Abstract
Background
Clinic blood pressure measurement (CBPM) is currently the most commonly used form of screening for hypertension, however it might have a problem detecting white coat hypertension (WCHT) and masked hypertension (MHT). Home blood pressure measurement (HBPM) may be an alternative, but its diagnostic performance is inconclusive relative to CBPM. Therefore, this systematic review aimed to estimate the performance of CBPM and HBPM compared with ambulatory blood pressure measurement(ABPM) and to pool prevalence of WCHT and MHT.
Methods
Medline, Scopus, Cochrane Central Register of Controlled Trials and WHO's International Clinical Trials Registry Platform databases were searched up to 23rd January 2020. Studies having diagnostic tests as CBPM or HBPM with reference standard as ABPM, reporting sensitivity and specificity of both tests and/or proportion of WCHT or MHT were eligible. Diagnostic performance of CBPM and HBPM were pooled using bivariate mixed-effect regression model. Random effect model was applied to pool prevalence of WCHT and MHT.
Results
Fifty-eight studies were eligible. Pooled sensitivity, specificity, and diagnostic odds ratio (DOR) of CBPM, when using 24-h ABPM as the reference standard, were 74% (95% CI: 65–82%), 79% (95% CI: 69%, 87%), and 11.11 (95% CI: 6.82, 14.20), respectively. Pooled prevalence of WCHT and MHT were 0.24 (95% CI 0.19, 0.29) and 0.29 (95% CI 0.20, 0.38). Pooled sensitivity, specificity, and DOR of HBPM were 71% (95% CI 61%, 80%), 82% (95% CI 77%, 87%), and 11.60 (95% CI 8.98, 15.13), respectively.
Conclusions
Diagnostic performances of HBPM were slightly higher than CBPM. However, the prevalence of MHT was high in negative CBPM and some persons with normal HBPM had elevated BP from 24-h ABPM. Therefore, ABPM is still necessary for confirming the diagnosis of HT.
Keywords: Clinic blood pressure measurement, Home blood pressure measurement, diagnostic performance, Hypertension, Systematic review, Meta-analysis
Background
Screening for hypertension (HT) is an important strategy for prevention of cardiovascular diseases (CVD). Currently, several approaches are being used for measuring blood pressure (BP) including office or clinic blood pressure measurement (CBPM) and out-off office blood pressure measurement (i.e. ambulatory blood pressure measurement (ABPM) and home blood pressure measurement (HBPM)). HBPM is the average of all BP measurements performed by a semiautomatic BP monitor, for at least 3 days with readings in the morning and the evening, while ABPM records BP periodically at regular intervals (typically every 15, 20 or 30 min) for a pre-defined period of time [1].
Among all BP measurement methods, CBPM is the most commonly used in routine clinical practice, albeit there are two major concerns with CBPM. First, patients may have falsely high BP only in the clinical setting, i.e., a phenomenon known as white coat hypertension(WCHT), or they may have normal BP in the clinic but have an elevated BP measured by out-off office blood pressure measurement (i.e. ABPM or HBPM), known as masked hypertension (MHT) [2]. WCHT increased risk of cardiovascular diseases (CVD) about 19% when compared to normotension [3, 4], whereas MHT significantly increased risk of CVD about 3 times when compared to normotension [5]. The lack of recognition of WCHT and MHT results in patients with WCHT receiving unnecessary treatments and patients with MHT receiving delayed proper treatments. Therefore, accurate diagnosis of HT is crucial in preventing complications of HT and avoiding unnecessary treatment. Guidelines by European Society of Cardiology (ESC) and the European Society of Hypertension (ESH) 2018 [6], and American Heart Association (AHA) 2017 [2] suggest the use of out-off office blood pressure measurement (i.e. HBPM and/or ABPM) to confirm the diagnosis of HT as both ABPM and HBPM have the different advantages and disadvantages of identifying WCHT and MHT. HBPM is less expensive and more available than ABPM. However, HBPM do not measure BP during routine daily activities and during sleep. Thus, HBPM may have the potential of measurement error and incorrect classification of BP status, especially in persons having high nocturnal BP [1].
A meta-analysis conducted in 2011 [7] assessed the diagnostic performance of CBPM (N = 7) and HBPM (N = 3) using day-time ABPM as the reference standard. This meta-analysis found the overall sensitivity of CBPM to be lower than HBPM (74.6% vs. 85.7%), yet the specificity was higher (74.6% vs. 62.4%). Another systematic review in 2015 found that positive predictive values of CBPM (i.e. probability of being diagnosed with HT by ABPM or HBPM in persons with an elevated BP by CBPM) ranged from 35 to 95% [8]. However, this study did not apply meta-analysis to pool the diagnostic accuracy of CBPM.
Since 2011, there have been several published studies regarding CBPM and HBPM to date. New information regarding the factors associated with HT diagnosis such as age, sex, measurement technique, and types of ABPM have become available [9–11]. Performing a subgroup analysis on these factors may be useful in guiding BP screening strategies. Therefore, this systematic review was conducted with following aims: (1) to update the diagnostic performances of CBPM and HBPM using ABPM as the standard test and, (2) to pool prevalence of WCHT among positive CBPM, and pool prevalence of MHT among negative CBPM, (3) to perform subgroup analysis by those potential factors associated with HT diagnosis. The results derived from this study will have practical application for primary care, internal medicine physicians and cardiologists regarding the appropriate measurement method to use for HT diagnosis.
Methods
The protocol of this systematic review has been registered in PROSPERO (CRD42018099647). The review protocol is available at https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018099647.
Selection of studies
Relevant studies were identified from Medline, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL) and WHO's International Clinical Trials Registry Platform (ICTRP) databases up to 23rd January 2020 using search terms and strategies described in the Additional file 1: Appendix. Reference lists of the included studies were searched to identify additional studies.
Study selection was manually performed by 2 independent reviewers (AK and TA). Studies were selected based on titles and abstracts and full articles were retrieved if more information was needed. The studies were eligible if (1) they included participants aged ≥ 18 years, (2) had diagnostic test as CBPM or HBPM with reference standard as ABPM, and (3) reported sensitivity and specificity and/or WCHT proportion among people with high BP from CBPM or MHT proportion among people with normal BP from CBPM. Endnote X9 was used to manage references during the process of study selection.
Study and standard tests
The study tests were CBPM and HBPM. CBPM was performed in a health care setting, whereas HBPM was self-performed in a household using manual or automatic sphygmomanometers. The thresholds used for defining HT were BP ≥ 140/90 mmHg for CBPM and BP ≥ 135/85 mmHg for HBPM or as defined by the included studies [12–14]. The reference standard test was ABPM which measured BP daytime (10–16 h) or 24 h.
Outcome of interest
The interested outcome was HT diagnosed by ABPM using the thresholds of ≥ 135/85, ≥ 120/70, and ≥ 130/80 mmHg for daytime, night-time, and 24-h, respectively or the thresholds as defined in the included studies. Diagnostic performance of studied tests (i.e., CBPM and HBPM) compared to the standard test (i.e. ABPM) was assessed by estimating sensitivity (i.e., probability of having positive CBPM among HT patients diagnosed by ABPM), specificity (i.e., probability of having negative CBPM among non-HT patients diagnosed by ABPM), likelihood ratio positive (LR + , i.e., sensitivity/(1-specificity)), likelihood ratio negative (LR-, i.e., (1-sensitivity)/specificity) and diagnostic odds ratio (DOR, i.e., LR + /LR-). WCHT was defined as normal BP measured by ABPM and/or HBPM among CBPM positive whereas MHT was defined as high BP measured by ABPM and/or HBPM among CBPM negative [15].
Data extraction
Two reviewers (AK and TA) independently extracted the data including study’s characteristics (i.e. study setting, study design), study participants (i.e. mean age, percent male, and underlying disease), study and standard tests (i.e. types, measurement device, time and duration of measurement, and cut-offs for HT diagnosis). Numbers of true positive, false positive, true negative, and false negative for each diagnostic test were extracted.
Risk of bias assessment
Risk of bias assessments were done independently by 2 reviewers (AK and TA) using the Quality of Diagnostic Accuracy Studies—2 (QUADAS-2) [16] including patient selection, index test, reference standard, and flow/timing domains. Each domain consists of two sections, i.e., risk of bias and applicability. Risk of bias comprised of 3 items (i.e., information used to support the risk of bias judgment, signaling questions and judgment) which was judged as low, high or unclear. Applicability was judged as low, unclear or high risk according to whether the study did or did not match the review question.
Statistical analysis
Diagnostic performances of CBPM/HBPM versus ABPM (i.e., sensitivity, specificity, area under receiver operating characteristic (ROC) curve, LR+, LR−, and DOR were estimated for individual studies. These were then pooled using a bivariate mixed-effect regression model according to the types of ABPM and thresholds used for defining HT (i.e. 24-h ABPM with threshold of ≥ 130/80 mmHg, daytime ABPM with threshold of ≥ 135/85 mmHg). For studies that applied both 24-h and daytime ABPMs as the reference standards, only the data that used 24-h ABPM were used for pooling diagnostic performance of CBPM and HBPM. The hierarchic summary ROC(HSROC) curve was also estimated and plotted if applicable (number of studies ≥ 4); this was classified as low, moderate or high accuracy if the HSROCs were 0.5 < x < 0 .7, 0.7 ≤ x ≤ 0.9, and 0.9 < x ≤ 1, respectively [17].
