Abstract
The global incidence of prostate cancer (PCa) has been increasing in recent years. Meanwhile, some studies have indicated the association between malignancies, such as lung and gastric cancer and PCa, and microRNAs (miRNAs). The present study was designed to assess the prognostic value of miR-92b-3p in patients with PCa and further investigate the biological function of miR-92b-3p. Real-time quantitative polymerase chain reaction was used to estimate the expression of miR-92b-3p in PCa tissues and cell lines compared with normal tissues and cells. Kaplan-Meier method was used to analyze the overall survival rate of patients with PCa. A Cox regression analysis was used to verify the prognostic value of miR-92b-3p. The biological function of miR-92b-3p was investigated using cell experiments. The findings of the present study revealed the upregulated expression of miR-92b-3p in PCa tissues and cells compared with normal tissues and cells. The overexpression of miR-92b-3p was significantly associated with the distant metastasis status and Tumor-Node-Metastasis stage of patients with PCa and predicted poor prognosis of PCa. In addition, the cell experiment results indicated that miR-92b-3p overexpression in PCa cells promoted cell proliferation, migration and invasion. The present study revealed that the overexpression of miR-92b-3p predicted poor prognosis in patients with PCa. Decreased expression of miR-92b-3p can suppress PCa cell proliferation, migration and invasion, which indicated that miR-92b-3p may function as an oncogene and serve as a novel therapeutic target for PCa.
Keywords: microRNA-92b-3p, prostate cancer, prognosis, proliferation, migration, invasion
Introduction
Prostate cancer (PCa) is the second most frequent malignancy found in men worldwide (1). There were 1.1 million new cases of PCa in 2012, accounting for 15% of male cancers (2). The incidence of PCa has been increasing worldwide in recent years, particularly in Asian countries, such as China, Japan, Korea and Indian (3). Statistical data indicates that PCa is the sixth leading cause of cancer-related deaths that occur in men, with an estimated 307,000 deaths in 2015, which accounted for 6.6% of total male cancer-associated mortality (4,5). There are some risk factors which may lead to PCa, such as obesity, smoking, alcohol consumption, a vasectomy and diet (6). Currently, early diagnosis and efficient treatment remain obstacles in PCa, owing to the unspecific clinical symptoms and complex disease pathogenesis (7). Although some advances have been made in tumor therapeutic strategies, such as surgery, chemotherapy and radiotherapy, the prognosis in patients with PCa remains poor (8). In addition, majority of patients with PCa suffer from severe pain, fractures and abnormal urination, which seriously reduce the quality of life of the patients (9). Therefore, it is necessary to develop novel reliable therapeutic strategies to improve the treatment of PCa.
MicroRNAs (miRNAs) are a group of non-coding small RNAs, approximately 18–22 nucleotides in length, that are involved in numerous important cell processes, such as cell proliferation, migration, invasion, differentiation and apoptosis (10). miRNAs can directly bind the 3′-untranslated region of target genes leading to inhibition in gene expression (11). Emerging studies report that miRNAs serve important regulatory roles in tumor progression making them potential therapeutic targets in various human cancers, such as lung and liver cancer (12,13). Notably, some aberrantly expressed miRNAs have also been detected in PCa, such as miR-215-5p (14) and miR-145 (15), which participate in disease pathogenesis and are associated with the prognosis of PCa. miR-92b-3p has been investigated in some human malignancies in previous studies. For instance, Long et al (16) indicated that miR-92b-3p acted as a tumor suppressor in pancreatic cancer by targeting Gabra3-associated oncogenic pathways. Notably, a study by Ma et al (17) found an overexpression of miR-92b-3p in PCa cell lines, which was associated with PCa metastasis, but this study did not investigate the clinical significance and biological function of miR-92b-3p in PCa.
To further improve PCa therapy, the present study aimed to detect the expression of miR-92b-3p in PCa tissues and cell lines and evaluate the clinical significance of miR-92b-3p. In addition, the biological function of miR-92b-3p was also investigated using gain- and loss-of-function experiments in PCa cells. The findings of the present study may provide a novel biomarker to predict PCa prognosis and a potential therapeutic target for improving the treatment of PCa.
