Abstract
OBJECTIVE
To study if prostatic ductal adenocarcinoma (PDA) controlled by Grade Group (GG), PSA, and tumor volume (TV) is an independent predictor of adverse radical prostatectomy (RP) outcomes.
MATERIALS
One-hundred and twenty-eight PDA and 1141 acinar continuous RPs were studied. Each tumor nodule (TN) was individually graded, staged, and its TV measured. Univariate analysis (UVA) identified features associated with lymph node metastasis (LN+), extraprostatic extension (EPE), positive surgical margins (SM+), and seminal vesicle invasion (SV+). We then assessed PDA effect on RP outcomes in a multivariate analysis (MVA).
RESULTS
In 127 cases PDA was present in 1 TN and no TN was pure PDA. One-hundred and twenty-three cases had PDA in TNs with highest grade, stage, and TV. Patients with PDA were older (65 vs 63 years, P < 0.001), had higher GG (P < 0.001), and LN+ (6.3% vs 2.7%, P = 0.049). Controlling these variables by GG eliminated statistical significance. Overall, there were 3249 separate TNs (129 PDA and 3120 acinar). In UVA, PDA predicted EPE (92/124 vs 517/3045), SV+ (28/ 1129 vs 116/3,120), and SM+ (51/129 vs 296/3120), all P < 0.001. In MVA, PDA lost its effect on EPE (OR = 0.88, P = 0.64), SM+ (OR = 0.86, P = 0.5), and SV+ (OR = 0.99, P = 0.98).
CONCLUSION
Controlled for grade and TV, PDA was not an independent predictor of adverse RP outcomes, but former 2 were. Hence, higher GG and TV associated with PDA TNs may be predictive of adverse RP outcomes rather than PDA by itself. These conclusions may be used in preoperative risk stratification and definitive therapy planning when PDA is identified on needle biopsy. UROLOGY 137: 108−114, 2020.
Prostatic ductal adenocarcinoma (PDA) was first described by Melicow et al in 1967 as a distinct malignant neoplasm that was microscopically reminiscent of uterine adenocarcinoma and authors hypothesized its Mullerian origin.1 PDA is no longer considered of Mullerian origin but of prostatic epithelial origin in either large periurethral or peripheral prostatic ducts. Pure PDA arising from the area of veru montanum with exophytic growth pattern and urinary retention symptoms at presentation is relatively rare and responsible for only 1.3% of prostate cancer (PCa).2 In contrast, carcinoma involving peripheral zone with PDA intimately admixed with prostatic acinar adenocarcinoma (PAA) is more prevalent and reported in ranges varying from 3.8% to 13.4% by different authors.3–7 PDA is considered a high-grade variant of PCa and assigned a Gleason pattern 4 or 5 if necrosis is present.8–10
Although several publications have suggested worse surgical outcomes for patients with PDA including extraprostatic extension (EPE), seminal vesicle invasion (SV+), higher incidence of positive surgical margins (SM+), higher rate of regional lymph node (LN) metastases, and earlier biochemical recurrence,4,5,10,11 a contemporary comprehensive analysis of cases with assessment of individual TNs and accounting for Grade Groups (GG) and tumor volume (TV) was not performed. Herein we present the results of a study where we performed such a detailed analysis with focused pathology re-review after the most recent updates on PCa grading in 2014.8,9,12,13 The conclusions of this study may be used in preoperative risk stratification and definitive therapy planning when PDA is identified on needle biopsy.
MATERIALS AND METHODS
We reviewed 1303 consecutive radical prostatectomies (RP) performed at the University of Miami from 2014 to 2019. The latest preoperative PSA was extracted from the charts. Every specimen was oriented, inked in 2 colors (right and left), and weighed without seminal vesicle.14–17 All prostates were serially sectioned from apex to base at 0.3 cm interval and entirely submitted for histologic examination as quadrants in regular size cassettes. The bladder neck and apex margins were submitted as perpendicular sections. The entire SV or its proximal portion, if larger than cassette size, was submitted for histological analysis. After manual dissection of LNs, the entire adipose tissue was submitted for histological analysis.18 All cases were reviewed by a single genitourinary pathologist (ONK). PSA density (PSAD) was calculated by dividing preoperative PSA by prostate weight without seminal vesicles.16
We defined separate TNs as those located at least 0.3 cm from each other in a plane section or at least 0.4 cm apart on adjacent sections.16,17 Each TN was mapped, staged, and its TV assessed by formula [TV = mm2 × 3 (tissue thickness) × 1.12 (shrinkage coefficient)].16,17,19 Invasion of the bladder neck was defined as the presence of cauterized carcinoma at the inked margin in an area with thick muscle bundles of muscularis propria in the perpendicularly submitted sections of the prostate base and considered a nonfocal EPE.8 TNs with SM+ at the apex without evidence of adipose tissue invasion, as well as tumors with SM+ in the area of intraprostatic incision where the presence of EPE could not be assessed, were staged as pT2+. SV+ was documented when PCa invaded muscular wall of the SV with at least three fourths of the latter outside the prostate. Patients with neoadjuvant therapy were excluded from the study.
