Abstract
The end of the Pleistocene in North America saw the extinction of 38 genera of mostly large mammals. As their disappearance seemingly coincided with the arrival of people in the Americas, their extinction is often attributed to human overkill, notwithstanding a dearth of archaeological evidence of human predation. Moreover, this period saw the extinction of other species, along with significant changes in many surviving taxa, suggesting a broader cause, notably, the ecological upheaval that occurred as Earth shifted from a glacial to an interglacial climate. But, overkill advocates ask, if extinctions were due to climate changes, why did these large mammals survive previous glacial−interglacial transitions, only to vanish at the one when human hunters were present? This question rests on two assumptions: that previous glacial−interglacial transitions were similar to the end of the Pleistocene, and that the large mammal genera survived unchanged over multiple such cycles. Neither is demonstrably correct. Resolving the cause of large mammal extinctions requires greater knowledge of individual species’ histories and their adaptive tolerances, a fuller understanding of how past climatic and ecological changes impacted those animals and their biotic communities, and what changes occurred at the Pleistocene−Holocene boundary that might have led to those genera going extinct at that time. Then we will be able to ascertain whether the sole ecologically significant difference between previous glacial−interglacial transitions and the very last one was a human presence.
Keywords: Pleistocene extinctions, human overkill, glacial−interglacial climate change, megafauna, North America
As the Pleistocene came to an end in North America, 38 genera of mammals vanished (Table 1). The majority are designated as megafauna, with a body mass over ∼45 kg, including several proboscideans (mammoth, mastodon, gomphothere) weighing more than 4,500 kg. Although five genera were much smaller (including the ∼2-kg rabbit Aztlanolagus and the ∼11-kg pronghorn Capromeryx), most of the attention is on the disappearance of the megafauna. Resolving why they vanished has long been complicated by the confluence of the profound climatic and ecological changes that occurred as Earth shifted from a glacial to an interglacial mode, and the spread of Clovis “big-game” hunters in North America. The latter first appeared ∼13.4 kya (all ages are in calibrated years), and, by the tenets of the Overkill Model, are said to have radiated across the continent, their intense predation driving large mammals to extinction in just centuries (1–3).
Table 1.
North American mammalian genera that went extinct at the end of the Pleistocene
| Genus | Common name | Genus | Common name |
| Pampatherium | Southern pampathere | Haringtonhippus | Harington’s horse |
| Holmesina | Northern pampathere | Tapirus | Vero tapir |
| Glyptotherium | Simpson’s glyptodont | Mylohyus | Long-nosed peccary |
| Megalonyx | Jefferson’s ground sloth | Platygonus | Flat-headed peccary |
| Eremotherium | Laurillard’s ground sloth | Camelops | Yesterday’s camel |
| Nothrotheriops | Shasta ground sloth | Hemiauchenia | Large-headed llama |
| Paramylodon | Harlan’s ground sloth | Paleolama | Stout-legged llama |
| Brachyprotoma | Short-faced skunk | Navahoceros | Mountain deer |
| Cuon | Dhole | Cervalces | Stag-moose |
| Tremarctos | Florida cave bear | Capromeryx | Diminutive pronghorn |
| Arctodus | Giant short-faced bear | Tetrameryx | Shuler’s pronghorn |
| Smilodon | Sabertooth | Stockoceros | Pronghorns |
| Homotherium | Scimitar cat | Saiga | Saiga |
| Miracinonyx | American cheetah | Euceratherium | Shrub ox |
| Castoroides | Giant beaver | Bootherium | Helmeted muskox |
| Hydrochoerus | Holmes’s capybara | Mixotoxodon | Toxodont |
| Neochoerus | Pinckney’s capybara | Cuvieronius | Cuvier’s gomphothere |
| Aztlanolagus | Aztlan rabbit | Mammut | American mastodon |
| Equus | Horses | Mammuthus | Mammoths |
North America serves as the iconic case for overkill, given the scale of its extinctions (far greater than in Africa and Eurasia), its apparent abruptness, and its kill sites showing that Clovis people hunted large mammals (4, 5). Substantial extinctions also occurred during the Late Pleistocene in South America, ∼50 megafaunal genera (6, 7), and in Sahul (the landmass formed when Australia, New Guinea, and Tasmania were joined during times of low Pleistocene sea level), which saw the extinction (earlier than in the Americas) of some two dozen genera of large mammals, reptiles, and birds (7–10). Yet, in both those cases, kill sites are lacking (or at best disputed), and the argument for overkill rests largely on the chronological coincidence of human arrival and megafaunal extinction. If there is a less circumstantial case to be made for overkill, it should be made in North America.
Why Humans Aren’t to Blame
There are, however, many reasons to be skeptical of the claim that humans were responsible for the extinction of those 38 genera in North America.
-
1)
It is estimated that when Clovis hunters arrived there were hundreds of millions of these large mammals on the landscape (1). Even so, there are only 16 occurrences in which humans killed or scavenged one of these animals (5, 11).
-
2)
Only five genera are among those 16 kill−scavenging occurrences: mammoth, mastodon, gomphothere, horse, and camel. There is no archaeological evidence that any of the other 33 genera were preyed upon by Clovis hunters (5, 11).
-
3)
That so few of the 38 genera appear to have been hunted may be because, so far at least, only 18 of them are known to have even survived up to the time Clovis people arrived in the Americas (12). Of the other 20 genera, 15 disappeared earlier, and 5 are undated. Many of those 20 taxa are rare in the fossil record, and dating of new finds might bring their last appearance closer to Clovis times. Or not. It is possible the reason some do not date to Clovis times is because they had already vanished. Extinctions were staggered by genera over the Pleistocene in other parts of the world, and there is no evidence to suggest North America was necessarily different (7, 12–16). That the majority of these animals may have disappeared by the time Clovis groups arrived would also explain why they so rarely appear in kill−scavenging sites.
-
4)
There is compelling evidence that humans arrived in the Americas at least ∼1,000 y prior to Clovis times (17, 18). Despite this longer overlap between people and megafauna, there are no pre-Clovis age kill−scavenging sites (5). Overkill advocates either dismiss evidence of a pre-Clovis human presence or consider it irrelevant, assuming Clovis groups were the first big-game hunters (1, 2).
-
5)
In contrast to the dearth of kill−scavenging occurrences of the extinct genera, there are, from the same period overkill is said to have occurred, ∼90 kill−scavenging occurrences of six extant large herbivores, including bison, elk, moose, and deer (11). Thus, where we have abundant archaeological evidence of large mammal hunting in the Late Pleistocene, it is of genera that survived to the present, while the supposed continent-wide slaughter of 38 extinct genera left scarcely an archaeological trace. Particularly telling is the record of bison. They were hunted starting in Clovis times (19), and, among the many hundreds of bison kill sites known from the subsequent ∼12,000 y, are sites where hundreds (20), and, in some sites, thousands (21), of these animals were slain. That long record of Indigenous hunting was capped by the slaughter of millions of bison by late 19th century Euro-American commercial hide hunters (22). Yet, bison survive today, even after millennia of intensive human predation.
