Abstract
Obesity rates continue to rise in children, and little guidance exists regarding the need for adjustment away from total body weight-based doses for those prescribing drugs to this population of children. A majority of drugs prescribed to children with obesity result in either sub-therapeutic or supra-therapeutic concentrations, placing these children at risk for treatment failure and drug toxicities. In this review, we highlight available obesity-specific pharmacokinetic and dosing information for the most frequently prescribed drugs to children in the inpatient and outpatient clinical settings. We also comment on available dosing recommendations for drugs prescribed to treat common pediatric obesity-related comorbidities. This review highlights that there is no safe or proven ‘rule of thumb,’ for dosing drugs for children with obesity, and a striking lack of pharmacokinetic data to support the creation of dosing guidelines for children with obesity for the most commonly prescribed drugs. It is important that those prescribing for children with obesity are aware of these gaps in knowledge and of potential drug treatment failure or adverse events related to drug toxicity as a result of these knowledge gaps. Until more data are available, we recommend close monitoring of drug response and adverse events in children with obesity receiving commonly prescribed drugs.
1. Background
The World Health Organization (WHO) declared childhood obesity one of the most serious global health problems of the 21st century, with an estimated 124 million children with obesity worldwide [1]. Obesity, as defined by the Centers for Disease Control (CDC), encompasses any child with a body mass index (BMI, kg/m2) of or greater than the 95th percentile for their age and sex [2]. In the United States (US), one in five children between the ages of 2–19 years meets BMI criteria for obesity [3]. In addition to common pediatric illnesses (e.g., otitis media, asthma, fever), these children also experience obesity-associated co-morbidities (e.g., hypercholesterolemia, hypertension, type 2 diabetes mellitus) that frequently require prescription drugs to control or cure symptoms [4]. However, optimal drug dose selection remains unclear for children with obesity [5, 6].
Drug dose selection in pediatrics is typically based on total body weight (TBW), with dosing recommendations derived from pharmacokinetic data in healthy adults or children. Sometimes allometric scaling is used to scale down drug doses from adult recommendations to children, who are generally smaller in size. However, this method is flawed for children with obesity, whose TBW may equal or even exceed that of an adult, while their organ anatomy and physiology remain developmentally immature and distinctly different from an adult [7–10]. Thus, neither TBW nor allometric scaling may adequately describe best dosing practices for children with obesity, despite frequent use of both strategies [7]. Additionally, in order to achieve the desired therapeutic effect (i.e., pharmacodynamics), the ‘best’ drug dose selection strategy must also account for potential variability in disease phenotype between children with and without obesity (e.g., asthma, discussed later in this manuscript) [11].
Upon recent review, approximately two-thirds of the drugs prescribed to children with obesity resulted in either sub-therapeutic or supra-therapeutic concentrations, placing these children at risk for treatment failure and drug toxicities [5]. Subsequently, drug dose individualization in obesity has appropriately received increased attention in the last decade [5–8, 10, 12–15]. However, agents prioritized for study have primarily focused on drugs with a narrow therapeutic index and high risk of serious toxicity, leaving a critical information gap for some of the most commonly utilized drugs in pediatrics. In this review, we highlight available obesity-specific pharmacokinetic, pharmacodynamic, and dosing information for the most frequently prescribed medications to children in the inpatient and outpatient clinical setting. We also comment on available dosing recommendations for common pediatric obesity-related comorbidities and discuss the lessons to be learned from the data available, identifying areas of focus for future research.
2. Search Strategy
To identify the most commonly prescribed drugs in the pediatric inpatient setting, we used the Pediatric Health Information System (PHIS), a nationally representative database of clinical/resource utilization data from over 45 pediatric hospitals in the US, to generate a list of the most frequently prescribed drugs in a single year (2017). This list was cross-referenced with medications identified as commonly prescribed in the pediatric hospital setting in a recent publication by Callaghan, to ensure inclusion of commonly prescribed drugs with known pharmacokinetic data available for review [7]. Outpatient drugs were selected from a publication of the most commonly prescribed outpatient drugs to children (2002–2010) by Chai et al. [16], which used two large outpatient prescription databases and, to our knowledge, represents the most complete outpatient drug list available to date. Intravenous (IV) fluids (e.g., normal saline, dextrose in water) and topical formulations (e.g., mupirocin, triamcinolone) were considered outside the scope of this review and were specifically excluded. Following removal of these formulations from the originally compiled inpatient and outpatient drug lists, the literature review ultimately included 22 inpatient (Table 1) and 15 outpatient drugs (Table 2).
Table 1.
Most frequently prescribed pediatric inpatient drugs
| Category | Drug | Summary of evidence |
|---|---|---|
| Antimicrobials | Cefazolin | No difference in CL or Vd [14, 61]. Dose based on TBW |
| Ceftriaxone | No specific studies identified; TBW suggested for cephalosporins as a drug class [7] | |
| Vancomycin | No clear difference in CL or Vd. Patients with obesity may be more likely to experience higher trough concentrations, but this is of uncertain clinical significance. Most recommend dosing based on TBW [14] | |
| Clindamycin | No clear difference in pharmacokinetic properties. Dose based on TBW [63] | |
| Analgesics/anesthetics | Acetaminophen | Obesity may result in decreased drug exposure, but changes in CYP2E1 metabolic pathways preclude any necessary dose adjustments [67] |
| Propofol | Mixed results regarding safest dosing; some suggest TBW-based for maintenance dosing [38] and other LBW for induction dosing to achieve desired exposure [40] | |
| Fentanyl | Increased drug clearance in adolescents with obesity compared with adults. Some studies recommend dosing based on IBW [43] or other adjusted body weight [50] | |
| Morphine sulfate | IBW has been suggested for dosing in children with obesity [7, 42] | |
| Rocuronium | No pediatric studies identified. Adult studies suggest dosing based on IBW [51, 52] | |
| Ibuprofen | No specific studies identified | |
| Ketorolac | No specific studies identified | |
| Oxycodone | No specific studies identified | |
| Hydromorphone | No specific studies identified | |
| Benzodiazepines | Midazolam | Increased peripheral Vd, necessitating possible higher initial doses to achieve desired exposure [45] |
| Lorazepam | No specific studies identified | |
| Steroids | Dexamethasone | No specific studies identified |
| Methylprednisolone | No specific studies identified | |
| Inhaled drugs | Albuterol | Increased likelihood of therapeutic failure using standard dosing (OR 1.24–1.38; 95% CI 1.03–1.7); dose escalation studies warranted [74] |
| Fluticasone | Increased likelihood of therapeutic failure using standard dosing in adults (twofold increase in morbidly obese adults compared with all other adults) [72] | |
| Other drugs | Ondansetron | No specific studies identified |
| Diphenhydramine | No specific studies identified | |
| Enoxaparin | Some studies noted higher doses needed to reach therapeutic concentrations for VTE prophylaxis in patients with obesity. However, dose based on TBW; treat to target (anti-Xa 0.1–0.3 IU/mL) [7] | |
| Ranitidine | No specific studies identified |
CI confidence interval, CL clearance, IBW ideal body weight, LBW lean body weight, OR odds ratio, TBW total body weight, Vd volume of distribution, reported in units per kg TBW
Table 2.
Most frequently prescribed pediatric outpatient drugs [16]
| Category | Drug | Summary of evidence |
|---|---|---|
| Antimicrobials | Amoxicillin | No specific studies identified |
| Azithromycin | No specific studies identified | |
| Cefdinir | No specific studies identified | |
| Cephalexin | No specific studies identified | |
| Trimethoprim/sulfamethoxazole | No specific studies identified | |
| Analgesics | Ibuprofen | No specific studies identified. Study in adults suggests increased Vd and CL in obesity [69] |
| Hydrocodone/acetaminophen | No specific studies identified | |
| ADHD drugs | Methylphenidate | No specific studies identified |
| Dextromethorphan/phenylphrine/chlorpheniramine | No specific studies identified | |
| Amphetamine/dextromethorphan | No specific studies identified | |
| Steroids | Prednisolone | No specific studies identified. However, studies of steroids in adults revealed poor oral absorption and increased CL leading to poor efficacy [70] |
| Prednisone | No specific studies identified | |
| Asthma treatment | Montelukast | Greater improvement in asthma symptom scores in pts with obesity compared with healthy weight [73] |
| Inhaled drugs | Albuterol | Increased likelihood of therapeutic failure using standard dosing (OR 1.24–1.38; 95% CI 1.03–1.7); dose escalation studies warranted [74] |
| Fluticasone | No specific studies identified. However, increased likelihood of therapeutic failure using standard dosing in adults (twofold increase in morbidly obese adults compared with all other adults) [72] |
ADHD attention deficit hyperactivity disorder, CI confidence interval, CL clearance, OR odds ratio, Vd volume of distribution
Once the lists of commonly prescribed inpatient (Table 1) and outpatient (Table 2) drugs were compiled, applicable articles were identified using PubMed MeSH term-based searches including the key words (1) weight, body size, and obesity, (2) pediatrics, children, or adolescents, and (3) generic and trade names for each drug included in Tables 1 and 2. Additionally, we expanded this search to include studies of drugs used specifically to treat obesity co-morbidities (e.g., hypercholesterolemia, hypertension, insulin resistance, etc.). Although these drugs (Table 3) may be less frequently prescribed to children in general, they are more likely to be prescribed to children with obesity specifically, and thus were deemed pertinent to include in this review.