While several morphologic patterns including single glands, cribriform structures, and solid tumor nests were described in PDA,3,5,9 the presence of tall pseudostratified columnar epithelium forming true papillary fronds usually within a cystic space was used as the most relevant diagnostic criterion for PDA (Fig. 1).3,9,10 The differential diagnosis included PIN-like ductal adenocarcinoma which was assigned a Gleason score (GS) 3 + 3 = 6 (GG1) and an intraductal spread of prostatic adenocarcinoma (not graded) when the morphologic findings met the corresponding diagnostic criteria.2,20 PDA was assigned pattern 4 or 5 if necrosis was present.3,8,9,13 For each TN grading, PDA was added to a percentage of pattern 4 or 5 to assign the GG base on contemporary recommendations.8,9,13 Tertiary pattern (minor high-grade component) was defined as a minor (<5%) Gleason pattern 5 component in GS 3 + 4 = 7 (GG2) and GS 4 + 3 = 7 (GG3) TNs only.8,9,21 We assigned GS 3 + 4 = 7 (GG2) for TNs where percentage of Gleason pattern 4 was < 5%.21
Figure 1.
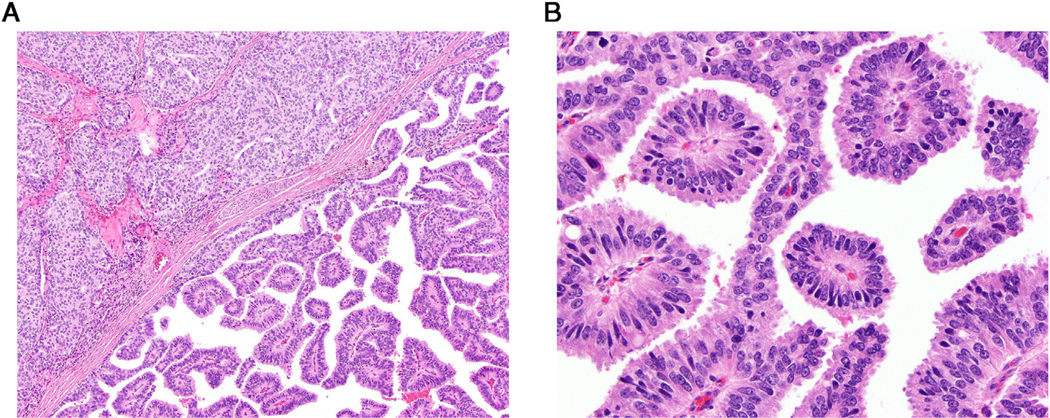
(A) Low-power view of contrasting acinar (left upper corner) and ductal (right lower) prostate cancers intermixed in the same tumor nodule. (B) High-power view of ductal adenocarcinoma forming true fibrovascular cores lined by tall pseudostratified columnar epithelium. (Color version available online.)
First, we assessed statistical differences between PDA and PAA groups based on patient age, prostate weight, number and location of TNs, and rate of regional LN metastases. We continued comparison between the 2 groups for all statistically significant variables stratifying for the patients’ TN with the highest GG per RP. Analysis of incidence of regional LN metastases between the 2 groups was performed after exclusion of all GS 3 + 3 = 6 (GG1) cancers with a notion that those contemporarily graded tumors are unlikely to metastasize.22 We conducted a univariate analysis where each TN was considered an independent variable to assess the association of preoperative PSA, PSAD, PDA, GG, and TV with EPE, SV+, and SM+ in each TN. We then conducted a multivariate analysis based on a multiple-effects generalized linear model because of the variable number of TNs in the RPs. In a multivariate analysis, we included all variables statistically significant in a univariate analysis and used each TN as an independent variable to assess PDA impact on EPE, SM+, and SV invasion controlled for TV and GG. Since PSAD is a ratio of PSA and prostate weigh, we tested 2 multivariate models including either PSAD or the latter 2 variables. Finally, we compared TNs with and without PDA by assigning a score of 1 for EPE, SV+, and SM+. The means of these summary scores were compared in grade-matched cohorts. GG4 and 5 we combined for analysis in view of sparsity of GG4 TNs with PDA. P values were obtained by Chi-square test and Student’s t-test. Statistical analysis was conducted using SAS v9.4. All tests were 2-sided and P < .05 designated statistical significance. The study was approved by the University of Miami Institutional Review Board (20140785).