-
6)
Large mammal extinctions were not the only significant change that took place on the Late Pleistocene landscape as Earth emerged from the grip of an Ice Age. Lost as well were multiple species of mammals whose genera survived in North America or elsewhere (e.g., the dire wolf, Canis dirus, and Dasypus bellus, the beautiful armadillo), some 20 genera of birds, several tortoises, a snake, and even a species of spruce (12, 14, 23–25).
-
7)
Nor did the extant large herbivores emerge unscathed. Bighorn sheep, bison, and elk decreased in size through the Late Pleistocene and into the Holocene; ultimately, a new bison species arose (26, 27). Other animals underwent sometimes-extensive range shifts (caribou and muskox no longer live in the southeastern United States, as each had in the Pleistocene), changes in abundance, and extirpation (14, 24, 28, 29). Ancient DNA evidence shows there were population bottlenecks and declining genetic diversity in the Late Pleistocene among a number of extant and extinct taxa, in some instances, beginning well before the first appearance of humans in North America (30–35).
Large mammal extinction at the end of the Pleistocene thus cannot be treated as an isolated phenomenon warranting its own unique explanation (4, 14, 24). Extinctions were not restricted to large animals, not all large animals went extinct, and the sweeping and complex changes that took place in animals, plants, and biotic communities indicate strong selective pressures in the Late Pleistocene environment. Those pressures were well beyond the influence of newly arrived human predators.
The Climate Challenge
To explain the suite of changes that occurred in North America at this time, we need to understand the climatic and ecological upheaval that took place as Earth shifted from a glacial to an interglacial mode, and particularly how that may have impacted the now-extinct mammals. By way of illustrating the explanatory challenge this poses, some of the extinct herbivores inhabited colder regions (e.g., Cervalces, Bootherium), others inhabited warmer ones (Euceratherium, Glyptotherium, Holmesina, Pampatherium), and others were not constrained to either (Homotherium, Megalonyx) (36–39). Some lived more solitary lives in the forests (Mylohyus, Mammut), scrub, or grassland (Capromeryx, Paramylodon), while others formed herds on open grasslands (Equus, Stockoceros) (36, 38, 40–44). Some occupied arid−semiarid environments (Nothrotheriops, Stockoceros), others were tied to marshy or semiaquatic habitats (Castoroides, Glyptotherium), and still others inhabited a wide range of ecological settings (Hemiauchenia, Megalonyx) (38, 45–48). Some were grazers (Equus, Glyptotherium, Mammuthus), others were browsers (Camelops, Eremotherium, Euceratherium, Mammut, Tapirus), and some were mixed feeders (Hemiauchenia, Mylohyus, Paleolama, Platygonus) (37, 40–43, 45, 49, 50). A few carnivores were omnivores or occasional scavengers (Arctodus); others had teeth and jaws finely honed to killing large animals (Homotherium, Smilodon) (51–53). Some carnivores may have been pack hunters (Cuon); others were solo predators who chased down their quarry (Miracinonyx), or stalked and ambushed their prey (Smilodon) (54–56).
Given how widely varied those now-extinct taxa were in their physiologies, adaptations, ecological and climatic tolerances, competitive interactions, and life histories and habitats, understanding what led to the extinction of each—and when—is no easy task. It requires, for instance, far greater knowledge than we now have of the magnitude and speed of changes at the end of the Pleistocene in temperature, precipitation, seasonality, and growing season and their impact on phenology (15, 57–61). Needed, as well, are measures of ecological community composition, productivity, diversity, and stability over time, and information on changing habitat availability, landscape structure (28, 59, 60, 62), and nutrient cycling and productivity with variations in greenhouse gases (49, 63–65). Important too will be an understanding of feedback effects of declining herbivore populations on vegetation growth and composition, or on predators dependent on them (53, 65–68), and, more broadly, the changing patterns of competition, predation, and grazing sequences (13, 29, 69, 70).
We need to understand each animal’s adaptive tolerances and thresholds in order to show how (and which) climatic and environmental changes affected them specifically (24, 28, 71). It will be necessary to couple high-resolution climatic and environmental information with evidence of population and demographic histories, in order to reveal when the extinctions of individual taxa began, rather than when the last member of a species died: Knowing the end point of a process that possibly played out over centuries or millennia may reveal very little of what caused it to start (4).
Accordingly, there will be no single climate “theory”—comparable to overkill’s one size fits all approach—that explains the disappearance of those 38 genera of mammals, along with all of the other extinctions and evolutionary and biotic changes that took place. Climate change may have been the ultimate driver, but the proximate factors that led, for example, to the demise of the mastodon in the midwestern forests are likely to have been quite distinct from those that caused horses and camels to vanish from western grasslands, or the glyptodont to disappear from southeastern swamps.
But if It Was Climate Change, Why Hadn’t It Happened Before?
All of which raises a longstanding question: If extinctions were caused by climate changes at the end of the Pleistocene, then why did all those animals survive multiple previous glacial−interglacial transitions (1, 3, 9, 72), only to vanish at the one transition when human hunters were on the landscape? The question, although often used by overkill advocates to criticize climate-based explanations, is a reasonable one. However, although easily asked, it is not readily answered.
Underlying the question are two assumptions: first, that previous glacial−interglacial transitions were similar to the Pleistocene−Holocene transition; and, second, that all 38 genera survived unchanged over multiple, previous glacial−interglacial transitions (1, 9). Neither assumption is demonstrably correct. I discuss each in turn [others explore this question from the opposite perspective: What circumstances might have made the Pleistocene−Holocene transition unique (15, 64, 70, 73)].
Not All Glacial−Interglacial Transitions Are Alike
There were scores of glacial−interglacial transitions over the ∼2.5 million years of the Pleistocene (74, 75). Of particular interest, however, are the 11 that occurred within the last 800,000 y, following a change in glacial−interglacial periodicity from 41,000- to ∼100,000-y cycles (76–78). This resulted in cycles of greater amplitude, duration, and asymmetry: Glacial periods lasted ∼90,000 y, and interglacial periods lasted ∼10,000 y, with the onset of interglacial warming occurring on the order of millennia (75, 77–79). Extinctions would have perhaps been more likely to have occurred in the last 800,000 y than during earlier, more moderate glacial−interglacial cycles. Surely, if the change from a glacial to an interglacial climate drove extinctions, there ought to have been prior episodes of megafaunal extinction on the earlier, people-free landscape? Yes and no.
