Abstract
Introduction
Sphingolipid accumulation has been linked to obesity, type 2 diabetes and non-alcoholic fatty liver disease (NAFLD). A recent study showed that depletion of dihydroceramide desaturase-1 (DES-1) in adipose and/or liver tissue decreases ceramide-to-dihydroceramide ratios (ceramide/dihydroceramide) in several tissues and improves the metabolic profile in mice. We tested the hypothesis that ceramide/dihydroceramide would also be elevated and relate positively to liver fat content and insulin resistance in humans.
Research design and methods
Thus, we assessed total and specific ceramide/dihydroceramide in various biosamples of 7 lean and 21 obese volunteers without or with different NAFLD stages, who were eligible for abdominal or bariatric surgery, respectively. Biosamples were obtained from serum, liver, rectus abdominis muscle as well as subcutaneous abdominal and visceral adipose tissue during surgery.
Results
Surprisingly, certain serum and liver ceramide/dihydroceramide ratios were reduced in both obesity and non-alcoholic steatohepatitis (NASH) and related inversely to liver fat content. Specifically, hepatic ceramide/dihydroceramide (species 16:0) related negatively to hepatic mitochondrial capacity and lipid peroxidation. In visceral adipose tissue, ceramide/dihydroceramide (species 16:0) associated positively with markers of inflammation.
Conclusion
These results failed to confirm the relationships of ceramide/dihydroceramide in humans with different degree of insulin resistance. However, the low hepatic ceramide/dihydroceramide favor a role for dihydroceramide accumulation in NASH, while a specific ceramide/dihydroceramide ratio in visceral adipose tissue suggests a role of ceramides in obesity-associated low-grade inflammation.
Keywords: insulin resistance, non-alcoholic fatty liver disease, inflammation, obesity
Significance of this study.
What is already known about this subject?
Sphingolipid accumulation is related to insulin resistance as well as progression of hepatic inflammation in persons with non-alcoholic fatty liver disease (NAFLD).
Recently, depletion of dihydroceramide desaturase-1 (DES-1) in adipose and/or liver tissue was linked to decrease of ceramide/dihydroceramide ratios and improvement of metabolic profile in mice.
What are the new findings?
Our data in humans with different stages of NAFLD suggest that—in contrast to the study in mice—decreased ceramide/dihydroceramide ratios lead to lower insulin sensitivity and liver fat content. This might rather reflect higher dihydroceramide production from de novo ceramide synthesis due to increased dietary intake of saturated lipids in obesity.
How might these results change the focus of research or clinical practice?
Our results underline the importance of dihydroceramides as potential biomarkers of disease as well as the importance of studies on tissue-specific differences of specific sphingolipid species in humans in order to answer the question whether DES-1 is a good drug target or not.
Introduction
Bioactive lipid metabolites such as diacylglycerols (DAGs), sphingolipids and acylcarnitines can interfere with insulin sensitivity and are linked to obesity and type 2 diabetes.1–3 Circulating ceramides have been associated with insulin resistance in humans without diabetes,4 and this effect has been proposed to be mediated by direct inhibition of insulin signaling in skeletal muscle.5 Obesity and insulin resistance also tightly relate to non-alcoholic fatty liver disease (NAFLD).6 In obese humans with NAFLD, the sn-1,2-DAG/protein kinase C-ε pathway correlates with hepatic insulin resistance.7–10 On the contrary, ceramides and dihydroceramides have been increased in livers of insulin-resistant humans8 and the ceramide/c-Jun N-terminal kinase pathway relates to hepatic oxidative stress and inflammation, that is, non-alcoholic steatohepatitis (NASH).11 12 Both acyl chain length and cellular localization of specific ceramide species have been recognized as important parameters for ceramide-induced deterioration of metabolic function.13 In line, ceramide 16 and 24 and certain sphingomyelin species were increased in methionine–choline-deficient mice, indicating upregulation of sphingolipid metabolism in NASH.14
