Significance
Competition for common resources can make some species groups thrive and others decline. Flowering plants rose to dominance between 125 and 80 Ma, undergoing an explosive radiation that is believed to have impacted long-established plant groups like gymnosperms. Here, we show that the decline of conifers is strongly and directly linked to the increasing diversity of flowering plants. Both the fossil record and molecular data converge in clarifying the effects of abiotic or biotic factors on the speciation and extinction rates of conifers. These results imply that long-term biological interactions through clade competition can play a more important role in the rise and demise of major organism groups than mass extinctions.
Keywords: competition, gymnosperms, macroevolution, paleoenvironment
Abstract
Competition among species and entire clades can impact species diversification and extinction, which can shape macroevolutionary patterns. The fossil record shows successive biotic turnovers such that a dominant group is replaced by another. One striking example involves the decline of gymnosperms and the rapid diversification and ecological dominance of angiosperms in the Cretaceous. It is generally believed that angiosperms outcompeted gymnosperms, but the macroevolutionary processes and alternative drivers explaining this pattern remain elusive. Using extant time trees and vetted fossil occurrences for conifers, we tested the hypotheses that clade competition or climate change led to the decline of conifers at the expense of angiosperms. Here, we find that both fossil and molecular data show high congruence in revealing 1) low diversification rates, punctuated by speciation pulses, during warming events throughout the Phanerozoic and 2) that conifer extinction increased significantly in the Mid-Cretaceous (100 to 110 Ma) and remained high ever since. Their extinction rates are best explained by the rise of angiosperms, rejecting alternative models based on either climate change or time alone. Our results support the hypothesis of an active clade replacement, implying that direct competition with angiosperms increased the extinction of conifers by pushing their remaining species diversity and dominance out of the warm tropics. This study illustrates how entire branches on the Tree of Life may actively compete for ecological dominance under changing climates.
Understanding how competition for resources can regulate the origination and extinction of species and lead to the rise and fall of entire clades represents an important goal of ecology and evolutionary biology (1). This is particularly challenging to study because each clade follows different diversity trajectories through time, which are determined by different speciation and extinction regimes that lead clades to rise, decline, or replace one another (2, 3). Paleontological data show evidence of several biotic replacements, with once-dominant groups disappearing and others rising to take their place (4–7).
Two major patterns of clade replacement may be recognized in the fossil record: a double-wedge pattern, in which one clade declines while the other thrives (e.g., brachiopods and bivalves) (4), and the mass extinction pattern, implying an extinction event that wipes out one group while allowing another to diversify (e.g., nonavian dinosaurs and mammals) (8). When two clades of organisms occupy similar habitats and the long-term diversity of one gradually increases while that of the other declines, we may naturally come to the conclusion that a competitive, or negative, interaction has taken place between the two (4). However, such a double-wedge pattern could conceivably be produced by differential responses to physical change or differential clade interactions (9). Identifying which abiotic and/or biotic factors control diversity changes is a key challenge in macroevolution (7, 10), and macroevolutionary models involving competition as a major driving factor remain disputed (1, 11).
When invoking the role of competition in clade replacements, two main process-based hypotheses can generally explain diversification dynamics of entire clades (1, 4, 9). The “passive replacement hypothesis” states that an incumbent clade prevents a competing clade from radiating by suppressing speciation, until the incumbent clade declines because of extrinsic factors, such as climate change, thus making the niche space vacant (8). In contrast, the “active displacement hypothesis” stipulates that the rise in diversity of a clade drives the decline of another clade by outcompeting it on limited resources and increasing its extinction rate (7). Distinguishing between these two hypotheses is even more challenging for groups that were once ecologically and taxonomically dominant at the global scale but have either gone extinct or drastically declined in abundance, reducing their ecological role to just a fraction of their past diversity (12–15).
In view of the different drivers that may affect the evolutionary history of a given clade, the question of whether physical changes or biotic interactions were responsible for clade replacements is probably best approached by studying individual cases. A notable example of clade replacement (9) stands within the seed plants (Spermatophyta), comprising the gymnosperms and its sister group, the flowering plants (angiosperms). Today, angiosperms represent nearly 90% of all extant plant species and dominate most of Earth’s terrestrial ecosystems. In contrast, gymnosperms account for ∼1% of the total plant diversity and are mostly confined to boreal regions and high-elevation environments, even in the tropics (16). How this major pattern of plant diversity came into existence over geological time is a long-standing puzzle—Darwin’s “abominable mystery” (17)—and remains a topic of intensive research (18–24). Although the timing of the origin of flowering plants is debated (25–31), there is consensus that they radiated in the Early Cretaceous (∼145 Ma) and are the most recently diversifying major clade among the land plants (25–31). In contrast, gymnosperms appeared well before angiosperms in the Devonian (∼380 Ma) and flourished in diversity during the Mesozoic (14, 32–34). The fossil record shows a sudden and rapid increase in diversity and geographic spread of angiosperms since the middle Cretaceous (18, 19, 35–40), which resulted in the ecological dominance, in terms of species richness, of flowering plants observed in most terrestrial ecosystems today (41–46). As a consequence, it is widely assumed that angiosperms underwent such an ecological and evolutionary diversification that they outcompeted and outnumbered other land plants in terms of richness (18, 19, 35–46). Despite decades of scientific debate on the pattern of biotic replacements (16–24, 35–39), the main underlying processes of speciation and extinction of gymnosperms have not been formally quantified.
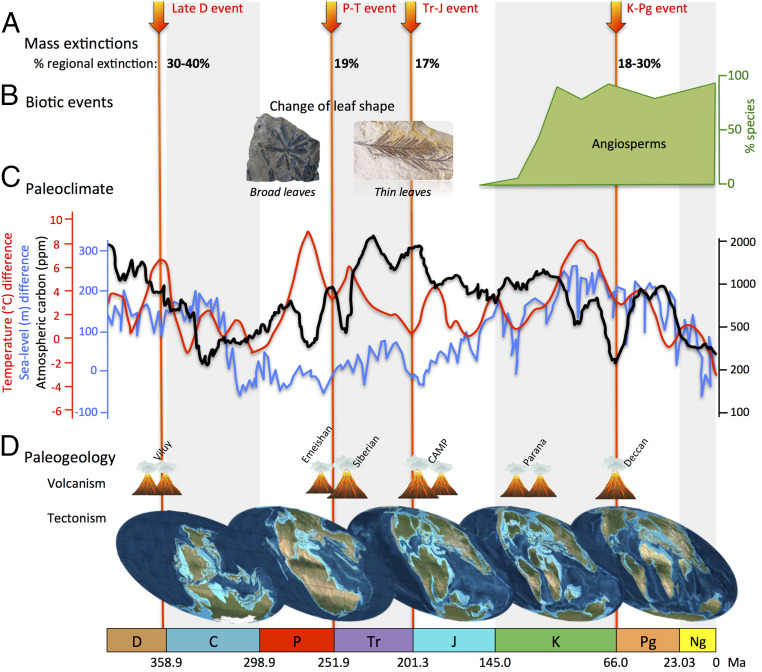
Among gymnosperms, conifers are currently the most diverse group with ∼615 to 630 species worldwide, mainly in boreal and temperate regions and at high elevations of terrestrial ecosystems (47, 48). The fossil record shows that conifers were a dominant component of the global flora during the Mesozoic, with the Triassic and Jurassic periods referred to as their heyday (34, 35, 38, 49). Studying the diversification of conifers may shed light on the competition between angiosperms and gymnosperms and how these interactions compare with alternative drivers including global change of biomes (36, 37, 50, 51), continental rearrangements (39, 52), and changes in key environmental parameters such as global temperatures (53, 54), which are sometimes interlinked (55). Mass extinctions are thought to have had limited impact on terrestrial plants compared with animal clades (35, 56, 57); nevertheless, these events acted as important drivers of plant diversity and turnover (58, 59). Each of these possible drivers, alone or in combination, could explain the wax and wane of conifer diversity through time (Fig. 1).
