Abstract
Background:
After deep vein thrombosis (DVT), many patients have impaired quality of life (QOL). We aimed to assess if pharmacomechanical catheter-directed thrombolysis (PCDT) improves short-term or long-term QOL in patients with proximal DVT and if QOL is related to extent of DVT.
Methods:
The ATTRACT Trial was an assessor-blinded randomized trial that compared PCDT with no PCDT in patients with DVT of the femoral, common femoral, or iliac veins. QOL was assessed at baseline and 1, 6, 12, 18 and 24 months using the VEINES-QOL/Sym disease-specific QOL measure and the SF-36 (PCS and MCS summary scores) general QOL measures. Change in QOL scores from baseline to assessment time were compared in the PCDT and No PCDT treatment groups overall, and in the iliofemoral DVT and femoral-popliteal DVT subgroups.
Results:
691 of 692 ATTRACT patients were analysed (mean age 53 years, 62% male, 57% iliofemoral DVT). VEINES-QOL change scores were greater (i.e. better) in PCDT vs. No PCDT from baseline to one month (difference 5.7; P=0.0006) and baseline to 6 months (5.1; P=0.0029), but not for other intervals. SF-36 PCS change scores were greater in PCDT vs. No PCDT from baseline to one month (difference 2.4; P=0.01), but not for other intervals. Among iliofemoral DVT patients, VEINES-QOL change scores from baseline to all assessments were greater in the PCDT vs. No PCDT group; this was statistically significant in the intention-to-treat analysis at 1 month (difference 10.0; P<0.0001) and 6 months (8.8; P<0.0001) and in the per-protocol analysis at 18 months (difference 5.8; P=0.0086) and 24 months (difference 6.6; P=0.0067). SF-36 PCS change scores were greater in PCDT vs. No PCDT from baseline to one month (difference 3.2; P=0.0010), but not for other intervals. In contrast, in femoral-popliteal DVT patients, change scores from baseline to all assessments were similar in the PCDT and No PCDT groups.
Conclusions:
Among patients with proximal DVT, PCDT leads to greater improvement in disease-specific QOL than No PCDT at 1 month and 6 months, but not later. In patients with iliofemoral DVT, PCDT led to greater improvement in disease-specific QOL over 24 months.
Clinical Trial Registration:
Keywords: deep vein thrombosis, quality of life, randomized trial, proximal DVT, catheter-directed thrombolysis, iliofemoral DVT, femoral-popliteal DVT
Table of Contents Summary
In the ATTRACT randomized trial, among patients with proximal deep vein thrombosis (DVT), early use of pharmacomechanical catheter-directed thrombolysis had a beneficial effect on quality of life (QOL) during the first 6 months post-treatment. In patients with iliofemoral DVT, this QOL benefit was apparent over 24 months post-treatment.
INTRODUCTION
Despite treatment with anticoagulation and compression stockings, 30–50% of patients with proximal deep vein thrombosis (DVT) develop the post-thrombotic syndrome (PTS), a chronic, burdensome complication.1, 2 PTS is characterised by limb pain, heaviness, swelling and skin changes, including, in severe cases, venous ulceration. Greater recognition of PTS, with an increased focus on using patient reported outcome measures to assess the impact of illness, has highlighted the importance of studying health-related quality of life (QOL) in patients with DVT.
QOL is impaired in the acute phase of DVT3, 4, and development of PTS reduces QOL in the months to years following DVT.5 In the Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis (ATTRACT) Trial, we showed that pharmacomechanical catheter-directed thrombolysis (PCDT) did not reduce the occurrence of PTS during 24 months follow-up but reduced the severity of PTS and accelerated resolution of acute symptoms.6 In the current analysis, we assessed the effect of PCDT on short-term and long-term QOL in all patients in ATTRACT and in predefined subgroups with (iliofemoral DVT) or without (femoral-popliteal DVT) involvement of the iliac or common femoral vein, and assessed if this effect differed over time.
METHODS
The ATTRACT Trial was an NHLBI (NIH)-sponsored, randomized controlled trial conducted at 56 U.S. clinical centers.6, 7 Patients with symptomatic proximal DVT of the femoral, common femoral, or iliac vein were potentially eligible. Patients were excluded if they were younger than 16 or older than 75 years; were pregnant; or had symptoms for more than 14 days, high bleeding risk, active cancer, established PTS, or ipsilateral DVT in the prior 2 years.
Patients were randomly assigned to receive PCDT (PCDT group) or not receive PCDT (No PCDT group). Randomization was stratified by clinical center and by whether there was involvement of the common femoral or iliac vein (“iliofemoral DVT”), or not (“femoral-popliteal DVT”), as per societal reporting guidelines 8, 9. Patients in both treatment groups received initial and long-term anticoagulation as recommended in published guidelines10, 11, and were provided with knee-high, 30–40 mmHg elastic compression stockings (initially at the 10-day follow-up visit, with replacement every 6 months). The stockings were sized-to-fit and their daily use was encouraged by study personnel at each follow-up visit throughout the 24 months. PCDT was performed consistent with published guidelines.12
Patients were assessed at baseline and 1 month (±7 days), 6 months (±1 month), 12 months (±1 month), 18 months (±1 month), and 24 months (±2 months) post-randomization. PTS, the primary outcome of the ATTRACT Trial, was defined as a Villalta score of 5 or higher or an ulcer in the leg with the index DVT, any time between 6 and 24 months.13, 14 Full eligibility criteria, study investigators, study sites and detailed description of PCDT methods are provided in the primary publication.6 The study was approved by the institutional review boards at all participating centers, and all patients provided informed consent.
Quality of life assessments
Validated, self-administered instruments were used to measure venous disease-specific and general QOL. Venous disease-specific QOL was measured using the Venous Insufficiency Epidemiological and Economic Study Quality of Life (VEINES-QOL/Sym), a patient self-assessment questionnaire.15 The instrument consists of 25 items that measure venous symptoms (heavy legs, aching legs, swelling, night cramps, heat or burning sensation, restless legs, throbbing, itching, tingling, intensity of leg pain), limitations in daily activities due to venous disease, psychological impact of venous disease, and change over the past year. Responses are rated on 2-point to 7-point Likert scales of intensity, frequency, or agreement. The VEINES/Sym is a validated subscale of the VEINES instrument (10 of the 25 items) that measures venous symptoms. The VEINES-QOL/Sym has undergone comprehensive and rigorous psychometric evaluation and is acceptable, reliable, valid, and responsive for use as a patient-reported measure of outcome in studies of chronic venous disease, including PTS and DVT.15, 16 General QOL was measured using the Medical Outcomes Study Short-Form Health Survey-36, Version 2 (SF-36v2), a validated, widely-used instrument.17, 18 The SF-36 has been used in other DVT studies to assess general QOL.5, 19–21 For all measures, lower scores indicate poorer QOL.
Administration of QOL instruments
The VEINES-QOL/Sym and SF-36 were combined into a single questionnaire document that took approximately 15–20 minutes for most patients to complete. Following a standard orientation, the patient filled in the questionnaire in a quiet office. The research nurse then checked for missing data and, without coercion, encouraged the patient to respond to all items. The nurse administering the questionnaire was blinded to the patient’s treatment allocation.
Scoring of QOL instruments
For the SF-36, an established computer scoring algorithm 22, 23 was used to generate summary scores for the Physical (PCS) and Mental (MCS) Component Scales (which reflect physical and mental health status, respectively). For VEINES-QOL, the intrinsic scoring method recently proposed by Bland24 was used, as the original “relative” scoring method15 has the disadvantage of always producing the same mean and standard deviation and, thus, cannot be used to study changes over time and to compare findings in different studies. Summary scores were computed for VEINES-QOL (impact of venous disease on QOL) and VEINES-Sym (venous symptom severity).
For SF-36 PCS and MCS, a change of 4 points is considered a minimal clinically important difference.25 For VEINES-QOL and VEINES-Sym scored using the intrinsic method, the minimal clinically important difference is uncertain, but is thought to be about 4 to 6 points, which is similar or a bit larger than for the original relative scoring method16.
Sample size and power
The total sample size required for the ATTRACT Trial’s primary outcome of PTS was 692 patients.6 For secondary outcomes including QOL scores, this sample size provided approximately 88% power to detect an effect size of 0.25 with continuous outcomes. An effect size of 0.25 translates into ability to detect a difference between groups of 1.25 points in the VEINES-QOL and VEINES-Sym and 2.5 points in the SF-36 PCS and MCS.
Statistical analysis
QOL analyses were performed using both modified intention-to-treat (ITT) and per-protocol analysis sets. The modified ITT analysis set consisted of all patients randomized except for those who did not have DVT at enrollment. The per-protocol analysis set excluded randomized patients who, within 7 days post-randomization, were assigned to receive PCDT but did not undergo the procedure, or who were assigned to No PCDT but underwent PCDT.
Group means and standard errors of the VEINES-QOL, VEINES-Sym, SF-36 PCS and MCS scores, and the mean differences and 95% confidence intervals (CIs) between treatment arms at each assessment were calculated. The repeated QOL scores over time (i.e. at baseline, 1, 6, 12, 18, 24 months) were analyzed with growth curve mixed models using piecewise-linear regression.26 The models took into account the correlation between the repeated observations. Models included both fixed effects: the pre-specified baseline factors (treatment, center, extent of DVT, sex), and continuous covariates (age at randomization, body mass index [BMI], and Villalta score); and random effects (actual visit dates, patient). Interaction terms (treatment x time for each visit) were assessed in each model, and a best-fit model was determined by removing non-significant (p>0.05) interaction terms. Change in QOL scores from baseline to 24 months, the pre-specified primary QOL outcome, were compared between treatment arms using estimates derived from the final growth curve models.
Sensitivity analyses for the VEINES (QOL and Sym) and SF-36 (PCS and MCS) outcomes used multiple imputation for missing baseline covariates and missing summary scores (except for deceased subjects), and the modelling structure described above. Missing data were assumed to be missing-at-random. The following auxiliary variables assisted in the imputation phase: for VEINES QOL/Sym scores, age, sex, BMI, extent of DVT and all available VEINES-QOL/Sym scores from previous visits; and for SF-36 MCS/PCS scores, age, sex, race, BMI, and all available SF-36 scores from previous visits. Imputation was performed separately within each treatment arm.
