Abstract
This manuscript on drug repurposing incorporates the broad experience of members of the Pulmonary Vascular Research Institute’s Innovative Drug Development Initiative as an open debate platform for academia, the pharmaceutical industry and regulatory experts surrounding the future design of clinical trials in pulmonary hypertension. Drug repurposing, use of a drug in a disease for which it was not originally developed, in pulmonary arterial hypertension has been a remarkable success story, as highlighted by positive large phase 3 clinical trials using epoprostenol, bosentan, iloprost, and sildenafil. Despite the availability of multiple therapies for pulmonary arterial hypertension, mortality rates have modestly changed. Moreover, pulmonary arterial hypertension patients are highly symptomatic and frequently end up on parental therapy and lung transplant waiting lists. Therefore, an unmet need for new treatments exists and drug repurposing may be an important avenue to address this problem.
Keywords: pulmonary hypertension, preclinical studies, drug repurposing
This position statement outlines the PVRI Drug Repurposing Committee’s views on the:
Innovation needed to create the best environment for drug repurposing.
Importance of both academic and industry contribution and collaboration.
Ways to enhance preclinical pipelines of drug discovery.
Importance of early stage trial design.
Critical role of current funding models and how they might facilitate change.
Take-home message
Drug repurposing is a potential method to develop novel therapies for pulmonary arterial hypertension (PAH), however multiple barriers exist. Here, we propose numerous methods to hopefully enhance the success of drug repurposing for PAH moving forward.
Introduction
Drug repurposing is the process of using a drug that failed in its initial indication, while drug repositioning is the use of an already-approved therapy in a different disease. These two terms are frequently used interchangeably in the literature, and for the purpose of this manuscript, we will use drug repurposing as the umbrella term encompassing both definitions.1,2 Drug repurposing is gaining attention in general drug discovery and development for multiple disease states.3 In pulmonary arterial hypertension (PAH), drug repurposing has been a major success story with multiple positive phase 3 trials. For instance, the survival benefit documented with intravenous epoprostenol was a major breakthrough in PAH treatment;4 however, continuous intravenous therapy comes with unique challenges and drawbacks including risk of infection, thrombosis/sudden obstruction and the burden on patients due to drug mixing and administration. Moreover, the era of pulmonary vasodilators was ushered in by repurposed oral drugs targeting the phosphodiesterase-5 (sildenafil)5 and the endothelin receptor pathways (bosentan).6
Although current PAH therapies mitigate symptom burden, enhance exercise capacity and slow the rate of clinical worsening, PAH patients are still highly symptomatic and life expectancy is poor.7 Therefore, new therapies are urgently needed to increase quality of life and enhance survival.8 Recently, a novel group of repurposed drugs, selected to modify the underlying disease processes, reverse pulmonary vascular remodelling and right ventricular dysfunction, were tested in small phase 2 clinical trials. These drugs targeted disrupted metabolism,9–11 excess inflammation12 and the bone morphogenic receptor type 2 (BMPR2) signalling pathway,13,14 and showed early signals of beneficial effects in select patients. However, transition to large phase 3 trials investigating these potentially disease-modifying drugs has been hampered by variable, ambiguous or conflicting results in the small trials, the difficulty to accurately assess the biological response of therapy, lack of funding for often already generic drugs and the insufficient collaboration between academia, governmental research organisations and industry. The only example of a large phase 3 trial studying a repurposed disease-modifying therapy in PAH was the use of the tyrosine kinase inhibitor imatinib, a drug currently approved for treatment of multiple types of cancer.15 Although imatinib reduced PAH severity, there were higher rates of subdural haematomas in patients on oral anticoagulation and high drop-out rates potentially due to inadequate dosing in the PAH population.16 These findings, in addition to imatinib coming to the end of its patented lifecycle, led to Novartis not pursuing further licensing studies.
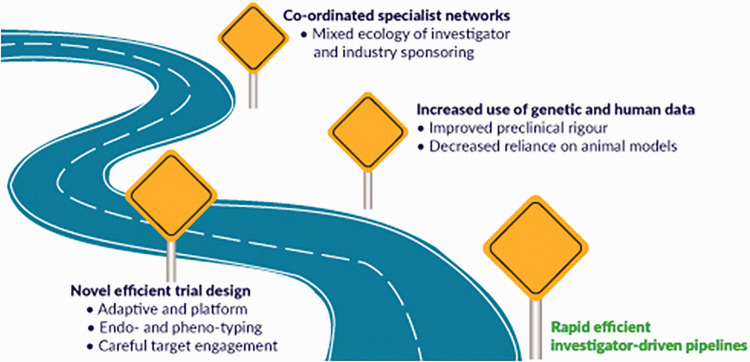
Drug repurposing to develop novel therapies for PAH remains an attractive option because it can combat the high costs of novel drug discovery and has an added safety benefit as already approved drugs have a known dosing and safety profile. Furthermore, technological advances have allowed for high throughput screening of existing drugs for novel mechanisms of action, which has led to several promising new candidates. Certainly, questions arise over how to best optimise drug repurposing. There is a need to evaluate what early experimental work should be academic-led and sponsored. There are structural questions about how to choose targets/therapies, and what the best pipelines for preclinical and clinical testing of repurposed drugs looks like. In this summary statement, we will briefly discuss the current landscape in repurposing and early phase studies in PAH, discuss the need for collaboration between academia, government and industry, outline ways to improve our preclinical pipeline and discuss the important of novel clinical trial design when using these therapies in a rare patient population. In summary, we are providing a roadmap (Fig. 1) that will hopefully increase the likelihood of successful drug repurposing in PAH.
Fig. 1.
Roadmap for successful drug repurposing in PAH.
Current repurposing landscape and academic and industrial contribution and collaboration
To clarify what repurposing looks like in the modern treatment era, we interrogated clinical trials registered on clinicaltrials.gov in the last decade (January 2010–2020), in addition, reviews as well as literature search sources were used. Only 13.2% of all therapies investigated were not repurposed drugs, and arguably even these therapies were derived directly or indirectly from repurposed predecessors (Table 1). Although all late stage studies leading to regulatory approval were industry-sponsored, many early clinical studies were academia driven and either funded by industry in a collaborative manner providing, drug substance or received governmental support. Therefore, the academia/investigator-driven research environment is a vital part of drug development and testing, accounting for most of the hypothesis finding and proof of principle trials in the contemporary era.
