Abstract
Clinical and animal studies show maternal alcohol consumption during pregnancy causes in offspring persistent alterations in neuroimmune and neurochemical systems known to increase alcohol drinking and related behaviors. Studies in lateral hypothalamus (LH) demonstrate in adolescent offspring that maternal oral administration of ethanol stimulates the neuropeptide, melanin-concentrating hormone (MCH), together with the inflammatory chemokine C-C motif ligand 2 (CCL2) and its receptor CCR2 which are increased in most MCH neurons. These effects, consistently stronger in females than males, are detected in embryos, not only in LH but hypothalamic neuroepithelium (NEP) along the third ventricle where neurons are born and CCL2 is stimulated within radial glia progenitor cells and their laterally projecting processes that facilitate MCH neuronal migration toward LH. With ethanol’s effects similarly produced by maternal peripheral CCL2 administration and blocked by CCR2 antagonist, we tested here using in utero intracerebroventricular (ICV) injections whether CCL2 acts locally within the embryonic NEP. After ICV injection of CCL2 (0.1 μg/μl) on embryonic day 14 (E14) when neurogenesis peaks, we observed in embryos just before birth (E19) a significant increase in endogenous CCL2 within radial glia cells and their processes in NEP. These auto-regulatory effects, evident only in female embryos, were accompanied by increased density of CCL2 and MCH neurons in LH, more strongly in females than males. These results support involvement of embryonic CCL2/CCR2 neuroimmune system in radial glia progenitor cells in mediating sexually dimorphic effects of maternal challenges such as ethanol on LH MCH neurons that colocalize CCL2 and CCR2.
Keywords: embryo, radial glia, hypothalamus, CCL2, CCR2, melanin-concentrating hormone
INTRODUCTION
Recent studies have demonstrated that the neuroimmune system in the brain, functioning beyond the signaling for immune responses, produces profound changes in the development of neurons that result in long-term behavioral consequences (Cui et al., 2014; Crews et al., 2015; Chang et al., 2015, 2018). The wide-ranging and long-lasting functionalities of neuroimmune signaling are evident in the progression of ethanol’s effects on neuronal development in the embryo and subsequent changes in the offspring’s behavior associated with increased alcohol consumption. While chronic high doses or binge drinking episodes of ethanol are found to affect inflammatory pathways involving such molecules as Toll-like receptors which cause considerable neurodegeneration that exacerbates existing alcohol use disorder (Lippai et al., 2013; Crews and Vetreno, 2014; Flores-Bastias and Karahanian, 2018), there is evidence that lower levels and shorter periods of ethanol exposure can have very different effects. Along with elevated neuroimmune activity, these include an increase in cell proliferation, neurogenesis and migration (Camarillo and Miranda, 2008; Mooney and Miller, 2010; Skorput and Yeh, 2015) and in the expression and density of neurons in the lateral hypothalamus (LH) that express melanin-concentrating hormone (MCH) (Chang et al., 2015, 2018), a neuropeptide that promotes alcohol drinking and other behaviors associated with alcohol use disorder (Duncan et al., 2005; Morganstern et al., 2010; Karlsson et al., 2016). These studies of MCH in the LH demonstrate in the rat that maternal intraoral administration of ethanol, at low-to-moderate doses from embryonic day 10 (E10) to E15 during the period of peak hypothalamic neurogenesis (Ifft, 1972), increases in adolescent offspring the density of neurons that express the inflammatory chemokine C-C motif ligand 2 (CCL2) and its main receptor CCR2, a neuroimmune system also positively linked to alcohol intake (Blednov et al., 2005; June et al., 2015; Valenta and Gonzales, 2016). It also stimulates the co-expression of CCL2 and CCR2 in up to 90% of the MCH neurons (Chang et al., 2015, 2018)
The possibility that the CCL2/CCR2 system actually mediates these stimulatory effects of ethanol on MCH neurons is supported by the additional findings that they are similarly produced by maternal peripheral administration of CCL2 and are blocked by maternal administration of the CCR2 antagonist INCB3344 during the period of ethanol exposure (Chang et al., 2018, 2019a). A recent study of the developmental origins of this CCL2/CCR2-mediated stimulatory effect on neuronal development has shown that ethanol’s effects on MCH neurons that colocalize CCL2 and CCR2 in LH are evident in the embryo and neonatal offspring and are reversed by maternal administration of a CCL2 antibody that neutralizes endogenous CCL2 and a CCR2 antagonist that blocks CCL2’s main receptor (Chang et al., 2019a). Also in the embryo, maternal ethanol administration stimulates CCL2 cells dense in the hypothalamic neuroepithelium (NEP), a primary source of neurons along the third ventricle (Bedont et al., 2015), and it also increases the colocalization of CCL2 within radial glia progenitor cells and their laterally projecting processes, effects that are mimicked by maternal CCL2 administration and accompanied by an increased number of MCH neurons close to the radial glia cells and positioned along their processes (Chang et al., 2019b). Further tests demonstrate that these effects of maternal ethanol and CCL2 administration on the development of CCL2 and MCH neurons are consistently stronger in females than males, as shown in the embryo as well as adolescent offspring (Chang et al., 2018; 2019a, 2019b) Thus, in response to inflammatory challenges such as ethanol, these studies suggest the involvement of endogenous CCL2-rich radial glia progenitor cells in embryos in promoting, in a sexually dimorphic manner, the genesis and migration of MCH neurons toward their final destination in the LH.
While bringing attention to the CCL2/CCR2 system and radial glia in the NEP as a possible mechanism underlying these strong stimulatory effects on neuronal development, these studies with maternal peripheral manipulations of ethanol or CCL2 in pregnant rats leave open the question as to whether these alterations in this neuroimmune mechanism result from direct effects on the embryonic brain or whether they are indirect consequences of effects in the mother produced by peripheral manipulations. To investigate this question, we employed here in utero intracerebroventricular (ICV) injections to directly manipulate the embryonic brain during the period of peak hypothalamic neurogenesis, while having minimal impact on maternal neuroimmune factors. This ICV technique, allowing delivery of substances directly into the microenvironment of developing neural systems, not only avoids the confounding variables of maternal peripheral injections but also reduces some uncertainty regarding the molecular dynamics of neuroimmune compounds crossing the fetal blood brain barrier. While studies over the years have employed ICV injections to alter brain microenvironments in adult, adolescent, and postnatal rodents (Lewinski et al., 1984; Jung et al., 1994; Fang et al., 2013; Li et al., 2016), this technique has more recently been used in the embryo to administer genetic material with electroporation to induce localized changes in gene expression in both superficial and deeper embryonic brain regions (Walantus et al., 2007; Vomund et al., 2013; Fekete et al., 2017; Rosin and Kurrasch, 2018). These investigations have reported good success in terms of both embryo survival rates and the effectiveness of gene transfer as revealed by measurements after birth and into adolescence. To our knowledge, there are only a few in utero ICV injection studies in rodent models that have administered non-genetic material without electroporation directly into the embryonic brain for purposes of providing pharmacological manipulations. In two such reports, direct injections of neuroimmune signaling molecules, the growth factor TGF-B1 that promotes radial glia differentiation (Stipursky et al., 2014) and maternal autoantibodies related to autism spectrum disorders (Camacho et al., 2014), have been performed, with little complication and significant success in demonstrating subsequent changes in target molecules and target regions.
In this manuscript, we use this technique of in utero ICV injection to accomplish two goals. The first is to determine if this technique is a viable method for investigating and directly manipulating mechanisms in the embryonic brain. The second is to test whether the effects in the offspring produced by maternal manipulations of a neuroimmune agent during pregnancy can be reproduced by delivering this agent into the embryonic brain, indicating that they reflect direct actions on the embryo rather than indirect consequences from changes in the mother. Specifically, using the in utero ICV injection technique, we delivered CCL2, a potent signaling molecule, directly into the third ventricle of the embryo at E14, when hypothalamic neurogenesis peaks (Ifft, 1972), and investigated in the embryo just before birth at E19 its effects on neuroimmune function in both the highly proliferative NEP surrounding the third ventricle and the more distant neuronal systems in the LH. In the NEP, we examined the CCL2-containing radial glia progenitor cells in addition to their processes in the medial hypothalamus (mHYP) that facilitate neuronal migration and then tested the CCL2 and MCH neurons in the LH, the site of their final destination. The results obtained with embryonic ICV CCL2 injection reveal very similar effects on the embryo as those produced by maternal peripheral administration of CCL2. In addition to validating this technique which negates potential confounding effects of maternal manipulations that indirectly alter the embryonic and amniotic environment, these results provide strong evidence for the involvement of a localized CCL2 system in the embryo brain in mediating the effects of maternal manipulations. They specifically demonstrate how CCL2 functions in an autoregulatory and sexually dimorphic manner to stimulate the radial glia progenitor cells and processes in which it colocalizes and the development of CCL2 neurons in the LH along with MCH neurons which themselves grow to colocalize CCL2 as well as CCR2.
EXPERIMENTAL PROCEDURES
All procedures were conducted in a fully accredited AAALAC facility (22°C, 12:12-h light-dark cycle with lights off at 8 am), in accordance with protocols approved by The Rockefeller University Animal Care and Use Committee and consistent with the NIH Guide to the Care and Use of Laboratory Animals.
Animals
Time-pregnant, Sprague-Dawley rats (220–240g) (Charles River Breeding Laboratories, Hartford, CT) arrived at the facility on embryonic day 5 (E5) and were acclimated to laboratory conditions until E14, at which time the experiments began as described in detail below. In all experiments, rodent chow (LabDiet Rodent Chow 5001, St. Louis, MO) and filtered water were available ad libitum. As described in the Experimental Design section below, female and male embryos were sacrificed at E19, the age before birth used in our prior study of the embryo (Chang et al., 2019a) when MCH neurons in the LH first exhibit an adult-like pattern (Brischoux et al., 2001). A total of 36 dams and 194 embryos were used in the experiments described below.
Embryonic third ventricular injection
Embryonic ICV injection was used to administer CCL2 (0.1 μg/μl) or a control solution directly into hypothalamic third ventricle of the embryo at E14, two hours after dark onset. It was performed as described (Haddad-Tovolli et al., 2013; Vomund et al., 2013) with minor modifications: 1) All surgical instruments and materials used in the procedure were autoclaved. 2) Glass micropipettes (1.2 mm in diameter) were prepared from 1.2 mm diameter glass microcapillaries (Harvard Apparatus Limited, GC120TF-10, 1.2 mm O.D. x 0.94 mm I.D.) by being pulled in a conventional Sutter P-80 device with the settings of P=103, Heat=719, and Velocity=0.45. 3) Prior to the injection, the pregnant rat at E14 (or in one experiment at E16) was anesthetized with isoflurane inhalation, with a flow rate of 3% for the duration of the surgery. The dam was then placed in a supine position on a 37° C-warmed heating pad, with a breathing mask connected to the anesthesia device (oxygen setting of 0.5 L/min and isoflurane at 1.5–3%) and her body fixed in place by taping her four legs to the table. The depth of anesthesia was frequently assessed by checking for a loss of response to reflex stimulation by toe or tail pinch with firm pressure. 4) To prepare for the laparotomy, the abdomen was shaved and disinfected with 70% ethanol followed by three times with iodine-iodophorpovidone, and the rat was then covered with sterile cloths, exposing only the shaved operation field. 5) A longitudinal incision (1.5 to 2 cm long) was then made on the abdominal skin, the peritoneum was cut, and a warm cotton gauze (37° C) made wet with sterile normal saline dispensed from a syringe was placed around the incision. 6) The uterus was then made visible, pulled out carefully with blunt forceps, and then placed onto the warm gauze frequently rinsed with normal saline to keep it moist throughout the surgery. Particular care during this entire procedure was taken to avoid pulling tightly on the mesometrium or uterus which can increase the risk for abortion. 7) Observing the embryo’s head from a dorsal view, the gap or fissure between the left and right cortical hemispheres was located visually, with the hemispheres easy to distinguish and the lateral ventricles inside them generally perceived as somewhat darker shapes. Injections were performed only in an embryo whose head was perfectly oriented for the injection, since reorienting the head can injure the embryo. With the head held stationary by one hand and the glass micropipette by the other at a 45° angle relative to the uterine wall, the head was gently punctured by the micropipette at the rostral end of the gap between the cortical hemispheres penetrated for about 2 mm, and a 1 μl injection was slowly administered into the third ventricle. This procedure was repeated in all embryos with good head positions, with the first and last embryos (from left to right) always kept without an injection to minimize chances of miscarriage. 8) All injections including the vehicle (normal saline) and CCL2 contained the dye (0.1% Fast Green) to give visual validation as to the success of the injection. The CCL2 (ProSpec Protein Specialist, Cat. # CHM-315) was diluted in 0.1% Fast Green solution for the final concentration of 0.1 μg/μl CCL2 per injection, a dose chosen based on a study in adult mice (LeThuc et al., 2016) and designed to be less than one-tenth the dose used in our recent study with maternal peripheral administration of CCL2 (Chang et al., 2018). Each litter yielded embryos from the following 3 groups: 2–3 embryos were injected with 1μl of the saline vehicle (“ICV Control”), 3–4 were injected with the 0.1 μg/μl of the CCL2 solution (“ICV CCL2”), and the remaining embryos were handled but received no injection (“No-ICV Control”). After the injection or being handled, each embryo was gently returned to the uterus in its original position before moving onto the next embryo. 9) The peritoneal cavity was moistened with the warmed normal saline solution before it was closed and sutured with surgical catgut (Chromic Gut 4–0, Ethicon), and then the skin was closed using the “interrupted stitching” method with a more resistant suture (Vicryl 3–0, Ethicon). After the incision and abdomen surface were disinfected with iodine-iodophorpovidone, the pregnant rat was removed from the anesthesia machine and placed into a clean recovery cage which was warmed by a heating pad (at middle gauge) until full recovery, which generally took approximately 1 hour. After this, the dam was moved to a new cage with regular diet and water, returned to the rat housing room, and monitored at least twice daily to insure successful recovering from the procedure without any signs of infection or pain. At E19, the embryos with about 85–90% survival rate were harvested, their tails were collected for genotyping to determine sex, and their brains were examined using quantitative real-time PCR and immunofluorescence histochemistry as described below. From each dam, we collected a total of 6 embryos, 1 embryo/sex/group, for a given experiment.
