Abstract
Background
In the past, most researchers paid more attention to the biological activity of tea infusion and tea polyphenols; however, the prebiotic role of tea leaf powder is still unknown. Green tea leaf powder is rich in dietary fiber and is suggested to be beneficial for human health. Only limited studies have looked at the effects of tea leaf powder (which mainly contains tea dietary fiber) on gut microbiota and health.
Objective
The purpose of our study was to determine the effects of green tea leaf powder in preventing hyperlipidemia and to understand its potential lipid-lowering mechanism.
Design
Mice in three treatment groups were fed high-fat diets (HFDs) by administering either 0.5, 1.0, or 2.0 g/kg•d dietary fiber-enriched green tea leaf powder of low, medium, or high, respectively, for 12 weeks. Serum biochemical analyses and mRNA gene expression levels of related energy and lipid metabolism biomarkers from the liver were investigated. In addition, 16S rRNA cecal microbiota and fecal short chain fatty acids (SCFAs) were tested.
Results
Green tea leaf powder reduced body weight and total cholesterol of HFD-fed mice in a dose-dependent manner. Green tea leaf powder also increased satiety hormone secretion and reduced systemic inflammation of HFD-fed mice. Real-time polymerase chain reaction (PCR) analyses reconfirmed that green tea leaf powder prevented dyslipidemia by enhancing hepatic mRNA expression levels of peroxisome proliferator-activated receptor alpha, cholesterol 7α-hydroxylase, and Adenosine triphosphate (ATP)-binding cassette transporter A1 and decreasing the expression of fatty acid synthase, sterol regulatory element-binding protein 1c, and liver X receptor. Green tea leaf powder promoted the growth of Blautia, Oscillibacter, Ruminiclostridium, Alloprevotella, and Butyrivibrio and inhibited the growth of Erysipelatoclostridium, Desulfovibrio, and Candidatus_Saccharimonas in the cecum of HFD-fed mice.
Conclusion
In summary, our results indicate that green tea leaf powder improves lipid metabolism of HFD-fed mice in a dose-dependent manner. The potential mechanism involves a synergistic role in reprogramming gut microbiota, increasing satiety hormone secretion, and reducing systemic inflammation.
Keywords: green tea leaf powder, dyslipidemia, gut microbiota, satiety, inflammation
Popular scientific summary
Green tea leaf powder improves lipid metabolism of high-fat diet-fed mice in a dose-dependent manner.
The potential mechanism involves a synergistic role in reprogramming gut microbiota, increasing satiety hormone secretion, and reducing systemic inflammation.
Consuming a high-fat diet (HFD) over a prolonged period seriously affects human health and leads to metabolic disorders and chronic diseases. In particular, excessive fat intake increases the risk of dyslipidemia, which is the primary reason for cardiovascular disease around the world. As an alternative to pharmacological medicine, dietary intervention such as prebiotics has been used to prevent or mitigate chronic metabolic disease. Increasing evidence supports that consuming foods rich in dietary fiber can reduce the incidence of metabolic disease (1). The recommended daily intakes of dietary fiber are 25 and 38 g for adult women and men, respectively (2). However, dietary fiber intake for most of the people across the world does not meet this recommendation.
Considerable research has shown that dietary fiber improves health. Its intake is known to increase satiety, affect energy intake, reduce weight, and control blood glucose and lipid metabolism (3). The interaction between dietary fiber and gut microbiota can have a major impact on host metabolism. Since dietary fiber cannot be digested by human enzymes, it directly reaches the large intestine. Dietary fiber can promote beneficial microbiota growth and inhibit harmful microbiota development, which help maintain the balance of intestinal flora. The major products of microbial fermentation with dietary fiber are SCFAs, including acetate, propionate, and butyrate. SCFAs serve as an energy resource for colonocytes and can also activate G-protein-coupled-receptors, which contribute to the modulation of host energy metabolism (4). In addition, SCFAs are known as histone deacetylase inhibitors, which result in anti-inflammatory effects (4).
Tea is one of the three most widely consumed beverages in the world and has high nutritional value and health function. Tea contains several nutritional and functional ingredients, including polyphenols and dietary fiber. In the past, most researchers have paid more attention to the biological activity of tea infusion and tea polyphenols; however, the prebiotic role of tea leaf powder, which is rich in dietary fiber, is still unknown. The traditional way of drinking tea results in almost all the dietary fiber being discarded as waste residue. Few studies have paid attention to the effects of tea leaf powder, which mainly includes tea dietary fiber, on gut microbiota and health. A previous article showed that tea dietary fiber could improve serum and hepatic lipid metabolism; however, whether the lipid-lowering effects were due to the modulation of gut microbiota, was not studied (5). Another article has also shown that green tea leaf powder significantly reduced the body fat content, hepatic triacylglycerol, and cholesterol accumulation in high-fat fed C57BL/6J mice (6). In addition, these effects of improving lipid metabolism are due to the increase of Akkermansia amounts in the small intestine using viable count, T-RFLP, and qPCR. However, high percentage of the gut microbiota has not been cultured. In addition, Terminal restriction fragment length polymorphism (T-RFLP) and qPCR require fluorescently labeled primer, have been proven to have low sensitivity, and being capable of detecting only abundant microbial taxa (>1%). Therefore, results of green tea leaf powder on total bacterial composition and diversity were lacking in HFD mice model. Green tea leaf powder is increasingly included as supplementary ingredients in foods, such as special drinks, biscuits, bread, desserts, etc. The comprehensive utilization of tea leaf powder can greatly improve the value of the tea industry, which is conducive to the development of the food industry and agriculture.
The purpose of our study was to determine the effects of green tea leaf powder in the prevention of hyperlipidemia and to understand its potential lipid-lowering mechanism. Our results suggest that green tea leaf powder could prevent HFD-induced dyslipidemia synergistically by modulating gut microbiota, increasing satiety hormone secretion, and reducing inflammation.
Materials and methods
Preparation of green tea leaf powder and analyses of its chemical composition and physiochemical properties
Green tea leaf powder was manufactured from green tea using hot water extraction, dehydration, and drying. Green tea raw materials were mixed in 90°C water for 30 min. The mixture was then dehydrated using a screw extrusion machine for 60 sec with a pressure of 0.5–1.2 MPa. Drying temperature was 100–130°C for 3–5 h. Next, the dehydrated green tea leaf was ground to powder with particles size 80–1,500 mesh and stored at 4°C prior to analysis.
Water, ash, protein, soluble, insoluble, and total fiber contents, and physicochemical properties including water-holding, swelling, and oil-holding capacity were determined as described previously (7). Cellulose, hemicellulose, lignin, and pectin were also determined as described previously (8). Tea polyphenol was determined according to GB/T 31740.2-2015.