Table 1.
Summary of repurposed or novel therapies investigated in pulmonary arterial hypertension.
| Repurposed therapies (n = 72) | Therapies where pulmonary hypertension arguably first indication (n = 11) |
| 7 approved therapies and 65 not approved | 8 approved therapies, 2 in development and 1 terminated |
| Trials in PAH not positive for primary endpoint, terminated early or other results:17 Terguride: 5-HT receptor antagonist18 Nilotinib: TK inhibitor (AMN107) NCT01179737 Aspirin and simvastatin: COX and HMG-CoA-reductase inhibitor19 Atorvastatin: HMG-CoA reductase inhibitor (APATH) Inhaled aviptadil: VI20 Imatinib: TK inhibitor (QTI571; IMPRES) Sorafenib: TK inhibitor21 Selonsertib: ASK1 inhibitor (ARROW)22 FK506: calcineurin inhibitor (tacrolimus)23 Pioglitazone: PPARgamma agonist NCT00825266 Ubenimex: aminopeptidase inhibitor (LIBERTY1/2) NCT02664558/ NCT02736149 Racecadotril: neprilysin inhibitor Anakinra: IL-1 inhibitor Ambrisentan plus spironolactone: ERA plus aldosterone antagonist NCT02253394 Fulvestrant: oestrogen antagonist NCT02911844 Tocilizumab: anti-IL-6 antibody (TRANSFORM-UK) NCT02676947 Selective PDHK 1 and 2 inhibitor (Acros) JTT-251 NCT03789643, withdrawn due to priority change Current ongoing clinical trials:17 Macitentan and tadalafil and selexipag versus macitentan and tadalafil in combination (TRITON) NCT02558231 Hormonal modulators: Anastrozole: aromatase inhibitor (PHANTOM) NCT03229499 Tamoxifen: oestrogen receptor inhibitor (T3PAH) NCT03528902 DHEA (EDIPHY) NCT03648385 Spironolactone: aldosterone antagonist NCT01712620 rhACE2: GSK2586881 NCT03177603 KAR5585: tryptophan hydroxylase 1 inhibitor NCT02746237 Escitalopram: SSRI NCT00190333 Fluoxetine: SSRI NCT03638908 PB1046: VIP analogue NCT03315507 GPCR pathways: Apelin (EXAP) NCT01590108 Mitochondrial and metabolic adaptations: Ranolazine: sodium channel inhibitor, partial FAO inhibitor NCT01839110 Ranolazine NCT02829034 Trimetazidine: FAO inhibitor NCT02102672 Metformin: biguanide, AMPK activator NCT03617458 Ferinject or CosmoFer: iron infusion NCT01447628 Epigenetic alterations and interaction with metabolic pathways: Olaparib: PARP inhibitor (OPTION) NCT03782818 Apabetalone: BRD4 inhibitor (APPRoAcH-p) NCT03655704 Oxidative stress related pathways: Bardoxolone methyl: IκB kinase and NF-κB inhibitor, Nrf2 activator (LARIAT) NCT02036970 Bardoxolone methyl (CATALYST) NCT02657356 Bardoxolone methyl (RANGER) NCT03068130 CXA-10: nitrated fatty acid compound (PRIMEx) NCT03449524 Inflammatory mediators: Rituximab: anti-CD20 antibody NCT01086540 Elafin: elastase-specific protease inhibitor NCT03522935 Transcriptional factors: ABI-009: mTOR inhibitor NCT02587325 Miscellaneous Carbonic anhydrase (CA) inhibitor: Acetazolamide, NCT02755259 (results not posted), Beta-agonist: Albuterol, APD811 NCT03270332, ongoing Benzbromarone (Medical University Graz) NCT02790450, phase 2 completed, not posted Carbon monoxide NCT01523548, not posted Heart failure: Non-selective beta adrenergic receptor blocker Carvedilol NCT01586156, safety study only24 Bisoprolol25 Nitro fatty acid CXA-10 NCT04053543 Alpha2 receptor agonist Dexmedetomidine NCT 01072643 use cautioned by26 H2 receptor agonist: Famotidine, NCT03554291, funded by NHLBI, study ongoing Selective serotonin reuptake inhibitor: Fluoxetine NCT00942708 Selective thromboxane receptor antagonist Ifetroban NCT02682511, study ongoing Phosphodiesterase 3 inhibitor Milrinone NCT04391478, study ongoing in PPHN L-Glutamine NCT01048905, completed, no results posted Calcium sensitizer Levosimendan,27 not pursued Beta-3 agonist: Mirabegron, NCT02775539 SPHERE-HF, not posted Pulsed nitric oxide (Bellerophon), NCT02725372, primary endpoint not met (stopped for futility) Tryptophan hydroxylase inhibitor Rodatristat RVT-1201 (Altavant), NCT03924154 (ELEVATE-1), terminated as not recruitable Thromboxane synthetase Inhibitor (Boehringer Ingelheim): Terbogrel, NCT02223481, phase 2 terminated due to leg pain,28 Non-selective ETA and ETB receptor antagonist Tezosentan (Idorsia), NCT01077297, terminated due to slow recruitment PDE-5 inhibitor Udenafil (Dong-A), NCT01553721, phase 2 completed29 Pirfenidone, NCT02951429 Nebivolol25,30 Vasopressin, NCT01370096, paediatric PH, terminated due to slow recruitment | Terminated: Prostacyclin receptor agonist QCC374, NCT02927366, Novartis, due to strategic reasons In development: Growth factor receptors: Sotatercept: activin receptor type 2A fusion protein acting as a ligand trap (SPECTRA) NCT03738150 Sotatercept (phase 2 successfully completed in PAH) Oral IP agonist (UT): Ralinepag, phase 2 completed, UT |
| Approved repurposed drugs (n = 7): • Beraprost – Japan only31–33 • Bosentan (Tracleer®)34 • Epoprostenol (Flolan®)35 • Inhaled iloprost (Ventavis®)36 • I.V. iloprost (Ilomedin®) – New Zealand only37–40 • Sildenafil (Revatio®)41 • Tadalafil (Adcirca®)42 | Approved non-repurposed drugs (n = 8): • Ambrisentan (Letairis®, Volibris®)43 • Macitentan (Opsumit®)44 • Riociguat (Adempas®)45 • Selexipag (Uptravi®)46 • Inhaled treprostinil (Tyvaso®)47 • Oral treprostinil (Orenitram®)48–51 • S.C. treprostinil (Remodulin®)52,53 • I.V. treprostinil (Remodulin®)54 |
PAH: pulmonary arterial hypertension; TK: tyrosine kinase; IL-1: interleukin-1; PDE-5: phosphodiesterase-5; PH: pulmonary hypertension; S.C.: subcutaneous; I.V.: intravenous.
