Abstract
Background
Previous studies have implicated reduced brain-derived neurotrophic factor (BDNF) expression and BDNF-TrkB receptor signaling as well as microglial activation and neuroinflammation in poststroke depression (PSD). However, the contributions of microglial BDNF-TrkB signaling to PSD pathogenesis are unclear.
Material/Methods
We compared depression-like behaviors as well as neuronal and microglial BDNF and TrkB expression levels in the amygdala, a critical mood-relating limbic structure, in rat models of stroke, depression, and PSD. Depression-like behaviors were assessed using the sucrose preference test, open-field test, and weight measurements, while immunofluorescence double staining was employed to estimate BDNF and TrkB expression by CD11b-positive amygdala microglia and NeuN-positive amygdala neuron. Another group of PSD model rats were examined following daily intracerebroventricular injection of proBDNF, tissue plasminogen activator (t-PA), or normal saline (NS) for 7 days starting 4 weeks after chronic unpredictable mild stress (CUMS).
Results
The numbers of BDNF/CD11b- and TrkB/CD11b-immunofluorescence-positive cells were lowest in the PSD group at 4 and 8 weeks after CUMS (P<0.05). PSD rats also showed reduced weight, sucrose preference, locomotion, and rearing compared with controls (P<0.05). The coexpression of BDNF/NeuN- and TrkB/NeuN-positive cells were not significantly different between groups at 4 and 8 weeks after CUMS (P>0.05). Injection of t-PA increased BDNF/CD11b- and TrkB/CD11b-positive cells in the amygdala of PSD rats and normalized behavior compared with NS or proBDNF injection (P<0.05). In contrast, proBDNF injection reduced BDNF and TrkB expression compared with NS (P<0.05).
Conclusions
These results suggest that decreased BDNF and TrkB expression by amygdala microglia may contribute to PSD pathogenesis and depression-like behaviors.
MeSH Keywords: Amygdala; Depression; Microglia; Receptor, trkB; Stroke
Background
Nearly one-third of stroke survivors develop depression [1]. Poststroke depression (PSD) is a complex neuropsychiatric disorder manifested by apathy, low self-esteem, slowness of thought, and darkened mood, symptoms that not only interfere with functional recovery but may also increase mortality and self-harm rates [2].
Studies have implicated neuroinflammation, dysfunctional neurotrophic factor signaling, and aberrant neuroplasticity in the pathogenesis of PSD [3,4]. Microglia are brain-residing macrophages distributed throughout the central nervous system, where they regulate neuroplasticity as well as local inflammatory responses. Activated microglia initiate opposing responses after stroke-related brain damage [5]. Activation and concomitant release of proinflammatory cytokines contribute to neurodegenerative processes and stress-related behavioral deficits associated with PSD [6]. Conversely, microglia protect neurons and sustain synaptic plasticity by releasing various neurotrophic factors such as brain-derived neurotrophic factor (BDNF) [7].
BDNF signaling through the tyrosine kinase receptor B (TrkB) is a crucial mediator of neural survival and plasticity. BDNF is expressed at high levels in regions of the brain known to be involved in the regulation of mood, including the prefrontal cortex, hippocampus, and amygdala [8]. Research has shown a clear association between PSD and changes in both BDNF and its precursor, proBDNF [9,10]. Specifically, reduced BDNF and elevated proBDNF are associated with PSD, while elevated BDNF expression can reduce nerve cell death associated with brain ischemia. Tissue-type plasminogen activator (tPA) is a proteolytic enzyme that participates in neurotransmission, synaptic plasticity, and the regulation of cognitive functions. ProBDNF can be converted to mature BDNF by plasmin through the activation of plasminogen, which is dependent on tPA [11]. These results suggest that changes in microglia-derived BDNF may contribute to PSD. However, the potential contribution of microglia-derived BDNF to PSD has not been explored.
In the current study, we evaluated BDNF and TrkB expression changes in amygdala microglia and neurons and associated behavioral changes in a rat model of PSD. Further, we examined if intracerebroventricular (ICV) injection of t-PA or proBDNF can alter PSD pathogenesis or behavioral expression.
Material and Methods
Animals
Adult Sprague-Dawley rats (250±50 g) were obtained from the Animal Experiment Center, Kunming Medical University. Animals were maintained (maximum 5 per cage) at 23±2°C under 55±5% humidity and a 12 h/12 h light/dark cycle with free access to food and water. The study was conducted in accordance with the guidelines of the China Animal Protection Law. The study protocol was approved by the Animal Ethics Committee of the First Affiliated Hospital of Dali University.
Middle cerebral artery occlusion
Briefly, animals were anesthetized with 2.5% isoflurane and the skin was prepared for surgery by routine disinfection. An intraluminal thread was introduced into the common carotid artery and advanced to permanently occlude the middle cerebral artery, followed by routine wound closure [12]. Rats were placed in a heated environment immediately after surgery. After waking, animals were returned to standard housing conditions and received intraperitoneal injections of penicillin (40 000 U/d) for 3 days to prevent postoperative infections. The Longa 5-grade standards and Horizontal Stick Experiment were used to assess functional neurological deficits following middle cerebral artery occlusion (MCAO). Three investigators blinded to MCAO evaluated neurological deficits, recorded the results, and determined the average scores.
