Abstract
Objectives:
To assess, by means of biological monitoring, exposure to polycyclic aromatic hydrocarbons (PAHs) and to metallic elements in Italian Navy workers operating near the industrial area in Taranto, and thereby estimate the health risk.
Methods:
A total of 450 workers in the Italian Navy were examined; they had office type jobs, and 150 of them worked near the industrial area in Taranto (exposed group), 150 in Taranto but far from this area (internal control group) and 150 in Brindisi (external control group). The recruited workers were administered a questionnaire inquiring about current and previous working activities, personal medical history, lifestyle and dietary habits, and their residence location. Then they collected a urine sample for the determination of 1-hydroxypyrene, 2-naphthol, cotinine and the metallic elements As, Cd, Co, Cr, Mn, Ni, Pb, Cu, Zn and Hg. The latter were measured in 110 workers in each group. In addition, in some of the work sites of the three groups, environmental samplings were carried out to determine PAHs and the 10 metallic elements, also taking into account the wind direction.
Results:
Airborne benzo(a)pyrene concentrations at the different sampling sites ranged from 0.02 to 0.06 ng/m3 and naphthalene between <25 and 65.3 ng/m3, regardless of the wind direction. Among the metallic elements, As, Cd, Co, Cr, Hg, Pb, Cu and Zn were present at concentrations below or just above the limit of detection (LOD). Mn and Ni were slightly higher in the work sites of the exposed group. The urinary concentrations of 1-hydroxypyrene, 2-naphthol and the single metallic elements were not higher in the exposed workers group than in the other 2 groups. Smokers had significantly higher urinary 1-hydroxypyrene and 2-naphthol concentrations, whereas cigarette smoking did not condition a higher urinary elimination of metallic elements in the three groups with the exception of Cd and Pb. Moreover, residence location conditioned Mn, Hg and As urinary excretion, consumption of shellfish and/or crustaceans in the 72 hours before urine collection conditioned As elimination, and consumption of legumes in the 72 hours before urine collection conditioned Ni elimination.
Conclusions:
This research did not find a higher urinary excretion of 1-hydroxypyrene, 2-naphthol and As, Cd, Co, Cr, Mn, Ni, Pb, Cu, Zn and Hg in the exposed workers group as compared to the internal control group working far from the industrial area of Taranto, nor in the group working in another city far away from Taranto, Brindisi. Therefore, it indicated that workers in the Italian Navy operating near the industrial area in Taranto were not exposed to a greater risk attributable to exposure to PAHs and metallic elements than the two control groups.
Key words: 1-hydroxypyrene, 2-naphthol, metallic elements, biological monitoring
Abstract
«Monitoraggio biologico dell’esposizione a idrocarburi policiclici aromatici e ad elementi metallici nei lavoratori della Marina Militare Italiana che lavoravano in prossimità dell’area industriale di Taranto (Sud-Italia)».
Obiettivi:
Valutare, attraverso monitoraggio biologico, l’esposizione ad idrocarburi policiclici aromatici (IPA) e ad elementi metallici dei lavoratori della Marina Militare Italiana che operavano in prossimità dell’area industriale di Taranto, per stimare il rischio per la salute.
Metodi:
Sono stati esaminati 450 lavoratori della Marina Militare Italiana, che svolgevano mansioni assimilabili a quelle d’ufficio, di cui 150 lavoravano in prossimità dell’area industriale di Taranto (gruppo esposti), 150 a Taranto ma a distanza da quest’area (gruppo controllo interno) e 150 a Brindisi. Ai lavoratori reclutati è stato somministrato un questionario con domande su attività lavorativa attuale e pregressa, storia sanitaria personale, abitudini di vita e alimentari e domicilio. Quindi i lavoratori hanno raccolto un campione di urine, nel quale sono stati determinati l’1-idrossipirene, il 2-naftolo, la cotinina e gli elementi metallici As, Cd, Co, Cr, Mn, Ni, Pb, Cu, Zn e Hg. Questi ultimi sono stati misurati soltanto in 110 lavoratori di ciascun gruppo. In alcune sedi dei tre gruppi sono stati eseguiti campionamenti ambientali, tenendo conto anche della direzione del vento, per la determinazione degli IPA e dei 10 elementi metallici anzidetti.
Risultati:
Il benzo(a)pirene aerodisperso nelle diverse sedi di campionamento dei tre gruppi ha mostrato concentrazioni variabili da 0,02 a 0,06 ng/m3 ed il naftalene comprese tra <25 e 65,3 ng/m3, prescindendo dalla direzione del vento. Degli elementi metallici, As, Cd, Co, Cr, Hg, Pb, Cu e Zn nelle sedi dei tre gruppi sono risultati in concentrazione da inferiore ad appena superiore al LOD. Mn e Ni sono risultati leggermente più elevati nel gruppo esposti. Le concentrazioni urinarie di 1-idrossipirene, di 2-naftolo e dei singoli elementi metallici non sono risultate più elevate nel gruppo esposti rispetto agli altri due gruppi. I fumatori hanno mostrato una più elevata eliminazione urinaria di 1-idrossipirene, di 2-naftolo, mentre l’abitudine al fumo di sigarette non determinava una più alta eliminazione urinaria di elementi metallici, ad eccezione del Cd e Pb. La residenza, inoltre, è risultata associata con l’eliminazione urinaria di Mn, Hg e As, il consumo di molluschi e/o crostacei nelle 72 ore precedenti la raccolta delle urine con l’eliminazione di As e il consumo di legumi nelle 72 ore precedenti la raccolta delle urine con l’eliminazione di Ni.
Conclusioni:
La ricerca non ha mostrato una più elevata eliminazione urinaria di 1-idrossipirene, 2-naftolo e di As, Cd, Co, Cr, Mn, Ni, Pb, Cu, Zn e Hg nei lavoratori che operavano in prossimità dell’area industriale di Taranto rispetto a quelli che lavoravano a Taranto, ma a distanza da quest’area, ed a quelli che lavoravano a Brindisi, a notevole distanza da Taranto. Essa, pertanto, ha evidenziato che i lavoratori della Marina Militare Italiana, che operavano in prossimità dell’area industriale di Taranto, non erano esposti ad un rischio per la salute da IPA e da elementi metallici più elevato rispetto a quello dei lavoratori dei due gruppi controllo.
Table A:
Urinary concentrations of 1-hydroxypyrene (μg/g creat) and 2-naphthol (μg/L) in the workers subdivided by group and by residence zone.
Tabella A: Concentrazioni urinarie di 1-idrossipirene (μg/g creat) e 2-naftolo (μg/L) nei lavoratori suddivisi per gruppo e per zona del domicilio.
Table B:
Urinary concentrations of 1-hydroxypyrene (μg/g creat) and 2-naphthol (μg/L) in the workers subdivided by group and residence area.
Tabella B: Concentrazioni urinarie di 1-idrossipirene (μg/g creat) e 2-naftolo (μg/L) nei lavoratori suddivisi per gruppo e per area del domicilio.
Table C:
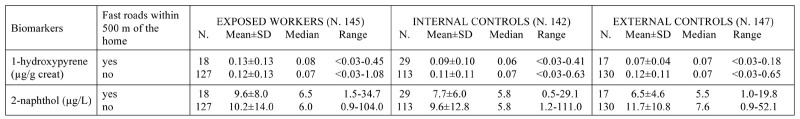
Urinary concentrations of 1-hydroxypyrene (μg/g creat) and 2-naphthol (μg/L) in the workers subdivided by group and by the presence of fast roads within 500 m of the home.
Tabella C: Concentrazioni urinarie di 1-idrossipirene (μg/g creat) e 2-naftolo (μg/L) nei lavoratori suddivisi per gruppo e per presenza di strade ad alto scorrimento entro 500 m dal domicilio.
Table D:
Urinary concentrations of 1-hydroxypyrene (μg/g creat) and 2-naphthol (μg/L) in the workers subdivided by group and by the consumption of smoked and/or grilled foods within 24 hours of urine collection.
Tabella D: Concentrazioni urinarie di 1-idrossipirene (μg/g creat) e 2-naftolo (μg/L) nei lavoratori suddivisi per gruppo e per consumo di cibi affumicati e/o cotti alla brace nelle 24 ore precedenti la raccolta delle urine.
Table E:
Correlation between urinary concentrations of 1-hydroxypyrene (μg/g creat) and 2-naphthol (μg/L) in the workers subdivided by group and by smoking habit verified on urinary cotinine: smokers: ≥200 ng/ml; non-smokers: <200 ng/ml.
Tabella E: Correlazione tra le concentrazioni urinarie di 1-idrossipirene (μg/g creat) e di 2-naftolo (μg/L) nei lavoratori suddivisi per gruppo e per abitudine tabagica verificata attraverso cotinina urinaria: fumatori: ≥200 ng/ml; non fumatori: <200 ng/ml.
Table F:
Urinary concentrations of the metallic elements (μg/L) in the workers subdivided by group and residence zone.
Tabella F: Concentrazioni urinarie degli elementi metallici (μg/L) nei lavoratori suddivisi per gruppo e per zona del domicilio.
Table G:
Urinary concentrations of the metallic elements (μg/L) in the workers subdivided by group and residence area.
Tabella G: Concentrazioni urinarie degli elementi metallici (μg/L) nei lavoratori suddivisi per gruppo e per area del domicilio.
Table H:
Urinary concentrations of mercury (μg/L) in the workers subdivided by group and by dental amalgam fillings.
Tabella H: Concentrazioni urinarie del mercurio (μg/L) nei lavoratori suddivisi per gruppo e per presenza di amalgami dentari.
Table I:
Urinary concentrations of arsenic (μg/L) in workers subdivided by group and by the consumption of shellfish and/or crustaceans.
Tabella I: Concentrazioni urinarie dell'arsenico (μg/L) nei lavoratori suddivisi per gruppo e per consumo di molluschi e/o crostacei.
Table L:
Correlations among the urinary concentrations of metallic elements (μg/L) in the workers subdivided by group.
Tabella L: Correlazioni tra le concentrazioni urinarie degli elementi metallici (μg/L) nei lavoratori suddivisi per gruppo.
Table M:
Reference values for urinary 1-hydroxypyrene (μg/g creat) and 2-naphthol (μg/L).
Tabella M: Valori di riferimento dell’1-idrossipirene (μg/g creat) e del 2-naftolo (μg/L) urinari.
Table N:
Reference values of the metallic elements (μg/L).
Tabella N: Valori di riferimento degli elementi metallici urinari (μg/L).