Prevalence of WCHT and MHT were separately pooled using a random-effect model if heterogeneity was present; otherwise a fixed-effect model was applied. Heterogeneity was assessed using a Q test (p < 0.1) and the I2 statistic (> 25%). Potential sources of heterogeneity (i.e. results from risk of bias assessment, mean age, sex, study settings and numbers of repeated BP measurements) were explored by adding variables one by one in a meta-regression model. If the variables could decrease I2 or tau2, a subgroup analysis was performed accordingly.
Publication bias was examined by Deeks’ funnel plot [18]. If there was asymmetry, a contour-enhanced funnel plot was further explored to distinguish whether an asymmetrical funnel was due to heterogeneity or publication bias. All statistical analyses were performed with STATA version 15.0 (StataCorp, College Station, Texas). P-values < 0.05 were considered statistically significant for all tests with the exception of heterogeneity and Egger’s tests, where a P-value < 0.10 was used.
Results
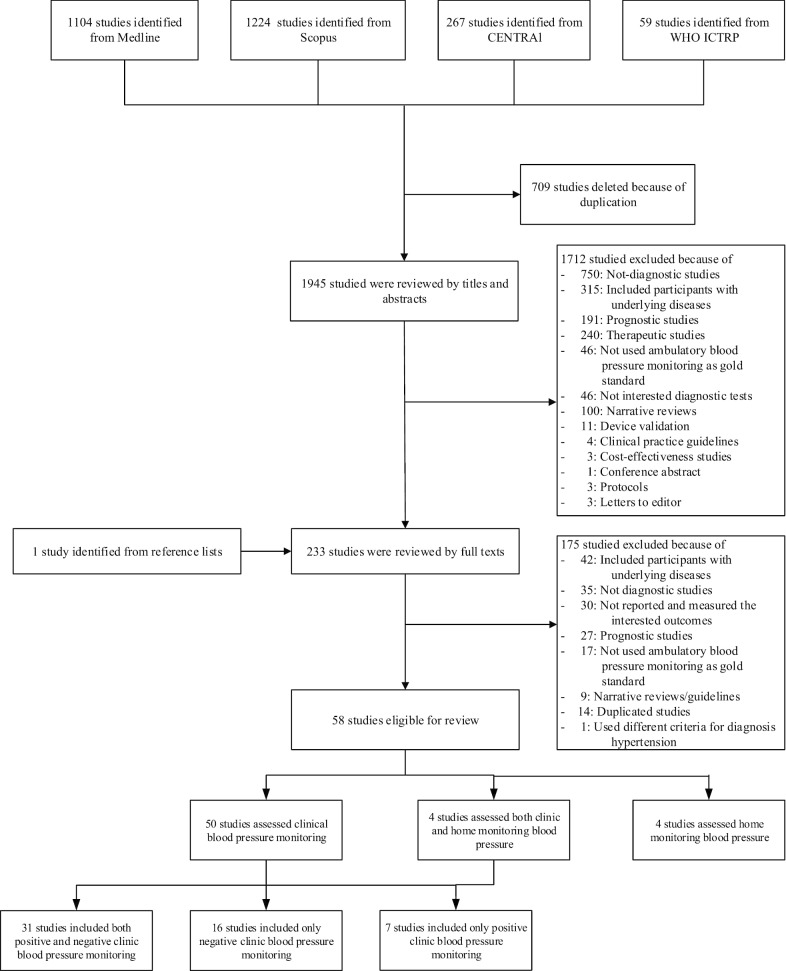
Searching from Medline, Scopus, CENTRAL and WHO ICTRP databases identified 1104, 1224, 267, and 59 articles, respectively. After deleting duplications, 1,945 studies were screened by titles and abstracts. A total of 233 full articles were reviewed. Fifty-eight studies met inclusion criteria and were included in the review. Among the included studies, 50 [9, 11, 19–66], 4 [67–70], and 4 [71–74] studies assessed CBPM, HBPM, and both CBPM and HBPM performances (see Fig. 1). Their characteristics are described in Table 1. Among the 58 studies, 32 and 26 studies recruited participants from hospital and community settings, correspondingly. Most studies included general population, whereas 4 studies included specific populations, i.e., white-collar workers [25, 66], male football players [40] and male military workers [37]. Twenty-one studies included participants who had not been prior diagnosed with HT, whereas 37 studies included both participant who had or had not been diagnosed with HT.
Fig. 1.
Flow chart of study selection
Table 1.
Characteristic of included studies
| Author (Year) | Country | Setting | Type of population | Previous HT | Taking anti-HT drug | Mean age (year) | %Male | CBPM; HBPM cut-off (mmHg) | No. of CBPM measurements per visit / No. of visit (interval) | No. of HBPM measurements per day/ No. of day | Time measuring ABPM | ABPM cut-off (mmHg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies with complete 2 × 2 diagnostic results of CBPM only | ||||||||||||
| Brueren M. 1995 [19] | Netherlands | Health care | Outpatient | Non-HT | No | 47 | 51.13 | 95(DBP) | 1/3 | – | Daytime (6 – 22.00) | 91(DBP) |
| Botomino A. 2004 [20] | Switzerland | Community | General population | Mixed | Unspecified | 53.7 | 42 | 140/90 | 2/1 | – | Daytime (6 – 18.00) | 135/85 |
| Ungar A 2004 [21] | Italy | Health care | General population | Mixed | No | 60 | 48.8 | 140/90 | 2/2 (1 day) | – | Daytime (7 – 22.00) | 135/85 |
| Ohkubo T. 2005 [22] | Japan | Community | General population | Mixed | Mixed | 61 | 40 | 140/90 | 2/1 | – | Daytime | 135/85 |
| Fagard R 2007 [23] | EU-countries | Community | General population | Mixed | No | 39 | 46.1 | 140/90 | 3/1 | – | Daytime (8 – 22.00) | 135/85 |
| Wang G 2007 [24] | China | Community | General population | Mixed | Mixed | (18 – 86) | 54.3 | 140/90 | 5/1 | – | Daytime (8 – 18.00) | 135/85 |
| Trudel X 2009 [25] | Canada | Community | White-collar workers | Non-HT | No | 44 | 38.8 | 140/90 | 3/1 | – | Daytime | 135/85 |
| Ishikawa J. 2010 [26] | Japan | Community | General population | Mixed | No | 59.9 | 52.7 | 140/90 | 2/1 | – | 24-h | 130/80 |
| Maseko M 2011 [27] | South Africa | Community | General population | Mixed | Unspecified | 43.9 | 35.6 | 140/90 | 5/1 | – | Daytime (9 – 19.00) | 140/85 |
| Afsar B. 2013 [28] | Turkey | Health care | Outpatient | Mixed | Unspecified | 57.46 | 67 | 140/90 | 2/1 | – | Daytime (7 – 23.00) | 135/85 |
| Berge H 2013 [40] | Norway | Community | Male professional football players | Mixed | No medication | 28.1 | – | 140/90 | 2/1 | – | Daytime (7 – 22.00) | 135/85 |
| Alwan H. 2014 [29] | Germany | Community | General population | Mixed | Mixed | 48 | 49 | 140/90 | 5/1 | – | Daytime (7 – 22.00) | 135/85 |
| Conen D. 2014 [9] | Europe, South America and Asia | Community | General population | Mixed | No | 45.74 | 48.48 | 140/90 | 2/1 | – |
Daytime Europe/America:10–20.00 Asian:8–18.00 |
135/85 |
| 24-h | 130/80 | |||||||||||
| Al-Hashimi K 2015 [30] | Oman | Health care | Outpatient | Mixed | Mixed | 46.15 | – | 140/90 | 1/1 | – | Daytime | 135/85 |
| Rhee M 2015 [11] | Korea | Community | General population | Mixed | Unspecified | 46.7 | 35.9 | 140/90 | 3/1 | – | 24-h | 130/80 |
| Mutlu S. 2016 [31] | Turkey | Health care | General in-patient | Mixed | Mixed | 44.4 | 54.4 | 140/90 | 1/1 | – | 24-h | 125 –130/80 |
| Scuteri A. 2016 [32] | Italy | Community | General population | Mixed | Mixed | 48.93 | 39.88 | 140/90 | NR | – | 24-h | 125/79 |
| Melgarejo J 2017 [33] | Europe and Asia | Community | General population | Mixed | No | – | – | 140/90 | 3/1 | – | 24-h | 130/80 |
| Fujita H. 2018 [34] | Japan | Community | General population | Non-HT | No | – | 51.5 | 140/90 | 3/1 | – | Daytime (8 – 20.00) | 135/85 |
| Erdogmus S. 2018 [61] | Turkey | Health care | General population | Mixed | Mixed | 55 | 42.3 | 140/90 | 3/1 | – | 24-h | 130/80 |
| Sheppard J.P. 2018 [65] | U.K | Health care | General population | Mixed | Mixed | 52.8 | 46.2 | NR | 1/1 | – | Daytime | 135/85 |
| Bhattarai M. 2019 [58] | Nepal | Health care | General population | Non-HT | Mixed | 43.82 | 55 | 140/90 | 3/1 | – | 24-h | 130/80 |
| Cai P. 2019 [59] | China | Health care | General population | Non-HT | No | 61.8 | 48.5 | 140/90 | 3/1 | – | 24-h | 130/80 |
| Kaul U. 2019 [62] | India | Health care | General population | Mixed | Mixed | NA | NA | 140/90 | 1/1 | – | 24-h | 130/80 |
| Michaud A. 2019 [63] | Canada | Health care | Outpatient | Mixed | Unspecified | 51.9 | 54 | 140/90 | 1/1 | – | 24-h | 130/80 |
| Trudel X. 2020 [66] | Canada | Community | Worker | Mixed | Mixed | 44.6 | 40.2 | 140/90 | 3/1 | – | Daytime (8–16.00) | 135/85 |
| Calvo-Vargus C. 2003 [60] | Mexico | Health care | General population | Mixed | Mixed | 54.7 | 82.2 | 140/90 | 3/2 (14 days) | – | Daytime (6–22.0) | 135/85 |
| Studies with complete 2 × 2 diagnostic results of HBPM only | ||||||||||||
| Almeida A. 2012 [67] | Brazil | Health care | General population | Mixed | Mixed | 50.6 | – | 135/85 | – | 12/5 | 24-h | 130/80 |