First, it is important to stress that while glacial−interglacial transitions were widespread, global phenomena, they were not all alike (78). They varied in the rapidity of the change from glacial cold to the peak of interglacial warmth: It was rapid in the case of the last interglacial period, Marine Isotope Stage 5 [MIS 5e, its onset at 130 kya (74, 77)], as well as at MIS 7 (243 kya), MIS 9 (337 kya), and MIS 19 (790 kya), but more gradual in the case of MIS 11 (424 kya), MIS 13 (533 kya), and MIS 17 (706 kya). Transitions varied, too, in the magnitude of changes in greenhouse gas concentrations: CO2 and methane spiked highest in MIS 9, and lower in MIS 7, MIS 13, MIS 15 (621 kya), and MIS 17. These transitions led into interglacial periods that varied in their intensity (the strongest were MIS 5e and MIS 11, and the weakest were MIS 13 and MIS 17); in air and sea surface temperatures (MIS 5e was particularly warm, and MIS 13 particularly cold); and, in their duration, ice volume, sea level histories, climatic stability, peak temperature duration, and regional expression (15, 77, 78, 80–85). The Pleistocene-to-Holocene transition (MIS 1) was similar, in certain respects, to some previous glacial−interglacial transitions, but not to all (15, 70, 78, 83). Indeed, there is some suspicion this most recent transition was distinctive, with unusually rapid interstadial and stadial oscillations (86). But, until we have comparably high-resolution data from all prior transitions, we will not know whether that is the case.
Second, much of the evidence of previous glacial−interglacial transitions is of limited relevance to understanding the cause of mammalian extinctions. That evidence is principally from deep ocean sediment cores, and Greenland and Antarctic ice cores. These provide measures of (or are proxies for) climate changes on a global scale, such as in atmosphere and ocean temperatures, concentrations of greenhouse gases (especially carbon dioxide and methane), and ice volume/sea level (78). Some of these changes had worldwide impact (e.g., greenhouse gases, which are well mixed and circulated in Earth’s atmosphere), but as for how these changes correlate with the local, terrestrial conditions of interest, the devil is in the details. Knowing, for example, that the Greenland ice cores indicate a general warming trend at a previous glacial−interglacial transition does not reveal how much and how rapidly warming played out across the varied regions of North America (57, 59). Or what environmental changes such warming would have triggered. Or how those changes would have impacted plant communities on which the now-extinct animals depended. Or how the animals responded.
In fact, we have very little evidence of what the terrestrial environment was like during prior glacial or interglacial periods to even begin to answer those questions (78). There are relatively few pollen and plant macrofossil (wood, leaves, seeds) records that date to the previous glacial−interglacial cycle, and there are virtually none for any of the earlier cycles (87). There are more sites (∼125) with vertebrate fossil remains (88), but, given the length of the Pleistocene, it is a limited record in a relative and absolute sense. Moreover, uncertainties in the dating of fossil remains, especially those older than the reliable limit of radiocarbon dating (∼50 kya), make it impossible to securely relate the appearances and disappearances of different taxa to previous glacial−interglacial transitions (7, 15).
Finally, a key takeaway from studies of fossil remains at the end of the Pleistocene is that each animal species—and this is true as well of the plants on which they depended—responded to climatic and ecological changes individualistically and on multiple time scales, depending, in part, on factors such as dispersal abilities, the rate and magnitude of climate change, and phenotypic or behavioral plasticity (12, 24, 28, 61, 62, 89–91). Stated simply, each species had to adapt/evolve, shift its range, or go extinct, and each did so on its own terms (92).
Yet, we are seldom certain which climatic aspects drove an observed biotic response (57). Climatic drivers may be direct, influencing the physiology of individual animals, or indirect, in which climate may affect, say, vegetation, which, in turn, acts as a filter through which the animals respond (26, 57, 59, 93). Other, more “local” factors can influence responses as well, such as vegetation composition, the effects of predation pressure and competition, and even stochastic events (13, 49, 57, 62, 70, 89, 94). As Diamond (ref. 94, p. 856) cautions, “How can we expect the fossil record to yield unambiguous interpretations of extinctions that happened 10,000 to 30,000 years ago, when we are often unable to understand declines of species being studied in the field today?”
Not All Animals Experienced All Glacial−Interglacial Transitions
Perhaps most important, of the 38 genera that went extinct at the end of the Pleistocene, it is not clear that all of them experienced multiple glacial−interglacial cycles, or that they went unchanged over the Pleistocene. Analysis of this issue has previously been done principally at the genus level, owing to the difficulty of identifying species in the fossil record, and of reliably distinguishing extinct forms from ancestors of extant species (12, 62, 72, 94).
Although genera are less susceptible to problems of taxonomic identification, using the genus as the unit of analysis nonetheless has significant disadvantages (94). Given that the Linnaean hierarchy routinely contains fewer genera than species, changes in the number of the former will generally have a greater quantitative impact than changes in the number of the latter (62). Also, large mammals typically have far fewer species per genus than small ones. The loss of just a few species can mean the disappearance of a large-mammal genus, where it might not in a multispecies small-mammal genus; this would make it appear, when looking only at genera, as if the rate of extinctions is higher in large mammals. Finally, it is more difficult to identify evolutionary relationships between genera than between species, thus preventing us from seeing speciation over time, which can be as telling a response to changing climatic circumstances as extinctions (12, 57, 62, 94).
While granting species are a more meaningful analytical unit, there remains the challenge of identifying them in the fossil record. It is often done by body size and morphological characters, but, if the samples are small and the gaps in the fossil record are large, what appear to be distinct taxa may prove, with additional fossil material, to be varieties of the same species (93). Ancient DNA analysis is now showing that some traditionally defined species were capable of introgression, and were not separate species in the classical sense (95, 96). These matters notwithstanding, species have far more potential to reflect responses to climatic and environmental change, for, unlike genera which are arbitrary taxonomic units and not under selection, species adapt/evolve, shift their range, and/or go extinct.
Ideally, the patterns of evolution and extinction over time would be tracked using individually dated specimens, but, owing to the dating uncertainties earlier noted, the ages of taxa are customarily given as ranges within or across North American Land Mammal Ages (NALMAs) (88). For the purposes here, these are the Late Blancan (∼2.5 Mya to ∼1.35 Mya); the Irvingtonian, its onset defined by the first appearance of Mammuthus south of 55°N (∼1.35 Mya to ∼210 kya); and the Rancholabrean, defined by the first appearance of Bison south of 55°N, and its end corresponding to the close of the Pleistocene [∼210 kya to ∼11.7 kya, although new genetic evidence suggests the initial arrival of bison in the Americas was ∼195 kya (97)].
