Abstract
Although CD133 is a representative cancer stem cell marker, its function in tumor aggressiveness under hypoxia remains unclear. Therefore, the present study aimed to investigate the associations between CD133, the epithelial-mesenchymal transition and distant metastasis in colorectal cancer. CD133+ and CD133− cells were isolated from a single colorectal cancer cell line LoVo, and their adhesive and migratory properties were compared under hypoxic conditions. Immunostaining analysis was performed to determine CD133 expression in clinical samples of primary tumors, as well as liver and peritoneal metastases. Under hypoxia, the expression levels of hypoxia-inducible factor (HIF)-1α and the epithelial-mesenchymal transition markers N-cadherin and vimentin were significantly higher in the CD133+ compared with those in the CD133− cells. Furthermore, the migratory ability of the CD133+ cells was higher compared with that of the CD133− cells under hypoxia. By contrast, the expression levels of β1 integrin were significantly lower in the CD133+ cells under hypoxia compared with those in the CD133− cells. Immunohistochemical analysis of clinical samples revealed that the levels of CD133 expression in metastatic tissues from the liver were significantly higher compared with those in the corresponding primary tumors, whereas CD133 expression levels in peritoneal metastatic tissues were significantly lower compared with those in the corresponding primary tumors. In conclusion, compared with the CD133− cells, the CD133+ colorectal cancer cells exhibited enhanced levels of HIF-1α expression and tumor cell migration during hypoxia. This was associated with an increased ability of epithelial-mesenchymal transition, possibly leading to the acquisition of an increased hematogenous metastatic potential and eventually resulting in liver metastasis. High β1 integrin expression levels in the CD133− cells under hypoxia may serve a key role in cell adhesion to the peritoneum, resulting in peritoneal metastasis.
Keywords: CD133, colorectal cancer, epithelial-mesenchymal transition, hypoxia, metastasis
Introduction
The cancer stem cell (CSC) hypothesis states that cancer originates from a small fraction of cancer cells termed CSCs that exhibit the abilities of self-renewal and pluripotency (1). Based on studies of leukemia initiation, the CSC hypothesis has been used to explain the development of a variety of solid tumors, such as lung and brain tumors (2–4). CSCs are often identified by cell surface markers, such as CD44 and CD24 (5,6); however, the role of these markers remains poorly understood. CD133, a cell surface marker, is a five-transmembrane domain glycoprotein present in lipid rafts, the cholesterol-rich domains on cell membranes (7). Although the precise functions of CD133 remain unclear, it has been identified as a candidate marker for CSCs in various types of solid tumors, including colorectal cancer (CRC) (8–10).
The metastatic routes of cancer include hematogenous, lymphatic and disseminated metastasis (11). These routes are important metastatic pathways in CRC, and the liver and lungs exhibit high frequencies of hematogenous metastasis (12). In Japan, ~17% of all colorectal cancer cases present with distant metastasis; 55.8% of them are liver metastases, 12.2% are lung metastases, and 22.9% are peritoneal dissemination (13). Hypoxia is a significant factor that contributes to the metastatic progression of cancer (14). A number of solid tumors often develop hypoxia due to insufficient blood flow during tumor growth, which leads to hematogenous metastasis as cancer cells form new vascular networks to survive and attempt to escape the unfavorable environment (15). Hypoxia-inducible factor (HIF)-1α is a key molecule induced under hypoxic conditions to fulfill this function (16). HIF-1 is a central transcription factor that enhances the transcription of various genes under hypoxic conditions and induces angiogenesis, cell proliferation, survival and migration, thus promoting cancer invasion and metastasis (17).
The epithelial-mesenchymal transition (EMT) is a normal physiological process during which epithelial cells lose their polarity and cell-cell adhesion, gain migratory and invasive properties, and transform into mesenchymal cells (18). The EMT is essential during organ formation and tissue growth; in cancer cells, this process enables them to invade the surrounding tissues and metastasize to distant sites, resulting in hematogenous metastasis (19). HIF-1α enhances the EMT in cancer cells (14), and hypoxia triggers the EMT in CRC cells (20).
Disseminated metastasis is a type of metastasis that occurs when cancer cells on the primary tumor surface detach from the tumor and adhere to other serosal surfaces such as the peritoneum; cell adhesion molecules are involved in disseminated metastasis (21). The integrin family of cell adhesion molecules comprises transmembrane receptors that facilitate cell-extracellular matrix attachment (22). Integrins have α and β subunits and serve an important role in cancer cell attachment to the peritoneum to induce peritoneal dissemination (21). In an animal model of surgical trauma, blocking β1 integrin reduced the number of tumor cells that attached to the peritoneum and subsequently impaired tumor outgrowth (23). This suggests an important role for β1 integrin in the adherence and proliferation of tumor cells in the peritoneum.
Our previous study demonstrated that the proliferative capacity of CD133+ cells was higher compared with that of CD133− cells, whereas resistance to chemotherapy in CD133− cells was higher compared with that of CD133+ cells in CRC (24). In addition, patients with CRC with liver metastasis without CD133 expression exhibited a significantly shorter overall and disease-free survival time (25). Thus, CD133 is not only an important CSC marker, but also serves an important role in cell proliferation and metastasis. Compared with CD133− pancreatic cancer cells, CD133+ pancreatic cancer cells are more likely to induce the EMT in a hypoxic environment (26,27). In addition, CD133 overexpression enhances the expression of HIF-1α in head and neck squamous cell carcinoma (28), suggesting that CSCs may promote the EMT. However, the separation of a cancer cell line into CD133+ and CD133− cells, and a direct comparison of their EMT-associated abilities to investigate the involvement of CSCs in this process has not yet been reported. Therefore, the present study isolated CD133+ and CD133− cell populations from a single CRC cell line LoVo and examined their EMT-associated abilities under hypoxia.
