Abstract
Pancreatic cancer is one of the most lethal of human malignancies. Nearly 100% cases of pancreatic cancer carry mutations in KRas. P-21-activated kinases (PAKs) are activated by and act downstream of KRas. Glaucarubinone, a natural product first isolated from the seeds of the tree Simarouba glauca, was originally developed as an antimalarial drug, and has more recently been recognised as an anticancer agent. The aims of this study were to determine whether glaucarubinone, alone or in combination with the front-line chemotherapeutic agent gemcitabine, would inhibit the growth of pancreatic cancer cells in vitro or in vivo and the mechanism involved. Growth of the human pancreatic cancer cell lines PANC-1 and MiaPaCa-2 was measured by 3H-thymidine incorporation in vitro, and by volume as xenografts in SCID mice. The expression and activities of the two serine/threonine kinases PAK1 and PAK4, which are key regulators of cancer progression, were measured by Western blotting. Here we report that glaucarubinone decreased proliferation and migration of pancreatic cancer cells in vitro, and reduced their growth as xenografts in vivo. Treatment with glaucarubinone and gemcitabine reduced proliferation in vitro and tumor growth in vivo more than treatment with either glaucarubinone or gemcitabine alone. Treatment with glaucarubinone reduced PAK1 and PAK4 activities, which were further decreased by the combination of glaucarubinone and gemcitabine. These results indicate that glaucarubinone reduced pancreatic cancer cell growth at least in part via inhibition of pathways involving PAK1 and PAK4. The synergistic inhibition by glaucarubinone and gemcitabine observed both in vitro and in vivo suggests that glaucarubinone may be a useful adjunct to current regimes of chemotherapy.
Keywords: Glaucarubinone, Gemcitabine, Pancreatic cancer, P21-activated kinases
1. Introduction
Pancreatic cancer is considered among the most aggressive and the least curable of all human malignancies. The 5 years survival rate is less than 5% because of its highly malignant phenotype, which is characterised by rapid progression, early metastasis and limited response to chemotherapy and radiotherapy [1]. Surgery is considered the only curative option; however, 80–90% of patients are diagnosed too late to permit surgical intervention because of distant metastases or major vessel involvement [2,3]. For these patients, treatment is confined to chemo- and/or radiotherapy. Even after surgery, the 5-years survival rate is only 10–25% due to a high rate of local recurrence, peritoneal dissemination, liver metastases, and lymph node recurrence [4]. Currently gemcitabine-based chemotherapy, which targets rapidly replicating cells, remains the main form of treatment for patients with advanced pancreatic cancer and as adjuvant therapy after surgical resection, but the response is varied, with only modest improvements in survival [5]. Tumor progression eventually occurs as cancer cells develop increasing resistance to chemotherapy. There is therefore an urgent need to improve treatment outcomes through the development of more effective chemotherapeutic regimens based on an increased understanding of the molecular mechanisms involved in the development of pancreatic adenocarcinoma.
Pancreatic cancer develops as a result of a series of genetic mutations, and progresses from non-invasive tumors to invasive and metastatic cancers [6]. The most frequent mutations in pancreatic cancer involve the Kras gene and are observed in more than 95% of cases. The product of the Kras gene is a protein of 21 kDa (P21), which binds to and activates the P21-activated kinases (PAKs). The PAK family of serine/threonine kinases are not only important in carcinogenesis, but also are major regulators of cytoskeletal dynamics, with significant roles in cell proliferation, survival and motility [7]. PAKs are divided into 2 groups (PAKs 1, 2 and 3, and PAKs 4, 5 and 6) based on sequence homologies. The PAK4 gene is amplified in some pancreatic cancers and amplification is associated with significantly higher kinase activity of the PAK4 protein [8]. PAK4 stimulates pancreatic cell migration/invasion [9]. In pancreatic cancer cells increased expression and activation of PAK1 is correlated with increased expression of MUC13, which stimulates xenograft growth of pancreatic cancer cells [10]. Conversely SMAD4 induces pancreatic cancer cell death by suppression of PAK1 [11].
Glaucarubinone, a quassinoid natural product first isolated from the seeds of Simarouba glauca, and later from numerous other species in the family Simaroubaceae, was originally developed as an antimalarial drug [12]. Although glaucarubinone has been shown to possess anti-cancer activity [13,14], the mechanisms involved are not fully understood. A recent report indicated that quassinoids including glaucarubinone inhibited the transcription factor activator protein-1 (AP-1), independently of their cytotoxicity [15]. Several independent lines of evidence suggest that glaucarubinone may act, at least in part, via inhibition of pathways involving PAK1 [16]. Thus suppression of PAK1 causes death in malaria parasites [17], as does glaucarubinone. We have previously shown that glaucarubinone inactivates NF-kappaB [15], whose activation requires PAK1. Furthermore, glaucarubinone may extend the lifespan of Caenorhabditis elegans, which is also increased by down-regulation of PAK1 [18]. Nearly 100% cases of pancreatic cancer carry mutations in KRas, and PAKs are activated by and act downstream of KRas. To determine the effects of glaucarubinone and/or gemcitabine on pancreatic cancer growth and the mechanism involved, we investigated the effects of glaucarubinone and/or gemcitabine on the growth and migration of pancreatic cancer cell lines, and on the expression and activation of PAK1 and PAK4 in the same cell lines in vitro and as xenografts in nude mice.
2. Material and methods
2.1. Cells and reagents
The human PANC-1 and MiaPaCa-2 pancreatic cancer cell lines (purchased from Sigma–Aldrich, Sydney, Australia) and the murine PAN02 pancreatic cancer cell line (obtained from National Cancer Institute (Frederick, MD, USA)) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% FBS (fetal bovine serum; HyClone Laboratories Inc., Melbourne, Australia) in a 37 °C incubator in a humidified atmosphere of 5% CO2. Cells tested negative for mycoplasma, and were passaged not more than 30 times. Glaucarubinone, prepared as prescribed previously [15], was obtained from the National Cancer Institute (NCI) Chemotherapeutics Repository, and gemcitabine was purchased from Sigma–Aldrich.
2.2. Cell proliferation
Cell proliferation was measured using a 3H-thymidine incorporation assay. Cells were seeded in a 96-well plate at 5 × 103 cells/well in DMEM containing 10% FBS, with 1 μCi/well [methyl-3H]-thymidine (Perkin Elmer, Boston, MA) in the presence of increasing concentrations of glaucarubinone. After 24 h, cells were harvested using a NUNC cell harvester (Nunc, Roskilde, Denmark). The amount of 3H-thymidine incorporated through DNA synthesis was detected with a β-counter (Packard, Meriden, CT). For assessment of combined effects on proliferation, cells were pretreated with glaucarubinone for 20 h, and the glaucarubinone was removed by washing. The cells were incubated with increasing concentrations of gemcitabine and 1 μCi/well [methyl-3H]-thymidine for a further 24 h. Cells were harvested and radioactivity detected as described above. Each treatment was done in duplicate and three independent experiments were collated.
2.3. Migration and invasion assay
Cell migration and invasion was measured using a Transwell Boyden Chamber assay as described previously [19]. Membranes (8-μm pore size, Becton Dickinson, NJ) were coated with 3 μg of human fibronectin on the lower surfaces and placed into a 24-well plate containing 600 μl/well serum-free DMEM containing 0.1% bovine serum albumin (BSA). Cells were added to the upper chambers at 5 × 104/100 μl with or without glaucarubinone at the concentrations indicated in the text. After 24 h non-migratory cells were removed by wiping the upper surface with a cotton swab. The membranes were fixed and stained with Quick-Dip (Fronine, Sydney, Australia). The cells that had migrated to the lower surface of the membranes were counted from 24 to 48 fields, at 40 times magnification using a NIKON Coolscope (Coherent Scientific, Adelaide, Australia). Each experiment was done in duplicate and three independent experiments were collated.
2.4. Combination index
The combined effects of gemcitabine and glaucarubinone were evaluated using the Chou–Talalay method [20,21]. The collated proliferation values for untreated cells or cells treated with glaucarubinone alone, gemcitabine alone, or glaucarubinone in combination with gemcitabine were used. The CalcuSyn program (T.C. Chou and M.P. Hayball; Biosoft, Cambridge, UK) was applied to calculate and analyse the combination index [8] using the mutually non-exclusive (α = 1) isobologram equation. The CI value is interpreted as follows: <1.0, synergistic; 1.0, additive and >1.0, antagonistic.
