Abstract
Objectives:
Major depressive disorder is a leading heterogeneous psychiatric illness manifested by persistent low mood, a feeling of sadness, and diminished interest in daily activities. Many biological, genetic, and social factors are thought to be linked with depression. But any suitable early risk assessment markers are absent for this illness. Therefore, we aimed to investigate the serum levels of IFN-γ in major depressive disorder patients to further investigate the association between serum levels of this cytokine and major depression.
Methods:
This prospective case-control study enrolled 120 major depressive disorder patients and 100 healthy controls matched by age, sex, and body mass index. A qualified psychiatrist diagnosed the major depressive disorder patients and evaluated healthy controls according to the Diagnostic and Statistical Manual of Mental Health Disorders (5th ed.; DSM-5). The Hamilton depression rating scale was applied for all the study participants to measure the severity of depression. Serum IFN-γ levels were measured by a commercially available enzyme-linked immunosorbent assay kit (Boster Biological Technology, Pleasanton, CA, USA).
Results:
This study observed that serum IFN-γ levels were significantly decreased in major depressive disorder patients compared to healthy controls. A significant negative correlation (r = −0.375; p < 0.001) was obtained between serum IFN-γ levels and Hamilton depression scores. Receiver operating characteristic analysis showed good diagnostic performance of lowered serum IFN-γ levels in depression with an area under the curve at 0.790.
Conclusion:
We suggest the altered serum IFN-γ levels are associated with the pathophysiology of depression. The reduced levels of serum IFN-γ might be used as an early risk assessment tool for major depression.
Keywords: Interferon-gamma, IFN-γ, major depressive disorder, major depressive disorder, serum, Bangladesh
Introduction
Major depressive disorder (MDD) is a heterogeneous, enervating psychiatric illness that persists for at least 2 weeks accompanied by depressed mood, loss of interest, and diminished thoughts in daily activities.1,2 The emergence of five or more symptoms mentioned in the Diagnostic and Statistical Manual of Mental Disorders (5th ed.; DSM-5) during the same 2 weeks is a salient feature in the diagnosis of MDD.2 Although the duration of these features may surprisingly fluctuate between weeks to even years.3 Globally, 4.4% of the total population is suffering from depressive disorders.4 MDD is predicted to become the leading cause of disability worldwide by 2030.5 Also, it has increased the risk of developing psychosocial and psychophysical disability along with the obesity, type 2 diabetes mellitus, cardiac disease, autoimmune diseases, neurodegenerative disorders, cancer, and intestinal disorders.6–10 Unfortunately, MDD patient has 20-fold greater tendency to suicide than others, and it is estimated about 50% of the 800,000 suicides per year across the world.11,12 In Bangladesh, the prevalence of psychiatric illness is about 16.05% among the adult population of which 28.7% suffer from MDD.13
To understand the exact pathophysiology of MDD, several hypotheses have been formulated including alterations in monoaminergic neurotransmission, imbalance of excitatory and inhibitory signaling in the brain, hyperactivity of the hypothalamic–pituitary–adrenal (HPA) axis, inflammation, and abnormalities in normal neurogenesis.14–18 The inflammatory hypothesis of depression suggested that hyperactivation in the immune system and uncontrolled cytokine production is involved in the pathogenesis of depression.19 Cytokines are cell signal–transducing proteins or polypeptides that produce and regulate immune responses and inflammation.20,21 They can cross the blood–brain barrier,22 thereby developing many aspects of MDD pathophysiology including neurotransmitter metabolism, neuroendocrine function, and neural plasticity.23 MDD is accompanied by significant alterations in cell-mediated inflammatory and immune responses, and these changes may be associated with the pathophysiology or pathogenesis of the illness, yet the data showed inconsistent outcomes in many recent studies.24–27 The pro-inflammatory cytokines have played a crucial role in the pathophysiology of psychiatric illnesses such as MDD.23 Pro-inflammatory cytokines and neuroinflammation are very important in producing inflammatory responses as well as in neurogenesis and neuroprotection. Persistent stress and subsequent secretion of pro-inflammatory cytokines lead to chronic inflammation that contributes to developing depression.28