RESULTS
We excluded patients with neoadjuvant hormonal therapy (n = 25, 2 of these had no hormonal therapy effect with 1 featuring the presence of PDA), no residual carcinoma at RP without neoadjuvant therapy/vanishing cancer (n = 5), no residual carcinoma after neoadjuvant hormonal therapy (n = 1), small cell carcinoma (n = 1), well differentiated neuroendocrine tumor/carcinoid tumor (n = 1), and peripheral gland adenosis and no PCa at RP (n = 1).23 This left a final cohort of 1269 treatment naïve patients with detailed pathological RP examination for the statistical analysis. PDA was present in 128 (10.1%) cases. One man had 2 separate TNs with PDA−smaller Gleason score 4 + 3 = 7 (0.21 cm3) TN involving left posterolateral zone and larger 3 + 4 = 7 (0.39 cm3) TN involving right-to-left anterior mid, both TNs were organ-confined. Thus, there was 129 TNs with PDA. One-thousand-one-hundred and forty-one men with PAA had 3120 discrete TNs (Table 1). None of the PDA TNs was centrally located in the periurethral area and no TN had pure ductal morphology. From a total of 129 discrete TNs with the PDA component, 23 (17.8%) were graded as GS 3 + 4 = 7 (GG2), 40 (31.0%) as GS 4 + 3 = 7 (GG3), 7 (5.4%) as GS 4 + 4 = 8 (GG4), and 59 (45.8%) as different combinations of patterns 4 and 5 (GG5). Tertiary pattern 5 (minor high-grade component) was present in 4 (17%) case of GS 3 + 4 = 7 and 9 (22.5%) case of GS 4 + 3 = 7. In no PDA TN, the high-grade component was composed of ductal cancer alone and there was always the presence of acinar pattern 4 and/or 5 in association with PDA. Overall, 32 of 129 (24.8%) TN’s with PDA were organ-confined. We found EPE in 91/129 (70.5%) TNs with PDA. Among PDA TNs, SV+ and SM+ were identified in 28 (21.7%) and 51 (39.5%), respectively. PDA TNs included 5 (3.9%) cases where SM+ was present in the area of intraprostatic incision (pT2+; Table 1). In all cases with carcinoma extending to the surgical resection margin at the prostate base (bladder neck) including both ductal and acinar cases, there were no prostatic glands (ie, intraprostatic incision) at the level of positive margin and all cases were considered a pT3a disease. In the majority of men (123/128, 96%), PDA was present in the TN with the largest TV, and highest stage and grade. In 1 man, nearly 4 times larger Gleason score 5 + 4 = 9 dominant TN had mucinous features and Gleason score 4 + 5 = 9 smaller contralateral TN had PDA component, both TNs had EPE. Four men had TNs without PDA with Gleason score 4 + 5 = 9 and PDA was in TNs with Gleason score 4 + 3 = 7 (n = 3) and 3 + 4 = 7 (n = 1). In 1 of these 4 cases, GS 4 + 3 = 7 (GG3) PDA TN had 10 times larger volume with associated EPE in contrast to organ-confined smaller volume GS 4 + 5 = 9 (GG5) TN. In 28 (21.9%) cases, PDA was present in anterior TNs, 57 (44.2%) PDA TNs involved the posterior/peripheral gland, 24 (18.6%) PDA TNs extended from anterior to posterior gland, 20 (15.5%) extensively involved the gland bilaterally. In univariate analysis comparing PDA and PAA TNs, the former were larger, had more frequent EPE, SV+, and M+, had higher grade even after Gleason score 3 + 3 = 6 (GG1) case were excluded, and had different spatial distribution in the gland with the tendency to a less compartmentalized disease (all P < .001).
Table 1.
Comparison of tumor nodules (TN) with prostatic ductal adenocarcinoma component and pure prostatic acinar adenocarcinoma
| Variable | Outcome | PDA (n = 129) | PAA (n = 3120) | P Value |
|---|---|---|---|---|
| EPE | Yes | 92 (71.3%) | 517 (16.6%) | <.001 |
| No | 32 (24.8%) | 2519 (81%) | ||
| pT2+ | 5 (3.9%) | 75 (2.4%) | ||
| SV+ | Yes | 28 (21.7%) | 116 (3.7%) | <.001 |
| No | 100 (78.3%) | 2995 (96.3%) | ||
| SM+ | Yes | 51 (39.5%) | 296 (9.5%) | <.001 |
| No | 77 (60.5%) | 2815 (90.5%) | ||
| Volume, cm3, mean (range) | 4.26 (.22–36.11) | .84 (.004–129.45) | <.001 | |
| Gleason score (Grade Group) | 3 + 3 = 6 (1) | 0 (0) | 1930 (61.8%) | <.001** |
| 3 + 4 = 7 (2)* | 23 (17.8%) | 710 (22.8%) | ||
| 4 + 3 = 7 (3)* | 40 (31.0%) | 202 (6.5%) | ||
| 8 (4) | 7 (5.4%) | 42 (1.3%) | ||
| 9–10 (5) | 59 (45.8%) | 236 (7.6%) | ||
| Tumor nodule location | Anterior | 28 (21.7%) | 1071 (34.3%) | <.001 |
| Posterior | 57 (44.2%) | 1784 (57.2%) | ||
| Anterior to posterior | 24 (18.6%) | 211 (6.8) | ||
| Extensive bilateral | 20 (15.5%) | 54 (1.7%) |
EPE, extraprostatic extension; PAA, prostatic acinar adenocarcinoma; PDA, prostatic ductal adenocarcinoma; SM+, positive surgical margin; SV+, seminal vesicle invasion.