Fig. 1.
An overview of hypothetical determinants of conifer diversification over time. Conifer evolution was punctuated by (A) four known mass extinction events (red arrows). Biotic events (B), such as the evolution of leaf shape and the appearance of competitor clades (e.g., angiosperms), are likely to be important drivers of diversification. Environmental (abiotic) changes may also impact diversity dynamics, including the paleoclimate (C), with global temperature (red curve highlighting the cooling and warming events), atmospheric carbon (black curve), or sea-level fluctuations (blue curve), as well as the paleogeology (D), such as plate tectonic movements (global paleogeographic changes) or volcanism. CAMP, Central Atlantic magmatic province. C, Carboniferous; D, Devonian; J, Jurassic; K, Cretaceous; Ng, Neogene; P, Permian; Pg, Paleogene; Tr, Triassic.
To assess a widely held hypothesis that the rise of angiosperms drove the decline of gymnosperms (here approximated with conifers), we estimate their diversification processes using an integrative approach that combines molecular phylogenetics and paleontological data. We analyze the available fossil record and comprehensive dated molecular phylogenies of conifers to tease apart the relative impact of angiosperm diversity and climate change (here approximated with global temperature and atmospheric carbon) on the diversification of conifers. Given the general difficulties involved in estimating macroevolutionary rates, we run comparable models of speciation and extinction using maximum likelihood phylogenetic and Bayesian fossil-based frameworks. We incorporate the phylogenetic and dating uncertainties for molecular data and the fossil preservation process, as well as the uncertainties associated with the age of each fossil occurrence. We explicitly integrate the putative effects of abiotic (climate) and biotic (relative diversity of angiosperms) factors as possible drivers of diversification and test their statistical fit. Our results show that angiosperms actively outcompeted gymnosperms during their rise to ecological and evolutionary dominance under global cooling.
Results and Discussion
Macroevolutionary Scenarios for Conifers.
Explaining diversity dynamics has typically relied on two types of models: the equilibrium model in which diversity is bounded and reaches a maximum carrying capacity imposed by ecological limits on diversification (60) and the nonequilibrium model in which diversity is unconstrained and expands toward the present, potentially limiting our ability to adequately characterize these processes through time (61, 62). Phylogenetic niche modeling has suggested that processes involving niche competition and niche partitioning, consistent with both of these diversification models, drive species accumulation in conifers (63). Yet, none of these models can explain the prevalence of ancient and depauperate lineages and the decline of clades (where extinction exceeds speciation), despite their pervasiveness in the fossil record (64). The limitation of these models is that such a process would lead to a third scenario, characterized by a hump-shaped or declining diversity curve through time (2, 3, 6, 7, 65).
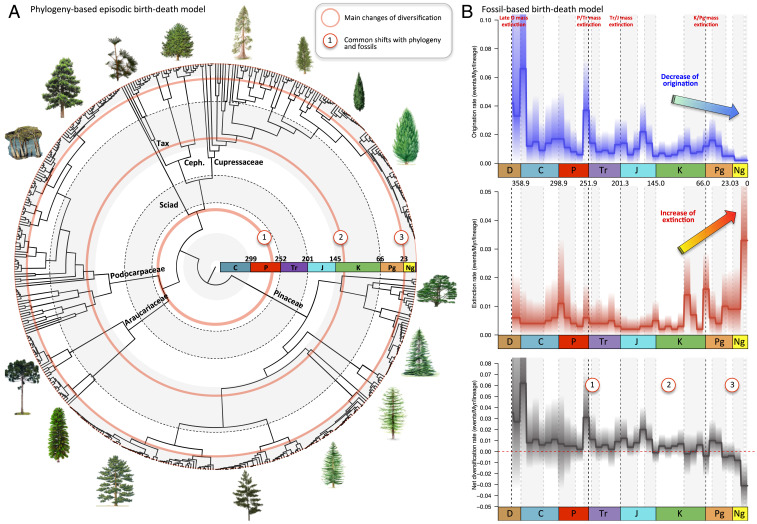
We tested the fit of these macroevolutionary scenarios as an explanation for the diversification of conifers. Using both the fossil record (including or not its sister group, the Cordaitales) and comprehensive dated phylogenies for conifers (33, 34), we estimated the temporal changes in rates of speciation (estimated with phylogeny) and origination (estimates with fossils) and/or extinction to explain the current diversity of conifers using episodic birth–death models (27, 66). Our phylogenetic results clearly reject the hypothesis of constant diversification and show that pulses of speciation and extinction have punctuated the evolution of conifers. We find evidence for significant changes in both speciation and extinction rates in three distinct geological periods (Fig. 2 and SI Appendix, Figs. S2 and S3 and Table S1). The fossil record of conifers shows high rates of origination in their early history, which then generally decreased through time except for peaks of origination at the Permian–Triassic mass extinction and the Middle Jurassic. The extinction rate of conifers, in contrast, increased significantly through time and particularly since the middle Cretaceous (the last 110 My) (SI Appendix, Fig. S4). Overall, net diversification rates (speciation/origination minus extinction) were very low throughout the whole period of conifer evolution and became negative in the Cenozoic, in particular since the Oligocene (34 Ma to the present). Therefore, our results support a macroevolutionary scenario involving a substantial role of extinction. Our analyses also provide evidence that the decline in diversity of conifers is controlled not only by an increase in extinction rate but also by the lack of a speciation rate high enough to maintain the overall diversity of the clade at constant levels (2).
Fig. 2.
Global diversification of conifers inferred from a molecular phylogeny and the fossil record. (A) Time-calibrated phylogeny of conifers and significant shifts in diversification rates (shown by red circles) inferred with an episodic birth–death model (SI Appendix, Table S1). Six shifts of diversification rates were identified: two shifts occurred at the end of the Permian (a single red circle for the two), one occurred during the Cretaceous terrestrial revolution (rise of angiosperms), one occurred at the end of the Oligocene, and two shifts were found in the Pliocene and Pleistocene (a single red circle for the two). (B) Rates of origination (blue), extinction (red), and net diversification rates (black; the difference between origination and extinction) inferred from a fossil-based analysis at the genus level, including Cordaitales, under the Bayesian approach implemented in PyRate (SI Appendix, Fig. S3 shows analyses without Cordaitales). Solid lines indicate mean posterior rates, and the shaded areas show 95% credibility intervals. Taken together, the phylogeny-based (A) and fossil-based (B) diversifications show 1) that diversification of conifers was low and punctuated by periods of extinction, 2) that the increase in extinction initiated in the Middle Cretaceous, and 3) the Cenozoic decline of conifers. The vertical dashed lines indicate the boundaries between geological boundaries and major mass extinction events. C, Carboniferous; Ceph., Cephalotaxaceae; D, Devonian; J, Jurassic; K, Cretaceous; Ng, Neogene; P, Permian; Pg, Paleogene; Sciad., Sciadopityaceae; Tax., Taxaceae; Tr, Triassic.
Mass extinctions could be responsible for the low extant diversity of conifers since they represent the surviving lineages of three known mass extinctions (at 251.9, 201.3, and 66 Ma). However, diversification analyses based both on the fossil record and the molecular phylogeny consistently rejected a strong effect of these events on conifer diversification, finding no peak of extinction during, or close to, general mass extinctions. On the contrary, the analyses unveiled a significant increase of diversification rates (driven by high rates of origination) around the time of the most severe of these three mass extinctions, at the Permian–Triassic boundary. Although previous phylogenetic analyses inferred a significant mass extinction event of conifers at ∼23 Ma (67), this event is not recovered from the fossil record (Fig. 2). Therefore, mass extinction events cannot explain the gradual increase of extinction in the last 100 My inferred here.