Table 3.
Drugs commonly prescribed for obesity co-morbid conditions
| Condition | Drug/drug class | Summary of evidence |
|---|---|---|
| Diabetes mellitus/insulin insensitivity/obesity | Metformin | Increased clearance in patients with obesity, leading to lower systemic exposure; dose escalation may be warranted [96] |
| Hypertension | Calcium channel blockers | Single small study showed children with obesity may require higher doses to achieve optimal BP control [86]. No studies evaluating drug distribution in children with obesity |
| β-Blockers | No specific studies identified. In adults, possible increased Vd in patients with obesity, especially in lipophilic drugs [21]. However, β-blockers preferentially bind lean body tissues, suggesting dosing based on lipophilicity of drugs alone is not adequate | |
| ACE inhibitors (ACEi) or angiotensin receptor blockers (ARBs) | No pharmacokinetic studies exist. Single study with very small sample size demonstrated no therapeutic difference for pts with obesity [85] | |
| Gastroesophageal reflux disease | Proton pump inhibitors | Data only available for pantoprazole: LBW [89, 90] Decreased CL/F; thus, no empiric dose escalation warranted, if using fixed dosing [91] |
| Histamine H2 receptor blockers (e.g., ranitidine) | No specific studies identified | |
| Hypercholesterolemia | Statins | Few pediatric studies suggest correlation between BMI and drug exposure, but results are mixed [81, 82] |
BMI body mass index, CL/F apparent total clearance of the drug from plasma, LBW lean body weight, Vd volume of distribution
3. Approaches to Drug Dose Selection
The lack of pharmacokinetic clinical trials for drug dose selection in children with obesity makes generalization of dosing recommendations difficult [6]. In general, two dosing strategies have been proposed for children with obesity: allometric scaling and physiologically-based dosing.
3.1. Anthropometrics-Based Dosing and Allometric Scaling
Allometric scaling refers to dosing based on body size scaled to a fixed exponent [8]. This exponent can be 1 (which defines a linear relationship between dose and body size), 0.75 (Klieber’s Law, commonly utilized in biology) or any other numerical value [17]. Recently, a scaling exponent specific to obesity based on the theory-based size descriptor of normal fat mass has been proposed by Anderson and Holford [8]. However, it remains unclear which anthropometric measure of size is most appropriate for scaling. Body weight, volume, and surface area have all been proposed.
TBW-based dosing is the most commonly utilized and appears to be appropriate for some drugs, but not others [5]. Differences are noted even within a given drug class (e.g., antimicrobials). For example, TBW-based dosing appears appropriate for clindamycin, vancomycin, cefazolin, and ceftriaxone [18], but leads to overdose and adverse events for gentamicin [7], azithromycin [19], and voriconazole [20]. To circumvent this variability in TBW-based dosing, some have proposed dosing based on the physiochemical properties of the drug. This approach assumes that a drug with high lipophilicity will have a larger volume of distribution (Vd) in obesity, requiring higher initial drug doses to achieve desired concentrations systemically and/or in the target organ. However, dosing is complicated by the potential for drug sequestering in, and unpredictable release from, adipose tissue, which may explain why, to date, no predictable, systematic relationship between the degree of drug lipophilicity and drug distribution has been identified [6, 10, 21]. For hydrophilic drugs, this pharmacokinetic relationship is even less clear [21].
Alternative anthropometric measures proposed for drug dose selection in obesity include BMI, body surface area (BSA), ideal body weight (IBW), and lean body weight (LBW), among others [14, 15]. Each of these indices of body composition comprises a calculation that incorporates a height component, which may be problematic, as obesity and its associated comorbidities (e.g., type 2 diabetes mellitus) can impact normal pediatric growth and development, including linear growth.
Historically, it has been noted that children with obesity/overweight tend to be taller than their normal-weight peers [22]. However, this trend does not continue into adulthood. Several studies note that children with obesity/overweight experience accelerated linear growth in childhood [23] and/or puberty [24, 25], followed by decreased height gain [23] that results in terminal height below [26] or equal to [23–25] peers without obesity. This altered pattern of linear growth affects BMI, BSA, IBW, and LBW calculations; therefore, some have proposed developing nomographs specific for children with obesity to aid drug dose selection [7]. We are currently exploring the utility of obesity-specific growth curves for dosing recommendations of metformin.
Obesity-associated alterations in organ size may also play a role in drug dose selection, particularly for pharmacologic agents that undergo biotransformation or metabolism in those organs. Liver volumes, for example, positively correlate with BSA [27], which is higher in obesity. Theoretically, larger liver volumes could alter the capacity for hepatic drug clearance and/or hepatic blood flow; however, the impact of intra-organ fat infiltration on these relationships has not been assessed in children with obesity.
Overall, the lack of evidence for dosing drugs based on TBW or other anthropometric measures for the vast majority of drugs prescribed to children with obesity makes generalization of dosing recommendations difficult [6], and it is likely that no single size measure is appropriate for all drugs [18]. Physiologically-based dosing, an alternative dosing approach increasingly recognized by regulatory agencies, focuses on organ function rather than size to help guide drug dose selection.
3.2. Physiologically-Based Dosing
An evolving approach to drug dose selection in obesity is based on the physiological changes that accompany obesity, and how these changes may impact drug absorption, distribution, metabolism, and excretion [10] (Fig. 1). For example, drug bioavailability may be altered due to differences in gastric emptying time, blood flow, drug transport, drug metabolizing enzyme expression and/or activity in individuals with versus those without obesity.
Fig. 1.