A previous study on the association between CD133 and peritoneal dissemination revealed that CD133+ cells of an ovarian cancer cell line were more likely to adhere to peritoneal mesothelial cells and cause peritoneal dissemination compared with CD133− cells (29). By contrast, CD133 has been demonstrated to not be associated with peritoneal dissemination in CRC (30), and the role of CD133 in peritoneal dissemination remains unclear. Our previous study reported that the expression levels of β1 integrin in CD133− cells were higher compared with those in CD133+ cells (24). Our other previous study analyzed the association between CD133 expression and postoperative recurrence of CRC in patients with peritoneal metastasis and reported that the recurrence rate in the CD133− group was higher compared with that in the CD133+ group (31). Therefore, we hypothesized that CD133+ cells may be important for distant metastasis due to the involvement of the EMT, whereas CD133− cells may be important for peritoneal metastasis due to the involvement of β1 integrin.
Materials and methods
Cell culture and reagents
The human CRC cell line LoVo (Japanese Cancer Research Resource Bank) was cultured in RPMI-1640 medium (Sigma-Aldrich; Merck KGaA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and a 1% antibiotic/antimycotic solution (Gibco; Thermo Fisher Scientific, Inc.) in a 5% CO2 incubator at 37°C (normoxia). For hypoxia, the cells were cultured in 1% O2, 5% CO2 and 94% N2 at 37°C in a humidified atmosphere using a multi-gas incubator (Juji Field, Inc.).
Patients and tissue samples
Primary and metastatic tumor tissue samples were collected from 88 consecutive patients with CRC and synchronous liver metastasis between 1998 and 2010, and from 58 consecutive patients with CRC and synchronous peritoneal metastasis between 1997 and 2017. These patients underwent complete resection of the primary and metastasized tumors at the University of Tokyo Hospital (Tokyo, Japan). None of the patients underwent preoperative chemo- and/or radiotherapy. The study was approved by the Ethics Committee of The University of Tokyo [approval no. 3252-(8)], and written informed consent was obtained from all participants.
Cell isolation by magnetic cell sorting
CD133+ and CD133− cells were isolated from a single CRC cell line as previously described (24). Briefly, LoVo cells (5×107 cells in 500 µl) were labeled with a biotin-conjugated anti-human CD133 monoclonal antibody (CD133/1-Biotin; dilution, 1:20; cat. no. 130-091-895; Miltenyi Biotec), washed twice with an isolation buffer and incubated with a microbead-conjugated biotin monoclonal antibody (dilution, 1:10; cat. no. 130-091-895; Miltenyi Biotec). The cells were isolated using a magnetic-activated cell sorting system (Miltenyi Biotec) according to the manufacturer's instructions. Magnetically labeled cells were passed through a LS column with a magnetic field. Labeled cells were isolated as the CD133+ population via positive selection, and unlabeled cells were isolated as the CD133− population via negative selection. Purity of the CD133+ and CD133− cells was confirmed by flow cytometry as described below.
Flow cytometric analysis
HIF-1α and vimentin expression was assessed by flow cytometry as previously described (32) with minor modifications. Briefly, isolated cells were harvested, fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 30 min at 4°C, permeabilized with 0.1% Tween-20 in PBS for 10 min at 4°C and stained using anti-HIF-1α (dilution, 1:50; cat. no. IC1935P; R&D Systems, Inc.) or anti-vimentin (dilution, 1:100; cat. no. 562337; BD Pharmingen) phycoerythrin-conjugated antibodies for 30 min at 4°C. Cell surface E-cadherin (dilution, 1:100; cat. no. 562870), N-cadherin (dilution, 1:100; cat. no. 561554) and β1 integrin (dilution, 1:25; cat. no. 561795) (all from BD Pharmingen) expression levels were assessed by incubating the cells with the antibodies for 30 min at 4°C and analyzing them using a BD FACScalibur flow cytometer (BD Biosciences) as previously described (20). The data were analyzed using Cell Quest software (version 3.0, BD Biosciences) and are presented as the mean fluorescence intensity.
Immunofluorescence analysis
LoVo cells (8×103 cells in 500 µl/well) were plated in 24-well plates for 24 h prior to incubation under hypoxic conditions. Then, the cells were fixed with 4% paraformaldehyde and permeabilized with 1% Triton X-100 in PBS for 10 min at 20–25°C, followed by incubation with 3% bovine serum albumin (Miltenyi Biotec) in PBS for blocking. The cells were incubated overnight at 4°C with a mouse polyclonal antibody against β-catenin (dilution, 1:500; cat. no. 610154; BD Pharmingen), washed and incubated with an Alexa Fluor 488-conjugated anti-mouse secondary antibody (dilution, 1:500; cat. no. 1907294, Thermo Fisher Scientific, Inc.) for 1 h at 20–25°C. Cell nuclei were stained using 4′,6-diamidino-2-phenylindole (Vector Laboratories, Inc.). Images were observed and acquired using a BZ-8100 confocal laser microscope (Keyence Corporation) at ×400 magnification in 10 fields per sample.
Migration assay
The chemokinetic activity of cells was evaluated using a modified Boyden chamber assay as previously described (20,33). Briefly, a polycarbonate filter with a collagen type I coating and 8-µm pores (Ieda Trading Corporation) was placed on a 96-well plate (Ieda Trading Corporation) containing RPMI-1640 medium supplemented with 5% fetal bovine serum loaded in the lower chamber. The cell suspension was prepared in RPMI-1640 medium containing 0.1% bovine serum albumin. LoVo cells (5×105 cells in 200 µl/well) were loaded into the upper chamber. Prior to the assay, the cells were precultured in either hypoxic or normoxic conditions for 24 h. Two chambers were prepared, and cells preincubated under hypoxia were incubated for another 12 h under hypoxia, whereas those preincubated in normoxia were incubated for another 12 h under normoxia. Subsequently, the filters were fixed with 99% methanol and stained using a Diff-Quick staining kit (Sysmex Corporation). The upper side of the filter was scraped with a polyethylene blade to eliminate non-migratory cells. The number of cells that migrated to the lower side of the filter was measured by recording the staining intensity using a 96-well microplate reader at 595 nm, which was defined as the migration index.