Underlying Abiotic and Biotic Drivers of Extinction.
Since a mass extinction event cannot explain the evolutionary decline of conifers, gradual environmental changes or competition with other clades may instead account for it. Conifers diversified against the backdrop of important environmental changes (34, 52, 54, 55). For instance, the global climate fluctuated between icehouse periods such as in the Late Carboniferous, early Permian, and Neogene to greenhouse periods including the late Permian, Cretaceous, and Paleogene (68, 69). The fossil record and phylogenies also show that angiosperms increased significantly in both taxonomic number and functional diversity (18–24, 35). Macroevolutionary studies further indicate that angiosperms experienced elevated positive net diversification rates throughout the Cretaceous and the Paleocene (20, 23, 27) and were rapidly accumulating familial and generic diversity in the Late Cretaceous (25–31, 70).
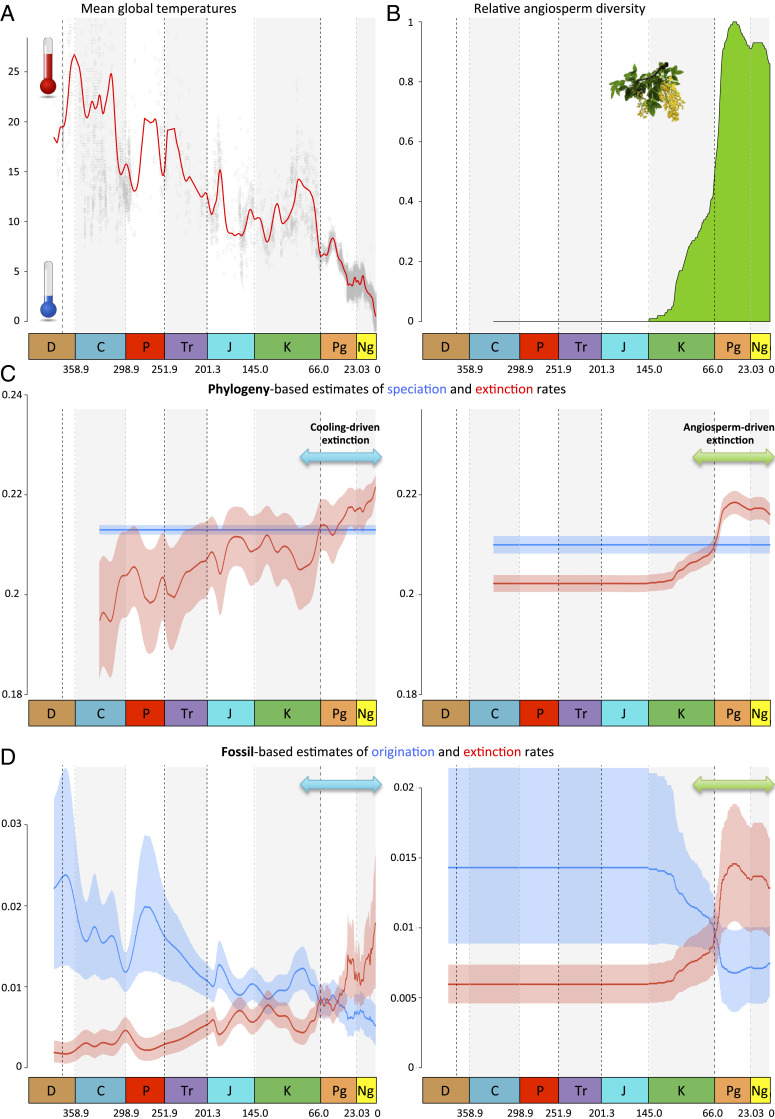
We tested competing hypotheses that could explain the factors underlying the identified macroevolutionary dynamics using a paleoenvironment-dependent diversification model (PDDM) applied to both the conifer phylogeny (71, 72) and the fossil record (6). As a baseline comparison, we first estimated time-dependent variations of speciation (estimated with phylogeny) or origination (estimates with fossils) rates and extinction rates with time-continuous birth–death models (65) (SI Appendix, Table S2). We then assessed the effect of global climate changes on the diversification of conifers, approximated with global variations of atmospheric carbon data (68) and on global oxygen isotopic data (69, 72) (Fig. 3A), hereafter denoted as the PDDM-climate model. We find evidence that global variations of past climates correlate significantly with changes in diversification rates (SI Appendix, Fig. S5 and Tables S3 and S4). Specifically, both fossil and phylogenetic datasets support a scenario where extinction rates correlate negatively with temperature (Fig. 3), indicating that warm climatic periods reduced extinction rates of conifers, while cold climatic periods fostered extinction. In both analyses, however, the most appropriate models indicate that the speciation and origination rates do not significantly correlate with paleoclimates, suggesting that climatic changes do not adequately explain the temporal dynamics of speciation (SI Appendix, Tables S3 and S4 show that phylogenetic models with varying speciation rates were outperformed). The rate-through-time plots (Fig. 3) show that extinction rates exceeded speciation/origination rates from the late Eocene until present, indicating that conifers have been in decline since the global shift to icehouse ∼34 Ma. These results support the hypothesis that gymnosperms suffered major extinctions when the climate changed. Specifically, the Eocene–Oligocene cooling event has been proposed as a major historical event that triggered important macroevolutionary changes worldwide (73), leading to a period of extinction in gymnosperms (55, 59).
Fig. 3.
Drivers of conifer diversification dynamics according to two putative causes of rate variation: global temperature changes (A) and angiosperm diversity (B). Based on oxygen isotopes (69), the mean global temperature curve is a proxy for the main climatic events in the last 350 My. The changes of angiosperm diversity through time show the rise of angiosperms during the Cretaceous as inferred by fossil-based analyses of vascular plants (27). Solid lines indicate mean parameter estimates (C) and mean posterior estimates (D) of the rates, and the shaded areas show CIs and 95% credibility intervals, respectively. Taken together, the phylogeny-based (C) and fossil-based (D) correlations show that 1) changes in angiosperm diversity correlate positively with extinction rates of conifers and 2) the variation of temperatures correlates negatively with extinction rate of conifers. C, Carboniferous; D, Devonian; J, Jurassic; K, Cretaceous; Ng, Neogene; P, Permian; Pg, Paleogene; Tr, Triassic.
An alternative explanation to climate variations as an underlying factor for the decline of conifers is the hypothesis of sequential clade replacement, which predicts that the diversification of a particular lineage is synchronized with the decline of a previously dominant one (1, 4, 7, 9). For plants, the diversification of angiosperms is generally believed to coincide with the decline of once-dominant groups like ferns, liverworts, mosses, and gymnosperms (16, 18, 19, 35–37, 50). We thus fitted a PDDM taking into account past variations of angiosperm diversity through time (27) (Fig. 3B), hereafter denoted the PDDM-angio model. Both the fossil record and phylogeny-based analyses showed that diversification rates correlate strongly with the dynamics of angiosperm diversity (SI Appendix, Fig. S5 and Table S5). In particular, we find that conifer extinction is significantly and positively linked to the rise to dominance of angiosperms, indicating that extinction rates of conifers increased when angiosperm diversity increased (Fig. 3 and SI Appendix, Table S5). Similar to the PDDM-climate model, the PDDM-angio model finds no evidence of a significant correlation between speciation/origination and past angiosperm diversity, suggesting that speciation was unaffected by angiosperms (phylogenetic models with varying speciation rates were outperformed) (SI Appendix, Table S5).