IFN-γ is a pleiotropic soluble pro-inflammatory cytokine produced by immune cells such as lymphocytes, cytotoxic lymphocytes, B cells, and antigen-presenting cells which orchestrates cellular programs via transcriptional and translational gene control mechanisms.29,30 IFN-γ is one of the major effectors of both innate and adaptive immune systems that have recently been postulated to play a crucial role in the pathophysiology of depression.31 Pro-inflammatory cytokine IFN-γ is mainly produced by natural killer (NK) cells and CD4+ T cells, but macrophages have also been revealed to secrete IFN-γ; the cellular source of IFN-γ in that study remains to be elucidated.32 The imbalance between serum IFN-γ levels in MDD patients and healthy controls (HCs) can be explained by several mechanisms of psychoneuroimmunology. Maes and colleagues14,33 first showed that activation of the inflammatory response system (IRS) in depression leading to HPA hyperactivity, indicating that pro-inflammatory cytokines induce HPA hyperactivity in depression. It has been demonstrated that patients with depression show hyperactivity of the HPA and a subsequent increase in cortisol, which in turn alters the production of cytokines.34–37 Accordingly, cytokines and monocytes can cross the blood–brain barrier, influencing behavior.38,39 All these findings recommend that there may be a neuro–immuno–endocrinological interaction, and hence, whatever disturbance in this interaction could be related to the psychiatric symptoms directly or indirectly.40 There is evidence that depression will affect the defensive mechanisms of the immune system by activating or interacting with cytokines.41
Despite extensive investigations, yet the pathophysiology of MDD is not clearly known. At present, the diagnosis of MDD mainly depends on patients’ self-report, clinical examination, and subjective evaluation of depressive symptoms. There are no established quantitative laboratory measurements are available to identify MDD. Based on the available evidence, the exploration of biological markers will be supportive in diagnosing, suggesting treatment, and in the management of MDD.42 Thus, this study aimed to observe the association between peripheral IFN-γ levels and major depression among the Bangladeshi population.
Methods
Study population
It was assumed the percentage exposed among controls and alpha risk will be 10% and 5%, respectively. This 1:1 matched case-control study intended to detect minimum odds ratio 2 with power 90%. Based on the above estimations, the theoretical sample size was 252 (126 cases and 126 controls).43 Therefore, this prospective case-control study recruited 120 MDD patients from the Department of Psychiatry, Dhaka Medical College and Hospital, Dhaka, Bangladesh. One hundred HCs were also enrolled from different areas of Dhaka city matched by age, sex, and body mass index (BMI). A qualified psychiatrist who is trained in the use of DSM-5 conducted the diagnosis of MDD patients and the evaluation of HCs. The duration of sample collection was 6 months from 10 August 2019 to 10 February 2020. The Hamilton depression (Ham-D) rating scale was used to quantify the severity of depression. Drug-naïve MDD patients with age range 18–60 years and BMI within 16–34 kg/m2 having a Ham-D score ⩾7 were included in this investigation. All the participants were carefully examined to observe any the previous history of illness, performed clinical tests for the complete evaluation, and evaluated the similar symptoms for differential diagnosis.44 Subjects with acute infections, neurological or immunological disorders, substance abuse, bipolar depression, panic disorder, and other comorbid psychiatric illnesses were excluded from this study. A structured questionnaire was developed to record the sociodemographic profiles of the study participants. Before data collection, a pilot test among 20 MDD patients was conducted, and finally, this pilot samples were excluded from the study. We used this questionnaire only in this study as a pilot (see the Supplemental Material).
Sample collection and storage
5 mL of the blood sample was withdrawn from the cephalic vein of each participant using a plastic syringe built-in with a stainless steel needle. The collected blood samples were kept in a falcon tube and allowed to clot at room temperature for an hour. The clotted samples were centrifuged to extract serum samples at 3000 r/min for 15 min. The serum samples were carefully separated from blood samples and stored at −80°C until further analysis.
Measurements of serum IFN-γ levels
The serum samples were analyzed for IFN-γ levels using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Boster Biological Technology, Pleasanton, CA, USA; Catalog no. EK0373) according to the manufacturer’s instructions. Each well in micro-plates was coated with antibodies directed toward an antigenic site of an IFN-γ molecule. The standard calibration curve of IFN-γ was constructed using different standard concentrations (15.63, 31.25, 62.50, 125, 250, 500, and 1000 pg/mL). The concentrations of serum IFN-γ were expressed as pg/mL, and the sensitivity of this assay was <2 pg/mL. All assays were carried out by the same persons to avoid inter-assay variations. The investigators who performed the assays were blind to the clinical data of the samples.
