Abstract
Objective:
The aim of this study was to investigate the association of extra-musculoskeletal manifestations (EMMs) with disease activity, functional status, and treatment patterns in a large population-based cohort of patients with axial spondyloarthritis (axSpA).
Methods:
A stratified random sample of patients with axSpA, drawn from health insurance data, received a survey on disease-related characteristics including history (ever presence) of the following EMMs: inflammatory bowel disease (IBD), psoriasis (PSO), and anterior uveitis (AU). Survey data were linked to health insurance data, gathering additional information on current occurrence (within one year) of EMMs and drug prescriptions. Separate multivariable linear regression models were calculated to determine the association of EMMs with disease activity (Bath Ankylosing Spondylitis Disease Activity Index), and functional status (Bath Ankylosing Spondylitis Functional Index) after adjustment for relevant parameters, including treatment.
Results:
A total of 1729 patients with axSpA were included in the analyses (response: 47%; mean age: 56 years; 46% female) of whom 6% (9%) had current (ever) IBD, 10% (15%) had current (ever) PSO, and 9% (27%) had current (ever) AU. Ever presence of IBD and history of PSO were significantly associated with higher level of disease activity. Ever presence of PSO was also associated with higher level of functional impairment, whereas current AU was significantly associated with lower disease activity. Patients with current IBD or PSO received more frequently biological and conventional synthetic disease-modifying anti-rheumatic drugs as well as systemic steroids. AU was associated with a higher use of conventional synthetic disease-modifying anti-rheumatic drugs only.
Conclusion:
Disease activity is higher in patients with axSpA with history of IBD or history of PSO. Functional impairment is also higher in patients with axSpA with history of PSO. The presence of different EMMs was associated with different treatment patterns in axSpA.
Keywords: anterior uveitis, axial spondyloarthritis, disease activity, functional status, inflammatory bowel disease, psoriasis, treatment patterns
Introduction
Axial spondyloarthritis (axSpA) is a chronic inflammatory rheumatic disease that predominantly affects the spine and/or sacroiliac joints and encompasses nonradiographic axSpA [without definite radiographic sacroiliitis (nr-axSpA)] and radiographic axSpA [also termed ankylosing spondylitis (AS) characterized by the presence of radiographic sacroiliitis according to the radiographic criterion of the modified New York criteria].1 In addition to spinal inflammation and structural damage, peripheral symptoms (peripheral arthritis, enthesitis, dactylitis) and extra-musculoskeletal manifestations (EMMs) such as inflammatory bowel disease (IBD), psoriasis (PSO), and anterior uveitis (AU) are common and contribute to the total burden of axSpA.2
Results from a recent meta-analysis found EMMs to be equally prevalent in AS and nr-axSpA, except for AU, which was slightly more prevalent in AS. The pooled prevalence of IBD was 4.1% in AS versus 6.4% in nr-axSpA, of PSO 10.2% in AS versus 10.9% in nr-axSpA, and of AU 23.0% in AS versus 15.9% in nr-axSpA.3 In contrast to studies on the prevalence of EMMs, studies on their association with disease outcomes and treatment pattern in axSpA are scarce. Some evidence on the effect of IBD was obtained within the DESIR cohort, a prospective observational cohort of patients with a high probability of early spondyloarthritis (SpA), where the authors found IBD to be associated with higher disease activity in early SpA.4 Results from the OASIS cohort, a longstanding AS cohort, showed, however, no association between the presence of EMMs and functional status over time.5
The objective of this population-based study was to investigate the association of IBD, PSO, and AU with disease activity, functional status, and treatment patterns in a large sample of patients with axSpA by taking advantage of data linkage within the Linking Patient-Reported Outcomes with CLAIms data for health services research in Rheumatology (PROCLAIR) network.
Patients and methods
Study design
Using data of a large health insurance fund in Germany, a total of 21,892 patients with a diagnosis of axSpA [International Classification of Diseases, 10th Revision (ICD-10) code M45] in at least two quarters of the year 2014 were identified. Of those, a random sample of 5000 patients, stratified by age and sex, was selected and received a survey by mail in 2015 (a reminder was sent out to those patients who had not answered within 4 weeks). The survey was developed in collaboration with a focus group and included validated, widely used self-report tools to collect data on disease-related characteristics in patients with axSpA. A description of the study design in more detail can be found in.7
Survey data comprised information on demographic, socioeconomic, and disease-related parameters including information on disease activity, assessed using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI),8 functional status, assessed using the Bath Ankylosing Spondylitis Functional Index (BASFI),9 psychological well-being, assessed using the five-item World Health Organization Well-Being Index (WHO-5),10 and diagnostic delay. The latter was calculated as the difference between the age at axSpA diagnosis and age at back pain onset. Moreover, patient-reported information on the history (ever presence) of IBD, PSO, and AU was gathered. Survey data were linked to health insurance data from 2015 to gather additional information, which was not collected by the survey, including drug prescriptions, comorbidities, and current/recent occurrence (i.e. within the 12 months in 2015) of IBD (ICD-10 codes K50-K51), PSO (ICD-10 code L40), and AU (ICD-10 code H20), where at least one outpatient claim had to be documented. Comorbidities were defined using the Elixhauser coding algorithms,11 slightly modified by excluding rheumatoid arthritis/collagen vascular diseases and including osteoporosis (ICD-10 codes M80-M82) and fibromyalgia (ICD-10 code M79.7). A more detailed description of this definition can be found in.12
Pharmacological treatment
Treatment of axSpA and EMMs was investigated using drug prescriptions in outpatient care in 2015 based on the pharmacological treatment group according to the Anatomical Therapeutic Chemical (ATC) classification. Pharmacological treatment included biological disease-modifying anti-rheumatic drugs (bDMARDs), nonsteroidal anti-inflammatory drugs (NSAIDs), opioids, nonopioid analgesics, systemic steroids, local (injectable) steroids, and conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs), although initiation of a bDMARD, csDMARD or systemic steroids could also be triggered by an EMM. Specific treatments of EMMs covered IBD-related treatment with intestinal topical steroids, PSO-related treatment with topical antipsoriatics (including steroids) and systemic oral antipsoriatics, and AU-related treatment with ophthalmologicals (including mydriatics and topical steroids). The respective ATC codes are shown in Supplemental Table S1. The proportion of prescriptions made by rheumatologists, gastroenterologists, dermatologists, and ophthalmologists for bDMARDs, csDMARDs, and systemic steroids is presented in Supplemental Table S2.
Statistical analysis
The total number of persons with linked survey and health insurance data was weighted according to the sex and age group distribution of the source population of 21,892 patients with a diagnosis of axSpA. Weighted subgroup analyses were performed on those with confirmed axSpA diagnosis. Missing data values were not imputed (variables had a maximum of 4% missing values, except for the variable HLA-B27 status having 31% missing values).
Differences between patients with axSpA with and without an EMM (separately for IBD, PSO, and AU) were examined using descriptive statistics [means, standard errors of the mean (SEMs), and percentages]. The SEM was used instead of the standard deviation to take the stratified nature of the study design into account. Significant differences were assessed using t-tests for continuous variables and Rao–Scott chi-square tests otherwise. Tests resulting in p-values <0.05 were considered statistically significant.
Univariable and multivariable linear regression models were calculated to analyze the association of (1) history of EMMs and (2) current/recent occurrence of EMMs with disease activity and functional impairment [separately for (a) IBD, (b) PSO, and (c) AU; with a focus on explanation]. Variables included in the multivariable models were chosen using backward selection whereby age, sex, and IBD [only model (a)], PSO [only model (b)], and AU [only model (c)] were always included in the respective models. Moreover, the set of tested variables comprised symptom duration, in rheumatologic care, HLA-B27, body mass index (BMI), lack of exercise, smoking, suffering from stress, physical therapy, and pharmacological treatment. A significance level of 0.05 was required for a variable to stay in the multivariable model. Parameter estimates (β) were calculated with 95% confidence intervals (CIs).
Data analyses were performed with SAS 9.4 (SAS Institute Inc., Cary, NC, USA) using procedures for complex survey designs.
Patient and public involvement
A focus group was set up and actively contributed to the development of the survey design.