Our analyses of the fossil record and the molecular phylogeny allowed for the teasing apart of the role of global temperature change and atmospheric carbon through time and the effect of the angiosperm radiation on the diversification of conifers. We performed a model comparison between the strongest models of each diversification series (constant rate, time-dependent, PDDM-climate, and PDDM-angio models) to select the most appropriate model. Model comparisons made with both phylogenetic-based analyses (Table 1) and fossil-based analyses (Table 2) indicate that the angiosperm-dependent (PDDM-angio) model best fits the conifer phylogeny and fossil record. These results remain robust regardless of the dated phylogenies used (33, 34); the strongest model is an angiosperm-driven extinction model (SI Appendix, Tables S6 and S7). Our results thus show that the extant conifer diversity pattern is most probably the outcome of ancient extinction events linked to the rise of angiosperms in the Cretaceous, with sustained extinction through the Cenozoic.
Table 1.
Best model for conifer diversification with the phylogeny-based diversification models
| Models | NP | logL | AICc | ΔAICc | ωAICc | λ | α | μ | β |
| BcstDcst | 2 | −1,778.72 ± 1.104 | 3,561.47 ± 2.208 | 2.46 | 0.164 | 0.2090 ± 0.0007 | — | 0.2027 ± 0.0007 | — |
| Bcst DTempVar | 3 | −1,777.54 ± 1.127 | 3,561.12 ± 2.253 | 2.11 | 0.195 | 0.2173 ± 0.0009 | — | 0.2222 ± 0.0012 | −0.0029 ± 8.19E-05 |
| Bcst DCarbonVar | 3 | −1,778.43 ± 1.114 | 3,562.91 ± 2.228 | 3.90 | 0.080 | 0.2131 ± 0.0008 | — | 0.2152 ± 0.0011 | −0.0001 ± 2.30E-06 |
| Bcst DAngioVar | 3 | −1,776.48 ± 1.123 | 3,559.01 ± 2.246 | 0 | 0.561 | 0.2161 ± 0.0007 | — | 0.2023 ± 0.0007 | 0.0772 ± 0.0012 |
Four models are compared, and each was previously selected in a reciprocal series of time-dependent models (a constant birth–death model [BcstDcst] was the best fit), of temperature-dependent models (a model with varying extinction [BcstDTempVar] was the best fit), or of atmospheric carbon-dependent models (a model with varying extinction [BcstDCarbonVar] was the best fit) and of angiosperm-dependent models (a model with varying extinction [BcstDAngioVar] was the best fit). Values are the means and SEs calculated from the fit of a random sample of 100 dated trees. The corrected Akaike information criterion (AICc), difference between AICc of two models (ΔAICc) and Akaike weight (ωAICc) allow comparison of the models to select the best-fit model. The bold line indicates the best-fitting model, which is the model with the extinction rate positively correlated with the proportion of angiosperms, suggesting that extinction increased when angiosperms diversified, whereas extinction decreased when angiosperms were absent (no effect on speciation). logL, log likelihood; NP, number of free parameters; α, rate of variation of the speciation according to the paleoenvironmental variable; β, rate of variation of the extinction according to the paleoenvironmental variable; λ, speciation rate; μ, extinction rate.
Table 2.
Best model for conifer diversification with the fossil-based diversification models
| Models | Marginal likelihood | Bayes factors | Rel. prob. | λ | α | μ | β |
| Constant BD | −1,004.62 | 24.06 | ∼0 | 0.0101 [0.008–0.0121] | — | 0.0069 [0.005–0.009] | — |
| Carbon-dep. BD (exp.) | −1,002.39 | 9.8 | ∼0 | 0.009 [0.0070–0.0120] | 0.0001 [−0.0002–0.0005] | 0.009 [0.0060–0.011] | −0.0005 [−0.001–0] |
| Carbon-dep. BD (lin.) | −1,002.69 | 10.1 | ∼0 | 0.009 [0.0070–0.0119] | 0.0002 [−0.0002–0.0006] | 0.009 [0.0063–0.0114] | −0.0005 [−0.0007 to −0.0001] |
| Temp.-dep. BD (exp.) | −999.14 | 13.1 | 0.0012 | 0.005 [0.0028–0.0077] | 0.033 [0.013–0.055] | 0.017 [0.0102–0.0266] | −0.053 [−0.079 to −0.027] |
| Temp.-dep. BD (linear) | −998.49 | 11.8 | 0.0023 | 0.006 [0.0035–0.0083] | 0.041 [0.009–0.0789] | 0.012 [0.0082–0.0157] | −0.022 [−0.027 to −0.014] |
| Angio-dep. BD (exp.) | −994.41 | 3.64 | 0.139 | 0.007 [0.005–0.0102] | −0.541 [−1.077 to −0.026] | 0.012 [0.009–0.016] | 1.487 [0.931–2.077] |
| Angio-dep. BD (linear) | −992.59 | 0 | 0.857 | 0.007 [0.005–0.0101] | −0.705 [−1.553–0.012] | 0.013 [0.009–0.016] | 0.901 [0.716–1.046] |
Six models are compared with the constant birth–death (BD) model: two carbon-dependent (Carbon-dep.) diversification models, two temperature-dependent (Temp.-dep.) diversification models, and two angiosperm-dependent (Angio.-dep.) diversification models. Rates could vary exponentially (exp.) or linearly (linear) with the variables. Values are the mean of the parameter, and the 95% credibility interval is denoted in brackets. The marginal likelihoods, Bayes factors, and relative probabilities (Rel. prob.) allow comparison of all models and selection of the best-fit model. The bold line indicates the best-fitting model, which is the model with the extinction rate positively correlated with the proportion of angiosperms, suggesting that extinction increased when angiosperms diversified, whereas extinction decreased when angiosperms were absent (no significant effect on speciation as the 95% credibility interval overlaps with zero). α, rate of variation of the speciation according to the paleoenvironmental variable; β, rate of variation of the extinction according to the paleoenvironmental variable; λ, baseline origination rate; μ, baseline extinction rate.
Rise to Dominance of Angiosperms and Decline of Conifers.
We found fossil and molecular evidence supporting the competition hypothesis that the rise of angiosperms led to higher conifer extinction, not only in the Cretaceous but also through the Cenozoic. These results are striking as we found remarkably consistent signals from the fossil and phylogenetic data, both providing strong support for the angiosperm-driven extinction model. Our results thus suggest that the Cretaceous rise of angiosperms, extended through the Cenozoic, indeed had a large effect on conifer diversification. Instead of a mass extinction model, this study provides support for the active displacement hypothesis, whereby the rise in diversity of one clade drives the decline of another by outcompeting it on limited resources (1, 9).
Two scenarios could lead to active displacement: 1) conifers had less opportunity to diversify as angiosperms increased in numbers (i.e., decreased origination rate), or 2) the decline of conifers was directly associated with the rise of angiosperms through increased extinction. Our data compilation and methodology allowed us to tease these scenarios apart. Both the fossil-based and the phylogeny-based analyses provide evidence that the rise of angiosperms had a significant negative effect on conifer diversity through millions of years from the Late Cretaceous onward, by directly acting on the extinction (and not on the speciation) of conifers. This result further supports the hypothesis of active displacement of conifers due to angiosperm-driven extinction.
In the Cenozoic, angiosperms came to dominate terrestrial ecosystems globally, in terms of diversification dynamics (23, 24, 27), geographic occupation with the exception of boreal regions (36, 38, 70), and ecological or physiological innovations (37, 44, 45). Previous studies have suggested that angiosperms prevailed ecologically over gymnosperms due to biological and physiological advantages such as a rapid growth strategy, animal pollination, new systems of chemical defense, and tolerance to climatic stress (16, 22, 41–43, 46). These advantages probably gave angiosperms a competitive edge over conifers from the Late Cretaceous onward, eventually leading to their decline.