Statistical analysis
All the statistical analyses were performed using statistical package for social sciences (SPSS) software, version 25.0 (IBM Corp., Armonk, NY, USA). We performed an independent sample t-test for non-categorical variables and Fisher’s exact test for categorical variables to find the group variances. Pearson’s correlation test was applied to find out the correlation between serum IFN-γ levels and Ham-D scores in MDD patients. Changes of serum IFN-γ levels between the MDD patients and HCs were presented by the Box plot graphs. A scatter plot graph was used to illustrate the correlation between serum IFN-γ levels and Ham-D scores in MDD patients. We presented data as mean values ± standard error of mean (SEM). Finally, receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic performance of the target cytokine and its accuracy to distinguish MDD patients and HCs.
Results
Study population and demographic data
The study population were classified according to their biophysical and sociodemographic characteristics. The demographic data of the study population are presented in Table 1. It was observed that patients with MDD and their corresponding HCs were similar in terms of age (patients: 32.19 ± 0.88; controls: 33.20 ± 0.89), BMI (patients: 25.28 ± 0.47; controls: 24.58 ± 0.35), sex (female/male: 67/53; female/male: 55/45 patients and controls, respectively), and smoking history (patients/controls: 77%/76%). Females constituted a higher percentage in both groups (56% and 55%, respectively). We found the BMI values were in the normal range for 52% of patients and 57% of HCs. Most of the MDD patients were literate (87.5%), non-smoker (77%), and low economic status (60%). People living in rural areas (58%) had a greater tendency to develop major depression compared to urban people.
Table 1.
Sociodemographic characteristics of the study population.
| Characteristics | MDD patients (n = 120) Mean ± SEM | Healthy controls (n = 100) Mean ± SEM | p-value |
|---|---|---|---|
| Age in years | 32.19 ± 0.88 | 33.20 ± 0.89 | 0.426 |
| 18–24 | 29 (24%) | 9 (9%) | |
| 25–34 | 44 (37%) | 49 (49%) | |
| 35–44 | 32 (27%) | 25 (25%) | |
| 45–60 | 15 (12%) | 17 (17%) | |
| Sex | 0.572 | ||
| Male | 53 (44%) | 45 (45%) | |
| Female | 67 (56%) | 55 (55%) | |
| Marital status | 0.967 | ||
| Married | 72 (60%) | 60 (60%) | |
| Unmarried | 48 (40%) | 40 (40%) | |
| BMI (kg/m2) | 25.28 ± 0.47 | 24.58 ± 0.35 | 0.244 |
| Below 18.5 (CED) | 6 (5%) | 2 (2%) | |
| 18.5–25 (normal) | 62 (52%) | 57 (57%) | |
| Above 25 (obese) | 52 (43%) | 41 (41%) | |
| Education level | 0.092 | ||
| Illiterate | 15 (12.5%) | 8 (8%) | |
| Primary level | 23 (19.2%) | 3 (3%) | |
| Secondary level | 57 (47.5%) | 50 (50%) | |
| Graduate and above | 25 (20.8%) | 39 (39%) | |
| Monthly income (KBDT) | 67.39 ± 3.02 | 79.62 ± 4.54 | 0.143 |
| Below 30 | 6 (5%) | 19 (19%) | |
| 30–60 | 53 (44%) | 32 (32%) | |
| 61–90 | 39 (33%) | 25 (25%) | |
| Above 90 | 22 (18%) | 24 (24%) | |
| Occupation | 0.146 | ||
| Service | 15 (12%) | 13 (13%) | |
| Business | 4 (4%) | 19 (19%) | |
| Student | 4 (4%) | 2 (2%) | |
| Jobless | 14 (11%) | 21 (21%) | |
| Others | 83 (69%) | 45 (45%) | |
| Economic status | 0.107 | ||
| Low | 73 (60%) | 59 (59%) | |
| Medium | 33 (28%) | 28 (28%) | |
| High | 14 (12%) | 13 (13%) | |
| Tobacco user | 0.643 | ||
| Yes | 28 (23%) | 24 (24%) | |
| No | 92 (77%) | 76 (76%) | |
| Area of residence | 0.653 | ||
| Rural | 70 (58%) | 70 (70%) | |
| Urban | 50 (42%) | 30 (30%) |
BMI: body mass index; CED: chronic energy deficiency; KBDT: kilo Bangladeshi taka; MDD: major depressive disorder; SEM: standard error mean.