The 38 currently identified extinct genera, the presently accepted species within each, and their ranges across the Pleistocene land mammal ages are detailed in SI Appendix, Table S1. Table 2 summarizes the patterns in these data, listing the extinct genera by NALMA range, and by the number of species recognized within each genus. Regarding the former (Table 2, rows), of the 38 genera that went extinct at the end of the Pleistocene, half of the genera (n = 19) were present throughout the entire Pleistocene, and survived multiple, previous glacial−interglacial cycles.
Table 2.
Age ranges of extinct genera sorted by NALMA (rows), and whether there is one species or two or more species per genus (columns) (data from SI Appendix, Table S1)
| One species recognized (n = 17) | Two+ species recognized (n = 21) | |
| Rancholabrean to the end of the Pleistocene (n = 6) | 1. Bootherium | 1. Cervalces |
| 2. Cuon | ||
| 3. Mixotoxodon | ||
| 4. Pampatherium | ||
| 5. Saiga | ||
| Irvingtonian to the end of the Pleistocene (n = 13) | 1. Brachyprotoma | 1. Camelops |
| 2. Cuvieronius | 2. Castoroides | |
| 3. Euceratherium | 3. Equus | |
| 4. Haringtonhippus | 4. Mammuthus | |
| 5. Mylohyus | 5. Nothrotheriops | |
| 6. Navahoceros | 6. Tetrameryx | |
| 7. Stockoceros | ||
| Blancan to the end of the Pleistocene (n = 19) | 1. Aztlanolagus | 1. Arctodus |
| 2. Hydrochoerus | 2. Capromeryx | |
| 3. Neochoerus | 3. Eremotherium | |
| 4. Paleolama | 4. Glyptotherium | |
| 5. Tremarctos | 5. Hemiauchenia | |
| 6. Holmesina | ||
| 7. Homotherium | ||
| 8. Mammut | ||
| 9. Megalonyx | ||
| 10. Miracinonyx | ||
| 11. Paramylodon | ||
| 12. Platygonus | ||
| 13. Smilodon | ||
| 14. Tapirus |
As for the other 19 genera, six only appeared after the onset of the Rancholabrean. This puts them in North America during the MIS 7 interglacial or (using the younger age for bison arrival) during the MIS 6 glacial period. Either way, these taxa experienced just one significant glacial−interglacial cycle prior to the terminal Pleistocene. The remaining 13 genera would have had to survive at least a dozen glacial−interglacial cycles, depending on when they were first on the Irvingtonian landscape. Thus, all 38 genera experienced at least one glacial−interglacial cycle, and all survived the higher-amplitude cycles of the last 800,000 y.
The more important question, however, is whether they survived unchanged. Extinctions are not the sole response of a species to changing climates; these can include changes in diet, size, and morphology; in population abundance and range; and in genetic changes that can lead to new species (57). Table 2 (columns) sorts the genera by those with only a single species, and those in which there are two or more species; the majority of the genera (n = 21) fall into the latter category.
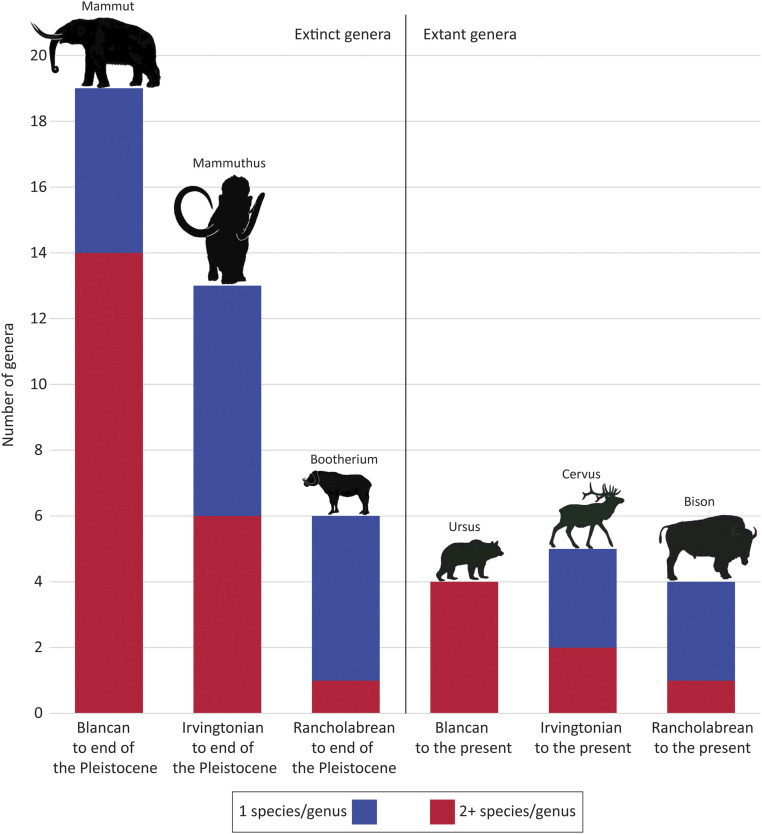
When those taxa are sorted by NALMA ranges, a distinctive pattern emerges. Contingency table analysis (Table 3) of those data indicates that there is a significant difference in the number of species within longer- versus shorter-lasting genera (as measured by the χ2 statistic). The former, genera that survived from the Blancan to the end of the Pleistocene, are significantly underrepresented by single-species taxa (n = 5), and overrepresented by genera having two or more species (n = 14), as determined by adjusted residuals. The reverse is true for genera present only during the Rancholabrean (Table 3 and Fig. 1, Left). In other words, the longer a genus survived, the greater the incidence of speciation and evolutionary change.
Table 3.
χ2 analysis of frequency counts of extinct genera, by NALMA and by the number of species within genera
| One species recognized | Two+ species recognized | Total | |
| Rancholabrean to the end of the Pleistocene | 5 (2.072) | 1 (−2.072) | 6 |
| Irvingtonian to the end of the Pleistocene | 7 (0.814) | 6 (−0.814) | 13 |
| Blancan to the end of the Pleistocene | 5 (−2.283) | 14 (2.283) | 19 |
| Total | 17 | 21 | 38 |
Calculated adjusted residuals are in parentheses, significant values are in bold (data from Table 2). χ2 = 6.659, df = 2, P = 0.036.
Fig. 1.
Histogram of frequency of extinct and extant genera by NALMA range and number of genera per species. Each column shows the total number of genera for the NALMA range denoted: the blue segments are the number of genera with only 1 species per genus; the red segments are the number of genera with 2+ species per genus. (Left) Extinct genera (data from Table 3). (Right) Extant genera (data from Table 5). Note the decline of multispecies genera (red segments) over shorter time spans for both extinct and extant taxa. The illustrated taxa are examples of genera in each NALMA column. Silhouettes courtesy of Anthony J. Stuart, reproduced with permission from ref. 7.