Quantitative real-time PCR
Quantitative real-time PCR (qRT-PCR) was used to measure the gene expression of brain lipid-binding protein (BLBP), a radial glia marker (Anthony et al., 2004), along with CCL2, CCR2 and MCH in the NEP, mHYP and LH of the embryos. They were sacrificed at E19, and their brains were immediately removed and placed on a microscope slide on top of an ice-filled petri dish, with the ventral surface facing up for slicing. With preliminary tests showing the NEP and mHYP areas to yield similar results, we combined these areas for the qRT-PCR analysis, which are referred to in the text as “NEP+mHYP”, and dissected them using gem razor blades (American Safety Razor Co., Verona, VA). Two coronal cuts were made, with the anterior cut 0.5 mm caudal to the posterior edge of the middle optic chiasm (Coronal Plate 10, E20) (Altman and Bayer, 1995) and the posterior cut 1.0 mm caudal to the anterior cut (Coronal Plate 10 to 13, E20). After putting this slice on a microscope glass with its posterior level plain facing up, the NEP+mHYP area and the LH were further microdissected. For the NEP+mHYP dissection, a lateral cut was made 0.6 mm bilateral to hypothalamic third ventricle, a ventral cut was then made at dorsal edge of the medial eminence, and a dorsal cut was made 0.1 mm ventral to the fornix. For the LH, a dissection was made 0.6 to 1.0 mm bilateral to hypothalamic ventricle, with the ventral border at 0.2 mm dorsal to the bottom of the hypothalamus and the dorsal border at the level of the fornix. Total RNA was then extracted from each microdissected sample, cDNA was synthesized, and qRT-PCR was performed, as previously described in our publications (Chang et al., 2012; Barson et al., 2013; Chang et al., 2018) and others (West et al., 2017; Adachi et al., 2018). The primers for BLBP, CCL2 and MCH, designed with ABI Primer Express Version 3.0 software from published sequences, and their sequence and concentrations are presented in Table 1. The mRNA levels of the target gene in each rat were normalized within subject relative to mRNA levels of the internal housekeeping gene, cyclophilin, in the same sample, and this ratio of target gene expression to housekeeping gene expression was calculated in each rat using the standard delta-delta Ct method. An ANOVA was then run on this ratio calculated for each subject, with the effect of ethanol on target gene expression determined by comparing the average ratio in the experimental groups to the control groups, as well as the control groups with each other. The mRNA gene expression data are presented as average ratio scores in the text and as fold changes in the figures.
Table 1:
Primers for measurements of mRNA levels using qRT-PCR
| Gene | GenBank Accession | Forward 5’−3’ | Reverse 5’−3’ | Concentration (nM) |
|---|---|---|---|---|
| Cyclophilin | NM_001004279 | TGTGCTGAATATTGGTGCTTGTAA | TGTGCTGAATATTGGTGCTTGTAA | 200 |
| BLBP | NM_030832 | TGATTCGGTTGGATGGAGACA | CGACATCCCCAAAGGTGAGA | 200 |
| CCL2 | NM_031530 | GTG CTG TCT CAG CCA GAT GCA GTT | AGT TCT CCA GCC GAC TCA TTG GG | 400 |
| CCR2 | NM_021866 | TAC CTG TTC AAC CTG GCC ATC T | AGA CCC ACT CAT TTG CAG CAT | 400 |
| MCH | M_29712 | CAAACAGGATGGCGAAGATGA | AGGCTTTCCCCATCCTGAAT | 50 |
Abbreviations: BLBP, brain lipid-binding protein; CCL2, C-C motif ligand 2; CCR2, receptor for CCL2; MCH, melanin-concentrating hormone.
Single- and double-label immunofluorescence histochemistry
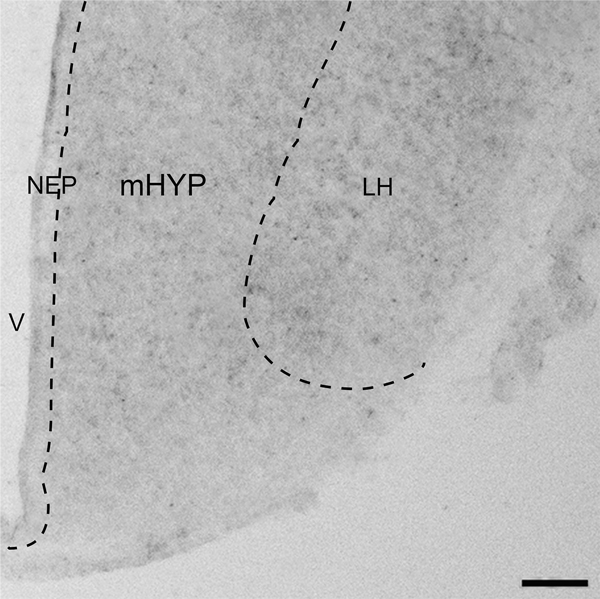
Immunofluorescence histochemistry (IF) was used, as previously described (Chang et al., 2013, 2015, 2018), to characterize the distribution pattern and quantify in the embryo at E19 the following: 1) BLBP-immunoreactive (BLBP+) radial glia progenitor cells and CCL2-immunoreactive (CCL2+) cells in the NEP, with CCR2+ cells not detected here at E19 using available antibodies; 2) BLBP+ and CCL2+ fibers or processes of these NEP cells, which are seen in the mHYP as they project laterally toward the LH and found to be longer and more clearly defined for the BLBP+ than CCL2+ processes; and 3) MCH-immunoreactive (MCH+) neurons, which in the embryo can be detected sparsely in the NEP, become denser in the mHYP, and are most dense in the LH. The precise areas examined, the NEP and mHYP as they relate to the LH, are illustrated in the photomicrographs (2.5x) of embryonic brain sections showing the relatively low levels of CCL2, BLBP and MCH immunoreactivity observed in control embryos at E19 (Fig. 1).
Figure 1:
DIC image (2.5x) illustrates in E19 embryo the three hypothalamic regions investigated in this manuscript. It shows from medial to lateral the hypothalamic neuroepithelium (NEP) along the third ventricle (V), the medial hypothalamus (mHYP) immediately lateral to the NEP, and the lateral hypothalamus (LH). Scale bar, 200 μm.
We first examined using single-labeling IF the BLBP+ and CCL2+ cells in the NEP in addition to their processes in the mHYP and also the CCL2+ and MCH+ cells in the LH, with the embryos killed by perfusing their dams intracardially with 0.9% normal saline followed by 4% paraformaldehyde in phosphate buffer (PB). The tails of the embryos were collected for genotyping to determine the sex, and their brains were removed and post-fixed for 4–6 h in the same fixative at 4° C, cryo-protected in 25% sucrose at 4° C for 72–96 h, and then frozen and stored at −80° C. Brains were cut at 30 μm with a cryostat, and free-floating coronal sections were processed with primary antibodies and their corresponding secondary antibodies listed in Table 2. Sections were viewed, and fluorescence images were captured using a Zeiss LSM 880 confocal microscope with 20x objective. For quantitation, the fluorescence image of the NEP, mHYP and LH as shown in Fig. 1 was outlined and analyzed. For each embryo, 8–10 images were collected at the anterior-posterior level of coronal plates 12 to 13 in E20 brains (Altman and Bayer, 1995). The density of single-label immunofluorescent cells in the NEP and LH was then quantified, using Image-Pro Plus software (Version 4.5; Media Cybernetics) as previously described (Chang et al., 2013, 2015, 2018). Only intact cells with an area of 50–100 μm2 were counted, and the population density of these cells in the NEP and LH is reported as cells/μm2. The measure in the mHYP recorded as objects/μm2 reflects mostly the processes or fibers of the BLBP+ and CCL2+ cells found to be dense in this area where few cells are evident, leading us to refer to these objects as “processes” in the text.
Table 2:
Antibodies used for single-labeling immunofluorescence histochemistry
| Primary antibody | Dilution | Vendor | Secondary antibody | Dilution | Vendor |
|---|---|---|---|---|---|
| Rabbit anti-CCL2 | 1:200 | Biorbyt, CA | Bio-Horse anti-Rabbit IgG + TSA Fluorescin | 1:100 | Vector, CA |
| Rabbit anti-BLBP | 1:200 | Abcam, MA | Cy3-Donkey anti-Rabbit IgG | 1:100 | Jackson ImmunoResearch Laboratories Inc, PA |
| Rabbit anti-MCH | 1:1000 | Phoenix | Cy3-Donkey anti-Rabbit IgG | 1:100 | Jackson ImmunoResearch |
| Pharmaceuticals, CA | Laboratories. Inc, PA | ||||
| Rabbit anti-Iba-1 | 1:1000 | Wako Chemicals USA, VA | Cy3-Donkey anti-Rabbit IgG | 1:100 | Jackson ImmunoResearch |
| Laboratories. Inc, PA | |||||
| Mouse anti-GFAP | 1:50 | EMD Millipore, MA | Cy3-Donkey anti-Mouse IgG | 1:100 | Jackson ImmunoResearch |
| Laboratories. Inc, PA | |||||
Abbreviations: CCL2, C-C motif ligand 2; BLBP, brain lipid-binding protein, marker for radial glia; MCH, melanin-concentrating hormone; Iba-1, marker for microglia; GFAP, marker for astrocytes.
Double-labeling IF was then used to determine whether CCL2 in the NEP and mHYP of the embryo colocalizes with the radial glial marker BLBP in its cells and processes. This double labeling was performed using a combination of primary antibodies and their corresponding secondary antibodies listed in Table 3, based on our procedures described previously (Chang et al., 2013, 2015, 2018). For this analysis of double-labeling, the images were captured by a Zeiss LSM 880 confocal microscope with 20x objective, and the double-labeling was further confirmed by Z-stack sectioning with a 40x oil-immersion lens, with the Z-stacks 30 μm thick and the step size of 0.7–0.8 μm for optimal stack collection and analysis. In all analyses, the cells and processes were counted in each section, and only the cells of a designated size (50–100 μm) were counted. The double-labeled cells counted in 20x images and double-labeled processes counted in 40x images are reported as the percentage of total single-labeled cells or processes, respectively. To confirm that the BLBP and CCL2+ cells in the NEP are epithelial cells, we used DAPI to stain the nucleus. Confocal Z-Stack sectioning (30μm thick and step size of 1.02 μm) of double-staining of DAPI with BLBP or CCL2 in the NEP showed with 40x objective that almost all of the radial glia cells and CCL2+ cells contain a nucleus, confirming that they are epithelial in nature (Chang et al., 2019b).
Table 3:
Antibodies used for double labeling immunofluorescence histochemistry
| Combination | Primary antibody | Dilution | Vendor | Secondary antibody | Dilution | Vendor |
|---|---|---|---|---|---|---|
| CCL2+BLBP | Rabbit anti-CCL2 | 1:200 | Biorbyt, CA | Bio-Horse anti-Rabbit IgG + TSA Fluorescein | 1:100 | Vector, CA |
| Mouse anti-BLBP | 1:200 | Abcam, MA | Cy3-Donkey anti-Mouse IgG | 1:100 | JacksonImmunoResearch Laboratories. Inc, PA | |
TUNEL, Iba-1 and GFAP immunofluorescence histochemistry
Immunofluorescence histochemistry (IF) was used, as previously described (Chang et al., 2012, 2013, 2018), to test in the NEP and LH the effect of the ICV injection procedure itself compared to no injection on cell apoptosis, evaluated by TUNEL using the In Situ Cell Death Detection kit, Fluorescein (Roche, Catalog # 11684795910), and also on the density of microglia and astrocytes, evaluated using antibodies for Iba-1 and GFAP, respectively.
