Abstract
Background:
The coastal area of Friuli Venezia Giulia (FVG) region, north-eastern Italy, was characterized by work activities in which asbestos was used until the early 1990s, particularly in shipbuilding. A public health surveillance program (PHSP) for asbestos-exposed workers was established, although limited evidence exists about the efficacy of such programs in reducing disease occurrence and mortality.
Objectives:
To compare mortality in a cohort of 2,488 men occupationally exposed to asbestos, enrolled in a PHSP in FVG between the early 1990s and 2008, with that of the general population of FVG and Italy.
Methods:
Standardized Mortality Ratios (SMR), with 95% Confidence Interval (95% CI), for all causes, all cancers, lung (LC) and pleural cancer (PC) were estimated in the cohort and in subgroups of workers with the first hire in shipbuilding that caused asbestos exposure (<1974, 1974-1984, 1985-1994).
Results:
A strong excess in mortality for PC with reference to FVG (SMR=6.87, 95% CI 4.45-10.17) and Italian population (SMR=13.95, 95% CI 9.02-20.64) was observed. For LC, the FVG-based SMR was 1.49 (95% CI 1.17-1.89) and the Italy-based 1.43 (95% CI 1.12-1.81). Mortality among workers with the first hire in shipbuilding before 1974 was high for PC (FVG-based SMR=8.98, 95% CI 5.56-13.75; Italy-based SMR=18.41, 95% CI 11.40-28.17) and for LC (FVG-based SMR =1.60, 95% CI 1.18-2.11; Italy-based SMR=1.54, 95% CI 1.14-2.03). Further, for LC between 1974 and 1984, the FVG-based SMR was 2.45 (95% CI 1.06-4.82), and the Italy-based SMR was 2.33 (95% CI 1.01-4.60).
Conclusions:
This cohort experienced an excess mortality for pleural and lung cancer, compared with regional and national populations. For lung cancer, the excess was stronger in workers with the first hire in shipbuilding before 1985, suggesting a key role of asbestos exposure.
Key words: Mortality, asbestos, mesothelioma, lung cancer, shipbuilding, epidemiology, Monfalcone
Abstract
«Mortalità in una coorte di lavoratori esposti all'amianto sotto sorveglianza sanitaria».
Introduzione:
La zona costiera del Friuli Venezia Giulia (FVG), Italia nord-orientale, è caratterizzata da attività produttive in cui l’amianto veniva utilizzato fino ai primi anni 1990, in particolare nell’industria navalmeccanica. L’efficacia dei programmi di sorveglianza sanitaria pubblica (PSSP) negli ex lavoratori esposti nel ridurre frequenza di malattia e mortalità non è ancora supportata da evidenza scientifica.
Obiettivi:
Confrontare la mortalità di una coorte di 2,488 uomini professionalmente esposti ad amianto, iscritti ad un PSSP in FVG dai primi anni ‘90 fino al 2008, con quella della popolazione generale di FVG e Italia.
Metodi:
I rapporti standardizzati di mortalità (SMR) e il relativo intervallo di confidenza (IC 95%), per tutte le cause, tutti tumori, tumore del polmone (TP) e tumore della pleura (TPL) sono stati calcolati nella coorte e in sottogruppi di lavoratori stratificati sulla base della data di prima assunzione che ha determinato esposizione ad amianto nella cantieristica navale (<1974, 1974-1984 e 1985-1994).
Risultati:
È stato osservato un eccesso di mortalità per TPL, rispetto alla popolazione regionale (SMR=6.87, IC 95% 4.45-10.17) e italiana (SMR=13.95, IC 95% 9.02-20.64). Per TP, l’SMR-FVG era 1.49 (IC 95% 1.17-1.89), e l’SMR-Italia era 1.43 (IC 95% 1.12-1.81). L’eccesso di mortalità tra i lavoratori assunti prima del 1974 nella costruzione navale era maggiore per TPL (SMR-FVG=8.98, IC 95% 5.56-13.75; SMR-Italia=18.41, IC 95% 11.40-28.17) e per TP (SMR-FVG=1.60, IC 95% 1.18-2.11; SMR-Italia=1.54, IC 95% 1.14-2.03). Inoltre per TP, nel periodo 1974-1984, l’SMR-FVG era 2.45 (IC 95% 1.06-4.82) mentre l’SMR-Italia era 2.33 (IC 95% 1.01-4.60).
Conclusioni:
La coorte indagata ha evidenziato un incremento di mortalità per tumore della pleura e del polmone. Per il tumore del polmone l’incremento è stato maggiore tra i lavoratori con prima data di assunzione nella costruzione navale prima del 1985, suggerendo un ruolo chiave dell’esposizione ad amianto.
Standardized Mortality Ratios for All Causes by calendar year. Observed (O) and Expected (E) Cases, SMR and 95% Confidence Interval (95% CI) Follow Up Period 1989 – 2011*
Standardized Mortality Ratios for All Cancers by calendar year. Observed (O) and Expected (E) Cases, SMR and 95% Confidence Interval (95% CI) Follow Up Period 1989 – 2011*
| Year | O | FVG Standard Rates1 | Italian Standard Rates2 | ||||
| E | SMR | 95% CI | E | SMR | 95% CI | ||
| 1989 | - | 0.39 | - | - | 0.32 | - | - |
| 1990 | - | 0.49 | - | - | 0.39 | - | - |
| 1991 | - | 0.57 | - | - | 0.46 | - | - |
| 1992 | - | 0.74 | - | - | 0.59 | - | - |
| 1993 | - | 0.86 | - | - | 0.70 | - | - |
| 1994 | - | 1.17 | - | - | 0.96 | - | - |
| 1995 | 0 | 1.58 | 0.00 | - | 1.34 | 0.00 | - |
| 1996 | 2 | 2.08 | 0.96 | 0.12-3.47 | 1.74 | 1.15 | 0.14-4.15 |
| 1997 | 1 | 2.45 | 0.41 | 0.01-2.27 | 2.20 | 0.46 | 0.01-2.53 |
| 1998 | 2 | 3.22 | 0.62 | 0.08-2.24 | 2.77 | 0.72 | 0.09-2.61 |
| 1999 | 2 | 3.93 | 0.51 | 0.06-1.84 | 3.33 | 0.60 | 0.07-2.17 |
| 2000 | 5 | 4.67 | 1.07 | 0.35-2.49 | 4.13 | 1.21 | 0.39-2.82 |
| 2001 | 10 | 6.52 | 1.53 | 0.74-2.82 | 5.69 | 1.76 | 0.84-3.23 |
| 2002 | 9 | 8.75 | 1.03 | 0.47-1.96 | 7.51 | 1.20 | 0.55-2.28 |
| 2003 | 12 | 11.27 | 1.06 | 0.55-1.86 | 10.12 | 1.19 | 0.61-2.08 |
| 2004 | 14 | 13.95 | 1.00 | 0.55-1.69 | 12.53 | 1.12 | 0.61-1.88 |
| 2005 | 11 | 14.69 | 0.75 | 0.37-1.34 | 13.27 | 0.83 | 0.41-1.48 |
| 2006 | 15 | 16.45 | 0.91 | 0.51-1.50 | 14.85 | 1.01 | 0.57-1.67 |
| 2007 | 22 | 17.08 | 1.29 | 0.81-1.95 | 15.95 | 1.38 | 0.86-2.08 |
| 2008 | 20 | 18.99 | 1.05 | 0.64-1.62 | 16.92 | 1.18 | 0.72-1.82 |
| 2009 | 18 | 18.93 | 0.95 | 0.56-1.50 | 17.53 | 1.03 | 0.61-1.62 |
| 2010 | 20 | 19.89 | 1.01 | 0.61-1.55 | 17.83 | 1.12 | 0.69-1.73 |
| 2011 | 18 | 20.31 | 0.89 | 0.53-1.40 | 18.21 | 0.99 | 0.59-1.56 |
1Standard rate: age-specific mortality rates of FVG for years 1989-2003 and 2006-2011, provided by the Istituto Superiore di Sanità (ISS); 2Standard rate: age-specific mortality rates of Italy for years 1989-2003 and 2006-2011, provided by the Istituto Superiore di Sanità (ISS). *Follow Up Period: 1989 – 2011. Cohort included 2,488 subjects, for 26,415.77 total person-years.
