Abstract
Pulmonary arterial hypertension is a complex disease resulting from the interplay of myriad biological and environmental processes that lead to remodeling of the pulmonary vasculature with consequent pulmonary hypertension. Despite currently available therapies, there remains significant morbidity and mortality in this disease. There is great interest in identifying and applying biomarkers to help diagnose patients with pulmonary arterial hypertension, inform prognosis, guide therapy, and serve as surrogate endpoints. An extensive literature on potential biomarker candidates is available, but barriers to the implementation of biomarkers for clinical use in pulmonary arterial hypertension are substantial. Various omic strategies have been undertaken to identify key pathways regulated in pulmonary arterial hypertension that could serve as biomarkers including genomic, transcriptomic, proteomic, and metabolomic approaches. Other biologically relevant components such as circulating cells, microRNAs, exosomes, and cell-free DNA have recently been gaining attention. Because of the size of the datasets generated by these omic approaches and their complexity, artificial intelligence methods are being increasingly applied to decipher their meaning. There is growing interest in imaging the lung with various modalities to understand and visualize processes in the lung that lead to pulmonary vascular remodeling including high resolution computed tomography, Xenon magnetic resonance imaging, and positron emission tomography. Such imaging modalities have the potential to demonstrate disease modification resulting from therapeutic interventions. Because right ventricular function is a major determinant of prognosis, imaging of the right ventricle with echocardiography or cardiac magnetic resonance imaging plays an important role in the evaluation of patients and may also be useful in clinical studies of pulmonary arterial hypertension.
Keywords: biomarker, genomics–metabolomics–proteomics, imaging, pharmacogenomics, pulmonary arterial hypertension
Introduction
Pulmonary arterial hypertension (PAH) is a pathologically and clinically heterogeneous group of diseases characterized by the remodeling of the small pulmonary vasculature and consequent precapillary pulmonary hypertension. The pathobiological processes that lead to disease development are diverse and underpinned by the influence of genetic, biological, and environmental stimuli. The interaction of therapy with the biological pathways that drive disease and clinical response to therapy are challenging to assess. As such, there has been growing interest in the potential role of biomarkers in the evaluation and treatment of PAH. An extensive range of biological features at all levels of cell structure, regulation, and metabolism including genetic markers, epigenetics, gene expression (RNA), microRNAs (miRNA), proteins, circulating cell populations, metabolites, cell-free DNA, and exosomes have been studied as potential biomarkers in PAH. Because lung biopsy is relatively contra-indicated in this patient population, there has been strong interest in developing non-invasive imaging modalities of the lung and, in particular, of the pulmonary vasculature to provide information about underlying disease processes in PAH. Right ventricular function has a major impact on prognosis in PAH; therefore, imaging of the right ventricle (RV) with echocardiography or cardiac magnetic resonance imaging (cMRI) may play an important role in clinical management and the evaluation of efficacy in clinical trials of PAH. Broadly, the role of biomarkers may be placed into one or more of the following categories:
For diagnosis
For prognosis
To predict therapeutic response
To serve as a surrogate endpoint
There have been extensive explorations of biomarkers for PAH diagnosis and to a lesser extent prognosis. The reader is referred to excellent references for consideration of PAH diagnostic biomarker candidates.1–4 This review will focus on the potential utility of biomarkers in clinical studies to signal therapeutic efficacy and the challenges of developing biomarkers as surrogate endpoints.
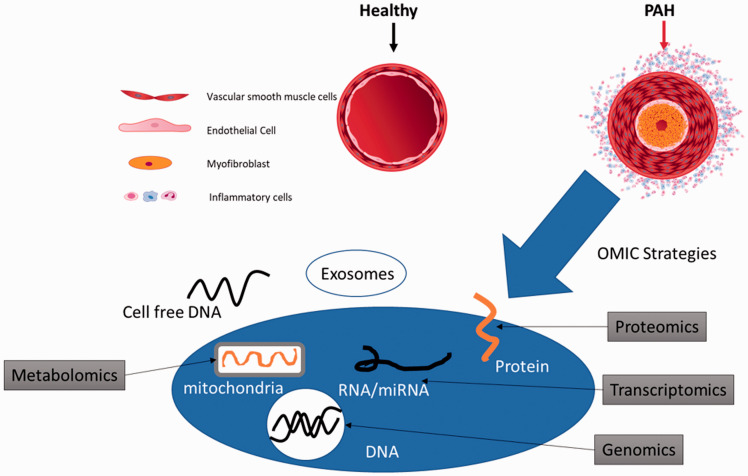
Various, and at times Quixotic, attempts have been made to identify circulating factors that reflect changes in the pulmonary vasculature during disease progression and under treatment. In addition, these circulating factors offer mechanistic insight into disease pathogenesis. Omic approaches (genomics, transcriptomics, proteomics, metabolomics, and volatilomics) to identify panels of biomarkers are increasingly employed to address the complexity of this disease (Fig. 1).
Table 1.
RVFWS from IMPRES echo sub-study.
| Time | Placebo N | Placebo mean | Placebo SE | Imatinib N | Imatinib mean | Imatinib SE |
|---|---|---|---|---|---|---|
| Baseline | 35 | –16 | 0.8 | 39 | –16.8 | 1.2 |
| 12 weeks | 33 | –16.3 | 0.9 | 29 | –18.4 | 1.5 |
| 24 weeks | 28 | –15.9 | 1.0 | 31 | –20.1 | 1.8 |
Fig. 1.
PAH is characterized by an abnormal proliferation of endothelial cells, and myofibroblasts within the pulmonary arterioles as well as perivascular inflammation that result in muscularization and hypertrophy of the vessel wall. Various “omic” strategies have been taken to decipher the underlying mechanisms of these pathologic processes.
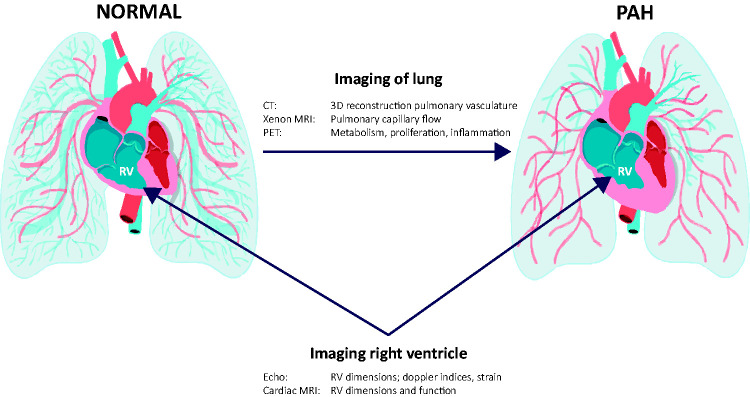
Advanced information technologies, including artificial intelligence/machine learning, have been deployed to identify leitmotifs within complex biological networks found in PAH blood samples, as well as to decipher non-intuitive signatures from imaging modalities. In addition to blood-based biomarkers, emerging technologies show promise in non-invasive, potentially mechanistic, imaging of the lung, including three-dimensional reconstructions of the pulmonary vasculature derived from computed tomographic (CT) data, Xenon magnetic resonance imaging (MRI) of the lung, which can evaluate the lung parenchyma and pulmonary capillary blood flow, and positron emission tomography (PET) imaging with tracers that are associated with altered metabolism (fluorine-18-labeled 2-fluoro-2-deoxyglucose (18FDG)), cell proliferation (18FLT), and inflammation (68-Gallium mannosylated albumin) (Fig. 2).
Fig. 2.
Imaging modalities of the lung include CT, Xenon MRI, and positron emission tomography (PET). Echocardiography and Cardiac MRI are useful in examining right ventricular dimensions and function in PAH.
Evaluation of the RV has been gaining increased attention because of the strong relationship between RV function and mortality in PAH. The imaging modalities of the RV of greatest interest are echocardiography and cMRI (Fig. 2).
The regulatory view of surrogate endpoints
The European Medicines Agency (EMA) has published a guideline for the use of biomarkers as surrogate endpoints (EMA Guideline Clinical Trials Small Populations 2006). The utility of surrogate endpoints was placed in the context of rare disorders where, for a given clinical endpoint, recruitment of a sufficient number of patients would be difficult or demonstration of this clinical endpoint would take an unreasonable length of time. In that scenario, use of other surrogate markers as substitutes for a clinical endpoint may be considered. However, the EMA guideline also pointed out that the term “surrogate endpoint” should only be used for biomarkers which have been validated. Thus, the question arises: what is required to validate a surrogate endpoint? Looking to the oncology literature, where such efforts have had a long history, it has been proposed that the coefficient of determination (R2) trial statistic be used.5 The R2 trial statistic requires a meta-analysis of multiple clinical trials where each trial serves as one data point. Linear regression is then performed to compare the change in the surrogate endpoint vs the change in the “hard” clinical endpoint. Cut-offs are chosen which are deemed acceptable for regulatory use. For example the German Institute for Quality and Efficiency in Health Care; Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen has indicated that there is lack of validity if upper bound of 95% CI R ≤ 0.7 (in this case R2 = 0.49) and that validity is proven if the lower bound of 95% CI R ≥ 0.85. Thus it is apparent that a “catch-22” emerges when we try to consider the use of a surrogate endpoint in lieu of a previously accepted clinical endpoint: the purpose of the surrogate endpoint is to address the limitations of a small population with a rare disease and the time it would take to demonstrate efficacy with clinical endpoints such as time to clinical worsening. However, to validate the surrogate endpoint would require performance of multiple clinical trials, which would obviously take even longer than performing just one phase 3 trial with a clinical primary endpoint.
While R2 cut-offs are arbitrary, for most trials in oncology, an R2 is not even available. In the oncology space, it has been noted that of the 25 drugs approved by accelerated pathway, 84% had no trial-level validation and that poorly validated surrogates can be misleading.5 With regard to PAH, there are currently no validated biomarkers. Indeed, even NT-proBNP, the well-respected biomarker for heart failure, has not been validated.
Based on these criteria, there is currently no biomarker in PAH that is a surrogate endpoint. Nevertheless, biomarkers that predict therapeutic response or track such response, especially early in treatment, may still have utility to guide clinical study design and development of therapeutic targets. Furthermore, newer endpoints, such as measures of ambulatory activity, are being viewed favorably and may not require this level of validation.