Immunohistochemistry analysis
For analysis of CD133 expression, consecutive 3-µm formalin-fixed paraffin-embedded sections were stained immunohistochemically as previously described (25,31). Following incubation with a mouse monoclonal anti-CD133 antibody (dilution, 1:100; cat. no. 130-090-422; Miltenyi Biotec), the Histofine SAB-PO (M) kit (Nichirei Corporation) was used to prevent non-specific binding, secondary antibody treatment and signal amplification. For chromogen development, the slides were incubated in 2% 3,3′-diaminobenzidine tetrahydrochloride and 50 mM Tris buffer (pH 7.6) containing 0.03% hydrogen peroxide. Meyer's hematoxylin (Sigma-Aldrich; Merck KGaA) was used 30 sec for counterstaining at 35–40°C.
A total of 1,000 tumor cells were counted manually using ImageJ software (National Institutes of Health, Bethesda, MD, USA). Positive CD133 expression was defined as the presence of CD133 staining in >5% cancer cells of the primary tumor samples (25,31). All images were evaluated independently at ×400 magnification by two investigators who were blinded to the clinical details of the specimens.
Statistical analyses
Data are presented as the mean ± SD. For the in vitro experiments, HIF-1α and EMT-related marker expression levels were compared using the unpaired two-tailed Student's t-test. Statistical significance of migratory differences among multiple groups was determined using two-way ANOVA followed by Tukey's post hoc test. For the clinical study, associations between patient characteristics and CD133 expression were determined using the χ2 or Fisher's exact test. Statistical analyses of tissue samples were performed using the paired Student's t-test. All analyses were performed using JMP Pro 14.0 software (SAS Institute). P<0.05 was considered to indicate a statistically significant difference.
Results
HIF-1α levels and EMT markers in CD133+ and CD133− cells under hypoxia
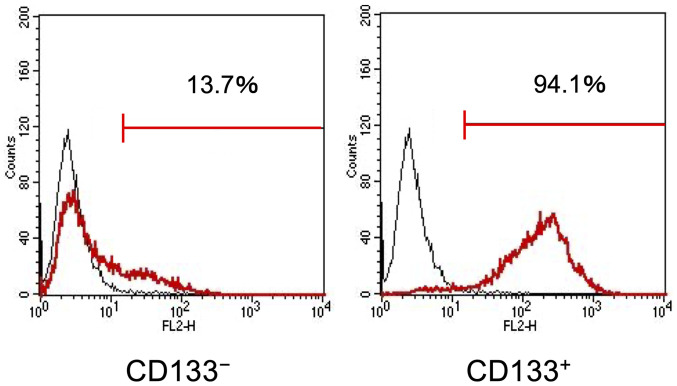
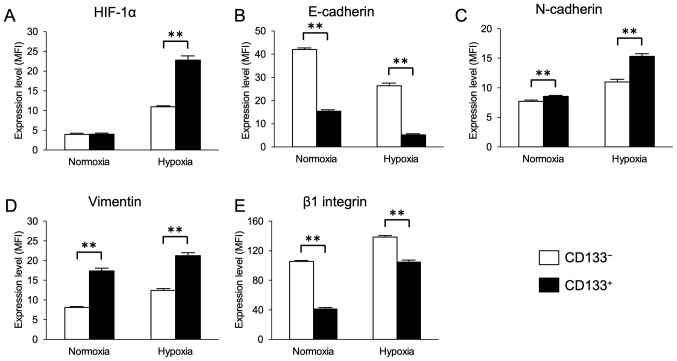
First, the present study investigated whether the CD133+ CRC cells exhibited enhanced HIF-1α and EMT-related marker expression under hypoxia. Magnetic-activated cell sorting was used to obtain the CD133+ and CD133− LoVo cell subpopulations with purities of 94.1% (Fig. 1). Flow cytometry analysis results demonstrated that the cell surface expression levels of HIF-1α appeared to be upregulated in the CD133+ and CD133− cells after 48-h exposure to hypoxia, and that the expression levels of HIF-1α in the CD133+ cells were significantly higher compared with those in the CD133− cells (Fig. 2A).
Figure 1.
Purity of CD133− and CD133+ cells. LoVo cells were separated into CD133− and CD133+ cell subpopulations using magnetic-activated cell sorting.
Figure 2.
Expression of HIF-1α, epithelial-mesenchymal transition markers and β1 integrin under normoxic and hypoxic conditions in CD133− and CD133+ cells. (A-E) Flow cytometry analysis results of the expression of (A) HIF-1α, (B) E-cadherin, (C) N-cadherin, (D) vimentin and (E) β1 integrin. Data are presented as the mean ± SD. **P<0.01. HIF-1α, hypoxia-inducible factor 1α; MFI, mean fluorescence intensity.
The expression levels of the EMT markers E-cadherin, N-cadherin and vimentin were next evaluated in the CD133+ and CD133− cells under hypoxia. The cell surface levels of E-cadherin expression appeared to be decreased, whereas the levels of N-cadherin and vimentin appeared to be increased in the CD133+ and CD133− cells under hypoxia compared with those in the corresponding cells under normoxia. Furthermore, under both normoxic and hypoxic conditions, the expression levels of E-cadherin in the CD133+ cells were significantly lower compared with those in the CD133− cells (Fig. 2B), and the expression levels of N-cadherin and vimentin in the CD133+ cells were significantly higher compared with those in the CD133− cells (Fig. 2C and D). Similarly, the expression levels of β1 integrin appeared to be increased in the CD133+ and CD133− cells under hypoxia compared with those in the corresponding cells under normoxia. However, under both normoxic and hypoxic conditions, the cell surface levels of β1 integrin expression in the CD133+ cells were significantly lower compared with those in the CD133− cells (Fig. 2E). The flow cytometry histogram plots for each protein under hypoxia are presented in Fig. S1.