2.5. Murine xenograft study
All mouse experiments were approved by the Austin Health Animal Research Ethics Committee. SCID mice were purchased from the Animal Resource Centre (Perth, Australia), and housed in micro-isolators. For the xenograft studies, 5 × 106 PANC-1 and MiaPaCa-2 cells in 100 μl DMEM were subcutaneously injected into the opposite flanks of each of 20 mice (6 weeks old). Tumor dimensions were measured every other day using callipers, and volumes calculated using the formula w2 × L/2, where w and L are the shortest and longest tumor diameters, respectively. Mice were divided into four groups (5 mice per group) as follows: (1) control, receiving intraperitoneal (i.p.) injection of saline every other day; (2) glaucarubinone, receiving glaucarubinone (i.p.) every other day at 1 mg/kg for the first week followed by 2 mg/kg for the remaining 5 weeks; (3) gemcitabine, receiving gemcitabine (i.p.) weekly at 20 mg/kg for 6 weeks; and (4) glaucarubinone and gemcitabine, receiving gemcitabine weekly as for group 3, and glaucarubinone every other day as for group 2. The dose chosen for glaucarubinone was based on preliminary experiments where the maximal amount that could be tolerated by mice without significant toxicity was 2 mg/kg. Mice were sacrificed on day 43, and tumor tissues were weighed, and extracted for immunoblotting or fixed and paraffin-embedded for immunostaining.
2.6. Western blot
Cells, treated with glaucarubinone and/or gemcitabine as indicated in the text, were lysed in SDS sample buffer and subjected to SDS–PAGE for immunoblotting. Proteins were detected with antibodies against PAK1, and its active form, phospho-PAK1 (Santa Cruz, USA), PAK4 and its active form, phospho-PAK4, and GAPDH (Genesearch, Melbourne, Australia). The bound antibodies were visualized using ECL reagents (GE Healthcare, Amersham, England), and the density of each band was analysed using Multigauge software (Berthold, Bundoora, Australia). Proteins were extracted from tumor tissues by homogenising and sonicating in 1% Triton X100 lysis buffer (50 mM HEPES, 150 mM NaCl, 10 mM EDTA, 10 mM Na4P2O7, 100 mM NaF, 5 μg/ml aprotinin, 5 μg/ml leupeptin and 1 mM PMSF), subjected to SDS–PAGE, and immunoblotted with the antibodies indicated in the text.
2.7. Immunohistochemistry (IHC)
The tumor tissues were stained with Ki67 antibody (Dakocytomation, Sydney, Australia) for proliferation, and active caspase 3 antibody (R&D systems, Minneapolis, MN) for apoptosis. The tumor tissues were imaged using a Nikon Coolscope and the ratio of positive cells to the total number of cells in each field calculated using the Image Pro-Plus 6.0 image analysis program (Media Cybernetics Inc., Silver Spring, MD).
2.8. Statistical analysis
All values are expressed as means ± standard error. Results were analysed by one-way analysis of variance or t-test as appropriate with the program SigmaStat (SPSS, Chicago, IL). Differences between two means with p < 0.05 were considered significant.
3. Results
3.1. Glaucarubinone inhibited pancreatic cancer cell proliferation and migration
The effect of glaucarubinone on pancreatic cancer cell proliferation was determined using a 3H-thymidine-incorporation assay. Glaucarubinone inhibited the proliferation of three pancreatic cancer cell lines in a dose-dependent manner (Fig. 1A–C). The IC50 values were 300 nM for PANC-1, 58 nM for MiaPaCa-2, and 960 nM for PAN02 (Table 1). The effect of glaucarubinone on pancreatic cancer cell migration/invasion was measured using a Boyden Chamber assay. Similarly, glaucarubinone inhibited the migration/invasion of these cell lines in a dose-dependent manner (Fig. 1A–C). The IC50 values were 210 nM for PANC-1, 44 nM for MiaPaCa-2, and 220 nM for PAN02 (Table 1). At the same concentrations used for the proliferation assay, glaucarubinone did not inhibit survival in the absence of serum for any of the 3 cell lines tested (Supplementary Fig. 1).
Fig. 1.
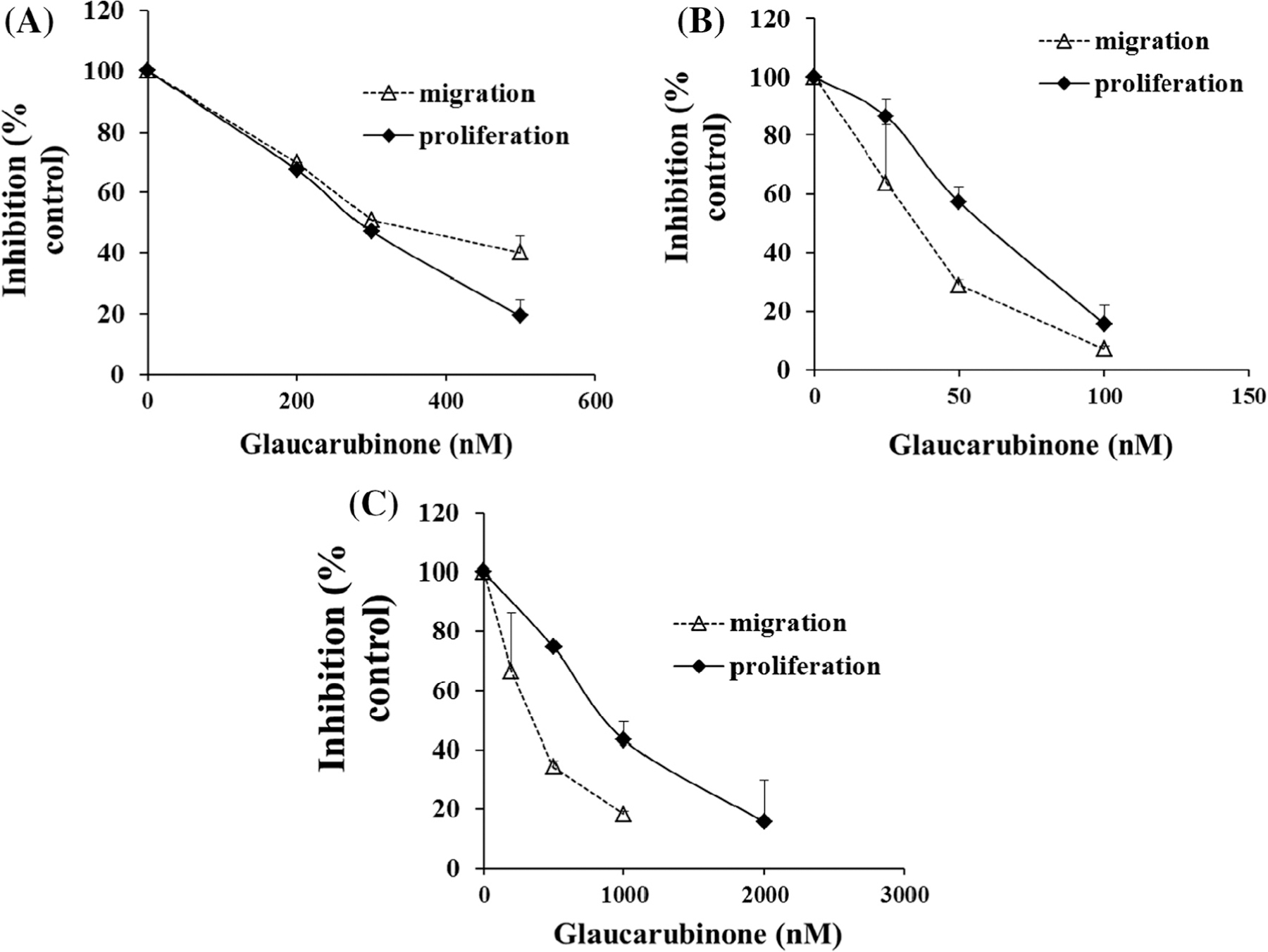
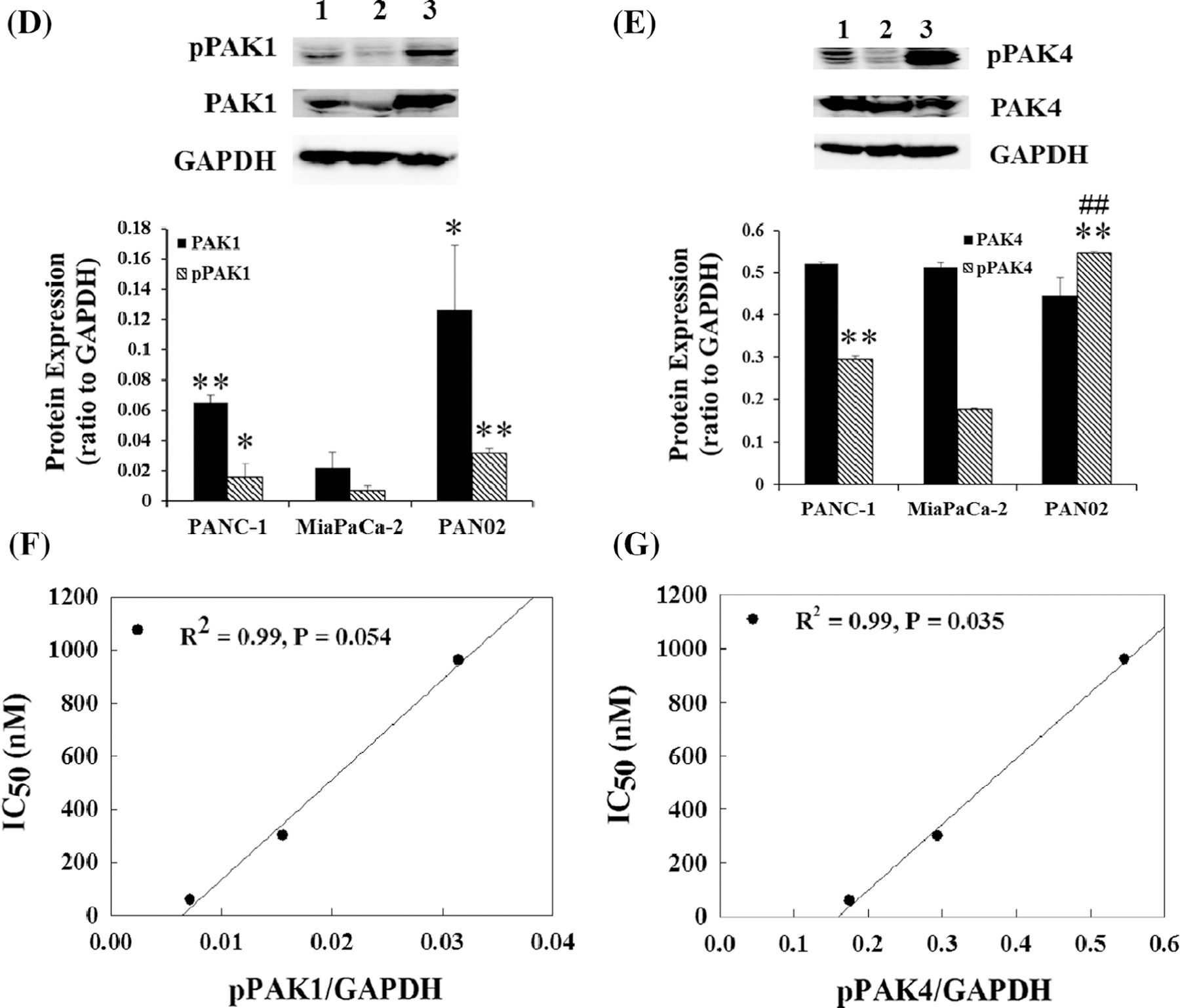