Results
A total of 2118 patients responded to the survey (47%), among whom 2082 patients gave their consent for linking survey data to health insurance data. Of those, a total 1776 patients confirmed their axSpA diagnosis (85%), among whom 1729 patients had valid data for the history of IBD, PSO, and AU and were therefore included in the analysis. The mean age was 56 years, and 46% were female. Both characteristics were comparable to those patients who did not respond to the survey. Furthermore, the mean number of comorbidities was also comparable between responders and nonresponders, while the mean number of prescribed pharmaceuticals was higher among responders.
Prevalence and specific pharmacological treatment of extra-musculoskeletal manifestations
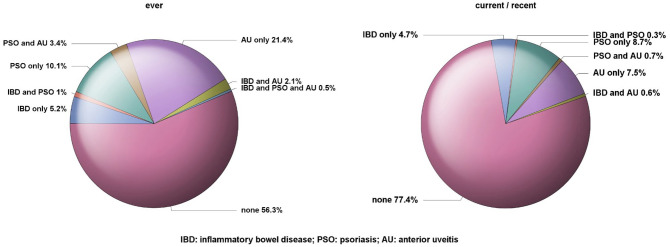
The prevalence for the ever (survey data) and current/recent occurrence (health insurance data for the 12 months of the period of interest) of EMMs is illustrated by Figure 1. A history of IBD was reported by 9% of the patients, among whom 6% received intestinal steroids (Table 1), and 6% had current IBD, among whom 10% received intestinal steroids (Table 2). Among the 15% of patients with a history of PSO, 26% received topical antipsoriatics and 0.8% systemic oral antipsoriatics respectively (Table 1), whereas among the 10% of patients with current PSO, 29% received topical antipsoriatics and 1.2% systemic oral antipsoriatics respectively (Table 2). A history of AU was reported by 27% of the patients with axSpA and 9% had current AU, among whom more than half received ophthalmologicals including 48% with ophthalmic topical steroids and 22% with mydriatics (Table 2). In 22.6% (43.7%) of the patients at least one EMM was currently (ever) present, including 1.6% (6.9%) with two EMMs currently (ever) present, and 0% (0.5%) with all three conditions currently (ever) present (Figure 1).
Figure 1.
Prevalence of inflammatory bowel disease, psoriasis, and anterior uveitis in patients with axial spondyloarthritis (n = 1729).
Table 1.
Main demographic, disease-related, and lifestyle characteristics of patients with axial spondyloarthritis (n = 1729) stratified by the history (ever presence; survey data) of inflammatory bowel disease, psoriasis, and anterior uveitis.
| Inflammatory bowel disease |
Psoriasis |
Anterior uveitis |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes (n = 150) | No (n = 1579) | p-value | Yes (n = 255) | No (n = 1474) | p-value | Yes (n = 463) | No (n = 1266) | p-value | |
| Sex, female (%) | 45.3 | 46.2 | 0.8380 | 47.7 | 45.8 | 0.5911 | 42.2 | 47.6 | 0.0475 |
| Age, years (mean ± SEM) | 54.6 ± 0.9 | 56.1 ± 0.1 | 0.1530 | 57.0 ± 0.7 | 55.8 ± 0.2 | 0.1227 | 58.1 ± 0.5 | 55.1 ± 0.2 | <0.0001 |
| Symptom duration, years (mean ± SEM) | 23.7 ± 1.1 | 25.4 ± 0.3 | 0.1425 | 26.4 ± 0.9 | 25.0 ± 0.3 | 0.1700 | 29.8 ± 0.6 | 23.5 ± 0.3 | <0.0001 |
| Diagnostic delay, years (mean ± SEM) | 5.6 ± 0.6 | 5.6 ± 0.2 | 0.9986 | 6.8 ± 0.6 | 5.4 ± 0.2 | 0.0171 | 5.6 ± 0.3 | 5.6 ± 0.2 | 0.9752 |
| In rheumatologic care (%) | 51.4 | 45.2 | 0.1543 | 56.6 | 43.8 | 0.0002 | 46.8 | 45.4 | 0.5958 |
| HLA-B27 positive (%) | 89.3 | 86.0 | 0.3656 | 80.2 | 87.5 | 0.0101 | 90.4 | 84.6 | 0.0097 |
| BASDAI, 0–10 (mean ± SEM) | 5.0 ± 0.2 | 4.4 ± 0.0 | 0.0024 | 4.9 ± 0.1 | 4.4 ± 0.1 | <0.0001 | 4.4 ± 0.1 | 4.5 ± 0.1 | 0.4266 |
| BASFI, 0–10 (mean ± SEM) | 4.6 ± 0.2 | 4.0 ± 0.1 | 0.0080 | 4.7 ± 0.2 | 4.0 ± 0.1 | <0.0001 | 4.2 ± 0.1 | 4.0 ± 0.1 | 0.1349 |
| Number of comorbiditiesa (mean ± SEM) | 2.7 ± 0.2 | 2.5 ± 0.0 | 0.3478 | 2.8 ± 0.1 | 2.4 ± 0.0 | 0.0045 | 2.5 ± 0.1 | 2.5 ± 0.1 | 0.8463 |
| Body mass index, kg/m2 (mean ± SEM) | 26.8 ± 0.4 | 27.0 ± 0.1 | 0.7071 | 27.9 ± 0.3 | 26.8 ± 0.1 | 0.0039 | 27.0 ± 0.2 | 27.0 ± 0.1 | 0.9744 |
| WHO-5, 0–100 (mean ± SEM) | 37.8 ± 1.8 | 45.6 ± 0.5 | <0.0001 | 40.0 ± 1.3 | 45.7 ± 0.6 | <0.0001 | 46.6 ± 1.0 | 44.2 ± 0.6 | 0.0409 |
| Smoking (current, %) | 22.9 | 18.5 | 0.2012 | 21.8 | 18.3 | 0.1970 | 17.6 | 19.3 | 0.4137 |
| Suffering from stress (%) | 46.0 | 38.8 | 0.0968 | 41.6 | 39.0 | 0.4494 | 37.5 | 40.1 | 0.3382 |
| Lack of exercise (%) | 26.8 | 23.9 | 0.4418 | 25.0 | 24.0 | 0.7321 | 21.0 | 25.3 | 0.0732 |
| Physical therapy (%) | 51.0 | 52.4 | 0.7428 | 51.7 | 52.4 | 0.8367 | 54.5 | 51.4 | 0.2704 |
| Pharmacological treatment (%) | |||||||||
| NSAIDs | 45.8 | 60.4 | 0.0006 | 60.9 | 58.8 | 0.5371 | 59.9 | 58.9 | 0.6905 |
| Nonopioid analgesics | 29.5 | 21.7 | 0.0309 | 24.0 | 22.0 | 0.4907 | 20.3 | 23.1 | 0.2316 |
| Opioids | 20.6 | 15.4 | 0.0989 | 18.1 | 15.4 | 0.2789 | 15.2 | 16.1 | 0.6767 |
| bDMARDs | 25.7 | 16.3 | 0.0034 | 19.8 | 16.7 | 0.2178 | 18.3 | 16.7 | 0.4271 |
| mAb against TNF (adalimumab, golimumab, infliximab) | 19.3 | 10.5 | 0.0011 | 12.4 | 11.1 | 0.5608 | 13.3 | 10.6 | 0.1102 |
| Certolizumab pegol | 2.8 | 1.2 | 0.0818 | 2.2 | 1.2 | 0.2202 | 1.3 | 1.4 | 0.9062 |
| Etanercept | 4.8 | 5.3 | 0.7745 | 6.5 | 5.1 | 0.3483 | 4.2 | 5.7 | 0.1972 |
| Secukinumab | – | 0.1 | – | 0.5 | – | – | 0.3 | – | – |
| Ustekinumab | 0.7 | – | – | – | 0.1 | – | 0.2 | – | – |
| Vedolizumab | 1.2 | – | – | – | 0.1 | – | – | 0.1 | – |
| Otherb | – | 0.1 | – | – | 0.1 | – | – | 0.1 | – |
| csDMARDs | 33.7 | 11.8 | <0.0001 | 21.3 | 12.4 | 0.0001 | 14.2 | 13.5 | 0.7305 |
| Methotrexate | 6.7 | 5.5 | 0.5695 | 11.7 | 4.6 | <0.0001 | 6.3 | 5.4 | 0.4843 |
| Sulfasalazine | 8.0 | 5.9 | 0.2945 | 7.3 | 5.9 | 0.3807 | 5.4 | 6.4 | 0.4356 |
| Mesalazine | 16.7 | 0.4 | <0.0001 | 2.1 | 1.8 | 0.7629 | 2.1 | 1.8 | 0.6938 |
| Azathioprine | 6.9 | 0.0 | <0.0001 | 0.8 | 0.6 | 0.7913 | 0.5 | 0.7 | 0.6000 |