Analyses for the change scores, similar to the above, were reported for the iliofemoral DVT and femoral-popliteal DVT subgroups. The growth curve mixed models were expanded to assess the treatment x time interactions within the extent of DVT subgroups (i.e. treatment x time x extent) using the data from all patients. Plots of the response trajectories (from baseline to 24 months) of the model-fitted VEINES-QOL change scores within each of the four groups defined by treatment (PCDT, No PCDT) and highest extent of DVT (iliofemoral, femoral-popliteal) were developed using locally-weighted scatterplot smoothing (LOESS), a non-parametric smoothing technique.27
Finally, forest plots were created to display model-fitted baseline-to-24-month change scores for PCDT vs. No PCDT within pre-specified subgroups defined by baseline age (<65, ≥65), sex, race (white, non-white), BMI (<25, 25–29, ≥30), DVT symptom duration pre-enrolment (<1 week, ≥1 week), DVT extent, and Villalta severity score (<5 points, 5–9, 10–14, ≥15). Linear regression models for the change scores were used to assess differential treatment effects within subgroups (i.e. subgroup x treatment interactions). Change scores using the growth-curve model-fitted estimates (with and without multiple imputation) were analyzed separately.
To account for the multiplicity of comparisons between treatment arms, statistical significance was declared only when P-values were less than 0.01. Statistical analyses were performed using SAS version 9.4 and the R version 3.5 programming language.
RESULTS
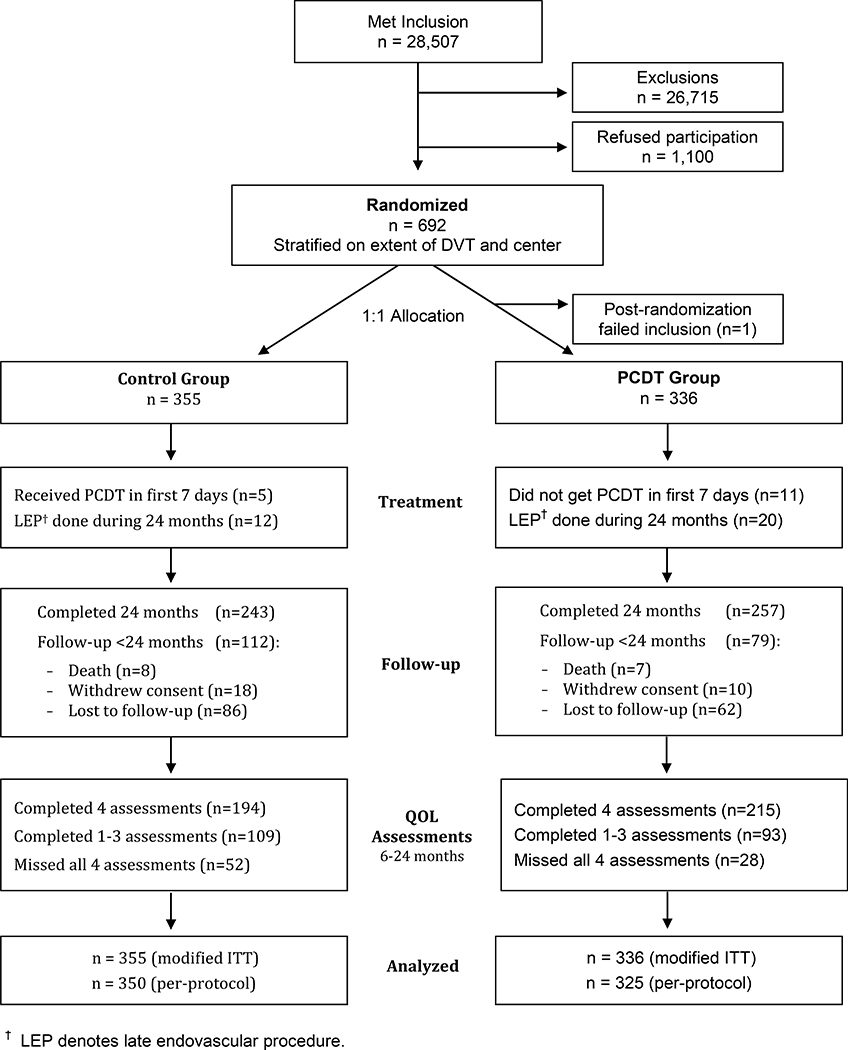
Between December 2009 and December 2014, 692 patients were randomized (337 to PCDT, 355 to No PCDT) and were followed for 2 years (Figure 1). One patient assigned to the PCDT group was found not to have a qualifying DVT and, therefore, was excluded from all analyses, leaving 691 patients in the modified ITT analysis set. Within 7 days of randomization, a further 11 patients who were assigned to receive PCDT but did not have PCDT, and 5 patients who were assigned to No PCDT but had PCDT were excluded from the per-protocol analysis (675 patients in per-protocol analysis set).
Figure 1:
CONSORT diagram for participants in QOL analyses
Baseline characteristics of the patients were similar in the PCDT and No PCDT groups (Table 1). Overall, median age was 53 years, 62% of patients were male, 78% were white, and median BMI was 31 kg/m2. The qualifying DVT was iliofemoral in 57% of patients and femoral-popliteal in 43% of patients.
Table 1.
Demographic and Clinical Characteristics at Baseline
| PCDT n = 336 | No PCDT n = 355 | Total N = 691 | |
|---|---|---|---|
| Age, years: median (IQR) | 52 (41, 62) | 53 (43, 62) | 53 (42, 62) |
| Male: n (%) | 205 (61) | 221 (62) | 426 (62) |
| Race: n (%) | |||
| White | 265 (79) | 276 (78) | 541 (78) |
| Black/African-American | 61 (18) | 62 (17) | 123 (18) |
| Other | 10 (3) | 17 (5) | 27 (31) |
| Weight, kg: median (IQR) | 95 (81, 111) | 92 (79, 110) | 93 (80, 110) |
| Body mass index, kg/m2: median (IQR) | 31 (27, 36) | 30 (26, 35) | 31 (27, 35) |
| DVT characteristics: n (%) | |||
| Left leg with index DVT | 207 (62) | 218 (61) | 425 (62) |
| Extends into common femoral and/or iliac vein | 195 (58) | 196 (55) | 391 (57) |
| Previous DVT or PE | 83 (25) | 87 (25) | 170 (25) |
| Previous ipsilateral DVT | 5 (1) | 14 (4) | 19 (3) |
| DVT risk factors: n (%)* | |||
| Major surgery | 27 (8) | 34 (10) | 61 (9) |
| Hospitalization | 26 (8) | 38 (11) | 64 (9) |
| Plaster cast immobilization | 8 (2) | 9 (3) | 17 (2) |
| Childbirth | 3 (1) | 5 (1) | 8 (1) |
| Outpatient when DVT diagnosed: n (%) | 268 (80) | 300 (85) | 568 (82) |
| DVT symptom duration (prior to randomization), days: median (IQR) | 6 (4, 10) | 6 (4, 9) | 6 (4, 10) |
Patients may contribute to more than one category
IQR, inter-quartile range; DVT, deep vein thrombosis; PE, pulmonary embolism
Quality of life
Detailed summaries of the raw QOL scores over time are presented in the Supplement (Tables A–I). The numbers of patients who completed QOL assessments at each visit are shown in Table J. In this section, we provide results for the change in QOL scores from baseline to each assessment time.
All Patients
In the modified ITT analysis set, model-fitted VEINES-QOL change scores from baseline to 24 months (primary outcome) was an average of 3.9 points higher in PCDT than No PCDT patients (P=0.043; Table 2). Difference in change scores in favor of PCDT achieved statistical significance at 1 month (5.7; P=0.0006) and at 6 months (5.1; P=0.0029), but not at 12 months and at 18 months (Table 2).
Table 2.
