Abstract
Collision carcinoma is a rare malignancy that generally occurs in cervical, esophageal, pulmonary, and squamous cell cancers. Few studies have been reported involving endometrial adenocarcinoma and fallopian tube carcinoma. We reported the case of a 58-year-old woman who presented because of irregular vaginal bleeding for more than 1 month. Cervical biopsy suggested moderately differentiated cervical adenocarcinoma, and the patient underwent radical hysterectomy under general anesthesia. However, postoperative pathology and immunohistochemical results indicated a collision tumor comprising endometrial adenocarcinoma (grade I) and primary serous fallopian tube carcinoma. According to the treatment principle of multiple primary tumors, a regimen of paclitaxel combined with carboplatin was administered. The patient also underwent local pelvic radiotherapy to treat lymph node metastasis. One month later, the patient developed brain metastases and died.
Keywords: Collision carcinoma, endometrial adenocarcinoma, primary serous fallopian tube carcinoma, immunohistochemistry, postoperative pathology, diagnosis
Background
Collision carcinoma is a type of malignant tumor that occurs in the same organs or parts of two or more different types of malignant tumor, with or without transition between the tumor tissues.1,2 Several studies have shown that collision tumor generally occurs in cervical, esophageal, pulmonary, and squamous cell cancers3–5 but is rare in the endometrium and fallopian tubes.
Herein, we present a case of endometrial adenocarcinoma (grade I) colliding with primary serous fallopian tube carcinoma. In this case report, we aimed to discuss the potential pitfalls in diagnosis and management of patients with collision carcinoma between the endometrium and fallopian tube.
Case report
A 58-year-old woman was admitted to our hospital due to irregular vaginal bleeding that had persisted for more than 1 month. Physical examination showed normal superficial lymph nodes, flat abdomen, no evidence of tumor in the vulva, cervical hypertrophy, a 3-cm cervical nodule, vaginal fornix involvement, and hysterauxesis with no neoplastic ovarian cyst. A cervical biopsy revealed a moderate to poorly differentiated adenocarcinoma. The patient was diagnosed with moderately differentiated cervical adenocarcinoma and underwent radical hysterectomy under general anesthesia, including bilateral adnexectomy, pelvic lymph node dissection, para-aortic lymphadenectomy, greater omentectomy, multi-point intestinal membrane biopsy, and intestinal adhesion lysis.
Postoperative pathology suggested endometrial adenocarcinoma (grade I, 6 × 3 ×1.5 cm), localized foci (grade II) accompanied by squamous cell differentiation, and high-grade serous carcinoma differentiation. The depth of myometrial invasion was more than one-half, and the depth of infiltration from the cervical mucous to the shallow layer interstitium was less than one-third. The cancer also transferred to or infiltrated the right common iliac (0/2, no metastases in 2 lymph nodes removed), right external iliac (0/1), right groin deep (0/4), right internal iliac and obturator (1/2), left common iliac (0/1), left external iliac (0/2), left groin deep (0/2), left internal iliac and obturator (0/2), as well as para-aortic (4/8) lymph nodes (Figure 1a). A high-grade primary serous carcinoma of the right fallopian tube (5 × 3 × 1 cm) infiltrated into muscular layer was diagnosed. The left fallopian tube and bilateral ovarian tissues showed suppurative inflammatory changes without abscess formation or cancer cells. Fibers and adipose tissues (omentum, left paracolic sulcus, right paracolic sulcus, retrograde peritoneum of bladder, uterine and rectal fossae) were also found (Figure 1b). As shown in Figure 2, hematoxylin and eosin staining and immunohistochemistry of monoclonal antibody and cancer gene markers showed the following: estrogen receptor (uterus ++50%, fallopian tube ++60%), progesterone receptor (uterus ++60%, fallopian tube ++30%), p53 (uterus + 10%, uterus ++50%, fallopian tube + 70%), p16 (uterus + 50%, fallopian tube +), cancer antigen 125 (CA125; uterus +, fallopian tube +), Wilms’ tumor protein (WT-1; part uterus +, part fallopian tube +), paired-box gene 8 (PAX-8; part uterus +, part fallopian tube +), and Ki-67 (uterus + 10%, fallopian tube + 70%), where + indicates staining intensity.