| Leflunomide | 0.7 | 0.9 | 0.7851 | 1.7 | 0.7 | 0.1470 | 0.4 | 1.0 | 0.1912 |
| Otherc | 1.4 | 0.4 | 0.0870 | 1.2 | 0.4 | 0.0874 | 0.9 | 0.3 | 0.1191 |
| Systemic steroids | 31.1 | 17.3 | <0.0001 | 23.1 | 17.8 | 0.0461 | 18.6 | 18.5 | 0.9804 |
| Local injectable steroids | 2.0 | 1.3 | 0.5069 | 0.4 | 1.6 | 0.1759 | 1.4 | 1.4 | 0.9180 |
| Intestinal topical steroids | 6.3 | 0.1 | <0.0001 | 0.8 | 0.7 | 0.7754 | – | 0.9 | – |
| Topical antipsoriatics | 16.6 | 12.4 | 0.1526 | 26.0 | 10.4 | <0.0001 | 12.3 | 12.9 | 0.7349 |
| Steroids | 16.6 | 12.2 | 0.1257 | 24.9 | 10.4 | <0.0001 | 12.3 | 12.6 | 0.8694 |
| Dithranol | – | 0.1 | – | 0.8 | – | – | 0.3 | 0.1 | 0.3024 |
| Vitamin D analogs | – | 0.5 | – | 2.4 | 0.1 | <0.0001 | 0.4 | 0.4 | 0.9814 |
| Systemic oral antipsoriatics | – | 0.2 | – | 0.8 | 0.1 | 0.0092 | – | 0.2 | – |
| Acitretin | – | 0.1 | – | – | 0.1 | – | – | 0.1 | – |
| Fumaric acid | – | 0.1 | – | 0.8 | – | – | – | 0.2 | – |
| Ophthalmologicals | 18.9 | 18.2 | 0.8319 | 20.3 | 17.9 | 0.3815 | 32.6 | 12.9 | <0.0001 |
| Ophthalmic topical steroids | 12.2 | 11.4 | 0.7638 | 9.9 | 11.8 | 0.3966 | 25.6 | 6.2 | <0.0001 |
| Mydriatics | 3.2 | 2.0 | 0.3194 | 2.1 | 2.1 | 0.9884 | 7.2 | 0.2 | <0.0001 |
| Ophthalmic NSAIDs | – | 0.9 | – | 0.4 | 0.9 | 0.4458 | 2.3 | 0.3 | 0.0002 |
| Antibiotics | 12.5 | 8.7 | 0.1246 | 11.0 | 8.7 | 0.2313 | 12.6 | 7.7 | 0.0019 |
| Otherd | 2.2 | 5.2 | 0.1295 | 8.1 | 4.4 | 0.0133 | 6.0 | 4.5 | 0.2198 |
defined by Elixhauser coding algorithms, excluding rheumatoid arthritis/collagen vascular diseases and including osteoporosis and fibromyalgia.
including abatacept, tocilizumab.
including mycophenolic acid, teriflunomide, ciclosporin, tacrolimus, lenalidomide.
including antivirals, glaucoma medication, antihistamines, artificial tears.
Significant differences were assessed using t-tests for continuous variables and using Rao–Scott chi-square tests otherwise. p-values <0.05 are shown in bold.
BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; bDMARDs, biological disease-modifying anti-rheumatic drugs; csDMARDs, conventional synthetic disease-modifying anti-rheumatic drugs; mAb, monoclonal antibody; NSAIDs, nonsteroidal anti-inflammatory drugs; SEM, standard error of the mean; TNF, tumor necrosis factor; WHO-5, five-item World Health Organization Well-Being Index.
Table 2.
Main demographic, disease-related, and lifestyle characteristics of patients with axial spondyloarthritis (n = 1729) stratified by the current presence (in 2015; health insurance data) of inflammatory bowel disease, psoriasis, and anterior uveitis.
| Inflammatory bowel disease |
Psoriasis |
Anterior uveitis |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes (n = 98) | No (n = 1631) | p-value | Yes (n = 165) | No (n = 1564) | p-value | Yes (n = 151) | No (n = 1578) | p-value | |
| Sex, female (%) | 46.4 | 46.1 | 0.9588 | 46.9 | 46.0 | 0.8394 | 39.3 | 46.8 | 0.0787 |
| Age, years (mean ± SEM) | 52.8 ± 1.2 | 56.1 ± 0.1 | 0.0115 | 57.7 ± 0.9 | 55.8 ± 0.2 | 0.0433 | 55.4 ± 1.0 | 56.0 ± 0.2 | 0.5716 |
| Symptom duration, years (mean ± SEM) | 24.3 ± 1.4 | 25.3 ± 0.3 | 0.5158 | 27.5 ± 1.2 | 25.0 ± 0.3 | 0.0415 | 27.0 ± 1.1 | 25.1 ± 0.3 | 0.1020 |
| Diagnostic delay, years (mean ± SEM) | 6.3 ± 0.9 | 5.6 ± 0.2 | 0.4081 | 8.4 ± 0.9 | 5.3 ± 0.2 | 0.0005 | 5.3 ± 0.5 | 5.7 ± 0.2 | 0.4822 |
| In rheumatologic care (%) | 49.9 | 45.5 | 0.4060 | 60.1 | 44.2 | 0.0001 | 54.6 | 44.9 | 0.0273 |
| HLA-B27 positive (%) | 84.7 | 86.4 | 0.6753 | 74.5 | 87.7 | <0.0001 | 95.1 | 85.3 | 0.0030 |
| BASDAI, 0–10 (mean ± SEM) | 4.6 ± 0.2 | 4.5 ± 0.0 | 0.3868 | 4.8 ± 0.2 | 4.4 ± 0.1 | 0.0221 | 4.0 ± 0.2 | 4.5 ± 0.1 | 0.0021 |
| BASFI, 0–10 (mean ± SEM) | 4.3 ± 0.3 | 4.1 ± 0.1 | 0.4752 | 4.8 ± 0.2 | 4.0 ± 0.1 | 0.0003 | 3.7 ± 0.2 | 4.1 ± 0.1 | 0.0282 |
| Number of comorbiditiesa (mean ± SEM) | 2.7 ± 0.2 | 2.5 ± 0.0 | 0.3264 | 3.1 ± 0.2 | 2.4 ± 0.0 | 0.0004 | 2.3 ± 0.1 | 2.5 ± 0.1 | 0.0955 |
| Body mass index, kg/m2 (mean ± SEM) | 26.0 ± 0.4 | 27.0 ± 0.1 | 0.0097 | 28.0 ± 0.4 | 26.9 ± 0.1 | 0.0087 | 26.6 ± 0.3 | 27.0 ± 0.1 | 0.2563 |
| WHO-5, 0–100 (mean ± SEM) | 41.0 ± 2.3 | 45.1 ± 0.5 | 0.0792 | 41.9 ± 1.7 | 45.2 ± 0.5 | 0.0710 | 47.7 ± 1.7 | 44.6 ± 0.5 | 0.0864 |
| Smoking (current, %) | 20.2 | 18.8 | 0.7355 | 22.7 | 18.5 | 0.1990 | 15.2 | 19.2 | 0.2297 |
| Suffering from stress (%) | 42.3 | 39.3 | 0.5638 | 39.8 | 39.4 | 0.9099 | 42.5 | 39.1 | 0.4206 |
| Lack of exercise (%) | 24.5 | 24.1 | 0.9289 | 24.2 | 24.1 | 0.9779 | 15.1 | 25.0 | 0.0070 |
| Pharmacological treatment (%) | |||||||||
| NSAIDs | 39.5 | 60.3 | <0.0001 | 65.0 | 58.5 | 0.1115 | 58.9 | 59.2 | 0.9565 |
| Nonopioid analgesics | 24.3 | 22.2 | 0.6406 | 22.5 | 22.3 | 0.9482 | 15.9 | 23.0 | 0.0488 |
| Opioids | 18.8 | 15.7 | 0.4218 | 20.2 | 15.3 | 0.1081 | 15.9 | 15.8 | 0.9771 |
| bDMARDs | 32.6 | 16.2 | <0.0001 | 23.3 | 16.5 | 0.0285 | 21.5 | 16.7 | 0.1341 |
| mAb against TNF (adalimumab, golimumab, infliximab) | 26.2 | 10.4 | <0.0001 | 14.9 | 10.9 | 0.1286 | 15.6 | 10.9 | 0.0815 |
| Certolizumab pegol | 3.7 | 1.3 | 0.0348 | 2.7 | 1.2 | 0.1022 | 2.8 | 1.2 | 0.1259 |
| Etanercept | 4.7 | 5.2 | 0.7844 | 7.4 | 5.0 | 0.1877 | 4.5 | 5.4 | 0.6258 |
| Secukinumab | – | 0.1 | – | 0.7 | – | – | – | 0.1 | – |
| Ustekinumab | – | 0.1 | – | – | 0.1 | – | – | 0.1 | – |
| Vedolizumab | 1.8 | – | – | – | 0.1 | – | – | 0.1 | – |
| Otherb | – | 0.1 | – | – | 0.1 | – | – | 0.1 | – |
| csDMARDs | 44.2 | 11.9 | <0.0001 | 23.9 | 12.6 | <0.0001 | 20.3 | 13.1 | 0.0162 |
| Methotrexate | 7.0 | 5.5 | 0.5499 | 13.8 | 4.7 | <0.0001 | 10.2 | 5.2 | 0.0099 |
| Sulfasalazine | 10.8 | 5.8 | 0.0478 | 8.9 | 5.8 | 0.1088 | 7.9 | 5.9 | 0.3662 |
