Abstract
Members of the ten-eleven translocation (TET) protein family of which three mammalian TET proteins have been discovered so far, catalyze the sequential oxidation of 5-methylcytosine to 5-hydroxymethylcytosine, 5-formylcytosine, and 5-carboxylcytosine which serve an important role in embryonic development and tumor progression. O-GlcNAcylation (O-linked β-N-acetylglucosaminylation) is a reversible post-translational modification known to serve important roles in tumorigenesis and metastasis especially in hematopoietic malignancies such as myelodysplastic syndromes, chronic myelomonocytic leukemia and acute myeloid leukemia. O-GlcNAcylation activity requires only two enzymes: O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA). OGT catalyzes attachment of GlcNAc sugar to serine, threonine and cytosine residues in proteins, while OGA hydrolyzes O-GlcNAc attached to proteins. Numerous recent studies have demonstrated that TETs can be O-GlcNAcylated by OGT, with consequent alteration of TET activity and stability. The present review focuses on the cellular, biological and biochemical functions of TET and its O-GlcNAcylated form and proposes a model of the role of TET/OGT complex in regulation of target proteins during cancer development. In addition, the present review provides directions for future research in this area.
Keywords: TET, O-GlcNAcylation
1. Introduction
Methylation of cytosines, a common epigenetic modification in eukaryotic cells, serves an important role in a variety of genetic processes, including gene stability and expression, chromosome accessibility and inactivation, and nucleosome positioning (1). 5-methylcytosine (5mC) is produced by DNA methyltransferase activity and is located in CG dinucleotides in DNA (2). Ten-eleven translocation (TET) family proteins participate in oxidation reactions of 5mC to 5-hydroxymethylcytosine, 5-formylcytosine, and 5-carboxylcytosine (5hmC, 5fC, 5caC), which further decrease DNA methylation patterns (2).
Post-translational modifications (PTMs) of proteins facilitate immediate responses of cells to intracellular or extracellular environmental stimuli by modifying functions of targeted proteins (3). PTMs are involved in various pathological processes such as proliferation, apoptosis and migration in tumors (3). O-linked β-N-acetylglucosaminylation (O-GlcNAcylation) is an atypical, dynamic and reversible PTM consisting of addition of N-acetyl-D-glucosamine, a unique non-elongated monosaccharide on proteins (4). Unlike classical glycosylation present in the endoplasmic reticulum and Golgi apparatus, O-GlcNAcylation takes place in the cytoplasm, nucleus and mitochondria and is implicated in a wide range of effects on cellular function and signaling in metabolic diseases and cancer (5). Compared to complex glycosyltransferase and glycosidase system of classical glycosylation, O-GlcNAcylation is only regulated by two enzymes: The glycosyltransferase OGT (O-GlcNAc transferase) and the glycoside hydrolase OGA (O-GlcNAcase) (3). Numerous recent studies indicate a close connection between OGT and TET (5–7). OGT can catalyze TET to form O-GlcNAcylated TET and can also interact with TET to form a complex with the ability to further modify chromatin which participated in regulating embryonic development and cancer progression (6,7). The present review focuses on the functional roles of TET family proteins and O-GlcNAcylation in cancer progression, with focus on the connection between TET proteins and OGT to clarify the effects of these proteins on cancer development.
2. TET family proteins
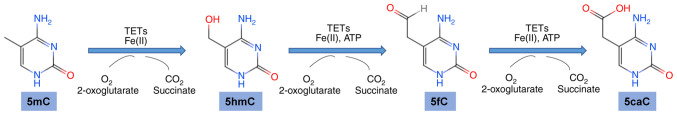
Epigenetic modifications, which include DNA methylation and histone modifications can alter gene expression but cannot change the primary sequence of DNA (8) Epigenetic modifications has been proven to be widely involved in tumor development (9–11). DNA hypermethylation is observed in myelodysplastic syndromes (MDS), acute myeloid leukemia (AML), colorectal cancer, hepatocellular carcinoma and ovarian cancer (12–14). TET family proteins function as DNA hydroxymethylases in vertebrates and can catalyze conversion of 5mC to 5hmC, and subsequently to 5fC and 5caC (15) (Fig. 1). These three versions of oxidized methylcytosines are all associated with DNA demethylation (16,17).
Figure 1.
TET proteins catalyze conversion of 5mC to 5hmC, 5fC and 5caC. Fe, ferrous; ATP, adenosine triphosphate; TETs, ten-eleven translocation family proteins; 5mC, 5-methylcytosine; 5hmC, 5-hydroxymethylcytosine; 5-fc, 5-formylcytosine; 5-caC, 5-carboxylcytosine.
TET proteins have a common cysteine-rich dioxygenase region and C-terminal region which binds to ferrous iron and α-ketoglutarate and catalyzes an oxidation reaction which involves hydroxylation of 5mC to 5hmC and further to 5fC and 5caC (12). The three TET proteins (TET1, TET2, TET3) have differing N-terminal regions (18). TET1 and TET3 have a CXXC-type zinc finger domain (19). TET2 has no CXXC DNA-binding domain, but can interact with a CXXC domain protein, inhibitor of disheveled Dvl and Axin complex (IDAX) (18).