Materials and methods
Patients and tissue collection
A total of 108 patients (average age of 66.7±9.1 years) who had been pathologically diagnosed as PCa in Shengli Oilfield Central Hospital (Dongying, China) from June 2010 to May 2013 were enrolled in the present study. PCa tissues and adjacent normal tissues (>2-cm from the edge of tumor) were obtained during resection and immediately stored in liquid nitrogen at −80°C for further use. The patients were enrolled in accordance with the following inclusion criteria: i) The tumor tissues were histopathologically diagnosed with PCa; ii) none of the patients had received any antitumor therapy prior to surgery; iii) the age range of the patients was 18–75 years; iv) had complete demographic and clinical data; and v) signed informed consent for the use of clinical samples and data. In addition, the following exclusion criteria were used: i) Cases with serious heart, liver, respiratory and kidney diseases; ii) cases with an age <18 or >75; and iii) cases that had incomplete clinical data or had no follow-up information. The collected PCa tissues were graded according to the Gleason grading system (18). In addition, the Tumor-Node-Metastasis (TNM) stage of the PCa tissues was determined using the criteria of the American Joint Committee on Cancer classification (19). In order to record the survival status of the patients, a 5-year follow-up survey was conducted, and each patient was followed up once a month by telephone. Among the 108 patients with PCa, 59 cases received antiandrogen therapy (flutamide) after surgery and 54 patients developed androgen-independent PCa. All patients had signed an informed consent form and the protocol of this study received approval from the Ethics Committee of Shengli Oilfield Central Hospital (Dongying, China; approval no. SLYTh100219).
Cell culture and transfection
Four PCa cell lines DU145, LNCaP, VCaP, 22Rv1 and one human prostate epithelial cell line RWPE1 were purchased from the Type Culture Collection of the Chinese Academy of Sciences. The PCa cells were cultured in RPMI-1640 medium (BioTek China) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific Inc.). RWPE1 cells were cultured in K-SMF medium (Gibco; Thermo Fisher Scientific Inc.) containing 5 ng/l epidermal growth factor (Gibco; Thermo Fisher Scientific Inc.) and 50 µg/ml bovine pituitary extract (Invitrogen; Thermo Fisher Scientific Inc.). All cells were maintained at 37°C in a humidified incubator with 5% CO2.
Cell lines LNCaP and DU145 were selected to perform the transfection experiments due to significantly higher expression of miR-92b-3p in the two cell lines compared with the normal cells. To regulate the expression of miR-92b-3p, 50 nM of miR-92b-3p mimic and mimic negative control (NC), and 100 nM of miR-92b-3p inhibitor and inhibitor NC were synthesized by Guangzhou RiboBio Co., Ltd.. The above sequences were separately transfected into PCa cells using Lipofectamine 2000® (Invitrogen; Thermo Fisher Scientific Inc.) at 37°C following the manufacturer's instructions. Untransfected cells were used as controls. The sequences of transfection vectors were as follows: miR-92b-3p Mimic 5′-UAUUGCACUUGUCCCGGCCUGU-3′; mimic NC 5′-UUCUCCGAACGUGUCACGU-3′; miR-92b-3p inhibitor 5′-ACAGGCCGGGACAAGUGCAAUA-3′; inhibitor NC 5′-CAGUACUUUUGUGUAGUACAA-3′. After 48 h of cell transfection, the subsequent cell experiments were performed.
RNA extraction and real-time quantitative (RT-q)PCR
Total RNA from the PCa tissues and all cell lines were extracted by using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific Inc.) according to the manufacturer's instructions. cDNA synthesis was performed using the PrimeScript RT reagent kit (Takara Bio Inc.) according to the manufacturer's instructions. The expression of miR-92b-3p was assessed using RT-qPCR, which was performed with the SYBR Green I Master Mix kit (Invitrogen; Thermo Fisher Scientific Inc.) on a 7500 Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific Inc.) with following thermocycling conditions: 95°C For 10 min and 40 cycles of 95°C for 20 sec, 58°C for 15 sec, 72°C for 20 sec. U6 was used as an internal control and the 2−ΔΔCq method (20) was used to calculate the final expression level of miR-92b-3p. The sequences of primers used were as follows: miR-92b-3p forward 5′-GCCGAGTATTGCACTTGTCC-3′, miR-92b-3p reverse 5′-CTCAACTGGTGTCGTGGA-3′; U6 forward 5′-CTCGCTTCGGCAGCACA-3′, U6 reverse 5′-AACGCTTCACGAATTTGCGT-3′.