Note: The information provided in Table 1 was derived from literature search, reviews and clinicaltrials.gov
However, investigator-led early phase studies are predominantly single-centre or regional in design and rarely cross international borders due to limited resources and funding mechanisms. These limitations may explain why no licensed therapy has come from an exclusively academic funded model, though historically successful therapies have a mixture of industry and academic in their genealogy. Adding to these difficulties, we are in a more challenging era for drug discovery in PAH as future trials will include patients on extensive background therapies, which will undoubtedly diminish effect sizes. Therefore, it is likely that future trials will require renewed collaborations between academia, government and industry to help overcome these roadblocks.
In addition to the modest change in long-term prognosis, the costs of current PAH treatments are high; as a recent pharmacoeconomic evaluation demonstrated, the discounted-quality-adjusted life year (QALY) costs range approximately £245,566 in oral therapies.55 Though the costs of oral therapies are coming down with generic availability, this is not the case for intravenous therapy and previous economic analyses are still valid with the cost-effectiveness ratio at £343,000/QALY.56 Healthcare utilisation, especially with intravenous therapy and transplant are not going to change in the foreseeable future. Thus, there remains an urgent need for academia, government and industry to renew their collaborative approaches, learning from each other’s best practices, to enhance our current treatment portfolio so we can both improve patient outcomes and mitigate the high economic burden of therapies.
Preclinical pipelines for drug repurposing
Targeted screening approaches
Targeted approaches using drugs with well-documented effects to combat underlying pathophysiology remain the dominant drug discovery model for PAH. Uses of targeted approaches to modulate the BMPR2 signalling are well documented. For instance, chloroquine, based on its known ability to inhibit lysosomal-mediated degradation of BMPR2 in vitro,57 has beneficial effects on pulmonary vascular disease severity in monocrotaline (MCT) rats.57 Moreover, etanercept, a tumour necrosis factor alpha inhibitor used in inflammatory diseases,58 prevents BMPR2 proteolytic cleavage and partially reverses PAH in Sugen-5416 hypoxia rats.59 These are just two specific examples showing available pharmaceuticals enhance BMPR2 signalling to mitigate PAH severity in relevant animal models. However, multiple publications showed numerous available drugs can combat multiple pathways implicated in PAH pathophysiology, which have previously been extensively reviewed.8,17,60
High throughput screening
Large drug screens can be used to identify potential compounds for PAH treatment using different physiological readouts in high throughput approaches. One example of a high throughput approach included use of a luciferase reporter-gene approach to probe 3756 Food and Drug Administration (FDA) approved medications that augmented BMPR2 signalling.61 FK506 (tacrolimus) was identified in the drug screen, and tacrolimus treatment effectively prevented and reversed pre-clinical PAH.61 These findings were initially translated in a compassionate use trial of tacrolimus in three end-stage PAH patients. Tacrolimus had important haemodynamic and functional improvements in these three patients,62 which eventually led to a single-centre study that evaluated the utility of tacrolimus in PAH patients.14 Although the trial was ultimately neutral, some patients responded to treatment, which suggests patient selection is crucial when using tacrolimus in PAH.
More recently, an unbiased screening approach that evaluated human pulmonary artery smooth muscle cell (PASMC) proliferation in vitro was used to analyse 5562 compounds.63 Emetine, an antiemetic and anti-protozoal drug, was identified as a modulator of PASMC proliferation in this experimental design. In rodent studies, emetine prevented pulmonary vascular remodelling in MCT rats.63 In a reversal approach in Sugen-5416 hypoxia rats, emetine improved haemodynamics and pulmonary vascular disease.63 However, emetine has cardiotoxic effects,64 and thus its translatability to PAH patients may be hampered.
Use of human tissue for screening
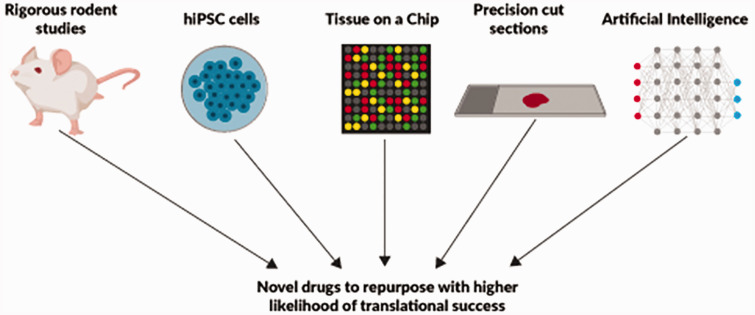
Another potential avenue to improve drug-screening approaches is to use human tissue via precision-cut lung sections (PCLS) and heart or lung on a chip approach. PCLS is a technique that permits culturing of human lung sections.65 In PAH research, PCLS were used to show the tyrosine kinase inhibitors, imatinib and nilotinib, promote vasodilation in preconstricted vessels,66 and that substance P promotes pulmonary vascular remodelling.67 However, use of PCLS for drug screening purposes in PAH has not been implemented yet.
Advances in cell biology and engineering have allowed for the development of organs on a chip, or microdevices that recapitulate the structure, environment and physiology of human organs.68 This technology for PAH could be quite useful as both the lung and right ventricle could be modelled and screened for novel drugs. Although not used explicitly in PAH, this technology probed lung physiology and identified angiopoietin-1 as a potential therapy for interleukin-2-mediated pulmonary oedema.69 Heart on a chip technology is also available,70 but to-date, analyses of right ventricular organ chips from PAH patients have not yet been performed.