Cases with SM+ in the area of intraprostatic incision are staged as pT2+.
Tertiary pattern 5 (minor high-grade component) was present in 4 (17%) case of GS 3 + 4 = 7 and 9 (23%) case of GS 4 + 3 = 7 TNs with PDA and 38 (5.4%) and 37 (18.4%) in PAA TNs, correspondingly.
Only GGs 2–5 are included in analysis because PDA by definition cannot be present in GG1 TNs.
In comparison to the 901 men in PAA group, when 240 (21%) patients with GS 3 + 3 = 6 (GG1) cancers were excluded, patients with PDA were older (median: 65 vs 63 years, P < .001), had more frequent extensive bilateral disease (15.6% vs 6%, P < .001), higher GG (P < .001), and more frequent LN metastases (6.3% vs 2.7%, P = .049). PSA (6.7 in PAA (n = 831) vs 7.7 in PDA (n = 124), median, P = .1) and PSAD (0.16 in PAA vs .18 in PDA, median, P = .2) were not significantly different between the 2 groups. However, after controlling for the GG of the TN with the highest grade per RP, all differences except GG distribution became insignificant (Table 2). Median prostate weight (44.2 vs 43.0, P = .14) and number of TNs (2, both, P = .09) per case did not differ significantly between the 2 groups. No LN metastases were present in patients with GS 3 + 3 = 6 (GG1) and GS 3 + 4 = 7 (GG2) cases.
Table 2.
Comparison of patients whose RP contained any foci of ductal carcinoma (PDA) with those with pure acinar adenocarcinoma (PAA)
| Grade Group, CI 95%, P Value | PDA (n = 128) | PAA (n = 901) | P Value | |
|---|---|---|---|---|
| Age, mean, years (range) | GG2-GG5 | 64.9 (40–85) | 62.5 (40–85) | <.001 |
| GG2 | 63.4 | 61.2 | .21 | |
| GG3 | 64.9 | 63.6 | .39 | |
| GG4–5 | 65.6 | 64.6 | .41 | |
| PSA, ng/ml (range) | GG2-GG5 | 11.6 (1–106) | 9.7 (.3–181.9) | .1 |
| PSAD, gn/ml/gr | GG2-GG5 | 0.25 (.03–1.31) | .22 (.1–3.12) | .2 |
| Extensive bilateral disease (%) | GG2-GG5, | 20/128 (15.6%) | 54/901 (6%) | <.001 |
| GG2 | 2/21 (9.5%) | 13/473 (2.8%) | .13 | |
| GG3 | 5/37 (13.5%) | 12/174 (6.9%) | .18 | |
| GG4–5 | 13/70 (18.6%) | 29/254 (11.4%) | .16 | |
| Regional lymph lode metastasis | GG2-GG5 | 8/127 (6.3%) | 24/900 (2.7%) | .049 |
| GG2 | 0/21 | 0/472 | N/A | |
| GG3 | 4/37 (11.1%) | 6/174(3.4%) | .73 | |
| GG4–5 | 4/70 (5.7%) | 17/254 (6.7%) | 1.0 | |
| Grade group distribution | GG2 | 21 (16.4%) | 473 (52.5) | <.001 |
| GG3 | 37 (28.9%) | 174 (19.3%) | ||
| GG4 | 7 (5.5%) | 29 (3.2%) | ||
| GG5 | 63 (49.2%) | 225 (25.0%) | ||
| Prostate weight, mean, grams (range) | GG2-GG5 (range) | 51.5 (19–171) | 48.1 (13.5–260) | .14 |
| Number of tumor nodules, mean (range) | GG2-GG5(range) | 2.6 (1–6) | 2.7 (1–7) | .09 |
PSA, prostate-specific antigen; PSAD, PSA density; RP, radical prostatectomy; SD, standard deviation.