Cell proliferation assay
DU145 and LNCaP cells were selected to perform the cell experiments following transfection with miR-92b-3p mimic, miR-92b-3p inhibitor or NCs as aforementioned. After the cells grew into a stable phase, they were seeded in 96-well plates at a density of 5×103 cells/well and cell proliferation was assessed using the MTT assay. The cells were incubated at 37°C for 3 days and 10 µl MTT (5 mg/ml; Sigma-Aldrich; Merck KGaA) was added every 24 h followed by subsequent 4 h incubation at 37°C. Subsequently, 150 µl of DMSO was added to each well and the absorbance of cells was measured using a microplate reader at 570 nm.
Cell migration and invasion assays
PCa cell migration and invasion abilities were measured using Transwell chambers (Corning Inc.). Membranes were precoated with Matrigel at 37°C for 6 h for invasion assay. Serum free RPMI-1640 medium without any drug treatment was added to the upper chambers and the lower chambers were filled with RPMI-1640 medium supplemented with 10% FBS. DU145 and LNCaP cells (5×105 cells/well) were seeded in the upper chambers. Following 48 h incubation at 37°C, the cells in the lower chambers were stained using 0.2% crystal violet for 10 min at room temperature and counted under an inverted light microscope (Olympus Corporation) with a magnification of ×200.
Statistical analysis
Data in the present study was analyzed using SPSS 21.0 software (IBM Corp.) and GraphPad Prism 7.0 software (GraphPad Software, Inc.) and were expressed as mean ± SD. Each experiment was repeated at least three times. A Chi-square test was performed to analyze the association between miR-92b-3p and the clinicopathological characteristics of patients with PCa. The differences between groups were assessed using a paired Student's t-test or one-way ANOVA followed by a post hoc Tukey's test. The Kaplan-Meier method was used to generate the survival curves of patients with PCa and the differences between survival curves were analyzed using the log-rank test. The prognostic significance of miR-92b-3p was evaluated using Cox regression analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-92b-3p is upregulated in PCa tissues and cell lines
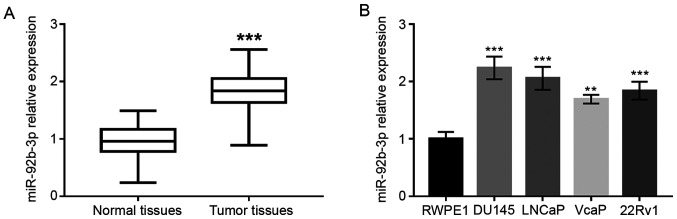
As shown in Fig. 1A, the expression of miR-92b-3p in PCa tissues was significantly upregulated compared to adjacent normal tissues (P<0.001). Upregulated expression of miR-92b-3p was also found in PCa cell lines compared with normal human prostate epithelial cells (P<0.01 or P<0.001; Fig. 1B).
Figure 1.
Expression of miR-92b-3p in patients with PCA and PCA cell lines. (A) Expression of miR-92b-3p in PCa tumor tissues was higher compared with that in adjacent normal tissues (the differences between the two groups were assessed using Student's t-test). (B) PCa cell lines had elevated miR-92b-3p expression compared with normal cells. **P<0.01; ***P<0.001. PCa, prostrate cancer; miRNA, microRNA.
Association of miR-92b-3p with clinicopathological characteristics of patients with PCa
Since miR-92b-3p was found to be upregulated in PCa tissues and cells, it was hypothesized that miR-92b-3p may be related to PCa development. Therefore, the association between miR-92b-3p expression and clinicopathological characteristics of patients with PCa were investigated. For this analysis, patients with PCa were divided into a low miR-92b-3p expression group (n=50) and high miR-92b-3p expression group (n=58) based on the mean expression value (1.824). The data were analyzed using a Chi-square test and presented in Table I. The expression of miR-92b-3p was associated with prostate-specific antigen (PSA), bone metastasis, Gleason score and TNM stage (all P<0.05; Table I). However, no relationship was found between miR-92b-3p and age (P>0.05; Table I).
Table I.
