Abstract
A dry-powder inhaled formulation of treprostinil (LIQ861) produced using PRINT® technology offers a substantial advantage over current nebulized therapy. Treprostinil is a synthetic prostacyclin analogue that is currently approved for inhalation administration to patients with pulmonary arterial hypertension via nebulized Tyvaso® inhalation solution. LTI-101 was a phase 1, placebo-controlled, double-blind, randomized, single-center study that evaluated the ascending single-dose pharmacokinetics of LIQ861 in healthy subjects. Six sequential, escalating doses (25, 50, 75, 100, 125, and 150 mcg) were studied to investigate treprostinil exposure from LIQ861 inhalation. Subjects (n = 57) were randomly assigned in a 3:1 ratio to receive a single dose of either LIQ861 (n = 43) or placebo (n = 14); 56 subjects completed all protocol-defined assessments. Following single-dose administration, treprostinil exposure from LIQ861 increased proportionally across the dose range studied, and the pharmacokinetics profile of treprostinil administered as LIQ861 was similar to prior reports of inhaled treprostinil. All doses of LIQ861 were generally well-tolerated with no deaths, serious adverse events, or dose-limiting toxicities. The most frequently reported treatment-emergent adverse events related to study drug administration were coughing and throat irritation, which are common to dry-powder formulations. Treatment-related treatment-emergent adverse events were reported more frequently at higher dose levels; however, all were assessed as mild in severity. We conclude that the pharmacokinetics profile of treprostinil using a dry-powder inhaled formulation increased in proportion to dose as anticipated and was similar to earlier reports of inhaled, nebulized treprostinil (Tyvaso®). Based on these results, a phase 3 study (INSPIRE; Clinicaltrials.gov Identifier NCT03399604) evaluating the long-term safety and tolerability of LIQ861 in patients with pulmonary arterial hypertension was initiated.
Keywords: inhaled prostacyclin, pharmacokinetics, pulmonary arterial hypertension
Introduction
The current management of pulmonary arterial hypertension (PAH) relies on targeted therapies to address the pathophysiologic abnormalities that damage the pulmonary vasculature and result in elevated pulmonary artery pressures, dyspnea, diminished exercise capacity, right heart failure, and, ultimately, death.1–3 The synthesis and release of prostacyclin are impaired in PAH,4,5 and the prostacyclin (prostaglandin I2 (PGI2)) pathway is a critical therapeutic target in the disease.6–8 Prostacyclin therapy provokes vasodilation and also has anti-proliferative, anti-thrombotic, and anti-inflammatory effects.2,3,9
Despite offering significant clinical benefits to patients with PAH, PGI2 therapies are underutilized in clinical practice,10–12 with only 34.1% of patients enrolled in the REVEAL Registry™ (Registry to Evaluate Early And Long-term PAH Disease Management) treated with a prostacyclin analogue.13 Lower rates of prostacyclin therapy are attributed, in part, to the complexities of dose up-titration coupled with challenges associated with diverse routes of administration.6,7 PGI2 therapies, currently approved for use in the United States, can be delivered by intravenous (IV), subcutaneous (SC),14,15 inhaled,16,17 and oral routes.18,19 IV and SC therapies place patients at increased risk of catheter occlusion, thrombosis, thromboembolism, infections, sepsis, and infusion site pain.7,12,14,20–27
Dosing errors due to variations in breathing patterns and the inability to up-titrate, as well as the number, frequency, and duration of inhalations, are limitations of currently-available inhaled prostacyclin therapies.7,22,28,29 Oral prostacyclins are associated with systemic side effects that may prompt treatment noncompliance or discontinuation.7,26,30 Novel drugs that target the PGI2 pathway and overcome the recognized limitations of the IV, SC, inhaled, and oral routes are needed to ensure that patients with PAH obtain the clinical benefits of prostacyclin therapy while reducing the risk of serious, dose-limiting, and systemic side effects.
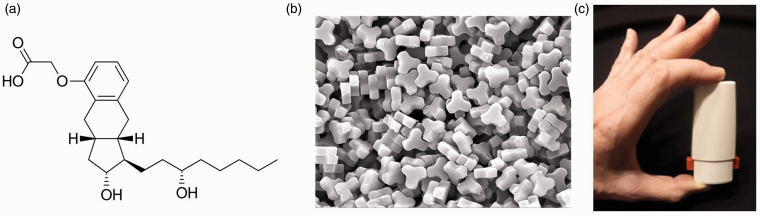
LIQ861 is an investigational, inhaled, dry-powder formulation of treprostinil designed using Liquidia’s PRINT® technology (Particle Replication in Nonwetting Templates), aiming to enhance deep-lung delivery using a convenient, palm-sized dry-powder inhaler (DPI), the Plastiape RS00 Model 8 Device, for the treatment of PAH (Fig. 1). PRINT® is a proprietary particle engineering platform that enables precise production of highly uniform drug particles with independent control over their size, shape, and chemical composition. Through this approach, PRINT® enables the development of drug particles that are engineered for optimal deposition in the lung following oral inhalation (Fig. 1).31–33 LIQ861 has the potential to overcome the limitations of current inhaled therapies and to maximize the therapeutic benefits of treprostinil for the treatment of PAH by safely delivering high doses into the lungs in one to two breaths. LTI-101 was a phase 1, placebo-controlled, double-blind, randomized, single-center, first-in-human study to evaluate the ascending single-dose pharmacokinetics (PK) of LIQ861 in healthy subjects.
