in a 6-well culture plate at a density of 5 × 105 cells/well. When reaching ∼50% confluence, cells were switched into serum-free Opti-MEM. siRNA (25 nmol/well) for negative control (si-NC), or si-FoxO3a or si-SREBP2 were transfected into cells using Lipofectamine RNAiMAX Transfection Reagent (Invitrogen). After 48 h transfection, HepG2 cells were switched into medium containing 2% FBS followed by ascorbic acid treatment (20 μm) for 12 h, and determination of protein expression by Western blotting. To investigate the involvement of FoxO3a or SREBP2 in ascorbic acid-regulated promoter activity, 293T cells were transfected with DNA for normal human PCSK9 promoter or LDLR promoter plus Renilla luciferase after 48 h of siRNA transfection. Cells were then treated with ascorbic acid for 12 h, followed by determination of promoter activity.
In vivo study
The animal study was approved by the Animal Ethics Committee of Hefei University of Technology and performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publications No. 8023, revised 1978) and the ARRIVE guidelines. C57BL/6J mice (males, ∼8-week–old) were purchased from the Animal Center of Nanjing University. Gulo−/− mice (C57BL/6J background) were obtained from the Zhejiang University (Hangzhou, China) (26). During the breeding and maintenance, Gulo−/− mice were supplemented with tap water containing 1 g/liter of ascorbic acid. The water was changed twice a week.
To determine the effect of the ascorbic acid supplement on LDLR/PCSK9 expression and lipid profiles in WT mice, C57BL/6J male mice (∼8-weeks old) were fed either normal chow or HFD containing 21% fat and 0.5% cholesterol, and randomly divided into two groups (5 mice/group). Mice were then i.p. injected with ascorbic acid (100 mg/day/kg bodyweight, AA group) or the same volume of saline (control group) for 1 week. The dose of ascorbic acid was chosen based on previous studies (42, 43). To determine the influence of ascorbic acid deficiency on PCSK9/LDLR expression and lipid profiles in vivo, male C57BL/6J (WT) or Gulo−/− mice (∼8-weeks old, 5 mice/group) were maintained for 3 weeks without the ascorbic acid supplement in the drinking water. To determine the effects of the ascorbic acid supplement on PCSK9/LDLR expression and lipid profiles in Gulo−/− mice, male Gulo−/− mice (∼8 weeks old) were fed a HFD and randomly divided into two groups (5 mice/group). Mice were then i.p. injected with ascorbic acid (100 mg/day/kg body weight, AA group) or the same volume of saline (control group) for 2 weeks.
All the mice were free to access food and drinking water during the treatment. At the end of experiment, all mice were anesthetized and euthanized in a CO2 chamber, followed by collection of liver and blood samples individually. The blood was kept for ∼2 h at room temperature. Serum samples were isolated after centrifugation for 20 min at 2,000 × g. Serum triglyceride, CHO, HDL, and LDL levels were analyzed using an automatic biochemical analyzer (model number 3100, Hitachi High-Technologies Corporation, Tokyo, Japan). Serum PCSK9 levels were determined by the ELISA kit. Liver samples were used to determine protein expression and ascorbic acid levels by Western blotting and the ascorbic acid assay kit, respectively.
Human serum samples
With a protocol approved by the Clinical Ethics Committee of the First Affiliated Hospital of Anhui Medical University (Hefei, Anhui, China) and adhered strictly to the Declaration of Helsinki Principle 2008, we recruited 24 volunteers at age ranges of 28-58 years old. All volunteers provided written informed consent and study protocol. Serum samples were prepared for determination of CHO, LDL, PCSK9, and ascorbic acid levels by automatic biochemical analyzer, ELISA, and ascorbic acid assay kits, respectively.
Statistical analysis
All experiments were repeated at least 5 times, and the representative results are presented. All values are expressed as mean ± S.E. All the data were initially subjected to a normal distribution analysis with SPSS software (1-sample K-S of nonparametric test). The data in normal distribution was then analyzed by ANOVA with post hoc test or t test. The significant difference was considered if p < 0.05 (n ≥ 5).
Data availability
All the data related to this work have been contained within the manuscript.