Chronic unpredictable mild stress plus isolation housing
One week after MCAO, PSD and depression group rats were housed individually and exposed randomly to 1 of 8 stressors each day for 21 days (3 weeks) [13]. Stressors included cage shaking (1 shake/s for 5 min), 45° cage tilting (15-h duration), food and water deprivation (24 h), 4°C swim (5 min), tail pinching (1 min), moist cage bedding (10-h duration), overnight illumination (12 h), and immobilization (2 h).
Behavioral tests
Body weight, sucrose preference, and open-field test activities were evaluated 1 day and 2, 4, and 8 weeks after chronic unpredictable mild stress (CUMS).
For the sucrose preference test, rats were adapted to sucrose prior to testing by placing 2 bottles of 1% (w/v) sucrose solution on the cage for 48 h. After 24-h food and water deprivation, animals were allowed to freely consume water or 1% sucrose solution for 1 h from separate bottles. Bottle positions were randomly varied in each sucrose preference test. After 1 h, the volumes of ingested sucrose solution and water were determined. Sucrose preference (%) was calculated based on the formula sucrose preference=vol. sucrose ingested/(vol. sucrose+vol. water ingested)×100.
For the open-field test, the test chamber was a wooden box (90×90×40 cm) with black walls and a white floor divided by black lines into 25 equal squares. A rat was placed in the center of the box and allowed free movement. Locomotor activity (at least 3 paws in a new quadrant) and rearing behavior (standing upright on hind legs) were quantified over a 5-min period. Both activities were manually recorded by 2 observers blinded to treatment group. The chamber was cleaned with 75% alcohol between tests to eliminate odors.
Immunofluorescence analysis
Animals were anesthetized with 2.5% isoflurane and perfused via the left ventricle with 50 mL of normal saline (NS) followed by 200 mL of 4% phosphate-buffered paraformaldehyde. The brain was removed and postfixed in 4% paraformaldehyde overnight, followed by transfer to 30% sucrose in 1× phosphate-buffered saline (PBS). Coronal sections were cut at a thickness of 5 μm using a cryostat vibratome (Leica, Germany) [14].
After being washed with 1×PBS, sections were blocked using 5% goat serum for 1 h and then treated with primary antibodies against CD11b (1: 300, Millipore Inc., USA) and BDNF (1: 500, Abcam Inc., USA) or CD11b and TrkB (1: 3000, Abcam, Inc.), as well as NeuN (1: 300, Abcam Inc.) and BDNF or NeuN and TrkB overnight at 4°C, respectively. Sections were then washed 3 times in 1×PBS and incubated with goat-anti-mouse IgG (1: 100, Dylight TM488, Cell Signaling Technology, Inc., Danvers, MA, USA) and goat-anti-rabbit IgG (1: 100, Dylight TM594, Cell Signaling Technology) at 37°C for 1 h in the dark. Following 3 washes in PBS, the sections were mounted with 70% glycerol and 30% PBS, and images were acquired using a fluorescence confocal microscope (Leica TCS SP8). Five slices were taken from the basolateral amygdala of every rat and 5 randomly chosen fields were scanned using a laser scanning confocal microscope with the HC PL APO 40×/0.95 CS lens. Photographs were analyzed with Image-Pro Plus 6.0 (Media Cybernetics, Silver Spring, MD, USA).
ICV administration of proBDNF, t-PA, or normal saline
Three weeks after CUMS, some PSD rats were anesthetized with 2.5% isoflurane and placed in a stereotaxic apparatus (68001R, RWD Life Sciences, Shenzhen, China). During the entire procedure, anesthetized rats were placed on a hot pad where their foot pinch reflex, breath, and body heat were monitored. A midline scalp incision was performed to expose the skull. According to the Watson and Paxinos Rat Brain Atlas [15], a hole was drilled at anteroposterior −0.90 mm, mediolateral 1.70 mm, and dorsoventral −4.00 mm, and a stainless-steel guide cannula (26 gauge) was placed 1 mm above the left lateral ventricle. The guide cannula was fixed to 2 stainless-steel screws anchored to the skull using a mixture of dental acrylic cement and cyanoacrylate glue. A stainless-steel cannula dummy was placed in the guide cannula to maintain patency. All animals were injected intraperitoneally with sodium penicillin (40 000 U) to prevent postoperative infection.
Animals were assigned to the NS group (N=8), t-PA group (N=8), or proBDNF group (N=8). Rats were infused with tPA (3 μg dissolved in 6 μL saline, Boehringer Ingelheim), proBDNF (3 μg dissolved in 6 μL saline, Abcam), or equal-volume NS once per day for 7 consecutive days using the syringe pump connecting to injection needles. Behavioral tests and immunofluorescence double staining were conducted as described above.
Statistical analysis
For all quantification procedures, observers were blind to treatment history. All data are expressed as mean±standard error (SE). Group means were compared by one-way ANOVA and S-N-K test. All calculations were performed using the SPSS 17.0 for Windows covariance software package. The level of significance was set at P<0.05.