Fig. 1.
LIQ861 particles and dry-powder inhaler.
Methods
Study design
This was a phase 1, placebo-controlled, double-blind, randomized, single-center study that evaluated the ascending single-dose PK of LIQ861 in healthy male and female subjects. Six sequential, escalating dose levels (25, 50, 75, 100, 125, and 150 mcg capsule strength) were investigated.
Subjects were screened within the 28 days (Days –28 to –1) prior to admission to the clinical research unit (CRU; PPD Development, Austin, TX) on Day 0 (the day prior to dosing) for baseline assessments. On Day 1, following the pre-dose collection of blood for PK, subjects were randomized in a 3:1, active-to-placebo ratio to receive a single dose of either LIQ861 or placebo at time zero. Placebo capsules contained a nearly identical preparation of excipient matrix compared with active LIQ861, with an equivalent mass of trehalose (the most abundant excipient) instead of treprostinil. In vitro aerosol performance testing was performed in accordance with General Chapter: <601> USP-29-NF-24, “Aerosol, Nasal Sprays, Metered Dose Inhalers and Dry Powder Inhalers” with a fine particle fraction of approximately 86% of emitted dose.34
All inhalations were conducted using the Plastiape RS00 Model 8 DPI and 2 inhalation breaths per capsule. Inhalations were made in immediate succession to minimize the amount of time needed to administer the complete dose. Variations in breathing patterns and skills were minimized by providing all subjects with in-depth training on the use of the device. An additional cohort of subjects at the 150-mcg dose was investigated due to capsule seating interference in the DPI device that occurred during drug administration for approximately half of the subjects in the original cohort. This cohort was repeated while the study remained blinded. Treatment administration by dose cohort is shown in Table 1.
Table 1.
Treatment administration by cohort.
| Cohort | Treprostinil dose, mcg |
LIQ861 |
Placebo |
||||
|---|---|---|---|---|---|---|---|
| Capsules administered, # × mcg | Powder weight, mg | n | Capsules administered, # × mcg | Powder weight, mg | n | ||
| 1 | 25 | 1 × 25 | 5 | 6 | 1 × 0 | 15 | 2 |
| 2 | 50 | 1 × 50 | 10 | 7 | 1 × 0 | 15 | 2 |
| 3 | 75 | 1 × 75 | 15 | 6 | 1 × 0 | 15 | 2 |
| 4 | 100 | 2 × 50 | 20 | 6 | 2 × 0 | 30 | 2 |
| 5 | 125 | 1 × 75 + 1 × 50 | 25 | 6 | 2 × 0 | 30 | 2 |
| 6 | 150 | 2 × 75 | 30 | 12 | 2 × 0 | 30 | 4 |
mcg: micrograms; n: number of subjects.
Subjects remained in the CRU from admission (Day 0) until discharge by the Investigator on the day after dosing (Day 2). Subjects returned to the CRU approximately two days after discharge (Day 4) for completion of final assessments.
Study participants
Healthy male and female volunteers between 18 and 45 years of age (inclusive), with a body mass index (BMI) of 18–32 kg/m2, who abstained from tobacco and nicotine use for at least two months prior to screening were eligible for enrollment. Eligible subjects were instructed not to take any prescription medication for 14 days or any dietary supplements or over-the-counter drugs for at least three days prior to CRU admission through completion of the study. Subjects provided written informed consent before participating in any study procedure.
Subjects were excluded if they had a history of asthma or other respiratory condition; a history of illicit drug or alcohol abuse or positive urine drug screen; were positive for human immunodeficiency virus, hepatitis B, and/or hepatitis C; in pregnant or lactating females; had donated plasma or blood within 7 or 30 days prior to CRU admission, respectively; had participated in another investigational drug study within the 30 days prior to CRU admission; or had surgery within six months of screening.
Pharmacokinetic analysis
Approximately 4 mL of whole blood was collected for each PK sample, yielding approximately 2 mL of plasma for analysis of treprostinil concentrations. Blood samples were collected via IV catheters implanted in peripheral arm veins and stored with Vacutainer® tubes with potassium EDTA (BD #367861 or equivalent) approximately one hour before dosing and at 5, 10, 15, 20, 25, 30, 45, 60, 90, 120, 150, 180, and 210 min and at 4, 6, and 8 h after study drug administration.
Treprostinil plasma concentrations were measured at PPD Laboratories (Middleton, WI) using a validated ultra-performance liquid chromatography-tandem mass spectrometry bioanalytical method (Bioanalytical Method P1313).35 The lower limit of quantification in the assay was 0.025 ng/mL and the upper limit of quantification was 10 ng/mL. Treprostinil concentrations were summarized using descriptive statistics.