Experimental Design and Statistical Analysis
Visual validation of in utero ICV injection technique:
To determine the success of the ICV injections and the spread of the injected solution, we included Fast Green dye (0.1%) in each injection and performed several control tests. In E16 embryos injected with the Fast Green vehicle solution (1 μl), brain sections were cut 2 hours after injection to examine the nature of the spread through the NEP and hypothalamus. Also, after injection in E14 embryos of the Fast Green vehicle solution compared to no injection (n = 7/group), we sacrificed the embryos at E19, the age studied in the experiments, and examined the effects of the injection procedure itself on the density of cells stained with TUNEL for apoptosis, Iba-1 for microglia, and GFAP for astrocytes.
Effect of ICV CCL2 injection at E14 on CCL2, CCR2, and BLBP mRNA in NEP+mHYP of female and male E19 embryos:
In all experiments, we performed a single in utero ICV injection, in embryos at E14 from dams (n = 7/group) administered 1 μl of 0.1 μg CCL2 (“ICV CCL2”) or of the saline control solution (“ICV Control”) or given no injection (“No-ICV Control”), with both solutions containing 0.1% Fast Green, and we then examined both female and male embryos at E19 (n = 7/sex/group). This first experiment used qRT-PCR to examine the effects of ICV CCL2 injection on gene expression of CCL2, CCR2 and BLBP in the NEP+mHYP as compared to the two control groups and to determine whether these effects differ between the sexes. The data analysis was performed using a two-way ANOVA and paired t-tests.
Effect of ICV CCL2 at E14 on single- and double-labeled CCL2+ and BLBP+ cells and processes in female and male E19 embryos:
Using the same experimental procedure as described above, we used IF in this experiment to examine a separate set of female and male embryos at E19 (n = 7/sex/group) the effect of ICV CCL2 injection (0.1 μg/μl) at E14 on the density of single-labeled CCL2+ and BLBP+ cells in the NEP and single-labeled CCL2+ and BLBP+ processes in the mHYP, in addition to double-labeled CCL2+/BLBP+ cells in the NEP and CCL2+/BLBP+ processes in the mHYP. We also tested for sex differences in these effects. The data analysis was again performed using a two-way ANOVA and paired t-tests.
Effect of ICV CCL2 at E14 on mRNA levels of CCL2, CCR2 and MCH in the LH of female and male E19 embryos:
This experiment used the same procedures and specifically qRT-PCR to further examine gene expression changes in the LH. In the same set of E19 embryos used to examine the NEP+mHYP (see above), we tested in females and males (n = 7/sex/group) the effect of ICV injection of CCL2 (0.1 μg/μl) at E14 on gene expression of CCL2, CCR2 and MCH in the LH and analyzed possible sex differences. The data analysis was performed using a two-way ANOVA and paired t-tests.
Effect of ICV CCL2 at E14 on the density of CCL2+ and MCH+ neurons in LH of female and male E19 embryos:
Here, we examined embryos at E19 from a separate set of dams (n = 7/sex/group) using IF the effect of ICV CCL2 injection (0.1 μg/μl) at E14 on the density of CCL2+ and MCH+ neurons in the LH, with the data analysis performed using a two-way ANOVA and paired t-tests.
Statistical analysis.
All data were presented as mean ± SEM and were analyzed using SPSS (Version 24), for normality using Shapiro-Wilk test, and homogeneity of variance using Levene’s test. After finding all data sets to be normally distributed and to have equal variances, we then determined significance using a two-way ANOVA, which tested within-subject main effects of maternal ethanol administration and their respective controls, between-subject main effects of sex, and the interactions between maternal treatment and sex. A significant interaction was interpreted using simple main effect analyses to test the differences between sexes as well as the differences within each sex. Paired t-tests were performed to directly compare the effects of CCL2 ICV injections vs control groups within each sex. All graphs were prepared using the GraphPad Prism software (Version 6).
RESULTS
Technique involving ICV injection of CCL2 at E14 and brain analyses at E19
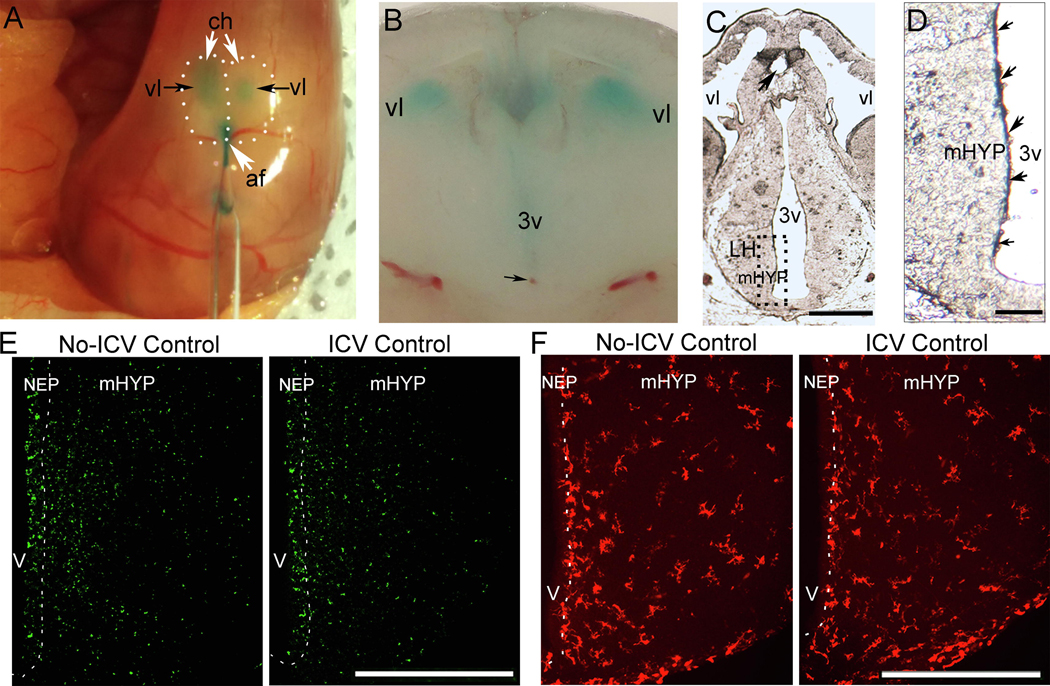
To more fully understand the technique of in utero ICV injection, we used a dye solution (0.1% Fast Green in normal saline) to reveal the nature and extent of its spread and performed histological analyses to verify the site of the injection and assess the extent of damage. As illustrated in the photomicrograph of an E16 embryo sacrificed 2 hours after injection (Fig. 2A), the injection through the anterior fissure of the dye solution (1 μl) shows from the surface of the skull how the dye is almost immediately seen filling the lateral ventricles of the embryo’s cortical hemispheres, a sign that the injection was successful. Also, as shown in a hand-cut coronal brain slice (Fig. 2B), a dense concentration of the dye solution is also clearly seen in the third ventricle, with the tip of the injection site (black arrow) evident along its ventral region. To further assess with histological analysis the extent of injury to the brain as well as spread of the dye, we then performed in E14 embryos from two dams (n = 7/dam) either a 1.0 μl injection of the dye solution (0.1% Fast Green in normal saline) or administered no injection and then sacrificed the embryos 2 hours later. A cryostat-cut section from a fresh brain at E14 reveals the point of entry of the injector (black arrow) in the dorsal region of the third ventricle (Fig. 2C) and illustrates at a higher magnification (Fig. 2D) a dense concentration of the dye solution within the NEP, as revealed by spots of purple or blue color along the third ventricle (small black arrows), that are not seen in the mHYP, suggesting that the effects of the injected solution, such as CCL2, may be greatest in the NEP. This is likely for the hypothalamus, with the injection occurring directly into the third ventricle, but also for other brain regions, with the injected solution spreading into the lateral ventricle. Tests with smaller volumes to reduce spread were found to increase the chances of an unsuccessful injection.
Figure 2:
A dye solution (0.1% Fast Green in normal saline) was used and histological analyses were performed to investigate the ICV injection technique, verify the injection site, and determine the nature and extent of spread of the injected solution. Immunofluorescence histochemistry (IF) was also performed to examine the effects of the injection procedure on cells in the NEP and LH. A, Photomicrograph of surface of embryo brain illustrates the dye solution injected through the anterior fissure as it fills the lateral ventricles of an E16 embryo. B, Coronal brain slice shows dense concentration of dye solution in the third ventricle and the tip of injection site (black arrow) in the ventral region. C, Cryostat-cut section from E14 brain shows point of entry of injector (black arrow) in the third ventricle. D, Higher magnification shows dense concentration of the dye solution within the NEP, indicated by blue and purple colored spots (small black arrows) along the third ventricle. E, Photomicrographs of ICV Control compared to No-ICV Control groups stained with TUNEL, a marker for apoptosis, show ICV injection of dye solution has no effect on TUNEL+ cells in the NEP and LH of E19 embryos (data in Table 4). F, Photomicrographs of ICV Control compared to No-ICV Control groups stained with Iba-1, marker for microglia, show ICV injection of dye solution has no effect on the density of Iba-1+ cells in the NEP and LH of E19 embryos (data in Table 4). Abbreviations: af: anterior fissure; ch, cortical hemisphere; IF, immunofluorescence; mHYP, medial hypothalamus; NEP, neuroepithelium; 3v, third ventricle; vl, lateral ventricle; Scale bars, 500 μm.
In an experiment using embryos sacrificed from 3 dams (n = 8–10/dam) at E19, the age studied in this report, we next used IF to examine the effects of the injection procedure itself on cells in the NEP and LH. Specifically, we tested whether ICV injection of the saline dye solution (1 μl) in embryos at E14 (“ICV Control”), as compared to embryos that were handled but received no injection (“No-ICV Control”), had effects on hypothalamic cells stained with TUNEL, a marker of apoptosis, and with the antibodies Iba-1 and GFAP, markers respectively of microglia and astrocytes. In all dams, 85–90% of the embryos were found to survive in both groups. Examination of these embryo brains showed that ICV injection of the saline dye solution compared to no injection (n = 7/group) had no effect on any of these measures in the NEP and LH (Table 4). The effects on GFAP could not be measured, as the immunostaining of this marker was too weak in the embryo to yield reliable results, as described in another report (Sancho-Tello et al., 1995). These results demonstrate that this experimental procedure, involving ICV injections into the third ventricle of the embryo at E14 and analyses of brain tissue at E19, allows a compound to be administered that remains most concentrated in the area of the NEP along the third ventricle, causes minimal damage at the needle entry site and tip of the injection site that are distant from the observation areas in the NEP as well as LH, and produces no signs of cellular damage or inflammation in these areas.
Table 4:
Density of TUNEL+and Iba-1+cells in NEP+mHYP and LH of the embryo brain
| Labeled Cells | Location | No-ICV Control (cells/μm2) | ICV Control (cells/μm2) |
|---|---|---|---|
| TUNEL | NEP+mHYP | 1.81E-4 ± 1.52E-5 | 1.80E-4 ± 4.52E-6 |
| LH | 1.33E-4 ± 2.35E-6 | 1.33E-4 ± 2.42E-6 | |
| Iba-1 | NEP+mHYP | 1.64E-4 ± 8.67E-6 | 1.67E-4 ± 6.36E-6 |
| LH | 4.74E-5 ± 4.08E-6 | 5.10E-5 ± 3.01E-7 | |
Abbreviations: CCL2, C-C motif ligand 2; BLBP, brain lipid-binding protein, radial glia marker.
Abbreviations: LH, lateral hypothalamus; NEP, neuroepithelium; mHYP, medial hypothalamus; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; Iba-1, marker for microglia; ICV, intracerebroventricular
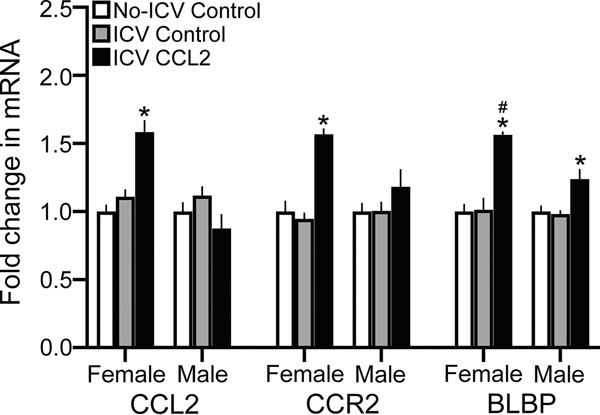
ICV CCL2 increases mRNA of CCL2, CCR2, and BLBP in NEP+mHYP of female E19 embryos
This experiment tested in the E19 embryo whether a single ICV injection of CCL2 (0.1 μg/μl), directly into the third ventricle at E14 when neurogenesis peaks, as compared to the ICV Control or No-ICV Control groups stimulates the expression of CCL2 and CCR2 in the NEP+mHYP measured using qRT-PCR. We found a significant main effect of ICV CCL2 injection on mRNA levels of both CCL2 (F(2,36) = 4.207, p = 0.023) and CCR2 (F(2,36) = 16.12, p < 0.0001), in addition to a significant effect of sex on CCL2 (F(1,36) = 29.06, p < 0.0001) and CCR2 (F(1,36) = 26.76, p < 0.0001) mRNA and a significant sex x treatment interaction on CCL2 (F(2,36) = 11.678, p < 0.0001) and CCR2 (F(2,36) = 6.04, p = 0.005) mRNA expression. There were no differences between the ICV Control and No-ICV Control groups for CCL2 mRNA in females ( p = 0.349) and males (p = 0.287) and for CCR2 mRNA in females (p = 0.417) and males (p = 0.728) (Table 5), indicating that the ICV injection procedure itself had little impact on gene expression. There were also no differences between female and male control groups in their mRNA levels of CCL2 (p = 0.277 for ICV Control and p = 0.247 for No-ICV Control) and CCR2 (p = 0.198 for ICV Control and p = 0.074 for No-ICV Control), indicating no sex differences in these baseline measures. In contrast, there were clear sex differences in the embryos given ICV CCL2 injections, with significantly higher mRNA levels in females than males of both CCL2 (p < 0.0001) and CCR2 (p < 0.0001) in the NEP+mHYP (Table 5). Furthermore, compared to the ICV Control and No-ICV Control groups, the ICV CCL2 injection significantly increased in females mRNA expression of CCL2 (p < 0.0001 and p < 0.0001, respectively) and CCR2 (p < 0.0001 and p < 0.0001, respectively) while having no effect in males on CCL2 (p = 0.358 and p = 0.079, respectively) and CCR2 (p = 0.191 and p = 0.178, respectively) (Fig. 3).