Standardized Mortality Ratios for Malignant Pleural cancer by calendar year. Observed (O) and Expected (E) Cases, SMR and 95% Confidence Interval (95% CI) Follow Up Period 1989 – 2011*
| Year | O | FVG Standard Rates1 | Italian Standard Rates2 | ||||
| E | SMR | 95% CI | E | SMR | 95% CI | ||
| 1989 | - | 0.00 | - | - | 0.00 | - | - |
| 1990 | - | 0.01 | - | - | 0.00 | - | - |
| 1991 | - | 0.00 | - | - | 0.00 | - | - |
| 1992 | - | 0.01 | - | - | 0.00 | - | - |
| 1993 | - | 0.01 | - | - | 0.01 | - | - |
| 1994 | - | 0.01 | - | - | 0.01 | - | - |
| 1995 | - | 0.02 | - | - | 0.01 | - | - |
| 1996 | 1 | 0.04 | 22.32 | 0.56-124.33 | 0.01 | 78.41 | 1.98-436.74 |
| 1997 | - | 0.03 | - | - | 0.02 | - | - |
| 1998 | - | 0.04 | - | - | 0.02 | - | - |
| 1999 | 1 | 0.06 | 17.73 | 0.45-98.77 | 0.03 | 32.01 | 0.81-178.30 |
| 2000 | - | 0.09 | - | - | 0.04 | - | - |
| 2001 | 2 | 0.14 | 13.81 | 1.67-49.86 | 0.05 | 37.27 | 4.51-134.55 |
| 2002 | 3 | 0.21 | 14.33 | 2.95-41.83 | 0.08 | 39.57 | 8.15-115.55 |
| 2003 | - | 0.14 | - | - | 0.10 | - | - |
| 2004 | 2 | 0.17 | 11.92 | 1.44-43.03 | 0.12 | 17.12 | 2.07-61.79 |
| 2005 | 1 | 0.32 | 3.13 | 0.08-17.45 | 0.15 | 6.77 | 0.17-37.68 |
| 2006 | 2 | 0.35 | 5.69 | 0.69-20.55 | 0.16 | 12.29 | 1.49-44.36 |
| 2007 | 3 | 0.31 | 9.73 | 2.00-28.41 | 0.18 | 16.51 | 3.40-48.21 |
| 2008 | 4 | 0.53 | 7.48 | 2.04-19.16 | 0.19 | 21.33 | 5.80-54.62 |
| 2009 | 3 | 0.43 | 7.01 | 1.44-20.48 | 0.21 | 14.62 | 3.01-42.69 |
| 2010 | 2 | 0.49 | 4.08 | 0.49-14.71 | 0.20 | 9.90 | 1.20-35.75 |
| 2011 | 1 | 0.22 | 4.59 | 0.12-25.59 | 0.20 | 4.93 | 0.12-27.47 |
1Standard rate: age-specific mortality rates of FVG for years 1989-2003 and 2006-2011 calculated from the data of mortality provided by the National Institute of Statistics (ISTAT); 2Standard rate: age-specific mortality rates of Italy for years 1989-2003 and 2006-2011 calculated from the data of mortality provided by the National Institute of Statistics (ISTAT); *Follow Up Period: 1989 – 2011. Cohort included 2,488 subjects, for 26,415.77 total person-years.
Standardized Mortality Ratios for Lung cancer by calendar year. Observed (O) and Expected (E) Cases, SMR and 95% Confidence Interval (95% CI) Follow Up Period 1989 – 2011*
Number of observed deaths since first surveillance enrollment, by months, for all causes of death, lung cancer and malignant neoplasm of the pleura
Abbreviations:
- Attributable Risk (AR)
- Computed tomography (CT)
- Friuli Venezia Giulia (FVG)
- Low-dose computed tomography (LDCT)
- Relative Risk (RR)
- Standardized Mortality Ratios (SMR)
Introduction
The increase in diseases of the respiratory system in former workers exposed to asbestos had been observed during the last century, around the seventies. The increased incidence and mortality were evident for asbestos-related respiratory diseases, mainly for cancers of the pleura and lung cancers (18). In particular, mesothelioma is closely associated with exposure to asbestos, whose pathogenicity of fibers is significantly modulated by their physical-chemical characteristics and follows the geography and history of asbestos exposure (13, 23). Asbestos was used in Italy since the early 1900s in several employment sectors, such as the metalworking industry, the textile industry, shipbuilding industry, electricity production industry, and several craft work activities and trades. Asbestos was generally used for thermal and acoustic insulation. Damage to human health arising from inhalation of the fibers has affected a large part of the exposed population, both workers and their spouses. For mesothelioma of the pleura, exposure to asbestos has a major causal role.
Various European regulations have been established for asbestos. In the years 1991 and 1992, Italian Legislative Decrees (D.Lgs.) were approved (8, 16) declaring the ‘extreme attention to risk’ and banning the use of asbestos in the work environment. The establishment of occupational health surveillance programs for formerly exposed workers, organized by health service agencies and managed by qualified and experienced personnel, is recommended, although very limited evidence exists on the efficacy of such programs in reducing disease occurrence and mortality (27).
The coastal area of FVG has been characterized by the presence of work activities where asbestos was used in large amount until the years 1990-1992, particularly in the shipbuilding industry, as well as within metalworking industries, in port or the cotton mills. A large number of workers were therefore exposed to asbestos; in the early months of 2014 over 9,100 individuals were enrolled in the FVG Register of Exposed to Asbestos (2). However, this list may underestimate the number of people formerly exposed to asbestos since enrollment in the register is voluntary (2, 29).
In a case-control study conducted in the general population of Trieste, the largest city in this coastal area and the location of shipbuilding industries, the RR of lung cancer, adjusted for smoking and air pollution, for certain occupational exposures to asbestos was 2.0 for squamous cell carcinoma (95% CI 1.28-3.11), 2.11 for small cell carcinoma (95% CI 1.31-3.39) and 2.16 for adenocarcinoma (95% CI 1.32- 3.53); the AR estimate for possible or definite exposure to asbestos was 20% (95% CI 11.5-28.5) (5). The interaction between asbestos and smoking regarding lung cancer risk is between additive and multiplicative; the association between asbestos exposure and lung cancer risk is essentially linear but may level off at very high exposures. The RR for lung cancer increases between 1% and 4% per fiber-year (f-y)/ml, corresponding to a doubling of the risk at 25-100 f-y/ml (22).
To evaluate whether male workers with previous occupational exposure to asbestos and enrolled in a public health surveillance program experienced an increase in mortality from all causes, all cancers, lung cancer, and malignant neoplasm of the pleura, we conducted an historical cohort study using as external comparisons the general population of FVG region and the whole Italian population. Moreover, we evaluated whether the period of the first hire at a job that caused asbestos exposure affected the occurrence of mortality in our cohort: trends in supply and consumption of asbestos in Italy peaked between the 1970s and the mid-1980s, with a rapid decrease since 1985 (32).
Methods
Setting
The FVG Region is located in North-East of Italy and has a total of 1,218,985 inhabitants (15). The Local Health Authority (LHA) located around the coastal city of Monfalcone provides health care, including preventive occupational medicine, to residents of part of the coastal area of FVG. Since the nineties, LHA has promoted a number of technical and health care activities, as part of an overall “asbestos project”, with the aim, on the one hand, to control the danger of asbestos-containing structures, landfills, instruments, and on the other, to take action limiting the health effects of past exposure, both on workers and on the general population.
As a part of this project, a public health surveillance program for persons with previous occupational exposure to asbestos has been carried out at a dedicated outpatient clinic of the LHA Occupational Medicine Center. Former workers exposed to asbestos and their spouses had voluntary access to this clinic from the early 1990s to the first decade of 2000s. Although some surveillance had started some years before, 91% of medical surveillance and examinations were provided after 1991 according to Italian legislation (8): this cohort study is based on subjects identified at this clinic until 2008. The periodicity of the examinations at the Center was defined by the doctor, but often also by the perceived needs of the subject. Exams varied individually and included physical examination, basal spirometry, alveolar diffusion of CO (DLCO), chest radiography and computerized chest tomography (CT).
Exposure assessment
The exposure to asbestos was individually confirmed by occupational health personnel during the surveillance examinations. The evaluation of the level of exposure to asbestos was carried out by an occupational doctor using a semi-quantitative method. Level of exposure was established individually based on work history and accessible documentation, such as the employment card, acknowledgment of exposure by National Institute for Insurance against Accidents at Work (INAIL), and/or declarations from colleagues. For each worker, the intensity of exposure was quantified by anamnestic data, information obtained during the interviews, and according to job-exposure matrices available in the literature (11, 12, 25). The intensity of exposure was considered high when there was evidence that the subject had direct or indirect contact with friable material containing asbestos in confined spaces; medium, when the subject had occasional contact with friable materials, worked with compact material containing asbestos or used continuously substrates containing asbestos; low, when the subject had occasional exposure to environmental materials containing asbestos.
Trends in supply and consumption of asbestos in Italy peaked between the 1970s and the mid-1980s, with a rapid decrease since 1985 (32). For this reason, the first hire with asbestos exposure year was considered to account for possible differences in the intensity of exposure.
Data collection
For each person, during surveillance program the following information was collected and electronically recorded by the occupational physician: demographics (name, surname, date of birth, residence and phone number); company name and industry sector; total years of exposure to asbestos; cigarette smoking habits (i.e., never, ex or current smoker); type, date and findings of the examination. Since only 96 women enlisted in the surveillance program, their evaluation will not be presented in this report.
Follow-up
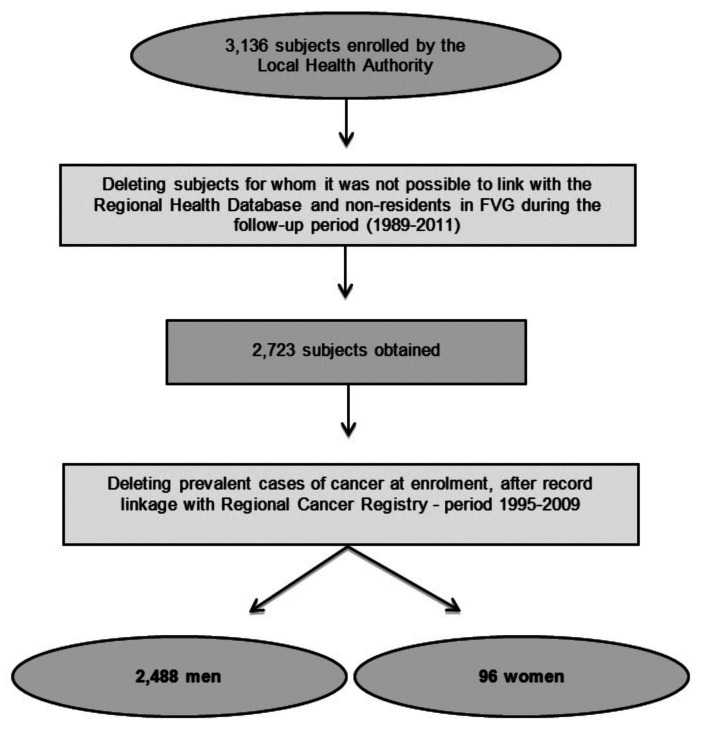
Selection cohort process is described in figure 1. For each member of the cohort, the residential status in FVG and the vital status, including the cause of death, between 1 January 1989 and 31 December 2011 were assessed through record linkage with the regional databases of residents and of mortality, respectively, of the Regional Health Database, using an anonymized unique personal identifier. Each member of the cohort was followed-up from the date of the first surveillance examination or 01/01/1989, whichever was last, to the date of death, end of residence in FVG or 31/12/2011, whichever was first.