Individual treprostinil PK parameters were calculated using Phoenix® WinNonlin® v6.3 (Certara, Princeton, NJ) and summarized with descriptive statistics. Plasma concentrations below the limit of quantification (BLQ) were set to zero, unless they fell between two quantifiable samples, in which case they were treated as missing. Figures were prepared using R version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
Dose proportionality assessments were conducted with Cmax and AUCinf using the confidence interval equivalence criterion with a power model.36 Given the exploratory nature of the study and the corresponding wide dose range with small sample sizes, a wider acceptance interval of 0.5–2.0 was applied to establish conclusion of proportionality within the tested dosing range.37 For both parameters, the estimate for the ratio of dose-normalized geometric mean, 90% confidence intervals, maximal proportional dose range, and threshold dose ratio to reject proportionality were calculated.
Safety assessments
Safety monitoring included an evaluation of adverse events (AEs) and assessments of electrocardiograms (ECG), respiratory signs, vital signs, clinical laboratory parameters (pre-dose and through 6–8 h post-dose), physical examinations, urinalysis, and concomitant medications. In addition, subjects in the 125-mcg and 150-mcg dose cohorts (cohorts 5 and 6) underwent continuous cardiac telemetry and were monitored by Advanced Cardiac Life Support-certified staff from 30 min prior to dosing through three hours after dose administration. This extra safety precaution was implemented due to the evidence of QTc prolongation in healthy subjects after administration of maintenance and supratherapeutic doses (54 and 84 mcg, respectively) of treprostinil (inhaled Tyvaso®).16
Following each dosing, and prior to each dose escalation, the Safety Review Committee (SRC), which consisted of the Principal Investigator, the Study Medical Monitor, and a representative from the Sponsor, convened by teleconference to review safety findings and interim PK results from each cohort. Treatment assignments remained blinded during these reviews and an unanimous agreement from voting members of the SRC was required prior to proceeding with the next sequential dose cohort. Stopping criteria were also prospectively defined in the study protocol. If a severe AE or serious adverse event (SAE) was observed in ≥2 subjects within a dose cohort, the SRC was required to stop escalation of the dose levels.
Results
Subject characteristics
Fifty-seven subjects were enrolled in the study, and 56 subjects completed all protocol-defined assessments. One subject in cohort 2 (50 mcg) withdrew consent from PK sample collections after the 10-min post-dose timepoint complaining of painful venipuncture and was replaced, which resulted in a total of seven subjects on active treatment in this cohort. The subject who was withdrawn consented to continued observation and safety assessments and was included in the safety population.
Demographic characteristics were well-balanced between the LIQ861 and placebo groups (Table 2). Similar proportions of male and female subjects were enrolled and subjects in both treatment groups were predominantly White. Mean age and BMI were similar in both treatment groups.
Table 2.
Demographic and clinical characteristics by treatment group.
| Characteristic |
Treatment group, n (%) |
|
|---|---|---|
| LIQ861 (n = 43) | Placebo (n = 14) | |
| Age at screening, years | 30.3 (6.7) | 26.1 (5.4) |
| Mean (SD) | 18, 44 | 19, 36 |
| Min, max | ||
| Sex, n (%) | ||
| Female | 21 (48.8) | 7 (50.0) |
| Male | 22 (51.2) | 7 (50.0) |
| Race, n (%) | ||
| American Indian or Alaska Native | 1 (2.3) | 0 (0.0) |
| Asian | 1 (2.3) | 0 (0.0) |
| Black or African American | 13 (30.2) | 4 (28.6) |
| White | 28 (65.1) | 10 (71.4) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 18 (41.9) | 8 (57.1) |
| Not Hispanic or Latino | 25 (58.1) | 6 (42.9) |
| BMI, kg/m2 | ||
| Mean (SD) | 25.8 (3.3) | 25.9 (2.8) |
| Min, max | 19.2, 30.6 | 22.1, 29.7 |
BMI: body mass index; max: maximum; min: minimum; n: number of subjects; SD: standard deviation.
PK of LIQ861
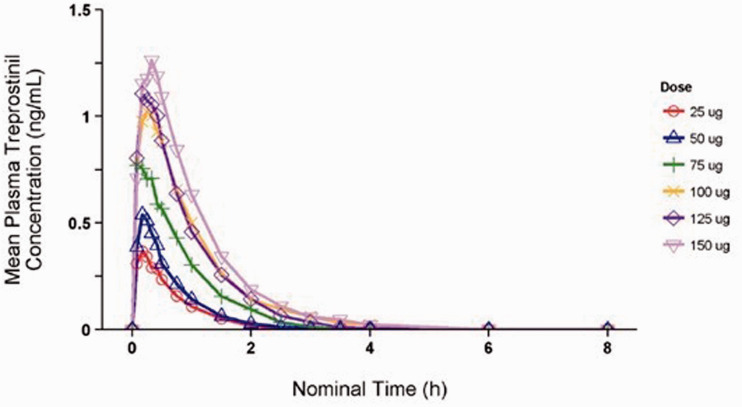
Plasma treprostinil concentrations in subjects who received LIQ861 were quantifiable by five minutes after dosing (time of first post-dose sample collection) in all but one subject. Absorption of treprostinil was rapid, with median Tmax ranging from 0.18 to 0.31 h across all dose levels (Table 3). Following peak concentrations, treprostinil plasma levels declined in a monophasic manner. Plasma concentrations of treprostinil were BLQ in the 25-, 50-, and 75-mcg dose cohorts by 3.5 h post-dose and in the 100-, 125-, and 150-mcg cohorts by six hours post-dose. Treprostinil plasma concentrations over time by dose are shown in Fig. 2.