Use of artificial intelligence to identify new drugs and enrich PAH populations
Another attractive approach to expand the PAH drug pipeline is the use of artificial intelligence (AI) to understand previously unrecognised drug actions. Recently, a network-based drug-disease analysis of over 900 FDA-approved drugs in 220 million patients provided insights into new purposes for available drugs.71 The authors identified multiple drug-disease networks and provided specific examples that carbamazepine is associated with increased risk of coronary artery disease while hydroxychloroquine decreases coronary artery disease risk.71 Although not explored in detail in the manuscript, there was a drug-pulmonary hypertension network.71 More recently, a network-based disease module identification and in silico drug repurposing methodology named Genome-wide Positioning Systems network was used to define new therapies for cancer.72 The authors showed that integration of patient DNA mutations and gene expression as quantified by RNAseq using approximately 5000 human tumour genomes mapped to the human protein–protein interactome allowed for individualisation of drug therapy. This in silico approach was validated in non-small cell lung cancer as ouabain modulated cell proliferation via hypoxia-inducible factor 1α and LEO1 Homolog signaling.72 The technology may be used for PAH drug development.
Another example of using AI is deep phenotyping to identify patients who may respond to a certain drug class. Recently, unsupervised machine learning was used to classify patients into four distinct immune categories after they had quantified the levels of 48 cytokines, chemokines and growth factors in a discovery cohort of 281 patients at Stanford University, USA, with a validation cohort of 104 patients at Sheffield University, UK.73 This approach identified low- and high-risk patients independent of other clinical phenotypes.73 This type of cluster analysis could be used to enrich patients for anti-inflammatory therapies with minimised risk of immunosuppressive side-effects. However, it is important to acknowledge that biomarker studies may have confounding variables such as diet, race, ethnicity, age and sex that need to be accounted for when implementing these approaches moving forward.
Summary comments and recommendations for drug and patient screening
Both targeted and high throughput drug screens should be used to identify potentially repurposable drugs.
Future studies using PCLS and organ-chips for drug screens should be explored as these techniques would use relevant human tissue and probably better predict how the drugs might work in human PAH.
Use of AI to expand the drug pipeline and define novel patient clusters that may enrich patients for precision medicine approaches should be investigated further.
Challenges of animal and cell modelling
The reproducibility crisis in preclinical science is well documented.74 As in many disease areas, the failure of early phase clinical trials in PAH may be due to the over reliance of data from imperfect rodent studies. Initially designed as proof-of-concept, animal studies have been adopted as preclinical validation of drug targets, which can be problematic. A pre-clinical rigour score was developed60 to judge the existing experimental evidence and likelihood that a therapeutic intervention may work in clinical PAH. Furthermore, a recent position paper from Circulation Research clearly laid out specific recommendations to improve pre-clinical rigour,75 which are briefly outlined below:
More robust statistical analysis: Power calculation prior to starting experiments, randomisation of treatment and blinded analysis.
More translational analyses used: Closed chest haemodynamic studies, advanced imaging to evaluate the right ventricle and use of human data to corroborate findings.
Inclusion of both sexes as PAH is a female predominant disease but most preclinical studies use male rodents.
Reversal/regression models rather than prevention models more likely recapitulate the disease state in PAH and thus reversal/regression models should be evaluated rather than prevention alone.
We acknowledge that current standards of study design and reporting in animal studies do not match human studies. Adopting concepts such as more transparent and appropriate power calculations, insistence on randomisation and blinded analyses, registration of preclinical studies to minimise publication/reporting bias will undoubtedly be a step in the right direction. Furthermore, multi-centre evaluations, such as those used in large phase 3 trials, may also help improve reproducibility in preclinical investigations. Such an approach was recently implemented in an international collaborative study that showed RVX-208, a bromodomain and extra-terminal motif inhibitor, partially reverses PAH and has beneficial effects on the right ventricle in pulmonary artery banded animals.76 The results have ultimately led to an ongoing phase 2, single-arm, open-label clinical trial evaluating RVX-208 in 10 PAH patients (NCT03655704). While this multi-laboratory, collaborative approach will likely improve translational success, the high demands impose significant costs and challenges. If such cross-Atlantic collaborations became the new standard needed for publication, it would probably shrink the actual research pool for PAH due to cost and feasibility. Moreover, this approach does not address the fundamental over-reliance on small animal data that is an important structural barrier to translation.
Although there are inherent problems with animal models, they are invaluable tools due to the ability to assess multiple physiological parameters relevant to PAH and the disease progression over time. In addition to the molecular data, both haemodynamic assessments and cardiac imaging can be performed in animal models. If possible, experiments in rodent models should also be supplemented by larger animal models (bovine, pig, rabbit). This is crucial to translatability as the strongest predictors of mortality in PAH patients are measures of right ventricular function.7
Summary comments and recommendations for preclinical work using animal models
Animal models continue to be necessary for drug repurposing analysis. Regression/reversal studies have the most clinical relevance and should be favoured over prevention studies.
For drugs that are moving forward to clinical trials, a multi-centre preclinical evaluation with positive results in regression/reversal models and evidence that the molecular pathway is altered in human tissue should take precedence.
Assessments of right ventricular systolic and diastolic function (either imaging or haemodynamic evaluation) in preclinical studies should be performed before transitioning to human studies.
Novel patient-based cellular preclinical models
The availability of inducible pluripotent stem cells (iPSCs) has allowed for the development of patient-centric therapies, which may increase the likelihood of translational success, as directly generated patient-derived cells are used. There have been successful demonstrations of modelling approaches using iPSCs to generate vascular cell lineages.77,78 This approach was, in part, used to confirm 4-PBA, a chemical chaperone, as a patient-specific therapy for the C118W BMPR2 mutation.79 iPSCs have some distinct and important advantages, such as allowing direct modelling of disease-causing mutations rather than relying on knock-in or knock-down systems which frequently do not recapitulate in vivo effects. Additionally, iPSCs free researchers from singular reliance on end-stage tissues and cells generated from transplant patients. All of these modelling approaches have their own pros and cons and ex vivo culture introduces additional potential biases such as changes to cells through passage or differences in culture conditions, but these techniques expand our toolbox for preclinical studies and are worthy of exploration.
Summary comments and recommendations for patient-based cellular models
iPSCs are a valuable tool for PAH and may serve as a way to test patient-specific or mutation-specific drugs.
iPSC may facilitate large-scale screenings in both biased and unbiased approaches.