TET proteins are highly expressed in embryonic stem cells (ESCs) (20). They are essential for ESC differentiation during embryogenesis and help regulate homeostasis of hematopoietic stem cells, mesenchymal stem celsl and progenitor cells (21). TET1 and TET2 are upregulated in ESCs and TET3 in oocytes (22). Expression of TET proteins is closely related to tumor malignancy (23–25); and their expression is significantly lower in tumor tissues compared with normal tissues (24,25). TET2 mutation is often observed in hematopoietic neoplasms including myelodysplastic syndromes and chronic myelomonocytic leukemia (26–28). TET2 expression enhances self-renewal, proliferation potential, osteoblast differentiation and hematopoietic supportive capacity of bone marrow stem cells in humans and mice (29). Li et al reported somatic mutation frequencies of the TET2 gene as 30% in MDS, 20% in myeloproliferative neoplasms, 42% in chronic myelomonocytic leukemia and 20% in AML (30). In breast cancer cells, TET2 occupies active enhancers and facilitates proper recruitment of estrogen receptor α, which then transcriptionally activates TET2 expression to establish a positive feedback loop between TET2 and estrogen signaling (31). TET2 also exerts tumor-promoting effects in melanoma and osteosarcoma cells (32,33). TET2 expression is enhanced in tumor-associated macrophages and myeloid-derived suppressor cells, and TET2 deletion in myeloid cells results in inhibition of melanoma growth (32). TET2 can target the promoter of interleukin-6 (IL-6) to increase its expression, and elevated IL-6 may promote lung cancer cell metastasis (33).
Studies on aberrant expression of the three types of TET proteins in various types of cancer are summarized in Table I.
Table I.
Aberrant expression of TET proteins in various cancers.
| Name of protein | Cancer types | Alteration | Consequent effects | (Refs.) |
|---|---|---|---|---|
| TET1 | Breast cancer, colon cancer, liver cancer | Decreased expression | Advanced cancer stage, nodal metastases and poor overall survival | (64,72) |
| TET2 | Leukemia, lymphoma | Mutation | Aberrant self-renewal and advanced-stage disease | (73–76) |
| TET3 | Ovarian cancer | Increased expression | Unfavorable prognosis | (77) |
| TET3 | Glioblastoma | Decreased expression | Promoted tumorigenesis | (78) |
TETs, ten-eleven translocation family proteins.
3. O-GlcNAcylation
O-GlcNAcylation is a reversible PTM that typically targets proteins in the cytoplasm, cell nuclei (34), or mitochondria (35). It can regulate cellular processes at various levels, such as transcription, translation, signal transduction or cell metabolism (36). In general, proteins modified by O-GlcNAcylation are phosphoproteins or parts of macromolecular complexes (phosphoglycerate kinase 1), transcription complexes (p53, c-myc), or nucleopores (transmembrane nucleoporin Pom121, nucleoporin 155) (37). O-GlcNAcylation has also been reported for numerous functional proteins, including epigenetic regulation factors including the TET proteins, the SIN3 transcription regulator family member A-histone deacetylases and the Polycomb group proteins that regulate DNA methylation, chromatin accessibility and chromatin modification (38).
O-GlcNAcylation often affects subcellular localization, stability and function of target proteins (7,39), and in some cases helps modulate protein phosphorylation status, protein stability, enzymatic activity, protein aggregation and interactions with other proteins or DNA (5,36,40). O-GlcNAc activity requires two enzymes: OGT and OGA (41). OGT catalyzes attachment of GlcNAc to serine (Ser), threonine (Thr) and cysteine residues in proteins (40,42).
OGT activity is highly sensitive to the uridine diphosphate GlcNAc level, and is altered by variations of glucose, glutamate or free fatty acid levels in cells (43). OGT activity is associated with epithelial-mesenchymal transition (EMT), p53, Wnt and TGF-β signaling pathways, inflammatory responses and apoptosis in cervical cancer cells (44). Knockdown of OGT in colon cells results in upregulation and altered glycosylation of E-cadherin, an important factor in EMT progression and may disrupt biosynthesis of glycosphingolipids (lactosylceramide, gangliosides and globosides), with consequent reduction of gangliosides (ganglioside 3 and ganglioside 2) but increase of globosides (globoside 3 and globoside 4) (45). Chronic lymphocytic leukemia (CLL) cells demonstrate high expression of O-GlcNAcylated proteins, including p53, c-Myc, and Akt and enhanced protein glycosylation alters intracellular signaling processes (p53 and PI3K/AKT/mTOR signaling pathways) in these cells (46). O-GlcNAcylation increases downstream signaling of toll-like receptors following cytokine stimulation in CLL cells (46). On the other hand, high baseline O-GlcNAc levels inhibit responses to such stimulation, resulting in increased resistance to TLR agonists, chemotherapeutic agents, B cell receptor crosslinking and mitogens (46). Hart et al (36) reported that increased O-GlcNAcylation of Thr58 on c-Myc inhibited c-Myc activity and reduced transformation of non-Hodgkin's lymphoma cells.