Recently, Chaurasia et al15 reported elegant mouse experiments targeting dihydroceramide desaturase-1 (DES-1), a rate-limiting enzyme for de novo ceramide synthesis. Global DES-1 depletion improved insulin sensitivity and hepatic steatosis, decreased free fatty acids (FFA) and fat mass and increased oxygen consumption. Liver-specific DES-1 ablation lowered ceramide/dihydroceramide ratios and resolved steatosis. There is also evidence for ceramide-mediated mitochondrial fission in murine models,16 suggesting that lower ceramide levels improve the mitochondrial capacity for fatty acid oxidation and thereby decrease steatosis and lipid-mediated insulin resistance.17 While these findings point to DES-1 as a promising therapeutic target,18 it remains to be tested whether lower ceramide/dihydroceramide, either in insulin target tissues or in the circulation of humans, indeed relate to tissue-specific insulin sensitivity and/or to hepatic oxidative capacity, particularly in the context of NAFLD. Several groups have demonstrated that dihydroceramides increase more than ceramides in insulin-resistant states. Dihydroceramides were better predictors of diabetes than other sphingolipids,19 and several plasma dihydroceramide subspecies closely correlated with waist circumference in a Mexican American population.20 Dihydroceramides are also excellent markers of coronary artery disease21 and may therefore have the potential as biomarkers in different disease areas. In this context, dihydroceramides could be a better readout of fatty acid flux through the ceramide biosynthesis pathway, owing to their lower abundance compared with ceramides. They are also slightly less effective substrates for ceramide-metabolizing enzymes, such as glucosylceramide synthase. Thus, they may build up more demonstrably than ceramides in response to fatty acids flowing through the pathway.
Research design and methods
Study participants
The research design and methods have been described in detail previously.11
In order to examine possible associations between ceramide/dihydroceramide and measures of insulin sensitivity, we used the absolute concentrations for ceramides and dihydroceramides, which were reported before in a recent study.10 We analyzed the ceramide/dihydroceramide for total and for specific sphingolipid species in all biosamples from 21 obese patients without (NAFL−) or with (NAFL+) non-alcoholic fatty liver or NASH as well as from seven healthy lean humans (control), who were all eligible for bariatric or abdominal surgery. Before surgery, they underwent hyperinsulinemic-euglycemic clamp tests with stable isotopically labeled glucose to assess tissue-specific insulin sensitivity.11 During surgery, biosamples were taken from serum, liver, skeletal muscle as well as visceral and subcutaneous fat tissues.10 Liver samples were used for measuring hepatic mitochondrial function by high-resolution respirometry and for histological NAFLD staging or immediately transferred in liquid nitrogen for further storage at −80°C. Participants’ characteristics are summarized in table 1. Of note, one patient in the NAFL+ group had type 2 diabetes mellitus and withdrew the oral glucose lowering medication for 3 days before the clamp test.
Table 1.
Participant characteristics11
| CON | NAFL− | NAFL+ | NASH | |
| Number (females) | 7 (5) | 7 (6) | 7 (4) | 7 (6) |
| Age (years) | 40±13 | 43±7 | 46±12 | 42±8 |
| BMI (kg/m2) | 25.2±3.3†§** | 49.5±8.3 | 56.1±7.0 | 51.4±7.1 |
| Waist circumference (cm) | 82.8±13.0*§¶ | 125.7±19.8†† | 144.5±16.2 | 129.2±17.0 |
| Fasting blood glucose (mg/dL) | 75±7 ‡ | 93±13 | 98±28 | 85±6 |
| Fasting plasma insulin (mIU/L) | 6.1 (5.1, 10.8)*‡ | 22.6 (12.3, 25.9) | 33.6 (32.4, 34.3) | 22.8 (16.3, 31.7) |
| HbA1c (%) | 5.3±0.3*‡ | 5.6±0.5 | 6.0±0.9 | 5.5±0.2 |
| HbA1c (mmol/mol) | 34±3*‡ | 38±6 | 42±10 | 37±2 |
| Peripheral insulin sensitivity (mg/kg/min) | 7.4±2.2*‡ | 3.1±1.7 | 1.8±0.3 | 2.8±0.6 |
| HCL (%) | 1 (0, 5)‡** | 2 (0, 5)‡‡§§ | 40 (10, 40)¶¶ | 45 (40, 65) |
Data are presented as mean±SD or median (q1, q3).
