Abstract
Background and Aims:
Therapeutic drug monitoring (TDM) of infliximab (IFX) and anti-infliximab antibodies (ATIs) is essential for treatment optimisation in inflammatory bowel disease (IBD) patients. The aim of this study was to estimate and compare the agreement and accuracy between a new rapid test and three established enzyme-linked immunosorbent assays (ELISAs) to quantify ATIs levels, and to evaluate the impact of exogenous IFX on the performance of these assays.
Methods:
We analysed 200 serum samples from 57 IBD outpatients in IFX induction or maintenance therapy at six IBD centres in Portugal. ATI levels were quantified using the rapid test Quantum Blue® (QB) Anti-Infliximab (Bühlmann) and three established ELISAs: In-House, Theradiag (Lisa Tracker Anti-Infliximab), and Immundiagnostik (IDKmonitor Infliximab). ATIs were quantified in patients’ serum samples and spiked samples with exogenous IFX, based on analytical and clinical cutoffs. Qualitative agreement and accuracy were estimated by Cohen’s kappa (k) with 95% confidence intervals.
Results:
ATIs quantification with clinical cutoffs showed a slight agreement between QB rapid test and In-House [k = 0.163 (0.051–0.276)] and Immundiagnostik [k = 0.085 (0.000–0.177)]. Regarding IFX/ATIs status, the QB rapid test showed a substantial agreement with Theradiag [k = 0.808 (0.729–0.888)] and a fair agreement with In-House [k = 0.343 (0.254–0.431)] and Immundiagnostik [k = 0.217 (0.138–0.297)]. The QB rapid test could not detect ATI-positive levels in samples with exogenous IFX at 5–300 µg/ml. Interference on ATIs detection was observed at exogenous IFX ⩾30 µg/ml for In-house and Immundiagnostik assays.
Conclusion:
QB rapid test is only suitable to detect ATI-positive levels in the absence of IFX.
Keywords: antibodies, drug monitoring, enzyme-linked immunosorbent assay, immunoassay, inflammatory bowel diseases, infliximab, point-of-care systems
Introduction
Infliximab (IFX) is a therapeutic monoclonal antibody against tumour necrosis factor alpha (TNF-α). IFX is effective in lowering disease activity and inducing clinical remission in patients with inflammatory bowel disease (IBD).1 However, up to 30% of patients fail to respond to induction treatment (primary loss of response), and 50% of patients may lose response during maintenance treatment (secondary loss of response), many during the first year.2,3 This loss of response to IFX therapy may occur due to several reasons, including the development of anti-drug antibodies (ADAs).4 ADAs may neutralise drug-target binding and increase drug clearance, resulting in suboptimal concentrations of active drug and shorter duration of response.5–7 Previous studies have shown that up to 44% of IBD patients treated with IFX develop anti-IFX antibodies (ATIs), depending on dosing schedules, concomitant use of steroids or immunomodulators, and measurement methods.8–12 When loss of response occurs, physicians usually change the therapeutic strategy by increasing the dosage or frequency of the current drug therapy, switch to another TNF-α antagonist, or switch to a different class of drug with another mode of action.13 However, this empirical approach increases the risk of irreversible tissue damage and healthcare costs, and could delay effective IBD treatment.1,14 Therefore, the assessment of drug and ADA levels, also known as therapeutic drug monitoring (TDM), is essential to define future therapeutic strategies. The specific assessment of IFX and ATI levels allows physicians to understand the reasons for unresponsiveness, identify patients that will most benefit from the dose adjustment of current IFX therapy or from switching to another drug,7,14,15 and reduce delays in effective treatment.1,14 TDM is thus essential to define therapeutic strategies in IBD patients, improving clinical outcomes and minimising IBD-related complications.
TDM has led to the development of methods for quantification of IFX and ATI levels with different applications and limitations. Of particular concern is that some methods quantify both IFX and ATIs, whereas others are specific for only one of these quantifications, which may have a significant impact on TDM’s results and interpretation.3,16,17 Several commercial kits measure IFX levels in the patient’s serum, most of them relying on enzyme-linked immunosorbent assays (ELISAs).16,18,19 However, these assays have a turnaround time of approximately 8 h, which might impair immediate adjustment of IFX therapy. In contrast, recent developments in rapid point-of-care tests allow the semi-quantitative assessment of ATI levels from the patient’s serum within minutes.20,21 In fact, the recent development of a rapid test to ATIs quantification (Quantum Blue® Anti-Infliximab, Bühlmann) promise a fast detection of ATIs (15–20 min turnaround time) on a single sample.20 These assays facilitate TDM and immediate adjustment of the IFX dosage. Nevertheless, the use of point-of-care tests for the quantification of ATIs in clinical practice is still limited by a lack of data and there is a need to evaluate their inter-assay heterogeneity and accuracy.22 Also, the impact of detectable IFX levels in patients’ serum on the TDM is understudied, particularly for the quantification of ATI levels by already established ELISAs.3,16,17,23 This indicates a need to evaluate and compare the performance of the various assays currently used in clinical practice to quantify ATI levels, to improve clinical decision-making based on TDM.
With this study we aimed to estimate and compare the accuracy and agreement between a new rapid test and three different established ELISAs for quantifying ATI levels in the serum of IBD patients. We also aimed to evaluate the impact of exogenous IFX on the performance of the four assays. We selected the recently commercially available rapid test Quantum Blue® Anti-Infliximab (Bühlmann) and the established In-House, Lisa Tracker Anti-Infliximab (Theradiag), and IDKmonitor Infliximab (Immundiagnostik) assays for quantification of ATI levels using analytical or clinical cutoff levels.
Methods
Patients and sample collection
This was a multicentre, non-interventional, retrospective study. From July 2016 to August 2019, 200 clinical samples were collected at six IBD centres in Portugal from 57 IBD patients attending routine outpatient consultations. The study population comprised patients who were adults (⩾18 years), male or female, diagnosed with moderate-to-severe active Crohn’s disease or ulcerative colitis, primary responders to IFX induction doses were assessed clinically and endoscopically, and received at least three IFX maintenance doses.
The clinical samples were obtained from patients undergoing the induction or maintenance treatment phase, and immediately before the infusion of a new IFX dose. Collected baseline sociodemographic and clinical data included birth date, date of diagnosis, sex, smoking status, diagnosis of Crohn’s disease or ulcerative colitis, and concomitant IBD-related medication. Blood samples were collected, centrifuged, and serum samples were kept at −80°C until being processed.