That pattern is also evident in North America’s large mammals that did not go extinct at the end of the Pleistocene. Table 4 lists these surviving genera by NALMA range (rows), and by the number of species within each genus (columns). The genera are more or less equally divided among the NALMAs, indicating the extant taxa also weathered multiple glacial−interglacial cycles, along with the purported wave of Clovis human hunters at the end of the Pleistocene. When those taxa are sorted by NALMA ranges, the longest-surviving genera are again those with more than one species, with genera originating in the Irvingtonian and Rancholabrean being mostly monospecific, although the statistical result is not significant (Table 5 and Fig. 1, Right).
Table 4.
Age ranges of extant genera by North American Land Mammal Age (rows), and whether there is one species or two or more species per genus (columns) (data from refs. 88, 98)
| One species recognized (n = 6) | Two+ species recognized (n = 7) | |
| Rancholabrean to the present (n = 4) | 1. Alces | 1. Bison |
| 2. Ovibos | ||
| 3. Rangifer | ||
| Irvingtonian to the present (n = 5) | 1. Antilocapra | 1. Oreamnos |
| 2. Cervus | 2. Panthera | |
| 3. Ovis | ||
| Blancan to the present (n = 4) | 1. Canis | |
| 2. Felis | ||
| 3. Odocoileus | ||
| 4. Ursus |
Table 5.
χ2 analysis of frequency counts of extant genera, by NALMA and by the number of species within genera; calculated adjusted residuals in parentheses (data from Table 4)
| One species recognized | Two+ species recognized | Total | |
| Rancholabrean to the present | 3 (1.390) | 1 (−1.390) | 4 |
| Irvingtonian to the present | 3 (0.791) | 2 (−0.791) | 5 |
| Blancan to the present | 0 (−2.225) | 4 (2.225) | 4 |
| Total | 6 | 7 | 13 |
χ2 = 5.154, df = 2, P = 0.076; the χ2 result is not significant.
Change is evident not just in the appearance of new species. Over the course of the Pleistocene, almost two dozen species within 17 of those genera disappeared, as determined by their presence in the Blancan and Irvingtonian, and their absence from Rancholabrean age faunas (SI Appendix, Table S1). However, whether those species disappeared as a result of evolutionary change or extinction is not clear, nor what role (if any) climate change may have played.
In effect, only by ignoring species within genera can one assert these 38 genera were unchanged over time. Of course, demonstrating that new species evolved within these genera—or that some species went extinct—is not itself an explanation for why those changes occurred. Nor does it demonstrate that species evolution, immigration or emigration, or extinction was in response to changing climates per se, or whether these took place during glacial−interglacial transitions; it only indicates that changes took place (57). Linking change to cause will require additional data.
Getting Past the Impasse
The question previously asked was, Why did 38 genera survive all previous glacial−interglacial transitions only to disappear at the last one? Yet, as not all of them ran that ∼2.5-million-year gauntlet, and human hunting at the end of the Pleistocene fails to explain their extinction, a different question should be asked. Namely, Since species changed (evolved and/or went extinct) multiple times over many hundreds of thousands of years, and did so long before people arrived, what climatic and environmental changes led to those genera going extinct during the last glacial−interglacial transition?
To answer that, we need to understand, as earlier noted, the physiology, habitats, and adaptations of each individual species (24, 71). That these went extinct only tells us their adaptive thresholds were breached, not what their tolerances were, nor what climatic and ecological variables may have been relevant in breaching those thresholds (4, 16, 24, 57, 62, 71). Building a case will require high-resolution chronologies and ancient DNA evidence to determine when each species began its downward demographic spiral, and track its population dynamics and range through to its extinction (7, 13, 31, 34, 35). It will also require detailed, region-specific records of the pace and magnitude of tclimate change, and of changes in the species and structure of the biotic community (drawn from pollen, macrofossils, ancient environmental DNA, and other proxies) (24, 28, 49, 64, 65, 67, 68, 99). Causal links between specific climatic or ecological changes, and the timing and process of the extinction of a species, can then be forged by investigating whether there is isotopic and ecomorphological evidence of changes in its diet, osteological and dental indicators of environmental stress, and/or genetic evidence of adaptive change and selection (including loss of genetic diversity) (5, 34).
Once such links are known, we can then investigate whether similar climatic and ecological changes were occurring at previous glacial−interglacial transitions (assuming we can acquire the necessary data); if so, whether to the same degree as at the end of the Pleistocene; and whether (or how) these may have impacted those 38 large mammal genera. Then we will be able to ascertain whether the sole significant difference between all previous glacial−interglacial transitions and the very last one was the presence of Clovis hunters.
Put another way, until we know what climatic and ecological factors were relevant to the extinction of those 38 genera at the end of the Pleistocene, a period for which we have relatively abundant data, we will not know what factors were relevant for previous glacial−interglacial transitions for which we have virtually no data. Asking why those 38 genera of animals did not go extinct during prior glacial−interglacial transitions remains a reasonable question to ask, but it is a hollow criticism to offer.
Supplementary Material
Acknowledgments
I am grateful to Ryan Breslawski, Steven Emslie, Philip Gibbard, Donald Grayson, Richard Klein, Ernest Lundelius, Daniel Mann, Jim Mead, James O’Connell, Cathy Whitlock, and Eske Willerslev for their helpful comments and advice. I appreciate, as well, the very thoughtful comments of my anonymous reviewers.
Footnotes
The author declares no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2015032117/-/DCSupplemental.
Data Availability.
All of the data are included in the manuscript and SI Appendix.