Use of Genome-wide association study data and Mendelian randomisation
Genome-wide association study (GWAS) data and Mendelian randomisation are in the process of being established as an alternative or adjunctive preclinical resource when assessing drug targets. The proposal that GWAS associations with disease might improve preclinical pipelines was published in 2012 and subsequently validated.80,81 Though GWAS have caused great excitement, the effect sizes vary and therefore it is not clear how much weight will be put on genetic data by pharmaceutical companies when making stop/go decisions on drugs. Moreover, the utility of this approach is unproven in rare diseases where the numbers of patients with genetic data is very unlikely to reach more than 10.5 This can be viewed as both a disadvantage from the perspective of a high false negative rate related to underpowering, but it can also be viewed as a positive factor as power will only be retained for large effects. Therefore, positive Mendelian randomisation data in the ‘large effect’ zone is likely to represent powerful evidence of effect.
In addition to common variation, the large effects of rare variants will probably not be tractable to Mendelian randomisation but are very useful for proof-of-concept studies. Consideration will need to be given as to whether treatments targeting pathways with rare variants should be generalised to heterogeneous patient groups or evaluated first in the context of the mutation carriers relevant to the therapy. This is directly relevant to PAH where we have a growing risk of very rare mutations causing disease, but no clear idea of the importance of the pathways related to these mutations to other PAH ontologies/classes.
Challenges in early stage trial design
The success rates of therapies in general drug discovery are modest when measured by the metric of successful licensing.82 Even for repurposed therapies, entering the drug pipeline at phase 2, only around a third will make it through to phase 3 and few of these will end up licensed in the clinic.82 A major hurdle in the post-vasodilator era is the extent to which trial design adopts a personalised medicine approach. It is already challenging to perform clinical trials in PAH, technically classified as rare diseases. The heterogeneous nature of the underlying aetiologies in PAH means that there are clear putative strategies that are more applicable to stratified approaches (BMPR2 modulation in heritable PAH, immunomodulation in CTD-PAH). The use of individual patient biological analysis may be a useful way to combat the heterogeneity in PAH, as it allows each patient to be their own control. For example, analysis of transpulmonary and transventricular gradients of both microRNAs and metabolites are related to important catheterisation-based measures of pulmonary vascular disease, right ventricular function and clinical outcome.83,84 Thus, the change in gradient of metabolite or miRNA in each patient may be useful for identifying responders to therapy.
Endotyping and phenotyping
To a modest extent, existing trials have already engaged with endotyping patients, even if this was never explicit. The first wave of trials included heterogeneous groups containing both PAH and chronic thromboembolic pulmonary hypertension (CTEPH) patients, but gradually transitioned to more restricted and refined populations. In CTEPH for example, successful trials were eventually contingent on more stringent screening of distal disease by expert panels.85 Vasodilation has been generally successful in the World Health Organization (WHO) Groups 1 and 4 pulmonary hypertension (PH), but they have often failed in adults with WHO Groups 286 and 387 PH. Of interest, Group 3 PH is currently being reinvigorated by two positive randomised controlled phase 2 trial using either inhaled prostacyclin (NCT02630316) or inhaled nitric oxide,88 again arguably based on better stratification of populations.
Target selection and demonstration of engagement
Vasodilatation in PAH has benefitted from a target engagement readout that is also an important physiological endpoint: pulmonary vascular resistance (PVR) and PVR/systemic vascular resistance ratio. As novel treatment strategies are proposed, we have not changed this model. Therefore, we have a gap opening up between target engagement and efficacy demonstration. We now need a suite of validated endpoints for demonstrating biological changes. More specifically, we need novel lung imaging that will allow us to develop in vivo readouts of common pathobiological targets such as proliferation, inflammation, and specific pathway targeting such as BMPR2. If direct imaging is not feasible, has unacceptable signal to noise ratio or cannot be adequately validated, then robust work will need to be undertaken to demonstrate that surrogate measures using biological specimens that assay pathways of interest can adequately replace these assessments. Focus should be on making sure new endpoints are assessed and developed to regulatory standards.
Recommendations
Development of novel endpoints that specifically quantify important biological aspects of the disease relevant to specific drug mechanisms such as proliferation and inflammation are needed.
Continuation of research on the utility of biological specimens to link the drug mechanism of action and patient outcome.
Funding trials
The paucity of transnational funding means that the academic community is poorly equipped to adopt some of the critical lessons from industry. Though investigator-initiated trials are more likely to be cutting edge and evaluate novel targets, they are rarely multicentre/multi-national, have limited monitoring capacities and are slow to recruit. To capitalise on the promise of personalised medicine, clinical trials will need to consider stratification of PAH populations. However, stratified trials will need to expand the number of sites to complete enrolment, which will require international collaboration and funding. Thus, there is a need for diverse funding sources and many of the current national funding agencies, like the US-based National Institutes of Health, do not fund studies in this manner. There are recent and notable recognitions of transnational funding opportunities, such as joint British, German and Dutch Heart Foundations collaborative grant, but these remain exceptions, fund only very few groups and to date have rarely been targeted at clinical trials. Collaboration will be vital to success, but currently funding structures and assessment metrics often act as a barrier, rather than an incentive, to setting up longer-term collaborative studies especially in experimental medicine. New funding sources will be required to complete modern clinical trials with more complex designs.
The complexity and expense of PAH clinical trials requires cooperation between the academic and non-academic medical community, pharmaceutical and other governmental sponsors. Each of them brings special expertise and important operational elements necessary for completion of studies. However, this complex interaction may collide with corporate sponsor perspectives concerning proprietary information that may require a degree of restricted access to data. Therefore, it is important to provide a platform for partnership between the academic, regulatory and industry communities. The PVRI is committed to facilitating multi-national cooperations in this area.
Industry sponsors can gain considerably from interaction with the medical and scientific community in terms of basic disease knowledge and ultimate early study design for clinical trials. On the other hand, the medical, scientific and PAH patient communities can gain from the research, operational and clinical trial knowledge as well as resources (including pharmacologic, toxicologic and others) that industry sponsors possess.
Recommendation
We need to engage funding agencies at national and international levels to change funding structures so that transnational experimental medicine projects can be launched.
Conclusions and recommendations
There is a need for innovation in preclinical and clinical studies if drug repurposing is going to be successfully adapted to the modern PAH research environment. Our roadmap (Fig. 1) demonstrates the main summary points.
The importance of a mixed ecology of investigator and industry-led and sponsored clinical trials
A need to revisit preclinical pipelines of drug discovery (Fig. 2).