Comparison of serum IFN-γ levels among the study population
The mean serum concentrations of IFN-γ were 3.85 ± 0.31 pg/mL for MDD patients and 8.95 ± 1.07 pg/mL for HCs (Figure 1). A significant decrease in serum IFN-γ levels was observed in MDD patients compared to HCs. For sex-specific comparison, females with depression or without depression showed lower serum IFN-γ levels compared to males among the study population. Moreover, significantly lower levels of serum IFN-γ were noticed in both male (4.22 ± 0.55) and female (3.60 ± 0.38) of MDD patients when compared with male (10.75 ± 1.61) and female (7.49 ± 1.45) in HCs, respectively (Table 2).
Figure 1.
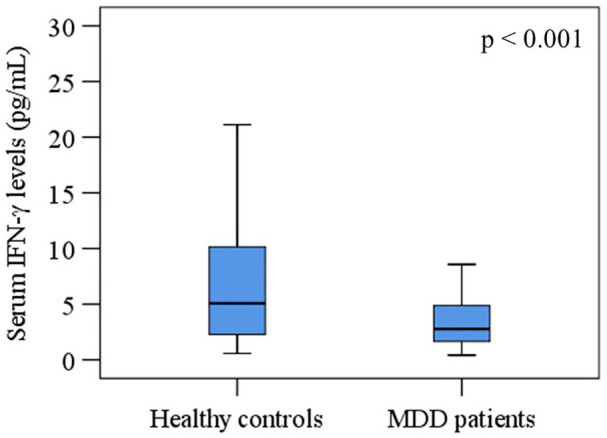
Variations of serum interferon-gamma (IFN-γ) among the study population. A significant difference between patient and control groups at a 95% confidence interval (CI).
Table 2.
Clinical information and laboratory findings of the study population.
| Parameters | MDD patients (n = 120) Mean ± SEM | Healthy controls (n = 100) Mean ± SEM | p-value |
|---|---|---|---|
| Ham-D score | 17.02 ± 0.32 | 3.50 ± 0.24 | <0.001 |
| Serum INF-γ level (pg/mL) | 3.85 ± 0.31 | 8.95 ± 1.07 | <0.001 |
| INF-γ in male (pg/mL) | 4.22 ± 0.55 | 10.75 ± 1.61 | <0.001 |
| INF-γ in female (pg/mL) | 3.60 ± 0.38 | 7.49 ± 1.45 | <0.001 |
Ham-D: 17-item Hamilton depression rating scale; INF-γ: interferon-gamma; KBDT: kilo Bangladeshi taka; MDD: major depressive disorder; SEM: standard error mean.
Correlation between various research parameters of study participants
Spearman’s correlation test was performed to find the relationship between serum IFN-γ levels and Ham-D scores. A significant negative correlation was obtained between serum IFN-γ levels and Ham-D scores in MDD patients (r = −0.375, p < 0.001). We observed no significant associations between reduced serum IFN-γ levels with the sociodemographic profile of MDD patients (p > 0.05). A scatter plot graph shows that individuals with greater Ham-D scores and lower serum IFN-γ levels are mostly females (Figure 2).
Figure 2.
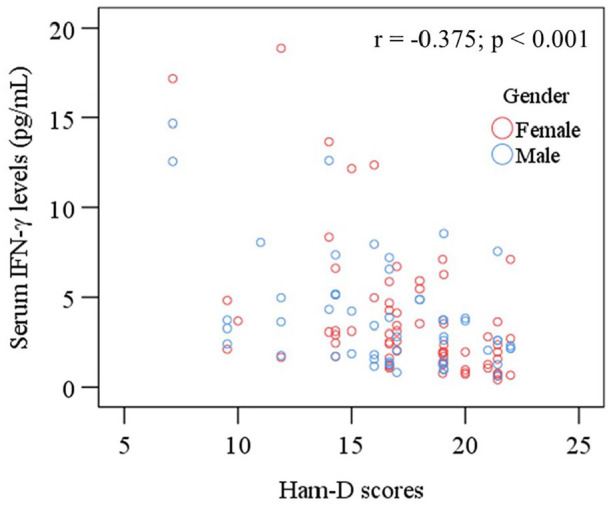
Scatter plot of serum interferon-gamma (IFN-γ) and Ham-D scores with sex differences among MDD patients.
Diagnostic performance evaluation of serum IFN-γ levels in MDD
The ROC curve of IFN-γ was plotted and the cut-off point for the diagnostic measure was determined as 1.49 pg/mL (Figure 3). The area under the ROC curve (AUC) was 0.790 which was significant (p < 0.05). Lower values were assigned as the disease condition. ROC analysis revealed that the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were 83.3%, 80.8%, 75.2%, and 76.4%, respectively.
Figure 3.