Nuclear β-catenin is considered to be a marker of the EMT (34). In the present study, β-catenin was localized in the cytoplasm under normoxia in the CD133+ and CD133− cells. Nuclear translocation of β-catenin was observed only in the CD133+ cells under hypoxia (Fig. S2).
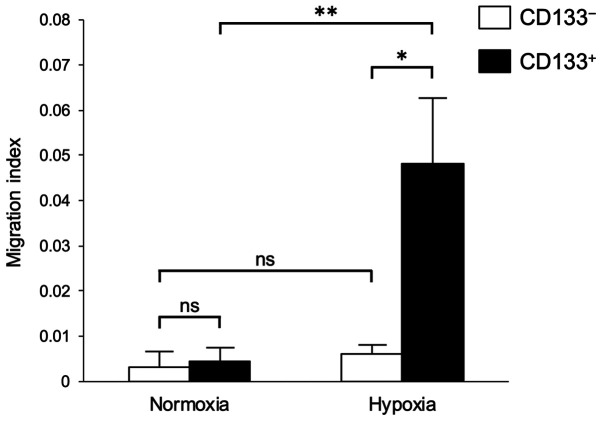
Migratory ability of the CD133+ and CD133− cells under hypoxia
Migration assays were performed to determine the hypoxia-induced differences in the migratory abilities of the CD133+ and CD133− cells. No significant differences were observed in the migratory abilities of the CD133− cells under normoxia and hypoxia (Fig. 3). However, the migratory ability of the CD133+ cells under hypoxia was significantly higher compared with those of the CD133+ cells under normoxia and the CD133− cells under hypoxia (Fig. 3).
Figure 3.
Effects of hypoxia on the migratory ability of CD133− and CD133+ cells. CD133− and CD133+ cells were pre-incubated under hypoxic or normoxic conditions. The cell migratory ability was analyzed following 12-h incubation under hypoxic or normoxic conditions using the Boyden chamber assay. Data are presented as the mean ± SD. *P<0.05 and **P<0.01; ns, not significant.
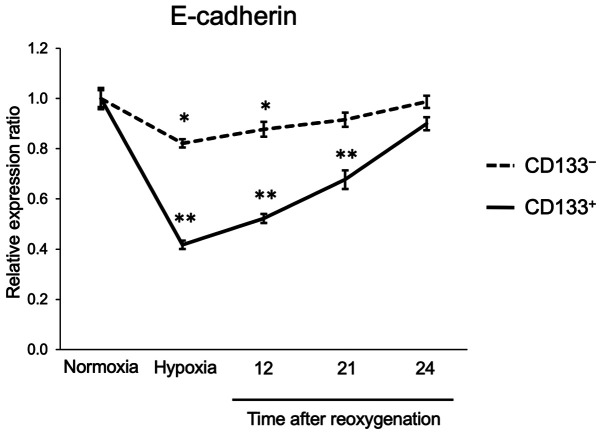
Recovery of E-cadherin expression after reoxygenation
The expression of E-cadherin in the CD133+ and CD133− cells subjected to reoxygenation after hypoxia was next assessed. The flow cytometry analysis results demonstrated that E-cadherin expression levels in the CD133− cells were reduced to 82% of those in normoxia following 24-h hypoxia; after reoxygenation, E-cadherin levels recovered gradually and almost reached pre-hypoxia levels within 24 h. In the CD133+ cells, E-cadherin expression levels were reduced to 42% of those under normoxia after 24-h hypoxia, and were restored to the pre-hypoxia levels within 24 h (Fig. 4). The flow cytometry histogram plots of the time course experiment are presented in Fig. S3. These results suggested that the CD133+ cells could recover their adhesive ability as rapidly as the CD133− cells following reoxygenation.
Figure 4.
Time-course of E-cadherin expression in CD133− and CD133+ cells following reoxygenation. CD133− and CD133+ cells were exposed to hypoxia for 24 h and reoxygenated, and the relative expression levels of E-cadherin were detected by flow cytometry. Data are presented as the mean ± SD. *P<0.05 and **P<0.01 vs. normoxia.
Expression of CD133 in primary tumors and synchronous liver or peritoneal metastasis
To assess the differences in CD133 expression in different patterns of tumor metastasis, CD133 expression levels were determined in tumor tissues from patients with CRC with liver or peritoneal metastasis. Immunohistochemical staining revealed CD133 expression on the luminal cell surface of CRC tissues (Fig. S4).
The associations between patient clinicopathological characteristics and CD133 expression are presented in Table I. CD133 expression exhibited no association with any clinicopathological factor, with the exception of histopathological differentiation in individuals with synchronous peritoneal metastasis.
Table I.
Clinicopathological characteristics of the patients enrolled in this study.