Genetics of PAH
Much progress has been made over the last several decades in the understanding of genetic risk for PAH. After the initial discovery that mutations in the bone morphogenetic receptor type 2 (BMPR2) underlie approximately 80% of heritable PAH (hPAH),6,7 there was a rapid appreciation that this mutation is incompletely penetrant and that about 10–15% of patients with idiopathic PAH (IPAH) also carry mutation in BMPR2.8 These data suggest that either there is a low penetrance or under-recognized disease within some families or that de novo mutations occur in IPAH patients with BMPR2 mutation and no family history of the disease. After expanding studies to related genes in transforming growth factor (TGF)-beta signaling, e.g. ALK19,10 and endoglin,11 in the late 1990s and early 2000s, genetic discoveries in PAH were limited by technology and the low prevalence of the disease, which limited traditional approaches to the identification of gene mutations associated with this disease. With the advent of next generation sequencing techniques, there has been rapid growth in identification of genes that appear to associate with and contribute to PAH in both IPAH and hPAH. These include CAV1,12 KCNK3,13 GDF2,14 SOX17,15 and TBX416 among others, and novel genes that underlie other forms of pulmonary vascular disease (PVD), such as EKFKA24 that underlie pulmonary veno-occlusive disease (PVOD).17,18 Moreover, PAH with known gene mutations appears to have a more advanced phenotype. hPAH with BMPR2 mutation presents at a younger age with more severe hemodynamics than other forms of PAH19 and is also associated with worse survival and more severe right ventricular dysfunction.20
Despite this expansion in understanding genetic underpinnings of PAH and how heritable forms of PAH may affect outcomes, there has been relatively little study of gene variants that predict drug responses to PAH therapies, which would be a highly useful property of biomarkers in this deadly disease. Benza and colleagues demonstrated that variants in endothelin metabolism may predict outcomes in PAH patients treated with endothelin receptor antagonists.21 It is presently unknown if any of the recently identified gene mutations in hPAH and IPAH may influence likelihood of drug responses in PAH, but this association has not previously been identified. It is possible that a single gene may not adequately predict treatment responses and a combination of genes may be required—or even a combination of genes with another protein or metabolomic biomarker. In prior whole exome testing, the hypothesis was generated that there is a genetic signature of vasodilator-responsive (VR)-IPAH which responds to calcium channel blocker therapy. Vascular smooth muscle contraction-related genes were found to be enriched in VR-IPAH, suggesting a potentially different genetic predisposition for this PAH subtype.22 Gene-based biomarkers of drug responsiveness are highly attractive, despite their limited study thus far. However, as DNA is stable over time and not influenced by the environment or drug exposure, it is well suited to use as a predictor of drug responses in patients regardless of their prior treatment course.
Single component biomarker candidates
An extensive list of single biomarker candidates for diagnosis and, to a lesser extent, prognosis may be found in the literature. The most well-known biomarker which reflects the degree of right ventricular failure in PAH is NT-proBNP. Reported candidates as diagnostic and/or prognostic biomarkers for PAH include cytokines such as interleukin (IL)-1, IL-2, IL-4, IL-6, IL-8, IL-10, tumor necrosis factor (TNF)-alpha, growth factors, such as vascular endothelial growth factors (VEGFs), platelet-derived growth factors (PDGFs), and TGF-beta, CC family chemokines, osteopontin, angiopoietins, matrix metalloproteinases, cardiac troponins, C reactive protein, von Willebrand factor (vWF), high density lipoprotein (HDL), thromboxane B2, fibrinogen, soluble fms-like tyrosine kinase-1 (sFLT1), placental growth factor, lymphotoxin-like inducible protein that competes with glycoprotein D for herpesvirus entry on T-lymphocytes (LIGHT), asymmetric dimethylarginine, GDF15, and hepatocyte growth factor (HDGF) among others.23–34 There is fairly extensive literature suggesting the potential utility of GDF15 as a biomarker for PAH.25,26,29,30,32,35,36 HDGF has also been reported as informative of prognosis above and beyond that provided by NT-proBNP.33 None of these biomarkers, however, meet the criteria for use as a surrogate endpoint. NT-proBNP is the only circulating biomarker currently employed in guidelines for risk assessment and is discussed in more detail below.37
NT-proBNP
NT-proBNP levels have been shown to correlate with cardiopulmonary hemodynamics, PaO2 at peak exercise, and peak minute ventilation.38 NT-proBNP has been shown to reflect changes in cMRI measured RV structure and function in PAH.39 The 2015 ESC/ERS guidelines indicate that an NT-proBNP level > 1400 ng/L is associated, among other parameters, with an increased risk of mortality in PAH.37 In the SERAPHIN hemodynamic substudy, macitentan improved NT-proBNP compared to placebo at six months. Absolute levels of NT-proBNP, but not their changes at baseline and six months, were associated with morbidity/mortality events.40 In the phase 3 GRIPHON study, NT-proBNP categories (low, medium, and high based on ESC/ERS 2015 guideline cut-offs) were prognostic for future morbidity/mortality events. In a time-dependent analysis, the risk of a morbidity/mortality event was 92% lower in the selexipag-treated group, and 90% lower in the placebo-treated group if the NT-proBNP level was low at baseline or follow-up.41 In the phase 3 study of imatinib in PAH, at week 24, NT-proBNP was lower in the imatinib group compared to placebo.42
Multiplex biomarker candidates
Given the limitations of single biomarkers in risk stratification and therapeutic response, there has been a growing interest in evaluating the utility of multiplex assays. The advent of advanced high throughput assay technologies has led to the omic approach at multiple levels of cellular structure and function as well as various strategies to make sense of the large resulting datasets, such as network analysis. Perhaps ironically, many such omic approaches attempt to whittle down the data to one or two candidates which are then subjected to more traditional and somewhat simplistic mechanistic studies. In recognition of this irony, mathematical modeling of complex systems, including machine learning, may be rising to the occasion. In some cases, omic approaches have focused on PAH lung tissue available at the time of transplant. Analyses are undertaken with cells obtained from PAH tissues (i.e. human pulmonary artery smooth muscle cells (HPASMCs)) grown in culture and other analyses have been performed on circulating cells or serum/plasma. Because there is particular interest in the ability to obtain biomarkers in the setting of clinical trials, this review will attempt to introduce a discussion about which pathways regulated in lung tissue or lung-specific cells from PAH patients intersect or overlap with pathways similarly regulated in circulating cells, serum, or plasma. A limitation of lung tissue analysis is that it represents end-stage disease because the tissue is typically obtained at the time of lung transplantation (as lung biopsies are not usually performed in this patient population). Thus, we can ask, is it reasonable and possible to examine the targets identified in samples from end-stage disease lungs earlier in the course of the disease by examining changes in circulating cells, serum, or plasma? This approach can be problematic because of the different tissue compartments under study and because the time profile of changes may not vary in a linear or monotonic manner. In this context, we review here some examples of currently available high throughput analyses at the level of gene, miRNA, and protein expression and some initial attempts to make sense of the data with a machine learning approach.
Transcriptomics
Gene expression
Several transcriptomic analyses related to PAH have been performed ranging from comparative studies to whole lung cell culture-based and, most recently, to single cell RNA sequencing approaches.
Sasagawa et al.43 performed a comparative transcriptome analysis of five mammalian datasets and identified 228 differentially expressed genes (DEGS) from the SU5416/hypoxia rat model, 379 DEGS from a mouse model of PAH associated with systemic sclerosis, 850 DEGS from a mouse PAH model associated with schistosomiasis, 1598 DEGS from one cohort of PAH subjects, and 4269 DEGS from a second cohort of PAH subjects. Comparative analysis identified four genes that were up- or downregulated across all the animal models and PAH patients: coiled-coil domain containing 80 (CCDC80) and anterior gradient were increased, and SMAD6 and granzyme A were decreased. Knockout of CCDC80 in Zebrafish showed the role of this gene in vascular development. A limitation of this study is the assumption that common variance across multiple animal models is necessary or sufficient to establish pathological importance in the human disease.
Stearman et al.44 performed a system analysis of the human PAH lung transcriptome. Pathway analysis was performed with Enrichr, Gene Ontology, and KEGG. Ingenuity Pathway Analysis (IPA) was used to visualize pathway over-representation. In addition, a novel network-based computational analysis, Evaluation of Differential DependencY (EDDY), was employed. IPA showed important roles for Estrogen Receptor 1, TNF, colony-stimulating factor 3, and IL-10. EDDY analysis demonstrated substantial rewiring across multiple reactomes. For example, EDDY identified TLR3 and TLR4 as central to the REACTOME pathway describing IFN regulatory factors-3 and 7.
In a search for circulating biomarkers, Grigoryev et al.45 sought to identify scleroderma PAH-associated genes regulated in PBMCs. Transcriptomics of PMBCs from IPAH patients were highly comparable with PBMCs from PAH-SSC patients. Gene ontology analysis showed significant changes in genes associated with angiogenesis, such as MMP9 and VEGF, according to the severity of PAH (perhaps counterintuitively higher in mild PAH compared to severe PAH). Other identified regulated genes included ADM, IL7R, ZFP36, GLUL, JUND, BCL6, adrenomedullin, and herpesvirus entry mediator genes. Novel genes, not previously associated with PAH, were also reported, such as EREG, CXCL2, and MMP25.
A meta-analysis of genome-wide expression profiling in the blood from PAH subjects was performed by Elinoff et al.46 The comparability of data across seven studies (which together included 156 PAH patients/110 healthy controls) was assessed and an attempt was made to derive a generalizable transcriptomic signature. IPAH compared with disease-associated PAH (APAH) displayed highly similar expression profiles with no DEGS between the two groups, even after substantially relaxing selection stringency. In contrast, using a false discovery rate I2 of ≤1% and <40% (low-to-moderate heterogeneity across studies), both IPAH and APAH differed markedly from healthy controls with the combined PAH cohort yielding 1269 differentially expressed, unique gene transcripts. Bioinformatic analyses, including gene-set enrichment which used all available data independent of gene selection thresholds, identified interferon, mammalian target of rapamycin/p70S6K, stress kinase, and Toll-like receptor signaling as enriched within the PAH gene signature. Enriched biological functions included tumorigenesis, autoimmunity, antiviral response, and cell death consistent with prevailing theories of PAH pathogenesis. Although otherwise indistinguishable, APAH (predominantly PAH due to systemic sclerosis) had a somewhat stronger interferon profile than IPAH.
Cheong et al.47 examined the effect of tadalafil on gene expression profiles in SSC-associated PAH. These authors found that genes associated with IL-12 and maintenance of the extracellular matrix were affected by treatment. In addition, genes encoding voltage-gated potassium channels and genes related to innate immunity were coordinately upregulated in subjects who improved with tadalafil treatment compared to those who did not. In contrast, upregulation of Golgi-related gene sets was associated with clinical worsening during the treatment period.