Association of miR-92b-3p expression and clinicopathological features of 108 patients with PCa.
| miR-92b-3p expression | |||||
|---|---|---|---|---|---|
| Features | Category | Total patients | Low, n=50 | High, n=58 | P-value |
| Age, years | 0.685 | ||||
| <60 | 41 | 20 | 21 | ||
| ≥60 | 67 | 30 | 37 | ||
| PSA, ng/ml | 0.009 | ||||
| <10 | 34 | 22 | 12 | ||
| ≥10 | 74 | 28 | 46 | ||
| Bone metastasis | 0.033 | ||||
| Negative | 55 | 31 | 24 | ||
| Positive | 53 | 19 | 34 | ||
| Gleason score | 0.001 | ||||
| ≤7 | 59 | 36 | 23 | ||
| >7 | 49 | 14 | 35 | ||
| TNM stage | 0.015 | ||||
| I–II | 47 | 28 | 19 | ||
| III–IV | 61 | 22 | 39 | ||
PSA, prostate-specific antigen; TNM, tumor-node-metastasis; PCa, prostate cancer.
Relationship between miR-92b-3p and overall survival of patients with PCa
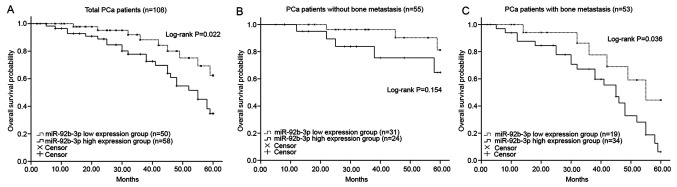
Kaplan-Meier curves revealed that patients with higher miR-92b-3p expression had a shorter overall survival rate compared with those with lower miR-92b-3p expression (log-rank P=0.022; Fig. 2A). In addition, the predictive value of miR-92b-3p for the prognosis of patients with PCa with different status of bone metastasis was evaluated. As shown in Fig. 2B and C, patients with PCa with high expression of miR-92b-3p had a shorter survival rate under both negative and positive metastasis status compared with those patients with low levels of miR-92b-3p, and high miR-92b-3p expression in patients with positive bone metastasis was significantly associated with poor overall survival (log-rank P=0.036; Fig. 2C). However, the difference of survival time between high and low miR-92b-3p groups was not statistically significant in patients with negative bone metastasis (log-rank P=0.154; Fig. 2B). Furthermore, the Cox regression analysis data revealed that the overexpression of miR-92b-3p was an independent prognostic factor for the overall survival rate of patients with PCa [hazard ratio (HR)=2.346; 95% confidence interval (CI)=1.185–5.276; P-value=0.025; Table II].
Figure 2.
Survival analysis of patients with PCa using the Kaplan-Meier method. (A) High miR-92b-3p expression was associated with shorter overall survival time in PCa patients (log-rank P=0.022). (B) Patients without bone metastasis had poor survival when the expression of miR-92b-3p was high, but the difference had no statistical significance (log-rank P=0.154). (C) In patients with bone metastasis, high miR-92b-3p was associated with poor overall survival (P=0.036). PCa, prostate cancer; miRNA, microRNA.
Table II.
Multivariate analysis for overall survival of patients with PCa using the Cox regression model.
| Variables | HR | 95% CI | P-value |
|---|---|---|---|
| Age | 1.431 | 0.589–3.425 | 0.421 |
| PSA | 1.715 | 0.845–3.503 | 0.140 |
| Bone metastasis | 1.985 | 1.018–3.742 | 0.049 |
| Gleason score | 2.161 | 1.069–4.370 | 0.032 |
| TNM stage | 2.085 | 1.042–4.185 | 0.040 |
| miR-92b-3p | 2.346 | 1.185–5.276 | 0.025 |
PSA, prostate-specific antigen; TNM, tumor-node-metastasis; PCa, prostate cancer; HR, hazard ratio; CI, confidence interval.