Potentially eligible samples were identified based on the previous quantification of ATI levels in our laboratory using our reference method (In-House assay). Samples were consecutively chosen to cover clinically relevant cutoff points for ATI negativity (<1.7 μg/ml) and positivity (⩾1.7 μg/ml) defined in the literature.17,24 ATI samples were included according to the following cutoff levels: negative, <1.7 μg/ml; low, 1.7–2.9 µg/ml; intermediate, 3.0–9.9 μg/ml; and high, ⩾10 μg/ml. Trough IFX concentrations were previously measured for all samples as part of the clinical routine using Quantum Blue® Infliximab (Bühlmann, Schönenbuch, Switzerland). More detailed information about the assays and protocols can be found in Supporting Information The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committees of each centre. All patients signed a written informed consent before their participation.
Quantification of ATI levels
All samples were analysed with the rapid point-of-care test Quantum Blue® Anti-Infliximab assay (Bühlmann, Schönenbuch, Switzerland), hereafter referred to as QB rapid test, according to the manufacturers’ instructions (Supporting Information). The ATI levels were calculated as IgG equivalents to the monoclonal reference antibody used for standardisation (μgeq/ml) and hereafter expressed as μg/ml. The following three ELISAs were used as comparators: In-House assay, Lisa Tracker anti-Infliximab (Theradiag, Croissy Beaubourg, France), and IDKmonitor Infliximab total ADA ELISA (Immundiagnostik, Bensheim, Germany). The quantifications using Theradiag and Immundiagnostik were performed following manufacturers’ instructions,25–27 whereas the In-House assays were carried out as previously described by Ben-Horin et al.17,28 (Supporting Information). The lower and upper detection limits for ATI levels described by the manufacturers were as follows: QB rapid test, 0.6–12 μg/ml; In-House assay, lower limit of 1.2 μg/ml; Theradiag, 0.01–0.2 μg/ml; and Immundiagnostik, higher average optical densities >10 antibody units (AU)/ml were classified as positive. All kits and samples were used and processed by the same technician.
Because the four assays tested have different technical characteristics, detection limits and expression of results, the test positivity cutoffs for a qualitative evaluation of the ATI levels are difficult to establish. Therefore, we used analytical and clinical cutoffs to test ATI-positive (ATI+) levels. The analytical cutoffs were based on the lower detection limits described by the manufacturers for each assay, while the clinical cutoffs used clinically relevant ATI+ levels defined in the literature.17,24 Using the analytical cutoffs, ATI+ levels were defined as ATI levels ⩾0.6 μg/ml for QB rapid test, ⩾1.2 μg/ml for In-House, ⩾0.01 μg/ml for Theradiag, and ⩾10 AU/ml for Immundiagnostik. Using the clinical cutoffs, ATI+ levels were defined as ATI levels ⩾1.7 µg/ml for QB rapid test, In-House and Theradiag, and ⩾10 AU/ml for Immundiagnostik.
Exogenous IFX in ATI-positive serum samples
To assess the impact of IFX in the quantification of ATI levels, exogenous IFX (Schering Plough, New Jersey, USA) was added to ATI+ serum samples with undetectable IFX concentrations (IFX–) <0.4 μg/ml. ATI+ serum samples with low, intermediate, and high ATI levels were selected – six different samples were selected for each ATIs group. Serum samples with ATI+ levels and IFX- concentrations were preincubated with several exogenous IFX concentrations (5, 10, 15, 30, 100 and 300 μg/ml) for 30 min at room temperature, as previously described by our group.23 The therapeutic range of IFX concentrations was considered to be between 0 and 100 μg/ml.29 ATI levels in samples with different IFX/ATI levels status were then quantified by the four assays as described above.
Statistical analyses
Categorical variables were described as absolute (n) and relative frequencies (%), and continuous variables were shown as the median and interquartile range (IQR). The quantitative agreement between assays could not be assessed because data was reported using different and arbitrary units (AU/ml). Therefore, the qualitative agreement of ATI levels or IFX/ATI levels status between pairs of assays was determined using Cohen’s kappa (k) coefficients and accuracy with 95% confidence intervals (CIs). The Cohen’s k coefficients were categorised according to the criteria of Landis and Koch: ⩽0.000 no agreement, 0.000–0.200 slight, 0.210–0.400 fair, 0.410–0.600 moderate, 0.610–0.800 substantial and 0.810–1.000 almost perfect agreement.30 Accuracy percentages of 0–4% were considered no accuracy, 4–15% minimal, 15–35% weak, 35–63% moderate, 64–81% strong and 82–100% almost perfect accuracy.31 Accuracy is the agreement between value found and an excepted reference value and the agreement refers to the closeness of two measured values, not to whether those values are correct or not (estimated by the kappa coefficient).
IFX/ATI levels status were stratified in four combinations of detectable (IFX+) or undetectable exogenous IFX and ATI-negative (ATI-) or ATI+ levels as follows: IFX+/ATI-, IFX+/ATI+, IFX-/ATI+, and IFX-/ATI-. To assess the impact of exogenous IFX concentrations on the quantification of ATI levels, graphical analyses plotted the mean of six measurements from six different samples (one measurement per sample), of ATI levels versus increasing exogenous IFX concentrations in spiked serum samples, by quantification assay, for each group of patients’ serum samples with low, intermediate, or high ATI+ levels. Statistical analysis was performed using SPSS version 24.0 (IBM Corp, Armonk, NY) and the graphical representation was performed using GraphPad Prism version 8.3.0 (GraphPad Software, Inc., San Diego, CA).
Results
Study population
This study analysed 200 serum samples collected from 57 IBD patients under IFX therapy. Table 1 shows the baseline demographic and clinical characteristics of the patients. Briefly, patients had a median age at diagnosis of 29 (19–36) years, 56.1% were female, 57.9% never smoked, 14.0% were current smokers, and 28.1% were former smokers. A total of 70.2% of patients had Crohn’s disease and 29.8% had ulcerative colitis; 22 patients (38.6%) were under concomitant immunosuppression (azathioprine or methotrexate).
Table 1.