| Daytime | 135/85 | |||||||||||
| Park J. 2017 [68] | Korea | Health care | Outpatient | Mixed | Unspecified | 51.8 | 46.5 | 135/85 | – | 3/6 | 24-h | 130/80 |
| de Almeida, 2014 [69] | Brazil | Health care | Outpatient | Mixed | Mixed | 50.6 | 46.8 | 135/85 | – | 12/3 | Daytime | 135/85 |
| Rhee M.Y., 2018 [70] | South Korea | Health care | General population | Non-HT | No | 52.2 | 47.1 | 135/85 | – | 3/7 | 24-h | 130/80 |
| Studies with complete 2 × 2 diagnostic results of both CBPM and HBPM | ||||||||||||
| Stergiou G 2000 [73] | Greece | Health care | Outpatient | Mixed | No | 48.4 | 54.9 | 140/90; 140/90 | 3/5 (14 – 21 days) | 2/6 | Daytime | 140/90 |
| Hanninen M 2010 [72] | Finland | Community | General population | Mixed | Mixed | 49.1 | 47.9 | 140/90; 135/85 | 2/4 (21 days) | 2/7 | Daytime (6 – 23.00) | 140/85 |
| Zhang L 2015 [71] | Belgium | Health care | General population | Mixed | No medication or wash out period > 2 weeks | 50.6 | 51.1 | 140 (SBP); 135/85 | 3/3 (7 days) | 6/7 | 24-h | 130/80 |
| Ntineri A. 2019 [74] | Greece, U.K., Finland | Health care | General population | Mixed | Mixed | 53.8 | 52.6 | 140/90: 135/85 | 3/3 (10–21 days) | 2/7 | 24-h | 130/80 |
| Studies with positive CBPM studies only | ||||||||||||
| Hoegholm A. 1999 [35] | Denmark | Community | General population | Mixed | No medication or wash out period > 5 weeks | 47.7 | 46.9 | 140/90 | 3/3 (7 days) | – | Daytime (7 – 23.00) | 135/85 |
| Martinez M 1999 [36] | Spain | Health care | General population | Non-HT | No medication or wash out period > 3 weeks | 52 | 47.8 | 140/90 | 2/3 (7–14 days) | – | Daytime (10 – 20.00) | 135/85 |
| Gan S 2003 [37] | Singapore | Community | Male military conscripts | Mixed | Unspecified | 20 | 100 | 140/90 | 2/2 (14 days) | – | 24-h | 135/85 |
| Tunckale A. 2004 [38] | Turkey | Health care | General patient | Non-HT | No medication or wash out period > 4 weeks | – | – | 140/90 | 1/3 | – | Daytime (6 – 24.00) | 135/85 |
| Shimbo D. 2009 [39] | U.S.A | Health care | Outpatient | Mixed | No medication | 52.5 | 46 | 140/90 | 3/2 (30 days) | – | Daytime (6 – 22.00) | 135/85 |
| Pengkeaw P. 2014 [41] | Thailand | Health care | Outpatient | Mixed | No medication or wash out period > 4 weeks | 42.29 | 74.2 | 140/90 | 2/1 | – | Daytime | 135/85 |
| Mancia G.2015 [42] | Italy | Community | General population | Unspecified | – | – | 140/90 | 3/2 | – | 24-h | 130/80 | |
| Studies with negative CBPM only | ||||||||||||
| Schoenthaler A 2010 [43] | U.S. (African or Latino) | Community | General population | Mixed | No medication | 35.9 | 39 | 140/90 | 3/3 (14 days) | – | Daytime | 135/85 |
| Viera A 2010 [44] | U.S | Community | General population | Non-HT | No medication | 49 | 44 | 140/90 | 3/1 | – | Daytime (6- 23.00) | 135/85 |
| 24-h | 130/80 | |||||||||||
| Bacaksiz A. 2013 [45] | Turkey | Health care | Outpatient | Non-HT | No medication | 35.8 | 51.1 | 140/90 | 2/1 | – | Daytime (9–21.00) | 135/85 |
| Sobrino J. 2013 [46] | Spain | Community | General population | Non-HT | No medication | 43.1 | 44.7 | 140/90 | 3/1 | – | 24-h | 130/80 |
| Daytime | 135/85 | |||||||||||
| Franklin S 2013 [47] | Europe Japan | Community | General population | Non-HT | Mixed | – | – | 140/90 | 2/1 | – | Daytime | 135/85 |
| Larsen T 2014 [48] | America | Health care | Outpatient | Non-HT | No medication | 49.8 | 47 | 140/90 | 1/3 | – | 24-h | 135/85 |
| Viera A 2015 [49] | America | Health care | Outpatient | Non-HT | No medication | 47 | 39 | 140/90 | 3/1 | – | 24-h | 130/80 |
| Trachsel L 2015 [50] | Switzerland | Community | General population | Non-HT | No medication | 42.8 | – | 140/90 | 3/1 | – | 24-h | 130/80 |
| Redmond N. 2016 [51] | America, | Community | General population | Mixed | Mixed | 59.1 | 30.7 | 140/90 | 2/1 | – | Daytime (10 – 20.00) | 135/85 |
| Viera A 2016 [52] | America | Health care | General population | Non-HT | No medication | 48 | 40 | 140/90 | 3/1 | – | Daytime | 135/85 |
| Piantanida. E 2016 [53] | Italy | Health care | General population | Non-HT | No medication | 46.3 | – | 140/90 | 3/1 | – | 24-h | 130/80 |
| Booth III J 2017 [54] | America | Health care | Outpatient | Non-HT | No medication | – | 140/90 | 2/1 | – | Daytime | 135/85 | |
| Anstey D 2017 [55] | America, | Community | General population | Mixed | Mixed | 56 | 35.7 | 140/90 | 2/1 | – | Daytime (10–20.00) | 135/85 |
| Ozkan S. 2018 [56] | Turkey | Health care | Outpatient | Non-HT | No medication | 58.8 | 25.1 | 140/90 | NR | – | 24-h | 130/80 |
| Gun T. 2018 [57] | Turkey | Health care | Outpatient | Non-HT | No medication | 55 | 46.5 | 140/90 | 1/1 | – | 24-h | 130/80 |
| Salazar M. R., 2019 [64] | Argentina | Health care | Outpatient | Mixed | Mixed | 51.4 | 40.7 | 140/90 | 3/1 | – | 24-h | Day:135/85 Night:120/70 |
ABPM ambulatory blood pressure measurement, CBPM clinic blood pressure measurement, DBP diastolic blood pressure, HBPM home blood pressure measurement, HT hypertension, NR not reported, SBP systolic blood pressure
Risk of bias assessment
Results of risk of bias assessment are presented in Additional file 1: Table 1. Almost all CBPM studies (94.44%) were low risk in all domains of applicability. Eight [20, 31, 38, 39, 44, 50, 52, 57] (16.7%) and 7 (12.9%) studies [30, 34, 41, 46, 49, 55, 58] were high or unclear bias in selection of study subjects, accordingly. Fifty-two studies (96.3%) [9, 11, 19–31, 33–38, 40–66, 70, 71, 74] applied the index/study test before the reference standard but with unclear explanation of blinding. Thirty-eight studies (70.4%) were high or unclear risk of bias in flows and timing. This is due to a lack of reporting the time interval between the study test and the reference standard or the exclusion of subjects with invalid test results or those lost to follow up. All HBPM studies were low risk of bias in all domains of applicability. Six studies (75%) applied the index test before the reference standard without blinding information, and 4 studies (50%) were high risk of bias in their flow and timing.
Pooling CBPM diagnostic performances
Among 54 CBPM studies, 31 studies [9, 11, 19–34, 40, 58–66, 71–73] reported 2 × 2 table data which could be assessed for diagnostic performance, while 7 [35–39, 41, 42] and 16 [43–57, 70] studies reported data for only positive and negative CBPM respectively (see Table 1). The mean age ranged from 28 to 62 years and percent male ranged from 35 to 100%. The number of CBPM measurements per visit ranged from 1 to 5 times (Table 1).