Animal experiments
All animal experiments were approved by the Institutional Animal Care and Use Committee of Nankai University. Eight-week-old male C57BL/6J mice were obtained from SPF Biotechnology Co., Ltd (Beijing, China) and housed at a constant temperature of 25°C and relative humidity of 50% in 12 h light–dark cycles. The mice had free access to water and food. After the acclimatization period of 1 week, the mice were randomly divided into five groups (seven mice per group). Negative control (NC) group were fed with a normal diet (AIN-93). Model control (MC) group were fed with an HFD (78.8% basal feed, 1% cholesterol, 10% yolk powder, 10% lard, and 0.2% cholate). The other three groups were fed with an HFD, but were also administered either 0.5 g/kg•d dietary fiber-enriched green tea leaf powder-low (DFL),1.0 g/kg•d dietary fiber-enriched green tea leaf powder-medium (DFM), or 2.0 g/kg•d dietary fiber-enriched green tea leaf powder-high (DFH) for 12 weeks by intragastric gavage. Body weight and food intake were assessed once a week. Feces of mice were collected before the end of the experiment for measuring SCFAs. SCFAs in feces were determined as previously described (9). Mice were sacrificed by anesthesia with somnopentyl after 12 h fasting. Blood, liver, and cecum contents were collected. Serum was centrifuged at 4,200 rpm for 15 min and stored at −20°C. The collected samples were immediately frozen in liquid nitrogen and stored at −80°C for further research.
Serum biochemical analysis
The levels of total triglyceride (TG), total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), and low-density lipoprotein-cholesterol (LDL-C) were determined using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Glucose and insulin concentrations were also determined using commercial kits (BioSino Biotechnology and Science Incorporation, Beijing, China).
Peptide YY (PYY) and glucagon-like peptide-1 (GLP-1) levels were determined as previously described (10). Leptin and adiponectin levels were determined by using ELISA kits (Bio-Swamp Life Science, Wuhan, China). Tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and lipopolysaccharides (LPS) were detected using commercial kits (Sinouk Institute of Biological Technology, Beijing, China).
Real-time quantitative PCR
Total RNA was extracted from the liver using RNeasy Mini Kit (Qiagen, Hilden, Germany) and quantified using a Nanophotometer (Implen, Germany) and a agarose gel electrophoresis. cDNA was synthesized using LunaScriptTM SuperMix Kit (NEB, MA, USA). Real-time qPCR was performed using the CFX Connect Real-Time System (BIO-RAD, USA). The primers used are listed in Table 1. The PCR procedure was as follows: initial denaturation at 95°C for 60 sec, 40 cycles at 95°C for 15 sec, and 60°C for 30 sec. Each gene expression was normalized to the reference β-actin gene.
Table 1.
Primer sequences used for real-time qPCR
| Gene | 5’ Primer | 3’ Primer |
|---|---|---|
| PPARα | TGCAGCCTCAGCCAAGTTGAA | TCCCGAACTTGACCAGCCA |
| SREBP1c | CTGGTGAGTGGAGGGACCAT | GACCGGTAGCGCTTCTCAAT |
| FAS | GAGGGTGTGCCATTCTGTCA | GCTATTCTCTACCGCTGGGG |
| LXR | AATGAAGCTGGTGAGCCTCC | CCATGTGGCCAACACAAAGG |
| CYP7A1 | CATAGCCAACTTGCCGCAG | AGACAAAGCACTTGCCCTTC |
| ABCA1 | TCCGTTGGCTTTCTCAGTCC | TTTGTTGTTGTTTTGTGGCCT |
| TLR4 | GGAAGACAAAAGAAAGACAGCCC | TGGGGAGATTCTTGATCTGCT |
| β-Actin | ACAGCAGTTGGTTGGAGCAA | ACGCGACCATCCTCCTCTTA |
PPARα, peroxisome proliferator-activated receptor alpha; SREBP1c, sterol regulatory element-binding protein 1c; FAS, fatty acid synthase; LXR, liver X receptor; CYP7A1, cholesterol 7α-hydroxylase; ABCA1, ATP-binding cassette transporter A1; TLR4, toll-like receptor 4.
Cecal microbiota analyses using 16S rDNA gene sequencing
Total genomic DNA, of the contents of the cecum, was extracted using QIAamp DNA Stool Minikit (Qiagen, Hilden, Germany). V3–V4 region of 16S rRNA gene was amplified using forward primer 341F (5’-CCTAYGGGRBGCASCAG-3’) and reverse primer 806R (5’-GGACTACNNGGGTATCTAAT-3’). PCR products were purified by GeneJETTM gel extraction kit (Thermo Scientific, MA, USA). Sequencing was performed on an Ion S5TM XL platform (Novogene Co. Ltd, Beijing, China).
Sequence analyses were done with Uparse software, and sequences with more than 97% similarity were assigned to an operational taxonomic unit. The Silva Database (Version 132) was used to categorize the taxonomic information (phylum, class, order, family, and genus). Alpha diversity (observed species, Chao1, Shannon, and PD_whole_tree) was analyzed. Unweighted unifrac, for principal coordinate analysis (PCoA), was used to investigate the differences between groups. Phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt) analysis was used to predict gut microbiota function, and the Kyoto encyclopedia of genes and genomes (KEGG) database was used for reference genomes. Linear discriminant analysis effect size (LEfSe) analysis was conducted to find representative microbiota between groups.
Statistics
Data are expressed as mean ± SEM. Differences with P-value < 0.05 were deemed to be significant. Analysis was performed using SPSS software.
Results
Chemical composition and physiochemical properties of green tea leaf powder
Green tea leaf powder consisted of 61.15 ± 1.24% total dietary fiber (54.62 ± 1.10% insoluble and 6.53 ± 0.19% soluble) (Table 2). Insoluble dietary fiber mainly contains 20.33 ± 1.39% cellulose, 10.91 ± 2.02% hemicellulose, and 12.77 ± 0.38% lignin. Soluble dietary fiber mainly contains 4.56 ± 0.10% pectin. In addition, green tea leaf powder exhibited higher water, oil-holding capacity, and swelling capacity.
Table 2.
Chemical composition and physiochemical properties of green tea leaf powder
| Chemical composition and physiochemical properties | Contents (average ± SD) |
|---|---|
| Chemical composition | |
| Water content | 10.91 ± 0.12% |
| Ash | 2.89 ± 0.34% |
| Protein | 20.61 ± 0.23% |
| Fat | 0.87 ± 0.04% |
| Tea polyphenol | 6.20 ± 0.07% |
| Total dietary fiber | 61.15 ± 1.24% |
| Soluble dietary fiber | 6.53 ± 0.19% |
| Insoluble dietary fiber | 54.62 ± 1.10% |
| Cellulose | 20.33 ± 1.39% |
| Hemicellulose | 10.91 ± 2.02% |
| Lignin | 12.77 ± 0.38% |
| Pectin | 4.56 ± 0.10% |
| Physiochemical properties | |
| Water-holding capacity | 3.49 ± 0.05 g/g |
| Swelling capacity | 2.53 ± 0.03 mL/g |
| Oil-holding capacity | 1.85 ± 0.21 g/g |
Effect of green tea leaf powder on body and liver weight
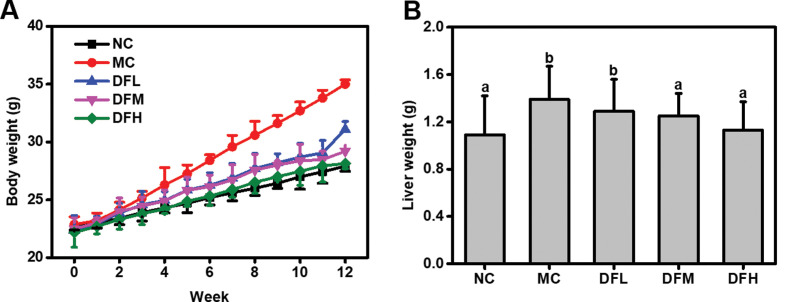
After 4 weeks, body weight of the MC group mice increased by 15.28% of the initial value, while the NC group mice only gained 6.73% (Fig. 1). The mice fed with green tea leaf powder showed a reduction in body weight compared to the MC group in a dose-dependent manner. In particular, a high dosage of green tea leaf powder was able to significantly reduce the body weight of HFD-fed mice (P < 0.05). In addition, green tea leaf powder also reduced live weight of HFD-fed mice.