All Patients: Change in Disease-specific and General QOL according to Treatment
| Outcome Measure | PCDT |
No PCDT |
PCDT – No PCDT Difference |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SE | n | Mean | SE | Est. | SE | 95% CI | p | ||
| VEINES-QOL: | |||||||||||
| Baseline to 1 month: | |||||||||||
| Raw data* | 314 | 14.2 | 1.4 | 314 | 8.5 | 1.2 | 5.7 | 1.8 | 2.1 | 9.3 | 0.0021 |
| Model fitted† | 14.7 | 1.3 | 9.0 | 1.3 | 5.7 | 1.7 | 2.5 | 9.0 | 0.0006 | ||
| Model fitted using MI‡ | 14.9 | 1.3 | 8.9 | 1.3 | 6.0 | 1.7 | 2.7 | 9.3 | 0.0003 | ||
| Baseline to 6 months: | |||||||||||
| Raw data* | 287 | 26.0 | 1.6 | 277 | 21.3 | 1.5 | 4.7 | 2.1 | 0.5 | 8.9 | 0.03 |
| Model fitted† | 26.5 | 1.3 | 21.5 | 1.3 | 5.1 | 1.7 | 1.7 | 8.4 | 0.0029 | ||
| Model fitted using MI‡ | 27.1 | 1.3 | 21.6 | 1.3 | 5.5 | 1.6 | 2.3 | 8.7 | 0.0008 | ||
| Baseline to 12 months: | |||||||||||
| Raw data* | 267 | 26.0 | 1.6 | 252 | 25.1 | 1.6 | 0.9 | 2.2 | −3.5 | 5.3 | 0.70 |
| Model fitted† | 26.8 | 1.3 | 25.3 | 1.4 | 1.5 | 1.7 | −1.8 | 4.8 | 0.38 | ||
| Model fitted using MI‡ | 27.2 | 1.3 | 25.7 | 1.3 | 1.5 | 1.7 | −1.8 | 4.7 | 0.37 | ||
| Baseline to 18 months: | |||||||||||
| Raw data* | 244 | 27.2 | 1.8 | 220 | 25.5 | 1.7 | 1.7 | 2.5 | −3.1 | 6.6 | 0.48 |
| Model fitted† | 27.4 | 1.3 | 24.7 | 1.3 | 2.7 | 1.7 | −0.6 | 5.9 | 0.11 | ||
| Model fitted using MI‡ | 28.1 | 1.3 | 25.3 | 1.3 | 2.7 | 1.6 | −0.5 | 5.9 | 0.10 | ||
| Baseline to 24 months: | |||||||||||
| Raw data* | 249 | 27.4 | 1.7 | 227 | 24.1 | 1.8 | 3.3 | 2.5 | −1.6 | 8.2 | 0.18 |
| Model fitted† | 28.1 | 1.5 | 24.2 | 1.5 | 3.9 | 1.9 | 0.1 | 7.6 | 0.04 | ||
| Model fitted using MI‡ | 28.9 | 1.6 | 25.0 | 1.5 | 3.9 | 2.0 | 0.1 | 7.8 | 0.04 | ||
| VEINES-Sym: | |||||||||||
| Baseline to 1 month: | |||||||||||
| Raw data* | 311 | 12.1 | 1.5 | 314 | 8.8 | 1.3 | 3.3 | 2.0 | −0.6 | 7.1 | 0.10 |
| Model fitted† | 12.9 | 1.5 | 9.6 | 1.5 | 3.3 | 2.1 | −0.8 | 7.4 | 0.11 | ||
| Model fitted using MI‡ | 13.1 | 1.5 | 9.3 | 1.5 | 3.8 | 2.1 | −0.4 | 8.0 | 0.08 | ||
| Baseline to 6 months: | |||||||||||
| Raw data* | 285 | 20.2 | 1.6 | 277 | 16.2 | 1.5 | 4.0 | 2.2 | −0.3 | 8.3 | 0.07 |
| Model fitted† | 21.1 | 1.5 | 16.8 | 1.5 | 4.3 | 2.1 | 0.1 | 8.4 | 0.05 | ||
| Model fitted using MI‡ | 21.5 | 1.5 | 16.8 | 1.5 | 4.7 | 2.2 | 0.4 | 9.1 | 0.03 | ||
| Baseline to 12 months: | |||||||||||
| Raw data* | 266 | 18.1 | 1.6 | 252 | 18.1 | 1.6 | 0.0 | 2.3 | −4.5 | 4.5 | 0.99 |
| Model fitted† | 19.1 | 1.5 | 18.2 | 1.5 | 0.9 | 2.1 | −3.3 | 5.1 | 0.68 | ||
| Model fitted using MI‡ | 19.2 | 1.5 | 18.4 | 1.5 | 0.8 | 2.2 | −3.5 | 5.1 | 0.72 | ||
| Baseline to 18 months: | |||||||||||
| Raw data* | 243 | 19.3 | 1.8 | 220 | 18.1 | 1.8 | 1.2 | 2.6 | −3.9 | 6.2 | 0.65 |
| Model fitted† | 20.2 | 1.5 | 18.2 | 1.5 | 2.0 | 2.1 | −2.2 | 6.1 | 0.35 | ||
| Model fitted using MI‡ | 20.6 | 1.5 | 18.7 | 1.5 | 2.0 | 2.1 | −2.1 | 6.1 | 0.34 | ||
| Baseline to 24 months: | |||||||||||
| Raw data* | 248 | 20.7 | 1.7 | 227 | 18.2 | 1.7 | 2.5 | 2.4 | −2.2 | 7.1 | 0.30 |
| Model fitted† | 21.2 | 1.6 | 18.2 | 1.7 | 3.0 | 2.3 | −1.5 | 7.6 | 0.19 | ||
| Model fitted using MI‡ | 22.1 | 1.7 | 18.9 | 1.6 | 3.2 | 2.3 | −1.3 | 7.7 | 0.16 | ||
| SF-36 PCS: | |||||||||||
| Baseline to 1 month: | |||||||||||
| Raw data* | 313 | 7.2 | 0.6 | 314 | 4.9 | 0.6 | 2.3 | 0.9 | 0.6 | 4.0 | 0.0077 |
| Model fitted† | 7.4 | 0.7 | 5.1 | 0.7 | 2.4 | 0.9 | 0.5 | 4.2 | 0.01 | ||
| Model fitted using MI‡ | 7.6 | 0.7 | 5.0 | 0.7 | 2.6 | 1.0 | 0.7 | 4.4 | 0.0072 | ||
| Baseline to 6 months: | |||||||||||
| Raw data* | 287 | 10.9 | 0.8 | 277 | 9.5 | 0.7 | 1.4 | 1.1 | −0.7 | 3.5 | 0.19 |
| Model fitted† | 11.5 | 0.7 | 9.7 | 0.7 | 1.8 | 0.9 | 0.0 | 3.6 | 0.05 | ||
| Model fitted using MI‡ | 11.8 | 0.7 | 9.6 | 0.7 | 2.2 | 1.0 | 0.3 | 4.1 | 0.02 | ||
| Baseline to 12 months: | |||||||||||
| Raw data* | 266 | 11.6 | 0.8 | 252 | 10.4 | 0.8 | 1.2 | 1.1 | −1.0 | 3.4 | 0.27 |
| Model fitted† | 11.5 | 0.7 | 10.0 | 0.7 | 1.5 | 0.9 | −0.3 | 3.4 | 0.10 | ||
| Model fitted using MI‡ | 11.8 | 0.7 | 10.0 | 0.7 | 1.8 | 0.9 | −0.1 | 3.6 | 0.06 | ||
| Baseline to 18 months: | |||||||||||
| Raw data* | 242 | 11.9 | 0.8 | 220 | 11.6 | 0.9 | 0.3 | 1.2 | −2.1 | 2.6 | 0.83 |
| Model fitted† | 11.6 | 0.7 | 10.3 | 0.7 | 1.3 | 1.0 | −0.7 | 3.3 | 0.21 | ||
| Model fitted using MI‡ | 11.9 | 0.7 | 10.5 | 0.7 | 1.3 | 1.0 | −0.6 | 3.3 | 0.18 | ||
| Baseline to 24 months: | |||||||||||
| Raw data* | 248 | 11.7 | 0.9 | 227 | 11.0 | 0.9 | 0.6 | 1.2 | −1.8 | 3.0 | 0.62 |
| Model fitted† | 11.7 | 0.8 | 10.7 | 0.8 | 1.0 | 1.1 | −1.2 | 3.3 | 0.37 | ||
| Model fitted using MI‡ | 11.9 | 0.8 | 11.0 | 0.8 | 0.9 | 1.1 | −1.3 | 3.1 | 0.42 | ||
| SF-36 MCS: | |||||||||||
| Baseline to 1 month: | |||||||||||
| Raw data* | 314 | −0.2 | 0.7 | 314 | −0.5 | 0.6 | 0.3 | 0.9 | −1.5 | 2.2 | 0.71 |
| Model fitted† | 0.3 | 0.1 | 0.4 | 0.1 | −0.1 | 0.1 | −0.3 | 0.1 | 0.42 | ||
| Model fitted using MI‡ | 0.3 | 0.1 | 0.5 | 0.1 | −0.2 | 0.1 | −0.4 | 0.0 | 0.11 | ||
| Baseline to 6 months: | |||||||||||
| Raw data* | 287 | 1.8 | 0.7 | 277 | 1.6 | 0.8 | 0.2 | 1.1 | −1.9 | 2.2 | 0.88 |
| Model fitted† | 1.8 | 0.5 | 2.4 | 0.5 | −0.6 | 0.7 | −1.9 | 0.8 | 0.42 | ||
| Model fitted using MI‡ | 1.8 | 0.5 | 2.9 | 0.5 | −1.1 | 0.7 | −2.4 | 0.3 | 0.11 | ||
| Baseline to 12 months: | |||||||||||
| Raw data* | 267 | 1.8 | 0.8 | 252 | 1.7 | 0.8 | 0.1 | 1.1 | −2.1 | 2.3 | 0.93 |
| Model fitted† | . | 2.2 | 0.4 | 2.8 | 0.5 | −0.6 | 0.6 | −1.7 | 0.5 | 0.30 | |
| Model fitted using MI‡ | . | 2.3 | 0.5 | 3.3 | 0.5 | −1.0 | 0.6 | −2.1 | 0.1 | 0.08 | |
| Baseline to 18 months: | |||||||||||
| Raw data* | 243 | 2.0 | 0.9 | 220 | 2.2 | 0.8 | −0.2 | 1.2 | −2.6 | 2.2 | 0.87 |
| Model fitted† | 2.5 | 0.5 | 3.2 | 0.5 | −0.6 | 0.6 | −1.8 | 0.5 | 0.29 | ||
| Model fitted using MI‡ | 2.7 | 0.5 | 3.6 | 0.5 | −1.0 | 0.6 | −2.2 | 0.2 | 0.11 | ||
| Baseline to 24 months: | |||||||||||
| Raw data* | 249 | 2.9 | 0.8 | 227 | 3.0 | 0.8 | −0.1 | 1.1 | −2.3 | 2.1 | 0.94 |
| Model fitted† | 2.9 | 0.6 | 3.6 | 0.6 | −0.7 | 0.7 | −2.1 | 0.8 | 0.36 | ||
| Model fitted using MI‡ | 3.1 | 0.6 | 4.0 | 0.6 | −0.9 | 0.7 | −2.4 | 0.6 | 0.22 | ||
statistical comparison using an unpaired t-test (based on the raw data)
statistical comparison using a Wald test using a growth curve model with piece-wise linear regression over time adjusted for stratification factors: extent of DVT (iliofemoral vs. femoral-popliteal) and center, and baseline covariates: age, sex, BMI, Villalta score.
VEINES-QOL score (0–100 range) – higher is better; SF-36 major scales (0–100 range): physical component score (PCS) and mental component score (MCS) – higher is better; a 4-point difference is considered to be clinically meaningful
Auxiliary variables used in multiple imputation (MI): for SF-36 (MCS and PCS), age (continuous), sex, race, BMI (continuous) and all available SF-36 scores from previous visits; for VEINES-QOL, age (continuous), sex, BMI, extent of index DVT and all available VEINES scores from previous visits
SE, standard error; Est, estimate; 95% CI, 95% confidence interval
For the VEINES symptom subscale (VEINES-Sym), there was a suggestion that the model-fitted change scores were greater in PCDT vs. No PCDT from baseline to 6 months (difference 4.3; P=0.045) but not at any other change interval (Table 2).
For SF-36 PCS, the model-fitted change score was greater in PCDT than No PCDT patients at 1 month (difference 2.4; P=0.012) but not at any other change interval. For SF-36 MCS, there were no differences between PCDT and No PCDT in model-fitted change scores at any assessment (Table 2).