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are generally produced as a result of incomplete combustion of organic materials. They are almost always present as a mixture, whose composition depends on the type of organic matter burned and on the combustion temperature (1). In the occupational setting, industries that could cause workers exposure to PAHs include the steel plants (cokeries, blast furnaces and steelworks), refineries, coal-fired gas and bitumen production plants, aluminum foundries and waste incinerators (20). In living environments, exposure to PAHs can be due to natural sources, such as fires or volcanic eruptions, or man-made sources such as industrial emissions, vehicle exhaust fumes and heating systems emissions. Other sources of environmental exposure to PAHs include cigarette smoke and some foods, especially if smoked or grilled (45). Among the PAHs, the International Agency for Research on Cancer (IARC) classifies benzo[a]pyrene in Group 1, and among the working activities in steelworks at highest risk of exposure to these pollutants, coke production and occupational exposure during iron and steel founding (8).
Metallic elements pollution can also pose an important public health problem, caused by exposure both at mining industries of the different metallic elements and at manufacturing plants of these elements, and in the living environment. The latter can be attributed to natural sources like volcanic eruptions or human activities, such as industrial emissions, dietary sources and drinking water (33). Prolonged exposure to high concentrations of metallic elements in occupational settings can cause chronic intoxication by the single metallic elements, that manifest in man as specific organ or tissue diseases associated with high concentrations of the element in biological samples, especially blood and urine. Prolonged exposure in living environments to such high concentrations of metallic elements as to cause chronic intoxication is very rare in the general population. Some metallic elements, such as arsenic (As), cadmium (Cd), hexavalent chromium (Cr) and nickel (Ni), are considered carcinogenic by the IARC and classified in group 1 (19).
The industrial area in Taranto (South Italy) is located in the north-west of the city, and includes large industrial plants located quite near the urban area, and in particular an integrated cycle steelworks numbered among the largest in Europe, a raw oil refinery as well as a cement factory, while there is a waste incinerator at a greater distance from the city, in the north-west direction. In the south of Taranto there is an important naval base of the Italian Navy, and a number of operative sites supporting this base are located inside the city, at various distances from the industrial area.
Biological monitoring has been widely employed to study the risk of an increased absorption of PAHs and metallic elements in populations resident near industrial areas, because the internal dose can provide more appropriate information for health risk assessment than environmental sampling (43,47). The aim of this research was therefore to study, on the basis of biomonitoring, environmental exposure to PAHs and metallic elements in workers operating at the Italian Navy sites located near the industrial area in Taranto.
Methods
Workers examined
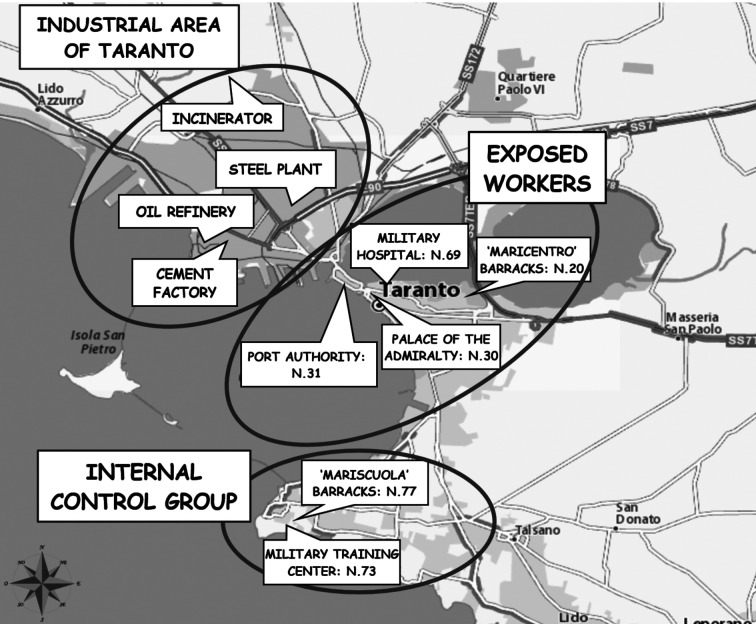
Examination was made of 150 workers based in Taranto, operating in the Italian Navy sites near the industrial area, from 2-6 km away (exposed group). They were compared with two control groups each also consisting of 150 workers in the Italian Navy, one group based in the southern part of the city of Taranto at a distance of 9-10 km from the industrial area (internal control group), and the other group in another city, Brindisi, at a considerable distance from Taranto (external control group) (figure 1).
Figure 1a.
Map of the Taranto area, showing the worksites of the exposed workers and internal control group where the study was carried out
Figure 1b.
Map of the Taranto and Brindisi area, showing the worksites of the external control group where the study was carried out
In order to recruit the workers, the military Commanders of each of the different sites of the three groups were asked to provide a list of male workers containing the following information: surname and first name, date of birth, residence location and address, date of enrollment in the Italian Navy, current job and seniority in that job, years of service at the current worksite. From the resulting list of 1425 workers, selection was made at each site of workers fulfilling the following inclusion criteria: age between 22 and 60 years; office type job for the last 3 years at least, with no occupational exposure to PAHs and metallic elements; at least 14 months of service at the current site; for the exposed workers and internal control groups, residence in the city of Taranto and the districts, San Vito, Lama and Talsano, located at a distance of 9 to 14 km away, to the south of the industrial area, and for the external control group, residence in Brindisi or San Vito dei Normanni. The latter municipality was included because it is near (about 14 Km) to the north of ‘Carlotto’ barracks. In the exposed and internal control groups, workers living outside Taranto were excluded to obtain two homogeneous groups of inhabitants in Taranto center or in one of the districts, that differed only as regards their work site in Taranto, and thus also provided information on the impact of PAHs and metallic elements emissions from the industrial area on workers resident in the center of Taranto compared to those resident in the suburban districts. Clearly, to achieve this objective, workers resident outside Taranto had to be excluded as their residence area could be a confounding factor. Finally, after obtaining their voluntary informed consent to take part in the study, 150 workers were enrolled in each group, randomly selected from the lists of workers.
The study was conducted between March and June 2016 on Wednesdays, Thursdays and Fridays in suitable premises made available by the military Commanders of each site. These weekdays were chosen to verify a possibly greater urinary excretion of PAHs metabolites and metallic elements after at least 3 days of working activities at the worksite, since all the workers examined did a daily work shift from Monday to Friday.
All the workers were administered a questionnaire collecting information about personal data, residence location, general characteristics, current and previous job descriptions, lifestyle, dietary habits and personal medical history.
Before enrollment each worker had been given an information leaflet illustrating the research aims and method, and informed consent to take part was obtained. The research was conducted in accordance with the Helsinki Declaration and was consistent with ethical public health practice, and in compliance with regulatory and statutory mandates to maintain, protect and restore community health.
Biological monitoring
All the workers collected a urine sample between 11.00 a.m. and 1.00 p.m. on the day of administration of the questionnaire. The urine samples, subdivided into 10 ml aliquots, were frozen to -20°C and transferred in containers on dry ice for analysis at the Industrial Toxicology Laboratory of the Occupational Medicine Operative Unit of the ASST ‘Spedali Civili’ Hospital of Brescia – University of Brescia.
Determination was made in the samples of 1-hydroxypyrene, 2-naphthol, cotinine and the following metallic elements: As, Cd, cobalt (Co), Cr, manganese (Mn), mercury (Hg), Ni, lead (Pb), copper (Cu) and zinc (Zn).
The first three biomarkers were determined in all 150 workers in each group, while the metallic elements were measured in 110 workers in each group, randomly selected, but representative of the age distribution and the worksites of each of the three groups.
In the urine samples, urinary creatinine was also determined to check the acceptability of each sample for biological monitoring, that according to WHO criteria should have a creatinine concentration ranging from 0.3 to 3.0 g/L, and to make suitable adjustments to some of the biomarkers measured (44).
The analytical methods employed to determine the different biomarkers are listed below.
Urinary 1-hydroxypyrene. The urine sample, extracted through a C18 column after prior enzymatic hydrolysis, was analyzed in HPLC with fluorimetric detection, in accordance with the method reported by Jongeneelen et al. (25), with some modifications. Gradient elution was done on a Supelcosil LC18 inverse phase column. A certified control was included in each analytical series. The limit of quantification (LOQ) was 0.03 μg/L, the measurement range 0.03-40 μg/L, and the mean imprecision among series, expressed as coefficient of variation (CV), was less than 9%.
Urinary 2-naphthol. The urine sample was subjected to enzymatic hydrolysis and analyzed in HPLC with fluorimetric detection. Elution was done in the isocratic phase using a Supelcosil LC18 inverse phase column, in accordance with the report by Kim et al. (26), with some modifications. A certified control was included in each analytical series. The LOQ was 0.5 μg/L, the measurement range 0.5-100 μg/L, and the CV less than 9%.
Urinary cotinine. This biomarker was determined by enzymatic immunodosage using the ILab 350 instrument. The analysis is based on the competitive binding of cotinine labeled with glucose-6-phosphate dehydrogenase (G6PDH) to the free cotinine contained in the sample, for a fixed number of cotinine-antibody specific binding sites. The enzymatic activity was spectrophotometrically determined at 340 nm. Two certified controls were included in each analytical series. The LOQ was 200 ng/ml, and the measurement range 200-3000 ng/ml.
Metallic elements in the urine (multielement analysis). The urine samples were diluted 10x with ultrapure bidistilled water (Fluka) and analyzed by inductively coupled plasma mass spectrometry with the dynamic reaction cell (ICP-MS-DRC) (6). Mn, Co, Cu, Zn, Cd, Hg, Pb were analyzed using the standard Total Quant method, while the method of dynamic reaction cell (DRC) filled with ammonia was used to analyze Cr. Certified controls were used to check the accuracy of the method. The limit of detection (LOD) ranged between 0.01 µg/L and 0.1 µg/L, and the CV was 10-15%.
Urinary Ni. The urine samples were diluted with bidistilled water and analyzed by Varian Zeeman atomic absorption spectrometry (SpectraAA400), applying the Zeeman effect to adjust for improved accuracy. The calibration curve was obtained adding between 2.5 µg/L and 10 µg/L. Certified controls were used to check the accuracy of the method. The LOD was 0.1 µg/L, the linearity range 0.1-150 µg/L, and the CV was 8.0%.