Fig. 1.
Green tea leaf powder reduces the body weight (A) and liver weight (B) of HFD-fed mice. Values were measured in mice from NC, MC, DFL, DFM, and DFH groups (n = 7).
Effect of green tea leaf powder on serum lipid and glucose levels
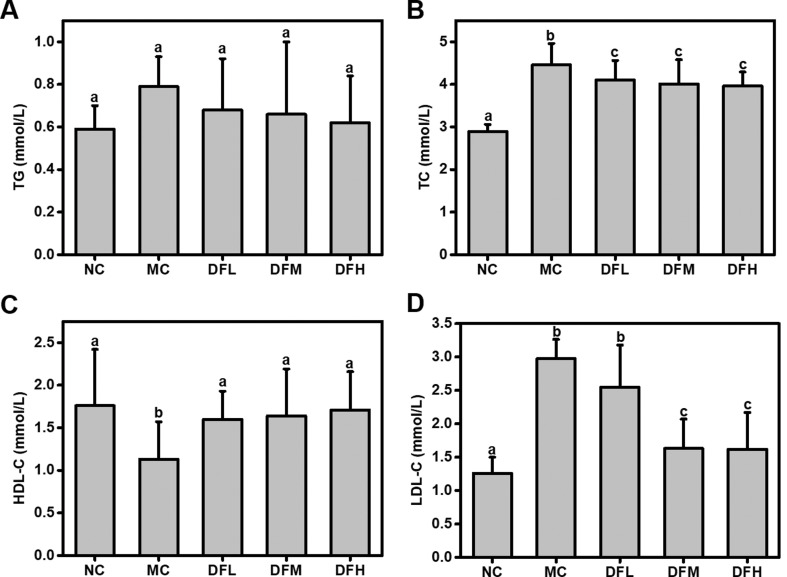
Green tea leaf powder significantly reduced TC and LDL-C levels, and significantly increased HDL-C levels in a dose-dependent manner (P < 0.05) (Fig. 2). In particular, when compared to the MC group, a high dosage of green tea leaf powder significantly increased HDL-C level (P < 0.05). We also found a reduction in TG; however, the difference was not statistically significant.
Fig. 2.
Effect of green tea leaf powder on the lipid metabolism. (A) TG, (B) TC, (C) HDL-C, and (D) LDL-C (n = 7). Significant differences (P < 0.05) are indicated using different letters (a, b, c).
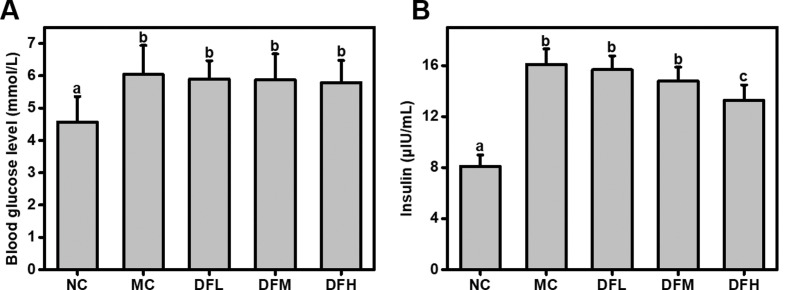
A high dosage of green tea leaf powder significantly reduced insulin levels of HFD-fed mice (P < 0.05) (Fig. 3). Green tea leaf powder also slightly reduced fasting blood glucose level; however, this difference was not statistically significant.
Fig. 3.
Effect of green tea leaf powder on glucose metabolism parameters. (A) Blood glucose levels and (B) insulin (n = 7). Significant differences (P < 0.05) are indicated using different letters (a, b, c).
Effect of green tea leaf powder on body metabolism and energy balance
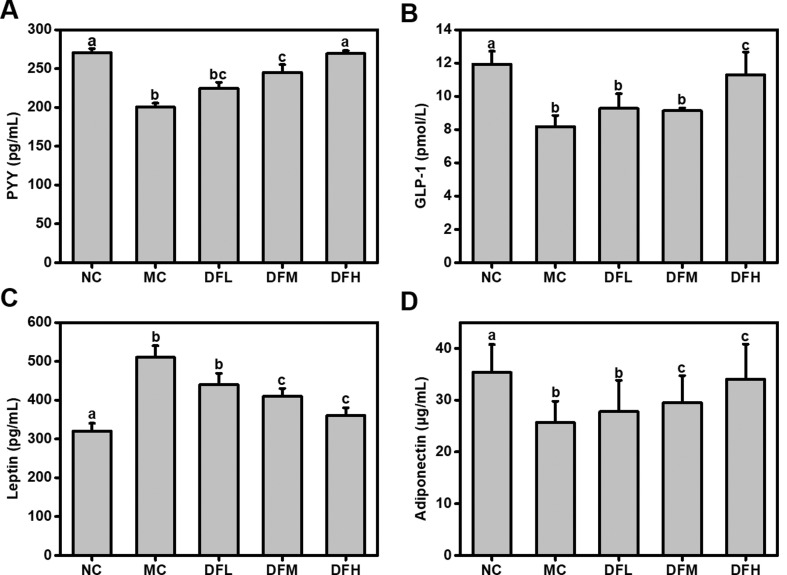
Green tea leaf powder significantly increased serum levels of the satiety hormones PYY and GLP-1 in a dose-dependent manner (Fig. 4). In particular, when compared to the MC group, a high dosage of green tea leaf powder significantly increased PYY and GLP-1 levels (P < 0.05). This shows that green tea leaf powder increased satiety hormone secretion and decreased appetite. In addition, a high dosage of green tea leaf powder could significantly increase serum adiponectin levels of HFD-fed mice (P < 0.05). Serum leptin levels were significantly increased in the MC group compared to the NC group, while the green tea leaf powder administration ameliorated this change.
Fig. 4.
Effect of green tea leaf powder on serum hormone concentrations. (A) PYY, (B) GLP-1, (C) leptin, and (D) adiponectin (n = 7). Significant differences (P < 0.05) are indicated using different letters (a, b, c).