Results
PSD model
Rats subjected to MCAO displayed focal neurological deficits after 24 h. These MCAO rats were then housed individually without further intervention (stroke group), while depression and PSD groups were subjected to the isolation-housing combined with CUMS protocol. After 3 weeks of CUMS, rats exhibited depression-like symptoms, including slow responses, drowsiness, and sluggishness, but without unexpected death. To evaluate the depressive-like symptoms, body weight, sucrose preference, and open-field activity were measured in all groups 1 day and 2, 4, and 8 weeks, after CUMS. We observed that PSD rats exhibited greater weight loss and lower sucrose intake (a sign of depression-like anhedonia), locomotor activity, and rearing in the open field, indicating successful establishment of the PSD model.
Behavioral tests
Changes in body weight
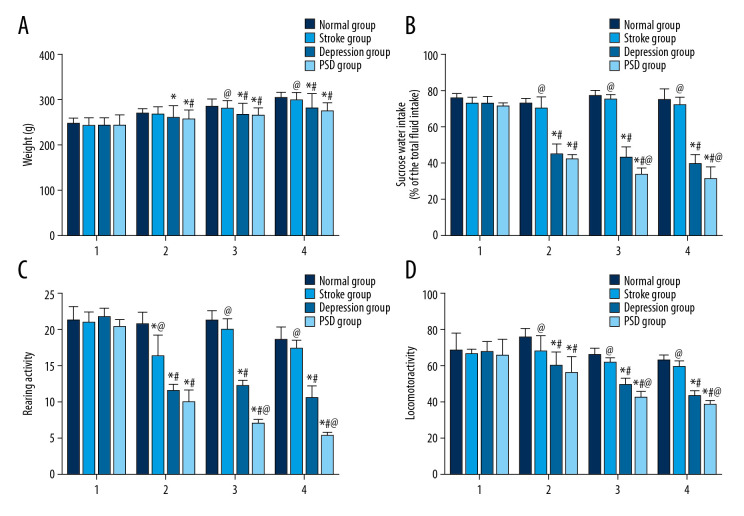
One day after CUMS, there was no significant difference in body weight among all groups (P>0.05). Two weeks after CUMS, however, body weight was significantly lower in the depression group and PSD group compared with the normal control group (P<0.05), while there was no significant difference between the stroke and normal control group (P>0.05). 4 and 8 weeks after CUMS, body weights were lower in the depression and PSD group than in the normal control group and stroke group (P<0.05) (Figure 1A).
Figure 1.
Behavioral testing results in each group at different time points after CUMS. Histograms show (A) body weight (indicative of feeding behavior), (B) sucrose preference, (C) rearing activity, and (D) locomotor activity. Data are presented as mean±SE. Intergroup comparisons evaluated by 1-way ANOVA. N=10 rats/group; 1 (first day after CUMS), 2 (2 weeks), 3 (4 weeks), 4 (8 weeks). * P<0.05 versus normal control rats; @ P<0.05 versus depression model rats (isolation-housing combined with CUMS); # P<0.05 versus stroke model rats (MCAO only). ANOVA – analysis of variance; CUMS – chronic unpredictable mild stress; MCAO – middle cerebral artery occlusion.
Changes in sucrose consumption
At 1 day after CUMS, there were no significant differences among intervention groups (P>0.05). At 2, 4, and 8 weeks after CUMS, sucrose consumption was significantly lower in the depression and PSD groups compared with the stroke and normal control groups (all P<0.05). Furthermore, at 4 and 8 weeks after CUMS, sucrose consumption in the PSD group was lower than in the depression group (P<0.05) (Figure 1B).
Rearing activity in the open-field test
At 1 day after CUMS, there was no significant difference in rearing activity among all groups (P>0.05). At 2, 4, and 8 weeks after CUMS, rearing activity scores were significantly lower in the PSD group and depression group than in the normal group and stroke group (P<0.05). Additionally, the PSD group exhibited significantly less rearing activity than the depression group at 4 and 8 weeks after CUMS (P<0.05), reaching lowest activity at 8 weeks after CUMS (Figure 1C).
Locomotor activity in the open-field test
One day after CUMS, there was no significant difference among all groups in the open-field test (P>0.05). Moreover, locomotor activity was significantly lower in the PSD group and depression group compared with the normal control group and stroke group at 2, 4, and 8 weeks after CUMS (P<0.05). Locomotor activity was also significantly lower in the PSD group compared with the depression group at 4 and 8 weeks after CUMS (P<0.05) (Figure 1D).
Behavioral scores in PSD rats after ICV administration of t-PA, proBDNF, or NS
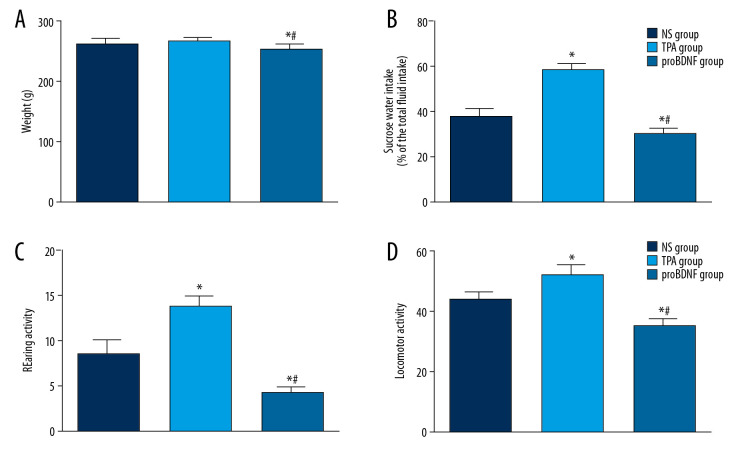
PSD rats exhibited significantly greater sucrose preference and activities in the open-field test (both rearing and locomotor activity) 1 week after ICV t-PA injection compared with PSD rats receiving ICV NS or proBDNF (P<0.05). PSD rats injected with t-PA were also heavier than PSD rats injected with proBDNF (P<0.05), while there was no significant difference between t-PA and NS injection groups (P>0.05). The results suggest that t-PA injection can significantly alleviate depressive-like behaviors in PSD model rats (Figure 2).