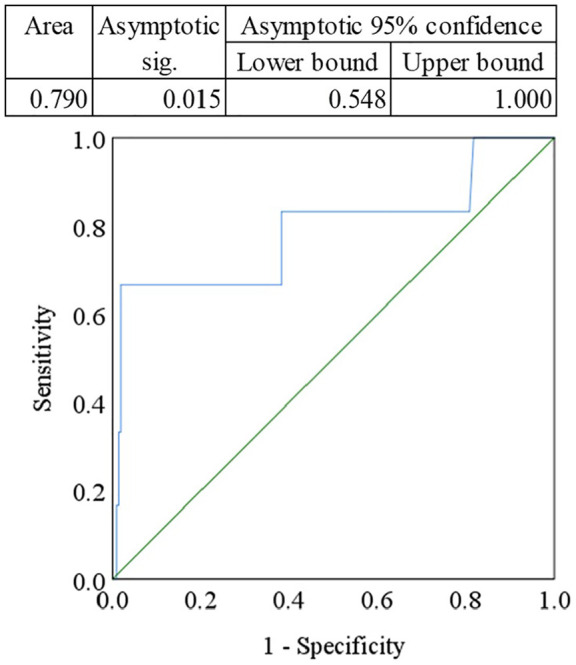
Receiver operating characteristic (ROC) curve for serum interferon-gamma (IFN-γ). The cut-off point was detected as 1.49 pg/mL.
Discussion
Numerous investigations especially on pro-inflammatory markers have been carried out over the last few years to establish an association between these markers and depression. Some pro-inflammatory cytokines have shown their role in the pathophysiology of depression but many studies reported inconsistent findings.44,45 Cytokine profiling in MDD patients is the most common measurement of immune biomarkers, and they are used to assess the treatment responses.46–49 In the current investigation, we found significantly decreased levels of serum IFN-γ in MDD patients compared to HCs. We also observed that the reduced levels of serum IFN-γ are negatively associated with the severity of depression. This decreased serum IFN-γ levels may contribute to activating the immune response in major depression. This study’s findings of altered serum IFN-γ levels in MDD patients can be explained by several psychoneuroimmunology mechanisms. Maes et al.14,33 first showed that activation of the IRS in depression leading to HPA hyperactivity, indicating that pro-inflammatory cytokines induce HPA hyperactivity in depression. Also, cytokine stimulation activates NF-κB; the signal transducer and activator of transcription (STAT) protein family of transcription factors is activated by cytokine receptor signaling to keep a balance between M1 and M2 macrophage activation. IFN-γ is produced by Th1 cells, binding with the type II IFN receptor to stimulate the Stat1 activation. Stat1 and NF-κB then combine to induce M1 macrophage activation.50,51 Alternatively, interleukin (IL)-4 and IL-13 produced by the Th2 cytokines stimulate Stat6 activation that exerts the M2-related gene expression, and the anti-inflammatory cytokine IL-10 is synthesized by M2 macrophages that stimulates Stat3 activation and inhibits the M1-related gene activation. Thus, pro-inflammatory cytokine IFN-γ has been implicated in the development of MDD.50,52
The results of this study are consistent with several past studies where serum IFN-γ level was found significantly lower in depression.53–55 Pallavi et al.55 observed lowered level of IFN-γ in MDD patients compared to HCs. Moreover, we found significantly lower levels of IFN-γ in both female and male MDD patients compared to female and male HCs, respectively. A recent meta-analysis by Köhler et al.54 extracted the data of IFN-γ from 17 investigations and obtained reduced levels of serum IFN-γ in MDD patients compared to HCs. Another review by Himmerich et al.55 reported the reduced IFN-γ levels in patients with MDD compared to HCs which is consistent with this study.
In addition, we conducted an ROC curve analysis to determine the diagnostic performance of reduced serum IFN-γ levels in MDD patients compared with HCs. Accuracy of a marker evaluated by the AUC in ROC analysis is as follows: 0.90–1.0 = excellent, 0.80–0.90 = good, 0.70–0.80 = fair, and 0.60–0.70 = poor and less than 0.6 = not useful.56 We observed a noteworthy finding from this analysis where the AUC was 0.790 which was considered significant as p < 0.05, lower values were assigned as the disease condition. According to the ROC analysis, the sensitivity, specificity, PPV, and NPV were 83.3%, 80.8%, 75.2%, and 76.4%, respectively, for the reduced serum IFN-γ levels in depression.