Glaucarubinone dose-dependently inhibited pancreatic cancer cell proliferation and migration/invasion, and the IC50 values correlated with the amount of active PAK4. The effect of glaucarubinone on proliferation and migration/invasion was measured in the pancreatic cancer cell lines PANC-1 (A), MiaPaCa-2 (B), and PAN02 (C) using thymidine incorporation and Boyden Chamber transwell assays. The values from untreated controls [28] were taken as 100% (A–C). The expression of the total and active forms of PAK1 (D) or PAK4 (E) was determined in untreated PANC-1, MiaPaCa-2, and PAN02 cell lines by Western blotting using anti-PAK1, anti-phospho-PAK1 (pPAK1), anti-PAK4 or anti-phospho-PAK4 (pPAK4) antibodies as described in Section 2. The IC50 values for inhibition of proliferation by glaucarubinone were significantly correlated with the amount of pPAK4 (G), and the correlation in the case of pPAK1 (F) approached significance. The data represent mean ± SEM, summarised from three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001, compared to control (A–C) or values from the MiaPaCa-2 cell line (D and E). ##p < 0.01 compared to the values from the PANC-1 cell line (E). 1, 2 and 3 in E and F represent PANC-1, MiaPaCa-2 and PAN02 cell lines.
Table 1.
IC50 values for glaucarubinone and gemcitabine.
| IC50 (nM) |
||||
|---|---|---|---|---|
| PANC-1 | MiaPaCa-2 | PAN02 | ||
| Glaucarubinone | Proliferation | 300 ± 42 | 58 ± 4 | 960 ± 130 |
| Migration/invasion | 210 ± 20 | 44 ± 23 | 220 ± 130 | |
| Gemcitabine | Proliferation | 40 ± 4 | 46 ± 2 | 100 ± 2 |
The effect of glaucarubinone or gemcitabine on pancreatic cancer cell proliferation was determined using a 3H-thymidine-incorporation assay (Fig. 1A). The effect of glaucarubinone on pancreatic cancer cell migration/invasion was measured using a Boyden Chamber assay (Fig. 1B). Values are the mean ± SEM from at least 3 independent experiments.
3.2. Increasing sensitivity to glaucarubinone correlated with reduced amounts of active PAK1 and PAK4
The IC50 values for inhibition of proliferation and migration by glaucarubinone varied among three pancreatic cancer cell lines. MiaPaCa-2 was most sensitive to glaucarubinone with the lowest IC50 values for both proliferation and migration, while PAN02 was least sensitive to glaucarubinone with the highest IC50 values (Table 1). Similar trends were observed in the expression and activation of PAK1 in untreated pancreatic cancer cell lines (Fig. 1D), as MiaPaCa-2 had the lowest expression of PAK1 and the lowest concentration of the active form of PAK1 (phospho-PAK1 (pPAK1)), while PAN02 had the highest expression of both PAK1 and phospho-PAK1 (Fig. 1D). Although there was no significant difference in PAK4 expression among untreated pancreatic cancer cell lines, MiaPaCa-2 had the lowest expression of the active form of PAK4 (phospho-PAK4 (pPAK4)), while PAN02 had the highest expression of phospho-PAK4 (Fig. 1E). The matching patterns of IC50 values and PAK1/4 activities suggested that the sensitivities of these pancreatic cancer cells to glaucarubinone might be correlated with the amount of active PAK1 and PAK4. In fact the IC50 values for inhibition of proliferation by glaucarubinone were significantly correlated with the amount of pPAK4 (Fig. 1G), and the correlation in the case of pPAK1 (Fig. 1F) approached significance. No correlation was observed between the IC50 values for inhibition of proliferation and the total amounts of PAK1 or PAK4, or between the IC50 values for inhibition of migration and either the amounts of pPAK1 or pPAK4, or the total amounts of PAK1 or PAK4 (data not shown).
3.3. Glaucarubinone and gemcitabine synergistically inhibited pancreatic cancer cell growth
The combined effects of glaucarubinone and gemcitabine on proliferation were measured by incubation of the pancreatic cancer cell lines with different concentrations of gemcitabine, alone or in combination with glaucarubinone, at concentrations close to the IC50 values for glaucarubinone given in Table 1. Gemcitabine on its own inhibited proliferation in the three pancreatic cancer cell lines in a dose-dependent manner (Fig. 2A–C). The IC50 values for gemcitabine were 40 nM for PANC-1, 46 nM for MiaPaCa-2, and 100 nM for PAN02 (Table 1). A further reduction of proliferation was observed in all three cell lines when the cells were treated with glaucarubinone and gemcitabine in combination, compared to gemcitabine alone (Fig. 2A–C). The combined effect of glaucarubinone and gemcitabine was shown to be synergistic rather than additive when using the Chou–Talalay method analysed [20] (Supplementary Fig. 2). These results demonstrated that glaucarubinone and gemcitabine synergistically inhibited pancreatic cancer cell growth.
Fig. 2.