Urinary As. The urine samples were treated with hydrochloric acid, potassium iodide solution, toluene and subjected to nitric acid extraction at 1%. Analyses were done with Varian Zeeman atomic absorption spectrometry (SpectraAA400), applying the Zeeman effect to improve the accuracy (5). The determination includes both the inorganic As compounds (As3+As5) and their methylated compounds (MMA+DMA). The matrix modifier was standard Palladium (1000 ppm) and standard Magnesium (1000 ppm) at a 1:1 ratio. Certified controls were used to check the accuracy of the method. The LOD was 1.0 µg/L, the linear range 1-150 µg/L, and the CV 4.5%.
Urinary creatinine. Creatinine was colorimetrically determined using the ILab 350 instrument (10). The investigation is based on the reaction of creatinine with picric acid in an alkaline environment and subsequent UV-VIS revealing of the complex. Two controls at different concentration levels were used in each analytical series. The LOQ was 0.1 g/L, and the measurement range 0.1-4 g/L.
Environmental monitoring
To obtain information on the environmental exposure to PAHs and metallic elements at the different sites, outdoor environmental samplings were carried out at most of them, to characterize the pollutants under study. Samplings were done according to the indications on the wind direction supplied by the Navy Hydrographic Institute (Geophysical Office, Oceanographic Section, METOC support), two at the military hospital and two at the ‘Maricentro’ barracks for the exposed workers group and two at the ‘Mariscuola’ barracks for the internal control group, on two different days, one when the wind was blowing from the north-west and one from the south, to verify a possibly greater concentration of PAHs and metallic elements in the Taranto area when there is a north-west wind blowing. For the external control group just one environmental sampling was done at the ‘Carlotto’ barracks, with a north wind blowing. The other navy sites where workers of the different groups operated were not sampled because they were not sufficiently far from those sampled.
Because the environmental sampling was referred to workers’ exposure while carrying out their normal working activities, PAHs were determined in particulate matter including inhalable dust+vapour-phase, and the metallic elements in inhalable dust, in accordance with the indications for monitoring occupational exposure to these pollutants, rather than in the PM10 fraction of the particulate matter, as is done to assess air quality in living environments. It should be noted that inhalable dust is the set of airborne particles with an aerodynamic diameter lower than 100 µm, whereas the PM10 fraction of the particle is the set of airborne particles with an aerodynamic diameter lower than 10 µm. Moreover, the inhalable dust concentration measured in workplaces is referred to a mean daily exposure of 8 hours, 40 hours per week, as the Threshold Limit Value - Time Weighted Average (TLV-TWA), with the aim of protecting the great majority of workers exposed to individual occupational risk factors, while the concentration determined in living environments is for a mean exposure of one calendar year as target value for air quality, aimed at protecting the general population exposed to the individual environmental risk factors (4,22).
In the air samples collected to determine the PAHs, the 16 compounds considered to be of greatest toxicological concern by the US-EPA were measured, namely acenaphthene, acenaphthylene, anthracene, benzo(a)anthracene, benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(a)pyrene, dibenzo(a,h)anthracene, benzo(g,h,i)perylene, chrysene, phenanthrene, fluoranthene, fluorene, indeno(1,2,3-c,d)pyrene, naphthalene and pyrene (15).
Air sampling lasted 8 hours and was done using fixed samplers according to the NIOSH 5506 method (30). Constant flow 2 L/min samplers were used. The PAHs were collected using a dual body sampling system composed of a PTFE membrane, in a 2 section transparent polystyrene membrane disk, with a diameter of 37 mm and porosity of 2 µm (sampling of the inhalable fraction of airborne dust particles), and a vial containing adsorbent XAD-2 100 mg/50 mg resin (sampling of the vapour phase) fitted to the tail of the membrane. The membranes were transferred into pyrex glass test tubes and, after adding 5 ml of acetonitrile for HPLC, they were immersed in an ultrasonic bath for 30 minutes. The solution was allowed to evaporate and the residue was revived with 1 ml of acetonitrile and injected into the gradient HPLC using an inverse phase column and fluorimetric detection. The resins contained in the vials were transferred into 2 pyrex glass vials, separating the front and back (resin + glass fiber). After processing the membranes, the gradient HPLC analysis was carried out using the inverse phase column and fluorimetric detection. The blanks were virgin membranes and vials treated in the same way as the samples. The calibration curve was built using the standard additions method. The LOD, expressed as absolute ng, was 20 for acenaphthene, 500 for acenaphthylene, 0.1 for anthracene, phenanthrene, chrysene and pyrene, 0.05 for benzo(a)anthracene and benzo(b)fluoranthene, 0.02 for benzo(k)fluoranthene and benzo(a)pyrene, 0.04 for dibenzo(a,h)anthracene, 0.03 for benzo(g,h,i)perylene, 0.2 for fluoranthene, 5 for fluorene, 0.4 for indeno(1,2,3-c,d)pyrene, and 25 for naphthalene.
Inhalable dust samplings to determine the metallic elements were done using constant flow 2 L/min SKC Intermediate fixed samplers, employing the UNI EN 481 method. The flows were calibrated before and after sampling with a primary precision flowmeter (Dry Cal, DC-lite, Bios International Corporation). The inhalable fraction of the airborne particles was collected by filtration, using the IOM (Institute of Occupational Medicine) selector on cellulose ester membranes (diameter 25 mm and porosity 0.8 µm) as indicated for the Unichim method 1998:2005 (41).
Analyses of the inhalable dust particles were done on the membranes, conditioned before and after collection, using the microgravimetric method, in an “Activa Climatic” box (for 24 hours at constant temperature and humidity), weighed before and after collection, using a precision scale to the fifth decimal figure. The dust particle volume was calculated in mg/m3 on the difference in weight in relation to the aspirated air volume. The LOD of the method was 0.03 mg and CV 0.2%.
To analyze the metallic elements (As, Cd, Co, Cr, Mn, Hg, Ni, Pb, Cu e Zn) the membranes were dissolved in concentrated ultrapure nitric acid and the solutions obtained were diluted with bidistilled water. Inductively coupled plasma mass spectrometry (ICP-MS) was employed for the analysis, with the dynamic cell reaction (ICP-MS-DRC) to dose chromium. The calibration curve was built using the external standard method. To verify the accuracy of the method, Nist 1640 certified controls were employed. The LOD ranged between 0.0001 and 0.0006 absolute µg and the CV between 6.5% and 9%.
The air samples collected to determine both the PAHs and the metallic elements were preserved at a temperature of +4° C and sent for analysis to the Industrial Hygiene Laboratory of the Toxicology and Occupational Diseases Prevention Operative Unit of the University of Brescia.
Statistical analysis
Statistical analyses were done using the SPSS program (version 14.0, Chicago, IL, USA). Analytical determinations below the LOD of the method were assigned a value of half the respective LOD in the data analyses. A normal distribution of the variables was verified using the Kolmogorov-Smirnov test. The variables were analyzed using non parametric tests or parametric tests after logarithmic transformation. A dependency relation of each normally distributed urinary biomarker on the independent variables was assessed by multiple linear regression models, while the dependence of not normally distributed urinary biomarkers on the independent variables was appraised through multiple logistic regression models, stratifying the dependent variable in four strata based on urinary concentrations quartiles. No regression analysis was applied to urinary Cr, due to the high number of determinations below the LOD. The level of significance was set at p<0.05.
Results
The differences in the number of workers at the various worksites in each group, shown in figure 1, depended on the total number of workers at each site that fulfilled the selection criteria for recruitment.
The general characteristics of the workers in the three groups are shown in table 1. The exposed and internal control groups were virtually always comparable, whereas the external control group were younger, had a shorter service record with the Italian Navy in general, but longer service at the current site. It is also evident that the exposed group had spent a significantly longer time (in minutes) in urban traffic during the 48 hours prior to urine collection that the other two groups.
Table 1.
General characteristics of the workers in the three groups examined
| General characteristics | Exposed workers (N. 150) | Internal controls (N. 150) | External controls (N. 150) | |||||||||
| N. / | Mean±SD | Median | Range | N. / | Mean±SD | Median | Range | N. / | Mean±SD | Median | Range | |
| Age (years)c | 45.6±6.0 | 45.8 | 31-59 | 45.2±5.8 | 45.3 | 30-60 | 41.2±6.0 | 41.2 | 8-58 | |||
| Body Mass Index (Kg/m2) | 26.4±3.1 | 26.0 | 21.1-37.9 | 26.9±3.2 | 26.4 | 9.5-40.1 | 26.5±2.9 | 26.0 | 20.9-34.7 | |||
| Years working at the Navyc | 25.5±6.5 | 25.5 | 12-42 | 25.7±6.2 | 26.1 | 1-41 | 20.9±6.2 | 21.5 | 5-38 | |||
| Years working at that sitec | 6.2±4.6 | 5.0 | 1-24 | 4.3±3.4 | 4.0 | 2-34 | 12.0±8.4 | 12.5 | 1-32 | |||
| Smoking habit (referred in the questionnaire) | ||||||||||||
| - smokers | 42 | 35 | 37 | |||||||||
| - non smokers | 65 | 83 | 79 | |||||||||
| - ex-smokers | 37 | 29 | 28 | |||||||||
| - electronic cigarettes | 3 | 1 | 2 | |||||||||
| - cigars | 3 | 2 | 4 | |||||||||
| Smokers | ||||||||||||
| - Number of cigarettes/dayb | 42 | 14.1±8.0 | 15.0 | 2-30 | 35 | 9.1±7.3 | 10.0 | 1-40 | 37 | 11.5±5.8 | 10.0 | 1-20 |
| - Number of cigarettes in the last 48 hoursa | 42 | 25.7±16.9 | 22.0 | 0-60 | 35 | 16.6±14.7 | 16.0 | 1-80 | 37 | 22.2±11.7 | 20.0 | 2-40 |
| Smoking habit on urinary cotinine | ||||||||||||
| - smokers ≥200 ng/ml | 44 | 34 | 44 | |||||||||
| - non smokers <200 ng/ml | 106 | 116 | 106 | |||||||||
| Alcohol consumptionb | ||||||||||||
| - teetotal | 44 | 62 | 40 | |||||||||
| - occasional (≤5 alcohol units/week) | 82 | 61 | 83 | |||||||||
| - habitual (>5 alcohol units/week) | 24 | 27 | 27 | |||||||||
| Residence zone | ||||||||||||
| - Taranto center | 88 | 63 | - | |||||||||
| - Taranto districts (S. Vito - Lama - Talsano) | 62 | 87 | - | |||||||||
| Residence area | ||||||||||||
| - urban (high habitation density) | 92 | 93 | 109 | |||||||||
| - rural (low habitation density) | 58 | 57 | 41 | |||||||||
| Overall time spent in urban traffic in the last 48 hours before urine collection (minutes)b | 142.7±119.7 | 20.0 | 0-960 | 116.6±114.7 | 90.0 | 0-720 | 107.6±98.9 | 82.5 | 0-600 | |||
| Fast roads within 500 m of the residence | ||||||||||||
| - yes | 19 | 30 | 19 | |||||||||
| - no | 131 | 120 | 131 | |||||||||
| Consumption of smoked and/or grilled foods in the last 24 hours before urine collection | ||||||||||||
| - Yes | 16 | 7 | 17 | |||||||||
| - No | 134 | 143 | 133 | |||||||||
| Dental amalgam fillings | ||||||||||||
| - Yes | 98 | 90 | 81 | |||||||||
| - No | 52 | 60 | 69 | |||||||||
| Consumption of shellfish and/or crustaceans | ||||||||||||
| - Last meal within 24 hours of urine collection | 11 | 7 | 19 | |||||||||
| - Last meal within 24-48 hours of urine collection | 3 | 15 | 7 | |||||||||
| - Last meal within 48-72 hours of urine collection | 8 | 8 | 11 | |||||||||
| - No consumption in the 72 hours before urine collection | 128 | 120 | 113 | |||||||||
Alcohol unit=12 g ethanol, equal to one glass of wine or a 330 ml can of beer or a shot of spirits;
ap<0.05,bp<0.01,cp<0.001
A smoking habit was investigated both through the questionnaire and by determining urinary cotinine, as shown in table 1, that showed an underestimation of the number of smokers based on the questionnaire results as compared to the urinary cotinine results. Therefore, in the statistical analyses only the biomarker results were applied.
