Abstract
Background
In December 2019, the coronavirus disease (COVID-19), caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), emerged in Wuhan, China, and has since spread throughout the world. This study aimed to investigate the association between the change in laboratory markers during the three days after pneumonia diagnosis and severe respiratory failure in COVID-19 patients.
Methods
Data of 23 COVID-19 patients with pneumonia, admitted to the Kumamoto City Hospital between February and April 2020 were retrospectively analyzed.
Results
Among the 23 patients, eight patients received mechanical ventilation (MV) (MV group), and the remaining 15 comprised the non-MV group. The levels of hemoglobin (Hb) and albumin (Alb) decreased in the MV group during the three days after pneumonia diagnosis more than in the non-MV group (median Hb: 1.40 vs. −0.10 g/dL, P = 0.015; median Alb: 0.85 vs. −0.30 g/dL, P = 0.020). Univariate logistic regression analysis showed that the decrease in Hb was associated with receiving MV care (odds ratio: 0.313, 95% confidence interval: 0.100–0.976, P = 0.045). Receiver operating characteristic curve analyses showed that the optimal cut-off value for the decrease in Hb level was −1.25 g/dL, with sensitivity and specificity values of 0.867 and 0.750, respectively.
Conclusions
The decrease in Hb level during the short period after pneumonia diagnosis might be a predictor of worsening pneumonia in COVID-19 patients.
Keywords: Coronavirus disease 2019, Severe acute respiratory syndrome coronavirus-2, Intermittent positive pressure ventilation, Hemoglobin, Albumin
Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; ARDS, acute respiratory distress syndrome; ICU, intensive care unit; MV, mechanical ventilation; Hb, hemoglobin, Alb albumin; CI, confidence interval; WBC, white blood cells; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; IL-6, interleukin-6; CRP, C-reactive protein; PCT, procalcitonin; CT, computed tomography; BUN, blood urea nitrogen; Plt, platelets; Cr, creatinine; ROC, receiver operating characteristic; AUC, area under the ROC curve; SpO2, oxygen saturation; AML, acute myeloid leukemia
1. Introduction
In December 2019, a previously unknown infectious disease was identified in Wuhan, China, rapidly spreading to the rest of the world. This new pathogen was a positive-sense, enveloped, non-segmented RNA virus, belonging to the genus Betacoronavirus. This genus includes the Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) and the Middle East Respiratory Syndrome Coronavirus (MERS-CoV). The new virus was named Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) by the International Committee on Taxonomy of Viruses [1]. This coronavirus disease 2019 (COVID-19) was declared a public health emergency of international concern by the World Health Organization [2]. Studies have shown that most COVID-19 patients present with mild disease symptoms such as fever, cough, sputum production, myalgia, and fatigue [3,4]. However, 12.2–29.0% of patients develop pneumonia and acute respiratory distress syndrome (ARDS) [4,5]. Thus, there is a need to explore risk factors that could predict which patients would require mechanical ventilation (MV). The treatment strategies for these patients should be distinct from mild COVID-19 patients.
Several retrospective reports on COVID-19 patients showed which laboratory factors at the time of hospitalization were associated with being admitted to the intensive care unit (ICU). These factors included elevated white blood cell (WBC) count, neutrophil count, D-dimer, alanine aminotransferase (AST), total bilirubin, lactate dehydrogenase (LDH), interleukin-6 (IL-6), C-reactive protein (CRP), and procalcitonin (PCT); and decreased levels of leukocytes and albumin (Alb) [4,6]. However, the clinical impact of change in laboratory factors after pneumonia diagnosis in COVID-19 patients remains unclear. Therefore, we conducted a retrospective study to investigate the clinical significance of the change in laboratory factors during a three-day period after pneumonia diagnosis in COVID-19 patients, comparing those who received MV care with those who did not.