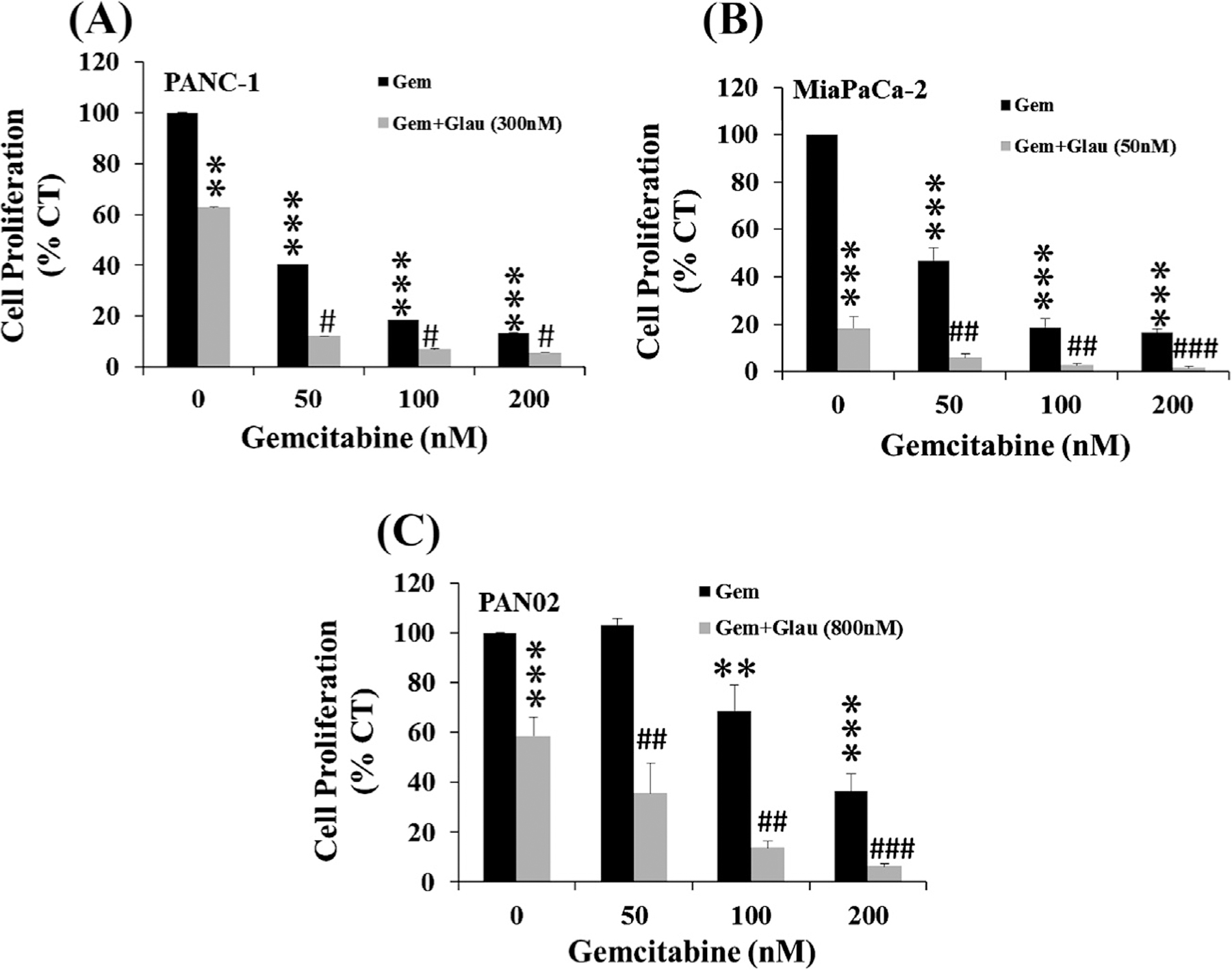
Glaucarubinone and gemcitabine synergistically inhibited pancreatic cancer cell proliferation. The effects of gemcitabine alone (Gem, black bars), and gemcitabine after 20 h pre-treatment with glaucarubinone (Glau, grey bars), on proliferation were tested in the pancreatic cancer cell lines PANC-1 (A), MiaPaCa-2 (B), and PAN02 (C) by thymidine incorporation assay. The concentrations of glaucarubinone used approximated the IC50 values determined in Fig. 1. The values obtained from untreated control cells were taken as 100%. All data represent mean ± SEM, summarised from three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001; compared to control, and #p < 0.05; ##p < 0.01; ###p < 0.001; compared to the corresponding gemcitabine treatment.
3.4. Inhibition of pancreatic cancer cell growth by glaucarubinone and gemcitabine was associated with reduced amounts of active PAK1 and PAK4
To investigate the mechanism involved in the inhibition of pancreatic cancer cell growth by gluacarubinone and gemcitabine, the effects of the two drugs on the expression and activity of PAK1 and PAK4 were determined. The human pancreatic cancer cell lines PANC-1 and MiaPaCa-2 were treated with glaucarubinone and/or gemcitabine at concentrations close to their IC50 values (Table 1). The total amounts of PAK1 and PAK4, and the amounts of active phospho-PAK1 and phospho-PAK4, were measured by Western blotting. In MiaPaCa-2 cells, glaucarubinone significantly decreased the amount of both active PAK1 (Fig. 3A) and active PAK4 (Fig. 3B) without affecting the expression of either PAK1 or PAK4. Gemcitabine alone had no significant effect on the amount of either active PAK1 (Fig. 3A) or active PAK4 (Fig. 3B), which stayed so even with gemcitabine at 100 nM that was twice more than its IC50 in a dose dependent assay (data not shown). However the amounts of both active PAK1 and PAK4 were further decreased by the combination of glaucarubinone and gemcitabine (Fig. 3A and B). Similar results were obtained in PANC-1 cells treated with glaucarubinone and gemcitabine (Fig. 3C and D), but not with glaucarubinone alone. These data suggested that glaucarubinone and gemcitabine synergistically reduced pancreatic cancer cell growth by reducing the amounts of active PAK1 and PAK4.
Fig. 3.
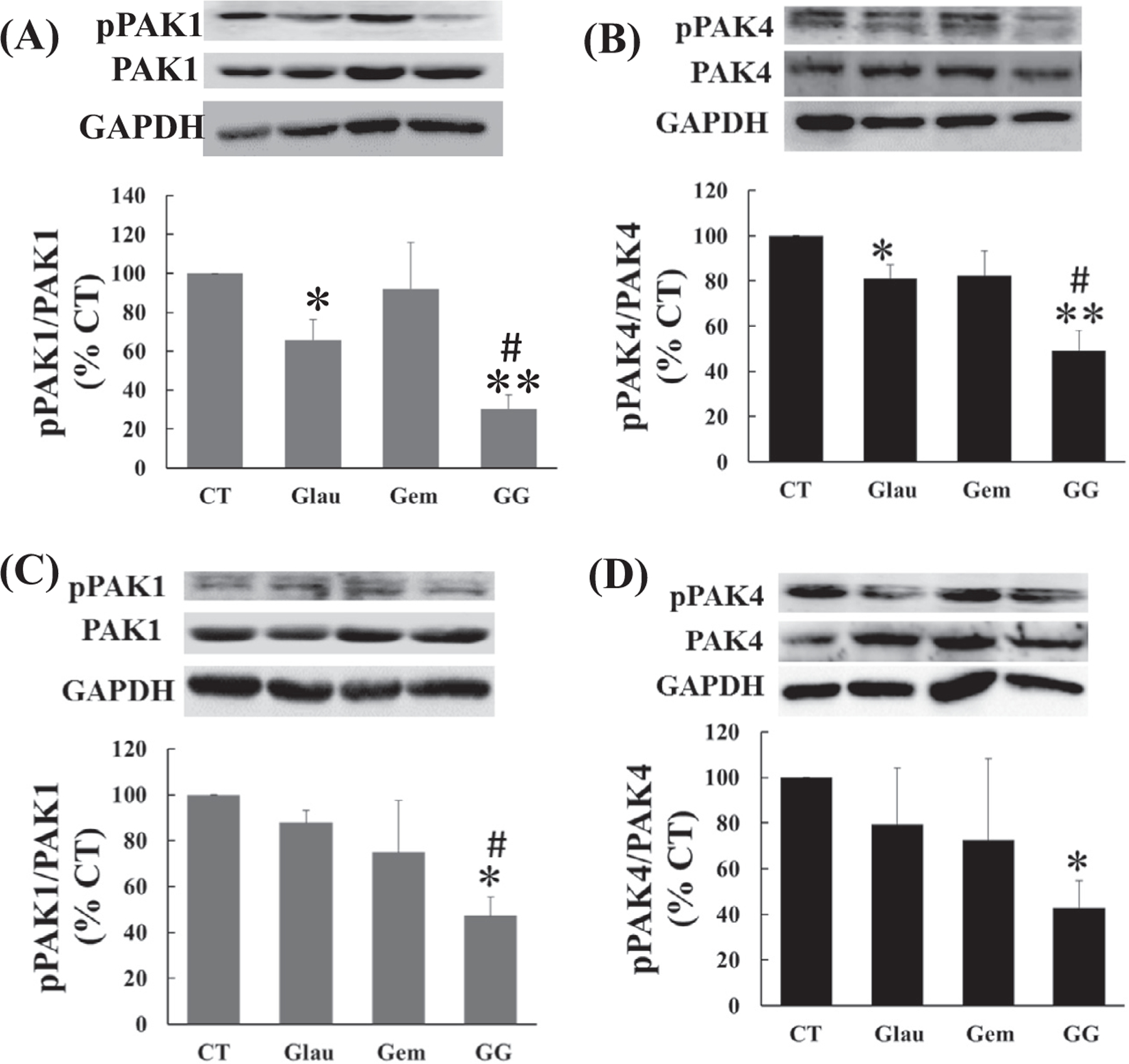
Glaucarubinone and gemcitabine synergistically reduced the amounts of active PAK1 and PAK4 in vitro. The effects of glaucarubinone (Glau) or gemcitabine (Gem) alone, or in combination (GG), on the amounts of total PAK1 and active phospho-PAK1 (pPAK1) (A and C) or total PAK4 and active phospho-PAK4 (pPAK4) (B and D) were determined in MiaPaCa-2 (A and B) or PANC-1 (C and D) cells by Western blotting using appropriate antibodies as described in Section 2. The concentrations of glaucarubinone used approximated the IC50 values calculated from the proliferation assays (Fig. 1), and were 50 nM and 300 nM for MiaPaCa-2 and PANC-1 cells, respectively. The concentrations of gemcitabine used approximated the IC50 values determined in similar proliferation assays (data not shown), and were 50 nM for both MiaPaCa-2 and PANC-1 cells. Variations in protein loading were corrected by GAPDH expression, and the corrected pPAK1/PAK1 or pPAK4/PAK4 ratios from untreated control cells [28] were taken as 100%. The data represent mean ± SEM, summarised from three independent Western blots. *p < 0.05; **p < 0.01 compared to control. #p < 0.05 compared to Glau treatment.