OGA has O-GlcNAc hydrolase and associated enzymatic activity of lysine acetyltransferase (47–49) and can therefore hydrolyze O-GlcNAc residues from attached proteins (41). Inhibition of OGA expression in rats and mice resulted in increased O-GlcNAcylation of all tissues (50). OGA shows high mRNA expression in lung, colon and breast cancers (49). In colon cell lines, O-GlcNAcylation level was increased by inhibition of OGA but decreased by inhibition of OGT (51). OGA serves an essential role in differentiation of ESCs (52,53). Blocking of O-GlcNAc cycling in mice by OGA knockdown resulted in anatomical defects and notable changes in expression of pluripotency markers such as Nanog, Sox2 and Orthodenticle homeobox 2 (53). OGA knockdown in mouse hematopoietic stem cells reduced progenitor pools, reduced cell stemness of cells, altered transcription of several crucial genes such as hypoxia inducible factor-1α and cyclin dependent kinase inhibitor 1C and increased apoptotic cell number in bone marrow (54).
4. O-GlcNAcylation of TETs
TET proteins mediate DNA demethylation, while OGT mediates protein O-GlcNAcylation (39,55). These two enzymatic activities may seem to be independent of each other. However, several recent studies have revealed the physical and functional interactions between TETs and OGT.
Firstly, TETs can be O-GlcNAcylated by OGT (6,56–58). Addition of a GlcNAc group to Ser and Thr residues of TET proteins inhibits TET phosphorylation, since Ser and Thr are potential phosphorylation sites (59). Cross-talk between modified Ser and Thr residues facilitates rapid adaptation of TET protein localization, activity, or targeting in response to altered environmental conditions or other external stimuli (6,59). Secondly, TETs preferentially associate with or bind to OGT in certain gene promoters located close to CpG-rich transcription start sites, hence regulating transcriptional levels of these genes through epigenetic modification (6). A large proportion of nuclear OGT is complexed with TETs (60). Such TET/OGT-occupied promoter regions are characterized by low levels of DNA modification, suggesting that TET demethylation activity serves a role in regulation of CpG island methylation (6). OGT in TET/OGT complexes also mediates O-GlcNAcylation of nearby histone H2B at Ser112, thereby facilitating lysine120 ubiquitination of H2B and transcriptional activation (61), particularly near transcription start sites (62). Thirdly, the TET/OGT complex can serve as a scaffold for epigenetic complexes, in addition to its own demethylation and O-GlcNAcylation activities (57,63). Host Cell Factor 1, a component of the H3K4 methyltransferase SET1/COMPASS complex (63), can be O-GlcNAcylated by OGT and bind further to the TET2/3/OGT complex to mediate transcriptional activation through methylation on histone 3 lysine 4 (6). TET/OGT complex can also interact with chromatin regulator SIN3 transcription regulator family member A and with several components of the nucleosome remodeling and deacetylase complex, hence enhancing expression of downstream genes, such as single stranded DNA binding protein 2 and LIM homeobox 2 regulated by TET and maintaining ESC pluripotency (57).
Although TET proteins and OGT have been hot topics of research in recent years, very limited knowledge of TET function and TET O-GlcNAcylation in cancer development and progression exists. TET/OGT complex contributes to certain epigenetic modifications, such as DNA demethylation, histone O-GlcNAcylation, histone methylation associated with positive regulation of gene expression, hence providing a direct link between epigenetics and cellular metabolism (62). Hsu et al (64) reported that TET1 demonstrated reduced expression in prostate and breast cancers, and suppresses cancer cell invasion by promoting expression of tissue inhibitors of metalloproteinases. In a study of cervical cancer cells, Guan et al (65) observed that nuclear localization and O-GlcNAcylation of TET3 were modulated by glucose metabolism, and that gene expression was regulated through TET/OGT-mediated epigenetic changes in response to nutrient availability. The role of O-GlcNAcylated TET proteins in cancer progression is an exciting topic for future study. Based on current finding in the field, a working model of the role of TET/OGT complex in regulation of target proteins during cancer development may be proposed (Fig. 2).
Figure 2.
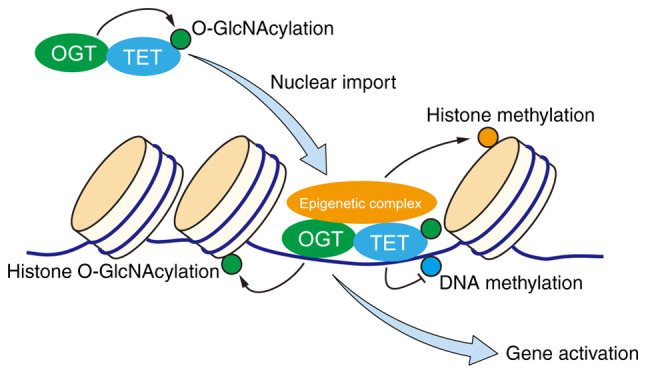
Proposed working model of the role of TET/OGT complex in regulation of target proteins during cancer development. TET/OGT complex can work as an epigenetic complex participating in the progress of DNA demethylation. Meanwhile, TET also facilitates the O-GlcNAc modification of histones regulated by OGT. The black arrow represents the product synthesized by the OGT, TET or TET/OGT complex. The blue arrow above represents nuclear import. The blue arrow below represents gene activation. TETs, ten-eleven translocation family proteins; OGT, O-GlcNAc transferase; O-GlcNAcylation, O-linked β-N-acetylglucosaminylation.