2. Patients and methods
2.1. Patients
Between February 1 and April 31, 2020, 40 consecutive COVID-19 patients were diagnosed and admitted to the Kumamoto City Hospital. Among these, 25 patients developed pneumonia, which was confirmed by computed tomography (CT). After excluding two patients who were lost to follow-up within a week from pneumonia diagnosis because they were transferred to another hospital, the remaining 23 patients were included in this study. The COVID-19 pneumonia was diagnosed following a published pictorial review [7]. All CT images were evaluated by two independent pulmonologists (M.A. and K.A.). The following baseline and clinical data were retrospectively reviewed in the included patients: age, sex, smoking status, clinical history, Body Mass Index (BMI), and laboratory and CT imaging findings. The institutional review board of the Kumamoto City Hospital approved this study (IRB-number 560). Because of its retrospective nature, there was no need for informed consent in this study.
2.2. Standards for introduction of intubation and MV
In severe respiratory failure cases that needed more than 5 L/min oxygen flow through a face mask, intubation and MV was considered. After intubation, the mechanical ventilator was set to positive end-expiratory pressure of more than 10 cmH2O. Non-invasive positive pressure ventilation was not considered in order to avoid potential promotion of aerosolization containing the SARS-CoV-2.
2.3. Outcome measure and statistical analysis
The primary outcome was identifying laboratory measures with significant change during a median of three days from pneumonia diagnosis. These values were compared between COVID-19 patients treated with MV (MV group) and those who were not (non-MV group).
Fisher's exact test and Mann-Whitney U test were used to compare the clinical values between the two patient groups. Univariate logistic regression analysis was performed to evaluate the association between the laboratory variables and MV treatment. In addition, a receiver operating characteristic (ROC) curve analysis was performed to determine the optimal cut-off value for predicting the introduction of MV care. All analyses were two-tailed, and differences with a P-value < 0.05 were considered statistically significant. Statistical analysis was performed using the SPSS software (version 23.0; IBM, Armonk, NY, USA).
3. Results
3.1. Patient characteristics
The characteristics and laboratory findings of the 23 COVID-19 patients at the time of pneumonia diagnosis are shown in Table 1, Table 2 , respectively. Eight of the 23 patients were treated by MV during hospitalization (MV group), while the remaining 15 patients were not (non-MV group). These groups were similar in age, sex, smoking status, clinical history, CT finding of pneumonia, the levels of WBC, neutrophils, lymphocyte, hemoglobin (Hb), Krebs von den Lungen-6, soluble interleukin-2 receptor (sIL-2R), blood urea nitrogen (BUN), and Alb. Platelets (Plt) level in the MV group was significantly lower than in the non-MV group (11.45 × 105/μL vs. 17.0 × 105/μL; P = 0.020). Additionally, the median level of the following measures was higher in the MV group: BMI, 27.16 kg/m2 vs. 23.12 kg/m2, P = 0.037; CRP, 7.54 mg/dL vs. 2.55 mg/dL, P = 0.028; LDH, 383.0 U/L vs. 240.0 U/L, P = 0.008; ferritin, 1534.0 ng/mL vs. 452.0 ng/mL, P = 0.009; PCT, 0.11 ng/mL vs. 0.06 ng/mL, P = 0.035; creatinine (Cr), 0.97 mg/dL vs. 0.74 mg/dL, P = 0.035; AST, 47.5 U/L vs. 24.0 U/L, P = 0.011; ALT, 41.5 U/L vs. 20.0 U/L, P = 0.035; D-dimer, 1.6 μg/mL vs. 1.0 μg/mL, P = 0.038. Regarding treatment strategies, there was no significant difference in the administration of antiviral agents such as lopinavir/ritonavir and favipiravir between the MV and non-MV groups, although the proportion of patients receiving corticosteroid and antibiotics therapy were significantly higher in the MV than the non-MV group (corticosteroid, 75.0% vs. 26.7%, P = 0.037; antibiotics, 100.0% vs. 53.3%, P = 0.026).
Table 1.