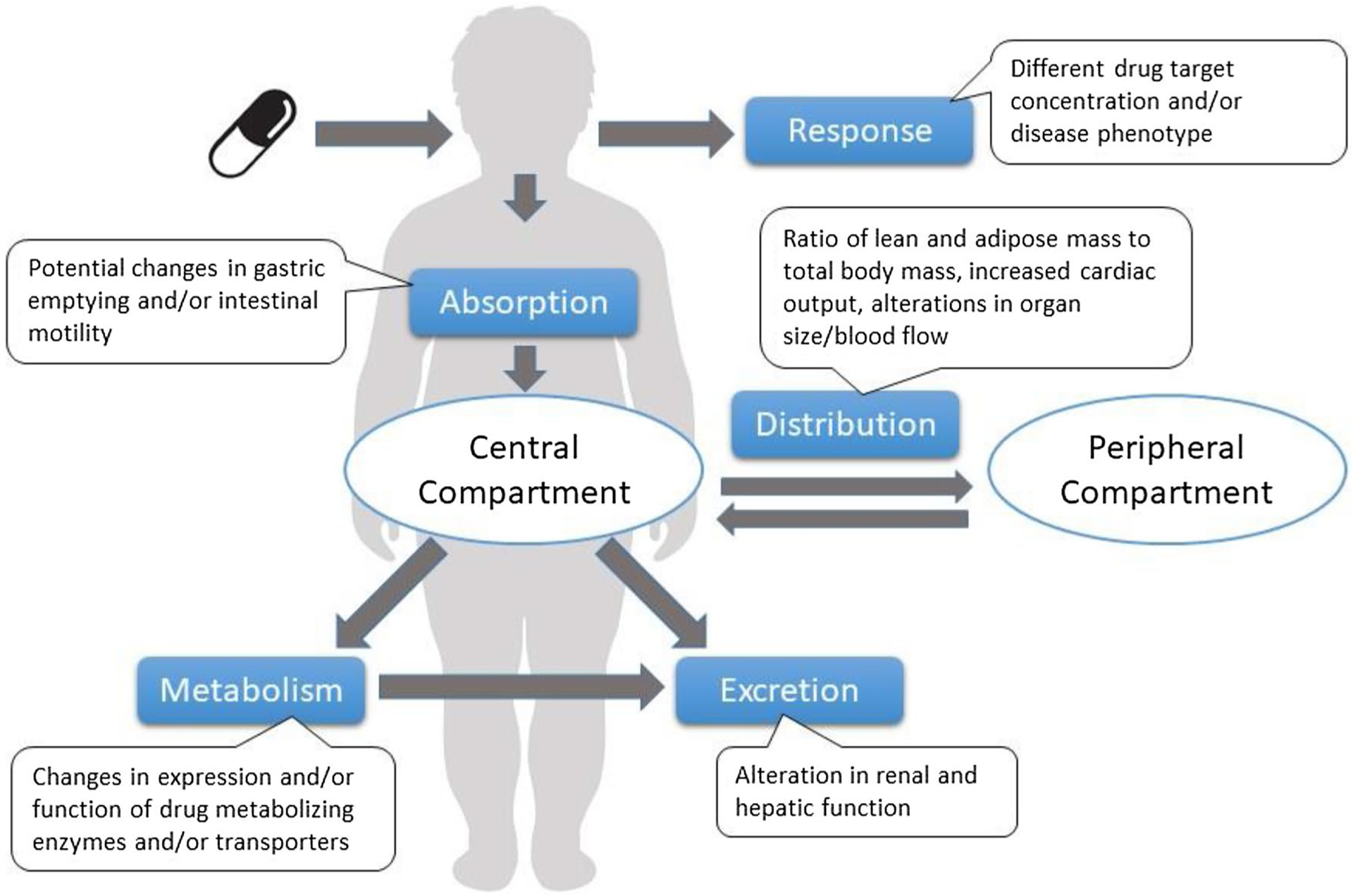
Effects of obesity on drug absorption, distribution, metabolism, and excretion
Specifically, obesity is associated with increased lean and total body mass, but decreased lean-to-total body mass ratio [12]; increased blood volume, cardiac output, splanchnic and hepatic blood flow [28]; and altered drug-binding protein concentrations for some drugs (e.g., propranolol and α1 acid glycoprotein) [29], but not others (e.g., phenytoin and albumin) [12, 29]. Combined, these obesity-related perturbations in physiology may account for the large amount of variability observed in the Vd for a given drug in obese individuals [6, 21, 28], unaccounted for by body size alone. Additionally, obesity-related hypertrophy of metabolically active organs (e.g., liver, kidneys) [6, 30], and subsequent alterations in blood flow to and from these organs [28], can also affect total drug clearance, as demonstrated by studies of the renally cleared drug vancomycin [31] and the hepatically cleared drug carbamazepine [32, 33]. Furthermore, individuals with obesity frequently have hepatic fat infiltration, a condition called non-alcoholic fatty liver disease, which can be associated with inflammation [34]. Inflammation, in turn, can affect hepatic metabolic activity, including drug metabolizing enzymes in the cytochrome P450 family (CYPs) [21]. Available studies to date suggest that activity of some hepatic CYPs appears increased in individuals with obesity (e.g., CYP2E1, CYP2D6), while the activity of others (e.g., CYP3A4, CYP2C19) appears decreased [35].
With the complex interplay of the many physiologic consequences of obesity, comprehensive, computer-based frameworks capable of synthesizing the available obesity physiology knowledge to study the pharmacokinetics and pharmacodynamics of drugs in obesity have gained popularity. These physiologically-based pharmacokinetic and pharmacodynamic models (PBPK-PD) show promise in pediatrics [36], but depend on the quality of the physiologic data available for obese children, which are often sparse [5]. Pharmacologic knowledge for children with obesity is urgently needed to provide optimal drug dosing recommendations, particularly for the most commonly prescribed drugs for children and for children with obesity in the inpatient and outpatient setting.
4. Summary of Data Available for Commonly Prescribed Inpatient Drugs
The number of studies examining pharmacokinetic data for drugs in children is growing, particularly for those drugs most commonly prescribed in the inpatient hospital setting. Areas of progress and focus include anesthetics and antimicrobials, with some studies specifically examining differences in drug metabolism in children with versus without obesity. The motivation for prioritizing these two drug classes for investigation likely stems from their narrow therapeutic index and the heightened risk of complications, adverse outcomes, or therapeutic failures if adequate systemic drug concentrations are not achieved [37]. Still, for the majority of the most frequently prescribed drugs to children during hospitalization (Table 1), data are severely lacking.
4.1. Anesthetics
Of the most commonly prescribed anesthetics, pharmacokinetic studies in children with obesity were identified for the following drugs: propofol [38–41], fentanyl [42–44], midazolam [42, 45, 46], and morphine [42]. Adult obesity data also exist for these drugs. Combined, these studies demonstrate significant alterations in the pharmacokinetics of anesthetics necessitating modifications to the routinely employed TBW-based drug dosing strategies. The recommendations in exactly how to alter dosing for patients with obesity varies by drug and from study to study. Of note, some studies included pharmacokinetic data for loading doses of the drug, and others for maintenance dosing; we chose to include information on both types of studies, as both types of dosing can be affected by obesity and would therefore affect drug dosing recommendations. We will clarify which studies were of induction dosing only throughout this section.
4.1.1. Propofol
The data for propofol in obese children are mixed. Diepstraten et al. proposed that an individual’s TBW is the most significant determinant of drug clearance, advocating that children with obesity receive TBW-based dosing to achieve maintenance anesthesia [39]. However, Olutoye et al. reported that children with obesity require a lower weight-based dose for anesthesia induction than healthy-weight children [40]. Adult literature report similarly mixed findings, with some reporting TBW-based dosing to be best [47], and others suggesting LBW [48] as a better metric to achieve adequate target anesthesia goals with propofol.
4.1.2. Fentanyl
Studies comparing fentanyl infusion in pediatric patients with obesity undergoing bariatric surgery found Vd values comparable to previously published studies in children without obesity, but increased drug clearance, likely secondary to increased hepatic blood circulation [43]. Based on these findings, a dosing strategy based on IBW or LBW, rather than TBW, is proposed for children with obesity, in line with recommendations from the adult bariatric surgery literature [43, 49]. One study in adults, by Shibutani et al., confirms that TBW-based dosing of fentanyl may result in overdose [50].
4.1.3. Midazolam
Studies of midazolam pharmacokinetics in children with obesity identify a marked increase in peripheral Vd, indicating a potential need for higher initial drug dose administration for continuous infusion, in order to achieve therapeutic exposures [45]. A study by van Rongen et al. describes higher observed clearance of midazolam in children with obesity compared with adults with obesity [45]. The authors propose that this observation may be due to decreased CYP3A activity in adults versus children and highlight the fact that extrapolation of adult obesity pharmacokinetic data to children can be fraught with problems [46].
4.1.4. Rocuronium
Although no pediatric data are available, adult data for rocuronium, a commonly prescribed muscle relaxant/paralytic during anesthesia, suggest the duration of action of rocuronium is prolonged in patients with obesity when prescribed according to their TBW [51]. Another study showed a shorter duration of action without prolonging onset time or complicating conditions necessary for successful intubation [52]. These studies suggest dosing of rocuronium should be based on a patient’s IBW for adults with obesity [51, 52].
4.2. Antimicrobials
Consequent to body weight being identified as a predictor of antibiotic treatment failure [53], antimicrobials have been another drug class of focus for pharmacokinetic studies in children with obesity. In the inpatient setting, the most robust information exists for vancomycin [54–60], one of the drugs identified as most commonly prescribed in the inpatient setting. We also identified two pediatric studies of cefazolin [61, 62] and one of clindamycin in obesity [63]. No studies of ceftriaxone were found.