Acknowledgments
We appreciate that Hua Wang, Yujue Zhang, and Derun Kong from the First Affiliated Hospital of Anhui Medical University (Hefei, China) for collection of human serum samples for this study.
Author contributions—D. W., Y. C., M. Y., and Y. G. data curation; D. W. and X. Y. formal analysis; X. Y. and Y. D. project administration; K. G. and X. W. methodology; H. H. and C. L. resources; J. H. and Y. D. conceptualization; J. H. supervision; J. H. and Y. D. funding acquisition; J. H. and Y. D. writing-original draft; J. H. and Y. D. writing-review and editing.
Funding and additional information—This work was supported by the International Science & Technology Cooperation Programs of China Grant 2017YFE0110100 (to J. H., Y. D., Y. C., and X. Y.), NSFC Grants 81722046 (to Y. D.), 81973316 and 81773727 (to J. H.), 81803517 (to X. Y.), and 31770863 (to Y. C.), and the Fundamental Research Funds for the Central Universities (to Y. D., X. Y., and Y. C.
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
- AA
- ascorbic acid
- CAT
- catalase
- FABP4
- fatty acid-binding protein 4
- FoxO3a
- forkhead box O3
- Gulo
- l-gulono-γ-lactone-oxidase
- HDL
- high-density lipoprotein cholesterol
- HFD
- high-fat diet
- HMGCR/HMGCS
- HMG-CoA reductase/synthase
- LDL
- low-density lipoprotein cholesterol
- LDLR
- LDL receptor
- PCSK9
- proprotein convertase subtilisin/kexin 9
- PPARγ
- peroxisome proliferator-activated receptor γ
- SOD1
- superoxide dismutase 1
- SREBP2
- sterol regulatory element-binding protein 2
- MFI
- mean fluorescence intensity
- qPCR
- quantitative PCR
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- SRE
- sterol regulatory element
- HNF1α
- hepatocyte nuclear 1α
- IRE
- insulin response element
- CHO
- cholesterol
- FBS
- fetal bovine serum
- HMG-CoA
- 3-hydroxy-3-methylglutaryl-CoA
- i.p
- intraperitoneal
- HRP
- horseradish peroxidase
- HSP
- heat shock protein
- Dil
- 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanide perchlorate.
References
- 1. Hunt K. J., Resendez R. G., Williams K., Haffner S. M., Stern M. P., and San Antonio Heart S, San Antonio Heart Study, (2004) National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio Heart Study. Circulation 110, 1251–1257 10.1161/01.CIR.0000140762.04598.F9 [DOI] [PubMed] [Google Scholar]
- 2. Kong W. J., Liu J., and Jiang J. D. (2006) Human low-density lipoprotein receptor gene and its regulation. J. Mol. Med. (Berl.)) 84, 29–36 10.1007/s00109-005-0717-6 [DOI] [PubMed] [Google Scholar]
- 3. Goldstein J. L., and Brown M. S. (2009) The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 29, 431–438 10.1161/ATVBAHA.108.179564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown M. S., and Goldstein J. L. (1997) The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89, 331–340 10.1016/S0092-8674(00)80213-5 [DOI] [PubMed] [Google Scholar]
- 5. Poirier S., and Mayer G. (2013) The biology of PCSK9 from the endoplasmic reticulum to lysosomes: new and emerging therapeutics to control low-density lipoprotein cholesterol. Drug Des. Dev. Ther. 7, 1135–1148 10.2147/DDDT.S36984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park S. W., Moon Y. A., and Horton J. D. (2004) Post-transcriptional regulation of low density lipoprotein receptor protein by proprotein convertase subtilisin/kexin type 9a in mouse liver. J. Biol. Chem. 279, 50630–50638 10.1074/jbc.M410077200 [DOI] [PubMed] [Google Scholar]