Figure 1.
Cohort Selection Process
Assessment of mortality
Observed deaths were identified through record linkage with the mortality database. In this database, the causes of death are codified according to the WHO International Classification of Diseases, 9th edition, (ICD-9). Deaths for all cancers were identified through ICD-9 codes 140 to 239; for malignant neoplasm of the pleura through code 163 and for lung cancer through code 162.
We calculated the time difference between surveillance enrollment and death, to estimate the possible weight of residual prevalent cases of an often rapidly lethal disease such as malignant neoplasm of pleura or lung cancer.
Statistical analysis
The number of observed deaths and calendar year- and age-specific mortality rates from 1989 to 2011 (Supplemental tables 1-4) were calculated for all causes of death, all cancers, malignant neoplasms of the pleura and lung cancer.
| Year | O | FVG Standard Rates1 | Italian Standard Rates2 | ||||
| E | SMR | 95% CI | E | SMR | 95% CI | ||
| 1989 | - | 0.97 | - | - | 0.84 | - | - |
| 1990 | - | 1.17 | - | - | 1.02 | - | - |
| 1991 | - | 1.40 | - | - | 1.21 | - | - |
| 1992 | - | 1.74 | - | - | 1.52 | - | - |
| 1993 | - | 1.98 | - | - | 1.78 | - | - |
| 1994 | - | 2.66 | - | - | 2.36 | - | - |
| 1995 | 2 | 3.75 | 0.53 | 0.06-1.93 | 3.37 | 0.59 | 0.07-2.14 |
| 1996 | 3 | 4.86 | 0.62 | 0.13-1.80 | 4.39 | 0.68 | 0.14-2.00 |
| 1997 | 6 | 5.93 | 1.01 | 0.33-2.21 | 5.52 | 1.09 | 0.36-2.37 |
| 1998 | 4 | 7.57 | 0.53 | 0.14-1.35 | 7.01 | 0.57 | 0.16-1.46 |
| 1999 | 5 | 9.13 | 0.55 | 0.18-1.28 | 8.40 | 0.60 | 0.19-1.39 |
| 2000 | 9 | 11.09 | 0.81 | 0.37-1.54 | 10.36 | 0.87 | 0.40-1.65 |
| 2001 | 16 | 14.99 | 1.07 | 0.61-1.73 | 13.97 | 1.14 | 0.65-1.85 |
| 2002 | 23 | 19.48 | 1.18 | 0.75-1.77 | 18.25 | 1.26 | 0.80-1.89 |
| 2003 | 20 | 26.53 | 0.75 | 0.46-1.16 | 24.51 | 0.82 | 0.50-1.26 |
| 2004 | 27 | 33.00 | 0.82 | 0.54-1.19 | 30.48 | 0.89 | 0.58-1.29 |
| 2005 | 30 | 32.56 | 0.92 | 0.62-1.32 | 30.92 | 0.97 | 0.65-1.39 |
| 2006 | 31 | 36.73 | 0.84 | 0.57-1.21 | 34.95 | 0.89 | 0.60-1.27 |
| 2007 | 44 | 38.65 | 1.14 | 0.83-1.53 | 37.96 | 1.16 | 0.85-1.55 |
| 2008 | 42 | 43.44 | 0.97 | 0.69-1.31 | 41.30 | 1.02 | 0.73-1.38 |
| 2009 | 41 | 45.20 | 0.91 | 0.65-1.23 | 43.54 | 0.94 | 0.67-1.28 |
| 2010 | 41 | 46.50 | 0.88 | 0.63-1.20 | 44.36 | 0.92 | 0.66-1.26 |
| 2011 | 44 | 47.97 | 0.92 | 0.67-1.23 | 46.36 | 0.95 | 0.69-1.27 |
1Standard rate: age-specific mortality rates of FVG for years 1989-2003 and 2006-2011, provided by the Istituto Superiore di Sanità (ISS); 2Standard rate: age-specific mortality rates of Italy for years 1989-2003 and 2006-2011, provided by the Istituto Superiore di Sanità (ISS). *Follow Up Period: 1989 – 2011. Cohort included 2,488 subjects, for 26,415.77 total person-years.
| Year | O | FVG Standard Rates1 | Italian Standard Rates2 | ||||
| E | SMR | 95% CI | E | SMR | 95% CI | ||
| 1989 | - | 0.12 | - | - | 0.11 | - | - |
| 1990 | - | 0.14 | - | - | 0.13 | - | - |
| 1991 | - | 0.16 | - | - | 0.15 | - | - |
| 1992 | - | 0.22 | - | - | 0.19 | - | - |
| 1993 | - | 0.26 | - | - | 0.23 | - | - |
| 1994 | - | 0.33 | - | - | 0.31 | - | - |
| 1995 | - | 0.44 | - | - | 0.43 | - | - |
| 1996 | - | 0.58 | - | - | 0.55 | - | - |
| 1997 | 1 | 0.73 | 1.37 | 0.03-7.62 | 0.69 | 1.45 | 0.04-8.06 |
| 1998 | - | 0.89 | - | - | 0.85 | - | - |
| 1999 | - | 1.06 | - | - | 1.03 | - | - |
| 2000 | 2 | 1.20 | 1.67 | 0.20-6.03 | 1.26 | 1.59 | 0.19-5.75 |
| 2001 | 2 | 1.74 | 1.15 | 0.14-4.16 | 1.71 | 1.17 | 0.14-4.23 |
| 2002 | 3 | 2.12 | 1.41 | 0.29-4.12 | 2.28 | 1.32 | 0.27-3.84 |
| 2003 | 5 | 2.87 | 1.74 | 0.57-4.06 | 3.01 | 1.66 | 0.54-3.87 |
| 2004 | 6 | 3.55 | 1.69 | 0.55-3.69 | 3.73 | 1.61 | 0.53-3.51 |
| 2005 | 7 | 3.73 | 1.88 | 0.75-3.86 | 3.86 | 1.81 | 0.73-3.73 |
| 2006 | 4 | 4.16 | 0.96 | 0.26-2.46 | 4.31 | 0.93 | 0.25-2.38 |
| 2007 | 10 | 4.15 | 2.41 | 1.16-4.43 | 4.56 | 2.19 | 1.05-4.04 |
| 2008 | 6 | 4.35 | 1.38 | 0.45-3.00 | 4.77 | 1.26 | 0.41-2.74 |
| 2009 | 9 | 4.36 | 2.06 | 0.95-3.92 | 4.88 | 1.84 | 0.84-3.50 |
| 2010 | 9 | 5.12 | 1.76 | 0.81-3.34 | 4.95 | 1.82 | 0.83-3.45 |
| 2011 | 6 | 4.79 | 1.25 | 0.41-2.73 | 4.98 | 1.20 | 0.39-2.63 |
1Standard rate: age-specific mortality rates of FVG for years 1989-2003 and 2006-2011, provided by the Istituto Superiore di Sanità (ISS); 2Standard rate: age-specific mortality rates of Italy for years 1989-2003 and 2006-2011, provided by the Istituto Superiore di Sanità (ISS). *Follow Up Period: 1989 – 2011. Cohort included 2,488 subjects, for 26,415.77 total person-years.
As measures of association, we calculated SMRs, with 95% Confidence Interval (95% CI), through the indirect method (6). SMR was calculated as the ratio of the number of deaths observed in the cohort to the number expected by the calendar year- age- and sex-specific rates among the general population in FVG and Italy.
To conduct sensitivity analyses, we used two external standards: the general population of FVG Region and of Italy. The sources of the age-specific mortality rates for men were as follows:
- for mortality for all causes and all cancers, including lung cancer but excluding malignant neoplasm of the pleura, the rates provided by Istituto Superiore di Sanità (14) for the FVG region and Italy (years 1989-2011), respectively;
- for malignant neoplasm of the pleura, the rates calculated from the data of mortality provided by the National Institute of Statistics (ISTAT) (15) for FVG and for Italy (years 1989-2011).
For the years 2004 and 2005, standard rates were not available. We thus used the available rates of the closest year: for the year 2004, we used the rates of the year 2003, for 2005 the rates available for the year 2006. Subgroup analyses restricted to subjects with the first hire in shipbuilding that determined asbestos exposure (from here on only ‘first hire’ or ‘hired’) ever employed in the shipbuilding industry were also conducted. To account for possible differences in the intensity of exposure over the years, the subgroup was further categorized according to the hiring period in the following three groups: (a) subjects hired for the first time before the year 1974, (b) those hired for the first time between 1974 and 1984 and (c) those who were hired for the first time between 1985 and 1994.
All analyses were performed using SAS© software, Version 9.3 (SAS Institute Inc., Cary, N.C., USA) and Microsoft®Excel 2013 spreadsheet.
Results
Table 1 shows the characteristics of the cohort. The cohort included 2,488 men, with 26,415.78 person-years of follow-up during period 1989-2011, and a mean follow-up of 10.62 years per subject. About 68% of the subjects had a medium exposure to asbestos, and 70% was represented by workers employed in the shipbuilding industry.