*p≤0.01, CON vs NAFL−.
†p<0.001, CON vs NAFL−.
‡p≤0.01, CON vs NAFL+.
§p<0.001, CON vs NAFL+.
¶p≤0.01, CON vs NASH.
**p<0.001, CON vs NASH.
††p≤0.05, NAFL− vs NAFL+.
‡‡p≤0.01, NAFL− vs NAFL+.
§§p<0.001, NAFL− vs NASH.
¶¶p≤0.05, NAFL+ vs NASH.
BMI, body mass index; CON, control; HCL, hepatocellular lipids; NAFL, non-alcoholic fatty liver; NASH, non-alcoholic steatohepatitis.
Hepatic lipid peroxidation and systemic inflammation
Thiobarbituric acid reactive substances (TBARS) were measured in serum and liver tissue as markers of lipid peroxidation.12 Interleukin-6 (IL-6), tumor necrosis factor alpha (TNFα), interleukin-1 receptor antagonist (IL-1ra) and fibroblast growth factor 21 (FGF-21) were quantified using Quantikine HS (IL-6, TNFα) or Quantikine (IL-1ra, FGF-21) ELISA kits (R&D Systems, Wiesbaden, Germany).
Sphingolipid measurements
Sphingolipids were quantified using liquid chromatography-mass spectrometry methodology as previously described.11 Briefly, lipid separation was achieved on a 2.1 (i.d.) × 150 mm Kinetex C8, 2.6 micron core-shell particle (Phenomenex, Torrance, California, USA) column. Sphingolipid species were identified based on exact mass and fragmentation patterns and verified by lipid standards. The concentration of each lipid metabolite was determined according to calibration curves using peak-area ratio of the analyte versus the corresponding internal standard. Calibration curves were generated using serial dilutions of each target analyte. The precision per cent coefficient of variation for sphingoid bases and ceramide panel is 5%–20%, whereas accuracy is 80%–120%. Data are expressed as picograms of metabolite per mg of wet tissue or mL of plasma. According to our in-house generated data and experience analyzing all kinds of tissues from mouse models, equivalent results were obtained when normalizing sphingolipid levels according to protein content in the sample. The absolute levels of total and specific sphingolipid species of this cohort have been previously reported. Briefly, insulin resistance was more prevalent in obese persons, while the degree of steatosis was highest in persons with NASH, who exclusively showed also increased total hepatic ceramides and dihydroceramide species.11
Statistical analysis
Data are presented as mean and SD (±SEM) or median (25th /75th percentiles) as appropriate. Linear regression models were used to calculate estimates (β) and p values of associations between metabolic parameters and sphingolipid species with and without adjustment for age, sex and body mass index (BMI). P values from two-sided tests ≤5% were considered to indicate significant differences. Analyses were performed using SAS V.9.4 (SAS Institute).