5. Discussion
The TET proteins (TET1, TET2, TET3) catalyze conversion of 5mC to 5hmC. O-GlcNAcylation is a reversible PTM and it can O-GlcNAcylate TETs (59). OGT and O-GlcNAcylation have been clearly demonstrated to serve an important role in tumorigenesis and metastasis (66). TET proteins can recruit the OGT to chromatin, which promotes post-transcriptional modifications of histones and facilitates gene expression (40). It was reported that TET2 mediates OGT modification on H2B Ser112 and is associated with highly transcribed genes (62). In addition, TET/OGT complex can serve as the scaffold for epigenetic complexes (7,63).
However, the present review had some limitations, such as the research of TET/OGT complex mainly focused on the function during embryonic development (6,7,55). The role of TETs and their O-GlcNAcylation in cancer development is largely unknown (60). The essential characteristic of cancer is uncontrolled cell proliferation resulting from accumulated alterations of cell metabolism and signaling pathways (67). One trait of cancer initiation is the dynamics of O-GlcNAcylation are highly sensitive to availability of nutrients and oxygen, determined by the cellular microenvironment (68). Aberrant glucose metabolism in cancer cells may alter O-GlcNAcylation of TET proteins and therefore affect their stability; conversely, TET loss-of function in cancer may influence the nuclear and/or cytoplasmic distribution of OGT, which in turn may affect the stability of tumor suppressors and oncogenes such as p53 (69), MYC (70), and β-catenin (71). The dysregulated expression and loss-of-function mutation of TET family proteins participated in the progress of a variety of cancers especially hematopoietic malignancies (29). Hence, it is logical to raise the question about whether TET/OGT is involved in cancer development and how they get involved. TETs can be post-translationally modified by the nutrient-sensing enzyme OGT, also suggesting a connection between metabolism and the epigenome (6,62). In addition to suggesting a broader role for the TET/OGT complex, the present review provides information about the interaction between OGT and TET proteins, which may provide new insights into the development of cancer.
6. Conclusions
TET proteins can interact with and undergo O-GlcNAcylation by OGT and O-GlcNAcylation can alter properties of TET enzymes (62). TET/OGT complexes are primarily targeted to promoter regions through interaction of TET with DNA, and TET-linked OGT can O-GlcNAcylate a wide variety of proteins (58). Relationships between OGT and TETs during cancer pathological processes remain to be elucidated. Identification of modified proteins present upstream and downstream of TET/OGT complex will be useful in this regard. The functions of TETs and their O-GlcNAcylation in cancer development is an important topic for future studies.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- TET protein
ten-eleven translocation family protein
- OGT
O-GlcNAc transferase
- OGA
O-GlcNAcase
- PTM
post-translational modification
- 5mC
5-methylcytosine
- 5hmC
5-hydroxymethylcytosine
- MDS
myelodysplastic syndromes
- AML
acute myeloid leukemia
- ESC
embryonic stem cell
- IL-6
interleukin-6
- Ser
serine
- Thr
threonine
- O-GlcNAcylation
O-linked β-N-acetylglucosaminylation
- EMT
epithelial-mesenchymal transition
- CLL
chronic lymphocytic leukemia
Funding
This study was supported by grants from the National Natural Science Foundation of China (grant nos. 81470294, 81770123 and 32071274), National Science and Technology Major Project of China (grant no. 2018ZX10302205), 13115 Key Projects of Scientific and Technical Innovation of Shaanxi Province (grant no. 2010ZDKG-53), Natural Science Foundation of Shaanxi Province (grant no. 2018JM3014), Youth Innovation Team of Shaanxi Universities, and Hundred-Talent Program of Shaanxi Province.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XL, XCP, HJL, and BXL designed the study and co-wrote the manuscript. YW, FG, and YY were involved in study conception and design, and revised the manuscript for important intellectual content. All authors have read and approved the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Schubeler D. Function and information content of DNA methylation. Nature. 2015;517:321–326. doi: 10.1038/nature14192. [DOI] [PubMed] [Google Scholar]
- 2.Scott-Browne JP, Lio CJ, Rao A. TET proteins in natural and induced differentiation. Curr Opin Genet Dev. 2017;46:202–208. doi: 10.1016/j.gde.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X, Qian K. Protein O-GlcNAcylation: Emerging mechanisms and functions. Nat Rev Mol Cell Biol. 2017;18:452–465. doi: 10.1038/nrm.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wells L, Vosseller K, Hart GW. Glycosylation of nucleocytoplasmic proteins: Signal transduction and O-GlcNAc. Science. 2001;291:2376–2378. doi: 10.1126/science.1058714. [DOI] [PubMed] [Google Scholar]