| Primary tumor with liver metastasis | Primary tumor with peritoneal metastasis | |||||
|---|---|---|---|---|---|---|
| Characteristics | CD133+, n (%) | CD133−, n (%) | P-value | CD133+, n (%) | CD133−, n (%) | P-value |
| Total, n | 44 | 40 | 33 | 25 | ||
| Age, median years (range) | 61 (41–81) | 59 (33–79) | 0.449 | 64 (41–80) | 68 (40–88) | 0.261 |
| Sex | 0.730 | 0.673 | ||||
| Male | 27 (61.4) | 26 (65.0) | 14 (42.4) | 12 (48.0) | ||
| Female | 17 (38.6) | 14 (35.0) | 19 (57.6) | 13 (52.0) | ||
| Site of primary tumor | 0.368 | 0.847 | ||||
| Right | 10 (22.7) | 6 (15.0) | 18 (54.5) | 13 (52.0) | ||
| Left | 34 (77.3) | 34 (85.0) | 15 (45.5) | 12 (48.0) | ||
| Differentiation | 0.084 | 0.037 | ||||
| High | 20 (45.5) | 14 (35.0) | 14 (42.4) | 6 (24.0) | ||
| Moderate | 24 (54.5) | 22 (55.0) | 16 (48.5) | 10 (40.0) | ||
| Other | 0 (0.0) | 4 (10.0) | 3 (12.0) | 9 (36.0) | ||
| T category | 0.896 | 0.105 | ||||
| T1-3 | 28 (63.7) | 26 (65.0) | 8 (24.2) | 2 (8.0) | ||
| T4 | 16 (36.3) | 14 (35.0) | 25 (75.8) | 23 (92.0) | ||
| N category | 0.641 | 0.308 | ||||
| N0 | 13 (29.6) | 10 (25.0) | 9 (27.3) | 4 (16.0) | ||
| N1-3 | 31 (70.4) | 30 (75.0) | 24 (72.7) | 21 (84.0) | ||
| Peritoneal cancer index | 0.915 | |||||
| <10 | 22 (66.7) | 17 (68.0) | ||||
| ≥10 | 11 (33.3) | 8 (32.0) | ||||
T, tumor; N, node.
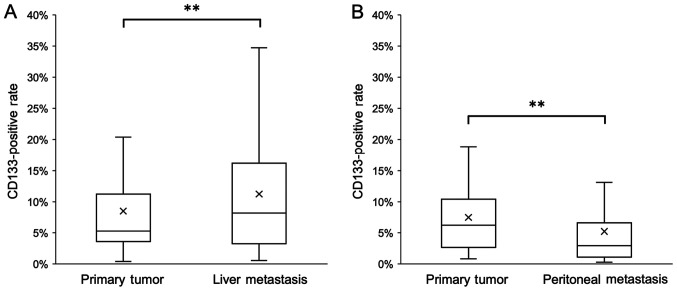
The percentage of CD133+ cells in liver metastatic tissues was significantly higher compared with that in the corresponding primary tumors (Fig. 5A). By contrast, the percentage of CD133+ cells in peritoneal metastatic tissues was significantly lower compared with that in the corresponding primary tumors (Fig. 5B).
Figure 5.
Percentage of CD133+ cells in tumor tissues from patients with colorectal cancer with synchronous liver or peritoneal metastasis. (A) CD133+ cells in the primary tumor and synchronous liver metastatic tissues. (B) CD133+ cells in the primary tumor and synchronous peritoneal metastatic tissues. X represents the mean; horizontal line represents the median. **P<0.01.
Discussion
A hypoxic environment is essential for cancer cells to acquire stem cell properties, and HIF pathways contribute to the proliferation of such CSCs (35). CD133, one of the CSC markers, has been reported to interact with HIF-1α. For example, Maeda et al (26) have reported that CD133 knockdown in pancreatic cancer cells inhibits HIF-1α expression. By contrast, under hypoxic conditions, HIF-1α expression levels in glioma cells increase, resulting in an increased proportion of CD133+ cells (36). These results suggest that CD133 expression may be associated with that of HIF-1α, and that these two molecules may promote each other's expression.
The present study is the first to report the separation of a single CRC cell line into CD133+ and CD133− cells and to directly compare the expression of HIF-1α and EMT-associated proteins in these two populations under hypoxic conditions. In the present study, HIF-1α expression in hypoxic CD133+ LoVo cells was higher compared with that in hypoxic CD133− LoVo cells. These results suggested that CD133 may promote HIF-1α expression in CRC cells.
Nuclear translocation of β-catenin was observed in the CD133+ cells only under hypoxia in the present study, and significant upregulation of the levels of EMT markers under hypoxia was observed in the CD133+ cells compared with those in CD133− cells. Under hypoxic conditions, HIF-1α promotes the nuclear translocation of β-catenin by activating the Wnt signaling pathway (37). Combining the nuclear translocation of β-catenin with HIF-1α activates the transcription of HIF-1α target genes such as VEGF and erythropoietin, which promotes the EMT (38). Therefore, the results of the present study suggested that the promotion of β-catenin nuclear translocation via CD133 and hypoxia-induced HIF-1α expression may be the intermediary steps that enhance the expression of EMT markers in CRC cell lines.
In the present study, although increased migration was observed in the CD133+ cells under hypoxia compared with that in the CD133− cells, no changes in the migratory ability were observed in the CD133− cells. Ding et al (39) have reported that CD133 knockdown reduces the migratory ability of pancreatic cancer cells, and that CD133 expression contributes to the promotion of migration. Additionally, promotion of the EMT enhances the invasive and migratory abilities of cancer cells (40). In the present study, hypoxia appeared to increase the expression of EMT markers in the CD133+ cells and enhance the migratory ability of these cells. Collectively, these results suggested that the promotion of the EMT induced the migration of CRC cells.
The EMT and the mesenchymal-epithelial transition (MET) serve important roles in cancer metastasis; although the EMT induces distant metastasis from primary lesions, the MET causes cancer cells in the metastatic site to revert to epithelial cells, re-expressing E-cadherin (40). This is considered to result in re-establishing adhesion between the cancer cells and the surrounding tissues (41), leading to the formation of macrometastasis (40). Accordingly, the MET following reoxygenation is also considered to enable cancer cells after undergoing the EMT under hypoxia to reverse the EMT and form colonies under normoxic conditions in distant organs (42). However, the MET in CD133+ cells has not yet been reported. In the present study, although the levels of E-cadherin expression in the CD133+ cells appeared to be downregulated by exposure to hypoxia compared with those under normoxia, they returned to pre-hypoxia levels 24 h after reoxygenation. Thus, the CD133+ CRC cells not only possessed high EMT ability, but may also achieve the MET after reoxygenation. This phenomenon may enable them to form macrometastasis at a metastatic site.