Data and resource availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Results
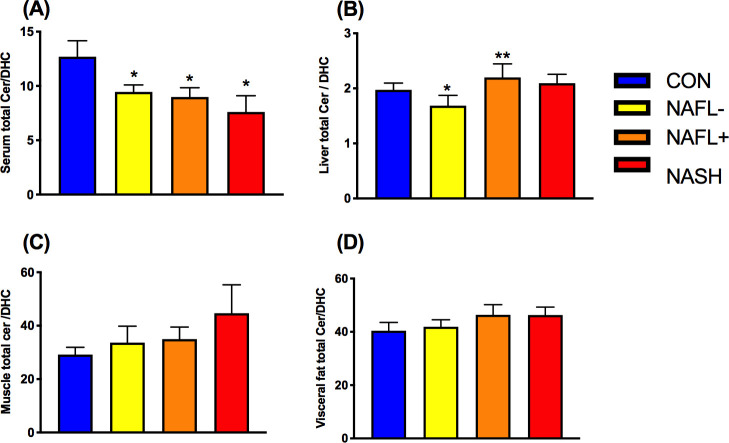
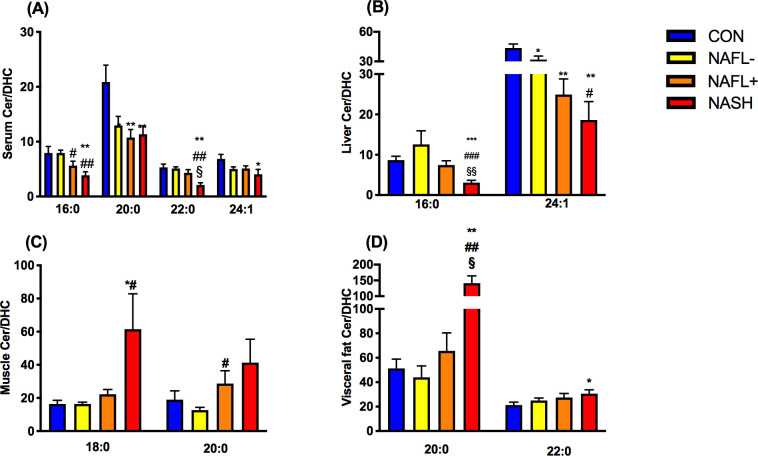
First, we tested the hypothesis that ceramide/dihydroceramide is elevated in insulin-resistant states and NAFLD as reported for certain mouse models. Total serum ceramide/dihydroceramide was lower in serum of all obese groups, higher in NAFL+ livers, but not different in skeletal muscle and visceral adipose tissue (figure 1A–D). Specific serum ceramide/dihydroceramide ratios were lower in all obese groups (20:0), NAFLD (16:0) or in NASH only (22:0, 24:1; figure 2A). Hepatic ceramide/dihydroceramide 16:0 and 24:1 were also lower in NASH compared with controls (figure 2B). Interestingly, muscle ceramide-to-dihydroceramide 18:0 and visceral adipose tissue ceramide/dihydroceramide 20:0 and 22:0 were increased in NASH (figure 2C and D). BMI (figure 3A) and waist circumference (β=−0.05, p=0.03) related negatively to total ceramide/dihydroceramide in serum and liver (β=−1.18, p=0.0005 and β=−0.49, p=0.006). BMI and waist circumference correlated positively with ceramide/dihydroceramide 16:0 in muscle (β=0.46, p=0.03 and β=0.27, p=0.02) and visceral adipose tissue (β=0.19, p=0.02 and β=0.10, p=0.03). Of note, reduction of ceramide 16:0 in mice with selective ceramide synthase 6 (CerS6) ablation associated with lower body weight and fat content.22
Figure 1.
Ratios of total ceramides to dihydroceramides (total ceramide/dihydroceramide) ratios in various tissues (A−D) of lean humans (controls (CON); blue) and obese persons without non-alcoholic fatty liver (NAFL−; yellow) or with NAFL (NAFL+) (orange) or with non-alcoholic steatohepatitis (NASH; red). Data are mean±SEM. *p<0.05 vs CON, **p<0.01 vs CON. DHC, dihydroceramide.
Figure 2.
Ratios of species of ceramides to the respective species of dihydroceramides (specific ceramide/dihydroceramide) ratios in various tissues (A−D) of lean humans (controls (CON); blue) and obese persons without non-alcoholic fatty liver (NAFL−; yellow) or with NAFL (NAFL+) (orange) or with non-alcoholic steatohepatitis (NASH; red). Data are mean±SEM. *p<0.05 vs CON, **p<0.01 vs CON, #p<0.05 vs NAFL−, ##p<0.01 vs NAFL, §p<0.05 vs NAFL+, §§p<0.01 vs NAFL+. DHC, dihydroceramide.