Assessment of urinary creatinine in the three groups demonstrated values outside the WHO range in 5 workers in the exposed group, 8 in the internal control group and 3 in the external control group. After checking that analyses of the biomarkers measured did not result significantly different when excluding these results, the anomalous findings in these workers were excluded from subsequent data analyses.
PAHs
Table 2 shows that the air concentrations of the different PAHs at the exposed group sites were virtually always comparable to those obtained at the internal control and external control groups sites, and that a north-west wind did not cause higher PAHs concentrations in the air than when the wind was blowing from a different direction. The PAHs measured at the different sites were present at concentrations ranging from below to just above the LOD, including those that the ACGIH recommends be kept at the lowest possible levels, namely benzo(a)anthracene, benzo(b)fluoranthene, benzo(a)pyrene and chrysene.
Table 2.
Environmental concentrations of the 16 PAHs (ng/m3) according to the US-EPA list, measured on the dates indicated in the different sampling sites for the three groups
| Exposed workers | Internal controls | External controls | |||||
| PAHs determined | Military Hospital Taranto - secondary avenue - north-east wind 4 May 2016 | Military Hospital Taranto - secondary avenue - south wind 11 May 2016 | Maricentro barracks Taranto - Magno building entrance - north-west wind 4 May 2016 | Maricentro barracks Taranto - Magno building entrance - south wind 11 May 2016 | Maricentro barracks Taranto S.Vito - sports field - north-west wind 29 April 2016 | Maricentro barracks Taranto S.Vito - sports field - south wind 21 May 2016 | ‘Carlotto’ barracks - Brindisi north-west wind 8 June 2016 |
| Acenaphthene | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
| Acenaphthylene | <500 | <500 | <500 | <500 | <500 | <500 | <500 |
| Anthracene | 0.1 | 0.4 | 0.5 | 0.2 | 0.1 | 1.3 | 0.3 |
| Benzo(a)anthracene | <0.05 | 0.12 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| Benzo(a)pyrene | 0.02 | 0.06 | 0.03 | 0.02 | 0.05 | 0.06 | 0.03 |
| Benzo(b)fluoranthrene | <0.05 | 0.05 | 0.09 | <0.05 | 0.05 | 0.05 | <0.05 |
| Benzo(g,h,i)perylene | 0.09 | 0.11 | 0.06 | 0.06 | 0.09 | 0.09 | 0.10 |
| Benzo(k)fluoranthrene | <0.02 | 0.03 | 0.03 | <0.02 | <0.02 | 0.04 | <0.02 |
| Chrysene | 0.1 | 0.1 | <0.1 | <0.1 | 0.1 | 0.1 | <0.1 |
| Dibenzo(a,h)anthracene | 0.05 | 0.07 | 0.04 | <0.04 | <0.04 | 0.04 | 0.10 |
| Phenanthrene | 0.4 | 4.2 | 0.3 | 3.4 | 3.9 | 0.2 | 3.9 |
| Fluoranthene | <0.2 | 2.7 | <0.2 | 2.3 | 11.3 | 2.5 | 3.3 |
| Fluorene | <5 | 15.1 | <5 | <5 | <5 | <5 | <5 |
| Indenopyrene | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 |
| Naphthalene | 30.0 | <25 | <25 | 64.1 | 65.3 | 33.0 | <25 |
| Pyrene | 1.1 | 0.6 | 0.3 | 0.5 | 0.7 | 1.1 | 0.6 |
Occupational limit values:
- ACGIH 2017 (as TLV-TWA): for benzo(a)anthracene, benzo(b)fluoranthene, benzo(a)pyrene and chrysene there is a note indicating that exposure to all the penetration routes must be carefully controlled at the lowest possible level; for naphthalene: 52 mg/m3
Environmental limit values:
- Target values for air quality: Law decree 155/10 (referred to the total quota present in the PM10 fraction of the particulate matter, calculated as the average over a calendar year): Benzo(a)pyrene: 1 ng/m3
The results for urinary 1-hydroxypyrene and 2-naphthol (table 3) are subdivided by smoking habit in each group, because reports in literature have demonstrated that cigarette smoke contains high quantities of PAHs, thus influencing urinary excretion of both metabolites in smokers (17,26). It can be seen from the table that no differences in urinary excretion of the two biomarkers were observed in smokers, non-smokers and total subjects when comparing the exposed group, internal control group and external control group. Urinary excretion of 1-hydroxypyrene and 2-naphthol was always significantly higher in smokers than non-smokers (p < 0.001).
Table 3.
Urinary concentrations of 1-hydroxypyrene (µg/g creat) and 2-naphthol (µg/L) in the workers subdivided by group and by smoking habit, verified on urinary cotinine value: smokers: ≥200 ng/ml; non smokers: <200 ng/ml.
| Biomarkers | Exposed workers | Internal controls | External controls | RVB | ||||||||||||
| N. | Median | 95th percentile | Range | N. cases >RVB | N. | Median | 95th percentile | Range | N. cases >RVB | N. | Median | 95th percentile | Range | N. cases >RVB | 5th - 95th percentile | |
| 1-hydroxypyrene (µg/g creat) | ||||||||||||||||
| - smokers | 41 | 0.21 | 0.51 | <0.03-1.08 | 1 | 32 | 0.21 | 0.52 | 0.03-0.63 | 0 | 42 | 0.20 | 0.47 | 0.03-0.65 | 0 | - |
| - Non smokers | 104 | 0.06 | 0.20 | <0.03-0.33 | 0 | 110 | 0.05 | 0.13 | <0.03-0.33 | 0 | 105 | 0.06 | 0.21 | <0.03-0.27 | 0 | - |
| - Total | 145 | 0.07 | 0.39 | <0.03-1.08 | 1 | 142 | 0.06 | 0.36 | <0.03-0.63 | 0 | 147 | 0.07 | 0.35 | <0.03-0.65 | 0 | 0.03-0.70 |
| 2-naphthol (µg/L) | ||||||||||||||||
| - smokers | 41 | 14.2 | 70.9 | 2.6-80.5 | 18 | 32 | 9.8 | 69.5 | 3.4-111.0 | 12 | 42 | 8.3 | 46.4 | 1.0-49.5 | 24 | - |
| - Non smokers | 104 | 5.3 | 18.9 | 0.9-104.0 | 6 | 110 | 5.5 | 20.3 | 0.5-40.6 | 8 | 105 | 5.6 | 25.5 | 0.9-52.1 | 13 | - |
| - Total | 145 | 6.0 | 30.9 | 0.9-104.0 | 24 | 142 | 5.8 | 28.8 | 0.5-111.0 | 20 | 147 | 7.4 | 32.6 | 0.9-52.1 | 37 | 0.5-15 |
RVB: Reference values Brescia laboratory
- 1-hydroxypyrene and 2-naphthol: Exposed workers vs Internal controls: total, smokers and non smokers all not significant; Exposed workers vs External controls: total, smokers and non smokers all not significant; Internal controls vs External controls: total, smokers and non smokers all not significant.
- 1-hydroxypyrene and 2-naphthol: smokers vs non smokers: exposed workers, internal controls and external controls all p<0.001
Then any relationship between alcohol consumption and the urinary excretion of 1-hydroxypyrene and 2-naphthol was studied in all three groups. The workers in each group were classified as teetotal, occasional drinkers and habitual drinkers (data not shown). No trend toward a relationship between the two biomarkers and alcohol consumption was found in any of the three groups.
For the exposed group and the internal control group, the urinary excretion of 1-hydroxypyrene and of 2-naphthol was also analyzed according to the residence location, in the center of Taranto or one of the more distant suburban districts (S. Vito, Lama and Talsano) (table A in supplementary materials). No difference was observed between the two groups when comparing the urinary excretion of the two PAHs metabolites in relation to a central or peripheral residence location in Taranto.
Similarly, comparison of the urinary excretion of the two metabolites and residence in an urban area with high population density or a rural area with low population density also showed no difference in the three groups of workers (table B in supplementary materials). Nor did the comparison of the presence of heavy traffic major roads within 500 m of the residence versus little traffic and no major roads nearby show differences in the urinary excretion of the two biomarkers (table C in supplementary materials).
Finally, the urinary excretion of 1-hydroxypyrene and of 2-naphthol was studied in all three groups in relation to the consumption of smoked or grilled foods within 24 hours before urine collection (table D in supplementary materials). The time lapse of 24 hours was chosen on the basis of literature reports (12). No increase in the urinary excretion of the two metabolites was found in any of the three groups according to consumption of these foods.
The correlation between the urinary excretion of 1-hydroxypyrene and 2-naphthol and a smoking habit was also studied (table E in supplementary materials). A significant positive correlation was found between the two biomarkers of exposure to PAHs in smokers in all three groups (exposed workers rho=0.68; internal controls rho=0.41; external controls rho=0.72), and in the whole group of subjects studied together in the exposed group (rho=0.31) and in the external control group (rho=0.43).