Patient characteristics.
| MV group (N = 8) | non-MV group (N = 15) | P- value | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Age, years | Median (range) | 67 (29–79) | 61 (23–83) | |||
| ≥60 | 5 | 62.5 | 8 | 53.3 | 0.510 | |
| <60 | 3 | 27.5 | 7 | 46.7 | ||
| Sex | Male | 6 | 75.0 | 8 | 53.3 | 0.290 |
| Female | 2 | 25.0 | 7 | 46.7 | ||
| Smoking status | Yes | 3 | 37.5 | 6 | 40.0 | 0.633 |
| No | 5 | 62.5 | 9 | 60.0 | ||
| Clinical history | ||||||
| DM | Yes | 5 | 62.5 | 4 | 26.7 | 0.110 |
| No | 3 | 37.5 | 11 | 73.3 | ||
| HT | Yes | 1 | 12.5 | 1 | 6.7 | 0.585 |
| No | 7 | 87.5 | 14 | 93.3 | ||
| BMI, kg/m2 | Median (range) | 27.16 (22.57–39.52) | 23.12 (19.13–30.67) | |||
| ≥25 | 6 | 75.0 | 4 | 26.7 | 0.037 | |
| <25 | 2 | 25.0 | 11 | 73.3 | ||
| CT findings | both lungs | 8 | 100.0 | 14 | 93.3 | 0.652 |
| One lung | 0 | 0.0 | 1 | 6.7 | ||
| GGO | 4 | 50.0 | 5 | 33.3 | 0.367 | |
| Others | 4 | 50.0 | 10 | 66.7 | ||
| Treatments | ||||||
| Intravenous Antibiotics |
Yes | 8 | 100.0 | 8 | 53.3 | 0.026 |
| No | 0 | 0.0 | 7 | 46.7 | ||
| LPV/RTV | Yes | 2 | 25.0 | 2 | 13.3 | 0.435 |
| No | 6 | 75.0 | 13 | 86.7 | ||
| Favipiravir | Yes | 7 | 87.5 | 13 | 86.7 | 0.606 |
| No | 1 | 12.5 | 2 | 13.3 | ||
| Systemic Corticosteroids |
Yes | 6 | 75.0 | 4 | 26.7 | 0.037 |
| No | 2 | 25.0 | 11 | 73.3 | ||
MV; mechanical ventilation, DM; diabetes mellitus, HT; hypertension, BMI; body mass index, CT; computed tomography, GGO; ground grass opacity, LPV/RTV; lopinavir/ritonavir.
Table 2.
Patient laboratory findings at pneumonia diagnosis.
| MV group (N = 8) | non-MV group (N = 15) |
P- value | ||||
|---|---|---|---|---|---|---|
| WBC,/μL | Median (range) | 4850.0 | (2900.0–9900.0) | 4500.0 | (2300.0–8200.0) | 0.418 |
| Neut,/μL | Median (range) | 3638.2 | (2130.0–9434.7) | 3066.0 | (1150.0–4544.1) | 0.302 |
| Lymph,/μL | Median (range) | 768.0 | (366.3–1702.4) | 959.1 | (423.3–3632.6) | 0.333 |
| Hb, g/dL | Median (range) | 15.05 | (11.8–16.9) | 15.3 | (11.5–17.4) | 0.923 |
| Plt, × 105/μL | Median (range) | 11.45 | (9.7–16.9) | 17.0 | (5.0–25.2) | 0.020 |
| CRP, mg/dL | Median (range) | 7.54 | (0.95–34.43) | 2.55 | (0.05–7.94) | 0.028 |