Figure 2.
Effects of t-PA, proBDNF, or NS administered by ICV injection on depression-like behavior in PSD models rats. Histograms show (A) body weight (feeding behavior), (B) sucrose preference, (C) rearing activity, and (D) locomotor activity in rats injected with NS, t-PA, or proBDNF as indicated. N=8 rats/group. * P<0.05 versus NS-treated rats; # P<0.05 versus t-PA-treated rats. BDNF – brain-derived neurotrophic factor; ICV – intracerebroventricular; NS – normal saline; PSD – poststroke depression; t-PA – tissue plasminogen activator.
Immunofluorescence analysis
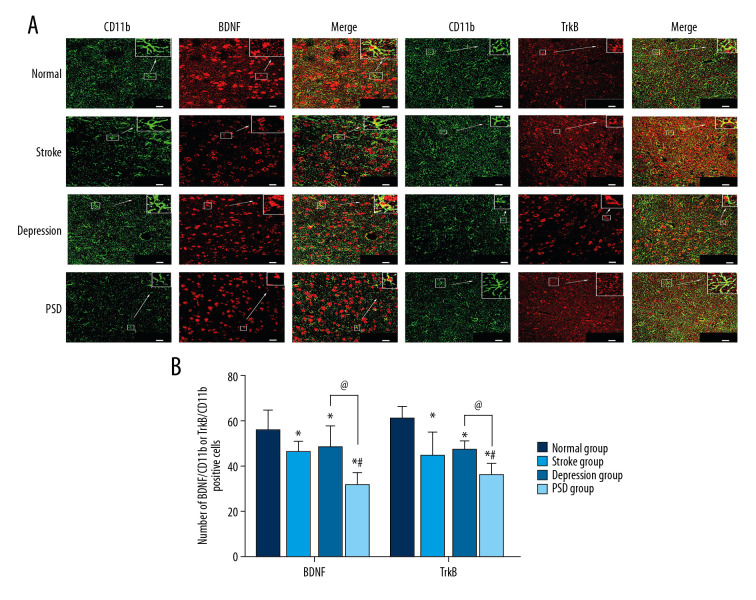
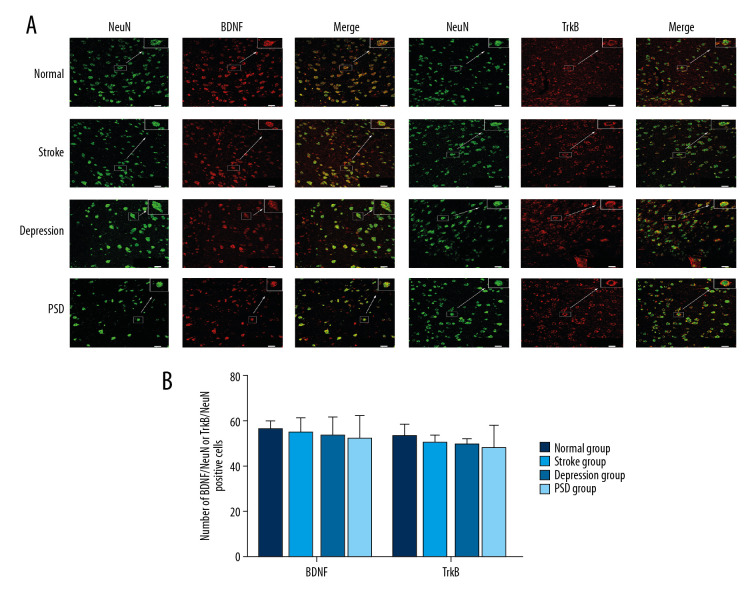
Immunolabeling with CD11b (green fluorescence) was used to identify microglia in the amygdala. Overlapping BDNF or TrkB immunolabeling (red fluorescence, merged yellow-green fluorescence) revealed microglial expression of these signaling factors. We examined changes in microglial expression levels of BDNF or TrkB at 4 and 8 weeks after CUMS. At 4 weeks after CUMS, the number of coexpression-positive cells of amygdala BDNF/CD11b and TrkB/CD11b were lower among stroke, depression, and PSD group rats compared with the normal control group (all P<0.05), and lower in the PSD group than the other treatment groups (all P<0.05), while there was no significant difference between depression and stroke groups (P>0.05) (Figure 3). The same reduction pattern was also observed 8 weeks after CUMS (Figure 4).
Figure 3.