Table 1.
Characteristics of the study cohort (N=2,488)
| N | % | ||
| Age at surveillance enrollment (years) | Mean (SD) | 56.76 (11.99) | - |
| 0-54 | 970 | 38.99 | |
| 55-59 | 491 | 19.73 | |
| 60-64 | 430 | 17.28 | |
| 65-69 | 284 | 11.41 | |
| 70-74 | 170 | 6.83 | |
| 75-79 | 96 | 3.86 | |
| 80+ | 47 | 1.89 | |
| Exposure level | Low | 477 | 19.17 |
| Medium | 1691 | 67.97 | |
| High | 320 | 12.86 | |
| Years of exposure | Mean (SD) | 18.28 (9.97) | - |
| <10 | 481 | 19.33 | |
| 10-19 | 837 | 33.64 | |
| 20-29 | 796 | 31.99 | |
| 30+ | 374 | 15.03 | |
| Industry sector | Metalworking | 294 | 11.89 |
| Shipbuilding | 1,740 | 70.39 | |
| Electric | 96 | 3.88 | |
| Insulation | 89 | 3.60 | |
| Other | 253 | 10.23 | |
| Person-years of observation | before year 2000 | 4,588.56 | 17.37 |
| 2000-2002 | 3,168.67 | 12.00 | |
| 2003-2005 | 5,650.95 | 21.39 | |
| 2006-2008 | 6,592.78 | 24.96 | |
| 2009-2011 | 6,414.82 | 24.28 |
Over 94% of deaths for all causes, 92% of deaths for malignant neoplasm of the pleura, and over 98% for lung cancer, occurred after 12 months from surveillance enrollment (Supplemental table 5). Measures of frequency and SMRs for all causes, all cancers, cancer of pleura, lung, and other individual cancers and for groups of non-neoplastic causes are presented in table 2. A total of 388 deaths were observed. The SMRs for all causes were 0.89 (95% CI 0.80-0.98) and 0.94 (95% CI 0.85-1.03), based on FVG and Italian rates, respectively. Mortality for non-neoplastic causes was lower than expected: SMR was 0.88 (95% CI 0.76-1.01), based on FVG rates and 0.90 (95% CI 0.78-1.03), based on Italian rates (data not shown). The observed deaths for all cancers did not exceed expected deaths: the SMR was 0.96 (95% CI 0.83-1.11) based on FVG rates and 1.07 (95% CI 0.92-1.24) based on Italian rates.
| Months since surveillance enrollment (N) by cause of death | N. of Obs. deaths (%) | Cumulative freq. (%) |
| All cause of death | ||
| ≤12 | 22 (5.67) | 22 (5.67) |
| 13 - 24 | 24 (6.19) | 46 (11.86) |
| 25 - 36 | 25 (6.44) | 71 (18.30) |
| 37 - 48 | 43 (11.08) | 114 (29.38) |
| 49 - 60 | 30 (7.73) | 144 (37.11) |
| 60+ | 244 (62.89) | 388 (100.00) |
| All cancers | ||
| ≤12 | 8 (4.42) | 8 (1.43) |
| 13 - 24 | 12 (6.63) | 20 (8.57) |
| 25 - 36 | 11 (6.08) | 31 (17.13) |
| 37 - 48 | 20 (11.05) | 51 (28.18) |
| 49 - 60 | 13 (7.18) | 64 (35.36) |
| 60+ 117 | (64.64) | 181 (100.00) |
| Lung cancer | ||
| ≤12 | 1 (1.43) | 1 (1.43) |
| 13 - 24 | 5 (7.14) | 6 (8.57) |
| 25 - 36 | - | - |
| 37 - 48 | 11 (15.71) | 17 (24.29) |
| 49 - 60 | 6 (8.57) | 23 (32.86) |
| 60+ 47 | (67.14) | 70 (100.00) |
| Malignant neoplasm of pleura | ||
| ≤12 | 2 (8.00) | 2 (8.00) |
| 13 - 24 | 2 (8.00) | 4 (16.00) |
| 25 - 36 | 2 (8.00) | 6 (24.00) |
| 37 - 48 | 4 (16.00) | 10 (40.00) |
| 49 - 60 | 2 (8.00) | 12 (48.00) |
| 60+ | 13 (52.00) | 25 (100.00) |
Table 2.
Observed (O) and Expected (E.) Deaths, Standardized Mortality Ratios (SMR) for Causes of Death and 95% Confidence Interval (95% CI) among 2,488 men enrolled in an asbestos surveillance program. Follow Up Period 1989 – 2011*
| Cause of Death | ICD9 | ICD10 | O | FVG Standard Rates1 | Italian Standard Rates2 | ||||
| E | SMR | 95% CI | E | SMR | 95% CI | ||||
| All Cause | 0-99 | A00-T98 | 388 | 437.30 | 0.89 | 0.80-0.98 | 414.38 | 0.94 | 0.85-1.03 |
| All Cancers | 140-239 | C00-D48 | 181 | 188.99 | 0.96 | 0.83-1.11 | 169.33 | 1.07 | 0.92-1.24 |
| Esophagus | 150 | C15 | 5 | 5.36 | 0.93 | 0.30-2.17 | 2.77 | 1.81 | 0.59-4.21 |
| Stomach | 151 | C16 | 11 | 12.63 | 0.87 | 0.43-1.56 | 10.63 | 1.03 | 0.52-1.85 |
| Colorectal | 153-154;159.0 | C18-C21 | 11 | 20.53 | 0.54 | 0.27-0.96 | 17.07 | 0.64 | 0.32-1.15 |
| Liver | 155.0-155.1 | C22 | 9 | 18.89 | 0.65 | 0.30-1.23 | 10.97 | 0.82 | 0.38-1.56 |
| Pancreas | 157 | C25 | 10 | 0.94 | 0.94 | 0.45-1.74 | 8.76 | 1.14 | 0.55-2.10 |
| Larynx | 161 | C32 | 1 | 3.19 | 0.31 | 0.01-1.74 | 3.04 | 0.33 | 0.01-1.83 |
| Trachea, bronchus, lung | 162 | C33-C34 | 70 | 47.05 | 1.49 | 1.17-1.89 | 48.99 | 1.43 | 1.12-1.81 |
| Malignant neoplasm of pleura3 | 163 | C45 | 25 | 3.64 | 6.87 | 4.45-10.17 | 1.79 | 13.95 | 9.02-20.64 |
| Prostate | 185 | C61 | 1 | 11.30 | 0.09 | 0.00-0.49 | 10.10 | 0.10 | 0.00-0.55 |
| Bladder | 188 | C67 | 3 | 5.31 | 0.56 | 0.12-1.65 | 6.73 | 0.45 | 0.09-1.30 |
| Kidney | 189 | C64 | 2 | 4.47 | 0.45 | 0.05-1.61 | 3.69 | 0.54 | 0.07-1.96 |
| Central Nervous System | 191 | C71 | 4 | 3.84 | 1.04 | 0.28-2.66 | 3.40 | 1.18 | 0.32-3.01 |
| Leukemia | 204-208 | C91-C95 | 2 | 4.17 | 0.48 | 0.06-1.73 | 4.99 | 0.40 | 0.05-1.45 |
| Mental disorders | 290-303 E 305-319 | F00-F99 | 4 | 6.41 | 0.62 | 0.17-1.60 | 4.14 | 0.97 | 0.26-2.47 |
| Diseases of nervous system and sense organs | 320-389 | G00-G99 | 7 | 11.03 | 0.63 | 0.25-1.31 | 11.43 | 0.61 | 0.25-1.26 |
| Diseases of digestive system | 520-579 | K00-K93 | 23 | 24.31 | 0.95 | 0.60-1.42 | 19.21 | 1.20 | 0.76-1.80 |
| Diseases of genitourinary system | 580-629 | N00-N99 | 5 | 4.88 | 1.02 | 0.33-2.39 | 5.80 | 0.86 | 0.28-2.01 |
| Symptoms, signs, and ill-defined conditions | 780-799 | R00-R99 | 5 | 3.78 | 1.32 | 0.43-3.08 | 3.84 | 1.30 | 0.42-3.03 |
| Injury and poisoning | 800-999 | S00-T98 | 14 | 19.42 | 0.72 | 0.39-1.21 | 16.27 | 0.86 | 0.47-1.45 |
| Diseases of circulatory system | 390-459 | I00-I99 | 112 | 128.60 | 0.87 | 0.73-1.05 | 133.34 | 0.84 | 0.70-1.01 |
| Diseases of respiratory system | 460-519 | J00-J99 | 28 | 27.14 | 1.03 | 0.69-1.50 | 26.37 | 1.06 | 0.71-1.54 |
*Cohort of study: 2,488 subjects for 26,415.77 total person-years; we used for standardization available rates of ICD-9 code (1980-2002). 1Standard rate: age-specific mortality rates of FVG for years 1989-2003 and 2006-2011, provided by the Istituto Superiore di Sanità (ISS); 2Standard rate: age-specific mortality rates of Italy for years 1989-2003 and 2006-2011, provided by the Istituto Superiore di Sanità (ISS); 3Standard rate: age-specific mortality rates of FVG and Italy for years 1989-2003 and 2006-2011 calculated from the data of mortality provided by the National Institute of Statistics (ISTAT).