| LDH, U/L | Median (range) | 383.0 | (217–782) | 240.0 | (154–824) | 0.008 |
| KL-6a, U/mL | Median (range) | 337.0 | (212–520) | 230.5 | (151–729) | 0.351 |
| Ferritinb, ng/mL | Median (range) | 1534.0 | (635.1–3070.0) | 452.0 | (186.3–1134) | 0.009 |
| sIL-2Rc, U/mL | Median (range) | 465.0 | (354.9–1648.0) | 772.0 | (410–1155) | 0.855 |
| PCT, ng/mL | Median (range) | 0.11 | (0.09–0.89) | 0.06 | (0.02–1.06) | 0.035 |
| BUN, mg/dL | Median (range) | 15.85 | (6.4–28) | 12.70 | (6.6–71.7) | 0.333 |
| Cr, mg/dL | Median (range) | 0.97 | (0.74–1.37) | 0.74 | (0.51–1.34) | 0.018 |
| AST, U/L | Median (range) | 47.5 | (25–213) | 24 | (16–145) | 0.011 |
| ALT, U/L | Median (range) | 41.5 | (19–267) | 20 | (12–55) | 0.030 |
| Alb, g/dL | Median (range) | 3.6 | (2.9–4.4) | 3.9 | (2.8–4.8) | 0.419 |
| D-dimer, μg/mL | Median (range) | 1.6 | (0.9–2.5) | 1.0 | (0.7–2.9) | 0.038 |
MV; mechanical ventilation, WBC; white blood cells, Neut; neutrophils, Lymph; lymphocytes, Hb; hemoglobin, Plt; platelets, CRP; C-reactive protein, LDH; lactase dehydrogenase, KL-6; Krebs von den Lungen-6, sIL-2R; soluble interleukin-2 receptor, PCT; procalcitonin, BUN; blood urea nitrogen, Cr; creatinine, AST; aspartate aminotransferase, ALT; alanine aminotransferase, Alb; albumin.
Two patients were not evaluated for KL-6.
Nine patients were not evaluated for ferritin.
Twelve patients were not evaluated for sIL-2R.
3.2. Time to initiation of MV care in the MV group
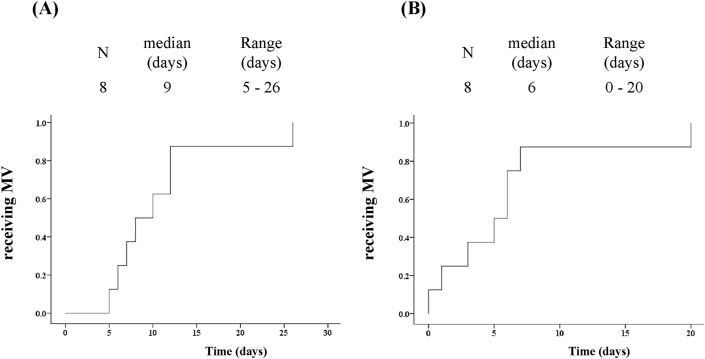
The median follow-up was 33 days (range: 16–102 days). In the MV group, the median days from the onset of illness and pneumonia diagnosis to the initiation of MV were nine days (range 5–26 days; Fig. 1A) and six days (range 0–20 days; Fig. 1 B), respectively. One patient received high-flow nasal cannula oxygen therapy before the introduction of MV, so the decision regarding intubation and MV was made only 26 and 20 days from the onset of illness and pneumonia diagnosis, respectively.
Fig. 1.
The time from onset of illness to MV initiation (A), and the diagnosis of pneumonia (B). MV, mechanical ventilation.