Finally, multiple linear regression models were applied to assess the dependence of 1-hydroxypyrene and of 2-naphthol on the following independent variables: exposure group, age, smoking habit, urinary creatinine, residence zone, residence area, alcohol consumption, overall time spent in urban traffic in the last 48 hours before urine collection, fast roads within 500 m of the residence, consumption of smoked and/or grilled foods in the last 24 hours before urine collection. As shown in table 4, both urinary 1-hydroxypyrene and 2-naphthol were significantly dependent on urinary creatinine and smoking habit.
Table 4.
Linear regression for the dependent variables 1-hydroxypyrene (µg/L) and 2-naphthol (µg/L) (after logarithmic transformation) in the exposed workers, internal control and external control groups.
| Independent variable | 1-hydroxypyrene (µg/L) | 2-naphthol (µg/L) | ||||
| beta | p | beta | p | |||
| Urinary creatinine (g/L) | 0.47 | <0.001 | 0.71 | <0.001 | ||
| Smoking habits (ref. non smokers*) | ||||||
| - smokers | 0.39 | 0.028 | 0.73 | <0.001 | ||
| Model | F 27.2 | p <0.001 | R2 0.48 | F 17.9 | p <0.001 | R2 0.37 |
Other independent variables considered in the models that resulted not significant: Exposure groups (ref. exposed); Age (years); Residence zone (ref. center); Residence area (ref. urban); Alcohol consumption (units/week); Consumption of smoked and/or grilled foods in the last 24 hours before urine collection (ref. no); Overall time spent in urban traffic in the last 48 hours before urine collection (minutes); Fast roads within 500 m of the residence (ref. no)
*non smokers: urinary cotinine <200 ng/ml
Metallic elements
The environmental concentrations of inhalable dust and of the metallic elements contained in it were measured at the various sites, as shown in table 5. Inhalable dust was not found in greater concentrations in the exposed group sites than in the other two groups sites. The concentrations ranged, according to the site location and the wind direction when the air sampling was carried out, from lower than 0.05 mg/m3, LOD of the analytical method employed, to 0.14 mg/m3. In detail, for the exposed group sites, when the wind was blowing from the north-west, concentrations of 0.11-0.14 mg/m3 were observed, and with a south wind, concentrations of <0.05-0.08 mg/m3; for the internal control group, with a north-west wind, concentrations of <0.05 mg/m3 and with a south wind, concentrations of 0.10 mg/m3, and for the external control group, with a north-east wind, concentrations of 0.12 mg/m3.
Table 5.
Environmental concentrations of inhalable dust (mg/m3) and the metallic elements it contains (ng/m3) at the different sampling sites of the three groups
| Groups and sampling sites | Inhalable dust (mg/m3) | Metallic elements (ng/m3) | |||||||||
| Cr | Mn | Co | Cu | Zn | Cd | Hg | Pb | As | Ni | ||
| Exposed workers | |||||||||||
| - Military Hospital Taranto - secondary avenue - north-west wind | 0.14 | 7.2 | 134 | 0.8 | 25 | 38 | <0.1 | <0.5 | 25 | <0.5 | 113 |
| - Military Hospital Taranto - secondary avenue - south wind | <0.05 | 5.4 | 68 | <0.2 | 20 | 90 | <0.1 | <0.5 | 11 | <0.5 | 19 |
| - ‘Maricentro’ barracks Taranto - Magno building entrance - north-west wind | 0.11 | <0.1 | 66 | <0.2 | 156 | 114 | <0.1 | <0.5 | 14 | <0.5 | 34 |
| - ‘Maricentro’ barracks Taranto - Magno building entrance - south wind | 0.08 | 2.7 | 28 | <0.2 | 21 | 18 | <0.1 | <0.5 | 9 | <0.5 | 60 |
| Internal controls | |||||||||||
| - ‘Mariscuola’ barracks Taranto S.Vito - sports field - norht-west wind | <0.05 | 2.4 | 14 | <0.2 | 15 | <0.5 | <0.1 | <0.5 | 7 | <0.5 | 44 |
| - ‘Mariscuola’ barracks Taranto S.Vito - sports field - south wind | 0.10 | 0.7 | 28 | <0.2 | 17 | 1 | <0.1 | <0.5 | 5 | <0.5 | 16 |
| External controls | |||||||||||
| - ‘Carlotto’ barracks - Brindisi - norht-east wind | 0.12 | 3.6 | 45 | <0.2 | 14 | 73 | <0.1 | <0.5 | 13 | <0.5 | 17 |
Occupational limit values:
- Law Decree 81/08 and subsequent modification and amendments (Attachment XXXVIII): metallic Cr and inorganic Cr compounds (II) and (III) 0.5 mg/m3;
Hg and inorganic compounds 0.02 mg/m3; inorganic Pb and compounds 0.15 mg/m3.
- ACGIH 2017 (as TLV-TWA): inhalable dust particles 10 mg/m3; metallic Cr and Cr compounds III 0.5 mg/m3; Mn element and inorganic compounds
0.1 mg/m3 on the inhalable fraction, 0.02 mg/m3 on the respirable fraction; Co and inorganic compounds (as Co) 0.02 mg/m3; Cu (dust and smog) 1 mg/m3;
Cd 0.01 mg/m3; elementary Hg and inorganic forms 0.025 mg/m3; Pb and inorganic compounds (as Pb) 0.05 mg/m3; As and inorganic compounds (as As)
0.01 mg/m3; Ni and inorganic compounds as elementary Ni 1.5 mg/m3.
Environmental limit values:
- Law Decree 155/10 limit values (as annual mean): lead 0.5 µg/m3;
- Target values for air quality: Law Decree 155/10 (referred to the total quota of each pollutant present in the PM10 fraction of the particle, calculated as the average over a calendar year): arsenic 6 ng/m3; cadmium 5 ng/m3; nickel 20 ng/m3.
- WHO guidelines (2000) (as annual average): manganese 0.15 µg/m3; mercury 1 µg/m3.
Values below the LOD are expressed as absolute ng.
As regards the airborne metallic elements determined at the work sites (Table 5), it should be noted that Cd, Hg, As and Co, (except the latter at the military hospital in Taranto with a north-west wind, when the concentration was 0.8 mg/m3) were all below the LOD of the analytical method in all the sites studied, for all three groups. Environmental Mn and Ni were found at concentrations of 134 ng/m3 and 113 ng/m3, respectively, at the military hospital in Taranto, corresponding to one higher order of magnitude than the concentrations observed at air sampling in the other sites of both the exposed group and the other two groups. Environmental Mn in the exposed workers group sites always showed higher concentrations when the wind was blowing from the north-west than from the south. The opposite was found for air sampling of the internal control group sites. Environmental Ni showed an inverse concentration depending on the wind direction at the two exposed group sites sampled, while at the internal controls sites the concentration was slightly higher when the wind was blowing from the north-west.
The urinary concentrations of the metallic elements measured in the workers in all three groups are shown in table 6. It can be seen that urinary Cr, Co and Cd were lower than the LOD of 0.05 µg/L in some workers in all three groups; Cr, in particular, was lower than the LOD in 35.8% of the exposed group, 31.1% of the internal control group and 49.5% of the external control group. Analyzing the median value for each metallic element in all three groups, a higher urinary excretion was not found in the exposed group than in the other two groups. Indeed, as compared to the other two groups, the highest values for Cr and Mn were found in the internal control group and for Cu, Zn, Hg, Pb, As and Ni in the external control group.
Table 6.
Urinary concentrations of the metallic elements (µg/L) in the workers subdivided by group
| Biomarkers (µg/L) | Exposed workers (N. 106) | Internal controls (N. 106) | External controls (N. 107) | SIVR | RVB | ||||||||||||
| Median | 95th percentile | Range | N. cases <LOD | N. cases >RVB* | Median | 95th percentile | Range | N. cases <LOD | N cases >RVB* | Median | 95th percentile | Range | N. cases <LOD | N. cases >RVB* | Reference values (List 2017) 5th -95th percentile | 5th -95th percentile | |
| Cr | 0.10 | 0.60 | <0.05-1.30 | 38 | 3 | 0.19 | 0.86 | <0.05-1.25 | 33 | 9 | 0.11 | 0.84 | <0.05-2.90 | 53 | 6 | 0.05-0.60 | 0.05-0.70 |
| Mn | 0.70 | 2.40 | 0.10-3.90 | 0 | 0 | 0.80 | 2.10 | 0.10-3.30 | 0 | 0 | 0.60 | 2.24 | 0.10-5.30 | 0 | 1 | 0.04-1.5 | 0.1-5.0 |
| Co | 0.42 | 0.94 | 0.08-3.40 | 0 | 2 | 0.37 | 1.24 | <0.05-2.44 | 2 | 2 | 0.42 | 1.12 | <0.05-1.80 | 2 | 1 | 0.08-2.2 | 0.1-1.5 |
| Cu | 11.9 | 23.8 | 2.4-49.0 | 0 | 1 | 11.6 | 27.8 | 1.0-32.8 | 0 | 0 | 5.3 | 27.8 | 2.3-37.8 | 0 | 2 | 5.01-24.0 | 5.8-36.0 |
| Zn | 257.0 | 968.9 | 21.0-1182.0 | 0 | 5 | 275.5 | 721.1 | 37.0-1088.0 | 0 | 2 | 313.0 | 84.0 | 20.0-1315.0 | 0 | 10 | nd-1048 | 200-900 |
| Cd | 0.21 | 0.82 | <0.05-1.50 | 8 | 0 | 0.25 | 0.82 | <0.05-1.23 | 4 | 0 | 0.26 | 0.84 | <0.05-3.90 | 6 | 2 | 0.1-0.9 | 0.1-1.5 |
| Hg | 0.42 | 2.06 | 0.10-5.20 | 0 | 1 | 0.35 | 1.63 | 0.10-4.20 | 0 | 0 | 0.57 | 2.37 | 0.10-3.60 | 0 | 0 | 0.1-5.0 | 0.1-5.0 |
| Pb | 0.42 | 1.42 | 0.10-3.00 | 0 | 0 | 0.41 | 0.95 | 0.10-1.38 | 0 | 0 | 0.49 | 1.35 | 0.10-3.90 | 0 | 0 | 0.17-2.64 | 0.4-7.0 |
| As | 3.6 | 19.7 | 1.0-57.0 | 0 | 7 | 3.4 | 16.5 | 1.0-33.0 | 0 | 5 | 6.8 | 30.1 | 1.0-64.6 | 0 | 21 | nd-16.1 | 1.0-15.0 |
| Ni | 0.60 | 2.87 | 0.10-4.70 | 0 | 2 | 0.60 | 3.20 | 0.10-4.40 | 0 | 2 | 0.70 | 2.30 | 0.10-4.60 | 0 | 1 | 0.37-4.44 | 0.1-4.0 |
*RVB: Reference Values Brescia Laboratory
Exposed workers vs Internal controls: Cr p=0.010; Exposed workers vs External controls: Cu p=0.003, Cd p=0.042, Hg p=0.007, As p<0.001;
Internal controls vs External controls: Cr p<0.001, Mn p<0.001, Cu p<0.001, Hg p<0.001, Pb=0.021, As p<0.001
To check for an association between a smoking habit and urinary excretion of the different metallic elements in the three groups, the subjects in each group were subdivided into smokers and non-smokers on the basis of urinary cotinine, as reported above. A significant association was found only between a smoking habit and urinary excretion of Pb in the internal control group (data not shown).