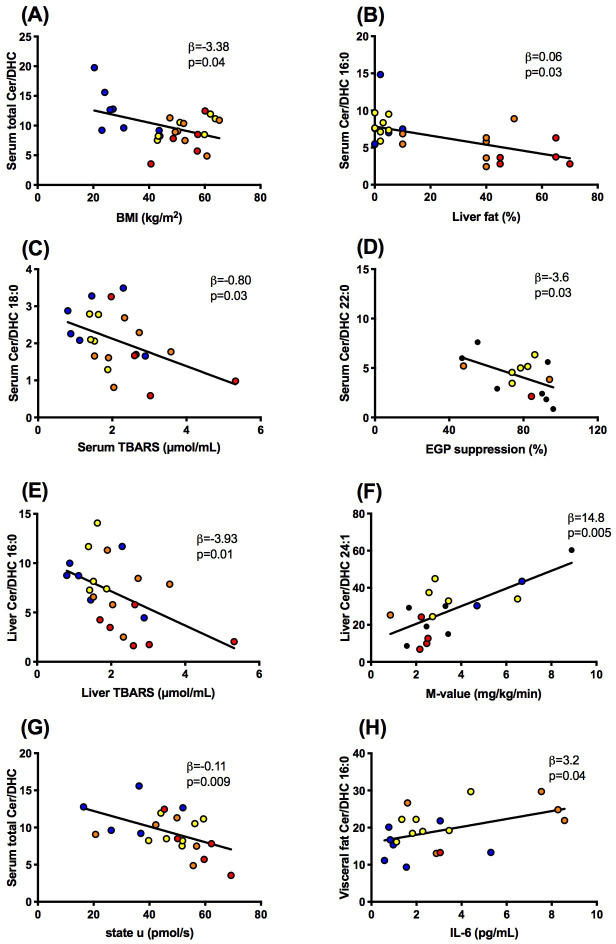
Figure 3.
Associations between ratios of ceramides to dihydroceramides (ceramide/dihydroceramide) in serum (A–D, G), liver (E, F) or visceral fat (H) and body mass index (BMI), liver fat content, thiobarbituric acid reactive substances (TBARS) in serum and liver, whole-body insulin sensitivity (M-value), state U respiration in liver tissue and interleukin-6 (IL-6) in lean humans (blue) and obese persons without non-alcoholic fatty liver (yellow) or with NAFL (orange) or with non-alcoholic steatohepatitis (red). DHC, dihydroceramide; EGP, endogenous glucose production; NAFL, non-alcoholic fatty liver.
Second, we tested the hypothesis that higher ceramide/dihydroceramide would associate with worse metabolic profile as reported in murine models.15 In contrast, patients with NAFLD featured lower serum total ceramide/dihydroceramide (β=−0.05, p=0.04), 16:0 (figure 3B) and 22:0 (β=−0.04, p=0.0004). There were neither associations of serum ceramide-to-dihydroceramide with whole-body insulin sensitivity (M-value) nor with circulating glucose or FFA. Hepatic total ceramide/dihydroceramide even correlated positively with M-value (β=26.0, p=0.01) and ceramide/dihydroceramide 16:0 negatively with liver fat content (β=−0.11, p=0.01). These findings fail to support that ceramide 16:0 is also involved in human obesity-induced insulin resistance. Similar to ceramide/dihydroceramide 16:0, hepatic ceramide/dihydroceramide 22:0 and 24:1 correlated negatively with liver fat content (β=−0.30, p=0.04, β=−0.39, p=0.0001) but positively with M-value (figure 3F). Only serum ceramide/dihydroceramide 22:0 related negatively to insulin-mediated suppression of endogenous glucose production (figure 3D), similar as in murine DES-1 deficiency.15 Furthermore, there were associations neither between hepatic ceramide/dihydroceramide and hepatic insulin sensitivity nor between muscle or adipose ceramide/dihydroceramide and glycemia, insulin sensitivity or hepatic fat content.
Third, we hypothesized that elevated ceramide/dihydroceramide relates to impaired mitochondrial function and greater oxidative stress, according to the recently postulated ceramide-mediated abnormal mitochondrial function favoring DAG-induced insulin resistance.16 Indeed, serum total ceramide/dihydroceramide correlated inversely with lower maximal uncoupled (state u) respiration from substrates of the tricarboxylic acid cycle (figure 3G). Similar negative associations were observed for serum ceramide/dihydroceramide 20:0 (β=−10.7, p=0.01), 22:0 (β=−1.88, p=0.04) and 24:1 (β=−2.71, p=0.006), hepatic ceramide/dihydroceramide 20:0 (β=−25.6, p=0.004), muscle total ceramide/dihydroceramide (β=−25.3, p=0.02), 20:0 and 24:0 (β=−34.2, p=0.02 and β=−24.9, p=0.02). The present analysis failed to detect any associations between ceramide/dihydroceramide 16:0 and hepatic mitochondrial capacity. Moreover, hepatic ceramide/dihydroceramide 16:0 rather related negatively to hepatic thiobarbituric acid reactive substances (TBARS), reflecting lipid peroxidation (figure 3E) with or without adjustment for age, sex and BMI. Similarly, serum and liver ceramide/dihydroceramide 18:0 (figure 3C; β=−0.55, p=0.04), 20:0 (β=−6.73, p=0.01) and 24:1 (β=−10.7, p=0.04) also correlated inversely with serum TBARS. Taken together, some ceramide/dihydroceramide may affect hepatic mitochondrial function, but without evidence for enhancing lipid peroxidation.