3.3. Clinical factors associated with receiving MV care during hospitalization
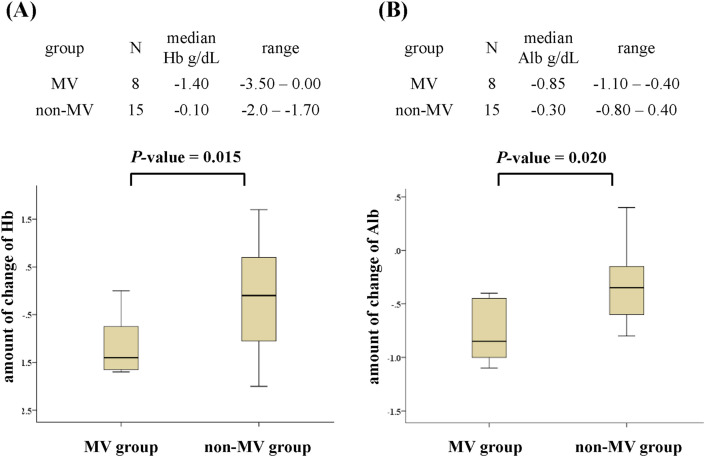
Table 3 presents comparisons of the rate of change in laboratory factors during the few days after pneumonia diagnosis between the MV and non-MV groups. The groups did not differ in the time between pneumonia diagnosis and the second evaluation [median days (range): 3 days (3–3) vs. 3 days (2–4), P = 1.000]. Over this short period, the levels of Hb and Alb in the MV group were significantly lower than in the non-MV group (median Hb change, −1.4 g/dL vs. −0.1 g/dL, P = 0.015, Fig. 2 A; median Alb change, −0.85 g/dL vs. −0.3 g/dL, P = 0.020, Fig. 2B). Additionally, there were 7 and 1 patients with increased Hb and Alb levels in the non-MV group, respectively. However, there were no patients with increased Hb and Alb levels in the MV group (Table S1, Supplementary Information). The groups were similar with regards to change in the other laboratory parameters including WBC, neutrophils, lymphocyte, Plt, CRP, LDH, PCT, BUN, Cr, AST, ALT, and D-dimer.
Table 3.
Rate of change in laboratory factors during the few days after pneumonia diagnosis between the MV and non-MV groups.
| MV group (N = 8) | non-MV group (N = 15) | P- value | ||||
|---|---|---|---|---|---|---|
| WBC,/μL | Median (range) | 1000.0 | (-3100.0–6700.0) | 200.0 | (-2700.0–2800.0) | 0.349 |
| Neut,/μL | Median (range) | 890.2 | (-2141.8–6435.1) | 73.0 | (-1548.9–3350.6) | 0.197 |
| Lymph,/μL | Median (range) | −108.2 | (-836.0–752.5) | −148.8 | (-2263.1–670.8) | 0.849 |
| Hb, g/dL | Median (range) | −1.4 | (-3.5 to −0.0) | −0.1 | (-2.0 – 1.7) | 0.015 |
| Plt, × 105/μL | Median (range) | 1.05 | (-3.8 – 5.1) | 1.80 | (-2.8 – 5.3) | 0.821 |
| CRP, mg/dL | Median (range) | 0.335 | (-17.95 to −8.88) | 0.17 | (-1.13 – 8.35) | 0.651 |
| LDH, U/L | Median (range) | 101.5 | (-167.0–130.0) | 28.0 | (-28.0–98.0) | 0.401 |
| PCT, ng/mL | Median (range) | 0.000 | (-0.05 – 0.21)-3 | −0.015 | (-0.1 – 0.06)-7 | 0.508 |
| BUN, mg/dL | Median (range) | 0.05 | (-8.3 – 40.0) | 0.60 | (-16.8–5.7) | 0.771 |
| Cr, mg/dL | Median (range) | 0.00 | (-0.26 – 0.71) | 0.01 | (-0.29 – 0.37) | 0.974 |
| AST, U/L | Median (range) | 6.5 | (-92.0–64.0) | 2.0 | (-57.0–21.0) | 0.561 |
| ALT, U/L | Median (range) | 0.5 | (-113.0–37.0) | 1.0 | (-4.0 – 16.0) | 0.846 |
| Alb, g/dL | Median (range) | −0.85 | (-1.1 to −0.4) | −0.3 | (-0.8 – 0.4) | 0.020 |
| D-dimer, μg/mL | Median (range) | 0.5 | (-0.1 – 5.2) | 0.3 | (-0.2 – 4.5) | 0.672 |
MV; mechanical ventilation, WBC; white blood cells, Neut; neutrophils, Lymph; lymphocytes, Hb; hemoglobin, Plt; platelets, CRP; C-reactive protein, LDH; lactase dehydrogenase, KL-6; Krebs von den Lungen-6, sIL-2R; soluble interleukin-2 receptor, PCT; procalcitonin, BUN; blood urea nitrogen, Cr; creatinine, AST; aspartate aminotransferase, ALT; alanine aminotransferase, Alb; albumin.