Characteristics of patients with IBD treated with infliximab.
| Patients (n = 57) | |
|---|---|
| Age at diagnosis, median (IQR), years | 29 (19–36) |
| Gender, n (%) | |
| Female | 32 (56.1) |
| Male | 25 (43.9) |
| Crohn’s disease, n (%) | 40 (70.2) |
| Ulcerative colitis, n (%) | 17 (29.8) |
| Smoking status, n (%) | |
| Never smoker | 33 (57.9) |
| Former smoker | 16 (28.1) |
| Current smoker | 8 (14.0) |
| Concomitant IBD-related medication, n (%) | |
| None | 21 (36.8) |
| Azathioprine | 19 (33.3) |
| Steroids | 9 (15.8) |
| Methotrexate | 3 (5.3) |
| Oral 5-aminosalicylates | 5 (8.8) |
| Time under biological therapy, median (min–max), months | 6 (1–20) |
| IFX mg/kg, median (min–max) | 6 (5–10) |
| Number of IFX received, median (min–max) | 3 (0–12) |
| Dose intervals, median (min–max) | 7 (5–8) |
| Dose optimization, n (%) | |
| No | 47 (82.5) |
| Yes | 10 (17.5) |
| Albumin g/l, median (min–max) | 41.9 (29.3–66.4) |
IBD, inflammatory bowel disease; IFX, infliximab, IQR, interquartile range; n, number of patients.
Agreement for ATI+ levels
Qualitative agreement and accuracy of the QB rapid test and three established ELISAs was determined by quantifying the ATI levels in patients’ serum samples and stratifying the results into analytical and clinical cutoffs.
When stratified by analytical cutoffs for ATI+ levels (QB rapid test ⩾0.6 µg/ml, In-House 1.2 µg/ml, Theradiag 0.01 µg/ml, and Immundiagnostik 10 AU/ml), ATI+ levels were detected in 48 (24.0%) samples with the QB rapid test, 161 samples (80.5%) with In-House, 65 (32.5%) samples with Theradiag and 158 (79.0%) samples with Immundiagnostik. As shown in Table 2, a moderate agreement was found between the QB rapid test and Theradiag (k = 0.489), while a slight agreement was observed between the QB rapid test and In-House (k = 0.160) and QB rapid test and Immundiagnostik (k = 0.139). Comparisons between the remaining assay pairs revealed fair agreements (Table 2).
Table 2.
Qualitative agreement between ATIs+ levels: comparison between assay pairs stratified by analytical and clinical cutoffs.
| Assay comparison | Accuracy (95% CI) |
Cohen’s kappa (95% CI) |
|---|---|---|
| Analytical cutoffs a | ||
| QB rapid test versus In-House | 51 (44–57) | 0.160 (0.102–0.217) |
| QB rapid test versus Theradiag | 75 (69–81) | 0.489 (0.384–0.595) |
| QB rapid test versus Immundiagnostik | 43 (36–50) | 0.139 (0.086–0.192) |
| In-House versus Theradiag | 70 (63–75) | 0.403 (0.301–0.505) |
| In-House versus Immundiagnostik | 80 (73–85) | 0.388 (0.235–0.541) |
| Theradiag versus Immundiagnostik | 67 (60–73) | 0.375 (0.276–0.474) |
| Clinical cutoffs b | ||
| QB rapid test versus In-House | 49 (41–56) | 0.163 (0.051–0.276) |
| QB rapid test versus Theradiag | 85 (79–90) | – |
| QB rapid test versus Immundiagnostik | 35 (29–42) | 0.085 (0.000–0.177) |
| In-House versus Theradiag | 34 (27–41) | – |
| In-House versus Immundiagnostik | 72 (65–78) | 0.289 (0.133–0.445) |
| Theradiag versus Immundiagnostik | 20 (15–26) | – |
ATIs+ levels: ⩾0.6 μg/ml for QB rapid test, ⩾1.2 μg/ml for In-House, ⩾0.01 μg/ml for Theradiag, and ⩾10 AU/ml for Immundiagnostik.
ATIs+ levels: ⩾1.7 µg/ml for QB rapid test, In-House and Theradiag, and ⩾10 AU/ml for Immundiagnostik.
ATIs+, anti-infliximab antibodies-positive; CI, confidence interval; QB, Quantum Blue.
Based on clinical cutoffs for ATI+ levels (QB rapid test, In-House, Theradiag ⩾1.7 μg/ml and Immundiagnostik > 10 AU/ml), QB rapid test detected 30 (15.0%) samples, Theradiag did not detect ATI+ samples, and In-House and Immundiagnostik detected the highest number of samples, 140 (70.0%) and 160 (80.0%), respectively. A total of 32% of the values negative with our threshold (<1.7 µg/ml) turn out positive with lower limit of quantification cutoff are under 0.010 µg/ml. In fact, these values might reflect only the intra-variability of the assay rather than represent the presence of antibodies. Although the In-House and Immundiagnostik assays detected an approximate number of ATI+ samples, not all samples matched. ATI+ levels were confirmed by both assays in 123 samples (61.5%). All 30 ATI+ samples identified by the QB rapid test were also positive in both the In-House and Immundiagnostik assays. As can be seen from Table 2, using the clinical cutoffs, a slight agreement was found between the QB rapid test and In-House (k = 0.163) or QB rapid test and Immundiagnostik (k = 0.085). The comparison of the In-House versus Immundiagnostik pair showed a fair agreement (k = 0.289). The k coefficient could not be calculated for the comparisons with Theradiag as this assay did not detect ATI+ samples.
Agreement for trough IFX and ATI levels status
The accuracy and agreement of IFX/ATI levels status between pairs of assays were also evaluated. The patients’ serum samples were divided into four IFX/ATI levels status, using both analytical and clinical cutoffs, resulting in IFX+ concentrations in 90 (45.0%) or 80 (40.0%) of the 200 samples, respectively. The number of IFX/ATIs levels status for each assay in addition to the comparisons between tests can be assessed in Supplementary Table S1.
As shown in Table 3, considering the analytical cutoffs, the QB rapid test did not detect IFX+/ATI+ samples and Theradiag detected only one, corresponding to one of the IFX+/ATI+ samples detected by In-House. All assays were able to detect samples with the remaining IFX/ATI levels status. Overall, a strong accuracy was found between the In-House and the Immundiagnostik assays (80%) with a substantial agreement (k = 0.661). A strong accuracy was also found between the Theradiag and the QB rapid test (75%) or In-House assays (70%) with a substantial agreement (k = 0.625) or a moderate agreement (k = 0.531), respectively (Table 3).
Table 3.