- 5.Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 6.Vella P, Scelfo A, Jammula S, Chiacchiera F, Williams K, Cuomo A, Roberto A, Christensen J, Bonaldi T, Helin K, Pasini D. Tet proteins connect the O-linked N-acetylglucosamine transferase Ogt to chromatin in embryonic stem cells. Mol Cell. 2013;49:645–656. doi: 10.1016/j.molcel.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 7.Hrit J, Goodrich L, Li C, Wang BA, Nie J, Cui X, Martin EA, Simental E, Fernandez J, Liu MY, et al. OGT binds a conserved C-terminal domain of TET1 to regulate TET1 activity and function in development. Elife. 2018;7:e34870. doi: 10.7554/eLife.34870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baylin SB, Jones PA. A decade of exploring the cancer epigenome-biological and translational implications. Nat Rev Cancer. 2011;11:726–734. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darılmaz Yüce G, Ortaç Ersoy E. Lung cancer and epigenetic modifications. Tuberk Toraks. 2016;64:163–170. doi: 10.5578/tt.10231. (In Turkish) [DOI] [PubMed] [Google Scholar]
- 10.Sasanakietkul T, Murtha TD, Javid M, Korah R, Carling T. Epigenetic modifications in poorly differentiated and anaplastic thyroid cancer. Mol Cell Endocrinol. 2018;469:23–37. doi: 10.1016/j.mce.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 11.Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet. 2017;174:651–660. doi: 10.1002/ajmg.b.32567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciesielski P, Jozwiak P, Krzeslak A. TET proteins and epigenetic modifications in cancers. Postepy Hig Med Dosw (Online) 2015;69:1371–1383. doi: 10.5604/17322693.1186346. (In Polish) [DOI] [PubMed] [Google Scholar]
- 13.Li D, Zeng Z. Epigenetic regulation of histone H3 in the process of hepatocellular tumorigenesis. Biosci Rep. 2019;39:BSR20191815. doi: 10.1042/BSR20191815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Losi L, Lauriola A, Tazzioli E, Gozzi G, Scurani L, D'Arca D, Benhattar J. Involvement of epigenetic modification of TERT promoter in response to all-trans retinoic acid in ovarian cancer cell lines. J Ovarian Res. 2019;12:62. doi: 10.1186/s13048-019-0536-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song J, Moscinski L, Zhang H, Zhang X, Hussaini M. Does SF3B1/TET2 double mutation portend better or worse prognosis Than Isolated SF3B1 or TET2 Mutation? Cancer Genomics Proteomics. 2019;16:91–98. doi: 10.21873/cgp.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen L, Wu H, Diep D, Yamaguchi S, D'Alessio AC, Fung HL, Zhang K, Zhang Y. Genome-wide analysis reveals TET- and TDG-dependent 5-methylcytosine oxidation dynamics. Cell. 2013;153:692–706. doi: 10.1016/j.cell.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko M, An J, Bandukwala HS, Chavez L, Aijö T, Pastor WA, Segal MF, Li H, Koh KP, Lähdesmäki H, et al. Modulation of TET2 expression and 5-methylcytosine oxidation by the CXXC domain protein IDAX. Nature. 2013;497:122–126. doi: 10.1038/nature12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Good CR, Madzo J, Patel B, Maegawa S, Engel N, Jelinek J, Issa JJ. A novel isoform of TET1 that lacks a CXXC domain is overexpressed in cancer. Nucleic Acids Res. 2017;45:8269–8281. doi: 10.1093/nar/gkx435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koh KP, Yabuuchi A, Rao S, Huang Y, Cunniff K, Nardone J, Laiho A, Tahiliani M, Sommer CA, Mostoslavsky G, et al. Tet1 and Tet2 regulate 5-hydroxymethylcytosine production and cell lineage specification in mouse embryonic stem cells. Cell Stem Cell. 2011;8:200–213. doi: 10.1016/j.stem.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawlaty MM, Breiling A, Le T, Barrasa MI, Raddatz G, Gao Q, Powell BE, Cheng AW, Faull KF, Lyko F, Jaenisch R. Loss of Tet enzymes compromises proper differentiation of embryonic stem cells. Dev Cell. 2014;29:102–111. doi: 10.1016/j.devcel.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu TP, Guo F, Yang H, Wu HP, Xu GF, Liu W, Xie ZG, Shi L, He X, Jin SG, et al. The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature. 2011;477:606–610. doi: 10.1038/nature10443. [DOI] [PubMed] [Google Scholar]
- 23.Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, He C, Zhang Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333:1300–1303. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng J, Guo S, Chen S, Mastriano SJ, Liu C, D'Alessio AC, Hysolli E, Guo Y, Yao H, Megyola CM, et al. An extensive network of TET2-targeting microRNAs regulates malignant hematopoiesis. Cell Rep. 2013;5:471–481. doi: 10.1016/j.celrep.2013.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang H, Liu Y, Bai F, Zhang JY, Ma SH, Liu J, Xu ZD, Zhu HG, Ling ZQ, Ye D, et al. Tumor development is associated with decrease of TET gene expression and 5-methylcytosine hydroxylation. Oncogene. 2013;32:663–669. doi: 10.1038/onc.