An updated approach including more rigorous study design and physiological assessments in pre-clinical research using imperfect animal models.
Implementation of human genetic data and novel cell systems will facilitate drug discovery.
The adoption of precision medicine approaches to clinical trial design including stratification, new methods for demonstrating target engagement and the use of endo- and phenotyping.
The critical role of current and new funding models and how they might facilitate change in the design and conduct of future PAH research.
Fig. 2.
Strategies to expand the pipelines for successful drug repurposing in PAH.
Acknowledgments
We thank Paul Corris for the general supervision of the Innovative Drug Development Initiative and their dedicated support of the Drug Repurposing workstream, as well as Georgie Sutton, PVRI Admin Manager, for her support in proofreading this manuscript.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Mark Toshner https://orcid.org/0000-0002-3969-6143
References
- 1.Langedijk J, Mantel-Teeuwisse AK, Slijkerman DS, et al. Drug repositioning and repurposing: terminology and definitions in literature. Drug Discov Today 2015; 20: 1027–1034. [DOI] [PubMed] [Google Scholar]
- 2.Pushpakom S, Iorio F, Eyers PA, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 2019; 18: 41–58. [DOI] [PubMed] [Google Scholar]
- 3.Pushpakom S, Iorio F, Eyers PA, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 2019; 18: 41–58. [DOI] [PubMed] [Google Scholar]
- 4.Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med 1996; 334: 296–301. [DOI] [PubMed] [Google Scholar]
- 5.Galiè N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005; 353: 2148–2157. [DOI] [PubMed] [Google Scholar]
- 6.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 2002; 346: 896–903. [DOI] [PubMed] [Google Scholar]
- 7.Thenappan T, Ormiston ML, Ryan JJ, et al. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ 2018; 360: j5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grinnan D, Trankle C, Andruska A, et al. Drug repositioning in pulmonary arterial hypertension: challenges and opportunities. Pulm Circ 2019; 9: 2045894019832226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michelakis ED, Gurtu V, Webster L, et al. Inhibition of pyruvate dehydrogenase kinase improves pulmonary arterial hypertension in genetically susceptible patients. Sci Transl Med 2017; 9: eaao4583. [DOI] [PubMed] [Google Scholar]
- 10.Khan SS, Cuttica MJ, Beussink-Nelson L, et al. Effects of ranolazine on exercise capacity, right ventricular indices, and hemodynamic characteristics in pulmonary arterial hypertension: a pilot study. Pulm Circ 2015; 5: 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liles JT, Hoyer K, Oliver J, et al. Ranolazine reduces remodeling of the right ventricle and provoked arrhythmias in rats with pulmonary hypertension. J Pharmacol Exp Ther 2015; 353: 480–489. [DOI] [PubMed] [Google Scholar]
- 12.Hernández-Sánchez J, Harlow L, Church C, et al. Clinical trial protocol for TRANSFORM-UK: a therapeutic open-label study of tocilizumab in the treatment of pulmonary arterial hypertension. Pulm Circ 2018; 8: 2045893217735820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Botros L, Szulcek R, Jansen SM, et al. The effects of mercaptopurine on pulmonary vascular resistance and BMPR2 expression in pulmonary arterial hypertension. Am J Respir Crit Care Med. Epub ahead of print 2020. [DOI] [PubMed] [Google Scholar]
- 14.Spiekerkoetter E, Sung YK, Sudheendra D, et al. Randomised placebo-controlled safety and tolerability trial of FK506 (tacrolimus) for pulmonary arterial hypertension. Eur Respir J 2017; 50: 1602449. [DOI] [PubMed] [Google Scholar]
- 15.Iqbal N. Imatinib: a breakthrough of targeted therapy in cancer. Chemother Res Pract 2014; 2014: 357027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoeper MM, Barst RJ, Bourge RC, et al. Imatinib mesylate as add-on therapy for pulmonary arterial hypertension: results of the randomized IMPRES study. Circulation 2013; 127: 1128–1138. [DOI] [PubMed] [Google Scholar]
- 17.Sommer N, Ghofrani HA, Pak O, et al. Current and future treatments of pulmonary arterial hypertension. Br J Pharmacol. Epub ahead of print 2020. [DOI] [PubMed] [Google Scholar]
- 18.Ghofrani HA, Al-Hiti H, Vonk-Noordegraaf A, et al. Proof-of-concept study to investigate the efficacy, hemodynamics and tolerability of terguride vs. placebo in subjects with pulmonary arterial hypertension: results of a double blind, randomised, prospective phase IIa study. In: B19 update in clinical trials of pulmonary arterial hypertension: American Thoracic Society, 2012, p.A2496-A.
- 19.Kawut SM, Bagiella E, Lederer DJ, et al. Randomized clinical trial of aspirin and simvastatin for pulmonary arterial hypertension: ASA-STAT. Circulation 2011; 123: 2985–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galie N, Boonstra A, Ewert R, et al. Effects of inhaled aviptadil (vasoactive intestinal peptide) in patients with pulmonary arterial hypertension (PAH). In: B16 pulmonary arterial hypertension: from assessing risk to therapeutic results: American Thoracic Society, 2010, p.A2516-A.