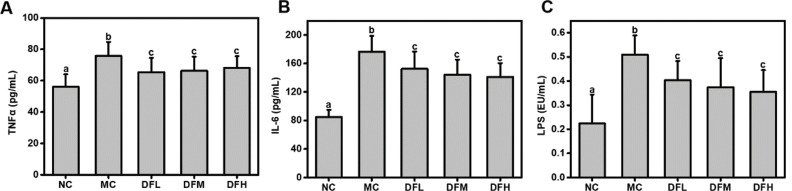
Effect of green tea leaf powder on systemic inflammation
We observed higher levels of TNF-α, IL-6, and LPS in the serum of the MC group compared to the NC group (Fig. 5). Green tea leaf powder was able to significantly reduce serum TNF-α, IL-6, and LPS levels of HFD-mice (P < 0.05). However, no significant differences in levels were observed between the groups fed on various dosages of green tea leaf powder. Thus, green tea leaf powder could reduce metabolic endotoxemia and significantly alleviated systemic inflammation.
Fig. 5.
Effect of green tea leaf powder on systemic inflammation. (A) TNF-α, (B) IL-6, and (C) LPS (n = 7). Significant differences (P < 0.05) are indicated using different letters (a, b, c).
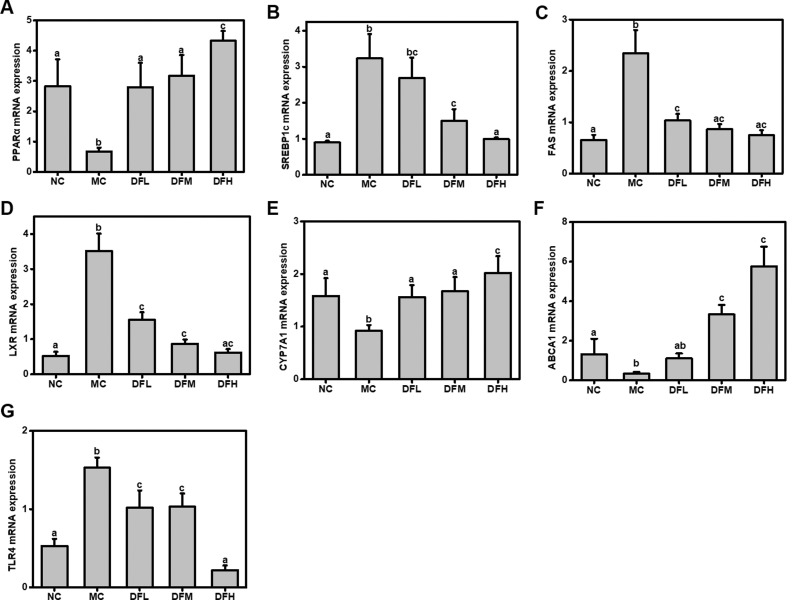
Effect of green tea leaf powder on lipid metabolism-related mRNA gene expression
Real-time PCR was performed to quantify the mRNA expression levels of genes that are biomarkers for energy and lipid metabolism. Compared to the NC group, the MC group had higher expression levels of lipid synthesis genes, including sterol regulatory element-binding protein 1c (SREBP1c), fatty acid synthase (FAS), and liver X receptor (LXR) and decreased the expression levels of PPARα, CYP7A1, and ABCA1 (Fig. 6). In contrast to the MC group, mice fed with green tea leaf powder showed significant (P < 0.05) dose-dependent increases in mRNA expression levels of PPARα, CYP7A1, and ABCA1. On the other hand, green tea leaf powder significantly suppressed mRNA expression of SREBP1c, FAS, LXR, and TLR4 (P < 0.05).
Fig. 6.
Effect of green tea leaf powder on mRNA expression levels of hepatic metabolic regulators. (A) PPARα, (B) SREBP1c, (C) FAS, (D) LXR, (E) CYP7A1, (F) ABCA1, and (G) TLR4 (n = 7). Significant differences (P < 0.05) are indicated using different letters (a, b, c).
Effect of green tea leaf powder on gut microbiota and SCFAs production
Richness and diversity analysis of gut microbiota
To assess the gut microbial community structure, richness and diversity were calculated using number of observed species, Chao1 estimator, Shannon index, and PD_whole_tree index (Table 3). With an increase in green tea leaf powder concentration, richness (observed species and estimated Chao1) was increased from 471 ± 49 and 582.7 ± 27.8 to 549 ± 44 and 679.4 ± 42.5, respectively. In contrast, the NC and MC groups had relatively low microbial community richness (observed species were 437 ± 31 and 398 ± 22, and Chao1 index was 540.3 ± 19.2 and 502.9 ± 27.0) (P < 0.05). Similarly, green tea leaf powder group had high microbial diversity (Shannon index varied from 5.2 ± 0.9 to 6.1 ± 0.7, and PD_whole_tree index between 29.6 ± 7.1 and 38.1 ± 6.9) compared to the NC and MC groups (Shannon index was 4.9 ± 0.8 and 4.4 ± 1.1, and PD_whole_tree index was 28.4 ± 6.6 and 25.3 ± 5.4) (P < 0.05).
Table 3.
Alpha diversity estimates of gut microbiota associated with mice from NC, MC, DFL, DFM, and DFH groups (n = 7)
| Groups | Observed species | Chao1 | Shannon | PD_whole_tree |
|---|---|---|---|---|
| NC | 437 ± 31 | 540.3 ± 19.2 | 4.9 ± 0.8 | 28.4 ± 6.6 |
| MC | 398 ± 22 | 502.9 ± 27.0 | 4.4 ± 1.1 | 25.3 ± 5.4 |
| DFL | 471 ± 49 | 582.7 ± 27.8 | 5.2 ± 0.9 | 29.6 ± 7.1 |
| DFM | 504 ± 37 | 614.6 ± 31.3 | 5.6 ± 0.5 | 35.9 ± 5.3 |
| DFH | 549 ± 44 | 679.4 ± 42.5 | 6.1 ± 0.7 | 38.1 ± 6.9 |
DFL, dietary fiber-enriched green tea leaf powder-low; DFM, dietary fiber-enriched green tea leaf powder-medium; DFH, dietary fiber-enriched green tea leaf powder-high; MC, model control; NC, negative control.
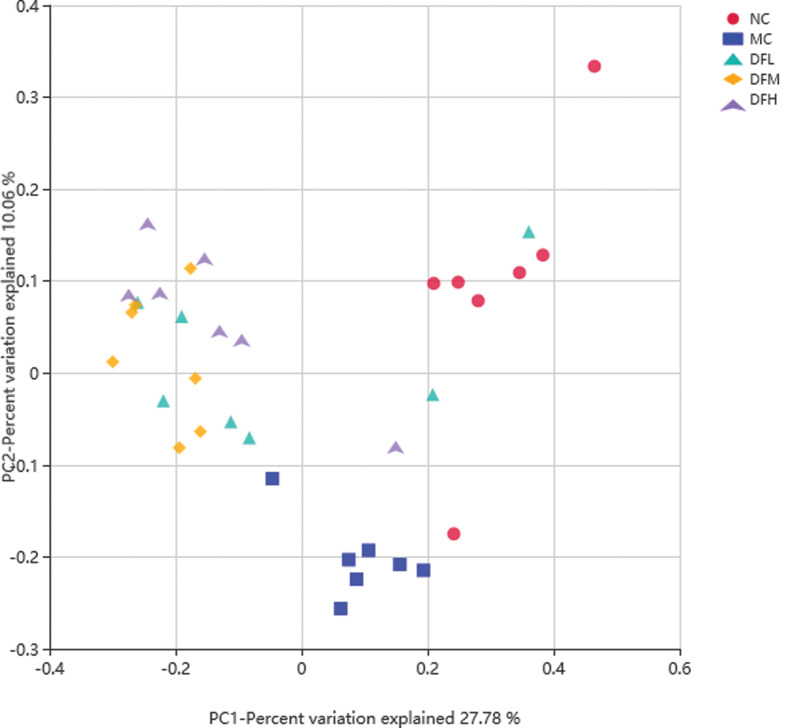
Green tea leaf powder modulates gut microbiota at different taxonomic levels
The phylogenetic differences between gut microbiota from NC, MC, DFL, DFM, and DFH groups of mice were assessed using PCoA analysis based on Bray-Curtis arithmetic (Fig. 7). Green tea leaf powder had a distinct microbiota composition that clustered separately from MC and NC groups.
