Abstract
Aims:
Thiopurines are believed to increase cancer risks, but data from Asian patients are sparse. We determined the risks of malignancies in thiopurine users with inflammatory bowel disease (IBD) or other indications from Hong Kong.
Methods:
All patients who had received thiopurines between 2005 and 2009 in Hong Kong were identified from local electronic healthcare database. Patients were followed from the start date of thiopurines until death or end of study in 2017. We excluded patients with baseline malignancy. Standardized incidence ratios (SIR) and the corresponding 95% confidence intervals (CI) of all malignancies were computed against matched local general population from the cancer registry. Patients in the same diagnosis category but not exposed to thiopurines were included as controls.
Results:
There were 7452 thiopurines users (median age 47.0 years), including 595 IBD patients, with a median follow-up of 11.2 years. Of them, 684 (9.2%) developed malignancies with an overall SIR of 2.30 (95% CI 2.13–2.48). The SIR in IBD patients who used thiopurines was 2.37 (95% CI 1.71–3.18) as compared with non-users (SIR 1.35, 95% CI 1.05–1.72). Highest risk of malignancies was observed in post-transplant patients (SIR 3.83, 95% CI 3.34–4.35), and lower risks were seen in patients with rheumatological diseases (SIR 1.46, 95% CI 1.02–2.02).
Conclusion:
IBD patients in Hong Kong who used thiopurines had 2.37-fold increase in risk of malignancies than the general population, which was higher than non-users and different from thiopurine users for other indications.
Keywords: inflammatory bowel disease, malignancy, thiopurines
Introduction
Thiopurines are frequently used as immunomodulators or steroid-sparing agents in patients with inflammatory bowel disease (IBD), autoimmune diseases and organ-allografting. Thiopurines are metabolized into 6-mercaptopurines and 6-thioguanine, which are subsequently converted into 6-thioGTP.1,2 However, thiopurines can lead to cell death and carcinogenesis.3 In 1987, the International Agency for Research on Cancer has labelled azathioprine as a human carcinogen.4 In particular, thiopurines are associated with an increase in risk of non-melanoma skin cancer in organ transplant recipients.5,6 Similarly, IBD patients receiving thiopurines have an elevated risk of malignancy, including lymphoma, urinary tract cancer and non-melanoma skin cancer.7–11 In particular, studies had shown an increase risk of lymphoma in IBD patients who used thiopurines with or without anti-tumor necrosis factor.8,10 In contrast, data from the Spanish registry failed to show any association between immunosuppressants use and extra-colonic cancer development.12 In the French CESAME cohort, it was also found that treatment with immunosuppressants were not associated with higher risk of new or recurrent cancer in IBD patients with history of cancer.13
While thiopurines are commonly used as immunosuppressant in IBD and other diseases including post-transplantation, rheumatological, and autoimmune diseases, the risks of malignancies related to the use of thiopurines in different disease groups remain largely unknown, particularly in Asian patients. In this study, we aimed to investigate the risk of malignancies in IBD patients from Hong Kong who used thiopurines and compared with non-users or thiopurine users for other indications.
Methods
Data source and patients
Study patients were identified from the Clinical Data Analysis and Reporting System of the Hospital Authority (CDARS) – an electronic database containing clinical information of all patients attending public hospitals and clinics in Hong Kong that has been widely used and described in previous territory-wide studies.14–17
In this study, we first retrieved all adult patients (⩾18 years old) who had received thiopurines including azathioprine or mercaptopurine between January 2005 and December 2009 in any public hospitals or clinics in Hong Kong. Their clinical diagnosis, and any diagnosis of malignancy based on pathological confirmation, were retrieved from the date of first prescription of azathioprine or mercaptopurine until the end of 2017 or death.
The International Statistical Classification of Diseases (9th Revision, ICD-9) was used as disease coding in the CDARS. Malignant neoplasms were identified using the ICD-9 codes and the details are listed in Supplemental Table S1.18 According to their underlying diseases and indications, thiopurine users were divided into four groups: IBD (including Crohn’s disease and ulcerative colitis), rheumatological diseases, post-transplantation and others. The details of “others”, which included both other autoimmune disease and other diagnoses are listed in Supplemental Table S2.
The occurrence of any malignant neoplasms that pre-dated the prescription date of azathioprine or mercaptopurine were excluded. To avoid delay on diagnosis entry, patients who had a diagnosis of cancer within 6 months of starting thiopurines were also excluded. Due to the frequent use of azathioprines in patients with myasthenia gravis and their causal association with thymoma,19–21 thymus cancer was not included in this analysis.
For estimation of risk of malignancies in different disease groups who had no thiopurine exposure, we retrieved additional control groups of adult patients who had the same diagnosis codes of the three main disease groups including IBD, rheumatological diseases, and post-transplantation during the same period of 2005–2009 from the CDARS for comparison.
The primary endpoint was the development of any malignancy. The starting point of the follow-up period was the first date of the prescription of thiopurines, and the end date was defined as the censor date (i.e., date of diagnosis of malignancy), death or the end of the follow-up period on 31 December 2017.
This study was approved by the Institutional Review Board of the University of Hong Kong and Hospital Authority Hong Kong West Cluster (UW 19-197). As this study involved anonymized data from the CDARS only, informed consent was not required.
Statistical analysis
The standardized incidence ratio (SIR) with 95% confidence interval (CI) was used to compute the risk of malignancies among thiopurine users when compared with the general population of Hong Kong. The SIR was calculated as the ratio of the observed number of malignancies to the expected number of malignancies in the general population. The expected number of malignancies was calculated according to the corresponding incidence rate in the general population of Hong Kong between 2005 and 2017 based on the Hong Kong Cancer Registry,22 standardized for both sex and age, and subsequently multiplied by the calculated person-years. Person-year was computed from the first prescription date of azathioprine or mercaptopurine to the end date of the study. As the ICD-10 coding systems are used in the classification of different types of neoplasms in the Hong Kong Cancer Registry, a conversion table is provided for the conversion to ICD-9 coding in the Hong Kong Cancer Registry.23
To analyze the dose and duration response of thiopurine exposures, a time-dependent multivariate Cox regression model was employed to study the hazard ratios (HRs) with different dosages and durations. Cumulative dosage was classified as the multiples of daily defined dosage (DDD) into categories.24 All prescriptions of thiopurines, including those given before start date of the study in 2005, were counted.