Both the fossil-based and phylogeny-based analyses further indicate that the extinction rates in the Late Cretaceous remained high and even increased toward the present, driving conifers to a stronger diversity decline. This continuing pattern of extinction is inferred with the episodic birth–death models (Fig. 2), as well as with the PDDM applied to the fossil record and the molecular phylogeny (Fig. 3). The ongoing conifer decline is in line with recent evidence that there is a widespread increase in dominance of Fagaceae at the expense of Pinaceae across northern temperate forests (74), despite the large functional differences between these families. Extant conifers are largely restricted to areas where growth of angiosperm competitors is reduced, such as high latitudes and elevations (cool environments) or nutrient-poor soils (43). Yet, contemporary forest dynamics where species of Pinaceae have been aggressive competitors capable of dominating entire regions, could lead to the exclusion of other conifers (46, 74).
Macroevolutionary evidence for the active displacement hypothesis has been scarce and, to our knowledge, it has never previously been shown to occur in plants. For instance, a recent study on ferns did not find evidence that fern diversification was affected by angiosperm diversity (75). Our study demonstrates that clade displacement previously reported for animals (5–7) can also apply to plants.
Limitations of the Data and Methodology.
Our estimates of diversification processes could be biased to some extent because of 1) the difficulty to estimate extinction rates from phylogenies and 2) the quality of the conifer fossil record (which is abundant in relation to many other plant groups but less so in relation to vertebrates and marine invertebrates).
Given the constraints in data availability at high resolution through the periods examined, we could not address here the precise ecological mechanisms underlying the competition between angiosperms and gymnosperms. Future paleoecological studies at finer spatial or temporal scales might allow testing whether the ecological niches of conifers and angiosperms overlapped during the ecological radiation of angiosperms in the Cretaceous, as they replaced conifers through time.
Our analyses support the angiosperm-driven diversification model, but they do not rule out the hypothesis that extinction also increased due to the global cooling at the end of the Paleogene (59). Both fossil and phylogenetic data show evidence for an increase of extinction through time that is positively correlated with the global cooling (Fig. 3), although this model has less support (SI Appendix, Tables S5 and S6). However, a combination of factors could have affected the diversification of conifers, with abiotic and biotic factors being intertwined (7). Floristic changes induced by the diversification of angiosperms, combined with climatic changes in the Cenozoic, might have jointly driven the decline in diversity of conifers. Our methodology only allowed the analysis of one factor at a time and a comparison among tests to select the most likely explanatory variable.
There could have been other untested variables. For instance, net diversification rates were not inferred to be very high, while evolutionary turnover was very elevated throughout the diversification of conifers (SI Appendix, Table S1). A low net diversification rate for such a long evolutionary history might be associated with the fact that conifers have long generation times, large genomes, and low rates of molecular evolution (76, 77). The low diversification rates of conifers likely contributed to the extreme differences between conifer and angiosperm species richness. In addition, the Cretaceous/Paleogene (K/Pg) event caused the final extinction of nonavian dinosaurs, which in turn, could have increased the extinction of conifers with which ecological interactions certainly existed: for instance, concerning the dispersal of conifer seeds (49, 78). Although the K/Pg event had a potential role in leading to the final extinction of specific clades, as exemplified by fossil data for the genus Podozamites, a recent study hypothesized that the rise of angiosperms is the most likely cause of their demise (79).
Our data and analyses focused on three candidates reflecting widespread environmental changes as likely factors having influenced the diversification of conifers. Additional factors could be at play, such as changes in land area, fragmentation of land masses, or changes in Earth’s biogeochemical cycles. Our choices depended on the availability of environmental and biological data spanning the appropriate time frame. Given the general difficulties around the estimation of birth–death models, we attempted to identify and test clear hypotheses under simplifying assumptions. These data showed that the extinction rate of conifers cannot be (solely) attributed to a sudden mass extinction event, nor the loss of dispersal agents and other consequences it had, but rather to a long-term driver affecting the probability of extinction.
Conclusions
Clade competition is a likely driver of diversification through geological time but remains difficult to demonstrate (4, 9). Previous studies on competition at the macroevolutionary scale have focused on the role of speciation to understand the factors responsible for early bursts of speciation followed by slowdowns, such as during cases of adaptive radiation (11). Meanwhile, fewer attempts have been made to understand the role of extinction, probably because of the perceived difficulties in estimating extinction rates. Yet, extinction is a major feature of biological evolution since the vast majority of species that ever lived are now extinct (64). The impact of past episodes of extinction in punctuating the evolutionary history of clades is often overlooked, which may lead to spurious conclusions on the actual macroevolutionary processes underlying current biodiversity patterns (65, 67, 71). These biases are particularly expected for ancient clades, which have survived and/or adapted to several events through their long history.
Our study of an ancient and relatively species-poor plant group deepens our understanding of how global diversity is regulated through time and in relation to multiple external factors. By integrating ecological models of macroevolution, phylogenetic, and fossil data, our study provides strong support for a widely held hypothesis of clade competition through deep time. Such a methodological framework could shed light on the diversification of many other branches on the Tree of Life. Here, the data compiled strongly indicate that angiosperms actively outcompeted gymnosperms during their rise to ecological and evolutionary dominance, under a period of global cooling. We found significant evidence that the extinction rate of conifers is linked to the rise of angiosperms and that Cenozoic climate cooling additionally contributed to driving extinction.
More than a third of all extant conifer species are threatened with extinction (48). Studying the impact of long-term environmental changes and competition by angiosperms on current conifer diversity may benefit from insights into what factors have influenced their past diversity. It becomes clear that interactions among organisms and environmental changes have strong and synergistic effects on the diversification of life. At least for conifers, but possibly for other major clades, long-term biotic and abiotic dynamics can play an even more important role in the rise and demise of species than mass extinction events.
Materials and Methods
Time-Calibrated Phylogeny and Fossil Occurrences.
We used two time-calibrated conifer phylogenies including 1) 489 of 615 species (∼80% of the living species diversity) (33) and 2) 578 of 615 species (∼94% of the living species diversity) (34). We studied the fossil record of conifers using a subset of the plant dataset compiled by Silvestro et al. (27) and updated with new occurrences available in the Paleobiology Database via FossilWorks. We checked for fossil occurrences using the name “Pinophyta,” and we specified the taxonomic criteria to look for family and genus names. We did not conduct analyses at the species level because this would greatly reduce the number of reliably identified taxa and the power of the analyses. We generated the conifer fossil dataset including the sister extinct lineages Cordaitales comprising 7,927 fossil occurrences representing 100 genera (31 extant and 69 extinct). For comparison, the conifer dataset of Silvestro et al. (27) contained 6,470 occurrences representing 55 genera (12 extant and 43 extinct). We also analyzed a dataset excluding Cordaitales, which resulted in 6,764 occurrences for 94 genera (31 extant and 63 extinct).
Estimation of Origination/Speciation and Extinction Rates.
We performed diversification analyses on the conifer time tree using TreePar (66), which was used to infer speciation and extinction rates through time. This method relaxes the assumption of constant diversification rates by allowing rates to change at specific points in time. Such a model is therefore suited for the detection of rapid changes in speciation and extinction rates, which may be expected in response to abiotic events such as the K/Pg mass extinction event or biotic factors such as floristic turnovers. We employed the “bd.shifts.optim” function to estimate discrete changes in speciation and extinction rates and mass extinction events in incompletely sampled phylogenies (66). TreePar estimates the maximum likelihood speciation and extinction rates together with the shift times in a phylogeny. We estimated possible shifts of diversification every 0.1 My while allowing a maximum of eight shifts and the diversification rate to be negative (i.e., periods of declining diversity). As the taxon sampling of the phylogeny was not complete, we used the analytical correction by setting the sampling fraction corresponding to the ratio of sampled species out of the total species diversity. We fitted nine different diversification scenarios using maximum likelihood to the two conifer time trees (33, 34) and computed the corrected Akaike information criterion corresponding to each scenario. We evaluated support for the selected model against all models and computed Akaike weights.