A total of 25 deaths for malignant neoplasm of the pleura were observed, compared with 3.64 and 1.79 expected according to the rates in FVG and general Italian population, respectively. The corresponding SMRs were 6.87 (95% CI 4.45-10.17) and 13.95 (95% CI 9.02-20.64). A total of 70 deaths for lung cancer were observed, and the SMRs were 1.49 (95% CI 1.17-1.89) and 1.43 (95% CI 1.12-1.81), respectively.
An increased, albeit not significant, mortality was also found for esophageal cancer standardizing for Italian rates. Colorectal cancer mortality was significantly lower than expected as compared with FVG general population. A highly significant reduction in prostate cancer mortality was also found: SMR= 0.09 (95% CI 0.00-0.49) based on FVG rates, SMR= 0.10 (95% CI 0.00-0.55) based on Italian rates.
The SMRs stratified by age group are presented in table 3. As expected, deaths under age 50 years were sparse. The SMRs for age 50-59 were lower than 1, with a borderline significant reduction of mortality for all cancers. A mortality excess was found at ages 50 and over for malignant pleural cancer, with an SMR from 4.84 (95% CI 1.58-10.54) based on FVG rates, to an SMR of 29.14 (95% CI 9.44-67.89) based on Italian rates. A mortality excess was also found at ages 70 and over for lung cancer, with an SMR from 1.55 (95% CI 1.03-2.25), to an SMR of 2.02 (95% CI 1.01-3.62) based on Italian rates.
Table 3.
Standardized Mortality Ratios by Cause of Death and Age Group. Observed (O) and Expected (E) Cases, SMR and 95% Confidence Interval (95% CI)
| Cause of Death by age group | O | FVG Standard Rates* | Italian Standard Rates** | ||||
| E | SMR | 95% CI | E | SMR | 95% CI | ||
| All Causes | |||||||
| ≤39 | 1 | 2.39 | 0.42 | 0.01-2.33 | 2.57 | 0.39 | 0.01-2.17 |
| 40-49 | 4 | 5.71 | 0.70 | 0.19-1.79 | 5.41 | 0.74 | 0.20-1.89 |
| 50-59 | 35 | 46.43 | 0.75 | 0.53-1.95 | 42.34 | 0.83 | 0.58-1.15 |
| 60-69 | 121 | 131.16 | 0.92 | 0.77-1.11 | 118.90 | 1.02 | 0.85-1.22 |
| 70-79 | 137 | 152.76 | 0.90 | 0.76-1.06 | 146.36 | 0.94 | 0.79-1.11 |
| 80+ | 90 | 98.84 | 0.91 | 0.74-1.13 | 98.79 | 0.91 | 0.74-1.13 |
| All Cancers | |||||||
| ≤39 | 0 | 0.31 | 0.00 | - | 0.33 | 0.00 | - |
| 40-49 | 2 | 2.01 | 0.99 | 0.12-3.58 | 1.78 | 1.12 | 0.14-4.05 |
| 50-59 | 13 | 22.22 | 0.59 | 0.31-1.00 | 19.97 | 0.65 | 0.35-1.11 |
| 60-69 | 74 | 68.40 | 1.08 | 0.85-1.37 | 60.04 | 1.23 | 0.97-1.56 |
| 70-79 | 67 | 69.11 | 0.97 | 0.76-1.23 | 61.73 | 1.08 | 0.85-1.38 |
| 80+ | 25 | 26.94 | 0.93 | 0.60-1.37 | 25.48 | 0.98 | 0.63-1.45 |
| Malignant Neoplasm of Pleura*** | |||||||
| ≤39 | - | 0.00 | - | - | 0.00 | - | - |
| 40-49 | - | 0.02 | - | - | 0.02 | - | - |
| 50-59 | 3 | 0.47 | 6.40 | 1.32-18.69 | 0.22 | 13.71 | 2.82-40.04 |
| 60-69 | 11 | 1.56 | 7.04 | 3.51-12.60 | 0.76 | 14.37 | 7.17-25.73 |
| 70-79 | 6 | 1.24 | 4.84 | 1.58-10.54 | 0.62 | 9.70 | 3.17-21.14 |
| 80+ | 5 | 0.35 | 14.45 | 4.68-33.68 | 0.17 | 29.14 | 9.44-67.89 |
| Lung Cancer | |||||||
| ≤39 | - | 0.06 | - | - | 0.03 | - | - |
| 40-49 | 1 | 0.40 | 2.49 | 0.06-13.85 | 0.42 | 2.40 | 0.06-13.38 |
| 50-59 | 4 | 5.57 | 0.72 | 0.20-1.84 | 6.06 | 0.66 | 0.18-1.69 |
| 60-69 | 26 | 17.66 | 1.47 | 0.96-2.16 | 18.97 | 1.37 | 0.89-2.01 |
| 70-79 | 28 | 17.82 | 1.57 | 1.04-2.28 | 18.06 | 1.55 | 1.03-2.25 |
| 80+ | 11 | 5.55 | 1.98 | 0.99-3.55 | 5.44 | 2.02 | 1.01-3.62 |
* Standard rate: age-specific mortality rates of FVG for years 1989-2003 and 2006-2011, provided by the Istituto Superiore di Sanità (ISS); ** Standard rate: age-specific mortality rates of Italy for years 1989-2003 and 2006-2011, provided by the Istituto Superiore di Sanità (ISS); *** Standard rate: age-specific mortality rates of FVG and Italy for years 1989-2003 and 2006-2011 calculated from the data of mortality provided by the National Institute of Statistics (ISTAT); Cohort included 2,488 subjects, for 26,415.77 total person-years.
Table 4 displays the results for subgroups of former workers exposed to asbestos in the shipbuilding industry, by the period at first hiring. The results pertaining to the group with the hypothesized highest level of exposure to asbestos (first hiring before 1985) and longer latency show a particularly high mortality for lung cancer (with an SMR from 1.54 (95% CI 1.14-2.03) based on Italian rates, to an SMR of 2.45 (95% CI 1.06-4.82) based on FVG rates) and malignant neoplasm of the pleura (with an SMR from 8.98 (95% CI 5.56-13.75) based on FVG rates, to an SMR of 18.41 (95% CI 11.40-28.17) based on Italian rates) but not for all causes and all cancers.
Table 4.
Standardized Mortality Ratios by Cause of Death, Industrial Sector and Year of Hire. Observed (O) and Expected (E) Cases, SMR and 95% Confidence Interval (95% CI)
| Cause of Death | O | FVG Standard Rates* | Italian Standard Rates** | ||||
| E | SMR | 95% CI | E | SMR | 95% CI | ||
| All Causes | |||||||
| All industrial sectors1 | 388 | 437.30 | 0.89 | 0.80-0.98 | 414.38 | 0.94 | 0.85-1.03 |
| Shipbuilding2 | 301 | 336.86 | 0.89 | 0.80-1.00 | 319.93 | 0.94 | 0.84-1.05 |
| Shipbuilding hired <19743 | 257 | 295.02 | 0.87 | 0.77-0.99 | 280.92 | 0.91 | 0.81-1.04 |
| Shipbuilding hired 1974-19844 | 30 | 27.78 | 1.08 | 0.73-1.54 | 25.89 | 1.16 | 0.78-1.66 |
| Shipbuilding hired 1985+5 | 14 | 14.06 | 1.00 | 0.54-1.67 | 13.12 | 1.07 | 0.58-1.79 |
| All Cancers | |||||||
| All industrial sectors1 | 181 | 188.99 | 0.96 | 0.83-1.11 | 169.33 | 1.07 | 0.92-1.24 |
| Shipbuilding2 | 144 | 143.01 | 1.01 | 0.85-1.19 | 128.17 | 1.12 | 0.95-1.33 |
| Shipbuilding hired <19743 | 124 | 123.86 | 1.00 | 0.83-1.20 | 111.19 | 1.12 | 0.93-1.34 |
| Shipbuilding hired 1974-19844 | 14 | 12.88 | 1.09 | 0.59-1.83 | 11.44 | 1.22 | 0.67-2.06 |
| Shipbuilding hired 1985+5 | 6 | 6.27 | 0.96 | 0.31-2.09 | 5.54 | 1.08 | 0.35-2.36 |
| Malignant neoplasm of pleura*** | |||||||
| All industrial sectors1 | 25 | 3.64 | 6.87 | 4.45-10.17 | 1.79 | 13.95 | 9.02-20.64 |
| Shipbuilding2 | 22 | 2.71 | 8.12 | 5.09-12.26 | 1.33 | 16.60 | 10.41-25.06 |
| Shipbuilding hired <19743 | 21 | 2.34 | 8.98 | 5.56-13.75 | 1.14 | 18.41 | 11.40-28.17 |
| Shipbuilding hired 1974-19844 | 0 | 0.25 | - | - | 0.13 | - | - |
| Shipbuilding hired 1985+5 | 1 | 0.12 | 8.40 | 0.21-46.81 | 0.06 | 16.88 | 0.43-94.04 |
| Lung Cancer | |||||||
| All industrial sectors1 | 70 | 47.05 | 1.49 | 1.17-1.89 | 48.99 | 1.43 | 1.12-1.81 |
| Shipbuilding2 | 59 | 35.56 | 1.66 | 1.28-2.16 | 36.86 | 1.60 | 1.23-2.08 |
| Shipbuilding hired <19743 | 49 | 30.71 | 1.60 | 1.18-2.11 | 31.79 | 1.54 | 1.14-2.03 |
| Shipbuilding hired 1974-19844 | 8 | 3.27 | 2.45 | 1.06-4.82 | 3.43 | 2.33 | 1.01-4.60 |
| Shipbuilding hired 1985+5 | 2 | 1.58 | 1.26 | 0.15-4.56 | 1.64 | 1.22 | 0.15-4.39 |
* Standard rate: age-specific mortality rates of FVG for years 1989-2003 and 2006-2011, provided by the Istituto Superiore di Sanità (ISS); ** Standard rate: age-specific mortality rates of Italy for years 1989-2003 and 2006-2011, provided by the Istituto Superiore di Sanità (ISS); *** Standard rate: age-specific mortality rates of FVG and Italy for years 1989-2003 and 2006-2011 calculated from the data of mortality provided by the National Institute of Statistics (ISTAT); 1Follow Up Period: 1989-2011. Cohort included 2,488 subjects, for 26,415.77 total person-years; 2Follow Up Period: 1989-2011. Cohort included 1,740 subjects, for 18,972.65 total person-years; 3Follow Up Period: 1989-2011. Cohort included 1,370 subjects, for 13,206.14 total person-years; 4Follow Up Period: 1989-2011. Cohort included 234 subjects, for 3,176.32 total person-years; 5Follow Up Period: 1989-2011. Cohort included 136 subjects, for 2,590.19 total person-years
Discussion
Strengths of the study
Under certain circumstances, epidemiologic studies of asbestos-exposed workers undergoing health surveillance may fulfill several criteria relevant to etiologic objectives: (a) the observation is conducted longitudinally, i.e., the direction of the observation goes from exposure to disease occurrence; (b) the hypothesis and related study inclusion criteria are exposure specific, i.e., cohort members are selected only under the condition of confirmed asbestos exposure; (c) the study allows for the evaluation of associations with multiple outcomes, i.e., mortality for malignant neoplasm of the pleura and lung cancer are the main outcomes, but the concurrent opportunity exists to estimate also all causes and all cancers mortality; (d) the cohort may be a representative sample of all the asbestos-exposed persons within a specific area and time period and, therefore, its disease experience may reflect the effects of the exposure in that area. In our case the group of exposed subjects (the study cohort) was established as population-based and all the asbestos-exposed workers residing in the territory of the LHA were eligible for inclusion in the surveillance program at the local Occupational Medicine Center; (e) a valid comparison group of unexposed subjects can be identified and its sex-, age- and time period-specific mortality rates are compared with the rates of the exposed. In this study we used two comparisons: the first one was the regional population that reflects the underlying morbidity and mortality of cancer due to factors other than asbestos exposure, the second was the whole Italian population which represents baseline rates (particularly of lung cancer and pleural neoplasms) not influenced by the asbestos experience of the index population.