Finally, ceramides could also induce insulin resistance by activating inflammatory pathways, triggered by toll-like receptor 4 recognition of saturated fatty acids and subsequently increased ceramide biosynthesis.23 24 Thus, we hypothesized that increased ceramide/dihydroceramide ratios would relate to increased levels of biomarkers of inflammation and possibly to lower circulating FFA, as a measure of inhibited lipolysis. In contrast, serum ceramide/dihydroceramide 20:0 related with lower systemic inflammation, as measured by IL-6 (β=−3.94, p=0.04), IL-1ra (β=−7.78, p=0.0001) and high-sensitivity C reactive protein (hsCRP; β=3.49, p=0.01). Total ceramide/dihydroceramide (β=−21.8, p=0.003), 20:0, 22:0 and 24:1 correlated similarly with IL-1ra (β=−11.2, p=0.03; β=−9.17, p=0.003 and β=−11.6, p=0.001, respectively) as well as ceramide/dihydroceramide 20:0 and 24:1 with hsCRP (β=−3.49, p=0.01 and β=−6.15, p=0.03, respectively). Interestingly, ceramide/dihydroceramide 16:0 in visceral adipose tissue related positively to IL-6 (figure 3H) and IL-1ra (β=5.1, p=0.006), supporting the concept of tissue-specific roles of these ratios.
Discussion
Given that circulating dihydroceramides are specifically increased in NASH despite comparable concentrations of total ceramides,11 the observation of lower hepatic ceramide/dihydroceramide ratios may rather reflect higher dihydroceramide production from de novo ceramide synthesis due to increased dietary intake of saturated lipids in obesity. Also, hepatic particularly dihydroceramides associated with whole-body insulin resistance in the same cohort11 and predict diabetes up to 9 years prior to its clinical diagnosis.19 This may also suggest that the increased dihydroceramide levels reflect an increased flow of fatty acids through the sphingolipid pathway in individuals with NAFLD or insulin resistance. Dihydroceramides could regulate oxidative stress, cell proliferation and apoptosis as well as mitochondrial function,25 26 which is underlined by their association with hepatic inflammation and oxidative stress in humans.11 Moreover, a recent study found increases in several hepatic ceramide and sphingomyelin species in a murine NASH model along with upregulation of ceramide synthases and DES-2, but not DES-1.22 This finding casts doubt on a key role of DES-1 for the pathogenesis of NAFLD not only in humans—as reported here—but also in mice.14
Strengths of this analysis are the translation of well-designed mouse model to humans, because these models may not reflect human pathology, as well as the inclusion of a broad range of human phenotypes including a lean healthy group. Although our cross-sectional study design does not allow direct conclusions as to causality, our analysis yielded novel observations regarding associations with insulin resistance and hepatic steatosis, which can be useful for designing prospective studies. We acknowledge that the ceramide-to-dihydroceramide ratio is only indicative and may not be an appropriate surrogate marker of DES-1 activity in vivo, which we did not directly measure. However, this analysis did not aim to investigate the therapeutic relevance of any enzyme inhibition.