Qualitative agreement regarding the IFX/ATIs levels status: comparison between assays pairs stratified by analytical and clinical cutoffs.
| Assay comparison |
n (%) |
Accuracy (95% CI) |
Cohen’s kappa (95% CI) |
|||
|---|---|---|---|---|---|---|
| IFX+/ATIs+ | IFX+/ATIs– | IFX–/ATIs+ | IFX–/ATIs– | |||
| Analytical cutoffs a | ||||||
| QB rapid test versus In-House | 0 (0.0%) | 42 (21.0%) | 46 (23.0%) | 3 (1.5%) | 45 (39–53) | 0.299 (0.211–0.388) |
| QB rapid test versus Theradiag | 0 (0.0%) | 89 (44.5%) | 46 (23.0%) | 15 (7.5%) | 75 (68–81) | 0.625 (0.535–0.715) |
| QB rapid test versus Immundiagnostik | 0 (0.0%) | 50 (25.0%) | 46 (23.0%) | 0 (0.0%) | 43 (36–50) | 0.275 (0.185–0.360) |
| In-House versus Theradiag | 1 (0.5%) | 42 (21.0%) | 94 (47.0%) | 2 (1.0%) | 70 (63–76) | 0.531 (0.433–0.629) |
| In-House versus Immundiagnostik | 30 (15.0%) | 22 (11.0%) | 107 (53.5%) | 0 (0.0%) | 80 (73–85) | 0.661 (0.568–0.753) |
| Theradiag versus Immundiagnostik | 0 (0.0%) | 49 (24.5%) | 95 (47.5%) | 0 (0.0%) | 68 (61–74) | 0.507 (0.407–0.606) |
| Clinical cutoffs b | ||||||
| QB rapid test versus In-House | 0 (0.0%) | 52 (26.0%) | 30 (15.0%) | 15 (7.5%) | 49 (41–57) | 0.343 (0.254–0.431) |
| QB rapid test versus Theradiag | 0 (0.0%) | 80 (40.0%) | 0 (0.0%) | 90 (45.5%) | 89 (84–93) | 0.808 (0.729–0.888) |
| QB rapid test versus Immundiagnostik | 0 (0.0%) | 41 (20.5%) | 30 (15.0%) | 1 (0.5%) | 35 (29–42) | 0.217 (0.138–0.297) |
| In-House versus Theradiag | 0 (0.0%) | 52 (26.0%) | 0 (0.0%) | 15 (7.5%) | 34 (27–41) | 0.219 (0.142–0.296) |
| In-House versus Immundiagnostik | 13 (6.5%) | 24 (12.0%) | 105 (52.5%) | 1 (0.5%) | 72 (65–78) | 0.531 (0.428–0.634) |
| Theradiag versus Immundiagnostik | 0 (0.0%) | 41 (20.5%) | 0 (0.0%) | 1 (0.5%) | 20 (15–26) | 0.129 (0.069–0.189) |
ATIs+ levels: ≥0.6 μg/ml for QB rapid test, ≥1.2 μg/ml for In-House, ≥0.01 μg/ml for Theradiag, and ≥10 AU/ml for Immundiagnostik. IFX+ levels: ≥0.4 µg/ml.
ATIs+ levels: ≥1.7 µg/ml for QB rapid test, In-House and Theradiag, and ≥10 AU/ml for Immundiagnostik. IFX+ levels: ≥0.4 µg/ml.
ATIs, anti-infliximab antibodies; ATIs+, ATIs-positive levels; ATIs -, ATIs-negative levels; CI, confidence interval; IFX, infliximab; IFX+, IFX -positive levels; IFX -, undetectable IFX levels; QB, Quantum Blue; n, number of matching samples between assays for each IFX/ATIs status in a total of 200 samples.
Regarding the clinical cutoffs for the quantification of ATI levels, only the In-House and Immundiagnostik assays detected IFX+/ATI+ samples (Table 3). Comparing with the analytical cutoffs, the In-House and Immundiagnostik assays identified a similar number of samples in each IFX/ATI status. Conversely, the QB rapid test and Theradiag identified a higher number of IFX-/ATI- samples and a lower number of IFX +/ATI– samples. All assays were able to identify the remaining IFX/ATI status samples except for the Theradiag, which did not detect IFX–/ATI+ samples. An almost perfect accuracy was found between the pair QB rapid test and Theradiag (89%) with an almost perfect agreement (k = 0.808). The pair In-House and Immundiagnostik showed a strong accuracy (72%) and a moderate agreement (k = 0.531).
Effect of exogenous IFX on ATI quantification
The impact of IFX on the quantification of ATI levels was evaluated by measuring spiked ATI+ serum samples (5, 10, 15, 30, 100 and 300 μg/ml IFX) with the four assays, based on the clinical cutoffs for ATI+ levels.
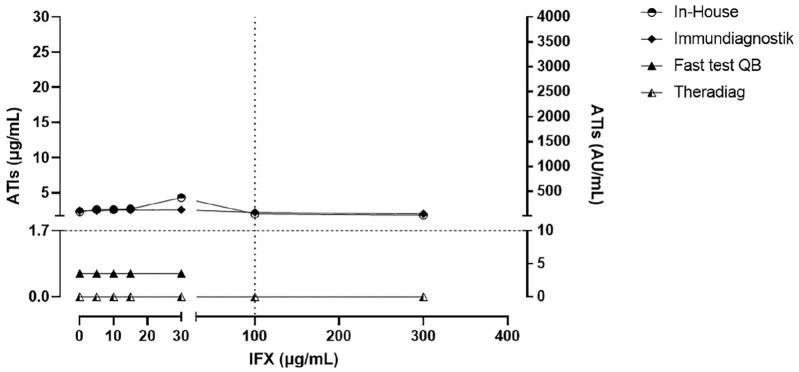
Figure 1 displays the results in the samples with low ATI levels (1.7–2.9 µg/ml). No impact of exogenous IFX was evident in the Immundiagnostik assay. An IFX concentration of 30 µg/ml influenced the In-House assay by an additive concentration-effect; however, this influence was not evident at higher concentrations. In contrast, both the QB rapid test and Theradiag assays could not detect ATI+ levels (>1.7 µg/ml) in samples with all IFX concentrations. Moreover, the QB rapid test indicated invalid values in the samples containing exogenous IFX concentrations >30 μg/ml.
Figure 1.
Low anti-infliximab antibodies levels (1.7–2.9 µg/ml) quantified by QB rapid test, In-House, Theradiag and Immundiagnostik assays in the presence of exogenous infliximab. The horizontal dotted line is the lower limit for positive levels of ATIs using the clinical cutoffs (1.7 μg/ml for QB rapid test, In-House and Theradiag, and 10 AU/ml for Immundiagnostik). The vertical dotted line is the upper limit of the therapeutic range of infliximab concentrations (0–100 μg/ml).
ATIs, anti-infliximab antibodies; IFX, infliximab; QB, Quantum Blue.