4.2.1. Vancomycin
Studies of vancomycin pharmacokinetics in children with obesity reveal mixed results, which appear to bear no clinical significance for children with versus those without obesity [54–57, 59]. There do not appear to be major differences in vancomycin clearance or Vd; although a few studies have identified differences in vancomycin troughs of questionable clinical significance between children with versus those without obesity [54, 59]. Heble et al. observed patients with obesity were more likely to experience higher initial trough concentrations when dosed based on TBW (median 14.4 μg/mL vs 10.5 μg/mL; p < 0.001), though all troughs were within a normal therapeutic range [54]. Madigan et al. used a higher daily dose of vancomycin to attempt to achieve goal trough concentrations in patients of all weight categories and found that adolescents with higher weights were more likely to experience elevated trough concentrations compared with all other groups of children [59]. Interpretation of these results was confounded by age, as significant differences in troughs were also observed based on age alone (i.e., adolescent vs child) [59]. Moffett et al. found a trend toward patients with obesity experiencing higher trough concentrations, but the differences between groups were not statistically or clinically significant [55]. Three additional studies found no evidence of differences in vancomycin pharmacokinetics between children with versus those without obesity [56–58]. Given the lack of clinical relevance of the sometimes statistically significant changes in vancomycin pharmacokinetics in children with obesity, some studies recommend dosing based on TBW for all pediatric patients, regardless of weight status [55].
4.2.2. Cefazolin
Cefazolin is a drug frequently prescribed in the hospital setting, especially peri-operatively. We identified two small studies examining cefazolin pharmacokinetics in pediatric patients with obesity [61, 62]. Cefazolin has hydrophilic properties; however, drug lipophilicity was not predictive of drug distribution within tissues and the studies found no differences in cefazolin clearance or Vd, adjusted for TBW, in children with versus those without obesity [61, 62]. Based on these limited data, it is recommended that cefazolin dosing be based on TBW for all patients, regardless of weight status (e.g., 30 mg/kg for perioperative prophylaxis [64]); however, the maximum safe total dose remains unknown. Maximum doses of both 2 g and 3 g have been proposed, based on studies in adults [62].
4.2.3. Clindamycin
Hypothesizing that clindamycin may require dose adjustment for patients with obesity due to the drug’s lipophilic properties, Smith et al. examined pharmacokinetic differences in children with and without obesity [63]. Similar to cefazolin, lipophilicity was not predictive of clindamycin pharmacokinetics, and TBW-based dosing was recommended for children of all weight statuses.
4.3. Other Commonly Prescribed Inpatient Drugs
4.3.1. Acetaminophen
In addition to its frequent over-the-counter use in the outpatient setting, we identified acetaminophen as the number one prescribed drug in the inpatient setting. Despite its frequent use, only one study of acetaminophen pharmacokinetics in children with obesity and non-alcoholic fatty liver disease (NAFLD) was identified [65]. Although this study did not find significant differences in circulating acetaminophen concentrations after a 5-mg/kg (up to 325 mg) single oral dose administration, it did identify significantly higher concentrations of the acetaminophen glucuronide metabolite in the plasma and urine of children with NAFLD, suggesting that hepatic glucuronosyltransferase (UGT) activity is upregulated in the presence of hepatic fat. This observation of increased UGT-mediated acetaminophen metabolism in obesity is supported by several adult studies [66–68]. Van Rongen et al. found that adults with obesity had significantly lower concentrations of acetaminophen after IV dose administration, putting them at risk for therapeutic failure; however, they also had higher concentrations of hepatotoxic CYP2E1-mediated acetaminophen metabolites, cysteine and mercapturate, putting them at higher risk for toxicity [67]. Thus, despite the potential need for higher initial doses of acetaminophen due to increased UGT activity, individuals with obesity may not tolerate the higher doses required, due to increased CYP2E1 activity leading to overproduction of hepatotoxic acetaminophen metabolites.
4.3.2. Other Inpatient Drugs
The data for other commonly prescribed pediatric inpatient drugs are even more sparse, with respect to pharmacokinetic investigations in children with obesity. No published information could be found for ondansetron, ibuprofen, diphenhydramine, ketorolac, ranitidine, oxycodone, ceftriaxone, hydromorphone, lorazepam, and methylprednisolone. For some of these drugs, data were available for adult patients with obesity; however, we have already highlighted the potential pitfalls in extrapolating pharmacokinetic data from adults with obesity to children (e.g., midazolam and CYP3A4) [46, 69].
5. Summary of Data Available for Commonly Prescribed Outpatient Drugs
Despite the frequency and the abundance of drugs prescribed to children in the outpatient setting [16] (Table 2), minimal information is available for drug dose recommendations for children with obesity. Specifically, we found no pharmacokinetic data to support evidenced-based drug dosing in children with obesity for the most frequently prescribed outpatient drugs: amoxicillin, amoxicillin-clavulanate, cefdinir, cephalexin, prednisolone, ibuprofen, trimethoprim/sulfamethoxazole, hydrocodone/acetaminophen, methylphenidate, dextromethorphan/phenylephrine/chlorpheniramine, prednisone, or amphetamine/dextromethorphan. Several studies of these agents were identified for adults with obesity (e.g., steroids [69–71]). Studies of oral steroids reported poor oral absorption and increased apparent drug clearance for subjects with obesity, leading to overall decreased efficacy [71]. Interestingly, one study by Milsap et al. pointed out that the relative baseline hypercortisolemia observed in patients with obesity may explain these observations of diminished drug response to exogenous steroids in obesity [70]. This hypothesis is also supported by evidence of attenuated cortisol responsiveness for the inhaled steroid budesonide in adults with obesity [72].
We did identify a single study on the pharmacokinetics of IV azithromycin in children with community-acquired pneumonia [19]. Although the study suggests that increased weight is associated with decreased clearance, it does not specifically comment on the obesity status of subjects in the patient demographics. We also identified a single study of montelukast pharmacodynamics for children with asthma, comparing patient outcomes using the Asthma Control Test for children with and without comorbid obesity [73]. Interestingly, patients with obesity had a significantly better response to a 24-week treatment course of montelukast than peers without obesity [73]. The authors propose that the leptin-induced leukotriene activation associated with obesity provides more asthma drug targets for montelukast, a leukotriene inhibitor. The results of a large pediatric clinical trial (>2500 children) published by McGarry et al. also provide supporting evidence that children with obesity benefit from montelukast for asthma control, suggesting that perhaps asthma phenotypes differ between children with versus those without obesity [74].
5.1. Asthma Inhalants
We identified compelling evidence that standard doses of inhaled asthma control medications are insufficient for children with obesity (e.g., poorer symptom control and pulmonary function testing scores) [74–76]. However, we could not find any published dosing studies of asthma inhalants used in children with obesity. In one of the largest pediatric clinical trials, involving over 1000 children with obesity and nearly 2000 children without obesity, asthma responsiveness to standard single-dose inhaled albuterol was inferior for children with versus those without obesity, as assessed by both objective (i.e., spirometry) and subjective (i.e., symptom relief) asthma metrics [74]. In this study, children with obesity were also more likely to need inhaled corticosteroids with long-acting β2 agonists (e.g., fluticasone/salmeterol) for asthma control [74]. However, a large retrospective analysis of four longitudinal adult trials concluded that individuals with overweight/obesity required longer to achieve peak spirometry on fluticasone/salmeterol treatment [75]. In another retrospective longitudinal study analysis of inhaled fluticasone alone or in combination with a steroid, adults with morbid obesity were at twofold greater risk for asthma exacerbation during treatment (39%) than all other BMI groups (n = 682) [76]. One commonly proposed mechanism for this observed therapeutic failure from standard inhaled drug doses in obesity is the increased inflammatory burden secondary to obesity, compounded by the already existing asthma-associated inflammation [74, 77]. We could not find pharmacokinetic or pediatric data to support these observations of altered pharmacodynamics in individuals with obesity and asthma.
6. Other Drugs Commonly Prescribed for Obesity Co-Morbid Conditions
Asthma is not the only chronic medical condition that disproportionately affects patients with obesity [4]. Other common obesity-comorbid conditions requiring prescription drug therapy include hypercholesterolemia, hypertension, insulin resistance, and gastroesophageal reflux disease [78]. In this section, we review the pharmacokinetics and dosing information available for the pharmacologic agents frequently used to treat these pediatric comorbid conditions (Table 3).