Investigation of any association between the consumption of alcohol and the urinary excretion of the different metallic elements according to alcohol consumption, classifying the subjects in each group as teetotal, occasional drinker or habitual drinkers, did not show a significant trend for any of the elements in any of the groups (data not shown).
To examine the role of the residence zone in conditioning the urinary excretion of the different metallic elements in the exposed and internal control groups, that were all resident in Taranto, the subjects in each group were subdivided according to whether they lived in the Center or one of the Districts (S. Vito, Lama, Talsano) of Taranto (table F in supplementary materials). No higher urinary excretion of the various elements was found in the residents in the center of Taranto as compared to those resident in the districts.
The role of the residence area, defined as urban with a high population density or rural, with a low population density, on conditioning the urinary excretion of the different metallic elements, was also studied (table G in supplementary materials). In general, a higher urinary excretion of the metallic elements was found in workers living in rural zones rather than in urban zones, that reached statistical significance in some cases.
Any relation between dental amalgam fillings and the urinary excretion of Hg was also studied in all three groups (table H in supplementary materials). There was a greater urinary excretion of Hg in all subjects with dental amalgam fillings in all three groups, although this did not reach statistical significance. The relation between the consumption of shellfish and/or crustaceans (seafood) and the urinary excretion of As was also studied, subdividing the workers according to whether the last seafood meal had been consumed within 24, 24-48 or 48-72 hours before urine collection (table I in supplementary materials). A significant trend toward a higher urinary excretion of As related to seafood consumption was found in the exposed group and in the external control group.
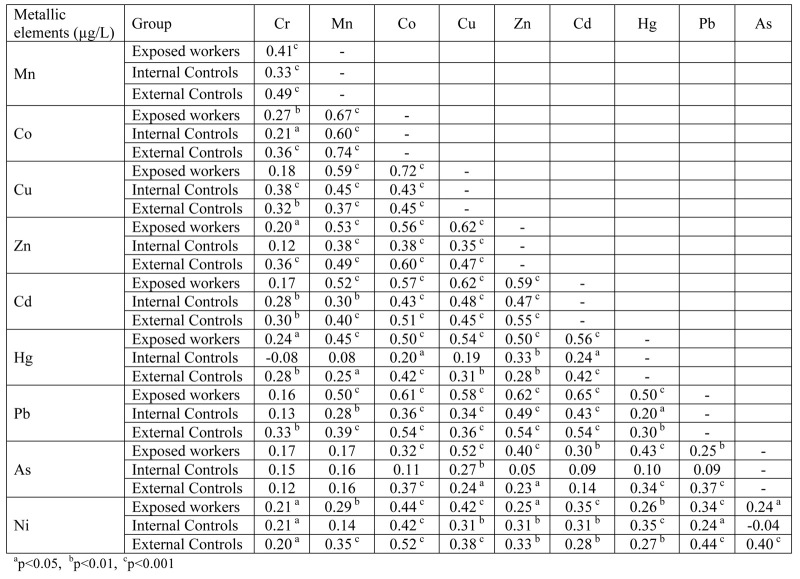
A correlation among the urinary excretion of the different metallic elements was also sought in the workers in all three groups (table L in supplementary materials). A significant positive correlation was found in all three groups, of Co with 8 other metallic elements (rho ranged from 0.20 to 0.74), of Cd, Pb, Ni, Cu and Zn with 7 elements (rho ranged from 0.20 to 0.72), of Mn and Hg with 5 elements (rho ranged from 0.20 to 0.56), of Cr with 3 elements (rho ranged from 0.21 to 0.49) and of As with just 1 metallic element (rho ranged from 0.24 to 0.52).
For the metallic elements Co, Cu, As, Pb and Zn, normally distributed after logarithmic transformation, multiple linear regression models were applied to assess their dependence on the following independent variables: exposure group, age, smoking habits, urinary creatinine, residence zone, residence area, alcohol consumption, overall time spent in urban traffic in the last 48 hours before urine collection, presence of fast roads within 500 m of the residence, consumption of shellfish and/or crustaceans in the last 72 hours before urine collection (only for As and Zn). All the metallic elements were significantly dependent on urinary creatinine; moreover, Co and Zn also showed a positive dependence on age, Pb on age and smoking habit, Cu on exposure group, and finally As showed also a negative dependence on exposure group, and a positive dependence on the consumption of shellfish and/or crustaceans in the 72 hours before urine collection (table 7).
Table 7.
Linear regression for the dependent variables Co, Cu, As, Pb and Zn (µg/L) (after logarithmic transformation) in the exposed workers, internal control and external control groups
Other independent variables considered in the models that resulted not significant: Residence area (ref. urban); Alcohol consumption (units/week); Overall time spent in urban traffic in the last 48 hours before urine collection (minutes); Presence of fast roads within 500 m of the residence (ref. no)
*non smokers: urinary cotinine >200 ng/ml
ns=not significant
For the metallic elements Mn, Cd, Hg and Ni, not normally distributed even after logarithmic transformation, multiple logistic regression models were used to appraise the association between their urinary concentrations and the following independent variables: exposure group, age, smoking habit, urinary creatinine, residence zone, residence area, alcohol consumption, overall time spent in urban traffic in the last 48 hours before urine collection, presence of fast roads within 500 m of the residence, presence of dental amalgam fillings (only for Hg), consumption of legumes in the last 72 hours before urine collection (only for Mn and Ni). In terms of concentrations quartiles, the analysis showed that urinary creatinine was clearly associated to all the metallic elements, while a positive dependence on residence zone was observed for urinary Mn in the 3rd and 4th quartiles, and for urinary Hg in the 4th quartile. Moreover, a positive dependence only in the 4th quartile was observed for urinary Cd on smoking habit, and for urinary Ni on consumption of legumes in the 72 hours before urine collection (table 8).
Table 8.
Logistic regression for the dependent variables Cr, Mn, Cd, Hg and Ni in the exposed workers, internal control and external control groups
Other independent variables considered in the models that resulted not significant: Exposure groups (ref. exposed); age (years); Overall time spent in urban traffic in the last 48 hours before urine collection (minutes); Presence of fast roads within 500 m of the residence (ref. no); Presence of dental amalgam fillings only for Hg (ref. no).
*non smokers: urinary cotinine >200 ng/ml; ns= not significant
Discussion
Our research did not demonstrate a higher urinary excretion of 1-hydroxypyrene, 2-naphthol and As, Cd, Co, Cr, Mn, Hg, Ni, Pb, Cu and Zn in the exposed workers group operating near the industrial area of Taranto compared to those operating in Taranto but at a distance from this area (internal control group), nor those operating in the city of Brindisi, at a considerable distance from Taranto (external control group). Nor were the environmental concentrations of PAHs and the metallic elements measured higher in the exposed workers than in the two control groups, except for Mn and Ni. Thus, no higher health risk emerged for the exposed workers as compared to the control groups workers.
PAHs
Although the number of samplings was limited, determination of the 16 PAHs of toxicological concern in the air at the exposed group sites and at the two control groups sites demonstrated very, very low concentrations of these, ranging from below to just above the LOD for each of the PAHs measured. In particular, for benzo(a)pyrene the environmental concentrations ranged from 0.02 to 0.06 ng/m3, that is two orders of magnitude less than the target value of 1 ng/m3 for air quality in living environments stipulated in Italy by Law Decree 155/10 as the annual average of the PM10 particulate fraction (22). Instead, for naphthalene, the highest airborne concentration was 65.3 ng/m3, markedly lower than both the TLV-TWA recommended by the ACGIH for occupational exposure, of 52 mg/m3, and the WHO proposed guideline value for indoor air quality, of 0.01 mg/m3 (4,46).
In our study, benzo(a)pyrene and naphthalene were present at much lower environmental concentrations than those observed in 2005 at personal sampling of 18 subjects resident in Taranto within 2 Km of the industrial area (benzo(a)pyrene: median value 1.5 ng/m3; naphthalene: median value 2055 ng/m3), by Campo et al. (13) and in 15 subjects resident in Alberobello, a rural village located about 50 km away from the industrial area (benzo(a)pyrene: median value 3.6 ng/m3; naphthalene: median value 986 ng/m3), with no significant differences between the environmental concentrations of these two PAHs sampled in the two areas. Moreover, the environmental concentrations of benzo(a)pyrene were lower even than those calculated as the average PM10 fraction for the entire year in 2014, based on the data collected by the Regional Agency for Environmental Protection (ARPA) monitoring stations present in the city of Taranto, that in turn were similar to, or lower than those reported for other Italian cities in the Italian Institute for Environmental Protection and Research (ISPRA) 2017 report (8,23). Thus, despite the small number of environmental samplings we did at the exposed and internal control groups sites, the results seem to confirm a low PAHs pollution in the Taranto area.
To interpret the results of the urinary biomarkers of exposure to PAHs determined in the workers in the three different groups, they were firstly compared with the 1-hydroxypyrene BEI of 2.5 µg/L proposed for 2017 by the ACGIH (4). This comparison demonstrated that the urinary 1-hydroxypyrene values in all three groups were well below this biological threshold limit value. The ACGIH-proposed BEI for urinary 1-hydroxypyrene, as indicated in the Documentation, was adjusted to the pyrene/benzo(a)pyrene ratio in the environmental PAHs mix, that ranged from 10 to 55 in the environmental sampling carried out at the exposed and internal control sites. It was not possible to compare 2-naphthol values with the respective BEI because this is not available, despite the many biomonitoring studies conducted in the occupational setting (34,36).