In a univariate analysis of 3249 discrete TNs (129 PDA and 3120 PAA), presence of PDA, higher GS (GG), preoperative PSA, PSAD, and increasing TV were directly associated with EPE, SV+, and SM+ (all P = .001). However, in a multivariate analysis based on a multiple-effects generalized linear model where the effect of PDA on adverse RP outcomes was controlled by GG, TV, and PSA or PSAD, the presence of PDA component and PSA-based variables lost their independent statistically significant impact on EPE, SV+, and SM+ but GG and TV retained their independent significance (Table 3). The results of a multivariate analysis are visually reflected in the forest plots in the supplement figure.
Table 3.
Generalized estimate equation: effect of prostatic ductal adenocarcinoma on EPE, SV+, and SM+
| UVA | MVA1 | MVA2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Variable | Category | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P Value |
| EPE | Ductal | No | Reference | Reference | Reference | |||
| Yes | 13.84 (9.10, 21.05) | 0.001 | .88 (.51, 1.51) | 0.64 | .88 (.51, 1.51) | .64 | ||
| Grade | GS6 | Reference | Reference | Reference | ||||
| GS7 (3 + 4) | 38.54 (25.15, 59.04) | 0.001 | 5.90 (3.64, 9.55) | 0.001 | 5.89 (3.64, 9.54) | .001 | ||
| GS7 (4 + 3) | 104.74 (65.26, 168.09) | 0.001 | 16.14 (9.26, 28.13) | 0.001 | 16.14 (9.26, 28.13) | .001 | ||
| GS8+ | 172.07 (108.44, 273.04) | 0.001 | 16.77 (9.83, 28.60) | 0.001 | 17.11 (9.99, 29.31) | .001 | ||
| Log of volume | Every one unit increased | 4.95 (4.31, 5.68) | 0.001 | 3.74 (3.15, 4.42) | 0.001 | 3.76 (3.17, 4.45) | .001 | |
| Log Prostate Weight | Every one unit increased | .79 (.63, 1.00) | 0.047 | NA | .78 (.54, 1.14) | .2 | ||
| Log Pre-PSA | Every one unit increased | 2.56 (2.20, 2.98) | 0.001 | NA | 1.08 (.82, 1.42) | .57 | ||
| Log PSAD | Every one unit increased | 2.49 (2.16, 2.88) | 0.001 | 1.14 (.90, 1.45) | 0.28 | NA | ||
| SV+ | Ductal | No | Reference | Reference | Reference | |||
| Yes | 7.19 (4.56, 11.33) | 0.001 | .99 (.58, 1.69) | 0.98 | .99 (.58, 1.69) | .98 | ||
| Grade | GS6 | NE | NE | NE | ||||
| GS7 (3 + 4) | ||||||||
| GS7 (4 + 3) | ||||||||
| GS8+ | ||||||||
| Log of volume | Every one unit increased | 4.75 (3.85, 5.86) | 0.001 | 4.52 (3.57, 5.72) | 0.001 | 4.49 (3.48, 5.79) | .001 | |
| Log of prostate weight | Every one unit increased | 1.41 (0.91, 2.18) | 0.123 | NA | 1.04 (0.61, 1.77) | .89 | ||
| Log of pre-PSA | Every one unit increased | 3.39 (2.68, 4.29) | 0.001 | NA | 1.02 (0.71, 1.47) | .92 | ||
| Log of PSAD | Every one unit increased | 2.81 (2.19, 3.61) | 0.001 | 1.00 (0.73, 1.37) | 0.979 | NA | ||
| SM | Ductal | No | Reference | Reference | Reference | |||
| Yes | 6.26 (4.31, 9.10) | 0.001 | .86 (.56, 1.32) | 0.5 | .86 (.56, 1.32) | .5 | ||
| Grade | GS6 | Reference | Reference | Reference | ||||
| GS7 (3 + 4) | 11.26 (7.54, 16.81) | 0.001 | 1.89 (1.17, 3.03) | 0.009 | 1.87 (1.16, 3.01) | .010 | ||
| GS7 (4 + 3) | 20.57 (13.00, 32.55) | 0.001 | 2.66 (1.51, 4.66) | 0.001 | 2.64 (1.51, 4.63) | .001 | ||
| GS8+ | 39.09 (25.96, 58.86) | 0.001 | 3.06 (1.78, 5.25) | 0.001 | 3.10 (1.81, 5.30) | 0.001 | ||
| Log of volume | Every one unit increased | 3.00 (2.68, 3.35) | 0.001 | 2.48 (2.15, 2.86) | 0.001 | 2.50 (2.16, 2.89) | .001 | |
| Log of prostate weight | Every one unit increased | 0.85 (0.64, 1.15) | 0.29 | NA | .73 (.51, 1.06) | .1 | ||
| Log of pre-PSA | Every one unit increased | 2.66 (2.19, 3.23) | 0.001 | NA | 1.19 (.94, 1.51) | .14 | ||
| Log of PSAD | Every one unit increased | 2.55 (2.12, 3.06) | 0.001 | 1.24 (1.00, 1.53) | 0.054 | NA | ||
EPE, extraprostatic extension; SM+, positive surgical margin; SV+, seminal vesicle invasion.