To access the effect of concomitant medications (other immunosuppressants and biologics) and disease groups, both univariate and multivariable Cox model were used to identify for risk factors of malignancies among thiopurine users. All concurrent medications from 2005 till the end of the study period were extracted for this analysis. Two-sided p values of less than 0.05 were regarded as statistically significant. All statistical analyses were performed by the R software, version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria, 2018).
Results
A total of 7452 patients (female 63.6%) were included in this study (Table 1; Supplemental Figure S1). The median follow up was 11.2 [interquartile range (IQR) 8.7–12.9] years with a total of 75,581 person-years follow up. The median duration of thiopurine use was 6.2 (IQR 2.0–10.8) years. The common indications for thiopurines were others (66.0%), followed by post-transplantation (20.5%), IBD (8.0%), and rheumatological diseases (5.5%). Among all thiopurine users, 180 (2.4%) patients had used anti-tumor necrosis factors (TNFs) and 3687 (49.5%) had used other immunomodulators. For IBD patients, 115 (19.3%) had used anti-TNFs and 58 (9.7%) had used methotrexate (Supplemental Table S3).
Table 1.
Baseline characteristics of all thiopurine users (n = 7452).
| Characteristics | Value |
|---|---|
| Median age, year (IQR) | 47.0 (36.0–57.0) |
| Male (%) | 2712 (36.4%) |
| Median follow-up duration, year (IQR) | 11.2 (8.7–12.9) |
| Median duration of thiopurine exposure (IQR) | 6.2 (2.0–10.8) |
| Total person-years | 75,581 |
| Diagnosis group (%) | |
| IBD | 595 (8.0) |
| Rheumatological Diseases | 411 (5.5) |
| Post-transplantation | 1531 (20.5) |
| Others | 4915 (66.0) |
| Biologics (%) | |
| Anti-tumor necrosis factor | 180 (2.4) |
| Adalimumab | 74 (1.0) |
| Etanercept | 37 (0.5) |
| Infliximab | 131 (1.8) |
| Other immunomodulators | 3687 (49.5) |
| Tacrolimus | 825 (11.1) |
| Ciclosporin | 2319 (31.1) |
| Methotrexate | 627 (8.4) |
| Mycophenolate | 1838 (24.7) |
IBD, inflammatory bowel disease; IQR, interquartile range.
Overall risk of malignancies
There were 684 (9.2%) thiopurine users who developed malignancies in the follow-up period, and the overall incidence rate of any malignancies was 8.81 per 1000 person-years. When compared with age- and sex-matched general population, the overall SIR of thiopurine users was 2.30 (95% CI, 2.13–2.48). The SIR in male and female thiopurine users was 2.56 (95% CI, 2.28–2.86) and 2.13 (95% CI, 1.92–2.35), respectively.
In general, thiopurine users of all age groups were found to have a higher risk of malignancies than matched general population (Table 2). Young (<44 years) thiopurines users had the highest SIR of 4.30 (95% CI 3.67–5.04), particularly among young male patients (SIR 6.52, 95% CI 4.98–8.53). Even among elderly patients (⩾65 years), the SIR was still higher than general population (SIR 1.50, 95% CI 1.29–1.74) but the difference between male and female patients was less obvious (SIR 1.52 and 1.47). Despite the higher SIR in younger patients, the absolute risk of malignancy was still highest in older patients with crude incidence risk rate of 15,245 per 100,000 populations.
Table 2.
SIRs of all malignancies in thiopurine users, stratified by sex and age.
| Male |
Female |
Total |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age group | Observed no. | Expected no. | SIR (95% CI) | Observed no. | Expected no. | SIR (95% CI) | Observed no. | Expected no. | SIR (95% CI) | Crude incidence rate (per 100,000 population) |
| 18–44 | 53 | 8.13 | 6.52 (4.98–8.53) | 100 | 27.5 | 3.64 (2.99–4.43) | 153 | 35.6 | 4.30 (3.67–5.04) | 4625.2 |
| 45–64 | 167 | 55.6 | 3.00 (2.58–3.49) | 196 | 93.3 | 2.10 (1.83–2.42) | 363 | 148.9 | 2.44 (2.20–2.70) | 11,932.9 |
| 65+ | 83 | 54.5 | 1.52 (1.23–1.89) | 85 | 57.8 | 1.47 (1.19–1.82) | 168 | 112.3 | 1.50 (1.29–1.74) | 15,245.0 |
| Sum | 303 | 118.3 | 2.56 (2.28–2.86) | 381 | 178.7 | 2.13 (1.92–2.35) | 684 | 297.1 | 2.30 (2.13–2.48) | 9178.7 |
CI, confidence interval; SIR, standardized incidence ratio.