The results of a whole blood RNA sequencing effort in PAH, which corrected for variance of total cell populations in samples, found enrichment in T cell receptor, PI3K signaling in B cells, and hypoxia signaling in PAH compared to controls.48 Specific targets that were differentially regulated included zinc finger transcription factors (TFs), HIF1-alpha, KLF10, TRPC1, Sestrin 1, and SMAD5. A Mendelian randomization exercise demonstrated the biological relevance of SMAD5 levels in the PAH population. An RNA LASSO model consisting of 25 genes was found to predict survival in PAH; 372 of the 507 top dysregulated genes from the whole blood RNAseq study were present in the lung tissue microarray study44 and 161/372 (43%) were also directionally consistent; 41/161 (25%) genes were nominally significant and 26 met false discovery rate (FDR) corrected significance. Sadly, only one gene was found to be similarly dysregulated in PAH patients in the whole blood RNA study, the lung transcriptomic study, and the genome-wide expression meta-analysis: AMD1 (encoding apolyamine biosynthesis intermediate enzyme, adenosylmethionine decarboxylase 1), which was consistently lower in PAH across all three studies.44,46,48
Recently Saygin et al.49 used single cell RNA sequencing in cells isolated from IPAH lungs to examine differential gene expression compared to control lungs. Transcriptomics were determined for endothelial cells, pericytes/smooth muscle cells, fibroblasts, and macrophage clusters. Gene ontology enrichments were determined. Interestingly, in genes associated with blood vessel and cardiovascular development, PDGF-beta was the most upregulated gene. PDGF was shown to be increased in PAH in a biomarker study of the effects of IV treprostinil, but more recently circulating PDGF levels were not found to be increased in an observational study, thus highlighting the potential difficulty of correlating local tissue specific regulation of PDGF signaling in PAH with peripheral biomarkers.50,51
Are there any “take home” messages from these recent transcriptomic efforts? One message seems to be that there is a connection between lung transcriptomics from PAH patients and peripheral blood mononuclear cells (PBMCs) in the circulation. For example, a PBMC regulatory network, identified using the Ingenuity program by Elinoff et al.,46 showed that TNF-alpha and granulocyte macrophage colony stimulating factor (GM-CSF) were significantly enriched upstream regulators. Similarly Stearman et al.44 identified a key role for TNF and CSF3 signaling in their lung transcriptomic analyses. Additional pathways identified in both the meta-analysis of PBMCs/blood, performed by Elinoff et al.,46 and the lung transcriptome analysis, performed by Stearman et al.,44 identified interferon and toll-like receptor (TLR) pathways as potentially important. However, when overlap was sought between the recent whole blood RNA sequencing effort of Rhodes et al.,48 the prior gene expression meta-analysis of Elinoff et al.,46 and the lung transcriptomic effort of Stearman et al.,44 there was only one target that was commonly dysregulated across all three studies. But do not be disheartened: it is likely that pathway regulation across these multiple studies may be more informative than identification of particular targets. For example, all three studies found differential regulation of Toll-like receptors which have been implicated in PAH pathobiology.52,53 Therapeutic or disease-monitoring strategies based on the networks, pathways, and gene products identified by these studies could be useful in future drug development for PAH.
The recent report of transcriptomics using single cell RNA sequencing demonstrated a marked upregulation of PDGF-BB in genes associated with blood vessel and cardiovascular development. This approach suggests an important role of cell-specific and localized pulmonary regulation that could be missed by less precise methods. Notably, imatinib, a kinase inhibitor with PDGFR activity, was shown to be effective in a phase 3 trial of PAH.54 Although further development of orally administered imatinib was hampered by a number of systemic side effects, there remains on-going interest in targeting this pathway.
MiRNAs
miRNAs are short noncoding RNAs that regulate gene expression by inhibiting translation or promoting degradation of target miRNAs. miRNAs are key regulators of a wide range of cellular processes and their discovery has generated a fair amount of interest in their potential role in PAH disease pathogenesis and/or progression.55,56 A variety of miRNAs have been associated with BMPR2 expression and signaling in pulmonary vascular cells in vitro and in vivo. Multiple miRNAs that directly regulate BMPR2 include the miR-17/92cluster, miR-302-367 cluster, and miR-21.56,57 The large number of miRNAs implicated in PAH at first glance appear to be a quagmire, but these can be organized into hierarchical motifs with convergent and divergent functions.56
Taking a multiomics approach, Chen et al.58 examined the effect of PDGF-BB on the pulmonary artery smooth muscle cell (PASMC) transcriptome and proteome. TF-target network analysis revealed that PDGF-BB regulated gene expression potentially via TFs including HIF1A, JUN, EST1, ETS1, SMAD1, FOS, SP1, STAT1, LEF1, and CEBPB. Notably, PDGF-BB led to BMPR2 downregulation. To understand the mechanism by which this crosstalk occurred, 1078 miRNAs were examined. It was found that 13 miRNAs changed in a time-dependent manner in PASMCs treated with PDGF-BB. Using the online prediction tool, Targetscan, multiple binding sites of miR-7, miR-245, and miR376b were found in the BMPR2 3’UTR. Western blot analysis showed that only miR-376b could decrease BMPR2 protein levels. An EdU incorporation assay showed that miR-376b promoted proliferation of PASMCs. Qian et al.59 found that miR-4632 was highly expressed in HPASMCs and significantly downregulated in PDGF-BB-stimulated HPASMCs. Functional studies revealed that miR-4632 inhibited proliferation and promoted apoptosis of HPASMCs. cJUN was identified as a direct target gene of miR-4632, and knockdown of cJUN was necessary for miR-4632-mediated HPASMC proliferation and apoptosis. In addition, the downregulation of miR-4632 by PDGF-BB was found to associate with histone deacetylation through the activation of PDGF receptor/phosphatidylinositol 3'-kinase/histone deacetylase 4 signaling. The expression of miR-4632 was reduced in the serum of patients with PAH. Another miRNA, miR-328, was also found to mediate PDGF-BB effects in PASMCs.60 In this study, the expression profiling of 1078 miRNAs was investigated in PASMCs with or without PDGF-BB stimulation. MiR-328 was found as a prominent downregulated miRNA, displaying a specific dose- and time-dependent downregulation upon PDGF-BB exposure. Functional analyses revealed that miR-328 could inhibit PASMCs proliferation and migration both with and without PDGF-BB treatment and the Ser/Thr-protein kinase-1 (PIM-1) was identified as a direct target of miR-328.
Rothman et al.61 identified a reduction in the levels of miR-140-5p in subjects with PAH compared to controls. Inhibition of miR-140-5p promoted PASMC proliferation and migration in vitro. In rat models of PAH, nebulized delivery of miR-140-5p mimic prevented the development of PAH and attenuated the progression of established PAH. Network and pathway analysis identified SMAD-specific E3 ubiquitin protein ligase 1 as a key miR-140-5p target and regulator of BMP signaling as well as with PDGFR-alpha.
It is apparent that the regulation of miRNAs in PAH is quite complex with a high level of redundancy. This level of complexity could represent a challenge to their utility as biomarkers in future studies. However, of particular interest are miRNAs that appear to play a role in the regulation of PDGF signaling and cross talk with the BMPR2 pathway.
Proteomics
Several proteomic analyses have been undertaken to identify novel biomarkers with potential diagnostic and/or prognostic utility.
In a plasma proteome analysis, Rhodes et al.62 identified a panel of nine proteins (ILR4, Epo, Factor D, IGFBP-1, TIMP2, TIMP1, Factor H, Plasminogen, and ApoE) that provided prognostic information independent of NT-proBNP levels and the REVEAL equation. The proteins were related to myocardial stress, inflammation, pulmonary vascular cellular dysfunction, iron status, and coagulation. Poor survival was preceded by a change in panel score and was validated in a follow-up cohort.
Lavoie et al.63 performed a proteomic analysis of outgrowth EPCs from PAH subjects with BMPR2 mutations compared to controls; 416 proteins were detected with two-dimensional PAGE in combination with liquid chromatography/tandem mass spectrometry analysis, of which 22 exhibited significantly altered abundance in blood-outgrowth ECs from patients with hPAH. One of these proteins, translationally controlled tumor protein (TCTP), was selected for further study because of its well-established role in promoting tumor cell growth and survival. Immunostaining showed marked upregulation of TCTP in lungs from patients with hPAH and IPAH, associated with remodeled vessels of complex lesions. Increased TCTP expression was also evident in the SU5416 rat model of severe and irreversible PAH, associated with intimal lesions, co-localizing with proliferating ECs and the adventitia of remodeled vessels but not in the vascular media. Furthermore, silencing of TCTP expression increased apoptosis and abrogated the hyperproliferative phenotype of blood-outgrowth ECs from patients with hPAH, raising the possibility that TCTP may be a link in the emergence of apoptosis-resistant, hyperproliferative vascular cells after EC apoptosis.
Meyrick et al.64 performed a proteomic analysis of transformed lymphocytes obtained from subjects with familial PAH (FPAH). The transformed blood lymphocyte protein extracts from four patients with FPAH, three obligate carriers, and three married-in control subjects from one family with a known BMPR2 mutation (exon 3 T354G) were labeled with either Cy3 or Cy5. Cy3/5 pairs were separated by standard two-dimensional differential gel electrophoresis using a Cy2-labeled internal standard of all patient samples. Log volume ratios were analyzed using a linear mixed-effects model. Proteins were identified by matrix-assisted laser desorption ionization, time-of-flight mass spectrometry (TOF MS) and tandem TOF/TOF MS/MS. Hierarchical clustering, heat-map, and principal components analyses revealed marked changes in protein expression in patients with FPAH when compared with obligate carriers. Significant changes were apparent in expression of 16 proteins (p < 0.05) when affected patients were compared with obligates: nine showed a significant increase and seven showed a significant reduction. These proteins could, potentially, have utility in predicting or tracking response to therapies in patients with PAH and BMPR2 mutations.
To understand the nature of insulin resistance often observed in PAH patients, Hemnes et al.65 performed an aptamer-based proteomic assay to measure abundance of 1139 proteins in fasting plasma from PAH patients and controls. By principal component analysis, PAH patients and controls were broadly separated by their plasma proteome. Forty different metabolic pathway proteins were examined for differential expression between PAH and control and it was found that 11 were different, at p < 0.05 by unpaired t test (FDR = 2). These differentially expressed proteins showed significant differences in insulin signaling (IGFBP6, IGF2R, IGFBP7, IGFBP2, pRKAA1, and ANGPTL4) and lipid signaling and transport (OLR1, APOA1, and CD36).
Proteomic analyses show promise to identify subsets of proteins that may provide prognostic information, identify the best candidates for particular indications, and predict response to therapeutic interventions. However, more rigorous efforts in multi-center studies may be needed to further qualify the use of particular proteins as biomarkers that could predict response to therapies.