Results were similar in sensitivity analyses with models using multiple imputation (Tables 2), and when analyzed using the per-protocol analysis set (Supplement; Tables D and E).
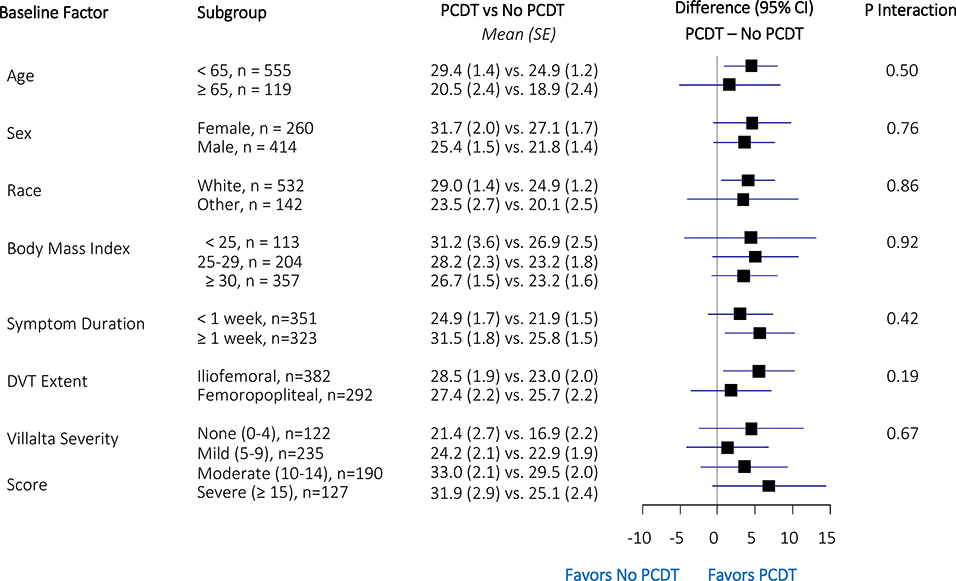
Subgroup Analysis
Forest plots of differences in model-fitted baseline-to-24-month VEINES-QOL change scores between PCDT and No PCDT patients according to subgroups are shown in Figure 2. For the change in QOL scores (VEINES-QOL, VEINES-Sym, SF-36 MCS, SF-36 PCS) from baseline to 24 months, none of the pre-specified subgroups, including for the iliofemoral vs. femoral-popliteal subgroups, showed statistically different (p<0.05) treatment effects. As a sensitivity analysis, we repeated the subgroup analysis using the change scores calculated from the raw data and the imputation-enhanced model-fitted estimates; none of these data sets showed any statistically significant subgroup effects.
Figure 2:
VEINES-QOL model-fitted change scores (Baseline to 24 months) treatment effects within subgroups. SE, standard error; CI, confidence interval
Iliofemoral DVT Subgroup
In the modified ITT analysis set, model-fitted VEINES-QOL change scores from baseline to all assessment times were greater in the PCDT group. Compared to the differences at 1 month (difference 10.0; P<0.0001) and at 6 months (difference 8.8; P<0.0001), the differences in favor of PCDT were about half as large at 12 months (difference 4.3; P=0.046), 18 months (difference 4.9; P=0.024), and at 24 months (difference 5.5; P=0.023). For VEINES-Sym, results were similar to those of VEINES-QOL (Table 3).
Table 3.
Iliofemoral DVT Subgroup: Change in Disease-specific and General QOL according to Treatment
| Outcome Measure | PCDT |
No PCDT |
PCDT – No PCDT Difference |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SE | n | Mean | SE | Est. | SE | 95% CI | p | ||
| VEINES-QOL: | |||||||||||
| Baseline to 1 month: | |||||||||||
| Raw data* | 180 | 15.5 | 1.7 | 169 | 5.3 | 1.8 | 10.2 | 2.5 | 5.3 | 15.1 | <0.0001 |
| Model fitted† | 16.1 | 1.6 | 6.1 | 1.7 | 10.0 | 2.2 | 5.7 | 14.2 | <0.0001 | ||
| Model fitted using MI‡ | 16.0 | 1.7 | 6.0 | 1.7 | 10.1 | 2.1 | 5.9 | 14.3 | <0.0001 | ||
| Baseline to 6 months: | |||||||||||
| Raw data* | 168 | 27.1 | 2.0 | 144 | 18.4 | 2.1 | 8.7 | 2.9 | 2.9 | 14.5 | 0.0032 |
| Model fitted† | 27.7 | 1.7 | 18.8 | 1.8 | 8.8 | 2.2 | 4.5 | 13.2 | <0.0001 | ||
| Model fitted using MI‡ | 28.1 | 1.7 | 18.9 | 1.7 | 9.2 | 2.1 | 5.0 | 13.3 | <0.0001 | ||
| Baseline to 12 months: | |||||||||||
| Raw data* | 153 | 26.8 | 2.2 | 133 | 24.4 | 2.2 | 2.4 | 3.1 | −3.8 | 8.6 | 0.44 |
| Model fitted† | 27.7 | 1.7 | 23.3 | 1.8 | 4.3 | 2.2 | 0.1 | 8.6 | 0.05 | ||
| Model fitted using MI‡ | 27.9 | 1.7 | 23.5 | 1.7 | 4.4 | 2.1 | 0.3 | 8.5 | 0.04 | ||
| Baseline to 18 months: | |||||||||||
| Raw data* | 139 | 28.3 | 2.4 | 122 | 25.8 | 2.4 | 2.4 | 3.4 | −4.2 | 9.0 | 0.47 |
| Model fitted† | 28.1 | 1.7 | 23.2 | 1.8 | 4.9 | 2.2 | 0.6 | 9.1 | 0.02 | ||
| Model fitted using MI‡ | 28.6 | 1.8 | 23.4 | 1.7 | 5.2 | 2.2 | 0.9 | 9.5 | 0.02 | ||
| Baseline to 24 months: | |||||||||||
| Raw data* | 141 | 28.3 | 2.3 | 128 | 23.3 | 2.6 | 5.0 | 3.4 | −1.8 | 11.8 | 0.15 |
| Model fitted† | 28.5 | 1.9 | 23.0 | 2.0 | 5.5 | 2.4 | 0.8 | 10.2 | 0.02 | ||
| Model fitted using MI‡ | 29.4 | 2.0 | 23.4 | 1.9 | 6.0 | 2.5 | 1.0 | 11.0 | 0.02 | ||
| VEINES-Sym: | |||||||||||
| Baseline to 1 month: | |||||||||||
| Raw data* | 177 | 12.7 | 1.9 | 169 | 5.7 | 1.9 | 7.1 | 2.7 | 1.7 | 12.4 | 0.0094 |
| Model fitted† | 13.6 | 1.7 | 6.6 | 1.8 | 7.1 | 2.2 | 2.7 | 11.4 | 0.0015 | ||
| Model fitted using MI‡ | 13.6 | 1.7 | 6.5 | 1.8 | 7.0 | 2.3 | 2.6 | 11.5 | 0.0020 | ||
| Baseline to 6 months: | |||||||||||
| Raw data* | 166 | 20.1 | 2.1 | 144 | 13.6 | 2.1 | 6.5 | 3.0 | 0.7 | 12.4 | 0.03 |
| Model fitted† | 21.8 | 1.8 | 13.9 | 1.8 | 7.9 | 2.2 | 3.5 | 12.3 | 0.0004 | ||
| Model fitted using MI‡ | 22.0 | 1.8 | 14.2 | 1.9 | 7.9 | 2.3 | 3.3 | 12.5 | 0.0008 | ||
| Baseline to 12 months: | |||||||||||
| Raw data* | 152 | 18.2 | 2.2 | 133 | 17.1 | 2.3 | 1.1 | 3.2 | −5.2 | 7.3 | 0.74 |
| Model fitted† | 19.7 | 1.7 | 15.8 | 1.8 | 4.0 | 2.2 | −0.3 | 8.3 | 0.07 | ||
| Model fitted using MI‡ | 19.7 | 1.7 | 16.1 | 1.9 | 3.7 | 2.3 | −0.8 | 8.2 | 0.11 | ||
| Baseline to 18 months: | |||||||||||
| Raw data* | 138 | 20.2 | 2.4 | 122 | 18.3 | 2.5 | 1.9 | 3.5 | −4.9 | 8.7 | 0.58 |
| Model fitted† | 20.7 | 1.7 | 16.0 | 1.8 | 4.7 | 2.2 | 0.5 | 8.9 | 0.03 | ||
| Model fitted using MI‡ | 21.2 | 1.7 | 16.5 | 1.9 | 4.7 | 2.2 | 0.3 | 9.0 | 0.04 | ||
| Baseline to 24 months: | |||||||||||
| Raw data* | 140 | 20.8 | 2.2 | 128 | 17.2 | 2.4 | 3.6 | 3.3 | −2.8 | 10.1 | 0.27 |
| Model fitted‡ | 21.6 | 1.9 | 16.2 | 1.9 | 5.4 | 2.4 | 0.7 | 10.1 | 0.02 | ||
| Model fitted using MI‡ | 22.7 | 1.9 | 17.0 | 2.0 | 5.6 | 2.4 | 0.8 | 10.4 | 0.02 | ||
| SF-36 PCS: | |||||||||||
| Baseline to 1 month: | |||||||||||
| Raw data* | 180 | 8.2 | 0.8 | 169 | 3.6 | 0.9 | 4.6 | 1.2 | 2.2 | 7.0 | 0.0002 |
| Model fitted† | 7.7 | 0.8 | 4.5 | 0.8 | 3.2 | 1.0 | 1.3 | 5.1 | 0.0010 | ||
| Model fitted using MI‡ | 7.8 | 0.8 | 4.5 | 0.8 | 3.3 | 1.0 | 1.4 | 5.2 | 0.0007 | ||
| Baseline to 6 months: | |||||||||||
| Raw data* | 168 | 11.0 | 1.0 | 144 | 8.1 | 1.1 | 2.9 | 1.5 | 0.0 | 5.8 | 0.05 |
| Model fitted† | 11.3 | 0.8 | 9.3 | 0.8 | 1.9 | 1.0 | 0.0 | 3.9 | 0.05 | ||
| Model fitted using MI‡ | 11.7 | 0.8 | 9.3 | 0.8 | 2.4 | 1.0 | 0.4 | 4.4 | 0.02 | ||
| Baseline to 12 months: | |||||||||||
| Raw data* | 153 | 11.4 | 1.1 | 133 | 10.4 | 1.2 | 1.0 | 1.6 | −2.2 | 4.3 | 0.53 |
| Model fitted† | 11.1 | 0.8 | 10.0 | 0.8 | 1.1 | 1.0 | −0.8 | 3.0 | 0.25 | ||
| Model fitted using MI‡ | 11.5 | 0.8 | 10.1 | 0.8 | 1.4 | 0.9 | −0.4 | 3.3 | 0.13 | ||
| Baseline to 18 months: | |||||||||||
| Raw data* | 138 | 11.5 | 1.2 | 122 | 11.9 | 1.3 | −0.4 | 1.7 | −3.8 | 2.9 | 0.81 |