Fig. 2.
Association between MV care and the change in Hb (A) and Alb (B) levels over the short period after the diagnosis of pneumonia. MV; mechanical ventilation, Hb; hemoglobin, Alb; albumin.
3.4. Univariate logistic regression analyses and ROC curve for receiving MV care during hospitalization
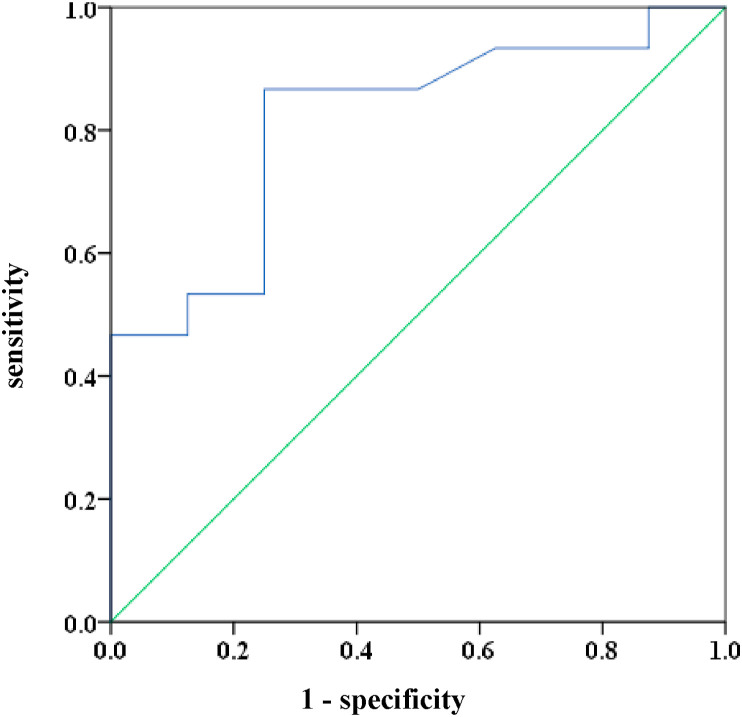
Univariate logistic regression analyses were used to assess the association between laboratory factors and receiving MV care during hospitalization. Results show that the rate of change in Hb level was the only parameter evaluated during the short period after pneumonia diagnosis that was significantly associated with receiving MV care (odds ratio: 0.313, 95% confidence interval [CI]: 0.100–0.976, P = 0.045; Table 4 ). The ROC curve analysis and the area under the ROC curve (AUC) were used to identify the optimal cut-off value in Hb level change during the first few days after pneumonia diagnosis for predicting who would require MV care during hospitalization. The results show that the AUC was 0.813 (95% CI: 0.629–0.996, P = 0.015). The optimal cut-off value for Hb level change was −1.25 g/dL (Fig. 3 ), and the sensitivity and specificity values were 0.867 and 0.750, respectively.
Table 4.