Table 5:
Effects of CCL2 ICV injection on mRNA expression in NEP+mHYP and LH of embryo brain
| NEP+mHYP | Female |
Male |
||||
|---|---|---|---|---|---|---|
| mRNA | No-ICV Control | ICV Control | ICV CCL2 | No-ICV Control | ICV Control | ICV CCL2 |
| CCL2 | 6.2E-4 ± 3.1E-5 | 6.9E-4 ± 3.7E-5 | 9.8E-4 ± 8.7E-5*# | 5.4E-4 ± 3.8E-5 | 6.1E-4 ± 4.3E-5 | 4.7E-4 ± 5.0E-5* |
| CCR2 | 3.3E-3 ± 1.9E-4 | 3.0E-3 ± 1.3E-4 | 4.9E-3 ± 2.2E-4*# | 2.4E-3 ± 1.6E-4 | 2.6E-3 ± 1.7E-4 | 3.0E-3 ± 3.9E-4* |
| BLBP | 5.6E-2 ± 3.2E-3 | 5.7E-2 ± 5.0E-3 | 8.2E-2 ± 4.5E-3* | 4.5E-2 ± 1.6E-3 | 4.8E-2 ± 1.3E-3 | 6.4E-2 ± 3.8E-3 |
| MCH | 6.6E-4 ± 3.9E-5 | 7.1E-4 ± 1.1E-5 | 1.1E-3 ± 6.1E-5* | 6.8E-4 ± 4.3E-5 | 7.2E-4 ± 3.2E-5 | 8.6E-4 ± 4.9E-5 |
| LH | Female |
Male |
||||
| mRNA | No-ICV Control | ICV Control | ICV CCL2 | No-ICV Control | ICV Control | ICV CCL2 |
| CCL2 | 6.8E-4 ± 4.7E-5 | 7.1E-4 ± 4.6E-5 | 1.2E-3 ± 5.0E-5*# | 7.1E-4 ± 6.0E-5 | 7.2E-4 ± 6.3E-5 | 8.2E-4 ± 4.6E-5* |
| CCR2 | 2.4E-3 ± 1.4E-4 | 2.5E-3 ± 1.5E-4 | 4.0E-3 ± 3.3E-4*# | 2.5E-3 ± 2.9E-4 | 2.6E-3 ±1.7E-4 | 2.8E-3 ± 9.8E-5* |
| MCH | 7.5E-3 ± 4.1E-4 | 7.5E-3 ± 3.9E-4 | 1.5E-2 ± 9.7E-4*# | 7.5E-3 ± 3.2E-4 | 6.91E-3 ± 5.0E-4 | 9.6E-3 ± 3.3E-4* |
Abbreviations: LH, lateral hypothalamus; NEP, neuroepithelium; mHYP, medial hypothalamus; CCL2, C-C motif ligand 2;
CCR2, C-C motif receptor 2; BLBP, brain lipid-binding protein, marker for radial glia; MCH, melanin-concentrating hormone;
ICV, intracerebroventricular
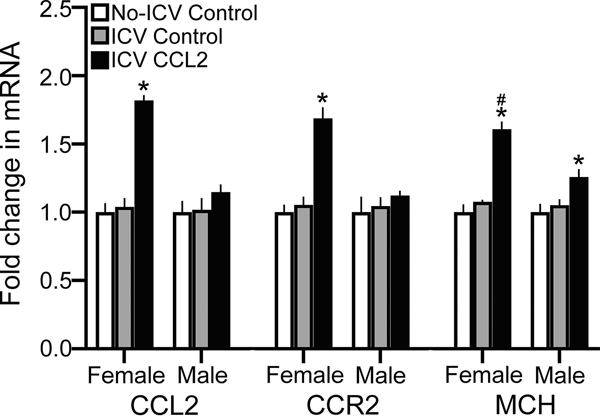
Figure 3:
ICV CCL2 administration (0.1 μg/μl, E14) compared to ICV Control and No-ICV Control groups (n = 7/sex/group) significantly increases mRNA levels (using qRT-PCR) of CCL2, CCR2, and BLBP in the NEP+mHYP of E19 embryos, with the data presented here as mRNA fold change (compared to No-ICV Control) based on average ratio scores given in Table 4. This stimulatory effect of CCL2 on endogenous CCL2 and CCR2 mRNA levels is evident only in female embryos, with the effect on BLBP mRNA significantly greater in females compared to that seen in males. Abbreviations: CCL2, C-C motif ligand 2; BLBP, brain lipid-binding protein; mHYP, medial hypothalamus; NEP, neuroepithelium. Data are mean ± SEM. *p < 0.05 versus control group. #, p < 0.05 versus males.
These CCL2-induced changes in expression of CCL2 and CCR2 in the NEP+mHYP were accompanied by changes in expression of the radial glial marker, BLBP. There was a significant main effect of ICV CCL2 injection on BLBP mRNA levels (F(2,36) = 27.65, p < 0.0001), along with a main effect of sex (F(1,36) = 28.26, p < 0.0001) and a sex x treatment interaction (F(2,36) = 5.47, p = 0.008) (Fig. 3). Again, while control females and males did not differ in their levels of BLBP mRNA in the ICV Control group (p = 0.066) and No-ICV Control group (p = 0.129), there was a significant sex difference in BLBP expression (p < 0.0001) in the CCL2-injected group (Table 5). The ICV CCL2 treatment compared to both the ICV Control and No-ICV Control groups caused a significant increase in BLBP mRNA in both female (p < 0.0001 and p < 0.0001, respectively) and male (p = 0.020 and p = 0.013, respectively) embryos (Fig. 3). Furthermore, direct comparisons between the CCL2-injected females and males (via paired t-test) showed this stimulatory effect to be significantly greater in females compared to the ICV Control (t(12) = 3.620, p = 0.006) and No-ICV Control (t(12) = 3.931, p = 0.003) groups. Thus, central injection of CCL2 directly into the embryonic third ventricle has stimulatory effects on expression of both CCL2 and CCR2 and also of the radial glia marker BLBP that are sexually dimorphic, with the CCL2/CCR2 neuroimmune system in the NEP highly responsive to CCL2 in females but unresponsive in males.
ICV CCL2 increases single-labeled CCL2 and BLBP cells and processes in female E19 embryos
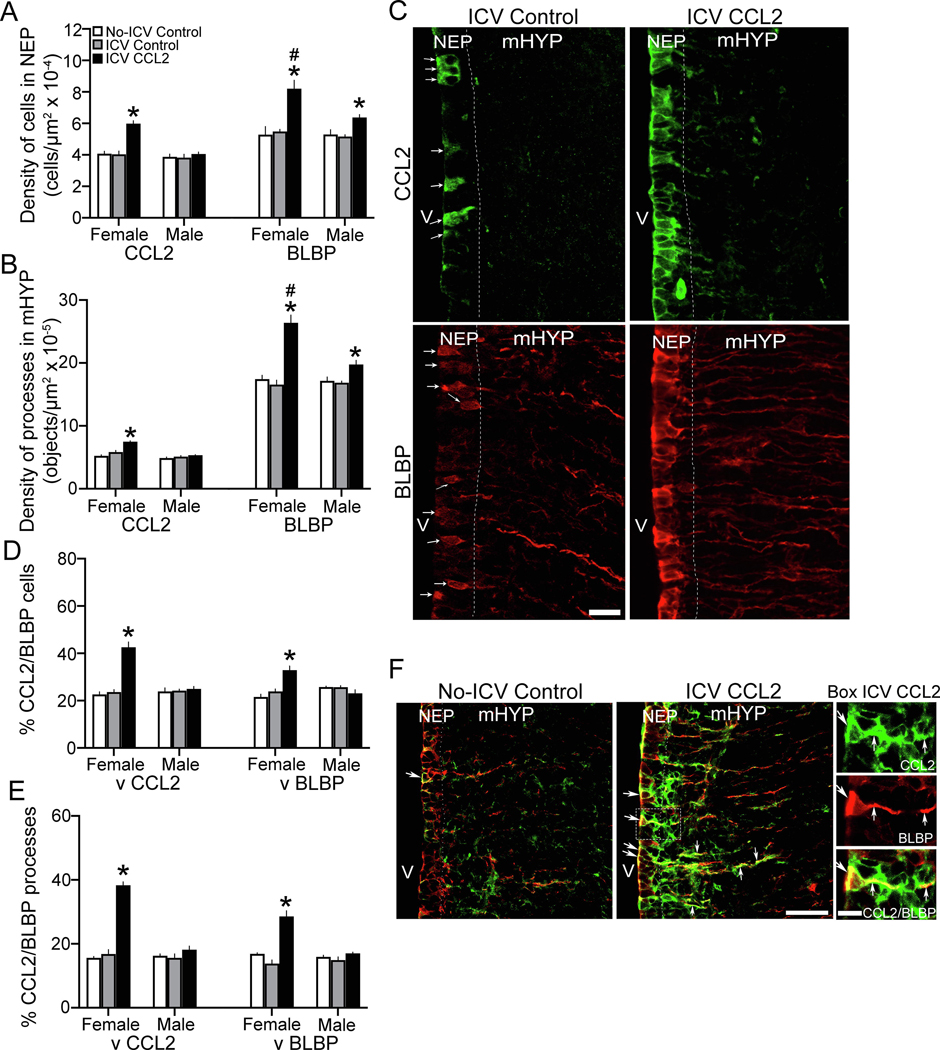
In our recent study of embryos at E19 (Chang et al., 2019b), we found that CCL2+ and BLBP+ cells are dense in the hypothalamic NEP, and they both have processes that project laterally through the mHYP. Here we tested in both female and male embryos at E19 whether ICV injection of CCL2 (0.1 μg/μl) at E14 affects these single-labeled CCL2+ and BLBP+ cells and processes. In the NEP, there was a significant main effect of ICV CCL2 injection on the density of single-labeled CCL2+ cells (F(2,36) = 18.42, p < 0.0001), along with a significant effect of sex (F(1,36) = 21.83, p < 0.0001) and a significant sex x treatment interaction (F(2,36) = 11.86, p = 0.00), and also a significant main effect of ICV CCL2 on the density of single-labeled BLBP+ cells (F(2,36) = 19.76, p < 0.0001), along with a significant effect of sex (F(4,36) = 5.75, p = 0.022) and a significant sex x treatment interaction (F(2,36) = 3.601, p = 0.038) (Fig. 4A). In addition, there was a significant main effect of ICV CCL2 injection on the density of single-labeled CCL2+ processes (F(2,36) = 17.23, p < 0.0001), along with a main effect of sex (F(1,36) = 31.14, p = 0.001) and significant sex x treatment interaction (F(2,36) = 8.27, p = 0.001), and also a significant main effect on the density of single-labeled BLBP+ processes (F(2,36) = 34.09, p < 0.0001), along with a main effect of sex (F(1,36) = 18.14, p < 0.0001) and a significant sex x treatment interaction (F(2,36) = 18.31, p < 0.0001) (Fig. 4B). There were no differences between the female and male control groups and between the two control groups within each sex. The female and male control embryos were similar in the density of single-labeled cells, both CCL2+ for the ICV Control (p = 0.7154) and No-ICV Control (p = 0.487) groups and BLBP+ for the ICV Control (p = 0.539) and No-ICV Control (p = 0.990) groups, and the density of single-labeled processes, both CCL2+ for the ICV Control (p = 0.134) and No-ICV Control (p = 0.329) groups and BLBP+ for the ICV Control (p = 0.801) and No-ICV Control (p = 0.790) groups. There were also no sex differences between the ICV Control and No-ICV Control groups in the density of single-labeled CCL2+ cells for females (p = 0.884) and males (p = 0.872) and BLBP+ cells for females (p = 0.702) and males (p = 0.806) and of single-labeled CCL2+ processes for females (p = 0.073) and males (p = 0.534) and BLBP+ processes for females (p = 0.431) and males (p = 0.797).