6.1. Statins
Similar to adults, childhood obesity is accompanied by hypercholesterolemia, a risk factor for developing cardiovascular disease later in life [79]. Statins, substrates for hepatic transporter SLCO1B1, are considered first-line pharmacotherapy for this condition in children as young as 8 years [80]. Pediatric [81, 82] and adult [83, 84] studies confirm that, within the statins drug class, the degree of drug lipophilicity alone fails to predict systemic drug exposure with respect to patient BMI. Recently, a SLCO1B1 genotype-stratified pharmacokinetic study of fixed-dose pravastatin demonstrated a weak positive correlation between systemic drug exposure and BMI Z-score in children [81]. However, in a subset of children with the wild-type SLCO1B1 genotype (c.521TT) and BMI Z-score >2.5 (n = 4), systemic pravastatin exposures were two- to fivefold in excess of all other children with the same genotype, suggesting that there may be differences in hepatic uptake of pravastatin in children with versus those without obesity [81, 82].
6.2. Anti-Hypertensives
Despite a known predominance of hypertension in patients with obesity, knowledge gaps remain with regard to appropriate drug dose selection of anti-hypertensives for patients with obesity. In a study of calcium channel blockers, where children with (n = 16) and without obesity (n = 17) were given identical mg/m2 drug doses, children with obesity demonstrated attenuated blood pressure response [85]. This observation is echoed in adult studies where findings of blunted blood pressure response to calcium channel blockers are thought to be secondary to higher drug Vd in the peripheral compartment in patients with obesity [86].
A trend towards higher Vd in patients with obesity was also observed for β-blockers, especially those with higher lipophilicity (e.g., propranolol, metoprolol) [21]. However, when Vd was corrected for TBW, the difference in Vd between obese and non-obese individuals diminished by approximately 15–35% [21]. Collectively, these observations highlight that dosing based solely on drug lipophilicity is inappropriate for β-blockers and suggest that these drugs preferentially bind to lean tissue. To our knowledge, studies of dosing based on LBW or IBW are lacking for β-blockers.
We were unable to identify any pharmacokinetic studies for angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) in obesity. A small pharmacodynamic study by Hanafy et al. observed no differences in therapeutic response to these agents in children with (n = 3) versus without obesity (n = 3) [85]. Overall, the lack of robust data, beyond a handful of small pharmacokinetic studies of anti-hypertensives in children with obesity, preclude firm dosing recommendations in this emerging patient population.
6.3. Proton Pump Inhibitors
Children with obesity are six times more likely than peers without obesity to have gastroesophageal reflux disease (GERD) [87], a chronic condition for which proton pump inhibitors (PPIs), potent acid suppressive medications, have become the mainstay of therapy [88]. Availability of pharmacokinetic PPI data in obesity is very limited, with published pediatric data focused on the PPI pantoprazole. In two independent prospective investigations, Shakhnovich et al. have demonstrated decreased apparent drug clearance for pantoprazole in obesity [89–91], advocating for LBW-based dosing [89, 90] and avoidance of empiric dose escalation [91] previously proposed in an adult study [92]. Given the growing concerns regarding association of PPI exposure with adverse events in children (e.g., infections, osteopenia) [93], to avoid unnecessary systemic PPI overexposure, studies are needed to investigate whether the decrease in CYP2C19-mediated apparent clearance for pantoprazole is a class effect for all PPIs in obesity.
6.4. Metformin
Metformin is approved for treating children with type 2 diabetes mellitus. It is also prescribed off-label for obesity and/or insulin resistance, as well as comorbidities such as NAFLD, polycystic ovary syndrome, and premature pubarche. Despite its wide use, pharmacokinetic studies of metformin for children with obesity are limited to one published abstract in children with type 2 diabetes mellitus [94] and two studies in children with obesity and insulin resistance [95, 96].
In pediatrics, metformin is typically prescribed as a fixed total daily dose of 2000 mg, regardless of patient weight; however, evidence is emerging that children with obesity may require higher drug doses to achieve systemic exposures comparable to non-obese peers. In a pharmacokinetic study of metformin concentrations over time for children with insulin resistance, drug clearance increased with TBW and LBW, both of which are higher in children with versus without obesity [96]. As a consequence of this increased clearance, lower systemic exposure to metformin was observed in children with obesity, risking therapeutic failure in this patient population unless drug doses are adjusted [96]. The authors hypothesized that the observed alterations in drug clearance may be secondary to obesity-related changes in kidney function, although differences in drug absorption and/or bioavailability in obesity offer an alternative explanation. A study of metformin in adult patients with type 2 diabetes mellitus supports the renal hypothesis [97]. In this study by Bardin et al., obesity and TBW did not affect metformin absorption rate, while Vd and clearance correlated positively with LBW, which is thought to be related to glomerular filtration rate and kidney function [97].
Metformin is primarily cleared by the kidneys, where it is actively secreted by renal transporters OCT2, and MATE-1/MATE-2K [98]. While metformin is not actively cleared in the liver, it serves as a substrate for the hepatic uptake transporter OCT1, the expression of which significantly correlates with BMI and percent fat mass [95]. Therefore, co-administration of metformin with other drug substrates for hepatic OCT1 (e.g., cimetidine, tramadol) to children with obesity can lead to unforeseen alterations in the pharmacokinetics and pharmacodynamics of all the pharmacologic agents administered [95].
7. Discussion
The amount of literature available regarding the pharmacokinetics and pharmacodynamics of drugs prescribed to children with obesity is growing; however, it focuses primarily on drugs with a narrow therapeutic index and a high risk of toxicity (e.g., chemotherapeutics, analgesics, and sedatives used intra-operatively, or in emergency/intensive care settings) [6, 15, 37]. While prioritization of such agents is unequivocally important, it leaves a critical information gap for the drugs most commonly prescribed to children in the inpatient and outpatient settings, including drugs specifically prescribed to treat obesity and its many comorbidities (e.g., hypercholesterolemia, hypertension, etc.). This lack of data currently impacts 124 million children with obesity worldwide [1].
More pharmacologic information is available for drugs used in the inpatient, compared with the outpatient, clinical setting. The most abundant pediatric data were identified for antibiotics; however, no information was available for antibiotics most commonly prescribed in the outpatient setting (e.g., amoxicillin, cefdinir, trimethoprim/sulfamethoxazole, etc.). Although some data were available for approximately 50% of the anesthetics/analgesics/sedatives used in the inpatient setting, no pediatric data were available for the commonly utilized steroids (dexamethasone and methylprednisolone; Table 1). No pediatric data were available for any of the analgesics or steroids prescribed in the outpatient setting, nor was any information available for drugs commonly prescribed to children for attention deficit hyperactivity disorder (Table 2).
The available information for oral and inhaled pharmacologic agents used in both the inpatient and outpatient setting to treat asthma, a common pediatric condition, suggests that montelukast is a beneficial primary and/or adjunct treatment option in obesity and that standard doses of all other agents appear inadequate for patients with obesity [73]. Surprisingly, no follow-up dose escalation studies were identified, despite available studies indicating the need for dose escalation in obesity [74]. Limited information was available for other comorbidities that disproportionately affect children with obesity (e.g., hypercholesterolemia, hypertension, insulin resistance, type 2 diabetes mellitus, GERD), but a common theme that adjustments to standard dosing are warranted for some drugs (e.g., metformin) but not others (e.g., pantoprazole) emerged (Table 3). Furthermore, when dose adjustments are warranted, dose escalation is not always the answer. For example, due to the decreased apparent clearance (L/h/kg TBW) of pantoprazole in obesity, children with obesity achieved higher systemic exposures for every mg/kg TBW drug received, placing them at risk for PPI-associated toxicities (e.g., infection, osteopenia) [82, 89–91].
Similarly, TBW-based dosing is not always the appropriate answer. Although at first glance, Table 1 may suggest that TBW is appropriate for dosing antibiotics for children with obesity, this observation cannot be extrapolated to all antibiotics. While the few studies we identified for these commonly prescribed antibiotics suggested TBW-based dosing may be appropriate, several of them lack robust pharmacokinetic evidence for children with obesity. Additionally, we found some evidence that TBW-based dosing is inappropriate for children with obesity receiving other antibiotics, like gentamicin [7] or azithromycin [19].