3.5. Glaucarubinone and gemcitabine inhibited pancreatic cancer cell xenograft growth in vivo by decreasing proliferation
To determine the effect of glaucarubinone and gemcitabine on pancreatic cancer growth in vivo, MiaPaCa-2 and PANC-1 cells were injected subcutaneously into opposite flanks of SCID mice. The tumor take rate (the number of mice with local tumor growth expressed as a percentage of the total number of mice injected) was 100% for both cell lines. The mice were treated with glaucarubinone and gemcitabine either alone or in combination as described in Section 2, and tumor dimensions were measured with callipers. From day 17, the tumor volume of the MiaPaCa-2 xenografts was significantly decreased by treatment with either glaucarubinone or gemcitabine alone (p < 0.05, Fig. 4A). The combined treatment with glaucarubinone and gemcitabine also decreased the tumor size of MiaPaCa-2 xenografts (p < 0.01, Fig. 4A). Importantly, from day 28, the combined treatment with glaucarubinone and gemcitabine decreased tumor volume significantly more than the gemcitabine treatment alone (p < 0.01, Fig. 4A), but not more than glaucarubinone treatment alone. Similarly by the end of the experiment, the tumor weights of MiaPaCa-2 xenografts were significantly reduced by glaucarubinone or gemcitabine alone to 28% and 44%, respectively, of untreated control tumors (Fig. 4C). The combined treatment with glaucarubinone and gemcitabine also reduced the tumor weight to 20% of control, and the reduction was significantly greater than for gemcitabine-treated tumors (p < 0.05, Fig. 4C), but not for glaucarubinone-treated tumors (Fig. 4C). Neither glaucarubinone nor gemcitabine alone had any significant effect on the tumor growth of PANC-1 xenografts (Fig. 4B and D). However, the combined treatment with glaucarubinone and gemcitabine significantly reduced the tumor volume from day 32 (p < 0.05, Fig. 4B), and the tumor weight to 37% of control (p < 0.05, Fig. 4D).
Fig. 4.
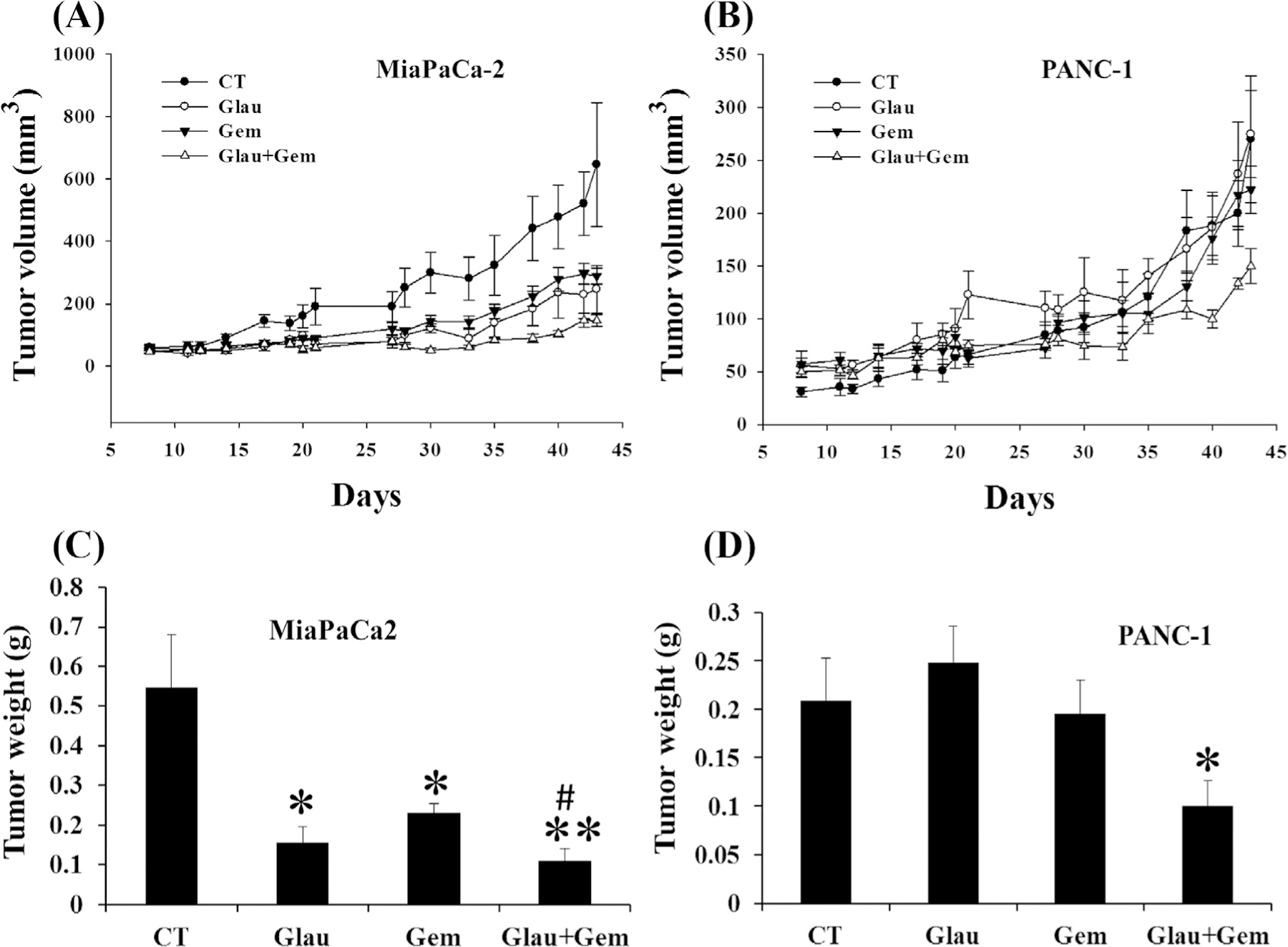
Glaucarubinone and gemcitabine synergistically inhibited pancreatic cancer cell growth in vivo. MiaPaCa-2 (A and C) and PANC-1 (B and D) cells were injected subcutaneously into opposite flanks of 6 week-old SCID mice. The animals were treated with saline [28], glaucarubinone (Glau, 1 mg/kg for the first week and 2 mg/kg thereafter), gemcitabine (Gem, 20 mg/kg), or the combination (Glau + Gem) by intraperitoneal injection every other day. Tumor volume (A and B) was calculated from tumor dimensions measured with callipers on the indicated days, and tumors were excised and weighed (C and D) at day 43. Both glaucarubinone (Glau) and gemcitabine (Gem) alone inhibited the growth of MiaPaCa-2 tumors (A and C), but not of PANC-1 tumors (B and D). The combination of glaucarubinone and gemcitabine not only further decreased the growth of MiaPaCa-2 tumors (A and C), but also inhibited the growth of PANC-1 tumors (B and D). **p < 0.001; *p < 0.05; compared to the values of untreated control [28] tumors. #p < 0.05 compared to treatment with glaucarubinone. Statistical significance is not shown on panels A and B for clarity.
Proliferation and apoptosis of cells within the xenografted tumors were measured by immunohistochemistry. For MiaPaCa-2 cells, there was no significant difference in proliferation between untreated control tumors and tumors treated with either glaucarubinone or gemcitabine alone. However the proliferation in tumors from the group treated with glaucarubinone and gemcitabine in combination was reduced to 75% of the value for untreated control tumors (Fig. 5A and B). There was no significant difference in apoptosis between tumors from the four groups (Fig. 5C and D). These results indicate that glaucarubinone and gemcitabine synergistically inhibited pancreatic cancer growth in vivo by reducing proliferation.