Table 3.
Treprostinil pharmacokinetic parameters following inhalation administration of LIQ861.
| Parameter |
LIQ861, mcg |
|||||
|---|---|---|---|---|---|---|
| 25 | 50 | 75 | 100 | 125 | 150 | |
| n | 6 | 6 | 6 | 6 | 6 | 12 |
| Cmax (ng/mL) | 0.31(0.19–0.54) | 0.45 (0.12–1.03) | 0.68 (0.43–1.11) | 1.04(0.73–1.54) | 1.08 (0.51–1.96) | 1.25 (0.70–2.34) |
| Tmax (h) | 0.21 (0.16–0.45) | 0.18 (0.08–0.42) | 0.25 (0.08–0.42) | 0.29 (0.17–0.50) | 0.24 (0.16–0.41) | 0.31 (0.08–0.47) |
| AUClast (h*ng/mL) | 0.25 (0.16–0.36) | 0.31 (0.07–0.73) | 0.68 (0.40–1.26) | 1.16 (0.78–1.44) | 1.01 (0.36–1.87) | 1.32 (0.58–2.84) |
| AUCinf (h*ng/mL) | 0.28 (0.18–0.39) | 0.34 (0.08–0.75) | 0.72 (0.45–1.29) | 1.20 (0.82–1.47) | 1.04 (0.38–1.91) | 1.36 (0.62–2.94) |
| t1/2 (h) | 0.50 (0.41–0.72) | 0.42 (0.25–0.52) | 0.60 (0.52–0.98) | 0.70 (0.47–0.97) | 0.52 (0.44–0.65) | 0.64 (0.46–0.95) |
| CL/F (L/h) | 90.9 (64.8–136.0) | 149.0 (66.6–624.0) | 105.0 (58.0–169.0) | 83.4 (67.9–122.0) | 120.0 (65.6–329.0) | 110.0 (51.0–243.0) |
Note: Data are geometric mean and range or median and range for Tmax.
AUCinf: area under the plasma concentration–time curve from 0 to infinity; AUClast: area under the plasma concentration–time curve from time 0 to time of last measurable plasma concentration; CL/F: clearance; Cmax: maximum plasma concentration; max: maximum; min: minimum; n: number of subjects; t1/2: terminal elimination half-life; Tmax: time to reach Cmax.
Fig. 2.
Mean plasma treprostinil concentration over time.
Geometric means for Cmax ranged from 0.31 to 1.25 ng/mL across the dose cohorts. Geometric means for AUCinf ranged from 0.28 h*ng/mL in cohort 1 (25 mcg) to 1.36 h*ng/mL in cohort 6 (150 mcg).
Dose proportionality was assessed based on Cmax and AUCinf over the tested dose range of 25–150 mcg treprostinil administered as LIQ861. The apparent clearance (CL/F) of treprostinil was observed to be independent of administered dose (geometric means ranged from 83.4 to 149.0 L/h).
Safety and tolerability
Overall, treprostinil administered by inhalation as LIQ861 was well-tolerated by study subjects. There were no deaths, SAEs, dose-limiting toxicities, or treatment-emergent adverse events (TEAEs) that led to study withdrawal. Approximately half of the subjects who received active treatment (55.8%) experienced a TEAE compared with 14.3% on placebo (Table 4). The majority of subjects on active treatment who reported a TEAE had an event that was considered related to study treatment by the Investigator (19/24 subjects; 79.2%).
Table 4.
Overall incidence of treatment-emergent adverse events by cohort and treatment.
| Cohort | n | Treprostinil dose, mcg |
Related to treatment |
Unrelated to treatment |
Overall |
|||
|---|---|---|---|---|---|---|---|---|
| No. (%) of subjects | No. of events | No. (%) of subjects | No. of events | No. (%) of subjects | No. of events | |||
| Cohort 1 | 6 | |||||||
| LIQ861 | 6 | 25 | 1 (16.7) | 1 | 2 (33.3) | 3 | 3 (50.0) | 4 |
| Placebo | 2 | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 |
| Cohort 2 | ||||||||
| LIQ861 | 7 | 50 | 2 (28.6) | 2 | 1 (14.3) | 2 | 3 (42.9) | 4 |
| Placebo | 2 | 0 | 0 (0.0) | 0 | 1 (50.0) | 1 | 1 (50.0) | 1 |
| Cohort 3 | ||||||||
| LIQ861 | 6 | 75 | 2 (33.3) | 3 | 0 (0.0) | 0 | 2 (33.3) | 3 |
| Placebo | 2 | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 |
| Cohort 4 | ||||||||
| LIQ861 | 6 | 100 | 2 (33.3) | 3 | 1 (16.7) | 2 | 3 (50.0) | 5 |
| Placebo | 2 | 0 | 0 (0.0) | 0 | 1 (50.0) | 1 | 1 (50.0) | 1 |
| Cohort 5 | ||||||||
| LIQ861 | 6 | 125 | 4 (66.7) | 12 | 0 (0.0) | 0 | 4 (66.7) | 12 |
| Placebo | 2 | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 |
| Cohort 6a | ||||||||
| LIQ861 | 6 | 150 | 4 (66.7) | 9 | 0 (0.0) | 0 | 4 (66.7) | 9 |
| Placebo | 2 | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 |
| Cohort 6b | ||||||||
| LIQ861 | 6 | 150 | 4 (66.7) | 10 | 1 (16.7) | 1 | 5 (83.3) | 11 |
| Placebo | 2 | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 |
| Total | ||||||||
| LIQ861 | 43 | 19 (44.2) | 40 | 5 (11.6) | 8 | 24 (55.8) | 48 | |
| Placebo | 14 | 0 (0.0) | 0 | 2 (14.3) | 2 | 2 (14.3) | 2 | |
Note: Percentages are based on the number of subjects in each respective cohort.