| Mesalazine | 22.9 | 0.6 | <0.0001 | 0.7 | 2.0 | 0.2558 | 1.4 | 1.9 | 0.7071 |
| Azathioprine | 10.9 | 0.0 | <0.0001 | – | 0.7 | – | 0.7 | 0.7 | 0.9818 |
| Leflunomide | – | 0.9 | – | 3.0 | 0.6 | 0.0016 | 1.2 | 0.8 | 0.5951 |
| Otherc | 1.0 | 0.4 | 0.4273 | 1.1 | 0.4 | 0.1994 | 0.7 | 0.5 | 0.6503 |
| Systemic steroids | 38.9 | 17.3 | <0.0001 | 25.1 | 17.8 | 0.0245 | 21.3 | 18.3 | 0.3645 |
| Local injectable steroids | 0.9 | 1.4 | 0.6246 | – | 1.6 | – | 2.0 | 1.3 | 0.4991 |
| Intestinal topical steroids | 9.7 | 0.1 | <0.0001 | – | 0.8 | – | – | 0.7 | – |
| Topical antipsoriatics | 12.6 | 12.8 | 0.9457 | 29.1 | 11.0 | <0.0001 | 14.6 | 12.6 | 0.4992 |
| Steroids | 12.6 | 12.5 | 0.9968 | 27.4 | 10.9 | <0.0001 | 14.6 | 12.3 | 0.4414 |
| Dithranol | – | 0.1 | – | 1.2 | – | – | – | 0.1 | – |
| Vitamin D analogs | – | 0.5 | – | 3.6 | 0.1 | <0.0001 | 0.5 | 0.4 | 0.8309 |
| Systemic oral antipsoriatics | – | 0.2 | – | 1.2 | 0.1 | 0.0006 | – | 0.2 | – |
| Acitretin | – | 0.1 | – | – | 0.1 | – | – | 0.1 | – |
| Fumaric acid | – | 0.1 | – | 1.2 | – | – | – | 0.1 | – |
| Dermatological topical steroids | 12.6 | 12.5 | 0.9968 | 27.4 | 10.9 | <0.0001 | 14.6 | 12.3 | 0.4414 |
| Ophthalmologicals | 17.3 | 18.3 | 0.7976 | 20.7 | 18.0 | 0.4069 | 52.3 | 15.0 | <0.0001 |
| Ophthalmic steroids | 12.2 | 11.4 | 0.8109 | 11.2 | 11.5 | 0.9226 | 48.3 | 7.9 | <0.0001 |
| Mydriatics | 2.8 | 2.1 | 0.6057 | 1.4 | 2.2 | 0.5056 | 22.2 | 0.2 | <0.0001 |
| Ophthalmic NSAIDs | 1.0 | 0.9 | 0.8632 | – | 1.0 | – | 6.2 | 0.3 | <0.0001 |
| Antibiotics | 10.4 | 8.9 | 0.6286 | 13.4 | 8.5 | 0.0424 | 15.0 | 8.4 | 0.0084 |
| Otherd | 2.4 | 5.1 | 0.2777 | 6.0 | 4.8 | 0.4802 | 5.5 | 4.9 | 0.7270 |
defined by Elixhauser coding algorithms, excluding rheumatoid arthritis/collagen vascular diseases and including osteoporosis and fibromyalgia.
including abatacept, tocilizumab.
including mycophenolic acid, teriflunomide, ciclosporin, tacrolimus, lenalidomide.
including antivirals, glaucoma medication, antihistamines, artificial tears.
Significant differences were assessed using t-tests for continuous variables and using Rao–Scott Chi-square tests otherwise. p-values <0.05 are shown in bold.
BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; bDMARDs, biological disease-modifying anti-rheumatic drugs; csDMARDs, conventional synthetic disease-modifying anti-rheumatic drugs; mAb, monoclonal antibody; NSAIDs, nonsteroidal anti-inflammatory drugs; SEM, standard error of the mean; TNF, tumor necrosis factor; WHO-5, five-item World Health Organization Well-Being Index.
Patient characteristics stratified by the presence of extra-musculoskeletal manifestations
The main demographic, disease-related, and socioeconomic characteristics of patients with axSpA stratified by the ever and current occurrence of EMMs are presented in Tables 1 and 2, respectively. Furthermore, the presence of comorbidities in patients with axSpA stratified by the ever and current occurrence of EMMs is shown in Supplemental Tables S3 and S4, respectively.
Patients with axSpA with a history of IBD were more often in rheumatological care (51% versus 45%), with higher disease activity (BASDAI: 5.0 versus 4.4) and functional impairment (BASFI: 4.6 versus 4.0) than patients with axSpA with no history of IBD. The mean number of comorbidities was also slightly increased in patients with axSpA with a history of IBD (2.7 versus 2.5), especially depression and fibromyalgia were more often present. Patients with and without a history of IBD were comparable regarding the lifestyle characteristics smoking, suffering from stress, lack of exercise, and BMI (Table 1), whereas the psychological well-being was significantly worse in patients with a history of IBD (WHO-5: 38 versus 46). Similar findings in terms of higher levels of disease activity and functional impairment among subjects with concurrent IBD were observed when examining the differences between patients with axSpA with and without current IBD (Table 2).
Patients with axSpA with a history of PSO were less often HLA-B27 positive and had a longer diagnostic delay than patients with axSpA with no history of PSO (Table 1). Furthermore, they were more often in rheumatologic care (57% versus 44%), with higher disease activity (BASDAI: 4.9 versus 4.4), functional impairment (BASFI: 4.7 versus 4.0), and number of comorbidities (2.8 versus 2.4) than patients with axSpA with no history of PSO. In particular, the number of patients with depression or fibromyalgia was increased among patients with axSpA with a history of PSO (Supplemental Table S3). Both groups were comparable in terms of the lifestyle characteristics smoking, suffering from stress, and lack of exercise (Table 1). However, patients with a history of PSO had a slightly higher BMI and a significantly lower psychological well-being (WHO-5: 40 versus 46). Comparing the groups of patients with and without current PSO led to similar results (Table 2).
Patients with axSpA with a history of AU were more frequently male and HLA-B27 positive than patients with axSpA with no history of AU. They were also older, had a longer symptom duration, and reported a better well-being as assessed by the WHO-5 index. Interestingly, patients with current/recent AU were more often in rheumatologic care (55% versus 45%) and had lower disease activity (BASDAI: 4.0 versus 4.5) and functional impairment (BASFI: 3.7 versus 4.1) than patients with axSpA with no current/recent AU. The mean number of comorbidities was also slightly decreased in patients with axSpA with current/recent AU (Table 2).