Metabolomics
Altered mitochondrial and cellular metabolisms have been well studied and are reviewed elsewhere.66,67 In short, there are multiple pathways that are affected including, among others, glucose oxidation, fatty acid oxidation, and glutamine metabolism. These pathways are altered in multiple tissues in PAH including the pulmonary vasculature and the RV. Given the broad disturbance in metabolism in PAH and the ready capacity to measure metabolites using mass-spectroscopy-based techniques, there is interest in using metabolomics as biomarkers of disease responsiveness. There is now evidence that metabolomics may be useful for this purpose based on the work of Rhodes and colleagues68 where, after identification of several metabolites that were different in PAH patients and healthy and controls, the authors found several metabolites in key pathways, including tRNA-specific modified nucleosides, tricarboxylic acid (TCA) intermediaries, glutamate, and fatty acid acylcarnitines among others, that correlated with increased risk of death. Importantly, the authors found that correction of several metabolites over time was associated with a better outcome in their PAH cohorts. These data provide a strong justification of using changes in plasma metabolites over time to identify drug responders. Integrating a proteomic and phosphoproteomic analysis of PAH endothelial cells, Xu et al.69 identified dysregulated pathways in PAH, including accelerated one carbon metabolism, abnormal TCA cycle flux and glutamate metabolism, dysfunctional arginine and nitric oxide pathways, and increased oxidative stress. Functional studies in cells confirmed abnormalities in glucose metabolism, mitochondrial oxygen consumption, and production of reactive oxygen species in PAH. Thus far, there have not been studies of which metabolites may predict responsiveness to a particular drug. Other studies have used a more reductionist approach, e.g. studying nitric oxide metabolites in prediction of response to either phosphodiesterase type 5 inhibitors or soluble guanylate cyclase stimulators. Changes to—and improvements in—metabolism may be detected in the blood, but there have also been studies demonstrating the feasibility of using exhaled breath, the so-called “volatome”, to distinguish PAH from control and may be useful for detecting responses to therapy.70 Blood-based or other metabolites may be early and sensitive markers of changes in signaling pathways that are relevant to PAH and affected by drug therapies. There are readily available mass screening assays to measure hundreds to thousands of metabolites for low cost compared to many omic analyses, making their study cost efficient and powerful.
In summary, use of metabolomic assays, both at baseline and after therapy, may offer the potential to identify markers of prognostic value at baseline and/or that are sensitive to change over the course of treatment. It is possible that changes in metabolites that correlate with important long-term outcomes could presage other pathophysiologic changes.
Circulating cells in PAH
Circulating mononuclear cells may play an important role for the vascular remodeling in PAH. Foris et al.71 used a comprehensive fluorescence-activated cell sorting analysis of circulating mononuclear cells in 20 PAH patients and 20 age-and-sex-matched controls, and additionally analyzed CD133(+) cells in the lung tissue of five PAH transplant recipients and five healthy controls (donor lungs). PAH patients were characterized by increased numbers of circulating CD133(+) cells and lymphopenia as compared with control. In PAH, CD133(+) subpopulations positive for CD117 or CD45 were significantly increased, whereas CD133(+) CD309(+), CD133(+) CXCR2(+), and CD133(+) CD31(+) cells were decreased. In CD133(+) cells, SOX2, Nanog, Ki67, and CXCR4 were not detected, but Oct3/4 mRNA was present in both PAH and controls. In lung tissue, CD133(+) cells included three main populations: type 2 pneumocytes, monocytes, and undifferentiated cells without significant differences between PAH and controls. The authors concluded that circulating CD133(+) progenitor cells were elevated in PAH and consisted of phenotypically different subpopulations that may be up- or downregulated. CD133(+) type 2 pneumocytes in the lung tissue were not associated with circulating CD133(+) mononuclear cells.
In a series that included 70 patients with pulmonary hypertension of various etiologies, a significant decrease in the relative numbers of circulating myeloid dendritic cells, but not plasmacytoid dendritic cells DCs, was found compared to the healthy controls. Flow cytometric analyses did not show significant changes between the relative number of circulating Tregs in patients with PH compared to healthy controls.72 In a study of circulating cells in PAH patients, significantly fewer CD8+ T cells but more CD25hi+ and FoxP3+CD4+ T cells were found in the peripheral blood of patients compared with controls. The percentage of FoxP3+ cells within the CD25hi+CD4+ T(reg) cells was similar. T(reg) cell functionality was equal in patients and controls.73 An increased CD4:CD8 ratio and deficiencies of NK cells and cytotoxic CD8+ T-lymphocytes were reported to be associated with increased one year mortality in PAH.74
In another series, IPAH patients were found to have abnormal circulating CD8+ T lymphocyte subsets, with a significant increase in CD45RA+ CCR7– peripheral cytotoxic effector-memory cells and reduction of CD45RA+ CCR7+ naive CD8+ cells versus controls. Furthermore, IPAH patients had a higher proportion of circulating regulatory T cells (T(reg)) and four-fold increases in the number of CD3+ and CD8+ cells in the peripheral lung compared with controls.75 CD45RA is a protein tyrosine phosphatase and is believed to be a marker for T-lymphocytes with greater cytolytic activity and greater propensity to travel to non-lymphoid tissues.75
In summary, studies of circulating cells in PAH are interesting, and elegant, but complex and have shown varying results. The reproducibility and implementation for multicenter studies to examine their potential role as biomarkers to predict therapeutic response may be challenging for both technical and operational reasons.
Exosomes and cell free DNA
There has been growing interest in the study of exosomes in PAH. Exosomes are nano-sized (30–150 nm) extracellular vesicles released from almost all types of cells, they can contain various bioactive molecules, such as nucleic acids (DNA and RNA), proteins, and lipids and, thus, can be involved in proximal and distal intercellular communication. Exosomes are believed to influence immune regulation, tumor invasion, and cellular degeneration.76 Exosomes and associated proteins could be a marker of PAH severity. For example, TCTP was previously identified in a proteomic analysis of outgrowth EPCs (see above). Ferrer et al.77 found that TCTP was also localized in extracellular vesicles, blood outgrowth endothelial cells (BOECs) isolated from patients harboring BMPR2 mutations released more exosomes than those derived from control subjects in proapoptotic conditions. Co-culture assays demonstrated that exosomes transferred TCTP from ECs to PASMCs, suggesting a role for endothelial-derived TCTP in conferring proliferation and apoptotic resistance. In an experimental model of PAH, rats treated with monocrotaline demonstrated increased concentrations of TCTP in the lung and plasma. Circulating TCTP levels were increased in patients with IPAH compared with control subjects. Based on these findings, TCTP was proposed as a potential biomarker for the onset and development of PAH.
De la Cuesta et al.78 performed a transcriptome analysis of HPASMCs and their extracellular vesicles. A total of 2417 gene transcripts were detected in HPASMC-EVs; 759 of these were enriched compared to their donor cells and included TGF-beta superfamily ligands, GDGF 11, and TGF-beta 3; 90 genes were differentially expressed in extracellular vesicles (EVs) from cells treated with TGF-beta compared to EVs under basal conditions and included bHLHE40 and paladin. Intercellular communication between human pulmonary artery endothelial cells (HPAECs) and HPASMC was shown via CRE-lox technology to be mediated by EVs.
Cell free DNA was shown to be increased in experimental acute thromboembolism.79 The idea of using cell free DNA as a biomarker for PAH disease severity is in the early stage of development.
In summary, there is growing interest in the study of exosomes and cell free DNA in clinical studies of PAH; these extracellular components may be of interest as exploratory endpoints in future studies.
Noninvasive imaging of the PAH lung
CT imaging
There are typical lung parenchymal changes in PAH that are seen on routine high-resolution CT or CT pulmonary angiography. Centrilobular ground glass nodularity seen in approximately 40% of patients with IPAH. In addition, some features are more typical of PVOD may be seen such as mediastinal lymphadenopathy and interlobular septal thickening.80 The presence of coexistent parenchymal lung diseases, such as interstitial fibrosis and emphysema, even if mild, can have a large effect on patient outcome.81
Advances in CT image acquisition, reconstruction, and post-processing have led to an increasing number of quantitative features that can be used to define the pulmonary vascular architecture, provide quantitative measures of pulmonary vascular remodeling, and monitor response to therapeutic intervention. In a proof of concept study, Rahaghi et al.82 measured changes in pulmonary vascular caliber and volume in response to inhaled nitric oxide on clinically available non-contrast CT scans. Hajian et al.83 applied a similar approach to quantify pulmonary blood vessel volume from CT data obtained in subjects with pulmonary hypertension and chronic obstructive pulmonary disease (COPD) before and after inhaled nitric oxide. In the future, these types of approaches could be deployed to examine the therapeutic response to anti-proliferative disease modifying therapies. Further work is required to evaluate the repeatability of these measurements and to understand what constitutes a meaningful change. The vessels detected by CT (>0.5 mm in diameter) are typically larger than the vessels most affected by PVD, but improvements in analytical methods and the use of artificial intelligence to evaluate CT scans from PAH patients may be on the horizon and might provide more detailed information about disease modification.
PET imaging
Zhao et al.84 performed dynamic PET imaging with 18FDG ligand with kinetic analysis in 20 patients with PAH. The lung parenchymal uptake was increased in the PAH group compared to controls (IPAH, n = 18, FDG score: 3.27 ± 1.22; connective tissue disease APAH, n = 2, FDG score: 5.07 and 7.11; controls FDG score: 2.02 ± 0.71; p < 0.05). Compartment analysis confirmed increased lung glucose metabolism in IPAH. Lung 18FDG uptake and metabolism varied within the IPAH population and within the lungs of individual patients, consistent with the recognized heterogeneity of vascular pathology in this disease. The monocrotaline rat PAH model also showed increased lung 18FDG uptake, which was reduced along with improvements in vascular pathology after treatment with dichloroacetate and two tyrosine kinase inhibitors, imatinib and sunitinib. Hyperproliferative pulmonary vascular fibroblasts isolated from IPAH patients exhibited upregulated glycolytic gene expression, along with increased cellular 18FDG uptake; both were reduced by dichloroacetate and imatinib.
In a series of 30 patients, Saygin et al.85 found that right ventricular end-diastolic and end-systolic volumes, right ventricular ejection fraction, and right ventricular FDG uptake by PET were significantly different between PH and healthy controls and strongly correlated with plasma NT-proBNP levels. In addition, lung standardized uptake value (SUV) was also found to be significantly higher in participants with PH than healthy controls. However, lung SUV did not show any significant correlations with NT-proBNP levels.
However, Ruiter et al.86 did not find a significant increase in 18FDG uptake in the lungs of PAH subjects, and 18FDG uptake in the lungs did not correlate with various parameters of disease severity, thus raising concerns regarding the utility of 18FDG-PET for the evaluation of this patient population.
Due to the relatively low signal-to-noise ratio, and heterogeneity of FDG uptake in PAH subjects, alternative imaging agents to evaluate PAH as a proliferative disease have been sought.
Ashek et al.87 performed dynamic 18FLT PET in eight patients with IPAH and applied in-depth kinetic analysis with a reversible two-compartment 4k model. There was a significantly increased lung 18FLT phosphorylation (k3) in patients with IPAH compared with non-PAH controls (0.086 ± 0.034 versus 0.054 ± 0.009 min-1; p < 0.05). There was heterogeneity in the lung 18FLT signal both between patients with IPAH and within the lungs of each patient, compatible with histopathologic reports of lungs from patients with IPAH. Consistent with 18FLT PET data, thymidine kinase 1 (TK1) expression was evident in the remodeled vessels in IPAH patient lung. In addition, hyperproliferative pulmonary vascular fibroblasts isolated from patients with IPAH exhibited upregulated expression of TK1 and the thymidine transporter, equilibrative nucleoside transporter 1. In the monocrotaline and Sugen hypoxia rat PAH models, increased lung 18FLT uptake was strongly associated with peripheral pulmonary vascular muscularization and the proliferation marker, Ki-67 score, together with prominent TK1 expression in remodeled vessels. The lung 18FLT uptake was attenuated by two antiproliferative treatments: dichloroacetate and the tyrosine kinase inhibitor, imatinib.