- 21.Gomberg-Maitland M, Maitland ML, Barst RJ, et al. A dosing/cross-development study of the multikinase inhibitor sorafenib in patients with pulmonary arterial hypertension. Clin Pharmacol Ther 2010; 87: 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boucherat O, Provencher S, Bonnet S. Therapeutic value of ASK1 inhibition in pulmonary arterial hypertension. Am J Respir Crit Care Med 2018; 197: 284–286. [DOI] [PubMed] [Google Scholar]
- 23.Spiekerkoetter E, Sung YK, Sudheendra D, et al. Randomised placebo-controlled safety and tolerability trial of FK506 (tacrolimus) for pulmonary arterial hypertension. Eur Respir J 2017; 50: 1602449. [DOI] [PubMed] [Google Scholar]
- 24.Farha S, Saygin D, Park MM, et al. Pulmonary arterial hypertension treatment with carvedilol for heart failure: a randomized controlled trial. JCI Insight 2017; 2: e95240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perros F, De Man Frances S, Bogaard Harm J, et al. Use of β-blockers in pulmonary hypertension. Circ Heart Fail 2017; 10: e003703. [DOI] [PubMed] [Google Scholar]
- 26.Nathan AT, Nicolson SC, McGowan FX. A word of caution: dexmedetomidine and pulmonary hypertension. Anesth Analg 2014; 119: 216–217. [DOI] [PubMed] [Google Scholar]
- 27.Hansen M, Andersen A, Nielsen-Kudsk J. EXPRESS: levosimendan in pulmonary hypertension and right heart failure. Pulm Circ 2018; 8: 204589401879090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langleben D, Christman BW, Barst RJ, et al. Effects of the thromboxane synthetase inhibitor and receptor antagonist terbogrel in patients with primary pulmonary hypertension. Am Heart J 2002; 143: E4. [DOI] [PubMed] [Google Scholar]
- 29.Kim H, Kim KH. Udenafil as a therapeutic option for pulmonary arterial hypertension. Korean Circ J 2019; 49: 361–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perros F, Ranchoux B, Izikki M, et al. Nebivolol for improving endothelial dysfunction, pulmonary vascular remodeling, and right heart function in pulmonary hypertension. J Am Coll Cardiol 2015; 65: 668–680. [DOI] [PubMed] [Google Scholar]
- 31.Nagaya N, Uematsu M, Okano Y, et al. Effect of orally active prostacyclin analogue on survival of outpatients with primary pulmonary hypertension. J Am Coll Cardiol 1999; 34: 1188–1192. [DOI] [PubMed] [Google Scholar]
- 32.Nagaya N, Shimizu Y, Satoh T, et al. Oral beraprost sodium improves exercise capacity and ventilatory efficiency in patients with primary or thromboembolic pulmonary hypertension. Heart 2002; 87: 340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galie N, Humbert M, Vachiery JL, et al. Effects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol 2002; 39: 1496–1502. [DOI] [PubMed] [Google Scholar]
- 34.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 2002; 346: 896–903. [DOI] [PubMed] [Google Scholar]
- 35.Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N Engl J Med 1996; 334: 296–302. [DOI] [PubMed] [Google Scholar]
- 36.Olschewski H, Simonneau G, Galie N, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med 2002; 347: 322–329. [DOI] [PubMed] [Google Scholar]
- 37.Scott JP, Higenbottam T, Wallwork J. The acute effect of the synthetic prostacyclin analogue iloprost in primary pulmonary hypertension. Br J Clin Pract 1990; 44: 231–234. [PubMed] [Google Scholar]
- 38.Groves BM, Badesch DB, Donnellan K, et al. Acute hemodynamic effects of iloprost in primary (unexplained) pulmonary hypertension. Sem Respir Crit Care Med 1994; 15: 230–237. [Google Scholar]
- 39.Gomez-Sanchez MA, De la Calzada CS, Gomez Pajuelo C, et al. Different hemodynamic responses between acute and chronic infusion of iloprost (prostacyclin-stable analogue) in severe pulmonary hypertension. Am Rev Respir Dis 1991; 144: 1404–1405. [DOI] [PubMed] [Google Scholar]
- 40.De la Mata J, Gomez-Sanchez MA, Aranzana M, et al. Long-term iloprost infusion therapy for severe pulmonary hypertension in patients with connective tissue diseases. Arthritis Rheum 1994; 37: 1528–1533. [DOI] [PubMed] [Google Scholar]
- 41.Galie N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005; 353: 2148–2157. [DOI] [PubMed] [Google Scholar]
- 42.Galiè N, Brundage BH, Ghofrani HA, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation 2009; 119: 2894–2903. [DOI] [PubMed] [Google Scholar]
- 43.Galie N, Olschewski H, Oudiz RJ, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation 2008; 117: 3010–3019. [DOI] [PubMed] [Google Scholar]
- 44.Channick RN, Delcroix M, Ghofrani HA, et al. Effect of macitentan on hospitalizations: results from the SERAPHIN trial. JACC Heart Fail 2015; 3: 1–8. [DOI] [PubMed] [Google Scholar]
- 45.Ghofrani HA, Galie N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013; 369: 330–340. [DOI] [PubMed] [Google Scholar]
- 46.Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 2015; 373: 2522–2533. [DOI] [PubMed] [Google Scholar]
- 47.McLaughlin VV, Benza RL, Rubin LJ, et al. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol 2010; 55: 1915–1922. [DOI] [PubMed] [Google Scholar]
- 48.Tapson VF, Torres F, Kermeen F, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trial. Chest 2012; 142: 1383–1390. [DOI] [PubMed] [Google Scholar]
- 49.Tapson VF, Jing ZC, Xu KF, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C2 study): a randomized controlled trial. Chest 2013; 144: 952–958. [DOI] [PubMed] [Google Scholar]
- 50.Jing Z-C, Parikh K, Pulido T, et al. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension. Circulation 2013; 127: 624–633. [DOI] [PubMed] [Google Scholar]
- 51.White RJ, Jerjes-Sanchez C, Bohns Meyer GM, et al. Combination therapy with oral treprostinil for pulmonary arterial hypertension. A double-blind placebo-controlled clinical trial. Am J Respir Crit Care Med 2020; 201: 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barst RJ, Galie N, Naeije R, et al. Long-term outcome in pulmonary arterial hypertension patients treated with subcutaneous treprostinil. Eur Respir J 2006; 28: 1195–1203. [DOI] [PubMed] [Google Scholar]
- 53.Simonneau G, Barst RJ, Galie N, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med 2002; 165: 800–804. [DOI] [PubMed] [Google Scholar]
- 54.McLaughlin VV, Gaine SP, Barst RJ, et al. Efficacy and safety of treprostinil: an epoprostenol analog for primary pulmonary hypertension. J Cardiovasc Pharmacol 2003; 41: 293–299. [DOI] [PubMed] [Google Scholar]
- 55.Coyle K, Coyle D, Blouin J, et al. Cost effectiveness of first-line oral therapies for pulmonary arterial hypertension: a modelling study. Pharmacoeconomics 2016; 34: 509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen YF, Jowett S, Barton P, et al. Clinical and cost-effectiveness of epoprostenol, iloprost, bosentan, sitaxentan and sildenafil for pulmonary arterial hypertension within their licensed indications: a systematic review and economic evaluation. Health Technol Assess 2009; 13: 1–320. [DOI] [PubMed] [Google Scholar]