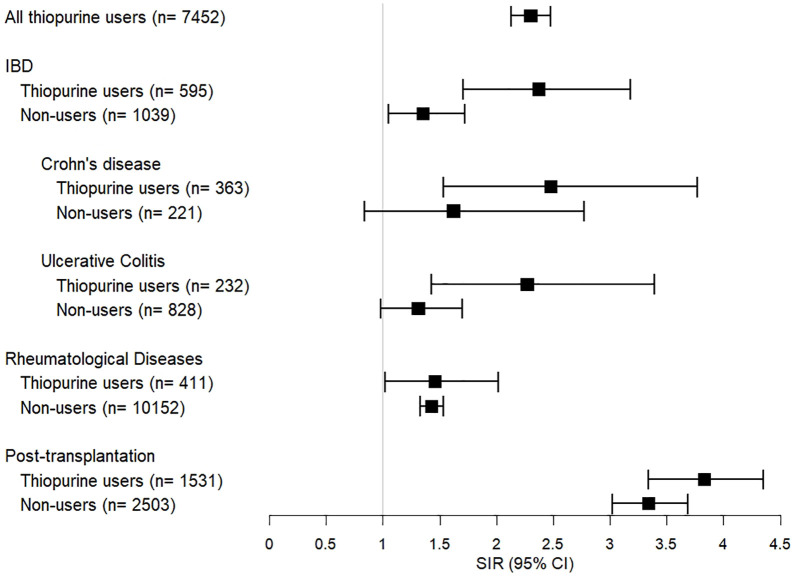
The risk of malignancies in each disease group of thiopurine users were all significantly higher than in the general population (Figure 1). The SIR in IBD patients was 2.37 (95% CI 1.71–3.18), which was lower than in post-transplant patients (SIR 3.83, 95% CI 3.34–4.35) but higher than in patients with rheumatological diseases (SIR 1.46, 95% CI 1.02–2.02) or other diseases (SIR 1.95, 95% CI 1.76–2.15). Among IBD patients who used thiopurines, both Crohn’s disease and ulcerative colitis had elevated SIR of 2.48 (95% CI 1.53–3.77) and 2.27 (95% CI 1.43–3.39), respectively (Supplemental Table S4).
Figure 1.
SIRs of all malignancies in thiopurine users and non-users according to disease groups.
CI, confidence interval; IBD, inflammatory bowel disease; SIR, standardized incidence ratio.
For control groups of patients without thiopurine exposure, the SIR of malignancy in IBD patients who did not use thiopurine was 1.35 (95% CI 1.05–1.72), which was much lower than thiopurine users (Figure 1). Non-thiopurine users with Crohn’s diseases (SIR 1.62, 95% CI 0.84–2.77) or ulcerative colitis (SIR 1.31, 95% CI 0.98–1.70) were not found to have significant increase in risk of malignancy, as compared with the general population. However, the SIRs of thiopurine non-users in the post-transplantation group (3.34, 95% CI 3.02–3.69) were still high, and only slightly lower than those of thiopurine users. In patients with rheumatological diseases, the SIRs were comparable between non-users (1.43, 95% CI 1.33–1.53) and users.
Risk factors of malignancies in thiopurine users
Older age (HR 1.05, 95% CI 1.04–1.05) and male sex (HR 1.24, 95% CI 1.05–1.45) were risk factors for developing malignancies among thiopurine users on multivariate analysis (Table 3). When compared with IBD patients, post-transplantation patients had higher risk of malignancies (HR 1.85, 95% CI 1.23–2.79) and patients with rheumatological diseases had lower risk (HR 0.53, 95% CI 0.32–0.88). Use of methotrexate (HR 2.09, 95% CI 1.58–2.77), but not anti-TNFs (HR 0.43, 95% CI 0.19–1.01), was found to be a risk factor of malignancies among thiopurine users.
Table 3.
Risk factors of malignancies among all thiopurine users: univariate and multivariate Cox regression model.
| Univariate model |
Multivariate model |
|||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age in year | 1.05 (1.04–1.05) | <0.001 | 1.05 (1.04–1.05) | <0.001 |
| Male sex | 1.45 (1.25–1.69) | <0.001 | 1.24 (1.05–1.45) | 0.010 |
| Indications: | ||||
| IBD | (Reference) | (Reference) | ||
| Rheumatological Disease | 1.38 (0.87–2.18) | 0.175 | 0.53 (0.32–0.88) | 0.014 |
| Post-Transplantation | 2.21 (1.58–3.09) | <0.001 | 1.69 (1.13–2.51) | 0.010 |
| Others | 1.25 (0.90–1.73) | 0.185 | 0.91 (0.64–1.28) | 0.597 |
| Concomitant Drug Usage among all thiopurine users: | ||||
| Anti-TNFs | 0.33 (0.15–0.74) | 0.007 | 0.43 (0.19–1.01) | 0.054 |
| Cyclosporin | 1.32 (1.13–1.54) | <0.001 | 1.04 (0.84–1.30) | 0.715 |
| Methotrexate | 1.31 (1.03–1.68) | 0.030 | 2.09 (1.58–2.77) | <0.001 |
| Tacrolimus | 0.98 (0.78–1.25) | 0.886 | 1.07 (0.82–1.40) | 0.603 |
| Mycophenolate | 0.77 (0.64–0.92) | 0.004 | 0.87 (0.72–1.09) | 0.256 |
CI, confidence interval; HR, hazard ratio; IBD, inflammatory bowel disease; SIR, standardized incidence ratio; TNF, tumor necrosis factor.
Dose and duration responses of thiopurine exposures and malignancies
Table 4 shows the time-dependent multivariate Cox regression model of risk of malignancies with different cumulative doses and durations of thiopurines. Thiopurine users with more than 5-year of cumulative prescription had a higher HR (1.64, 95% CI 1.32–2.04). Patients with higher cumulative doses of 500–1500 and >1500 DDD also had elevated HR of 1.38 (1.15–1.65) and 1.50 (1.18–1.91), respectively.
Table 4.
Time-dependent Cox regression analysis on cumulative prescription period and dosage of thiopurines among thiopurine users†.
| HR (95% CI)‡ | p value | |
|---|---|---|
| Cumulative Prescription Period (years) | ||
| <2 | Reference | |
| 2–5 | 1.16 (0.92–1.46) | 0.19 |
| >5 | 1.64 (1.32–2.04) | <0.001 |
| Cumulative Dosage § | ||
| <500 DDD | Reference | |
| 500–1500 DDD | 1.38 (1.15–1.65) | <0.001 |
| >1500 DDD | 1.50 (1.18–1.91) | 0.001 |
19 individuals were not included in the analysis since prescription records have unclear treatment duration.
adjusted for age, sex, use of concomitant immunosuppressants.