Integrins, which are cell adhesion molecules, facilitate the attachment between cancer cells and the extracellular matrix (22). The HIF-1 pathway is one of the signaling pathways that regulate integrin functions under hypoxic conditions (43). Hypoxia induces the transcription of various integrin family molecules in a number of cancer cell lines, including colorectal and breast cancer (44–46). Previous studies have reported that integrin expression induces CD133 expression and increases the ratio of CD133+ cells in colorectal and liver cancer cell lines (47,48). Thus, although the expression of a variety of integrin family molecules contributes to CD133 expression levels, the mechanisms underlying the interplay between CD133 and integrins remain unclear. Our previous study demonstrated that β1 integrin expression levels and the adhesive ability of the CD133− LoVo cells were higher compared with those in the CD133+ LoVo cells (24). In the present study, compared with those in the CD133+ cells, the CD133− cells exhibited higher expression levels of β1 integrin under both normoxic and hypoxic conditions. These results suggested that the adhesive ability of the CD133− cells may be stronger compared with that of the CD133+ cells, which are considered to be CSCs.
Adhesive ability serves an important role in the peritoneal dissemination of cancer cells. Specifically, cancer cells released from the primary lesion need to adhere to the peritoneal surface to penetrate the peritoneum and form a colony (21). Cell adhesion molecules, such as β1 integrin and E-cadherin, assist in the adhesion of cancer cells to the peritoneal surface and facilitate the interaction of cancer cells with the extracellular matrix (21). Cancer cells with strong peritoneal metastatic capability can upregulate the expression of β1 integrin in gastric (49), ovarian (50) and pancreatic (51) cancer. In addition, blocking β1 integrin reduces the number of tumor cells adhering to the peritoneum ex vivo (23). In the present study, β1 integrin expression levels in the CD133− cells were higher compared with those in the CD133+ cells. These results suggest that CD133− cells with high β1 integrin expression may be involved in the occurrence of peritoneal metastasis.
Accordingly, we hypothesized that the CD133+ cells may induce hematogenous metastasis owing to their high EMT and MET recovery abilities, whereas CD133− cells may induce peritoneal metastasis due to their high β1 integrin content. In our previous study, positive CD133 expression was defined as the presence of CD133 staining in >5% of the tumor cells, and the results demonstrated that CD133 positivity in liver metastasis was slightly higher compared with that in primary lesions (25). However, the specific ratio of CD133+ cells could not be evaluated in the previous study. In the present study, the percentages of CD133+ cells in primary tumor tissues and liver metastatic lesions were compared; the percentage of CD133+ cells in metastatic liver tissues was significantly higher compared with that in the corresponding primary tumors. A previous in vivo study used a mouse liver metastasis model to demonstrate the high liver metastatic potential of CD133+ human lung cancer cells compared with CD133− cells (52). In a clinical sample-based study, CD133 mRNA expression levels in metastatic liver lesions were significantly higher compared with those in colorectal tumors (53), thus corroborating the results of the present study. However, not all liver metastatic cells were CD133+ in the present study. The CD133+ cells may have undergone MET transformation at the metastatic site and differentiation to CD133− cells after liver metastasis occurred, as reported previously (24). Thus, the number of CD133− cells may increase with tumor growth. This is supported by the observation that CD133 protein levels in colorectal metastatic liver tissues decrease as the tumor grows (54).
Our previous study demonstrated that CD133 expression levels in peritoneal metastatic tissues were lower compared with those in primary lesions, which was contrary to the expression pattern observed in individuals with liver metastasis (31). However, the specific positivity ratio was not evaluated. In the present study, significantly lower CD133 expression levels were identified in peritoneal metastatic tissues compared with those in the corresponding primary tumors. Although a CD133+ ovarian cancer cell line has been reported to be more likely to adhere to peritoneal mesothelial cells and cause peritoneal dissemination compared with CD133− cells (29), another study has demonstrated that CD133 expression levels in CRC with peritoneal metastasis are lower compared with those in CRC with liver metastasis (30). The present results also suggested that the CD133− cells contributed to peritoneal metastasis in CRC.
In conclusion, the results of the present study demonstrated that the CD133+ CRC cells exhibited a high EMT potential under hypoxic conditions, which may lead to the development of liver metastasis. By contrast, CD133− cells may potentiate the development of peritoneal metastasis via expression of β1 integrin.
Supplementary Material
Acknowledgements
Not applicable.
Funding
This study was supported by Grants-in-Aid for Scientific Research (grant nos. 17K10620, 7K 10621, 17K10623, 18K07194, 19K09114 and 19K09115) and Challenging Research (Exploratory) grant (grant no. 20K21626) from the Japan Society for The Promotion of Science, and the Project for Cancer Research and Therapeutic Evolution from the Japan Agency for Medical Research and Development. (grant no. JP 19cm0106502).