| Model fitted† | 11.0 | 0.8 | 10.7 | 0.9 | 0.3 | 1.0 | −1.7 | 2.2 | 0.78 | ||
| Model fitted using MI‡ | 11.3 | 0.8 | 10.9 | 0.9 | 0.5 | 1.0 | −1.5 | 2.4 | 0.64 | ||
| Baseline to 24 months: | |||||||||||
| Raw data* | 141 | 11.2 | 1.2 | 128 | 11.2 | 1.3 | 0.0 | 1.8 | −3.5 | 3.4 | 0.99 |
| Model fitted† | . | 10.8 | 0.9 | 11.4 | 0.9 | −0.5 | 1.1 | −2.7 | 1.7 | 0.63 | |
| Model fitted using MI‡ | . | 11.2 | 0.9 | 11.7 | 1.0 | −0.5 | 1.1 | −2.7 | 1.7 | 0.66 | |
| SF-36 MCS: | |||||||||||
| Baseline to 1 month: | |||||||||||
| Raw data* | 180 | 0.1 | 0.9 | 169 | 0.3 | 1.0 | −0.1 | 1.3 | −2.7 | 2.5 | 0.93 |
| Model fitted‡ | 0.3 | 0.1 | 0.4 | 0.1 | −0.1 | 0.1 | −0.3 | 0.2 | 0.56 | ||
| Model fitted using MI‡ | 0.3 | 0.1 | 0.5 | 0.1 | −0.2 | 0.1 | −0.4 | 0.1 | 0.21 | ||
| Baseline to 6 months: | |||||||||||
| Raw data* | 168 | 1.9 | 0.9 | 144 | 3.1 | 1.1 | −1.2 | 1.5 | −4.0 | 1.7 | 0.43 |
| Model fitted† | 1.8 | 0.6 | 2.2 | 0.6 | −0.5 | 0.8 | −2.0 | 1.1 | 0.56 | ||
| Model fitted using MI‡ | 1.8 | 0.6 | 2.8 | 0.6 | −0.9 | 0.8 | −2.4 | 0.5 | 0.21 | ||
| Baseline to 12 months: | |||||||||||
| Raw data* | 153 | 1.9 | 1.0 | 133 | 3.4 | 1.2 | −1.5 | 1.6 | −4.6 | 1.6 | 0.34 |
| Model fitted† | 2.1 | 0.5 | 2.6 | 0.5 | −0.5 | 0.7 | −1.9 | 0.8 | 0.41 | ||
| Model fitted using MI‡ | 2.2 | 0.5 | 3.2 | 0.5 | −0.9 | 0.6 | −2.2 | 0.3 | 0.15 | ||
| Baseline to 18 months: | |||||||||||
| Raw data* | 138 | 1.5 | 1.2 | 122 | 3.4 | 1.2 | −1.8 | 1.7 | −5.2 | 1.5 | 0.27 |
| Model fitted† | 2.4 | 0.5 | 3.1 | 0.5 | −0.6 | 0.6 | −1.9 | 0.6 | 0.33 | ||
| Model fitted using MI‡ | 2.6 | 0.5 | 3.5 | 0.5 | −0.9 | 0.6 | −2.2 | 0.3 | 0.16 | ||
| Baseline to 24 months: | |||||||||||
| Raw data* | 141 | 3.7 | 1.1 | 128 | 3.9 | 1.2 | −0.2 | 1.6 | −3.3 | 2.9 | 0.88 |
| Model fitted† | 2.8 | 0.6 | 3.5 | 0.6 | −0.7 | 0.7 | −2.2 | 0.7 | 0.32 | ||
| Model fitted using MI‡ | 3.0 | 0.6 | 3.9 | 0.6 | −0.9 | 0.7 | −2.4 | 0.6 | 0.23 | ||
statistical comparison using an unpaired t-test (based on the raw data)
statistical comparison using a Wald test using a growth curve model with piece-wise linear regression over time adjusted for stratification factors: extent of DVT (iliofemoral vs. femoral-popliteal) and center, and baseline covariates: age, sex, BMI, Villalta score.
VEINES-QOL score (0–100 range) – higher is better; SF-36 major scales (0–100 range): physical component score (PCS) and mental component score (MCS) – higher is better; a 4-point difference is considered to be clinically meaningful
Auxiliary variables used in multiple imputation (MI): for SF-36 (MCS and PCS), age (continuous), sex, race, BMI (continuous) and all available SF-36 scores from previous visits; for VEINES-QOL, age (continuous), sex, BMI, extent of index DVT and all available VEINES scores from previous visits
SE, standard error; Est, estimate; 95% CI, 95% confidence interval
For SF-36 PCS, model-fitted change scores at 1 month were greater in the PCDT group (difference 3.2; P=0.0010), but change scores from baseline to other assessment times, including 24 months, did not differ (Table 3). For SF-36 MCS, there were no differences between PCDT and No PCDT in model-fitted change in scores from baseline to any assessment, including 24 months (Table 3).
Results were substantively similar in the sensitivity analyses with models using multiple imputation (Tables 3). When analyzed using the per-protocol analysis set (Supplement; Tables F and G), the above noted differences between PCDT and no PCDT were greater, particularly for change in VEINES-QOL scores from baseline to 18 months (difference 5.8; P=0.0086) and baseline to 24 months (difference 6.6; P=0.0067) (Table F).
Femoral-popliteal DVT Subgroup
VEINES-QOL, VEINES-Sym, SF-36 PCS and SF-36 MCS change scores from baseline to each assessment were similar in the PCDT and No PCDT groups (Table 4). Results were similar in sensitivity analyses with models using multiple imputation and when analyzed using the per-protocol analysis set (Supplement; Tables H and I).
Table 4.
Femoral-popliteal DVT Subgroup: Change in Disease-specific and General QOL according to Treatment
| Outcome Measure | PCDT |
No PCDT |
PCDT – No PCDT Difference |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SE | n | Mean | SE | Est. | SE | 95% CI | p | ||
| VEINES-QOL: | |||||||||||
| Baseline to 1 month: | |||||||||||
| Raw data* | 134 | 12.4 | 2.2 | 145 | 12.3 | 1.6 | 0.1 | 2.7 | −5.2 | 5.4 | 0.97 |
| Model fitted† | 13.0 | 1.9 | 12.5 | 1.8 | 0.5 | 2.4 | −4.2 | 5.2 | 0.83 | ||
| Model fitted using MI‡ | 13.5 | 1.9 | 12.6 | 1.8 | 0.9 | 2.5 | −3.9 | 5.7 | 0.71 | ||
| Baseline to 6 months: | |||||||||||
| Raw data* | 119 | 24.4 | 2.4 | 133 | 24.3 | 2.0 | 0.0 | 3.1 | −6.1 | 6.1 | 0.75 |
| Model fitted† | 25.0 | 1.9 | 24.4 | 1.9 | 0.6 | 2.4 | −4.1 | 5.4 | 0.32 | ||
| Model fitted using MI‡ | 25.8 | 1.9 | 24.8 | 1.9 | 1.0 | 2.4 | −3.6 | 5.7 | 0.28 | ||
| Baseline to 12 months: | |||||||||||
| Raw data* | 114 | 24.9 | 2.4 | 119 | 25.9 | 2.1 | −1.0 | 3.2 | −7.3 | 5.3 | 0.83 |
| Model fitted† | 25.5 | 1.9 | 27.9 | 1.9 | −2.4 | 2.4 | −7.0 | 2.3 | 0.90 | ||
| Model fitted using MI‡ | 26.1 | 1.9 | 28.6 | 1.9 | −2.5 | 2.3 | −7.1 | 2.1 | 0.82 | ||
| Baseline to 18 months: | |||||||||||
| Raw data* | 105 | 25.8 | 2.7 | 98 | 25.0 | 2.5 | 0.8 | 3.7 | −6.5 | 8.1 | 0.99 |
| Model fitted† | 26.5 | 2.0 | 26.8 | 1.9 | −0.3 | 2.4 | −5.0 | 4.5 | 0.79 | ||
| Model fitted using MI‡ | 27.2 | 2.0 | 27.7 | 1.9 | −0.6 | 2.4 | −5.3 | 4.2 | 0.67 | ||
| Baseline to 24 months: | |||||||||||
| Raw data* | 108 | 26.3 | 2.6 | 99 | 25.2 | 2.3 | 1.1 | 3.5 | −5.8 | 8.1 | 0.75 |
| Model fitted† | 27.4 | 2.2 | 25.7 | 2.1 | 1.8 | 2.7 | −3.5 | 7.1 | 0.51 | ||
| Model fitted using MI‡ | 28.3 | 2.2 | 26.9 | 2.2 | 1.4 | 2.8 | −4.1 | 6.9 | 0.61 | ||
| VEINES-Sym: | |||||||||||
| Baseline to 1 month: | |||||||||||
| Raw data* | 134 | 11.2 | 2.3 | 145 | 12.5 | 1.8 | −1.3 | 2.9 | −6.9 | 4.4 | 0.66 |