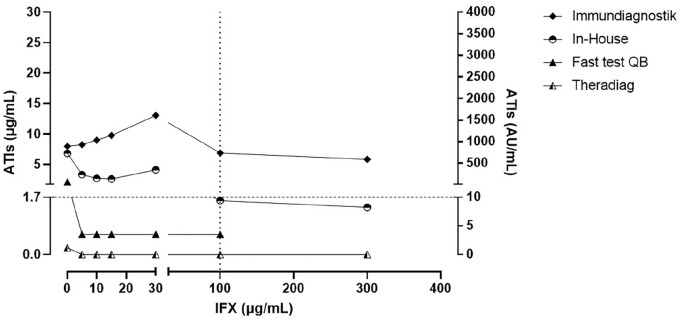
Figure 2 presents the results in the samples with intermediate ATI levels (3.0–9.9 μg/ml). The impact of exogenous IFX was more evident in the Immundiagnostik and In-House assays with a decrease in ATI levels. In the presence of 30 μg/ml IFX, these assays were influenced by an additive concentration-effect, however, only the Immundiagnostik assay could detect ATI+ levels at the higher concentrations of exogenous IFX. The QB rapid test and Theradiag assays could not detect ATI+ levels with IFX concentrations from 5 to 30 µg/ml or with all concentrations, respectively. The QB rapid test indicated invalid values in samples containing IFX concentrations >100 µg/ml.
Figure 2.
Intermediate anti-infliximab antibodies levels (3.0–9.9 µg/ml) quantified by QB rapid test, In-House, Theradiag, and Immundiagnostik assays in the presence of exogenous infliximab. The horizontal dotted line is the lower limit for positive levels of ATIs using the clinical cutoffs (1.7 μg/ml for QB rapid test, In-House and Theradiag, and 10 AU/ml for Immundiagnostik). The vertical dotted line is the upper limit of the therapeutic range of infliximab concentrations (0–100 μg/ml). QB rapid test indicated invalid values in some samples in the 100 µg/ml IFX concentrations.
ATIs anti-infliximab antibodies; IFX, infliximab; QB, Quantum Blue.
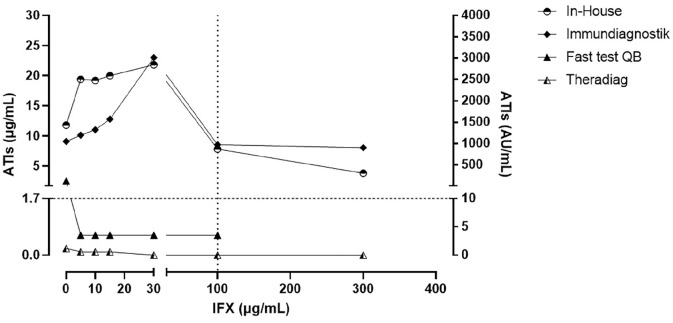
Figure 3 shows the results in the samples with high ATI levels (⩾10 μg/ml). The In-House and Immundiagnostik assays were able to detect ATI+ levels at all exogenous IFX concentrations. As described above, both assays showed an additive effect at 30 μg/ml IFX and ATI levels decreased at 100 μg/ml IFX. Similarly, the QB rapid test and Theradiag assays could not detect ATI+ levels in samples with IFX concentrations from 5 to 300 µg/ml or with all concentrations, respectively.
Figure 3.
High anti-infliximab antibodies levels (⩾10 µg/ml) quantified by QB rapid test, In-House, Theradiag, and Immundiagnostik assays in the presence of exogenous infliximab. The horizontal dotted line is the lower limit for positive levels of ATIs using the clinical cutoffs (1.7 μg/ml for QB rapid test, In-House and Theradiag, and 10 AU/ml for Immundiagnostik). The vertical dotted line is the upper limit of the therapeutic range of infliximab concentrations (0–100 μg/ml).
ATIs, anti-infliximab antibodies; IFX, infliximab; QB, Quantum Blue.
Discussion
IFX is an effective therapy to the treatment of IBD.32–34 However many patients may lose response to treatment due to ATI.35 The ATIs measurement is crucial to adjust the therapy or switch to another drug. The most commonly used assays to evaluate ATI levels are ELISAs,36 which are very time-consuming. Therefore, the development of a rapid anti-IFX test allows a rapid quantification of ATIs, increasing the effectiveness of TDM and the immediate adjustment of the drug.20,21 In these present study, we evaluated and compared the qualitative agreement and accuracy of one rapid point-of-care test and three established ELISAs. Moreover, the impact of IFX on the quantification of ATI levels by the four assays was evaluated. IFX and ATI levels were measured in 200 serum samples from 57 IBD patients undergoing induction or maintenance therapy with IFX.
By using analytical and clinical-based cutoffs for defining ATI+ levels, we showed that the In-House and Immundiagnostik assays detected similar numbers of ATI+ samples with both cutoffs. On the other hand, the QB rapid test and Theradiag assays detected a higher number of ATI+ samples using the analytical cutoffs compared with the clinical ones. These results suggest a high prevalence of false negatives for the QB rapid test and Theradiag assays using clinical cutoffs. This finding is consistent with previous data obtained with the Theradiag assay.17,27,37 Several factors could explain these observations, such as drug interference underestimating ATI levels.16,17,38 The presence of IFX in the patient’s serum interferes with the binding of the marked IFX to the captured ATI, leading to false-negative results.17,27,37 The assays’ inability to detect ATI in the presence of IFX may render inconclusive test results.17,27,37
We next focused on the quantification of ATI levels in patients’ serum samples with different status for trough IFX and ATI levels (detectable or undetectable). We showed that the In-House and Immundiagnostik assays were more accurate and could detect ATI+ samples in the presence of IFX. However, in the presence of IFX, the QB rapid test did not accurately detect ATI+ levels using both analytical and clinical cutoffs. A kappa analysis to the IFX-/ATIs samples was also performed and the QB rapid test improves its capacity to detect ATIs in the absence of the drug (Supplementary Table S2). Furthermore, there was a disagreement between the QB rapid test and the In-House or Immundiagnostik assays in the quantification of ATI+ samples. These findings have clinical relevance and reinforces that the QB rapid test is affected by drug interference. Then, our results show that the QB rapid test and Theradiag measure only free ATIs s detecting a lower amount of ATI+ samples when compared with In-House Immundiagnostik assays. The ability to detect ATI in the presence of the IFX is important, as it was shown that IBD patients with both good IFX trough levels (⩾3 μg/ml) and ATI+ levels have significantly higher levels of C-reactive protein and less mucosal healing during treatment,7,39 which indicates a reduced control of inflammation mediated by these antibodies even when drug levels are adequate. Our results show that the disagreement increase when the samples had a double-positive or double-negative status, probably related to the specific limitations of each assay. This disagreement can also occur due to the cutoff point chosen to discriminate the ATIS positive from the ATIs negative. This led us to define two different approaches – clinical and analytical approach. The clinical approach seems to highlight the assays’ differences. Disagreement increased when samples had double-negative status, probably related to the fact that the QB rapid test detect a greater number of ATI- than the remaining assays. This disagreement could be explained due to the specific limitations and characteristics of each assay.