Treatment patterns stratified by the presence of extra-musculoskeletal manifestations
The number of patients receiving pharmacological treatment was increased among patients with axSpA with a history of IBD compared to patients with axSpA with no history of IBD, although those with a history of IBD were less often treated with NSAIDs (Table 1). There were also differences in pharmacological treatment with a higher frequency of bDMARDs (33% versus 16%), csDMARDs (44% versus 12%), and systemic steroids (39% versus 17%) in patients with axSpA with current IBD compared to those without current IBD. In particular, patients with axSpA with current IBD received considerably more often monoclonal antibodies against tumor necrosis factor (adalimumab, golimumab, infliximab), mesalazine, and azathioprine than patients with axSpA without current IBD (Table 2).
The number of patients receiving pharmacological treatment was increased among patients with axSpA with a history of PSO compared to those with no history of PSO, where the increase was statistically significant for csDMARDs (21% versus 12%), which was mainly attributable to an increased prescription of methotrexate (Table 1), and systemic steroids (23% versus 18%). In addition to treatment with csDMARDs and systemic steroids, the treatment with bDMARDs was also significantly higher in patients with axSpA with current PSO than in those without current PSO.
Patients with axSpA with and without history of AU were comparable in terms of pharmacological treatment, excluding ophthalmologicals (Table 1). The presence of current/recent AU (recorded within the 12 months of the period of interest) was, however, associated with a higher frequency of csDMARDs, especially the prescription of methotrexate was increased, and a lower frequency of nonopioid analgesics prescriptions (Table 2).
Treatment with bDMARDs was predominantly prescribed by rheumatologists, except for vedolizumab, which was only prescribed by gastroenterologists (Supplemental Table S2). Most csDMARDs were more frequently prescribed by rheumatologists than by gastroenterologists. Only the prescriptions of mesalazine and azathioprine were increased among gastroenterologists compared to rheumatologists. Systemic steroids were mainly prescribed by general practitioners/internal medicine specialist followed by rheumatologists. They were less often prescribed by gastroenterologists, dermatologists, and ophthalmologists.
Association of extra-musculoskeletal manifestations with disease activity and functional impairment
The results from multivariable linear regression models analyzing the association of ever and current occurrence of EMMs with disease activity and functional impairment in patients with axSpA are outlined in Tables 3 and 4, respectively. History of IBD was significantly associated with higher disease activity (BASDAI increase of 0.37 points) after adjustment for other relevant parameters including treatment. History of PSO was associated with both higher level of disease activity (BASDAI increase of 0.31 points) and higher level of functional impairment (BASFI increase of 0.37 points), whereas history of AU showed neither a strong association with disease activity nor with functional status (Table 3).
Table 3.
Association of history (ever presence) of inflammatory bowel disease, psoriasis, and anterior uveitis with disease activity and functional impairment in patients with axial spondyloarthritis (n = 1729): results from multivariable linear regression models.
| Reference | Model 1: inflammatory bowel disease |
Model 2: psoriasis |
Model 3: anterior uveitis |
||||
|---|---|---|---|---|---|---|---|
| BASDAI β (95% CI) |
BASFI β (95% CI) |
BASDAI β (95% CI) |
BASFI β (95% CI) |
BASDAI β (95% CI) |
BASFI β (95% CI) |
||
| Inflammatory bowel disease, present | not present | 0.37 (0.01, 0.73) | 0.35 (–0.07, 0.77) | – | – | – | – |
| Psoriasis, present | not present | – | – | 0.31 (0.05, 0.58) | 0.37 (0.05, 0.68) | – | – |
| Anterior uveitis, present | not present | – | – | – | – | –0.10 (–0.31, 0.11) | –0.01 (–0.26, 0.24) |
| Age | per 10 years | 0.18 (0.11, 0.25) | 0.62 (0.54, 0.70) | 0.18 (0.10, 0.25) | 0.61 (0.53, 0.69) | 0.19 (0.12, 0.26) | 0.62 (0.54, 0.70) |
| Sex, female | male | 0.59 (0.41, 0.77) | 0.00 (–0.22, 0.22) | 0.58 (0.39, 0.76) | –0.01 (–0.23, 0.21) | 0.58 (0.39, 0.77) | 0.00 (–0.23, 0.22) |
| Rheumatological care | no | 0.31 (0.10, 0.52) | 0.57 (0.34, 0.81) | 0.29 (0.08, 0.50) | 0.55 (0.32, 0.78) | 0.31 (0.10, 0.53) | 0.58 (0.35, 0.81) |
| Body mass index, kg/m² | per unit | 0.04 (0.02, 0.06) | 0.09 (0.07, 0.12) | 0.04 (0.02, 0.06) | 0.09 (0.06, 0.11) | 0.04 (0.02, 0.06) | 0.09 (0.07, 0.11) |
| Lack of exercise | no | – | 0.62 (0.37, 0.88) | – | 0.63 (0.37, 0.88) | – | 0.62 (0.37, 0.88) |
| Smoking (current) | no | 0.30 (0.06, 0.54) | 0.54 (0.28, 0.80) | 0.30 (0.06, 0.54) | 0.54 (0.28, 0.80) | 0.31 (0.06, 0.55) | 0.55 (0.29, 0.81) |
| Suffering from stress | no | 0.76 (0.56, 0.95) | 0.25 (0.03, 0.48) | 0.76 (0.56, 0.95) | 0.25 (0.03, 0.48) | 0.76 (0.57, 0.96) | 0.26 (0.03, 0.49) |
| Physical therapy | no | 0.36 (0.17, 0.55) | 0.44 (0.22, 0.66) | 0.36 (0.17, 0.55) | 0.44 (0.22, 0.66) | 0.36 (0.17, 0.55) | 0.43 (0.22, 0.65) |
| NSAIDs | no | 0.41 (0.22, 0.60) | – | 0.39 (0.20, 0.58) | – | 0.39 (0.20, 0.58) | – |
| Nonopioid analgesics | no | 0.39 (0.16, 0.61) | 0.47 (0.19, 0.75) | 0.40 (0.17, 0.63) | 0.48 (0.20, 0.76) | 0.39 (0.17, 0.62) | 0.48 (0.20, 0.76) |
| Opioids | no | 1.13 (0.86, 1.39) | 1.74 (1.42, 2.06) | 1.13 (0.87, 1.39) | 1.74 (1.43, 2.06) | 1.14 (0.87, 1.40) | 1.75 (1.43, 2.07) |
| bDMARDs | no | –0.47 (–0.74, –0.20) | – | –0.45 (–0.72, –0.19) | – | –0.45 (–0.72, –0.18) | – |
| Systemic steroids | no | 0.27 (0.03, 0.51) | 0.34 (0.05, 0.62) | 0.29 (0.04, 0.53) | 0.35 (0.06, 0.63) | 0.30 (0.05, 0.54) | 0.36 (0.07, 0.65) |
Variables tested in backward selection: IBD (ever), PSO (ever), AU (ever), age, sex, symptom duration, in rheumatologic care, HLA-B27, body mass index, lack of exercise, smoking, suffering from stress, physical therapy, NSAIDs, nonopioid analgesics, opioids, bDMARDs, csDMARDs, systemic steroids, local injectable steroids, intestinal topical steroids, topical antipsoriatics, systemic oral antipsoriatics, and ophthalmologicals. Variables not included in the above table were not chosen by backward selection in any multivariable model. Age, sex, and IBD (only model 1), PSO (only model 2), AU (only model 3) were always included in the respective models. Significant associations are shown in bold.
AU, anterior uveitis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; bDMARDs, biological disease-modifying anti-rheumatic drugs; CI, confidence interval; IBD, inflammatory bowel disease; NSAIDs, nonsteroidal anti-inflammatory drugs; PSO, psoriasis.
Table 4.