Dual-immunofluorescence detection of BDNF or TrkB expression by amygdala microglia 4 weeks after CUMS. (A) BDNF or TrkB expression (red fluorescence) in CD11b-positive microglia (green fluorescence, merged yellow fluorescence) (bar=20 μm). (B) Histograms show the number of BDNF/CD11b- or TrkB/CD11b-positive cells in amygdala microglia of each group. N=5 rats/group. * P<0.05 versus normal control rats; @ P<0.05 versus depression model rats; # P<0.05 versus stroke model rats. BDNF – brain-derived neurotrophic factor; CUMS – chronic unpredictable mild stress; TrkB – tyrosine kinase receptor B.
Figure 4.
Dual-immunofluorescence detection of CD11b expression (green fluorescence) and BDNF or TrkB expression (red fluorescence) by amygdala microglia 8 weeks after CUMS. (A) BDNF or TrkB expression in CD11b-positive microglia (bar=20 μm). (B) Histograms show the number of BDNF/CD11b- or TrkB/CD11b-positive cells in amygdala of each group. N=5 rats/group. * P<0.05 versus normal control rats; @ P<0.05 versus depression model rats; # P<0.05 versus stroke model rats. BDNF – brain-derived neurotrophic factor; CUMS – chronic unpredictable mild stress; TrkB – tyrosine kinase receptor B.
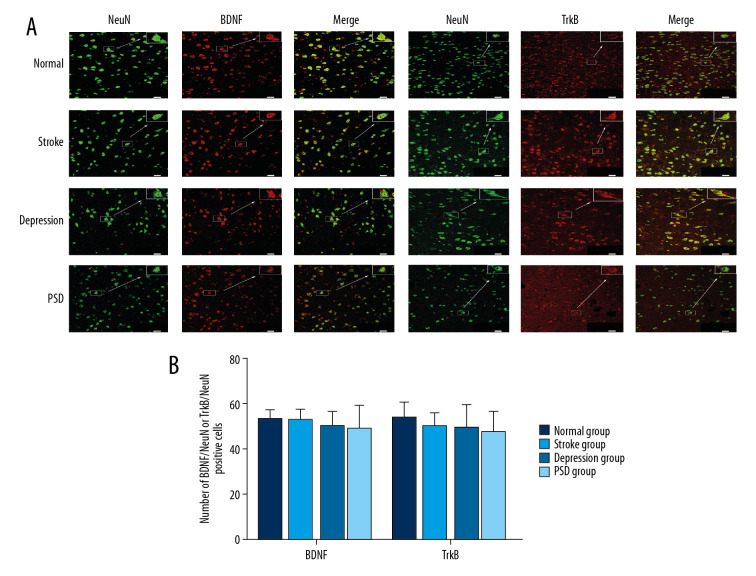
Similarly, immunolabeling with NeuN (green fluorescence) was used to identify neurons, and overlapping BDNF and TrkB immunolabeling (red fluorescence, merged yellow-green fluorescence) represented these signaling factors expressed by the same neuron. We also measured the level of BDNF or TrkB labeled by NeuN at 4 and 8 weeks after CUMS. Results showed that the number of BDNF/NeuN- and TrkB/NeuN-immunofluorescence-positive cells was lower in PSD group than that in the normal group at both 4 and 8 weeks after CUMS, but there were no significant differences among all groups (all P>0.05) (Figures 5, 6).
Figure 5.
Dual-immunofluorescence detection of BDNF or TrkB expression by amygdala neuron 4 weeks after CUMS. (A) BDNF or TrkB expression (red fluorescence) in NeuN-positive neuron (green fluorescence, merged yellow fluorescence) (bar=20 μm). (B) Histograms show the number of BDNF/NeuN- or TrkB/NeuN-positive cells in amygdala of each group. N=5 rats/group. * P<0.05 versus normal control rats; @ P<0.05 versus depression model rats;# P<0.05 versus stroke model rats. BDNF – brain-derived neurotrophic factor; CUMS – chronic unpredictable mild stress; TrkB – tyrosine kinase receptor B.
Figure 6.
Dual-immunofluorescence detection of NeuN expression (green fluorescence) and BDNF or TrkB expression (red fluorescence) by amygdala neuron 8 weeks after CUMS. (A) BDNF or TrkB expression in NeuN-positive neuron (bar=20 μm). (B) Histograms show the number of BDNF/NeuN- or TrkB/NeuN-positive cells in amygdala of each group. N=5 rats/group. * P<0.05 versus normal control rats; @ P<0.05 versus depression model rats; # P<0.05 versus stroke model rats. BDNF – brain-derived neurotrophic factor; CUMS – chronic unpredictable mild stress; TrkB – tyrosine kinase receptor B.