Fig. 7.
Beta diversity estimates for the gut microbiota from NC, MC, DFL, DFM, and DFH groups of mice (n = 7).
To determine whether different concentrations of green tea leaf powder had an effect on the composition of cecal microbiota for HFD-fed mice, a taxon-dependent analysis was conducted.
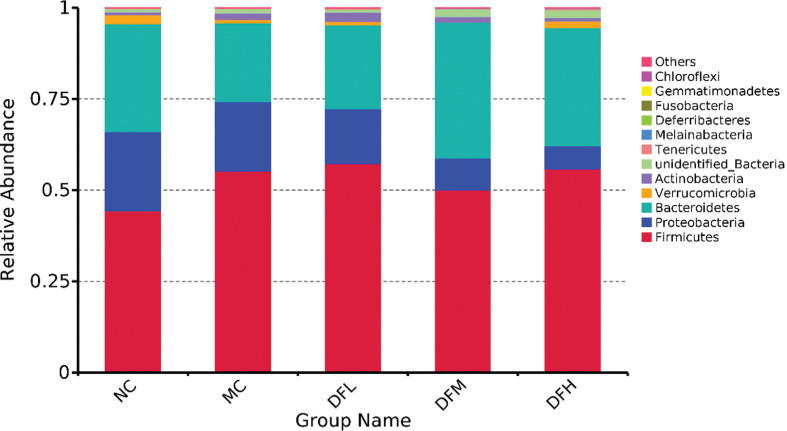
At the phylum level (Fig. 8), an HFD showed a dramatic decrease in the relative abundance of Bacteroidetes and a trend toward an increase in the relative abundance of Firmicutes in the cecum compared to the NC group (P < 0.05). Green tea leaf powder significantly increased the relative abundance of Bacteroidetes and Verrucomicrobia and decreased the relative abundance of Proteobacteria and the ratio of Firmicutes/Bacteroidetes (F/B) (P < 0.05) in the cecum of HFD-mice. The numbers of Actinobacteria did not significantly change.
Fig. 8.
The average relative abundance of phyla in the cecum microbiota of mice fed with green tea leaf powder (n = 7).
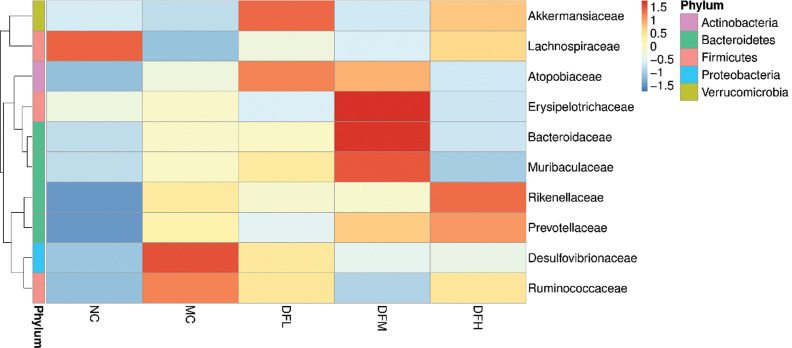
The 10 most abundant families identified are shown in Fig. 9. Compared to the MC group, a high dosage of green tea leaf powder significantly increased the relative abundance of Lachnospiraceae, Prevotellaceae, and Rikenellaceae and significantly decreased the relative abundance of Desulfovibrionaceae and Erysipelotrichaceae in the cecum (P < 0.05). Green tea leaf powder also promoted the growth of Akkermansiaceae; however, we did not find any significant differences between dosage levels.
Fig. 9.
Heatmap of the average relative abundance of the 10 most abundant families in the cecum microbiota of mice fed with green tea leaf powder (n = 7).
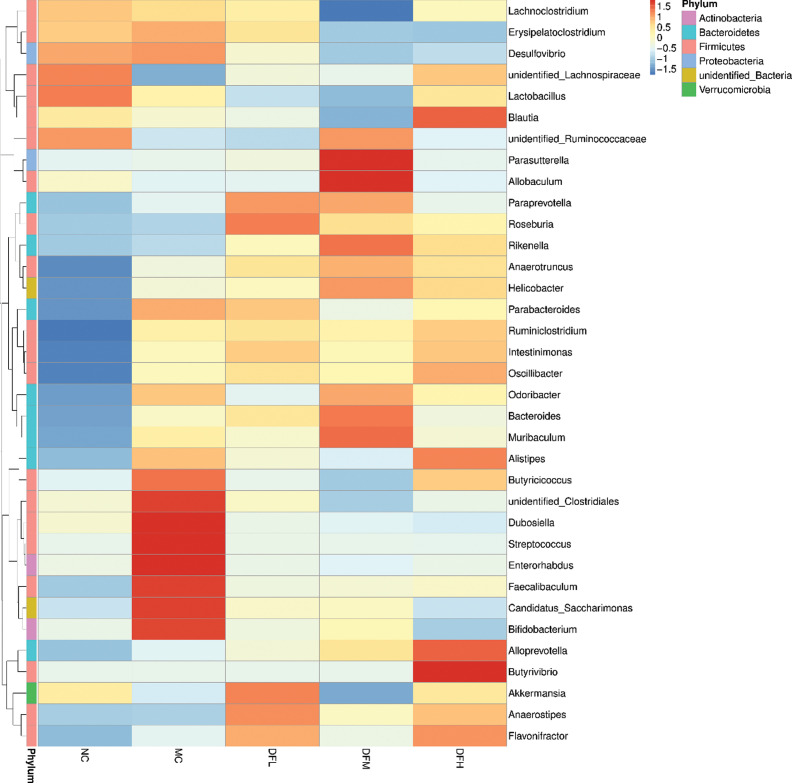
At the genus level (Fig. 10), compared to the MC group, a high dosage of green tea leaf powder significantly increased the relative abundance of Blautia, Oscillibacter, Ruminiclostridium, Alloprevotella, and Butyrivibrio and decreased the relative abundance of Erysipelatoclostridium, Desulfovibrio, and Candidatus_Saccharimonas (P < 0.05). A medium dosage of green tea leaf powder significantly increased the relative abundance of Parasutterella, Allobaculum, and Anaerotruncus (P < 0.05). A low dosage of green tea leaf powder significantly increased the relative abundance of Roseburia, Akkermansia, and Anaerosipes (P < 0.05).