More recently, evidence for macrophage-mediated inflammation was demonstrated in PAH subjects using (68)Ga-2-(p-isothiocyanatobenzyl)-1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA) mannosylated human serum albumin ((68)Ga-NOTA-MSA) PET imaging: pulmonary uptake of (68)Ga-NOTA-MSA was significantly higher in patients with PAH than normal subjects or than those with pulmonary hypertension due to left heart disease.88 Other PET-based imaging agents, such as agents that quantify adrenomedullin receptors in the lung, have been proposed but remain in the nascent stage of development.89
In summary, PET imaging to evaluate underlying processes implicated in PAH pulmonary vascular remodeling and inflammation holds some promise, but the studies thus far have been small with mixed results. Issues related to mechanism of action under study, signal-to-noise ratios, availability of imaging agents, and PET imagers need to be considered if this technology is to be implemented in larger clinical studies of PAH.
Xenon MRI
Xenon MRI has recently been applied to evaluate patients with PVD.
Wang et al.90 compared 129 Xenon MRI images and dynamic spectroscopy in healthy volunteers (n = 23) and patients with COPD (n = 8), idiopathic pulmonary fibrosis (IPF) (n = 12), left heart failure (LHF) (n = 6), and PAH (n = 10). For each patient, three-dimensional maps were generated to depict ventilation, barrier uptake (129Xe dissolved in interstitial tissue), and red blood cell (RBC) transfer (129Xe dissolved in RBCs). Dynamic 129Xe spectroscopy was used to quantify cardiogenic oscillations in the RBC signal amplitude and frequency shift. Compared with healthy volunteers, all patient groups exhibited decreased ventilation and RBC transfer (both p ≤ 0.01). Patients with COPD demonstrated more ventilation and barrier defects compared with all other groups (both p ≤ 0.02). In contrast, IPF patients demonstrated elevated barrier uptake compared with all other groups (p ≤ 0.007) and increased RBC amplitude and shift oscillations compared with healthy volunteers (p = 0.007 and p ≤ 0.01, respectively). Patients with COPD and PAH both exhibited decreased RBC amplitude oscillations (p = 0.02 and p = 0.005, respectively) compared with healthy volunteers. LHF was distinguishable from PAH by enhanced RBC amplitude oscillations (p = 0.01). 129Xe red blood cell chemical shift to measure pulmonary blood oxygenation in vivo has been shown to be feasible.90 There is also potential to estimate pulmonary interstitial thickness which is of particular interest in interstitial lung disease and associated vascular disease.91
In summary, RBC amplitude oscillations measured with Xenon MRI represent a non-invasive physiologically meaningful assessment of localized pulmonary capillary blood flow and therefore could be a useful parameter to evaluate the response to therapeutic interventions in PAH subjects.
Noninvasive imaging of the RV
Echocardiography
Echocardiographic parameters that have traditionally been studied in the PAH population include right atrium (RA) and RV dimensions, RV fractional area change, tricuspid annular plane systolic excursion, the left ventricle (LV) eccentricity index, and presence of a pericardial effusion. In particular, an enlarged RA and presence of a pericardial effusion are risk factors that contribute to poor prognosis based on the 2015 ESC/ERS guidelines. The LV eccentricity index captures the change in morphology of the LV in the setting of PAH. As pulmonary pressures increase, the interventricular septum tends to flatten and bulge into the LV. This change can be expressed as the ratio of the minor axis of the LV parallel to the septum (D2) divided by the minor axis perpendicular to the septum (D1).91 As the septum flattens and bulges into the LV, the LV eccentricity index increases. This parameter as well as the RV area/LV area ratio were found to be useful in early clinical trials of endothelin receptor antagonists.92
In 1996, Dr. Tei reported an index of RV myocardial performance that has become known as the Tei Index.93 This index is the ratio of isovolumic contraction and relaxation time divided by the RV ejection time. Intuitively one can appreciate that, in the face of increased afterload due to pulmonary hypertension (PH), isovolumic contraction and relaxation times would be increased and RV ejection time decreased. Thus, the RV Tei Index is elevated in PAH compared to normal subjects.
More recently, it has been shown that changes in the RV Tei Index correlated with changes in PVR (r = 0.706, p = 0.002, n = 16).94
There is growing interest in the measurement of RV strain to evaluate changes in RV function in the setting of PH. Strain refers to mechanical deformation and can be measured using the echocardiographic technique of speckle tracking. With respect to evaluation of RV strain, it can be measured in three aspects: longitudinal (also referred to as free wall); circumferential; and radial. Global RV strain, as well as strain rates (the rate of change of strain), can be measured. RV free wall strain (RVFWS) appears to be the most robust measurement. RVFWS measured by echo correlated with RVEF measured by CMR (r = 0.83, p < 0.005, n = 66). In a multivariate analysis, RVFWS > –14% was associated with worse outcomes (HR: 4.66, CI: 1.25–17.37, p = 0.022). In a multivariate analysis, the only echo parameter associated with a clinical composite endpoint (six hospitalizations and nine deaths) was RVFWS.95
RV global longitudinal peak systolic strain (RV GLS) was found to correlate with PVR (r = –0.549, p < 0.05).96
In a prospective series of subjects with known or suspected PH (n = 575, n = 300 with WHO group 1 PAH), RVFWS correlated with functional class, six-minute walk distance, NT-proBNP, and right heart failure, with HR 1.46 (1.05–2.12) for death per every 6.7% decline in RV strain. RV strain predicted survival when adjusted for pulmonary pressure, PVR, and RAP.97
Siddiqui et al.98 found that RV GLS was associated with hospitalization or death (n = 98, HR: 1.54 per 1 SD (5.31), 95% CI: 2.12, p = 0.008). In a meta-analysis of 11 studies to examine RV strain n = 1169 PH patients, a 19% reduction in RVLS had a higher risk of combined endpoint HR 1.22, and a 22% reduction in RV GLS had a higher risk of all-cause mortality HR 2.96.99 In another meta-analysis, which examined 10 published studies (n = 1001 of which 76% had PAH), RV strain when treated as a continuous variable had a HR of 1.14 (95% CI: 1.11–1.8, p < 0.001) per 1% change for mortality.100 In the echo substudy of the IMPRES trial, RV Tei Index improved significantly compared to placebo at week 24 (n = 74, Imatinib change from baseline: −0.11 ± 0.18; placebo 0.5 ± 0.18, p = 0.007).101 In another analysis of the IMPRES echo substudy, right ventricular free wall longitudinal strain (FWLS) and LV global circumferential strain were measured at baseline, 12 weeks, and 24 weeks in 68 patients with advanced PAH randomized to imatinib or placebo. Improvement in PVR at 24 weeks correlated with a change in RVFWLS (r = 0.39, p = 0.02, n = 37) and LV global circumferential strain (r = 0.61, p < 0.001, n = 36). Using a repeated measure mixed effects model, the improvement in RVFWS in the imatinib group corrected for baseline was significant compared to the placebo group (p = 0.03). This analysis included the echo data from weeks 12 and 24.102 The strain analysis of echo data from the IMPRES trial was not pre-specified. The interesting results were considered hypothesis generating. The table 1 summarizes the RFWS data at baseline, 12 weeks, and 24 weeks.
Various attempts have been made to develop formulae from echocardiographic parameters that correlate with PVR measured by right heart catheterization (RHC). For example, Kasai et al.103 found that TRPG/COLVOT correlated highly with PVR measured at RHC (r = 0.807, p < 0.001). The data were based on echocardiograms of 40 subjects with chronic thromboembolic pulmonary hypertension (CTEPH), which may or may not be applicable to cardiopulmonary hemodynamics of subjects with WHO Group 1 PAH. Abbas et al.104 correlated TRV2/TVIRVOT with PVR from RHC (r = 0.79, p < 0.001) in 150 subjects not restricted to PAH. However, in subjects with a PVR > 6 Wood units, the r value fell to 0.33–0.48. In a retrospective study of 45 subjects, Sinha et al.105 reported that pulmonary artery elastance adjusted for hemoglobin correlated with PVR measured at RHC (r = 0.89, p = 0.008). The utility of these formulae to assess the effect of a therapeutic intervention in the setting of a clinical trial is not known.
In summary, measurement of changes in RV Tei Index and RVFWS correlate with RVEF measured by cMRI as well as PVR and are likely to provide meaningful information with regard to clinical outcomes including survival.
cMRI
Noninvasive imaging is central to assessment of disease severity and progression in patients with PAH.106 The current ERS/ESC guidelines focus assessment on right atrial area and the presence of pericardial effusion by echocardiography or cMRI; however, indicators of RV performance have demonstrated relationships to clinical outcomes, such as mortality, and provide additional information that permits risk stratification and assessment of therapeutic insertion.107
The SERAPH study was the first study to use MRI-derived RV mass as a primary endpoint in a PAH trial, showing regression of RV hypertrophy with sildenafil.108 More recently, the REPAIR study (https://clinicaltrials.gov/ct2/show/NCT02310672) used cMRI-derived stroke volume as a co-primary endpoint along with PVR in a trial assessing the effect of Macitentan in PAH as the first study to use cMRI as a primary endpoint in a clinical trial. This study uses pulmonary arterial forward flow, akin to stroke volume as the primary endpoint. RV volume and function are secondary endpoints. A meta-analysis of studies showed the prognostic value of RV ejection fraction and volume measurements in PAH.109 However, the relative magnitude of prognostic significance is difficult to compare. Larger individual studies have since been published suggesting that both RV ejection fraction and RV end-systolic volume are strong predictors of mortality.107 Thresholds for RVEF and RV end-systolic volume have been described, with prognostic value demonstrated at baseline and follow-up assessments.106 Relatively higher RV volume to mass ratio has been proposed as an important prognostic marker, and further work is warranted to evaluate the significance of RV mass:volume phenotypes.110 Clinical studies used RV end-diastolic mass as a primary endpoint to assess treatment effects as this was shown to be also of prognostic value.108,111,112
Right atrial volume has been shown to be a prognostic in PAH,113 and this is reflected in the ESC/ERS guidelines, further study of the prognostic importance of the RV relative to the RA, or even incremental value of both chambers is also warranted.
Studies have shown the prognostic value of the pulsatility of the proximal pulmonary vasculature, with incremental prognostic value when added to the RV.114,115 A limitation is out of plane motion of the main pulmonary artery, and assessment of the right main pulmonary artery may be more robust.115 Such parameters may also have sensitivity to the detection of early PH. In future, 3D dynamic imaging may provide a superior assessment of the pulsatility of the proximal vasculature.