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
KK and SI conceived of the study and contributed to the design of the study. MO, HSh, HNa and JK carried out the experiments with support from KK and HSo. JK, HNa and KH contributed to sample preparation. MO, KK, HSo, HSh, HNa, HNo, KS, MK, KM, SE, YI, HI, YY, HA and SI contributed to the interpretation of the results. MO drafted the manuscript and designed the figures. KK, HSo, HSh, JK, HNa, HNo, KS, MK, KM, SE, YI, HI, YY, HA, KH and SI managed the literature search and revised the manuscript critically. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of The University of Tokyo [approval no. 3252-(8)], and written informed consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 3.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 4.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 5.Li F, Tiede B, Massagué J, Kang Y. Beyond tumorigenesis: Cancer stem cells in metastasis. Cell Res. 2007;17:3–14. doi: 10.1038/sj.cr.7310118. [DOI] [PubMed] [Google Scholar]
- 6.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 7.Shmelkov SV, St Clair R, Lyden D, Rafii S. AC133/CD133/Prominin-1. Int J Biochem Cell Biol. 2005;37:715–719. doi: 10.1016/j.biocel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 10.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 11.Paduch R. The role of lymphangiogenesis and angiogenesis in tumor metastasis. Cell Oncol (Dordr) 2016;39:397–410. doi: 10.1007/s13402-016-0281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Japanese Society for Cancer of the Colon and Rectum: Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma: The 3d English Edition [Secondary Publication] J Anus Rectum Colon. 2019;3:175–195. doi: 10.23922/jarc.2019-018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M, et al. Japanese Society for Cancer of the Colon Rectum Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1–42. doi: 10.1007/s10147-019-01485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: Drivers of tumour metastasis. Nat Rev Cancer. 2014;14:430–439. doi: 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–361. doi: 10.1016/S1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 16.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/MCB.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 18.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 20.Hongo K, Tsuno NH, Kawai K, Sasaki K, Kaneko M, Hiyoshi M, Murono K, Tada N, Nirei T, Sunami E, et al. Hypoxia enhances colon cancer migration and invasion through promotion of epithelial-mesenchymal transition. J Surg Res. 2013;182:75–84. doi: 10.1016/j.jss.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 21.de Cuba EM, Kwakman R, van Egmond M, Bosch LJ, Bonjer HJ, Meijer GA, te Velde EA. Understanding molecular mechanisms in peritoneal dissemination of colorectal cancer: Future possibilities for personalised treatment by use of biomarkers. Virchows Arch. 2012;461:231–243. doi: 10.1007/s00428-012-1287-y. [DOI] [PubMed] [Google Scholar]
- 22.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 23.Oosterling SJ, van der Bij GJ, Bögels M, ten Raa S, Post JA, Meijer GA, Beelen RH, van Egmond M. Anti-beta1 integrin antibody reduces surgery-induced adhesion of colon carcinoma cells to traumatized peritoneal surfaces. Ann Surg. 2008;247:85–94. doi: 10.1097/SLA.0b013e3181588583. [DOI] [PubMed] [Google Scholar]
- 24.Hongo K, Tanaka J, Tsuno NH, Kawai K, Nishikawa T, Shuno Y, Sasaki K, Kaneko M, Hiyoshi M, Sunami E, et al. CD133(−) cells, derived from a single human colon cancer cell line, are more resistant to 5-fluorouracil (FU) than CD133(+) cells, dependent on the β1-integrin signaling. J Surg Res. 2012;175:278–288. doi: 10.1016/j.jss.2011.03.076. [DOI] [PubMed] [Google Scholar]
- 25.Kishikawa J, Kazama S, Oba K, Hasegawa K, Anzai H, Harada Y, Abe H, Matsusaka K, Hongo K, Oba M, et al. CD133 expression at the metastatic site predicts patients' outcome in colorectal cancer with synchronous liver metastasis. Ann Surg Oncol. 2016;23:1916–1923. doi: 10.1245/s10434-016-5099-1. [DOI] [PubMed] [Google Scholar]
- 26.Maeda K, Ding Q, Yoshimitsu M, Kuwahata T, Miyazaki Y, Tsukasa K, Hayashi T, Shinchi H, Natsugoe S, Takao S. CD133 modulate HIF-1alpha expression under hypoxia in EMT phenotype pancreatic cancer stem-like cells. Int J Mol Sci. 2016;17:1025. doi: 10.3390/ijms17071025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salnikov AV, Liu L, Platen M, Gladkich J, Salnikova O, Ryschich E, Mattern J, Moldenhauer G, Werner J, Schemmer P, et al. Hypoxia induces EMT in low and highly aggressive pancreatic tumor cells but only cells with cancer stem cell characteristics acquire pronounced migratory potential. PLoS One. 2012;7:e46391. doi: 10.1371/journal.pone.0046391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen YS, Wu MJ, Huang CY, Lin SC, Chuang TH, Yu CC, Lo JF. CD133/Src axis mediates tumor initiating property and epithelial-mesenchymal transition of head and neck cancer. PLoS One. 2011;6:e28053. doi: 10.1371/journal.pone.0028053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitsui H, Shibata K, Suzuki S, Umezu T, Mizuno M, Kajiyama H, Kikkawa F. Functional interaction between peritoneal mesothelial cells and stem cells of ovarian yolk sac tumor (SC-OYST) in peritoneal dissemination. Gynecol Oncol. 2012;124:303–310. doi: 10.1016/j.ygyno.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Neumann J, Löhrs L, Albertsmeier M, Reu S, Guba M, Werner J, Kirchner T, Angele M. Cancer stem cell markers are associated with distant hematogenous liver metastases but not with peritoneal carcinomatosis in colorectal cancer. Cancer Invest. 2015;33:354–360. doi: 10.3109/07357907.2015.1047507. [DOI] [PubMed] [Google Scholar]