In conclusion, the present analyses suggest that in obesity and during NAFLD progression to NASH, enhanced de novo ceramide synthesis may lead to dihydroceramide accumulation and in turn decreased ceramide/dihydroceramide ratios. In contrast to DES-1-deficient mice, the decreased ceramide/dihydroceramide ratios lead to lower insulin sensitivity or intrahepatic fat accumulation. Nevertheless, the negative relationship of certain hepatic ceramide/dihydroceramide ratios with hepatic mitochondrial function could result in enhanced lipid oxidation, thereby preventing further deterioration of insulin sensitivity. Thus, whether DES-1 is a good drug target or not is difficult to answer. Finally, these data underline the importance of future studies on tissue-specific differences of specific sphingolipid species in humans.
Acknowledgments
We would like to thank all volunteers, Dr Chrysi Koliaki and Dr Julia Szendroedi, PhD, for contributing to the clinical studies, Kai Tinnes, Myrko Esser and Ulrike Partke for their excellent technical support. MR is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Contributors: MR initiated the investigation, designed and lead the clinical experiments and wrote the manuscript; MA obtained and analyzed data and wrote the manuscript; PES led the sphingolipid measurements; RG performed and analyzed the sphingolipid measurements; SG performed the clinical experiments; KS performed statistical analysis; MS obtained tissue samples; IE performed histological examinations; CH analyzed inflammatory markers. All authors reviewed and edited the manuscript and gave final approval of the version to be published.
Funding: This study was supported in part by the Ministry of Science and Research of the State of North Rhine-Westfalia (MIWF NRW), the German Federal Ministry of Health (BMG) as well as by a grant of the Federal Ministry for Research (BMBF) to the German Center for Diabetes Research (DZD e.V.), the German Research Foundation (DFG, CRC 1116/2), German Diabetes Association (DDG), the Schmutzler-Stiftung as well as funds from the Touchstone Diabetes Center at UT Southwestern Medical Center (to PES).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study was conducted after the approval of the ethics board of Heinrich Heine University Düsseldorf (reference number 3516) and has been performed in accordance with the ethical standards as set down in the 1964 Declaration of Helsinki and its last amendments of 2013 or comparable ethical standards.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. The data sets generated during and/or analyzed during the current study are not publicly available, since they are subject to national data protection laws and restrictions imposed by the ethics committee to ensure data privacy of the study participants. However, they can be applied for through an individual project agreement with the principal investigator of the German Diabetes Study.
References
- 1.Xia JY, Holland WL, Kusminski CM, et al. Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Cell Metab 2015;22:266–78. 10.1016/j.cmet.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen MC, Shulman GI. Roles of diacylglycerols and ceramides in hepatic insulin resistance. Trends Pharmacol Sci 2017;38:649–65. 10.1016/j.tips.2017.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gancheva S, Jelenik T, Álvarez-Hernández E, et al. Interorgan metabolic crosstalk in human insulin resistance. Physiol Rev 2018;98:1371–415. 10.1152/physrev.00015.2017 [DOI] [PubMed] [Google Scholar]
- 4.Lemaitre RN, Yu C, Hoofnagle A, et al. Circulating sphingolipids, insulin, HOMA-IR, and HOMA-B: the strong heart family study. Diabetes 2018;67:1663–72. 10.2337/db17-1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Summers SA, Goodpaster BH. Crosstalk proposal: intramyocellular ceramide accumulation does modulate insulin resistance. J Physiol 2016;594:3167–70. 10.1113/JP271676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tilg H, Moschen AR, Roden M. Nafld and diabetes mellitus. Nat Rev Gastroenterol Hepatol 2017;14:32–42. 10.1038/nrgastro.2016.147 [DOI] [PubMed] [Google Scholar]