| Model fitted† | 12.4 | 2.0 | 12.7 | 1.9 | −0.3 | 2.5 | −5.1 | 4.5 | 0.91 | ||
| Model fitted using MI‡ | 12.8 | 2.0 | 12.4 | 1.9 | 0.4 | 2.5 | −4.5 | 5.4 | 0.86 | ||
| Baseline to 6 months: | |||||||||||
| Raw data* | 119 | 20.3 | 2.4 | 133 | 19.1 | 2.2 | 1.2 | 3.3 | −5.2 | 7.7 | 0.70 |
| Model fitted† | 20.8 | 2.0 | 19.6 | 1.9 | 1.1 | 2.4 | −3.7 | 5.9 | 0.65 | ||
| Model fitted using MI‡ | 21.2 | 2.0 | 19.6 | 1.9 | 1.7 | 2.5 | −3.2 | 6.6 | 0.50 | ||
| Baseline to 12 months: | |||||||||||
| Raw data* | 114 | 18.0 | 2.4 | 119 | 19.2 | 2.3 | −1.2 | 3.3 | −7.6 | 5.3 | 0.72 |
| Model fitted† | 18.9 | 2.0 | 20.9 | 1.9 | −2.1 | 2.4 | −6.8 | 2.6 | 0.39 | ||
| Model fitted using MI‡ | 18.9 | 2.0 | 20.9 | 1.9 | −2.1 | 2.3 | −6.6 | 2.5 | 0.38 | ||
| Baseline to 18 months: | |||||||||||
| Raw data* | 105 | 18.0 | 2.8 | 98 | 17.9 | 2.7 | 0.1 | 3.8 | −7.5 | 7.7 | 0.97 |
| Model fitted† | 20.0 | 2.0 | 20.6 | 2.0 | −0.7 | 2.4 | −5.4 | 4.1 | 0.79 | ||
| Model fitted using MI‡ | 20.3 | 2.0 | 20.9 | 1.9 | −0.6 | 2.3 | −5.1 | 3.9 | 0.79 | ||
| Baseline to 24 months: | |||||||||||
| Raw data* | 108 | 20.4 | 2.7 | 99 | 19.5 | 2.1 | 0.9 | 3.5 | −5.9 | 7.7 | 0.78 |
| Model fitted† | 21.1 | 2.2 | 20.3 | 2.1 | 0.8 | 2.7 | −4.5 | 6.1 | 0.78 | ||
| Model fitted using MI‡ | 21.7 | 2.1 | 20.8 | 2.1 | 0.9 | 2.6 | −4.3 | 6.0 | 0.74 | ||
| SF-36 PCS: | |||||||||||
| Baseline to 1 month: | |||||||||||
| Raw data* | 133 | 5.9 | 1.0 | 145 | 6.4 | 0.8 | −0.5 | 1.2 | −2.9 | 2.0 | 0.70 |
| Model fitted† | 6.3 | 0.9 | 6.6 | 0.8 | −0.3 | 1.1 | −2.4 | 1.8 | 0.77 | ||
| Model fitted using MI‡ | 6.4 | 0.9 | 6.4 | 0.9 | 0.0 | 1.1 | −2.2 | 2.1 | 0.97 | ||
| Baseline to 6 months: | |||||||||||
| Raw data* | 119 | 10.7 | 1.2 | 133 | 11.0 | 1.0 | −0.3 | 1.5 | −3.3 | 2.7 | 0.86 |
| Model fitted† | 10.4 | 0.9 | 11.0 | 0.9 | −0.6 | 1.1 | −2.7 | 1.5 | 0.59 | ||
| Model fitted using MI‡ | 10.8 | 0.9 | 10.7 | 0.9 | 0.0 | 1.1 | −2.1 | 2.1 | 0.98 | ||
| Baseline to 12 months: | |||||||||||
| Raw data* | 113 | 12.0 | 1.1 | 119 | 10.5 | 1.0 | 1.5 | 1.5 | −1.4 | 4.5 | 0.31 |
| Model fitted† | 10.9 | 0.9 | 11.0 | 0.9 | −0.2 | 1.1 | −2.2 | 1.9 | 0.88 | ||
| Model fitted using MI‡ | 11.1 | 0.9 | 10.9 | 0.8 | 0.2 | 1.0 | −1.8 | 2.2 | 0.85 | ||
| Baseline to 18 months: | |||||||||||
| Raw data* | 104 | 12.4 | 1.2 | 98 | 11.2 | 1.2 | 1.2 | 1.7 | −2.2 | 4.5 | 0.50 |
| Model fitted† | 11.3 | 0.9 | 11.1 | 0.9 | 0.3 | 1.1 | −1.9 | 2.5 | 0.82 | ||
| Model fitted using MI‡ | 11.5 | 0.9 | 11.1 | 0.9 | 0.4 | 1.1 | −1.7 | 2.5 | 0.73 | ||
| Baseline to 24 months: | |||||||||||
| Raw data* | 107 | 12.3 | 1.2 | 99 | 10.9 | 1.2 | 1.4 | 1.7 | −2.0 | 4.8 | 0.41 |
| Model fitted† | 11.8 | 1.0 | 11.1 | 1.0 | 0.7 | 1.3 | −1.8 | 3.2 | 0.59 | ||
| Model fitted using MI‡ | 11.8 | 1.0 | 11.3 | 1.0 | 0.5 | 1.2 | −1.9 | 2.9 | 0.66 | ||
| SF-36 MCS: | |||||||||||
| Baseline to 1 month: | |||||||||||
| Raw data* | 134 | −0.6 | 1.0 | 145 | −1.4 | 0.8 | 0.8 | 1.3 | −1.7 | 3.4 | 0.53 |
| Model fitted† | 0.3 | 0.1 | 0.4 | 0.1 | −0.1 | 0.1 | −0.3 | 0.2 | 0.56 | ||
| Model fitted using MI‡ | 0.3 | 0.1 | 0.5 | 0.1 | −0.2 | 0.1 | −0.4 | 0.1 | 0.21 | ||
| Baseline to 6 months: | |||||||||||
| Raw data* | 119 | 1.5 | 1.1 | 133 | 0.0 | 1.0 | 1.5 | 1.5 | −1.5 | 4.6 | 0.33 |
| Model fitted† | 1.8 | 0.6 | 2.2 | 0.6 | −0.5 | 0.8 | −2.0 | 1.1 | 0.56 | ||
| Model fitted using MI‡ | 1.8 | 0.6 | 2.8 | 0.6 | −0.9 | 0.8 | −2.4 | 0.5 | 0.21 | ||
| Baseline to 12 months: | |||||||||||
| Raw data* | 114 | 1.6 | 1.3 | 119 | −0.2 | 1.0 | 1.9 | 1.6 | −1.3 | 5.1 | 0.26 |
| Model fitted† | 2.1 | 0.5 | 2.6 | 0.5 | −0.5 | 0.7 | −1.9 | 0.8 | 0.41 | ||
| Model fitted using MI‡ | 2.2 | 0.5 | 3.2 | 0.5 | −0.9 | 0.6 | −2.2 | 0.3 | 0.15 | ||
| Baseline to 18 months: | |||||||||||
| Raw data* | 105 | 2.6 | 1.2 | 98 | 0.7 | 1.1 | 1.9 | 1.7 | −1.4 | 5.2 | 0.26 |
| Model fitted† | 2.4 | 0.5 | 3.1 | 0.5 | −0.6 | 0.6 | −1.9 | 0.6 | 0.33 | ||
| Model fitted using MI‡ | 2.6 | 0.5 | 3.5 | 0.5 | −0.9 | 0.6 | −2.2 | 0.3 | 0.16 | ||
| Baseline to 24 months: | |||||||||||
| Raw data* | 108 | 1.8 | 1.1 | 99 | 1.8 | 1.2 | 0.1 | 1.6 | −3.1 | 3.3 | 0.95 |
| Model fitted† | 2.8 | 0.6 | 3.5 | 0.6 | −0.7 | 0.7 | −2.2 | 0.7 | 0.32 | ||
| Model fitted using MI‡ | 3.0 | 0.6 | 3.9 | 0.6 | −0.9 | 0.7 | −2.4 | 0.6 | 0.23 | ||
statistical comparison using an unpaired t-test (based on the raw data)
statistical comparison using a Wald test using a growth curve model with piece-wise linear regression over time adjusted for stratification factors: extent of DVT (iliofemoral vs. femoral-popliteal) and center, and baseline covariates: age, sex, BMI, Villalta score.
VEINES-QOL score (0–100 range) – higher is better; SF-36 major scales (0–100 range): physical component score (PCS) and mental component score (MCS) – higher is better; a 4-point difference is considered to be clinically meaningful
Auxiliary variables used in multiple imputation (MI): for SF-36 (MCS and PCS), age (continuous), sex, race, BMI (continuous) and all available SF-36 scores from previous visits; for VEINES-QOL, age (continuous), sex, BMI, extent of index DVT and all available VEINES scores from previous visits
SE, standard error; Est, estimate; 95% CI, 95% confidence interval
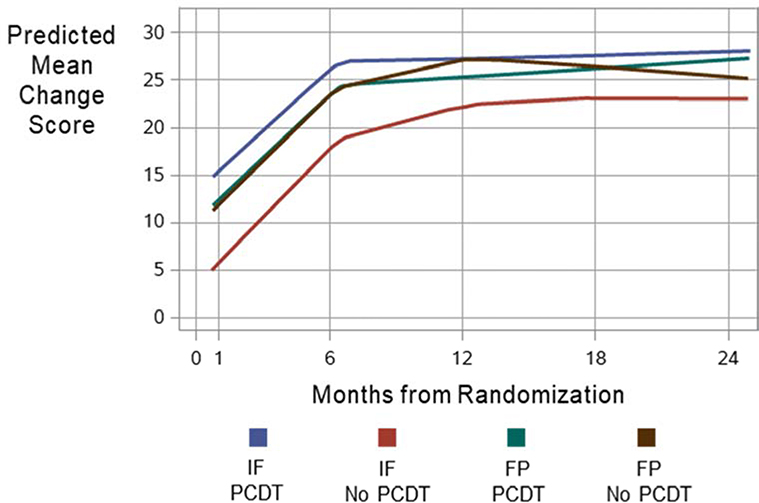
Trajectories of the VEINES-QOL scores in Iliofemoral and Femoral-popliteal DVT Subgroups
Figure 3 shows LOESS-smoothed estimates of the model predicted VEINES-QOL change scores from baseline to each assessment in the four groups defined by treatment (PCDT, No PCDT) and extent of DVT (iliofemoral, femoral-popliteal). All groups showed substantial improvement in VEINES-QOL change scores during follow-up. The change in the PCDT iliofemoral subgroup was greater than in the No PCDT iliofemoral subgroup, particularly during the first 6 months. The change in PCDT and No PCDT femoral-popliteal groups were similar at all time points.