Fig. 5.
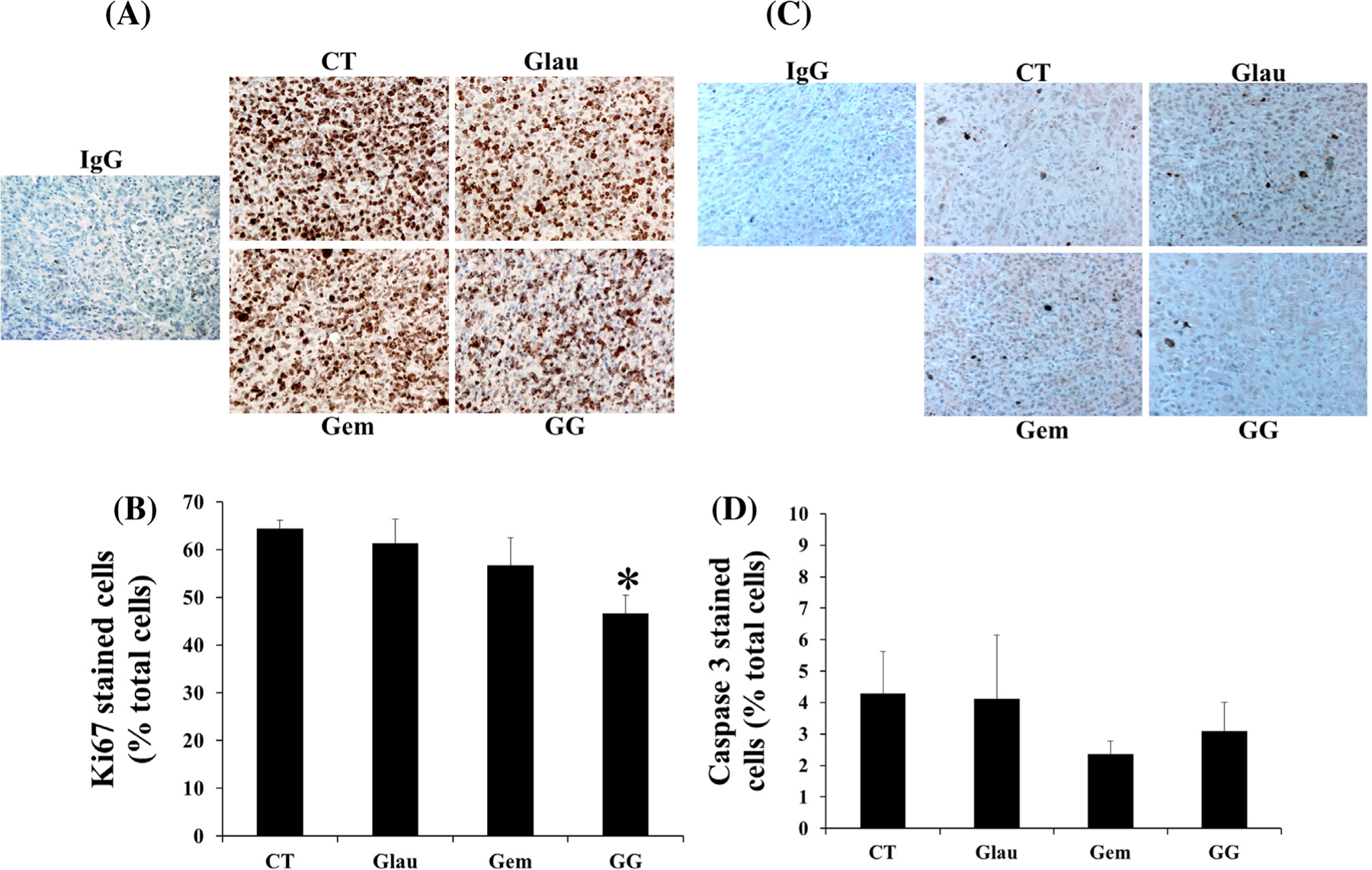
Glaucarubinone and gemcitabine inhibited pancreatic cancer cell growth in vivo by decreasing proliferation without affecting apoptosis. MiaPaCa-2 cells were injected subcutaneously into 6 week-old SCID mice, and the animals were treated with saline [28], glaucarubinone (Glau), gemcitabine (Gem), or the combination (GG) as described in Fig. 4 legend. Proliferation (A and B) in the excised tumors was measured by Ki67 immunohistochemistry (A), and the ratios of positively stained cells to total cells were calculated (B). Anti-rabbit IgG was used as an isotype control. *p < 0.05; compared to the values from untreated control [28] tumors. Apoptosis in excised tumors was measured by caspase 3 immunohistochemistry (C). The ratios of positively stained cells to total cells were calculated (D).
3.6. Glaucarubinone and gemcitabine decreased the amounts of active PAK1 and PAK4 in vivo
To determine the effects of glaucarubinone and gemcitabine on the expression and activity of PAK1 and PAK4, proteins were extracted from tumor tissues. The total concentrations of PAK1 and PAK4, and of active pPAK1 and pPAK4, were measured by Western blotting as described in Section 2. Similarly to the in vitro results, glaucarubinone alone decreased the amounts of active PAK1 in both MiaPaCa-2 and PANC-1 tumors, without affecting the expression of PAK1 (Fig. 6A and C). Treatment with glaucarubinone and gemcitabine in combination further reduced the amounts of active PAK1 in both MiaPaCa-2 and PANC-1 tumors (Fig. 6A and C). However, in contrast to the in vitro results, only treatment with glaucarubinone and gemcitabine in combination significantly reduced the amounts of active PAK4 in both MiaPaCa-2 and PANC-1 tumors (Fig. 6B and D). Neither glaucarubinone nor gemcitabine affected the amount of active PAK4 in MiaPaCa-2 tumors (Fig. 6B). While gemcitabine reduced the amount of active PAK4 in PANC-1 tumors, glaucarubinone had no significant effect on the amount of active PAK4 in PANC-1 tumors (Fig. 6D). These results suggested that glaucarubinone and gemcitabine synergistically reduced pancreatic cancer growth in vivo at least partially through a reduction in the amounts of active PAK1 and PAK4.
Fig. 6.