- 57.Long L, Yang X, Southwood M, et al. Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circ Res 2013; 112: 1159–1170. [DOI] [PubMed] [Google Scholar]
- 58.Mease PJ. Tumour necrosis factor (TNF) in psoriatic arthritis: pathophysiology and treatment with TNF inhibitors. Ann Rheum Dis 2002; 61: 298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hurst LA, Dunmore BJ, Long L, et al. TNFα drives pulmonary arterial hypertension by suppressing the BMP type-II receptor and altering NOTCH signalling. Nat Commun 2017; 8: 14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prins KW, Thenappan T, Weir EK, et al. Repurposing medications for treatment of pulmonary arterial hypertension: what’s old is new again. J Am Heart Assoc 2019; 8: e011343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spiekerkoetter E, Tian X, Cai J, et al. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest 2013; 123: 3600–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spiekerkoetter E, Sung YK, Sudheendra D, et al. Low-dose FK506 (tacrolimus) in end-stage pulmonary arterial hypertension. Am J Respir Crit Care Med 2015; 192: 254–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Siddique MAH, Satoh K, Kurosawa R, et al. Identification of emetine as a therapeutic agent for pulmonary arterial hypertension: novel effects of an old drug. Arterioscler Thromb Vasc Biol 2019; 39: 2367–2385. [DOI] [PubMed] [Google Scholar]
- 64.Yang WC, Dubick M. Mechanism of emetine cardiotoxicity. Pharmacol Ther 1980; 10: 15–26. [DOI] [PubMed] [Google Scholar]
- 65.Liu G, Betts C, Cunoosamy DM, et al. Use of precision cut lung slices as a translational model for the study of lung biology. Respir Res 2019; 20: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rieg AD, Bünting NA, Cranen C, et al. Tyrosine kinase inhibitors relax pulmonary arteries in human and murine precision-cut lung slices. Respir Res 2019; 20: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Springer J, Fischer A. Substance P-induced pulmonary vascular remodelling in precision cut lung slices. Eur Respir J 2003; 22: 596–601. [DOI] [PubMed] [Google Scholar]
- 68.Esch EW, Bahinski A, Huh D. Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov 2015; 14: 248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huh D, Leslie DC, Matthews BD, et al. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med 2012; 4: 159ra47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Agarwal A, Goss JA, Cho A, et al. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip 2013; 13: 3599–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng F, Desai RJ, Handy DE, et al. Network-based approach to prediction and population-based validation of in silico drug repurposing. Nat Commun 2018; 9: 2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheng F, Lu W, Liu C, et al. A genome-wide positioning systems network algorithm for in silico drug repurposing. Nat Commun 2019; 10: 3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sweatt AJ, Hedlin HK, Balasubramanian V, et al. Discovery of distinct immune phenotypes using machine learning in pulmonary arterial hypertension. Circ Res 2019; 124: 904–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Begley CG, Ioannidis JP. Reproducibility in science: improving the standard for basic and preclinical research. Circ Res 2015; 116: 116–126. [DOI] [PubMed] [Google Scholar]
- 75.Provencher S, Archer SL, Ramirez FD, et al. Standards and methodological rigor in pulmonary arterial hypertension preclinical and translational research. Circ Res 2018; 122: 1021–1032. [DOI] [PubMed] [Google Scholar]
- 76.Van der Feen DE, Kurakula K, Tremblay E, et al. Multicenter preclinical validation of BET inhibition for the treatment of pulmonary arterial hypertension. Am J Respir Crit Care Med 2019; 200: 910–920. [DOI] [PubMed] [Google Scholar]
- 77.Kiskin FN, Chang CH, Huang CJZ, et al. Contributions of BMPR2 mutations and extrinsic factors to cellular phenotypes of pulmonary arterial hypertension revealed by induced pluripotent stem cell modeling. Am J Respir Crit Care Med 2018; 198: 271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sa S, Gu M, Chappell J, et al. Induced pluripotent stem cell model of pulmonary arterial hypertension reveals novel gene expression and patient specificity. Am J Respir Crit Care Med 2017; 195: 930–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dunmore BJ, Yang X, Crosby A, et al. 4PBA restores signalling of a cysteine-substituted mutant BMPR2 receptor found in patients with PAH. Am J Respir Cell Mol Biol. Epub ahead of print 2020. [DOI] [PubMed] [Google Scholar]
- 80.Sanseau P, Agarwal P, Barnes MR, et al. Use of genome-wide association studies for drug repositioning. Nat Biotechnol 2012; 30: 317–320. [DOI] [PubMed] [Google Scholar]
- 81.Nelson MR, Tipney H, Painter JL, et al. The support of human genetic evidence for approved drug indications. Nat Genet 2015; 47: 856–860. [DOI] [PubMed] [Google Scholar]
- 82.Harrer S, Shah P, Antony B, et al. Artificial intelligence for clinical trial design. Trends Pharmacol Sci 2019; 40: 577–591. [DOI] [PubMed] [Google Scholar]
- 83.Chouvarine P, Geldner J, Giagnorio R, et al. Trans-right-ventricle and transpulmonary microRNA gradients in human pulmonary arterial hypertension. Pediatr Crit Care Med 2020; 21: 340–349. [DOI] [PubMed] [Google Scholar]
- 84.Chouvarine P, Giera M, Kastenmüller G, et al. Trans-right ventricle and transpulmonary metabolite gradients in human pulmonary arterial hypertension. Heart. Epub ahead of print 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ghofrani HA, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013; 369: 330–340. [DOI] [PubMed] [Google Scholar]
- 86.Guay CA, Morin-Thibault LV, Bonnet S, et al. Pulmonary hypertension-targeted therapies in heart failure: a systematic review and meta-analysis. PLoS One 2018; 13: e0204610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Prins KW, Duval S, Markowitz J, et al. Chronic use of PAH-specific therapy in World Health Organization Group III Pulmonary Hypertension: a systematic review and meta-analysis. Pulm Circ 2017; 7: 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nathan SD, Flaherty KR, Glassberg MK, et al. A randomized, double-blind, placebo-controlled study to assess the safety and efficacy of pulsed, inhaled nitric oxide at a dose of 30 µg/kg ideal body weight/hr in subjects at risk of pulmonary hypertension associated with pulmonary fibrosis receiving oxygen therapy. Chest. Epub ahead of print 2020. [DOI] [PubMed] [Google Scholar]