The use of machine learning may assist with optimizing the reproducibility of measurements made of cMRI and maximizing the prognostic value. Machine learning using convolutional neural networks to automate RV contours has shown promise and automatic mortality prediction from RV motion has been shown to have strong prognostic value, in comparison to human measurements.116,117 In addition, a tensor-based machine learning approach has been proposed for automatic extraction of disease features in PAH, with diagnostic and prognostic utility.118 The value of such approaches require comparison with standard measurements derived by human observers.
In summary, MRI parameters of RV function are well suited to use in clinical studies of PAH as potential biomarkers of response to therapeutic interventions. Because these studies are non-invasive, may be performed on scanners that are widely available, have parameters which are reproduceable, and correlate with clinical outcomes, they could have utility in future clinical studies of PAH.
Artificial intelligence applied to understanding the complexity of PAH pathogenesis
In a prospective observational study of WHO group 1 PAH patients, Sweatt et al.119 measured a circulating proteomic panel of 48 cytokines, chemokines, and factors using multiplex immunoassay. Unsupervised machine learning (consensus clustering) was applied in two cohorts independently to classify patients into proteomic immune clusters, without guidance from clinical features. To identify central proteins in each cluster, partial correlation network analysis was performed. Clinical characteristics and outcomes were subsequently compared across clusters. Four PAH clusters with distinct proteomic immune profiles were identified in the discovery cohort. Cluster 2 (n = 109) had low cytokine levels similar to controls. Other clusters had unique sets of upregulated proteins central to immune networks-cluster 1 (n = 58; TNF-related apoptosis-inducing ligand (TRAIL), C-C motif chemokine ligand 5 (CCL5), CCL7, CCL4, macrophage migration inhibitory factor (MIF)), cluster 3 (n = 77; IL-12, IL-17, IL-10, IL-7, VEGF), and cluster 4 (n = 37; IL-8, IL-4, PDGF-beta, IL-6, CCL11). Demographics, PAH clinical subtypes, comorbidities, and medications were similar across clusters. Noninvasive and hemodynamic surrogates of clinical risk identified cluster 1 as high-risk and cluster 3 as low-risk groups. Five-year transplant-free survival rates were unfavorable for cluster 1 (47.6%; 95% CI: 35.4–64.1%) and favorable for cluster 3 (82.4%; 95% CI: 72.0–94.3%; across-cluster p < 0.001). Findings were replicated in a validation cohort, where machine learning classified four immune clusters with comparable proteomic, clinical, and prognostic features.
In summary, the use of artificial intelligence to analyze complex datasets and to identify novel regulated pathways predictive of outcomes could also be leveraged to identify biomarkers of therapeutic response in clinical trials.
Publicly available databases
Tissue and blood repositories available for research
There are several repositories established for biological samples from PAH subjects. Most involve blood samples. One repository, the Pulmonary Hypertension Breakthrough Initiative (PHBI) sponsored by the NHLBI, has stored lung tissue preserved in various formats. Researchers affiliated with academic institutions may access the PHBI resources through an application process (phbi.org). The NIH/NHLBI launched an initiative, “Redefining Pulmonary Hypertension through Pulmonary Vascular Disease Phenomics (PVDOMICS)”, that aims to augment the current PH classification based on shared biological features. PVDOMICS will enroll 1500 participants with PH due to various etiologies (WHO Groups 1–5) and healthy comparators. Enrollees will undergo deep clinical phenotyping and blood will be acquired for comprehensive omic analyses that will focus on discovery of molecular-based subtypes of PVD through application of high dimensional model-based clustering methods. In addition to an updated molecular classification of PVD, the phenomic data generated will be a rich resource to the broad community of heart and lung disease investigators.118 In future clinical studies of PAH subjects, it is hoped that academic centers and pharmaceutical industry sponsors will continue to collaborate to optimize the use of samples collected for analyses of biomarkers and translational research toward the common goal of improving diagnosis, prognosis, and therapies available for patients with PAH.
Summary and conclusions
There has been extensive work to identify potential biomarkers for PAH. Omic strategies have been employed to examine changes at the level of gene and protein expression as well as changes in miRNAs and metabolic profiles. Changes in circulating cell subtypes, cell free DNA, and exosomes have been examined. Most studies attempt to distinguish PAH from normal control subjects and to identify prognostic markers. Transcriptomics of lung and blood/PBMCs suggest some degree of overlap of regulated networks involved in inflammation and other pathways that could be useful in evaluating response to future therapies targeting these networks. An example from the field of proteomics has been described wherein a panel of nine proteins showed prognostic power independent of the REVEAL score. There is potential utility of using biomarkers to predict response to a therapeutic intervention. However, there is also evidence that local cell type-specific pathway perturbations occur in PAH lung that are not easily discernible from analysis of peripheral biomarkers. Because right ventricular function is closely associated with prognosis in PAH, imaging the RV with either echocardiography or cMRI is of growing interest. Newer techniques to examine pulmonary vasculature remodeling with 3D CT reconstruction or Xenon MRI have been explored in small studies. In addition, PET imaging with agents associated with inflammation and cell proliferation have been reported. From a regulatory perspective, there are currently no validated biomarker surrogate endpoints that meet criteria for use as approvable endpoints in PAH. Indeed, even NT-proBNP does not meet criteria for use as a surrogate endpoint based on the EMA guidelines. Nevertheless, NT-proBNP and potentially other biomarkers have utility to examine the effects of therapy, as well as use as prognostic indicators. Finally, there is a growing interest in the use of artificial intelligence/machine learning with potential applications to imaging and analyses of large omic databases.
Take-home message
There is potential utility of using biomarkers to predict response to a therapeutic intervention and utilize these to enrich a clinical trial population or use as an endpoint.
A wide variety of genomic, proteomic, and metabolomic approaches are being proposed and are actively under consideration.
Right ventricular function is closely associated with prognosis in pulmonary arterial hypertension, leading to a potential role for imaging the right ventricle with either echocardiography or cardiac magnetic resonance imaging as a principal endpoint. Newer techniques to examine pulmonary vasculature remodeling with 3D computed tomographic reconstruction or Xenon magnetic resonance imaging are being explored in small studies. In addition, positron emission tomography imaging with agents associated with inflammation and cell proliferation have been reported. From a regulatory perspective, there are currently no validated biomarker surrogate endpoints that meet criteria for use as approvable endpoints in Pulmonary arterial hypertension. Indeed, even NT-proBNP does not meet criteria for use as a surrogate endpoint based on the European Medicines Agency guidelines.
Nevertheless, NT-proBNP and potentially other biomarkers have utility to examine the effects of therapy, help stratify patient selection pre randomization as well as playing a role as prognostic indicators.
Acknowledgments
We thank Paul Corris and Sylvia Nikkho for the general supervision of the Innovative Drug Development Initiative and their dedicated support of the Biomarker workstream, as well as Georgie Sutton, PVRI Admin Manager, for her support in proofreading this manuscript.
Footnotes
Conflict of interest: The author(s) declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Halliday SJ, Hemnes AR. Identifying “super responders” in pulmonary arterial hypertension. Pulm Circ 2017; 7: 300–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hemnes AR. Using omics to understand and treat pulmonary vascular disease. Front Med (Lausanne) 2018; 5: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kan M, Shumyatcher M, Himes BE. Using omics approaches to understand pulmonary diseases. Respir Res 2017; 18: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kedzierski P, Torbicki A. Precision medicine: the future of diagnostic approach to pulmonary hypertension? Anatol J Cardiol 2019; 22: 168–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kemp R, Prasad V. Surrogate endpoints in oncology: when are they acceptable for regulatory and clinical decisions, and are they currently overused? BMC Med 2017; 15: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nichols WC, Koller DL, Slovis B, et al. Localization of the gene for familial primary pulmonary hypertension to chromosome 2q31-32. Nat Genet 1997; 15: 277–280. [DOI] [PubMed] [Google Scholar]
- 7.Lane KB, Machado RD, Pauciulo MW, et al. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. The International PPH Consortium. Nat Genet 2000; 26: 81–84. [DOI] [PubMed] [Google Scholar]
- 8.Thomson JR, Machado RD, Pauciulo MW, et al. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-beta family. J Med Genet 2000; 37: 741–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trembath RC, Thomson JR, Machado RD, et al. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med 2001; 345: 325–334. [DOI] [PubMed] [Google Scholar]
- 10.Harrison RE, Flanagan JA, Sankelo M, et al. Molecular and functional analysis identifies ALK-1 as the predominant cause of pulmonary hypertension related to hereditary haemorrhagic telangiectasia. J Med Genet 2003; 40: 865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaouat A, Coulet F, Favre C, et al. Endoglin germline mutation in a patient with hereditary haemorrhagic telangiectasia and dexfenfluramine associated pulmonary arterial hypertension. Thorax 2004; 59: 446–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Austin ED, Ma L, LeDuc C, et al. Whole exome sequencing to identify a novel gene (caveolin-1) associated with human pulmonary arterial hypertension. Circ Cardiovasc Genet 2012; 5: 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma L, Roman-Campos D, Austin ED, et al. A novel channelopathy in pulmonary arterial hypertension. N Engl J Med 2013; 369: 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graf S, Haimel M, Bleda M, et al. Identification of rare sequence variation underlying heritable pulmonary arterial hypertension. Nat Commun 2018; 9: 1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhodes CJ, Batai K, Bleda M, et al. Genetic determinants of risk in pulmonary arterial hypertension: international genome-wide association studies and meta-analysis. Lancet Respir Med 2019; 7: 227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu N, Gonzaga-Jauregui C, Welch CL, et al. Exome sequencing in children with pulmonary arterial hypertension demonstrates differences compared with adults. Circ Genom Precis Med 2018; 11: e001887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Best DH, Sumner KL, Austin ED, et al. EIF2AK4 mutations in pulmonary capillary hemangiomatosis. Chest 2014; 145: 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eyries M, Montani D, Girerd B, et al. EIF2AK4 mutations cause pulmonary veno-occlusive disease, a recessive form of pulmonary hypertension. Nat Genet 2014; 46: 65–69. [DOI] [PubMed] [Google Scholar]