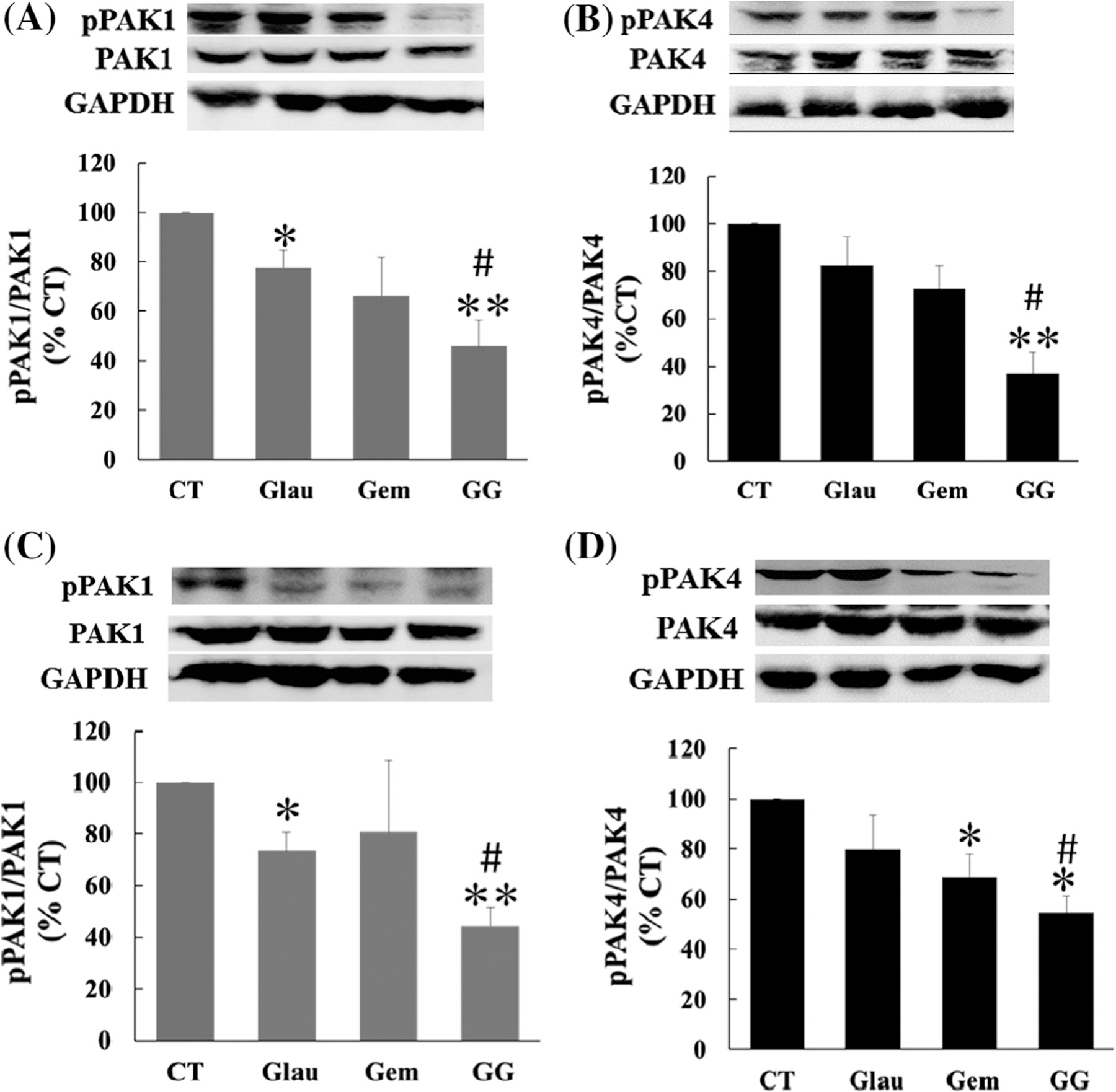
Glaucarubinone and gemcitabine synergistically reduced the amounts of active PAK1 and PAK4 in vivo. MiaPaCa-2 or PANC-1 cells were injected subcutaneously into 6 week-old SCID mice, and the animals were treated with saline [28], glaucarubinone (Glau), gemcitabine (Gem), or the combination (GG), as described in Fig. 4 legend. The amounts of total PAK1 and active phospho-PAK1 (pPAK1) (A and C) or total PAK4 and active phospho-PAK4 (pPAK4) (B and D) were determined in extracts from excised MiaPaCa-2 (A and B) or PANC-1 (C and D) tumors by Western blotting using appropriate antibodies. Variations in protein loading were corrected by GAPDH expression, and the corrected pPAK1/PAK1 or pPAK4/PAK4 ratios from untreated control tumors [28] were taken as 100%. The data represent mean ± SEM, summarised from 5 mice for each group. *p < 0.05; ***p < 0.01 compared to control. #p < 0.05 compared to treatment with glaucarubinone.
4. Discussion
In the current study, we have demonstrated that glaucarubinone decreased proliferation and migration leading to growth inhibition of pancreatic cancer cells in vitro and in vivo, but did not affect either cell survival in vitro or apoptosis in vivo. Furthermore pretreatment with glaucarubinone enhanced the inhibition by gemcitabine of pancreatic cancer cell growth in vitro and treatment with glaucarubinone and gemcitabine in combination suppressed the growth of pancreatic cancer cell lines as xenografts in vivo.
As summarised in Section 1, PAKs are a likely target for the anticancer activity of quassinoids. Almost 100% of pancreatic cancers carry KRas mutations [6], and PAKs are among the major downstream effectors of Ras. In particular, PAK1 is required for Ras-induced malignant transformation of cells [22], and PAK4 activity is necessary for Ras-driven, anchorage-independent growth of colorectal cancer cells [23]. We therefore tested the hypothesis that a reduction in the amounts of active PAKs might contribute to the effects of glaucarubinone on the growth of pancreatic cancer cells. Our observation that treatment with glaucarubinone reduced the amounts of active PAK1 and PAK4 in human MiaPaCa-2 cells in vitro (Fig. 3A and B) indicates that glaucarubinone reduced pancreatic cancer cell proliferation and migration at least partly through inhibition of pathways involving PAK1 and PAK4. Further investigation of the mechanism by which glaucarubinone regulates the amounts of active PAKs is clearly warranted. Although gemcitabine itself did not affect the amounts of either active PAK1 or PAK4, in combination with glaucarubinone it caused a significantly greater reduction in the amounts of active PAK1 and PAK4 than that caused by glaucarubinone alone in both MiaPaCa-2 and PANC-1 cells (Fig. 3A–D). This reduction likely contributes to the synergistic inhibition of pancreatic cancer cell growth that results from combined treatment with gemcitabine and glaucarubinone.
Although glaucarubinone decreased the activity of PAK1, it did not effects the activities of either ERK or AKT (data not shown), which was different to our previous report that PAK1 konckdown suppressed colorectal cancer cell growth via inactivation of both ERK and AKT [19]. The discrepancy between the observation with pancreatic cancer cells and our previous observations with colorectal cancer cells may be caused by (1) different cancer types, pancreatic vs. colorectal, or (2) different cell lines as inhibition of PAK1 suppressed colorectal cancer (CRC) cell growth and survival by inactivation of ERK and AKT in DLD1 [19], but not in HCT116 [24].
Among the three pancreatic cell lines used in this study, MiaPaCa-2 was the most sensitive, and PAN02 the least sensitive, to glaucarubinone (Table 1). It may be more than coincidental that MiaPaCa-2 cells had the lowest expression of both total and active PAK1 and PAN02 cells the highest (Fig. 1D). Although the PAK4 expression levels were similar in these three lines, PAN02 cells had the highest expression of active PAK4 (Fig. 1E). In fact the IC50 values for inhibition of proliferation by glaucarubinone were significantly correlated with the amount of pPAK4 (Fig. 1G), and the correlation in the case of pPAK1 (Fig. 1F) approached significance. These observations are consistent with the suggestion that glaucarubinone inhibited pancreatic cancer cell growth via inhibition of pathways involving PAK1 and PAK4.
The in vivo study showed that glaucarubinone alone (2 mg/Kg, i.p., daily) and gemcitabine alone (20 mg/Kg, i.p., twice weekly) inhibited the tumor growth of MiaPaCa-2, but not PANC-1 cells. Although the IC50 values for inhibition of MiaPaCa-2 in vitro by glaucarubinone and gemcitabine were similar, glaucarubinone seemed to be more potent than gemcitabine as an inhibitor of the xenograft growth of MiaPaCa-2 cells. This difference is presumably because the in vivo growth of cancer cells in Scid mice occurs in a completely different micro-environment from their culture conditions in vitro. The inhibition by both glaucarubinone and gemcitabine of cell growth under the in vitro cell culture conditions was measured by their effects on the proliferation and migration rates of these cancer cells. Unlike in the in vitro setting, the inhibition by both glaucarubinone and gemcitabine of cancer cell xenograft growth in vivo was affected not only by the proliferation and migration rate, but also by the colonising ability of the cancer cells and the extent of tumor angiogenesis. Furthermore the combination of glaucarubinone with gemcitabine further decreased the tumor growth of MiaPaCa-2 cells, and suppressed the tumor growth of PANC-1 cells as well (Fig. 4). Glaucarubinone reduced the activities of PAK1 in tumors grown from both MiaPaCa-2 and PANC-1 cells, and gemcitabine further enhanced the reduction in the amount of active PAK1 by glaucarubinone in vivo. Although glaucarubinone alone did not significantly affect the amount of active PAK4, the combination of glaucarubinone with gemcitabine decreased the amount of active PAK4 in tumors grown from both MiaPaCa-2 and PANC-1 cells. These data indicate that glaucarubinone and gemcitabine synergistically reduced pancreatic cancer growth in vivo likely via inhibition of pathways involving PAK1 and PAK4 though a contribution from the suppression of DNA synthesis by gemcitabine cannot be ruled out.
Gemcitabine has long been the first-line chemotherapeutic agent in pancreatic cancer. However, a large number of patients do not respond to the drug due to both intrinsic and acquired resistance. To improve the efficacy, systemically administered gemcitabine has been combined with a second cytotoxic agent, such as a platinum analogue, fluoropyrimidine, or a targeted cytotoxic agent [25]. A recent meta-analysis including 26 high-quality randomized trials comparing gemcitabine mono-therapy and combination therapy has demonstrated that gemcitabine-based combination therapy provides a modest improvement of survival, but is associated with increased toxicity compared with gemcitabine mono-therapy [26]. Our findings that glaucarubinone and gemcitabine synergistically inhibited pancreatic cancer cell growth in vitro and in vivo, at least in part via inhibition of pathways involving PAK1 and PAK4, could provide a new alternative for pancreatic cancer therapy.