QB rapid test and Theradiag are drug-sensitive ATI assays, while In-House and Immundiagnostik are drug-tolerant ATI assays. Drug-sensitive ATI assays measures only free antibodies not bound to infliximab, detecting a lower amount of ATI+ samples when compared with drug-tolerant ATI assays. A recent study shows evidence that there is a different clinical interpretation of results when using drug-sensitive versus drug-tolerant assays.40 The choice of the cutoff to discriminate positive versus negative also enhances disagreement.
To better understand the impact of IFX on the quantification of ATI, we performed additional experiments using IFX- serum samples incubated with different concentrations of exogenous IFX. We were able to evaluate which IFX concentrations decreased each assays’ ability to quantify ATI+ levels. Notably, the addition of exogenous IFX concentrations corresponding to concentrations detected in clinical practice resulted in undetectable ATI levels by the QB rapid test. Using clinical cutoffs, this test could not detect ATIs in serum with intermediate (3.0–9.9 µg/ml) and high (⩾10 µg/ml) ATI+ levels in the presence of 5–300 µg/ml exogenous IFX concentrations. In contrast, the Immundiagnostik and In-house assays were slightly affected by the lowest concentrations of exogenous IFX. Also, these assays were able to detect ATI up to 300 μg/ml of IFX in serum with low, intermediate, and high ATI+ levels. We have previously described the same drug concentration dependency in these assays.23
These results show that the ATIs detected are affected by the drug. In this sense, our results show that the QB rapid test and Theradiag are drug-sensitive assays and the In-House and Immundiagnostik are drug-tolerant assays. Clinicians who use these data should have a general understanding of the assay methods to be able to interpret and implement the results. Therefore, these assays should not be interchangeably, and their results should not be directly compared.
The main limitation of this study was the measurement of ATIs levels performed on a single plate and only once, not allowing conclusions about inter and intra assays variability. Furthermore, all the ATI assays used in this study are non-functional assays (not detecting the neutralizing antibodies). In this study, patients were not followed up and it was not possible take conclusions about the relationship between the drug response and the ATI status. Moreover, with emerging reports on transient antibodies, it would be prudent to first ascertain the antibody persistence before making clinical decisions based on a single measurement of ATI levels.41 Further prospective studies with larger patient cohorts are needed to confirm and validate the findings of this study. Although the findings should be interpreted with caution, a key strength of this study is the large number of serum samples obtained from a multicentric and real-world heterogeneous cohort of IBD patients. Finally, the wide range of ATI+ levels allowed to evaluate the assays’ performance both at low and high levels. However, it is important to distinguish clinically between patients with ATIs < 3.7 µg/ml and >10 µg/ml, since patients with low ATIs levels are more susceptible to dose optimisation while patients with high ATIs levels usually require switch to another drug.
The findings of this study have several important implications for future practice. Clinicians should be aware that treatment optimisation may differ according to the assay used for TDM. The QB rapid test could not accurately detect ATI+ samples in the presence of IFX. In the optimization of the treatment, clinicians should be aware that the results of the different IFX/ATI status may differ according to the assays. The choice of the assay will probably have little influence on therapeutic decisions in the IFX+/ATIs– (change of drug class) and IFX–/ATIs+ status (change of anti-TNFα antibody drug), since agreement between assays is significantly higher in these circumstances. However, the agreement between assays was weaker when patients had double-negative (IFX–/ATI–) or double-positive (IFX+/ATI+) status. In these situations, erroneous therapeutic decisions may occur. Dose optimisation, shorter interval (IFX–/ATI–) and change of drug class or concomitant use of immunomodulators (IFX+/ATI+) should take into account the fact that the results are assay dependent. A reasonable approach to tackle this issue could be using the QB rapid test to quantify ATI levels only in IFX- samples, after performing another rapid point-of-care test to quantify the IFX levels in the patients’ serum samples.
In conclusion, we have shown that the QB rapid test can be used for the quantification of ATI levels in serum samples with undetectable IFX levels but should not be used in samples with IFX concentrations ⩾0.4 µg/ml. The comparison of qualitative agreements and accuracies between the QB rapid test and the In-House, Theradiag, and Immundiagnostik ELISAs suggest that these assays are not interchangeable for the quantification of ATI levels in IBD patients’ serum. These findings are particularly relevant for physicians when making clinical decisions about IBD treatment optimization based on ATIs quantification assays.
Supplemental Material
Supplemental material, sj-pdf-1-tag-10.1177_1756284820965790 for Rapid test detection of anti-infliximab antibodies: performance comparison with three different immunoassays by Cátia Rocha, Paula Lago, Samuel Fernandes, Luís Correia, Francisco Portela, Ana Isabel Vieira, Marta Patita, Bruno Arroja, Paula Ministro, Catarina Alves, Cláudia Camila Dias and Fernando Magro in Therapeutic Advances in Gastroenterology
Supplemental material, sj-pdf-2-tag-10.1177_1756284820965790 for Rapid test detection of anti-infliximab antibodies: performance comparison with three different immunoassays by Cátia Rocha, Paula Lago, Samuel Fernandes, Luís Correia, Francisco Portela, Ana Isabel Vieira, Marta Patita, Bruno Arroja, Paula Ministro, Catarina Alves, Cláudia Camila Dias and Fernando Magro in Therapeutic Advances in Gastroenterology
Acknowledgments
The authors would like to thank Bühlmann (Schönenbuch, Switzerland) for kindly providing the Quantum Blue® Anti-Infliximab kits used in this study and all investigators at the hospitals who provided samples. Moreover, the authors would like to express their gratitude to Sandra Dias for her involvement as the GEDII coordinator and all the help during data collection. The authors would also like to acknowledge the medical writers Ana Morgado, and Sofia Nunes, from Scientific ToolBox Consulting (Lisbon, Portugal) for providing editorial assistance, which was funded by GEDII. CR acknowledge their grant (PDE/BDE/114583/2016) from Portuguese national funds by Fundação para a Ciência e Tecnologia (FCT).