8. Conclusion
Overall, this review of the literature highlights that there is no safe or proven ‘rule of thumb,’ for dosing medications for children with obesity. Drug pharmacokinetics, pharmacodynamics, and dosing recommendations should be investigated in this patient population and will likely reflect an interplay between drug physiochemical properties, body size, and the impact of obesity on human physiology, rather than any one of these determinants alone. Currently, it is important that those prescribing for children with obesity are aware of these gaps in knowledge and are cognizant of potential drug treatment failure as a result of inadequate target organ/tissue exposure, or adverse events related to drug toxicity and systemic overexposure. Until more data are available, we recommend close monitoring of drug response and adverse events in children with obesity, and consideration of dose escalation for inhaled asthma agents.
Key Points.
This review highlights that there is no safe or proven ‘rule of thumb’ for dosing drugs for children with obesity.
It is important that those prescribing for children with obesity are aware of these gaps in knowledge and of potential drug treatment failure or adverse events related to drug toxicity as a result of these knowledge gaps.
Until more data are available, we recommend close monitoring of drug response and adverse events in children with obesity receiving commonly prescribed drugs; empiric dose escalation for asthma inhalants may be warranted.
Funding
VS receives funding support from the NASPGHAN Foundation and NCATS L40 TR000598.
Footnotes
Conflict of interest KEK, JW, CH-C, KW and VS have no conflicts of interest to disclose.
References
- 1.WHO | Report of the Commission on Ending Childhood Obesity. WHO; http://www.who.int/end-childhood-obesity/publications/echo-report/en/. Accessed April 26, 2019. [Google Scholar]
- 2.Defining Childhood Obesity | Overweight & Obesity | CDC. 2019. https://www.cdc.gov/obesity/childhood/defining.html. Accessed June 25, 2019.
- 3.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US Children, 1999–2016. Pediatrics. 2018. 10.1542/peds.2017-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collaborators TG 2015 O. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. 10.1056/nejmoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harskamp-van Ginkel MW, Hill KD, Becker KC, et al. Drug dosing and pharmacokinetics in children with obesity: a systematic review. JAMA Pediatr. 2015;169(7):678–85. 10.1001/jamapediatrics.2015.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowe S, Siegel D, Benjamin DK. Gaps in drug dosing for obese children: a systematic review of commonly prescribed emergency care medications. Clin Ther. 2015;37(9):1924–32. 10.1016/j.clinthera.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callaghan LC. Prescribing in paediatric obesity: methods to improve dosing safety in weight-based dose calculations. Arch Dis Child Educ Pract. 2018. 10.1136/archdischild-2016-311491. [DOI] [PubMed] [Google Scholar]
- 8.Anderson BJ, Holford NH. What is the best size predictor for dose in the obese child? Paediatr Anaesth. 2017;27(12):1176–84. 10.1111/pan.13272. [DOI] [PubMed] [Google Scholar]
- 9.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–67. 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 10.Kendrick JG, Carr RR, Ensom MHH. Pediatric obesity: pharmacokinetics and implications for drug dosing. Clin Ther. 2015;37(9):1897–923. 10.1016/j.clinthera.2015.05.495. [DOI] [PubMed] [Google Scholar]
- 11.Sandritter TL, McLaughlin M, Artman M, Lowry J. The interplay between pharmacokinetics and pharmacodynamics. Pediatr Rev. 2017;38(5):195–206. 10.1542/pir.2016-0101. [DOI] [PubMed] [Google Scholar]
- 12.Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49(2):71–87. 10.2165/11318100-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 13.Matson KL, Horton ER, Capino AC, Advocacy Committee for the Pediatric Pharmacy Advocacy Group. Medication dosage in overweight and obese children. J Pediatr Pharmacol Ther. 2017;22(1):81–3. 10.5863/1551-6776-22.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Natale S, Bradley J, Nguyen WH, et al. Pediatric obesity: pharmacokinetic alterations and effects on antimicrobial dosing. Pharmacotherapy. 2017;37(3):361–78. 10.1002/phar.1899. [DOI] [PubMed] [Google Scholar]
- 15.Ross EL, Jorgensen J, DeWitt PE, et al. Comparison of 3 body size descriptors in critically Ill obese children and adolescents: implications for medication dosing. J Pediatr Pharmacol Ther. 2014;19(2):103–10. 10.5863/1551-6776-19.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chai G, Governale L, McMahon AW, Trinidad JP, Staffa J, Murphy D. Trends of outpatient prescription drug utilization in US children, 2002–2010. Pediatrics. 2012;130(1):23–31. 10.1542/peds.2011-2879. [DOI] [PubMed] [Google Scholar]
- 17.Savage VM, Deeds EJ, Fontana W. Sizing up allometric scaling theory. PLoS Comput Biol. 2008;4(9):e1000171 10.1371/journal.pcbi.1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross EL, Heizer J, Mixon MA, et al. Development of recommendations for dosing of commonly prescribed medications in critically ill obese children. Am J Health Syst Pharm. 2015;72(7):542–56. 10.2146/ajhp140280. [DOI] [PubMed] [Google Scholar]
- 19.Zheng Y, Liu S-P, Xu B-P, et al. Population pharmacokinetics and dosing optimization of azithromycin in children with community-acquired pneumonia. Antimicrob Agents Chemother. 2018. 10.1128/aac.00686-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moriyama B, Jarosinski PF, Figg WD, et al. Pharmacokinetics of intravenous voriconazole in obese patients: implications of CYP2C19 homozygous poor metabolizer genotype. Pharmacotherapy. 2013;33(3):e19–22. 10.1002/phar.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheymol G Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet. 2000;39(3):215–31. 10.2165/00003088-200039030-00004. [DOI] [PubMed] [Google Scholar]
- 22.Forbes GB. Nutrition and growth. J Pediatr. 1977;91(1):40–2. [DOI] [PubMed] [Google Scholar]
- 23.He Q, Karlberg J. Bmi in childhood and its association with height gain, timing of puberty, and final height. Pediatr Res. 2001;49(2):244–51. 10.1203/00006450-200102000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Johnson W, Stovitz SD, Choh AC, Czerwinski SA, Towne B, Demerath EW. Patterns of linear growth and skeletal maturation from birth to 18 years of age in overweight young adults. Int J Obes (Lond). 2012;36(4):535–41. 10.1038/ijo.2011.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Simone M, Farello G, Palumbo M, et al. Growth charts, growth velocity and bone development in childhood obesity. Int J Obes Relat Metab Disord. 1995;19(12):851–7. [PubMed] [Google Scholar]
- 26.Vignolo M, Naselli A, Di Battista E, Mostert M, Aicardi G. Growth and development in simple obesity. Eur J Pediatr. 1988;147(3):242–4. [DOI] [PubMed] [Google Scholar]
- 27.Small BG, Wendt B, Jamei M, Johnson TN. Prediction of liver volume—a population-based approach to meta-analysis of paediatric, adult and geriatric populations—an update. Biopharm Drug Dispos. 2017;38(4):290–300. 10.1002/bdd.2063. [DOI] [PubMed] [Google Scholar]
- 28.Knibbe CAJ, Brill MJE, van Rongen A, Diepstraten J, van der Graaf PH, Danhof M. Drug disposition in obesity: toward evidence-based dosing. Annu Rev Pharmacol Toxicol. 2015;55:149–67. 10.1146/annurev-pharmtox-010814-124354. [DOI] [PubMed] [Google Scholar]
- 29.Benedek IH, Blouin RA, McNamara PJ. Serum protein binding and the role of increased alpha 1-acid glycoprotein in moderately obese male subjects. Br J Clin Pharmacol. 1984;18(6):941–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung UJ, Choi M-S. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15(4):6184–223. 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bauer LA, Black DJ, Lill JS. Vancomycin dosing in morbidly obese patients. Eur J Clin Pharmacol. 1998;54(8):621–5. [DOI] [PubMed] [Google Scholar]
- 32.Caraco Y, Zylber-Katz E, Berry EM, Levy M. Significant weight reduction in obese subjects enhances carbamazepine elimination. Clin Pharmacol Ther. 1992;51(5):501–6. [DOI] [PubMed] [Google Scholar]
- 33.Caraco Y, Zylber-Katz E, Berry EM, Levy M. Carbamazepine pharmacokinetics in obese and lean subjects. Ann Pharmacother. 1995;29(9):843–7. 10.1177/106002809502900902. [DOI] [PubMed] [Google Scholar]
- 34.Angulo P Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16):1221–31. 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 35.Brill MJE, Diepstraten J, van Rongen A, van Kralingen S, van den Anker JN, Knibbe CAJ. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51(5):277–304. 10.2165/11599410-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 36.Ghobadi C, Johnson TN, Aarabi M, et al. Application of a systems approach to the bottom-up assessment of pharmacokinetics in obese patients: expected variations in clearance. Clin Pharmacokinet. 2011;50(12):809–22. 10.2165/11594420-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Pediatric Trials Network | PTN. https://pediatrictrials.org/. Accessed 22 Mar 2019.