References
- 1.Martin P. S., Twilight of the Mammoths: Ice Age Extinctions and the Rewilding of America (University of California Press, 2005). [Google Scholar]
- 2.Surovell T. A., Pelton S. R., Anderson-Sprecher R., Myers A. D., Test of Martin’s overkill hypothesis using radiocarbon dates on extinct megafauna. Proc. Natl. Acad. Sci. U.S.A. 113, 886–891 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haynes G., “The evidence for human agency in the Late Pleistocene megafaunal extinctions” in Encyclopedia of the Anthropocene, DellaSalla D., Goldstein M., Eds. (Elsevier, 2018), pp. 219–226. [Google Scholar]
- 4.Meltzer D. J., Pleistocene overkill and North American mammalian extinctions. Annu. Rev. Anthropol. 44, 33–53 (2015). [Google Scholar]
- 5.Grayson D. K., Meltzer D. J., Revisiting Paleoindian exploitation of extinct North American mammals. J. Archaeol. Sci. 56, 177–193 (2015). [Google Scholar]
- 6.Barnosky A. D., Lindsey E. L., Timing of Quaternary megafaunal extinction in South America in relation to human arrival and climate change. Quat. Int. 217, 10–29 (2010). [Google Scholar]
- 7.Stuart A. J., Late Quaternary megafaunal extinctions on the continents: A short review. Geol. J. 50, 338–363 (2015). [Google Scholar]
- 8.Wroe S., et al. , Climate change frames debate over the extinction of megafauna in Sahul (Pleistocene Australia-New Guinea). Proc. Natl. Acad. Sci. U.S.A. 110, 8777–8781 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koch P. L., Barnosky A. D., Late Quaternary extinctions: State of the debate. Annu. Rev. Ecol. Evol. Syst. 37, 215–250 (2006). [Google Scholar]
- 10.Peters K. J., et al. , FosSahul 2.0, an updated database for the Late Quaternary fossil records of Sahul. Sci. Data 6, 272 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grayson D. K., Meltzer D. J., Breslawski R. P., Overkill and the North American archaeological record—Not guilty by association? J. Archaeol. Sci. In press. [Google Scholar]
- 12.Grayson D. K., Giant Sloths and Sabertooth Cats: Extinct Mammals and the Archaeology of the Ice Age Great Basin (The University of Utah Press, 2016). [Google Scholar]
- 13.Widga C., et al. , Late Pleistocene proboscidean population dynamics in the North American Midcontinent. Boreas 46, 772–782 (2017). [Google Scholar]
- 14.Grayson D. K., The archaeological record of human impacts on animal populations. J. World Prehist. 15, 1–68 (2001). [Google Scholar]
- 15.Mann D. H., Groves P., Gaglioti B. V., Shapiro B. A., Climate-driven ecological stability as a globally shared cause of Late Quaternary megafaunal extinctions: The plaids and stripes hypothesis. Biol. Rev. Camb. Philos. Soc. 94, 328–352 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grayson D. K., Meltzer D. J., A requiem for North American overkill. J. Archaeol. Sci. 30, 585–593 (2003). [Google Scholar]
- 17.Davis L. G., et al. , Late Upper Paleolithic occupation at Cooper’s Ferry, Idaho, USA, ∼16,000 years ago. Science 365, 891–897 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Jenkins D. L., et al. , Clovis age Western Stemmed projectile points and human coprolites at the Paisley Caves. Science 337, 223–228 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Haynes C. V., Huckell B. B., Murray Springs: A Clovis Site with Multiple Activity Areas in the San Pedro Valley (University of Arizona Press, 2007). [Google Scholar]
- 20.Wheat J. B., The Olsen-Chubbuck Site: A Paleo-Indian Bison Kill (Memoirs of the Society for American Archaeology , Cambridge University Press, 1972), vol. 26. [Google Scholar]
- 21.Reher C., Frison G. C., The Vore site, 48CK302, A stratified buffalo jump in the Wyoming Black Hills. Plains Anthropol. Mem. 25, xvi−xxxi (1980). [Google Scholar]
- 22.Roe F., The North American Buffalo: A Critical Study of the Species in its Wild State (The University of Toronto Press, ed. 2, 1970). [Google Scholar]
- 23.Grayson D. K., Pleistocene avifaunas and the overkill hypothesis. Science 195, 691–693 (1977). [DOI] [PubMed] [Google Scholar]
- 24.Grayson D. K., Deciphering North American Pleistocene extinctions. J. Anthropol. Res. 63, 185–213 (2007). [Google Scholar]
- 25.Jackson S. T., Weng C., Late Quaternary extinction of a tree species in eastern North America. Proc. Natl. Acad. Sci. U.S.A. 96, 13847–13852 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill M. E., Hill M. G., Widga C. C., Late Quaternary bison diminution on the Great Plains of North America: Evaluating the role of human hunting versus climate change. Quat. Sci. Rev. 27, 1752–1771 (2008). [Google Scholar]
- 27.Boulanger M. T., Lyman R. L., Northeastern North American Pleistocene megafauna chronologically overlapped minimally with Paleoindians. Quat. Sci. Rev. 85, 35–46 (2014). [Google Scholar]
- 28.Graham R. W., Quaternary mammal communities: Relevance of the individualistic response and non-analogue faunas. Paleontol. Soc. Pap. 11, 141–158 (2005). [Google Scholar]
- 29.Blois J. L., McGuire J. L., Hadly E. A., Small mammal diversity loss in response to late-Pleistocene climatic change. Nature 465, 771–774 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Campos P. F., et al. , Ancient DNA analyses exclude humans as the driving force behind late Pleistocene musk ox (Ovibos moschatus) population dynamics. Proc. Natl. Acad. Sci. U.S.A. 107, 5675–5680 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karpinski E., et al. , American mastodon mitochondrial genomes suggest multiple dispersal events in response to Pleistocene climate oscillations. Nat. Commun. 11, 4048 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lorenzen E. D., et al. , Species-specific responses of Late Quaternary megafauna to climate and humans. Nature 479, 359–364 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mann D. H., Groves P., Kunz M. L., Reanier R. E., Gaglioti B. V., Ice-age megafauna in Arctic Alaska: Extinction, invasion, survival. Quat. Sci. Rev. 70, 91–108 (2013). [Google Scholar]
- 34.Orlando L., Cooper A., Using ancient DNA to understand evolutionary and ecological processes. Annu. Rev. Ecol. Evol. Syst. 45, 573–598 (2014). [Google Scholar]
- 35.Shapiro B., et al. , Rise and fall of the Beringian steppe bison. Science 306, 1561–1565 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Hoganson J. W., McDonald H. G., First report of Jefferson’s ground sloth (Megalonyx jeffersonii) in North Dakota: Paleobiogeographical and paleoecological significance. J. Mammal. 88, 73–80 (2007). [Google Scholar]