The choice of analyzing workers undergoing health surveillance also avoids the limitations that may be present when an occupational cohort coincides with the workers of a single company. In the latter case, sometimes subjects’ inclusion in the cohort study fully depends on the company collaboration and on data completeness, i.e., access to complete personnel records. Indeed, in the study area such access has not been possible for some decades, and the study of workers under public health surveillance has now overcome this limitation. In addition, a population-based approach to asbestos-exposed workers also allows for the inclusion of individuals whose job has been outsourced (subcontracted) and not included directly under the main shipyard payroll. Outsourcing jobs in different areas and historical periods may represent a large proportion of exposed workers.
Possible limitations
The use of asbestos-exposed workers undergoing health surveillance has also limitations. In particular, selection bias may arise whenever observation-time in the study does not include the whole pool of data related to every person who ever experienced occupational exposure to asbestos in the study area, with each person followed to the end of life (30). It is well known that most studies use a subset of this person-time experience, and hence bias may occur (7). Indeed, in our study, follow-up started only when asbestos-exposed workers entered health surveillance. Therefore, the follow-up period (observation) from age at hiring in the first job entailing asbestos exposure till entry in health surveillance is missing. It might have occurred that subjects who died or left the study area before the establishment of the health surveillance program, or before the start of follow-up, experienced an exposure that was qualitatively (type of asbestos materials, other carcinogens or toxicants) or quantitatively (concentration or duration) different as compared with the subjects who enrolled in the surveillance program. This selection bias might under or overestimate mortality rates of conditions with short induction period (injuries, cardiovascular diseases, respiratory diseases, certain neoplasms). Also, the exclusion of an important part (18%) of the persons initially enrolled by the LHA because of the impossibility of linkage with the Regional Health Database information might cause a selection bias.
The cohort appears left truncated, hence the observed person-time from the beginning of the exposure to the beginning of the follow up is reduced, and the rates of occurrence of the cohort give rise to an overestimation of the effects. Left-truncation bias is not a major issue when latency analysis is not the goal of the study, as in our case.
About the main outcomes of interest, i.e., mortality for lung and pleural cancers, we assume that the induction period for the study subjects was not completed at time of surveillance enrollment particularly for those exposed for the first time since the middle 1980s: the descriptive epidemiology of mesothelioma showed, as of 2014, increasing mortality rates in the study area and the whole country (28). Regarding possible differential exclusion from follow-up of individuals due to migration out of the study area, this might have occurred, although the FVG enjoyed a period of growth and prosperity from the 1960s to the year 2008; therefore, it is unlikely that many of them moved, thus becoming ineligible for enrollment in the surveillance program. Another possible selection bias to consider is enrollment in the surveillance program depending on health status rather than on likelihood of asbestos exposure. Bias might have occurred if subjects enrolled because symptomatic or because they had received a diagnosis of any cancer, or specifically lung or pleural cancers. To avoid such a bias, we excluded subjects who had received any diagnosis of cancer during the period of operation of the regional cancer registry (1995-2009). In addition, for the purpose to estimate the weight of latent cancers, we conducted a sensitivity analysis that calculated the number of deaths occurring within the first 12, 24, 36, 48 and 60 months from enrollment in the surveillance program (Supplemental table 5). It is well known that the prognosis of lung and pleural cancers is poor. Therefore, it is unlikely that selection bias due to preferential selection associated with a latent cancer condition played a role in the study results and that large overestimation of the SMRs is present.
On the other hand, it is possible that, while we excluded from the cohort prevalent cancer cases, in this way we may have reduced for other cancers the comparability of the cohort versus the general population mortality rates. In fact, the general population (which is an external comparator) does include persons with a long-lasting diagnosis of cancer, especially as it relates to less lethal sites such as colon and prostate. All these things considered, we may conclude that the SMR for all cancers in this analysis may be slightly underestimated, lending even more credit to the interpretation that occupational exposure to asbestos in this cohort is causally associated both to mesothelioma and to lung cancer. Instead, results about other cancer sites should be interpreted with caution.
From this cohort of asbestos-exposed workers undergoing health surveillance, 25 deaths for malignant neoplasm of the pleura were observed, and a consequent large excess of mortality was estimated with mortality ratios between 7 and 14, according to the two populations (local and national) that we used as comparisons. In addition, a total of 70 deaths for lung cancer was observed displaying SMRs around 1.5 in the overall cohort.
Internal consistency
Although these results are consistent in all subgroups within the cohort, the increase in mortality from lung cancer and malignant neoplasm of the pleura showed to be stronger among former workers exposed to asbestos in the shipbuilding industry. In our study results are even stronger among subjects with known hiring in shipbuilding before the year 1974 for malignant neoplasm of the pleura and between 1974 and 1984 for lung cancer. The excess of mortality from malignant neoplasm of the pleura is not statistically significant in the cohort of former workers hired for the first time in shipbuilding between 1985 and 1994: this fact may be explained by the low number of subjects of the subcohort and the low number of observed cases (cohort=136 men; observed=1). These results can also be explained by the long latency of the malignant neoplasm of the pleura: likely, former workers exposed to asbestos, hired for the first time since the mid-1980s, could become cases until 2020 or later.
Results for cancers other than lung and pleura showed some mortality decrease for several sites, notably stomach, colorectal, prostate, and leukemia as well as a non-statistically significant excess mortality observed for esophageal cancer (based on Italian, but not on local rates). The latter result on the esophagus may be due to the strong and the well-known mortality excesses for esophageal cancer of the whole FVG region when compared to the rest of Italy. These results may be explained by the regional higher prevalence of risky behaviors related to alcohol drinking (10, 34). Therefore, it is unlikely that this reportedly positive association be explained as an effect of asbestos exposure. Rather, it is worth noting that in this cohort we did not find an association with cancer of the larynx, another target organ of both asbestos and alcohol exposure. More evident, on the other hand, is the reduced mortality for stomach, colorectal, and especially prostate cancer as compared both with local and Italian rates.
Mortality for all causes did not differ from that of the general population, not even when standardization was performed on subcohorts of former workers exposed to asbestos in shipbuilding industries, in different periods. This finding may be partially explained by a healthy worker effect; in fact, 58.27% of the cohort was in working-age with less than 60 years, at first examination.
External consistency
Two preliminary, descriptive reports about workers from the same local shipyard who were hired in 1942 and 1950-1959 were published previously, showing high mesothelioma occurrence (3, 4). One Italian historical cohort mortality study was conducted among 3,984 shipyard workers in the harbor of Genoa, Italy, between 1960 and 1981 (28). In this cohort, SMRs were computed using male residents in the Province of Genoa as the referent population. For the whole cohort, significantly increased SMRs were detected for all causes, all cancers, liver, larynx, lung, pleural and bladder cancers, respiratory tract diseases, and cirrhosis of the liver. In particular, for malignant pleural neoplasm, the observed number of deaths was 60, the expected number was 11.5 with an SMR of 5.24, 95% CI: 4.00-6.74. For lung cancer, the observed number of deaths was 298, the expected number was 168.7, with an SMR of 1.77, 95% CI: 1.57-1.98.