DDD = 150 mg azathioprine.
CI, confidence interval; DDD, daily defined dosage; HR, hazard ratio.
Risks of site-specific malignancies in all thiopurine users
The detailed risks of malignancies among all thiopurine users when compared with the general population is shown in Table 5. Among all thiopurine users, the highest SIR was observed in lymphoid and hematological malignancies (SIR 7.73, 95% CI 6.46–9.16), particularly non-Hodgkin’s lymphoma (SIR 11.0, 95% CI 8.93–13.4). It was followed by malignant bone and cartilage tumor (SIR 7.70, 95% CI 1.92–20.0) and cancer at vagina, vulva, and other female genital organs (SIR 7.28, 95% CI 2.89–14.8). Both melanoma (SIR 5.13, 95% CI 1.59–11.9) and non-melanoma skin cancers (SIR 4.17, 95% CI 2.95–5.69) were found to be increased in thiopurine users. Moreover, urinary tract cancers (SIR 5.11, 95% CI 3.79–6.72) including kidney (SIR 4.92, 95% CI 3.32–6.97) and bladder (5.41, 95% CI 3.33–8.22) cancers, were higher than general population. Common cancers including colorectal (SIR 2.16, 95% CI 1.76–2.61), lung (SIR 1.36, 95% CI 1.04–1.73), and prostate (SIR 1.64, 95% CI 1.01–2.49) were all increased but there was no significant increase in breast cancer (SIR 1.03, 95% CI 0.77–1.34). Other cancers that were found to be significantly increased in thiopurine users were cancers of lip, oral cavity and pharynx, and thyroid.
Table 5.
Site-specific risks of malignancies in all thiopurine users.
| SIR (95% CI) | |||||
|---|---|---|---|---|---|
| All thiopurine users (n = 7452) | IBD (n = 595) | Rheumatological diseases (n = 411) | Post-transplantation (n = 1531) | Others (n = 4915) | |
| All sites | 2.30 (2.13–2.48) | 2.37 (1.71–3.18) | 1.46 (1.02–2.02) | 3.83 (3.34–4.35) | 1.95 (1.76–2.15) |
| Lip, oral cavity and pharynx | 4.02 (2.62–5.85) | 4.87 (1.22–19.5) | 2.51 (0.14–11.04) | 5.57 (2.79–11.1) | 3.49 (1.92–5.75) |
| Nasopharynx | 1.69 (1.01–2.63) | N/A | N/A | 1.36 (0.51–3.62) | 2.29 (1.33–3.94) |
| Digestive organs | 1.86 (1.59–2.16) | 2.84 (1.64–4.53) | 1.01 (0.43–1.95) | 2.66 (1.99–3.47) | 1.62 (1.31–1.97) |
| Esophagus | 2.30 (1.10–4.14) | 3.72 (0.21–16.4) | 3.89 (0.22–17.1) | 1.01 (0.06–4.43) | 2.5 (0.99–5.06) |
| Stomach | 0.91 (0.46–1.60) | N/A | 1.16 (0.16–8.20) | 2.76 (1.24–6.14) | 0.41 (0.13–1.28) |
| Small intestine | 1.02 (0.06–4.48) | N/A | N/A | N/A | 1.58 (0.22–11.19) |
| Colorectum | 2.16 (1.76–2.61) | 4.28 (2.22–7.33) | 0.53 (0.09–1.63) | 3.86 (2.72–5.29) | 1.67 (1.26–2.18) |
| Liver | 1.66 (1.12–2.33) | 1.57 (0.26–4.84) | 1.77 (0.29–5.45) | 0.43 (0.07–1.34) | 2.19 (1.41–3.22) |
| Gallbladder and extrahepatic bile duct | 1.44 (0.52–3.09) | 6.00 (0.84–42.6) | N/A | 5.20 (1.68–16.1) | 0.42 (0.06–2.95) |
| Pancreas | 1.40 (0.64–2.60) | N/A | 2.03 (0.29–14.4) | 2.68 (0.87–8.32) | 1.05 (0.39–2.8) |
| Nasal cavity | 1.54 (0.09–6.79) | N/A | N/A | 6.57 (0.93–46.7) | N/A |
| Larynx | 2.46 (0.76–5.72) | 8.12 (1.14–57.7) | N/A | N/A | 3.10 (1.00–9.63) |
| Trachea, bronchus and lung | 1.36 (1.04–1.73) | 0.80 (0.13–2.48) | 1.17 (0.36–2.71) | 1.47 (0.81–2.43) | 1.39 (1.00–1.87) |
| Bone and soft tissue | 1.53 (0.47–3.55) | N/A | N/A | 1.76 (0.25–12.5) | 1.77 (0.57–5.5) |
| Bone and cartilage | 7.70 (1.92–20.0) | N/A | N/A | 11.8 (1.66–83.8) | 8.00 (2.00–32.0) |
| Melanoma of skin | 5.13 (1.59–11.9) | N/A | N/A | 6.18 (0.87–43.9) | 5.86 (1.89–18.2) |
| Non-melanoma skin | 4.17 (2.95–5.69) | 2.09 (0.12–9.21) | 1.32 (0.08–5.81) | 15.5 (10.20–22.4) | 1.56 (0.75–2.8) |
| Breast | 1.03 (0.77–1.34) | 1.01 (0.17–3.10) | 0.24 (0.01–1.07) | 1.65 (0.88–2.76) | 1.00 (0.7–1.36) |
| Vagina, vulva and other female organs | 7.28 (2.89–14.8) | N/A | 10.9 (1.54–77.5) | 41.0 (15.4–109.1) | 1.65 (0.23–11.7) |
| Cervix uteri | 1.84 (0.98–3.08) | N/A | N/A | 2.19 (0.55–8.77) | 2.08 (1.12–3.86) |
| Corpus uteri | 1.48 (0.9–2.27) | N/A | 0.99 (0.14–7.02) | 1.60 (0.52–4.97) | 1.59 (0.94–2.69) |
| Ovary | 1.55 (0.83–2.60) | 9.10 (2.26–23.6) | 3.38 (0.56–10.4) | 3.55 (1.10–8.25) | 0.53 (0.13–1.36) |
| Prostate | 1.64 (1.01–2.49) | 2.42 (0.40–7.45) | 1.65 (0.09–7.26) | 1.83 (0.66–3.94) | 1.48 (0.77–2.53) |
| Urinary organs | 5.11 (3.79–6.72) | 5.10 (1.27–13.2) | 1.49 (0.09–6.56) | 10.8 (6.89–15.6) | 3.56 (2.25–5.31) |
| Kidney and other urinary organs | 4.92 (3.32–6.97) | 7.79 (2.51–24.2) | N/A | 11.0 (6.62–18.2) | 2.82 (1.52–5.24) |