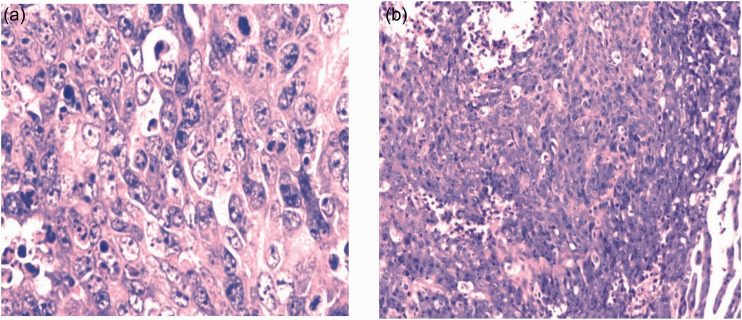
Figure 1.
Postoperative pathology showing hematoxylin and eosin staining results of (a) endometrial adenocarcinoma, and (b) fallopian tube carcinoma.
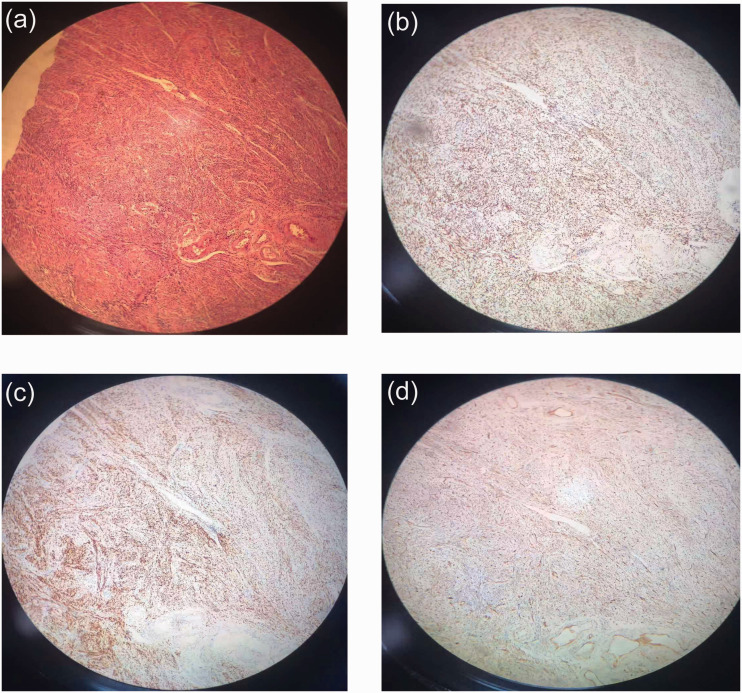
Figure 2.
(a) Hematoxylin and eosin staining of specimen, and immunohistochemical results of (b) estrogen receptor, (c) progesterone receptor, and (d) Wilms’ tumor 1 protein (WT-1) under electron microscope (10×).
Macroscopic assessment showed that the endometrial adenocarcinoma and primary serous fallopian tube carcinoma were continuous and in close proximity (Figure 3). The patient was diagnosed with a collision tumor comprising endometrial adenocarcinoma and primary serous fallopian tube carcinoma. She received paclitaxel (200 mg, d1) and carboplatin (area under the curve = 5; d1, q3w), as well as intensity-modulated radiation therapy (tumor-absorbed dose [DT]: CTV 45Gy/25F) in 10v-x (10 megabytes of radiation energy) pelvic lymph node drainage area.6 One month after pelvic radiotherapy, the patient developed brain metastases and died.
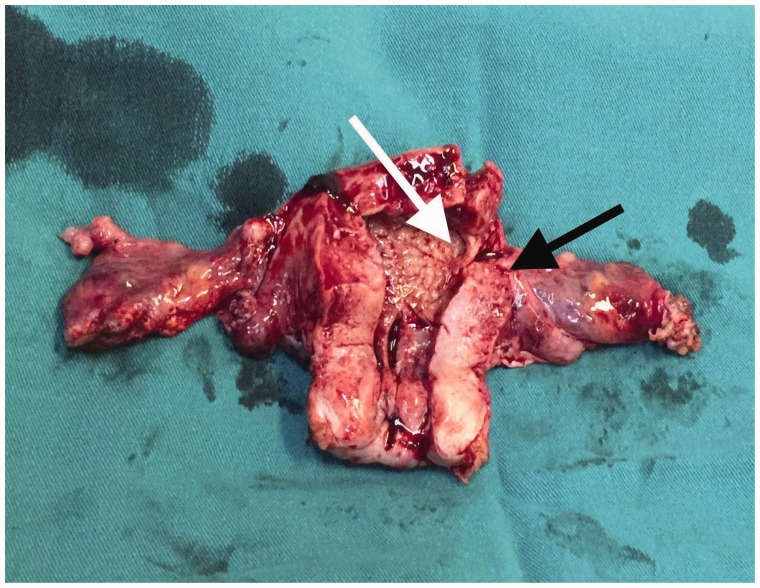
Figure 3.