- 7.Magkos F, Su X, Bradley D, et al. Intrahepatic diacylglycerol content is associated with hepatic insulin resistance in obese subjects. Gastroenterology 2012;142:e1442:1444–6. 10.1053/j.gastro.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luukkonen PK, Zhou Y, Sädevirta S, et al. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J Hepatol 2016;64:1167–75. 10.1016/j.jhep.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 9.Ter Horst KW, Gilijamse PW, Versteeg RI, et al. Hepatic Diacylglycerol-Associated protein kinase Cε translocation links hepatic steatosis to hepatic insulin resistance in humans. Cell Rep 2017;19:1997–2004. 10.1016/j.celrep.2017.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruby MA, Massart J, Hunerdosse DM, et al. Human carboxylesterase 2 reverses obesity-induced diacylglycerol accumulation and glucose intolerance. Cell Rep 2017;18:636–46. 10.1016/j.celrep.2016.12.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apostolopoulou M, Gordillo R, Koliaki C, et al. Specific hepatic sphingolipids relate to insulin resistance, oxidative stress, and inflammation in nonalcoholic steatohepatitis. Diabetes Care 2018;41:1235–43. 10.2337/dc17-1318 [DOI] [PubMed] [Google Scholar]
- 12.Koliaki C, Szendroedi J, Kaul K, et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab 2015;21:739–46. 10.1016/j.cmet.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 13.Turpin-Nolan SM, Brüning JC. The role of ceramides in metabolic disorders: when size and localization matters. Nat Rev Endocrinol 2020;16:224–33. 10.1038/s41574-020-0320-5 [DOI] [PubMed] [Google Scholar]
- 14.Somm E, Montandon SA, Loizides-Mangold U, et al. The GLP-1R agonist liraglutide limits hepatic lipotoxicity and inflammatory response in mice fed a methionine-choline deficient diet. Transl Res 2020. 10.1016/j.trsl.2020.07.008. [Epub ahead of print: 22 Jul 2020]. [DOI] [PubMed] [Google Scholar]
- 15.Chaurasia B, Tippetts TS, Mayoral Monibas R, et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science 2019;365:386–92. 10.1126/science.aav3722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammerschmidt P, Ostkotte D, Nolte H, et al. CerS6-Derived sphingolipids interact with Mff and promote mitochondrial fragmentation in obesity. Cell 2019;177:e1523:1536–52. 10.1016/j.cell.2019.05.008 [DOI] [PubMed] [Google Scholar]
- 17.Samuel VT, Shulman GI. Nonalcoholic fatty liver disease, insulin resistance, and ceramides. N Engl J Med 2019;381:1866–9. 10.1056/NEJMcibr1910023 [DOI] [PubMed] [Google Scholar]
- 18.Kusminski CM, Scherer PE. Lowering ceramides to overcome diabetes. Science 2019;365:319–20. 10.1126/science.aax6594 [DOI] [PubMed] [Google Scholar]
- 19.Wigger L, Cruciani-Guglielmacci C, Nicolas A, et al. Plasma Dihydroceramides are diabetes susceptibility biomarker candidates in mice and humans. Cell Rep 2017;18:2269–79. 10.1016/j.celrep.2017.02.019 [DOI] [PubMed] [Google Scholar]
- 20.Mamtani M, Kulkarni H, Dyer TD, et al. Waist circumference is genetically correlated with incident type 2 diabetes in Mexican-American families. Diabet Med 2014;31:31–5. 10.1111/dme.12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poss AM, Maschek JA, Cox JE, et al. Machine learning reveals serum sphingolipids as cholesterol-independent biomarkers of coronary artery disease. J Clin Invest 2020;130:1363–76. 10.1172/JCI131838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raichur S, Brunner B, Bielohuby M, et al. The role of C16:0 ceramide in the development of obesity and type 2 diabetes: CerS6 inhibition as a novel therapeutic approach. Mol Metab 2019;21:36–50. 10.1016/j.molmet.2018.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holland WL, Bikman BT, Wang L-P, et al. Lipid-Induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest 2011;121:1858–70. 10.1172/JCI43378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chavez JA, Knotts TA, Wang L-P, et al. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem 2003;278:10297–303. 10.1074/jbc.M212307200 [DOI] [PubMed] [Google Scholar]
- 25.Barbarroja N, Rodriguez-Cuenca S, Nygren H, et al. Increased dihydroceramide/ceramide ratio mediated by defective expression of degs1 impairs adipocyte differentiation and function. Diabetes 2015;64:1180–92. 10.2337/db14-0359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Cuenca S, Barbarroja N, Vidal-Puig A. Dihydroceramide desaturase 1, the gatekeeper of ceramide induced lipotoxicity. Biochim Biophys Acta 2015;1851:40–50. 10.1016/j.bbalip.2014.09.021 [DOI] [PubMed] [Google Scholar]