Finally, when we compared the means of the summary scores (1 score assigned for EPE, SV+, and SM+), there were differences between GG2 and 3 TNs with and without PDA. However, in these 2 groups the average TV was close to twice larger in PDA TNs (5.18 cm3 vs 3.48 cm3). When we accounted for the TV, the presence of PDA was not associated with a higher likelihood of adverse RP outcomes.
DISCUSSION
PDA is considered a high-grade prostatic malignancy associated with adverse surgical outcomes in RP and aggressive clinical behavior.4,5,10,11 Unlike other histological variants of PCa (eg, mucinous, atrophic, foamy etc.), where the cancer is graded based on the underlying histological architecture, PDA is graded at least pattern 4 and pattern 5 is assigned in the presence of necrosis.8–10,13 In addition to a distinct morphology, PDA may retain scatted basal cells which are usually not present in PAA and differences in immunohistochemical expression have also been reported.7 However, in this study we demonstrate that PDA does not have a significant independent risk of EPE, SV+, SM+, and LN+ at RP by itself but rather its adverse surgical outcomes are determined by higher GG and TV of TNs harboring PDA.
We assigned a GS for each TN and included PDA in the percentage of pattern 4 or 5 per TN. We did not use the percentage of PDA as a separate variable. However, in several studies, other authors undertook such analysis with somewhat contradictory results. Samaratunga et al, analyzed34 cases with PDA comprising from 5% to 100% of the total TV, and concluded that the percentage of PDA did not correlate with likelihood of EPE (pT3).5 In contrast, Jang et al, after analysis of 101 PDA cases, suggested higher T-stage and shorter biochemical recurrence period for mixed tumors with a PDA component exceeding 30%.4 Although both studies performed a multivariate analysis, the latter did not include TV in the multivariate analysis and the former included GS6 (GG1) cases in the control group and did not separate GS 3 + 4 = 7 (GG2) and GS 4 + 3 = 7 (GG3) as distinct groups which may have resulted in the different conclusions. In our cohort, all TNs with PDA demonstrated an intimately admixed high grade PAA component and 97% of those TNs with PDA component had the highest GG, stage, and largest TV in the RP specimen.
We found PDA in 10.1% of patients on consecutive RPs that exceed previously reported data.3 Although some reports indicate an increased incidence5,6,24,25 of PDA more recently, a confounding factor may be that fewer RPs are performed for very low, low, and favorable intermediate risk PCa thus enriching the pool of radical prostatectomy patients with those more likely to harbor PDA. Another feature of PDA that has not been described before is its anterior location in 21.9% (28/128) of RPs with PDA.
Although demographic observations in several studies suggest that men with PDA tend to be older and have advanced stage disease at presentation,4,6,10,24–28 we did not find a statistically significant difference between patients with PDA and PAA of the same GGs (P > .05) in terms of age, TV, number of TNs, disease extension, or incidence of lymph node metastases.
Similar to prior studies, in a univariate analysis of discrete 3249 TNs, the presence of PDA component, higher GG, and increasing TV predicted EPE, seminal vesicle invasion, and positive surgical margins (all P values <.0001). However, when we assessed the impact of PDA after adjusting for the effects of GG and TV, PDA lost statistically significant risk for all adverse surgical outcomes, while GG and TV retained their significance (P < .0001). In other words, our data demonstrate that higher GG and larger TV of TNs in which PDA is present determine adverse RP outcomes in these TNs rather than PDA alone. Molecular data may be supporting this by demonstrating that PDA has a similar molecular profile to high-grade PAA.29 This phenomenon is not unique to PCa. Likewise, the presence of divergent squamous or glandular differentiation in bladder cancer was considered an independent adverse prognostic feature, but in stage and grade matched cases their presence does not exercise an independent effect on outcome indicating that merely their presence is more common in high-grade high-stage cancer30—a conclusion which we arrived at in our study with respect to PDA. Although one may suggest that PDA determined the high-grade of TNs harboring it and thus determined the adverse outcomes, we feel that this was not the case because in no TN was the high-grade component (patterns 4 or 5) composed of purely ductal cancer and comparable grade acinar component was always present. Moreover, multivariate analysis supports this conclusion showing that the mere presence of PDA has no impact on adverse RP outcomes after controlling for TV and Gleason score (GG).