We analyzed the fossil record using a Bayesian model to simultaneously infer the temporal dynamics of origination and extinction rates, as well as preservation (80). This approach, implemented in PyRate (80), uses all fossil occurrences that can be assigned to a taxon, in this case genera, to jointly model the preservation and diversification processes. The preservation process infers the individual origination and extinction times of each taxon based on all fossil occurrences and on an estimated preservation rate (expressed as expected occurrences per taxon per million years). We followed the approach developed by Silvestro et al. (27), which includes several modifications appropriate for inferring the variation in origination and extinction at global scale and large temporal ranges. We used a homogeneous Poisson process of preservation and accounted for varying preservation rates across taxa with gamma-distributed rate heterogeneity (80). To accommodate the variability in preservation rates across taxa, we used eight rate categories for the gamma distribution. We also dissected the birth–death process into time intervals, defined by the geological epochs of the stratigraphic timescale, and estimated origination and extinction rates within these intervals. The estimation of origination and extinction rates within time intervals improved the mixing of the Markov chain Monte Carlo (MCMC) and allowed for inferring general trends of rate variation throughout long timescales (27).
Origination and extinction rates are measured as the expected number of origination and extinction events per lineage per million years. One potential problem in fixing a priori the number of rate shifts is overparameterization. We overcame this issue by assuming that the rates of origination and extinction are part of two families of parameters following a common prior distribution, with parameters estimated from the data using hyperpriors (27, 80). We ran PyRate for 10 million MCMC generations on each of the 10 randomly replicated datasets. We monitored chain mixing and effective sample sizes by examining the log files. After excluding the first 20% of the samples as burn-in period, we combined the posterior estimates of the origination and extinction rates across all replicates to generate rates-through-time plots. We examined the marginal posterior distributions of origination and extinction rates through the mass extinction events documented in geological history, the major climate change, and the major environmental changes like the Cretaceous rise of angiosperms.
PDDM.
To identify causal mechanisms of conifer diversification, we examined the link between past environmental variables and speciation/extinction rates. There are several possible global or regional phenomena that occurred during the conifer evolution. We focused on the role of one abiotic factor and one biotic factor, which could be linked to changes in biodiversity. One of the most important abiotic effects on biodiversity over time is climate change (72), of which the global fluctuations in temperatures (69) and in atmospheric carbon (68) are the main components. We focused on the impact of these climatic variables. In addition, ecological interaction with rapidly expanding clades is recognized as an important macroevolutionary biotic driver (5–7). Conifers experienced a drastic floristic change in the Mid-Cretaceous associated in time with the origin and rapid radiation of angiosperms (18–30). The rise and dominance of angiosperms could have directly contributed to higher competition with conifers or indirectly by altering plant ecosystems, which in turn, affected the ability of conifers to diversify and their probability of extinction. We calculated the range through diversity trajectory for angiosperms based on the estimates from Silvestro et al. (27) to obtain the temporal variations of angiosperms.
We used a birth–death method to quantify the potential effect of environmental variables on diversification rates (71, 72). This PDDM builds on time-dependent diversification models (65) and allows speciation and extinction rates to depend not only on time but also, on an external variable (which may vary through time). This approach assumes that clades evolve under a birth–death process, in which speciation (λ) and extinction (μ) rates can vary through time, and both can be influenced by one or several environmental variables. Although the measurements describing environmental variables are often discrete, the approach uses a smoothing function to allow the modeling of diversification rates in continuous time. The approach can be used to derive likelihoods for λ and μ with an exponential function of temperature T(t), such that , or a linear function, such that . λ0 and α are the two parameters to estimate. The estimation of a positive α would indicate that higher temperatures increase speciation rates, whereas a negative α would indicate that higher temperatures decrease speciation rates. The same rationale applies to the extinction but with the parameter β quantifying the correlation between changes in extinction rates and temperature variations. We fitted and compared diversification models with constant rates, time (SI Appendix, Table S2), temperature (SI Appendix, Table S3), and angiosperm (SI Appendix, Table S4) dependence.
A similar birth–death model has been incorporated in PyRate to test for a correlation between speciation and extinction rates and changes in environmental variables using fossil data (6). In PyRate, speciation and extinction rates for a given time frame t are calculated based on the aforementioned equations, while the correlation parameters α and β are equivalent to those in the phylogenetic analogous method (6). We relied on the posterior estimates of the episodic birth–death of the conifer fossil record and calculated the times of speciation and of extinction as the mean of the posterior samples from each replicate. Thus, we obtained 10 posterior estimates of the times of speciation and extinction for all genera and used them as input data in all subsequent analyses. These analyses focused therefore exclusively on the estimation of birth–death parameters (i.e., without remodeling preservation or reestimating times of speciation and extinction). This procedure reduced drastically the computational burden while allowing us to account for the preservation process and the uncertainties associated with the fossil ages.
We used the estimated times of speciation and extinction of all taxa to test whether speciation and extinction dynamics correlate with abiotic factors using the global temperature or instead, correlate with biotic factors via competition and/or positive interaction between species through the rise of angiosperms. We ran the PDDM model using default gamma priors on the baseline speciation and extinction rates and normal priors on the correlation parameters α and β for speciation and extinction, respectively (6). We ran 10 million MCMC iterations with sampling frequency of 1,000 and combined the posterior samples of the parameters after excluding the first 20% of the samples as burn-in. We monitored chain mixing and effective sample sizes by examining the log files. Posterior samples of the parameters were summarized over all replicates as mean values and 95% credibility interval. We considered the correlation to be statistically significant when zero was not included within the 95% credibility interval of α and β.
Supplementary Material
Acknowledgments
We thank three anonymous reviewers who provided insightful comments to improve the study. We are grateful to Andrew Leslie and Michael Donoghue for discussions. We thank John Alroy, Allister Rees, Bruce Tiffney, Hallie Sims, and Carlos Jaramillo for their contributions of paleobotanical data to the Paleobiology Database. This study benefited from Marie Curie International Outgoing Fellowship 627684 (to F.L.C.), Carl Tryggers Stiftelse Grant 12:24 (to F.L.C.), Swiss National Science Foundation PCEFP3_187012, FN-1749 (to D.S.), Swedish Research Council Grants 2019-04739 (to D.S.) and 2019-05191 (to A.A.), the Swedish Foundation for Strategic Research (A.A.), the Biodiversity and Ecosystems in a Changing Climate Programme (A.A.), the Knut and Alice Wallenberg Foundation (A.A.), and the Royal Botanic Gardens, Kew (A.A.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2005571117/-/DCSupplemental.
Data Availability.
Phylogenetic trees and fossil occurrence data have been made freely available in a publicly accessible database (https://doi.org/10.6084/m9.figshare.12037593.v1).