Footnotes
Author contributions: FM supervised the study and contributed to study conception or design. PL, SF, LC, FP, AV, MP, BA, and PM were involved in the recruitment of patients and collection of samples. CR contributed to quantification assays. CR, CCD, and FM contributed to the analysis and interpretation of data. CR and FM contributed to the drafting of the manuscript. PL, SF, LC, FP, AV, MP, BA, PM, CA, CCD, and FM critical revised the manuscript. All authors approved the final version of the manuscript, including the authorship list and take responsibility for the accuracy and integrity of any part of the work.
Conflict of interest statement: Fernando Magro served as a speaker and received honoraria from Abbvie, Biogen, Falk, Ferring, Hospira, Laboratorios Vitoria, Merck Sharp & Dohme, and Vifor Pharma. All other authors declare no conflicts of interest.
Data sharing and data accessibility: All the data generated is not suitable for sharing beyond that contained within the report. Further information can be obtained from the corresponding author.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded in part by the Portuguese IBD Study Group (GEDII), and in part by PDE/BDE/114583/2016, from Portuguese national funds by Fundação para a Ciência e Tecnologia (FCT).
Guarantor: Fernando Magro
ORCID iDs: Cláudia Camila Dias  https://orcid.org/0000-0001-9356-3272
https://orcid.org/0000-0001-9356-3272
Fernando Magro  https://orcid.org/0000-0003-2634-9668
https://orcid.org/0000-0003-2634-9668
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Cátia Rocha, Department of Biomedicine, Unit of Pharmacology and Therapeutics, Faculty of Medicine, University of Porto, Porto, Portugal; Faculty of Medicine, University of Lisbon, Lisbon, Portugal; Institute of Environmental Health, Faculty of Medicine, University of Lisbon, Lisbon, Portugal.
Paula Lago, Department of Gastroenterology, Centro Hospitalar do Porto, Porto, Portugal.
Samuel Fernandes, Department of Gastroenterology and Hepatology, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria, Lisbon, Portugal.
Luís Correia, Department of Gastroenterology and Hepatology, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria, Lisbon, Portugal.
Francisco Portela, Department of Gastroenterology, Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal.
Ana Isabel Vieira, Department of Gastroenterology, Hospital Garcia de Orta, Almada, Portugal.
Marta Patita, Department of Gastroenterology, Hospital Garcia de Orta, Almada, Portugal.
Bruno Arroja, Department of Gastroenterology, Hospital de Braga, Braga, Portugal.
Paula Ministro, Department of Gastroenterology, Centro Hospitalar Tondela-Viseu, Viseu, Portugal.
Catarina Alves, Faculty of Medicine, University of Porto, Porto, Portugal.
Cláudia Camila Dias, Center for Health Technology and Services Research (CINTESIS), Faculty of Medicine, University of Porto, Porto, Portugal; Health Information and Decision Sciences Department, Faculty of Medicine, University of Porto, Porto, Portugal.
Fernando Magro, Department of Biomedicine, Unit of Pharmacology and Therapeutics, Faculty of Medicine, University of Porto, Alameda Prof. Hernâni Monteiro, Porto, 4200-319, Portugal; Portuguese IBD Study Group (GEDII), Porto, Portugal; Department of Gastroenterology, São João Hospital Centre, Porto, Portugal.
References
- 1. Bendtzen K. Personalized medicine: theranostics (therapeutics diagnostics) essential for rational use of tumor necrosis factor-alpha antagonists. Discov Med 2013; 15: 201–211. [PubMed] [Google Scholar]
- 2. Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther 2011; 33: 987–995. [DOI] [PubMed] [Google Scholar]
- 3. Steenholdt C, Ainsworth MA, Tovey M, et al. Comparison of techniques for monitoring infliximab and antibodies against infliximab in Crohn’s disease. Ther Drug Monit 2013; 35: 530–538. [DOI] [PubMed] [Google Scholar]
- 4. Moss AC. Optimizing the use of biological therapy in patients with inflammatory bowel disease. Gastroenterol Rep 2015; 3: 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hindryckx P, Novak G, Vande Casteele N, et al. Incidence, prevention and management of anti-drug antibodies against therapeutic antibodies in inflammatory bowel disease: a practical overview. Drugs 2017; 77: 363–377. [DOI] [PubMed] [Google Scholar]
- 6. Roda G, Jharap B, Neeraj N, et al. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol 2016; 7: e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yanai H, Lichtenstein L, Assa A, et al. Levels of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin Gastroenterol Hepatol 2015; 13: 522–530.e2. [DOI] [PubMed] [Google Scholar]
- 8. Farrell RJ, Alsahli M, Jeen YT, et al. Intravenous hydrocortisone premedication reduces antibodies to infliximab in Crohn’s disease: a randomized controlled trial. Gastroenterology 2003; 124: 917–924. [DOI] [PubMed] [Google Scholar]
- 9. Ainsworth MA, Bendtzen K, Brynskov J. Tumor necrosis factor-alpha binding capacity and anti-infliximab antibodies measured by fluid-phase radioimmunoassays as predictors of clinical efficacy of infliximab in Crohn’s disease. Am J Gastroenterol 2008; 103: 944–948. [DOI] [PubMed] [Google Scholar]
- 10. Wolbink GJ, Aarden LA, Dijkmans BAC. Dealing with immunogenicity of biologicals: assessment and clinical relevance. Curr Opin Rheumatol 2009; 21: 211–215. [DOI] [PubMed] [Google Scholar]
- 11. Vermeire S, Noman M, Van Assche G, et al. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut 2007; 56: 1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van Assche G, Haens GD, Carbonez A, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med 2003; 348: 601–608. [DOI] [PubMed] [Google Scholar]
- 13. Gomollón F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis 2017; 11: 3–25. [DOI] [PubMed] [Google Scholar]
- 14. Steenholdt C, Bendtzen K, Brynskov J, et al. Clinical implications of measuring drug and anti-drug antibodies by different assays when optimizing infliximab treatment failure in Crohn’s disease: post hoc analysis of a randomized controlled trial. Am J Gastroenterol 2014; 109: 1055–1064. [DOI] [PubMed] [Google Scholar]
- 15. Roblin X, Rinaudo M, Del Tedesco E, et al. Development of an algorithm incorporating pharmacokinetics of adalimumab in inflammatory bowel diseases. Am J Gastroenterol 2014; 109: 1250–1256. [DOI] [PubMed] [Google Scholar]
- 16. Vande Casteele N, Buurman DJ, Sturkenboom MGG, et al. Detection of infliximab levels and anti-infliximab antibodies: a comparison of three different assays. Aliment Pharmacol Ther 2012; 36: 765–771. [DOI] [PubMed] [Google Scholar]
- 17. Kopylov U, Mazor Y, Yavzori M, et al. Clinical utility of antihuman lambda chain-based enzyme-linked immunosorbent assay (ELISA) versus double antigen ELISA for the detection of anti-infliximab antibodies. Inflamm Bowel Dis 2012; 18: 1628–1633. [DOI] [PubMed] [Google Scholar]
- 18. Li M, Li H, Gao K, et al. A simple and cost-effective assay for measuring anti-drug antibody in human patients treated with Adalimumab. J Immunol Methods 2018; 452: 6–11. [DOI] [PubMed] [Google Scholar]
- 19. Spencer EA, Dubinsky MC. Therapeutic drug monitoring in inflammatory bowel disease: history and future directions. Pediatr Clin North Am 2017; 64: 1309–1326. [DOI] [PubMed] [Google Scholar]
- 20. Corp BD. Anti-Infliximab Antibodies. https://buhlmannlabs.com/products-solutions/quantum-blue/quantum-blue-tdm/anti-infliximab-antibodies/ (2020, accessed 1 April 2020).