To our knowledge, we present the largest group of 128 PDA cases with detailed contemporary pathological analysis of RP specimens entirely submitted for histological analysis. Comparing our data to prior studies, one needs to be alert that in 133133 of 710 (18.7%) GS 3 + 4 = 7 (GG2) TNs without PDA, the percentage of pattern 4 was less than 5% which was not infrequently considered as GS 3 + 3 = 6 (GG1) with tertiary pattern 4 in the past.21 None of PDA GS 3 + 4 = 7 (GG2) TNs had pattern 4 less than 5%. Tertiary pattern 5 was observed in 3 of 18 (16.7%) of PDA GS 3 + 4 = 7 (GG2) TNs and 8 of 35 (22.9%) of PDA GS 4 + 3 = 7 (GG2) TNs. Although studies using Surveillance, Epidemiology, and End Results (SEER) Program data without pathology re-review suggested an increased mortality risk6,24 with the presence of PDA and some authors recommended that PDA should not be followed like acinar carcinoma of the same GG.11 We do not feel we have enough evidence to support or reject this. We are extracting follow-up information on these men to assess the impact of PDA on more long term outcomes like biochemical recurrence and metastasis, which will be the focus of a separate paper.
CONCLUSION
In our RP cohort, 10.1% of RP had PDA, which always occurred admixed with acinar cancer. Most of the cases (127/128) had a PDA component in 1 TN per RP. In slightly more than 20% of the patients, the PDA was present in the anterior TNs. Similarly to previous studies, we demonstrated a significant association of PDA with EPE, SV+, SM+, and LN metastases when GG and TV of corresponding TN were not taken into consideration. However, after controlling for the effect of GG and TV on adverse RP outcomes, PDA alone was no longer an independent predictor of these adverse RP outcomes. Hence, higher GG and TV of TNs in which PDA is present are likely to explain the more adverse cancer found at RP rather than the presence of PDA alone. These findings are relevant to men with PDA diagnosed on needle biopsy for their preoperative risk stratification and definitive therapy planning.
Supplementary Material
Acknowledgments
The data were in part presented at the 108th Annual Meeting of the United States and Canadian Academy of Pathology (USCAP), March 16–21, 2019, National Harbor, Maryland, USA.
Footnotes
SUPPLEMENTARY MATERIALS
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.urology.2019.10.014.
References
- 1.Melicow MM, Pachter MR. Endometrial carcinoma of proxtatic utricle (uterus masculinus). Cancer. 1967;20:1715–1722. [DOI] [PubMed] [Google Scholar]
- 2.Zhou M. High-grade prostatic intraepithelial neoplasia, PIN-like carcinoma, ductal carcinoma, and intraductal carcinoma of the prostate. Mod Pathol. 2018;31:S71–S79. [DOI] [PubMed] [Google Scholar]
- 3.Epstein JI. Prostatic ductal adenocarcinoma: a mini review. Med Princ Pract. 2010;19:82–85. [DOI] [PubMed] [Google Scholar]
- 4.Jang WS, Shin SJ, Yoon CY, et al. Prognostic significance of the proportion of ductal component in ductal adenocarcinoma of the prostate. J Urol. 2017;197:1048–1053. [DOI] [PubMed] [Google Scholar]
- 5.Samaratunga H, Duffy D, Yaxley J, Delahunt B. Any proportion of ductal adenocarcinoma in radical prostatectomy specimens predicts extraprostatic extension. Hum Pathol. 2010;41:281–285. [DOI] [PubMed] [Google Scholar]
- 6.Wu YP, Chen SH, Wang ST, et al. Prognostic values of clinicopathological characteristics and survival outcomes in prostate infiltrating ductal carcinoma: a population-based study. Oncotarget. 2017;8:29048–29055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seipel AH, Samaratunga H, Delahunt B, et al. Immunohistochemical profile of ductal adenocarcinoma of the prostate. Virchows Arch. 2014;465:559–565. [DOI] [PubMed] [Google Scholar]
- 8.Braunhut BL, Punnen S, Kryvenko ON. Updates on grading and staging of prostate cancer. Surg Pathol Clin. 2018;11:759–774. [DOI] [PubMed] [Google Scholar]
- 9.Kryvenko ON, Epstein JI. Prostate cancer grading: a decade after the 2005 modified gleason grading system. Arch Pathol Lab Med. 2016;140:1140–1152. [DOI] [PubMed] [Google Scholar]
- 10.Seipel AH, Delahunt B, Samaratunga H, Egevad L. Ductal adenocarcinoma of the prostate: histogenesis, biology and clinicopathological features. Pathology. 2016;48:398–405. [DOI] [PubMed] [Google Scholar]
- 11.Bergamin S, Eade T, Kneebone A, et al. Ductal carcinoma of the prostate: an uncommon entity with atypical behaviour. Clin Oncol (R Coll Radiol). 2019;31:108–114. [DOI] [PubMed] [Google Scholar]