In all three groups, the 95th percentile of the 1-hydroxypyrene concentrations observed in non-smokers was within the reference top limit of the Italian Reference Values Society (SIVR) (0.23 µg/g creat) and those of other European and extra-European nations, while in smokers it virtually overlapped the top limit of the SIVR values (0.46 µg/g creat) and was within the reference range of the other European and extra-European nations (table M in supplementary materials) (24, 29, 39, 48). Instead, for 2-naphthol, the reference values of the NHANES study were employed (29). In regard to these reference values, in non-smokers in all three groups the 95th percentile of 2-naphthol concentrations was slightly higher than the NHANES 95th percentile (15.8 µg/L), whereas in the smokers it was within the NHANES 95th percentile (51.6 µg/L) only in the external control group. Comparison with the reference values for the Korean population showed a comparable trend in smokers, while in non-smokers the 95th percentile in our study was almost always within the reference values (table M in supplementary materials) (39). Moreover, the 95th percentiles of 1-hydroxypyrene were always lower than those observed in 2005 in 18 residents in Taranto near the plant and 15 residents far away from the plant, while the 95th percentile levels of 1-hydroxypyrene and 2-naphthol were always higher in all three groups investigated in our study, both in smokers and non-smokers, than those recently reported in 1016 residents in a city in central Italy (13,40).
A smoking habit was found to be the most important source of non-occupational exposure to PAHs in the smokers. In fact, in all three groups the urinary concentrations of 1-hydroxypyrene and 2-naphthol were higher in smokers than non-smokers, and both biomarkers were dependent on the concentrations of urinary cotinine. This confirms what has been reported in previous studies, and demonstrates the need to consider smokers and non-smokers separately when interpreting the results of biomonitoring of exposure to PAHs (34,42). Unlike in previous reports in literature, in our study the other main sources of non-occupational exposure to PAHs, namely road traffic, and the consumption of smoked and/or grilled foods did not reveal any influence on the urinary excretion of 1-hydroxypyrene and 2-naphthol (1,31).
Metallic elements
The environmental concentrations of inhalable dust we observed at the sites of all three groups were invariably 2-3 orders of magnitude lower than the occupational limit value of 10 mg/m3 proposed for 2017 by the ACGIH, regardless of the wind direction on the day of sampling (4). Similarly, As, Cd, Co, Cr, Cu, Hg and Pb were several orders of magnitude lower than the occupational limit value for each of these elements specified by Italian Law Decree 81/08 and subsequent modification and amendments and/or the ACGIH for 2017 (4,21). Moreover, for As, Cd, Hg and Pb they were also lower than the limit value for air quality indicated in Italian Law Decree 155/10 or by the WHO (22,45). In particular, the environmental concentrations of As and Cd at the exposed and internal control groups sites were lower than the mean value for 2016 observed by the ARPA environmental monitoring for air quality in the Taranto area. For Pb the environmental concentrations at the sites of these two groups seemed to be comparable to the ARPA 2016 data for the internal control group and slightly higher in the exposed group (9).
In the exposed group, environmental Mn tended to show higher concentrations than in the other two groups, in particular in the measurements made at the site nearest to the industrial area and with the wind blowing from the north-west. In any case, the values were contained not only within the ACGIH occupational limit value for 2017 of 20 µg/m3, but also within the WHO guideline value for air quality of 150 ng/m3 (4,45). It should also be taken into account that the levels of environmental Mn in the areas near steelworks plants are taken as tracers of Mn emissions (3). In fact, the metal is always present in steel alloys, apart from being used as an additive in the production of some special steels (14).
Ni was always present at environmental concentrations several orders of magnitude lower than the TLV-TWA of the ACGIH for 2017, but in some samplings at the sites of the exposed and internal control groups they were higher than the target value for air quality of 20 ng/m3 specified by Italian Law Decree 155/10 (4,22). Ni is a metallic element present in the production of steel, especially stainless steel, and can therefore be detected in steelworks emissions, apart from deriving from the combustion of fossil fuels and from waste incinerator emissions (2). Unlike our observations in environmental sampling in the exposed and internal control groups sites, the ARPA 2016 data on the annual average Ni concentrations in the Taranto area were always at least one order of magnitude lower than the target air quality value (9). Therefore, the higher concentrations of both Ni and Mn found at these sites could also be due to the fact that our results were obtained for the inhalable dust fraction, not the PM10 fraction to which the ARPA 2016 data are referred, bearing in mind that the individual metallic elements examined could tend to distribute differently in the specific granulometric dust fractions. Finally, the interpretation of the environmental results should also consider the limited number of samplings performed in this study.
The urinary concentrations of Cr, Co, Cd and Hg were always considerably lower than the BEI proposed by the ACGIH, while only urinary As demonstrated higher concentrations than the BEI in one worker in the exposed group and two workers in the external control group. For most of the metallic elements, the 95th percentile of urinary excretion was within the SIVR reference range in all three groups (24). The exceptions were Mn and As, that were slightly higher than the SIVR 95th percentile in all three groups, Cr and Cu, that were higher than the SIVR percentile in the internal control group and Zn, that was higher only in the external control group. This observation of some results exceeding the SIVR 95th percentile is also partly confirmed by comparing our results with those reported in literature for other European nations and the US, except for Cr and Zn, that were within the reference range in the French population (table N in supplementary materials) (18, 28, 29, 32).
The urinary concentrations of Mn corresponding to the 95th percentile were virtually comparable, especially in the exposed and internal control groups, to those at the 95th percentile obtained in 2005 in a group of 50 subjects from the general population of Taranto with no occupational exposure to the metal element (38). Mn is normally present in the air, ground and water, and food is considered to be the main source of intake in the general population, in particular due to the consumption of nuts, cereals and legumes, while exposure to airborne Mn is negligible (3). However, urinary Mn seems to be little affected by the oral intake of Mn, while most studies conducted in the occupational medicine setting indicate that this marker is valid as a biomarker of exposure at group level but is poorly correlated to the airborne concentrations of Mn (6,35). The finding of higher urinary Mn values than the SIVR reference values also in the external control group suggests that these urinary Mn levels are not caused by specific pollution situations in the city of Taranto. However, logistic regression analysis showed a dependence of urinary Mn on residence zone in Taranto in the 3rd and 4th quartiles. Our results, therefore, indicate the need for further studies to clarify the significance of urinary Mn levels in biomonitoring studies of the general population.
The 95th percentiles for the metal elements other than Mn were always lower than those observed in 2005 in the previously mentioned 50 subjects from the general population of Taranto, with the exception of Cr and As in the external control group (38). In the absence of recent biomonitoring studies investigating urinary elimination of metallic elements in the general population living near the steel foundries, comparison was made with the results observed in areas potentially polluted by metallic elements due to the presence of waste incinerators (11,16). Particularly, the 95th percentiles of urinary Mn and of urinary Hg observed in our study were lower and higher, respectively, than those observed in the areas near the waste incinerators, while other metallic elements did not show univocal results.
In subjects belonging to the general population, urinary As is strongly conditioned by the consumption of shellfish and/or crustaceans in the 72 hours before urine collection, as illustrated in the literature (27,37). In our study there was a significant trend toward a greater excretion of As related to the consumption of seafood within 72 hours before the urine collection, in the exposed group and also in the external control group, confirmed also by the regression analyses.
A limitation of the study is that the method used for the determination of urinary cotinine does not have a very sensitive LOD (200 ng/ml) compared to the limit used in other studies to classify smokers and non smokers. However, although we cannot exclude an underestimation of the number of smokers, it should be minor and should not profoundly modify the observed results.
In conclusion, the research demonstrated that in the exposed and internal control groups, all resident in Taranto or its suburban districts (Lama, San Vito and Talsano), working near the industrial area of Taranto and/or living in the city center did not result in a greater urinary excretion of 1-hydroxypyrene, 2-naphthol, As, Cd, Co, Cr, Mn, Hg, Ni, Pb, Cu and Zn than working at a site located far away from the Taranto industrial area, or living in one of the suburban districts of the city. This conclusion is based on the recruitment criteria applied for the enrollment of the exposed workers and internal control groups, that included residence in the center of Taranto or one of the suburban districts rather than in other municipalities in the province of Taranto or other provinces in Apulia.
No potential conflict of interest relevant to this article was reported by the authors
Acknowledgements:
We wish to thank the Ministry of Defense and the Department for Navy Health Care, that strongly promoted this research to gain data on exposure of workers operating in Taranto near the industrial area. Thanks go also to all the Commanders of the different sites who allowed the research to be carried out. Special mention is due to all the workers at the Italian Navy operating in Taranto and Brindisi, who patiently underwent the study protocol.
Special thanks are reserved to the medical C.F. Dr. Maurizio Di Bella (coordinator of all the activities in the field), medical T.V. Dr. Francesco Lippolis and the medical C.F. Dr. Giuseppe Mollo, referral doctors of the three workers groups examined, for their contribution to individuating the research sites, maintaining contacts with the sites Commanders and recruiting the workers.