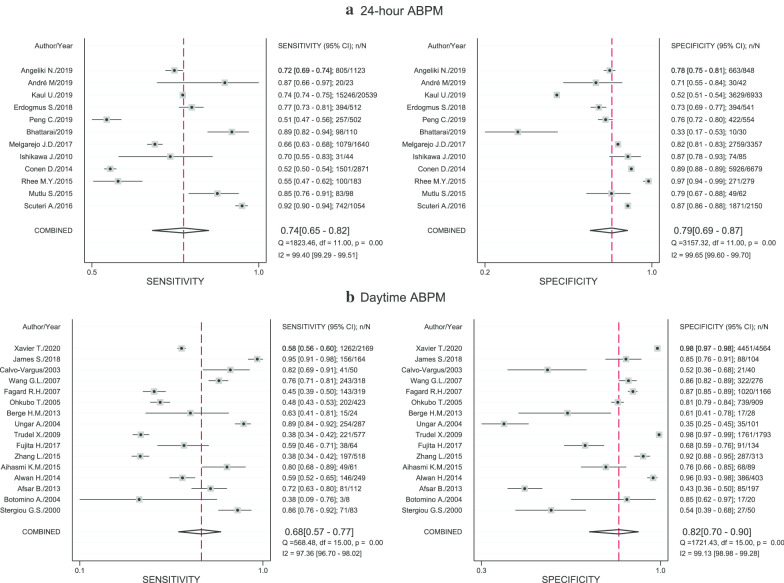
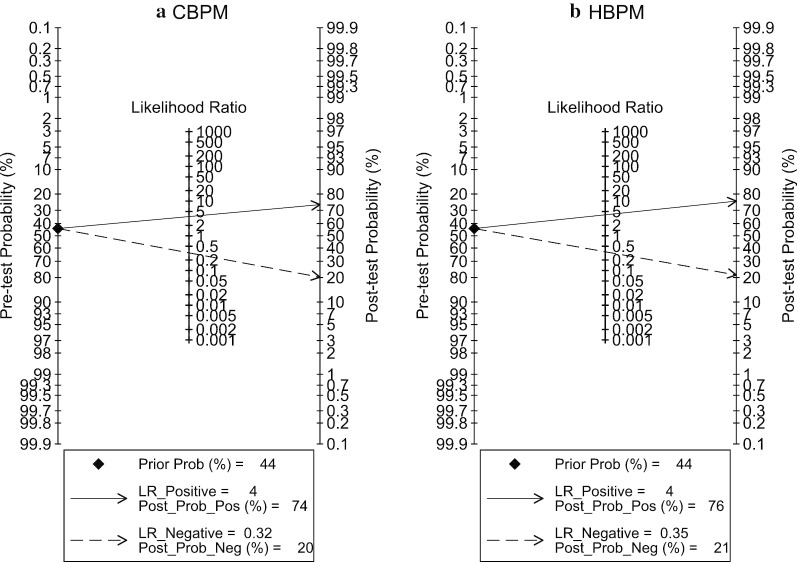
Among the studies that reported 2 × 2 data (n = 66,767), 29 studies [9, 11, 20–34, 40, 58–63, 66, 72–74] used a CBPM cutoff threshold as ≥ 140/90 for diagnosis of HT, while one study [19] used the threshold of DBP > 95 mmHg and one study did not reported the threshold. The 24-h ABPM had a cut-off of ≥ 130/80 mmHg for 12 studies [11, 26, 31–33, 58, 59, 61–63, 74] and daytime ABPM had a cut-off of ≥ 135/85 mmHg for 16 studies [20–25, 28–30, 34, 60, 65, 66, 73]. Two [27, 72] and one [19] studies applied daytime ABPM with cut-offs of ≥ 140/85 and DBP ≥ 95 mmHg, respectively (see Additional file 1: Table 2). When using the 24-h ABPM with the cut-off of ≥ 130/80 mmHg as the reference standard, the diagnostic performance of CBPM were 0.74 (95% CI 0.65–0.82; I2 = 99.4%), 0.79 (95% CI 0.69, 0.87; I2 = 99.65%), 3.6 (95% CI 2.4, 5.3; I2 = 99.67%) and 0.32 (95% CI 0.24, 0.44; I2 = 99.58%) for sensitivity, specificity, LR + and LR-, respectively (see Fig. 2a and Additional file 1: Fig. 1). These diagnostic characteristics all require setting a threshold and trading off sensitivity for specificity or LR + for LR- hence they must be judged in pairs. For example, given a pretest probability of HT of 44%, the post-test probability was increased to 74% if CBPM was positive or reduced to 20% if CBPM was negative (see Fagan’s plot Fig. 3a). Alternatively, a single measure of diagnostic performance, i.e., the DOR was 11.11 (95% CI 6.44, 19.160; I2 = 100%), see Additional file 1:Fig. 1c. The HSROC reflects diagnostic performance across the entire range of possible threshold values; in this case, the pooled HSROC was 0.83 (95% CI 0.82, 0.85) indicating moderately good discrimination for judging presence of HT (Additional file 1: Fig. 2a).
Fig. 2.
Pooled sensitivity and specificity of clinic blood pressure measurements compared with 24-h and daytime ambulatory blood pressure measurements. “n” referred to number of hypertensive patients who had positive clinic blood pressure measurement and number of non-hypertensive patients who had negative clinic blood pressure measurement for pooling sensitivity and specificity, respectively. “N” referred to number of hypertensive patients and non-hypertensive patients for pooling sensitivity and specificity, respectively. Reference line referred to pooled sensitivity or pooled specificity
Fig. 3.
Fagan’s plot of clinic and home blood pressure measurements compared with 24-h ambulatory blood pressure measurement
When using daytime ABPM with cut-off of 135/85 mmHg as the reference standard, the pooled sensitivity and specificity were 68% (95% CI 57, 77; I2 = 97.36%) and 82% (95% CI 70, 90; I2 = 99.13%), see Fig. 2b. In addition, LR + , LR- and DOR of CBPM were 3.7 (95% CI 2.3, 6.0; I2 = 98.57%), 0.39 (95% CI 0.30, 0.52; I2 = 94.54%) and 9.46 (95% CI 5.39, 16.60; I2 = 100%), accordingly (see Additional file 1: Fig. 3). When all ABPMs with no restriction on the cutoffs as the reference standards were used, the pooled sensitivity and specificity were 70% (95% CI 63%, 76%; I2 = 98.56%) and 81% (95% CI 73%, 87%; I2 = 99.47%) and pooled LR + and LR- were 3.67 (95% CI 2.69, 5.00; I2 = 99.35%) and 0.37 (95% CI 0.31–0.44; I2 = 98.57%). For publication bias, Deeks’ funnel plot showed no evidence of publication bias (Additional file 1: Fig. 4a).
Subgroup analysis
Subgroup analyses were performed by results of risk of bias assessment, age group (< 50 and ≥ 50 years), percent males (< 50% and ≥ 50%), number of repeated measurements of CBPM (1, 2–5 times), setting of studies (community and hospital-based) and type of patients (no HT, mixed HT with non-HT). When considering only studies with low risk of bias in the domain of flow and timing (N = 7), pooled sensitivity, specificity, LR + and DOR of CBPM were 73% (95% CI 60, 83), 75% (95% CI 51, 89), 2.9 (95% CI 1.5, 5.3), and 8 (95% CI 5, 14), respectively. The degrees of heterogeneity (I2) did not decrease for each sub-group of these factors (Additional file 1: Table 3), but performances of CBPM improved in some sub-groups including age group ≤ 50 year, percent male ≤ 50% and community-based setting with the LR + of 5.1 (95% CI 3.0, 8.7), 5.8 (95% CI 3.5, 9.8), and 6.0 (95% CI 3.9, 9.3), respectively.
Pooling HBPM diagnostic performances
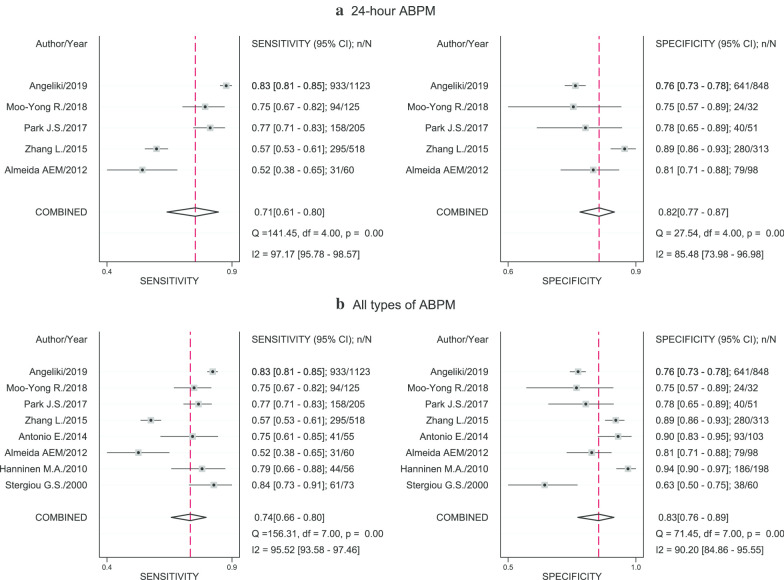
Eight HBPM studies [67–74] reported 2 × 2 data (Additional file 1: Table 4) with cutoff threshold of 135/85 [67–71, 74] (N = 7) and 140/90 [73] (N = 1) mmHg and measurement duration of about 3 to 7 days. The number of measurements per day ranged from 2 to 12 times (see Table 1). Mean age and percent male ranged from 48.1 to 51.8 years and 46.5% to 54.9% respectively. Among them, five and three studies applied 24-h and daytime ABPM, respectively.