Overexpression of miR-92b-3p promotes PCa cell proliferation
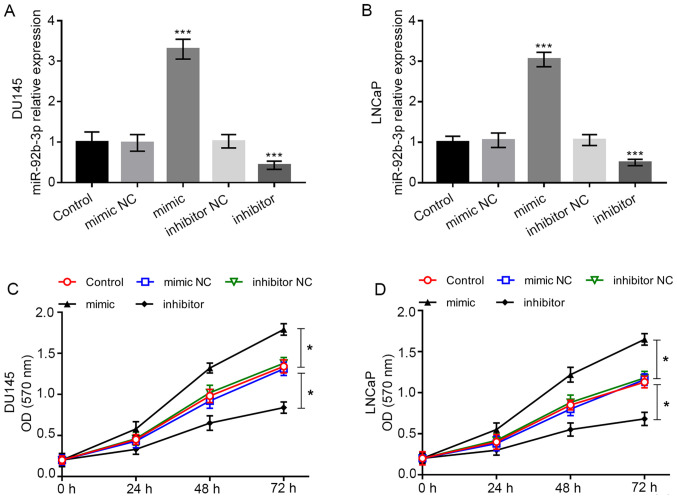
To further investigate the biological function of miR-92b-3p in PCa cells, cell experiments were conducted. Cell lines DU-145 and LNCaP were included in cell transfection as they had significantly high miR-92b-3p expression compared with the normal cell line RWPE1. The expression of miR-92b-3p was dramatically higher in cells transfected with miR-92b-3p mimic, while it was dramatically lower in cells transfected with miR-92b-3p inhibitor compared with the cells in control group (all P<0.001; Fig. 3A and B). Using the MTT assay, it was found that the overexpression of miR-92b-3p promoted tumor cell proliferation, whereas downregulation of miR-92b-3p inhibited the cell proliferation of DU-145 and LNCaP cells (all P<0.05; Fig. 3C and D).
Figure 3.
Regulatory effect of miR-92b-3p on cell proliferation of DU145 and LNCaP cells. (A and B) Expression of miR-92b-3p was upregulated by the miR-92b-3p mimic, but was downregulated by the miR-92b-3p inhibitor compared with cells in the control group. (C and D) Overexpression of miR-92b-3p in PCa cells led to increased cell proliferation, while the downregulation of miR-92b-3p inhibited cell proliferation. *P<0.05, ***P<0.001 compared with the untransfected control group. PCa, prostate cancer; miRNA, microRNA; OD, optical density; NC, negative control.
Overexpression of miR-92b-3p promotes cell migration and invasion of PCa cells
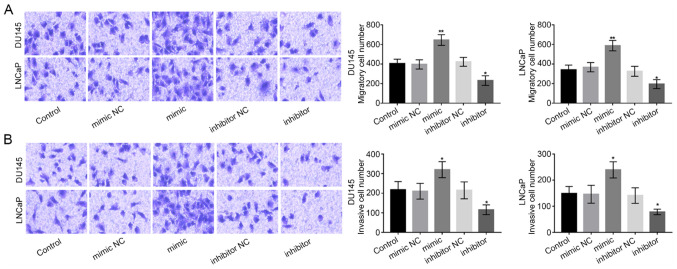
Subsequently, Transwell chambers were used to measure the migration and invasion abilities of DU-145 and LNCaP cells. The migration ability of PCa cells was promoted by miR-92b-3p expression overexpression, while was inhibited by the downregulation of miR-92b-3p (P<0.05 or P<0.01; Fig. 4A). As for the invasion ability, we found that the overexpression of miR-92b-3p significantly boosted PCa cell invasion, while the downregulation of miR-92b-3p significantly reduced PCa cell invasion (all P<0.05; Fig. 4B).
Figure 4.
miR-92b-3p accelerated PCa cell migration and invasion abilities in DU145 and LNCaP cells. (A) PCa cell migration was enhanced by the overexpression of miR-92b-3p, but was inhibited by the silencing of miR-92b-3p. (B) The cell invasion was promoted in PCa cells with overexpression of miR-92b-3p, but was suppressed in cells with decreased miR-92b-3p. *P<0.05 and **P<0.01 compared with untransfected control group. PCa, prostate cancer; miRNA, microRNA; NC, negative control.
Discussion
There is growing evidence that indicates that miRNAs, which can transmit signals and regulate intracellular gene expression, serve important roles in tumor development and progression (21). Additionally, some studies have also found that miRNAs serve as tumor suppressors or oncogenes involved in tumor progression (22,23). For example, a study by Wang et al (24) revealed that miR-66a-3p expression in gastric cancer tissues and cells was upregulated, which may function as an oncogene by targeting the Hippo pathway. Additionally, miR-506 was downregulated in cervical cancer tissues, which further showed that miR-506 was able to suppress tumor cell proliferation and can serve as a novel therapeutic target of cervical cancer (25). Similarly, in PCa tissues, some miRNAs with ectopic expression have also been found. For instance, a study by Zhang et al (26) found that downregulation of miR-410-3p can accelerate PCa cell apoptosis and suppress cell proliferation, migration and epithelial-mesenchymal transition progress and exert oncogenic functions by downregulating PTEN. A study also showed that the downregulation of miR-375 presented better discriminating performance compared with prostate-specific antigen indicating that miR-375 had stronger diagnostic accuracy and can be used as a non-invasive biomarker for PCa screening (27). The aforementioned studies demonstrated the importance of identifying novel miRNAs that affect tumor progression to improve the treatment of PCa.