- 7. Li H., Dong B., Park S. W., Lee H. S., Chen W., and Liu J. (2009) Hepatocyte nuclear factor 1alpha plays a critical role in PCSK9 gene transcription and regulation by the natural hypocholesterolemic compound berberine. J. Biol. Chem. 284, 28885–28895 10.1074/jbc.M109.052407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duan Y., Chen Y., Hu W., Li X., Yang X., Zhou X., Yin Z., Kong D., Yao Z., Hajjar D. P., Liu L., Liu Q., and Han J. (2012) Peroxisome proliferator-activated receptor γ activation by ligands and dephosphorylation induces proprotein convertase subtilisin kexin type 9 and low density lipoprotein receptor expression. J. Biol. Chem. 287, 23667–23677 10.1074/jbc.M112.350181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tao R., Xiong X., DePinho R. A., Deng C. X., and Dong X. C. (2013) FoxO3 transcription factor and Sirt6 deacetylase regulate low density lipoprotein (LDL)-cholesterol homeostasis via control of the proprotein convertase subtilisin/kexin type 9 (Pcsk9) gene expression. J. Biol. Chem. 288, 29252–29259 10.1074/jbc.M113.481473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dong B., Wu M., Li H., Kraemer F. B., Adeli K., Seidah N. G., Park S. W., and Liu J. (2010) Strong induction of PCSK9 gene expression through HNF1α and SREBP2: mechanism for the resistance to LDL-cholesterol lowering effect of statins in dyslipidemic hamsters. J. Lipid Res. 51, 1486–1495 10.1194/jlr.M003566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abdollahzad H., Eghtesadi S., Nourmohammadi I., Khadem-Ansari M., Nejad-Gashti H., and Esmaillzadeh A. (2009) Effect of vitamin C supplementation on oxidative stress and lipid profiles in hemodialysis patients. Int. J. Vitam. Nutr. Res. 79, 281–287 10.1024/0300-9831.79.56.281 [DOI] [PubMed] [Google Scholar]
- 12. Linster C. L., and Van Schaftingen E. (2007) Vitamin C: biosynthesis, recycling and degradation in mammals. FEBS J. 274, 1–22 10.1111/j.1742-4658.2006.05607.x [DOI] [PubMed] [Google Scholar]
- 13. Michels A. J., Hagen T. M., and Frei B. (2013) Human genetic variation influences vitamin C homeostasis by altering vitamin C transport and antioxidant enzyme function. Annu. Rev. Nutr. 33, 45–70 10.1146/annurev-nutr-071812-161246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frikke-Schmidt H., and Lykkesfeldt J. (2009) Role of marginal vitamin C deficiency in atherogenesis: in vivo models and clinical studies. Basic Clin. Pharmacol. Toxicol. 104, 419–433 10.1111/j.1742-7843.2009.00420.x [DOI] [PubMed] [Google Scholar]
- 15. Björkhem I., and Kallner A. (1976) Hepatic 7α-hydroxylation of cholesterol in ascorbate-deficient and ascorbate-supplemented guinea pigs. J. Lipid Res. 17, 360–365 [PubMed] [Google Scholar]
- 16. Nakata Y., and Maeda N. (2002) Vulnerable atherosclerotic plaque morphology in apolipoprotein E-deficient mice unable to make ascorbic acid. Circulation 105, 1485–1490 10.1161/01.CIR.0000012142.69612.25 [DOI] [PubMed] [Google Scholar]
- 17. Zaid A., Roubtsova A., Essalmani R., Marcinkiewicz J., Chamberland A., Hamelin J., Tremblay M., Jacques H., Jin W., Davignon J., Seidah N. G., and Prat A. (2008) Proprotein convertase subtilisin/kexin type 9 (PCSK9): hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration. Hepatology 48, 646–654 10.1002/hep.22354 [DOI] [PubMed] [Google Scholar]
- 18. Tavori H., Giunzioni I., and Fazio S. (2015) PCSK9 inhibition to reduce cardiovascular disease risk: recent findings from the biology of PCSK9. Curr. Opin. Endocrinol. Diabetes Obes. 22, 126–132 10.1097/MED.0000000000000137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park J. K., Ki M. R., Lee H. R., Hong I. H., Ji A. R., Ishigami A., Park S. I., Kim J. M., Chung H. Y., Yoo S. E., and Jeong K. S. (2010) Vitamin C deficiency attenuates liver fibrosis by way of up-regulated peroxisome proliferator-activated receptor-γ expression in senescence marker protein 30 knockout mice. Hepatology 51, 1766–1777 10.1002/hep.23499 [DOI] [PubMed] [Google Scholar]