The international epidemiological evidence of health problems caused by asbestos exposure among shipyard workers is profuse. In particular, the association between asbestos and pleural and lung neoplasms have been the subject of a number of original studies and multiple reviews (17, 20, 27, 31). Supported by strong evidence from studies among shipyard workers, the association between occupational asbestos exposure and malignant neoplasm of the pleura has been demonstrated (21) and findings suggest that the association between asbestos exposure and lung cancer risk is linear (22).
According to ‘The Helsinki Declaration on Management and Elimination of Asbestos-Related Diseases’ adopted by the International Conference on Monitoring and Surveillance of Asbestos-Related Diseases, for medical, legal, and social reasons asbestos-related diseases should be diagnosed at the earliest possible stage (33) for minimizing the adverse health effects of asbestos, as well as for compensation of disease and disability. In addition, since new scientific evidence provides support to the health benefits of low-dose computed tomographic screening in people with a high risk of smoking-related lung cancer (1), by analogy, the Helsinki Declaration also recommended such screening programs for workers with a history of asbestos exposure who are at high risk of lung cancer. Presently, such a recommendation is based on the evidence that CT scan screening in asbestos-exposed workers is effective in detecting asymptomatic lung cancer (9, 24), although evidence of reduced mortality among asbestos-exposed workers enrolled in organized screening programs is scanty. Pesch et al. (2010) estimated the cancer risk of asbestos in a surveillance cohort of 576 German workers highly exposed to asbestos in a variety of industrial sectors (26). They were selected for high-resolution computerized tomography of the chest in 1993-1997 and were followed up to 15 years. In this cohort SMRs were 28.1 (95% CI: 15.73-46.36) for pleural mesothelioma, 0.39 (95% CI: 0.17-0.77) for lung cancer, 0.59 (95% CI: 0.49-0.70) for all causes and 0.82 (95% CI: 0.63-1.07) for all cancers.
Mastrangelo et al. (2013) showed preliminary results of mortality from post-occupational health surveillance of asbestos workers recruited in the Veneto Region (about 1,700 asbestos workers) and monitored either with chest x-ray or LDCT (19). Compared to the general population the SMRs for lung cancer were 1.05 (95% CI: 0.48-1.99) in workers without pleural plaques, 1.05 (95% CI: 0.48-2.00) in workers with pleural plaques but not asbestosis and 4.62 (95% CI: 0.56-16.7) in workers with asbestosis. Pleural mesothelioma mortality was not reported.
In summary, whereas results from both our cohort and the German cohort described by Pesch et al. (2010) are consistent in demonstrating a still very strong association with neoplasm of the pleura during asbestos-exposed surveillance, lung cancer relative mortality varies across monitored cohorts.
Conclusions
When compared to the general population of FVG and of Italy, this cohort of subjects enrolled in a surveillance program for exposure to asbestos showed a very strong excess of mortality for malignant neoplasm of the pleura. Lung cancer mortality was also significantly increased. Relative mortality was stronger when we considered subcohorts of former workers exposed to asbestos in shipbuilding industries: for lung cancer, the excess was stronger in workers with the first hire in shipbuilding between 1974-1984, suggesting a key role of asbestos exposure. Mortality for all causes did not differ from that of the general population; this finding may be partially explained by a healthy worker effect. Future internal analyses of this cohort should evaluate whether any of the surveillance activities conducted within subgroups such as periodic LDCT demonstrate any relative reduction in lung cancer and mesothelioma mortality.
Conflit of interests
F. Barbone, M. Bovenzi and F.E. Pisa have acted as expert witnesses for the public prosecutor in criminal trials on asbestos-related cancers.
References
- 1.Aberle DR, Adams AM, Berg CD, et al. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asbestos Regional Commission: Friuli Venezia Giulia Region, Italy: 2014 [Google Scholar]
- 3.Bianchi C, Bianchi T. Mesothelioma among Shipyard Workers in Monfalcone, Italy. Indian J Occup Environ Med. 2012;16:119–123. doi: 10.4103/0019-5278.111753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianchi C, Bianchi T. Shipbuilding and Mesothelioma in Monfalcone, Italy. Indian J Occup Environ Med. 2012;16:14–17. doi: 10.4103/0019-5278.99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bovenzi M, Stanta G, Antiga G, et al. Occupational Exposure and Lung Cancer Risk in a Coastal Area of Northeastern Italy. Int Arch Occup Environ Health. 1993;65:35–41. doi: 10.1007/BF00586056. [DOI] [PubMed] [Google Scholar]
- 6.Breslow NE, Day NE. Statistical Methods in Cancer Research. Volume II--the Design and Analysis of Cohort Studies. IARC Sci Publ. 1987:1–406. [PubMed] [Google Scholar]
- 7.Checkoway H, Pearce NE, Kriebel D. Research Methods in Occupational Epidemiology. 2004 [Google Scholar]
- 8.D. Lgs n 277: D. Lgs N. 277 of August 15, 1991. Implementation of Directives N. 80/1107 / Eec, N. 82/605 / Eec, N. 83/477 / Eec, N. 86/188 / Eec and N. 88/642 / Eec, Concerning the Protection of Workers against the Risks Related to Exposure to Chemical, Physical and Biological Agents During Work , a Provision of Art. 7 of the Law of 30 July 1990, N. 212. 1991 [Google Scholar]
- 9.Fasola G, Belvedere O, Aita M, et al. Low-Dose Computed Tomography Screening for Lung Cancer and Pleural Mesothelioma in an Asbestos-Exposed Population: Baseline Results of a Prospective, Nonrandomized Feasibility Trial--an Alpe-Adria Thoracic Oncology Multidisciplinary Group Study (Atom 002) Oncologist. 2007;12:1215–1224. doi: 10.1634/theoncologist.12-10-1215. [DOI] [PubMed] [Google Scholar]
- 10.Franceschi S, Talamini R, Barra S, et al. Smoking and Drinking in Relation to Cancers of the Oral Cavity, Pharynx, Larynx, and Esophagus in Northern Italy. Cancer Res. 1990;50:6502–6507. [PubMed] [Google Scholar]
- 11.Goldberg M, Imbernon M. The Use of Job Exposure Matrices for Cancer Epidemiology Research and Surveillance. Arch Pub Health. 2002;60:173–185. [Google Scholar]
- 12.Goldberg M, Kromhout H, Guenel P, et al. Job Exposure Matrices in Industry. Int J Epidemiol. 1993;22(Suppl 2):S10–S15. doi: 10.1093/ije/22.supplement_2.s10. [DOI] [PubMed] [Google Scholar]
- 13.International Agency for Research on Cancer: Iarc Monographs on the Evaluation of Carcinogenic Risks to Humans. A Review of Human Carcinogens: Metals, Arsenic, Dusts, and Fibres. WHO press 2011 [Google Scholar]
- 14.Istituto Superiore di Sanità, Italy. Available At: http://www.Iss.It/Site/Mortalita/Scripts/Selcause.Asp. [Last Access: 03/11/2015] [Google Scholar]
- 15.ISTAT: Italian National Institute of Statistics, 15th Census of Population and Housing, 2011. Available At: http://Dati-Censimentopopolazione.Istat.It/ [Last Access: 03/11/2015] [Google Scholar]
- 16.Italian Law n 257: Italian Law N. 257 of March 27, 1992. Rules Relating to the Cessation of Asbestos. 1992 [Google Scholar]
- 17.Krstev S, Stewart P, Rusiecki J, Blair A. Mortality among Shipyard Coast Guard Workers: A Retrospective Cohort Study. Occup Environ Med. 2007;64:651–658. doi: 10.1136/oem.2006.029652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magnani C, Fubini B, Mirabelli D, et al. Pleural Mesothelioma: Epidemiological and Public Health Issues. Report from the Second Italian Consensus Conference on Pleural Mesothelioma. Med Lav. 2013;104:191–202. [PubMed] [Google Scholar]
- 19.Mastrangelo G, Marangi G, Ballarin MN, et al. Post-Occupational Health Surveillance of Asbestos Workers. Med Lav. 2013;104:351–358. [PubMed] [Google Scholar]