The present study focused on the expression and functional role of miR-92b-3p in PCa. miR-92b-3p has been previously investigated in some cancers. For example, the overexpression of miR-92b-3p was detected in gastric cancer SGC-7901 cells, which inhibited SGC-7901 cell proliferation, migration and invasion via downregulating matrix metalloproteinase-2/9 expression and targeting homeobox D10 (28). Another study by Gong et al (29) revealed that miR-92b-3p inhibition prevented colorectal carcinoma cell proliferation, invasion, and migration by upregulating F-box with WD repeated domain-containing 7 (FBXW7). Notably, a previous study by Ma et al has reported miR-92b-3p was deregulated in PCa cells and this may be related with chemoresistance of tumor cells (17). Nevertheless, the expression of miR-92b-3p in PCa tissues and its clinical and functional role in PCa progression remain largely elusive. In the present study, miR-92b-3p was upregulated in PCa tissues and cell lines compared with normal tissues and normal cells, and the expression of miR-92b-3p was associated with PSA, bone metastasis, TNM stage and Gleason score of patients with PCa, which suggested that miR-92b-3p might be involved in PCa development. In addition, the recorded 5-year follow-up survival information analyzed by Kaplan-Meier survival curves demonstrated that the patients with higher miR-92b-3p expression had shorter overall survival rates compared with those patients with lower miR-92b-3p levels. Besides, in patients with PCA who had positive bone metastasis, high levels of miR-92b-3p were also associated with shorter overall survival time when compared to patients with low levels of miR-92b-3p. Although the survival time in patients without bone metastasis was also shorter when they had high expression of miR-92b-3p, but the difference did not reach statistically significance, which may due to the limited sample size. In addition, Cox regression analysis further revealed that miR-92b-3p was an independent prognostic factor for PCa. According to these findings of the present study, miR-92b-3p may serve as a biomarker for PCa prognosis.
A number of studies have provided evidence for the therapeutic potential of miRNAs in a wide variety of human cancers, including PCa (30,31). The proposed functional miRNAs exert therapeutic potential by regulating tumor cell biological processes, such as cell proliferation, migration and invasion (32). Thus, cell experiments were conducted in the present study to investigate the functional role of miR-92b-3p in PCa progression. The expression of miR-92b-3p was regulated by miR-92b-3p mimic or inhibitor following transfection. The MTT assay findings of the present study revealed that the overexpression of miR-92b-3p promoted cell proliferation, migration and invasion, while the downregulation of miR-92b-3p led to opposite results, which suggested that miR-92b-3p may function as an oncogene in PCa progression. The oncogenic role of miR-92b-3p has also been demonstrated in other malignancies, such as colorectal carcinoma and gastric cancer (28,29). FBXW7 has been identified as a tumor suppressor in the progression of non-small cell lung carcinoma (NSCLC), and was related with the chemoresistance of NSCLC (33,34). Whether miR-92b-3p could regulate FBXW7 in NSCLC is unclear, and whether miR-92b-3p could be involved in the chemoresistance of NSCLC through targeting FBXW7 is also uncertain.
There were some limitations to the present study. First, the sample size was relatively small, which may limit the accuracy of analysis results, such as the Kaplan-Meier survival analysis for patients with PCa without bone metastasis. Second, although the potential target genes of miR-92b-3p were identified, the exact target of miR-92b-3p in PCa was not explored. Thus, the results and conclusion should be confirmed and improved by further studies with a larger study population and mechanism-related investigations.
In conclusion, the present study found that miR-92b-3p was upregulated in PCa tissues and cells compared with normal controls. The overexpression of miR-92b-3p predicted poor prognosis of patients with PCa and can be used as an independent prognostic biomarker. Downregulation of miR-92b-3p is able to suppress cell proliferation, migration and invasion of PCa cells. Based on these findings, miR-92b-3p may act as a potential therapeutic target for patients with PCa.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and material
All data analyzed during this study are included in the published article.
Authors' contributions
GW, BC and WL conducted this study, analyzed the clinical data and wrote the manuscript. RJ and BT performed the cell experiments and analyzed the corresponding data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
All patients provided signed informed consent and the present study received approval from the Ethics Committee of Shengli Oilfield Central Hospital (Dongying, China; approval no. SLYTh100219).