Association of current/recent presence (recorded within the one-year period of interest) inflammatory bowel disease, psoriasis, and anterior uveitis with disease activity and functional impairment in patients with axial spondyloarthritis (n = 1729): results from multivariable linear regression models.
| Model 1: inflammatory bowel disease |
Model 2: psoriasis |
Model 3: anterior uveitis |
|||||
|---|---|---|---|---|---|---|---|
| Reference | BASDAI β (95% CI) | BASFI β (95% CI) | BASDAI β (95% CI) | BASFI β (95% CI) | BASDAI β (95% CI) | BASFI β (95% CI) | |
| Inflammatory bowel disease, present | not present | 0.30 (–0.10, 0.71) | 0.33 (–0.14, 0.80) | – | – | – | – |
| Psoriasis, present | not present | – | – | 0.07 (–0.27, 0.40) | 0.30 (–0.09, 0.70) | – | – |
| Anterior uveitis, present | not present | – | – | – | – | –0.44 (–0.77, –0.10) | –0.38 (–0.77, 0.02) |
| Age | per 10 years | 0.18 (0.11, 0.26) | 0.62 (0.54, 0.70) | 0.18 (0.11, 0.25) | 0.62 (0.53, 0.70) | 0.18 (0.11, 0.25) | 0.62 (0.54, 0.70) |
| Sex | male | 0.59 (0.40, 0.77) | 0.00 (–0.22, 0.22) | 0.58 (0.40, 0.77) | –0.01 (–0.23, 0.22) | 0.57 (0.39, 0.76) | –0.02 (–0.24, 0.21) |
| Rheumatologic care | no | 0.32 (0.10, 0.53) | 0.58 (0.35, 0.81) | 0.31 (0.09, 0.51) | 0.56 (0.33, 0.79) | 0.33 (0.11, 0.54) | 0.59 (0.36, 0.82) |
| Body mass index, kg/m² | per unit | 0.04 (0.02, 0.06) | 0.09 (0.07, 0.12) | 0.04 (0.02, 0.06) | 0.09 (0.07, 0.11) | 0.04 (0.02, 0.06) | 0.09 (0.07, 0.11) |
| Lack of exercise | no | – | 0.62 (0.37, 0.88) | – | 0.63 (0.37, 0.88) | – | 0.61 (0.35, 0.86) |
| Smoking (current) | no | 0.31 (0.06, 0.55) | 0.55 (0.29, 0.81) | 0.30 (0.06, 0.55) | 0.54 (0.28, 0.80) | 0.29 (0.05, 0.54) | 0.54 (0.28, 0.80) |
| Suffering from stress | no | 0.76 (0.57, 0.96) | 0.26 (0.03, 0.49) | 0.76 (0.57, 0.96) | 0.26 (0.03, 0.48) | 0.77 (0.58, 0.97) | 0.27 (0.04, 0.49) |
| Physical therapy | no | 0.35 (0.16, 0.54) | 0.43 (0.21, 0.65) | 0.36 (0.17, 0.55) | 0.43 (0.21, 0.65) | 0.35 (0.17, 0.54) | 0.43 (0.21, 0.65) |
| NSAIDs | no | 0.40 (0.21, 0.60) | – | 0.39 (0.19, 0.58) | – | 0.39 (0.19, 0.58) | – |
| Nonopioid analgesics | no | 0.40 (0.17, 0.62) | 0.48 (0.20, 0.76) | 0.40 (0.17, 0.63) | 0.48 (0.21, 0.76) | 0.38 (0.16, 0.61) | 0.47 (0.19, 0.74) |
| Opioids | no | 1.13 (0.87, 1.40) | 1.75 (1.43, 2.06) | 1.13 (0.87, 1.40) | 1.74 (1.43, 2.06) | 1.14 (0.88, 1.40) | 1.76 (1.44, 2.07) |
| bDMARDs | no | –0.47 (–0.74, –0.20) | – | –0.46 (–0.72, –0.19) | – | –0.45 (–0.72, –0.18) | – |
| Systemic steroids | no | 0.27 (0.03, 0.52) | 0.33 (0.05, 0.62) | 0.29 (0.05, 0.54) | 0.35 (0.06, 0.63) | 0.30 (0.06, 0.55) | 0.36 (0.08, 0.65) |
Variables tested in backward selection: IBD (current), PSO (current), AU (current), age, sex, symptom duration, in rheumatologic care, HLA-B27, body mass index, lack of exercise, smoking, suffering from stress, physical therapy, NSAIDs, nonopioid analgesics, opioids, bDMARDs, csDMARDs, systemic steroids, local injectable steroids, intestinal topical steroids, topical antipsoriatics, systemic oral antipsoriatics, ophthalmologicals. Variables not included in the above table were not chosen by backward selection in any multivariable model. Age, sex, and IBD (only model 1), PSO (only model 2), AU (only model 3) were always included in the respective models. Significant associations are shown in bold.
AU, anterior uveitis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; bDMARDs, biological disease-modifying anti-rheumatic drugs; CI, confidence interval; IBD, inflammatory bowel disease; NSAIDs, nonsteroidal anti-inflammatory drugs; PSO, psoriasis.
The current presence of IBD and the current presence of PSO showed no significant association with disease activity and functional status, respectively (Table 4), whereas current AU was significantly associated with lower disease activity (BASDAI decrease of 0.44 points) after adjustment for other relevant parameters including treatment.
Discussion
The objective of this population-based study was to examine the association of EMMs with disease activity, functional status, and treatment patterns in a large well-characterized cohort of patients with axSpA. We found that a history (ever presence) of IBD was associated with higher disease activity in axSpA, while adjusting for relevant parameters, including treatment. A history of PSO was associated with both higher disease activity and higher functional impairment, whereas a history of AU showed no strong association with either disease activity or functional status. Furthermore, we observed that current presence of AU was associated with lower disease activity, despite adjusting for relevant parameters. The presence of EMMs had a clear impact on the treatment pattern of axSpA: patients with current IBD or PSO received more frequently bDMARDs and csDMARDs as well as systemic steroids. Current AU was associated with a higher use of csDMARDs only. Moreover, despite the high disease activity of patients with axSpA with current IBD or PSO, there was a considerable proportion among these patients without pharmacological treatment.
In our study, history of IBD was present in 9% of the patients with axSpA, which is comparable to what has been reported in previous studies.3,13,14 Patients with IBD history were less often treated with NSAIDs whereas their use of bDMARDs, csDMARDs, and steroids was increased compared to patients without IBD history. A less frequent use of NSAIDs and a more frequent use of bDMARDs, csDMARDs, and steroids is to be expected in case of IBD history and was also observed in other studies.4,15 However, with 39% is the proportion of patients receiving NSAIDs among the patients with axSpA with current IBD still high and emphasizes the need for coordinated management of these patients. We demonstrated that history of IBD was associated with higher disease activity assessed by BASDAI. This association had been observed previously among patients with a high probability of early SpA4 and is also suggested by findings of another study which demonstrated that higher disease activity is independently associated with microscopic gut inflammation in axSpA.16
We observed a prevalence of 15% for the history of PSO in patients with axSpA. A similar prevalence was found in recent studies.3,13,14 Patients with history of PSO were less often HLA-B27 positive and had a longer diagnostic delay than patients without PSO. Furthermore, we demonstrated that history of PSO was associated with a BASDAI increase of 0.31 points and a BASFI increase of 0.37 points. Associations between PSO and increased diagnostic delay,17 indicating a need for specific referral strategies on the level of dermatologists, and between absence of HLA-B27 and higher frequency of PSO18–20 had been reported previously. Moreover, an association between diagnostic delay and worse outcomes in axSpA21 and significantly higher BASDAI and BASFI scores in HLA-B27-negative patients with axSpA18,19 had been described. Altogether, these findings support our data and argue for a close relation between PSO and worse disease outcomes regarding BASDAI and BASFI. Patients with PSO were more frequently treated by csDMARDs, bDMARDs and systemic steroids that might be related to both, the primary skin affection treatment and treatment of a more active and/or severe axSpA. The higher number of patients with PSO receiving systemic steroids is in line with findings from a previous study showing that psoriasis is often treated with systemic steroids in Germany, mainly prescribed by general practitioners/internal medicine specialist.22
AU was the most frequent EMM as had been shown in previous studies.3,14,23 Around one-third of patients with axSpA included in the study had a history of AU and in around one-tenth, AU was currently or recently (since AU occurs normally in acute flares) present. We found patients with AU to be more often male and HLA-B27 positive, thus, in this sense our data are in agreement with results previously reported suggesting AU to occur more commonly among male patients24–26 and its presence to be related to HLA-B27.20,27,28 We also observed an association between current/recent AU and lower disease activity assessed by BASDAI. This intriguing association might be indicative of a disconnection between ocular and musculoskeletal inflammation. It could also be argued that patients with AU would primarily focus on their AU diminishing their perception of axSpA. These results suggest the need for further longitudinal investigations to evaluate the impact of AU on disease outcomes in axSpA. The presence of AU had only a relatively small impact on the treatment pattern of SpA that might be related to solely local treatment of an acute flare of AU in the majority of cases.