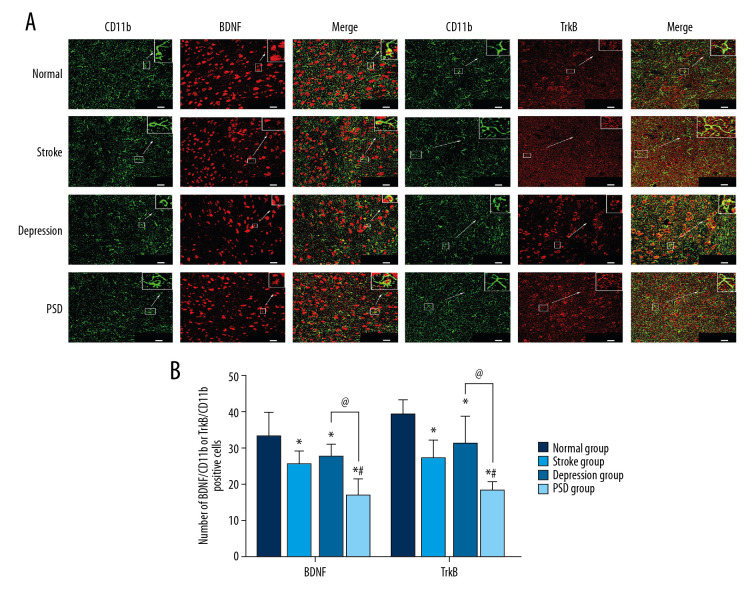
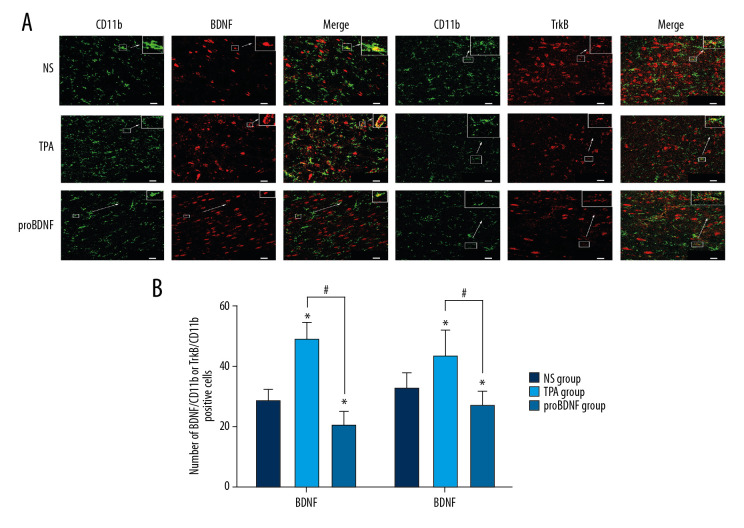
The number of BDNF/CD11b- or TrkB/CD11b-positive cells was significantly higher in the t-PA injection group compared to the NS and proBDNF injection groups (both P<0.05). Conversely, the coexpression of BDNF/CD11b- or TrkB/CD11b-positive cells was lower in the proBDNF injection group compared with the NS and t-PA groups (P<0.05) (Figure 7).
Figure 7.
Effects of t-PA, proBDNF, and NS ICV injection on microglial expression of BDNF or TrkB in the amygdala 1 week after injection. (A) BDNF or TrkB expression (red fluorescence) in CD11b-positive microglia (green fluorescence) (bar=20 μm). (B) Histograms show the number of BDNF/CD11b- or TrkB/CD11b-positive cells in microglia of PSD model rats 1 week after ICV injection. N=5 rats/group. * P<0.05 versus NS injection; # p<0.05 versus tPA injection. BDNF – brain-derived neurotrophic factor; ICV – intracerebroventricular; NS – normal saline; PSD – poststroke depression; t-PA – tissue plasminogen activator; TrkB – tyrosine kinase receptor B.
Discussion
PSD is a common neuropsychiatric complication that greatly impedes patient rehabilitation and impairs quality of life. Depression exacerbates infarction size, while stroke increases the risk of depression, so the depression-stroke association appears to be bidirectional [16].
In the present study, PSD rats exhibited significantly lower sucrose preference, rearing activity, and locomotor activity compared with stroke alone, depression alone, and control groups at 4 and 8 weeks after CUMS, indicating a more severe depression-like phenotype. This finding is consistent with our previous research and literature search results [17,18].
Microglia, the resident brain macrophages, account for approximately 10% of all human brain cells. Microglia are involved in multiple functions, including regulation of stem cell proliferation, tissue remodeling, pruning of synapses, promoting nerve regeneration, and monitoring neuronal activity [19,20]. Under normal conditions, microglia display a ramified morphology with a small cell soma and fine processes, referred to as the “resting state.” In this state, microglia serve a vital role in immune surveillance [19]. Under pathological conditions such as infection, inflammation, and neuronal injury, microglia are transformed to activated states characterized by retraction and thickening of processes and hypertrophy of the cell body [5].
Our results showed that the coexpression of amygdala BDNF/CD11b and TrkB/CD11b in PSD rats was lower than in the other control groups, whereas there was no equivalent result for the coexpression of BDNF/NeuN and TrkB/NeuN in PSD rats. Microglial BDNF can lead to the activation of microglia, and activated microglia can further induce the expression of BDNF [21]. Thus, small changes in microglial BDNF levels may cause obvious physiological effects, which suggests that microglia have a significant role in BDNF expression in PSD.