Although the current investigation sought to be comprehensive, there were some limitations to be considered. The main limitation was the single measurement of serum IFN-γ in MDD patients may not be able to explore the whole inflammatory process in the central nervous system. This was a case-control study that itself is a limitation. We did not consider the effect of the food habits of the study participant in our study parameter. The better-clarified result would be found if we could evaluate the multiple pro-inflammatory cytokines in a longitudinal study among a more homogeneous study population by strictly controlling the confounding factors. Despite having the above limitations, this study has some substantial advantages. To the best of our knowledge, this is the first-ever study carried out in Bangladesh with such a large sample where age and sex were strictly matched for cases and controls. Furthermore, this study enrolled MDD patients from a leading tertiary care teaching hospital, so homogeneity and diversification were obtained for this study. Also, this study performed a gender-specific investigation of serum cytokine levels. Finally, we simultaneously investigated the serum IFN-γ levels in both MDD patients and HCs under the same experimental settings.
Conclusion
This study suggests that the depleted levels of IFN-γ are involved in the pathophysiology of depression. Thus, the reduced serum levels of IFN-γ might be served as an early risk assessment marker for MDD. The decreased serum levels, the correlation with the severity of the disease, and the diagnostic value support IFN-γ as a promising marker, but still, some controversial factors understanding the actual pathophysiology of depression. Therefore, further studies are recommended with a large and more homogeneous study population to explore the exact role of IFN-γ in the pathophysiology of depression.
Supplemental Material
Supplemental material, sj-pdf-1-smo-10.1177_2050312120974169 for Serum interferon-gamma level is associated with drug-naïve major depressive disorder by Sohel Daria, Maliha Afrin Proma, Mohammad Shahriar, Sardar Mohammad Ashraful Islam, Mohiuddin Ahmed Bhuiyan and Md. Rabiul Islam in SAGE Open Medicine
Acknowledgments
The authors are thankful to the physicians and staff at the Department of Psychiatry, Dhaka Medical College and Hospital for their support in data collection.
Footnotes
Author contributions: S.D. and M.A.P. conceived, designed, and conducted the experiment, performed analyses, and wrote the initial draft of the manuscript. M.S., S.M.A.I., and M.A.B. analyzed the experiment, wrote, and revised the manuscript. M.R.I. supervised the whole work, performed computational analyses, gave important intellectual content in the manuscript, edited the data, and revised the manuscript. Finally, all authors carefully read the manuscript and approved the final version to submit.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: The study was conducted according to the World Medical Association Declaration of Helsinki. All study participants gave written consent to participate in this study. The study protocol was approved by the ethical review committee of the Department of Psychiatry, Dhaka Medical College and Hospital, Dhaka, Bangladesh. Approval no. MEU-DMC/ECC/2019/125.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was self-funded.
Informed consent: We obtained written consent from each participant after a well brief to them about the purpose of the study. The legally authorized primary caregiver gave the sign on behalf of patients if independent thinking capacity was suspected.
ORCID iD: Md. Rabiul Islam  https://orcid.org/0000-0003-2820-3144
https://orcid.org/0000-0003-2820-3144
Availability of data: Data will be available upon reasonable request from the corresponding author.
Supplemental material: Supplemental material for this article is available online.
References
- 1. Böttcher C, Fernández-Zapata C, Snijders GJL, et al. Single-cell mass cytometry of microglia in major depressive disorder reveals a non-inflammatory phenotype with increased homeostatic marker expression. Transl Psychiatry 2020; 10(1): 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Psychiatric Association. Diagnostic statistical manual of mental disorders, 5th ed. Arlington, VA: American Psychiatric Association, 2013. [Google Scholar]
- 3. American Psychiatric Association. Aripiprazole (Abilify): depression, major depressive disorder (MDD). Ottawa (ON): Canadian Agency for Drugs and Technologies in Health, 2016, https://www.ncbi.nlm.nih.gov/books/NBK409752/ (accessed 20 July 2020). [PubMed] [Google Scholar]
- 4. Depression: let’s talk. The world health organization report, 2017, https://www.who.int/news-room/detail/30-03-2017–depression-let-s-talk-says-who-as-depression-tops-list-of-causes-of-ill-health (accessed 14 July 2020).