- 19.Girerd B, Montani D, Eyries M, et al. Absence of influence of gender and BMPR2 mutation type on clinical phenotypes of pulmonary arterial hypertension. Respir Res 2010; 11: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brittain EL, Pugh ME, Wheeler LA, et al. Shorter survival in familial versus idiopathic pulmonary arterial hypertension is associated with hemodynamic markers of impaired right ventricular function. Pulm Circ 2013; 3: 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benza RL, Gomberg-Maitland M, Demarco T, et al. Endothelin-1 pathway polymorphisms and outcomes in pulmonary arterial hypertension. Am J Respir Crit Care Med 2015; 192: 1345–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemnes AR, Zhao M, West J, et al. Critical genomic networks and vasoreactive variants in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2016; 194: 464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Naamani N, Palevsky HI, Lederer DJ, et al. Prognostic significance of biomarkers in pulmonary arterial hypertension. Ann Am Thorac Soc 2016; 13: 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andreassen AK, Wergeland R, Simonsen S, et al. N-terminal pro-B-type natriuretic peptide as an indicator of disease severity in a heterogeneous group of patients with chronic precapillary pulmonary hypertension. Am J Cardiol 2006; 98: 525–529. [DOI] [PubMed] [Google Scholar]
- 25.Foris V, Kovacs G, Tscherner M, et al. Biomarkers in pulmonary hypertension: what do we know? Chest 2013; 144: 274–283. [DOI] [PubMed] [Google Scholar]
- 26.Garfield BE, Crosby A, Shao D, et al. Growth/differentiation factor 15 causes TGFbeta-activated kinase 1-dependent muscle atrophy in pulmonary arterial hypertension. Thorax 2019; 74: 164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heresi GA. Clinical perspective: biomarkers in pulmonary arterial hypertension. Int J Clin Pract Suppl 2011; 169: 5–7. [DOI] [PubMed] [Google Scholar]
- 28.Hickey PM, Lawrie A, Condliffe R. Circulating protein biomarkers in systemic sclerosis related pulmonary arterial hypertension: a review of published data. Front Med (Lausanne) 2018; 5: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li G, Li Y, Tan XQ, et al. Plasma growth differentiation factor-15 is a potential biomarker for pediatric pulmonary arterial hypertension associated with congenital heart disease. Pediatr Cardiol 2017; 38: 1620–1626. [DOI] [PubMed] [Google Scholar]
- 30.Nickel N, Kempf T, Tapken H, et al. Growth differentiation factor-15 in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2008; 178: 534–541. [DOI] [PubMed] [Google Scholar]
- 31.Odler B, Foris V, Gungl A, et al. Biomarkers for pulmonary vascular remodeling in systemic sclerosis: a pathophysiological approach. Front Physiol 2018; 9: 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhodes CJ, Wharton J, Wilkins MR. Pulmonary hypertension: biomarkers. Handb Exp Pharmacol 2013; 218: 77–103. [DOI] [PubMed] [Google Scholar]
- 33.Yang J, Nies MK, Fu Z, et al. Hepatoma-derived growth factor predicts disease severity and survival in pulmonary arterial hypertension. Am J Respir Crit Care Med 2016; 194: 1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zelniker T, Uhlmann L, Spaich S, et al. Novel biomarkers for risk stratification in pulmonary arterial hypertension. ERJ Open Res 2015; 1: 1(2): 00008–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meadows CA, Risbano MG, Zhang L, et al. Increased expression of growth differentiation factor-15 in systemic sclerosis-associated pulmonary arterial hypertension. Chest 2011; 139: 994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nickel N, Jonigk D, Kempf T, et al. GDF-15 is abundantly expressed in plexiform lesions in patients with pulmonary arterial hypertension and affects proliferation and apoptosis of pulmonary endothelial cells. Respir Res 2011; 12: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 38.Berghaus TM, Kutsch J, Faul C, et al. The association of N-terminal pro-brain-type natriuretic peptide with hemodynamics and functional capacity in therapy-naive precapillary pulmonary hypertension: results from a cohort study. BMC Pulm Med 2017; 17: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gan CT, McCann GP, Marcus JT, et al. NT-proBNP reflects right ventricular structure and function in pulmonary hypertension. Eur Respir J 2006; 28: 1190–1194. [DOI] [PubMed] [Google Scholar]
- 40.Galie N, Jansa P, Pulido T, et al. SERAPHIN haemodynamic substudy: the effect of the dual endothelin receptor antagonist macitentan on haemodynamic parameters and NT-proBNP levels and their association with disease progression in patients with pulmonary arterial hypertension. Eur Heart J 2017; 38: 1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chin KM, Rubin LJ, Channick R, et al. Association of N-terminal pro brain natriuretic peptide and long-term outcome in patients with pulmonary arterial hypertension. Circulation 2019; 139: 2440–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoeper MM, Barst RJ, Bourge RC, et al. Imatinib mesylate as add-on therapy for pulmonary arterial hypertension: results of the randomized IMPRES study. Circulation 2013; 127: 1128–1138. [DOI] [PubMed] [Google Scholar]
- 43.Sasagawa S, Nishimura Y, Sawada H, et al. Comparative transcriptome analysis identifies CCDC80 as a novel gene associated with pulmonary arterial hypertension. Front Pharmacol 2016; 7: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stearman RS, Bui QM, Speyer G, et al. Systems analysis of the human pulmonary arterial hypertension lung transcriptome. Am J Respir Cell Mol Biol 2019; 60: 637–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grigoryev DN, Mathai SC, Fisher MR, et al. Identification of candidate genes in scleroderma-related pulmonary arterial hypertension. Transl Res 2008; 151: 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elinoff JM, Mazer AJ, Cai R, et al. Meta-analysis of blood genome-wide expression profiling studies in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2020; 318: L98–L111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheong FY, Gower AC, Farber HW. Changes in gene expression profiles in patients with pulmonary arterial hypertension associated with scleroderma treated with tadalafil. Semin Arthritis Rheum 2017; 46: 465–472. [DOI] [PubMed] [Google Scholar]
- 48.Rhodes CJ, Otero-Nunez P, Wharton J, et al. Whole blood RNA profiles associated with pulmonary arterial hypertension and clinical outcome. Am J Respir Crit Care Med 2020; 202: 586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saygin D, Tabib T, Bittar HET, et al. Transcriptional profiling of lung cell populations in idiopathic pulmonary arterial hypertension. Pulm Circ 2020; 10(1): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hiremath J, Thanikachalam S, Parikh K, et al. Exercise improvement and plasma biomarker changes with intravenous treprostinil therapy for pulmonary arterial hypertension: a placebo-controlled trial. J Heart Lung Transplant 2010; 29: 137–149. [DOI] [PubMed] [Google Scholar]
- 51.Saleby J, Bouzina H, Ahmed S, et al. Plasma receptor tyrosine kinase RET in pulmonary arterial hypertension diagnosis and differentiation. ERJ Open Res 2019; 5(4): 00037–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turton HA, Thompson AAR, Farkas L. RNA signaling in pulmonary arterial hypertension – a double-stranded sword. Int J Mol Sci 2020; 21(9): 3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hilbert T, Dornbusch K, Baumgarten G, et al. Pulmonary vascular inflammation: effect of TLR signalling on angiopoietin/TIE regulation. Clin Exp Pharmacol Physiol 2017; 44: 123–131. [DOI] [PubMed] [Google Scholar]
- 54.Hoeper MM, Barst RJ, Bourge RC, et al. Imatinib mesylate as add-on therapy for pulmonary arterial hypertension: results of the randomized IMPRES study. Circ 2013; 127(10): 1128–1138. [DOI] [PubMed]
- 55.Rothman AM, Chico TJ, Lawrie A. MicroRNA in pulmonary vascular disease. Prog Mol Biol Transl Sci 2014; 124: 43–63. [DOI] [PubMed] [Google Scholar]
- 56.Negi V, Chan SY. Discerning functional hierarchies of microRNAs in pulmonary hypertension. JCI Insight 2017; 2: e91327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee A, McLean D, Choi J, et al. Therapeutic implications of microRNAs in pulmonary arterial hypertension. BMB Rep 2014; 47: 311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen J, Cui X, Qian Z, et al. Multi-omics analysis reveals regulators of the response to PDGF-BB treatment in pulmonary artery smooth muscle cells. BMC Genomics 2016; 17: 781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qian Z, Li Y, Chen J, et al. miR-4632 mediates PDGF-BB-induced proliferation and antiapoptosis of human pulmonary artery smooth muscle cells via targeting cJUN. Am J Physiol Cell Physiol 2017; 313: C380–C391. [DOI] [PubMed] [Google Scholar]
- 60.Qian Z, Zhang L, Chen J, et al. MiR-328 targeting PIM-1 inhibits proliferation and migration of pulmonary arterial smooth muscle cells in PDGFBB signaling pathway. Oncotarget 2016; 7: 54998–55011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rothman AM, Arnold ND, Pickworth JA, et al. MicroRNA-140-5p and SMURF1 regulate pulmonary arterial hypertension. J Clin Invest 2016; 126: 2495–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rhodes CJ, Wharton J, Ghataorhe P, et al. Plasma proteome analysis in patients with pulmonary arterial hypertension: an observational cohort study. Lancet Respir Med 2017; 5: 717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lavoie JR, Ormiston ML, Perez-Iratxeta C, et al. Proteomic analysis implicates translationally controlled tumor protein as a novel mediator of occlusive vascular remodeling in pulmonary arterial hypertension. Circulation 2014; 129: 2125–2135. [DOI] [PubMed] [Google Scholar]
- 64.Meyrick BO, Friedman DB, Billheimer DD, et al. Proteomics of transformed lymphocytes from a family with familial pulmonary arterial hypertension. Am J Respir Crit Care Med 2008; 177: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hemnes AR, Luther JM, Rhodes CJ, et al. Human PAH is characterized by a pattern of lipid-related insulin resistance. JCI Insight 2019; 4: e123611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Culley MK, Chan SY. Mitochondrial metabolism in pulmonary hypertension: beyond mountains there are mountains. J Clin Invest 2018; 128: 3704–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maron BA, Leopold JA, Hemnes AR. Metabolic syndrome, neurohumoral modulation, and pulmonary arterial hypertension. Br J Pharmacol 2020; 177: 1457–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rhodes CJ, Ghataorhe P, Wharton J, et al. Plasma metabolomics implicates modified transfer RNAs and altered bioenergetics in the outcomes of pulmonary arterial hypertension. Circulation 2017; 135: 460–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu W, Comhair SAA, Chen R, et al. Integrative proteomics and phosphoproteomics in pulmonary arterial hypertension. Sci Rep 2019; 9: 18623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cohen-Kaminsky S, Nakhleh M, Perros F, et al. A proof of concept for the detection and classification of pulmonary arterial hypertension through breath analysis with a sensor array. Am J Respir Crit Care Med 2013; 188: 756–759. [DOI] [PubMed] [Google Scholar]