After stroke, activated microglia can give rise to elevated levels of proinflammatory cytokines, including interleukin (IL)-1, IL-6, and tumor necrosis factor-α [22]. These cytokines have been shown to stimulate indoleamine-2,3-dioxygenase in glial cells and lead to conversion of tryptophan to kynurenine, which is further converted to neurotoxic quinolinic acid. Binding of quinolinic acid to N-methyl-D-aspartate receptors results in reduced levels of serotonin and increased glutamatergic activity, long known to be associated with depression [23]. Further, these cytokines can reduce the release of BDNF or interrupt its binding to TrkB, which may contribute to depression-like behaviors [24]. Parkhurst et al. [25] has also suggested that microglia-derived BDNF plays an integral part in homeostatic neuroplasticity mechanisms. In addition, microglia can interfere with the formation of dendritic spines by regulating the level of BDNF. The amygdala is involved in emotional behavior, locomotor activity, and integration of endocrine functions. Damage to the amygdala can lead to aberrant motional behavior, decreased aggression, increased passivity, and mood disorders [26]. Neuronal atrophy and cell loss in the amygdala have been demonstrated in animal models of depression [27] and following stroke [26].
BDNF and its receptor TrkB contribute to PSD [25] and may also mediate the therapeutic response to various treatments. Indeed, BDNF and TrkB expression levels are decreased in depression and upregulated by antidepressants [28]. The precursor of mature BDNF, proBDNF, can induce neuronal cell apoptosis, long-term depression, neurite collapse, and synaptic retraction, thus opposing the actions of BDNF [29]. Clinical and experimental studies have demonstrated that serum proBDNF is upregulated in depression patients [30] and rat models of depression [31]. Benraiss et al. [32] reported that BDNF injected into the lateral ventricles could increase neurogenesis in several brain regions, while Yang et al. [33] found that anti-proBDNF antibody injection could reverse depression-like behavior in a rat model of chronic stress.
The proteolytic enzyme t-PA, which converts plasminogen to plasmin and promotes conversion of proBDNF to BDNF, can also influence depression [34]. In addition to being secreted by endothelial cells, t-PA has also been shown to be secreted by neurons and microglia of the central nervous system, where it is highly expressed and is involved in neuronal synaptic plasticity and stress reaction [35]. The activity of t-PA is an important regulator of structural plasticity of the amygdala [36]. Indeed, some researchers have suggested that the overexpression of t-PA may have a potential therapeutic effect on depression, especially for comorbidities related to cardiovascular disease [8]. Our research initially verified this view and further clarified the effect of exogenous administration of t-PA on expression of microglial BDNF.
In this study, we found that numbers of BDNF/CD11b- and TrkB/CD11b-positive cells were greatly increased after injection of t-PA in PSD model rats compared with NS and proBDNF injection. Moreover, t-PA injection enhanced sucrose consumption and open-field activity by PSD rats (i.e., reduced depression-related anhedonia and locomotor retardation) compared with NS or proBDNF injection. Duman and Monteggia [27] reported that downregulation of BDNF expression blocked neural protection and repair, leading to neuronal cell apoptosis and eventual depression. Our results suggest that downregulation of BDNF expression by microglia contributes to the development of depression after stroke.
Conclusions
In a rat model, the coexpression of BDNF/CD11b and TrkB/CD11b was lower in the PSD group compared with the stroke, depression, and control groups at 4 and 8 weeks after CUMS. Behavioral test scores were also significantly lower in PSD rats than in stroke and depression groups at 4 and 8 weeks after CUMS, suggesting that downregulation of BDNF and TrkB expression by amygdala microglia may be relevant in the development of PSD. These findings provide a foundation for further studies on the contributions of microglia-derived BDNF to depression following stroke.
Footnotes
Source of support: This study was supported by the National Natural Science Foundation of China (grant no. 81360208)
References
- 1.Hackett ML, Yapa C, Parag V, Anderson CS. Frequency of depression after stroke: A systematic review of observational studies. Stroke. 2005;36:1330–40. doi: 10.1161/01.STR.0000165928.19135.35. [DOI] [PubMed] [Google Scholar]
- 2.Su Q, Cheng Y, Jin K, et al. Estrogen therapy increases BDNF expression and improves post-stroke depression in ovariectomy-treated rats. Exp Ther Med. 2016;12:1843–48. doi: 10.3892/etm.2016.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Setiawan E, Wilson AA, Mizrahi R, et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry. 2015;72:268–75. doi: 10.1001/jamapsychiatry.2014.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang C, Zhang YP, Li YY, et al. Minocycline ameliorates depressive behaviors and neuro-immune dysfunction induced by chronic unpredictable mild stress in the rat. Behav Brain Res. 2019;356:348–57. doi: 10.1016/j.bbr.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Ma Y, Wang J, Wang Y, Yang GY. The biphasic function of microglia in ischemic stroke. Prog Neurobiol. 2017;157:247–72. doi: 10.1016/j.pneurobio.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Khansari PS, Sperlagh B. Inflammation in neurological and psychiatric diseases. Inflammopharmacology. 2012;20:103–7. doi: 10.1007/s10787-012-0124-x. [DOI] [PubMed] [Google Scholar]