- 37.Kropf M., Mead J. I., Scott Anderson R., Dung, diet, and the paleoenvironment of the extinct shrub-ox (Euceratherium collinum) on the Colorado Plateau, USA. Quat. Res. 67, 143–151 (2007). [Google Scholar]
- 38.Schubert B. W., Graham R. W., McDonald H. G., Grimm E. C., Stafford T. W., Latest Pleistocene paleoecology of Jefferson’s ground sloth (Megalonyx jeffersonii) and elk-moose (Cervalces scotti) in northern Illinois. Quat. Res. 61, 231–240 (2004). [Google Scholar]
- 39.Widga C., Fulton T. L., Martin L. D., Shapiro B., Homotherium serum and Cervalces from the great lakes region, USA: Geochronology, morphology and ancient DNA. Boreas 41, 546–556 (2012). [Google Scholar]
- 40.McDonald H. G., Morgan G. S., Ground sloths of New Mexico. New Mexico Mus. Nat. Hist. Sci. Bull. 53, 652–663 (2011). [Google Scholar]
- 41.McDonald H. G., Pelikan S., Mammoths and mylodonts: Exotic species from two different continents in North American Pleistocene faunas. Quat. Int. 142–143, 229–241 (2006). [Google Scholar]
- 42.Metcalfe J. Z., Longstaffe F. J., Environmental change and seasonal behavior of mastodons in the Great Lakes region inferred from stable isotope analysis. Quat. Res. 82, 366–377 (2014). [Google Scholar]
- 43.Teale C. L., Miller N. G., Mastodon herbivory in mid-latitude late-Pleistocene boreal forests of eastern North America. Quat. Res. 78, 72–81 (2012). [Google Scholar]
- 44.White R., Morgan G. S., Capromeryx (Artiodactyla: Antilocapridae) from the Rancholabrean Tramperos Creek fauna, Union County, New Mexico, with a review of the occurrence and paleobiology of Capromeryx in the Rancholabrean of New Mexico. New Mexico Mus. Nat. Hist. Sci. Bull. 53, 641–651 (2011). [Google Scholar]
- 45.McDonald H. G., Paleoecology of extinct xenarthrans and the great American biotic interchange. Bull. Fla. Mus. Nat. Hist. 45, 313–333 (2005). [Google Scholar]
- 46.McDonald H. G., Jefferson G. T., Distribution of Pleistocene Nothrotheriops (Xenarthra, Nothrotheridae) in North America. Nat. Hist. Mus. Los Angel. Cty. Sci. Ser. 41, 313–331 (2008). [Google Scholar]
- 47.Plint T., Longstaffe F. J., Zazula G., Giant beaver palaeoecology inferred from stable isotopes. Sci. Rep. 9, 7179 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yann L. T., DeSantis L. R. G., Koch P. L., Lundelius E. L., Dietary ecology of Pleistocene camelids: Influences of climate, environment, and sympatric taxa. Palaeogeogr. Palaeoclimatol. Palaeoecol. 461, 389–400 (2016). [Google Scholar]
- 49.DeSantis L. R. G., Feranec R. S., MacFadden B. J., Effects of global warming on ancient mammalian communities and their environments. PLoS One 4, e5750 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cammidge T. S., Kooyman B., Theodor J. M., Diet reconstructions for end-Pleistocene Mammut americanum and Mammuthus based on comparative analysis of mesowear, microwear, and dental calculus in modern Loxodonta africana. Palaeogeogr. Palaeoclimatol. Palaeoecol. 538, 109403 (2020). [Google Scholar]
- 51.DeSantis L. R. G., “Dietary ecology of Smilodon” in Smilodon: The Iconic Sabertooth , Werdelin L., McDonald H. G., Shaw C. A., Eds. (Johns Hopkins University Press, 2018), pp. 153–170. [Google Scholar]
- 52.Schubert B. W., Late Quaternary chronology and extinction of North American giant short-faced bears (Arctodus simus). Quat. Int. 217, 188–194 (2010). [Google Scholar]
- 53.Van Valkenburgh B., Hayward M. W., Ripple W. J., Meloro C., Roth V. L., The impact of large terrestrial carnivores on Pleistocene ecosystems. Proc. Natl. Acad. Sci. U.S.A. 113, 862–867 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown C., Balisi M., Shaw C. A., Van Valkenburgh B., Skeletal trauma reflects hunting behaviour in extinct sabre-tooth cats and dire wolves. Nat. Ecol. Evol. 1, 0131 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Lewis M. E., “The postcranial morphology of Smilodon” in Smilodon: The Iconic Sabertooth, Werdelin L., McDonald H. G., Shaw C. A., Eds. (Johns Hopkins University Press, 2018), pp. 171–195. [Google Scholar]
- 56.Van Valkenburgh B., Grady F., Kurten B., The Plio-Pleistocene cheetah-like cat (Miracinonyx inexpectatus) of North America. J. Vertebr. Paleontol. 10, 434–454 (1990). [Google Scholar]
- 57.Blois J. L., Hadly E. A., Mammalian response to Cenozoic climatic change. Annu. Rev. Earth Planet. Sci. 37, 181–208 (2009). [Google Scholar]
- 58.Blois J. L., Zarnetske P. L., Fitzpatrick M. C., Finnegan S., Climate change and the past, present, and future of biotic interactions. Science 341, 499–504 (2013). [DOI] [PubMed] [Google Scholar]
- 59.Botta F., Dahl-Jensen D., Rahbek C., Svensson A., Nogués-Bravo D., Abrupt change in climate and biotic systems. Curr. Biol. 29, R1045–R1054 (2019). [DOI] [PubMed] [Google Scholar]
- 60.Guthrie R. D., “Mosaics, allochemics and nutrients: an ecological theory of Late Pleistocene megafaunal extinctions” in Quaternary Extinctions: A Prehistoric Revolution, Martin P. S., Klein R. G., Eds. (University of Arizona Press, 1984), pp. 259–298. [Google Scholar]
- 61.Williams J. E., Blois J. L., Range shifts in response to past and future climate change: Can climate velocities and species’ dispersal capabilities explain variation in mammalian range shifts? J. Biogeogr. 45, 2175–2189 (2018). [Google Scholar]
- 62.Graham R. W., Lundelius E. L., “Coevolutionary disequilibrium and Pleistocene extinctions” in Quaternary Extinctions: A Prehistoric Revolution, Martin P. S., Klein R. G., Eds. (University of Arizona Press, 1984), pp. 223–249. [Google Scholar]
- 63.Ehleringer J. R., “The influence of atmospheric CO2, temperature, and water on the abundance of C3/C4 taxa” in A History of Atmospheric CO2 and Its Effects on Plants, Animals, and Ecosystems, Ecological Studies, Baldwin I. T., et al., Eds. (Springer, New York, NY, 2005), pp. 214–231. [Google Scholar]
- 64.Faith J. T., Late Pleistocene climate change, nutrient cycling, and the megafaunal extinctions in North America. Quat. Sci. Rev. 30, 1675–1680 (2011). [Google Scholar]
- 65.Zobel M., et al. , Ancient environmental DNA reveals shifts in dominant mutualisms during the late Quaternary. Nat. Commun. 9, 139 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gill J. L., Ecological impacts of the late Quaternary megaherbivore extinctions. New Phytol. 201, 1163–1169 (2014). [DOI] [PubMed] [Google Scholar]