aOriginal cohort at 150 mcg treprostinil.
bRepeated cohort at 150 mcg treprostinil with six additional patients.
n: number of subjects per treatment group.
The most common TEAEs related to study drug administration were cough (25.6%) and throat irritation (20.9%) (Table 5). Painful respiration and dizziness were both experienced by 14% of subjects on active treatment. Treatment-related TEAEs were reported more frequently at higher dose levels (Table 4). All treatment-related events were reported as mild in severity. TEAEs considered unrelated to study treatment that were reported during the study were associated with vasovagal symptoms that occurred during or around the time of PK venipuncture and included presyncope (n = 5), dizziness (n = 1), feeling hot (n = 1), and headache (n = 1) in subjects who received LIQ861 and rhinorrhea (n = 1) and vessel puncture site pain (n = 1) in subjects who received placebo.
Table 5.
Summary of related and unrelated treatment-emergent adverse events by system organ class and preferred term.
| System organ class and preferred term |
Group |
|||||
|---|---|---|---|---|---|---|
|
LIQ861 (n = 43) |
Placebo (n = 14) |
Overall (n = 57) |
||||
| No. (%) of subjects | No. of events | No. (%) of subjects | No. of events | No. (%) of subjects | No. of events | |
| Related events | ||||||
| Gastrointestinal disorders | ||||||
| Nausea | 3 (7.0) | 3 | 0 (0.0) | 0 | 3 (5.3) | 3 |
| Nervous system disorders | ||||||
| Dizziness | 6 (14.0) | 6 | 0 (0.0) | 0 | 6 (10.5) | 6 |
| Headache | 4 (9.3) | 4 | 0 (0.0) | 0 | 4 (7.0) | 4 |
| Respiratory, thoracic, and mediastinal disorders | ||||||
| Cough | ||||||
| Painful respiration | 11 (25.6) | 11 | 0 (0.0) | 0 (0.0) | 11 (19.3) | 11 |
| Throat irritation | 6 (14.0) | 6 | 0 (0.0) | 0 (0.0) | 6 (10.5) | 6 |
| 9 (20.9) | 9 | 0 (0.0) | 0 (0.0) | 9 (15.8) | 9 | |
| Vascular disorders | ||||||
| Hot flush | 1 (2.3) | 1 | 0 (0.0) | 0 (0.0) | 1 (1.8) | 1 |
| Unrelated events | ||||||
| General disorders and administration site conditions | ||||||
| Feeling hot | ||||||
| Vessel puncture site pain | 1 (2.3) | 1 | 0 | 0 | 1 (1.8) | 1 |
| 0 (0.0) | 0 | 1 (7.1) | 1 | 1 (1.8) | 1 | |
| Nervous system disorders | ||||||
| Dizziness | 1 (2.3) | 1 | 0 (0.0) | 0 | 1 (1.8) | 1 |
| Headache | 1 (2.3) | 1 | 0 (0.0) | 0 | 1 (1.8) | 1 |
| Presyncopea | 5 (11.6) | 5 | 0 (0.0) | 0 | 5 (8.8) | 5 |
| Respiratory, thoracic, and mediastinal disorders | ||||||
| Rhinorrhea | ||||||
| 0 (0.0) | 0 | 1 (7.1) | 1 | 1 (1.8) | 1 | |
aFive subjects reported vasovagal symptoms that were coded as presyncope and were all attributed by the Investigator as related to venipuncture for PK collection and not related to the study drug.
There were no clinically significant changes in laboratory parameters, vital signs, physical examination findings, or ECG parameters observed during the study in either treatment group. A slight prolongation in QTc interval, which is consistent with the known pharmacodynamic effects of treprostinil, was observed in subjects administered more than 100 mcg, but no subjects had a prolongation of more than 60 msec from baseline or a QTc interval that exceeded 450 msec.