References
- 1.Agency for Toxic Substances and Disease Registry: Toxicological profile for Polycyclic Aromatic Hydrocarbons. Atlanta, GA, US: Department of Health and Human Services, Public Health Service: ATSDR, 1995. [PubMed] [Google Scholar]
- 2.Agency for Toxic Substances and Disease Registry: Toxicological profile for Nickel. Atlanta, GA, US: Department of Health and Human Services, Public Health Service: ATSDR, 2005 [Google Scholar]
- 3.Agency for Toxic Substances and Disease Registry: Toxicological profile for Manganese. Atlanta, GA: US Department of Health and Human Services, Public Health Service: ATSDR, 2012. [PubMed] [Google Scholar]
- 4.American Conference of Governmental Industrial Hygienists: TLVs and BEIs for chemical substances and physical agents. Cincinnati, OH, US: ACGIH, 2017 [Google Scholar]
- 5.Apostoli P, Bartoli D, Alessio L, Buchet JP. Biological monitoring of occupational exposure to inorganic arsenic. Occup Environ Med. 1999;56:825–832. doi: 10.1136/oem.56.12.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apostoli P, Lucchini R, Alessio L. Are current biomarkers suitable for the assessment of manganese exposure in individual workers. Am J Ind Med. 2000;37:283–290. doi: 10.1002/(sici)1097-0274(200003)37:3<283::aid-ajim6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 7.Apostoli P. Elements in environmental and occupational medicine. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;778:63–97. doi: 10.1016/s0378-4347(01)00442-x. [DOI] [PubMed] [Google Scholar]
- 8.Apulia Regional Agency for Environmental Protection: Relazione annuale sulla Qualità dell’aria in Puglia. Anno 2014. Bari, Italy: ARPA, 2014. Available at http://www.arpa.puglia.it/web/guest/rapporti_annuali_qa . [Google Scholar]
- 9.Bari, Italy: ARPA; 2016. Apulia Regional Agency for Environmental Protection: Valutazione metalli nel PM10 ex D.Lgs. 155/2010 – anno 2016 - siti di monitoraggio in provincia di Taranto. Available at file:///D:/Download/report%20metalli%20PM10%20Taranto_2016%20(1).pdf. [Google Scholar]
- 10.Benedict SR, Behre JA. Some application of a new color reaction for creatinine. Journal of Biological Chemistry. 1936;114:515–532. [Google Scholar]
- 11.Bocca B, Bena A, Pino A, et al. Human biomonitoring of metals in adults living near a waste-to-energy incinerator in ante-operam phase: Focus on reference values and health-based assessments. Environ Res. 2016;148:338–350. doi: 10.1016/j.envres.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Buckley TJ, Lioy PJ. An examination of the time course from human dietary exposure to polycyclic aromatic hydrocarbons to urinary elimination of 1-hydroxypyrene. Br J Ind Med. 1992;49:113–124. doi: 10.1136/oem.49.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campo L, Vimercati L, Carrus A, et al. Environmental and biological monitoring of PAHs exposure in coke-oven workers at the Taranto plant compared to two groups from the general population of Apulia, Italy. Med Lav. 2012;103:347–360. [PubMed] [Google Scholar]
- 14.Clarke C, Upson S. A global portrait of the manganese industry-A socioeconomic perspective. Neurotoxicology. 2017;58:173–179. doi: 10.1016/j.neuro.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Environmental Protection Agency. List of priority pollutants, Code of Federal Regulations 40 CFR 401.15. US: EPA, 2010 [Google Scholar]
- 16.Gatti MG, Bechtold P, Campo L, et al. Human biomonitoring of polycyclic aromatic hydrocarbons and metals in the general population residing near the municipal solid waste incinerator of Modena, Italy. Chemosphere. 2017;186:546–557. doi: 10.1016/j.chemosphere.2017.07.122. [DOI] [PubMed] [Google Scholar]
- 17.Hansen AM, Mathiesen L, Pedersen M, Knudsen LE. Urinary 1-hydroxypyrene (1-HP) in environmental and occupational studies - a review. Int J Hyg Environ Health. 2008;211:471–503. doi: 10.1016/j.ijheh.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Hoet P, Jacquerye C, Deumer G, et al. Reference values and upper reference limits for 26 trace elements in the urine of adults living in Belgium. Clin Chem Lab Med. 2013;51:839–849. doi: 10.1515/cclm-2012-0688. [DOI] [PubMed] [Google Scholar]
- 19.International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. A review of human carcinogens: Arsenic, metals, fibres, and dusts. Lyon, France: IARC, 2012: 100C. [PMC free article] [PubMed] [Google Scholar]
- 20.International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. A Review of Human Carcinogens: Chemical agents and related occupations. Lyon, France: IARC, 2012: 100F. [PMC free article] [PubMed] [Google Scholar]
- 21.Italian Government: Legislative Decree of 9 April 2008, n.81. Attuazione dell’articolo 1 della legge 3 agosto 2007, n. 123, in materia di tutela della salute e della sicurezza nei luoghi di lavoro. Off J Ital Rep of 30 April 2008; n. 108, Ordinary Supplement n. 101. Available at: http://www.lavoro.gov.it/sicurezzalavoro/MS/Normativa/Pages/default.aspx . [Google Scholar]
- 22.Italian Government: Legislative Decree of 13 August 2010, n. 155. Attuazione della direttiva 2008/50/CE relativa alla qualità dell’aria ambiente e per un’aria più pulita in Europa. Off J Ital Rep of 15 September 2010, n. 216, Ordinary Supplement n. 217 [Google Scholar]
- 23.Italian Institute for Environmental Protection and Research: Qualità dell’ambiente urbano. XIII Rapporto. Edizione 2017. Roma, Italy: ISPRA, 2017 [Google Scholar]
- 24.Italian Society of Reference Values: 4a lista dei valori di riferimento per elementi, composti organici e loro metaboliti. Edizione 2017. SIVR, 2017. Available at http://www.valoridiriferimento.it/ [Google Scholar]
- 25.Jongeneelen FJ, Anzion RB, Henderson PT. Determination of hydroxylated metabolites of polycyclic aromatic hydrocarbons in urine. J Chromatogr. 1987;413:227–232. doi: 10.1016/0378-4347(87)80230-x. [DOI] [PubMed] [Google Scholar]
- 26.Kim H, Kim YD, Lee H, et al. Assay of 2-naphthol in human urine by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1999;734:211–217. doi: 10.1016/s0378-4347(99)00350-3. [DOI] [PubMed] [Google Scholar]
- 27.Lovreglio P, D’Errico MN, Gilberti ME, et al. The influence of diet on intra and inter-individual variability of urinary excretion of arsenic species in Italian healthy individuals. Chemosphere. 2012;86:898–905. doi: 10.1016/j.chemosphere.2011.10.050. [DOI] [PubMed] [Google Scholar]
- 28.Morton J, Tan E, Leese E, Cocker J. Determination of 61 elements in urine samples collected from a non-occupationally exposed UK adult population. Toxicol Lett. 2014;231:179–193. doi: 10.1016/j.toxlet.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 29.National Health and Nutrition Examination Survey (NHANES): Fourth National Report on Human Exposure to Environmental Chemicals. Updated tables. Volume one and two. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, January 2017 [Google Scholar]
- 30.National Institute for Occupational Safety and Health: Polynuclear Aromatic Hydrocarbons by HPLC: Method 5506, Issue 3. In Eller PM, Cassinelli ME (eds): NIOSH Manual of Analytical Methods, 4th ed. Cincinnati, OH, US: NIOSH Department of Health and Human Services, Centers for Disease Control and Prevention, 1998 [Google Scholar]
- 31.Nielsen T, Jørgensen HE, Larsen JC, Poulsen M. City air pollution of polycyclic aromatic hydrocarbons and other mutagens: occurrence, sources and health effects. Sci Total Environ. 1996;189-190:41–49. doi: 10.1016/0048-9697(96)05189-3. [DOI] [PubMed] [Google Scholar]
- 32.Nisse C, Tagne-Fotso R, Howsam M, et al. Blood and urinary levels of metals and metalloids in the general adult population of Northern France: The IMEPOGE study, 2008-2010. Int J Hyg Environ Health. 2017;220:341–363. doi: 10.1016/j.ijheh.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 33.Nordberg GF, Fowler BA, Nordberg M, Friberg LT. 4th edn. Burlington, US: Elsevier; 2015. Handbook on the toxicology of metals. [Google Scholar]
- 34.Preuss R, Angerer J, Drexler H. Naphthalene – an environmental and occupational toxicant. Int Arch Occup Environ Health. 2003;76:556–576. doi: 10.1007/s00420-003-0458-1. [DOI] [PubMed] [Google Scholar]
- 35.Smith D, Gwiazda R, Bowler R, et al. Biomarkers of Mn exposure in humans. Am J Ind Med. 2007;50:801–811. doi: 10.1002/ajim.20506. [DOI] [PubMed] [Google Scholar]
- 36.Sobus JR, Mc Clean MD, Herrick RF, et al. Investigation of PAH biomarkers in the urine of workers exposed to hot asphalt. Ann Occup Hyg. 2009;53:551–560. doi: 10.1093/annhyg/mep041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soleo L, Lovreglio P, Iavicoli S, et al. Significance of urinary arsenic speciation in assessment of seafood ingestion as the main source of organic and inorganic arsenic in a population resident near a coastal area. Chemosphere. 2008;73:291–299. doi: 10.1016/j.chemosphere.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 38.Soleo L, Lovreglio P, Panuzzo L, et al. Health risk assessment of exposure to metals in the workers of the steel foundry and in the general population of Taranto (Italy) G Ital Med Lav Ergon. 2012;34:381–391. [PubMed] [Google Scholar]
- 39.Sul D, Ahn R, Im H, et al. Korea National Survey for Environmental Pollutants in the human body 2008: 1-hydroxypyrene, 2-naphthol, and cotinine in urine of the Korean population. Environ Res. 2012;118:25–30. doi: 10.1016/j.envres.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 40.Tombolini F, Pigini D, Tranfo G, et al. Levels of urinary metabolites of four PAHs and cotinine determined in 1016 volunteers living in Central Italy. Environ Sci Pollut Res Int. 2018 doi: 10.1007/s11356-018-1650-x. doi: 10.1007/s11356-018-1650-x. [DOI] [PubMed] [Google Scholar]
- 41.UNICHIM: Method 1998:05. Workplaces - Determination of the inhalable fraction - Gravimetric Method. UNICHIM, 2005 [Google Scholar]
- 42.Van Rooij JG, Veeger MM, Bodelier-Bade MM, et al. Smoking and dietary intake of polycyclic aromatic hydrocarbons as sources of interindividual variability in the baseline excretion of 1-hydroxypyrene in urine. Int Arch Occup Environ Health. 1994;66:55–65. doi: 10.1007/BF00386580. [DOI] [PubMed] [Google Scholar]
- 43.Vimercati L, Baldassarre A, Gatti MF, et al. Non-occupational exposure to heavy metals of the residents of an industrial area and biomonitoring. Environ Monit Assess. 2016;188:673. doi: 10.1007/s10661-016-5693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.World Health Organization: Biological Monitoring of Chemical Exposure in the Workplace, vol. 1. Geneva, Switzerland: WHO, 1996 [Google Scholar]
- 45.World Health Organization: Air quality guidelines for Europe. 2nd Edition. Copenhagen, Denmark: WHO Regional Office for Europe, 2000 [Google Scholar]
- 46.World Health Organization: Guidelines for Indoor air quality: selected pollutants. Copenhagen, Denmark: WHO Regional Office for Europe, 2010. [PubMed] [Google Scholar]
- 47.Wilhelm M, Eberwein G, Hölzer J, et al. Influence of industrial sources on children’s health-hot spot studies in North Rhine Westphalia, Germany. Int J Hyg Environ Health. 2007;210:591–599. doi: 10.1016/j.ijheh.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Wilhelm M, Hardt J, Schulz C, Angerer J. Human Biomonitoring Commission of the German Federal Environment Agency. New reference value and the background exposure for the PAHs metabolites 1-hydroxypyrene and 1- and 2-naphthol in urine of the general population in Germany: basis for validation of human biomonitoring data in environmental medicine. Int J Hyg Environ Health. 2008;211:447–453. doi: 10.1016/j.ijheh.2007.09.002. [DOI] [PubMed] [Google Scholar]