Fig. 10.
Heatmap of the average relative abundance of the 35 most abundant genera in the cecum microbiota of mice fed with green tea leaf powder (n = 7).
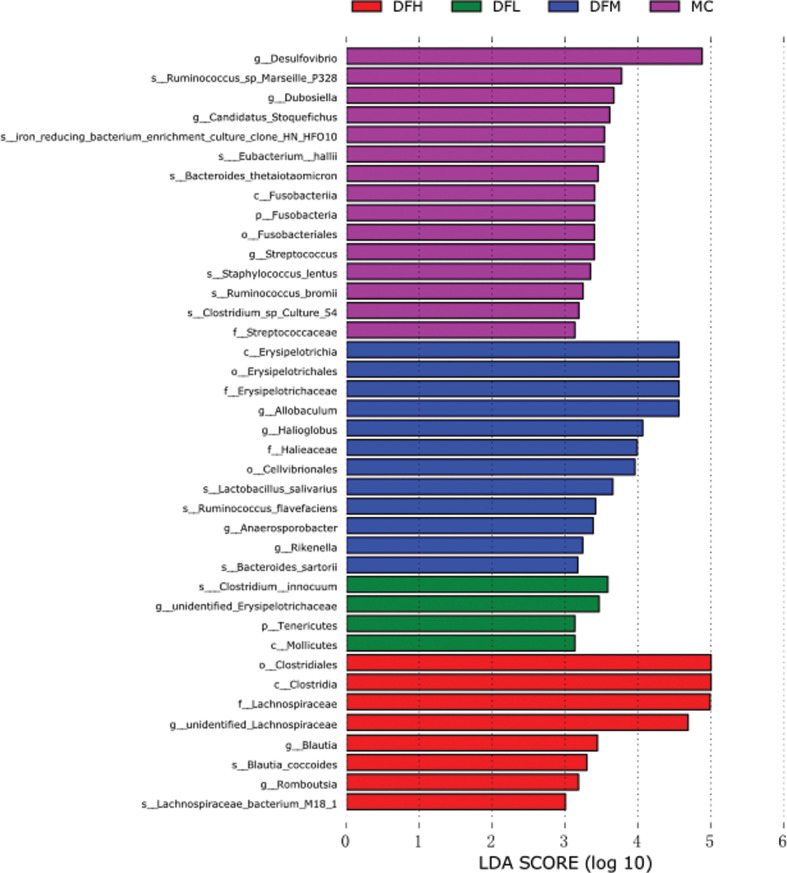
To identify the representative microbiota of the groups, gut microbiota from the different treatment groups were compared using LEfSe analysis. The LEfSe presented the enrichment of microbiota taxa, from phylum to species level. We found substantial differences between the different dosage levels of dietary treatments (Fig. 11). Desulfovibrio was significantly more abundant in the MC group. Allobaculum and Erysipelotrichaceae were more abundant in DFM group. Lachnospiraceae were more abundant in the DFH group.
Fig. 11.
LEfSe analysis of gut microbiota between groups. Red shaded areas indicate DFH-enriched taxa, green shaded areas indicate DFL-enriched taxa, blue shaded areas indicate DFM-enriched taxa, and purple shaded areas indicate MC-enriched taxa (LDA > 3).
Functional prediction of gut microbiota
The 12 KEGG biological pathways were significantly influenced in the gut microbiota with different dosages of green tea leaf powder (Table 4). Green tea leaf powder administration significantly increased fatty acid metabolism, butanoate metabolism, lipid metabolism, carbohydrate metabolism, energy metabolism, xenobiotics biodegradation and metabolism, cofactors and vitamins metabolism, cellular processes and signaling, and genetic information processing of gut microbiota. Meanwhile, green tea leaf powder administration significantly decreased metabolic disease, infectious diseases, and lipopolysaccharide biosynthesis of gut microbiota.
Table 4.
List of significantly different KEGG pathways with greater abundance in MC, DFL, DFM, and DFH groups
| KEGG pathway | Abundance mean, MC | Abundancemean, DFL | Abundance mean, DFM | Abundance mean, DFH |
|---|---|---|---|---|
| Fatty acid metabolism | 22,120a | 22,882a | 27,899b | 35,277c |
| Butanoate metabolism | 32,107a | 44,287b | 56,333b,c | 96,513c |
| Lipid metabolism | 20,136a | 22,457a | 50,098b | 76,501c |
| Carbohydrate metabolism | 49,045a | 89,764b | 100,927b,c | 152,826c |
| Energy metabolism | 8,276a | 8,255a | 8,410a | 9,745b |
| Metabolic diseases | 2,750a | 2,681a | 1,863b | 1,872b |
| Infectious diseases | 2,654a | 2,702a | 1,893b,c | 1,508c |
| Lipopolysaccharide biosynthesis | 1,100a | 923b | 805b | 789c |
| Xenobiotic biodegradation and metabolism | 973a | 1,028b | 1,142b | 1,047b |
| Cofactor and vitamin metabolism | 1,340a | 1,229a | 1,341a | 1,506b |
| Cellular processes and signaling | 31,997a | 34,901b | 34,178b | 33,793b |
| Genetic information processing | 23,022a | 22,015a | 25,608b | 24,435b |
Significant differences (P < 0.05) are indicated using different letters (a, b, c).
DFL, dietary fiber-enriched green tea leaf powder-low; DFM, dietary fiber-enriched green tea leaf powder-medium; DFH, dietary fiber-enriched green tea leaf powder-high; MC, model control; NC, negative control.
Green tea leaf powder increases SCFAs production
Acetate, propionate, butyrate, and iso-butyrate levels in the feces were significantly reduced in the MC group compared to the NC group (P < 0.05) (Table 5). Compared to the MC group, green tea leaf powder administration increased acetate and butyrate levels in the feces in a dose-dependent manner. In particular, a high-dosage of green tea leaf powder significantly increased acetate and butyrate levels in the feces of HFD-mice (P < 0.05). The concentrations of other SCFAs did not show significant differences.
Table 5.
Fecal SCFAs in C57BL/6J mice from NC, MC, DFL, DFM, and DFH groups (n = 7)
| SCFAs (μmol/g) | NC | MC | DFL | DFM | DFH |
|---|---|---|---|---|---|
| Acetate | 36.02 ± 2.37a | 21.91 ± 3.32b | 25.13 ± 3.81b | 32.25 ± 5.06a | 40.33 ± 6.48a |
| Propionate | 1.23 ± 0.19a | 0.89 ± 0.02b | 0.95 ± 0.10a,b | 1.28 ± 0.16a,b | 1.43 ± 0.21a,b |
| Butyrate | 4.39 ± 0.22a | 3.51 ± 0.86b | 3.98 ± 0.91a,b | 5.02 ± 0.78c | 6.02 ± 0.75c |
| Valerate | 0.14 ± 0.02a | 0.13 ± 0.06a | 0.15 ± 0.03a | 0.14 ± 0.04a | 0.16 ± 0.05a |
| Iso-butyrate | 0.68 ± 0.10a | 0.15 ± 0.01b | 0.26 ± 0.02b | 0.33 ± 0.08a,b | 0.47 ± 0.07a |
Significant differences (P < 0.05) are indicated using different letters (a, b, c).