The pooled sensitivity, specificity, DOR, LR + and LR- of HBPM were respectively 0.71 (95% CI 0.61, 080; I2 = 97.17%), 0.82 (95% CI 0.77, 0.87; I2 = 85.48), 11.60 (95% CI 8.98, 15.13; I2 = 100%), 4.02 (95% CI 3.38, 4.78; I2 = 19.39%) and 0.35 (95% CI 0.26, 0.46; I2 = 95.69%), when the 24-h ABPM with cut-off of ≥ 130/80 mmHg was applied as the reference standard (see Fig. 4a and Additional file 1: Fig. 5). In addition, among persons having HBPM positive, 14% had normotension from 24-h ABPM. In contrast, 40% of those having negative HBPM were diagnosed with hypertension from 24-h ABPM. Again, given a pretest-probability of 44%, a positive HBPM would result in a post-test probability of 76%, while a negative HBPM would reduce the probability to 21% (Fig. 3b). The pooled HSROC was 0.85 (95% CI 0.82, 0.88), reiterating moderately good discrimination, see Additional file 1: Fig. 2b.
Fig. 4.
Pooled sensitivity and specificity of home blood pressure measurements compared with 24-h and all types ambulatory blood pressure measurements. “n” referred to number of hypertensive patients who had positive home blood pressure measurement and number of non-hypertensive patients who had negative home blood pressure measurement for pooling sensitivity and specificity, respectively. “N” referred to number of hypertensive patients and non-hypertensive patients for pooling sensitivity and specificity, respectively. Reference line referred to pooled sensitivity or pooled specificity
When all types ABPM with all cut-offs as the reference standard were applied, the overall pooled sensitivity and specificity were respectively 74% (95% CI 66%, 80%; I2 = 95.52%) and 83% (95% CI 76%, 89%; I2 = 90.20%), see Fig. 4b. The pooled DOR, LR + and LR-were 13.73 (95% CI 8.55, 22.03; I2 = 99.99%), 4.36 (95% CI 3.04, 6.27; I2 = 75.06%), and 0.32 (95% CI 0.25, 0.41; I2 = 94.34%), respectively. Analysis using daytime ABPM as the reference standard and subgroup analysis of HBPM could not be performed due to the small number of included studies. Deeks’ funnel plot indicated no evidence of publication bias, see Additional file 1: Fig. 4b.
Pooling prevalence of WCHT and MHT by CBPM
Seven [35–39, 41, 42] and 16 studies [43–57, 64] reported only data of WCHT and MHT, see Table 1. These studies were then combined with 31 CBPM studies with 2 × 2 data above yielding a total of 38 and 47 studies for pooling proportions of WCHT and MHT, respectively. Among the 38 studies with WCHT, time of ABPM measures were 24-h (N = 14) and daytime (N = 24). Among the 47 studies with MHT, ABPM measurements were 24-h ABPM (N = 20) and daytime ABPM (N = 27). Four studies compared the performance of CBPM with both HBPM and ABPM but only two studies provided the number of people who had negative CBPM but had high blood pressure from either ABPM or HBPM. Therefore, most studies (N = 45) used only ABPM as the reference standard for pooling the prevalence of MHT.
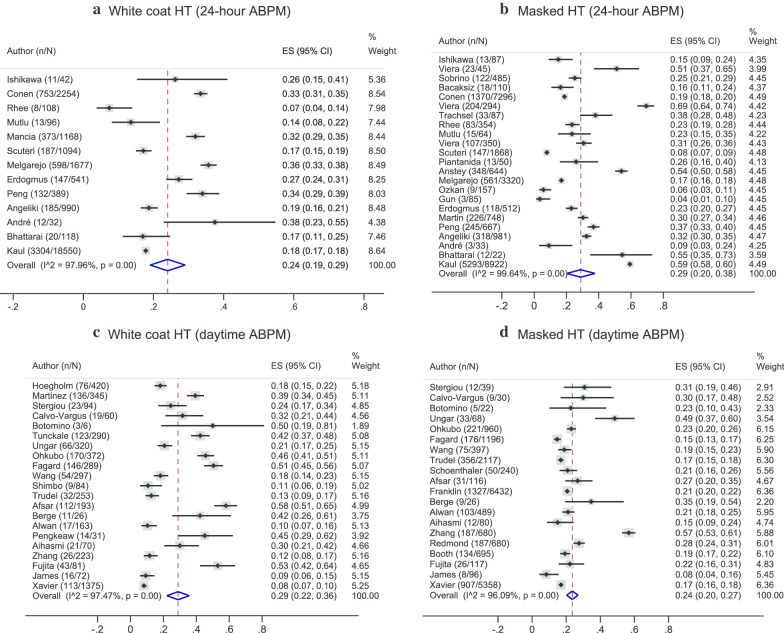
Using the 24-h ABPM with a cut-off of 130/80 mmHg as the reference standard (N = 23), the pooled prevalence of WCHT and MHT were 0.24 (95% CI 0.19, 0.29; I2 = 97.96%) and 0.29 (95% CI 0.20, 0.38; I2 = 99.64%), see Fig. 5a and 5b. If daytime ABPM with cut-off of 135/85 mmHg was applied as the reference standard, the pooled prevalence of WCHT (N = 21) and MHT (N = 20) would be 0.29 (95% CI 0.22, 0.36; I2 = 97.47%) and 0.24 (95% CI 0.20, 0.27; I2 = 96.09%), see Fig. 5c and 5d.
Fig. 5.
Pooled prevalence of white coat hypertension and masked hypertension using daytime and 24-h ambulatory blood pressure measurements as the reference standards. “n” referred to number of false positive and false negative clinic blood pressure measurements for pooling prevalence of white coat and masked hypertension, respectively. “N” referred to number of positive and negative clinic blood pressure measurements for pooling prevalence of white coat and masked hypertension, respectively. Reference line referred to pooled prevalence of white coat or masked hypertension
When all types of ABPM were applied with any cut-offs as the reference standard, the pooled prevalence of WCHT (N = 38; n = 32,685) and MHT (N = 47; n = 47,713) were 0.28 (95% CI 0.25, 0.32) and 0.27 (95% CI 0.22, 0.32). Subgroup analyses were performed, but none of the co-variables could decrease the degree of heterogeneity (see Additional file 1: Table 7). However, subgroup of repeated measures of CBPM 4–5 times and 24-h ABPM could respectively reduce the pooled WCHT from 0.28 to 0.23 (95% CI 0.16, 0.31) and 0.23 (95% CI 0.18, 0.28). Likewise, repeated CBPM measure could reduce the pooled MHT from 0.27 to 0.15 (95% CI 0.10, 0.19) whereas the 24-h ABPM conversely increased the prevalence to 0.33 (95% CI 0.22, 0.43).
Discussion
The findings suggest that when using 24-h ABPM as the reference standard, diagnostic performances of HBPM were slightly higher than those of CBPM. The pooled sensitivity, specificity, DOR, LR + and area under ROC for HBPM were respectively 71%, 82%, 11.60, 4.02 and 0.85, while these corresponding values for CBPM were respectively 74%, 79%, 11.11, 3.6, and 0.83.
To date, there has been only one relevant meta-analysis published in 2011 [7], which included fewer studies than ours (i.e., 7 versus 31 for CBPM and 3 versus 8 for HBPM). Overall sensitivities found in our study were lower than the previous review (i.e., 74% vs. 75% for CBPM and 71% vs. 86% for HBPM), while the specificities were higher (i.e., 79% vs. 75% for CBPM and 82% vs. 62% for HBPM). Our pooled estimates are more precise than the previous review which was limited by the small number of included studies. In addition, our results indicated that roughly 29% of those who are positive on CBPM may have WCHT and roughly 24% of those who are negative on CBPM may have MHT, when using daytime ABPM as the reference standard.
However, when using 24-h ABPM as the reference standard, the percent of people having WCHT reduced from 29 to 24%, while the percent of people having MHT increased from 24 to 29%. This reinforces the belief that 24-h ABPM yields the best detection for HT because it can capture the nighttime and morning surge BP. The number of repeated measurements of CBPM also affected the diagnostic performance, i.e., there was a lower WCHT and MHT if repeatedly measuring CBPMs over 4–5 visits.
The misclassification of patients who actually do not have hypertension is an important issue for diagnosis and treatment of hypertension because previous evidence from meta-analyses found a similar risk of cardiovascular disease between those with WCHT and normotension [75–77]. Unnecessary treatments of WCHT have several disadvantages including potential adverse drug events and costs. Measuring BP in the patient’s own environment using HBPM could reduce stress and decrease over-diagnosis of HT.
In contrast to WCHT, detection of MHT is important for CVD prevention. Our results found that prevalence of MHT was high in normal CBPM (29%). Even in people with negative HBPM, 40% of them had high BP when performing 24-h ABPM. Thus, ABPM is still necessary for confirming the diagnosis of MHT. Nonetheless, screening all individuals with normal CBPM is impractical; so prioritizing people who are high risk of CVD to screen with ABPM is important. According to the 2018 ESC/ESH guideline, persons with high normal office BP or with normal office BP but having hypertension-mediated organ damage or at high total cardiovascular risk are indicated for ABPM or HBPM monitoring [1].