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haferlach T, Nagata Y, Grossmann V, Okuno Y, Bacher U, Nagae G, Schnittger S, Sanada M, Kon A, Alpermann T, et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia. 2014;28:241–247. doi: 10.1038/leu.2013.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez-Mercado M, Yip BH, Pellagatti A, Davies C, Larrayoz MJ, Kondo T, Pérez C, Killick S, McDonald EJ, Odero MD, et al. Mutation patterns of 16 genes in primary and secondary acute myeloid leukemia (AML) with normal cytogenetics. PLoS One. 2012;7:e42334. doi: 10.1371/journal.pone.0042334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shih AH, Abdel-Wahab O, Patel JP, Levine RL. The role of mutations in epigenetic regulators in myeloid malignancies. Nat Rev Cancer. 2012;12:599–612. doi: 10.1038/nrc3343. [DOI] [PubMed] [Google Scholar]
- 29.Li R, Zhou Y, Cao Z, Liu L, Wang J, Chen Z, Xing W, Chen S, Bai J, Yuan W, et al. TET2 loss dysregulates the behavior of bone marrow mesenchymal stromal cells and accelerates Tet2−/−Driven myeloid malignancy progression. Stem Cell Reports. 2018;10:166–179. doi: 10.1016/j.stemcr.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z, Cai X, Cai CL, Wang J, Zhang W, Petersen BE, Yang FC, Xu M. Deletion of Tet2 in mice leads to dysregulated hematopoietic stem cells and subsequent development of myeloid malignancies. Blood. 2011;118:4509–4518. doi: 10.1182/blood-2010-12-325241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Ozark PA, Smith ER, Zhao Z, Marshall SA, Rendleman EJ, Piunti A, Ryan C, Whelan AL, Helmin KA, et al. TET2 coactivates gene expression through demethylation of enhancers. Sci Adv. 2018;4:eaau6986. doi: 10.1126/sciadv.aau6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan W, Zhu S, Qu K, Meeth K, Cheng J, He K, Ma H, Liao Y, Wen X, Roden C, et al. The DNA Methylcytosine Dioxygenase Tet2 sustains immunosuppressive function of Tumor-infiltrating myeloid cells to promote melanoma progression. Immunity. 2017;47:284–297.e5. doi: 10.1016/j.immuni.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Itoh H, Kadomatsu T, Tanoue H, Yugami M, Miyata K, Endo M, Morinaga J, Kobayashi E, Miyamoto T, Kurahashi R, et al. TET2-dependent IL-6 induction mediated by the tumor microenvironment promotes tumor metastasis in osteosarcoma. Oncogene. 2018;37:2903–2920. doi: 10.1038/s41388-018-0160-0. [DOI] [PubMed] [Google Scholar]
- 34.Levine ZG, Walker S. The Biochemistry of O-GlcNAc Transferase: Which functions make it essential in mammalian cells? Annu Rev Biochem. 2016;85:631–657. doi: 10.1146/annurev-biochem-060713-035344. [DOI] [PubMed] [Google Scholar]
- 35.Ma J, Banerjee P, Whelan SA, Liu T, Wei AC, Ramirez-Correa G, McComb ME, Costello CE, O'Rourke B, Murphy A, Hart GW. Comparative proteomics reveals dysregulated mitochondrial O-GlcNAcylation in diabetic hearts. J Proteome Res. 2016;15:2254–2264. doi: 10.1021/acs.jproteome.6b00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: Roles in signaling, transcription, and chronic disease. Annu Rev Biochem. 2011;80:825–858. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Love DC, Hanover JA. The hexosamine signaling pathway: Deciphering the ‘O-GlcNAc code’. Sci STKE. 2005;2005:re13. doi: 10.1126/stke.3122005re13. [DOI] [PubMed] [Google Scholar]
- 38.Gambetta MC, Muller J. A critical perspective of the diverse roles of O-GlcNAc transferase in chromatin. Chromosoma. 2015;124:429–442. doi: 10.1007/s00412-015-0513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bond MR, Hanover JA. O-GlcNAc cycling: A link between metabolism and chronic disease. Annu Rev Nutr. 2013;33:205–229. doi: 10.1146/annurev-nutr-071812-161240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanover JA, Krause MW, Love DC. Bittersweet memories: Linking metabolism to epigenetics through O-GlcNAcylation. Nat Rev Mol Cell Biol. 2012;13:312–321. doi: 10.1038/nrm3334. [DOI] [PubMed] [Google Scholar]
- 41.Mulloy B, Dell A, Stanley P, James HP. Structural analysis of glycans. In: Essentials of Glycobiology 3rd. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, et al., editors. Cold Spring Harbor; NY: 2015. pp. 639–652. [Google Scholar]
- 42.Maynard JC, Burlingame AL, Medzihradszky KF. Cysteine S-linked N-acetylglucosamine (S-GlcNAcylation), A new post-translational modification in mammals. Mol Cell Proteomics. 2016;15:3405–3411. doi: 10.1074/mcp.M116.061549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berthier A, Vinod M, Porez G, Steenackers A, Alexandre J, Yamakawa N, Gheeraert C, Ploton M, Maréchal X, Dubois-Chevalier J, et al. Combinatorial regulation of hepatic cytoplasmic signaling and nuclear transcriptional events by the OGT/REV-ERBα complex. Proc Natl Acad Sci USA. 2018;115:E11033–E11042. doi: 10.1073/pnas.1805397115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao J, Yang Y, Qiu R, Zhang K, Teng X, Liu R, Wang Y. Proteomic analysis of the OGT interactome: Novel links to epithelial-mesenchymal transition and metastasis of cervical cancer. Carcinogenesis. 2018;39:1222–1234. doi: 10.1093/carcin/bgy097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biwi J, Clarisse C, Biot C, Kozak RP, Madunic K, Mortuaire M, Wuhrer M, Spencer DIR, Schulz C, Guerardel Y, et al. OGT Controls the expression and the glycosylation of E-cadherin, and affects glycosphingolipid structures in human colon cell lines. Proteomics. 2019;19:e1800452. doi: 10.1002/pmic.201800452. [DOI] [PubMed] [Google Scholar]