- 21. Bantleon F, Schneider M, Ricken B, et al. P202 Quantum blue anti-infliximab: development and evaluation of a point of care rapid test for measuring anti-infliximab antibodies in human serum. J Crohns Colitis 2019; 13(Suppl. 1): S194–S195. [Google Scholar]
- 22. Shankar G, Devanarayan V, Amaravadi L, et al. Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products. J Pharm Biomed Anal 2008; 48: 1267–1281. [DOI] [PubMed] [Google Scholar]
- 23. Afonso J, Lopes S, Gonçalves R, et al. Detection of anti-infliximab antibodies is impacted by antibody titer, infliximab level and IgG4 antibodies: a systematic comparison of three different assays. Therap Adv Gastroenterol 2016; 9: 781–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ungar B, Anafy A, Yanai H, et al. Significance of low level infliximab in the absence of anti-infliximab antibodies. World J Gastroenterol 2015; 21: 1907–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Stappen T, Billiet T, Vande Casteele N, et al. An optimized anti-infliximab bridging enzyme-linked immunosorbent assay for harmonization of anti-infliximab antibody titers in patients with inflammatory bowel diseases. Inflamm Bowel Dis 2015; 21: 2172–2177. [DOI] [PubMed] [Google Scholar]
- 26. Detrez I, Dreesen E, Van Stappen T, et al. Variability in golimumab exposure: a ‘Real-Life’ Observational Study in active ulcerative colitis. J Crohns Colitis 2016; 10: 575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Imaeda H, Andoh A, Fujiyama Y. Development of a new immunoassay for the accurate determination of anti-infliximab antibodies in inflammatory bowel disease. J Gastroenterol 2012; 47: 136–143. [DOI] [PubMed] [Google Scholar]
- 28. Ben-Horin S, Yavzori M, Katz L, et al. The immunogenic part of infliximab is the F(ab’)2, but measuring antibodies to the intact infliximab molecule is more clinically useful. Gut 2011; 60: 41–48. [DOI] [PubMed] [Google Scholar]
- 29. Adedokun OJ, Sandborn WJ, Feagan BG, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology 2014; 147: 1296–1307.e5. [DOI] [PubMed] [Google Scholar]
- 30. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–174. [PubMed] [Google Scholar]
- 31. Mchugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012; 22: 276–282. [PMC free article] [PubMed] [Google Scholar]
- 32. Caviglia R, Ribolsi M, Rizzi M, et al. Maintenance of remission with infliximab in inflammatory bowel disease: efficacy and safety long-term follow-up. World J Gastroenterol 2007; 13: 5238–5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hyams J, Crandall W, Kugathasan S, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn’s disease in children. Gastroenterology 2007; 132: 863–873. [DOI] [PubMed] [Google Scholar]
- 34. Hyams J, Damaraju L, Blank M, et al. Induction and maintenance therapy with infliximab for children with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol 2012; 10: 391–399.e1. [DOI] [PubMed] [Google Scholar]
- 35. Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with Inflammatory Bowel Disease (IBD): a meta-analysis. Am J Gastroenterol 2012; 108: 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Freeman K, Connock M, Auguste P, et al. Clinical effectiveness and cost-effectiveness of use of therapeutic monitoring of tumour necrosis factor alpha (TNF-α) inhibitors [LISA-TRACKER® enzyme-linked immunosorbent assay (ELISA) kits, TNF-α-Blocker ELISA kits and Promonitor® ELISA kits] versus standard care in patients with Crohn’s disease: systematic reviews and economic modelling. Health Technol Assess 2016; 20: 1–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pérez I, Fernández L, Sánchez-ramón S, et al. Reliability evaluation of four different assays for therapeutic drug monitoring of infliximab levels. Therap Adv Gastroenterol 2018; 11: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Atiqi S, Hooijberg F, Loeff FC, et al. Immunogenicity of TNF-inhibitors. Front Immunol 2020; 11: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vande Casteele N, Khanna R, Levesque BG, et al. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn’s disease. Gut 2015; 64: 1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Van Stappen T, Vande Casteele N, Van Assche G, et al. Clinical relevance of detecting anti-infl iximab antibodies with a drug-tolerant assay: post hoc analysis of the TAXIT trial. Gut 2018; 67: 818–826. [DOI] [PubMed] [Google Scholar]
- 41. Vande Casteele N, Ballet V, Van Assche G, et al. Early serial trough and antidrug antibody level measurements predict clinical outcome of infliximab and adalimumab treatment. Gut 2012; 61: 321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tag-10.1177_1756284820965790 for Rapid test detection of anti-infliximab antibodies: performance comparison with three different immunoassays by Cátia Rocha, Paula Lago, Samuel Fernandes, Luís Correia, Francisco Portela, Ana Isabel Vieira, Marta Patita, Bruno Arroja, Paula Ministro, Catarina Alves, Cláudia Camila Dias and Fernando Magro in Therapeutic Advances in Gastroenterology
Supplemental material, sj-pdf-2-tag-10.1177_1756284820965790 for Rapid test detection of anti-infliximab antibodies: performance comparison with three different immunoassays by Cátia Rocha, Paula Lago, Samuel Fernandes, Luís Correia, Francisco Portela, Ana Isabel Vieira, Marta Patita, Bruno Arroja, Paula Ministro, Catarina Alves, Cláudia Camila Dias and Fernando Magro in Therapeutic Advances in Gastroenterology