A major strength of this study was the large population-based sample of patients with axSpA available from the linkage of survey data to health insurance data. This allowed for investigation of the associations between EMMs and disease outcomes adjusting for important variables including treatment. Patients in specialist and nonspecialist care were included, thus providing a real-life setting, where patients in nonspecialist care tend to receive less pharmacological treatment, in particular bDMARDs.6
This study used a cross-sectional design and did not allow conclusions to be drawn on the direction of causation. Moreover, the current disease outcome measures used (BASDAI and BASFI) are dominated by subjective scores like pain and fatigue, which could also be affected by other factors such as depression and fibromyalgia. Both were more often present in patients with axSpA with a history of IBD or PSO. All diagnoses were obtained from health insurance data, which are normally collected for administrative rather than for scientific purposes. We specifically validated the diagnosis of axSpA by asking a question about the diagnosis made; this has not been done for EMMs. However, differences in the application of specific treatments between patients with and without EMMs indicates validity of the approach on the group level at least for the current/recent EMMs. Patients who responded to the survey and, thus, were included in this study were comparable in terms of age, female sex, and number of comorbidities to those who did not respond. However, there was some nonresponse bias regarding the prescription of pharmacological treatment, which was increased among responders.
Another limitation is that no information on activity/severity of IBD, PSO and AU was available for the analysis; furthermore, no information on the frequency of uveitis flares within the period of interest could be obtained. Moreover, it was not possible to define clearly, whether the observed treatment pattern (e.g. higher use of bDMARDs) was attributable to the presence of EMM itself, to higher disease activity/severity of axSpA or to both. Finally, the study used data from German patients gathered in 2015; the epidemiology of EMMs and treatment patterns may vary across countries and the latter may have changed over time due to broader treatment options. The IL-17 inhibitor secukinumab, for instance, became available in Germany for the treatment of AS in December 2015.
In conclusion, using a large and well-characterized population-based sample, we have provided evidence that history of IBD or history of PSO (but not AU) are associated with higher disease activity and history of PSO is associated with higher level of functional limitations in axSpA. All three EMMs showed an impact on the treatment pattern in axSpA.
Supplemental Material
Supplemental material, sj-pdf-1-tab-10.1177_1759720X20972610 for The impact of extra-musculoskeletal manifestations on disease activity, functional status, and treatment patterns in patients with axial spondyloarthritis: results from a nationwide population-based study by Imke Redeker, Britta Siegmund, Kamran Ghoreschi, Uwe Pleyer, Johanna Callhoff, Falk Hoffmann, Ursula Marschall, Hildrun Haibel, Joachim Sieper, Angela Zink and Denis Poddubnyy in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-pdf-2-tab-10.1177_1759720X20972610 for The impact of extra-musculoskeletal manifestations on disease activity, functional status, and treatment patterns in patients with axial spondyloarthritis: results from a nationwide population-based study by Imke Redeker, Britta Siegmund, Kamran Ghoreschi, Uwe Pleyer, Johanna Callhoff, Falk Hoffmann, Ursula Marschall, Hildrun Haibel, Joachim Sieper, Angela Zink and Denis Poddubnyy in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-pdf-3-tab-10.1177_1759720X20972610 for The impact of extra-musculoskeletal manifestations on disease activity, functional status, and treatment patterns in patients with axial spondyloarthritis: results from a nationwide population-based study by Imke Redeker, Britta Siegmund, Kamran Ghoreschi, Uwe Pleyer, Johanna Callhoff, Falk Hoffmann, Ursula Marschall, Hildrun Haibel, Joachim Sieper, Angela Zink and Denis Poddubnyy in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-pdf-4-tab-10.1177_1759720X20972610 for The impact of extra-musculoskeletal manifestations on disease activity, functional status, and treatment patterns in patients with axial spondyloarthritis: results from a nationwide population-based study by Imke Redeker, Britta Siegmund, Kamran Ghoreschi, Uwe Pleyer, Johanna Callhoff, Falk Hoffmann, Ursula Marschall, Hildrun Haibel, Joachim Sieper, Angela Zink and Denis Poddubnyy in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
We would like to thank the participating patients who took the time to complete the survey, the patients of the focus group who provided valuable input for the survey design, and the BARMER Statutory Health Insurance for providing data for this study.
Footnotes
Author contributions: All authors have substantially contributed to conducting the underlying research and drafting this manuscript.
Conflict of interest statement: I. Redeker, J. Callhoff, F. Hoffmann, A. Zink declare no conflicts of interest. U. Marschall is an employee of BARMER. H. Haibel reports consulting fees or members of speakers’ bureau from AbbVie, Janssen, MSD, Novartis, Pfizer, and Roche. B. Siegmund, has served as a consultant for AbbVie, Boehringer, Celgene, Falk, Janssen, Lilly, Pfizer, Prometheus, Takeda and received speaker’s fees from AbbVie, CED Service GmbH, Falk, Ferring, Janssen, Novartis, Takeda (BS served as representative of the Charité). J. Sieper reports consulting fees or members of speakers’ bureau from AbbVie, Janssen, Lilly, MSD, Novartis, and Pfizer and grants from AbbVie, MSD, and Pfizer. D. Poddubnyy reports consulting fees or members of speaker’s bureau from AbbVie, BMS, Celgene, Lilly, MSD, Novartis, Pfizer, Roche, and UCB and grants from AbbVie, MSD, Novartis and Pfizer.
Ethics approval: The study was approved by the ethics committee of the Charité - Universitätsmedizin Berlin, Berlin, Germany (EA1/051/15).
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Federal Ministry of Education and Research within the research network PROCLAIR (01EC1405). The funder had no role in study design, data collection, data analysis, manuscript preparation and publication decisions.
Patient consent: Patients’ written informed consent on the linkage of survey data to health insurance data was obtained in all cases.
ORCID iDs: Imke Redeker  https://orcid.org/0000-0003-3377-2683
https://orcid.org/0000-0003-3377-2683
Denis Poddubnyy  https://orcid.org/0000-0002-4537-6015
https://orcid.org/0000-0002-4537-6015
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Imke Redeker, Epidemiology Unit, German Rheumatism Research Centre, Charitéplatz 1, Berlin, 10117, Germany.
Britta Siegmund, Department of Gastroenterology, Infectious Diseases and Rheumatology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
Kamran Ghoreschi, Department of Dermatology, Venereology and Allergology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
Uwe Pleyer, Department of Ophthalmology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
Johanna Callhoff, Epidemiology Unit, German Rheumatism Research Centre, Berlin, Germany.
Falk Hoffmann, Department of Health Services Research, Carl von Ossietzky University, Oldenburg, Germany.
Ursula Marschall, BARMER Institute for Health Systems Research, BARMER Statutory Health Insurance, Wuppertal, Germany.
Hildrun Haibel, Department of Gastroenterology, Infectious Diseases and Rheumatology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
Joachim Sieper, Department of Gastroenterology, Infectious Diseases and Rheumatology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
Angela Zink, Epidemiology Unit, German Rheumatism Research Centre, Berlin, Germany; Department of Rheumatology and Clinical Immunology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
Denis Poddubnyy, Epidemiology Unit, German Rheumatism Research Centre, Berlin, Germany; Department of Gastroenterology, Infectious Diseases and Rheumatology, Charité – Universitätsmedizin Berlin, Berlin, Germany.