- 20. Briot A., Decaunes P., Volat F., Belles C., Coupaye M., Ledoux S., and Bouloumié A. (2018) Senescence alters PPARγ (peroxisome proliferator-activated receptor γ)-dependent fatty acid handling in human adipose tissue microvascular endothelial cells and favors inflammation. Arterioscler. Thromb. Vasc. Biol. 38, 1134–1146 10.1161/ATVBAHA.118.310797 [DOI] [PubMed] [Google Scholar]
- 21. Fasano C., Disciglio V., Bertora S., Lepore Signorile M., and Simone C. (2019) FOXO3a from the nucleus to the mitochondria: A round trip in cellular stress response. Cells 8, 1110 10.3390/cells8091110] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brunet A., Bonni A., Zigmond M. J., Lin M. Z., Juo P., Hu L. S., Anderson M. J., Arden K. C., Blenis J., and Greenberg M. E. (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857–868 10.1016/S0092-8674(00)80595-4 [DOI] [PubMed] [Google Scholar]
- 23. Abifadel M., Varret M., Rabès J. P., Allard D., Ouguerram K., Devillers M., Cruaud C., Benjannet S., Wickham L., Erlich D., Derré A., Villeger L., Farnier M., Beucler I., Bruckert E., et al. (2003) Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 34, 154–156 10.1038/ng1161 [DOI] [PubMed] [Google Scholar]
- 24. Abifadel M., Rabès J. P., Devillers M., Munnich A., Erlich D., Junien C., Varret M., and Boileau C. (2009) Mutations and polymorphisms in the proprotein convertase subtilisin kexin 9 (PCSK9) gene in cholesterol metabolism and disease. Hum. Mutat. 30, 520–529 10.1002/humu.20882 [DOI] [PubMed] [Google Scholar]
- 25. Sotiriou S., Gispert S., Cheng J., Wang Y., Chen A., Hoogstraten-Miller S., Miller G. F., Kwon O., Levine M., Guttentag S. H., and Nussbaum R. L. (2002) Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat. Med. 8, 514–517 10.1038/0502-514 [DOI] [PubMed] [Google Scholar]
- 26. Ji X., Hu X., Zou C., Ruan H., Fan X., Tang C., Shi W., Mei L., Zhu H., Hussain M., Zeng L., Zhang X., and Wu X. (2017) Vitamin C deficiency exacerbates diabetic glomerular injury through activation of transforming growth factor-β signaling. Biochim. Biophys. Acta 1861, 2186–2195 10.1016/j.bbagen.2017.06.018 [DOI] [PubMed] [Google Scholar]
- 27. Qing Z., Xiao-Hui W., Xi-Mei W., and Chao-Chun Z. (2018) Vitamin C deficiency aggravates tumor necrosis factor α-induced insulin resistance. Eur. J. Pharmacol. 829, 1–11 10.1016/j.ejphar.2018.03.044 [DOI] [PubMed] [Google Scholar]
- 28. Martin A., and Frei B. (1997) Both intracellular and extracellular vitamin C inhibit atherogenic modification of LDL by human vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 17, 1583–1590 10.1161/01.ATV.17.8.1583 [DOI] [PubMed] [Google Scholar]
- 29. Zhang K., Dong J., Cheng X., Bai W., Guo W., Wu L., and Zuo L. (2012) Association between vitamin C deficiency and dialysis modalities. Nephrology 17, 452–457 10.1111/j.1440-1797.2012.01595.x [DOI] [PubMed] [Google Scholar]
- 30. McRae M. P. (2008) Vitamin C supplementation lowers serum low-density lipoprotein cholesterol and triglycerides: a meta-analysis of 13 randomized controlled trials. J. Chiropr. Med. 7, 48–58 10.1016/j.jcme.2008.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Afkhami-Ardekani M., and Shojaoddiny-Ardekani A. (2007) Effect of vitamin C on blood glucose, serum lipids & serum insulin in type 2 diabetes patients. Indian J. Med. Res. 126, 471–474 [PubMed] [Google Scholar]
- 32. Lee H., Ahn J., Shin S. S., and Yoon M. (2019) Ascorbic acid inhibits visceral obesity and nonalcoholic fatty liver disease by activating peroxisome proliferator-activated receptor alpha in high-fat-diet-fed C57BL/6J mice. Int. J. Obes. (Lond.) 43, 1620–1630 10.1038/s41366-018-0212-0 [DOI] [PubMed] [Google Scholar]