- 5. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006; 3(11): e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levitan RD, Davis C, Kaplan AS, et al. Obesity comorbidity in unipolar major depressive disorder: refining the core phenotype. J Clin Psychiatry 2012; 73(8): 1119–1124. [DOI] [PubMed] [Google Scholar]
- 7. Katon WJ. The comorbidity of diabetes mellitus and depression. Am J Med 2008; 121(11 Suppl. 2): S8–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Halaris A. Comorbidity between depression and cardiovascular disease. Int Angiol 2009; 28(2): 92–99. [PubMed] [Google Scholar]
- 9. Kayser MS, Dalmau J. The emerging link between autoimmune disorders and neuropsychiatric disease. J Neuropsychiatry Clin Neurosci 2011; 23(1): 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brintzenhofe-Szoc KM, Levin TT, Li Y, et al. Mixed anxiety/depression symptoms in a large cancer cohort: prevalence by cancer type. Psychosomatics 2009; 50(4): 383–391. [DOI] [PubMed] [Google Scholar]
- 11. Suicide: the world health organization report, 2016, http://www.who.int/topics/suicide/en/ (accessed 14 July 2020).
- 12. Chesney E, Goodwin GM, Fazel S. Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry 2014; 13(2): 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Islam MR, Shafique A. Prescribing practice of antidepressant drugs at outpatient department of a tertiary care teaching hospital in Bangladesh. Open J Depress 2017; 6(1): 61002. [Google Scholar]
- 14. Maes M, Bosmans E, Meltzer HY, et al. Interleukin-1 beta: a putative mediator of HPA axis hyperactivity in major depression. Am J Psychiatry 1993; 150(8): 1189–1193. [DOI] [PubMed] [Google Scholar]
- 15. Varghese FP, Brown ES. The hypothalamic-pituitary-adrenal axis in major depressive disorder: a brief primer for primary care physicians. Prim Care Companion J Clin Psychiatry 2001; 3(4): 151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Islam MR, Ali S, Karmoker JR, et al. Evaluation of serum amino acids and non-enzymatic antioxidants in drug-naïve first-episode major depressive disorder. BMC Psychiatry 2020; 20(1): 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Milenkovic VM, Stanton EH, Nothdurfter C, et al. The role of chemokines in the pathophysiology of major depressive disorder. Int J Mol Sci 2019; 20(9): 2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Emon MPZ, Das R, Nishuty NL, et al. Reduced serum BDNF levels are associated with the increased risk for developing MDD: a case-control study with or without antidepressant therapy. BMC Res Notes 2020; 13(1): 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies. Neurosci Biobehav Rev 2005; 29(4–5): 891–909. [DOI] [PubMed] [Google Scholar]
- 20. Murray RZ, Stow JL. Cytokine secretion in macrophages: SNAREs, rabs, and membrane trafficking. Front Immunol 2014; 5: 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferreira VL, Borba HHL, de F Bonetti A, et al. Cytokines and interferons: types and functions. Open Access. Epub ahead of print 13 March 2018. DOI: 10.5772/intechopen.74550. [DOI] [Google Scholar]
- 22. Yarlagadda A, Alfson E, Clayton AH. The blood brain barrier and the role of cytokines in neuropsychiatry. Psychiatry 2009; 6(11): 18–22. [PMC free article] [PubMed] [Google Scholar]
- 23. Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 2009; 65(9): 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Levada OA, Troyan AS. Poststroke depression biomarkers: a narrative review. Front Neurol 2018; 9: 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Elomaa AP, Niskanen L, Herzig KH, et al. Elevated levels of serum IL-5 are associated with an increased likelihood of major depressive disorder. BMC Psychiatry 2012; 12: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kakeda S, Watanabe K, Katsuki A, et al. Relationship between interleukin (IL)-6 and brain morphology in drug-naïve, first-episode major depressive disorder using surface-based morphometry. Sci Rep 2018; 8(1): 10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee CH, Giuliani F. The role of inflammation in depression and fatigue. Front Immunol 2019; 10: 1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim YK, Na KS, Myint AM, et al. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog Neuropsychopharmacol Biol Psychiatry 2016; 64: 277–284. [DOI] [PubMed] [Google Scholar]
- 29. Schroder K, Hertzog PJ, Ravasi T, et al. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol 2004; 75(2): 163–189. [DOI] [PubMed] [Google Scholar]
- 30. Meyer O. Interferons and autoimmune disorders. Joint Bone Spine 2009; 76(5): 464–473. [DOI] [PubMed] [Google Scholar]