Macroscopic assessment of the specimen indicating the fallopian tube carcinoma (black arrow) and the endometrial adenocarcinoma (white arrow).
Discussion
At present, the pathogenesis of collision carcinoma remains unclear. Fujii et al.7 indicated that collision tumor is derived from two independent neoplastic clones in one organ. Jang et al.2 suggested that in collision tumor, each tumor has a well-defined boundary separated by a non-neoplastic stroma. The patient described here had a collision tumor consisting of endometrial adenocarcinoma (grade I) and primary serous fallopian tube carcinoma, which, to our knowledge, has not been reported before.
Endometrial adenocarcinoma is a prevalent gynecological tumor in postmenopausal women that presents with abnormal vaginal bleeding.8 Primary fallopian tube cancer is a rare gynecological malignancy, accounting for 0.14% to 1.8% cases of female genital cancers,9 of which primary serous fallopian tube carcinoma is the most common type.10 Additionally, it is more likely to occur in advanced cases with poor prognosis due to insidious lesions and lack of recommended screening methods.6 To our knowledge, metastatic malignancies may have the same or similar immunohistochemical phenotypes as the primary lesion. In our case, the patient had two types of malignant tumor components, and we were able to differentiate between the endometrial carcinoma and the fallopian tube carcinoma by expression of p53 and p16, and thus could exclude mutual metastasis. However, this patient did not have multiple primary carcinoma, as generally defined, but a collision carcinoma. The distinction is that the former refers to two pathological types of tumor found simultaneously or successively in different sites in the same patient,11 whereas the latter means that two tumor tissues are close to each other, with no transitional changes or mixing between them.12 According to our macroscopic assessment and pathological and immunohistochemical results of the postoperative specimen, the findings in our patient were consistent with the diagnosis of collision carcinoma.
In terms of treatment, because of the low incidence of collision cancer itself, there is no standard or optimal treatment scheme. Currently, the treatment for endometrial adenocarcinoma is surgical resection followed by radiotherapy, which can only reduce, not eliminate, the risk of local recurrence.13 Previous studies have reported that platinum combined with other chemotherapeutic drugs, such as paclitaxel, for epithelial ovarian malignancies has become a general choice for postoperative fallopian tube cancer.14,15 In this case, the endometrial adenocarcinoma was a low-grade malignant tumor (grade I), which is mainly treated with surgery and postoperative chemoradiotherapy combined with hormone therapy, whereas the primary serous fallopian tube carcinoma is highly malignant, and is treated by surgery combined with chemotherapy. According to the treatment principle of multiple primary tumors, paclitaxel combined with platinum regimen was administered. Local pelvic radiotherapy was also adopted for treatment of lymph node metastasis.
Compared with a single cancer, a collision tumor consisting of endometrial adenocarcinoma and primary serous fallopian tube carcinoma is not specific in clinical manifestations, physical examination, or tumor indicators, which can lead to the misdiagnosis of collision tumor as direct invasion of adjacent tissues by malignant tumor. This was the case in our patient. She was diagnosed as having cervical adenocarcinoma on the basis of history, physical examination, and local pathology before surgery. At this stage of diagnosis, the surgeon should consider whether further relevant examinations, such as fractional curettage, are needed. After surgery, the tumor was found to be endometrial adenocarcinoma, and postoperative pathology indicated the existence of tubal cancer. Therefore, multiple samples of pathological tissues should be collected in clinical work to improve the accuracy of diagnosis, and postoperative pathology is an important part of the diagnosis. When the preoperative pathological examination gives inconsistent results, clinical diagnosis and other examination methods are needed. Multidisciplinary discussion among clinicians, surgeons, and pathologists should be used when necessary to reduce the risk of misdiagnosis and missed diagnosis.