- 38.Diepstraten J, Chidambaran V, Sadhasivam S, et al. Propofol clearance in morbidly obese children and adolescents: influence of age and body size. Clin Pharmacokinet. 2012;51(8):543–51. 10.2165/11632940-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 39.Diepstraten J, Chidambaran V, Sadhasivam S, et al. An integrated population pharmacokinetic meta-analysis of propofol in morbidly obese and nonobese adults, adolescents, and children. CPT Pharmacometrics Syst Pharmacol. 2013;2:e73 10.1038/psp.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olutoye OA, Yu X, Govindan K, et al. The effect of obesity on the ED(95) of propofol for loss of consciousness in children and adolescents. Anesth Analg. 2012;115(1):147–53. 10.1213/ANE.0b013e318256858f. [DOI] [PubMed] [Google Scholar]
- 41.Chidambaran V, Sadhasivam S, Diepstraten J, et al. Evaluation of propofol anesthesia in morbidly obese children and adolescents. BMC Anesthesiol. 2013;13:8 10.1186/1471-2253-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaughns JD, Ziesenitz VC, van den Anker JN. Clinical pharmacology of frequently used intravenous drugs during bariatric surgery in adolescents. Curr Pharm Des. 2015;21(39):5650–9. [DOI] [PubMed] [Google Scholar]
- 43.Vaughns JD, Ziesenitz VC, Williams EF, et al. Use of fentanyl in adolescents with clinically severe obesity undergoing bariatric surgery: a pilot study. Paediatr Drugs. 2017;19(3):251–7. 10.1007/s40272-017-0216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gish EC, Harrison D, Gormley AK, Johnson PN. Dosing evaluation of continuous intravenous fentanyl infusions in overweight children: a pilot study. J Pediatr Pharmacol Ther. 2011;16(1):39–46. 10.5863/1551-6776-16.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Rongen A, Vaughns JD, Moorthy GS, Barrett JS, Knibbe CAJ, van den Anker JN. Population pharmacokinetics of midazolam and its metabolites in overweight and obese adolescents. Br J Clin Pharmacol. 2015;80(5):1185–96. 10.1111/bcp.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Rongen A, Brill MJE, Vaughns JD, et al. Higher midazolam clearance in obese adolescents compared with morbidly obese adults. Clin Pharmacokinet. 2018;57(5):601–11. 10.1007/s40262-017-0579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Kralingen S, Diepstraten J, Peeters MYM, et al. Population pharmacokinetics and pharmacodynamics of propofol in morbidly obese patients. Clin Pharmacokinet. 2011;50(11):739–50. 10.2165/11592890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 48.Dong D, Peng X, Liu J, Qian H, Li J, Wu B. Morbid obesity alters both pharmacokinetics and pharmacodynamics of propofol: dosing recommendation for anesthesia induction. Drug Metab Dispos. 2016;44(10):1579–83. 10.1124/dmd.116.071605. [DOI] [PubMed] [Google Scholar]
- 49.Samuels PJ, Sjoblom MD. Anesthetic considerations for pediatric obesity and adolescent bariatric surgery. Curr Opin Anaesthesiol. 2016;29(3):327–36. 10.1097/ACO.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 50.Shibutani K, Inchiosa MA, Sawada K, Bairamian M. Pharmacokinetic mass of fentanyl for postoperative analgesia in lean and obese patients. Br J Anaesth. 2005;95(3):377–83. 10.1093/bja/aei195. [DOI] [PubMed] [Google Scholar]
- 51.Leykin Y, Pellis T, Lucca M, Lomangino G, Marzano B, Gullo A. The pharmacodynamic effects of rocuronium when dosed according to real body weight or ideal body weight in morbidly obese patients. Anesth Analg. 2004;99(4):1086–9. 10.1213/01.ane.0000120081.99080.c2. [DOI] [PubMed] [Google Scholar]
- 52.Meyhoff CS, Lund J, Jenstrup MT, et al. Should dosing of rocuronium in obese patients be based on ideal or corrected body weight? Anesth Analg. 2009;109(3):787–92. 10.1213/ane.0b013e3181b0826a. [DOI] [PubMed] [Google Scholar]
- 53.Longo C, Bartlett G, Macgibbon B, et al. The effect of obesity on antibiotic treatment failure: a historical cohort study. Pharmacoepidemiol Drug Saf. 2013;22(9):970–6. 10.1002/pds.3461. [DOI] [PubMed] [Google Scholar]
- 54.Heble DE, McPherson C, Nelson MP, Hunstad DA. Vancomycin trough concentrations in overweight or obese pediatric patients. Pharmacotherapy. 2013;33(12):1273–7. 10.1002/phar.1321. [DOI] [PubMed] [Google Scholar]
- 55.Moffett BS, Kim S, Edwards MS. Vancomycin dosing in obese pediatric patients. Clin Pediatr (Phila). 2011;50(5):442–6. 10.1177/0009922810393500. [DOI] [PubMed] [Google Scholar]
- 56.Eiland LS, Sonawane KB. Vancomycin dosing in healthy-weight, overweight, and obese pediatric patients. J Pediatr Pharmacol Ther. 2014;19(3):182–8. 10.5863/1551-6776-19.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Le J, Capparelli EV, Wahid U, et al. Bayesian estimation of vancomycin pharmacokinetics in obese children: matched case–control study. Clin Ther. 2015;37(6):1340–51. 10.1016/j.clinthera.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nassar L, Hadad S, Gefen A, et al. Prospective evaluation of the dosing regimen of vancomycin in children of different weight categories. Curr Drug Saf. 2012;7(5):375–81. [DOI] [PubMed] [Google Scholar]
- 59.Madigan T, Sieve RM, Graner KK, Banerjee R. The effect of age and weight on vancomycin serum trough concentrations in pediatric patients. Pharmacotherapy. 2013;33(12):1264–72. 10.1002/phar.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Camaione L, Elliott K, Mitchell-Van Steele A, Lomaestro B, Pai MP. Vancomycin dosing in children and young adults: back to the drawing board. Pharmacotherapy. 2013;33(12):1278–87. 10.1002/phar.1345. [DOI] [PubMed] [Google Scholar]
- 61.Koshida R, Nakashima E, Taniguchi N, Tsuji A, Benet LZ, Ichimura F. Prediction of the distribution volumes of cefazolin and tobramycin in obese children based on physiological pharmacokinetic concepts. Pharm Res. 1989;6(6):486–91. [DOI] [PubMed] [Google Scholar]
- 62.Schmitz ML, Blumer JL, Cetnarowski W, Rubino CM. Determination of appropriate weight-based cutoffs for empiric cefazolin dosing using data from a phase 1 pharmacokinetics and safety study of cefazolin administered for surgical prophylaxis in pediatric patients aged 10 to 12 years. Antimicrob Agents Chemother. 2015;59(7):4173–80. 10.1128/AAC.00082-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith MJ, Gonzalez D, Goldman JL, et al. Pharmacokinetics of clindamycin in obese and nonobese children. Antimicrob Agents Chemother. 2017. 10.1128/aac.02014-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70(3):195–283. 10.2146/ajhp120568. [DOI] [PubMed] [Google Scholar]
- 65.Barshop NJ, Capparelli EV, Sirlin CB, Schwimmer JB, Lavine JE. Acetaminophen pharmacokinetics in children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2011;52(2):198–202. 10.1097/MPG.0b013e3181f9b3a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abernethy DR, Divoll M, Greenblatt DJ, Ameer B. Obesity, sex, and acetaminophen disposition. Clin Pharmacol Ther. 1982;31(6):783–90. [DOI] [PubMed] [Google Scholar]
- 67.van Rongen A, Välitalo PAJ, Peeters MYM, et al. Morbidly obese patients exhibit increased CYP2E1-mediated oxidation of acetaminophen. Clin Pharmacokinet. 2016;55(7):833–47. 10.1007/s40262-015-0357-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goday Arno A, Farré M, Rodríguez-Morató J, et al. Pharmacokinetics in morbid obesity: influence of two bariatric surgery techniques on paracetamol and caffeine metabolism. Obes Surg. 2017;27(12):3194–201. 10.1007/s11695-017-2745-z. [DOI] [PubMed] [Google Scholar]