Figure 4:
ICV CCL2 administration (0.1 μg/μl, E14) compared to ICV Control and No-ICV Control groups (n = 7/sex/group) increases the density of single-labeled CCL2 and BLBP cells in the NEP and processes in the mHYP and the percentage of double-labeled CCL2+/BLBP+ radial glia cells and processes in NEP and mHYP of E19 embryos. A, ICV CCL2 compared to control groups increases in the NEP the density of CCL2+ cells, in female but not males embryos, and of BLBP+ cells, in both sexes but significantly greater in females. B, ICV CCL2 compared to control groups increases in the mHYP the density of CCL2+ processes, in female but not male embryos, and BLBP+ processes, in both sexes but significantly greater in females. C, These effects in ICV CCL2 compared to ICV Control embryos are illustrated in representative immunostaining images (40x) of single-labeled CCL2+ cells and processes (green) and BLBP+ cells and processes (red) in female embryos, with individual cells in Control embryo identified by white arrows.. D, ICV CCL2 compared to control groups significantly increases in the NEP the percentage of double-labeled CCL2+/BLBP+ radial glia cells, relative to single-labeled CCL2+ or BLBP+ cells, in female but not male embryos. E, ICV CCL2 compared to control groups significantly increases in the mHYP the percentage of double-labeled CCL2+/BLBP+ radial glia processes relative to single-labeled CCL2+ and BLBP+ processes, in female but not male embryos. F, This effect of ICV CCL2 on double-labeled CCL2+/BLBP+ cells and processes is illustrated in the 20x images, with those in the box enlarged to the right (40x) showing (white arrows) the single-labeled CCL2+ (green) and BLBP+ (red) cells and processes and the double-labeled CCL2+/BLBP+ cells and processes (yellow). Abbreviations: CCL2, C-C motif ligand 2; BLBP, brain lipid-binding protein; mHYP, medial hypothalamus; NEP, neuroepithelium; V, third ventricle. Scale bars, 200 μm. *p < 0.05 versus control group.
In contrast, ICV CCL2-injected embryos showed significant sex differences in the single-labeled cells in the NEP (Fig. 4A) and their processes in the mHYP (Fig. 4B). The females had a markedly higher density than male embryos of both single-labeled CCL2+ (p < 0.0001) and BLBP+ (p = 0.001) cells and single-labeled CCL2+ (p < 0.0001) and BLBP+ (p < 0.0001) processes. Further, ICV injection of CCL2 in female embryos caused a significant increase in the density of single-labeled CCL2+ cells compared to their ICV Control (p < 0.0001) and No-ICV Control (p < 0.0001) groups and of single-labeled CCL2+ processes compared to their ICV Control (p < 0.0001) and No-ICV Control (p = 0.043) groups. This is in contrast to the ICV CCL2-injected male embryos, which exhibited no change in the density of CCL2+ cells compared to their control groups (p = 0.421 and p = 0.872, respectively) and CCL2+ processes compared to their control groups (p = 0.499 and p = 0.198, respectively). The ICV CCL2-injected embryos also showed significant sex differences in the single-labeled BLBP+ cells and processes, with the females having a higher density than males of single-labeled BLBP+ cells (p = 0.001) and processes (p < 0.0001). Also, while ICV CCL2 injection increased in both females and males the density of single-labeled BLBP+ cells compared to the ICV Control (p < 0.0001 for female and p = 0.024 for male) and No-ICV Control (p < 0.0001 for female and p = 0.042 for male) groups and of BLBP+ processes compared to the ICV Control (p < 0.0001 for female and p = 0.015 for male) and No-ICV Control (p < 0.0001 for female and p = 0.028 for male) groups, direct comparisons between the CCL2-injected females and CCL2-injected males (via paired t-test) showed the stimulatory effect of CCL2 to be significantly greater in females than males on the BLBP+ cells when compared to ICV Control (t(12) = 2.554, p = 0.035) and No-ICV Control (t(12) = 3.107, p = 0.009) groups and the BLBP+ processes when compared to the ICV Control (t(12) = 4.626, p = 0.001) and No-ICV Control (t(12) = 4.251, p = 0.002) groups. These effects of ICV CCL2 injection detected only in female embryos are clearly illustrated in the photomicrographs (Fig. 4C). While sparse under control conditions, the CCL2+ and BLBP+ cells in the NEP of embryos given ICV CCL2 injections become very dense along the third ventricle, as do their CCL2+ and BLBP+ processes extending into the mHYP with are shorter and fewer in number for CCL2.
ICV CCL2 increases double-labeling of CCL2 within BLBP cells and processes in female E19 embryos
Further analyses in the same embryos of the CCL2+ and BLBP+ cells and processes showed CCL2 to exist in the BLBP-labeled radial glia cells of the NEP and their processes in the mHYP, as previously described (Chang et al., 2019b). There was a significant main effect of ICV CCL2 injection (0.1 μg/μl) on the density of double-labeled CCL2+/BLBP+ cells relative to the single-labeled CCL2+ (F(2,36) = 75.49, p < 0.0001) and BLBP+ (F(2,36) = 31.63, p < 0.0001) cells, along with a significant effect of sex on these double-labeled cells relative to single-labeled CCL2+ (F(1,36) = 54.86 p < 0.0001) and BLBP+ (F(1,36) = 17.92, p < 0.0001) cells and a significant sex x treatment interaction for CCL2+/BLBP+ cells relative to single-labeled CCL2+ (F(2,36) = 50.52, p < 0.0001) and BLBP+ (F(2,36) = 18.85, p < 0.0001) cells (Fig. 4D). It similarly showed a significant main effect of ICV CCL2 injection on the density of double-labeled CCL2+/BLBP+ processes relative to the single-labeled CCL2+ (F(2,36) = 75.49, p < 0.0001) and BLBP+ (F(2,36) = 31.63, p < 0.0001) processes, along with a significant effect of sex on these double-labeled processes relative to single-labeled CCL2+ (F(1,36) = 54.86, p < 0.0001) and BLBP+ (F(1,36) = 17.92, p < 0.0001) processes and a significant sex x treatment interaction for CCL2+/BLBP+ processes relative to single-labeled CCL2+ (F(2,36) = 5.052, p < 0.0001) and BLBP+ (F(2,36) = 18.85, p < 0.0001) processes (Fig. 4E). Double-labeled cells and processes in control groups showed no differences between the female and male embryos and between the control groups within each sex. In contrast to control embryos, the ICV CCL2-injected embryos exhibited strong sex differences in the colocalization of CCL2 with the BLBP+ radial glia cells and processes. Female embryos compared to males had a significantly greater percentage of double-labeled CCL2+/BLBP+ cells, relative to single-labeled CCL2+ (p < 0.0001) and BLBP+ (p < 0.0001) cells, and double-labeled CCL2+/BLBP+ processes, relative to single-labeled CCL2+ (p < 0.0001) and BLBP+ (p < 0.0001) processes. Furthermore, ICV CCL2 injection in female embryos compared to controls significantly increased the percentage of double-labeled CCL2+/BLBP+ cells, relative to single-labeled CCL2+ (p < 0.0001 for ICV Control and p < 0.0001 for No-ICV Control) and BLBP+ (p < 0.0001 for ICV Control and p < 0.0001 for No-ICV Control) cells, and of double-labeled CCL2+/BLBP+ processes, relative to single-labeled CCL2+ (p < 0.0001 for ICV Control and p < 0.0001 for No-ICV Control) and BLBP+ (p < 0.0001 for ICV Control and p < 0.0001 for No-ICV Control) processes (Fig. 4E). This is in contrast to ICV CCL2 injection in male embryos, which had no effect on double-labeled CCL2+/BLBP+ cells, relative to single-labeled CCL2+ (p = 0.119 for ICV Control and p = 0.246 for No-ICV Control) and BLBP+ (p = 0.187 for ICV Control and p = 0.489 for No-ICV Control) cells, and on double-labeled CCL2+/BLBP+ processes, relative to single-labeled CCL2+ (p = 0.735 for ICV Control and p = 0.635 for No-ICV Control) and BLBP+ (p = 0.188 for ICV Control and p = 0.182 for No-ICV Control) processes. These effects of ICV CCL2 injection in female embryos are illustrated in the photomicrographs (Fig. 4F), which show the marked increase in percentage of radial glia progenitor cells in the NEP and processes in the mHYP that colabel CCL2, an effect that is not evident in male embryos.
ICV CCL2 increases mRNA levels of CCL2, CCR2 and MCH in LH of female and male E19 embryos
This experiment tested whether the stimulatory effect of ICV CCL2 injection at E14 on CCL2 within radial glia in the NEP is accompanied by a change in expression of CCL2, CCR2 and MCH at E19 in the LH where their cells are densest and the gene expression of CCL2 and CCR2 mRNA is 5-fold lower in concentration than that in the NEP+mHYP. This analysis revealed a significant main effect of ICV CCL2 injection (0.1 μg/μl) on gene expression of CCL2 (F(2,54) = 14.61, p < 0.0001), CCR2 (F(2,54) = 12.459, p < 0.0001) and MCH (F(2,54) = 65.28, p < 0.0001), along with an effect of sex on CCL2 (F(1,54) = 12.40, p = 0.001),CCR2 (F(1,54) = 4.141, p = 0.047) and MCH (F(1,54) = 28.06, p < 0.0001) and a sex x CCL2 treatment interaction for CCL2 (F(2,54) = 15.60, p < 0.0001), CCR2 (F(2,54) = 6.525, p = 0.003) and MCH (F(2,54) = 16.14, p < 0.0001). There were no differences between the female and male control groups and also between the ICV Control and No-ICV Control groups (Table 5). In embryos given an ICV injection of CCL2 at E14, however, there were clear differences between the females and males. The CCL2-injected females compared to CCL2-injected males had significantly higher mRNA levels in the LH of both CCL2 (p < 0.0001) and CCR2 (p < 0.0001). Also, ICV CCL2 injection compared to control groups significantly increased in the LH of female embryos mRNA levels of both CCL2 (p < 0.0001 for ICV Control and p < 0.0001 for No-ICV Control) and CCR2 (p < 0.0001 for ICV Control and p < 0.0001 for No-ICV Control). However, ICV CCL2 had no effect in male embryos on the expression of CCL2 (p = 0.195, for ICV Control and p = 0.259 for No-ICV Control) and CCR2 (p = 0.312 for ICV Control and p = 0.534 for No-ICV Control) (Fig. 5). Similarly, ICV CCL2 injection significantly increased MCH gene expression in the LH of both females and males as compared to the ICV Control (p < 0.0001 for females and p = 0.001 for males) and No-ICV Control (p < 0.0001 for females and p = 0.001 for males) groups. However, the direct comparisons between the female and male embryos injected with CCL2 showed the stimulatory effect on MCH in the LH to be significantly greater in the females compared to the ICV Control (t(18) = 2.655, p = 0.016) and No-ICV Control (t(18) = 2.577, p = 0.019) groups (Fig. 5). Further analyses of MCH mRNA levels in the NEP+mHYP, while 10-fold lower than in the LH, revealed a similar stimulatory effect of ICV CCL2 injection, with a significant main effect of ICV CCL2 treatment on MCH expression (F(2,36) = 27.573, p < 0.0001), along with no significant effect of sex (F(1,36) = 2.569, p = 0.118) and a significant sex x CCL2 treatment interaction (F(2,36) = 4.481, p = 0.018) (Table 5). While ICV CCL2 injection significantly increased MCH gene expression in both females and males as compared to the ICV Control ( p < 0.0001 for females and p = 0.023 for males) and No-ICV Control (p < 0.0001 for females and p = 0.005 for males) groups, direct comparisons of CCL2’s effect in female and male embryos showed it to be significantly greater in the females compared to the ICV Control (t(12) = 2.389, p = 0.034) and No-ICV Control (t(12) = 2.709, p = 0.019) groups. These results demonstrate that ICV injection of CCL2 has similar stimulatory effects on CCL2, CCR2 and MCH in the LH as observed in the NEP.
Figure 5:
ICV CCL2 administration (1 μg/μl, E14) compared to ICV Control and No-ICV Control groups (n = 7/sex/group) stimulates in the LH of E19 embryos the expression of CCL2, CCR2, and MCH measured using qRT-PCR, with the data presented here as mRNA fold change (compared to No-ICV Control) based on average ratio scores given in Table 5. This stimulatory effect on CCL2, CCR2, and MCH mRNA levels is evident in both the female and male embryos but is significantly greater in the female embryos. Abbreviations: CCL2, C-C motif ligand 2; MCH, melanin-concentrating hormone; LH, lateral hypothalamus. Data are mean ± SEM. *p < 0.05 versus control group. #, p < 0.05 versus males.