References
- 1.Benton M. J., Progress and competition in macroevolution. Biol. Rev. Camb. Philos. Soc. 62, 305–338 (1987). [Google Scholar]
- 2.Quental T. B., Marshall C. R., How the Red Queen drives terrestrial mammals to extinction. Science 341, 290–292 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Žliobaitė I., Fortelius M., Stenseth N. C., Reconciling taxon senescence with the Red Queen’s hypothesis. Nature 552, 92–95 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Sepkoski J. J., Jr, “Competition in macroevolution: The double wedge revisited” in Evolutionary Paleobiology, Jablonski D., Erwin D. H., Lipps J. H., Eds. (University of Chicago Press, Chicago, IL, 1996), pp. 211–255. [Google Scholar]
- 5.Liow L. H., Reitan T., Harnik P. G., Ecological interactions on macroevolutionary time scales: Clams and brachiopods are more than ships that pass in the night. Ecol. Lett. 18, 1030–1039 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Silvestro D., Antonelli A., Salamin N., Quental T. B., The role of clade competition in the diversification of North American canids. Proc. Natl. Acad. Sci. U.S.A. 112, 8684–8689 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Condamine F. L., Romieu J., Guinot G., Climate cooling and clade competition likely drove the decline of lamniform sharks. Proc. Natl. Acad. Sci. U.S.A. 116, 20584–20590 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Archibald J. D., Extinction and Radiation: How the Fall of Dinosaurs Led to the Rise of Mammals (JHU Press, Baltimore, MD, 2011). [Google Scholar]
- 9.Benton M. J., “Extinction, biotic replacements, and clade interactions” in The Unity of Evolutionary Biology: Proceedings of the Fourth International Congress of Systematic and Evolutionary Biology, Dudley E. C., Ed. (Dioscorides Press, Portland, OR, 1991), pp. 89–102. [Google Scholar]
- 10.Benton M. J., The Red Queen and the Court Jester: Species diversity and the role of biotic and abiotic factors through time. Science 323, 728–732 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Aristide L., Morlon H., Understanding the effect of competition during evolutionary radiations: An integrated model of phenotypic and species diversification. Ecol. Lett. 22, 2006–2017 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Schneider H., et al. , Ferns diversified in the shadow of angiosperms. Nature 428, 553–557 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Schuettpelz E., Pryer K. M., Evidence for a Cenozoic radiation of ferns in an angiosperm-dominated canopy. Proc. Natl. Acad. Sci. U.S.A. 106, 11200–11205 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagalingum N. S., et al. , Recent synchronous radiation of a living fossil. Science 334, 796–799 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Laenen B., et al. , Extant diversity of bryophytes emerged from successive post-Mesozoic diversification bursts. Nat. Commun. 5, 5134 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Crepet W. L., Niklas K. J., Darwin’s second ‘abominable mystery’: Why are there so many angiosperm species? Am. J. Bot. 96, 366–381 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Friedman W. E., The meaning of Darwin’s ‘abominable mystery’. Am. J. Bot. 96, 5–21 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Lidgard S., Crane P. R., Quantitative analyses of the early angiosperm radiation. Nature 331, 344–346 (1988). [Google Scholar]
- 19.Lupia R., Lidgard S., Crane P. R., “Angiosperm diversification and mid-Cretaceous environmental change” in Biotic Response to Global Change: The Last 145 Million Years, Culver S. J., Rawson P. F., Eds. (Cambridge University Press, Cambridge, United Kingdom, 2000), pp. 207–222. [Google Scholar]
- 20.Davies T. J., et al. , Darwin’s abominable mystery: Insights from a supertree of the angiosperms. Proc. Natl. Acad. Sci. U.S.A. 101, 1904–1909 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vamosi J. C., Vamosi S. M., Key innovations within a geographical context in flowering plants: Towards resolving Darwin’s abominable mystery. Ecol. Lett. 13, 1270–1279 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Augusto L., Davies T. J., Delzon S., De Schrijver A., The enigma of the rise of angiosperms: Can we untie the knot? Ecol. Lett. 17, 1326–1338 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Magallón S., Sánchez-Reyes L. L., Gómez-Acevedo S. L., Thirty clues to the exceptional diversification of flowering plants. Ann. Bot. 123, 491–503 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onstein R. E., Darwin’s second ‘abominable mystery’: Trait flexibility as the innovation leading to angiosperm diversity. New Phytol., 10.1111/nph.16294 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Bell C. D., Soltis D. E., Soltis P. S., The age and diversification of the angiosperms re-revisited. Am. J. Bot. 97, 1296–1303 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Magallón S., Gómez-Acevedo S., Sánchez-Reyes L. L., Hernández-Hernández T., A metacalibrated time-tree documents the early rise of flowering plant phylogenetic diversity. New Phytol. 207, 437–453 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Silvestro D., Cascales-Miñana B., Bacon C. D., Antonelli A., Revisiting the origin and diversification of vascular plants through a comprehensive Bayesian analysis of the fossil record. New Phytol. 207, 425–436 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foster C. S. P., et al. , Evaluating the impact of genomic data and priors on Bayesian estimates of the angiosperm evolutionary timescale. Syst. Biol. 66, 338–351 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Barba-Montoya J., Dos Reis M., Schneider H., Donoghue P. C. J., Yang Z., Constraining uncertainty in the timescale of angiosperm evolution and the veracity of a Cretaceous Terrestrial Revolution. New Phytol. 218, 819–834 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H. T., et al. , Origin of angiosperms and the puzzle of the Jurassic gap. Nat. Plants 5, 461–470 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Coiro M., Doyle J. A., Hilton J., How deep is the conflict between molecular and fossil evidence on the age of angiosperms? New Phytol. 223, 83–99 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Burleigh J. G., Barbazuk W. B., Davis J. M., Morse A. M., Soltis P. S., Exploring diversification and genome size evolution in extant gymnosperms through phylogenetic synthesis. J. Bot. 2012, 292857 (2012). [Google Scholar]
- 33.Leslie A. B., et al. , Hemisphere-scale differences in conifer evolutionary dynamics. Proc. Natl. Acad. Sci. U.S.A. 109, 16217–16221 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leslie A. B., et al. , An overview of extant conifer evolution from the perspective of the fossil record. Am. J. Bot. 105, 1531–1544 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Niklas K. J., Tiffney B. H., Knoll A. H., Patterns in vascular land plant diversification. Nature 303, 614–616 (1983). [Google Scholar]
- 36.Coiffard C., Gomez B., Daviero-Gomez V., Dilcher D. L., Rise to dominance of angiosperm pioneers in European Cretaceous environments. Proc. Natl. Acad. Sci. U.S.A. 109, 20955–20959 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Boer H. J., Eppinga M. B., Wassen M. J., Dekker S. C., A critical transition in leaf evolution facilitated the Cretaceous angiosperm revolution. Nat. Commun. 3, 1221 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peralta-Medina E., Falcon-Lang H. J., Cretaceous forest composition and productivity inferred from a global fossil wood database. Geology 40, 219–222 (2012). [Google Scholar]
- 39.Chaboureau A. C., Sepulchre P., Donnadieu Y., Franc A., Tectonic-driven climate change and the diversification of angiosperms. Proc. Natl. Acad. Sci. U.S.A. 111, 14066–14070 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jud N. A., Michael D. D., Williams S. A., Mathews J. C., Tremaine K. M., Bhattacharya J., A new fossil assemblage shows that large angiosperm trees grew in North America by the Turonian (Late Cretaceous). Sci. Adv. 4, eaar8568 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wing S. L., Boucher L. D., Ecological aspects of the Cretaceous flowering plant radiation. Annu. Rev. Earth Planet. Sci. 26, 379–421 (1998). [Google Scholar]