Our study has some strengths. We estimated the diagnostic performance of CBPM and HBPM relative to ABPM by additionally pooling data from 31 and 8 studies with prevalence of WCHT and MHT. However, our study also faced limitations. Firstly, our pooling was based on high heterogeneity across studies, particularly for pooling prevalence of WCHT and MHT. Although we attempted to explore the sources of heterogeneity by performing subgroup analyses according to age group, sex, and results from risk of bias assessment, none of them was identified as a source of heterogeneity. A small number of HBPM studies was available compared to the large number of CBPM studies, and estimation of diagnostic performance yielded imprecision. Thus, results need to be updated when more studies are available, or individual patient data (IPD) meta-analysis should be considered to allow for more sub-group analysis of specific factors. Although most included studies had low risk of bias in subject selection and index test, most of them (70.4% for CBPM and 50% for HBPM) had high and unclear risk of bias in flow and timing due to long/unclear time interval between performing index and standard tests.
The long interval may lead to misclassification of disease due to improvement or worsening of the BP condition [16]. For instance, patients with high BP by CBPM/HBPM may be prescribed anti-hypertensive drugs to lowering BP before performing ABPM. This might underestimate the diagnostic performance of CBPM and HBPM. Finally, we did not identify relevant studies from grey/unpublished databases. Although there was no evidence of publication bias suggested by Deeks’ funnel plot [18] for both pooled estimates of CBPM and HBPM, potential publication bias could not be ruled out and overestimated diagnostic accuracy of CBPM and HBPM might be present. However, some previous systematic review and meta-analyses found that including unpublished studies might have a minimal effect on the overall estimates, so they should not impact the overall findings [78, 79].
Conclusion
In conclusion, diagnostic performances of HBPM were slightly higher than the performance of CBPM. However, the prevalence of MHT was high in negative CBPM and some persons with normal HBPM had elevated BP from 24-h ABPM. Therefore, ABPM is still necessary for confirming the diagnosis of HT, especially in people who have high normal CBPM/HBPM or normal CBPM/HBPM with hypertension-mediated organ damage or at high CVD risk.
Supplementary information
Additional file 1. Additional appendix, tables, and figures.
Acknowledgements
We would like to express our gratitude to Dr. Natasha Chawala to help us to edit the English language of this manuscript.
Abbreviations
- ABPM
Ambulatory blood pressure measurement
- AHA
American Heart Association
- BP
Blood pressure
- CBPM
Clinic blood pressure measurement
- CI
Confidence interval
- CKD
Chronic kidney disease
- CVD
Cardiovascular diseases
- DBP
Diastolic blood pressure
- DM
Diabetes mellitus
- DOR
Diagnostic odds ratio
- HBPM
Home blood pressure measurement
- HT
Hypertension
- LR
Likelihood ratio
- MHT
Masked hypertension
- NM
Normotension
- QUADAS-2
Quality of Diagnostic Accuracy Studies-2
- ROC
Receiver operating characteristic
- SBP
Systolic blood pressure
- SD
Standard deviation
- SE
Standard error
- WCHT
White coat hypertension
Authors’ contributions
AK, TA and AT were responsible for designing the study. AK and TA were responsible for searching the databases, selecting the studies, and extracting the data. AK, TA, and AT analyzed the data. AK and TA drafted the manuscript. CD, AT, UC, and JA critically revised the manuscript.
Funding
This study had no funding support.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its Additional information files.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Auttakiat Karnjanapiboonwong, Email: auttakiat@yahoo.com.
Thunyarat Anothaisintawee, Email: thunyarat.ano@mahidol.ac.th.
Usa Chaikledkaew, Email: usa.cha@mahidol.ac.th.
Charungthai Dejthevaporn, Email: charungthaid@gmail.com.
John Attia, Email: john.attaia@newcastle.edu.au.
Ammarin Thakkinstian, Email: ammarin.tha@mahidol.ac.th.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12872-020-01736-2.
References
- 1.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH) Eur Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017;71(6):e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 3.Briasoulis A, Androulakis E, Palla M, Papageorgiou N, Tousoulis D. White-coat hypertension and cardiovascular events: a meta-analysis. J Hypertens. 2016;34(4):593–599. doi: 10.1097/HJH.0000000000000832. [DOI] [PubMed] [Google Scholar]
- 4.Huang Y, Huang W, Mai W, Cai X, An D, Liu Z, Huang H, Zeng J, Hu Y, Xu D. White-coat hypertension is a risk factor for cardiovascular diseases and total mortality. J Hypertens. 2017;35(4):677–688. doi: 10.1097/HJH.0000000000001226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palla M, Saber H, Konda S, Briasoulis A. Masked hypertension and cardiovascular outcomes: an updated systematic review and meta-analysis. Integr Blood Pressure Control. 2018;11:11–24. doi: 10.2147/IBPC.S128947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement D, Coca A, De Simone G, Dominiczak A, et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2018;36(12):2284–2309. doi: 10.1097/HJH.0000000000001961. [DOI] [PubMed] [Google Scholar]
- 7.Hodgkinson J, Mant J, Martin U, Guo B, Hobbs FD, Deeks JJ, Heneghan C, Roberts N, McManus RJ. Relative effectiveness of clinic and home blood pressure monitoring compared with ambulatory blood pressure monitoring in diagnosis of hypertension: systematic review. BMJ. 2011;342:d3621. doi: 10.1136/bmj.d3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piper MA, Evans CV, Burda BU, Margolis KL, O'Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. preventive services task force. Ann Internal Med. 2015;162(3):192–204. doi: 10.7326/M14-1539. [DOI] [PubMed] [Google Scholar]
- 9.Conen D, Aeschbacher S, Thijs L, Li Y, Boggia J, Asayama K, Hansen TW, Kikuya M, Bjorklund-Bodegard K, Ohkubo T, et al. Age-specific differences between conventional and ambulatory daytime blood pressure values. Hypertension. 2014;64(5):1073–1079. doi: 10.1161/HYPERTENSIONAHA.114.03957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etyang AO, Warne B, Kapesa S, Munge K, Bauni E, Cruickshank JK, Smeeth L, Scott JA. Clinical and epidemiological implications of 24-hour ambulatory blood pressure monitoring for the diagnosis of hypertension in kenyan adults: a population-based study. J Am Heart Assoc. 2016;5:12. doi: 10.1161/JAHA.116.004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhee MY, Kim SW, Choi EH, Kim JH, Nah DY, Shin SJ, Gu N. Prevalence of masked hypertension: a population-based survey in a large city by using 24-hour ambulatory blood pressure monitoring. Korean Circ J. 2016;46(5):681–687. doi: 10.4070/kcj.2016.46.5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaques H. National Institute for H, Clinical E: NICE guideline on hypertension. Eur Heart J. 2013;34(6):406–408. [PubMed] [Google Scholar]
- 13.Ritchie LD, Campbell NC, Murchie P. New NICE guidelines for hypertension. BMJ. 2011;343:d5644. doi: 10.1136/bmj.d5644. [DOI] [PubMed] [Google Scholar]
- 14.Dixon DL, Salgado TM, Caldas LM, Van Tassell BW, Sisson EM: The, American College of Cardiology/American Heart Association hypertension guideline and opportunities for community pharmacists. J Am Pharm Assoc. 2017;2003:2018. doi: 10.1016/j.japh.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 15.de la Sierra A. Definition of white coat hypertension: ambulatory blood pressure, self-measured blood pressure, or both? Hypertension (Dallas, Tex: 1979) 2013;62(1):16–17. doi: 10.1161/HYPERTENSIONAHA.113.01565. [DOI] [PubMed] [Google Scholar]
- 16.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM. Group Q-: QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 17.Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240(4857):1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 18.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58(9):882–893. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Brueren MM, Dinant GJ, Schouten BJ, van Ree JW. Hypertension diagnosis by the family physician: measurements according to the NHG-standard (Dutch College of General Practitioners) compared with ambulatory blood pressure determination. Ned Tijdschr Geneeskd. 1995;139(6):278–282. [PubMed] [Google Scholar]
- 20.Botomino A, Martina B, Ruf D, Bruppacher R, Hersberger KE. White coat effect and white coat hypertension in community pharmacy practice. Blood Press Monit. 2005;10(1):13–18. doi: 10.1097/00126097-200502000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Ungar A, Pepe G, Monami M, Lambertucci L, Torrini M, Baldasseroni S, Tarantini F, Marchionni N, Masotti G. Isolated ambulatory hypertension is common in outpatients referred to a hypertension centre. J Hum Hypertens. 2004;18(12):897–903. doi: 10.1038/sj.jhh.1001756. [DOI] [PubMed] [Google Scholar]