- 69.Abernethy DR, Greenblatt DJ. Drug disposition in obese humans. An update. Clin Pharmacokinet. 1986;11(3):199–213. 10.2165/00003088-198611030-00002. [DOI] [PubMed] [Google Scholar]
- 70.Milsap RL, Plaisance KI, Jusko WJ. Prednisolone disposition in obese men. Clin Pharmacol Ther. 1984;36(6):824–31. [DOI] [PubMed] [Google Scholar]
- 71.Goleva E, Covar R, Martin RJ, Leung DYM. Corticosteroid pharmacokinetic abnormalities in overweight and obese corticosteroid resistant asthmatics. J Allergy Clin Immunol Pract. 2016;4(2):357.e2–360.e2. 10.1016/j.jaip.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anderson WJ, Lipworth BJ. Does body mass index influence responsiveness to inhaled corticosteroids in persistent asthma? Ann Allergy Asthma Immunol. 2012;108(4):237–42. 10.1016/j.anai.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 73.Farzan S, Khan S, Elera C, Tsang J, Akerman M, DeVoti J. Effectiveness of montelukast in overweight and obese atopic asthmatics. Ann Allergy Asthma Immunol. 2017;119(2):189–90. 10.1016/j.anai.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 74.McGarry ME, Castellanos E, Thakur N, et al. Obesity and bronchodilator response in black and Hispanic children and adolescents with asthma. Chest. 2015;147(6):1591–8. 10.1378/chest.14-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Camargo CA, Boulet L-P, Sutherland ER, et al. Body mass index and response to asthma therapy: fluticasone propionate/salmeterol versus montelukast. J Asthma. 2010;47(1):76–82. 10.3109/02770900903338494. [DOI] [PubMed] [Google Scholar]
- 76.Camargo CA, Sutherland ER, Bailey W, et al. Effect of increased body mass index on asthma risk, impairment and response to asthma controller therapy in African Americans. Curr Med Res Opin. 2010;26(7):1629–35. 10.1185/03007995.2010.483113. [DOI] [PubMed] [Google Scholar]
- 77.Pelaia G, Vatrella A, Busceti MT, et al. Cellular mechanisms underlying eosinophilic and neutrophilic airway inflammation in asthma. Mediators Inflamm. 2015;2015:879783 10.1155/2015/879783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet. 2002;360(9331):473–82. 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]
- 79.Chang C-J, Jian D-Y, Lin M-W, Zhao J-Z, Ho L-T, Juan C-C. Evidence in obese children: contribution of hyperlipidemia, obesity-inflammation, and insulin sensitivity. PLoS One. 2015;10(5):e0125935 10.1371/journal.pone.0125935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lamaida N, Capuano E, Pinto L, Capuano E, Capuano R, Capuano V. The safety of statins in children. Acta Paediatr. 2013;102(9):857–62. 10.1111/apa.12280. [DOI] [PubMed] [Google Scholar]
- 81.Wagner JB, Abdel-Rahman S, Van Haandel L, et al. Impact of SLCO1B1 genotype on pediatric simvastatin acid pharmacokinetics. J Clin Pharmacol. 2018;58(6):823–33. 10.1002/jcph.1080. [DOI] [PubMed] [Google Scholar]
- 82.Wagner JB, Abdel-Rahman S, Gaedigk R, et al. Impact of genetic variation on pravastatin systemic exposure in pediatric hypercholesterolemia. Clin Pharmacol Ther. 2018. 10.1002/cpt.1330. [DOI] [PubMed] [Google Scholar]
- 83.DeGorter MK, Tirona RG, Schwarz UI, et al. Clinical and pharmacogenetic predictors of circulating atorvastatin and rosuvastatin concentrations in routine clinical care. Circ Cardiovasc Genet. 2013;6(4):400–8. 10.1161/CIRCGENETICS.113.000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shitara Y, Sugiyama Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMGCoA) reductase inhibitors: drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol Ther. 2006;112(1):71–105. 10.1016/j.pharmthera.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 85.Hanafy S, Pinsk M, Jamali F. Effect of obesity on response to cardiovascular drugs in pediatric patients with renal disease. Pediatr Nephrol. 2009;24(4):815–21. 10.1007/s00467-008-1064-y. [DOI] [PubMed] [Google Scholar]
- 86.Sankaralingam S, Kim RB, Padwal RS. The impact of obesity on the pharmacology of medications used for cardiovascular risk factor control. Can J Cardiol. 2015;31(2):167–76. 10.1016/j.cjca.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 87.Koebnick C, Getahun D, Smith N, Porter AH, Der-Sarkissian JK, Jacobsen SJ. Extreme childhood obesity is associated with increased risk for gastroesophageal reflux disease in a large population-based study. Int J Pediatr Obes. 2011;6(2–2):e257–63. 10.3109/17477166.2010.491118. [DOI] [PubMed] [Google Scholar]
- 88.Gold BD. Gastroesophageal reflux disease: could intervention in childhood reduce the risk of later complications? Am J Med. 2004;117(Suppl 5A):23S–9S. [DOI] [PubMed] [Google Scholar]
- 89.Shakhnovich V, Abdel-Rahman S, Friesen CA, et al. Lean body weight dosing avoids excessive systemic exposure to proton pump inhibitors for children with obesity. Pediatr Obes. 2019. 10.1111/ijpo.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shakhnovich V, Smith PB, Guptill JT, et al. Obese children require lower doses of pantoprazole than nonobese peers to achieve equal systemic drug exposures. J Pediatr. 2018;193(102–108):e1 10.1016/j.jpeds.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shakhnovich V, Brian Smith P, Guptill JT, et al. A population-based pharmacokinetic model approach to pantoprazole dosing for obese children and adolescents. Paediatr Drugs. 2018;20(5):483–95. 10.1007/s40272-018-0305-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen W-Y, Chang W-L, Tsai Y-C, Cheng H-C, Lu C-C, Sheu B-S. Double-dosed pantoprazole accelerates the sustained symptomatic response in overweight and obese patients with reflux esophagitis in Los Angeles grades A and B. Am J Gastroenterol. 2010;105(5):1046–52. 10.1038/ajg.2009.632. [DOI] [PubMed] [Google Scholar]
- 93.Stark CM, Nylund CM. Side effects and complications of proton pump inhibitors: a pediatric perspective. J Pediatr. 2016;168:16–22. 10.1016/j.jpeds.2015.08.064. [DOI] [PubMed] [Google Scholar]
- 94.Atabek ME, Pirgon O. Use of metformin in obese adolescents with hyperinsulinemia: a 6-month, randomized, double-blind, placebo-controlled clinical trial. J Pediatr Endocrinol Metab. 2008;21(4):339–48. [DOI] [PubMed] [Google Scholar]
- 95.Sam WJ, Roza O, Hon YY, et al. Effects of SLC22A1 polymorphisms on metformin-induced reductions in adiposity and metformin pharmacokinetics in obese children with insulin resistance. J Clin Pharmacol. 2017;57(2):219–29. 10.1002/jcph.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van Rongen A, van der Aa MP, Matic M, et al. Increased metformin clearance in overweight and obese adolescents: a pharmacokinetic substudy of a randomized controlled trial. Paediatr Drugs. 2018;20(4):365–74. 10.1007/s40272-018-0293-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bardin C, Nobecourt E, Larger E, Chast F, Treluyer J-M, Urien S. Population pharmacokinetics of metformin in obese and non-obese patients with type 2 diabetes mellitus. Eur J Clin Pharmacol. 2012;68(6):961–8. 10.1007/s00228-011-1207-0. [DOI] [PubMed] [Google Scholar]
- 98.Sánchez-Infantes D, Díaz M, López-Bermejo A, Marcos MV, de Zegher F, Ibáñez L. Pharmacokinetics of metformin in girls aged 9 years. Clin Pharmacokinet. 2011;50(11):735–8. 10.2165/11593970-000000000-00000. [DOI] [PubMed] [Google Scholar]