ICV CCL2 increases density of CCL2+ and MCH+ cells in LH of female and male embryos
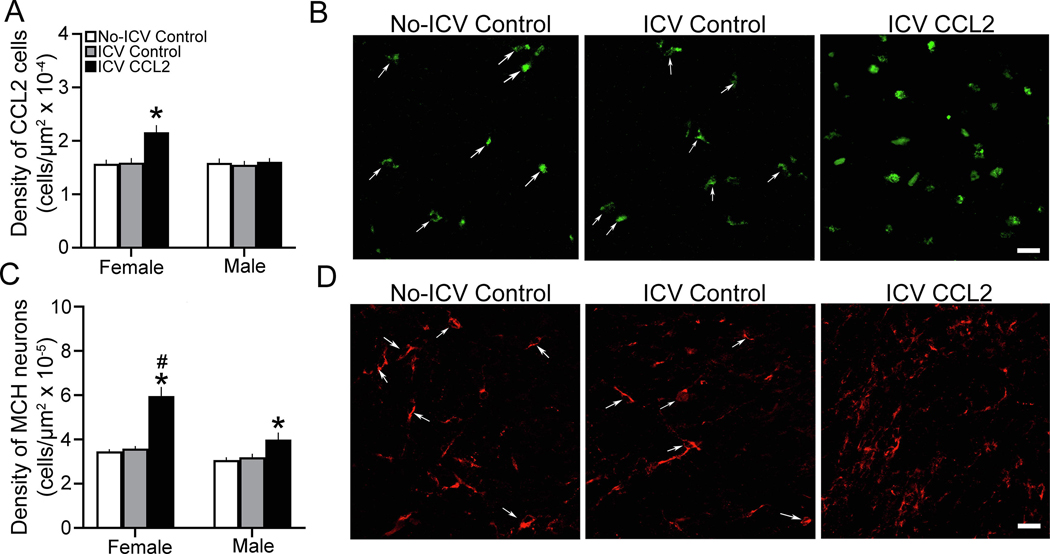
Here we used single-labeled IF to test whether ICV CCL2 injection at E14 affects the density of CCL2+ and MCH+ neurons in the LH of E19 embryos. There was a significant main effect of ICV CCL2 administration (0.1 μg/μl) compared to control groups on the density of CCL2+ cells in the LH (F(2,36) = 7.84, p = 0.001), together with a significant effect of sex (F(1,36) = 6.093, p = 0.005) and a significant interaction between sex and ICV CCL2 treatment (F(2,36) = 6.093, p = 0.005). There were no differences between ICV control and No-ICV control groups for females and males and between the females and males. In contrast, there were significant differences between the female and male embryos that received ICV injection of CCL2 (Fig. 6A). The ICV CCL2-injected females had significantly greater CCL2+ cell density in the LH than the males (p < 0.0001), and the ICV CCL2 treatment significantly increased in female embryos the density of CCL2+ cells compared to the ICV Control (p < 0.0001) and No-ICV Control (p < 0.0001) groups (Fig 6A), as illustrated in the photomicrographs (Fig. 6B). The male embryos exhibited no response to the ICV CCL2 injection compared to both their ICV Control (p = 0.604) and No-ICV Control (p = 0.711) groups (Fig. 6A).
Figure 6:
ICV CCL2 administration (0.1 μg/μl, E14) compared to ICV Control and No-ICV Control groups (n = 7/sex/group) increases the density of CCL2+ and MCH+ cells in the LH of E19 embryos. A, CCL2 ICV compared to control groups significantly increases the density of CCL2+ cells in the LH of female but not male embryos. B, Photomicrographs (20x) illustrate the CCL2+ cells in the LH of E19 embryos in the three groups, with individual cells in Control embryos identified by white arrows. C, ICV CCL2 compared to control groups significantly increases the density of MCH+ neurons in the LH of both female and male embryos, with the females showing a significantly greater effect. D, Photomicrographs (20x) illustrate the MCH+ neurons (and their processes) in the LH of female embryos in the three groups, with individual cells in Control embryos identified by white arrows. Abbreviations: CCL2, C-C motif ligand 2; MCH, melanin-concentrating hormone; mHYP, medial hypothalamus; NEP, neuroepithelium. Scale bars, 100 μm. Data are mean ± SEM. *p < 0.05 versus control group. #, p < 0.05 versus males
This sexually dimorphic effect of ICV-injected CCL2 on endogenous CCL2 in the LH was accompanied by changes in the density of MCH+ neurons in this area that were also sexually dimorphic (Fig. 6C). There was a significant main effect of ICV CCL2 treatment (F(2,36) = 18.96, p < 0.0001), along with a significant effect of sex (F(1,36) = 12.33, p = 0.001) and a significant interaction between sex and ICV CCL2 treatment (F(2,36) = 3.303, p = 0.0048). While there were no differences between the ICV control and No-ICV control groups for females and males and between the females and males, the females had a significantly higher density than males of MCH+ neurons (p < 0.0001). Whereas ICV CCL2 treatment caused a significant increase in MCH+ cell density in both the females compared to their ICV Control (p < 0.0001) and No-ICV Control (p < 0.0001) groups and the males compared to their ICV Control (p < 0.0001) and No-ICV Control (p < 0.0001) groups, direct comparisons (via paired t-test) between females and males of the CCL2-induced increase in MCH+ neurons showed this effect to be significantly stronger in the females than males when compared to the ICV Control (t(12) = 2.836, p = 0.015) and No-ICV Control (t(12) = 2.185, p = 0.049) groups (Fig. 6C). This effect on MCH+ neurons in the LH of female embryos is illustrated in the photomicrographs (Fig. 6D). These single-labeled CCL2+ and MCH+ cells, while too few in number in the embryonic hypothalamus to reveal significant colocalization using double-labeling IF, are most concentrated in the same area of the LH (see Materials and Methods) where they are shown to exhibit significant CCL2+/MCH+ colabeling in the neonate as well as adolescent offspring (Chang et al., 2018, 2019a). These results, consistent with measurements of gene expression, show ICV CCL2 injection to increase the density of CCL2+ cells in the LH along with MCH+ neurons in the same area, supporting a close relationship between this chemokine and neuropeptide.
DISCUSSION
Studies investigating prenatal exposure to teratogenic compounds are generally limited to maternal peripheral manipulations, precluding an analysis of disturbances that reflect actions directly within the fetal brain. Such functional clarity at the fetal level, however, is crucial for a comprehensive understanding of embryonic mechanisms mediating the effects observed subsequently in the offspring, as well as for developing effective therapeutic interventions. The current study demonstrates the use of in utero ICV injections for delivering pharmacological agents, first to directly manipulate in the embryo specific neuroimmune signaling in a particular region, as shown here for the CCL2/CCR2 axis in the NEP where neurons are born, and then to investigate the anatomical and functional relationship of this CCL2/CCR2 system and local cell development to the density of neurons in a distant area, as shown here for the LH where both CCL2 and CCR2 are found to exist in neurons and colocalize with the orexigenic neuropeptide, MCH. After several practice tests, we garnered an 85–90% survival rate with direct injections into the third ventricle at E14. We also performed control tests that allow us to exclude possible confounding effects of the injection procedure itself, showing that the vehicle injection compared to no injection has no apparent impact on cell development or inflammation in the embryonic brain. Further, by delaying nearly a week after the injection before extracting the offspring brains just prior to the usual birth date, we further mitigated any confounding variables that may arise, not only from the injection procedure itself but also from the birth process and postnatal environment. To determine the extent of spread of the injected substance, we tracked the progression of an injected Fast Green solution and found it to fill the lateral ventricles of the cortical hemispheres, an indication that the ICV injection was successful, and also the hypothalamic third ventricle as demonstrated in a fresh tissue dissection shortly after injection. Further histological analyses revealed a very dense concentration of Fast Green specifically in the NEP itself, with much lower concentrations evident within the hypothalamus, and they showed little dye beyond the adjacent ventricles, suggesting that the injected solution remained confined within the embryonic brain and did not spread to the amniotic fluid or maternal brain. With the present experiments injecting a CCL2 solution directly into the third ventricle at E14 during peak hypothalamic neurogenesis, the success of this single ICV injection and the likelihood that CCL2 is directly affecting the hypothalamic CCL2/CCR2 neuroimmune system in an auto-regulatory manner are indicated by the marked changes in the embryonic NEP and LH observed days later at E19.
Our results demonstrate that ICV CCL2 injection significantly stimulates CCL2/CCR2 signaling in the NEP. While the vehicle injection procedure itself causes no apparent apoptosis or inflammatory response, administration of CCL2 into the third ventricle significantly increases in female embryos mRNA expression of both CCL2 and CCR2 and the density of CCL2 cells, some of which are shown to be neurons (Chang et al., 2019a, 2019b). Whereas endogenous CCL2 has been implicated in alcohol-induced neurotoxicity (Ren et al., 2017; Zhang and Luo, 2019), the cytotoxic effects produced by CCL2 administration have yet to be investigated and can be directly tested in the embryo brain with ICV injection of CCL2. With a moderate dose of prenatal ethanol exposure that stimulates CCL2 found to have no effect on apoptosis (Chang et al., 2012) and a study with CCL2 siRNA suggesting that CCL2 reduces cellular apoptosis while increasing cell proliferation (Lu et al., 2017), CCL2 at least at low-to-moderate concentrations may be more involved in neuronal development than apoptosis. There are numerous in vitro studies showing CCL2 to be a potent neuromodulator, causing an increase in the proliferation, differentiation and migration of neurons (Liu et al., 2007; Turbic et al., 2011; Poon et al., 2014), in neuronal excitability (Gao, 2009; Takeda et al., 2018), and in neurotransmitters and neuropeptides (van Gassen et al., 2005). There is also a recent in vivo study showing peripheral CCL2 administration to increase the density of CCL2 neurons that co-express CCR2 in the LH (Chang et al., 2019a) and in vitro evidence that CCL2 acting on its own cell containing CCR2 stimulates its own production and secretion (Sakai et al., 2006; Harris et al., 2013; Cazareth et al., 2014). These different reports consistent with the present study suggest that CCL2 has a potent stimulatory effect on neural systems in the brain and that it functions under autocrine regulation, properties that need to be further investigated with direct pharmacological manipulation of the fetal brain. The present results obtained with in utero ICV CCL2 injection focus attention on the NEP as a site where this auto-regulatory loop functions, with CCL2 activating its own signaling within cells along the third ventricle and promoting the development of neurons that express CCL2 as well as CCR2.
Further tests here reveal a close relationship of this CCL2/CCR2 system in the hypothalamic NEP to radial glia progenitor cells that are dense in the NEP and have processes that branch through the mHYP toward the LH. Radial glia have a diverse set of functionalities based on their subpopulation distinction, which include serving as the primary neural progenitor cells for the brain and providing extended processes or scaffolds that guide migrating neurons from their site of differentiation to their terminal locations, determining their distribution and patterning (LaMonica et al., 2013; Barry et al., 2014; Taverna et al., 2014; Bedont et al., 2015). With direct pharmacological manipulation of the embryonic brain, we show here that CCL2 injected into the third ventricle at E14, in addition to upregulating the endogenous CCL2/CCR2 system and increasing CCL2 cell proliferation in the NEP, stimulates at E19 the expression and density of BLBP-labeled radial glia progenitor cells and their processes projecting laterally through the mHYP toward the LH. Consistent with evidence showing CCL2 to co-express with BLBP-labeled radial glia-like cells in a neural stem cell niche of the spinal cord (Knerlich-Lukoschus et al., 2010), we additionally demonstrate that ICV CCL2 injection markedly stimulates the colocalization of CCL2 within radial glia cells and processes. This close anatomical relationship suggests that the CCL2/CCR2 system in the hypothalamic NEP has a function within radial glia in promoting neurogenesis and providing processes to facilitate the migration of neurons to distant hypothalamic regions such as the LH. This is further supported by in vitro evidence that CCL2 strongly stimulates the migration of primary hypothalamic neurons (Poon et al., 2014) and in vivo evidence that CCR2 receptors in the embryo are required for the stimulatory effect on radial glia and local neurons produced by ethanol (Chang et al., 2019b). Together, these findings indicate a potential functional pathway through which endogenous CCL2 acts in an auto-regulatory fashion to stimulate CCL2/CCR2 signaling in the NEP and increase the proliferation and migration of neurons toward the LH, including those that colocalize CCL2 and CCR2.
Our findings additionally suggest that these stimulatory effects of CCL2 in the proliferative NEP are accompanied by changes in neuronal patterning in an outer region of the hypothalamus, the LH. Direct manipulation by ICV CCL2 injection in the developing brain at E14 markedly increases in this area both mRNA expression of CCL2 and CCR2 and the density of CCL2 cells in E19 embryos. These cells are clustered in the middle of the LH, where they have been identified as neurons colabeling NeuN and found to colocalize CCR2 (Chang et al., 2015, 2018, 2019a). With this stimulatory effect in the embryonic LH similar to that observed in the NEP and this same effect of ethanol shown to be blocked by a CCR2 antagonist (Chang et al., 2019a), the results here suggest that the CCL2 cells originate in the NEP, deriving specifically from CCL2-colocalizing radial glia cells, and they act within radial glia processes that provide scaffolds to facilitate cell migration, similar to that described for neuroepithelial radial glia in the cortex that generate neurons in outer cortical regions (Noctor et al., 2007; Franco et al., 2012; Barry et al., 2014). This idea receives additional support from our tests showing that the injected dye, and likely CCL2, is highly concentrated within the NEP while weaker throughout the hypothalamus, the mRNA levels of both CCL2 and CCR2 in the NEP are 5-fold higher than those in the LH, and the CCL2 cells in the NEP are distinct in having laterally projecting processes which likely participate in guiding the migration of neurons toward the LH.