- 20.McCormack V, Peto J, Byrnes G, et al. Estimating the Asbestos-Related Lung Cancer Burden from Mesothelioma Mortality. Br J Cancer. 2012;106:575–584. doi: 10.1038/bjc.2011.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neumann V, Loseke S, Nowak D, et al. Malignant Pleural Mesothelioma: Incidence, Etiology, Diagnosis, Treatment, and Occupational Health. Dtsch Arztebl Int. 2013;110:319–326. doi: 10.3238/arztebl.2013.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen LS, Baelum J, Rasmussen J, et al. Occupational Asbestos Exposure and Lung Cancer--a Systematic Review of the Literature. Arch Environ Occup Health. 2014;69:191–206. doi: 10.1080/19338244.2013.863752. [DOI] [PubMed] [Google Scholar]
- 23.NIOSH: National Institute for Occupational Safety and Health. Department of Health and Human Services, Centers for Disease Control and Prevention. Current Intelligence Bulletin: Asbestos Fibers and Other Elongate Mineral Particles: State of the Science and Roadmap for Research, Version 4. April 2011 [Google Scholar]
- 24.Ollier M, Chamoux A, Naughton G, et al. Chest Ct Scan Screening for Lung Cancer in Asbestos Occupational Exposure: A Systematic Review and Meta-Analysis. Chest. 2014;145:1339–1346. doi: 10.1378/chest.13-2181. [DOI] [PubMed] [Google Scholar]
- 25.Orlowski E, Pohlabeln H, Berrino F, et al. Retrospective Assessment of Asbestos Exposure--II. At the Job Level: Complementarity of Job-Specific Questionnaire and Job Exposure Matrices. Int J Epidemiol. 1993;22(Suppl 2):S96–S105. doi: 10.1093/ije/22.supplement_2.s96. [DOI] [PubMed] [Google Scholar]
- 26.Pesch B, Taeger D, Johnen G, et al. Cancer Mortality in a Surveillance Cohort of German Males Formerly Exposed to Asbestos. Int J Hyg Environ Health. 2010;213:44–51. doi: 10.1016/j.ijheh.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Pinto C, Novello S, Torri V, et al. Second Italian Consensus Conference on Malignant Pleural Mesothelioma: State of the Art and Recommendations. Cancer Treat Rev. 2013;39:328–339. doi: 10.1016/j.ctrv.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Puntoni R, Merlo F, Borsa L, et al. A Historical Cohort Mortality Study among Shipyard Workers in Genoa, Italy. Am J Ind Med. 2001;40:363–370. doi: 10.1002/ajim.1110. [DOI] [PubMed] [Google Scholar]
- 29.Regional Law no. 22: Measures on Surveillance, Prevention and Information from Asbestos Risk Situations and Regional Interventions Related to It. Text Coordinated with Regional Law 21/2005. 2001 [Google Scholar]
- 30.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 2008 [Google Scholar]
- 31.Tomioka K, Natori Y, Kumagai S, Kurumatani N. An Updated Historical Cohort Mortality Study of Workers Exposed to Asbestos in a Refitting Shipyard, 1947-2007. Int Arch Occup Environ Health. 2011;84:959–967. doi: 10.1007/s00420-011-0655-2. [DOI] [PubMed] [Google Scholar]
- 32.Virta RL. Worldwide Asbestos Supply and Consumption Trends from 1900 through 2003: U.S. Geological Survey Circular 1298. Available At: http://Pubs.Usgs.Gov/Circ/2006/1298/C1298.pdf. [Last Access: 03/11/2015]: p. 80. [Google Scholar]
- 33.Wolff H, Vehmas T, Oksa P, et al. Asbestos, Asbestosis, and Cancer, the Helsinki Criteria for Diagnosis and Attribution 2014: Recommendations. Scand J Work Environ Health. 2015;41:5–15. doi: 10.5271/sjweh.3462. [DOI] [PubMed] [Google Scholar]
- 34.Zambon P, Talamini R, La Vecchia C, et al. Smoking, Type of Alcoholic Beverage and Squamous-Cell Oesophageal Cancer in Northern Italy. Int J Cancer. 2000;86:144–149. doi: 10.1002/(sici)1097-0215(20000401)86:1<144::aid-ijc23>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Standardized Mortality Ratios for All Causes by calendar year. Observed (O) and Expected (E) Cases, SMR and 95% Confidence Interval (95% CI) Follow Up Period 1989 – 2011*
Standardized Mortality Ratios for All Cancers by calendar year. Observed (O) and Expected (E) Cases, SMR and 95% Confidence Interval (95% CI) Follow Up Period 1989 – 2011*
| Year | O | FVG Standard Rates1 | Italian Standard Rates2 | ||||
| E | SMR | 95% CI | E | SMR | 95% CI | ||
| 1989 | - | 0.39 | - | - | 0.32 | - | - |
| 1990 | - | 0.49 | - | - | 0.39 | - | - |
| 1991 | - | 0.57 | - | - | 0.46 | - | - |
| 1992 | - | 0.74 | - | - | 0.59 | - | - |
| 1993 | - | 0.86 | - | - | 0.70 | - | - |
| 1994 | - | 1.17 | - | - | 0.96 | - | - |
| 1995 | 0 | 1.58 | 0.00 | - | 1.34 | 0.00 | - |
| 1996 | 2 | 2.08 | 0.96 | 0.12-3.47 | 1.74 | 1.15 | 0.14-4.15 |
| 1997 | 1 | 2.45 | 0.41 | 0.01-2.27 | 2.20 | 0.46 | 0.01-2.53 |
| 1998 | 2 | 3.22 | 0.62 | 0.08-2.24 | 2.77 | 0.72 | 0.09-2.61 |
| 1999 | 2 | 3.93 | 0.51 | 0.06-1.84 | 3.33 | 0.60 | 0.07-2.17 |
| 2000 | 5 | 4.67 | 1.07 | 0.35-2.49 | 4.13 | 1.21 | 0.39-2.82 |
| 2001 | 10 | 6.52 | 1.53 | 0.74-2.82 | 5.69 | 1.76 | 0.84-3.23 |
| 2002 | 9 | 8.75 | 1.03 | 0.47-1.96 | 7.51 | 1.20 | 0.55-2.28 |
| 2003 | 12 | 11.27 | 1.06 | 0.55-1.86 | 10.12 | 1.19 | 0.61-2.08 |
| 2004 | 14 | 13.95 | 1.00 | 0.55-1.69 | 12.53 | 1.12 | 0.61-1.88 |
| 2005 | 11 | 14.69 | 0.75 | 0.37-1.34 | 13.27 | 0.83 | 0.41-1.48 |
| 2006 | 15 | 16.45 | 0.91 | 0.51-1.50 | 14.85 | 1.01 | 0.57-1.67 |
| 2007 | 22 | 17.08 | 1.29 | 0.81-1.95 | 15.95 | 1.38 | 0.86-2.08 |
| 2008 | 20 | 18.99 | 1.05 | 0.64-1.62 | 16.92 | 1.18 | 0.72-1.82 |
| 2009 | 18 | 18.93 | 0.95 | 0.56-1.50 | 17.53 | 1.03 | 0.61-1.62 |
| 2010 | 20 | 19.89 | 1.01 | 0.61-1.55 | 17.83 | 1.12 | 0.69-1.73 |
| 2011 | 18 | 20.31 | 0.89 | 0.53-1.40 | 18.21 | 0.99 | 0.59-1.56 |
1Standard rate: age-specific mortality rates of FVG for years 1989-2003 and 2006-2011, provided by the Istituto Superiore di Sanità (ISS); 2Standard rate: age-specific mortality rates of Italy for years 1989-2003 and 2006-2011, provided by the Istituto Superiore di Sanità (ISS). *Follow Up Period: 1989 – 2011. Cohort included 2,488 subjects, for 26,415.77 total person-years.
Standardized Mortality Ratios for Malignant Pleural cancer by calendar year. Observed (O) and Expected (E) Cases, SMR and 95% Confidence Interval (95% CI) Follow Up Period 1989 – 2011*
| Year | O | FVG Standard Rates1 | Italian Standard Rates2 | ||||
| E | SMR | 95% CI | E | SMR | 95% CI | ||
| 1989 | - | 0.00 | - | - | 0.00 | - | - |
| 1990 | - | 0.01 | - | - | 0.00 | - | - |
| 1991 | - | 0.00 | - | - | 0.00 | - | - |
| 1992 | - | 0.01 | - | - | 0.00 | - | - |
| 1993 | - | 0.01 | - | - | 0.01 | - | - |
| 1994 | - | 0.01 | - | - | 0.01 | - | - |
| 1995 | - | 0.02 | - | - | 0.01 | - | - |
| 1996 | 1 | 0.04 | 22.32 | 0.56-124.33 | 0.01 | 78.41 | 1.98-436.74 |
| 1997 | - | 0.03 | - | - | 0.02 | - | - |
| 1998 | - | 0.04 | - | - | 0.02 | - | - |
| 1999 | 1 | 0.06 | 17.73 | 0.45-98.77 | 0.03 | 32.01 | 0.81-178.30 |
| 2000 | - | 0.09 | - | - | 0.04 | - | - |
| 2001 | 2 | 0.14 | 13.81 | 1.67-49.86 | 0.05 | 37.27 | 4.51-134.55 |
| 2002 | 3 | 0.21 | 14.33 | 2.95-41.83 | 0.08 | 39.57 | 8.15-115.55 |
| 2003 | - | 0.14 | - | - | 0.10 | - | - |
| 2004 | 2 | 0.17 | 11.92 | 1.44-43.03 | 0.12 | 17.12 | 2.07-61.79 |
| 2005 | 1 | 0.32 | 3.13 | 0.08-17.45 | 0.15 | 6.77 | 0.17-37.68 |
| 2006 | 2 | 0.35 | 5.69 | 0.69-20.55 | 0.16 | 12.29 | 1.49-44.36 |
| 2007 | 3 | 0.31 | 9.73 | 2.00-28.41 | 0.18 | 16.51 | 3.40-48.21 |
| 2008 | 4 | 0.53 | 7.48 | 2.04-19.16 | 0.19 | 21.33 | 5.80-54.62 |
| 2009 | 3 | 0.43 | 7.01 | 1.44-20.48 | 0.21 | 14.62 | 3.01-42.69 |
| 2010 | 2 | 0.49 | 4.08 | 0.49-14.71 | 0.20 | 9.90 | 1.20-35.75 |
| 2011 | 1 | 0.22 | 4.59 | 0.12-25.59 | 0.20 | 4.93 | 0.12-27.47 |
1Standard rate: age-specific mortality rates of FVG for years 1989-2003 and 2006-2011 calculated from the data of mortality provided by the National Institute of Statistics (ISTAT); 2Standard rate: age-specific mortality rates of Italy for years 1989-2003 and 2006-2011 calculated from the data of mortality provided by the National Institute of Statistics (ISTAT); *Follow Up Period: 1989 – 2011. Cohort included 2,488 subjects, for 26,415.77 total person-years.
Standardized Mortality Ratios for Lung cancer by calendar year. Observed (O) and Expected (E) Cases, SMR and 95% Confidence Interval (95% CI) Follow Up Period 1989 – 2011*
Number of observed deaths since first surveillance enrollment, by months, for all causes of death, lung cancer and malignant neoplasm of the pleura