Discussion
Study LTI-101 is the first clinical trial to evaluate the safety and PK of treprostinil administered as LIQ861, a novel dry-powder formulation, to healthy subjects. Our results showed that administration of single doses of LIQ861 resulted in rapid absorption of treprostinil (Tmax 0.18–0.31 h) and median values for Tmax that were consistent across the dose range. Approximately 50% of subjects in the higher dose cohorts (100, 125, and 150 mcg) continued to show measurable concentrations of treprostinil at 4 h post-dosing with all doses well-tolerated. Observed exposures of treprostinil after inhalation of LIQ861 increased proportionally with doses between 25 and 150 mcg. Mean maximum observed plasma concentration (Cmax) and area under the plasma concentration versus time curve from time 0 (pre-dose) to infinity (AUCinf) values from this study were compared with mean exposure levels reported in the published literature and in the Drug Approval Reviews from the US Food and Drug Administration for Tyvaso®.16,38,39 Commonalities between the exposures observed in study LTI-101 and those reported for Tyvaso® were used to project a Tyvaso® transition dose for LIQ861 for patients participating in phase 3 clinical trials.
A systemic exposure was achieved with fewer inhalations than required for Tyvaso® and no unexpected safety issues, with subjects experiencing only mild, class-related AEs. LIQ861 may improve the therapeutic profile of treprostinil by enhancing deep-lung delivery and achieving higher dose levels than current inhaled therapies while overcoming some of the limitations of prostacyclin therapies delivered by the IV, SC, and oral routes.
There is a clear need for new, more convenient PAH therapies that offer sustained long-term benefits and improved safety and tolerability profiles while increasing the utilization of drugs that target the prostacyclin pathway.40 Oral agents may overcome some of the limitations associated with IV, SC, and inhaled PGI2 therapy. Treprostinil18 and selexipag19 are oral prostacyclin agonists that are approved in the US and recommended for the treatment of Group 1 PAH World Health Organization/Functional class II and III patients.8 They offer the advantages of oral therapy, but side effects such as headaches and nausea limit the ability of patients to reach therapeutic doses and contribute to treatment discontinuation in 14.3% and 19.0% of patients taking selexipag41 and inhaled treprostinil, respectively.42
Improvement in devices for inhaled therapies has the potential to enhance and maintain treatment adherence as well as clinical response. Inhaled therapy may also reduce the risk of systemic vasodilation and ventilation-perfusion mismatch.28,43,44 However, variations in breathing patterns between patients make it difficult to control dosing, and delivery systems that are difficult to operate or cumbersome may increase the likelihood of errors in dosing.7,28 Inhaled therapies are not administered during sleep, which can place patients at risk of sub-therapeutic plasma levels.7 In addition, the number, frequency, and length of inhalations combined with the time required for drug preparation and maintenance of inhaler devices impose considerable burdens on patients and their caregivers and may reduce treatment compliance and persistence.7,22,29 However, a recent systematic review and meta-analysis examined the efficacy and safety profiles of prostacyclin mimetics with regard to their route of administration. All agents improved six-minute walk distance, although the authors suggested that the modest gains in exercise capacity for some of the agents might have been due to underdosing. Of note, inhaled therapies were associated with a more favorable impact on patient quality of life when compared with oral agents.26
Currently available inhaled prostacyclins include iloprost (Ventavis®) and treprostinil (Tyvaso®). Ventavis® has a half-life of approximately 20 min and requires at least six administrations/day consisting of 35 mcg of iloprost. Ventavis® is delivered using an inhaler device that must be assembled each morning, with 10 parts listed in the patient user guide.17 Tyvaso® requires use of the Tyvaso® Inhalation System, an ultrasonic, pulsed delivery device that consists of 13 parts as reported in the patient starter kit. Tyvaso® has a half-life of three to four hours and requires four separate, equally spaced treatment sessions per day. The maximum recommended target dose is nine breaths per treatment, four times daily for an approximate total dose of 54 mcg.16
Prostacyclin analogues must reach the lungs in order to be effective. Therefore, localized dosing to the lungs through inhalation allows more drug to be delivered directly to the site of action, while limiting the occurrence of systemic side effects that are typically associated with oral and parenteral therapies. Results from in vitro studies31 suggest that the precise size, trefoil-like shape, and uniformity of each dry-powder particle of LIQ861 may provide deep-lung delivery of treprostinil and may reduce deposition in the upper airway where cough, throat irritation, pharyngolaryngeal pain, and bronchospasm have been reported for inhaled prostacyclins.16,17
In this study, LIQ861 doses ranging from 25 to 150 mcg capsule strength were well-tolerated by subjects and resulted in the occurrence of only mild TEAEs with no dose-limiting side effects. Cough and throat irritation were the most frequently reported TEAEs and are characteristic of other inhaled prostacyclin formulations.16,17 Each of the five events of presyncope were attributed by the Investigator to the venipunctures for the PK collection and not related to the study drug. These results suggest that patients may tolerate higher inhaled doses of treprostinil when delivered as a PRINT® dry powder. Consequently, doses above 150 mcg of LIQ861, which represent treprostinil plasma levels > 85 mcg, the maximum tolerated dose for Tyvaso®, may be achievable. A phase 3 study45 (INSPIRE; Clinicaltrials.gov Identifier NCT03399604) to evaluate the long-term safety and tolerability of LIQ861 in patients with PAH is under way. Interim safety and tolerability results are consistent with those reported for this phase 1 PK trial.46
Conclusions
LIQ861 demonstrates a dose proportionality based on Cmax and AUCinf over the tested dose range of 25–150 mcg, and all doses were well-tolerated by subjects. LIQ861 achieved higher dose levels than currently approved inhaled therapies while overcoming some of the limitations of prostacyclin therapies delivered by the IV, SC, and oral routes.