Of particular interest is that these effects produced by direct pharmacological manipulation of the embryonic brain via ICV injection are similar to those produced by maternal peripheral administration of CCL2, which as recently reported include a stimulatory effect of maternal CCL2 on the CCL2/CCR2 system in the NEP and LH along with the radial glia cells and processes (Chang et al., 2018, 2019a, 2019b). In addition to revealing a similarity in these effects of peripheral and central administration, the present findings are notable in showing a single ICV injection of CCL2 at E14 during peak hypothalamic neurogenesis to be sufficient in producing effects similar in magnitude to those caused by 5 days of maternal peripheral injections of CCL2 from E10 to E15. This demonstrates how vulnerable the embryonic brain is to direct challenges, such as that produced by neuroimmune factors (Akhtar et al., 2017), and how the effects of maternal peripheral administration are significantly buffered, requiring a higher dose and longer period of exposure. With proper controls for confounding effects of in utero ICV manipulations and a lack of confounding variables from maternal manipulations, the present findings lead us to conclude that the peripheral manipulations in pregnant dams are, in fact, acting directly on chemokine systems in the embryonic brain rather than indirectly through effects on the mother. Studies with maternal administration of ethanol, at a moderate dose and during the same embryonic period, reveal similar stimulatory effects on this neuroimmune system and show them to be blocked by maternal administration of a CCR2 antagonist and to be evident not only in the embryo but also in adolescent offspring (Chang et al., 2015, 2018, 2019a). Together, this evidence suggests that the endogenous CCL2/CCR2 system in radial glia of the embryo is necessary for, and a strong mediator of, a CCL2- or ethanol-induced increase in cell proliferation in the NEP and the migration through the mHYP of neurons that terminate in the LH, effects that persist into adolescence.
Along with the increased expression and density of CCL2 cells in the LH, our study demonstrates that ICV injection of CCL2 in the embryo at E14 has a stimulatory effect on the neuropeptide MCH, increasing its expression and density of MCH neurons in the LH. These MCH-expressing neurons, also stimulated in embryos and adolescent offspring by maternal peripheral administration of CCL2 or ethanol, are clustered in the middle part of the LH where the CCL2 cells are dense and up to 90% of MCH neurons are found to colocalize CCL2 as well as CCR2 (Chang et al., 2015, 2018, 2019a). These results are consistent with in vitro reports, showing CCL2 to increase the expression and density of other peptide-expressing neurons (Poon et al., 2014) and stimulate the differentiation of neurons (Edman et al., 2008) and also to have lasting impact on the excitability and function of surrounding proinflammatory mediators (Cerri et al., 2016). Together with this evidence, the close relationship between CCL2 and MCH in LH neurons suggests a specific mechanism through which this neuroimmune system acts intracellularly to stimulate and contribute to changes in development of specific peptide-expressing neurons induced by challenges such as ethanol that stimulate CCL2. After initial priming by embryonic ethanol exposure, this mechanism linking CCL2 to MCH appears to be dynamic in nature without further exposure, with the percent of MCH neurons colocalizing CCL2 that are very low in E19 embryo when cells are relatively sparse markedly increased a few days later to approximately 40% in the neonate and to almost 90% in adolescent offspring (Chang et al., 2015, 2018). The additional finding, that ICV injection of CCL2 stimulates MCH mRNA in the NEP as well as the LH, supports the possibility that MCH neurons in the LH originate from the NEP, as previously suggested (Chang et al., 2019b). With knock-down of a similar pro-inflammatory chemokine, CXCL12, shown to disrupt radial glia migratory scaffolds and ultimately alter migratory patterns (Abe et al., 2015; Ding et al., 2015; Zhu et al., 2015), we propose that CCL2 by acting within radial glia cells in the NEP and their processes in the mHYP promotes the genesis and migration specifically of MCH neurons to the LH. This is substantiated by evidence that maternal ethanol administration which stimulates CCL2 increases the number of MCH neurons within the NEP, where the radial glia cells colocalize CCL2, and also within the mHYP, where the MCH neurons are positioned along the CCL2-upregulated processes of the radial glia that appear to be guiding the MCH neurons into the LH (Chang et al., 2019b).
An important finding of the present study is that the effects of ICV CCL2 injection examined in both female and male embryos are sexually dimorphic. As shown with maternal peripheral administration of CCL2 or ethanol (Chang et al., 2018; 2019a, 2019b), the stimulatory effects of ICV CCL2 injection on radial glia in the NEP and MCH neurons in the LH are consistently stronger in female embryos, although also evident in males. Of particular note is that the positive, auto-inducing effects of ICV CCL2 injection on the hypothalamic CCL2/CCR2 system, both the expression of CCL2 and CCR2 and the density of CCL2 cells in the NEP and LH, occurs only in female embryos, with no such effects detected in male embryos. This dramatic sex difference evident in the embryonic NEP and LH is consistent with two other studies in the embryo which measured CCL2 cell density in the LH (Chang et al., 2019a) and CCL2 gene expression in the cortex and hippocampus (Terasaki and Schwarz, 2016). While these sex differences in response to CCL2 or ethanol likely reflect the effects of sex hormones and enzymes known to stimulate neurogenesis (Pellegrini et al., 2016; Brocca and Garcia-Segura, 2019), specific evidence that male testes are active in the embryo while female ovaries are quiescent (McCarthy et al., 2018), leading to high intracerebral levels of estrogen in male embryonic brain (Bakker et al., 2006), suggests that the neuroprotective effects of this steroid (Chakrabarti et al., 2016; McEwen and Milner, 2017) may serve to diminish the auto-inducing effects of CCL2 in males. While there is limited evidence suggesting sexual dimorphism in neuroimmune systems that affect neuronal differentiation (Alfonso-Loeches et al., 2013; Terasaki and Schwarz, 2016; Pascual et al., 2017), these results with central administration of CCL2 similar to peripheral CCL2 or ethanol focus attention specifically on the hypothalamic CCL2/CCR2 system in the NEP as a key mechanism contributing to the sexual dimorphic, stimulatory effects on CCL2-labeled radial glia and also on MCH neurons. With males still exhibiting a small increase in MCH despite no effect on embryonic CCL2/CCR2 system in the NEP, additional factors are likely to be involved in the increased density of MCH neurons. These include other neuroimmune systems known to be stimulated by ethanol (Crews et al., 2015) and other signals that contribute to the greater responsiveness in females of MCH neurons to metabolic challenges (Fukushima et al., 2015).
These results obtained with direct manipulations of embryonic CCL2/CCR2 systems further advance our understanding of the neuroimmune mechanisms in the embryonic brain and how they mediate effects on neuronal development produced by maternal challenges such as ethanol. This mechanism involves the production and dispersion of endogenous CCL2, which through CCR2 acts within neuroepithelial radial glia cells to promote neural differentiation and migration toward LH of MCH neurons that colocalize CCL2 and CCR2. This neuroimmune system in embryos, found to be strongly stimulated in females while unresponsive in males, likely mediates the sexually dimorphic effects of CCL2 and ethanol on MCH neurons and possibly contributes to the higher levels of adolescent risk factors for alcohol use disorders described in women (Foster et al., 2015; Peltier et al., 2019).
Highlights:
Intracerebroventricular (ICV) injection in utero of CCL2 has similar effects on embryo as maternal CCL2 administration.
ICV CCL2 stimulates endogenous CCL2 in hypothalamic neuroepithelial radial glia progenitor cells.
These autoregulatory effects are accompanied by increased CCL2 and MCH neurons in lateral hypothalamus that colocalize CCL2.
These effects are sexually dimorphic, with neuroepithelial CCL2/CCR2 system auto-regulated in female but not male embryos.
These results support involvement of embryonic CCL2/CCR2 system in mediating effects of maternal challenges such as ethanol.
ACKNOWLEDGEMENTS
This research was supported by National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Number R01AA024798. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors would like to thank Ms. Gazal Gulati for her assistance with the literature, references and figures. We also extend gratitude to The Rockefeller University’s Bio-Imaging Resource Center and Translational Technology Core Laboratory for the use of their equipment.
Abbreviations
- CCL2
Chemokine C-C motif ligand 2
- CCR2
CCL2 receptor
- MCH
Melanin-concentrating hormone
- ICV
intracerebroventricular injection
- LH
Lateral hypothalamus
- E10, E14, E19
Embryonic day 10, embryonic day 14, embryonic day 19
- qRT-PCR
Quantitative real-time polymerase chain reaction
- NEP
Neuroepithelium
- mHYP
Medial hypothalamus
- IF
Immunofluorescence histochemistry
- BLBP
Brain lipid-binding protein
- Iba-1
Microglial marker
- CXCL12
C-X-C motif chemokine 12
Footnotes
Declaration of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abe P, Molnar Z, Tzeng YS, Lai DM, Arnold SJ, Stumm R (2015) Intermediate progenitors facilitate intracortical progression of thalamocortical axons and interneurons through cxcl12 chemokine signaling. J Neurosci 35:13053–13063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi N, Suzuki S, Matsuoka H, Fushimi S, Ono J, Ohta KI, Hirai Y, Miki T, Koshimizu H (2018) Corticotropin-releasing hormone-binding protein is up-regulated by brain-derived neurotrophic factor and is secreted in an activity-dependent manner in rat cerebral cortical neurons. J Neurochem 146:99–110 [DOI] [PubMed] [Google Scholar]
- Akhtar F, Rouse CA, Catano G, Montalvo M, Ullevig SL, Asmis R, Kharbanda K, Maffi SK (2017) Acute maternal oxidant exposure causes susceptibility of the fetal brain to inflammation and oxidative stress. J Neuroinflammation 14:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual M, Guerri C (2013) Gender differences in alcohol-induced neurotoxicity and brain damage. Toxicology 311:27–34 [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA (1995) Atlas of prenatal rat brain development: CRC press [Google Scholar]
- Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C (2006) Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci 9:220–226 [DOI] [PubMed] [Google Scholar]
- Barry DS, Pakan JM, McDermott KW (2014) Radial glial cells: Key organisers in cns development. Int J Biochem Cell Biol 46:76–79 [DOI] [PubMed] [Google Scholar]
- Barson JR, Fagan SE, Chang GQ, Leibowitz SF (2013) Neurochemical heterogeneity of rats predicted by different measures to be high ethanol consumers. Alcohol Clin Exp Res 37:141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedont JL, Newman EA, Blackshaw S (2015) Patterning, specification, and differentiation in the developing hypothalamus. Wiley Interdiscip Rev Dev Biol 4:445–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Bergeson SE, Walker D, Ferreira VM, Kuziel WA, Harris RA (2005) Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behav Brain Res 165:110–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischoux F, Fellmann D, Risold PY (2001) Ontogenetic development of the diencephalic mch neurons: A hypothalamic ‘mch area’ hypothesis. Eur J Neurosci 13:1733–1744 [DOI] [PubMed] [Google Scholar]
- Brocca ME, Garcia-Segura LM (2019) Non-reproductive functions of aromatase in the central nervous system under physiological and pathological conditions. Cell Mol Neurobiol 39:473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho J, Jones K, Miller E, Ariza J, Noctor S, de Water JV, Martinez-Cerdeno V (2014) Embryonic intraventricular exposure to autism-specific maternal autoantibodies produces alterations in autistic-like stereotypical behaviors in offspring mice. Behav Brain Res 266:46–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarillo C, Miranda RC (2008) Ethanol exposure during neurogenesis induces persistent effects on neural maturation: Evidence from an ex vivo model of fetal cerebral cortical neuroepithelial progenitor maturation. Gene Expr 14:159–171 [PMC free article] [PubMed] [Google Scholar]