| Bladder | 5.41 (3.33–8.22) | N/A | 3.61 (0.51–25.7) | 10.4 (4.94–21.8) | 4.67 (2.58–8.42) |
| Brain and nervous system | 0.91 (0.15–2.82) | N/A | N/A | N/A | 1.44 (0.36–5.78) |
| Thyroid gland | 2.24 (1.43–3.32) | N/A | 1.60 (0.22–11.3) | 2.93 (1.22–7.05) | 2.31 (1.42–3.78) |
| Lymphoid and hematopoietic tissue | 7.73 (6.46–9.16) | 6.55 (2.81–12.7) | 7.70 (3.70–13.9) | 14.1 (10.51–18.5) | 5.81 (4.48–7.38) |
| Multiple myeloma | 4.47 (2.24–7.85) | N/A | 5.36 (0.75–38.0) | 6.72 (2.17–20.8) | 3.38 (1.41–8.12) |
| Leukemia | 4.36 (2.75–6.5) | 11.9 (3.68–27.5) | 6.19 (1.03–19.1) | 2.96 (0.74–7.68) | 3.82 (2.04–6.40) |
| Hodgkin lymphoma | 2.90 (0.48–8.94) | N/A | 31.3 (4.41–222.2) | N/A | 2.23 (0.31–15.8) |
| Non-Hodgkin lymphoma | 11.0 (8.93–13.4) | 5.54 (1.38–14.4) | 7.97 (2.86–17.1) | 23.5 (17.11–31.4) | 7.88 (5.78–10.5) |
CI, confidence interval; IBD, inflammatory bowel disease; SIR, standardized incidence ratio.
Risks of site-specific malignancies in IBD patients and other disease groups
The risks of site-specific malignancies in each disease group are also shown in Table 5. Among IBD patients, the risks of malignancies in descending order were leukemia (SIR 11.9, 95% CI 3.68–27.5), ovarian cancer (SIR 9.10, 95% CI 2.26–23.6), laryngeal cancer (SIR 8.12, 95% CI 1.14–57.7), kidney and other urinary tract cancers (SIR 7.79, 95% CI 2.51–24.2), non-Hodgkin’s lymphoma (SIR 5.54, 95% CI 1.38–14.4), and colorectal cancer (SIR 4.28, 95% CI 2.22–7.33). The risks of site-specific malignancies in patients with Crohn’s disease and ulcerative colitis are shown in Supplemental Table S4.
When compared with other diseases, the risk of colorectal cancer was highest among IBD patients who used thiopurines (Table 5). On the other hand, the risk of lymphoid and hematological cancers was highest among post-transplant patients (SIR, 14.1; 95% CI 10.5–18.5). The risks of certain cancers were also much higher among post-transplant patients, including non-melanoma skin cancer (SIR 15.5, 95% CI 10.2–22.4), urinary tract cancers (SIR 10.8, 95% CI 6.89–16.0), and female genital organ cancers (SIR 41.0, 95% CI 15.4–109.1). For patients with rheumatological diseases, the risk of vaginal cancers (SIR 10.9, 95%CI 1.5–77.5), and lymphoid and hematological cancers (7.70, 95% CI 3.7–13.9) were elevated significantly.
Sensitivity analysis was performed in the subgroup patients with other autoimmune diseases only, with consistent results as compared with the Others group (Supplemental Table S5).
Discussion
In this territory-wide study of thiopurine users from Hong Kong, we found that there was a 2.3-fold increase in risk of malignancies among thiopurine users of all indications. We further demonstrated the association between the risk of malignancies and longer cumulative duration (>5 year) or higher cumulative dose of thiopurines (>500 DDD). When comparing thiopurine users of different indications, IBD patients appeared to have intermediate risk of cancers with SIR of 2.37, which was lower than post-transplantation patients (SIR 3.83), but higher than patients with rheumatological diseases (SIR 1.46) and other diseases (SIR 1.95). As compared with IBD patients without thiopurine exposures, the SIR among IBD patients who used thiopurines was higher (SIR 1.35 versus 2.37). In contrast, the SIRs were comparable in the two disease groups (post-transplantation and rheumatological diseases) with or without thiopurine exposures. Although carcinogenicity of thiopurines has been widely discussed,1,25 previous cohort studies focus mainly on cancer risk in thiopurine users with a specific indication (e.g. IBD or post-transplantation) in western populations.6,26
Notably, younger (18–44 years) thiopurine users were actually found to have the highest risk (SIR 4.3) when compared with matched population in this study. The risk was up to 6.5-fold higher among young male thiopurine users than age-matched general population. On the other hand, the absolute risk of young thiopurine users, as expressed in crude incidence rate, was actually much lower than older thiopurine users (Table 2). Since most IBD patients present at young age, the increase in cancer risk in the younger age group is of clinical importance as the risk and benefits should be carefully weighed when prescribing thiopurines to younger patients.