DFL, dietary fiber-enriched green tea leaf powder-low; DFM, dietary fiber-enriched green tea leaf powder-medium; DFH, dietary fiber-enriched green tea leaf powder-high; MC, model control; NC, negative control.
Discussion
Green tea leaf powder had a preventive effect on dyslipidemia. From previous studies, the most effective active ingredient in green tea infusions is tea polyphenols, such as catechins. The tea polyphenols act as antioxidants and scavenging of free radicals for promoting health. For our study, the main bioactive ingredient of green tea leaf powder was dietary fiber. Green tea leaf powder could significantly ameliorate the HFD-induced dyslipidemia in a dose-dependent manner, which is possible by the synergistic modulation of gut microbiota, the action of gut satiety hormones, and enhancing anti-inflammatory cytokine production.
Satiety hormones can regulate appetite and play an important role in regulating body metabolism and energy balance. GLP-1 is a gut hormone from enteroendocrine L cells and it has many physiological functions, including promoting glucose-dependent insulin secretion as an incretin hormone, increasing pancreatic β-cell development, and inducing satiety via hypothalamus (11). PYY is also a gut anorexigenic hormone and acts by suppressing appetite in the brain, increasing gut transit rate, and reducing energy intake (12). In our study, food intake in the green tea leaf powder group was significantly lower than the MC group (data not shown), and the body weights of the mice in the green tea leaf powder group were significantly lower. Thus, the underlying mechanism which resulted in an improvement of dyslipidemia may be due to the reduction of food consumption and increase in satiety. High levels of leptin, an adipose tissue hormone, were observed in the MC group. This showed that the HFD-mice had developed leptin resistance, which probably resulted in insulin resistance and the development fatty liver disease (13). Green tea leaf powder reduced serum leptin levels in our study, and leptin resistance and sensitivity were improved. Increasing serum adiponectin levels using green tea leaf powder has been found to correlate with lipoprotein metabolism, resulting in an increase in HDL-C and a decrease in TG levels (14).
Diet and gut microbiota play an important role in low-grade inflammation characterized HFD-induced metabolic disease. Chronic and low-grade inflammation are characterized by elevated proinflammatory markers, including TNF-α and IL-6 (15). HFD-induced gut dysbiosis resulted in damage to gut integrity and the release of LPS from intestinal gram-negative bacteria into the blood, which could lead to chronic inflammation. Particularly, LPS could activate TLR4-induced insulin resistance (16). In our study, green tea leaf powder reduced metabolic endotoxemia and significantly alleviated systemic inflammation. Thus, another mechanism of improving dyslipidemia may be attributed to the alleviation of low-grade chronic inflammation.
It has been reported that the expression of genes related to hepatic fatty acid synthesis and oxidation could be altered by an HFD. Therefore, SREBP1c, FAS, PPARα, LXR, CYP7A1, and ABCA1 mRNA expression levels were evaluated in our study to investigate whether green tea leaf powder could influence the dyslipidemia in HFD-fed mice. SREBP1-c is a critical transcription factor, which participates in liver adipocyte differentiation and adipogenesis. Meanwhile, SREBP-1c was proved to be involved in hepatic cholesterol synthesis (17). Green tea leaf powder decreased SREBP-1c mRNA expression levels and could thus reduce fat and cholesterol synthesis. These results corresponded with lower TC levels of the green tea leaf powder group. FAS could enhance fatty acid synthesis and accumulate TG. LXR also plays an important role in lipogenesis. Green tea leaf powder could decrease the mRNA expression levels of FAS and LXR, thus it could reduce fatty acid synthesis. The mRNA levels of PPARα and CYP7A1 in the green tea leaf powder group were significantly higher than in the MC group. PPARα expression could promote fatty acid catabolism and reduce fat mass. CYP7A1 expression could improve hepatic steatosis and obesity by inhibiting hepatic lipogenesis (18). ABCA1 expression is vital for HDL-C formation and serves as a cellular efflux transporter of liver lipid and cholesterol. Therefore, the serum HDL-C level of the green tea leaf powder group is significantly higher than the MC group.
Increased ratios of F/B in the cecum are associated with obesity and metabolic disease. In our study, green tea leaf powder increased the relative abundance of Bacteroides and decreased the ratio of F/B. Thus, green tea leaf powder can potentially reduce weight and alleviate metabolic disease. Verrucomicrobia contains Akkermansia, a mucin-degrading microbiota, which was reported to play an important role in preventing obesity and metabolic disease (19).
Lachnospiraceae is involved in the production of butyrate (20). Erysipelotrichaceae is related to inflammation gastrointestinal diseases and is enriched in colorectal cancer (21). Desulfovibrionaceae is potentially an endotoxin LPS producer, which could induce a potent inflammation reaction (22). A lower abundance of the Desulfovibrionaceae was discovered in the green tea leaf powder group compared to the MC group, which may help in alleviating inflammation. Green tea leaf powder intervention increased Prevotellaceae and Rikenellaceae, which are hydrogen-producing bacteria and are similar to lactulose intervention (23). Hydrogen could ameliorate the symptoms of inflammatory bowel disease by reducing oxidative stress.
A high dosage of green tea leaf powder could significantly increase the relative abundance of Blautia, Oscillibacter, Ruminiclostridium, Alloprevotella, and Butyrivibrio. Blautia was reported to have the ability to improve glucose and lipid homeostasis (24). Oscillibacter is related to reduce body weight (25). Ruminiclostridium was reported to degrade cellulose and lignin in nature (26). Alloprevotella, an SCFA-producing bacteria, was negatively related to obesity and metabolic syndrome (27). Butyrivibrio is a well-known butyrate-producing bacteria (28), and our results also showed that a high dosage of green tea leaf powder significantly increased butyrate concentrations, whereas a high dosage of green tea leaf powder significantly decreased the abundance of Erysipelatoclostridium, Desulfovibrio, and Candidatus_Saccharimonas. Erysipelatoclostridium was considered an opportunistic pathogen in the gut (29). Desulfovibrio is a gram-negative bacteria and also a potential LPS producer (22). Candidatus_Saccharimonas was found in a high proportion of the feces of HFD-fed mice, which is consistent with our results (30). Thus, green tea leaf powder can decrease the relative abundance of pathogenic microbiota and improve the gut homeostasis.
Conclusion
Our results show that green tea leaf powder supplementation in HFD-mice improves dyslipidemia in a dosedependent manner, which is possible by the synergistic modulation of gut microbiota, the action of gut satiety hormones, and enhancing anti-inflammatory cytokine production. These results imply that green tea leaf powder intervention may be useful in modulating some of the adverse effects that accompany HFD-induced dyslipidemia.
Acknowledgments
This work was financially supported by the Fundamental Research Funds for the Central Universities, Nankai University (No. 63201219).
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
Authors’ contributions
JW and PL conceived, designed and wrote the study. SL and HM responsible for project development, data collection, and data analysis. BZ and YH modified the language. SW reviewed the manuscript. All authors read and approved the final manuscript.