- 31. Litteljohn D, Nelson E, Hayley S. IFN-γ differentially modulates memory-related processes under basal and chronic stressor conditions. Front Cell Neurosci 2014; 8: 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hou R, Garner M, Holmes C, et al. Peripheral inflammatory cytokines and immune balance in generalised anxiety disorder: case-controlled study. Brain Behav Immun 2017; 62: 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maes M, Scharpé S, Meltzer HY, et al. Relationships between interleukin-6 activity, acute phase proteins, and function of the hypothalamic-pituitary-adrenal axis in severe depression. Psychiatry Res 1993; 49(1): 11–27. [DOI] [PubMed] [Google Scholar]
- 34. Pavón L, Sandoval-López G, Hernández ME, et al. Th2 cytokine response in major depressive disorder patients before treatment. J Neuroimmunol 2006; 172(1-2): 156–165. [DOI] [PubMed] [Google Scholar]
- 35. Islam MR, Islam MR, Ahmed I, et al. Elevated serum levels of malondialdehyde and cortisol are associated with major depressive disorder: a case-control study. SAGE Open Med 2018; 6: 2050312118773953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tian R, Hou G, Li D, et al. A possible change process of inflammatory cytokines in the prolonged chronic stress and its ultimate implications for health. Scientific World J 2014; 2014: 780616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation 2009; 16(5): 300–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ménard C, Hodes GE, Russo SJ. Pathogenesis of depression: insights from human and rodent studies. Neuroscience 2016; 321: 138–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hodes GE, Kana V, Menard C, et al. Neuroimmune mechanisms of depression. Nat Neurosci 2015; 18(10): 1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pérez-Sánchez G, Becerril-Villanueva E, Arreola R, et al. Inflammatory profiles in depressed adolescents treated with fluoxetine: an 8-week follow-up open study. Mediators Inflamm 2018; 2018: 4074051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weisse CS. Depression and immunocompetence: a review of the literature. Psychol Bull 1992; 111(3): 475. [DOI] [PubMed] [Google Scholar]
- 42. Hacimusalar Y, Eşel E. Suggested biomarkers for major depressive disorder. Noro Psikiyatr Ars 2018; 55(3): 280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lemeshow S, Hosmer DW, Klar J. Adequacy of sample size in health studies. Chichester: John Wiley & Sons, 1990, p.19. [Google Scholar]
- 44. Rickards H. Depression in neurological disorders: Parkinson’s disease, multiple sclerosis, and stroke. J Neurol Neurosurg Psychiatry 2005; 76(Suppl. 1): i48–i52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Farooq RK, Asghar K, Kanwal S, et al. Role of inflammatory cytokines in depression: focus on interleukin-1β. Biomed Rep 2017; 6(1): 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 2006; 27(1): 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cassano P, Bui E, Rogers AH, et al. Inflammatory cytokines in major depressive disorder: a case-control study. Aust N Z J Psychiatry 2017; 51(1): 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Anjum S, Qusar Shahriar M, Islam SMA, et al. Altered serum interleukin-7 and interleukin-10 are associated with drug-free major depressive disorder. Ther Adv Psychopharmacol 2020; 10: 2045125320916655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nishuty NL, Khandoker MMH, Karmoker JR, et al. Evaluation of serum interleukin-6 and C-reactive protein levels in drug-naïve major depressive disorder patients. Cureus 2019; 11(1): e3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Munder M, Eichmann K, Morán JM, et al. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol 1999; 163(7): 3771–3777. [PubMed] [Google Scholar]
- 51. Mills CD, Kincaid K, Alt JM, et al. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 2000; 164(12): 6166–6173. [DOI] [PubMed] [Google Scholar]
- 52. Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage M1–M2 polarization balance. Front Immunol 2014; 5: 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pallavi P, Sagar R, Mehta M, et al. Serum cytokines and anxiety in adolescent depression patients: gender effect. Psychiatry Res 2015; 229(1–2): 374–380. [DOI] [PubMed] [Google Scholar]
- 54. Köhler CA, Freitas TH, Maes MD, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatrica Scandinavica 2017; 135(5): 373–387. [DOI] [PubMed] [Google Scholar]
- 55. Himmerich H, Patsalos O, Lichtblau N, et al. Cytokine research in depression: principles, challenges, and open questions. Front Psychiatry 2019; 10: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Won GH, Kim JW, Choi TY, et al. Theta-phase gamma-amplitude coupling as a neurophysiological marker in neuroleptic-naïve schizophrenia. Psychiatry Res 2018; 260: 406–411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-smo-10.1177_2050312120974169 for Serum interferon-gamma level is associated with drug-naïve major depressive disorder by Sohel Daria, Maliha Afrin Proma, Mohammad Shahriar, Sardar Mohammad Ashraful Islam, Mohiuddin Ahmed Bhuiyan and Md. Rabiul Islam in SAGE Open Medicine