For IBD patients on thiopurines, the risks of non-Hodgkin lymphoma (SIR 5.5) and leukemia (SIR 11.9) were increased significantly. This finding is compatible with previous Western studies, which demonstrate an increase in lymphoma risk in thiopurine users with IBD.8,10,11 Similar increase in this lymphoma risk was also found in thiopurine uses of all indications in this study, and particularly in post-transplant patients (SIR 23.5). Apart from lymphoid malignancies, we also found an increase in leukemia risk among IBD patients who were treated with thiopurines. A previous study had revealed the potential role of azathioprine as a leukemogenic agent.27 Case reports also showed that acute leukemia arose in thiopurine users with Crohn’s disease, connective tissue diseases, chronic hepatitis, and renal transplantation.28–30
Compared with the general population, there was a 5-fold increase in urinary tract cancers among IBD patients who used thiopurines. A recently published cohort study also showed a 3.4-fold (95% CI 1.47–6.71) increase in SIR of urinary tract cancer among IBD patients with azathioprine usage.31 Another cohort study from Demark identified a 2.84-fold (95% CI 1.24–6.51) increase in risk in azathioprine users after adjusting for the exposure of other medications. Similar increase in risk of urinary tract cancers was also observed among thiopurine users with other indications.
Besides urinary tract cancers, the risk of colorectal cancer was also found to be increased in IBD patients. In fact, the relationship between colorectal cancer and thiopurines usage in IBD patients are conflicting. Several European cohort studies showed that thiopurine therapy could reduce the risk in colorectal neoplasia among IBD patients.32,33 We suspected that our findings may be related to the adoption of a bottom-up approach in which only severe IBD patients would be given thiopurines.
Despite an overall increase in risk of melanoma and non-melanoma skin cancer among thiopurines users, the risk was limited to patients with transplantation or other diseases in our population. Our IBD patients who used thiopurines appeared to have lower risk of skin cancers. Several cohort studies and one meta-analysis from the West have shown a significant increase in the risk of non-melanoma skin cancer among thiopurine users.26,34 However, our study population was mainly Asians with darker skin complexion and a relatively lower skin cancer risk as reported,35 which may account for the discrepancies seen in skin cancer risks. Studies have also proposed the potential role of thiopurines therapy in organ transplant recipients in causing non-melanoma skin cancer.6,36 In a recent meta-analysis, organ transplant recipients receiving azathioprine were shown to have an elevated risk of squamous cell carcinoma.5 Similarly, a retrospective cohort study showed a significant increase in squamous cell carcinoma of the skin in thiopurines users who suffered from rheumatological diseases.37 Studies have proposed the accumulation of 6-thioguainine, a metabolite of azathioprine, could react with ultraviolet A radiation, predisposing to photosensitive reactions and oxidative damages to the skin and leading to skin malignancy.38,39
Based on multivariate analysis, we showed that older age and male thiopurine users had higher risk of malignancies. Moreover, post-transplant patients had the highest risk when compared with thiopurine users with other indications. While concomitant use of other immunosuppressants and biologics is not uncommon, methotrexate was the only medication found to increase cancer risk among thiopurine users (HR 1.69). As these agents were likely used sequentially in the treatment algorithm, further studies may be warranted to study the potential cumulative effects of thiopurines and methotrexate on cancer development. Although anti-TNFs have been shown to elevate the cancer risk in thiopurine users,40,41 we failed to show any additional risk with the combined use of anti-TNFs and thiopurines in this study (Table 3). However, this may be due to the low usage rate of anti-TNFs as these agents were not usually covered by the public health care during the study period.
The strength of this study was the comprehensive analysis of a large cohort of thiopurines users in Hong Kong. We also included control disease groups without thiopurine exposure for comparison. Since the risks of malignancies may differ in different diseases as well as racial groups due to variation in genetic factors and external environments, a more direct and specific monitoring and cancer screening program should be considered based on these new data from Asian patients.
However, several limitations exist for this study. First, categorization of patient groups was restricted by the ICD-9 diseases codes, which may have limited information in differentiating the etiologies of some diagnoses. In particular, the largest “Others” group comprise patients with heterogeneous indications for thiopurines. Second, some risk factors for cancer are not available in the electronic health database, such as family history of cancer, EBV infection status, smoking or drinking habits. Moreover, the IBD disease activity was not available in the database, which may give rise to the impression that IBD patients with thiopurine exposure had a higher risk of colorectal cancer. Third, about two-thirds of our patients were women due to the inclusion of rheumatological and autoimmune diseases with female predominance, which may underestimate the effect size of malignancies in this cohort.
In conclusion, this population-based study has further delineated the risks of malignancies in Asian thiopurine users, including IBD and other indications. IBD patients who used thiopurines had an overall 2.37-fold increase in risks of malignancies, which was higher than IBD patients without thiopurine exposures but lower than post-transplantation patients. The risk was proportional to the cumulative duration and dosage of thiopurine uses, and the risk was particularly high among young male patients. The results of the study might infer the needs of periodic screening of specific malignancies in these patients.
Supplemental Material
Supplemental material, sj-pdf-1-tag-10.1177_1756284820967275 for Risk of malignancies in patients with inflammatory bowel disease who used thiopurines as compared with other indications: a territory-wide study by Kelvin Y. C. Zheng, Chuan-Guo Guo, Irene O. L. Wong, Lijia Chen, Ho Yin Chung, Ka Shing Cheung and Wai K. Leung in Therapeutic Advances in Gastroenterology
Acknowledgments
We thank the Hospital Authority of Hong Kong for the use the Clinical Data Analysis and Reporting System.