- 67.Malhi Y., et al. , Megafauna and ecosystem function from the Pleistocene to the Anthropocene. Proc. Natl. Acad. Sci. U.S.A. 113, 838–846 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bakker E. S., et al. , Combining paleo-data and modern exclosure experiments to assess the impact of megafauna extinctions on woody vegetation. Proc. Natl. Acad. Sci. U.S.A. 113, 847–855 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ripple W. J., Van Valkenburgh B., Linking top-down forces to the Pleistocene megafaunal extinctions. Bioscience 60, 516–526 (2010). [Google Scholar]
- 70.Scott E. C., Extinctions, scenarios, and assumptions: Changes in latest Pleistocene large herbivore abundance and distribution in western North America. Quat. Int. 217, 225–239 (2010). [Google Scholar]
- 71.Price G. J., Louys J., Faith J. T., Lorenzen E., Westaway M. C., Big data little help in megafauna mysteries. Nature 558, 23–25 (2018). [DOI] [PubMed] [Google Scholar]
- 72.Martin P. S., “Prehistoric overkill” in Pleistocene Extinctions: The Search for a Cause, Martin P. S., Wright H., Eds. (Yale University Press, 1967), pp. 75–120. [Google Scholar]
- 73.Cooper A., et al. , PALEOECOLOGY. Abrupt warming events drove Late Pleistocene Holarctic megafaunal turnover. Science 349, 602–606 (2015). [DOI] [PubMed] [Google Scholar]
- 74.Lisiecki L. E., Raymo M. E., A Pliocene-Pleistocene stack of 57 globally distributed benthic δ 18O records. Paleoceanography 20, PA1003 (2005). [Google Scholar]
- 75.Railsback L. B., Gibbard P. L., Head M. J., Voarintsoa N. R. G., Toucanne S., An optimized scheme of lettered marine isotope substages for the last 1.0 million years, and the climatostratigraphic nature of isotope stages and substages. Quat. Sci. Rev. 111, 94–106 (2015). [Google Scholar]
- 76.Elderfield H., et al. , Evolution of ocean temperature and ice volume through the mid-Pleistocene climate transition. Science 337, 704–709 (2012). [DOI] [PubMed] [Google Scholar]
- 77.Hughes P. D., Gibbard P. L., Global glacier dynamics during 100 ka Pleistocene glacial cycles. Quat. Res. 90, 222–243 (2018). [Google Scholar]
- 78.Past Interglacials Working Group of PAGES , Interglacials of the last 800,000 years. Rev. Geophys. 54, 162–219 (2016). [Google Scholar]
- 79.Head M. J., Gibbard P. L., Early–Middle Pleistocene transitions: Linking terrestrial and marine realms. Quat. Int. 389, 7–46 (2015). [Google Scholar]
- 80.Clark P. U., et al. , Oceanic forcing of penultimate deglacial and last interglacial sea-level rise. Nature 577, 660–664 (2020). [DOI] [PubMed] [Google Scholar]
- 81.Ganopolski A., Winkelmann R., Schellnhuber H. J., Critical insolation−CO2 relation for diagnosing past and future glacial inception. Nature 529, 200–203 (2016). [DOI] [PubMed] [Google Scholar]
- 82.Herold N., Yin Q. Z., Karami M. P., Berger A., Modelling the climatic diversity of the warm interglacials. Quat. Sci. Rev. 56, 126–141 (2012). [Google Scholar]
- 83.Martrat B., Jimenez-Amat P., Zahn R., Grimalt J. O., Similarities and dissimilarities between the last two deglaciations and interglaciations in the North Atlantic region. Quat. Sci. Rev. 99, 122–134 (2014). [Google Scholar]
- 84.Ruddiman W. F., et al. , Late Holocene climate: Natural or anthropogenic? Rev. Geophys. 54, 93–118 (2016). [Google Scholar]
- 85.Yin Q., Berger A., Interglacial analogues of the Holocene and its natural near future. Quat. Sci. Rev. 120, 28–46 (2015). [Google Scholar]
- 86.Lowe J. J., Walker M., Reconstructing Quaternary Environments (Routledge, ed. 3, 2014). [Google Scholar]
- 87.Williams J. W., et al. , The Neotoma Paleoecology Database, a multiproxy, international, community-curated data resource. Quat. Res. 89, 156–177 (2018). [Google Scholar]
- 88.Bell C., Lundelius E., Barnosky A., “The Blancan, Irvingtonian, and Rancholabrean mammal ages” in Late Cretaceous and Cenozoic Mammals of North America, Woodburne M., Ed. (Columbia University Press, 2004), pp. 232–314. [Google Scholar]
- 89.Graham R. W., et al. , Spatial response of mammals to Late Quaternary environmental fluctuations. Science 272, 1601–1606 (1996). [DOI] [PubMed] [Google Scholar]
- 90.Grimm E. C., Jacobson G. L., “Late Quaternary vegetation history of the eastern United States” in The Quaternary Period in the United States , Gillespie A. R., Porter S. C., Atwater B. F., Eds. (Elsevier, 2004), vol. 1, pp. 381–402. [Google Scholar]
- 91.Williams J. W., et al. , Model systems for a no-analog future: Species associations and climates during the last deglaciation. Ann. N. Y. Acad. Sci. 1297, 29–43 (2013). [DOI] [PubMed] [Google Scholar]
- 92.Davis M. B., Shaw R. G., Range shifts and adaptive responses to Quaternary climate change. Science 292, 673–679 (2001). [DOI] [PubMed] [Google Scholar]
- 93.Martin J. M., Mead J. I., Barboza P. S., Bison body size and climate change. Ecol. Evol. 8, 4564–4574 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Diamond J., “Historic extinctions: A Rosetta Stone for understanding prehistoric extinctions” in Quaternary Extinctions: A Prehistoric Revolution, Martin P. S., Klein R. G., Eds. (University of Arizona Press, 1984), pp. 824–862. [Google Scholar]
- 95.Heintzman P. D., et al. , A new genus of horse from Pleistocene North America. eLife 6, e29944 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Enk J., et al. , Mammuthus population dynamics in Late Pleistocene North America: Divergence, phylogeography, and introgression. Front. Ecol. Evol. 4, 42 (2016). [Google Scholar]
- 97.Froese D., et al. , Fossil and genomic evidence constrains the timing of bison arrival in North America. Proc. Natl. Acad. Sci. U.S.A. 114, 3457–3462 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mead J. I., Taylor L., “Pleistocene (irvingtonian) artiodactyla from porcupine cave” in Biodiversity Response to Climate Change in the Middle Pleistocene: The Porcupine Cave Fauna from Colorado, Barnosky A. D., Ed. (University of California Press, 2004), pp. 280–292. [Google Scholar]
- 99.Willerslev E., et al. , Fifty thousand years of Arctic vegetation and megafaunal diet. Nature 506, 47–51 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All of the data are included in the manuscript and SI Appendix.