- 46.Shi Y, Tomic J, Wen F, Shaha S, Bahlo A, Harrison R, Dennis JW, Williams R, Gross BJ, Walker S, et al. Aberrant O-GlcNAcylation characterizes chronic lymphocytic leukemia. Leukemia. 2010;24:1588–1598. doi: 10.1038/leu.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayakawa K, Hirosawa M, Tabei Y, Arai D, Tanaka S, Murakami N, Yagi S, Shiota K. Epigenetic switching by the metabolism- sensing factors in the generation of orexin neurons from mouse embryonic stem cells. J Biol Chem. 2013;288:17099–17110. doi: 10.1074/jbc.M113.455899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toleman C, Paterson AJ, Whisenhunt TR, Kudlow JE. Characterization of the histone acetyltransferase (HAT) domain of a bifunctional protein with activable O-GlcNAcase and HAT activities. J Biol Chem. 2004;279:53665–53673. doi: 10.1074/jbc.M410406200. [DOI] [PubMed] [Google Scholar]
- 49.Singh JP, Qian K, Lee JS, Zhou J, Han X, Zhang B, Ong Q, Ni W, Jiang M, Ruan HB, et al. O-GlcNAcase targets pyruvate kinase M2 to regulate tumor growth. Oncogene. 2020;39:560–573. doi: 10.1038/s41388-019-0975-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macauley MS, Shan X, Yuzwa SA, Gloster TM, Vocadlo DJ. Elevation of Global O-GlcNAc in rodents using a selective O-GlcNAcase inhibitor does not cause insulin resistance or perturb glucohomeostasis. Chem Biol. 2010;17:949–958. doi: 10.1016/j.chembiol.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fuentes-García G, Castañeda-Patlan MC, Vercoutter-Edouart AS, Lefebvre T, Robles-Flores M. O-GlcNAcylation Is Involved in the regulation of stem cell markers expression in colon cancer cells. Front Endocrinol (Lausanne) 2019;10:289. doi: 10.3389/fendo.2019.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jang H, Kim TW, Yoon S, Choi SY, Kang TW, Kim SY, Kwon YW, Cho EJ, Youn HD. O-GlcNAc regulates pluripotency and reprogramming by directly acting on core components of the pluripotency network. Cell Stem Cell. 2012;11:62–74. doi: 10.1016/j.stem.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 53.Olivier-Van Stichelen S, Wang P, Comly M, Love DC, Hanover JA. Nutrient-driven O-linked N-acetylglucosamine (O-GlcNAc) cycling impacts neurodevelopmental timing and metabolism. J Biol Chem. 2017;292:6076–6085. doi: 10.1074/jbc.M116.774042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abramowitz LK, Harly C, Das A, Bhandoola A, Hanover JA. Blocked O-GlcNAc cycling disrupts mouse hematopoeitic stem cell maintenance and early T cell development. Sci Rep. 2019;9:12569. doi: 10.1038/s41598-019-48991-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Delatte B, Fuks F. TET proteins: On the frenetic hunt for new cytosine modifications. Brief Funct Genomics. 2013;12:191–204. doi: 10.1093/bfgp/elt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ito R, Katsura S, Shimada H, Tsuchiya H, Hada M, Okumura T, Sugawara A, Yokoyama A. TET3-OGT interaction increases the stability and the presence of OGT in chromatin. Genes Cells. 2014;19:52–65. doi: 10.1111/gtc.12107. [DOI] [PubMed] [Google Scholar]
- 57.Shi FT, Kim H, Lu W, He Q, Liu D, Goodell MA, Wan M, Songyang Z. Ten-eleven translocation 1 (Tet1) is regulated by O-linked N-acetylglucosamine transferase (Ogt) for target gene repression in mouse embryonic stem cells. J Biol Chem. 2013;288:20776–20784. doi: 10.1074/jbc.M113.460386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Q, Liu X, Gao W, Li P, Hou J, Li J, Wong J. Differential regulation of the ten-eleven translocation (TET) family of dioxygenases by O-linked β-N-acetylglucosamine transferase (OGT) J Biol Chem. 2014;289:5986–5996. doi: 10.1074/jbc.M113.524140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bauer C, Gobel K, Nagaraj N, Colantuoni C, Wang M, Müller U, Kremmer E, Rottach A, Leonhardt H. Phosphorylation of TET proteins is regulated via O-GlcNAcylation by the O-linked N-acetylglucosamine transferase (OGT) J Biol Chem. 2015;290:4801–4812. doi: 10.1074/jbc.M114.605881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh JP, Zhang K, Wu J, Yang X. O-GlcNAc signaling in cancer metabolism and epigenetics. Cancer Lett. 2015;356:244–250. doi: 10.1016/j.canlet.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fujiki R, Hashiba W, Sekine H, Yokoyama A, Chikanishi T, Ito S, Imai Y, Kim J, He HH, Igarashi K, et al. GlcNAcylation of histone H2B facilitates its monoubiquitination. Nature. 