Univariate logistic regression analysis for rate of change in laboratory factors during the few days after pneumonia diagnosis between the MV and non-MV groups.
| Reference | Odds rates | 95% CI | P-value | |
|---|---|---|---|---|
| WBC,/μL | Continuous variable | 1.000 | (1.000–1.001) | 0.288 |
| Lymph,/μL | Continuous variable | 1.000 | (0.999–1.002) | 0.825 |
| Hb, g/dL | Continuous variable | 0.313 | (0.100–0.976) | 0.045 |
| Plt, × 105/μL | Continuous variable | 0.939 | (0.677–1.302) | 0.706 |
| CRP, mg/dL | Continuous variable | 0.932 | (0.784–1.108) | 0.428 |
| LDH, U/L | Continuous variable | 1.002 | (0.989–1.015) | 0.783 |
| PCT, ng/mL | Continuous variable | 3.914 × 104 | (0.000–1.671 × 1013) | 0.297 |
| Alb, g/dL | Continuous variable | 0.475 | (0.103–2.187) | 0.339 |
| D-dimer, μg/mL | Continuous variable | 1.073 | (0.601–1.915) | 0.812 |
MV; mechanical ventilation, 95% CI; 95% confidence interval, WBC; white blood cells, Lymph; lymphocytes, Hb; hemoglobin, Plt; platelets, CRP; C-reactive protein, LDH; lactase dehydrogenase, PCT; procalcitonin, Alb; albumin.
Fig. 3.
ROC curve for the change in Hb as a predictor for receiving MV care. The AUC was 0.813 (95% CI: 0.629–0.996) and the optimal cut-off value for predicting receiving MV care was −1.25 (sensitivity 86.7%; specificity 75.0%). ROC; receiver operating characteristic, AUC; area under the curve, CI; confidence interval, MV; mechanical ventilation.
4. Discussion
In December 2019, new viral pneumonia had emerged in Wuhan, China, and has since spread across the globe. The cause of the disease, named COVID-19, is the SARS-CoV-2, a member of the Betacoronavirus genus [1]. The treatment strategies include antiviral agents such as remdesivir and favipiravir [8,9], and immunosuppression therapies with corticosteroids such as dexamethasone and methylprednisolone, and interleukin-6 receptor (IL-6R) antagonist such as tocilizumab, for patients with advanced disease [10,11]. However, these and other therapies are still in the developmental stage. Several studies have reported that the mortality rate of COVID-19 pneumonia ranges from 4.3% to 14.6% [4,12,13]. Additionally, Richardson et al. reported that 12.2% of COVID-19 patients received MV care, and that the mortality rate among these patients during hospitalization was as high as 88.1% [5]. Thus, there is a need to identify risk factors that could predict the need to administer MV care in COVID-19 patients.
Several studies have reported that some laboratory parameters at the time of hospitalization were associated with mortality and severe disease that required MV care and admission into the ICU [3,4,14]. Some laboratory findings in the current study were similar to those reported in the abovementioned studies. These included elevated levels of CRP, LDH, PCT, AST, and D-dimer, and a decreased Plt level.
To the best of our knowledge, this is the first report to investigate the association between the rate of change in laboratory parameters over the first few days since pneumonia diagnosis in COVID-19 patients and their need to receive MV care. This study shows that Hb level change in the days after pneumonia diagnosis in COVID-19 patients is associated with the need to be treated with MV. Thus, a decrease in Hb level after pneumonia diagnosis in COVID-19 patients might be a predictive factor for severe respiratory failure and the need for MV care.
The most commonly reported reason for mortality or the development of a severe disease that requires MV care is the cytokine storm syndrome caused by SARS-CoV-2 [4]. Particularly, elevated IL-6 level was reported to be part of a larger cytokine storm, and associated with poor prognosis [15]. Another study found that IL-6 level in COVID-19 patients with oxygen saturation (SpO2) < 90% was significantly higher than in those with SpO2 > 90% (median IL-6, 51.69 vs. 6.69 pg/mL, P < 0.001) [6]. Thus, IL-6 level is thought to play an important role in the cytokine network associated with severe respiratory failure, including ARDS, in COVID-19 patients.