- Cazareth J, Guyon A, Heurteaux C, Chabry J, Petit-Paitel A (2014) Molecular and cellular neuroinflammatory status of mouse brain after systemic lipopolysaccharide challenge: Importance of ccr2/ccl2 signaling. J Neuroinflammation 11:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerri C, Genovesi S, Allegra M, Pistillo F, Puntener U, Guglielmotti A, Perry VH, Bozzi Y, Caleo M (2016) The chemokine ccl2 mediates the seizure-enhancing effects of systemic inflammation. J Neurosci 36:3777–3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti M, Das A, Samantaray S, Smith JA, Banik NL, Haque A, Ray SK (2016) Molecular mechanisms of estrogen for neuroprotection in spinal cord injury and traumatic brain injury. Rev Neurosci 27:271–281 [DOI] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Leibowitz SF (2013) Prenatal exposure to nicotine stimulates neurogenesis of orexigenic peptide-expressing neurons in hypothalamus and amygdala. J Neurosci 33:13600–13611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Leibowitz SF (2015) Prenatal exposure to ethanol stimulates hypothalamic ccr2 chemokine receptor system: Possible relation to increased density of orexigenic peptide neurons and ethanol drinking in adolescent offspring. Neuroscience 310:163–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Devi Sai Sri Kavya B, Leibowitz SF (2019a) Ccl2/ccr2 chemokine system in embryonic hypothalamus: Involvement in sexually dimorphic stimulatory effects of prenatal ethanol exposure on peptide-expressing neurons. Neuroscience [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Boorgu D, Leibowitz SF (2019b) Radial glia progenitor cells and ccl2/ccr2 system in embryonic neuroepithelium: Involvement in sexually dimorphic, stimulatory effects of maternal ethanol administration on development of hypothalamic melanin-concentrating hormone neurons in offspring. Journal of Neuroinflammation [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Liang SC, Barson JR, Leibowitz SF (2012) Prenatal ethanol exposure stimulates neurogenesis in hypothalamic and limbic peptide systems: Possible mechanism for offspring ethanol overconsumption. Neuroscience 222:417–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Halkina V, Edelstien J, Ramirez E, Leibowitz SF (2018) Hypothalamic ccl2/ccr2 chemokine system: Role in sexually dimorphic effects of maternal ethanol exposure on melanin-concentrating hormone and behavior in adolescent offspring. J Neurosci 38:9072–9090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP (2014) Neuroimmune basis of alcoholic brain damage. Int Rev Neurobiol 118:315–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Sarkar DK, Qin L, Zou J, Boyadjieva N, Vetreno RP (2015) Neuroimmune function and the consequences of alcohol exposure. Alcohol Res 37:331–341, 344–351 [PMC free article] [PubMed] [Google Scholar]
- Cui C, Shurtleff D, Harris RA (2014) Neuroimmune mechanisms of alcohol and drug addiction. Int Rev Neurobiol 118:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Jin GH, Zou LQ, Zhang XQ, Li HM, Tao XL, Zhang XH, Qin JB, Tian ML (2015) Stromal derived factor-1alpha in hippocampus radial glial cells in vitro regulates the migration of neural progenitor cells. Cell Biol Int 39:750–758 [DOI] [PubMed] [Google Scholar]
- Duncan EA, Proulx K, Woods SC (2005) Central administration of melanin-concentrating hormone increases alcohol and sucrose/quinine intake in rats. Alcohol Clin Exp Res 29:958–964 [DOI] [PubMed] [Google Scholar]
- Edman LC, Mira H, Arenas E (2008) The beta-chemokines ccl2 and ccl7 are two novel differentiation factors for midbrain dopaminergic precursors and neurons. Exp Cell Res 314:2123–2130 [DOI] [PubMed] [Google Scholar]
- Fang CZ, Yang YJ, Wang QH, Yao Y, Zhang XY, He XH (2013) Intraventricular injection of human dental pulp stem cells improves hypoxic-ischemic brain damage in neonatal rats. PLoS One 8:e66748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete CD, Goz RU, Dinallo S, Miralles CP, Chiou TT, Bear J Jr., Fiondella CG, LoTurco JJ, De Blas AL (2017) In vivo transgenic expression of collybistin in neurons of the rat cerebral cortex. J Comp Neurol 525:1291–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Bastias O, Karahanian E (2018) Neuroinflammation produced by heavy alcohol intake is due to loops of interactions between toll-like 4 and tnf receptors, peroxisome proliferator-activated receptors and the central melanocortin system: A novel hypothesis and new therapeutic avenues. Neuropharmacology 128:401–407 [DOI] [PubMed] [Google Scholar]
- Foster KT, Hicks BM, Iacono WG, McGue M (2015) Gender differences in the structure of risk for alcohol use disorder in adolescence and young adulthood. Psychol Med 45:3047–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco SJ, Gil-Sanz C, Martinez-Garay I, Espinosa A, Harkins-Perry SR, Ramos C, Muller U (2012) Fate-restricted neural progenitors in the mammalian cerebral cortex. Science 337:746–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima A, Hagiwara H, Fujioka H, Kimura F, Akema T, Funabashi T (2015) Sex differences in feeding behavior in rats: The relationship with neuronal activation in the hypothalamus. Frontiers in neuroscience 9:88–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XB (2009) Electrophysiological effects of mch on neurons in the hypothalamus. Peptides 30:2025–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad-Tovolli R, Szabo NE, Zhou X, Alvarez-Bolado G (2013) Genetic manipulation of the mouse developing hypothalamus through in utero electroporation. J Vis Exp [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris Q, Seto J, O’Brien K, Lee PS, Kondo C, Heard BJ, Hart DA, Krawetz RJ (2013) Monocyte chemotactic protein-1 inhibits chondrogenesis of synovial mesenchymal progenitor cells: An in vitro study. Stem Cells 31:2253–2265 [DOI] [PubMed] [Google Scholar]
- Ifft JD (1972) An autoradiographic study of the time of final division of neurons in rat hypothalamic nuclei. J Comp Neurol 144:193–204 [DOI] [PubMed] [Google Scholar]
- June HL, Liu J, Warnock KT, Bell KA, Balan I, Bollino D, Puche A, Aurelian L (2015) Crf-amplified neuronal tlr4/mcp-1 signaling regulates alcohol self-administration. Neuropsychopharmacology 40:1549–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JS, Song DK, Suh HW, Kim YH (1994) Effects of intraventricular injection of morphine and betaendorphin on serotonin release from the spinal cord in rats. Pharmacol Biochem Behav 49:1037–1042 [DOI] [PubMed] [Google Scholar]
- Karlsson C, Rehman F, Damadzic R, Atkins AL, Schank JR, Gehlert DR, Steensland P, Thorsell A, Heilig M (2016) The melanin-concentrating hormone-1 receptor modulates alcohol-induced reward and darpp-32 phosphorylation. Psychopharmacology (Berl) 233:2355–2363 [DOI] [PubMed] [Google Scholar]
- Knerlich-Lukoschus F, von der Ropp-Brenner B, Lucius R, Mehdorn HM, Held-Feindt J (2010) Chemokine expression in the white matter spinal cord precursor niche after force-defined spinal cord contusion injuries in adult rats. Glia 58:916–931 [DOI] [PubMed] [Google Scholar]
- LaMonica BE, Lui JH, Hansen DV, Kriegstein AR (2013) Mitotic spindle orientation predicts outer radial glial cell generation in human neocortex. Nat Commun 4:1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinski A, Konopacki J, Pawlikowski M, Lewinska MK, Smith NK, Reiter RJ (1984) Effects of intraventricular injections of 6-hydroxydopamine on anterior pituitary cell proliferation. Anat Rec 208:421–426 [DOI] [PubMed] [Google Scholar]
- Li P, Chaudhary N, Gemmete JJ, Thompson BG, Hua Y, Xi G, Pandey AS (2016) Intraventricular injection of noncellular cerebrospinal fluid from subarachnoid hemorrhage patient into rat ventricles leads to ventricular enlargement and periventricular injury. Acta Neurochir Suppl 121:331–334 [DOI] [PubMed] [Google Scholar]
- Lippai D, Bala S, Csak T, Kurt-Jones EA, Szabo G (2013) Chronic alcohol-induced microrna-155 contributes to neuroinflammation in a tlr4-dependent manner in mice. PLoS One 8:e70945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Zhang ZG, Zhang RL, Gregg SR, Wang L, Yier T, Chopp M (2007) Chemokine ligand 2 (ccl2) induces migration and differentiation of subventricular zone cells after stroke. J Neurosci Res 85:2120–2125 [DOI] [PubMed] [Google Scholar]
- Lu B, Zhou Y, Su Z, Yan A, Ding P (2017) Effect of ccl2 sirna on proliferation and apoptosis in the u251 human glioma cell line. Mol Med Rep 16:3387–3394 [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Herold K, Stockman SL (2018) Fast, furious and enduring: Sensitive versus critical periods in sexual differentiation of the brain. Physiol Behav 187:13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Milner TA (2017) Understanding the broad influence of sex hormones and sex differences in the brain. J Neurosci Res 95:24–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney SM, Miller MW (2010) Prenatal exposure to ethanol affects postnatal neurogenesis in thalamus. Exp Neurol 223:566–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganstern I, Chang GQ, Karatayev O, Leibowitz SF (2010) Increased orexin and melanin-concentrating hormone expression in the perifornical lateral hypothalamus of rats prone to overconsuming a fat-rich diet. Pharmacol Biochem Behav 96:413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Kriegstein AR (2007) Neural stem and progenitor cells in cortical development. Novartis Found Symp 288:59–98 [PubMed] [Google Scholar]
- Pascual M, Montesinos J, Montagud-Romero S, Forteza J, Rodriguez-Arias M, Minarro J, Guerri C (2017) Tlr4 response mediates ethanol-induced neurodevelopment alterations in a model of fetal alcohol spectrum disorders. J Neuroinflammation 14:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini E, Diotel N, Vaillant-Capitaine C, Perez Maria R, Gueguen MM, Nasri A, Cano Nicolau J, Kah O (2016) Steroid modulation of neurogenesis: Focus on radial glial cells in zebrafish. J Steroid Biochem Mol Biol 160:27–36 [DOI] [PubMed] [Google Scholar]
- Peltier MR, Verplaetse TL, Mineur YS, Petrakis IL, Cosgrove KP, Picciotto MR, McKee SA (2019) Sex differences in stress-related alcohol use. Neurobiol Stress 10:100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon K, Ho HT, Barson JR, Leibowitz SF (2014) Stimulatory role of the chemokine ccl2 in the migration and peptide expression of embryonic hypothalamic neurons. J Neurochem [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z, Wang X, Yang F, Xu M, Frank JA, Wang H, Wang S, Ke ZJ, Luo J (2017) Ethanol-induced damage to the developing spinal cord: The involvement of ccr2 signaling. Biochim Biophys Acta 1863:2746–2761 [DOI] [PubMed] [Google Scholar]
- Rosin JM, Kurrasch DM (2018) In utero electroporation induces cell death and alters embryonic microglia morphology and expression signatures in the developing hypothalamus. J Neuroinflammation 15:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai N, Wada T, Furuichi K, Shimizu K, Kokubo S, Hara A, Yamahana J, Okumura T, Matsushima K, Yokoyama H (2006) Mcp-1/ccr2-dependent loop for fibrogenesis in human peripheral cd14-positive monocytes. Journal of leukocyte biology 79:555–563 [DOI] [PubMed] [Google Scholar]
- Sancho-Tello M, Valles S, Montoliu C, Renau-Piqueras J, Guerri C (1995) Developmental pattern of gfap and vimentin gene expression in rat brain and in radial glial cultures. Glia 15:157–166 [DOI] [PubMed] [Google Scholar]
- Skorput AG, Yeh HH (2015) Effects of ethanol exposure in utero on cajal-retzius cells in the developing cortex. Alcohol Clin Exp Res 39:853–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipursky J, Francis D, Dezonne RS, Bergamo de Araujo AP, Souza L, Moraes CA, Alcantara Gomes FC (2014) Tgf-beta1 promotes cerebral cortex radial glia-astrocyte differentiation in vivo. Front Cell Neurosci 8:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M, Nasu M, Kanazawa T, Takahashi M, Shimazu Y (2018) Chemokine ligand 2/chemokine receptor 2 signaling in the trigeminal ganglia contributes to inflammatory hyperalgesia in rats. Neurosci Res 128:25–32 [DOI] [PubMed] [Google Scholar]
- Taverna E, Gotz M, Huttner WB (2014) The cell biology of neurogenesis: Toward an understanding of the development and evolution of the neocortex. Annu Rev Cell Dev Biol 30:465–502 [DOI] [PubMed] [Google Scholar]
- Terasaki LS, Schwarz JM (2016) Effects of moderate prenatal alcohol exposure during early gestation in rats on inflammation across the maternal-fetal-immune interface and later-life immune function in the offspring. Journal of Neuroimmune Pharmacology 11:680–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turbic A, Leong SY, Turnley AM (2011) Chemokines and inflammatory mediators interact to regulate adult murine neural precursor cell proliferation, survival and differentiation. PLoS One 6:e25406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta JP, Gonzales RA (2016) Chronic intracerebroventricular infusion of monocyte chemoattractant protein-1 leads to a persistent increase in sweetened ethanol consumption during operant self-administration but does not influence sucrose consumption in long-evans rats. Alcohol Clin Exp Res 40:187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gassen KL, Netzeband JG, de Graan PN, Gruol DL (2005) The chemokine ccl2 modulates ca2+ dynamics and electrophysiological properties of cultured cerebellar purkinje neurons. Eur J Neurosci 21:2949–2957 [DOI] [PubMed] [Google Scholar]
- Vomund S, Sapir T, Reiner O, Silva MA, Korth C (2013) Generation of topically transgenic rats by in utero electroporation and in vivo bioluminescence screening. J Vis Exp:e50146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walantus W, Castaneda D, Elias L, Kriegstein A (2007) In utero intraventricular injection and electroporation of e15 mouse embryos. J Vis Exp:239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West NR et al. (2017) Oncostatin m drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat Med 23:579–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Luo J (2019) Role of mcp-1 and ccr2 in alcohol neurotoxicity. Pharmacol Res 139:360–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Matsumoto T, Nagasawa T, Mackay F, Murakami F (2015) Chemokine signaling controls integrity of radial glial scaffold in developing spinal cord and consequential proper position of boundary cap cells. J Neurosci 35:9211–9224 [DOI] [PMC free article] [PubMed] [Google Scholar]