2011;480:557–560. doi: 10.1038/nature10656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Q, Chen Y, Bian C, Fujiki R, Yu X. TET2 promotes histone O-GlcNAcylation during gene transcription. Nature. 2013;493:561–564. doi: 10.1038/nature11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deplus R, Delatte B, Schwinn MK, Defrance M, Mendez J, Murphy N, Dawson MA, Volkmar M, Putmans P, Calonne E, et al. TET2 and TET3 regulate GlcNAcylation and H3K4 methylation through OGT and SET1/COMPASS. EMBO J. 2013;32:645–655. doi: 10.1038/emboj.2012.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hsu CH, Peng KL, Kang ML, Chen YR, Yang YC, Tsai CH, Chu CS, Jeng YM, Chen YT, Lin FM, et al. TET1 suppresses cancer invasion by activating the tissue inhibitors of metalloproteinases. Cell Rep. 2012;2:568–579. doi: 10.1016/j.celrep.2012.08.030. [DOI] [PubMed] [Google Scholar]
- 65.Guan W, Guyot R, Samarut J, Flamant F, Wong J, Gauthier KC. Methylcytosine dioxygenase TET3 interacts with thyroid hormone nuclear receptors and stabilizes their association to chromatin. Proc Natl Acad Sci USA. 2017;114:8229–8234. doi: 10.1073/pnas.1702192114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Phoomak C, Silsirivanit A, Park D, Sawanyawisuth K, Vaeteewoottacharn K, Wongkham C, Lam EW, Pairojkul C, Lebrilla CB, Wongkham S. O-GlcNAcylation mediates metastasis of cholangiocarcinoma through FOXO3 and MAN1A1. Oncogene. 2018;37:5648–565. doi: 10.1038/s41388-018-0366-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liberti MV, Locasale JW. The warburg effect: How does it benefit cancer cells? Trends Biochem Sci. 2016;41:211–228. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma Z, Vosseller K. Cancer metabolism and elevated O-GlcNAc in oncogenic signaling. J Biol Chem. 2014;289:34457–34465. doi: 10.1074/jbc.R114.577718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang WH, Kim JE, Nam HW, Ju JW, Kim HS, Kim YS, Cho JW. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat Cell Biol. 2026;8:1074–1083. doi: 10.1038/ncb1470. [DOI] [PubMed] [Google Scholar]
- 70.Itkonen HM, Minner S, Guldvik IJ, Sandmann MJ, Tsourlakis MC, Berge V, Svindland A, Schlomm T, Mills IG. O-GlcNAc transferase integrates metabolic pathways to regulate the stability of c-MYC in human prostate cancer cells. Cancer Res. 2013;73:5277–5287. doi: 10.1158/0008-5472.CAN-13-0549. [DOI] [PubMed] [Google Scholar]
- 71.Olivier-Van Stichelen S, Guinez C, Mir AM, Perez-Cervera Y, Liu C, Michalski JC, Lefebvre T. The hexosamine biosynthetic pathway and O-GlcNAcylation drive the expression of β-catenin and cell proliferation. Am J Physiol Endocrinol Metab. 2012;302:E417–E424. doi: 10.1152/ajpendo.00390.2011. [DOI] [PubMed] [Google Scholar]
- 72.Thomson JP, Ottaviano R, Unterberger EB, Lempiäinen H, Muller A, Terranova R, Illingworth RS, Webb S, Kerr AR, Lyall MJ, et al. Loss of Tet1-Associated 5-hydroxymethylcytosine is concomitant with aberrant promoter hypermethylation in liver cancer. Cancer Res. 2016;76:3097–3108. doi: 10.1158/0008-5472.CAN-15-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Delhommeau F, Dupont S, Della Valle V, James C, Trannoy S, Massé A, Kosmider O, Le Couedic JP, Robert F, Alberdi A, et al. Mutation in TET2 in myeloid cancers. N Engl J Med. 2029;360:2289–2301. doi: 10.1056/NEJMoa0810069. [DOI] [PubMed] [Google Scholar]
- 74.Itzykson R, Kosmider O, Renneville A, Gelsi-Boyer V, Meggendorfer M, Morabito M, Berthon C, Adès L, Fenaux P, Beyne-Rauzy O, et al. Prognostic score including gene mutations in chronic myelomonocytic leukemia. J Clin Oncol. 2013;31:2428–2436. doi: 10.1200/JCO.2012.47.3314. [DOI] [PubMed] [Google Scholar]
- 75.Nibourel O, Kosmider O, Cheok M, Boissel N, Renneville A, Philippe N, Dombret H, Dreyfus F, Quesnel B, Geffroy S, et al. Incidence and prognostic value of TET2 alterations in de novo acute myeloid leukemia achieving complete remission. Blood. 2010;116:1132–1135. doi: 10.1182/blood-2009-07-234484. [DOI] [PubMed] [Google Scholar]
- 76.Dominguez PM, Ghamlouch H, Rosikiewicz W, Kumar P, Béguelin W, Fontán L, Rivas MA, Pawlikowska P, Armand M, Mouly E, et al. TET2 deficiency causes germinal center hyperplasia, impairs plasma cell differentiation, and promotes B-cell lymphomagenesis. Cancer Discov. 2018;8:1632–1653. doi: 10.1158/2159-8290.CD-18-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cao T, Pan W, Sun X, Shen H. Increased expression of TET3 predicts unfavorable prognosis in patients with ovarian cancer-a bioinformatics integrative analysis. J Ovarian Res. 2019;12:101. doi: 10.1186/s13048-019-0575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carella A, Tejedor JR, García MG, Urdinguio RG, Bayón GF, Sierra M, López V, García-Toraño E, Santamarina-Ojeda P, Pérez RF, et al. Epigenetic downregulation of TET3 reduces genome-wide 5hmC levels and promotes glioblastoma tumorigenesis. Int J Cancer. 2020;146:373–387. doi: 10.1002/ijc.32520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.