- 7.Streit WJ, Xue QS. Alzheimer’s disease, neuroprotection, and CNS immunosenescence. Front Pharmacol. 2012;3:138. doi: 10.3389/fphar.2012.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai SJ. Role of tissue-type plasminogen activator and plasminogen activator inhibitor-1 in psychological stress and depression. Oncotarget. 2017;8:113258–68. doi: 10.18632/oncotarget.19935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo L, Li C, Du X, et al. Effect of aerobic exercise on BDNF/proBDNF expression in the ischemic hippocampus and depression recovery of rats after stroke. Behav Brain Res. 2019;362:323–31. doi: 10.1016/j.bbr.2018.11.037. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Zhao YD, Zeng JW, et al. Serum brain-derived neurotrophic factor levels in post-stroke depression. J Affect Disord. 2014;168:373–79. doi: 10.1016/j.jad.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Pang PT, Teng HK, Zaitsev E, et al. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–91. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- 12.Hein M, Zoremba N, Bleilevens C, et al. Levosimendan limits reperfusion injury in a rat middle cerebral artery occlusion (MCAO) model. BMC Neurol. 2013;13:106. doi: 10.1186/1471-2377-13-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farooq RK, Isingrini E, Tanti A, et al. Is unpredictable chronic mild stress (UCMS) a reliable model to study depression-induced neuroinflammation? Behav Brain Res. 2012;231(1):130–37. doi: 10.1016/j.bbr.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Cotto B, Li H, Tuma RF, et al. Cocaine-mediated activation of microglia and microglial MeCP2 and BDNF production. Neurobiol Dis. 2018;117:28–41. doi: 10.1016/j.nbd.2018.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 2008. [Google Scholar]
- 16.Villa RF, Ferrari F, Moretti A. Post-stroke depression: mechanisms and pharmacological treatment. Pharmacol Ther. 2018;184:131–44. doi: 10.1016/j.pharmthera.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Peng C, Guo X, et al. Expression of brain-derived neurotrophic factor and tyrosine kinase B in cerebellum of poststroke depression rat model. Chin Med J (Engl) 2015;128(21):2926–31. doi: 10.4103/0366-6999.168058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ifergane G, Boyko M, Frank D, et al. Biological and behavioral patterns of post-stroke depression in rats. Can J Neurol Sci. 2018;45(4):451–61. doi: 10.1017/cjn.2017.302. [DOI] [PubMed] [Google Scholar]
- 19.Kalkman HO, Feuerbach D. Antidepressant therapies inhibit inflammation and microglial m1-polarization. Pharmacol Ther. 2016;163:82–93. doi: 10.1016/j.pharmthera.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Loane DJ, Kumar A. Microglia in the TBI brain: The good, the bad, and the dysregulated. Exp Neurol. 2016;275(Pt 3):316–27. doi: 10.1016/j.expneurol.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Zeng L, Yu T, et al. Positive feedback loop of autocrine BDNF from microglia causes prolonged microglia activation. Cell Physiol Biochem. 2014;34(3):715–23. doi: 10.1159/000363036. [DOI] [PubMed] [Google Scholar]
- 22.Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab. 2012;32(9):1677–98. doi: 10.1038/jcbfm.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young JJ, Bruno D, Pomara N. A review of the relationship between proinflammatory cytokines and major depressive disorder. J Affect Disord. 2014;169:15–20. doi: 10.1016/j.jad.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 24.Felger JC, Lotrich FE. Inflammatory cytokines in depression: Neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parkhurst CN, Yang G, Ninan I, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155(7):1596–609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowley MP, Drevets WC, Ongur D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2002;52:404–12. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- 27.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–27. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Kim YR, Kim HN, Hong KW, et al. Anti-depressant effects of phosphodiesterase 3 inhibitor cilostazol in chronic mild stress-treated mice after ischemic stroke. Psychopharmacology. 2016;233:1055–66. doi: 10.1007/s00213-015-4185-6. [DOI] [PubMed] [Google Scholar]
- 29.Li JY, Liu J, Manaph NPA, et al. ProBDNF inhibits proliferation, migration and differentiation of mouse neural stem cells. Brain Res. 2017;1668:46–55. doi: 10.1016/j.brainres.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Zhou L, Xiong J, Lim Y, et al. Upregulation of blood proBDNF and its receptors in major depression. J Affect Disord. 2013;150:776–84. doi: 10.1016/j.jad.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Bai YY, Ruan CS, Yang CR, et al. ProBDNF signaling regulates depression-like behaviors in rodents under chronic stress. Neuropsychopharmacology. 2016;41:2882–92. doi: 10.1038/npp.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benraiss A, Chmielnicki E, Lerner K, et al. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci. 2001;21:6718–31. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang CR, Bai YY, Ruan CS, et al. Injection of anti-proBDNF in anterior cingulate cortex (ACC) reverses chronic stress-induced adverse mood behaviors in mice. Neurotox Res. 2017;31:298–308. doi: 10.1007/s12640-016-9687-4. [DOI] [PubMed] [Google Scholar]
- 34.Rahman M, Luo H, Sims NR, et al. Investigation of mature BDNF and proBDNF signaling in a rat photothrombotic ischemic model. Neurochem Res. 2018;43:637–49. doi: 10.1007/s11064-017-2464-9. [DOI] [PubMed] [Google Scholar]
- 35.Melchor JP, Strickland S. Tissue plasminogen activator in central nervous system physiology and pathology. Thromb Haemost. 2005;93(4):655–60. doi: 10.1160/TH04-12-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McEwen BS. Structural plasticity of the adult brain: how animal models help us understand brain changes in depression and systemic disorders related to depression. Dialogues Clin Neurosci. 2004;6(2):119–33. doi: 10.31887/DCNS.2004.6.2/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]