References
- 1. Rudwaleit M, van der Heijde D, Landewe R, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009; 68: 777–783. [DOI] [PubMed] [Google Scholar]
- 2. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet 2017; 390: 73–84. [DOI] [PubMed] [Google Scholar]
- 3. de Winter JJ, van Mens LJ, van der Heijde D, et al. Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: a meta-analysis. Arthritis Res Ther 2016; 18: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wendling D, Guillot X, Prati C, et al. Effect of gut involvement in patients with high probability of early spondyloarthritis: data from the DESIR cohort. J Rheumatol. Epub ahead of print 1 June 2019. DOI: 10.3899/jrheum.181326. [DOI] [PubMed] [Google Scholar]
- 5. Essers I, Ramiro S, Stolwijk C, et al. Do extra-articular manifestations influence outcome in ankylosing spondylitis? 12-year results from OASIS. Clin Exp Rheumatol 2016; 34: 214–221. [PubMed] [Google Scholar]
- 6. Haibel H, Redeker I, Zink A, et al. Health care and disease burden in persons with axial spondyloarthritis in Germany. Z Rheumatol 2019; 78: 865–874. [DOI] [PubMed] [Google Scholar]
- 7. Redeker I, Hoffmann F, Callhoff J, et al. Determinants of psychological well-being in axial spondyloarthritis: an analysis based on linked claims and patient-reported survey data. Ann Rheum Dis 2018; 77: 1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garrett S, Jenkinson T, Kennedy LG, et al. A new approach to defining disease status in ankylosing spondylitis: the bath ankylosing spondylitis disease activity index. J Rheumatol 1994; 21: 2286–2291. [PubMed] [Google Scholar]
- 9. Calin A, Garrett S, Whitelock H, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the bath ankylosing spondylitis functional index. J Rheumatol 1994; 21: 2281–2285. [PubMed] [Google Scholar]
- 10. Topp CW, Ostergaard SD, Sondergaard S, et al. The WHO-5 well-being index: a systematic review of the literature. Psychother Psychosom 2015; 84: 167–176. [DOI] [PubMed] [Google Scholar]
- 11. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43: 1130–1139. [DOI] [PubMed] [Google Scholar]
- 12. Redeker I, Callhoff J, Hoffmann F, et al. The prevalence and impact of comorbidities on patients with axial spondyloarthritis: results from a nationwide population-based study. Arthritis Res Ther 2020; 22: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao S, Jones GT, Macfarlane GJ, et al. Associations between smoking and extra-axial manifestations and disease severity in axial spondyloarthritis: results from the BSR Biologics Register for Ankylosing Spondylitis (BSRBR-AS). Rheumatology (Oxford) 2019; 58: 811–819. [DOI] [PubMed] [Google Scholar]
- 14. Stolwijk C, van Tubergen A, Castillo-Ortiz JD, et al. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann Rheum Dis 2015; 74: 65–73. [DOI] [PubMed] [Google Scholar]
- 15. Resende GG, Lanna CC, Bortoluzzo AB, et al. Enteropathic arthritis in Brazil: data from the Brazilian registry of spondyloarthritis. Rev Bras Reumatol 2013; 53: 452–459. [DOI] [PubMed] [Google Scholar]
- 16. Van Praet L, Van den Bosch FE, Jacques P, et al. Microscopic gut inflammation in axial spondyloarthritis: a multiparametric predictive model. Ann Rheum Dis 2013; 72: 414–417. [DOI] [PubMed] [Google Scholar]
- 17. Redeker I, Callhoff J, Hoffmann F, et al. Determinants of diagnostic delay in axial spondyloarthritis: an analysis based on linked claims and patient-reported survey data. Rheumatology (Oxford) 2019; 58: 1634–1638. [DOI] [PubMed] [Google Scholar]
- 18. Chung HY, Machado P, van der Heijde D, et al. HLA-B27 positive patients differ from HLA-B27 negative patients in clinical presentation and imaging: results from the DESIR cohort of patients with recent onset axial spondyloarthritis. Ann Rheum Dis 2011; 70: 1930–1936. [DOI] [PubMed] [Google Scholar]
- 19. Arevalo M, Gratacos Masmitja J, Moreno M, et al. Influence of HLA-B27 on the ankylosing spondylitis phenotype: results from the REGISPONSER database. Arthritis Res Ther 2018; 20: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Derakhshan MH, Dean L, Jones GT, et al. Predictors of extra-articular manifestations in axial spondyloarthritis and their influence on TNF-inhibitor prescribing patterns: results from the British society for rheumatology biologics register in ankylosing spondylitis. RMD Open 2020; 6: e001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seo MR, Baek HL, Yoon HH, et al. Delayed diagnosis is linked to worse outcomes and unfavourable treatment responses in patients with axial spondyloarthritis. Clin Rheumatol 2015; 34: 1397–1405. [DOI] [PubMed] [Google Scholar]
- 22. Augustin M, Schäfer I, Reich K, et al. Systemic treatment with corticosteroids in psoriasis–health care provision far beyond the S3-guidelines. J Dtsch Dermatol Ges 2011; 9: 833–838. [DOI] [PubMed] [Google Scholar]
- 23. Essers I, Ramiro S, Stolwijk C, et al. Characteristics associated with the presence and development of extra-articular manifestations in ankylosing spondylitis: 12-year results from OASIS. Rheumatology (Oxford) 2015; 54: 633–640. [DOI] [PubMed] [Google Scholar]
- 24. Webers C, Essers I, Ramiro S, et al. Gender-attributable differences in outcome of ankylosing spondylitis: long-term results from the outcome in ankylosing spondylitis international study. Rheumatology (Oxford) 2016; 55: 419–428. [DOI] [PubMed] [Google Scholar]
- 25. Mitulescu TC, Popescu C, Naie A, et al. Acute anterior uveitis and other extra-articular manifestations of spondyloarthritis. J Med Life 2015; 8: 319–325. [PMC free article] [PubMed] [Google Scholar]
- 26. Braakenburg AM, de Valk HW, de Boer J, et al. Human leukocyte antigen-B27-associated uveitis: long-term follow-up and gender differences. Am J Ophthalmol 2008; 145: 472–479. [DOI] [PubMed] [Google Scholar]
- 27. D’Ambrosio EM, La Cava M, Tortorella P, et al. Clinical features and complications of the HLA-B27-associated acute anterior uveitis: a metanalysis. Semin Ophthalmol 2017; 32: 689–701. [DOI] [PubMed] [Google Scholar]
- 28. Valls Pascual E, Fontanilla Ortega P, Vicens Bernabeu E, et al. Clinical characteristics, treatment and ocular complications of HLA-B27-related anterior uveitis and HLA-B27-non related anterior uveitis. Reumatol Clin 2016; 12: 244–247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tab-10.1177_1759720X20972610 for The impact of extra-musculoskeletal manifestations on disease activity, functional status, and treatment patterns in patients with axial spondyloarthritis: results from a nationwide population-based study by Imke Redeker, Britta Siegmund, Kamran Ghoreschi, Uwe Pleyer, Johanna Callhoff, Falk Hoffmann, Ursula Marschall, Hildrun Haibel, Joachim Sieper, Angela Zink and Denis Poddubnyy in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-pdf-2-tab-10.1177_1759720X20972610 for The impact of extra-musculoskeletal manifestations on disease activity, functional status, and treatment patterns in patients with axial spondyloarthritis: results from a nationwide population-based study by Imke Redeker, Britta Siegmund, Kamran Ghoreschi, Uwe Pleyer, Johanna Callhoff, Falk Hoffmann, Ursula Marschall, Hildrun Haibel, Joachim Sieper, Angela Zink and Denis Poddubnyy in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-pdf-3-tab-10.1177_1759720X20972610 for The impact of extra-musculoskeletal manifestations on disease activity, functional status, and treatment patterns in patients with axial spondyloarthritis: results from a nationwide population-based study by Imke Redeker, Britta Siegmund, Kamran Ghoreschi, Uwe Pleyer, Johanna Callhoff, Falk Hoffmann, Ursula Marschall, Hildrun Haibel, Joachim Sieper, Angela Zink and Denis Poddubnyy in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-pdf-4-tab-10.1177_1759720X20972610 for The impact of extra-musculoskeletal manifestations on disease activity, functional status, and treatment patterns in patients with axial spondyloarthritis: results from a nationwide population-based study by Imke Redeker, Britta Siegmund, Kamran Ghoreschi, Uwe Pleyer, Johanna Callhoff, Falk Hoffmann, Ursula Marschall, Hildrun Haibel, Joachim Sieper, Angela Zink and Denis Poddubnyy in Therapeutic Advances in Musculoskeletal Disease