Figure 3:
LOESS-smoothed estimates of the model-predicted VEINES-QOL mean change-from-baseline scores at each assessment for the 4 groups defined by extent of DVT and treatment arm. IF, iliofemoral DVT; FP, isolated femoral-popliteal DVT
Interpretation of the growth curve model QOL results
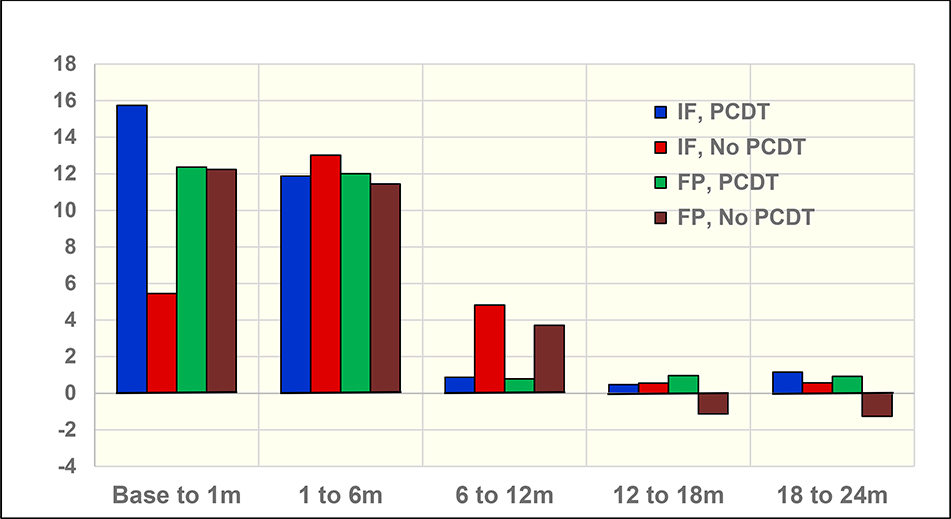
In addition to evaluating QOL improvement with PCDT from baseline to individual timepoints through 2 years, our analysis sought to determine the pattern of change over time. With the inclusion of the extent of DVT subgroups in the growth curve model, we observed that the improvement in VEINES-QOL/Sym and SF-36 PCS QOL with PCDT was statistically significantly greater in the iliofemoral DVT group compared with the femoral-popliteal DVT group during the first month post-randomization, but not during the intervals from 1 to 6 months, 6 to 12 months, 12 to 18 months, or 18 to 24 months (Supplement, Appendix A). The incremental changes in VEINES-QOL scores between assessments are shown in Figure 4. For all four groups, there are substantial QOL incremental improvements from baseline to 1 month, and from 1 month to 6 months, but not beyond that. For patients with iliofemoral DVT, the largest interval improvement occurs in PCDT vs. No PCDT patients from baseline to 1 month. However, for the between-visit intervals beyond 1 month, differences in the degree of QOL change between treatment arms are not apparent. For femoral-popliteal DVT patients, the treatment differences were negligible for all incremental changes.
Figure 4.
VEINES-QOL Incremental Change by Group (Model-fitted Estimates). IF, iliofemoral DVT; FP, isolated femoral-popliteal DVT
DISCUSSION
In our original publication describing the main results of the ATTRACT Trial, we evaluated QOL scores at two time-points (baseline and 24 months post-randomization) in the overall study population, and reported no difference in the degree of change in QOL between patients who were assigned, versus not assigned, to PCDT. In the current, more detailed analysis of QOL outcomes in the ATTRACT Trial, we used more sophisticated analytic methods that allowed us to more fully utilize all available data from all follow-up assessments, enabling us to assess time-dependent patterns of QOL change during different time periods within the 24 months of study follow-up.
We report four main findings. First, regardless of treatment group, venous disease-specific QOL and general QOL improved markedly during the 24 months after diagnosis of proximal DVT, with most of this improvement occurring during the first 6 months after diagnosis. Second, patients with iliofemoral DVT had poorer QOL scores over the 24 months of follow-up than patients with femoral-popliteal DVT. Third, in the total study population, patients in the PCDT group had greater improvement in venous disease-specific QOL during the first 1 month and 6 months after randomization compared with the No PCDT group, but this benefit was no longer apparent by 12, 18 or 24 months, and there was no difference in the extent of improvement with PCDT in overall physical or mental general QOL at any time point. Fourth, the greater improvement in disease-specific QOL with PCDT during the first 6 months was only observed in patients who had iliofemoral DVT, and not in patients with femoral-popliteal DVT. Our results also suggest that in patients with iliofemoral DVT, but not those with femoral-popliteal DVT, disease-specific QOL change scores were also better with PCDT at 12, 18, and 24 months. In patients with iliofemoral DVT, the improvement in disease-specific QOL with PCDT vs. No PCDT was large enough to be considered clinically important during the first 6 months, but of uncertain clinical importance subsequently.
Our observation that general and venous disease-specific QOL improves over 24 months after DVT and that most of this improvement occurs in the first 6 months after DVT is consistent with previous reports by our group5, 21 and by the CAVENT (Catheter-Directed Venous Thrombolysis in Acute Iliofemoral Vein Thrombosis) trial investigators 28.
We found that patients with iliofemoral DVT have worse QOL than patients with femoral-popliteal DVT, which is consistent with previous observations that QOL is poorer after proximal DVT than after isolated distal DVT 5, and that PTS is more common and more severe after iliofemoral DVT than after femoral-popliteal or more distal DVT.29, 30
Why might PCDT have improved venous QOL even though it did not prevent PTS? First, most of the improvement in QOL was in the first 6 months and the ATTRACT trial did find that PCDT reduced clot burden and reduced early leg pain and swelling to a greater extent than No PCDT, and was associated with a reduced point prevalence of PTS at the 6-month visit (but not thereafter)6. Second, although PCDT did not prevent PTS it did reduce its severity, and less severe PTS is likely to be associated with improved QOL. Third, although both measures ask about leg pain, heaviness, cramping, itching, and pins and needles sensation, the VEINES-QOL instrument used to measure venous QOL may have captured different clinical characteristics than the Villalta scale used to measure PTS. We did not utilize a self-reported QOL measure as the study’s primary outcome because of the possibility of response bias that could stem from patients’ knowledge of their treatment allocation in this open-label study. Further work to compare the performance and correlation of these and other outcome measures would be of interest.
Strengths of our study include that we assessed both disease-specific and general QOL repeatedly during 24 months using validated measures. Although patients and healthcare providers were not blinded to treatment, bias was minimized by having central randomization, allocation concealment, blinded outcome assessment, and comparable use of anticoagulants and compression stockings during follow-up in both groups. Our modelling techniques enabled us to use all of the data during follow-up, to assess if effects on QOL differed over time, and to adjust for baseline factors that may influence QOL. Consistency of findings in sensitivity analysis that used multiple imputation to address missing data and in per-protocol analyses increase confidence in the validity of our findings. Stratification of randomization by whether the iliofemoral outflow tract was involved, which is known to influence the risk of PTS and its severity, supports separate reporting of findings in the iliofemoral and femoral-popliteal subgroups, as recommended by societal consensus guidelines.8, 9
Our study also has limitations. During the ATTRACT Trial, a number of measures were taken to ensure that patients attended follow-up visits, including electronic reminders to study sites of upcoming patient visits, and routine education of study teams on best practices for patient retention at investigator meetings, teleconferences, and via electronic communications. In some instances, patients who had moved out of town were permitted to be seen at different study sites. Nevertheless, we had missing QOL responses primarily due to missed visits, which increased over time and were greater in the No PCDT group, for reasons that are unclear. However, as noted in the preceding paragraph, sensitivity analyses suggest that our findings are robust. We also acknowledge that our analysis has limited power to detect differences in treatment effects between subgroups and, particularly, within each of the femoral-popliteal and iliofemoral subgroups.
In conclusion, PCDT leads to better disease-specific QOL at 1 month and 6 months in patients with iliofemoral DVT, but not in patients with femoral-popliteal DVT. PCDT also appears to lead to greater improvement in disease-specific QOL over 24 months in patients with iliofemoral DVT.
Supplementary Material
Article Highlights.
Type of Research:
Analysis of a multicenter randomized trial
Key Findings:
In this analysis of the ATTRACT Study, among patients with proximal deep vein thrombosis (DVT), pharmacomechanical catheter-directed thrombolysis (PCDT) with anticoagulation, compared with anticoagulation alone, had a beneficial effect on quality of life (QOL) during the first 6 months post-treatment (e.g. VEINES-QOL change scores were greater in PCDT vs. No PCDT from baseline to one month (difference 5.7; P=0.0006) and baseline to 6 months (5.1; P=0.0029). Further, among proximal DVT patients with iliofemoral DVT, this benefit was apparent over 24 months post-treatment.
Take Home Message:
Patients with iliofemoral DVT have a worse long-term prognosis (poorer QOL) than patients with femoral-popliteal DVT. Early use of pharmacomechanical catheter-directed thrombolysis improves QOL in patients with acute iliofemoral DVT and may be reasonable to consider in selected patients who have severe symptoms, low bleeding risk, and a willingness to undergo a catheter-based procedure, after careful discussion of the benefits and risks.
Acknowledgements
The study’s development and conduct were supported by the Society of Interventional Radiology Foundation. Dr. Kahn is a Tier 1 Canada Research Chair holder and is an investigator of the Canadian Institutes of Health Research-funded CanVECTOR Network. Dr. Kearon is supported by an Investigator Award from the Heart and Stroke Foundation of Canada and the Jack Hirsh Professorship in Thromboembolism. The authors wish to thank the entire network of investigators and study staff at the coordinating centers, core laboratories, and clinical centers (see Appendix B).