- 42.Regal P. J., Ecology and evolution of flowering plant dominance. Science 196, 622–629 (1977). [DOI] [PubMed] [Google Scholar]
- 43.Bond W. J., The tortoise and the hare: Ecology of angiosperm dominance and gymnosperm persistence. Biol. J. Linn. Soc. Lond. 36, 227–249 (1989). [Google Scholar]
- 44.Lusk C. H., Wright I., Reich P. B., Photosynthetic differences contribute to competitive advantage of evergreen angiosperm trees over evergreen conifers in productive habitats. New Phytol. 160, 329–336 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Friis E. M., Pedersen K. R., Crane P. R., Cretaceous angiosperm flowers: Innovation and evolution in plant reproduction. Palaeogeogr. Palaeoclimatol. Palaeoecol. 232, 251–293 (2006). [Google Scholar]
- 46.Brodribb T. J., Pittermann J., Coomes D. A., Elegance versus speed: Examining the competition between conifer and angiosperm trees. Int. J. Plant Sci. 173, 673–694 (2012). [Google Scholar]
- 47.Farjon A., Filer D., An Atlas of the World’s Conifers. An Analysis of Their Distribution, Biogeography, Diversity and Conservation Status (Brill, Leiden, the Netherlands, 2013). [Google Scholar]
- 48.Fragnière Y., Bétrisey S., Cardinaux L., Stoffel M., Kozlowski G., Fighting their last stand? A global analysis of the distribution and conservation status of gymnosperms. J. Biogeogr. 42, 809–820 (2015). [Google Scholar]
- 49.Farjon A., A Natural History of Conifers (Timber Press, Portland, OR, 2008). [Google Scholar]
- 50.Lupia R., Lidgard S., Crane P. R., Comparing palynological abundance and diversity: Implications for biotic replacement during the Cretaceous angiosperm radiation. Paleobiology 25, 305–340 (1999). [Google Scholar]
- 51.van de Schootbrugge B., et al. , Floral changes across the Triassic/Jurassic boundary linked to flood basalt volcanism. Nat. Geosci. 2, 589–594 (2009). [Google Scholar]
- 52.Mao K., et al. , Distribution of living Cupressaceae reflects the breakup of Pangea. Proc. Natl. Acad. Sci. U.S.A. 109, 7793–7798 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morley R. J., “Cretaceous and tertiary climate change and the past distribution of megathermal rainforests” in Tropical Rainforest Responses to Climatic Change, Bush M. B., Flenley J. R., Gosling W. D., Eds. (Springer, Berlin, Germany, 2011), 1–34. [Google Scholar]
- 54.Willis K. J., McElwain J. C., The Evolution of Plants (Oxford University Press, Oxford, United Kingdom, ed. 2, 2014). [Google Scholar]
- 55.Pittermann J., Stuart S. A., Dawson T. E., Moreau A., Cenozoic climate change shaped the evolutionary ecophysiology of the Cupressaceae conifers. Proc. Natl. Acad. Sci. U.S.A. 109, 9647–9652 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cascales-Miñana B., Cleal C. J., The plant fossil record reflects just two great extinction events. Terra Nova 26, 195–200 (2014). [Google Scholar]
- 57.Nowak H., Schneebeli-Hermann E., Kustatscher E., No mass extinction for land plants at the Permian-Triassic transition. Nat. Commun. 10, 384 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McElwain J. C., Punyasena S. W., Mass extinction events and the plant fossil record. Trends Ecol. Evol. 22, 548–557 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Crisp M. D., Cook L. G., Cenozoic extinctions account for the low diversity of extant gymnosperms compared with angiosperms. New Phytol. 192, 997–1009 (2011). [DOI] [PubMed] [Google Scholar]
- 60.Rabosky D. L., Hurlbert A. H., Species richness at continental scales is dominated by ecological limits. Am. Nat. 185, 572–583 (2015). [DOI] [PubMed] [Google Scholar]
- 61.Harmon L. J., Harrison S., Species diversity is dynamic and unbounded at local and continental scales. Am. Nat. 185, 584–593 (2015). [DOI] [PubMed] [Google Scholar]
- 62.Marshall C. R., Quental T. B., The uncertain role of diversity dependence in species diversification and the need to incorporate time-varying carrying capacities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 371, 20150217 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Larcombe M. J., Jordan G. J., Bryant D., Higgins S. I., The dimensionality of niche space allows bounded and unbounded processes to jointly influence diversification. Nat. Commun. 9, 4258 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raup D. M., Extinction: Bad Genes or Bad Luck? (WW. Norton & Co., 1991). [PubMed] [Google Scholar]
- 65.Morlon H., Parsons T. L., Plotkin J. B., Reconciling molecular phylogenies with the fossil record. Proc. Natl. Acad. Sci. U.S.A. 108, 16327–16332 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stadler T., Mammalian phylogeny reveals recent diversification rate shifts. Proc. Natl. Acad. Sci. U.S.A. 108, 6187–6192 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.May M. R., Höhna S., Moore B. R., A Bayesian approach for detecting the impact of mass-extinction events on molecular phylogenies when rates of lineage diversification may vary. Methods Ecol. Evol. 7, 947–959 (2016). [Google Scholar]
- 68.Foster G. L., Royer D. L., Lunt D. J., Future climate forcing potentially without precedent in the last 420 million years. Nat. Commun. 8, 14845 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prokoph A., Shields G. A., Veizer J., Compilation and time-series analysis of a marine carbonate δ18O, δ13C, 87Sr/86Sr and δ34S database through Earth history. Earth Sci. Rev. 87, 113–133 (2008). [Google Scholar]
- 70.Ramírez-Barahona S., Sauquet H., Magallón S., The delayed and geographically heterogeneous diversification of flowering plant families. Nat. Ecol. Evol. 4, 1232–1238 (2020). [DOI] [PubMed] [Google Scholar]
- 71.Condamine F. L., Rolland J., Morlon H., Macroevolutionary perspectives to environmental change. Ecol. Lett. 16 (suppl. 1), 72–85 (2013). [DOI] [PubMed] [Google Scholar]
- 72.Condamine F. L., Rolland J., Morlon H., Assessing the causes of diversification slowdowns: Temperature-dependent and diversity-dependent models receive equivalent support. Ecol. Lett. 22, 1900–1912 (2019). [DOI] [PubMed] [Google Scholar]
- 73.Pearson P. N., et al. , Extinction and environmental change across the Eocene-Oligocene boundary in Tanzania. Geology 36, 179–182 (2008). [Google Scholar]
- 74.Alfaro Reyna T., Retana J., Martínez-Vilalta J., Is there a substitution of Pinaceae by Fagaceae in temperate forests at the global scale? Global Planet. Change 166, 41–47 (2018). [Google Scholar]
- 75.Lehtonen S., et al. , Environmentally driven extinction and opportunistic origination explain fern diversification patterns. Sci. Rep. 7, 4831 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lanfear R., et al. , Taller plants have lower rates of molecular evolution. Nat. Commun. 4, 1879 (2013). [DOI] [PubMed] [Google Scholar]
- 77.Prunier J., Verta J. P., MacKay J. J., Conifer genomics and adaptation: At the crossroads of genetic diversity and genome function. New Phytol. 209, 44–62 (2016). [DOI] [PubMed] [Google Scholar]
- 78.Sander P. M., Gee C. T., Hummel J., Clauss M., “Mesozoic plants and dinosaur herbivory” in Plants in Mesozoic Time: Morphological Innovations, Phylogeny, Ecosystems, Gee C. T., Ed. (Indiana University Press, Bloomington, IN, 2010), pp. 331–360. [Google Scholar]
- 79.Pole M., et al. , The rise and demise of Podozamites in east Asia-An extinct conifer life style. Palaeogeogr. Palaeoclimatol. Palaeoecol. 464, 97–109 (2016). [Google Scholar]
- 80.Silvestro D., Salamin N., Antonelli A., Meyer X., Improved estimation of macroevolutionary rates from fossil data using a Bayesian framework. Paleobiology 45, 546–570 (2019). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Phylogenetic trees and fossil occurrence data have been made freely available in a publicly accessible database (https://doi.org/10.6084/m9.figshare.12037593.v1).