References
- 1.Kaczmarczyk MM, Miller MJ, Freund GG. The health benefits of dietary fiber: beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism 2012; 61: 1058–66. doi: 10.1016/j.metabol.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahl WJ, Stewart ML. Position of the academy of nutrition and dietetics: health implications of dietary fiber. J Acad Nutr and Diet 2015; 115: 1861–70. doi: 10.1016/j.jand.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Hamaker BR, Tuncil YE. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J Mol Biol 2014; 426: 3838–50. doi: 10.1016/j.jmb.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 2016; 165: 1332–45. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 5.Guo W, Shu Y, Yang X. Tea dietary fiber improves serum and hepatic lipid profiles in mice fed a high cholesterol diet. Plant Food Hum Nutr 2016; 71: 145–50. doi: 10.1007/s11130-016-0536-7. [DOI] [PubMed] [Google Scholar]
- 6.Axling U, Olsson C, Jie X, Fernandez C, Larsson S, Strom K, et al. . Green tea powder and Lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice. Nutr Metab 2012; 9: 105. doi: 10.1186/1743-7075-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lan G, Chen H, Chen S, Tian J. Chemical composition and physicochemical properties of dietary fiber from Polygonatum odoratum as affected by different processing methods. Food Res Int 2012; 49: 406–10. doi: 10.1016/j.foodres.2012.07.047. [DOI] [Google Scholar]
- 8.Ferreira DS, Poppi RJ, Lima Pallone JA. Evaluation of dietary fiber of brazilian soybean (Glycine max) using near-infrared spectroscopy and chemometrics. J Cereal Sci 2015; 64: 43–7. doi: 10.1016/j/jcs.2015.04.004. [DOI] [Google Scholar]
- 9.Nakajima A, Nakatani A, Hasegawa S, Irie J, Ozawa K, Tsujimoto G, et al. . The short chain fatty acid receptor GPR43 regulates inflammatory signals in adipose tissue M2-type macrophages. PLoS One 2017; 12: e0179696. doi: 10.1371/journal.pone.0179696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan RJ, Greenwood CE. Influence of dietary carbohydrates and glycaemic response on subjective appetite and food intake in healthy elderly persons. Int J Food Sci Nutr 2002; 53: 305–16. doi: 10.1080/09637480220138160. [DOI] [PubMed] [Google Scholar]
- 11.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, et al. . Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012; 61: 364–71. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, et al. . Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G proteincoupled receptor, Gpr41. PNAS 2008; 105: 16767–72. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Muzafar HM, Amin KA. Probiotic mixture improves fatty liver disease by virtue of its action on lipid profiles, leptin, and inflammatory biomarkers. BMC Complem Altern M 2017; 17: 43. doi: 10.1186/s12906-016-1540-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yanai H, Yoshida H. Beneficial effects of adiponectin on glucose and lipid metabolism and atherosclerotic progression: mechanisms and perspectives. Int J Mol Sci 2019; 20: 1190. doi: 10.3390/ijms20051190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chassaing B, Gewirtz AT. Gut microbiota, low-grade inflammation, and metabolic syndrome. Toxicol Pathol 2014; 42: 49–53. doi: 10.1177/0192623313508481. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez-Tapia M, Aguilar-Lopez M, Perez-Cruz C, Pichardo-Ontiveros E, Wang M, Donovan SM, et al. . Nopal (Opuntia ficus indica) protects from metabolic endotoxemia by modifying gut microbiota in obese rats fed high fat/sucrose diet. Sci Rep 2017; 7: 4716. doi: 10.1038/s41598-017-05096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xia X, Li G, Song J, Zheng J, Kan J. Hypocholesterolaemic effect of whole-grain highland hull-less barley fed a high-fat diet. Brit J Nutr 2018; 119: 1102–10. doi: 10.1017/s0007114518000831. [DOI] [PubMed] [Google Scholar]
- 18.Li T, Owsley E, Matozel M, Hsu P, Novak CM, Chiang JYL. Transgenic expression of cholesterol 7 alpha-hydroxylase in the liver prevents high-fat diet induced obesity and insulin resistance in mice. Hepatology 2010; 52: 678–90. doi: 10.1002/hep.23721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. . Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. PNAS 2013; 110: 9066–71. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li T, Gao J, Du M, Mao X. Milk fat globule membrane supplementation modulates the gut microbiota and attenuates metabolic endotoxemia in high-fat diet-fed mice. J Funct Foods 2018; 47: 56–65. doi: 10.1016/j.jff.2018.05.038. [DOI] [Google Scholar]
- 21.Kaakoush NO. Insights into the role of Erysipelotrichaceae in the human host. Front Cell Infect Microbiol 2015; 5: 84. doi: 10.3389/fcimb.2015.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao S, Fei N, Pang X, Shen J, Wang L, Zhang B, et al. . A gut microbiota-targeted dietary intervention for amelioration of chronic inflammation underlying metabolic syndrome. Fems Microbiol Ecol 2014; 87: 357–67. doi: 10.1111/1574-6941.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhai S, Zhu L, Qin S, Li L. Effect of lactulose intervention on gut microbiota and short chain fatty acid composition of C57BL/6J mice. Microbiologyopen 2018; 7: e00612. doi: 10.1002/mbo3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong X, Xu J, Lian F, Yu X, Zhao Y, Xu L, et al. . Structural alteration of gut microbiota during the amelioration of human type 2 diabetes with hyperlipidemia by metformin and a traditional Chinese herbal formula: a multicenter, randomized, open label clinical trial. mBio 2018; 9: e02392–17. doi: 10.1128/mBio.02392-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim BS, Song MY, Kim H. The anti-obesity effect of Ephedra sinica through modulation of gut microbiota in obese korean women. J Ethnopharmacol 2014; 152: 532–539. doi: 10.1016/j.jep.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 26.Yoav S, Barak Y, Shamshoum M, Borovok I, Lamed R, Dassa B, et al. . How does cellulosome composition influence deconstruction of lignocellulosic substrates in Clostridium (Ruminiclostridium) thermocellum DSM 1313? Biotechnol Biofuels 2017; 10: 222. doi: 10.1186/s13068-017-0909-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shang Q, Song G, Zhang M, Shi J, Xu C, Hao J, et al. . Dietary fucoidan improves metabolic syndrome in association with increased Akkermansia population in the gut microbiota of high-fat diet-fed mice. J Funct Foods 2017; 28: 138–46. doi: 10.1016/j/jff.2016.11.002. [DOI] [Google Scholar]
- 28.Damms-Machado A, Mitra S, Schollenberger AE, Kramer KM, Meile T, Koenigsrainer A, et al. . Effects of surgical and dietary weight loss therapy for obesity on gut microbiota composition and nutrient absorption. Biomed Res Int 2015; 2015: 806248. doi: 10.1155/2015/806248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shao T, Shao L, Li H, Xie Z, He Z, Wen C. Combined signature of the fecal microbiome and metabolome in patients with gout. Front Microbiol 2017; 8: 268. doi: 10.3389/fmicb.2017.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin H, An Y, Hao F, Wang Y, Tang H. Correlations of fecal metabonomic and microbiomic changes induced by high-fat diet in the pre-obesity state. Sci Rep 2016; 6: 21618. doi: 10.1038/srep21618. [DOI] [PMC free article] [PubMed] [Google Scholar]