- 71.Foris V, Kovacs G, Marsh LM, et al. CD133+ cells in pulmonary arterial hypertension. Eur Respir J 2016; 48: 459–469. [DOI] [PubMed] [Google Scholar]
- 72.Rohm I, Grun K, Muller LM, et al. Cellular inflammation in pulmonary hypertension: detailed analysis of lung and right ventricular tissue, circulating immune cells and effects of a dual endothelin receptor antagonist. Clin Hemorheol Microcirc 2019; 73: 497–522. [DOI] [PubMed] [Google Scholar]
- 73.Ulrich S, Nicolls MR, Taraseviciene L, et al. Increased regulatory and decreased CD8+ cytotoxic T cells in the blood of patients with idiopathic pulmonary arterial hypertension. Respiration 2008; 75: 272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Edwards AL, Gunningham SP, Clare GC, et al. Professional killer cell deficiencies and decreased survival in pulmonary arterial hypertension. Respirology 2013; 18: 1271–1277. [DOI] [PubMed] [Google Scholar]
- 75.Austin ED, Rock MT, Mosse CA, et al. T lymphocyte subset abnormalities in the blood and lung in pulmonary arterial hypertension. Respir Med 2010; 104: 454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu JY, Chen GH, Yang YJ. Exosomes: a rising star in falling hearts. Front Physiol 2017; 8: 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferrer E, Dunmore BJ, Hassan D, et al. A potential role for exosomal translationally controlled tumor protein export in vascular remodeling in pulmonary arterial hypertension. Am J Respir Cell Mol Biol 2018; 59: 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De la Cuesta F, Passalacqua I, Rodor J, et al. Extracellular vesicle cross-talk between pulmonary artery smooth muscle cells and endothelium during excessive TGF-beta signalling: implications for PAH vascular remodelling. Cell Commun Signal 2019; 17: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Uzuelli JA, Dias-Junior CA, Izidoro-Toledo TC, et al. Circulating cell-free DNA levels in plasma increase with severity in experimental acute pulmonary thromboembolism. Clin Chim Acta 2009; 409: 112–116. [DOI] [PubMed] [Google Scholar]
- 80.Rajaram S, Swift AJ, Condliffe R, et al. CT features of pulmonary arterial hypertension and its major subtypes: a systematic CT evaluation of 292 patients from the ASPIRE Registry. Thorax 2015; 70: 382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lewis RA, Thompson AAR, Billings CG, et al. Mild parenchymal lung disease and/or low diffusion capacity impacts survival and treatment response in patients diagnosed with idiopathic pulmonary arterial hypertension. Eur Respir J 2020; 55: 2000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rahaghi FN, Winkler T, Kohli P, et al. Quantification of the pulmonary vascular response to inhaled nitric oxide using noncontrast computed tomography imaging. Circ Cardiovasc Imaging 2019; 12: e008338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hajian B, De Backer J, Vos W, et al. Pulmonary vascular effects of pulsed inhaled nitric oxide in COPD patients with pulmonary hypertension. Int J Chron Obstruct Pulmon Dis 2016; 11: 1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao L, Ashek A, Wang L, et al. Heterogeneity in lung (18)FDG uptake in pulmonary arterial hypertension: potential of dynamic (18)FDG positron emission tomography with kinetic analysis as a bridging biomarker for pulmonary vascular remodeling targeted treatments. Circulation 2013; 128: 1214–1224. [DOI] [PubMed] [Google Scholar]
- 85.Saygin D, Highland KB, Farha S, et al. Metabolic and functional evaluation of the heart and lungs in pulmonary hypertension by gated 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography. Pulm Circ 2017; 7: 428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ruiter G, Wong YY, Raijmakers P, et al. Pulmonary 2-deoxy-2-[(18)F]-fluoro-d-glucose uptake is low in treated patients with idiopathic pulmonary arterial hypertension. Pulm Circ 2013; 3: 647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ashek A, Spruijt OA, Harms HJ, et al. 3'-Deoxy-3'-[18F]fluorothymidine positron emission tomography depicts heterogeneous proliferation pathology in idiopathic pulmonary arterial hypertension patient lung. Circ Cardiovasc Imaging 2018; 11: e007402. [DOI] [PubMed] [Google Scholar]
- 88.Park JB, Suh M, Park JY, et al. Assessment of inflammation in pulmonary artery hypertension by (68)Ga-mannosylated human serum albumin. Am J Respir Crit Care Med 2020; 201: 95–106. [DOI] [PubMed] [Google Scholar]
- 89.Alonso Martinez LM, Harel F, Letourneau M, et al. SPECT and PET imaging of adrenomedullin receptors: a promising strategy for studying pulmonary vascular diseases. Am J Nucl Med Mol Imaging 2019; 9: 203–215. [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Z, Bier EA, Swaminathan A, et al. Diverse cardiopulmonary diseases are associated with distinct xenon magnetic resonance imaging signatures. Eur Respir J 2019; 54: 1900831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Howard LS, Grapsa J, Dawson D, et al. Echocardiographic assessment of pulmonary hypertension: standard operating procedure. Eur Respir Rev 2012; 21: 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Galie N, Hinderliter AL, Torbicki A, et al. Effects of the oral endothelin-receptor antagonist bosentan on echocardiographic and doppler measures in patients with pulmonary arterial hypertension. J Am Coll Cardiol 2003; 41: 1380–1386. [DOI] [PubMed] [Google Scholar]
- 93.Tei C, Dujardin KS, Hodge DO, et al. Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr 1996; 9: 838–847. [DOI] [PubMed] [Google Scholar]
- 94.Ogihara Y, Yamada N, Dohi K, et al. Utility of right ventricular Tei-index for assessing disease severity and determining response to treatment in patients with pulmonary arterial hypertension. J Cardiol 2014; 63: 149–153. [DOI] [PubMed] [Google Scholar]
- 95.Da Costa Junior AA, Ota-Arakaki JS, Ramos RP, et al. Diagnostic and prognostic value of right ventricular strain in patients with pulmonary arterial hypertension and relatively preserved functional capacity studied with echocardiography and magnetic resonance. Int J Cardiovasc Imaging 2017; 33: 39–46. [DOI] [PubMed] [Google Scholar]
- 96.Li Y, Xie M, Wang X, et al. Right ventricular regional and global systolic function is diminished in patients with pulmonary arterial hypertension: a 2-dimensional ultrasound speckle tracking echocardiography study. Int J Cardiovasc Imaging 2013; 29: 545–551. [DOI] [PubMed] [Google Scholar]
- 97.Fine NM, Chen L, Bastiansen PM, et al. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circ Cardiovasc Imaging 2013; 6: 711–721. [DOI] [PubMed] [Google Scholar]
- 98.Siddiqui I, Rajagopal S, Brucker A, et al. Clinical and echocardiographic predictors of outcomes in patients with pulmonary hypertension. Am J Cardiol 2018; 122: 872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hulshof HG, Eijsvogels TMH, Kleinnibbelink G, et al. Prognostic value of right ventricular longitudinal strain in patients with pulmonary hypertension: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging 2019; 20: 475–484. [DOI] [PubMed] [Google Scholar]
- 100.Shukla M, Park JH, Thomas JD, et al. Prognostic value of right ventricular strain using speckle-tracking echocardiography in pulmonary hypertension: a systematic review and meta-analysis. Can J Cardiol 2018; 34: 1069–1078. [DOI] [PubMed] [Google Scholar]
- 101.Shah AM, Campbell P, Rocha GQ, et al. Effect of imatinib as add-on therapy on echocardiographic measures of right ventricular function in patients with significant pulmonary arterial hypertension. Eur Heart J 2015; 36: 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Querejeta Roca G, Campbell P, Claggett B, et al. Impact of lowering pulmonary vascular resistance on right and left ventricular deformation in pulmonary arterial hypertension. Eur J Heart Fail 2015; 17: 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kasai H, Matsumura A, Sugiura T, et al. Noninvasive assessment of pulmonary vascular resistance by echocardiography in chronic thromboembolic pulmonary hypertension. Respir Investig 2015; 53: 210–216. [DOI] [PubMed] [Google Scholar]
- 104.Abbas AE, Franey LM, Marwick T, et al. Noninvasive assessment of pulmonary vascular resistance by Doppler echocardiography. J Am Soc Echocardiogr 2013; 26: 1170–1177. [DOI] [PubMed] [Google Scholar]
- 105.Sinha N, Devabhaktuni S, Kadambi A, et al. Can echocardiographically estimated pulmonary arterial elastance be a non-invasive predictor of pulmonary vascular resistance? Arch Med Sci 2014; 10: 692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kiely D, Levin D, Hassoun P, et al. EXPRESS: statement on imaging and pulmonary hypertension from the Pulmonary Vascular Research Institute (PVRI). Pulm Circ 2019; 9: 2045894019841990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lewis RA, Johns CS, Cogliano M, et al. Identification of cardiac magnetic resonance imaging thresholds for risk stratification in pulmonary arterial hypertension. Am J Respir Crit Care Med 2020; 201: 458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wilkins MR, Paul GA, Strange JW, et al. Sildenafil versus endothelin receptor antagonist for pulmonary hypertension (SERAPH) study. Am J Respir Crit Care Med 2005; 171: 1292–1297. [DOI] [PubMed] [Google Scholar]
- 109.Baggen VJ, Leiner T, Post MC, et al. Cardiac magnetic resonance findings predicting mortality in patients with pulmonary arterial hypertension: a systematic review and meta-analysis. Eur Radiol 2016; 26: 3771–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Badagliacca R, Poscia R, Pezzuto B, et al. Right ventricular remodeling in idiopathic pulmonary arterial hypertension: adaptive versus maladaptive morphology. J Heart Lung Transplant 2015; 34: 395–403. [DOI] [PubMed] [Google Scholar]
- 111.Hassoun PM, Zamanian RT, Damico R, et al. Ambrisentan and tadalafil up-front combination therapy in scleroderma-associated pulmonary arterial hypertension. Am J Respir Crit Care Med 2015; 192: 1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Van Wolferen SA, Marcus JT, Boonstra A, et al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J 2007; 28: 1250–1257. [DOI] [PubMed] [Google Scholar]
- 113.Darsaklis K, Dickson ME, Cornwell W, 3rd, et al. Right atrial emptying fraction non-invasively predicts mortality in pulmonary hypertension. Int J Cardiovasc Imaging 2016; 32: 1121–1130. [DOI] [PubMed] [Google Scholar]
- 114.Swift AJ, Rajaram S, Condliffe R, et al. Pulmonary artery relative area change detects mild elevations in pulmonary vascular resistance and predicts adverse outcome in pulmonary hypertension. Invest Radiol 2012; 47: 571–577. [DOI] [PubMed] [Google Scholar]
- 115.Gan CT, Lankhaar JW, Westerhof N, et al. Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest 2007; 132: 1906–1912. [DOI] [PubMed] [Google Scholar]
- 116.Dawes TJW, De Marvao A, Shi W, et al. Machine learning of three-dimensional right ventricular motion enables outcome prediction in pulmonary hypertension: a cardiac MR imaging study. Radiology 2017; 283: 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bello GA, Dawes TJW, Duan J, et al. Deep learning cardiac motion analysis for human survival prediction. Nat Mach Intell 2019; 1: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Swift AJ, Lu H, Uthoff J, et al. A machine learning cardiac magnetic resonance approach to extract disease features and automate pulmonary arterial hypertension diagnosis. Eur Heart J Cardiovasc Imaging. Epub ahead of print 2020, Jan 30: jeaa001.. [DOI] [PMC free article] [PubMed]
- 119.Sweatt AJ, Hedlin HK, Balasubramanian V, et al. Discovery of distinct immune phenotypes using machine learning in pulmonary arterial hypertension. Circ Res 2019; 124: 904–919. [DOI] [PMC free article] [PubMed] [Google Scholar]