Acknowledgements
Carole Alison Chrvala, PhD, and Philippe Brudi, MD, are acknowledged for their assistance with the preparation of this manuscript.
Footnotes
Conflict of interest: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Robert Roscigno, Toby Vaughn, and Stephanie Anderson are employees of Liquidia Technologies. William Wargin declares that there is no conflict of interest. Thomas Hunt is an employee of PPD, a contract research organization for Liquidia Technologies. Nicholas S. Hill is a consultant and scientific medical advisor, and has received grant/research support payments to his institution from Liquidia Technologies.
Ethical approval: Salus IRB. IRB Registration #: 00006833. Chair: John C. Lewis, DVM.
Guarantor: Nicholas S. Hill
Contributorship: All authors contributed to the conception and design of the study; the acquisition, analysis, and interpretation of data; reviewed and revised the manuscript; gave final approval for submission; and agreed to be accountable of all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Support for this study and development of this manuscript was provided by Liquidia Technologies. Authors were not compensated for the development of the manuscript.
References
- 1.Ataya A, Cope J, Alnuaimat H. A review of targeted pulmonary arterial hypertension-specific pharmacotherapy. J Clin Med 2016; 5: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badlam JB, Bull TM. Steps forward in the treatment of pulmonary arterial hypertension: latest developments and clinical opportunities. Ther Adv Chronic Dis 2017; 8: 47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes H, Yeoh HL, Fothergill T, et al. Prostacyclin for pulmonary arterial hypertension. Cochrane Database Syst Rev 2019; 5: CD012785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christman BW, McPherson CD, Newman JH, et al. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med 1992; 327: 70–75. [DOI] [PubMed] [Google Scholar]
- 5.Tuder RM, Cool CD, Geraci MW, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med 1999; 159: 1925–1932. [DOI] [PubMed] [Google Scholar]
- 6.Del Pozo R, Hernandez Gonzalez I, Escribano-Subias P. The prostacyclin pathway in pulmonary arterial hypertension: a clinical review. Expert Rev Respir Med 2017; 11: 491–503. [DOI] [PubMed] [Google Scholar]
- 7.Farber HW, Gin-Sing W. Practical considerations for therapies targeting the prostacyclin pathway. Eur Respir Rev 2016; 25: 418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galiè N, Humbert M, Vachiery JL, et al. ; ESC Scientific Document Group. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (ESC) and the European respiratory society (ERS): endorsed by: Association for European paediatric and congenital cardiology (AEPC), international society for heart and lung transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 9.Gomberg-Maitland M, Olschewski H. Prostacyclin therapies for the treatment of pulmonary arterial hypertension. Eur Respir J 2008; 31: 891–901. [DOI] [PubMed] [Google Scholar]
- 10.Farber HW, Miller DP, Meltzer LA, et al. Treatment of patients with pulmonary arterial hypertension at the time of death or deterioration to functional class IV: insights from the REVEAL registry. J Heart Lung Transplant 2013; 32: 1114–1122. [DOI] [PubMed] [Google Scholar]
- 11.McGoon MD, Miller DP. REVEAL: a contemporary US pulmonary arterial hypertension registry. Eur Respir Rev 2012; 21: 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLaughlin VV, Palevsky HI. Parenteral and inhaled prostanoid therapy in the treatment of pulmonary arterial hypertension. Clin Chest Med 2013; 34: 825–840. [DOI] [PubMed] [Google Scholar]
- 13.Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL registry. Chest 2010; 137: 376–387. [DOI] [PubMed] [Google Scholar]
- 14.United Therapeutics Corp. Remodulin (package insert). Durham, NC: United Therapeutics Corp, 2018. [Google Scholar]
- 15.Actelion Pharmaceuticals US, Inc. Veletri (package insert) South San Francisco, CA: Actelion Pharmaceuticals US, Inc., 2018.