Patient consent for publication
Consent for publication was obtained from the patients.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Castillejos-Molina RA, Gabilondo-Navarro FB. Prostate cancer. Salud Publica Mex. 2016;58:279–284. doi: 10.21149/spm.v58i2.7797. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Pu YS, Chiang HS, Lin CC, Huang CY, Huang KH, Chen J. Changing trends of prostate cancer in Asia. Aging Male. 2004;7:120–132. doi: 10.1080/13685530412331284687. [DOI] [PubMed] [Google Scholar]
- 4.Kimura T, Egawa S. Epidemiology of prostate cancer in Asian countries. Int J Urol. 2018;25:524–531. doi: 10.1111/iju.13593. [DOI] [PubMed] [Google Scholar]
- 5.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 6.Perdana NR, Mochtar CA, Umbas R, Hamid AR. The risk factors of prostate cancer and its prevention: A literature review. Acta Med Indones. 2016;48:228–238. [PubMed] [Google Scholar]
- 7.Redman JM, Gulley JL, Madan RA. Combining immunotherapies for the treatment of prostate cancer. Urol Oncol. 2017;35:694–700. doi: 10.1016/j.urolonc.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gasnier A, Parvizi N. Updates on the diagnosis and treatment of prostate cancer. Br J Radiol. 2017;90:20170180. doi: 10.1259/bjr.20170180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powell Gray B, Kelly L, Ahrens DP, Barry AP, Kratschmer C, Levy M, Sullenger BA. Tunable cytotoxic aptamer-drug conjugates for the treatment of prostate cancer. Proc Natl Acad Sci USA. 2018;115:4761–4766. doi: 10.1073/pnas.1717705115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rupaimoole R, Calin GA, Lopez-Berestein G, Sood AK. miRNA deregulation in cancer cells and the tumor microenvironment. Cancer Discov. 2016;6:235–246. doi: 10.1158/2159-8290.CD-15-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esquela-Kerscher A, Slack FJ. Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 12.Markou A, Zavridou M, Lianidou ES. miRNA-21 as a novel therapeutic target in lung cancer. Lung Cancer (Auckl) 2016;7:19–27. doi: 10.2147/LCTT.S60341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu HM, Kim SG. miRNA-324, a potential therapeutic target for paracetamol-induced liver injury. Stem Cell Investig. 2016;3:67. doi: 10.21037/sci.2016.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen JY, Xu LF, Hu HL, Wen YQ, Chen D, Liu WH. miRNA-215-5p alleviates the metastasis of prostate cancer by targeting PGK1. Eur Rev Med Pharmacol Sci. 2020;24:639–646. doi: 10.26355/eurrev_202001_20040. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Wu J. Prognostic role of microRNA-145 in prostate cancer: A systems review and meta-analysis. Prostate Int. 2015;3:71–74. doi: 10.1016/j.prnil.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long M, Zhan M, Xu S, Yang R, Chen W, Zhang S, Shi Y, He Q, Mohan M, Liu Q, Wang J. miR-92b-3p acts as a tumor suppressor by targeting Gabra3 in pancreatic cancer. Mol Cancer. 2017;16:167. doi: 10.1186/s12943-017-0723-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma H, Wang LY, Yang RH, Zhou Y, Zhou P, Kong L. Identification of reciprocal microRNA-mRNA pairs associated with metastatic potential disparities in human prostate cancer cells and signaling pathway analysis. J Cell Biochem. 2019;120:17779–17790. doi: 10.1002/jcb.29045. [DOI] [PubMed] [Google Scholar]
- 18.Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA, Grading Committee The 2014 international society of urological pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma: Definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40:244–252. doi: 10.1097/PAS.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 19.Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, Borgen PI, Clark GM, Edge SB, Hayes DF, et al. Staging system for breast cancer: Revisions for the 6th edition of the AJCC cancer staging manual. Surg Clin North Am. 2003;83:803–819. doi: 10.1016/S0039-6109(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y, Song Y, Yao L, Song G, Teng C. Circulating microRNAs: Promising biomarkers involved in several cancers and other diseases. DNA Cell Biol. 2017;36:77–94. doi: 10.1089/dna.2016.3426. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg BA, Fang JC. Long-term outcomes of acute heart failure: Where are we now? J Am Coll Cardiol. 2017;70:2487–2489. doi: 10.1016/j.jacc.2017.08.075. [DOI] [PubMed] [Google Scholar]