Conclusions
Preoperative diagnosis of collision tumor is difficult. We present a case of a collision tumor consisting of endometrial adenocarcinoma and primary serous fallopian tube carcinoma confirmed by immunohistochemical studies. Sampling of macroscopically heterogeneous tumors and immunohistochemical detection of monoclonal antibodies and cancer genes together represent a useful approach to confirm this difficult diagnosis.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Ethical statement and informed consent: This study was approved by the Ethic Committee of the Huzhou Central Hospital (2019121301), and written informed consent was obtained from the patient. All clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki.
Funding: This study was supported by Young Professionals Program of Zhejiang Provincial Medical and Health Science and Technology Project (2019RC285).
ORCID iDs: Hai-Hong Liao https://orcid.org/0000-0002-5722-1301
References
- 1.Schoolmeester JK, Keeney GL. Collision tumor of the ovary: adult granulosa cell tumor and endometrioid carcinoma. Int J Gynecol Pathol 2012; 31: 538–540. DOI: 10.1097/PGP.0b013e31824d354f. [DOI] [PubMed] [Google Scholar]
- 2.Jang KS, Lee WM, Kim YJ, et al. Collision of three histologically distinct endometrial cancers of the uterus. J Korean Med Sci 2012; 27: 89–92. DOI: 10.3346/jkms.2012.27.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coupland SE, Dodson A, Liu H, et al. Intraocular collision tumour: case report and literature review. Graefes Arch Clin Exp Ophthalmol 2013; 251: 1383–1388. DOI: 10.1007/s00417-012-2216-0. [DOI] [PubMed] [Google Scholar]
- 4.Yamasaki M, Takenaka T, Matsumoto N, et al. Primary pulmonary collision tumor comprising squamous cell carcinoma and mucosa-associated lymphoid tissue lymphoma. Lung Cancer 2019; 129: 107–109. DOI: 10.1016/j.lungcan.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Jia X, Li G, Dong H, et al. Apparent early-stage esophageal collision tumor. Gastrointest Endosc 2016; 83: 841–842; discussion 842. DOI: 10.1016/j.gie.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Ma Y, Duan W. Clinical and survival analysis of 36 cases of primary fallopian tube carcinoma. World J Surg Oncol 2014; 12: 311. DOI: 10.1186/1477-7819-12-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujii H, Zhu XG, Matsumoto T, et al. Genetic classification of combined hepatocellular-cholangiocarcinoma. Hum Pathol 2000; 31: 1011–1017. DOI: 10.1053/hupa.2000.9782. [DOI] [PubMed] [Google Scholar]
- 8.Bacalbasa N, Balescu I, Dragan I, et al. Endometrial adenocarcinoma presenting as hematometra with underlying thickened endometrial lining in a postmenopausal woman - a case report. Anticancer Res 2016; 36: 2353. [PubMed] [Google Scholar]
- 9.Ul'Rikh EA, Papunidi MD, Urmancheeva AF, et al. [Fallopian tube carcinoma: clinical and morphological features, analysis of 69 cases]. Vopr Onkol 2014; 60: 375–378. [PubMed] [Google Scholar]
- 10.Pectasides D, Pectasides E, Economopoulos T. Fallopian tube carcinoma: a review. Oncologist 2006; 11: 902–912. DOI: 10.1634/theoncologist.11-8-902. [DOI] [PubMed] [Google Scholar]
- 11.Chai W, Gong F, Zhang W, et al. Multiple primary cancer in the female genital system: Two rare case reports and a literature review. Medicine (Baltimore) 2017; 96: e8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geng MN, Da YJ, Yuan JI, et al. Survival analysis of pancreatic and periampullary collision cancers. J Dig Dis 2010; 11: 231–236. [DOI] [PubMed] [Google Scholar]
- 13.Kölbl AC, Birk AE, Kuhn C, et al. Influence of VEGFR and LHCGR on endometrial adenocarcinoma. Oncol Lett 2016; 12: 2092–2098. DOI: 10.3892/ol.2016.4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Webber K, Friedlander M. Chemotherapy for epithelial ovarian, fallopian tube and primary peritoneal cancer. Best Pract Res Clin Obstet Gynaecol 2017; 41: 126–138. DOI: 10.1016/j.bpobgyn.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Cheng M, Lee HH, Chang WH, et al. Weekly dose-dense paclitaxel and triweekly low-dose cisplatin: a well-tolerated and effective chemotherapeutic regimen for first-line treatment of advanced ovarian, fallopian tube, and primary peritoneal cancer. Int J Environ Res Public Health 2019; 16: 4794. DOI: 10.3390/ijerph16234794. [DOI] [PMC free article] [PubMed] [Google Scholar]