Sources of funding
The ATTRACT Trial was supported by grants from the National Heart, Lung, and Blood Institute (NHLBI) for the clinical coordinating center (U01-HL088476 to Washington University in St. Louis) and data coordinating center (U01-HL088118 to McMaster University, Hamilton, ON); the Washington University Center for Translational Therapies in Thrombosis, which is supported by a grant from the NHLBI (U54-HL112303); the Washington University Institute of Clinical and Translational Sciences, which is supported by a grant from the National Center for the Advancement of Translational Sciences (UL1-TR00044810); Boston Scientific; Covidien (now Medtronic); Genentech; the Society of Interventional Radiology Foundation; the Canada Research Chairs Program (Tier 1 support to Dr. Kahn); the CanVECTOR Network (funded by Canadian Institutes of Health Research CDT-142654, to Dr. Kahn); the Heart and Stroke Foundation of Canada (Investigator Award to Dr. Kearon); and a Jack Hirsh Professorship in Thrombosis (to Dr. Kearon). BSN Medical donated the compression stockings.
Disclosures (in order of authorship)
Susan R. Kahn: Advisory board fees from BMS Pfizer, Sanofi, and Aspen.
Jim A. Julian: None.
Clive Kearon: None.
Chu-Shu Gu: None.
David J. Cohen: Grant support from Abbott Vascular and Boston Scientific; consulting fees, Cardinal Health; grant support and consulting fees, Medtronic.
Elizabeth A. Magnuson: None.
Anthony J. Comerota: Consulting fees from Medtronic.
Samuel Z. Goldhaber: Grant support from BiO2 Medical; grant support and consulting fees, Boehringer Ingelheim, BMS, Daiichi Sankyo, Janssen, Portola, Bayer, and BTG/Ekos.
Michael R. Jaff: Holds equity in Embolitech and Venarum; uncompensated advisor, Boston Scientific, Cordis Corporation, and Medtronic; consultant, Volcano/Phillips.
Mahmood K. Razavi: Consulting fees from Abbott, Boston Scientific, Medtronic, Veniti, and Volcano/Phillips
Andrei L. Kindzelski: None.
Joseph Schneider: None.
Paul Kim: None.
Rabih Chaer: Speaker, Boston Scientific; medical advisory board, Abbott.
Akhilesh Sista: Unrestricted research grant to NYU, Penumbra, Inc.; Unpaid scientific advisory board member, Thrombolex.
Robert McLafferty: DSMB member for clinical trial, Veniti.
John A. Kaufman: Medical Advisory Board, Argon Medical; Consultant, Novate; Medical Advisory Board and Ownership Interest, Bio2 Medical; Ownership Interest, Veniti; Consultant, Cook Medical.
Brandt Wible: Amirsys-Elsevier for work performed on STATdx, RADPrimer, and the textbook
Diagnostic Imaging: Interventional Procedures, 2nd edition.
Morey Blinder: Honoraria from Janssen Pharmaceuticals.
Suresh Vedantham: Grant support from Cook Medical.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rabinovich A, Kahn SR. How I treat the post-thrombotic syndrome. Blood. 2018;pii: blood-2018–01-785956. doi: 10.1182/blood-2018-01-785956. [Epub ahead of print]. [DOI] [Google Scholar]
- 2.Kahn SR, Comerota AJ, Cushman M, Evans NS, Ginsberg JS, Goldenberg NA, et al. The Postthrombotic Syndrome: Evidence-Based Prevention, Diagnosis, and Treatment Strategies: A Scientific Statement From the American Heart Association. Circulation. 2014;130(18):1636–61. [DOI] [PubMed] [Google Scholar]
- 3.van Korlaar I, Vossen C, Rosendaal F, Cameron L, Bovill E, Kaptein A. Quality of life in venous disease. Thromb Haemost. 2003;90(1):27–35. [PubMed] [Google Scholar]
- 4.Kahn SR, Ducruet T, Lamping DL, Arsenault L, Miron MJ, Roussin A, et al. Prospective evaluation of health-related quality of life in patients with deep venous thrombosis. Arch Intern Med. 2005;165(10):1173–8. [DOI] [PubMed] [Google Scholar]
- 5.Kahn SR, Shbaklo H, Lamping DL, Holcroft CA, Shrier I, Miron MJ, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6(7):1105–12. [DOI] [PubMed] [Google Scholar]
- 6.Vedantham S, Goldhaber SZ, Julian JA, Kahn SR, Jaff MR, Cohen DJ, et al. Pharmacomechanical Catheter-Directed Thrombolysis for Deep-Vein Thrombosis. New England Journal of Medicine. 2017;377(23):2240–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vedantham S, Goldhaber SZ, Kahn SR, Julian J, Magnuson E, Jaff MR, et al. Rationale and design of the ATTRACT Study: A multicenter randomized trial to evaluate pharmacomechanical catheter-directed thrombolysis for the prevention of postthrombotic syndrome in patients with proximal deep vein thrombosis. American Heart Journal. 2013;165(4):523–30.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, et al. Management of Massive and Submassive Pulmonary Embolism, Iliofemoral Deep Vein Thrombosis, and Chronic Thromboembolic Pulmonary Hypertension. Circulation. 2011;123(16):1788–830. [DOI] [PubMed] [Google Scholar]
- 9.Vedantham S, Grassi CJ, Ferral H, Patel NH, Thorpe PE, Antonacci VP, et al. Reporting standards for endovascular treatment of lower extremity deep vein thrombosis. J Vasc Interv Radiol. 2006;17(3):417–34. [DOI] [PubMed] [Google Scholar]
- 10.Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149(2):315–52. [DOI] [PubMed] [Google Scholar]
- 11.Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic Therapy for VTE Disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S–94S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vedantham S, Sista AK, Klein SJ, Nayak L, Razavi MK, Kalva SP, et al. Quality Improvement Guidelines for the Treatment of Lower-Extremity Deep Vein Thrombosis with Use of Endovascular Thrombus Removal. Journal of Vascular and Interventional Radiology. 2014;25(9):1317–25. [DOI] [PubMed] [Google Scholar]
- 13.Kahn SR. Measurement properties of the Villalta scale to define and classify the severity of the post-thrombotic syndrome. J Thromb Haemost. 2009;7:884–8. [DOI] [PubMed] [Google Scholar]
- 14.Kahn SR, Partsch H, Vedantham S, Prandoni P, Kearon C. Definition of post-thrombotic syndrome of the leg for use in clinical investigations: a recommendation for standardization. J Thromb Haemost. 2009;7:879–83. [DOI] [PubMed] [Google Scholar]
- 15.Lamping DL, Schroter S, Kurz X, Kahn SR, Abenhaim L. Evaluating outcomes in chronic venous disorders of the leg: Development of a scientifically rigorous, patient-reported measure of symptoms and quality of life. J Vasc Surg. 2003;37(2):410–9. [DOI] [PubMed] [Google Scholar]
- 16.Kahn SR, Lamping DL, Ducruet T, Arsenault L, Miron MJ, Roussin A, et al. VEINES-QOL/Sym questionnaire was a reliable and valid disease-specific quality of life measure for deep venous thrombosis. J Clin Epidemiol. 2006;59(10):1049–56. [DOI] [PubMed] [Google Scholar]
- 17.Ware JE, Snow KK, Kosinski MA, Gandek B. SF-36 Health Survey: Manual and interpretation guide. Boston: The Health Institute, New England Medical Center Hospitals; 1993. [Google Scholar]
- 18.Ware J, Kosinski M, Bjorner J, Turner-Bowker D, Gandek B, Maruish M. Development. User’s Manual for the SF-36v2® Health Survey. Lincoln (RI): QualityMetric Incorporated; 2007. [Google Scholar]
- 19.Kahn SR, Hirsch A, Shrier I. Effect of post-thrombotic syndrome on health-related quality of life after deep venous thrombosis. Arch Intern Med. 2002;162:1144–8. [DOI] [PubMed] [Google Scholar]
- 20.Kahn SR, Kearon C, Julian JA, MacKinnon B, Kovacs MJ, Wells P, et al. Predictors of the post-thrombotic syndrome during long-term treatment of proximal deep vein thrombosis. J Thromb Haemost. 2005;3(4):718–23. [DOI] [PubMed] [Google Scholar]
- 21.Kahn SR, Shapiro S, Wells PS, Rodger MA, Kovacs MJ, Anderson DR, et al. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383(9920):880–8. [DOI] [PubMed] [Google Scholar]
- 22.Ware JE, Kosinski MA, Keller SD. SF-36 physical and mental component summary measures: A user’s manual. Boston: The Health Institute, New England Medical Center; 1994. [Google Scholar]
- 23.Streiner DL, Norman GR. Health measurement scales: A practical guide to their development and use (2nd edition). Oxford: Oxford University Press; 1995. [Google Scholar]
- 24.Bland JM, Dumville JC, Ashby RL, Gabe R, Stubbs N, Adderley U, et al. Validation of the VEINES-QOL quality of life instrument in venous leg ulcers: repeatability and validity study embedded in a randomised clinical trial. BMC cardiovascular disorders. 2015;15(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ware JE, Kosinski M, Lincoln : Quality M. SF-36 physical and mental health summary scores: A manual for users of version 1, 2nd2001. [Google Scholar]
- 26.Fairclough DL. Design and Analysis of Quality of Life Studies in Clinical Trials, Second Edition; Chapman and Hall/CRC Press; Boca Raton, Florida: 2010. ISBN 9781420061178. Page 85. [Google Scholar]
- 27.Cleveland WS, Devlin SJ. Locally Weighted Regression: An Approach to Regression Analysis by Local Fitting. Journal of the American Statistical Association. 1988;83(403):596–610. [Google Scholar]
- 28.Enden T, Wik HS, Kvam AK, Haig Y, Klow NE, Sandset PM. Health-related quality of life after catheter-directed thrombolysis for deep vein thrombosis: secondary outcomes of the randomised, non-blinded, parallel-group CaVenT study. BMJ Open. 2013;3(8):e002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galanaud JP, Monreal M, Kahn SR. Predictors of the post-thrombotic syndrome and their effect on the therapeutic management of deep vein thrombosis. Journal of vascular surgery Venous and lymphatic disorders. 2016;4(4):531–4. [DOI] [PubMed] [Google Scholar]
- 30.Kahn SR, Shrier I, Julian JA, Ducruet T, Arsenault L, Miron MJ, et al. Determinants and time course of the post-thrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149(10):698–707. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.