- 22.Ohkubo T, Kikuya M, Metoki H, Asayama K, Obara T, Hashimoto J, Totsune K, Hoshi H, Satoh H, Imai Y. Prognosis of "masked" hypertension and "white-coat" hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol. 2005;46(3):508–515. doi: 10.1016/j.jacc.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 23.Fagard RH, Van Den Broeke C, De Cort P. Prognostic significance of blood pressure measured in the office, at home and during ambulatory monitoring in older patients in general practice. J Hum Hypertens. 2005;19(10):801–807. doi: 10.1038/sj.jhh.1001903. [DOI] [PubMed] [Google Scholar]
- 24.Wang GL, Li Y, Staessen JA, Lu L, Wang JG. Anthropometric and lifestyle factors associated with white-coat, masked and sustained hypertension in a Chinese population. J Hypertens. 2007;25(12):2398–2405. doi: 10.1097/HJH.0b013e3282efeee7. [DOI] [PubMed] [Google Scholar]
- 25.Trudel X, Brisson C, Larocque B, Milot A. Masked hypertension: different blood pressure measurement methodology and risk factors in a working population. J Hypertens. 2009;27(8):1560–1567. doi: 10.1097/HJH.0b013e32832cb036. [DOI] [PubMed] [Google Scholar]
- 26.Ishikawa J, Hoshide S, Eguchi K, Schwartz JE, Pickering TG, Shimada K, Kario K. Masked hypertension defined by ambulatory blood pressure monitoring is associated with an increased serum glucose level and urinary albumin-creatinine ratio. J Clin Hypertens (Greenwich) 2010;12(8):578–587. doi: 10.1111/j.1751-7176.2010.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maseko MJ, Woodiwiss AJ, Majane OH, Molebatsi N, Norton GR. Marked underestimation of blood pressure control with conventional vs. ambulatory measurements in an urban, developing community of African ancestry. Am J Hypertens. 2011;24(7):789–795. doi: 10.1038/ajh.2011.48. [DOI] [PubMed] [Google Scholar]
- 28.Afsar B. Comparison of demographic, clinical, and laboratory parameters between patients with sustained normotension, white coat hypertension, masked hypertension, and sustained hypertension. J Cardiol. 2013;61(3):222–226. doi: 10.1016/j.jjcc.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Alwan H, Pruijm M, Ponte B, Ackermann D, Guessous I, Ehret G, Staessen JA, Asayama K, Vuistiner P, Younes SE, et al. Epidemiology of masked and white-coat hypertension: the family-based SKIPOGH study. PLoS ONE. 2014;9(3):e92522. doi: 10.1371/journal.pone.0092522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Hashmi K, Al-Busaidi N, Amina B, Jaju D, Al-Waili K, Al-Rasadi K, Al-Sabti H, Al-Abri M. White coat hypertension and masked hypertension among omani patients attending a tertiary hospital for ambulatory blood pressure monitoring. Oman Med J. 2015;30(2):90–94. doi: 10.5001/omj.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mutlu S, Sari O, Arslan E, Aydogan U, Doganer YC, Koc B. Comparison of ambulatory blood pressure measurement with home, office and pharmacy measurements: is arterial blood pressure measured at pharmacy reliable? J Eval Clin Pract. 2016;22(1):40–45. doi: 10.1111/jep.12424. [DOI] [PubMed] [Google Scholar]
- 32.Scuteri A, Morrell CH, Orru M, AlGhatrif M, Saba PS, Terracciano A, Ferreli LA, Loi F, Marongiu M, Pilia MG, et al. Gender specific profiles of white coat and masked hypertension impacts on arterial structure and function in the SardiNIA study. Int J Cardiol. 2016;217:92–98. doi: 10.1016/j.ijcard.2016.04.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melgarejo JD, Maestre GE, Thijs L, Asayama K, Boggia J, Casiglia E, Hansen TW, Imai Y, Jacobs L, Jeppesen J, et al. Prevalence, treatment, and control rates of conventional and ambulatory hypertension across 10 populations in 3 continents. Hypertension. 2017;70(1):50–58. doi: 10.1161/HYPERTENSIONAHA.117.09188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujita H, Matsuoka S, Awazu M. Masked isolated nocturnal hypertension in children and young adults. Pediatr Cardiol. 2018;39(1):66–70. doi: 10.1007/s00246-017-1728-0. [DOI] [PubMed] [Google Scholar]
- 35.Hoegholm A, Kristensen KS, Bang LE, Gustavsen PH. White coat hypertension and blood pressure variability. Am J Hypertens. 1999;12(10):966–972. doi: 10.1016/S0895-7061(99)00109-0. [DOI] [PubMed] [Google Scholar]
- 36.Martinez MA, Garcia-Puig J, Martin JC, Guallar-Castillon P, Aguirre de Carcer A, Torre A, Armada E, Nevado A, Madero RS. Frequency and determinants of white coat hypertension in mild to moderate hypertension: a primary care-based study. Monitorizacion Ambulatoria de la Presion Arterial (MAPA)-Area 5 Working Group. Am J Hypertens. 1999;12(3):251–259. doi: 10.1016/S0895-7061(98)00262-3. [DOI] [PubMed] [Google Scholar]
- 37.Gan SK, Loh CY, Seet B. Hypertension in young adults–an under-estimated problem. Singapore Med J. 2003;44(9):448–452. [PubMed] [Google Scholar]
- 38.Tunçkale A, Aran SN, Karpuz H, Dirican A. Relationship between insulin resistance and end-organ damage in white coat hypertension. Am J Hypertens. 2004;17(11):1011–1016. doi: 10.1016/j.amjhyper.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Shimbo D, Kuruvilla S, Haas D, Pickering TG, Schwartz JE, Gerin W. Preventing misdiagnosis of ambulatory hypertension: algorithm using office and home blood pressures. J Hypertens. 2009;27(9):1775–1783. doi: 10.1097/HJH.0b013e32832db8b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berge HM, Andersen TE, Solberg EE, Steine K. High ambulatory blood pressure in male professional football players. Br J Sports Med. 2013;47(8):521–525. doi: 10.1136/bjsports-2013-092354. [DOI] [PubMed] [Google Scholar]
- 41.Pengkeaw P, Suwannakarn S. Prevalence of hypertension in suspected hypertensive patients in Rajavithi Hospital using ambulatory blood pressure monitoring. J Med Assoc Thail. 2014;97(Suppl 11):S25–30. [PubMed] [Google Scholar]
- 42.Mancia G, Facchetti R, Grassi G, Bombelli M. Adverse Prognostic value of persistent office blood pressure elevation in white coat hypertension. Hypertension. 2015;66(2):437–444. doi: 10.1161/HYPERTENSIONAHA.115.05367. [DOI] [PubMed] [Google Scholar]
- 43.Schoenthaler AM, Schwartz J, Cassells A, Tobin JN, Brondolo E. Daily interpersonal conflict predicts masked hypertension in an urban sample. Am J Hypertens. 2010;23(10):1082–1088. doi: 10.1038/ajh.2010.141. [DOI] [PubMed] [Google Scholar]
- 44.Viera AJ, Hinderliter AL, Kshirsagar AV, Fine J, Dominik R. Reproducibility of masked hypertension in adults with untreated borderline office blood pressure: comparison of ambulatory and home monitoring. Am J Hypertens. 2010;23(11):1190–1197. doi: 10.1038/ajh.2010.158. [DOI] [PubMed] [Google Scholar]
- 45.Bacaksiz A, Erdogan E, Sonmez O, Sevgili E, Tasal A, Onsun N, Topukcu B, Kulac B, Uysal O, Goktekin O. Ambulatory blood pressure monitoring can unmask hypertension in patients with psoriasis vulgaris. Med Sci Monitor. 2013;19:501–509. doi: 10.12659/MSM.889197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sobrino J, Domenech M, Camafort M, Vinyoles E, Coca A. Prevalence of masked hypertension and associated factors in normotensive healthcare workers. Blood Press Monit. 2013;18(6):326–331. doi: 10.1097/MBP.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 47.Franklin SS, Thijs L, Li Y, Hansen TW, Boggia J, Liu Y, Asayama K, Bjorklund-Bodegard K, Ohkubo T, Jeppesen J, et al. Masked hypertension in diabetes mellitus: treatment implications for clinical practice. Hypertension. 2013;61(5):964–971. doi: 10.1161/HYPERTENSIONAHA.111.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsen TR, Gelaye A, Waanbah B, Assad H, Daloul Y, Williams F, Williams M, Steigerwalt S. Prevalence of masked hypertension in African Americans. J Clin Hypertens (Greenwich) 2014;16(11):801–804. doi: 10.1111/jch.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Viera AJ, Lin FC, Tuttle LA, Shimbo D, Diaz KM, Olsson E, Stankevitz K, Hinderliter AL. Levels of office blood pressure and their operating characteristics for detecting masked hypertension based on ambulatory blood pressure monitoring. Am J Hypertens. 2015;28(1):42–49. doi: 10.1093/ajh/hpu099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trachsel LD, Carlen F, Brugger N, Seiler C, Wilhelm M. Masked hypertension and cardiac remodeling in middle-aged endurance athletes. J Hypertens. 2015;33(6):1276–1283. doi: 10.1097/HJH.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 51.Redmond N, Booth JN, 3rd, Tanner RM, Diaz KM, Abdalla M, Sims M, Muntner P, Shimbo D. Prevalence of masked hypertension and its association with subclinical cardiovascular disease in African Americans: results from the Jackson heart study. J Am Heart Assoc. 2016;5(3):e002284. doi: 10.1161/JAHA.115.002284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Viera AJ, Lin FC, Tuttle LA, Olsson E, Girdler SS, Hinderliter AL. Examination of several physiological and psychosocial factors potentially associated with masked hypertension among low-risk adults. J Clin Hypertens (Greenwich) 2016;18(8):784–789. doi: 10.1111/jch.12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piantanida E, Gallo D, Veronesi G, Pariani N, Masiello E, Premoli P, Sassi L, Lai A, Tanda ML, Ferrario M, et al. Masked hypertension in newly diagnosed hypothyroidism: a pilot study. J Endocrinol Invest. 2016;39(10):1131–1138. doi: 10.1007/s40618-016-0488-7. [DOI] [PubMed] [Google Scholar]