- 31.Nagata H, Ishihara S, Kishikawa J, Sonoda H, Murono K, Emoto S, Kaneko M, Sasaki K, Otani K, Nishikawa T, et al. CD133 expression predicts post-operative recurrence in patients with colon cancer with peritoneal metastasis. Int J Oncol. 2018;52:721–732. doi: 10.3892/ijo.2018.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawai K, Tsuno NH, Kitayama J, Okaji Y, Yazawa K, Asakage M, Hori N, Watanabe T, Takahashi K, Nagawa H. Epigallocatechin gallate, the main component of tea polyphenol, binds to CD4 and interferes with gp120 binding. J Allergy Clin Immunol. 2003;112:951–957. doi: 10.1016/S0091-6749(03)02007-4. [DOI] [PubMed] [Google Scholar]
- 33.Iida Y, H Tsuno N, Kishikawa J, Kaneko K, Murono K, Kawai K, Ikeda T, Ishihara S, Yamaguchi H, Sunami E, et al. Lysophosphatidylserine stimulates chemotactic migration of colorectal cancer cells through GPR34 and PI3K/Akt pathway. Anticancer Res. 2014;34:5465–5472. [PubMed] [Google Scholar]
- 34.Banyard J, Bielenberg DR. The role of EMT and MET in cancer dissemination. Connect Tissue Res. 2015;56:403–413. doi: 10.3109/03008207.2015.1060970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soeda A, Park M, Lee D, Mintz A, Androutsellis-Theotokis A, McKay RD, Engh J, Iwama T, Kunisada T, Kassam AB, et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alpha. Oncogene. 2009;28:3949–3959. doi: 10.1038/onc.2009.252. [DOI] [PubMed] [Google Scholar]
- 37.Vu T, Datta PK. Regulation of EMT in colorectal cancer: a culprit in metastasis. Cancers (Basel) 2017;9:171. doi: 10.3390/cancers9120171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valenta T, Hausmann G, Basler K. The many faces and functions of β-catenin. EMBO J. 2012;31:2714–2736. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding Q, Miyazaki Y, Tsukasa K, Matsubara S, Yoshimitsu M, Takao S. CD133 facilitates epithelial-mesenchymal transition through interaction with the ERK pathway in pancreatic cancer metastasis. Mol Cancer. 2014;13:15. doi: 10.1186/1476-4598-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 41.Bukholm IK, Nesland JM, Børresen-Dale AL. Re-expression of E-cadherin, alpha-catenin and beta-catenin, but not of gamma-catenin, in metastatic tissue from breast cancer patients [seecomments] J Pathol. 2000;190:15–19. doi: 10.1002/(SICI)1096-9896(200001)190:1<15::AID-PATH489>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 42.Manzo G. Similarities between embryo development and cancer process suggest new strategies for research and therapy of tumors: a new point of view. Front Cell Dev Biol. 2019;7:20. doi: 10.3389/fcell.2019.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ata R, Antonescu CN. Integrins and cell metabolism: an intimate relationship impacting cancer. Int J Mol Sci. 2017;18:189. doi: 10.3390/ijms18010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koike T, Kimura N, Miyazaki K, Yabuta T, Kumamoto K, Takenoshita S, Chen J, Kobayashi M, Hosokawa M, Taniguchi A, et al. Hypoxia induces adhesion molecules on cancer cells: A missing link between Warburg effect and induction of selectin-ligand carbohydrates. Proc Natl Acad Sci USA. 2004;101:8132–8137. doi: 10.1073/pnas.0402088101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ju JA, Godet I, Ye IC, Byun J, Jayatilaka H, Lee SJ, Xiang L, Samanta D, Lee MH, Wu PH, et al. Hypoxia selectively enhances integrin α5β1 receptor expression in breast cancer to promote metastasis. Mol Cancer Res. 2017;15:723–734. doi: 10.1158/1541-7786.MCR-16-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryu MH, Park HM, Chung J, Lee CH, Park HR. Hypoxia-inducible factor-1alpha mediates oral squamous cell carcinoma invasion via upregulation of alpha5 integrin and fibronectin. Biochem Biophys Res Commun. 2010;393:11–15. doi: 10.1016/j.bbrc.2010.01.060. [DOI] [PubMed] [Google Scholar]
- 47.Wu X, Cai J, Zuo Z, Li J. Collagen facilitates the colorectal cancer stemness and metastasis through an integrin/PI3K/AKT/ Snail signaling pathway. Biomed Pharmacother. 2019;114:108708. doi: 10.1016/j.biopha.2019.108708. [DOI] [PubMed] [Google Scholar]
- 48.Chen WC, Chang YS, Hsu HP, Yen MC, Huang HL, Cho CY, Wang CY, Weng TY, Lai PT, Chen CS, et al. Therapeutics targeting CD90-integrin-AMPK-CD133 signal axis in liver cancer. Oncotarget. 2015;6:42923–42937. doi: 10.18632/oncotarget.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakashio T, Narita T, Akiyama S, Kasai Y, Kondo K, Ito K, Takagi H, Kannagi R. Adhesion molecules and TGF-beta1 are involved in the peritoneal dissemination of NUGC-4 human gastric cancer cells. Int J Cancer. 1997;70:612–618. doi: 10.1002/(SICI)1097-0215(19970304)70:5<612::AID-IJC20>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 50.Strobel T, Cannistra SA. Beta1-integrins partly mediate binding of ovarian cancer cells to peritoneal mesothelium in vitro. Gynecol Oncol. 1999;73:362–367. doi: 10.1006/gyno.1999.5388. [DOI] [PubMed] [Google Scholar]
- 51.Hosono J, Narita T, Kimura N, Sato M, Nakashio T, Kasai Y, Nonami T, Nakao A, Takagi H, Kannagi R. Involvement of adhesion molecules in metastasis of SW1990, human pancreatic cancer cells. J Surg Oncol. 1998;67:77–84. doi: 10.1002/(SICI)1096-9098(199802)67:2<77::AID-JSO2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 52.Zhang H, Yang N, Sun B, Jiang Y, Hou C, Ji C, Zhang Y, Liu Y, Zuo P. CD133 positive cells isolated from A549 cell line exhibited high liver metastatic potential. Neoplasma. 2014;61:153–160. doi: 10.4149/neo_2014_021. [DOI] [PubMed] [Google Scholar]
- 53.Jing F, Kim HJ, Kim CH, Kim YJ, Lee JH, Kim HR. Colon cancer stem cell markers CD44 and CD133 in patients with colorectal cancer and synchronous hepatic metastases. Int J Oncol. 2015;46:1582–1588. doi: 10.3892/ijo.2015.2844. [DOI] [PubMed] [Google Scholar]
- 54.Huang X, Sheng Y, Guan M. Co-expression of stem cell genes CD133 and CD44 in colorectal cancers with early liver metastasis. Surg Oncol. 2012;21:103–107. doi: 10.1016/j.suronc.2011.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.