Footnotes
Author contributions: WKL was responsible for the conception and design of this study. LC, KSC and KYCZ were involved in data collection. KYCZ, CGG, IOLW, KSC and HYC were involved in data analysis and interpretation. KYCZ, CGG and WKL drafted the manuscript. All authors approved the final version of the article.
Declaration of conflicting interests: WKL has received speaker fee from Eisai, Ipsen and honorarium for attending advisory board for Janssen and Pfizer. Other authors have no conflict of interest to declare.
Ethics: This study was approved by the Institutional Review Board of the University of Hong Kong and Hospital Authority Hong Kong West Cluster (UW 19-197).
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Guarantor: Wai K. Leung
ORCID iD: Kelvin Y. C. Zheng  https://orcid.org/0000-0001-5742-0370
https://orcid.org/0000-0001-5742-0370
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Kelvin Y. C. Zheng, Department of Medicine, Li Ka Shing Faculty of Medicine, University of Hong Kong, Hong Kong, China
Chuan-Guo Guo, Department of Medicine, Li Ka Shing Faculty of Medicine, University of Hong Kong, Hong Kong, China.
Irene O. L. Wong, School of Public Health, Li Ka Shing Faculty of Medicine, University of Hong Kong, Hong Kong, China
Lijia Chen, Department of Medicine, Li Ka Shing Faculty of Medicine, University of Hong Kong, Hong Kong, China.
Ho Yin Chung, Department of Medicine, Li Ka Shing Faculty of Medicine, University of Hong Kong, Hong Kong, China.
Ka Shing Cheung, Department of Medicine, Li Ka Shing Faculty of Medicine, University of Hong Kong, Hong Kong, China.
Wai K. Leung, Department of Medicine, University of Hong Kong, Queen Mary Hospital, 102 Pokfulam Road, Hong Kong, China.
References
- 1. Karran P. Thiopurines, DNA damage, DNA repair and therapy-related cancer. Br Med Bull 2006; 79–80: 153–170. [DOI] [PubMed] [Google Scholar]
- 2. Swann PF, Waters TR, Moulton DC, et al. Role of postreplicative DNA mismatch repair in the cytotoxic action of thioguanine. Science 1996; 273: 1109–1111. [DOI] [PubMed] [Google Scholar]
- 3. Brem R, Karran P. Multiple forms of DNA damage caused by UVA photoactivation of DNA 6-thioguanine. Photochem Photobiol 2012; 88: 5–13. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Supplement. Lyon: International Agency for Research on Cancer, 1979. [Google Scholar]
- 5. Jiyad Z, Olsen CM, Burke MT, et al. Azathioprine and risk of skin cancer in organ transplant recipients: systematic review and meta-analysis. Am J Transplant 2016; 16: 3490–3503. [DOI] [PubMed] [Google Scholar]
- 6. Moloney FJ, Comber H, O’Lorcain P, et al. A population-based study of skin cancer incidence and prevalence in renal transplant recipients. Br J Dermatol 2006; 154: 498–504. [DOI] [PubMed] [Google Scholar]
- 7. Armstrong RG, West J, Card TR. Risk of cancer in inflammatory bowel disease treated with azathioprine: a UK population-based case-control study. Am J Gastroenterol 2010; 105: 1604–1609. [DOI] [PubMed] [Google Scholar]
- 8. Kotlyar DS, Lewis JD, Beaugerie L, et al. Risk of lymphoma in patients with inflammatory bowel disease treated with azathioprine and 6-mercaptopurine: a meta-analysis. Clin Gastroenterol Hepatol 2015; 13: 847–858.e844; quiz e848–850. [DOI] [PubMed] [Google Scholar]
- 9. Smith MA, Irving PM, Marinaki AM, et al. Review article: malignancy on thiopurine treatment with special reference to inflammatory bowel disease. Aliment Pharmacol Ther 2010; 32: 119–130. [DOI] [PubMed] [Google Scholar]
- 10. Lemaitre M, Kirchgesner J, Rudnichi A, et al. Association between use of thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA 2017; 318: 1679–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pasternak B, Svanstrom H, Schmiegelow K, et al. Use of azathioprine and the risk of cancer in inflammatory bowel disease. Am J Epidemiol 2013; 177: 1296–1305. [DOI] [PubMed] [Google Scholar]
- 12. Chaparro M, Ramas M, Benítez JM, et al. Extracolonic cancer in inflammatory bowel disease: data from the GETECCU Eneida Registry. Am J Gastroenterol 2017; 112: 1135–1143. [DOI] [PubMed] [Google Scholar]
- 13. Beaugerie L, Carrat F, Colombel JF, et al. Risk of new or recurrent cancer under immunosuppressive therapy in patients with IBD and previous cancer. Gut 2014; 63: 1416–1423. [DOI] [PubMed] [Google Scholar]
- 14. Leung WK, Wong IO, Cheung KS, et al. Effects of Helicobacter pylori treatment on incidence of gastric cancer in older individuals. Gastroenterology 2018; 155: 67–75. [DOI] [PubMed] [Google Scholar]
- 15. Cheung KS, Chan EW, Wong AY, et al. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: a population-based study. Gut 2018; 67: 28–35. [DOI] [PubMed] [Google Scholar]
- 16. Guo CG, Cheung KS, Zhang F, et al. Risks of hospitalization for upper gastrointestinal bleeding in users of selective serotonin reuptake inhibitors after Helicobacter pylori eradication therapy: a propensity score matching analysis. Aliment Pharmacol Ther 2019; 50: 1001–1008. [DOI] [PubMed] [Google Scholar]
- 17. Guo C-G, Cheung KS, Zhang F, et al. Incidences, temporal trends and risks of hospitalisation for gastrointestinal bleeding in new or chronic low-dose aspirin users after treatment for Helicobacter pylori: a territory-wide cohort study. Gut 2020; 69: 445–452. [DOI] [PubMed] [Google Scholar]
- 18. World Health Organization. Manual of the international statistical classification of diseases, injuries, and causes of death: based on the recommendations of the ninth revision conference, 1975, and adopted by the twenty-ninth World Health Assembly. Geneva: World Health Organization, 1977. [Google Scholar]
- 19. Suster S, Moran CA. Thymoma classification: current status and future trends. Am J Clin Pathol 2006; 125: 542–554. [DOI] [PubMed] [Google Scholar]
- 20. Tormoehlen LM, Pascuzzi RM. Thymoma, myasthenia gravis, and other paraneoplastic syndromes. Hematol Oncol Clin North Am 2008; 22: 509–526. [DOI] [PubMed] [Google Scholar]
- 21. Kumar R. Myasthenia gravis and thymic neoplasms: a brief review. World J Clin Cases 2015; 3: 980–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hong Kong Hospital Authority. Hong Kong Cancer Registry, https://www3.ha.org.hk/cancereg/hkcar.html (2019, accessed December 2019).