- 54.Booth JN, 3rd, Muntner P, Diaz KM, Viera AJ, Bello NA, Schwartz JE, Shimbo D. Evaluation of criteria to detect masked hypertension. J Clin Hypertens (Greenwich) 2016;18(11):1086–1094. doi: 10.1111/jch.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anstey DE, Booth JN, Abdalla M, Spruill TM, Min YI, Muntner P, Shimbo D. Predicted atherosclerotic cardiovascular disease risk and masked hypertension among blacks in the Jackson heart Study. Circul Cardiovasc Qual Outcomes. 2017;10:7. doi: 10.1161/CIRCOUTCOMES.116.003421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ozkan S, Ata N, Yavuz B. Increased masked hypertension prevalence in patients with obesity. Clin Exp Hypertens (New York, NY) 2018;2018:1–4. doi: 10.1080/10641963.2018.1431262. [DOI] [PubMed] [Google Scholar]
- 57.Gun T, Ozkan S, Yavuz B. Is tinnitus an early voice of masked hypertension? High masked hypertension rate in patients with tinnitus. Clin Exp Hypertens (New York, NY) 2018;2018:1–4. doi: 10.1080/10641963.2018.1465077. [DOI] [PubMed] [Google Scholar]
- 58.Bhattarai M, Sainju NK, Bhandari B, Kc V, Karki DB. Prevalence of white coat hypertension among the patients visiting in a tertiary care center, Kathmandu, Nepal. Kathmandu Univ Med J. 2019;17(66):119–122. [PubMed] [Google Scholar]
- 59.Cai P, Peng Y, Chen Y, Li L, Chu W, Wang Y, Wang X. Association of thyroid function with white coat hypertension and sustained hypertension. J Clin Hypertens (Greenwich, Conn) 2019;21(5):674–683. doi: 10.1111/jch.13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Calvo-Vargas C, Padilla-Rios V, Troyo-Sanromán R. Loaned self-measurement equipment model compared with ambulatory blood pressure monitoring. Blood Pressure Monit. 2003;8(2):63–70. doi: 10.1097/00126097-200304000-00002. [DOI] [Google Scholar]
- 61.Erdogmus S, Kutlay S, Celebi ZK, Aydin T, Ors Sendogan D, Kumru G, Keven K, Nergizoglu G, Erturk S, Sengul S. Clinical correlates of ambulatory blood pressure phenotypes at a tertiary care hospital in Turkey. Kidney Blood Pressure Res. 2018;43(3):690–700. doi: 10.1159/000489742. [DOI] [PubMed] [Google Scholar]
- 62.Kaul U, Arambam P, Rao S, Kapoor S, Swahney JPS, Sharma K, Nair T, Chopda M, Hiremath J, Ponde CK, et al. Usefulness of ambulatory blood pressure measurement for hypertension management in India: the India ABPM study. J Hum Hypertens. 2019;71:91. doi: 10.1038/s41371-019-0243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Michaud A, Lamarre-Cliche M, Cloutier L. Screening for hypertension: An elevated office blood pressure measurement is valuable, adding an automated one is even better. Blood Pressure Monit. 2019;24(3):123–129. doi: 10.1097/MBP.0000000000000382. [DOI] [PubMed] [Google Scholar]
- 64.Salazar MR, Espeche WG, Balbin E, Leiva Sisnieguez CE, Minetto J, Leiva Sisnieguez BC, Maciel PM, Stavile RN, Carbajal HA. Prevalence of isolated nocturnal hypertension according to 2018 European Society of Cardiology and European Society of Hypertension office blood pressure categories. J Hypertens. 2019;6:914. doi: 10.1097/HJH.0000000000002278. [DOI] [PubMed] [Google Scholar]
- 65.Sheppard JP, Martin U, Gill P, Stevens R, Hobbs FR, Mant J, Godwin M, Hanley J, McKinstry B, Myers M, et al. Prospective external validation of the Predicting Out-of-OFfice Blood Pressure (PROOF-BP) strategy for triaging ambulatory monitoring in the diagnosis and management of hypertension: observational cohort study. BMJ (Clinical research ed) 2018;361:k2478. doi: 10.1136/bmj.k2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trudel X, Brisson C, Gilbert-Ouimet M, Vezina M, Talbot D, Milot A. Long working hours and the prevalence of masked and sustained hypertension. Hypertension. 2020;75(2):532–538. doi: 10.1161/HYPERTENSIONAHA.119.12926. [DOI] [PubMed] [Google Scholar]
- 67.Almeida AE, Stein R, Gus M, Nascimento JA, Arevalo JR, Fuchs FD, Ribeiro JP. Improved diagnostic accuracy of a 3-day protocol of home blood pressure monitoring for the diagnosis of arterial hypertension. Blood Pressure Monit. 2013;18(2):119–126. doi: 10.1097/MBP.0b013e32835ebb18. [DOI] [PubMed] [Google Scholar]
- 68.Park JS, Rhee MY, Namgung J, Lee SY, Cho DK, Choi TY, Kim SY, Kim JY, Park SM, Choi JH, et al. Comparison of optimal diagnostic thresholds of hypertension with home blood pressure monitoring and 24-hour ambulatory blood pressure monitoring. Am J Hypertens. 2017;30(12):1170–1176. doi: 10.1093/ajh/hpx115. [DOI] [PubMed] [Google Scholar]
- 69.de Almeida AEM, Stein R, Gus M, Nascimento JA, Belli KC, Arévalo JRG, Fuchs FD, Ribeiro JP. Relevance to home blood pressure monitoring protocol of blood pressure measurements taken before first-morning micturition and in the afternoon. Arq Bras Cardiol. 2014;103(4):338–347. doi: 10.5935/abc.20140139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rhee MY, Kim JY, Kim JH, Namgung J, Lee SY, Cho DK, Choi TY, Kim SY. Optimal schedule of home blood-pressure measurements for the diagnosis of hypertension. Hypertens Res. 2018;41(9):738–747. doi: 10.1038/s41440-018-0069-6. [DOI] [PubMed] [Google Scholar]
- 71.Zhang L, Li Y, Wei FF, Thijs L, Kang YY, Wang S, Xu TY, Wang JG, Staessen JA. Strategies for classifying patients based on office, home, and ambulatory blood pressure measurement. Hypertension. 2015;65(6):1258–1265. doi: 10.1161/HYPERTENSIONAHA.114.05038. [DOI] [PubMed] [Google Scholar]
- 72.Hanninen MR, Niiranen TJ, Puukka PJ, Jula AM. Comparison of home and ambulatory blood pressure measurement in the diagnosis of masked hypertension. J Hypertens. 2010;28(4):709–714. doi: 10.1097/HJH.0b013e3283369faa. [DOI] [PubMed] [Google Scholar]
- 73.Stergiou GS, Skeva II, Baibas NM, Kalkana CB, Roussias LG, Mountokalakis TD. Diagnosis of hypertension using home or ambulatory blood pressure monitoring: comparison with the conventional strategy based on repeated clinic blood pressure measurements. J Hypertens. 2000;18(12):1745–1751. doi: 10.1097/00004872-200018120-00007. [DOI] [PubMed] [Google Scholar]
- 74.Ntineri A, Niiranen TJ, McManus RJ, Lindroos A, Jula A, Schwartz C, Kollias A, Andreadis EA, Stergiou GS. Ambulatory versus home blood pressure monitoring: frequency and determinants of blood pressure difference and diagnostic disagreement. J Hypertens. 2019;37(10):1974–1981. doi: 10.1097/HJH.0000000000002148. [DOI] [PubMed] [Google Scholar]
- 75.Franklin SS, Thijs L, Hansen TW, O'Brien E, Staessen JA. White-coat hypertension: new insights from recent studies. Hypertension (Dallas, Tex: 1979) 2013;62(6):982–987. doi: 10.1161/HYPERTENSIONAHA.113.01275. [DOI] [PubMed] [Google Scholar]
- 76.Verdecchia P, Reboldi GP, Angeli F, Schillaci G, Schwartz JE, Pickering TG, Imai Y, Ohkubo T, Kario K. Short- and long-term incidence of stroke in white-coat hypertension. Hypertension (Dallas, Tex: 1979) 2005;45(2):203–208. doi: 10.1161/01.HYP.0000151623.49780.89. [DOI] [PubMed] [Google Scholar]
- 77.Stergiou GS, Asayama K, Thijs L, Kollias A, Niiranen TJ, Hozawa A, Boggia J, Johansson JK, Ohkubo T, Tsuji I, et al. Prognosis of white-coat and masked hypertension: international database of home blood pressure in relation to cardiovascular outcome. Hypertension. 2014;63(4):675–682. doi: 10.1161/HYPERTENSIONAHA.113.02741. [DOI] [PubMed] [Google Scholar]
- 78.Schmucker CM, Blümle A, Schell LK, Schwarzer G, Oeller P, Cabrera L, von Elm E, Briel M, Meerpohl JJ. on behalf of the Oc: Systematic review finds that study data not published in full text articles have unclear impact on meta-analyses results in medical research. PLoS ONE. 2017;12(4):e0176210. doi: 10.1371/journal.pone.0176210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fergusson D, Laupacis A, Salmi LR, McAlister FA, Huet C. What should be included in meta-analyses? An exploration of methodological issues using the ISPOT meta-analyses. Int J Technol Assess Health Care. 2000;16(4):1109–1119. doi: 10.1017/S0266462300103150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Additional appendix, tables, and figures.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its Additional information files.