- 23.Zhao L, Yu H, Yi S, Peng X, Su P, Xiao Z, Liu R, Tang A, Li X, Liu F, Shen S. The tumor suppressor miR-138-5p targets PD-L1 in colorectal cancer. Oncotarget. 2016;7:45370–45384. doi: 10.18632/oncotarget.9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Li B, Zhang L, Li Q, He Z, Zhang X, Huang X, Xu Z, Xia Y, Zhang Q, et al. miR-664a-3p functions as an oncogene by targeting Hippo pathway in the development of gastric cancer. Cell Prolif. 2019;52:e12567. doi: 10.1111/cpr.12567. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Gong M, Chen C, Zhao H, Sun M, Song M. miR-506 suppresses cervical cancer cell proliferation both in vitro and in vivo. Neoplasma. 2018;65:331–338. doi: 10.4149/neo_2018_170112N25. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Zhang D, Lv J, Wang S, Zhang Q. miR-410-3p promotes prostate cancer progression via regulating PTEN/AKT/mTOR signaling pathway. Biochem Biophys Res Commun. 2018;503:2459–2465. doi: 10.1016/j.bbrc.2018.06.176. [DOI] [PubMed] [Google Scholar]
- 27.Kachakova D, Mitkova A, Popov E, Popov I, Vlahova A, Dikov T, Christova S, Mitev V, Slavov C, Kaneva R. Combinations of serum prostate-specific antigen and plasma expression levels of let-7c, miR-30c, miR-141, and miR-375 as potential better diagnostic biomarkers for prostate cancer. DNA Cell Biol. 2015;34:189–200. doi: 10.1089/dna.2014.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C, Huo B, Wang Y, Cheng C. Downregulation of microRNA-92b-3p suppresses proliferation, migration, and invasion of gastric cancer SGC-7901 cells by targeting Homeobox D10. J Cell Biochem. 2019;120:17405–17412. doi: 10.1002/jcb.29005. [DOI] [PubMed] [Google Scholar]
- 29.Gong L, Ren M, Lv Z, Yang Y, Wang Z. miR-92b-3p promotes colorectal carcinoma cell proliferation, invasion, and migration by inhibiting FBXW7 in vitro and in vivo. DNA Cell Biol. 2018;37:501–511. doi: 10.1089/dna.2017.4080. [DOI] [PubMed] [Google Scholar]
- 30.Arisan ED, Rencuzogullari O, Freitas IL, Radzali S, Keskin B, Kothari A, Warford A, Uysal-Onganer P. Upregulated Wnt-11 and miR-21 expression trigger epithelial mesenchymal transition in aggressive prostate cancer cells. Biology (Basel) 2020;9:52. doi: 10.3390/biology9030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krebs M, Solimando AG, Kalogirou C, Marquardt A, Frank T, Sokolakis I, Hatzichristodoulou G, Kneitz S, Bargou R, Kübler H, et al. miR-221-3p regulates VEGFR2 expression in high-risk prostate cancer and represents an escape mechanism from sunitinib in vitro. J Clin Med. 2020;9:670. doi: 10.3390/jcm9030670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng C, Guo K, Chen B, Wen Y, Xu Y. miR-214-5p inhibits human prostate cancer proliferation and migration through regulating CRMP5. Cancer Biomark. 2019;26:193–202. doi: 10.3233/CBM-190128. [DOI] [PubMed] [Google Scholar]
- 33.Hidayat M, Mitsuishi Y, Takahashi F, Tajima K, Yae T, Miyahara K, Hayakawa D, Winardi W, Ihara H, Koinuma Y, et al. Role of FBXW7 in the quiescence of gefitinib-resistant lung cancer stem cells in EGFR-mutant non-small cell lung cancer. Bosn J Basic Med Sci. 2019;19:355–367. doi: 10.17305/bjbms.2019.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao G, Li Y, Wang M, Li X, Qin S, Sun X, Liang R, Zhang B, Du N, Xu C, et al. FBXW7 suppresses epithelial-mesenchymal transition and chemo-resistance of non-small-cell lung cancer cells by targeting snai1 for ubiquitin-dependent degradation. Cell Prolif. 2018;51:e12473. doi: 10.1111/cpr.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed during this study are included in the published article.