- 33. Lakoski S. G., Lagace T. A., Cohen J. C., Horton J. D., and Hobbs H. H. (2009) Genetic and metabolic determinants of plasma PCSK9 levels. J. Clin. Endocrinol. Metab. 94, 2537–2543 10.1210/jc.2009-0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miserez A. R., Muller P. Y., Barella L., Barella S., Staehelin H. B., Leitersdorf E., Kark J. D., and Friedlander Y. (2002) Sterol-regulatory element-binding protein (SREBP)-2 contributes to polygenic hypercholesterolaemia. Atherosclerosis 164, 15–26 10.1016/S0021-9150(01)00762-6 [DOI] [PubMed] [Google Scholar]
- 35. Tao R., Wei D., Gao H., Liu Y., DePinho R. A., and Dong X. C. (2011) Hepatic FoxOs regulate lipid metabolism via modulation of expression of the nicotinamide phosphoribosyltransferase gene. J. Biol. Chem. 286, 14681–14690 10.1074/jbc.M110.201061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tikhanovich I., Cox J., and Weinman S. A. (2013) Forkhead box class O transcription factors in liver function and disease. J. Gastroenterol. Hepatol. 28, 125–131 10.1111/jgh.12021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang W., Yang X., Chen Y., Hu W., Liu L., Zhang X., Liu M., Sun L., Liu Y., Yu M., Li X., Li L., Zhu Y., Miao Q. R., Han J., et al. (2018) Activation of hepatic Nogo-B receptor expression-A new anti-liver steatosis mechanism of statins. Biochim. Biophys. Acta 1863, 177–190 10.1016/j.bbalip.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen Y., Duan Y., Kang Y., Yang X., Jiang M., Zhang L., Li G., Yin Z., Hu W., Dong P., Li X., Hajjar D. P., and Han J. (2012) Activation of liver X receptor induces macrophage interleukin-5 expression. J. Biol. Chem. 287, 43340–43350 10.1074/jbc.M112.403394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yu M., Jiang M., Chen Y., Zhang S., Zhang W., Yang X., Li X., Li Y., Duan S., Han J., and Duan Y. (2016) Inhibition of macrophage CD36 expression and cellular oxidized low density lipoprotein (oxLDL) accumulation by tamoxifen: a peroxisome proliferator-activated receptor (PPAR) γ-dependent mechanism. J. Biol. Chem. 291, 16977–16989 10.1074/jbc.M116.740092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang B., Zhang Z., Xia S., Xing C., Ci X., Li X., Zhao R., Tian S., Ma G., Zhu Z., Fu L., and Dong J. T. (2013) KLF5 activates microRNA 200 transcription to maintain epithelial characteristics and prevent induced epithelial-mesenchymal transition in epithelial cells. Mol. Cell Biol. 33, 4919–4935 10.1128/MCB.00787-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sun L., Yang X., Li Q., Zeng P., Liu Y., Liu L., Chen Y., Yu M., Ma C., Li X., Li Y., Zhang R., Zhu Y., Miao Q. R., Han J., et al. (2017) Activation of adiponectin receptor regulates proprotein convertase subtilisin/kexin type 9 expression and inhibits lesions in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 37, 1290–1300 10.1161/ATVBAHA.117.309630 [DOI] [PubMed] [Google Scholar]
- 42. Liang T., Chen X., Su M., Chen H., Lu G., and Liang K. (2014) Vitamin C exerts beneficial hepatoprotection against concanavalin A-induced immunological hepatic injury in mice through inhibition of NF-κB signal pathway. Food Funct. 5, 2175–2182 10.1039/c4fo00224e [DOI] [PubMed] [Google Scholar]
- 43. Walia V., Garg C., and Garg M. (2019) Nitrergic signaling modulation by ascorbic acid treatment is responsible for anxiolysis in mouse model of anxiety. Behav. Brain Res. 364, 85–98 10.1016/j.bbr.2019.02.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data related to this work have been contained within the manuscript.