- 23. Hong Kong Hospital Authority. International classification of diseases, https://www3.ha.org.hk/cancereg/icd10.html (2019, accessed December 2019).
- 24. Pedersen EG, Pottegård A, Hallas J, et al. Use of azathioprine for non-thymoma myasthenia and risk of cancer: a nationwide case–control study in Denmark. Eur J Neurol 2013; 20: 942–948. [DOI] [PubMed] [Google Scholar]
- 25. Bo J, Schrøder H, Kristinsson J, et al. Possible carcinogenic effect of 6-mercaptopurine on bone marrow stem cells: relation to thiopurine metabolism. Cancer 1999; 86: 1080–1086. [DOI] [PubMed] [Google Scholar]
- 26. Setshedi M, Epstein D, Winter TA, et al. Use of thiopurines in the treatment of inflammatory bowel disease is associated with an increased risk of non-melanoma skin cancer in an at-risk population: a cohort study. J Gastroenterol Hepatol 2012; 27: 385–389. [DOI] [PubMed] [Google Scholar]
- 27. Kwong YL. Azathioprine: association with therapy-related myelodysplastic syndrome and acute myeloid leukemia. J Rheumatol 2010; 37: 485–490. [DOI] [PubMed] [Google Scholar]
- 28. Silvergleid AJ, Schrier SL. Acute myelogenous leukemia in two patients treated with azathioprine for nonmalignant diseases. Am J Med 1974; 57: 885–888. [DOI] [PubMed] [Google Scholar]
- 29. Alexson E, Brandt KD. Acute leukemia after azathioprine treatment of connective tissue disease. Am J Med Sci 1977; 273: 335–340. [PubMed] [Google Scholar]
- 30. Yenson PR, Forrest D, Schmiegelow K, et al. Azathioprine-associated acute myeloid leukemia in a patient with Crohn’s disease and thiopurine S-methyltransferase deficiency. Am J Hematol 2008; 83: 80–83. [DOI] [PubMed] [Google Scholar]
- 31. Bourrier A, Carrat F, Colombel JF, et al. Excess risk of urinary tract cancers in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Aliment Pharmacol Ther 2016; 43: 252–261. [DOI] [PubMed] [Google Scholar]
- 32. Gordillo J, Cabré E, Garcia-Planella E, et al. Thiopurine therapy reduces the incidence of colorectal neoplasia in patients with ulcerative colitis. Data from the ENEIDA Registry. J Crohns Colitis 2015; 9: 1063–1070. [DOI] [PubMed] [Google Scholar]
- 33. van Schaik FDM, van Oijen MGH, Smeets HM, et al. Thiopurines prevent advanced colorectal neoplasia in patients with inflammatory bowel disease. Gut 2012; 61: 235–240. [DOI] [PubMed] [Google Scholar]
- 34. Peyrin–Biroulet L, Khosrotehrani K, Carrat F, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology 2011; 141: 1621–1628.e1625. [DOI] [PubMed] [Google Scholar]
- 35. Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg 2018; 44: 903–910. [DOI] [PubMed] [Google Scholar]
- 36. Baccarani U, Adani GL, Montanaro D, et al. De novo malignancies after kidney and liver transplantations: experience on 582 consecutive cases. Transplant Proc 2006; 38: 1135–1137. [DOI] [PubMed] [Google Scholar]
- 37. van den Reek JM, van Lumig PP, Janssen M, et al. Increased incidence of squamous cell carcinoma of the skin after long-term treatment with azathioprine in patients with auto-immune inflammatory rheumatic diseases. J Eur Acad Dermatol Venereol 2014; 28: 27–33. [DOI] [PubMed] [Google Scholar]
- 38. Perrett CM, Walker SL, O’Donovan P, et al. Azathioprine treatment photosensitizes human skin to ultraviolet A radiation. Br J Dermatol 2008; 159: 198–204. [DOI] [PubMed] [Google Scholar]
- 39. O’Donovan P, Perrett CM, Zhang X, et al. Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science 2005; 309: 1871–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bongartz T, Sutton AJ, Sweeting MJ, et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 2006; 295: 2275–2285. [DOI] [PubMed] [Google Scholar]
- 41. Solomon DH, Kremer JM, Fisher M, et al. Comparative cancer risk associated with methotrexate, other non-biologic and biologic disease-modifying anti-rheumatic drugs. Semin Arthritis Rheum 2014; 43: 489–497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tag-10.1177_1756284820967275 for Risk of malignancies in patients with inflammatory bowel disease who used thiopurines as compared with other indications: a territory-wide study by Kelvin Y. C. Zheng, Chuan-Guo Guo, Irene O. L. Wong, Lijia Chen, Ho Yin Chung, Ka Shing Cheung and Wai K. Leung in Therapeutic Advances in Gastroenterology