Zhang et al. have demonstrated using immunocompromised mice transplanted with human primary acute myeloid leukemia (AML) that IL-6 impedes erythroid differentiation at the proerythroblast stage in AML [16]. This result indicates that elevated IL-6 could result in progressive anemia. In the current study, we did not evaluate IL-6 level in our COVID-19 patients. However, the progression of anemia in these patients is thought to be due to elevated IL-6. One possible reason is the fact that the patients in the current study had no evidence of hemorrhagic diathesis. Additionally, previous studies have reported that IL-6 induces the liver to synthesize acute phase proteins, including CRP [17]. In the current study, the CRP level in the MV group was significantly higher than in the non-MV group, which indicates that IL-6 level may have exhibited similar tendencies. Thus, the decrease in Hb level might be associated with elevated IL-6 level, and as such, can be useful for predicting poor prognosis (Fig. S1, Supplementary Information).
In the current study, we evaluated the rate of change in laboratory factors over the three days after pneumonia diagnosis, in search of predictors for development of respiratory failure. Previous studies have shown that the duration from the onset of illness to ARDS in COVID-19 patients was 8–9 days [4,18], which is similar to the time from the onset of illness to severe respiratory failure in our study. We need to evaluate the rate of change in laboratory factors before severe respiratory failure had occurred, so we can predict early on which patient might develop it and act accordingly.
The present study has several limitations. First, this is a retrospective single-center study that included a small number of Japanese patients. Further studies with greater sample size including other ethnic groups are warranted to evaluate the clinical significance of the decrease in Hb level. Second, five of the 23 patients were diagnosed with pneumonia by CT scans performed in another hospital the day before they were hospitalized at our hospital. For the purpose of analysis, their laboratory data on admission to our hospital were considered as the data at pneumonia diagnosis. Third, the decision regarding intubation and initiation of MV care was left to the attending physician, which may have affected the results. However, because all patients receiving MV care had developed severe respiratory failure and needed more than 5 L/min oxygen flow through a face mask, the influence of the attending physician's decision was likely minimal. Additionally, therapeutic strategies including corticosteroid administration may also have affected our results. Although the proportion of patients receiving corticosteroid therapy was significantly higher in the MV than the non-MV group, corticosteroid therapy was reported to not contribute to the progression of anemia [19]. Therefore, the influence on our results was likely minimal.
In conclusion, the present study shows that the decrease in Hb level during the first few days after pneumonia diagnosis in COVID-19 patients was associated with the development of severe respiratory failure that required MV care. This Hb decrease might be useful for predicting the occurrence of severe respiratory failure requiring MV care in these patients.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors are grateful to Ms. Tamura and Ms. Tashiro, who are secretaries at department of respiratory medicine at Kumamoto University Hospital, for their support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resinv.2020.10.009.
Appendix ASupplementary data
The following are the Supplementary data to this article:
References
- 1.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. Jama. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu F., Li L., Xu M., Wu J., Luo D., Zhu Y. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye Z., Zhang Y., Wang Y., Huang Z., Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. 2020;30:4381–4389. doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C. Remdesivir for the treatment of covid-19 - final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coomes E.A., Haghbayan H. Favipiravir, an antiviral for COVID-19? J Antimicrob Chemother. 2020;75:2013–2014. doi: 10.1093/jac/dkaa171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Jiang W., He Q., Wang C., Wang B., Zhou P. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5:57. doi: 10.1038/s41392-020-0158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. Jama. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du R.H., Liang L.R., Yang C.Q., Wang W., Cao T.Z., Li M. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55 doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients with coronavirus disease 2019. Clin Infect Dis. 2020;71:1061–1069. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang T.Y., Dutta R., Benard B., Zhao F., Yin R., Majeti R. IL-6 blockade reverses bone marrow failure induced by human acute myeloid leukemia. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.aax5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt-Arras D., Rose-John S. IL-6 pathway in the liver: from physiopathology to therapy. J Hepatol. 2016;64:1403–1415. doi: 10.1016/j.jhep.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchman A.L. Side effects of corticosteroid therapy. J Clin Gastroenterol. 2001;33:289–294. doi: 10.1097/00004836-200110000-00006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.