- 16.United Therapeutics Corp. Tyvaso (package insert). Durham, NC: United Therapeutics Corp, 2017. [Google Scholar]
- 17.Actelion Pharmaceuticals US, Inc. Ventavis (package insert). South San Francisco, CA: Actelion Pharmaceuticals US, Inc, 2017. [Google Scholar]
- 18.United Therapeutics Corp. Orenitram (package insert). Durham, NC: United Therapeutics Corp, 2019. [Google Scholar]
- 19.Actelion Pharmaceuticals US, Inc. Uptravi (package insert). South San Francisco, CA: Actelion Pharmaceuticals US, Inc, 2019. [Google Scholar]
- 20.Hohsfield R, Archer-Chicko C, Housten T, et al. Pulmonary arterial hypertension emergency complications and evaluation: practical guide for the advanced practice registered nurses in the emergency department. Adv Emerg Nurs J 2018; 40: 246–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.GlaxoSmithKline. Flolan (Package insert). Zebulon, NC: GlaxoSmithKline, 2018. [Google Scholar]
- 22.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med 2004; 351: 1425–1436. [DOI] [PubMed] [Google Scholar]
- 23.Kingman M, Archer-Chicko C, Bartlett M, et al. Management of prostacyclin side effects in adult patients with pulmonary arterial hypertension. Pulm Circ 2017; 7: 598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeVarge BL. Prostanoid therapies in the management of pulmonary arterial hypertension. Ther Clin Risk Manag 2015; 11: 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mubarak KK. A review of prostaglandin analogs in the management of patients with pulmonary arterial hypertension. Respir Med 2010; 104: 9–21. [DOI] [PubMed] [Google Scholar]
- 26.Picken C, Fragkos KC, Eddama M, et al. Adverse events of prostacyclin mimetics in pulmonary arterial hypertension: a systematic review and Meta-analysis. J Clin Med 2019; 8: 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simonneau G, Barst RJ, Galiè N, et al. ; Treprostinil Study Group. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med 2002; 165: 800–804. [DOI] [PubMed] [Google Scholar]
- 28.Hill NS, Preston IR, Roberts KE. Inhaled therapies for pulmonary hypertension. Respir Care 2015; 60: 794–802. [DOI] [PubMed] [Google Scholar]
- 29.Badesch DB, McLaughlin VV, Delcroix M, et al. Prostanoid therapy for pulmonary arterial hypertension. J Am Coll Cardiol 2004; 43: 56S–61S. [DOI] [PubMed] [Google Scholar]
- 30.El Yafawi R, Wirth JA. What is the role of oral prostacyclin pathway medications in pulmonary arterial hypertension management? Curr Hypertens Rep 2017; 19: 97. [DOI] [PubMed] [Google Scholar]
- 31.Maynor BW. 21st century particle engineering: particle design, manufacture and scale-up of PRINT® technology. Respir Drug Deliv 2018; 1: 211–220. [Google Scholar]
- 32.Mack P, Tully J, Herlihy KP, et al. Formulation and in vivo evaluation of treprostinil dry powder for inhalation, fabricated using the PRINT® particle technology. Respir Drug Deliv 2014; 2: 473–476. [Google Scholar]
- 33.Garcia A, Mack P, Williams S, et al. Microfabricated engineered particle systems for respiratory drug delivery and other pharmaceutical applications. J Drug Deliv 2012; 2012: 941243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roscigno R, Vaughn T, Wargin W, et al. Phase 1 safety and pharmacokinetic study of inhaled LIQ861: a new dry powder formulation of treprostinil In: Presented at: PHA’s international pulmonary hypertension conference, Orlando, FL, 28 June–1 July 2018. [Google Scholar]
- 35.Moein MM, El Beqqali A, Abdel-Rehim M. Bioanalytical method development and validation: critical concepts and strategies. J Chromatogr B Analyt Technol Biomed Life Sci 2017; 1043: 3–11. [DOI] [PubMed] [Google Scholar]
- 36.Smith BP, Vandenhende FR, DeSante KA, et al. Confidence interval criteria for assessment of dose proportionality. Pharm Res 2000; 17: 1278–1283. [DOI] [PubMed] [Google Scholar]
- 37.Hummel J, McKendrick S, Brindley C, et al. Exploratory assessment of dose proportionality: review of current approaches and proposal for a practical criterion. Pharm Stat 2009; 8: 38–49. [DOI] [PubMed] [Google Scholar]
- 38.Kumar P, Thudium E, Laliberte K, et al. A comprehensive review of treprostinil pharmacokinetics via four routes of administration. Clin Pharmacokinet 2016; 55: 1495–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Channick RN, Voswinckel R, Rubin LJ. Inhaled treprostinil: a therapeutic review. Drug Des Devel Ther 2012; 6: 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lang IM, Gaine SP. Recent advances in targeting the prostacyclin pathway in pulmonary arterial hypertension. Eur Respir Rev 2015; 24: 630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sitbon O, Channick R, Chin KM, et al. ; GRIPHON Investigators. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 2015; 373: 2522–2533. [DOI] [PubMed] [Google Scholar]
- 42.Benza RL, Seeger W, McLaughlin VV, et al. Long-term effects of inhaled treprostinil in patients with pulmonary arterial hypertension: the treprostinil sodium inhalation used in the management of pulmonary arterial hypertension (TRIUMPH) study open-label extension. J Heart Lung Transplant 2011; 30: 1327–1333. [DOI] [PubMed] [Google Scholar]
- 43.Parikh KS, Rajagopal S, Fortin T, et al. Safety and tolerability of high-dose inhaled treprostinil in pulmonary hypertension. J Cardiovasc Pharmacol 2016; 67: 322–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voswinckel R, Reichenberger F, Enke B, et al. Acute effects of the combination of sildenafil and inhaled treprostinil on haemodynamics and gas exchange in pulmonary hypertension. Pulm Pharmacol Ther 2008; 21: 824–832. [DOI] [PubMed] [Google Scholar]
- 45.ClinicalTrials.gov. Investigation of the safety and pharmacology of dry-powder inhalation of treprostinil (INSPIRE), https://clinicaltrials.gov/ct2/show/NCT03399604 (2018, accessed 6 August 2019).
- 46.Hill NS, Feldman JP, Sahay S, et al. INSPIRE: a phase 3 open-label, multicenter study to evaluate the safety and tolerability of LIQ861 in pulmonary arterial hypertension (PAH). J Heart Lung Transplant 2019; 38: S11. [Google Scholar]