- 12.Epstein JI, Amin MB, Reuter VE, Humphrey PA. Contemporary gleason grading of prostatic carcinoma: an update with discussion on practical issues to implement the 2014 international society of urological pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma. Am J Surg Pathol. 2017;41:e1–e7. [DOI] [PubMed] [Google Scholar]
- 13.Epstein JI, Egevad L, Amin MB, et al. The 2014 international society of urological pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40:244–252. [DOI] [PubMed] [Google Scholar]
- 14.Samaratunga H, Montironi R, True L, et al. International society of urological pathology (ISUP) consensus conference on handling and staging of radical prostatectomy specimens. Working group 1: specimen handling. Mod Pathol. 2011;24:6–15. [DOI] [PubMed] [Google Scholar]
- 15.Tjionas GA, Epstein JI, Williamson SR, et al. Average weight of seminal vesicles: an adjustment factor for radical prostatectomy specimens weighed with seminal vesicles. Int J Surg Pathol. 2015;23: 617–622. [DOI] [PubMed] [Google Scholar]
- 16.Kryvenko ON, Carter HB, Trock BJ, Epstein JI. Biopsy criteria for determining appropriateness for active surveillance in the modern era. Urology. 2014;83:869–874. [DOI] [PubMed] [Google Scholar]
- 17.Sundi D, Kryvenko ON, Carter HB, Ross AE, Epstein JI, Schaeffer EM. Pathological examination of radical prostatectomy specimens in men with very low risk disease at biopsy reveals distinct zonal distribution of cancer in black American men. J Urol. 2014;191:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon JY, Kryvenko ON, Ghani KR, Bertucci C, Menon M, Gupta NS. Characteristics of pelvic lymph node metastases in prostatic adenocarcinoma: a study of 83 cases. Int J Surg Pathol. 2012;20:449–454. [DOI] [PubMed] [Google Scholar]
- 19.Kryvenko ON, Lyapichev K, Chinea FM, et al. Radical prostatectomy findings in white Hispanic/Latino men with NCCN very lowrisk prostate cancer detected by template biopsy. Am J Surg Pathol. 2016;40:1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paulk A, Giannico G, Epstein JI. PIN-like (ductal) adenocarcinoma of the prostate. Am J Surg Pathol. 2018;42:1693–1700. [DOI] [PubMed] [Google Scholar]
- 21.Kryvenko ON, Epstein JI. Re: Clinical significance of prospectively assigned gleason tertiary pattern 4 in contemporary Gleason score 3 + 3 = 6 prostate cancer. Prostate. 2016;76:1130–1131. [DOI] [PubMed] [Google Scholar]
- 22.Ross HM, Kryvenko ON, Cowan JE, Simko JP, Wheeler TM, Epstein JI. Do adenocarcinomas of the prostate with Gleason score (GS) </=6 have the potential to metastasize to lymph nodes? Am J Surg Pathol. 2012;36:1346–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lotan TL, Epstein JI. Diffuse adenosis of the peripheral zone in prostate needle biopsy and prostatectomy specimens. Am J Surg Pathol. 2008;32:1360–1366. [DOI] [PubMed] [Google Scholar]
- 24.Morgan TM, Welty CJ, Vakar-Lopez F, Lin DW, Wright JL. Ductal adenocarcinoma of the prostate: increased mortality risk and decreased serum prostate specific antigen. J Urol. 2010;184:2303–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meeks JJ, Zhao LC, Cashy J, Kundu S. Incidence and outcomes of ductal carcinoma of the prostate in the USA: analysis of data from the Surveillance, Epidemiology, and End Results program. BJU Int. 2012;109:831–834. [DOI] [PubMed] [Google Scholar]
- 26.Packiam VT, Patel SG, Pariser JJ, et al. Contemporary population-based comparison of localized ductal adenocarcinoma and high-risk acinar adenocarcinoma of the prostate. Urology. 2015;86:777–782. [DOI] [PubMed] [Google Scholar]
- 27.Tu SM, Lopez A, Leibovici D, et al. Ductal adenocarcinoma of the prostate: clinical features and implications after local therapy. Cancer. 2009;115:2872–2880. [DOI] [PubMed] [Google Scholar]
- 28.Vinceneux A, Bruyere F, Haillot O, et al. Ductal adenocarcinoma of the prostate: clinical and biological profiles. Prostate. 2017;77:1242–1250. [DOI] [PubMed] [Google Scholar]
- 29.Seipel AH, Whitington T, Delahunt B, et al. Genetic profile of ductal adenocarcinoma of the prostate. Hum Pathol. 2017;69:1–7. [DOI] [PubMed] [Google Scholar]
- 30.Kryvenko ON, Epstein JI. Latest novelties on the World Health Organization morphological classifications of genitourinary cancers. Eur Urol Suppl. 2017;16:199–209. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