Supplementary Material
Acknowledgements
We thank the Drug Synthesis & Chemistry Branch, NCI, for supplying the glaucarubinone used in this study, and Hiroshi Maruta for stimulating discussions. This work was supported by National Health and Medical Research Council (NHMRC) of Australia Grants 508908 (HH) [27], 1041831 (GB & AS), and 1020983 (GB), by the Austin Hospital Medical Research Foundation, and by the Intramural Research Program of the NIH National Cancer Institute Center for Cancer Research. Dannel Yeo is supported by the Ph.D scholarship from Australian Rotary Health.
Footnotes
Conflict of Interest
There is no conflict of interests from the authors of this paper titled “Glaucarubinone and gemcitabine synergistically reduce pancreatic cancer growth via down-regulation of P21-activated kinases”.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.canlet.2014.01.001.
References
- [1].Hidalgo M, Pancreatic cancer, N. Engl. J. Med 362 (2010) 1605–1617. [DOI] [PubMed] [Google Scholar]
- [2].Yeo TP, Hruban RH, Leach SD, Wilentz RE, Sohn TA, Kern SE, Iacobuzio-Donahue CA, Maitra A, Goggins M, Canto MI, Abrams RA, Laheru D, Jaffee EM, Hidalgo M, Yeo CJ, Pancreatic cancer, Curr. Probl. Cancer 26 (2002) 176–275. [DOI] [PubMed] [Google Scholar]
- [3].Allison DC, Piantadosi S, Hruban RH, Dooley WC, Fishman EK, Yeo CJ, Lillemoe KD, Pitt HA, Lin P, Cameron JL, DNA content and other factors associated with ten-year survival after resection of pancreatic carcinoma, J. Surg. Oncol 67 (1998) 151–159. [DOI] [PubMed] [Google Scholar]
- [4].Kayahara M, Nagakawa T, Ueno K, Ohta T, Takeda T, Miyazaki I, An evaluation of radical resection for pancreatic cancer based on the mode of recurrence as determined by autopsy and diagnostic imaging, Cancer 72 (1993) 2118–2123. [DOI] [PubMed] [Google Scholar]
- [5].Castellanos E, Berlin J, Cardin DB, Current treatment options for pancreatic carcinoma, Curr. Oncol. Rep 13 (2011) 195–205. [DOI] [PubMed] [Google Scholar]
- [6].Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, Morsberger LA, Latimer C, McLaren S, Lin ML, McBride DJ, Varela I, Nik-Zainal SA, Leroy C, Jia M, Menzies A, Butler AP, Teague JW, Griffin CA, Burton J, Swerdlow H, Quail MA, Stratton MR, Iacobuzio-Donahue C, Futreal PA, The patterns and dynamics of genomic instability in metastatic pancreatic cancer, Nature 467 (2010) 1109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Molli PR, Li DQ, Murray BW, Rayala SK, Kumar R, PAK signaling in oncogenesis, Oncogene 28 (2009) 2545–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen S, Auletta T, Dovirak O, Hutter C, Kuntz K, El-ftesi S, Kendall J, Han H, Von Hoff DD, Ashfaq R, Maitra A, Iacobuzio-Donahue CA, Hruban RH, Lucito R, Copy number alterations in pancreatic cancer identify recurrent PAK4 amplification, Cancer Biol. Ther 7 (2008) 1793–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kimmelman AC, Hezel AF, Aguirre AJ, Zheng H, Paik JH, Ying H, Chu GC, Zhang JX, Sahin E, Yeo G, Ponugoti A, Nabioullin R, Deroo S, Yang S, Wang X, McGrath JP, Protopopova M, Ivanova E, Zhang J, Feng B, Tsao MS, Redston M, Protopopov A, Xiao Y, Futreal PA, Hahn WC, Klimstra DS, Chin L, DePinho RA, Genomic alterations link Rho family of GTPases to the highly invasive phenotype of pancreas cancer, Proc. Natl. Acad. Sci. USA 105 (2008) 19372–19377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chauhan SC, Ebeling MC, Maher DM, Koch MD, Watanabe A, Aburatani H, Lio Y, Jaggi M, MUC13 mucin augments pancreatic tumorigenesis, Mol. Cancer Ther 11 (2012) 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang Z, Oh E, Clapp DW, Chernoff J, Thurmond DC, Inhibition or ablation of p21-activated kinase (PAK1) disrupts glucose homeostatic mechanisms in vivo, J. Biol. Chem 286 (2011) 41359–41367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Monjour L, Rouquier F, Alfred C, Polonsky J, Therapeutic trials of experimental murine malaria with the quassinoid, glaucarubinone, C. R. Acad. Sci 304 (1987) 129–132. [PubMed] [Google Scholar]
- [13].Valeriote FA, Corbett TH, Grieco PA, Moher ED, Collins JL, Fleck TJ, Anticancer activity of glaucarubinone analogues, Oncol. Res 10 (1998) 201–208. [PubMed] [Google Scholar]
- [14].Fiaschetti G, Grotzer MA, Shalaby T, Castelletti D, Arcaro A, Quassinoids: from traditional drugs to new cancer therapeutics, Curr. Med. Chem 18 (2011) 316–328. [DOI] [PubMed] [Google Scholar]
- [15].Beutler JA, Kang MI, Robert F, Clement JA, Pelletier J, Colburn NH, McKee TC, Goncharova E, McMahon JB, Henrich CJ, Quassinoid inhibition of AP-1 function does not correlate with cytotoxicity or protein synthesis inhibition, J. Nat. Prod 72 (2009) 503–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Maruta H, Herbal therapeutics that block the oncogenic kinase PAK1: a practical approach towards PAK1-dependent diseases and longevity, Phytother. Res.: PTR (2013). [DOI] [PubMed]
- [17].Sicard A, Semblat JP, Doerig C, Hamelin R, Moniatte M, Dorin-Semblat D, Spicer JA, Srivastava A, Retzlaff S, Heussler V, Waters AP, Doerig C, Activation of a PAK-MEK signalling pathway in malaria parasite-infected erythrocytes, Cell. Microbiol 13 (2011) 836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yanase S, Luo Y, Maruta H, PAK1-deficiency/down-regulation reduces brood size, activates HSP16.2 gene and extends lifespan in Caenorhabditis elegans, Drug Discov. Therap 7 (2013) 29–35. [DOI] [PubMed] [Google Scholar]
- [19].Huynh N, Liu KH, Baldwin GS, He H, P21-activated kinase 1 stimulates colon cancer cell growth and migration/invasion via ERK- and AKT-dependent pathways, Biochim. Biophys. Acta 2010 (1803) 1106–1113. [DOI] [PubMed] [Google Scholar]
- [20].Chou TC, Talalay P, Quantitative analysis of dose–effect relationships: the combined effects of multiple drugs or enzyme inhibitors, Adv. Enzyme Regul 22 (1984) 27–55. [DOI] [PubMed] [Google Scholar]
- [21].Chou TC, Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies, Pharmacol. Rev 58 (2006) 621–681. [DOI] [PubMed] [Google Scholar]
- [22].He H, Hirokawa Y, Gazit A, Yamashita Y, Mano H, Kawakami Y, Kawakami C.Y. Hsieh, Kung HJ, Lessene G, Baell J, Levitzki A, Maruta H, The Tyr-kinase inhibitor AG879, that blocks the ETK-PAK1 interaction, suppresses the RAS-induced PAK1 activation and malignant transformation, Cancer Biol. Ther 3 (2004) 96–101. [DOI] [PubMed] [Google Scholar]
- [23].Callow MG, Clairvoyant F, Zhu S, Schryver B, Whyte DB, Bischoff JR, Jallal B, Smeal T, Requirement for PAK4 in the anchorage-independent growth of human cancer cell lines, J. Biol. Chem 277 (2002) 550–558. [DOI] [PubMed] [Google Scholar]
- [24].Tabusa H, Brooks T, Massey AJ, Knockdown of PAK4 or PAK1 inhibits the proliferation of mutant KRAS colon cancer cells independently of RAF/MEK/ERK and PI3K/AKT signaling, Mol. Cancer Res.: MCR 11 (2013) 109–121. [DOI] [PubMed] [Google Scholar]
- [25].Michl P, Gress TM, Current concepts and novel targets in advanced pancreatic cancer, Gut 62 (2013) 317–326. [DOI] [PubMed] [Google Scholar]
- [26].Sun C, Ansari D, Andersson R, Wu DQ, Does gemcitabine-based combination therapy improve the prognosis of unresectable pancreatic cancer?, World J Gastroenterol.: WJG 18 (2012) 4944–4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vincent J, Hochhalter AK, Broglio K, Avots-Avotins AE, Survey respondents planning to have screening colonoscopy report unique barriers, Permanente J 15 (2011) 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vincent F, Duncton MA, TRPV4 agonists and antagonists, Curr. Top. Med. Chem 11 (2011) 2216–2226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


